The Structure of FADD and Its Mode of Interaction with Procaspase-8
|
|
|
- Marylou Thompson
- 6 years ago
- Views:
Transcription
1 Molecular Cell 22, , June 9, 2006 ª2006 Elsevier Inc. DOI /j.molcel The Structure of FADD and Its Mode of Interaction with Procaspase-8 Paul E. Carrington, 1,2 Cristinel Sandu, 1,2 Yufeng Wei, 1 Justine M. Hill, 1,3 Gaku Morisawa, 1 Ted Huang, 1 Evridipis Gavathiotis, 1 Yu Wei, 1 and Milton H. Werner 1, * 1 Laboratory of Molecular Biophysics The Rockefeller University 1230 York Avenue, Box 42 New York, New York Summary The structure of FADD has been solved in solution, revealing that the death effector domain (DED) and death domain (DD) are aligned with one another in an orthogonal, tail-to-tail fashion. Mutagenesis of FADD and functional reconstitution with its binding partners define the interaction with the intracellular domain of CD95 and the prodomain of procaspase-8 and reveal a self-association surface necessary to form a productive complex with an activated death receptor. The identification of a procaspase-specific binding surface on the FADD DED suggests a preferential interaction with one, but not both, of the DEDs of procaspase-8 in a perpendicular arrangement. FADD selfassociation is mediated by a hydrophobic patch in the vicinity of F25 in the DED. The structure of FADD and its functional characterization, therefore, illustrate the architecture of key components in the deathinducing signaling complex. Introduction The extrinsic cell death pathway is mediated by type I transmembrane receptors of the tumor necrosis factor (TNF) receptor superfamily (Ashkenazi and Dixit, 1998; Wu, 2004). The subgroup of TNF receptors that contain a death domain (DD) within the intracellular region have been termed death receptors (DRs). DRs engage trimeric ligands, which organize the receptors into trimers and stimulate the assembly of an intracellular complex containing the Fas-associated DD protein, FADD (Mort-1) (Boldin et al., 1995; Chinnaiyan et al., 1995), and the initiator procaspases 8 (Flice, MACH-1) (Boldin et al., 1996; Muzio et al., 1996) and 10 (Wang et al., 2001; Kischkel et al., 2001). The assembly of activated receptor, FADD, and the procaspases is commonly known as a death-inducing signaling complex or DISC. FADD is the nucleus of this assembly, as it is responsible for sensing the death stimulus at a receptor and recruiting the procaspases into the nascent DISC. FADD is constructed from two w90 amino acid motifs that are structurally similar to one another (Eberstadt et al., 1998; Jeong et al., 1999; Berglund et al., 2000) but can be readily distinguished by amino acid *Correspondence: mwerner@portugal.rockefeller.edu 2 These authors contributed equally to this work. 3 Present address: Institute for Molecular Bioscience, The University of Queensland, Brisbane QLD 4072, Australia. sequence. The N-terminal death effector domain (DED) and the C-terminal DD each adopt a six a-helical bundle structure that is characteristic of a structural family of death motifs, which includes the caspase-recruitment domain (CARD) and the pyrin domain (Fesik, 2000). The FADD DD is responsible for receptor engagement, while the DED contains the binding site for procaspase-8 and/ or -10 (Chinnaiyan et al., 1995, 1996). However, mutagenesis of FADD (Thomas et al., 2002, 2004; Hill et al., 2004) and functional analysis in multiple contexts (Thomas et al., 2002, 2004; Sandu et al., 2006) demonstrated that the DED must be linked to the DD for a productive interaction with a DR (Thomas et al., 2002, 2004). Although FADD DD can act as an inhibitor at CD95 and DR5 under overexpression conditions in cell culture, an isolated FADD DD cannot be simultaneously shown to bind membrane bound receptor by coimmunoprecipitation (CoIP) with activated receptor (Zhang and Winoto, 1996; Sandu et al., 2006). These observations argue for a physical and functional relationship between the two domains of FADD. The three-dimensional structure of intact FADD illustrates that the DED and DD adopt a well-defined orientation in solution. Combined with functional identification of the CD95 and procaspase-8 binding surfaces under physiological conditions using FADD-deficient cells, the structure illustrates how CD95 and procaspase-8 are likely to be oriented as a building block for DISC assembly. Biochemical analysis in vitro and in cell culture reveals that the DR dependence on the FADD DED for DISC assembly is an obligatory self-association between FADD molecules. Our data define the surface of self-association to be localized to a hydrophobic patch in the vicinity of F25 in the DED, which contrasts a recent report (Muppidi et al., 2006). FADD self-associates at a surface that is independent of the binding site for procaspase-8 in the DED and is likely to occur only in the context of the activated DR at the cell membrane surface. In combination with the recently described structure of the molluscum contagiosum viral FLICE-inhibitory protein (v-flip) MC159 (Li et al., 2005; Yang et al., 2005), we describe a molecular model for the interaction between FADD and procaspase-8, which argues for a physical interaction between the FADD DED and one, but not both, of the DEDs resident in the prodomain of caspase-8. Results Structure Determination The three-dimensional structure of FADD has been determined for residues (of 208), using a single mutation, F25Y, to suppress self-aggregation of the protein at neutral ph in solution (Figure 1). The structure was solved using 2934 experimental restraints that included 1906 nuclear Overhauser effects (NOEs), J NHa coupling constants (Kuboniwa et al., 1994), D NH residual dipolar couplings (RDCs; Bax et al., 2001) collected in bacteriophage Pf1 (Hansen et al., 1998), and D NH couplings in CTAB-doped DLPC/CHAPSO bicelles (Wang et al., 1998). F and J angle restraints were
2 Molecular Cell 600 Figure 1. Three-Dimensional Structure of FADD (A) Stereo superposition of the backbone atoms for the 25 members of the FADD structure family refined in explicit solvent (Supplemental Data). (B) A ribbon representation of FADD illustrating the orthogonal, tail-to-tail orientation of the two domains in the same orientation as in (A). The twelve a helices have been numbered sequentially from the N terminus to the C terminus of the protein. (C) Side view of FADD, illustrating the shape of the molecule. (D) Sequencealignment of the FADD DED and DD. a helices are shaded gray and numbered above the sequence. Residues that permit structural superposition of the two domains are shaded green. Black dots below the sequenceindicate residuesof FADD DD implicated in the interaction with CD95 (Jeong et al., 1999; Bang et al., 2000; Hill et al., 2004). Open circles above the DED indicate residues forming the procaspase-8 binding surface (see text). Asterisks above the DED identify residues whose mutation interferes with the interaction between FADD and CD95 or DR5 (Thomas et al., 2002, 2004).
3 FADD Interaction with Procaspase included from the analysis of 13 C a/b chemical shifts using the program TALOS (Delaglio et al., 1995). To determine the structure, the unambiguously assigned NOEs and 3 J NHa coupling constants were used to fold the molecule from a fully extended chain using a protocol provided with Xplor-NIH (version ) (Schwieters et al., 2003) (see the Supplemental Data available with this article online). Following initial folding, iterative rounds of NOE assignment/refinement were conducted, and pseudopotentials were included for F, J, and c angles (Kuszewski and Clore, 2000), 3 J NHa coupling constants (Garrett et al., 1994), and 13 C a/b chemical shifts (Kuszewski et al., 1995). The radius of gyration was approximated from the number of amino acids to aid in the internal packing of each FADD domain (Kuszewski et al., 1999). The domain orientation resulting from the twomedia RDC refinement was established by alignment tensor projection analysis as described (Al-Hashimi et al., 2000). Of 270 calculated structures, the 100 lowest-energy structures having the best agreement with experimental restraints were subsequently refined in explicit solvent (Spronk et al., 2002) to improve the local geometry, electrostatics, and packing quality for each domain of the intact protein. The 25 lowest-energy structures comprise a family with a root mean square deviation (rmsd) from the mean structure of 1.0 Å for the nonhydrogen backbone atoms between residues (1.4 Å rmsd from the mean for all nonhydrogen atoms) (Table 1 and Supplemental Data). The Structure of FADD FADD is a tandem repeat of structurally homologous domains (Figures 1A and 1B) oriented with the N and C termini facing one another (Figures 1B and 1C). Although the sequence similarity between each domain is quite low (%12%), structurally conserved positions in each domain can be aligned by sequence (Figure 1D), permitting the superposition of the two domains to a rmsd of 2.6 Å over 23 C a positions. The structure of each FADD domain observed in the intact molecule is very similar to the structures observed for the isolated domains (Eberstadt et al., 1998; Jeong et al., 1999; Berglund et al., 2000). The rmsd between the FADD DED in the intact protein and the isolated DED (Eberstadt et al., 1998) is 1.79 Å. The rmsd between the FADD DD in the intact protein and the isolated FADD DD (Berglund et al., 2000) is 2.6 Å (superimposed for residues in helices 2 5), which is similar to the 2.3 Å rmsd observed between the two reported structures of the isolated FADD DD (Jeong et al., 1999; Berglund et al., 2000). The major distinction in the DD structure from the intact protein relative to the isolated domain is the connection between the FADD DED and first helix of the DD. This connection alters the position of this helix in the intact protein relative to that observed in the structures of the isolated domain. Table 1. Structural Statistics for FADD In Vacuum In Solvent b Rmsd from experimental restraints a NOEs (1906) Å Å Intraresidue (487) Å Å Sequential (588) Å Å Medium (1 <ji jj % 5) (472) Å Å Long (1 <ji jj > 5) (191) Å Å Hydrogen bond (168) Å Å F, J, c 1 (480) º º 3 J NHa (120) Hz 13 C a ppm 13 C b ppm 1 D phage NH (123) Hz Hz 1 D bicelle NH (137) Hz Hz Deviations from Idealized Covalent Geometry Bonds (Å) Angles (º) Impropers (º) Coordinate Precision c Backbone DED + DD (residues 5 183) Å Å DED (residues 5 86) Å Å DD (residues ) Å Å All nonhydrogen DED + DD (residues 5 183) Å Å DED (residues 5 86) Å Å DD (residues ) Å Å Quality Factors d Percent residues in most 79.7% 88.5% favorable Ramachandran Bad contacts/structure a Numbers in parentheses indicate total number of restraints. Deviations calculated for residues No NOEs were included for atoms separated by three chemical bonds in the refinement, with the exception of those involving backbone atoms. b Refined in explicit solvent as described in Spronk et al. (2002), using NOE, dihedral, and RDC restraints only. c Calculated with the programs PROCHECK and PROCHECK_NMR (Laskowski et al., 1996) relative to the mean structure of the 25 final family members. d Calculated with PROCHECK_NMR. Less than 1% of residues appear in generously allowed regions, and less than 0.1% of residues appear in unfavored regions of the Ramachandran plot. Bad contacts calculated with PROCHECK. The Domains of FADD Are Oriented Spin relaxation analysis of FADD (Figure S1) indicated that FADD tumbles with an apparent rotational correlation time (t c ) of ns calculated from modelfree analysis of the 15 N{ 1 H} backbone dynamics (Lipari and Szabo, 1982a, 1982b). This is comparable to the t c computed for each of the domains in the intact molecule (DED, t c is ns; DD, ns), suggesting that the individual domains of FADD tumble as a single entity in solution. A plot of R 2 /R 1 versus occurrence for each of the two FADD domains indicates that the two domains of FADD are likely to be oriented in solution (see Figure S2). A similar conclusion could be drawn from a plot of the RDCs measured in Pf1 phage as a function of occurrence (see Figure S2) (Braddock et al., 2001). Since the RDCs can report directly on the relative orientation of protein segments in solution (Al-Hashimi et al., 2000; Bax et al., 2001), a projection analysis was used to interpret the experimental data collected from two alignment media in order to determine whether a single relative orientation of the FADD domains existed for the proteins in solution (Al-Hashimi et al., 2000). To define that orientation, the structure of FADD was refined against both sets of experimental RDCs and the alignment tensor measured in bicelles projected into the
4 Molecular Cell 602 Figure 2. The Interdomain Interface of FADD (A) 13 C-edited (top) and 15 N-edited (bottom) NOESY spectra of FADD. The indicated strips were taken at the 13 C chemical shift of H59-CD2 and the 15 N chemical shift for the side chain of N155. These strips display NOEs (indicated) that could be inferred as interdomain contacts. Note that the 13 C-edited NOESY displays 13 C shifts in lieu of 1 H shifts to take advantage of the larger chemical shift range of 13 C. (B) The interface between the FADD DED (cyan) and DD (blue) is shown by a cylinder representation for one member of the structure family. The orientation of selected side chains for the entire family is shown for those residues (red) forming salt bridges and/or hydrogen bonds across the interface. Donor and acceptor atoms are shown in black. (C) Stereo representation of the interface colored as in (B) for each domain. A close-up view of the domain interface is shown as a molecular surface in stereo with selected side chains from one member of the family in red and the putative donor or acceptor atom in black. frame of reference of the phage (see Figure S2). This resulted in a single orientational solution for the domains of FADD (see Figure S2) (Al-Hashimi et al., 2000). This approach rigorously defined the relative orientation of the two FADD domains but could not define how closely packed the two domains were in the intact protein. Chemical shift degeneracies precluded the identification of many unambiguous interdomain NOEs that could help define the spatial relationship between the FADD domains. However, two unambiguous NOEs were observed (Figure 2A), as were several NOEs between the ends of the six-residue linker (residues 87 92) and each folded domain. Collectively, these restraints sterically hindered the orientation of the interdomain linker, defining the spatial relationship between the two FADD domains (Figures 2B and 2C). The small interface Figure 3. Mapping Functionally Deficient Mutants onto the Structure of FADD (A) The structure of FADD is shown in a surface representation and colored as described in Figure 1. Highlighted in red are residues whose mutation results in a loss of association with CD95. (B) Additional mutants, which reside on the opposite face of the DD (D106, K125, R142, and R146), are defective in CD95 association (Hill et al., 2004), but not TRADD association (Sandu et al., 2005), as discussed in the text. (C) Mapping of self-association-deficient mutants onto the structure of FADD. The structure of FADD has been rotated 90º about the vertical axis relative to (A). Highlighted in orange are residues of the DED whose mutation disrupts receptor interaction (Thomas et al., 2002, 2004) (see Supplemental Data). Highlighted in red are residues whose mutation disrupts the interaction with the receptor (DD mutants). Highlighted in tan are positions in the DED whose mutation disrupts both receptor and procaspase interaction (see text). (D) Further rotation of 150º about the vertical axis reveals the location of the procaspasespecific mutation sites in the FADD DED. These residues are highlighted in green and labeled (see text).
5 FADD Interaction with Procaspase Figure 4. Identification of the FADD Binding Surface for Procaspase-8 (A) GST-CD95 intracellular domain (IC) pull-down experiment with 35 S-labeled FADD and procaspase-8 (Hill et al., 2004). S12, R38, D44, and E51 were defective for the interaction with procaspase-8, but not CD95 IC. Note that a truncated form of FADD (residues 1 183) did not disrupt the interaction between FADD and CD95 or FADD and procaspase-8 in this context. Procaspase-8 failed to associate with GST-CD95 IC on its own or with GST itself (data not shown) in this assay. (B) FADD-deficient Jurkat I2.1 cells stably transfected with wild-type or mutant FADD, as indicated (each with an HA epitope tag) were assessed by CoIP following stimulation with antibody CH-11 (150 ng/ml for 3 hr). F25R was unable to associate with CD95, but D44R retains CD95 interaction despite its lower level of expression. (C) Ectopic expression of FADD D44R is robust in MCF7-Fas cells (Jaattela et al., 1995) and coimmunoprecipitates with CD95 as well as wild-type FADD in this context. (D) Flow cytometry analysis of stably transfected Jurkat I2.1 cells following CH-11 stimulation as in (B). Cells expressing FADD F25R and D44R are completely defective in programmed cell death. (E and F) To ensure that the apoptotic defect for D44R in Jurkat I2.1 cells was not an artifact of the level of protein expression, FADD or FADD D44R was ectopically expressed in the same cells, confirming that FADD D44R is defective in cell death. In this instance, the level of FADD or FADD D44R expression was robust (F). between the domains (buried accessible surface of 515 Å 2 ) is established through interactions that may be inferred between residues in the interhelical loops of a4/a5 and a10/a11 (Figures 2B and 2C). The side chains of E56 and H59 in the DED are in proximity to E154 and N155, respectively, and display NOEs between them in each case (Figure 2A). These residues probably form salt bridges or hydrogen bonds to establish the spatial relationship between the domains observed for FADD in solution. Interaction with the CD95 DD FADD has been extensively mutated in the DD in an effort to identify the surface of FADD responsible for interaction with activated CD95 (Jeong et al., 1999; Bang et al., 2000; Hill et al., 2004). The majority of FADD residues whose mutation disrupted the interaction with CD95 reside along a contiguous surface of the FADD DD (Jeong et al., 1999; Bang et al., 2000; Hill et al., 2004) (Figure 3). This surface (Figure 3A) is composed of a patch of positive charge (K110, R113, R114, R117, R127) and a predominantly nonpolar surface in the vicinity of the a11/a12 interhelical loop. An additional CD95 binding surface has been described (Hill et al., 2004) that forms a small basic patch (K125, R142, and R146) on the opposite side of the domain. Although the mutant R142E disrupts the interaction with CD95 (Hill et al., 2004), it has no effect on interaction between FADD and TRADD (Sandu et al., 2005). Thus, this patch of basic residues appears to play a role particular to receptor association, possibly forming contacts to a neighboring CD95 DD in the activated receptor complex.
6 Molecular Cell 604 Figure 5. FADD Self-Associates through Its DED (A) Flag-tagged full-length FADD was ectopically expressed in HEK293 cells with HA-tagged full-length FADD or HA-tagged individual FADD domains. HA-tagged full-length FADD and HA-tagged FADD DED coimmunoprecipitated with Flag-tagged full-length FADD, indicating that FADD can self-associate through the DED. (B) The DED surface responsible for FADD self-association was identified by mutagenesis at the indicated positions. Flag-tagged full-length FADD was used to coimmunoprecipitate mutant, HA-tagged full-length FADD, illustrating that a region that includes F25 and K33 forms the self-association surface. (C) The NMR peak patterns of the F25Y and F25Y/K33E are similar to one another, indicating only local chemical shift perturbations in the vicinity of K33E. By contrast, F25Y/R71E results in significant line broadening for many peaks in the DED and many spectral changes. The extent of shift perturbation is shown as a function of sequence in the histogram plots to the right of each spectrum. Asterisks indicate residues that lose more than 50% of the signal intensity in the double mutant relative to F25Y. The shift differences are represented as an increased thickness on a backbone worm representation of the FADD DED. Red indicates the position of the most shifted residue, yellow the secondmost shifted signals,
7 FADD Interaction with Procaspase Identification of a Procaspase-Specific Binding Surface on the DED To define the binding surface for procaspase-8, an initial screen introduced mutants into the DED that were neutral to the interaction between CD95 and FADD in a GST pull-down assay, but disrupted the interaction between CD95/FADD complexes and procaspase-8 (Figure 4A). This screen identified S12, R38, D44, and E51 as potential participants in the interaction with procaspase-8, which localized to a DED surface encompassing helices a1 and a4 (Figures 3D and 4A). F25R and K33E, two residues that reside on either end of the loop between a2 and a3, also failed to bind CD95 in this assay (data not shown). F25 and K33 were previously implicated in binding either procaspase-8 (Eberstadt et al., 1998) or the FLICE-inhibitory protein c-flip (Kaufmann et al., 2002) in a pull-down assay. To elucidate the role of these DED positions in procaspase interaction, F25R and D44R were stably transfected into FADD-deficient Jurkat I2.1 cells. CoIP with anti-cd95 demonstrated that D44R, but not F25R, retained the ability to coassociate with activated CD95 (Figure 4B), even though both mutants disrupted the ability of FADD to respond to FasL (Figure 4D). These observations suggested that F25R and D44R impact different steps in the signal transduction process. A potential issue was the relatively low level of stable D44R expression in Jurkat I2.1 cells, which could not be improved despite repeated attempts. To ensure that the loss of FasL response was not solely due to the expression level, FADD D44R was ectopically expressed in both MCF7-Fas cells (Jaattela et al., 1995) (Figure 4C) and Jurkat I2.1 cells (Figure 4F). Just as for the stably transfected cells, D44R retained the ability to coimmunoprecipitate with CD95 from MCF7-Fas cells (Figure 4C) and failed to respond to a FasL stimulus in Jurkat I2.1 cells (Figure 4E). Thus, the loss of function for D44R was most likely due to a disruption in the interaction with the procaspases, as suggested by Figures 4A and 4B. F25R, on the other hand, introduced a more fundamental defect. FADD Self-Associates through the DED F25, in conjunction with L28 and K33, forms a small cluster of DED residues between a2 and a3 whose mutation disrupts both the interaction with CD95 (Figure 4B and Sandu et al. [2006]) and the interaction with procaspase-8 or c-flip (Eberstadt et al., 1998; Kaufmann et al., 2002; Sandu et al., 2006). Mutation of Q34 and K35 also disrupts the interaction with procaspase-8 and c-flip (Kaufmann et al., 2002). We reasoned that since mutation of F25 to tyrosine was necessary to suppress FADD aggregation in solution (see also Eberstadt et al. [1998]), the simplest explanation for the role some amino acids play between F25 and K35 was that they mediated FADD-FADD interaction in a functional context. To test this hypothesis, Flag-tagged full-length FADD was coexpressed with either HA-tagged full-length FADD or the corresponding individual FADD domains in HEK293 cells. CoIP with FADD could only be achieved with full-length FADD or the FADD DED in this context (Figure 5A). Thus, the FADD DED mediates self-association. To identify the surface of self-association, mutants were introduced into full-length FADD, and CoIP was performed between wild-type, Flag-tagged FADD, and mutant HA-tagged FADD (Figure 5B). This experiment revealed that F25 and K33 form part of a FADD selfassociation surface. Other regions, in particular those in the RXDLF motif (Hill et al., 2002) ina6 (R71, D74, R77) and along the procaspase binding surface (D44), do not impinge upon FADD-FADD interaction in this assay. One of these tested positions, R71, has been implicated in modulating the interaction between receptor and FADD (Thomas et al., 2002, 2004; Supplemental Data), and several mutations in the RXDLF motif between residues 72 and 76 (R72A, R72A/D74A, or L75A/ L76A) disrupt the FRET response between FADD molecules overexpressed as YFP or CFP fusions in cell culture (Muppidi et al., 2006). The disruption in FRET led to the suggestion that it was the RXDLF motif that was the site of self-association in FADD (Yang et al., 2005; Muppidi et al., 2006), in contrast to our results (Figure 5B). To resolve this discrepancy, the impact of RXDLF mutation was examined by NMR spectroscopy in either the FADD DED or the FADD NMR construct (residues 2 191). The NMR spectrum of F25Y/K33E DED (Figure 5C) illustrates the following: (1) the pattern of peaks is similar for F25Y and F25Y/K33E, indicating a similar threedimensional fold for the two species; (2) chemical shift changes in F25Y/K33E are localized to the site of the mutation, suggesting only a local impact of the mutation on the protein structure; and (3) minimal changes in line width are observed for residues in other regions of the domain, indicating a minimal effect of K33E on the global fold of the domain. By contrast, the F25Y/R71E DED mutant displays a significant number of exchange-broadened peaks in the NMR spectrum and substantial chemical shift changes throughout the domain (Figure 5C). Although the similarity in peak pattern of F25Y/R71E relative to F25Y DED suggests that the F25Y/R71E domain is folded, the broadened lines indicate that the mutation induces dynamic disorder in the DED. The R72A mutation, which was sufficient to disrupt the FRET response between FADD-CFP and FADD-YFP in cell culture (Muppidi et al., 2006), had a more global impact on the protein structure. Introduction of F25Y/R72A into the NMR construct (residues 2 191) demonstrates that FADD is substantially disordered. Many NMR peaks exchange broaden, and none of the DED resonances are visible (Figure 5D), even though the protein was physically intact by SDS-PAGE (Figure 5D, inset) and retained much of its helicity as measured by CD (Figure 5D). Since several and cyan the least shifted signals. Note that the sample of F25Y displays some unfolded impurity as weak, sharp signals at w8 ppm that are not seen in samples of the mutant. (D) Overlay of NMR spectra for FADD (residues 2 191) F25Y versus F25Y/R72A. Exchange-broadened signals of F25Y/R72A in the DED are indicated by circled residues/labels for peaks in the F25Y spectrum. SDS-PAGE indicated that F25Y/R72A is intact (see inset). Shown in blue are selected residues in helical regions of the DD, which appear in the same positions in both spectra, suggesting that at least parts of the protein are folded. CD spectra of the FADD F25Y and F25Y/R72A (residues 2 191) NMR samples are shown to the right, indicating a similar helical content for the two proteins.
8 Molecular Cell 606 Figure 6. Stabilization of the DED Fold Stereo view of the 1.2 Å X-ray crystal structure of DED2 from MC159 (Yang et al., 2005). Residues forming salt bridges stabilizing the a1/a2 and a5/a6 helix-loop-helix elements are shown as sticks and labeled. The equivalent residues in the FADD DED are labeled with parentheses. Evidence for a hydrogen-bonded Arg side chain is observed in NMR spectra of the DED of FADD and PEA-15, wherein a single Arg N 3 proton is observed at 9 ppm, indicative of hydrogen bonding to an Arg side chain (data not shown). A trio of hydrophobic residues contributes to this charge-stabilized interaction. All six of these residues are highly conserved in the sequences of the FADD, procaspase, PEA-15, and MC159 DEDs (B). Blue shading indicates the six residues displayed in (A), green shading the additional residues forming part of the RXDLF motif and residues in a2 contributing to the stabilization of the domain fold. For comparison purposes, the hydrophobic patch is shaded yellow. peaks for helical residues in the FADD DD are unchanged in chemical shift (Figure 5D), at least some of the F25Y/ R72A protein retains its original tertiary structure. The loss in FADD self-association (Muppidi et al., 2006) or function (Yang et al., 2005) by mutation within RXDLF, therefore, most likely occurs secondary to a loss in structural integrity. The R71E and D74A mutations have a lower impact on overall protein order, accounting for the observation that self-association is still observed between wild-type and either the R71E or D74A mutant proteins in the CoIP experiment (Figure 5B). The role RXDLF plays in stabilizing the protein fold can readily be appreciated from the 1.2 Å crystal structure of v-flip MC159 (Figure 6)(Yang et al., 2005). Discussion A Diverse Organization for Tandem Death Motifs The three-dimensional structure of intact FADD reveals a tandem DED and DD oriented in an orthogonal, tailto-tail fashion. FADD exists in an extended conformation, leaving its surfaces for interaction with CD95, with the procaspases, and with itself sterically unhindered (Figure 3). The extended structure of FADD is contrasted by the more compact structure of tandem DEDs observed in the v-flip MC159 (Figure 7A) (Li et al., 2005; Yang et al., 2005). A predominantly hydrophobic interface is observed between DED1 and DED2 of MC159, which includes burial of F30 (equivalent to FADD F25) at the DED interface. Given the sequence similarity between the FADD and MC159 DEDs (>50%), one wonders why FADD isn t structurally more similar to MC159. The principal difference between the two proteins appears in a6 in FADD and in the corresponding a6/a7 helices in MC159 DED1. MC159 a6 (M71-F77) and a7 (K81- E86) are orthogonal and fold back on one another to form a series of extensive contacts with a5 in the domain. This pulls the irregularly structured portion of the linker (L87 L93) up toward DED1, inserting F92 into a pocket formed by three helices of DED 1 (a2, a4, and a5) (Figure 7A). FADD both lacks the hydrophobic residues found in the interdomain linker of MC159 and displays a continuous a6 helix from K73 to A86 (Figure 7A). Thus, FADD would be unable to form a DEDto-DD arrangement homologous to the tandem DEDs in MC159. A Model for the FADD/Procaspase-8 Complex The sequence similarity between the DEDs of MC159 and FADD suggests that the structure of MC159 might provide an insight into how FADD engages one or more DEDs in the procaspase prodomain. Residues from helices a2 and a5 in DED1 engage residues in helices a1 and a4 of DED2 in MC159 (Figures 7A and 7B) (Li et al., 2005; Yang et al., 2005). The procaspase binding-deficient mutants in the FADD DED reside in a1 and a4 and overlap with residues of MC159 DED2 forming the interface with DED1 (Figure 7B), suggesting that the FADD DED may be homologous to MC159 DED2 in its approach to a procaspase prodomain. This implies that one of the DEDs of the procaspase prodomain should be equivalent to MC159 DED1. To meet this criteria, a prodomain DED must display two hydrophobic residues at the interdomain interface in MC159, namely F30 and L31. The DEDs of procaspase-8 display a pair of conserved hydrophobic residues at structurally
9 FADD Interaction with Procaspase Figure 7. A Model for the Interaction between FADD and Procaspase-8 (A) The structure of FADD is compared with v-flip MC159, illustrating the structural distinctions between these proteins (see text). The insertion of the linker residue F92 in MC159 into DED1 is indicated in red and can be compared to the position of P92 in FADD (also red). (B) The structure of MC159 is shown with DED2 in cyan and DED1 in green. Highlighted in DED2 are helices a1 (red) and a4 (orange). Yellow bands represent positions in MC159 DED2 that are structurally equivalent to S12, R38, D44, and E51 in FADD. Blue bands highlight residues in MC159 DED2 that form contacts with DED1 (E105, R135, and N145) (Li et al., 2005; Yang et al., 2005). These comparisons suggest that the procaspase-8 binding surface of the FADD DED, formed by the a1 and a4 helices, engages procaspase-8 in a manner that is related to the interaction between MC159 DED2 and DED1. The FADD DED would be equivalent to MC159 DED2 (see text). (C) A docking model for FADD bound to procaspase-8, constructed with the program HADDOCK (Dominguez et al., 2000; seefigure S4). The resultant model has been colorized to highlight the a1 and a4 helices as in (B). Arrows emphasize the helix orientation at the FADD/procaspase-8 and procaspase-8 DED1/DED2 interfaces. Note that the a1/a4 helices in FADD are nearly perpendicular to the a1/a4 helices in procaspase-8 DED2. While the model suggests that the orientation of the FADD DED and DD remains unchanged in the presence of procaspase-8, this has yet to be determined. equivalent positions to MC159 F30 and L31: F24/L25 in DED1 and F122/L123 in DED2. However, only the residues in procaspase DED2 would be accessible for an interaction with FADD since F24 and L25 in procaspase DED1 would be buried at the DED1/DED2 interface. We conclude, therefore, that the procaspase-specific binding surface in FADD engages procaspase-8 DED2 in the vicinity of F122 and L123 (Figure 7C). Mutagenesis at these positions of procaspase-8 disrupts CoIP between FADD and procaspase-8 from 293T cells (Yang et al., 2005), in support of our view. The model constructed on the basis of the structure/function analysis (see Figure S4) suggests that the procaspase-8 prodomain would be oriented perpendicular to the FADD DED (see arrows in Figure 7C). While the model implies that the long axis of FADD and the prodomain are perpendicular to one another, we do not know if any reorientation of the FADD domains occurs upon binding CD95 or procaspase-8. An Emerging Picture of the DISC The structure/function correlates described for FADD and the structure of the v-flip MC159 (Li et al., 2005; Yang et al., 2005) lead to a convergent hypothesis for the interaction between FADD and procaspase-8. FADD DED mutants specifically deficient in the interaction with procaspase-8 structurally superimpose with residues at the interface between the two DEDs in MC159. This observation argues strongly that FADD should engage a procaspase-8 DED in a manner that is topologically similar to the DED-to-DED interaction in MC159. A similar hypothesis led to the identification of mutants in procaspase-8 DED2 that interfere with FADD binding (Yang et al., 2005). It is now apparent that the FADD DED is required to be covalently attached to the DD for a stable interaction with CD95 (Sandu et al., 2006). The observation that an exposed hydrophobic patch (Eberstadt et al., 1998) in the vicinity of F25 mediates FADD self-association
10 Molecular Cell 608 (Figure 5B) resolves a long-standing conundrum on the role of this patch in FADD function. Mutations within this patch disrupt all FADD functions without simultaneously disrupting the protein fold (Figure 5). By contrast, mutations in a5 and a6, which include the RXDLF motif (Hill et al., 2002; Kaufmann et al., 2002), lead to a loss in structural integrity for FADD (Figures 5C and 5D) as well as for the DED of the phosphoprotein enriched in astrocytes, PEA-15 (Hill et al., 2002). Although FRET suggested that FADD self-association is mediated by the RXDLF motif (Muppidi et al., 2006), NMR spectroscopy demonstrates that mutation of this motif is not tolerated by either PEA-15 (Hill et al., 2002) or FADD (Figure 5). Loss of function for the RXDLF mutants appears to be the consequence, therefore, of dynamic disorder introduced into the FADD DED. It is interesting to note that, while FADD and PEA-15 both contain the RXDLF motif and share 55% sequence similarity between their DEDs (Figure 6B), PEA-15 does not self-associate (Hill et al., 2002). FADD, on the other hand, self-associates via the hydrophobic patch in the vicinity of F25 (Figure 5), a feature absent in the DED of PEA-15 (Figure 6B). The emerging picture of the DISC as a complex of simultaneous FADD-FADD and FADD-receptor interactions can now explain why FADD does not spontaneously activate cell death in the absence of a ligand stimulus. Although FADD, procaspase-8, and procaspase-10 are constitutively present in cells, the affinity of FADD/CD95 or FADD/FADD interactions must be relatively low in isolation. This explains why the interaction between isolated FADD and CD95 DDs is neither easily reconstituted (Berglund et al., 2000) nor seen by CoIP from cells expressing the FADD DD (Zhang and Winoto, 1996; Sandu et al., 2006). To assemble a DISC requires a membrane-localized, activated receptor, in which the receptors cluster into higher order states in a FADDdependent manner (Siegel et al., 2004; C. Wu, C.S., and M.H.W., unpublished data). Since the procaspases appear to be recruited to the DISC as a preformed dimer (Boatright et al., 2003), the building block of the DISC should require two molecules of FADD, one for each molecule of procaspase. Thus, we speculate that a FADD homodimer forms at, or between, activated DRs to create the structural context necessary for procaspase recruitment and activation. This may explain why FADD/FADD and FADD/CD95 interactions are simultaneously required for productive DISC assembly. Given the close sequence similarity between c-flip, procaspase-8, and procaspase-10, it is likely that they will each bind the same surface of the FADD DED identified in this study. MC159 appears to be an exception, for it does not appear to compete with procaspase-8 for FADD binding sites in a pull-down assay (Yang et al., 2005), and MC159 must simultaneously associate with TRAF3 to act as an inhibitor of apoptosis (Thurau et al., 2006). Experimental Procedures Proteins and Nucleic Acids The design, construction, and expression of FADD and FADD mutants for protein interaction assays, NMR spectroscopy, and cellbased assays were conducted as previously described (Hill et al., 2004; Sandu et al., 2005). All of the FADD mutants tested for either self-association (Figure 5) or interaction with procaspase-8 (Figure 4) were previously studied for their effect on CD95 (Hill et al., 2004) or TRADD interaction (Sandu et al., 2005). NMR samples contained mm protein at ph 6.8 in either 10 mm sodium phosphate/ 300 mm NaCl or in 200 mm sodium phosphate buffer. Both buffers contained 1 mm benzamidine HCl, 1 mm DTT, and 50 mm NaN 3. For the mutations in the FADD DED (residues 2 88), the protein was expressed from pet28b and purified by Ni 2+ -chelate chromatography. NMR samples were prepared in 50 mm potassium phosphate (ph 4), 150 mm NaCl, and 1 mm DTT as previously described (Eberstadt et al., 1998). The F25Y/R72A mutant DED was poorly expressed at 20ºC or 37ºC, unstable at neutral ph, and found to be unfolded at ph 4 in the NMR tube; hence, this mutant was expressed as residues from pqe9 (Qiagen) with an N-terminal His 6 tag in the F25Y background and assessed under the same conditions used for the structure determination. Cell Culture and Transient and Stable Transfection Transient transfection of MCF7-Fas cells (Jaattela et al., 1995) with FADD and FADD D44R was performed as previously described (Hill et al., 2004). Jurkat I2.1 cells were stably transfected with various FADD constructs as previously described (Thomas et al., 2002, 2004; Sandu et al., 2005). Briefly, cells were transfected with 40 mg of PvuI linearized pcdna3.1-puro(+) vector encoding different FADD mutants/constructs. The cells were electroporated using a Bio-Rad Gene Pulser at 270V and 960 mf, and the cells were selected for puromycin resistance. The expression of FADD constructs was tested by Western blotting with rabbit anti-fadd polyclonal antibody (Santa Cruz Biotechnology) or an anti-ha peroxidase (3F10, Roche Diagnostics GmbH). Jurkat I2.1 cells were transiently transfectedwithdmrie-creagent (Invitrogen) using 4 mg ofanexpression vector individually encoding GFP and FADD or FADD mutant. Apoptosis Assay For stably transfected cells, Jurkat cells/ml were treated with an agonistic antibody (CH-11, 150 ng/ml) for 3 hr at 37ºC. Cells were stained with the FITC-conjugated Annexin V (FITC-Annexin V) and propidium iodide (Sigma-Aldrich). Cells ( ) were acquired and analyzed on a FACSCalibur flow cytometer (Becton Dickinson) using the CellQuest software (BD Biosciences). Apoptotic cells were quantified as the percentage of cells stained with Annexin V. For transiently transfected cells, the cells were stained with AnnexinV-Cy3, and GFP-positive cells were counted 18 hr posttransfection and 3 hr after activation with CH-11 (150 ng/ml). Coimmunoprecipitation IP of CD95 with FADD from MCF7-Fas cells was performed as previously described for overexpressed cells (Hill et al., 2004). IP of CD95 with FADD mutants from Jurkat I2.1 cells was performed by suspending cells stably expressing FADD or FADD mutants in 3 ml of RMPI medium (2FBS) and stimulated with 3 mg of CH-11 anti-human CD95 IgM for 10 min at 37ºC. The cells were harvested and washed with cold PBS and resuspended in 1 ml of lysis buffer, incubated on ice for 10 min, and cleared by centrifugation. Nine hundred microliters of supernatant was mixed with 40 ml of goat anti- IgM-agarose beads (Sigma-Aldrich) and nutated for 3 hr at 4ºC. The beads were washed five times with lysis buffer and resuspended in 30 ml of SDS sample loading buffer prior to SDS-PAGE. Circular Dichroism CD spectra were acquired with an AVIV model 62DS spectrometer, using a 0.1 cm path-length cell. The NMR samples of Figure 5D were diluted with 50 mm sodium phosphate (ph 7.0) to 8 mm final concentration. Spectra were acquired between 195 and 260 nm in 1.0 nm steps with a 3 s delay time for three scans at 15ºC. The data were baseline corrected by solvent subtraction. Molar ellipticity was calculated from the average of reported ellipticity of the three scans using the equation ([Q] = 3298 D3). NMR Spectroscopy All NMR experiments were acquired at 15ºC using either a Bruker DMX 600, Avance 600, or Avance 800, all equipped with a cryoprobe. RDCs were measured in the NMR sample buffer to which the
11 FADD Interaction with Procaspase filamentous phage Pf1 was added to a final concentration of w9 mg/ ml (Hansen et al., 1998) or by 1:1 dilution of a 10% stock solution of DLPC/CHAPSO/CTAB (4.2:1:0.1 molar ratios) (Wang et al., 1998) with FADD stock solution (1 mm). Initial values for the axial component of the dipolar coupling tensor and for the rhombicity were determined from a normalized histogram of the RDCs (Clore et al., 1998). NMR spectra were processed with NMRPipe/NMRDraw (Delaglio etal., 1995) andanalyzedusing PIPP andstapp (Garrett et al., 1991). Structure Calculations The structures were calculated with the program X-PLOR-NIH adapted to incorporate pseudopotentials for 3 J NHa coupling constants (Garrett et al., 1994), secondary 13 C a and 13 C b chemical shifts (Kuszewski et al., 1995), a conformational database potential for dihedral angles (Kuszewski and Clore, 2000), and a harmonic potential for the refinement against RDCs (Tjandra et al., 1997). Initial refinement began from an extended polypeptide chain and the fold established with the unambiguous set of NOEs, F and J angles extracted from TALOS (Cornilescu et al., 1999), and 3 J NHa couplings constants. The two interdomain NOEs and/or the RDCs measured in Pf1 bacteriophage were included in separate folding trials as described in Supplemental Data. These folding experiments established that the interdomain NOEs and RDCs reinforced one another rather than biased the initial model, leading to a single model following iterative rounds of NOE analysis and refinement employing a torsion angle restraint protocol provided in the Xplor-NIH distribution. Both sets of RDCs were included following the initial folding trials with the force constant set to a final value of 1.0. The agreement between expected and calculated RDC values was determined by SVD analysis with the program DC (Delaglio etal., 1995). Structure quality was assessed with PROCHECK and PROCHECK_NMR (Laskowski et al., 1996). Structures were displayed and analyzed using the program PYMOL (DeLano 2002). Supplemental Data Supplemental Data include supplemental text and four figures and can be found with this article online at content/full/22/5/599/dc1/. Acknowledgments We thank Vishva Dixit, Michael Lenardo, and Marcus Peter for cdnas of human CD95, FADD, and caspase-8; and Marcus Peter for MCF7-Fas cells and antibodies. We further would like to acknowledge Yigong Shi, Hao Wu, David Fushman, Ranajeet Ghose, Joel Tolman, and G. Marius Clore for many useful discussions or the sharing of unpublished results over the course of this work. This work was supported by fellowships from the Human Frontier Science Program, the NHMRC of Australia, and the Norman and Rosita Winston Foundation (to J.M.H.) and by the National Science Foundation, the National Institutes of Health, and Irma T. Hirschl Trust grants (to M.H.W.). M.H.W. is a Distinguished Young Scholar of the W.M. Keck Foundation. Received: December 8, 2005 Revised: March 15, 2006 Accepted: April 14, 2006 Published: June 8, 2006 References Al-Hashimi, H.M., Valafar, H., Terrell, M., Zartler, E.R., Eidsness, M.K., and Prestegard, J.H. (2000). Variation of molecular alignment as a means of resolving orientational ambiguities in protein structures from dipolar couplings. J. Magn. Reson. 143, Ashkenazi, A., and Dixit, V.M. (1998). Death receptors: signaling and modulation. Science 281, Bang, S., Jeong, E.-J., Kim, I.-K., Jung, Y.-K., and Kim, K.-S. (2000). Fas- and tumor necrosis factor-mediated apoptosis uses the same binding surface of FADD to trigger signal transduction. J. Biol. Chem. 275, Bax, A., Kontaxis, G., and Tjandra, N. (2001). Dipolar couplings in macromolecular structure determination. Methods Enzymol. 339, Berglund, H., Olerenshaw, D., Sankar, A., Federwisch, M., McDonald, N.Q., and Driscoll, P.C. (2000). The three-dimensional solution structure and dynamic properties of the human FADD death domain. J. Mol. Biol. 302, Boatright, K.M., Renatus, M., Scott, F.L., Sperandio, S., Shin, H., Pedersen, I.M., Ricci, J.E., Edris, W.A., Sutherlin, D.P., Green, D.R., and Salvesen, G.S. (2003). A unified model for apical caspase activation. Mol. Cell 11, Boldin, M.P., Varfolomeev, E.E., Pancer, Z., Mett, I.L., Camonis, J.H., and Wallach, D. (1995). A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270, Boldin, M.P., Goncharov, T.M., Goltsev, Y.V., and Wallach, D. (1996). Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85, Braddock, D.T., Cai, M.L., Baber, J.L., Huang, Y., and Clore, G.M. (2001). Rapid identification of medium- to large-scale interdomain motion in modular proteins using dipolar couplings. J. Am. Chem. Soc. 123, Chinnaiyan, A.M., O Rourke, K., Tewari, M., and Dixit, V.M. (1995). FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, Chinnaiyan, A.M., Tepper, C.G., Seldin, M.F., O Rourke, K., Kischkel, F.C., Hellbardt, S., Krammer, P.H., Peter, M.E., and Dixit, V.M. (1996). FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J. Biol. Chem. 271, Clore, G.M., Gronenborn, A.M., and Bax, A. (1998). A robust method for determining the magnitude of the fully asymmetric alignment tensor of oriented macromolecules in the absence of structural information. J. Magn. Reson. 133, Cornilescu, G., Delaglio, F., and Bax, A. (1999). Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13, Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. (1995). NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, DeLano, W.L. (2002) The PyMOL molecular graphics system. ( Dominguez, C., Boelens, R., and Bonvin, A.M.J.J. (2000). HAD- DOCK: a protein-protein docking approach based on biochemical and/or biophysical information. J. Am. Chem. Soc. 125, Eberstadt, M., Huang, B.H., Chen, Z.H., Meadows, R.P., Ng, S.C., Zheng, L.X., Lenardo, M.J., and Fesik, S.W. (1998). NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature 392, Fesik, S.W. (2000). Insights into programmed cell death through structural biology. Cell 103, Garrett, D.S., Powers, R., Gronenborn, A.M., and Clore, G.M. (1991). A common sense approach to peak picking in two-, three-, and fourdimensional spectra using automatic computer analysis of contour diagrams. J. Magn. Reson. 95, Garrett, D.S., Kuszewski, J., Hancock, T.J., Lodi, P.J., Vuister, G.W., Gronenborn, A.M., and Clore, G.M. (1994). The impact of direct refinement against three-bond HN-CaH coupling constants on protein structure determination by NMR. J. Magn. Reson. B. 104, Hansen, M., Mueller, L., and Pardi, A. (1998). Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions. Nat. Struct. Biol. 5, Hill, J.M., Ramos, J., Ginsberg, M., and Werner, M.H. (2002). The interaction of the death motif protein PEA-15 with ERK MAP kinase reveals a common docking site for protein interaction with the death domain/death effector domain fold. EMBO J. 21, Hill, J.M., Morisawa, G., Kim, T., Huang, T., Wei, Y., Wei, Y.F., and Werner, M.H. (2004). Identification of an expanded binding surface
Theory and Applications of Residual Dipolar Couplings in Biomolecular NMR
 Theory and Applications of Residual Dipolar Couplings in Biomolecular NMR Residual Dipolar Couplings (RDC s) Relatively new technique ~ 1996 Nico Tjandra, Ad Bax- NIH, Jim Prestegard, UGA Combination of
Theory and Applications of Residual Dipolar Couplings in Biomolecular NMR Residual Dipolar Couplings (RDC s) Relatively new technique ~ 1996 Nico Tjandra, Ad Bax- NIH, Jim Prestegard, UGA Combination of
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets
 Supporting information Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets Wan-Na Chen, Christoph Nitsche, Kala Bharath Pilla, Bim Graham, Thomas
Supporting information Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets Wan-Na Chen, Christoph Nitsche, Kala Bharath Pilla, Bim Graham, Thomas
Supplementary Figure 1.
 a b c d e f g 1 Supplementary Figure 1. Identification of unfolded regions in the Chz1-H2A.Z-H2B complex and structure and dynamics of Chz.core-sH2B_H2A.Z. (a) 1 H- 15 N HSQC spectrum of Chz1. All backbone
a b c d e f g 1 Supplementary Figure 1. Identification of unfolded regions in the Chz1-H2A.Z-H2B complex and structure and dynamics of Chz.core-sH2B_H2A.Z. (a) 1 H- 15 N HSQC spectrum of Chz1. All backbone
Table S1. Primers used for the constructions of recombinant GAL1 and λ5 mutants. GAL1-E74A ccgagcagcgggcggctgtctttcc ggaaagacagccgcccgctgctcgg
 SUPPLEMENTAL DATA Table S1. Primers used for the constructions of recombinant GAL1 and λ5 mutants Sense primer (5 to 3 ) Anti-sense primer (5 to 3 ) GAL1 mutants GAL1-E74A ccgagcagcgggcggctgtctttcc ggaaagacagccgcccgctgctcgg
SUPPLEMENTAL DATA Table S1. Primers used for the constructions of recombinant GAL1 and λ5 mutants Sense primer (5 to 3 ) Anti-sense primer (5 to 3 ) GAL1 mutants GAL1-E74A ccgagcagcgggcggctgtctttcc ggaaagacagccgcccgctgctcgg
I690/B680 Structural Bioinformatics Spring Protein Structure Determination by NMR Spectroscopy
 I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
Protein Structure Determination Using NMR Restraints BCMB/CHEM 8190
 Protein Structure Determination Using NMR Restraints BCMB/CHEM 8190 Programs for NMR Based Structure Determination CNS - Brünger, A. T.; Adams, P. D.; Clore, G. M.; DeLano, W. L.; Gros, P.; Grosse-Kunstleve,
Protein Structure Determination Using NMR Restraints BCMB/CHEM 8190 Programs for NMR Based Structure Determination CNS - Brünger, A. T.; Adams, P. D.; Clore, G. M.; DeLano, W. L.; Gros, P.; Grosse-Kunstleve,
NMR, X-ray Diffraction, Protein Structure, and RasMol
 NMR, X-ray Diffraction, Protein Structure, and RasMol Introduction So far we have been mostly concerned with the proteins themselves. The techniques (NMR or X-ray diffraction) used to determine a structure
NMR, X-ray Diffraction, Protein Structure, and RasMol Introduction So far we have been mostly concerned with the proteins themselves. The techniques (NMR or X-ray diffraction) used to determine a structure
PROTEIN'STRUCTURE'DETERMINATION'
 PROTEIN'STRUCTURE'DETERMINATION' USING'NMR'RESTRAINTS' BCMB/CHEM'8190' Programs for NMR Based Structure Determination CNS - Brünger, A. T.; Adams, P. D.; Clore, G. M.; DeLano, W. L.; Gros, P.; Grosse-Kunstleve,
PROTEIN'STRUCTURE'DETERMINATION' USING'NMR'RESTRAINTS' BCMB/CHEM'8190' Programs for NMR Based Structure Determination CNS - Brünger, A. T.; Adams, P. D.; Clore, G. M.; DeLano, W. L.; Gros, P.; Grosse-Kunstleve,
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/1/eaau413/dc1 Supplementary Materials for Structure and dynamics conspire in the evolution of affinity between intrinsically disordered proteins Per Jemth*, Elin
advances.sciencemag.org/cgi/content/full/4/1/eaau413/dc1 Supplementary Materials for Structure and dynamics conspire in the evolution of affinity between intrinsically disordered proteins Per Jemth*, Elin
Residual Dipolar Couplings BCMB/CHEM 8190
 Residual Dipolar Couplings BCMB/CHEM 8190 Recent Reviews Prestegard, A-Hashimi & Tolman, Quart. Reviews Biophys. 33, 371-424 (2000). Bax, Kontaxis & Tjandra, Methods in Enzymology, 339, 127-174 (2001)
Residual Dipolar Couplings BCMB/CHEM 8190 Recent Reviews Prestegard, A-Hashimi & Tolman, Quart. Reviews Biophys. 33, 371-424 (2000). Bax, Kontaxis & Tjandra, Methods in Enzymology, 339, 127-174 (2001)
Supplemental Information
 Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
Supporting Information
 Supporting Information Ellena et al. 10.1073/pnas.0908317106 SI Experimental Procedures Protein Expression and Sample Preparation. Syb(1 96) and Syb(1 116) from Rattus norvegicus were expressed in BL21(DE3)
Supporting Information Ellena et al. 10.1073/pnas.0908317106 SI Experimental Procedures Protein Expression and Sample Preparation. Syb(1 96) and Syb(1 116) from Rattus norvegicus were expressed in BL21(DE3)
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
Orientational degeneracy in the presence of one alignment tensor.
 Orientational degeneracy in the presence of one alignment tensor. Rotation about the x, y and z axes can be performed in the aligned mode of the program to examine the four degenerate orientations of two
Orientational degeneracy in the presence of one alignment tensor. Rotation about the x, y and z axes can be performed in the aligned mode of the program to examine the four degenerate orientations of two
Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS
 Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS CNS input file generatemetal.inp: remarks file generate/generatemetal.inp
Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS CNS input file generatemetal.inp: remarks file generate/generatemetal.inp
Dipolar Couplings in Partially Aligned Macromolecules - New Directions in. Structure Determination using Solution State NMR.
 Dipolar Couplings in Partially Aligned Macromolecules - New Directions in Structure Determination using Solution State NMR. Recently developed methods for the partial alignment of macromolecules in dilute
Dipolar Couplings in Partially Aligned Macromolecules - New Directions in Structure Determination using Solution State NMR. Recently developed methods for the partial alignment of macromolecules in dilute
Magnetic Resonance Lectures for Chem 341 James Aramini, PhD. CABM 014A
 Magnetic Resonance Lectures for Chem 341 James Aramini, PhD. CABM 014A jma@cabm.rutgers.edu " J.A. 12/11/13 Dec. 4 Dec. 9 Dec. 11" " Outline" " 1. Introduction / Spectroscopy Overview 2. NMR Spectroscopy
Magnetic Resonance Lectures for Chem 341 James Aramini, PhD. CABM 014A jma@cabm.rutgers.edu " J.A. 12/11/13 Dec. 4 Dec. 9 Dec. 11" " Outline" " 1. Introduction / Spectroscopy Overview 2. NMR Spectroscopy
Nature Structural & Molecular Biology: doi: /nsmb.3194
 Supplementary Figure 1 Mass spectrometry and solution NMR data for -syn samples used in this study. (a) Matrix-assisted laser-desorption and ionization time-of-flight (MALDI-TOF) mass spectrum of uniformly-
Supplementary Figure 1 Mass spectrometry and solution NMR data for -syn samples used in this study. (a) Matrix-assisted laser-desorption and ionization time-of-flight (MALDI-TOF) mass spectrum of uniformly-
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Timescales of Protein Dynamics
 Timescales of Protein Dynamics From Henzler-Wildman and Kern, Nature 2007 Dynamics from NMR Show spies Amide Nitrogen Spies Report On Conformational Dynamics Amide Hydrogen Transverse Relaxation Ensemble
Timescales of Protein Dynamics From Henzler-Wildman and Kern, Nature 2007 Dynamics from NMR Show spies Amide Nitrogen Spies Report On Conformational Dynamics Amide Hydrogen Transverse Relaxation Ensemble
SUPPLEMENTARY INFORMATION
 Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
Residual Dipolar Couplings Measured in Multiple Alignment Media.
 Residual Dipolar Couplings Measured in Multiple Alignment Media. We have already seen that the orientational degeneracy inherent to a single measured coupling can be raised by measuring different directions
Residual Dipolar Couplings Measured in Multiple Alignment Media. We have already seen that the orientational degeneracy inherent to a single measured coupling can be raised by measuring different directions
Timescales of Protein Dynamics
 Timescales of Protein Dynamics From Henzler-Wildman and Kern, Nature 2007 Summary of 1D Experiment time domain data Fourier Transform (FT) frequency domain data or Transverse Relaxation Ensemble of Nuclear
Timescales of Protein Dynamics From Henzler-Wildman and Kern, Nature 2007 Summary of 1D Experiment time domain data Fourier Transform (FT) frequency domain data or Transverse Relaxation Ensemble of Nuclear
Structure of the α-helix
 Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Interpreting and evaluating biological NMR in the literature. Worksheet 1
 Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Introduction solution NMR
 2 NMR journey Introduction solution NMR Alexandre Bonvin Bijvoet Center for Biomolecular Research with thanks to Dr. Klaartje Houben EMBO Global Exchange course, IHEP, Beijing April 28 - May 5, 20 3 Topics
2 NMR journey Introduction solution NMR Alexandre Bonvin Bijvoet Center for Biomolecular Research with thanks to Dr. Klaartje Houben EMBO Global Exchange course, IHEP, Beijing April 28 - May 5, 20 3 Topics
Chapter 6. The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR
 The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
Useful background reading
 Overview of lecture * General comment on peptide bond * Discussion of backbone dihedral angles * Discussion of Ramachandran plots * Description of helix types. * Description of structures * NMR patterns
Overview of lecture * General comment on peptide bond * Discussion of backbone dihedral angles * Discussion of Ramachandran plots * Description of helix types. * Description of structures * NMR patterns
SUPPLEMENTARY INFORMATION
 5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
5 N 4 8 20 22 24 2 28 4 8 20 22 24 2 28 a b 0 9 8 7 H c (kda) 95 0 57 4 28 2 5.5 Precipitate before NMR expt. Supernatant before NMR expt. Precipitate after hrs NMR expt. Supernatant after hrs NMR expt.
Experimental Techniques in Protein Structure Determination
 Experimental Techniques in Protein Structure Determination Homayoun Valafar Department of Computer Science and Engineering, USC Two Main Experimental Methods X-Ray crystallography Nuclear Magnetic Resonance
Experimental Techniques in Protein Structure Determination Homayoun Valafar Department of Computer Science and Engineering, USC Two Main Experimental Methods X-Ray crystallography Nuclear Magnetic Resonance
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
Introduction to Comparative Protein Modeling. Chapter 4 Part I
 Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Examples of Protein Modeling. Protein Modeling. Primary Structure. Protein Structure Description. Protein Sequence Sources. Importing Sequences to MOE
 Examples of Protein Modeling Protein Modeling Visualization Examination of an experimental structure to gain insight about a research question Dynamics To examine the dynamics of protein structures To
Examples of Protein Modeling Protein Modeling Visualization Examination of an experimental structure to gain insight about a research question Dynamics To examine the dynamics of protein structures To
NMR study of complexes between low molecular mass inhibitors and the West Nile virus NS2B-NS3 protease
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
BMB/Bi/Ch 173 Winter 2018
 BMB/Bi/Ch 173 Winter 2018 Homework Set 8.1 (100 Points) Assigned 2-27-18, due 3-6-18 by 10:30 a.m. TA: Rachael Kuintzle. Office hours: SFL 220, Friday 3/2 4:00-5:00pm and SFL 229, Monday 3/5 4:00-5:30pm.
BMB/Bi/Ch 173 Winter 2018 Homework Set 8.1 (100 Points) Assigned 2-27-18, due 3-6-18 by 10:30 a.m. TA: Rachael Kuintzle. Office hours: SFL 220, Friday 3/2 4:00-5:00pm and SFL 229, Monday 3/5 4:00-5:30pm.
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN
 THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
ml. ph 7.5 ph 6.5 ph 5.5 ph 4.5. β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS
 a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
Evaluation of the Utility of NMR Structures Determined from Minimal NOE-Based Restraints for Structure-Based Drug Design, Using MMP-1 as an Example
 Biochemistry 2000, 39, 13365-13375 13365 Evaluation of the Utility of NMR Structures Determined from Minimal NOE-Based Restraints for Structure-Based Drug Design, Using MMP-1 as an Example Xuemei Huang,
Biochemistry 2000, 39, 13365-13375 13365 Evaluation of the Utility of NMR Structures Determined from Minimal NOE-Based Restraints for Structure-Based Drug Design, Using MMP-1 as an Example Xuemei Huang,
Solving the three-dimensional solution structures of larger
 Accurate and rapid docking of protein protein complexes on the basis of intermolecular nuclear Overhauser enhancement data and dipolar couplings by rigid body minimization G. Marius Clore* Laboratory of
Accurate and rapid docking of protein protein complexes on the basis of intermolecular nuclear Overhauser enhancement data and dipolar couplings by rigid body minimization G. Marius Clore* Laboratory of
CAP 5510 Lecture 3 Protein Structures
 CAP 5510 Lecture 3 Protein Structures Su-Shing Chen Bioinformatics CISE 8/19/2005 Su-Shing Chen, CISE 1 Protein Conformation 8/19/2005 Su-Shing Chen, CISE 2 Protein Conformational Structures Hydrophobicity
CAP 5510 Lecture 3 Protein Structures Su-Shing Chen Bioinformatics CISE 8/19/2005 Su-Shing Chen, CISE 1 Protein Conformation 8/19/2005 Su-Shing Chen, CISE 2 Protein Conformational Structures Hydrophobicity
T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y. jgp
 S u p p l e m e n ta l m at e r i a l jgp Lee et al., http://www.jgp.org/cgi/content/full/jgp.201411219/dc1 T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y S u p p l e m e n ta l D I S C U S
S u p p l e m e n ta l m at e r i a l jgp Lee et al., http://www.jgp.org/cgi/content/full/jgp.201411219/dc1 T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y S u p p l e m e n ta l D I S C U S
Protein Structure Determination from Pseudocontact Shifts Using ROSETTA
 Supporting Information Protein Structure Determination from Pseudocontact Shifts Using ROSETTA Christophe Schmitz, Robert Vernon, Gottfried Otting, David Baker and Thomas Huber Table S0. Biological Magnetic
Supporting Information Protein Structure Determination from Pseudocontact Shifts Using ROSETTA Christophe Schmitz, Robert Vernon, Gottfried Otting, David Baker and Thomas Huber Table S0. Biological Magnetic
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10657 Supplementary Text Introduction. All retroviruses contain three genes: namely, gag, pol and env, which code for structural, enzymatic and glycoprotein receptor proteins, respectively.
doi:10.1038/nature10657 Supplementary Text Introduction. All retroviruses contain three genes: namely, gag, pol and env, which code for structural, enzymatic and glycoprotein receptor proteins, respectively.
Structural characterization of NiV N 0 P in solution and in crystal.
 Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.1299 Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy Ishita Sengupta 1, Philippe S. Nadaud 1, Jonathan J. Helmus 1, Charles D. Schwieters 2
DOI: 10.1038/NCHEM.1299 Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy Ishita Sengupta 1, Philippe S. Nadaud 1, Jonathan J. Helmus 1, Charles D. Schwieters 2
Expanded View Figures
 The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
Molecular Modeling lecture 2
 Molecular Modeling 2018 -- lecture 2 Topics 1. Secondary structure 3. Sequence similarity and homology 2. Secondary structure prediction 4. Where do protein structures come from? X-ray crystallography
Molecular Modeling 2018 -- lecture 2 Topics 1. Secondary structure 3. Sequence similarity and homology 2. Secondary structure prediction 4. Where do protein structures come from? X-ray crystallography
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure Determination Using NMR Restraints BCMB/CHEM 8190
 Protein Structure Determination Using NMR Restraints BCMB/CHEM 8190 Programs for NMR Based Structure Determination CNS - Brunger, A. T.; Adams, P. D.; Clore, G. M.; DeLano, W. L.; Gros, P.; Grosse-Kunstleve,
Protein Structure Determination Using NMR Restraints BCMB/CHEM 8190 Programs for NMR Based Structure Determination CNS - Brunger, A. T.; Adams, P. D.; Clore, G. M.; DeLano, W. L.; Gros, P.; Grosse-Kunstleve,
Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate
 SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Aligned sequences of yeast IDH1 (top) and IDH2 (bottom) with isocitrate dehydrogenase from Escherichia coli [ICD, pdb 1PB1, Mesecar, A. D., and Koshland,
Protein Dynamics. The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron.
 Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
Supplementary Information. The protease GtgE from Salmonella exclusively targets. inactive Rab GTPases
 Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Supporting Information
 Supporting Information German Edition: DOI: Sampling of Glycan-Bound Conformers by the Anti-HIV Lectin Oscillatoria agardhii agglutinin in the Absence of Sugar** Marta G. Carneiro, Leonardus M. I. Koharudin,
Supporting Information German Edition: DOI: Sampling of Glycan-Bound Conformers by the Anti-HIV Lectin Oscillatoria agardhii agglutinin in the Absence of Sugar** Marta G. Carneiro, Leonardus M. I. Koharudin,
Three-dimensional structure of a viral genome-delivery portal vertex
 Three-dimensional structure of a viral genome-delivery portal vertex Adam S. Olia 1, Peter E. Prevelige Jr. 2, John E. Johnson 3 and Gino Cingolani 4 1 Department of Biological Sciences, Purdue University,
Three-dimensional structure of a viral genome-delivery portal vertex Adam S. Olia 1, Peter E. Prevelige Jr. 2, John E. Johnson 3 and Gino Cingolani 4 1 Department of Biological Sciences, Purdue University,
SUPPLEMENTARY MATERIAL FOR
 SUPPLEMENTARY MATERIAL FOR THE LIPID-BINDING DOMAIN OF WILD TYPE AND MUTANT ALPHA- SYNUCLEIN: COMPACTNESS AND INTERCONVERSION BETWEEN THE BROKEN- AND EXTENDED-HELIX FORMS. Elka R. Georgieva 1, Trudy F.
SUPPLEMENTARY MATERIAL FOR THE LIPID-BINDING DOMAIN OF WILD TYPE AND MUTANT ALPHA- SYNUCLEIN: COMPACTNESS AND INTERCONVERSION BETWEEN THE BROKEN- AND EXTENDED-HELIX FORMS. Elka R. Georgieva 1, Trudy F.
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supporting Information
 Supporting Information Micelle-Triggered b-hairpin to a-helix Transition in a 14-Residue Peptide from a Choline-Binding Repeat of the Pneumococcal Autolysin LytA HØctor Zamora-Carreras, [a] Beatriz Maestro,
Supporting Information Micelle-Triggered b-hairpin to a-helix Transition in a 14-Residue Peptide from a Choline-Binding Repeat of the Pneumococcal Autolysin LytA HØctor Zamora-Carreras, [a] Beatriz Maestro,
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Supplemental Data. Structure of the Rb C-Terminal Domain. Bound to E2F1-DP1: A Mechanism. for Phosphorylation-Induced E2F Release
 Supplemental Data Structure of the Rb C-Terminal Domain Bound to E2F1-DP1: A Mechanism for Phosphorylation-Induced E2F Release Seth M. Rubin, Anne-Laure Gall, Ning Zheng, and Nikola P. Pavletich Section
Supplemental Data Structure of the Rb C-Terminal Domain Bound to E2F1-DP1: A Mechanism for Phosphorylation-Induced E2F Release Seth M. Rubin, Anne-Laure Gall, Ning Zheng, and Nikola P. Pavletich Section
Physiochemical Properties of Residues
 Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Supporting information for. Towards automatic protein backbone assignment using proton-detected 4D solid-state NMR data
 Supporting information for Towards automatic protein backbone assignment using proton-detected 4D solid-state NMR data Shengqi Xiang 1, Veniamin Chevelkov 1,2, Stefan Becker 1, Adam Lange 1,2,3 * 1 Max
Supporting information for Towards automatic protein backbone assignment using proton-detected 4D solid-state NMR data Shengqi Xiang 1, Veniamin Chevelkov 1,2, Stefan Becker 1, Adam Lange 1,2,3 * 1 Max
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Crystal Structure of RAIDD Death Domain Implicates Potential Mechanism of PIDDosome Assembly
 doi:10.1016/j.jmb.2005.12.082 J. Mol. Biol. (2006) 357, 358 364 COMMUNICATION Crystal Structure of RAIDD Death Domain Implicates Potential Mechanism of PIDDosome Assembly Hyun Ho Park and Hao Wu* Department
doi:10.1016/j.jmb.2005.12.082 J. Mol. Biol. (2006) 357, 358 364 COMMUNICATION Crystal Structure of RAIDD Death Domain Implicates Potential Mechanism of PIDDosome Assembly Hyun Ho Park and Hao Wu* Department
Alpha-helical Topology and Tertiary Structure Prediction of Globular Proteins Scott R. McAllister Christodoulos A. Floudas Princeton University
 Alpha-helical Topology and Tertiary Structure Prediction of Globular Proteins Scott R. McAllister Christodoulos A. Floudas Princeton University Department of Chemical Engineering Program of Applied and
Alpha-helical Topology and Tertiary Structure Prediction of Globular Proteins Scott R. McAllister Christodoulos A. Floudas Princeton University Department of Chemical Engineering Program of Applied and
Supplementary Information
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2017 Supplementary Information Probing the excited-state chemical shifts and exchange
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2017 Supplementary Information Probing the excited-state chemical shifts and exchange
Supporting Information
 Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION
 THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
Protein NMR spectroscopy
 Protein NMR spectroscopy Perttu Permi National Biological NMR Center, Institute of Biotechnology, University of elsinki Protein NMR spectroscopy course, 19th January 2009 1 spectrum of 20 kda Ca-binding
Protein NMR spectroscopy Perttu Permi National Biological NMR Center, Institute of Biotechnology, University of elsinki Protein NMR spectroscopy course, 19th January 2009 1 spectrum of 20 kda Ca-binding
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
NMR in Medicine and Biology
 NMR in Medicine and Biology http://en.wikipedia.org/wiki/nmr_spectroscopy MRI- Magnetic Resonance Imaging (water) In-vivo spectroscopy (metabolites) Solid-state t NMR (large structures) t Solution NMR
NMR in Medicine and Biology http://en.wikipedia.org/wiki/nmr_spectroscopy MRI- Magnetic Resonance Imaging (water) In-vivo spectroscopy (metabolites) Solid-state t NMR (large structures) t Solution NMR
Dihedral Angles. Homayoun Valafar. Department of Computer Science and Engineering, USC 02/03/10 CSCE 769
 Dihedral Angles Homayoun Valafar Department of Computer Science and Engineering, USC The precise definition of a dihedral or torsion angle can be found in spatial geometry Angle between to planes Dihedral
Dihedral Angles Homayoun Valafar Department of Computer Science and Engineering, USC The precise definition of a dihedral or torsion angle can be found in spatial geometry Angle between to planes Dihedral
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6
 Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Structure and RNA-binding properties. of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex
 Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Nature Structural and Molecular Biology: doi: /nsmb.2938
 Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
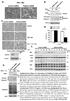 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Supplementary Information. Overlap between folding and functional energy landscapes for. adenylate kinase conformational change
 Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Introduction to" Protein Structure
 Introduction to" Protein Structure Function, evolution & experimental methods Thomas Blicher, Center for Biological Sequence Analysis Learning Objectives Outline the basic levels of protein structure.
Introduction to" Protein Structure Function, evolution & experimental methods Thomas Blicher, Center for Biological Sequence Analysis Learning Objectives Outline the basic levels of protein structure.
Modeling for 3D structure prediction
 Modeling for 3D structure prediction What is a predicted structure? A structure that is constructed using as the sole source of information data obtained from computer based data-mining. However, mixing
Modeling for 3D structure prediction What is a predicted structure? A structure that is constructed using as the sole source of information data obtained from computer based data-mining. However, mixing
Name: BCMB/CHEM 8190, BIOMOLECULAR NMR FINAL EXAM-5/5/10
 Name: BCMB/CHEM 8190, BIOMOLECULAR NMR FINAL EXAM-5/5/10 Instructions: This is an open book, limited time, exam. You may use notes you have from class and any text book you find useful. You may also use
Name: BCMB/CHEM 8190, BIOMOLECULAR NMR FINAL EXAM-5/5/10 Instructions: This is an open book, limited time, exam. You may use notes you have from class and any text book you find useful. You may also use
Solution Structure and Backbone Dynamics of the TGFβ Type II Receptor Extracellular Domain,
 026 Biochemistry 2003, 42, 026-039 Solution Structure and Backbone Dynamics of the TGFβ Type II Receptor Extracellular Domain, Shashank Deep, Kerfoot P. Walker, III, Zhanyong Shu, and Andrew P. Hinck*,
026 Biochemistry 2003, 42, 026-039 Solution Structure and Backbone Dynamics of the TGFβ Type II Receptor Extracellular Domain, Shashank Deep, Kerfoot P. Walker, III, Zhanyong Shu, and Andrew P. Hinck*,
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Outline. Levels of Protein Structure. Primary (1 ) Structure. Lecture 6:Protein Architecture II: Secondary Structure or From peptides to proteins
 Lecture 6:Protein Architecture II: Secondary Structure or From peptides to proteins Margaret Daugherty Fall 2003 Outline Four levels of structure are used to describe proteins; Alpha helices and beta sheets
Lecture 6:Protein Architecture II: Secondary Structure or From peptides to proteins Margaret Daugherty Fall 2003 Outline Four levels of structure are used to describe proteins; Alpha helices and beta sheets
Apoptosis in Mammalian Cells
 Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
Supplementary information
 Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
HADDOCK: High Ambiguity
 Determination of Protein-Protein complexes HADDOCK: High Ambiguity Driven DOCKing A protein-protein docking approach based on biochemical and/or biophysical data In PDB: >15000 protein structures but
Determination of Protein-Protein complexes HADDOCK: High Ambiguity Driven DOCKing A protein-protein docking approach based on biochemical and/or biophysical data In PDB: >15000 protein structures but
NMR in Structural Biology
 NMR in Structural Biology Exercise session 2 1. a. List 3 NMR observables that report on structure. b. Also indicate whether the information they give is short/medium or long-range, or perhaps all three?
NMR in Structural Biology Exercise session 2 1. a. List 3 NMR observables that report on structure. b. Also indicate whether the information they give is short/medium or long-range, or perhaps all three?
Supplementary Materials belonging to
 Supplementary Materials belonging to Solution conformation of the 70 kda E.coli Hsp70 complexed with ADP and substrate. Eric B. Bertelsen 1, Lyra Chang 2, Jason E. Gestwicki 2 and Erik R. P. Zuiderweg
Supplementary Materials belonging to Solution conformation of the 70 kda E.coli Hsp70 complexed with ADP and substrate. Eric B. Bertelsen 1, Lyra Chang 2, Jason E. Gestwicki 2 and Erik R. P. Zuiderweg
CONFOCHECK. Innovation with Integrity. Infrared Protein Analysis FT-IR
 CONFOCHECK Infrared Protein Analysis Innovation with Integrity FT-IR CONFOCHECK: FT-IR System for Protein Analytics FT-IR Protein Analysis Infrared spectroscopy measures molecular vibrations due to the
CONFOCHECK Infrared Protein Analysis Innovation with Integrity FT-IR CONFOCHECK: FT-IR System for Protein Analytics FT-IR Protein Analysis Infrared spectroscopy measures molecular vibrations due to the
