SUPPLEMENTARY INFORMATION
|
|
|
- Easter Foster
- 5 years ago
- Views:
Transcription
1 SUPPLEMENTARY INFORMATION doi: /nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved SP motif residues of XylE, G83A, E153A, R160A, G340A, R341A, and E397A, led to complete loss of function in both cell-based uptake and proteoliposome-based counterflow assays. Gly 83 and Gly 340, the highly conserved residues in the TM2-3 and TM8-9 motifs, are located on the tight turns connecting TMs 2-3 and TMs 8-9, respectively (Supplementary Figs. 1b & 2). Change of either Gly to Ala may cause disturbance of the local structural integrity, thereby leading to complete loss of transport activity. The charged residues, Glu 153 and Arg 160 on the TM4-5 motif, Arg 341 on the TM 8-9 motif, and Glu 397 on the TM10-11 motif, are involved in the inter-domain contacts (Supplementary Fig. 6). The observation that single point mutation of any of these residues led to complete abrogation of transport activity supports our speculation that the interactions between the intracellular domain and the TMs are functionally critical for XylE. Mutation of Arg404 resulted in more than 75% loss of the transport activity in both assays. Structural examination shows that Arg 404 contributes to the local, but not the inter-domain, H-bond network in the present conformation of XylE (Supplementary Fig. 6). Mutation of the residues in the TM12C motif, E465A or K467A, was largely tolerated, probably because these two residues are barely involved in the interaction network (Supplementary Fig. 6). Notably, the XylE variant containing E222A exhibited little change in transport activities. Supporting 1
2 RESEARCH SUPPLEMENTARY INFORMATION this functional observation, Glu222 is located at one side of the structure and not involved in any H-bond formation in the present conformation. The biochemical characterizations suggested that the SP motifs as well as the intracellular helix domain play an essential role for the function of XylE. Given that extensive H-bonds are formed by these structural elements, some of the residues may be involved in proton-symport. On the other hand, single missense mutation of some residues, such as Glu153, Arg160, Arg341, and Glu397, led to complete loss of activity even in the counterflow assay, suggesting that these residues may play an important role in the structural changes required for the completion of a transport cycle. Elucidation of the functional mechanism of the SP motifs awaits further biochemical, biophysical, and simulation analyses. 2
3 SUPPLEMENTARY INFORMATION RESEARCH Supplementary Figures and Legends 3
4 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 XylE is a bacterial homologue of GLUT1-4. a, Phylogenetic tree of GLUT1-4 and their homologues. Multiple sequence alignments were performed with ClustalW and the result was presented with PHYLIP. Sequences of 84 members in the sugar porter family 2.A.1.1 in the Transporter Classification Database (TCDB), excluding 6 redundant sequences for the same proteins, were used for the alignment. b, Sequence alignment of XylE with human GLUT1-4. Secondary structural elements of XylE are indicated above the sequence alignment. Invariant and highly conserved amino acids are shaded yellow and grey, respectively. The conserved SP family signature motifs are underscored with red lines or stars. The residues that are H-bonded to D-xylose are shaded red. The aromatic residues that surround the bound ligands in the crystal structures are shaded orange. Gln 175 and Gly 388 in XylE as well as their corresponding amino acids in GLUT1-4, which are involved in binding to D-glucose, but not D-xylose, are shaded blue. c, Sequence conservation between the E. coli XylE and the human proteins GLUT1-4. The pair-wise sequence comparison was performed with Blastp (NCBI. Basic Local Alignment Search Tool). 4
5 SUPPLEMENTARY INFORMATION RESEARCH Supplementary Figure 2 Sequence alignment of the E. coli XylE with homologs from other organisms. Secondary structural elements of the E. coli XylE are indicated above the sequence alignment. Conserved amino acids are shaded orange, yellow, and grey with decreasing degree of conservations. The conserved SP family signature motifs are underscored with red lines or dots. The listed XylE homologs, from top to bottom, are from Escherichia coli O157:H7 str. EDL933, GI: ; Cyanothece sp. PCC 7822, GI: ; Saccharomyces cerevisiae EC1118, GI: ; Plasmodium knowlesi strain H, GI: ; Arabidopsis thaliana, GI: ; and GLUT1 of Homo sapiens, GI: The sequences were aligned with ClustalW. 5
6 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 3 Recombinant XylE binds to and transports D-xylose. a, ITC titration for WT XylE with 10 mm D-xylose at ph 6.5. The titration signals were fitted by Origin 7. b, XylE transports D-xylose in a ph-dependent manner in the proteoliposome-based counterflow assay. The proteoliposomes were preloaded with 20 mm cold D-xylose at indicated ph. At time point zero, 2 μl concentrated proteoliposomes were diluted into 100 μl KPM buffer (same ph as the proteoliposomes were prepared with) containing 0.83 μm [ 3 H]- D-Xylose. Reaction was stopped at indicated time points by immediately filtering the solution through 0.22 μm filter membranes and washed with ice-cold buffer. The filter was then taken for liquid scintillation counting. All Error bars represent the standard deviation of three independent experiments. c, Transport activity of XylE was measured using a liposome-based counterflow assay at ph 6.5. Details of the experiments can be found in Methods. 6
7 SUPPLEMENTARY INFORMATION RESEARCH Supplementary Figure 4 Structural determination of XylE bound to D-xylose. a, Up to four Hg atoms were found. The anomalous signal for Hg, shown as magenta mesh, was contoured at 5 σ. The refined positions of Hg atoms are represented by grey spheres. b, A stereo view of the 2Fo-Fc electron density for one representative slab is contoured at 1.2 σ. 7
8 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 5 The structure of D-xylose-bound XylE is outward-facing and partly occluded. a, The N- and C-domains of XylE share a similar fold. The two distinct domains can be superimposed with a root-mean-square deviation (rmsd) of 3.11 Å over 153 Cα atoms. b, TM7 and TM10 are bent helices. c, D-xylose resides in the center of the structure, occluded from intracellular side, but solvent accessible from extracellular side of the membrane. A halfway, cut-open view of the surface electrostatic potential is shown here. The van der Waals surface of XylE was calculated with the program HOLE and shown in the right panel, which revealed that the bound D-xylose is accessible to solvent on the extracellular side, but insulated from cytoplasm. The radii of the potential water path are tabulated. The surface electrostatic potential was calculated with PyMol. 8
9 SUPPLEMENTARY INFORMATION RESEARCH Supplementary Figure 6 The polar interactions between the intracellular helices and the TMs of XylE. The residues that mediate the polar interactions between the intracellular helices and the TMs are shown in sticks. The invariant residues between XylE and GLUT1-4 are colored and labeled green. H-bonds are represented by red, dashed lines. A stereoview is shown. IC: intracellular α-helix. 9
10 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 7 D-xylose is coordinated by residues mainly from the C-domain. a, The N- and C-domains are colored green and cyan, respectively. TM7, which is a discontinuous helix, is highlighted in blue. Right panel: D-xylose stands 10
11 SUPPLEMENTARY INFORMATION RESEARCH against the C-domain. D-xylose is shown as white ball-and-sticks. b, D-xylose is bound to XylE by both polar and aromatic residues. A stereoview is shown. Otherwise, the panel is the same as Fig. 2b. c, Tyr298 and Gln415 of XylE contribute to D-xylose coordination through water-mediated H-bonds. The direct H-bonds discussed in the main text are shown as black, dashed lines. Water-mediated H-bonds are highlighted in red. The water molecules are shown as red spheres. A stereoview is shown. Supplementary Figure 8 XylE selectively binds to D-glucose. a, The chemical structures of the sugars examined in the competition assay. b, The binding affinity between D-glucose and XylE was measured with ITC at ph 6.5. The details of the experiment can be found in online Methods. 11
12 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 9 Electron densities for the ligands bound in the three structures. a. The 2Fo-Fc electron density of 6-bromo-6-deoxy-D-glucose (6-Br-D-glucose), shown in cyan mesh, is contoured at 1σ. The anomalous signal for bromide, shown in magenta mesh, is contoured at 5σ. b-d, The omit electron densities, observed in the structures of XylE obtained in the presence of (b) D-xylose, (c) D-glucose, and (d) 6-Br-D-glucose, are contoured at 3σ and shown in blue meshes. The ligands and water molecules, which were not built when calculating the omit densities, are displayed to better illustrate the positions and orientations of the ligands. 12
13 SUPPLEMENTARY INFORMATION RESEARCH Supplementary Figure 10 D-xylose and D-glucose bind to XylE in a similar manner. a, Coordination of D-glucose by XylE. A stereoview is shown. Otherwise, the panel is the same as Fig. 3b. b, D-xylose (black) and D-glucose (silver), which exhibit similar configurations, are located at the same position within XylE. Two perpendicular views are shown. c-d, Comparison of the coordination of D-xylose and D-glucose by XylE. Water-mediated H-bonds are represented by red, dashed lines, and the direct H-bonds discussed in the main text are colored black. The water molecules are shown as red spheres. 13
14 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Table 1. Statistics of data collection and refinement Data D-xylose-bound XylE D-glucose- Hg-SAD Pt-SAD Native bound XylE 6-BrGlcbound XylE Space Group P P P P P Unit Cell (Å) 95.1, , 96.1, , 95.3, , 95.0, , 95.2, Unit Cell ( ) 90, 90, 90 90, 90, 90 90, 90, 90 90, 90, 90 90, 90, 90 Wavelength (Å) Resolution (Å) ( ) ( ) ( ) ( ) ( ) R merge (%) 8.6 (52.7) 6.3 (59.9) 6.5 (61.9) 8.2 (69.0) 8.4 (71.6) I/σ 19.2 (2.6) 21.8 (2.40) 27 (2.5) 24.4 (2.2) 29.3 (3.8) Completeness (%) 98.7 (89.7) (96.8) 98.1 (97.9) 99.6 (97.9) (100.0) Number of 80,336 91, , , ,030 measured reflections Number of unique 19,816 28,438 19,996 17,572 24,981 reflections Redundancy 4.1 (3.2) 3.2 (3.2) 5.4 (4.0) 6.1 (6.0) 7.8 (8.1) Wilson B factor (Å 2 ) R work / R free (%) 22.75/ / /24.64 Number of atoms Overall Protein Ligand Water Other entities Average B value (Å 2 ) Overall Protein Ligand Water Other entities R.m.s. deviations Bonds (Å) Angle ( ) Ramachandran plot statistics (%) Most favourable Additionally allowed Generously allowed Disallowed Values in parentheses are for the highest resolution shell. R merge =Σ h Σ i I h,i -I h /Σ h Σ i I h,i, where I h is the mean intensity of the i observations of symmetry related reflections of h. R=Σ F obs -F calc /ΣF obs, where F calc is the calculated protein structure factor from the atomic model (R free was calculated with 5% of the reflections selected). 14
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
SUPPLEMENTARY INFORMATION
 Table of Contents Page Supplementary Table 1. Diffraction data collection statistics 2 Supplementary Table 2. Crystallographic refinement statistics 3 Supplementary Fig. 1. casic1mfc packing in the R3
Table of Contents Page Supplementary Table 1. Diffraction data collection statistics 2 Supplementary Table 2. Crystallographic refinement statistics 3 Supplementary Fig. 1. casic1mfc packing in the R3
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
SUPPLEMENTARY INFORMATION. doi: /nature07461
 Figure S1 Electrophysiology. a ph-activation of. Two-electrode voltage clamp recordings of Xenopus oocytes expressing in comparison to waterinjected oocytes. Currents were recorded at 40 mv. The ph of
Figure S1 Electrophysiology. a ph-activation of. Two-electrode voltage clamp recordings of Xenopus oocytes expressing in comparison to waterinjected oocytes. Currents were recorded at 40 mv. The ph of
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
SUPPLEMENTARY INFORMATION
 Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
SUPPLEMENTARY INFORMATION
 Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
SUPPLEMENTARY INFORMATION
 www.nature.com/nature 1 Figure S1 Sequence alignment. a Structure based alignment of the plgic of E. chrysanthemi (ELIC), the acetylcholine binding protein from the snail Lymnea stagnalis (AchBP, PDB code
www.nature.com/nature 1 Figure S1 Sequence alignment. a Structure based alignment of the plgic of E. chrysanthemi (ELIC), the acetylcholine binding protein from the snail Lymnea stagnalis (AchBP, PDB code
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11744 Supplementary Table 1. Crystallographic data collection and refinement statistics. Wild-type Se-Met-BcsA-B SmCl 3 -soaked EMTS-soaked Data collection Space
SUPPLEMENTARY INFORMATION doi:10.1038/nature11744 Supplementary Table 1. Crystallographic data collection and refinement statistics. Wild-type Se-Met-BcsA-B SmCl 3 -soaked EMTS-soaked Data collection Space
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
SUPPLEMENTARY INFORMATION
 Dph2 SeMet (iron-free) # Dph2 (iron-free) Dph2-[4Fe-4S] Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell dimensions a, b, c (Å) 58.26, 82.08, 160.42 58.74, 81.87, 160.01 55.70, 80.53,
Dph2 SeMet (iron-free) # Dph2 (iron-free) Dph2-[4Fe-4S] Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell dimensions a, b, c (Å) 58.26, 82.08, 160.42 58.74, 81.87, 160.01 55.70, 80.53,
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary Information. The protease GtgE from Salmonella exclusively targets. inactive Rab GTPases
 Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase Cwc27
 Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Supplementary Figure 3 a. Structural comparison between the two determined structures for the IL 23:MA12 complex. The overall RMSD between the two
 Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Supplementary figure 1. Comparison of unbound ogm-csf and ogm-csf as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine
 Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
SUPPLEMENTARY INFORMATION
 Supplementary Table S1 Kinetic Analyses of the AMSH-LP mutants AMSH-LP K M (μm) k cat x 10-3 (s -1 ) WT 71.8 ± 6.3 860 ± 65.4 T353A 76.8 ± 11.7 46.3 ± 3.7 F355A 58.9 ± 10.4 5.33 ± 0.30 proximal S358A 75.1
Supplementary Table S1 Kinetic Analyses of the AMSH-LP mutants AMSH-LP K M (μm) k cat x 10-3 (s -1 ) WT 71.8 ± 6.3 860 ± 65.4 T353A 76.8 ± 11.7 46.3 ± 3.7 F355A 58.9 ± 10.4 5.33 ± 0.30 proximal S358A 75.1
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08 Å resolution: comparison with the Azotobacter vinelandii MoFe protein
 Acta Cryst. (2015). D71, 274-282, doi:10.1107/s1399004714025243 Supporting information Volume 71 (2015) Supporting information for article: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08
Acta Cryst. (2015). D71, 274-282, doi:10.1107/s1399004714025243 Supporting information Volume 71 (2015) Supporting information for article: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08
SUPPLEMENTARY INFORMATION
 Data collection Supplementary Table 1 Statistics of data collection, phasing and refinement Native Se-MAD Space group P2 1 2 1 2 1 P2 1 2 1 2 1 Cell dimensions a, b, c (Å) 50.4, 94.2, 115.4 49.8, 94.2,
Data collection Supplementary Table 1 Statistics of data collection, phasing and refinement Native Se-MAD Space group P2 1 2 1 2 1 P2 1 2 1 2 1 Cell dimensions a, b, c (Å) 50.4, 94.2, 115.4 49.8, 94.2,
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Diphthamide biosynthesis requires a radical iron-sulfur enzyme. Pennsylvania State University, University Park, Pennsylvania 16802, USA
 Diphthamide biosynthesis requires a radical iron-sulfur enzyme Yang Zhang, 1,4 Xuling Zhu, 1,4 Andrew T. Torelli, 1 Michael Lee, 2 Boris Dzikovski, 1 Rachel Koralewski, 1 Eileen Wang, 1 Jack Freed, 1 Carsten
Diphthamide biosynthesis requires a radical iron-sulfur enzyme Yang Zhang, 1,4 Xuling Zhu, 1,4 Andrew T. Torelli, 1 Michael Lee, 2 Boris Dzikovski, 1 Rachel Koralewski, 1 Eileen Wang, 1 Jack Freed, 1 Carsten
Supplementary information
 Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN
 THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
FW 1 CDR 1 FW 2 CDR 2
 Supplementary Figure 1 Supplementary Figure 1: Interface of the E9:Fas structure. The two interfaces formed by V H and V L of E9 with Fas are shown in stereo. The Fas receptor is represented as a surface
Supplementary Figure 1 Supplementary Figure 1: Interface of the E9:Fas structure. The two interfaces formed by V H and V L of E9 with Fas are shown in stereo. The Fas receptor is represented as a surface
SUPPLEMENTARY INFORMATION
 Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Supporting Information
 Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b).
 Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b). Crystal contacts at B-C loop are magnified and stereo view of A-weighted
Supplementary Figure 1 Crystal packing of ClR and electron density maps. Crystal packing of type A crystal (a) and type B crystal (b). Crystal contacts at B-C loop are magnified and stereo view of A-weighted
Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets
 Supporting information Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets Wan-Na Chen, Christoph Nitsche, Kala Bharath Pilla, Bim Graham, Thomas
Supporting information Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets Wan-Na Chen, Christoph Nitsche, Kala Bharath Pilla, Bim Graham, Thomas
Supplementary information for:
 SUPPLEMETARY IFRMATI Supplementary information for: Structure of a β 1 -adrenergic G protein-coupled receptor Tony Warne, Maria J. Serrano-Vega, Jillian G. Baker#, Rouslan Moukhametzianov, Patricia C.
SUPPLEMETARY IFRMATI Supplementary information for: Structure of a β 1 -adrenergic G protein-coupled receptor Tony Warne, Maria J. Serrano-Vega, Jillian G. Baker#, Rouslan Moukhametzianov, Patricia C.
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1: The HpUreI crystal used for collection of native diffraction data. The crystal belongs to spacegroup P4 2 2 1 2 and has an approximate maximal dimension of 0.25 mm. Supplementary
Supplementary Figure 1: The HpUreI crystal used for collection of native diffraction data. The crystal belongs to spacegroup P4 2 2 1 2 and has an approximate maximal dimension of 0.25 mm. Supplementary
Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps
 Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Chemical structure of LPS and LPS biogenesis in Gram-negative bacteria. a. Chemical structure of LPS. LPS molecule consists of Lipid A, core oligosaccharide and O-antigen. The polar
Supplementary Figure 1 Chemical structure of LPS and LPS biogenesis in Gram-negative bacteria. a. Chemical structure of LPS. LPS molecule consists of Lipid A, core oligosaccharide and O-antigen. The polar
Bacterial protease uses distinct thermodynamic signatures for substrate recognition
 Bacterial protease uses distinct thermodynamic signatures for substrate recognition Gustavo Arruda Bezerra, Yuko Ohara-Nemoto, Irina Cornaciu, Sofiya Fedosyuk, Guillaume Hoffmann, Adam Round, José A. Márquez,
Bacterial protease uses distinct thermodynamic signatures for substrate recognition Gustavo Arruda Bezerra, Yuko Ohara-Nemoto, Irina Cornaciu, Sofiya Fedosyuk, Guillaume Hoffmann, Adam Round, José A. Márquez,
CH 3 CH 2 OH +H 2 O CHO. 2e + 2H + + O 2 H 2 O +HCOOH
 2 4 H CH 3 2e + 2H + + 2 H 2 2 H CH 2 H 2e + 2H + + 2 H 2 2 H +H 2 CH 2e + 2H + + 2 H 2 2 H +HCH Supplemental Figure S. The three-step 4DM reaction, each step requires two reducing equivalents from ADPH
2 4 H CH 3 2e + 2H + + 2 H 2 2 H CH 2 H 2e + 2H + + 2 H 2 2 H +H 2 CH 2e + 2H + + 2 H 2 2 H +HCH Supplemental Figure S. The three-step 4DM reaction, each step requires two reducing equivalents from ADPH
Structure and RNA-binding properties. of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex
 Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Supporting Information
 Supporting Information Micelle-Triggered b-hairpin to a-helix Transition in a 14-Residue Peptide from a Choline-Binding Repeat of the Pneumococcal Autolysin LytA HØctor Zamora-Carreras, [a] Beatriz Maestro,
Supporting Information Micelle-Triggered b-hairpin to a-helix Transition in a 14-Residue Peptide from a Choline-Binding Repeat of the Pneumococcal Autolysin LytA HØctor Zamora-Carreras, [a] Beatriz Maestro,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10458 Active Site Remodeling in the Bifunctional Fructose-1,6- bisphosphate aldolase/phosphatase Juan Du, Rafael F. Say, Wei Lü, Georg Fuchs & Oliver Einsle SUPPLEMENTARY FIGURES Figure
doi:10.1038/nature10458 Active Site Remodeling in the Bifunctional Fructose-1,6- bisphosphate aldolase/phosphatase Juan Du, Rafael F. Say, Wei Lü, Georg Fuchs & Oliver Einsle SUPPLEMENTARY FIGURES Figure
Supplementary Information. Viral immunoevasin targeting of a Natural Killer cell receptor family
 Supplementary Information Viral immunoevasin targeting of a Natural Killer cell receptor family Richard Berry 1, Natasha Ng 1, Philippa M. Saunders 2, Julian P. Vivian 1, Jie Lin 2, Felix A. Deuss 1, Alexandra
Supplementary Information Viral immunoevasin targeting of a Natural Killer cell receptor family Richard Berry 1, Natasha Ng 1, Philippa M. Saunders 2, Julian P. Vivian 1, Jie Lin 2, Felix A. Deuss 1, Alexandra
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R)
 Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Plasmid Relevant features Source. W18N_D20N and TrXE-W18N_D20N-anti
 Table S1. E. coli plasmids Plasmid Relevant features Source pdg680 T. reesei XynII AA 2-190 with C-terminal His 6 tag optimized for E. coli expression in pjexpress401 Wan et al. (in press) psbn44d psbn44h
Table S1. E. coli plasmids Plasmid Relevant features Source pdg680 T. reesei XynII AA 2-190 with C-terminal His 6 tag optimized for E. coli expression in pjexpress401 Wan et al. (in press) psbn44d psbn44h
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
Supplementary Information
 Supplementary Information The direct role of selenocysteine in [NiFeSe] hydrogenase maturation and catalysis Marta C. Marques a, Cristina Tapia b, Oscar Gutiérrez-Sanz b, Ana Raquel Ramos a, Kimberly L.
Supplementary Information The direct role of selenocysteine in [NiFeSe] hydrogenase maturation and catalysis Marta C. Marques a, Cristina Tapia b, Oscar Gutiérrez-Sanz b, Ana Raquel Ramos a, Kimberly L.
SI Text S1 Solution Scattering Data Collection and Analysis. SI references
 SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
NB-DNJ/GCase-pH 7.4 NB-DNJ+/GCase-pH 7.4 NB-DNJ+/GCase-pH 4.5
 SUPPLEMENTARY TABLES Suppl. Table 1. Protonation states at ph 7.4 and 4.5. Protonation states of titratable residues in GCase at ph 7.4 and 4.5. Histidine: HID, H at δ-nitrogen; HIE, H at ε-nitrogen; HIP,
SUPPLEMENTARY TABLES Suppl. Table 1. Protonation states at ph 7.4 and 4.5. Protonation states of titratable residues in GCase at ph 7.4 and 4.5. Histidine: HID, H at δ-nitrogen; HIE, H at ε-nitrogen; HIP,
Nature Structural & Molecular Biology doi: /nsmb Supplementary Figure 1. CRBN binding assay with thalidomide enantiomers.
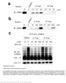 Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/1/eaau413/dc1 Supplementary Materials for Structure and dynamics conspire in the evolution of affinity between intrinsically disordered proteins Per Jemth*, Elin
advances.sciencemag.org/cgi/content/full/4/1/eaau413/dc1 Supplementary Materials for Structure and dynamics conspire in the evolution of affinity between intrinsically disordered proteins Per Jemth*, Elin
Stabilizing the CH2 domain of an Antibody by Engineering in an Enhanced Aromatic Sequon
 Stabilizing the CH2 domain of an Antibody by Engineering in an Enhanced Aromatic Sequon Wentao Chen,, Leopold Kong, Stephen Connelly, Julia M. Dendle,, Yu Liu,, Ian A. Wilson,#, Evan T. Powers, *, Jeffery
Stabilizing the CH2 domain of an Antibody by Engineering in an Enhanced Aromatic Sequon Wentao Chen,, Leopold Kong, Stephen Connelly, Julia M. Dendle,, Yu Liu,, Ian A. Wilson,#, Evan T. Powers, *, Jeffery
Supplementary Information. Structural basis for precursor protein-directed ribosomal peptide macrocyclization
 Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
SUPPLEMENTARY INFORMATION
 doi: 10.108/nature0608 a c pmol L-[ H]Leu / mg LeuT pmol L-[ H]Leu / min / mg LeuT 900 50 600 450 00 150 200 150 100 0 0.0 2.5 5.0.5 10.0.5 50 N Cl CMI IMI DMI H C CH N N H C CH N Time (min) 0 0 100 200
doi: 10.108/nature0608 a c pmol L-[ H]Leu / mg LeuT pmol L-[ H]Leu / min / mg LeuT 900 50 600 450 00 150 200 150 100 0 0.0 2.5 5.0.5 10.0.5 50 N Cl CMI IMI DMI H C CH N N H C CH N Time (min) 0 0 100 200
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1. MhsT and LeuT architecture.
 Supplementary Figure 1 MhsT and LeuT architecture. a, Cartoon structure representation and topology diagram for MhsT in the occluded inward-facing state and b, LeuT in the occluded outward-facing state
Supplementary Figure 1 MhsT and LeuT architecture. a, Cartoon structure representation and topology diagram for MhsT in the occluded inward-facing state and b, LeuT in the occluded outward-facing state
Physiochemical Properties of Residues
 Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
Cryo-EM data collection, refinement and validation statistics
 1 Table S1 Cryo-EM data collection, refinement and validation statistics Data collection and processing CPSF-160 WDR33 (EMDB-7114) (PDB 6BM0) CPSF-160 WDR33 (EMDB-7113) (PDB 6BLY) CPSF-160 WDR33 CPSF-30
1 Table S1 Cryo-EM data collection, refinement and validation statistics Data collection and processing CPSF-160 WDR33 (EMDB-7114) (PDB 6BM0) CPSF-160 WDR33 (EMDB-7113) (PDB 6BLY) CPSF-160 WDR33 CPSF-30
Cks1 CDK1 CDK1 CDK1 CKS1. are ice- lobe. conserved. conserved
 Cks1 d CKS1 Supplementary Figure 1 The -Cks1 crystal lattice. (a) Schematic of the - Cks1 crystal lattice. -Cks1 crystallizes in a lattice that contains c 4 copies of the t - Cks1 dimer in the crystallographic
Cks1 d CKS1 Supplementary Figure 1 The -Cks1 crystal lattice. (a) Schematic of the - Cks1 crystal lattice. -Cks1 crystallizes in a lattice that contains c 4 copies of the t - Cks1 dimer in the crystallographic
Table S1. Primers used for the constructions of recombinant GAL1 and λ5 mutants. GAL1-E74A ccgagcagcgggcggctgtctttcc ggaaagacagccgcccgctgctcgg
 SUPPLEMENTAL DATA Table S1. Primers used for the constructions of recombinant GAL1 and λ5 mutants Sense primer (5 to 3 ) Anti-sense primer (5 to 3 ) GAL1 mutants GAL1-E74A ccgagcagcgggcggctgtctttcc ggaaagacagccgcccgctgctcgg
SUPPLEMENTAL DATA Table S1. Primers used for the constructions of recombinant GAL1 and λ5 mutants Sense primer (5 to 3 ) Anti-sense primer (5 to 3 ) GAL1 mutants GAL1-E74A ccgagcagcgggcggctgtctttcc ggaaagacagccgcccgctgctcgg
Supplemental Data SUPPLEMENTAL FIGURES
 Supplemental Data CRYSTAL STRUCTURE OF THE MG.ADP-INHIBITED STATE OF THE YEAST F 1 C 10 ATP SYNTHASE Alain Dautant*, Jean Velours and Marie-France Giraud* From Université Bordeaux 2, CNRS; Institut de
Supplemental Data CRYSTAL STRUCTURE OF THE MG.ADP-INHIBITED STATE OF THE YEAST F 1 C 10 ATP SYNTHASE Alain Dautant*, Jean Velours and Marie-France Giraud* From Université Bordeaux 2, CNRS; Institut de
Introduction to Comparative Protein Modeling. Chapter 4 Part I
 Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
SUPPLEMENTARY INFORMATION
 doi:10.108/nature11899 Supplementar Table 1. Data collection and refinement statistics (+TPMP, native) (-TPMP, native) (+TPMP, recombinant) (MgCl ) (MgSO ) Data collection Space group C P 1 C P 1 1 P 1
doi:10.108/nature11899 Supplementar Table 1. Data collection and refinement statistics (+TPMP, native) (-TPMP, native) (+TPMP, recombinant) (MgCl ) (MgSO ) Data collection Space group C P 1 C P 1 1 P 1
of the Guanine Nucleotide Exchange Factor FARP2
 Structure, Volume 21 Supplemental Information Structural Basis for Autoinhibition of the Guanine Nucleotide Exchange Factor FARP2 Xiaojing He, Yi-Chun Kuo, Tyler J. Rosche, and Xuewu Zhang Inventory of
Structure, Volume 21 Supplemental Information Structural Basis for Autoinhibition of the Guanine Nucleotide Exchange Factor FARP2 Xiaojing He, Yi-Chun Kuo, Tyler J. Rosche, and Xuewu Zhang Inventory of
SUPPLEMENTARY INFORMATION
 UPPEER ORO doi:10.1038/nature10753 D D D D P E ntracellular C1 W P P C EC1 D Q R H C D W D R C C2 D E D E C R Q Q W P W W R P P EC2 EC3 P C C P W P W W P C W H R C R E C3 P R R P P P C Extracellular embrane
UPPEER ORO doi:10.1038/nature10753 D D D D P E ntracellular C1 W P P C EC1 D Q R H C D W D R C C2 D E D E C R Q Q W P W W R P P EC2 EC3 P C C P W P W W P C W H R C R E C3 P R R P P P C Extracellular embrane
Three-dimensional structure of a viral genome-delivery portal vertex
 Three-dimensional structure of a viral genome-delivery portal vertex Adam S. Olia 1, Peter E. Prevelige Jr. 2, John E. Johnson 3 and Gino Cingolani 4 1 Department of Biological Sciences, Purdue University,
Three-dimensional structure of a viral genome-delivery portal vertex Adam S. Olia 1, Peter E. Prevelige Jr. 2, John E. Johnson 3 and Gino Cingolani 4 1 Department of Biological Sciences, Purdue University,
Advanced Certificate in Principles in Protein Structure. You will be given a start time with your exam instructions
 BIRKBECK COLLEGE (University of London) Advanced Certificate in Principles in Protein Structure MSc Structural Molecular Biology Date: Thursday, 1st September 2011 Time: 3 hours You will be given a start
BIRKBECK COLLEGE (University of London) Advanced Certificate in Principles in Protein Structure MSc Structural Molecular Biology Date: Thursday, 1st September 2011 Time: 3 hours You will be given a start
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits. AhpC and AhpF from Escherichia coli
 Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits AhpC and AhpF from Escherichia coli Phat Vinh Dip 1,#, Neelagandan Kamariah 2,#, Malathy Sony Subramanian Manimekalai
Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits AhpC and AhpF from Escherichia coli Phat Vinh Dip 1,#, Neelagandan Kamariah 2,#, Malathy Sony Subramanian Manimekalai
Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached
 Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached to HpUreI. Urea hydrolysis products 2NH 3 and 1CO 2
Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached to HpUreI. Urea hydrolysis products 2NH 3 and 1CO 2
Supplemental Information. Molecular Basis of Spectral Diversity. in Near-Infrared Phytochrome-Based. Fluorescent Proteins
 Chemistry & Biology, Volume 22 Supplemental Information Molecular Basis of Spectral Diversity in Near-Infrared Phytochrome-Based Fluorescent Proteins Daria M. Shcherbakova, Mikhail Baloban, Sergei Pletnev,
Chemistry & Biology, Volume 22 Supplemental Information Molecular Basis of Spectral Diversity in Near-Infrared Phytochrome-Based Fluorescent Proteins Daria M. Shcherbakova, Mikhail Baloban, Sergei Pletnev,
Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate
 SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
SUPPLEMENTARY INFORMATION
 5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
ml. ph 7.5 ph 6.5 ph 5.5 ph 4.5. β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS
 a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
Supplementary Figure 1 Schematic overview of ASTNs in neuronal migration. (a) Schematic of roles played by ASTNs 1 and 2. ASTN-1-mediated adhesions
 Supplementary Figure 1 Schematic overview of ASTNs in neuronal migration. (a) Schematic of roles played by ASTNs 1 and 2. ASTN-1-mediated adhesions undergo endocytosis into clathrin-coated vesicles dependent
Supplementary Figure 1 Schematic overview of ASTNs in neuronal migration. (a) Schematic of roles played by ASTNs 1 and 2. ASTN-1-mediated adhesions undergo endocytosis into clathrin-coated vesicles dependent
Supplemental Information
 Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
The structure of a nucleolytic ribozyme that employs a catalytic metal ion. Yijin Liu, Timothy J. Wilson and David M.J. Lilley
 SUPPLEMENTARY INFORMATION The structure of a nucleolytic ribozyme that employs a catalytic metal ion Yijin Liu, Timothy J. Wilson and David M.J. Lilley Cancer Research UK Nucleic Acid Structure Research
SUPPLEMENTARY INFORMATION The structure of a nucleolytic ribozyme that employs a catalytic metal ion Yijin Liu, Timothy J. Wilson and David M.J. Lilley Cancer Research UK Nucleic Acid Structure Research
SUPPLEMENTARY INFORMATION
 SUPPLMTARY IFORMATIO a doi:10.108/nature10402 b 100 nm 100 nm c SAXS Model d ulers assigned to reference- Back-projected free class averages class averages Refinement against single particles Reconstructed
SUPPLMTARY IFORMATIO a doi:10.108/nature10402 b 100 nm 100 nm c SAXS Model d ulers assigned to reference- Back-projected free class averages class averages Refinement against single particles Reconstructed
Acta Crystallographica Section F
 Supporting information Acta Crystallographica Section F Volume 70 (2014) Supporting information for article: Chemical conversion of cisplatin and carboplatin with histidine in a model protein crystallised
Supporting information Acta Crystallographica Section F Volume 70 (2014) Supporting information for article: Chemical conversion of cisplatin and carboplatin with histidine in a model protein crystallised
Nature Structural and Molecular Biology: doi: /nsmb.2783
 Supplementary Figure 1: Crystallized chimera construct (mhv1cc). (a) Sequence alignment between mhv1cc and other VSDs. These sequences (mhv1cc, Kv1.2 Kv2.1; shaker family voltage gated potassium channel
Supplementary Figure 1: Crystallized chimera construct (mhv1cc). (a) Sequence alignment between mhv1cc and other VSDs. These sequences (mhv1cc, Kv1.2 Kv2.1; shaker family voltage gated potassium channel
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12242 C. thermophilum 666 RPAVLDNVYIRPALE-GKRVPGKVEIHQNGIRYQSPLSTTQRVDVLFSNIRHLFFQPCQN S. pombe 659 RPAHINDVYVRPAID-GKRLPGFIEIHQNGIRYQSPLRSDSHIDLLFSNMKHLFFQPCEG
SUPPLEMENTARY INFORMATION doi:10.1038/nature12242 C. thermophilum 666 RPAVLDNVYIRPALE-GKRVPGKVEIHQNGIRYQSPLSTTQRVDVLFSNIRHLFFQPCQN S. pombe 659 RPAHINDVYVRPAID-GKRLPGFIEIHQNGIRYQSPLRSDSHIDLLFSNMKHLFFQPCEG
Structurale, Université Grenoble Alpes, CNRS, CEA, Grenoble, France
 Supplementary Information to Lysine relay mechanism coordinates intermediate transfer in vitamin B6 biosynthesis Matthew J. Rodrigues 1,2, Volker Windeisen 1,3, Yang Zhang 4, Gabriela Guédez 3, Stefan
Supplementary Information to Lysine relay mechanism coordinates intermediate transfer in vitamin B6 biosynthesis Matthew J. Rodrigues 1,2, Volker Windeisen 1,3, Yang Zhang 4, Gabriela Guédez 3, Stefan
Structural insights into Aspergillus fumigatus lectin specificity - AFL binding sites are functionally non-equivalent
 Acta Cryst. (2015). D71, doi:10.1107/s1399004714026595 Supporting information Volume 71 (2015) Supporting information for article: Structural insights into Aspergillus fumigatus lectin specificity - AFL
Acta Cryst. (2015). D71, doi:10.1107/s1399004714026595 Supporting information Volume 71 (2015) Supporting information for article: Structural insights into Aspergillus fumigatus lectin specificity - AFL
Impact of the crystallization condition on importin-β conformation
 Supporting information Volume 72 (2016) Supporting information for article: Impact of the crystallization condition on importin-β conformation Marcel J. Tauchert, Clément Hémonnot, Piotr Neumann, Sarah
Supporting information Volume 72 (2016) Supporting information for article: Impact of the crystallization condition on importin-β conformation Marcel J. Tauchert, Clément Hémonnot, Piotr Neumann, Sarah
Nature Structural and Molecular Biology: doi: /nsmb.2938
 Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
Structural characterization of NiV N 0 P in solution and in crystal.
 Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
RNA Polymerase I Contains a TFIIF-Related DNA-Binding Subcomplex
 Molecular Cell, Volume 39 Supplemental Information RNA Polymerase I Contains a TFIIFRelated DNABinding Subcomplex Sebastian R. Geiger, Kristina Lorenzen, Amelie Schreieck, Patrizia Hanecker, Dirk Kostrewa,
Molecular Cell, Volume 39 Supplemental Information RNA Polymerase I Contains a TFIIFRelated DNABinding Subcomplex Sebastian R. Geiger, Kristina Lorenzen, Amelie Schreieck, Patrizia Hanecker, Dirk Kostrewa,
ENZYME MECHANISMS, PROTEASES, STRUCTURAL BIOLOGY
 Supplementary Information SUBJECT AREAS: ENZYME MECHANISMS, PROTEASES, STRUCTURAL BIOLOGY Correspondence and requests for materials should be addressed to N.T. (ntanaka@pharm.showa-u.ac.jp) or W.O. (owataru@vos.nagaokaut.ac.jp)
Supplementary Information SUBJECT AREAS: ENZYME MECHANISMS, PROTEASES, STRUCTURAL BIOLOGY Correspondence and requests for materials should be addressed to N.T. (ntanaka@pharm.showa-u.ac.jp) or W.O. (owataru@vos.nagaokaut.ac.jp)
Rational Design of Thermodynamic and Kinetic Binding Profiles by. Optimizing Surface Water Networks Coating Protein Bound Ligands
 SUPPORTING INFORMATION Rational Design of Thermodynamic and Kinetic Binding Profiles by Optimizing Surface Water Networks Coating Protein Bound Ligands Stefan G. Krimmer,, Jonathan Cramer,, Michael Betz,
SUPPORTING INFORMATION Rational Design of Thermodynamic and Kinetic Binding Profiles by Optimizing Surface Water Networks Coating Protein Bound Ligands Stefan G. Krimmer,, Jonathan Cramer,, Michael Betz,
A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and. Disfavors Mn Binding.
 Supporting information for A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and Disfavors Mn Binding. Anne-Frances Miller* and Ting Wang Department of Chemistry, University
Supporting information for A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and Disfavors Mn Binding. Anne-Frances Miller* and Ting Wang Department of Chemistry, University
Experimental and Computational Mutagenesis to Investigate the. Positioning of a General Base within an Enzyme Active Site
 Experimental and Computational Mutagenesis to Investigate the Positioning of a General Base within an Enzyme Active Site Jason P. Schwans, Philip Hanoian, Benjamin J. Lengerich, Fanny Sunden, Ana Gonzalez
Experimental and Computational Mutagenesis to Investigate the Positioning of a General Base within an Enzyme Active Site Jason P. Schwans, Philip Hanoian, Benjamin J. Lengerich, Fanny Sunden, Ana Gonzalez
NMR study of complexes between low molecular mass inhibitors and the West Nile virus NS2B-NS3 protease
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
Bioinformatics. Macromolecular structure
 Bioinformatics Macromolecular structure Contents Determination of protein structure Structure databases Secondary structure elements (SSE) Tertiary structure Structure analysis Structure alignment Domain
Bioinformatics Macromolecular structure Contents Determination of protein structure Structure databases Secondary structure elements (SSE) Tertiary structure Structure analysis Structure alignment Domain
