ml. ph 7.5 ph 6.5 ph 5.5 ph 4.5. β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS
|
|
|
- Shanna Bridges
- 6 years ago
- Views:
Transcription
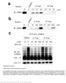
1 a UV28 absorption (mau) β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b β 2 AR-Gs complex β 2 AR-Gs complex + foscarnet β 2 AR-Gs complex + pyrophosphate β 2 AR-Gs complex c ph 7.5 ph 6.5 ph 5.5 ph 4.5 d UV28 absorption (mau) T4L-β 2 AR-Gs complex T4L-β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex excess GTPγS Nb35 T4L-β 2 AR-Gs complex + Nb35 T4L-β 2 AR-Gs complex + Nb35 + GTPγS β 2 AR-Gs-Nb35 complex dissociated complex Nb T4L-β 2 AR-Gs complex + Nb35 + Nb37 β 2 AR-Gs-Nb35-Nb37 complex Nb35 & Nb Figure S1: Effect of nucleotide analogs, ph, and nanobodies on the stability of the β 2 AR-Gs complex. a) Analytical gel filtration showing that nucleotides GDP and GTPγS (.1 mm) cause dissociation of the β 2 AR-Gs complex. b) The phosphates pyrophosphate and foscarnet (used at 5 mm) resemble the nucleotide phosphate groups, but do not cause disruption of the complex. When used as additives they improved crystal growth of both the T4L-β2AR-Gs complex (without nanobodies), T4L-β2AR-Gs-Nb37, and T4L-β2AR-Gs-Nb35. c) The ph limit was determined to guide the preparation of crystallization screens. For the same purpose the effect of ionic strength (data not shown) was determined using NaCl at various concentrations. The complex is stable in,, and 5 mm but dissociates at 2.5 M NaCl. d) Nanobody 35 (Nb35, red dashed line) binds to the β 2 AR-Gs complex (blue solid line) to form the β 2 AR-Gs-Nb35 complex (red solid line) which is insensitive to GTPγS treatment (green solid line) in contrast to the treated β 2 AR-Gs complex alone (green dashed line). Nb35 and Nb37 binds separate epitopes on the Gs heterotrimer to form a β 2 AR-Gs-Nb35-Nb37 complex (purple solid line). Nb37 binding also prevents GTPγS from dissociating the β 2 AR-Gs complex (data not shown).
2 µm Figure S2: Crystals of the T4L-β2AR-Gs-Nb35 complex in sponge-like mesophase
3 a b c TM3 TM7 D13 R131 R131 P323 TM3 Y391 N322 Y132 α5 Y326 d e D13 TM3 β1 TM2 T68 Y141 αn IL2 α1 F139 α5 P-loop Figure S3: Views of electron density for residues in the β 2 AR-Gs interface. a) The D/ERY motif at the cytoplasmic end of TM3. b) Packing interaction between Arg131 of the E/DRY motif and Tyr391 of C-terminal Gαs. c) The NPxxY in the cytoplasmic end of TM7. d) Interactions of Thr68 and Tyr141 with Asp13 of the E/DRY motif. Phe139 of IL2 is buried in a hydrophobic pocket in Gαs. e) The β1-α1 loop (P-loop) of Gαs involved in nucleotide binding.
4 β 2 AR GαsAH (β 2 AR-Gs) GαsAH (GTPγS bound) Gβ Figure S4: Rigid body motion of the alpha helical domain The GαsAH domain of the GTPγS bound structure (1AZT, Sunahara et al.) was aligned with GαsAH domain of the nucleotide-free β 2 AR-Gs structure to show that the displacement of GαsAH is a rigid body movement without significant intradomain conformational changes.
5 T4L β 2 AR GαsAH GαsRas Nb35 Figure S5: Packing interactions of GαsAH domain Shown in blue are residues in the GαsAH domain that are within 5Å of neighboring lattice contacts on Nb35, the GαsRas domain, and the β 2 AR.
6 a β 2 AR Aligned GαsRas (β2ar-gs) GαsRas (GTPγS bound) GαsAH (GTPγS bound) Nb35 Gβ (β 2 AR-Gs) b Aligned Gαi AH GαsRas (β2ar-gs) (GDP bound G i ) Gαi Ras (GDP bound G i ) Nb35 Gβ (β 2 AR-Gs) Gβ (GDP bound G i ) c Gαi AH GαsRas (β2ar-gs) (GDP bound G i ) Gαi Ras (GDP bound G i ) Nb35 Aligned Gβ (β 2 AR-Gs) Gβ (GDP bound G i ) Figure S6: Displacement of GαsAH domain is not caused by a steric clash with Nb35 The GαsRas domain of the β 2 AR-Gs structure was aligned with the GαsRas domain of the GTPγS bound structure (1AZT, Sunahara et al.) in (a) and with GαiRas domain of GDP bound Gi structure (1GP2, Wall et al.) in (b). c) The Gβ of the β 2 AR-Gs structure was aligned with Gβ of the GDP bound Gi structure. In all three alignments, Nb35 (in red) does not overlap with the space occupied by the nucleotide bound state of the alpha helical domain in the Gαs-GTPγS and Gi-GDP structures.
7 β 2 AR purification Nanobody purification Sf9 insect cells Solubilization anti FLAG alprenolol anti FLAG E. coli periplasmic expression Lysis Ni MonoS bind agonist Gs heterotrimer purification dephosphorylation & deglycosylation Sf9 insect cells Solubilization Ni MonoQ SEC β 2 AR-Gs : Nb mixing ratio determination β 2 AR - Gs complex purification dephosphorylation anti FLAG SEC SEC Complex formation & detergent exchange Figure S7: Flow-chart of the purification procedures for preparing β 2 AR-Gs complex with Nb35
8 a kda T4L-β 2 AR-Gs mixture Bio Rad Precision Standards T4L-β 2 AR M1 flow through T4L-β 2 AR α subunit β subunit b M1 & SEC pure T4L-β 2 AR-Gs M1 pure T4L-β 2 AR-Gs UV28 absorption (mau) pooled fractions T4L-β 2 AR-Gs complex excess T4L-β 2 AR c UV28 absorption (mau) γ subunit d UV28 absorption (mau) Without prior λppase treatment of β 2 AR and Gs heterotrimer 8 8 % 1M NaCl Figure S8: Purity and homogeneity of the β 2 AR-Gs complex a) Analytical SDS-PAGE/Coomassie blue stain of samples obtained at various stages of β 2 AR-Gs purification. BI-1677 agonist bound, dephosphorylated, and deglycosylated receptor is used in excess of Gs heterotrimer for optimal coupling efficiency with the functional fraction of the G protein. Functional purification of Gs is achieved through its interaction with the immobilized receptor on M1 resin while non-functional/non-binding Gs is not retained. b) A representative elution profile of one of four consecutive preparative size exclusion chromatography (SEC) runs with fractionation indicated in red. SEC fractions containing the β 2 AR-Gs complex (within the indicated dashed lines) were pooled, spin concentrated, and analyzed for purity and homogeneity by SDS-PAGE/Coomassie blue (a, lane 6), gel filtration (c), and by anion exchange chromotography (d). d) Upper panel shows elution profile from an analytical ion exchange chromatography (IEC) run of β 2 AR-365-Gs complex that was treated with λ phosphatase prior to complex formation. Lower panel shows IEC of complex which was not dephosphorylated resulting in a heterogeneous preparation. Off-peak fractions from the preparative SEC (b) were used for analytical gel filtration experiments shown in figures S1 and S12.
9 a b Pooled fractions UV28 absorption (mau) 15 5 Nb % 1M NaCl UV28 absorption (mau) 5 3 T4L-β 2 AR-Gs-Nb35 complex Nb Figure S9: Purification of Nb35 and determination of β 2 AR:Gs:Nb mixing ratio a) Preparative ion exchange chromotography following nickel affinity chromotography purification of Nb35. The nanobody eluted in two populations (shown in red) as a minor peak and a major homogeneous peak which was collected, spin concentrated, and used for crystallography following determination of proper mixing ratio with the β 2 AR-Gs complex as shown in (b). b) The β 2 AR-Gs complex was mixed with slight excess of Nb35 (1 to 1.2 molar ratio of β 2 AR-Gs complex to Nb35) on the basis of their protein concentrations and verified by analytical gel filtration.
10 1) High affinity agonist binding β 2 AR BI-1677 bound β 2 AR in DDM α β γ GDP bound Gs heterotrimer in DDM 2) Apyrase treatment β 2 AR α β GDP γ High affinity nucleotide-free β 2 AR-Gs interaction GMP 3) Detergent exchange P i Apyase β 2 AR α β γ Stable β 2 AR-Gs complex in MNG-3 Figure S: Formation of a stable β 2 AR-Gs complex. A stable complex was achieved by the combined effects of: 1) binding a high affinity agonist to the receptor with an extremely slow dissociation rate (as described in Rasmussen et al., 11); 2) formation of a nucleotide free complex in the presence of apyrase, which hydrolyses released GDP preventing it from rebinding and causing dissociation of the β 2 AR-Gs complex; and 3) detergent exchange of DDM for MNG-3 which stabilizes the complex.
11 a UV28 absorption (mau) DDM MNG-3 MNG-3 analog 1 β 2 AR-Gs complex dissociated β 2 AR dissociated Gs heterotrimer 15 5 DDM MNG-3 analog 2 β 2 AR-Gs complex dissociated β 2 AR dissociated Gs heterotrimer b [ 3 H]-DHA bind ing ( percent of t=) 8 β 2 AR/MNG3 β 2 AR/DDM 3 Time below CMC (minutes) Time below CMC (hours) Figure S11: Stabilizing effect of MNG-3 on the β 2 AR-Gs complex a) Analytical gel filtration of β 2 AR-Gs complexes purified in DDM (in black), MNG-3 (in blue), or two MNG-3 analogs (in red and green) following incubation for 48 hrs at 4ºC. In contrast to DDM, the β 2 AR-Gs complexes are stable in the MNG detergents. b) Effect of diluting unliganded purified β 2 AR in either DDM or MNG-3 below the critical micelle concentration (CMC) of the detergent. Functional activity of the receptor was determined by [ 3 H]-dihydro alprenolol ([ 3 H]-DHA) saturation binding. Diluting β 2 AR maintained in DDM by -fold below the CMC causes loss of [ 3 H]-DHA binding (black data points) after sec. In contrast, β 2 AR in MNG-3 diluted -fold below the CMC maintained full ability to bind [ 3 H]-DHA after 24 hrs.
12 UV28 absorption (mau) a cysteine mediated cross-linking of β2ar-gs complex Control at t= After 7 days at o C : β 2 AR-Gs complex b 5 3 Untreated control Iodoacetamide treatment β 2 AR-Gs complex c 5 3 Control (.1 mm TCEP) 1 mm TCEP mm TCEP mm β-me Figure S12: Effect of alkylating and reducing agents on the stability and aggregation of the β 2 AR-Gs complex. a) Disulfide-mediated aggregation of the β 2 AR-Gs complex was observed by size exclusion chromatography (SEC) following incubation at º C for 7 days in buffer containing.1 mm tris(2-carboxyethyl)phosphine (TCEP). b) Treatment of the complex with iodoacetamide (5 mm for hrs at º C) led to dissociation of the complex. Alkylating free cysteines with iodoacetic acid and cadmium chloride also led to dissociation. c) Disulfide-mediated aggregation of the complex could be prevented by higher concentrations of reducing agents. Treatment of the β 2 AR-Gs complex with.1, 1, and mm TCEP for 1 hr at º C, or mm betamercaptoethanol (β-me, 1 hr at º C) did not lead to dissociation or aggregation. Crystallization setups were performed using 1 to 5 mm TCEP, which was essential for optimal crystal growth.
13 Supplementary Table 1: Potential intermolecular interactions within the β 2 AR-Gs interface atom in β 2 AR atom in Gαs distance (Å) TM3 IL2 TM5 [ ARG 131 CG ] [ TYR 391 CE2 ] 3.6 [ ALA 134 O ] [ HIS 387 ND1 ] 3.1 [ ALA 134 CB ] [ HIS 387 ND1 ] 3.8 [ ALA 134 CB ] [ HIS 387 CG ] 3.8 [ ILE 135 O ] [ GLN 384 NE2 ] 2.9 α5 [ ILE 135 CD1 ] [ LEU 388 CD1 ] 3.5 [ ILE 135 CD1 ] [ LEU 393 CD1 ] 3.5 [ THR 136 O ] [ ARG 38 NH2 ] 3. [ PRO 138 O ] [ ILE 383 CD1 ] 3.4 [ PRO 138 CG ] [ GLN 384 CG ] 3.3 β1 α4 [ PHE 139 CD2 ] [ HIS 41 NE2 ] 3.6 [ PHE 139 CB ] [ VAL 217 CG2 ] 3.7 [ PHE 139 CE1 ] [ PHE 376 CZ ] 3.3 [ PHE 139 CZ ] [ CYS 379 C ] 3.9 [ PHE 139 CZ ] [ ARG 38 N ] 3.2 α5 [ TYR 141 CD2 ] [ HIS 387 NE2 ] 3.9 [ GLN 142 OE1 ] [ HIS 387 NE2 ] 3.5 αn [ SER 143 OG ] [ ALA 39 CB ] 3.6 [ VAL 222 CG1 ] [ LEU 393 CD1 ] 3.7 [ GLU 225 OE2 ] [ GLN 384 NE2 ] 3. [ ALA 226 CA ] [ LEU 388 CD2 ] 3.6 [ ALA 226 CB ] [ LEU 393 O ] 3.8 [ GLN 229 NE2 ] [ ASP 381 OD1 ] 3.6 α5 [ GLN 229 NE2 ] [ GLN 384 OE1 ] 2.8 [ GLN 229 OE1 ] [ ARG 385 NE ] 3.4 [ GLN 229 CG ] [ LEU 388 CD2 ] 3.9 [ LEU 23 CG ] [ LEU 394 CD1 ] 3.5 [ LYS 232 NZ ] [ ASP 381 OD1 ] 3.1 β6 α5 α4 [ ILE 233 CD1 ] [ TYR 358 OH ] 3.4 [ ILE 233 CG1 ] [ ARG 385 NH1 ] 3.5 [ ARG 239 NE ] [ THR 35 OG1 ] 3.5 TM6 [ ALA 271 CB ] [ LEU 393 O ] 3. [ THR 274 CG2 ] [ GLU 392 O ] 3.4 α5 [ THR 274 OG1 ] [ LEU 393 CD2 ] 3.3 [ LEU 275 CD2 ] [ LEU 393 CD2 ] 3.6
14 Supplementary Table 2: Data collection and refinement statistics Data collection* Number of crystals Space group P 2 1 Cell dimensions a, b, c (Å) 119.3, 64.6, 131.2,, ( ) 9., 91.7, 9. Resolution (Å) ( ) R merge (%) 15.6 (55.3) <I>/< I>.8 (1.8) Completeness (%) 91.2 (53.9) Redundancy 6.5 (5.) Refinement Resolution (Å) No. reflections 375 (1557 in test set) R work /R free (%) 22.6 / 27.8 No. atoms 275 No. protein residues 1318 Anisotropic B tensor B 11 = -6.4 / B 22 = 3.8 / B 33 = 2.6 / B 13 = 1.9 Unmodelled sequences a 2 adrenergic receptor 29 b, , 2-264, G s 1-8, -88, 3-4, G s 1-4, T4 lysozyme 161 c Average B-factors (Å 2 ) 2 adrenergic receptor G s ras domain 81.4 G s helical domain G s 63. G s 83.6 Nanobody T4 lysozyme R.m.s. deviation from ideality Bond length (Å).7 Bond angles ( ).71 Ramachandran statistics d Favored regions (%) 95.4 Allowed regions (%) 4.6 Outliers (%) * Highest shell statistics are in parentheses. a These regions were omitted from the model due to poorly resolved electron density. Unmodeled purification tags are not
15 included in these residue ranges. b Residues 1-28 of the 2 AR were omitted from the construct and T4L was fused to the amino terminus of transmembrane helix 1 to facilitate crystallization. c Residue 1 of T4L was omitted from the construct d As defined by MolProbity 38. Supplementary Table 3: Data collection statistics by resolution shell Resolution Shell (Å) <I>/< I> R merge (%) Completeness (%) Overall
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Exam I Answer Key: Summer 2006, Semester C
 1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
Supplementary figure 1. Comparison of unbound ogm-csf and ogm-csf as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine
 Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
type GroEL-GroES complex. Crystals were grown in buffer D (100 mm HEPES, ph 7.5,
 Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Secondary Structure. Bioch/BIMS 503 Lecture 2. Structure and Function of Proteins. Further Reading. Φ, Ψ angles alone determine protein structure
 Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
Physiochemical Properties of Residues
 Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Supplementary Figure 3 a. Structural comparison between the two determined structures for the IL 23:MA12 complex. The overall RMSD between the two
 Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Introduction to Comparative Protein Modeling. Chapter 4 Part I
 Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
FW 1 CDR 1 FW 2 CDR 2
 Supplementary Figure 1 Supplementary Figure 1: Interface of the E9:Fas structure. The two interfaces formed by V H and V L of E9 with Fas are shown in stereo. The Fas receptor is represented as a surface
Supplementary Figure 1 Supplementary Figure 1: Interface of the E9:Fas structure. The two interfaces formed by V H and V L of E9 with Fas are shown in stereo. The Fas receptor is represented as a surface
Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition
 Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/310/5751/1159/dc1 Supporting Online Material for Structure of the Quaternary Complex of Interleukin-2 with Its α, β, and γ c Receptors Xinquan Wang, Mathias Rickert,
www.sciencemag.org/cgi/content/full/310/5751/1159/dc1 Supporting Online Material for Structure of the Quaternary Complex of Interleukin-2 with Its α, β, and γ c Receptors Xinquan Wang, Mathias Rickert,
SUPPLEMENTARY INFORMATION
 UPPEER ORO doi:10.1038/nature10753 D D D D P E ntracellular C1 W P P C EC1 D Q R H C D W D R C C2 D E D E C R Q Q W P W W R P P EC2 EC3 P C C P W P W W P C W H R C R E C3 P R R P P P C Extracellular embrane
UPPEER ORO doi:10.1038/nature10753 D D D D P E ntracellular C1 W P P C EC1 D Q R H C D W D R C C2 D E D E C R Q Q W P W W R P P EC2 EC3 P C C P W P W W P C W H R C R E C3 P R R P P P C Extracellular embrane
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Supplementary Information. The protease GtgE from Salmonella exclusively targets. inactive Rab GTPases
 Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Any protein that can be labelled by both procedures must be a transmembrane protein.
 1. What kind of experimental evidence would indicate that a protein crosses from one side of the membrane to the other? Regions of polypeptide part exposed on the outside of the membrane can be probed
1. What kind of experimental evidence would indicate that a protein crosses from one side of the membrane to the other? Regions of polypeptide part exposed on the outside of the membrane can be probed
1. What is an ångstrom unit, and why is it used to describe molecular structures?
 1. What is an ångstrom unit, and why is it used to describe molecular structures? The ångstrom unit is a unit of distance suitable for measuring atomic scale objects. 1 ångstrom (Å) = 1 10-10 m. The diameter
1. What is an ångstrom unit, and why is it used to describe molecular structures? The ångstrom unit is a unit of distance suitable for measuring atomic scale objects. 1 ångstrom (Å) = 1 10-10 m. The diameter
Protein Data Bank Contents Guide: Atomic Coordinate Entry Format Description. Version Document Published by the wwpdb
 Protein Data Bank Contents Guide: Atomic Coordinate Entry Format Description Version 3.30 Document Published by the wwpdb This format complies with the PDB Exchange Dictionary (PDBx) http://mmcif.pdb.org/dictionaries/mmcif_pdbx.dic/index/index.html.
Protein Data Bank Contents Guide: Atomic Coordinate Entry Format Description Version 3.30 Document Published by the wwpdb This format complies with the PDB Exchange Dictionary (PDBx) http://mmcif.pdb.org/dictionaries/mmcif_pdbx.dic/index/index.html.
SUPPLEMENTARY INFORMATION
 Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
SUPPLEMENTARY INFORMATION
 5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
What binds to Hb in addition to O 2?
 Reading: Ch5; 158-169, 162-166, 169-174 Problems: Ch5 (text); 3,7,8,10 Ch5 (study guide-facts); 1,2,3,4,5,8 Ch5 (study guide-apply); 2,3 Remember Today at 5:30 in CAS-522 is the second chance for the MB
Reading: Ch5; 158-169, 162-166, 169-174 Problems: Ch5 (text); 3,7,8,10 Ch5 (study guide-facts); 1,2,3,4,5,8 Ch5 (study guide-apply); 2,3 Remember Today at 5:30 in CAS-522 is the second chance for the MB
B O C 4 H 2 O O. NOTE: The reaction proceeds with a carbonium ion stabilized on the C 1 of sugar A.
 hbcse 33 rd International Page 101 hemistry lympiad Preparatory 05/02/01 Problems d. In the hydrolysis of the glycosidic bond, the glycosidic bridge oxygen goes with 4 of the sugar B. n cleavage, 18 from
hbcse 33 rd International Page 101 hemistry lympiad Preparatory 05/02/01 Problems d. In the hydrolysis of the glycosidic bond, the glycosidic bridge oxygen goes with 4 of the sugar B. n cleavage, 18 from
Supplementary information for:
 SUPPLEMETARY IFRMATI Supplementary information for: Structure of a β 1 -adrenergic G protein-coupled receptor Tony Warne, Maria J. Serrano-Vega, Jillian G. Baker#, Rouslan Moukhametzianov, Patricia C.
SUPPLEMETARY IFRMATI Supplementary information for: Structure of a β 1 -adrenergic G protein-coupled receptor Tony Warne, Maria J. Serrano-Vega, Jillian G. Baker#, Rouslan Moukhametzianov, Patricia C.
Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase Cwc27
 Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Central Dogma. modifications genome transcriptome proteome
 entral Dogma DA ma protein post-translational modifications genome transcriptome proteome 83 ierarchy of Protein Structure 20 Amino Acids There are 20 n possible sequences for a protein of n residues!
entral Dogma DA ma protein post-translational modifications genome transcriptome proteome 83 ierarchy of Protein Structure 20 Amino Acids There are 20 n possible sequences for a protein of n residues!
Biochemistry Quiz Review 1I. 1. Of the 20 standard amino acids, only is not optically active. The reason is that its side chain.
 Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
SUPPLEMENTARY INFORMATION
 Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Bahnson Biochemistry Cume, April 8, 2006 The Structural Biology of Signal Transduction
 Name page 1 of 6 Bahnson Biochemistry Cume, April 8, 2006 The Structural Biology of Signal Transduction Part I. The ion Ca 2+ can function as a 2 nd messenger. Pick a specific signal transduction pathway
Name page 1 of 6 Bahnson Biochemistry Cume, April 8, 2006 The Structural Biology of Signal Transduction Part I. The ion Ca 2+ can function as a 2 nd messenger. Pick a specific signal transduction pathway
Signal Transduction Phosphorylation Protein kinases. Misfolding diseases. Protein Engineering Lysozyme variants
 Signal Transduction Phosphorylation Protein kinases Misfolding diseases Protein Engineering Lysozyme variants Cells and Signals Regulation The cell must be able to respond to stimuli Cellular activities
Signal Transduction Phosphorylation Protein kinases Misfolding diseases Protein Engineering Lysozyme variants Cells and Signals Regulation The cell must be able to respond to stimuli Cellular activities
Major Types of Association of Proteins with Cell Membranes. From Alberts et al
 Major Types of Association of Proteins with Cell Membranes From Alberts et al Proteins Are Polymers of Amino Acids Peptide Bond Formation Amino Acid central carbon atom to which are attached amino group
Major Types of Association of Proteins with Cell Membranes From Alberts et al Proteins Are Polymers of Amino Acids Peptide Bond Formation Amino Acid central carbon atom to which are attached amino group
Problem Set 1
 2006 7.012 Problem Set 1 Due before 5 PM on FRIDAY, September 15, 2006. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. For each of the following parts, pick
2006 7.012 Problem Set 1 Due before 5 PM on FRIDAY, September 15, 2006. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. For each of the following parts, pick
7.012 Problem Set 1 Solutions
 ame TA Section 7.012 Problem Set 1 Solutions Your answers to this problem set must be inserted into the large wooden box on wheels outside 68120 by 4:30 PM, Thursday, September 15. Problem sets will not
ame TA Section 7.012 Problem Set 1 Solutions Your answers to this problem set must be inserted into the large wooden box on wheels outside 68120 by 4:30 PM, Thursday, September 15. Problem sets will not
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
Packing of Secondary Structures
 7.88 Lecture Notes - 4 7.24/7.88J/5.48J The Protein Folding and Human Disease Professor Gossard Retrieving, Viewing Protein Structures from the Protein Data Base Helix helix packing Packing of Secondary
7.88 Lecture Notes - 4 7.24/7.88J/5.48J The Protein Folding and Human Disease Professor Gossard Retrieving, Viewing Protein Structures from the Protein Data Base Helix helix packing Packing of Secondary
Supporting Information
 Supporting Information Micelle-Triggered b-hairpin to a-helix Transition in a 14-Residue Peptide from a Choline-Binding Repeat of the Pneumococcal Autolysin LytA HØctor Zamora-Carreras, [a] Beatriz Maestro,
Supporting Information Micelle-Triggered b-hairpin to a-helix Transition in a 14-Residue Peptide from a Choline-Binding Repeat of the Pneumococcal Autolysin LytA HØctor Zamora-Carreras, [a] Beatriz Maestro,
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Information. Viral immunoevasin targeting of a Natural Killer cell receptor family
 Supplementary Information Viral immunoevasin targeting of a Natural Killer cell receptor family Richard Berry 1, Natasha Ng 1, Philippa M. Saunders 2, Julian P. Vivian 1, Jie Lin 2, Felix A. Deuss 1, Alexandra
Supplementary Information Viral immunoevasin targeting of a Natural Killer cell receptor family Richard Berry 1, Natasha Ng 1, Philippa M. Saunders 2, Julian P. Vivian 1, Jie Lin 2, Felix A. Deuss 1, Alexandra
BCH 4053 Exam I Review Spring 2017
 BCH 4053 SI - Spring 2017 Reed BCH 4053 Exam I Review Spring 2017 Chapter 1 1. Calculate G for the reaction A + A P + Q. Assume the following equilibrium concentrations: [A] = 20mM, [Q] = [P] = 40fM. Assume
BCH 4053 SI - Spring 2017 Reed BCH 4053 Exam I Review Spring 2017 Chapter 1 1. Calculate G for the reaction A + A P + Q. Assume the following equilibrium concentrations: [A] = 20mM, [Q] = [P] = 40fM. Assume
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Structural insights into energy regulation of light-harvesting complex from spinach CP29
 SUPPLEMENTARY INFORMATION Structural insights into energy regulation of light-harvesting complex from spinach CP29 Xiaowei Pan 1, Mei Li 1, Tao Wan 1,2, Longfei Wang 1,2, Chenjun Jia 1,2, Zhiqiang Hou
SUPPLEMENTARY INFORMATION Structural insights into energy regulation of light-harvesting complex from spinach CP29 Xiaowei Pan 1, Mei Li 1, Tao Wan 1,2, Longfei Wang 1,2, Chenjun Jia 1,2, Zhiqiang Hou
Heterotrimeric G proteins and the role of lipids in signaling. John Sondek, Ph.D. Depts. of Pharmacology and Biochemistry & Biophyscis
 Heterotrimeric G proteins and the role of lipids in signaling John Sondek, Ph.D. Depts. of Pharmacology and Biochemistry & Biophyscis The GTPase cycle molecular switch A GTPases is NOT a kinase Two major
Heterotrimeric G proteins and the role of lipids in signaling John Sondek, Ph.D. Depts. of Pharmacology and Biochemistry & Biophyscis The GTPase cycle molecular switch A GTPases is NOT a kinase Two major
Peptides And Proteins
 Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11744 Supplementary Table 1. Crystallographic data collection and refinement statistics. Wild-type Se-Met-BcsA-B SmCl 3 -soaked EMTS-soaked Data collection Space
SUPPLEMENTARY INFORMATION doi:10.1038/nature11744 Supplementary Table 1. Crystallographic data collection and refinement statistics. Wild-type Se-Met-BcsA-B SmCl 3 -soaked EMTS-soaked Data collection Space
Ramachandran Plot. 4ysz Phi (degrees) Plot statistics
 B Ramachandran Plot ~b b 135 b ~b ~l l Psi (degrees) 5-5 a A ~a L - -135 SER HIS (F) 59 (G) SER (B) ~b b LYS ASP ASP 315 13 13 (A) (F) (B) LYS ALA ALA 315 173 (E) 173 (E)(A) ~p p ~b - -135 - -5 5 135 (degrees)
B Ramachandran Plot ~b b 135 b ~b ~l l Psi (degrees) 5-5 a A ~a L - -135 SER HIS (F) 59 (G) SER (B) ~b b LYS ASP ASP 315 13 13 (A) (F) (B) LYS ALA ALA 315 173 (E) 173 (E)(A) ~p p ~b - -135 - -5 5 135 (degrees)
Table S1. Theoretical and apparent molecular weights of the proteins and protein complexes used for ITC analysis
 Table S1. Theoretical and apparent molecular weights of the proteins and protein complexes used for ITC analysis Sample Theoretical molecular weight (without glycosylation) Apparent molecular weight R-2
Table S1. Theoretical and apparent molecular weights of the proteins and protein complexes used for ITC analysis Sample Theoretical molecular weight (without glycosylation) Apparent molecular weight R-2
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached
 Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached to HpUreI. Urea hydrolysis products 2NH 3 and 1CO 2
Supplementary Figure S1. Urea-mediated buffering mechanism of H. pylori. Gastric urea is funneled to a cytoplasmic urease that is presumably attached to HpUreI. Urea hydrolysis products 2NH 3 and 1CO 2
Bacterial protease uses distinct thermodynamic signatures for substrate recognition
 Bacterial protease uses distinct thermodynamic signatures for substrate recognition Gustavo Arruda Bezerra, Yuko Ohara-Nemoto, Irina Cornaciu, Sofiya Fedosyuk, Guillaume Hoffmann, Adam Round, José A. Márquez,
Bacterial protease uses distinct thermodynamic signatures for substrate recognition Gustavo Arruda Bezerra, Yuko Ohara-Nemoto, Irina Cornaciu, Sofiya Fedosyuk, Guillaume Hoffmann, Adam Round, José A. Márquez,
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.108/nature1099 Supplementary Tables: Supplementary Table 1. Data collection and refinement statistics. Highest resolution shell is shown in parentheses. Structure Data
SUPPLEMENTARY INFORMATION doi:10.108/nature1099 Supplementary Tables: Supplementary Table 1. Data collection and refinement statistics. Highest resolution shell is shown in parentheses. Structure Data
Model Mélange. Physical Models of Peptides and Proteins
 Model Mélange Physical Models of Peptides and Proteins In the Model Mélange activity, you will visit four different stations each featuring a variety of different physical models of peptides or proteins.
Model Mélange Physical Models of Peptides and Proteins In the Model Mélange activity, you will visit four different stations each featuring a variety of different physical models of peptides or proteins.
Viewing and Analyzing Proteins, Ligands and their Complexes 2
 2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08 Å resolution: comparison with the Azotobacter vinelandii MoFe protein
 Acta Cryst. (2015). D71, 274-282, doi:10.1107/s1399004714025243 Supporting information Volume 71 (2015) Supporting information for article: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08
Acta Cryst. (2015). D71, 274-282, doi:10.1107/s1399004714025243 Supporting information Volume 71 (2015) Supporting information for article: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
What makes a good graphene-binding peptide? Adsorption of amino acids and peptides at aqueous graphene interfaces: Electronic Supplementary
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 21 What makes a good graphene-binding peptide? Adsorption of amino acids and
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 21 What makes a good graphene-binding peptide? Adsorption of amino acids and
Supporting information to: Time-resolved observation of protein allosteric communication. Sebastian Buchenberg, Florian Sittel and Gerhard Stock 1
 Supporting information to: Time-resolved observation of protein allosteric communication Sebastian Buchenberg, Florian Sittel and Gerhard Stock Biomolecular Dynamics, Institute of Physics, Albert Ludwigs
Supporting information to: Time-resolved observation of protein allosteric communication Sebastian Buchenberg, Florian Sittel and Gerhard Stock Biomolecular Dynamics, Institute of Physics, Albert Ludwigs
Ranjit P. Bahadur Assistant Professor Department of Biotechnology Indian Institute of Technology Kharagpur, India. 1 st November, 2013
 Hydration of protein-rna recognition sites Ranjit P. Bahadur Assistant Professor Department of Biotechnology Indian Institute of Technology Kharagpur, India 1 st November, 2013 Central Dogma of life DNA
Hydration of protein-rna recognition sites Ranjit P. Bahadur Assistant Professor Department of Biotechnology Indian Institute of Technology Kharagpur, India 1 st November, 2013 Central Dogma of life DNA
NAME IV. /22. I. MULTIPLE CHOICE. (48 points; 2 pts each) Choose the BEST answer to the question by circling the appropriate letter.
 NAME Exam I I. /48 September 25, 2017 Biochemistry I II. / 4 BI/CH 421/621 III. /26 IV. /22 TOTAL /100 I. MULTIPLE CHOICE. (48 points; 2 pts each) Choose the BEST answer to the question by circling the
NAME Exam I I. /48 September 25, 2017 Biochemistry I II. / 4 BI/CH 421/621 III. /26 IV. /22 TOTAL /100 I. MULTIPLE CHOICE. (48 points; 2 pts each) Choose the BEST answer to the question by circling the
Introduction to the Ribosome Overview of protein synthesis on the ribosome Prof. Anders Liljas
 Introduction to the Ribosome Molecular Biophysics Lund University 1 A B C D E F G H I J Genome Protein aa1 aa2 aa3 aa4 aa5 aa6 aa7 aa10 aa9 aa8 aa11 aa12 aa13 a a 14 How is a polypeptide synthesized? 2
Introduction to the Ribosome Molecular Biophysics Lund University 1 A B C D E F G H I J Genome Protein aa1 aa2 aa3 aa4 aa5 aa6 aa7 aa10 aa9 aa8 aa11 aa12 aa13 a a 14 How is a polypeptide synthesized? 2
Protein Structures: Experiments and Modeling. Patrice Koehl
 Protein Structures: Experiments and Modeling Patrice Koehl Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins:
Protein Structures: Experiments and Modeling Patrice Koehl Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins:
Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4.
 Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4. Field Desorption 5. MS MS techniques Matrix assisted
Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4. Field Desorption 5. MS MS techniques Matrix assisted
Bis sulfone Reagents. Figure 1.
 Bis sulfone Reagents An intact IgG molecule has four accessible inter chain disulfide bonds that can be reduced to form eight free cysteine thiols, which can serve as sites for conjugation. The reaction
Bis sulfone Reagents An intact IgG molecule has four accessible inter chain disulfide bonds that can be reduced to form eight free cysteine thiols, which can serve as sites for conjugation. The reaction
Nature Structural & Molecular Biology doi: /nsmb Supplementary Figure 1. CRBN binding assay with thalidomide enantiomers.
 Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Comparison between Bacteriorhodopsin and Halorhodopsin. Halorhodopsin (HR) and Bacteriorhodopsin (BR) belong to a subfamily of
 Comparison between Bacteriorhodopsin and Halorhodopsin Halorhodopsin (HR) and Bacteriorhodopsin (BR) belong to a subfamily of heptahelical membrane proteins, the archaeal rhodopsins. They are found in
Comparison between Bacteriorhodopsin and Halorhodopsin Halorhodopsin (HR) and Bacteriorhodopsin (BR) belong to a subfamily of heptahelical membrane proteins, the archaeal rhodopsins. They are found in
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10458 Active Site Remodeling in the Bifunctional Fructose-1,6- bisphosphate aldolase/phosphatase Juan Du, Rafael F. Say, Wei Lü, Georg Fuchs & Oliver Einsle SUPPLEMENTARY FIGURES Figure
doi:10.1038/nature10458 Active Site Remodeling in the Bifunctional Fructose-1,6- bisphosphate aldolase/phosphatase Juan Du, Rafael F. Say, Wei Lü, Georg Fuchs & Oliver Einsle SUPPLEMENTARY FIGURES Figure
7.05 Spring 2004 February 27, Recitation #2
 Recitation #2 Contact Information TA: Victor Sai Recitation: Friday, 3-4pm, 2-132 E-mail: sai@mit.edu ffice ours: Friday, 4-5pm, 2-132 Unit 1 Schedule Recitation/Exam Date Lectures covered Recitation #2
Recitation #2 Contact Information TA: Victor Sai Recitation: Friday, 3-4pm, 2-132 E-mail: sai@mit.edu ffice ours: Friday, 4-5pm, 2-132 Unit 1 Schedule Recitation/Exam Date Lectures covered Recitation #2
Potassium channel gating and structure!
 Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Supplementary Information. Broad Spectrum Anti-Influenza Agents by Inhibiting Self- Association of Matrix Protein 1
 Supplementary Information Broad Spectrum Anti-Influenza Agents by Inhibiting Self- Association of Matrix Protein 1 Philip D. Mosier 1, Meng-Jung Chiang 2, Zhengshi Lin 2, Yamei Gao 2, Bashayer Althufairi
Supplementary Information Broad Spectrum Anti-Influenza Agents by Inhibiting Self- Association of Matrix Protein 1 Philip D. Mosier 1, Meng-Jung Chiang 2, Zhengshi Lin 2, Yamei Gao 2, Bashayer Althufairi
3. Results Results Crystal structure of the N-terminal domain of human SHBG in complex with DHT
 3. Results 33 3. Results 3.1. Crystal structure of the N-terminal domain of human SHBG in complex with DHT 3.1.1. Crystallization For crystallization experiments the amino-terminal laminin G-like domain
3. Results 33 3. Results 3.1. Crystal structure of the N-terminal domain of human SHBG in complex with DHT 3.1.1. Crystallization For crystallization experiments the amino-terminal laminin G-like domain
Protein Struktur (optional, flexible)
 Protein Struktur (optional, flexible) 22/10/2009 [ 1 ] Andrew Torda, Wintersemester 2009 / 2010, AST nur für Informatiker, Mathematiker,.. 26 kt, 3 ov 2009 Proteins - who cares? 22/10/2009 [ 2 ] Most important
Protein Struktur (optional, flexible) 22/10/2009 [ 1 ] Andrew Torda, Wintersemester 2009 / 2010, AST nur für Informatiker, Mathematiker,.. 26 kt, 3 ov 2009 Proteins - who cares? 22/10/2009 [ 2 ] Most important
Sequential resonance assignments in (small) proteins: homonuclear method 2º structure determination
 Lecture 9 M230 Feigon Sequential resonance assignments in (small) proteins: homonuclear method 2º structure determination Reading resources v Roberts NMR of Macromolecules, Chap 4 by Christina Redfield
Lecture 9 M230 Feigon Sequential resonance assignments in (small) proteins: homonuclear method 2º structure determination Reading resources v Roberts NMR of Macromolecules, Chap 4 by Christina Redfield
1. Amino Acids and Peptides Structures and Properties
 1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
Bioinformatics Practical for Biochemists
 Bioinformatics Practical for Biochemists Andrei Lupas, Birte Höcker, Steffen Schmidt WS 2013/14 03. Sequence Features Targeting proteins signal peptide targets proteins to the secretory pathway N-terminal
Bioinformatics Practical for Biochemists Andrei Lupas, Birte Höcker, Steffen Schmidt WS 2013/14 03. Sequence Features Targeting proteins signal peptide targets proteins to the secretory pathway N-terminal
Translation. A ribosome, mrna, and trna.
 Translation The basic processes of translation are conserved among prokaryotes and eukaryotes. Prokaryotic Translation A ribosome, mrna, and trna. In the initiation of translation in prokaryotes, the Shine-Dalgarno
Translation The basic processes of translation are conserved among prokaryotes and eukaryotes. Prokaryotic Translation A ribosome, mrna, and trna. In the initiation of translation in prokaryotes, the Shine-Dalgarno
Full wwpdb X-ray Structure Validation Report i
 Full wwpdb X-ray Structure Validation Report i Feb 17, 2018 01:16 am GMT PDB ID : 1IFT Title : RICIN A-CHAIN (RECOMBINANT) Authors : Weston, S.A.; Tucker, A.D.; Thatcher, D.R.; Derbyshire, D.J.; Pauptit,
Full wwpdb X-ray Structure Validation Report i Feb 17, 2018 01:16 am GMT PDB ID : 1IFT Title : RICIN A-CHAIN (RECOMBINANT) Authors : Weston, S.A.; Tucker, A.D.; Thatcher, D.R.; Derbyshire, D.J.; Pauptit,
Biochemistry 3100 Sample Problems Chemical and Physical Methods
 (1) Describe the function of the following compounds in chemical sequencing, synthesis and modification: (a) Dithiothreitol (c) t-butyloxycarbonyl chloride (b) Dicyclohexyl carbodiimide (d) Cyanogen Bromide
(1) Describe the function of the following compounds in chemical sequencing, synthesis and modification: (a) Dithiothreitol (c) t-butyloxycarbonyl chloride (b) Dicyclohexyl carbodiimide (d) Cyanogen Bromide
Biophysical Journal, Volume 96. Supporting Material
 Biophysical Journal, Volume 96 Supporting Material NMR dynamics of PSE-4 β-lactamase: an interplay of ps-ns order and μs-ms motions in the active site Sébastien Morin and Stéphane M. Gagné NMR dynamics
Biophysical Journal, Volume 96 Supporting Material NMR dynamics of PSE-4 β-lactamase: an interplay of ps-ns order and μs-ms motions in the active site Sébastien Morin and Stéphane M. Gagné NMR dynamics
Chapter 4: Amino Acids
 Chapter 4: Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. lipid polysaccharide enzyme 1940s 1980s. Lipids membrane 1960s. Polysaccharide Are energy metabolites and many of
Chapter 4: Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. lipid polysaccharide enzyme 1940s 1980s. Lipids membrane 1960s. Polysaccharide Are energy metabolites and many of
Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate
 SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
Supplementary Figure 1. Stability constants of metal monohydroxides. The log K values are summarized according to the atomic number of each element
 Supplementary Figure 1. Stability constants of metal monohydroxides. The log K values are summarized according to the atomic number of each element as determined in a previous study 1. The log K value
Supplementary Figure 1. Stability constants of metal monohydroxides. The log K values are summarized according to the atomic number of each element as determined in a previous study 1. The log K value
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Detailed description of overall and active site architecture of PPDC- 3dThDP, PPDC-2HE3dThDP, PPDC-3dThDP-PPA and PPDC- 3dThDP-POVA
 Online Supplemental Results Detailed description of overall and active site architecture of PPDC- 3dThDP, PPDC-2HE3dThDP, PPDC-3dThDP-PPA and PPDC- 3dThDP-POVA Structure solution and overall architecture
Online Supplemental Results Detailed description of overall and active site architecture of PPDC- 3dThDP, PPDC-2HE3dThDP, PPDC-3dThDP-PPA and PPDC- 3dThDP-POVA Structure solution and overall architecture
Computational Protein Design
 11 Computational Protein Design This chapter introduces the automated protein design and experimental validation of a novel designed sequence, as described in Dahiyat and Mayo [1]. 11.1 Introduction Given
11 Computational Protein Design This chapter introduces the automated protein design and experimental validation of a novel designed sequence, as described in Dahiyat and Mayo [1]. 11.1 Introduction Given
HIV protease inhibitor. Certain level of function can be found without structure. But a structure is a key to understand the detailed mechanism.
 Proteins are linear polypeptide chains (one or more) Building blocks: 20 types of amino acids. Range from a few 10s-1000s They fold into varying three-dimensional shapes structure medicine Certain level
Proteins are linear polypeptide chains (one or more) Building blocks: 20 types of amino acids. Range from a few 10s-1000s They fold into varying three-dimensional shapes structure medicine Certain level
Supplementary Information. Structural basis for precursor protein-directed ribosomal peptide macrocyclization
 Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Supplementary Information
 Supplementary Information Structural analysis of leader peptide binding enables leaderfree cyanobactin processing Jesko Koehnke 1,2, Greg Mann 1,2, Andrew F Bent 1,2, Hannes Ludewig 1, Sally Shirran 1,
Supplementary Information Structural analysis of leader peptide binding enables leaderfree cyanobactin processing Jesko Koehnke 1,2, Greg Mann 1,2, Andrew F Bent 1,2, Hannes Ludewig 1, Sally Shirran 1,
Objective: Students will be able identify peptide bonds in proteins and describe the overall reaction between amino acids that create peptide bonds.
 Scott Seiple AP Biology Lesson Plan Lesson: Primary and Secondary Structure of Proteins Purpose:. To understand how amino acids can react to form peptides through peptide bonds.. Students will be able
Scott Seiple AP Biology Lesson Plan Lesson: Primary and Secondary Structure of Proteins Purpose:. To understand how amino acids can react to form peptides through peptide bonds.. Students will be able
Advanced Certificate in Principles in Protein Structure. You will be given a start time with your exam instructions
 BIRKBECK COLLEGE (University of London) Advanced Certificate in Principles in Protein Structure MSc Structural Molecular Biology Date: Thursday, 1st September 2011 Time: 3 hours You will be given a start
BIRKBECK COLLEGE (University of London) Advanced Certificate in Principles in Protein Structure MSc Structural Molecular Biology Date: Thursday, 1st September 2011 Time: 3 hours You will be given a start
THE UNIVERSITY OF MANITOBA. PAPER NO: _1_ LOCATION: 173 Robert Schultz Theatre PAGE NO: 1 of 5 DEPARTMENT & COURSE NO: CHEM / MBIO 2770 TIME: 1 HOUR
 THE UNIVERSITY OF MANITOBA 1 November 1, 2016 Mid-Term EXAMINATION PAPER NO: _1_ LOCATION: 173 Robert Schultz Theatre PAGE NO: 1 of 5 DEPARTMENT & COURSE NO: CHEM / MBIO 2770 TIME: 1 HOUR EXAMINATION:
THE UNIVERSITY OF MANITOBA 1 November 1, 2016 Mid-Term EXAMINATION PAPER NO: _1_ LOCATION: 173 Robert Schultz Theatre PAGE NO: 1 of 5 DEPARTMENT & COURSE NO: CHEM / MBIO 2770 TIME: 1 HOUR EXAMINATION:
Native State of Complement Protein C3d Analyzed via Hydrogen Exchange and Conformational Sampling
 Devaurs et al. Native State of Complement Protein C3d Analyzed via Hydrogen Exchange and Conformational Sampling Didier Devaurs 1, Malvina Papanastasiou 2, Dinler A Antunes 1, Jayvee R Abella 1, Mark Moll
Devaurs et al. Native State of Complement Protein C3d Analyzed via Hydrogen Exchange and Conformational Sampling Didier Devaurs 1, Malvina Papanastasiou 2, Dinler A Antunes 1, Jayvee R Abella 1, Mark Moll
SUPPLEMENTARY INFORMATION. doi: /nature07461
 Figure S1 Electrophysiology. a ph-activation of. Two-electrode voltage clamp recordings of Xenopus oocytes expressing in comparison to waterinjected oocytes. Currents were recorded at 40 mv. The ph of
Figure S1 Electrophysiology. a ph-activation of. Two-electrode voltage clamp recordings of Xenopus oocytes expressing in comparison to waterinjected oocytes. Currents were recorded at 40 mv. The ph of
Section Week 3. Junaid Malek, M.D.
 Section Week 3 Junaid Malek, M.D. Biological Polymers DA 4 monomers (building blocks), limited structure (double-helix) RA 4 monomers, greater flexibility, multiple structures Proteins 20 Amino Acids,
Section Week 3 Junaid Malek, M.D. Biological Polymers DA 4 monomers (building blocks), limited structure (double-helix) RA 4 monomers, greater flexibility, multiple structures Proteins 20 Amino Acids,
