SUPPLEMENTARY INFORMATION
|
|
|
- Maryann Preston
- 5 years ago
- Views:
Transcription
1 Data collection Supplementary Table 1 Statistics of data collection, phasing and refinement Native Se-MAD Space group P P Cell dimensions a, b, c (Å) 50.4, 94.2, , 94.2, 116.2,, (º) 90,90,90 90,90,90 Peak Inflection Remote Wavelength (Å) Resolution (Å) 50~3.6 (3.73~3.6) 50~3.8 (3.94~3.8) 50~3.8 (3.94~3.8) 50~3.8 (3.94~3.8) R merge(%) 0.065(0.831) I/ I 32.2(2.2) 31.9(1.8) 29.8(1.4) 31.6(1.8) Completeness (%) 99.7(100) 99.8(100) 99.8(100) 99.8(100) Redundancy Refinement Resolution (Å) 50~3.6 No. reflections 46,786 R work/ R free 0.265/0.289 No. atoms 2660 Protein 2606 Ligand 54 Water 0 B-factors(Å 2 ) Protein Ligand Water R.m.s deviations Bond lengths (Å) Bond angles (º) One crystal was used for each data set listed above. Highest resolution shell is shown in parenthesis. WWW NATURE.COM/NATURE 1
2 Supplementary Figure 1 RibU is the S component of the ECF transporter for riboflavin. a, Riboflavin is bound to RibU from Staphylococcus aureus (S. aureus). WWW NATURE.COM/NATURE 2
3 Shown here is an absorption spectrum of recombinant RibU protein, which indicates the presence of riboflavin. The wavelength scan of riboflavin alone (blue line) is shown as the control. In addition, mass spectrometric analysis of RibU confirmed the presence of riboflavin (data not shown). b, RibU formed a stable complex with the T, A, and A components of the putative ECF-type transporter for riboflavin. RibU and its corresponding T, A, and A proteins, all derived from S. aureus, were co-expressed in E. coli. The Gene ID and predicted molecular weight are: RibU: GI: , 21.1 kda; A: GI: , 32.9 kda; A : GI: , 30.0 kda; T: GI: , 30.8 kda. These four proteins formed a stable complex, which was co-purified by three sequential steps: affinity chromatography, anion exchange, and gel filtration. Shown here is the gel filtration chromatogram of the quaternary complex (indicated by the red line). The peak fractions from gel filtration were visualized on SDS-PAGE by coomassie staining. The protein bands corresponding to A, A, and T components were excised from the gel and subjected to complete proteolysis by trypsin. The trypsinized fragments were separated by HPLC and analyzed by mass spectroscopy. 36, 29, and 16 peptide fragments exactly matched the sequences of A, A, and T, respectively (data not shown). These results unambiguously confirmed the identity of the components. The A and A components could be co-expressed and co-purified by affinity chromatography and gel filtration (indicated by the blue line). See Method for details. c, The presence of all four components (RibU, T, A, and A, all from S. aureus) allowed the riboflavin-auxotrophic E. coli mutant strain BSV11 to grow in LB without added riboflavin. By contrast, the individual presence of RibU, T, RibU+T, or A+A, failed to support the growth of the mutant E. coli in LB without added riboflavin. An equal volume of the culture (10 μl) was dispensed onto the LB plates, occupying the upper half of each plate. The lower half of each plate was used WWW NATURE.COM/NATURE 3
4 to streak the culture from the upper half. The plates were incubated at 37 o C overnight. See the Method for details. Similar results were obtained for the E. coli mutant strain BSV13. BSV11 and BSV13 are E. coli mutant strains that have lost the ability to synthesize riboflavin de novo. WWW NATURE.COM/NATURE 4
5 Supplementary Figure 2 Electron density maps of RibU. a, A stereo view of the anomalous density map, contoured at 4.0, in each asymmetric unit. The four strongest peaks, each above 5.0, correspond to Met20 and Met123 in the two RibU molecules. The other two peaks shown, each above 3.0 _, correspond to Met9 and Met79 in one of the two RibU molecules. b, A stereo view of the 2Fo-Fc electron density map, contoured at 1.0, in each asymmetric unit. The protein main chain is colored yellow. c, A stereo view of the 2Fo-Fc electron density map, contoured at 1.0, around TM1 and TM2. d, A stereo view of the 2Fo-Fc electron density map, contoured at 1.0, around TM3 and TM4. e, A stereo view of the 2Fo-Fc electron density map, contoured at 1.0, around TM5 and TM6. f-g, Stereo views of the WWW NATURE.COM/NATURE 5
6 OMIT electron density map, contoured at 2.5, around the modeled riboflavin molecules in the two RibU molecules. WWW NATURE.COM/NATURE 6
7 Supplementary Figure 3 Structure of RibU in one asymmetric unit. a, Three mutually perpendicular views of the RibU homo-dimer in one asymmetric unit. The two molecules are colored blue and green, with their N- and C-termini labeled and the bound riboflavin molecules shown in yellow. b, Surface electrostatic potential of the RibU homo-dimer in one asymmetric unit. The left, middle, and right panels correspond to those in panel a. If the RibU dimer were biologically relevant, the membrane-spanning distance was only approximately 20 Å and patches of highly charged surface would be buried within the lipid membrane (middle panel). These features do not support the scenario that RibU may form a homo-dimer in lipid membrane as observed in the crystals. WWW NATURE.COM/NATURE 7
8 Supplementary Figure 4 Structure of a RibU molecule. a, Four mutually perpendicular views of the RibU molecule. The N- and C-termini are colored blue and red, respectively. The bound riboflavin molecule is shown in ball-and-stick. b, Surface electrostatic potential of the RibU molecule. The four views correspond to those in panel a. The outer surface of the RibU cylinder is predominantly hydrophobic, consistent with the notion that this surface may be in contact with the non-polar interior of the lipid bilayer. By contrast, both ends of the cylinder-shaped RibU are highly charged. WWW NATURE.COM/NATURE 8
9 Supplementary Figure 5 Sequence alignment of RibU from representative bacterial species. The amino acid sequences of RibU homologs from 8 bacterial species are aligned, with the secondary structural elements indicated above the sequences. Invariant residues are highlighted in red whereas conserved amino acids are boxed. Residues that may be hydrogen-bonded to riboflavin through side chain and main chain atoms are denoted by magenta and green triangles, respectively. Residue that may bind riboflavin through van der Waals interactions are identified by blue squares. Positively charged amino acids in L4 loop and the C-termini are shaded green. The 17 amino acids in the L1 loop are shaded blue. The RibU homologs are from Staphylococcus aureus (GI: ), Lactococcus lactis (GI: ), Clostridium acetobutylicum (GI: ), Streptococcus pyogenes (GI: ), Leuconostoc mesenteroides (GI: ), Enterococcus faecalis (GI: ), Pediococcus pentosaceus (GI: ), Symbiobacterium thermophilum (GI: ). WWW NATURE.COM/NATURE 9
10 Supplementary Figure 6 Structure of RibU is dissimilar to those of the transmembrane domains of the ABC transporters. Shown here is a structural comparison of RibU with three classes of ABC transporters. The N- and C-termini are indicated. Two perpendicular views of each structure are shown in the upper and bottom panels. WWW NATURE.COM/NATURE 10
11 Supplementary Figure 7 Structural comparison of RibU with hits of the DALI search. a, A stereo view of the structural overlay of RibU (blue) with particulate methane monooxygenase (pmmo) from Methylosinus trichosporium OB3B (colored orange, PDB accession code 3CHX). b, A stereo view of the structural overlay of RibU (blue) with pmmo from Methylococcus capsulatus (colored magenta, PDB accession code 1YEW). C, Structures of RibU and pmmo. The N- and C-termini are indicated. WWW NATURE.COM/NATURE 11
12 Supplementary Figure 8 Mapping of conserved amino acids onto the structure of RibU. Based on the sequence alignment of 12 RibU homologs, residues that are conserved in 7-9 and bacterial species are colored yellow and orange, respectively. Invariant residues are highlighted in red. A ribbon diagram and a surface representation are shown. WWW NATURE.COM/NATURE 12
13 Supplementary Figure 9 Features of the riboflavin-binding pocket. a, A stereo view of the MAD experimental electron density map for riboflavin. The electron density map, colored magenta, is contoured at 0.7. The final model of riboflavin is shown here as a reference. b, Features of the riboflavin-binding pocket. All buried amino acids (left panels) are divided into two groups: non-polar (middle panels) and polar/charged (right panels). The polar/charged amino acids are predominantly located in a small region of RibU (identified by magenta circle in the right panel), around the L1 loop and TM3. By contrast, the hydrophobic residues are mainly located in TM4-6. Altogether, the L1 loop and the N-terminal portion of TM4 have a high density of polar and charged amino acids in the substrate-binding pocket, whereas the C- terminal portion of TM5 and the N-terminal portion of TM6 are predominantly WWW NATURE.COM/NATURE 13
14 hydrophobic. These structural features only support one way of orienting riboflavin into the binding pocket. WWW NATURE.COM/NATURE 14
15 Supplementary Figure 10 Riboflavin is recognized by conserved amino acids from L1 and TM 4-6. Detailed interactions are indicated in the two stereo panels. Residues in L1, TM4, TM5, and TM6 are shown in green, cyan, blue, and magenta, respectively. Hydrogen bonds are represented by red, dashed lines. WWW NATURE.COM/NATURE 15
16 Supplementary Figure 11 FMN, but not FAD, can be modeled into RibU. a, A slice of the riboflavin-bound RibU is shown for comparison. The surface of RibU is represented by blue mesh. The main chain of RibU is shown in blue ribbon. b, A close-up view of RibU with riboflavin replaced by FMN. The model was subjected to rigid body refinement. As can be seen, the extra phosphate group can be accommodated. c, FAD cannot be modeled into RibU. Modeling the adenine dinucleotide portion of FAD requires major structural rearrangements in RibU. WWW NATURE.COM/NATURE 16
17 Supplementary Figure 12 A working model of RibU. In this model, the L1 loop is predicted to regulate substrate binding: it remains open in the apo-transporter and closes down upon binding to substrate in the periplasm. TM1-3 are thought to move away from TM4-6, likely driven by ATP hydrolysis of the A component, and allows the substrate to be released into the cytoplasm. WWW NATURE.COM/NATURE 17
18 Supplementary Figure 13 The S components of ECF transporters are predicted WWW NATURE.COM/NATURE 18
19 to contain 6 TMs. The amino acid sequences of 17 ECF transporters are aligned with those of two RibU proteins from Staphylococcus aureus and Bacillus subtilis. Of the 17 transporters, 4 are specific for folate (FolT, vitamin B 9 ), 7 for thiamine precursor (HmpT, vitamin B 1 ), and 6 for cobalamin precursor (CblT, vitamin B 12 ). The corresponding secondary structural elements are shown for RibU. The conserved amino acids are highlighted in yellow, and the extent of sequence conservation is shown above the sequences by color-coded vertical bars. The two RibU proteins are from Staphylococcus aureus (RibU-Sa, GI: ) and Bacillus subtilis (RibU- Bs, GI: ). The 4 folate transporters are from Thermoanaerobacter tengcongensis (FolT-Tt, GI: ), Enterococcus faecalis (FolT-Ef, GI: ), Lactobacillus gasseri (FolT-Lg, GI: ), and Pediococcus pentosaceus (FolT-Pp, GI: ). The 7 thiamine transporters are from Thermoanaerobacter tengcongensis (HmpT-Tt, GI: ), Alkaliphilus metalliredigens (HmpT-Am, GI: ), Bacillus sp. B14905 (HmpT-Bb, GI: ), Clostridium acetobutylicum (HmpT-Ca, GI: ), Enterococcus faecalis (HmpT-Ef,GI: ), Lactococcus lactis (HmpT-Ll, GI: ), and Streptococcus pyogenes (HmpT-Sy, GI: ). The 6 cobalamin precursor transporters are from Listeria monocytogenes (CblT-Lm, GI: ), Alkaliphilus metalliredigens (CblT-Am, GI: ), Bacillus sp. B14905 (CblT-Bb, GI: ), Clostridium botulinum (CblT-Cb, GI: ), Desulfitobacterium hafniense Y51 (CblT-Ds, GI: ), and Geobacillus thermodenitrificans (CblT- Gt, GI: ). WWW NATURE.COM/NATURE 19
20 Supplementary Figure 14 Alignment of ligand-specific ECF transporters reveals WWW NATURE.COM/NATURE 20
21 candidate sequences that are involved in ligand binding. a, Alignment of the S components of five folate transporters. The predicted secondary structural elements are shown above the sequences. The conserved amino acids are highlighted in yellow, and the extent of sequence conservation is indicated above the sequences by colorcoded vertical bars. The amino acid sequences that correspond to those of the riboflavin-binding site in RibU are indicated by thick magenta lines below the sequences. The five folate transporters are from Alkaliphilus metalliredigens (FolT- Am, GI: ), Enterococcus faecalis (FolT-Ef, GI: ), Lactobacillus gasseri (FolT-Lg, GI: ), Pediococcus pentosaceus (FolT-Pp, GI: ), and Thermoanaerobacter tengcongensis (FolT-Tt, GI: ). b, Alignment of the S component of eight cobalamin precursor transporters. The eight cobalamin precursor transporters are from Alkaliphilus metalliredigens (CblT-Am, GI: ), Bacillus sp. B14905 (CblT-Bb, GI: ), Clostridium botulinum (CblT-Cb, GI: ), Desulfitobacterium hafniense Y51 (CblT-Ds, GI: ), Geobacillus thermodenitrificans (CblT-Gt, GI: ), Listeria monocytogenes (CblT-Lm, GI: ), Moorella thermoacetica (CblT-Mt, GI: ), and Thermoanaerobacter tengcongensis (CblT-Tt, GI: ). WWW NATURE.COM/NATURE 21
22 Supplementary Figure 15 The S components of the group II ECF transporters within the same bacterial species share some sequence features. Sequences of the predicted L4 loop and the C-terminus of the S components from Clostridium tetani and Streptococcus mutans are shown here. Clostridium tetani and Streptococcus mutans were chosen because, compared to other species, there are a large number of group II S components that share the same A-T module. The positively charged amino acids are colored green. WWW NATURE.COM/NATURE 22
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary information
 Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
SUPPLEMENTARY INFORMATION
 Table of Contents Page Supplementary Table 1. Diffraction data collection statistics 2 Supplementary Table 2. Crystallographic refinement statistics 3 Supplementary Fig. 1. casic1mfc packing in the R3
Table of Contents Page Supplementary Table 1. Diffraction data collection statistics 2 Supplementary Table 2. Crystallographic refinement statistics 3 Supplementary Fig. 1. casic1mfc packing in the R3
SUPPLEMENTARY INFORMATION
 Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1: The HpUreI crystal used for collection of native diffraction data. The crystal belongs to spacegroup P4 2 2 1 2 and has an approximate maximal dimension of 0.25 mm. Supplementary
Supplementary Figure 1: The HpUreI crystal used for collection of native diffraction data. The crystal belongs to spacegroup P4 2 2 1 2 and has an approximate maximal dimension of 0.25 mm. Supplementary
SUPPLEMENTARY INFORMATION
 Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Chemical structure of LPS and LPS biogenesis in Gram-negative bacteria. a. Chemical structure of LPS. LPS molecule consists of Lipid A, core oligosaccharide and O-antigen. The polar
Supplementary Figure 1 Chemical structure of LPS and LPS biogenesis in Gram-negative bacteria. a. Chemical structure of LPS. LPS molecule consists of Lipid A, core oligosaccharide and O-antigen. The polar
SUPPLEMENTARY INFORMATION
 Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
SI Text S1 Solution Scattering Data Collection and Analysis. SI references
 SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Supporting Information
 Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supplementary Information. Structural basis for precursor protein-directed ribosomal peptide macrocyclization
 Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
SUPPLEMENTARY INFORMATION
 www.nature.com/nature 1 Figure S1 Sequence alignment. a Structure based alignment of the plgic of E. chrysanthemi (ELIC), the acetylcholine binding protein from the snail Lymnea stagnalis (AchBP, PDB code
www.nature.com/nature 1 Figure S1 Sequence alignment. a Structure based alignment of the plgic of E. chrysanthemi (ELIC), the acetylcholine binding protein from the snail Lymnea stagnalis (AchBP, PDB code
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11744 Supplementary Table 1. Crystallographic data collection and refinement statistics. Wild-type Se-Met-BcsA-B SmCl 3 -soaked EMTS-soaked Data collection Space
SUPPLEMENTARY INFORMATION doi:10.1038/nature11744 Supplementary Table 1. Crystallographic data collection and refinement statistics. Wild-type Se-Met-BcsA-B SmCl 3 -soaked EMTS-soaked Data collection Space
SUPPLEMENTARY INFORMATION
 Dph2 SeMet (iron-free) # Dph2 (iron-free) Dph2-[4Fe-4S] Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell dimensions a, b, c (Å) 58.26, 82.08, 160.42 58.74, 81.87, 160.01 55.70, 80.53,
Dph2 SeMet (iron-free) # Dph2 (iron-free) Dph2-[4Fe-4S] Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell dimensions a, b, c (Å) 58.26, 82.08, 160.42 58.74, 81.87, 160.01 55.70, 80.53,
Cryo-EM data collection, refinement and validation statistics
 1 Table S1 Cryo-EM data collection, refinement and validation statistics Data collection and processing CPSF-160 WDR33 (EMDB-7114) (PDB 6BM0) CPSF-160 WDR33 (EMDB-7113) (PDB 6BLY) CPSF-160 WDR33 CPSF-30
1 Table S1 Cryo-EM data collection, refinement and validation statistics Data collection and processing CPSF-160 WDR33 (EMDB-7114) (PDB 6BM0) CPSF-160 WDR33 (EMDB-7113) (PDB 6BLY) CPSF-160 WDR33 CPSF-30
Transmembrane Domains (TMDs) of ABC transporters
 Transmembrane Domains (TMDs) of ABC transporters Most ABC transporters contain heterodimeric TMDs (e.g. HisMQ, MalFG) TMDs show only limited sequence homology (high diversity) High degree of conservation
Transmembrane Domains (TMDs) of ABC transporters Most ABC transporters contain heterodimeric TMDs (e.g. HisMQ, MalFG) TMDs show only limited sequence homology (high diversity) High degree of conservation
SUPPLEMENTARY INFORMATION. doi: /nature07461
 Figure S1 Electrophysiology. a ph-activation of. Two-electrode voltage clamp recordings of Xenopus oocytes expressing in comparison to waterinjected oocytes. Currents were recorded at 40 mv. The ph of
Figure S1 Electrophysiology. a ph-activation of. Two-electrode voltage clamp recordings of Xenopus oocytes expressing in comparison to waterinjected oocytes. Currents were recorded at 40 mv. The ph of
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS
 Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS CNS input file generatemetal.inp: remarks file generate/generatemetal.inp
Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS CNS input file generatemetal.inp: remarks file generate/generatemetal.inp
Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits. AhpC and AhpF from Escherichia coli
 Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits AhpC and AhpF from Escherichia coli Phat Vinh Dip 1,#, Neelagandan Kamariah 2,#, Malathy Sony Subramanian Manimekalai
Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits AhpC and AhpF from Escherichia coli Phat Vinh Dip 1,#, Neelagandan Kamariah 2,#, Malathy Sony Subramanian Manimekalai
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
SUPPLEMENTARY INFORMATION
 Supplementary Table S1 Kinetic Analyses of the AMSH-LP mutants AMSH-LP K M (μm) k cat x 10-3 (s -1 ) WT 71.8 ± 6.3 860 ± 65.4 T353A 76.8 ± 11.7 46.3 ± 3.7 F355A 58.9 ± 10.4 5.33 ± 0.30 proximal S358A 75.1
Supplementary Table S1 Kinetic Analyses of the AMSH-LP mutants AMSH-LP K M (μm) k cat x 10-3 (s -1 ) WT 71.8 ± 6.3 860 ± 65.4 T353A 76.8 ± 11.7 46.3 ± 3.7 F355A 58.9 ± 10.4 5.33 ± 0.30 proximal S358A 75.1
Supplementary Information. The protease GtgE from Salmonella exclusively targets. inactive Rab GTPases
 Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08 Å resolution: comparison with the Azotobacter vinelandii MoFe protein
 Acta Cryst. (2015). D71, 274-282, doi:10.1107/s1399004714025243 Supporting information Volume 71 (2015) Supporting information for article: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08
Acta Cryst. (2015). D71, 274-282, doi:10.1107/s1399004714025243 Supporting information Volume 71 (2015) Supporting information for article: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08
ml. ph 7.5 ph 6.5 ph 5.5 ph 4.5. β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS
 a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
Cks1 CDK1 CDK1 CDK1 CKS1. are ice- lobe. conserved. conserved
 Cks1 d CKS1 Supplementary Figure 1 The -Cks1 crystal lattice. (a) Schematic of the - Cks1 crystal lattice. -Cks1 crystallizes in a lattice that contains c 4 copies of the t - Cks1 dimer in the crystallographic
Cks1 d CKS1 Supplementary Figure 1 The -Cks1 crystal lattice. (a) Schematic of the - Cks1 crystal lattice. -Cks1 crystallizes in a lattice that contains c 4 copies of the t - Cks1 dimer in the crystallographic
CH 3 CH 2 OH +H 2 O CHO. 2e + 2H + + O 2 H 2 O +HCOOH
 2 4 H CH 3 2e + 2H + + 2 H 2 2 H CH 2 H 2e + 2H + + 2 H 2 2 H +H 2 CH 2e + 2H + + 2 H 2 2 H +HCH Supplemental Figure S. The three-step 4DM reaction, each step requires two reducing equivalents from ADPH
2 4 H CH 3 2e + 2H + + 2 H 2 2 H CH 2 H 2e + 2H + + 2 H 2 2 H +H 2 CH 2e + 2H + + 2 H 2 2 H +HCH Supplemental Figure S. The three-step 4DM reaction, each step requires two reducing equivalents from ADPH
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
It s really this simple.
 Background Light harvesting complexes exist to facilitate and maximize the absorption capacity of the reaction centers (RC) as well as PSI and PSII Purple bacteria utilize these functions by having an
Background Light harvesting complexes exist to facilitate and maximize the absorption capacity of the reaction centers (RC) as well as PSI and PSII Purple bacteria utilize these functions by having an
Structure and mechanism of the ECF-type ABC transporter for thiamin Erkens, Guus Bjorn
 University of Groningen Structure and mechanism of the ECF-type ABC transporter for thiamin Erkens, Guus Bjorn IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
University of Groningen Structure and mechanism of the ECF-type ABC transporter for thiamin Erkens, Guus Bjorn IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
Structure and RNA-binding properties. of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex
 Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN
 THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
Supplemental Data SUPPLEMENTAL FIGURES
 Supplemental Data CRYSTAL STRUCTURE OF THE MG.ADP-INHIBITED STATE OF THE YEAST F 1 C 10 ATP SYNTHASE Alain Dautant*, Jean Velours and Marie-France Giraud* From Université Bordeaux 2, CNRS; Institut de
Supplemental Data CRYSTAL STRUCTURE OF THE MG.ADP-INHIBITED STATE OF THE YEAST F 1 C 10 ATP SYNTHASE Alain Dautant*, Jean Velours and Marie-France Giraud* From Université Bordeaux 2, CNRS; Institut de
SUPPLEMENTARY INFORMATION
 doi:10.108/nature11899 Supplementar Table 1. Data collection and refinement statistics (+TPMP, native) (-TPMP, native) (+TPMP, recombinant) (MgCl ) (MgSO ) Data collection Space group C P 1 C P 1 1 P 1
doi:10.108/nature11899 Supplementar Table 1. Data collection and refinement statistics (+TPMP, native) (-TPMP, native) (+TPMP, recombinant) (MgCl ) (MgSO ) Data collection Space group C P 1 C P 1 1 P 1
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R)
 Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
Potassium channel gating and structure!
 Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate
 SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
Review. Membrane proteins. Membrane transport
 Quiz 1 For problem set 11 Q1, you need the equation for the average lateral distance transversed (s) of a molecule in the membrane with respect to the diffusion constant (D) and time (t). s = (4 D t) 1/2
Quiz 1 For problem set 11 Q1, you need the equation for the average lateral distance transversed (s) of a molecule in the membrane with respect to the diffusion constant (D) and time (t). s = (4 D t) 1/2
Diphthamide biosynthesis requires a radical iron-sulfur enzyme. Pennsylvania State University, University Park, Pennsylvania 16802, USA
 Diphthamide biosynthesis requires a radical iron-sulfur enzyme Yang Zhang, 1,4 Xuling Zhu, 1,4 Andrew T. Torelli, 1 Michael Lee, 2 Boris Dzikovski, 1 Rachel Koralewski, 1 Eileen Wang, 1 Jack Freed, 1 Carsten
Diphthamide biosynthesis requires a radical iron-sulfur enzyme Yang Zhang, 1,4 Xuling Zhu, 1,4 Andrew T. Torelli, 1 Michael Lee, 2 Boris Dzikovski, 1 Rachel Koralewski, 1 Eileen Wang, 1 Jack Freed, 1 Carsten
Acta Cryst. (2014). D70, doi: /s
 Acta Cryst. (2014). D70, doi:10.1107/s1399004714021166 Supporting information Volume 70 (2014) Supporting information for article: Elucidation of the bicarbonate binding site and insights into the carboxylation
Acta Cryst. (2014). D70, doi:10.1107/s1399004714021166 Supporting information Volume 70 (2014) Supporting information for article: Elucidation of the bicarbonate binding site and insights into the carboxylation
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1. Experimental approach for enhancement of unbiased Fo Fc maps.
 Supplementary Figure 1 Experimental approach for enhancement of unbiased Fo Fc maps. a, c, Unbiased Fo-Fc maps of the Tth 70S post-catalysis complex at 2.55 Å resolution with (a) or without (c) bulk solvent
Supplementary Figure 1 Experimental approach for enhancement of unbiased Fo Fc maps. a, c, Unbiased Fo-Fc maps of the Tth 70S post-catalysis complex at 2.55 Å resolution with (a) or without (c) bulk solvent
Supporting Information
 Supporting Information Wilson et al. 10.1073/pnas.0804276105 Fig. S1. Sites of oxazolidinone resistance mutations in bacteria and archaea. (a) Secondary structure of the peptidyltransferase ring of the
Supporting Information Wilson et al. 10.1073/pnas.0804276105 Fig. S1. Sites of oxazolidinone resistance mutations in bacteria and archaea. (a) Secondary structure of the peptidyltransferase ring of the
for Molecular Biology and Neuroscience and Institute of Medical Microbiology, Rikshospitalet-Radiumhospitalet
 SUPPLEMENTARY INFORMATION TO Structural basis for enzymatic excision of N -methyladenine and N 3 -methylcytosine from DNA Ingar Leiros,5, Marivi P. Nabong 2,3,5, Kristin Grøsvik 3, Jeanette Ringvoll 2,
SUPPLEMENTARY INFORMATION TO Structural basis for enzymatic excision of N -methyladenine and N 3 -methylcytosine from DNA Ingar Leiros,5, Marivi P. Nabong 2,3,5, Kristin Grøsvik 3, Jeanette Ringvoll 2,
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Cryo-EM structure and model of the C. thermophilum 90S preribosome. a, Gold standard FSC curve showing the average resolution of the 90S preribosome masked and unmasked (left). FSC
Supplementary Figure 1 Cryo-EM structure and model of the C. thermophilum 90S preribosome. a, Gold standard FSC curve showing the average resolution of the 90S preribosome masked and unmasked (left). FSC
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10458 Active Site Remodeling in the Bifunctional Fructose-1,6- bisphosphate aldolase/phosphatase Juan Du, Rafael F. Say, Wei Lü, Georg Fuchs & Oliver Einsle SUPPLEMENTARY FIGURES Figure
doi:10.1038/nature10458 Active Site Remodeling in the Bifunctional Fructose-1,6- bisphosphate aldolase/phosphatase Juan Du, Rafael F. Say, Wei Lü, Georg Fuchs & Oliver Einsle SUPPLEMENTARY FIGURES Figure
Supplementary Information
 Supplementary Information The direct role of selenocysteine in [NiFeSe] hydrogenase maturation and catalysis Marta C. Marques a, Cristina Tapia b, Oscar Gutiérrez-Sanz b, Ana Raquel Ramos a, Kimberly L.
Supplementary Information The direct role of selenocysteine in [NiFeSe] hydrogenase maturation and catalysis Marta C. Marques a, Cristina Tapia b, Oscar Gutiérrez-Sanz b, Ana Raquel Ramos a, Kimberly L.
genome analysis, amino acid biosynthesis and transport, T-box, bacteria
 236 Part 5 Chapter # EVOLUTIONAL AND FUNCTIONAL ANALYSIS OF T-BOX REGULON IN BACTERIA: IDENTIFICATION OF NEW GENES INVOLVED IN AMINO ACID METABOLISM Vitreschak A.G. *1, Lyubetsky V.A. 1, Gelfand M.S. 1
236 Part 5 Chapter # EVOLUTIONAL AND FUNCTIONAL ANALYSIS OF T-BOX REGULON IN BACTERIA: IDENTIFICATION OF NEW GENES INVOLVED IN AMINO ACID METABOLISM Vitreschak A.G. *1, Lyubetsky V.A. 1, Gelfand M.S. 1
Structural insights into Aspergillus fumigatus lectin specificity - AFL binding sites are functionally non-equivalent
 Acta Cryst. (2015). D71, doi:10.1107/s1399004714026595 Supporting information Volume 71 (2015) Supporting information for article: Structural insights into Aspergillus fumigatus lectin specificity - AFL
Acta Cryst. (2015). D71, doi:10.1107/s1399004714026595 Supporting information Volume 71 (2015) Supporting information for article: Structural insights into Aspergillus fumigatus lectin specificity - AFL
Supplementary Information for
 Supplementary Information for Structural basis for the inhibition of Mycobacterium tuberculosis L,D-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains Hyoun Sook Kim
Supplementary Information for Structural basis for the inhibition of Mycobacterium tuberculosis L,D-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains Hyoun Sook Kim
Supplementary figure 1. Comparison of unbound ogm-csf and ogm-csf as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine
 Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
Supplemental Information. Molecular Basis of Spectral Diversity. in Near-Infrared Phytochrome-Based. Fluorescent Proteins
 Chemistry & Biology, Volume 22 Supplemental Information Molecular Basis of Spectral Diversity in Near-Infrared Phytochrome-Based Fluorescent Proteins Daria M. Shcherbakova, Mikhail Baloban, Sergei Pletnev,
Chemistry & Biology, Volume 22 Supplemental Information Molecular Basis of Spectral Diversity in Near-Infrared Phytochrome-Based Fluorescent Proteins Daria M. Shcherbakova, Mikhail Baloban, Sergei Pletnev,
Introduction to Comparative Protein Modeling. Chapter 4 Part I
 Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
The structure of vanadium nitrogenase reveals an unusual bridging ligand
 SUPPLEMENTARY INFORMATION The structure of vanadium nitrogenase reveals an unusual bridging ligand Daniel Sippel and Oliver Einsle Lehrstuhl Biochemie, Institut für Biochemie, Albert-Ludwigs-Universität
SUPPLEMENTARY INFORMATION The structure of vanadium nitrogenase reveals an unusual bridging ligand Daniel Sippel and Oliver Einsle Lehrstuhl Biochemie, Institut für Biochemie, Albert-Ludwigs-Universität
SUPPLEMENTARY INFORMATION
 Parallel Allostery by camp and PDE Coordinates Activation and Termination Phases in camp Signaling Srinath Krishnamurthy, 1 Nikhil Kumar Tulsian, 1 Arun Chandramohan, 1 and Ganesh S. Anand 1, * 1 Department
Parallel Allostery by camp and PDE Coordinates Activation and Termination Phases in camp Signaling Srinath Krishnamurthy, 1 Nikhil Kumar Tulsian, 1 Arun Chandramohan, 1 and Ganesh S. Anand 1, * 1 Department
Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached
 Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached to HpUreI. Urea hydrolysis products 2NH 3 and 1CO 2
Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached to HpUreI. Urea hydrolysis products 2NH 3 and 1CO 2
Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b).
 Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b). Crystal contacts at B-C loop are magnified and stereo view of A-weighted
Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b). Crystal contacts at B-C loop are magnified and stereo view of A-weighted
Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps
 Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Crystal Structure of Fibroblast Growth Factor 9 (FGF9) Reveals Regions. Implicated in Dimerization and Autoinhibition
 JBC Papers in Press. Published on November 1, 2000 as Manuscript M006502200 Crystal Structure of Fibroblast Growth Factor 9 (FGF9) Reveals Regions Implicated in Dimerization and Autoinhibition 1 Copyright
JBC Papers in Press. Published on November 1, 2000 as Manuscript M006502200 Crystal Structure of Fibroblast Growth Factor 9 (FGF9) Reveals Regions Implicated in Dimerization and Autoinhibition 1 Copyright
SUPPLEMENTARY INFORMATION
 SUPPLMTARY IFORMATIO a doi:10.108/nature10402 b 100 nm 100 nm c SAXS Model d ulers assigned to reference- Back-projected free class averages class averages Refinement against single particles Reconstructed
SUPPLMTARY IFORMATIO a doi:10.108/nature10402 b 100 nm 100 nm c SAXS Model d ulers assigned to reference- Back-projected free class averages class averages Refinement against single particles Reconstructed
The Fic protein Doc uses an inverted substrate to phosphorylate and. inactivate EF-Tu
 The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu Daniel Castro-Roa 1, Abel Garcia-Pino 2,3 *, Steven De Gieter 2,3, Nico A.J. van Nuland 2,3, Remy Loris 2,3, Nikolay
The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu Daniel Castro-Roa 1, Abel Garcia-Pino 2,3 *, Steven De Gieter 2,3, Nico A.J. van Nuland 2,3, Remy Loris 2,3, Nikolay
Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis
 Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis Xiuying Liu 1,3, Yan Yin 1,2,3, Jinjun Wu 1 and Zhenfeng Liu 1 1 National Laboratory of Biomacromolecules,
Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis Xiuying Liu 1,3, Yan Yin 1,2,3, Jinjun Wu 1 and Zhenfeng Liu 1 1 National Laboratory of Biomacromolecules,
Kang, Lin-Woo, Ph.D. Professor Department of Biological Sciences Konkuk University Seoul, Korea nd Semester
 Kang, Lin-Woo, Ph.D. Professor Department of Biological Sciences Konkuk University Seoul, Korea 2018. 2 nd Semester Absorbance Assay (280 nm) Considerations for use Absorbance assays are fast and
Kang, Lin-Woo, Ph.D. Professor Department of Biological Sciences Konkuk University Seoul, Korea 2018. 2 nd Semester Absorbance Assay (280 nm) Considerations for use Absorbance assays are fast and
Acta Crystallographica Section D
 Supporting information Acta Crystallographica Section D Volume 70 (2014) Supporting information for article: Structural characterization of the virulence factor Nuclease A from Streptococcus agalactiae
Supporting information Acta Crystallographica Section D Volume 70 (2014) Supporting information for article: Structural characterization of the virulence factor Nuclease A from Streptococcus agalactiae
Supplementary Information
 Supplementary Information Structural analysis of leader peptide binding enables leaderfree cyanobactin processing Jesko Koehnke 1,2, Greg Mann 1,2, Andrew F Bent 1,2, Hannes Ludewig 1, Sally Shirran 1,
Supplementary Information Structural analysis of leader peptide binding enables leaderfree cyanobactin processing Jesko Koehnke 1,2, Greg Mann 1,2, Andrew F Bent 1,2, Hannes Ludewig 1, Sally Shirran 1,
Targeting protein-protein interactions: A hot topic in drug discovery
 Michal Kamenicky; Maria Bräuer; Katrin Volk; Kamil Ödner; Christian Klein; Norbert Müller Targeting protein-protein interactions: A hot topic in drug discovery 104 Biomedizin Innovativ patientinnenfokussierte,
Michal Kamenicky; Maria Bräuer; Katrin Volk; Kamil Ödner; Christian Klein; Norbert Müller Targeting protein-protein interactions: A hot topic in drug discovery 104 Biomedizin Innovativ patientinnenfokussierte,
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Plasmid Relevant features Source. W18N_D20N and TrXE-W18N_D20N-anti
 Table S1. E. coli plasmids Plasmid Relevant features Source pdg680 T. reesei XynII AA 2-190 with C-terminal His 6 tag optimized for E. coli expression in pjexpress401 Wan et al. (in press) psbn44d psbn44h
Table S1. E. coli plasmids Plasmid Relevant features Source pdg680 T. reesei XynII AA 2-190 with C-terminal His 6 tag optimized for E. coli expression in pjexpress401 Wan et al. (in press) psbn44d psbn44h
CAP 5510 Lecture 3 Protein Structures
 CAP 5510 Lecture 3 Protein Structures Su-Shing Chen Bioinformatics CISE 8/19/2005 Su-Shing Chen, CISE 1 Protein Conformation 8/19/2005 Su-Shing Chen, CISE 2 Protein Conformational Structures Hydrophobicity
CAP 5510 Lecture 3 Protein Structures Su-Shing Chen Bioinformatics CISE 8/19/2005 Su-Shing Chen, CISE 1 Protein Conformation 8/19/2005 Su-Shing Chen, CISE 2 Protein Conformational Structures Hydrophobicity
Nature Structural and Molecular Biology: doi: /nsmb.2783
 Supplementary Figure 1: Crystallized chimera construct (mhv1cc). (a) Sequence alignment between mhv1cc and other VSDs. These sequences (mhv1cc, Kv1.2 Kv2.1; shaker family voltage gated potassium channel
Supplementary Figure 1: Crystallized chimera construct (mhv1cc). (a) Sequence alignment between mhv1cc and other VSDs. These sequences (mhv1cc, Kv1.2 Kv2.1; shaker family voltage gated potassium channel
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Expanded View Figures
 The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
The structure of a nucleolytic ribozyme that employs a catalytic metal ion. Yijin Liu, Timothy J. Wilson and David M.J. Lilley
 SUPPLEMENTARY INFORMATION The structure of a nucleolytic ribozyme that employs a catalytic metal ion Yijin Liu, Timothy J. Wilson and David M.J. Lilley Cancer Research UK Nucleic Acid Structure Research
SUPPLEMENTARY INFORMATION The structure of a nucleolytic ribozyme that employs a catalytic metal ion Yijin Liu, Timothy J. Wilson and David M.J. Lilley Cancer Research UK Nucleic Acid Structure Research
Protein Structure Basics
 Protein Structure Basics Presented by Alison Fraser, Christine Lee, Pradhuman Jhala, Corban Rivera Importance of Proteins Muscle structure depends on protein-protein interactions Transport across membranes
Protein Structure Basics Presented by Alison Fraser, Christine Lee, Pradhuman Jhala, Corban Rivera Importance of Proteins Muscle structure depends on protein-protein interactions Transport across membranes
Supplementary materials. Crystal structure of the carboxyltransferase domain. of acetyl coenzyme A carboxylase. Department of Biological Sciences
 Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Structural basis of PROTAC cooperative recognition for selective protein degradation
 SUPPLEMENTARY INFORMATION Structural basis of PROTAC cooperative recognition for selective protein degradation Morgan S. Gadd 1, Andrea Testa 1, Xavier Lucas 1, Kwok-Ho Chan, Wenzhang Chen, Douglas J.
SUPPLEMENTARY INFORMATION Structural basis of PROTAC cooperative recognition for selective protein degradation Morgan S. Gadd 1, Andrea Testa 1, Xavier Lucas 1, Kwok-Ho Chan, Wenzhang Chen, Douglas J.
Nature Structural & Molecular Biology doi: /nsmb Supplementary Figure 1. CRBN binding assay with thalidomide enantiomers.
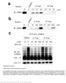 Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
SUPPLEMENTARY INFORMATION
 5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
Structural insights into WcbI, a novel polysaccharide-biosynthesis enzyme
 Volume 1 (2014) Supporting information for article: Structural insights into WcbI, a novel polysaccharide-biosynthesis enzyme Mirella Vivoli, Emily Ayres, Edward Beaumont, Michail N. Isupov and Nicholas
Volume 1 (2014) Supporting information for article: Structural insights into WcbI, a novel polysaccharide-biosynthesis enzyme Mirella Vivoli, Emily Ayres, Edward Beaumont, Michail N. Isupov and Nicholas
Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase Cwc27
 Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Crystal lattice Real Space. Reflections Reciprocal Space. I. Solving Phases II. Model Building for CHEM 645. Purified Protein. Build model.
 I. Solving Phases II. Model Building for CHEM 645 Purified Protein Solve Phase Build model and refine Crystal lattice Real Space Reflections Reciprocal Space ρ (x, y, z) pronounced rho F hkl 2 I F (h,
I. Solving Phases II. Model Building for CHEM 645 Purified Protein Solve Phase Build model and refine Crystal lattice Real Space Reflections Reciprocal Space ρ (x, y, z) pronounced rho F hkl 2 I F (h,
Sunhats for plants. How plants detect dangerous ultraviolet rays
 Sunhats for plants How plants detect dangerous ultraviolet rays Anyone who has ever suffered sunburn will know about the effects of too much ultraviolet (UV) radiation, in particular UV-B (from 280-315
Sunhats for plants How plants detect dangerous ultraviolet rays Anyone who has ever suffered sunburn will know about the effects of too much ultraviolet (UV) radiation, in particular UV-B (from 280-315
Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6
 Supplementary Information for: Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6 Yawei Dai 1, 2, Markus Seeger 3, Jingwei Weng 4, Song Song 1, 2, Wenning Wang 4, Yan-Wen 1, 2,
Supplementary Information for: Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6 Yawei Dai 1, 2, Markus Seeger 3, Jingwei Weng 4, Song Song 1, 2, Wenning Wang 4, Yan-Wen 1, 2,
of the Guanine Nucleotide Exchange Factor FARP2
 Structure, Volume 21 Supplemental Information Structural Basis for Autoinhibition of the Guanine Nucleotide Exchange Factor FARP2 Xiaojing He, Yi-Chun Kuo, Tyler J. Rosche, and Xuewu Zhang Inventory of
Structure, Volume 21 Supplemental Information Structural Basis for Autoinhibition of the Guanine Nucleotide Exchange Factor FARP2 Xiaojing He, Yi-Chun Kuo, Tyler J. Rosche, and Xuewu Zhang Inventory of
Interpreting and evaluating biological NMR in the literature. Worksheet 1
 Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
