Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1. Definition and assessment of ciap1 constructs.
|
|
|
- Gwendoline McCormick
- 5 years ago
- Views:
Transcription
1 Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations and deletions are indicated with magenta lines. The mutations in the ciap1-b3r construct reduce proteolysis and non-native disulfide
2 formation 1. (b) The binding of ciap1-b3r (blue) and ciap1-b3r MF-AA (gray) to monobiotinylated ubiquitin was tested using biolayer interferometry. Equilibrium response values are plotted against ciap1 concentration. No detectable binding is observed by the ciap1- B3R MF-AA construct. ciap1-b3r binds ubiquitin with a K D of approximately 20 M as determined by a single-site binding isotherm fit, in good agreement with past studies 2. Recent reports have suggested that this mutation destabilizes ciap1 3, which might contribute to the very slight decrease in E2 binding affinity we observe in the MF-AA variants. The use of ciap1 MF-AA in E2 binding studies removes the complicating effects of direct ciap1-ubiquitin binding in the case of the E2-Ub conjugate. (c) The binding of ciap1-b3r (blue) and ciap1-b3r- CARD (green) to E2 SRCK was assessed using biolayer interferometry. Equilibrium response values were plotted as a function of E2 concentration, and the resulting curves were fit to a single-site binding isotherm. The affinities determined are approximately 27 M (ciap1-b3r) and 19 M (ciap1-b3r- CARD), recapitulating the relative increase in affinity upon CARD deletion observed in the case of ciap1-b3r MF-AA.
3 Supplementary Figure 2 UbcH5c does not induce dimerization of ciap1.
4 (a) ciap1-b3r responds to SMAC mimetics by dimerizing, as detected by native gel electrophoresis. Lane 1 shows a native molecular weight marker (Native Mark, Invitrogen) with molecular weights indicated at left. Lane 2 shows monomeric ciap1 (64 M). Lane 3 shows dimerized ciap1 in the presence of AVPW (1 mm). Binding of the bivalent SMAC mimetic BV6 (1 mm) induces stronger and more compact dimerization because it can engage two BIR3 domains simultaneously. Lanes 4-7 demonstrate that UbcH5c (64 M) does not affect the dimerization state of ciap1. Lanes 8-10 demonstrate that the gel mobility of UbcH5c is not affected by the SMAC mimetics. All samples contain 1% DMSO. (b) ciap1-b3r (64 M) was incubated with UbcH5c at 0 to 128 M. No detectable dimer is formed. ciap1 bound to BV6 is included as a dimerization control.
5 .
6 Supplementary Figure 3 Methionine methyl groups assigned by mutagenesis. 1 H- 13 C HMQC spectra of each methionine to leucine point mutant (blue) overlaid with the ciap1-b3r spectrum (red). The M466L mutant is also shown with the contours drawn four times lower. The M266L, M391L, M392L, and M402L spectra display secondary chemical shift changes that may be due to structural perturbations as a result of the mutation.
7 Supplementary Figure 4 Dispersion profiles of UBA α3. The residues in UBA α3 were fit to a single exchange process with k ex = 970 ± 100 s -1 and p B = 2.2 ± 0.1 %. Data and fits at 900 MHz are shown in red, 800 MHz data and fits are in blue. Error bars were determined by treating the spectral noise level as the uncertainty in peak heights. The two residues in UBA α3 that do not have detectable R 2 dispersion, Q411 and L418, are severely overlapped in the 1 H- 15 N spectra. As shown in Fig. 4a, R 2 dispersion was also detected at additional residues in the UBA (A388, V389, M390, A399, F404, K425, D429 and I430) as well as at five additional residues outside the UBA domain (L359, T534, I548, V581 and I608);
8 however, as the residues do not cluster into large, contiguous surfaces we did not fit the data to extract kinetic or equilibrium parameters describing the exchange processes.
9 Supplementary Figure 5 Characterization of the conformational states of ciap1 variants. (a) Size-exclusion multi-angle light scattering (SEC-MALS) traces of ciap1 constructs with and without SMAC mimetics. Apo-proteins are shown in black, AVPW-bound protein in blue, and BV6 bound proteins (as a dimerization control) in red. The UV absorbance for each peak was normalized to 1. As expected, ciap1-b3r (top) dimerizes in response to both AVPW and BV6, while ciap1-b3r- CARD (center) is constitutively open and dimerizes only in the presence of BV6. The L617E construct (bottom) blocks AVPW-induced dimerization without disrupting the closed conformation. (b) L617 is positioned in the RING dimerization interface. The structure of the
10 ciap2 RING dimer (PDB code: 3EB5, ref. 4) is shown in cartoon representation with the homologous residue to L617, L603, colored magenta and shown as spheres. Zinc ions are shown as light gray spheres.
11
12 Supplementary Figure 6 Standard SAXS models. Averaged, filtered SAXS models from 10 independent ab initio calculations are shown for ciap1-b3r, ciap1-b3r- C7 and ciap1- B3R L617E with and without AVPW. Fits (dark blue lines) of the raw scattering data (red circles) to the best ab initio model from each set are shown. Chi values for those fits are also shown. Models were generated as described (see Methods) using GASBOR.
13
14 Supplementary Figure 7 Molecular models based on standard ciap1 SAXS data. (a) The best of ten molecular models of ciap1-b3r- C7 is shown superposed with the averaged, filtered ab initio model. A normalized spatial discrepancy (NSD) for the aligment is indicated. The fit (dark blue line) of the molecular model to the experimental data (red circles) is shown below the model, and the Chi value describing the fit is indicated. I stands for scattering intensity and q is proportional to the scattering angle (q = 4 sin( )/, where 2 = the angle between the incident X-ray beam and the detector, and = the X-ray wavelength in Ångstroms). (b) The same data as in (a) is shown for ciap1-b3r- C7 in the presence of 1 mm AVPW. (c, d) Constraints used in the generation of closed monomeric molecular models are shown. All constraints are based on mutational data 1. (e) The best of ten molecular models of ciap1-b3r is shown superposed with the averaged, filtered ab initio model, in two views. The NSD between the two models is indicated. (f) The fit of the best ciap1-b3r molecular model to the experimental data is shown. All colors and variables are as in (a). (g) A subset of calculated molecular models for ciap1-b3r is shown aligned by their BIR3 domains to demonstrate the heterogeneity of the position of the CARD. The protein likely exists as an ensemble of states not fully reflected by any single model. (h) The distributions of R g values in the ensembles of molecular models generated by EOM for ciap1-b3r (black), ciap1- B3R L617E (orange), ciap1-b3r- C7 (violet) and ciap1-b3r L617E + AVPW (green) are shown. The initial, unoptimized pools are displayed as dotted lines, and the optimized ensembles as solid lines. Note that the closed states (ciap1-b3r and ciap1-b3r L617E ) adopt tighter and smaller distributions than the open states (ciap1-b3r- C7 and ciap1-b3r L617E + AVPW), reflecting the more rigid conformation of the closed states.
15 Supplementary Figure 8
16 Controls for TR-SAXS measurements. (a,b) Time-resolved SAXS (TR-SAXS) experiments of ciap1-b3r and ciap1-b3r L617E mixed with buffer instead of AVPW. (c, d) TR- SAXS experiment of ciap1-b3r- C7 mixed (c) with buffer and (d) with AVPW. (e) TR-SAXS analysis of ciap1-b3r L617E with a higher concentration of AVPW (1 mm). The relative populations of the monomeric, open, and dimeric conformations of ciap1 were extracted from the TR-SAXS data by deconvolution using the static scattering curves of each state as reference (See Methods). Each point represents and average from three experiments, plus or minus standard deviation. Data are fit to first order integrated rate equations. The closed monomer fraction is shown as blue squares, open fraction as hot pink circles, dimer fraction as purple diamonds.
17 Supplementary Tables Supplementary Table 1. Kinetic constants for the association of ciap1 with E2 and E2- Ub. E2 type: E2SRCK E2SRCK- Ub Construct AVPW koff (s - 1 ) kon (M - 1 s - 1 ) koff (s - 1 ) kon (M - 1 s - 1 ) ciap1- B3RMF- AA 0.78 ± ,000 ± 2, ± ,000 ± 20,000 ciap1- B3RMF- AA- ΔCARD 0.8 ± ,000 ± 5, ± ,000 ± 40,000 ciap1- B3RMF- AA ± ,000 ± 7, ± ,000 ± 50,000 ciap1- B3RMF- AA- ΔCARD ± ,000 ± 7, ± ,000 ± 100,000 Off- rates were measured directly from the dissociation phases of biolayer interferometry experiments. Reported values are the averages and standard deviations of three independent experiments. The association phases were too rapid to make meaningful direct measurement of kon values, and so the on- rates reported here are calculated from the measured koff values and the measured equilibrium KD values. Supplementary Table 2. Standard SAXS statistics. ciap1- B3R ciap1- B3RL617E ciap1- B3R- ΔC7 AVPW MWSAXS (MWactual) (kda) 40.8 (39.2) 80.2 (78.5) 40.8 (39.2) 47.9 (39.2) 41.5 (38.4) 40.5 (38.4) Rg (Å) Dmax (Å) Porod volume (Å 3 ) χ 2 (GASBOR, mean) 1.60 ± ± ± ± ± ± 0.1 χ 2 (GASBOR, best) NSD(DAMAVER, mean) 1.10 ± ± ± ± ± ± 0.07 χ 2 (BUNCH, mean) 3.0 ± 0.3 ND ND ND 1.21 ± ± 0.1 χ 2 (BUNCH, best) 2.65 ND ND ND All reported statistics are from 1 mg/ml samples. Values are calculated using the listed ATSAS programs. SAXS- derived molecular weights were calculated using SAXS MoW 3. ND = not determined. Where errors are reported, values reflect averages of ten independent calculations and errors reflect standard deviations.
18 Supplementary Table 3: EOM statistics Construct AVPW Avg. Rg (Å) initial pool Avg. Rg (Å) optimized ensemble Avg. NSD optimized ensemble (DAMVER) χ 2 (EOM) ciap1- B3R ± ND ND ND ND ciap ± B3RL617E ± ciap1- B3R ± ΔC ± Chi values and average Rg values (for the random initial pools and optimized ensembles) were returned by EOM. Also shown are average NSDs of the final optimized pools (n = 20 ± s.d.), calculated using DAMAVER. Larger NSDs are consistent with more flexible systems. ND=not determined.
19 Supplementary References 1. Dueber, E. C. et al. Antagonists induce a conformational change in ciap1 that promotes autoubiquitination. Science 334, (2011). 2. Dynek, J. N. et al. c- IAP1 and UbcH5 promote K11- linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 29, (2010). 3. Budhidarmo, R. & Day, C. L. The Ubiquitin- Associated Domain of Cellular Inhibitor of Apoptosis Proteins Facilitates Ubiquitylation. J. Biol. Chem. in press (2014). 4. Mace, P. D. et al. Structures of the ciap2 RING domain reveal conformational changes associated with ubiquitin- conjugating enzyme (E2) recruitment. J. Biol. Chem. 283, (2008).
Supplemental Information. Structural and Mechanistic Paradigm. of Leptin Receptor Activation Revealed
 Structure, Volume 22 Supplemental Information Structural and Mechanistic Paradigm of Leptin Receptor Activation Revealed by Complexes with Wild-Type and Antagonist Leptins Kedar Moharana, Lennart Zabeau,
Structure, Volume 22 Supplemental Information Structural and Mechanistic Paradigm of Leptin Receptor Activation Revealed by Complexes with Wild-Type and Antagonist Leptins Kedar Moharana, Lennart Zabeau,
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Supplementary Information. Overlap between folding and functional energy landscapes for. adenylate kinase conformational change
 Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Structural characterization of NiV N 0 P in solution and in crystal.
 Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
Supplementary figure 1. Comparison of unbound ogm-csf and ogm-csf as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine
 Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
SUPPLEMENTARY INFORMATION
 Supplementary Table S1 Kinetic Analyses of the AMSH-LP mutants AMSH-LP K M (μm) k cat x 10-3 (s -1 ) WT 71.8 ± 6.3 860 ± 65.4 T353A 76.8 ± 11.7 46.3 ± 3.7 F355A 58.9 ± 10.4 5.33 ± 0.30 proximal S358A 75.1
Supplementary Table S1 Kinetic Analyses of the AMSH-LP mutants AMSH-LP K M (μm) k cat x 10-3 (s -1 ) WT 71.8 ± 6.3 860 ± 65.4 T353A 76.8 ± 11.7 46.3 ± 3.7 F355A 58.9 ± 10.4 5.33 ± 0.30 proximal S358A 75.1
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R)
 Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Modelling against small angle scattering data. Al Kikhney EMBL Hamburg, Germany
 Modelling against small angle scattering data Al Kikhney EMBL Hamburg, Germany Validation of atomic models CRYSOL Rigid body modelling SASREF BUNCH CORAL Oligomeric mixtures OLIGOMER Flexible systems EOM
Modelling against small angle scattering data Al Kikhney EMBL Hamburg, Germany Validation of atomic models CRYSOL Rigid body modelling SASREF BUNCH CORAL Oligomeric mixtures OLIGOMER Flexible systems EOM
The Fic protein Doc uses an inverted substrate to phosphorylate and. inactivate EF-Tu
 The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu Daniel Castro-Roa 1, Abel Garcia-Pino 2,3 *, Steven De Gieter 2,3, Nico A.J. van Nuland 2,3, Remy Loris 2,3, Nikolay
The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu Daniel Castro-Roa 1, Abel Garcia-Pino 2,3 *, Steven De Gieter 2,3, Nico A.J. van Nuland 2,3, Remy Loris 2,3, Nikolay
Supplementary materials. Crystal structure of the carboxyltransferase domain. of acetyl coenzyme A carboxylase. Department of Biological Sciences
 Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Interpreting and evaluating biological NMR in the literature. Worksheet 1
 Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
BM29 biosaxs data processing tutorial
 HERCULES 2014 BM29 biosaxs data processing tutorial Page 2 OUTLINE Sample Changer Primary Data Processing Model Validation HPLC-SAXS Primary Data Processing Model Validation Ab Initio Model Software in
HERCULES 2014 BM29 biosaxs data processing tutorial Page 2 OUTLINE Sample Changer Primary Data Processing Model Validation HPLC-SAXS Primary Data Processing Model Validation Ab Initio Model Software in
SI Text S1 Solution Scattering Data Collection and Analysis. SI references
 SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS
 Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS CNS input file generatemetal.inp: remarks file generate/generatemetal.inp
Supporting Protocol This protocol describes the construction and the force-field parameters of the non-standard residue for the Ag + -site using CNS CNS input file generatemetal.inp: remarks file generate/generatemetal.inp
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN
 THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
Molecular Modeling lecture 2
 Molecular Modeling 2018 -- lecture 2 Topics 1. Secondary structure 3. Sequence similarity and homology 2. Secondary structure prediction 4. Where do protein structures come from? X-ray crystallography
Molecular Modeling 2018 -- lecture 2 Topics 1. Secondary structure 3. Sequence similarity and homology 2. Secondary structure prediction 4. Where do protein structures come from? X-ray crystallography
NMR in Medicine and Biology
 NMR in Medicine and Biology http://en.wikipedia.org/wiki/nmr_spectroscopy MRI- Magnetic Resonance Imaging (water) In-vivo spectroscopy (metabolites) Solid-state t NMR (large structures) t Solution NMR
NMR in Medicine and Biology http://en.wikipedia.org/wiki/nmr_spectroscopy MRI- Magnetic Resonance Imaging (water) In-vivo spectroscopy (metabolites) Solid-state t NMR (large structures) t Solution NMR
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
Nature Structural & Molecular Biology: doi: /nsmb.3194
 Supplementary Figure 1 Mass spectrometry and solution NMR data for -syn samples used in this study. (a) Matrix-assisted laser-desorption and ionization time-of-flight (MALDI-TOF) mass spectrum of uniformly-
Supplementary Figure 1 Mass spectrometry and solution NMR data for -syn samples used in this study. (a) Matrix-assisted laser-desorption and ionization time-of-flight (MALDI-TOF) mass spectrum of uniformly-
Supplementary Figures. Supplementary Figure 1. 2D-GIWAXS of PEDOT:PSS with varying EG content in dispersion.
 Supplementary Figures Supplementary Figure 1. 2D-GIWAXS of PEDOT:PSS with varying EG content in dispersion. 1 Supplementary Figure 2. Contrast of PEDOT to PSS. a. Optical constants (n=1-δ+iβ) for Na:PSS
Supplementary Figures Supplementary Figure 1. 2D-GIWAXS of PEDOT:PSS with varying EG content in dispersion. 1 Supplementary Figure 2. Contrast of PEDOT to PSS. a. Optical constants (n=1-δ+iβ) for Na:PSS
BSA pegylation PG Journal of chromatographic A, 1147 (2007)
 BSA pegylation BSA 5mg/mL, pi= 4.7, Sigma A796, 65-7% free sulfyhydryl, cysteine 34 of BSA is not paired with another cysteine in the protein structure 1. PEG 1kD, kd, 3 kd 1mg/mL PEG:BSA=1.2:1 (molar
BSA pegylation BSA 5mg/mL, pi= 4.7, Sigma A796, 65-7% free sulfyhydryl, cysteine 34 of BSA is not paired with another cysteine in the protein structure 1. PEG 1kD, kd, 3 kd 1mg/mL PEG:BSA=1.2:1 (molar
ID14-EH3. Adam Round
 Bio-SAXS @ ID14-EH3 Adam Round Contents What can be obtained from Bio-SAXS Measurable parameters Modelling strategies How to collect data at Bio-SAXS Procedure Data collection tests Data Verification and
Bio-SAXS @ ID14-EH3 Adam Round Contents What can be obtained from Bio-SAXS Measurable parameters Modelling strategies How to collect data at Bio-SAXS Procedure Data collection tests Data Verification and
Introduction to Biological Small Angle Scattering
 Introduction to Biological Small Angle Scattering Tom Grant, Ph.D. Staff Scientist BioXFEL Science and Technology Center Hauptman-Woodward Institute Buffalo, New York, USA tgrant@hwi.buffalo.edu SAXS Literature
Introduction to Biological Small Angle Scattering Tom Grant, Ph.D. Staff Scientist BioXFEL Science and Technology Center Hauptman-Woodward Institute Buffalo, New York, USA tgrant@hwi.buffalo.edu SAXS Literature
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Nature Structural and Molecular Biology: doi: /nsmb.2938
 Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
SOCS3 binds specific receptor JAK complexes to control cytokine signaling by direct kinase inhibition SUPPLEMENTARY INFORMATION
 SOCS3 binds specific receptor JAK complexes to control cytokine signaling by direct kinase inhibition Nadia J. Kershaw 1,2, James M. Murphy 1,2, Nicholas P.D. Liau 1,2, Leila N. Varghese 1,2, Artem Laktyushin
SOCS3 binds specific receptor JAK complexes to control cytokine signaling by direct kinase inhibition Nadia J. Kershaw 1,2, James M. Murphy 1,2, Nicholas P.D. Liau 1,2, Leila N. Varghese 1,2, Artem Laktyushin
ion mobility spectrometry IR spectroscopy
 Debasmita Gho 29.10.2016 Introducti on Owing to its accuracy, sensitivity, and speed, mass spectrometry (MS) coupled to fragmentation techniques is the method of choice for determining the primary structure
Debasmita Gho 29.10.2016 Introducti on Owing to its accuracy, sensitivity, and speed, mass spectrometry (MS) coupled to fragmentation techniques is the method of choice for determining the primary structure
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
SUPPLEMENTARY INFORMATION Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis Sergio de Cima, Luis M. Polo, Carmen Díez-Fernández, Ana I. Martínez, Javier
Small-Angle X-ray Scattering (SAXS) SPring-8/JASRI Naoto Yagi
 Small-Angle X-ray Scattering (SAXS) SPring-8/JASRI Naoto Yagi 1 Wikipedia Small-angle X-ray scattering (SAXS) is a small-angle scattering (SAS) technique where the elastic scattering of X-rays (wavelength
Small-Angle X-ray Scattering (SAXS) SPring-8/JASRI Naoto Yagi 1 Wikipedia Small-angle X-ray scattering (SAXS) is a small-angle scattering (SAS) technique where the elastic scattering of X-rays (wavelength
ELECTRONIC SUPPLEMENTARY INFORMATION
 This journal is The Royal Society of Chemistry 1 ELECTRONIC SUPPLEMENTARY INFORMATION More than one non-canonical phosphodiester bond into the G-tract: formation of unusual parallel G-quadruplex structures
This journal is The Royal Society of Chemistry 1 ELECTRONIC SUPPLEMENTARY INFORMATION More than one non-canonical phosphodiester bond into the G-tract: formation of unusual parallel G-quadruplex structures
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
Structural basis for catalytically restrictive dynamics of a high-energy enzyme state
 Supplementary Material Structural basis for catalytically restrictive dynamics of a high-energy enzyme state Michael Kovermann, Jörgen Ådén, Christin Grundström, A. Elisabeth Sauer-Eriksson, Uwe H. Sauer
Supplementary Material Structural basis for catalytically restrictive dynamics of a high-energy enzyme state Michael Kovermann, Jörgen Ådén, Christin Grundström, A. Elisabeth Sauer-Eriksson, Uwe H. Sauer
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
SUPPLEMENTARY INFORMATION
 Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
SUPPLEMENTARY INFORMATION
 Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS)
 nature methods Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS) Greg L Hura, Angeli L Menon, Michal Hammel, Robert P Rambo, Farris L Poole II, Susan E Tsutakawa,
nature methods Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS) Greg L Hura, Angeli L Menon, Michal Hammel, Robert P Rambo, Farris L Poole II, Susan E Tsutakawa,
SUPPLEMENTARY INFORMATION
 Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supporting Information
 Supporting Information Oxaliplatin binding to human copper chaperone Atox1 and protein dimerization Benny D. Belviso, 1 Angela Galliani, 2 Alessia Lasorsa, 2 Valentina Mirabelli, 1,3 Rocco Caliandro, 1
Supporting Information Oxaliplatin binding to human copper chaperone Atox1 and protein dimerization Benny D. Belviso, 1 Angela Galliani, 2 Alessia Lasorsa, 2 Valentina Mirabelli, 1,3 Rocco Caliandro, 1
The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar mass
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2016 The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2016 The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits. AhpC and AhpF from Escherichia coli
 Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits AhpC and AhpF from Escherichia coli Phat Vinh Dip 1,#, Neelagandan Kamariah 2,#, Malathy Sony Subramanian Manimekalai
Structure, mechanism and ensemble formation of the Alkylhydroperoxide Reductase subunits AhpC and AhpF from Escherichia coli Phat Vinh Dip 1,#, Neelagandan Kamariah 2,#, Malathy Sony Subramanian Manimekalai
SUPPLEMENTARY INFORMATION
 SUPPLMTARY IFORMATIO a doi:10.108/nature10402 b 100 nm 100 nm c SAXS Model d ulers assigned to reference- Back-projected free class averages class averages Refinement against single particles Reconstructed
SUPPLMTARY IFORMATIO a doi:10.108/nature10402 b 100 nm 100 nm c SAXS Model d ulers assigned to reference- Back-projected free class averages class averages Refinement against single particles Reconstructed
Biological Small Angle X-ray Scattering (SAXS) Dec 2, 2013
 Biological Small Angle X-ray Scattering (SAXS) Dec 2, 2013 Structural Biology Shape Dynamic Light Scattering Electron Microscopy Small Angle X-ray Scattering Cryo-Electron Microscopy Wide Angle X- ray
Biological Small Angle X-ray Scattering (SAXS) Dec 2, 2013 Structural Biology Shape Dynamic Light Scattering Electron Microscopy Small Angle X-ray Scattering Cryo-Electron Microscopy Wide Angle X- ray
Purification, SDS-PAGE and cryo-em characterization of the MCM hexamer and Cdt1 MCM heptamer samples.
 Supplementary Figure 1 Purification, SDS-PAGE and cryo-em characterization of the MCM hexamer and Cdt1 MCM heptamer samples. (a-b) SDS-PAGE analysis of the hexamer and heptamer samples. The eluted hexamer
Supplementary Figure 1 Purification, SDS-PAGE and cryo-em characterization of the MCM hexamer and Cdt1 MCM heptamer samples. (a-b) SDS-PAGE analysis of the hexamer and heptamer samples. The eluted hexamer
Supporting Information
 Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supplementary Information
 Supplementary Information Supplementary Table 1. Atomic details for the crystal structures of silver closo-boranes. See Table 1 for further details. α Ag 2 B 10 H 10 Wyckoff x y z U / Å 2 Occ. Ag 4d 0.250
Supplementary Information Supplementary Table 1. Atomic details for the crystal structures of silver closo-boranes. See Table 1 for further details. α Ag 2 B 10 H 10 Wyckoff x y z U / Å 2 Occ. Ag 4d 0.250
Supplementary Figure 1 Comparison of the temperature factors of the AR-LBD in the monomeric and homodimeric crystal forms.
 Supplementary Figure 1 Comparison of the temperature factors of the AR-LBD in the monomeric and homodimeric crystal forms. (a) The AR-LBD dimer is represented as a cartoon colored according to the average
Supplementary Figure 1 Comparison of the temperature factors of the AR-LBD in the monomeric and homodimeric crystal forms. (a) The AR-LBD dimer is represented as a cartoon colored according to the average
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Quantitation of the binding of pro53 peptide to sorla Vps10p measured by the AP reporter assay. The graph shows tracings of the typical chromogenic AP reaction observed with AP-pro53
Supplementary Figure 1 Quantitation of the binding of pro53 peptide to sorla Vps10p measured by the AP reporter assay. The graph shows tracings of the typical chromogenic AP reaction observed with AP-pro53
Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps
 Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Protein dynamics from NMR Relaxation data
 Protein dynamics from NMR Relaxation data Clubb 3/15/17 (S f2 ) ( e ) Nitrogen-15 relaxation ZZ-exchange R 1 = 1/T 1 Longitudinal relaxation (decay back to z-axis) R 2 = 1/T 2 Spin-spin relaxation (dephasing
Protein dynamics from NMR Relaxation data Clubb 3/15/17 (S f2 ) ( e ) Nitrogen-15 relaxation ZZ-exchange R 1 = 1/T 1 Longitudinal relaxation (decay back to z-axis) R 2 = 1/T 2 Spin-spin relaxation (dephasing
type GroEL-GroES complex. Crystals were grown in buffer D (100 mm HEPES, ph 7.5,
 Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Impact of the crystallization condition on importin-β conformation
 Supporting information Volume 72 (2016) Supporting information for article: Impact of the crystallization condition on importin-β conformation Marcel J. Tauchert, Clément Hémonnot, Piotr Neumann, Sarah
Supporting information Volume 72 (2016) Supporting information for article: Impact of the crystallization condition on importin-β conformation Marcel J. Tauchert, Clément Hémonnot, Piotr Neumann, Sarah
Tracking Protein Allostery in Evolution
 Tracking Protein Allostery in Evolution Glycogen phosphorylase frees sugars to provide energy GP orthologs diverged 600,000,000 years can respond to transcription controls, metabolite concentrations and
Tracking Protein Allostery in Evolution Glycogen phosphorylase frees sugars to provide energy GP orthologs diverged 600,000,000 years can respond to transcription controls, metabolite concentrations and
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
Supplementary Information. Structural basis for precursor protein-directed ribosomal peptide macrocyclization
 Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6
 Supplementary Information for: Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6 Yawei Dai 1, 2, Markus Seeger 3, Jingwei Weng 4, Song Song 1, 2, Wenning Wang 4, Yan-Wen 1, 2,
Supplementary Information for: Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6 Yawei Dai 1, 2, Markus Seeger 3, Jingwei Weng 4, Song Song 1, 2, Wenning Wang 4, Yan-Wen 1, 2,
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb97 P ( μm, hours) 1 2 4 P DMSO Figure S1 unningham et al. E 97 65 27 MFN1 GFP-Parkin Opa1 ctin GPDH HEK293 GFP-Parkin 19 115 97 65 27 Mitochondrial Fraction SH-SY5Y GFP-Parkin Mito DMSO Mito
DOI:.38/ncb97 P ( μm, hours) 1 2 4 P DMSO Figure S1 unningham et al. E 97 65 27 MFN1 GFP-Parkin Opa1 ctin GPDH HEK293 GFP-Parkin 19 115 97 65 27 Mitochondrial Fraction SH-SY5Y GFP-Parkin Mito DMSO Mito
Use of SEC-MALS. (Size Exclusion Chromatography - Multi Angle. Light Scattering) for protein quality and characterization
 Use of SEC-MALS (Size Exclusion Chromatography - Multi Angle Light Scattering) for protein quality and characterization Methods for protein characterization Analytical SEC is a common method to characterize
Use of SEC-MALS (Size Exclusion Chromatography - Multi Angle Light Scattering) for protein quality and characterization Methods for protein characterization Analytical SEC is a common method to characterize
Acta Crystallographica Section D
 Supporting information Acta Crystallographica Section D Volume 70 (2014) Supporting information for article: Structural characterization of the virulence factor Nuclease A from Streptococcus agalactiae
Supporting information Acta Crystallographica Section D Volume 70 (2014) Supporting information for article: Structural characterization of the virulence factor Nuclease A from Streptococcus agalactiae
Intrinsic beam emittance of laser-accelerated electrons measured by x-ray spectroscopic imaging
 Intrinsic beam emittance of laser-accelerated electrons measured by x-ray spectroscopic imaging G. Golovin 1, S. Banerjee 1, C. Liu 1, S. Chen 1, J. Zhang 1, B. Zhao 1, P. Zhang 1, M. Veale 2, M. Wilson
Intrinsic beam emittance of laser-accelerated electrons measured by x-ray spectroscopic imaging G. Golovin 1, S. Banerjee 1, C. Liu 1, S. Chen 1, J. Zhang 1, B. Zhao 1, P. Zhang 1, M. Veale 2, M. Wilson
How to judge data quality
 SSRL Workshop: Small-Angle X-ray Scattering and Diffraction Studies, March 28-30, 2016 How to judge data quality Tsutomu Matsui SSRL Lab / Dept. of Chemistry Stanford University Subject of this session
SSRL Workshop: Small-Angle X-ray Scattering and Diffraction Studies, March 28-30, 2016 How to judge data quality Tsutomu Matsui SSRL Lab / Dept. of Chemistry Stanford University Subject of this session
Structure and RNA-binding properties. of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex
 Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
1 Supplementary Figures Supplementary Figure 1 Type I FGFR1 inhibitors (a) Chemical structures of a pyrazolylaminopyrimidine inhibitor (henceforth referred to as PAPI; PDB-code of the FGFR1-PAPI complex:
Expanded View Figures
 The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2013. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201301472 Reconfigurable Infrared Camouflage Coatings from a Cephalopod
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2013. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201301472 Reconfigurable Infrared Camouflage Coatings from a Cephalopod
Cryo-EM data collection, refinement and validation statistics
 1 Table S1 Cryo-EM data collection, refinement and validation statistics Data collection and processing CPSF-160 WDR33 (EMDB-7114) (PDB 6BM0) CPSF-160 WDR33 (EMDB-7113) (PDB 6BLY) CPSF-160 WDR33 CPSF-30
1 Table S1 Cryo-EM data collection, refinement and validation statistics Data collection and processing CPSF-160 WDR33 (EMDB-7114) (PDB 6BM0) CPSF-160 WDR33 (EMDB-7113) (PDB 6BLY) CPSF-160 WDR33 CPSF-30
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
NOTES. Fusion Protein Separation. Highlighted FACTS:
 Fusion Protein Separation NOTES Highlighted FACTS: Fusion proteins are structural hybrids that contain two different proteins, which retain the functional properties from the original proteins. Zenix and
Fusion Protein Separation NOTES Highlighted FACTS: Fusion proteins are structural hybrids that contain two different proteins, which retain the functional properties from the original proteins. Zenix and
New insight into the dynamical system of αb-crystallin oligomers
 Supplementary Materials New insight into the dynamical system of αb-crystallin oligomers Rintaro Inoue*, Takumi Takata, Norihiko Fujii, Kentaro Ishii, Susumu Uchiyama, Nobuhiro Sato, Yojiro Oba, Kathleen
Supplementary Materials New insight into the dynamical system of αb-crystallin oligomers Rintaro Inoue*, Takumi Takata, Norihiko Fujii, Kentaro Ishii, Susumu Uchiyama, Nobuhiro Sato, Yojiro Oba, Kathleen
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
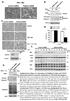 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
What binds to Hb in addition to O 2?
 Reading: Ch5; 158-169, 162-166, 169-174 Problems: Ch5 (text); 3,7,8,10 Ch5 (study guide-facts); 1,2,3,4,5,8 Ch5 (study guide-apply); 2,3 Remember Today at 5:30 in CAS-522 is the second chance for the MB
Reading: Ch5; 158-169, 162-166, 169-174 Problems: Ch5 (text); 3,7,8,10 Ch5 (study guide-facts); 1,2,3,4,5,8 Ch5 (study guide-apply); 2,3 Remember Today at 5:30 in CAS-522 is the second chance for the MB
Structural insights into WcbI, a novel polysaccharide-biosynthesis enzyme
 Volume 1 (2014) Supporting information for article: Structural insights into WcbI, a novel polysaccharide-biosynthesis enzyme Mirella Vivoli, Emily Ayres, Edward Beaumont, Michail N. Isupov and Nicholas
Volume 1 (2014) Supporting information for article: Structural insights into WcbI, a novel polysaccharide-biosynthesis enzyme Mirella Vivoli, Emily Ayres, Edward Beaumont, Michail N. Isupov and Nicholas
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
Real-Time Structural Evolution at the Interface of an. Organic Transistor During Thermal Annealing
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for: Real-Time Structural Evolution
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for: Real-Time Structural Evolution
MEASURING PROTEIN AGGREGATION WITH THE VISCOTEK SEC-MALS 20
 MEASURING PROTEIN AGGREGATION WITH THE VISCOTEK SEC-MALS 20 Introduction Protein aggregation is recognised as a major issue in the biopharmaceutical industry. Proteins have a tendency to aggregate over
MEASURING PROTEIN AGGREGATION WITH THE VISCOTEK SEC-MALS 20 Introduction Protein aggregation is recognised as a major issue in the biopharmaceutical industry. Proteins have a tendency to aggregate over
NB-DNJ/GCase-pH 7.4 NB-DNJ+/GCase-pH 7.4 NB-DNJ+/GCase-pH 4.5
 SUPPLEMENTARY TABLES Suppl. Table 1. Protonation states at ph 7.4 and 4.5. Protonation states of titratable residues in GCase at ph 7.4 and 4.5. Histidine: HID, H at δ-nitrogen; HIE, H at ε-nitrogen; HIP,
SUPPLEMENTARY TABLES Suppl. Table 1. Protonation states at ph 7.4 and 4.5. Protonation states of titratable residues in GCase at ph 7.4 and 4.5. Histidine: HID, H at δ-nitrogen; HIE, H at ε-nitrogen; HIP,
Development of Novel Small- Angle X-ray Scattering Data Analysis Methods for Study of Flexible Proteins. Michael Kachala EMBL-Hamburg, Germany
 Development of Novel Small- Angle X-ray Scattering Data Analysis Methods for Study of Flexible Proteins Michael Kachala EMBL-Hamburg, Germany 60 mkl >1 mg/ml Monocromatic X-ray beam Sample Mono- or polydisperse
Development of Novel Small- Angle X-ray Scattering Data Analysis Methods for Study of Flexible Proteins Michael Kachala EMBL-Hamburg, Germany 60 mkl >1 mg/ml Monocromatic X-ray beam Sample Mono- or polydisperse
Small Angle X-Ray Solution Scattering of Biological Macromolecules
 Small Angle X-Ray Solution Scattering of Biological Macromolecules Emre Brookes UltraScan Workshop 15 June 2014 Overview Experimental method Sample preparation Experimental data analysis Experimental method
Small Angle X-Ray Solution Scattering of Biological Macromolecules Emre Brookes UltraScan Workshop 15 June 2014 Overview Experimental method Sample preparation Experimental data analysis Experimental method
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Supporting Information
 Supporting Information C-terminal truncated -synuclein fibrils contain strongly twisted β-sheets Aditya Iyer, Steven J. Roeters ǂ, Vladimir Kogan Ί, Sander Woutersen *ǂ, Mireille M.A.E Claessens * and
Supporting Information C-terminal truncated -synuclein fibrils contain strongly twisted β-sheets Aditya Iyer, Steven J. Roeters ǂ, Vladimir Kogan Ί, Sander Woutersen *ǂ, Mireille M.A.E Claessens * and
RNA Polymerase I Contains a TFIIF-Related DNA-Binding Subcomplex
 Molecular Cell, Volume 39 Supplemental Information RNA Polymerase I Contains a TFIIFRelated DNABinding Subcomplex Sebastian R. Geiger, Kristina Lorenzen, Amelie Schreieck, Patrizia Hanecker, Dirk Kostrewa,
Molecular Cell, Volume 39 Supplemental Information RNA Polymerase I Contains a TFIIFRelated DNABinding Subcomplex Sebastian R. Geiger, Kristina Lorenzen, Amelie Schreieck, Patrizia Hanecker, Dirk Kostrewa,
Supplementary Materials for
 www.advances.sciencemag.org/cgi/content/full/1/7/e1500263/dc1 Supplementary Materials for Newton s cradle proton relay with amide imidic acid tautomerization in inverting cellulase visualized by neutron
www.advances.sciencemag.org/cgi/content/full/1/7/e1500263/dc1 Supplementary Materials for Newton s cradle proton relay with amide imidic acid tautomerization in inverting cellulase visualized by neutron
JBC Papers in Press. Published on September 10, 2008 as Manuscript M
 JBC Papers in Press. Published on September 10, 2008 as Manuscript M804753200 The latest version is at http://www.jbc.org/cgi/doi/10.1074/jbc.m804753200 STRUCTURES OF THE ciap2 RING DOMAIN REVEAL CONFORMATIONAL
JBC Papers in Press. Published on September 10, 2008 as Manuscript M804753200 The latest version is at http://www.jbc.org/cgi/doi/10.1074/jbc.m804753200 STRUCTURES OF THE ciap2 RING DOMAIN REVEAL CONFORMATIONAL
Carbazole Derivatives Binding to c-kit G-quadruplex DNA
 Supplementary Materials Carbazole Derivatives Binding to c-kit G-quadruplex DNA Agata Głuszyńska 1, *, Bernard Juskowiak 1, Martyna Kuta-Siejkowska 2, Marcin Hoffmann 2 and Shozeb Haider 3 1 Laboratory
Supplementary Materials Carbazole Derivatives Binding to c-kit G-quadruplex DNA Agata Głuszyńska 1, *, Bernard Juskowiak 1, Martyna Kuta-Siejkowska 2, Marcin Hoffmann 2 and Shozeb Haider 3 1 Laboratory
Supporting Information
 Supporting Information Wiley-VCH 2012 69451 Weinheim, Germany Bound Cations Significantly Stabilize the Structure of Multiprotein Complexes in the Gas Phase** Linjie Han, Suk-Joon Hyung, and Brandon T.
Supporting Information Wiley-VCH 2012 69451 Weinheim, Germany Bound Cations Significantly Stabilize the Structure of Multiprotein Complexes in the Gas Phase** Linjie Han, Suk-Joon Hyung, and Brandon T.
Supplementary Materials: Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1
 S1 of S8 Supplementary Materials: Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1 Katsiaryna Tarasava, Jens Loebus and Eva Freisinger Figure S1. Deconvoluted
S1 of S8 Supplementary Materials: Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1 Katsiaryna Tarasava, Jens Loebus and Eva Freisinger Figure S1. Deconvoluted
Sunhats for plants. How plants detect dangerous ultraviolet rays
 Sunhats for plants How plants detect dangerous ultraviolet rays Anyone who has ever suffered sunburn will know about the effects of too much ultraviolet (UV) radiation, in particular UV-B (from 280-315
Sunhats for plants How plants detect dangerous ultraviolet rays Anyone who has ever suffered sunburn will know about the effects of too much ultraviolet (UV) radiation, in particular UV-B (from 280-315
Supplemental Materials and Methods
 Supplemental Materials and Methods Time-resolved FRET (trfret) to probe for changes in the Box A/A stem upon complex assembly U3 MINI was folded and the decay of Fl fluorescence was measured at 20 ºC (see
Supplemental Materials and Methods Time-resolved FRET (trfret) to probe for changes in the Box A/A stem upon complex assembly U3 MINI was folded and the decay of Fl fluorescence was measured at 20 ºC (see
Dr. Yonca Yuzugullu PERG (Protein Engineering Research Group)
 Dr. Yonca Yuzugullu PERG (Protein Engineering Research Group) BSc, 1997 Ankara University, Turkey PhD, 2010 Middle East Technical University, Turkey Lecturer in Department of Biology Kocaeli University,
Dr. Yonca Yuzugullu PERG (Protein Engineering Research Group) BSc, 1997 Ankara University, Turkey PhD, 2010 Middle East Technical University, Turkey Lecturer in Department of Biology Kocaeli University,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
Overview & Applications. T. Lezon Hands-on Workshop in Computational Biophysics Pittsburgh Supercomputing Center 04 June, 2015
 Overview & Applications T. Lezon Hands-on Workshop in Computational Biophysics Pittsburgh Supercomputing Center 4 June, 215 Simulations still take time Bakan et al. Bioinformatics 211. Coarse-grained Elastic
Overview & Applications T. Lezon Hands-on Workshop in Computational Biophysics Pittsburgh Supercomputing Center 4 June, 215 Simulations still take time Bakan et al. Bioinformatics 211. Coarse-grained Elastic
In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes
 Supporting information for In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes Yuting Zhou, Jianmin Song, Lei Wang*, Xuting Xue, Xiaoman Liu, Hui Xie*, and Xin Huang* MIIT Key Laboratory
Supporting information for In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes Yuting Zhou, Jianmin Song, Lei Wang*, Xuting Xue, Xiaoman Liu, Hui Xie*, and Xin Huang* MIIT Key Laboratory
Supplemental Data SUPPLEMENTAL FIGURES
 Supplemental Data CRYSTAL STRUCTURE OF THE MG.ADP-INHIBITED STATE OF THE YEAST F 1 C 10 ATP SYNTHASE Alain Dautant*, Jean Velours and Marie-France Giraud* From Université Bordeaux 2, CNRS; Institut de
Supplemental Data CRYSTAL STRUCTURE OF THE MG.ADP-INHIBITED STATE OF THE YEAST F 1 C 10 ATP SYNTHASE Alain Dautant*, Jean Velours and Marie-France Giraud* From Université Bordeaux 2, CNRS; Institut de
Chris C. Broomell, Henrik Birkedal, Cristiano L. P. Oliveira, Jan Skov Pedersen, Jan-André Gertenbach, Mark Young, and Trevor Douglas*
 Chris C. Broomell, Henrik Birkedal, Cristiano L. P. Oliveira, Jan Skov Pedersen, Jan-André Gertenbach, Mark Young, and Trevor Douglas* Protein Cage Nanoparticles as Secondary Building Units for the Synthesis
Chris C. Broomell, Henrik Birkedal, Cristiano L. P. Oliveira, Jan Skov Pedersen, Jan-André Gertenbach, Mark Young, and Trevor Douglas* Protein Cage Nanoparticles as Secondary Building Units for the Synthesis
