Cell. Molecular. FLIP Structure and Function. Volume 20 Number 6 December 22, 2005
|
|
|
- Michael Burns
- 5 years ago
- Views:
Transcription
1 Molecular Volume 20 Number 6 December 22, 2005 Cell FLIP Structure and Function
2 Molecular Cell, Vol. 20, , December 22, 2005, Copyright ª2005 by Elsevier Inc. DOI /j.molcel Crystal Structure of MC159 Reveals Molecular Mechanism of DISC Assembly and FLIP Inhibition Jin Kuk Yang, 1,3 Liwei Wang, 1,3 Lixin Zheng, 2 Fengyi Wan, 2 Misonara Ahmed, 1 Michael J. Lenardo, 2 and Hao Wu 1, * 1 Department of Biochemistry Weill Medical College of Cornell University 1300 York Avenue New York, New York Laboratory of Immunology National Institute of Allergy and Infectious Diseases National Institutes of Health Bethesda, Maryland Summary The death-inducing signaling complex (DISC) comprising Fas, Fas-associated death domain (FADD), and caspase-8/10 is assembled via homotypic associations between death domains (DDs) of Fas and FADD and between death effector domains (DEDs) of FADD and caspase-8/10. Caspase-8/10 and FLICE/ caspase-8 inhibitory proteins (FLIPs) that inhibit caspase activation at the DISC level contain tandem DEDs. Here, we report the crystal structure of a viral FLIP, MC159, at 1.2 Å resolution. It reveals a noncanonical fold of DED1, a dumbbell-shaped structure with rigidly associated DEDs and a different mode of interaction in the DD superfamily. Whereas the conserved hydrophobic patch of DED1 interacts with DED2, the corresponding region of DED2 mediates caspase-8 recruitment and contributes to DISC assembly. In contrast, MC159 cooperatively assembles with Fas and FADD via an extensive surface that encompasses the conserved charge triad. This interaction apparently competes with FADD self-association and disrupts higher-order oligomerization required for caspase activation in the DISC. Introduction Death receptors (DRs) in the Tumor Necrosis Factor (TNF) Receptor (TNFR) superfamily mediate the extrinsic pathway of apoptosis and play critical roles in embryonic development, cellular homeostasis, and immune regulation (French and Tschopp, 2003; Locksley et al., 2001; Peter and Krammer, 2003; Wajant, 2002). Current members of the DR subfamily include Fas (also known as CD95 and APO-1), TNF-R1, DR3, TRAIL-R1, TRAIL-R2, DR6, EDA-R, and NGF-R (French and Tschopp, 2003). Signaling by these receptors is triggered by interaction with one or more specific ligands that are members of the TNF superfamily. Genetic mutations or abnormal expression of DRs and their ligands have been associated with many human diseases including the Autoimmune Lymphoproliferative Syndrome (ALPS), cancer, and tissue destructive diseases such as graft-versus-host disease, multiple sclerosis, and stroke *Correspondence: haowu@med.cornell.edu 3 These authors contributed equally to this work. (French and Tschopp, 2003). Highly effective therapeutics for modulating DR signaling have emerged. These include antagonistic antibodies in tissue destructive diseases and agents that selectively trigger DR-mediated cell death in cancer cells (French and Tschopp, 2003). Therefore, a detailed understanding of the signaling and regulatory mechanisms of DRs has broad medical and biological importance. DR signaling is mediated by highly specific proteinprotein associations that generate oligomeric signaling assemblies such as the DISC (Kischkel et al., 1995; Wajant, 2002). DRs contain a DD in their intracellular regions (Itoh and Nagata, 1993; Tartaglia et al., 1993; Wajant, 2002). For Fas, its DD recruits the FADD adaptor protein via a homotypic interaction with the C-terminal DD of FADD (Chinnaiyan et al., 1995; Kischkel et al., 1995). FADD also contains an N-terminal DED that interacts homotypically with the tandem DEDs in the prodomains of caspase-8 or -10 (Boldin et al., 1996; Muzio et al., 1996). These interactions form the ternary DISC that contains Fas, FADD, and caspase-8 or -10. Recruitment of procaspases into the DISC initiates proteolytic autoprocessing (Medema et al., 1997; Salvesen and Dixit, 1999; Shi, 2004). This liberates active caspase-8 or -10 into the cytoplasm to cleave and activate effector caspases such as caspase-3 and caspase-7, leading to a cascade of events in apoptotic cell death (Salvesen, 2002). In addition to caspase-8 and -10, most FLIPs are also tandem DED-containing proteins, which regulate DISC assembly and caspase activation (Thome and Tschopp, 2001). Cellular FLIPs (cflips), comprising the long and short isoforms cflip(l) and cflip(s), are tightly regulated in expression in T cells and might be involved in the control of both T cell activation and death (Goltsev et al., 1997; Han et al., 1997; Hu et al., 1997b; Inohara et al., 1997; Irmler et al., 1997; Rasper et al., 1998; Shu et al., 1997; Srinivasula et al., 1997; Thome and Tschopp, 2001). Viral FLIPs (vflips) appear to have evolved to inhibit apoptosis of infected host cells and are present in the poxvirus Molluscum contagiosum virus (MCV) (Bertin et al., 1997; Garvey et al., 2002b; Hu et al., 1997a; Shisler and Moss, 2001; Thome et al., 1997) and g-herpesviruses such as equine herpesvirus-2 (EHV-2), herpesvirus saimiri (HVS), the Kaposi-associated human herpesvirus-8 (HHV-8), and rhesus rhadinovirus (RRV) (Bertin et al., 1997; Hu et al., 1997a; Searles et al., 1999; Thome et al., 1997; Thome and Tschopp, 2001). The DDs and DEDs involved in DR signaling are members of the DD superfamily, which also includes the caspase recruitment domains (CARDs) involved in the intrinsic cell death pathway and the Pyrin domains (PYDs) involved in inflammatory signaling (Kohl and Grutter, 2004; Reed et al., 2004). The DD superfamily contains homotypic protein-protein interaction modules. NMR structures of the first DD (Huang et al., 1996), DED (Eberstadt et al., 1998), CARD (Chou et al., 1998), and PYD (Hiller et al., 2003) have all revealed a common six-helical bundle structure. Despite their biological importance, there are currently no reported structures of tandem
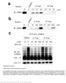
3 Molecular Cell 940 Figure 1. Cooperative Assembly of Fas, FADD, and MC159 (A) Schematic domain organization of Fas, FADD, and MC159 is shown. (B) His-tag pull-down of MC159, Fas DD, and FADD is shown. (C) His-tag pull-down of Fas DD and FADD. (D) His-tag pull-down of MC159 and FADD DED. (E) His-tag pull-down of Fas DD and FADD DD. Contaminants are marked by an asterisk. DEDs. Both the architectural features of the individual DEDs in tandem DEDs and the mode of their association are entirely unknown. Therefore, the molecular basis for the recruitment of caspase-8, caspase-10, and FLIPs remains to be revealed. To elucidate the molecular basis for the functions of tandem DED-containing proteins, we determined the crystal structure of MC159, a vflip from MCV, at 1.2 Å resolution. It reveals a dumbbell-shaped structure with rigidly associated DED1 and DED2 via hydrophobic interactions. DED1 is highly divergent from DED2 and other DED structures. The DED1/DED2 interaction is topologically different from the previously observed CARD/CARD and DD/DD interactions (Qin et al., 1999; Xiao et al., 1999). A hydrogen-bonded charge triad is present on the surface of DED1 and DED2, which may represent a general characteristic feature of DEDs, but not other members of the DD superfamily. We found that the conserved hydrophobic patch of DED2 mediates caspase-8 recruitment and DISC assembly. Similar mechanisms might be used by cflips and herpesvirus vflips to compete with caspase-8 recruitment. In contrast to hydrophobic interactions, MC159 uses an extensive charge surface that encompasses the charge triad to cooperatively assemble with Fas and FADD. This interaction does not compete with caspase-8 recruitment, but apparently competes with FADD self-association and disrupts higher order oligomerization required for caspase activation in the DISC. Together, these structural, biochemical, and mutational studies reveal important insights into the molecular mechanism of DISC assembly and FLIP inhibition. Results Fas, FADD, and MC159 Are Cooperatively Assembled into a Ternary Complex We used recombinant proteins and a His-tag pull-down assay to establish an in vitro system for assessing the recruitment of MC159. MC159 interacted effectively with Fas DD and FADD, as shown by the pull-down of an apparent ternary complex with His-tagged MC159 (Figure 1B, lane 4). However, in the absence of Fas DD, His-tagged MC159 only weakly pulled down FADD (Figure 1B, lane 3) and, in the absence of MC159, Fas DD only weakly interacted with FADD (Figure 1C, lanes 3 and 7). These data suggest that the binary Fas DD/ FADD and FADD/MC159 interactions are weak and that the ternary complex is cooperatively assembled. Because isolated FADD DED interacted strongly with MC159 (Figure 1D, lane 4) and isolated FADD DD interacted strongly with Fas DD (Figure 1E, lane 2), it appears that the DED in full-length FADD autoinhibits its DD from interacting effectively with Fas DD and that the DD in full-length FADD autoinhibits its DED from interacting strongly with MC159. This autoinhibition is relieved when the third component of the complex is present. High-Resolution Structure of MC159: Noncanonical Fold of DED1 and the Charge Triad To elucidate the molecular basis of DISC assembly and FLIP-mediated inhibition of caspase activation, we determined the crystal structure of MC159, a vflip from MCV (Figure 2, Table 1). The structure of full-length MC159 was first solved at 3.8 Å resolution by singlewavelength anomalous diffraction and 4-fold noncrystallographic symmetry averaging (Figure 2A). It revealed that more than fifty residues at the C-terminal tail of MC159 were disordered in the crystal. By removing this region of MC159, a different crystal form that diffracted to 1.2 Å resolution was obtained (Figure 2B). The original atomic model built from the low-resolution crystal form was used to determine the structure of the high-resolution crystal form. Structural analyses are based on information from the high-resolution structure. The tandem DEDs of MC159 form a dumbbell-shaped structure with each DED as the weight at either end (Figure 2C). Although all members of the DD superfamily appear to exhibit the fold of a six-helical bundle, DED1 is highly divergent from DED2 and the known NMR
4 Crystal Structure of Viral FLIP MC Figure 2. Crystal Structure of MC159 (A) MC159 tetramer structure from the P222 1 crystal form is shown in cyan, magenta, yellow, and green, respectively, for each monomer. (B) Stereo ribbon drawing of the high-resolution structure of MC159 from the P crystal form. DED1 is shown in cyan and DED2 in magenta. Helices H0-H7 of DED1 and H1-H6 of DED2 are labeled. H3 is absent in DED1. (C) Electrostatic surface representation of MC159, showing the dumbbell-shaped structure and the rich charges on one face of MC159. The top panel is related to (B) by an w180º rotation along the vertical axis followed by a 90º rotation on the page. The lower panel is related to (B) by an w90º rotation on the page. (D) Stereo cylinder drawing showing the superposition of MC159 DED1 (cyan) with FADD DED (red) and DED2 (magenta) with FADD DED (yellow). For the superposition between DED1 and FADD, helices H0 and H7 are only present in DED1, whereas H3 is only present in FADD. (E) Stick model for the hydrogen bonding interactions in the charge triad of DED1 is shown. structures of single DEDs from FADD (Eberstadt et al., 1998) and PEA-15 (Hill et al., 2002)(Figure 2D, see Table S1 in the Supplemental Data available with this article online). In particular, the topologically equivalent helix H3 is missing, which is replaced by a short loop connecting helices H2 and H4. This loop is mostly hydrophilic and does not contribute to the hydrophobic core of DED1. Two additional helices are present, helix H0 at the N terminus and helix H7. Helix H7 covers helix H5 and helps to close the hydrophobic core of the domain. It also brings the chain to the beginning of DED2. In addition, DED1 has a shorter helix H2 and a different orientation in helix H6. Only about half of the residues in DED1 were aligned within 3 Å in Ca distance to DED2, FADD DED, and PEA-15 DED (Table S1). In fact, a DALI structural homology search (Holm and Sander, 1995) showed that DED1 structure is almost as different to these DED structures as to other structures in the DD superfamily such as those of DDs, PYDs, and CARDs (Table S1). On the other hand, DED2 is a bona fide DED. It showed extensive structural homology with the known DED structures from FADD and PEA-15, which were also the top two matches from a structural homology search with DALI (Figure 2D, Table S1). Among the 97 residues in DED2, 74 residues were aligned with FADD and PEA- 15 to within 3.0 Å in Ca distance, giving rise to RMSDs of 1.4 Å in both cases (Table S1). All six canonical helices are present in DED2. In comparison with FADD DED, the helices H1, H4, H5, and H6 of DED2 are more conserved structurally, whereas helices H2 and H3 show more
5 Molecular Cell 942 Table 1. Crystallographic Statistics Data Collection SeMet (Residues 1 241) Native (Residues 1 187) Space group P222 1 P Cell dimensions a, b, c 84.0 Å, Å, Å 35.0 Å, 62.9 Å, 75.8 Å Resolution Å Å R sym 8.4% (35.3%) 5.9% (30.7%) I/sI 20.0 (3.5) 48.5 (4.4) Completeness 99.7 (98.6%) 99.6% (97.9%) Redundancy 6.5 (4.5) 6.7 (4.2) Refinement Resolution Å Number of reflections 51,463 R work /R free 21.2%/23.3% Number atoms Protein/water and 1492/322 other small molecule Average B factors Protein/water and 15.8 Å 2 /33.5 Å 2 other small molecule Rms deviations Bond lengths/angles Å/0.99º Ramachandran plot Most favored/ additionally allowed 93.9%/6.1% Highest resolution shell is shown in parenthesis. variations in position. Helix H6 is exceptionally long in DED2; it comprises 21 residues and runs to the end of the construct at residue 187. Approximately half of helix H6 does not pack against the core of DED2; instead it protrudes prominently. In the low-resolution crystal form, helix H6 is involved in the organization of four MC159 molecules in an almost perfect 222 noncrystallographic symmetry (Figure 2A). However, MC159 is a monomer in solution and the tetrameric association does not appear to have any biological significance. Previous truncation studies have shown that a large part of helix H6 could be truncated (up to residue 179) without affecting its apoptosis protection function (Garvey et al., 2002a), and mutations of exposed residues on the remaining part of H6 did not affect ternary complex formation with Fas and FADD (see below). The high-resolution structure of MC159 revealed a hydrogen-bonded charge triad on the surface of DED1 and DED2 (Figure 2E, Table S2), which contributes to the highly charged features on one face of the structure (Figure 2C). We refer the charge triad as the E/D-RxDL motif ( x for any amino acid). It involves the Arg and Asp residues in the RxDL motif in helix H6 (residues in DED1 and residues in DED2) and a negatively charged residue in helix H2 (E24 in DED1 and E111 in DED2). The RxDL motif was previously shown to be functionally important (Garvey et al., 2002b). Extensive hydrogen bonding interactions are observed among these charged side chains. In DED1, for example, Arg69 situates in between Asp71 and Glu24. All three polar atoms in the Arg69 side chain make hydrogen bonds: N3 and Nh2 with the Od1 and Od2 atoms of Asp71 and Nh1 with O31 and O32 of Glu24 with perfect hydrogen bonding distances. These hydrogen bonds likely help to maintain a precise organization of the side chains, which may be functionally important (see below). It is also possible that they play a local structural role in maintaining the conformation of this region of the DEDs. The charge triad is highly conserved in most single and tandem DEDs (Figure 3A), but is not present in other members of the DD superfamily, suggesting that it is a characteristic feature of DEDs alone. Interestingly, the charge triad is conspicuously altered or missing in three of the four DEDs in caspase-8 and caspase-10. In caspase-8 DED2, the E/D-RxDL motif has a Glu, Lys, and Ser at the three charged residue positions, and x is missing. It is possible that the change from Arg to Lys lowers the hydrogen bonding potential so it now only interacts with one negatively charged residue in the triad. For caspase-10, the E/D-RxDL motif is missing in both DED1 and DED2. It is possible that this motif does not play an important role in caspase recruitment but is important in other functions of DED-containing proteins (see below). The Interaction between DED1 and DED2 Reveals a Conserved Rigid Tandem DED Association and a Different Mode of Interaction in the DD Superfamily Unlike beads on a string, the structure of MC159 revealed that DED1 and DED2 are rigidly associated with each other in a compact structure (Figures 2B and 2C). The DED1/DED2 interface is extensive, burying approximately 1380 Å 2 of surface area. The two DEDs are related, very approximately, by a translation across the contact interface so that one side of DED1 is contacting the equivalently opposite side of DED2. The translational relationship between DED1 and DED2 is made possible by helix H7 of DED1. The interaction at the DED1/DED2 interface is mostly hydrophobic, mediated by helices H2 and H5 of DED1 and helices H1 and H4 of DED2 (Figures 3B and 3C). There are a total of 195 interfacial atomic contacts, among which 117 are between nonpolar atoms, 17 are between polar atoms, and 61 are mixed contacts between polar and nonpolar atoms. The surface patch formed by H2 and H5 of DED1 is equivalent to the conserved hydrophobic patch identified on the surface of FADD DED that is important for caspase-8 interaction (Eberstadt et al., 1998) (Figure 3A). The H1 and H4 surface of DED2 is equivalent to the second hydrophobic patch identified in the FADD DED structure on the opposite side, which does not appear to be important in FADD signaling (Eberstadt et al., 1998). In addition to hydrophobic interactions, three interfacial hydrogenbonding networks are observed (Figure 3C). The first one is the salt bridge between Asp34, localized between H2 and H4 of DED1, and Arg97 in H1 of DED2. The second one involves the side chains of H23 and S26 in H2 of DED1, Glu105 in H1 of DED2, and Arg135 in the H3-H4 loop of DED2. The last one is the salt bridge between Glu62 in H5 of DED1 and Lys98 in H1 of DED2. The interfacial residues, especially those that are completely buried at the DED1/DED2 interface and contribute large surface areas, such as F30, L31, F92, L93, and R97, are mostly conserved in tandem DEDs (Figure 3A). This suggests that all known tandem DEDs form a similar rigid compact structure as MC159. The observed interaction between DED1 and DED2 represents an example of a DED/DED interaction.
6 Crystal Structure of Viral FLIP MC Figure 3. Mode of Interaction between DED1 and DED2 (A) Sequence alignment of MC159 with other tandem DED-containing proteins and structure-based sequence alignment with FADD. Residues with more than 40 Å 2 surface area buried at the DED1/DED2 interface are shaded in red (completely buried) and magenta (partially buried) and residues with Å 2 surface area buried at the DED1/DED2 interface are shaded in brown. Residues at the charge triad E/D/-RxDL motif positions are shaded in green, and residues at the conserved hydrophobic patch of DED2 are shaded in blue. Residues of MC159 involved in FADD interaction are marked by an asterisk. Residues of caspase-8 involved in FADD interaction are marked by #. Sequences identical to MC159 are shaded in yellow. Secondary structures of MC159 and FADD are shown above and below the sequences, respectively. Different shades of blue bars indicate whether a residue is buried (dark blue) or exposed (light blue). Abbreviations are as follows: CASP-8, caspase-8; CASP-10, caspase-10; cflip, cflip(s); EHV-2 E8, vflip from equine herpesvirus-2. (B) Regions of MC159 involved in the interaction are shown in blue for DED1 (residues and 57 66), red for DED2 (residues and ), and green for the DED1-DED2 linker (residues 86 94). (C) Detailed interaction between DED1 and DED2. Potential hydrogen bonds are shown by dotted lines. (D and E) Comparison of DED1/DED2 interaction with the Apaf-1 CARD/pro-caspase-9 CARD interaction is shown. Apaf-1 and pro-caspase-9 in the Apaf-1/pro-caspase-9 complex are each superimposed with DED1, respectively, in (D) and (E). (F and G) Comparison of DED1/DED2 interaction with the Pelle DD/Tube DD interaction. Pelle and Tube in the Pelle/Tube complex are each superimposed with DED1, respectively, in (F) and (G). However, because DED1 is highly divergent from a typical DED fold, the interaction may not be representative of other DED/DED interactions. The interaction also differs from the two known modes of interaction within the DD superfamily, the interaction seen in the Pelle DD/ Tube DD complex (Xiao et al., 1999) and that in the Apaf-1 CARD/pro-caspase-9 CARD complex (Qin et al., 1999) (Figures 3D, 3E, 3F, and 3G). The interaction between Pelle and Tube is completely different, involving the H4 region of Pelle and the H6 region of Tube as well as an interaction at the C-terminal tail of Tube. The Apaf-1 CARD/pro-caspase-9 CARD interaction is somewhat similar; instead of the H2 and H5 helices as in DED1, the H2 and H3 helices of Apaf-1 interact with the H1 and H4 helices of pro-caspase-9. Interestingly, these three cases of homotypic interactions within the DD superfamily are all asymmetric, in contrast to what might have been expected for homotypic interactions.
7 Molecular Cell 944 MC159 Mutants that Fail to Protect Cells from Apoptosis Do Not Form a Ternary Complex with Fas and FADD Previous mutational studies on MC159 have suggested that FADD recruitment does not seem sufficient for its anti-apoptotic function because almost all MC159 mutants that were deficient in protecting cells from Fas and TNFR1-induced apoptosis appear to have retained their abilities to interact with FADD (Garvey et al., 2002a). Having established that FADD does not interact strongly with MC159 in the absence of Fas, we considered the possibility that the binary FADD/MC159 interaction may not serve as a good indicator for MC159 recruitment to the DISC. Instead, we used our in vitro assay of ternary complex formation with Fas and FADD to characterize these previously generated MC159 mutants (Table 2). Strikingly, all tested mutants that failed to protect cells from apoptosis, including M2, M3, M5, M6, M8, M16, M17, M21, and M24, were also unable to form ternary complexes with Fas and FADD (Figure S1, lanes 2, 4, 6, 11, 15, 13, 17, 19, and 24). In contrast, MC159 mutants that retained full or partial ability to protect cells, such as M7, M9, M10, M18, and M27, were able to form the ternary complex (Figure S1, lanes 28, 30, 32, 37, and 39). Therefore, the ability of MC159 to protect cells from apoptosis is closely correlated with its recruitment to Fas and FADD. Hence, in contrast to previous suggestion, it appears that DISC recruitment of MC159 is both necessary and sufficient for its apoptotic protection function. The DED2 Hydrophobic Patch of Caspase-8 Is Required for FADD Interaction The MC159 structure provides a template for examining the structure and function relationships of tandem DEDcontaining proteins. Because earlier mutagenesis experiments on FADD showed that its hydrophobic patch, comprised of the conserved F25 and L26 residues, is important for caspase-8 recruitment (Eberstadt et al., 1998), we speculated that the same hydrophobic patch on caspase-8 may be involved in FADD association. Because the corresponding hydrophobic patch on DED1 is buried at the DED1/DED2 interface, only one conserved hydrophobic patch is present on tandem DED structures (Figures 2C and 3A). To elucidate whether this conserved hydrophobic patch of DED2 is involved in interaction with FADD, we performed an immunoprecipitation between caspase-8 and FADD (Figure 4A). An active site mutant of caspase-8 (C360S) was used to avoid caspase-8 autoprocessing. As predicted, the single site hydrophobic patch mutant of caspase-8, F122G, exhibited weakened interaction with FADD. The double mutant, F122G and L123G, completely abolished its interaction with FADD, demonstrating that these surfaceexposed hydrophobic residues are absolutely required for the interaction of caspase-8 with FADD. The DED2 Hydrophobic Patch of MC159 Is Not Required for Ternary Complex Formation, and MC159 Does Not Compete with Caspase-8 for DISC Recruitment To determine whether the same hydrophobic patch on DED2 of MC159 is also important for MC159 s recruitment, we examined the ability of the hydrophobic-patch Table 2. Characterization of Previously Generated, Denoted as M Followed by a Number, and Additional, Denoted as MC Followed by a Number, MC159 Mutants for Ternary Complex Formation with Fas and FADD Name Mutation Sites a Ternary Complex Formation M2 H23A, E24A, D25A 2 2 M3 H33A, D34A 2 2 M5 E62A 2 2 M6 R69A, D71A 2 2 M7 K81A, E82A + + M8 R95A, R97A 2 2 M9 E105A, E106A 6 6 M10 R135A, E138A + + M16 E18A, E19A, D21A 2 2 M17 F30A 2 2 M18 E111A, R113A 6 + M21 L72A, L73A 2 2 M24 L31A 2 2 M27 M160A, L161A + 6 M29 L157A 2 6 MC1 S9A, L10A, P11A, F12A + MC2 E24D + MC3 E24Q 2 MC4 P37A + MC5 T40A, T41A, T43A, Q44A 2 MC6 C47A, S48A + MC7 S50A, Q51A, Q52A + MC8 Q51A, Q52A + MC9 T56A, L57A 2 MC10 Q68A + MC11 R69K 2 MC12 D71N 2 MC13 K74A, S75A + MC14 L79A, S80A 2 MC15 Q86A, L87A, L88A + MC16 T94A, R95A 2 MC17 D108A, S109A, S110A + MC18 E111Q 6 MC19 L117G + MC20 F118G + MC21 L117G, F118G + MC22 C120A, N121A, L122A, 2 N123A, P124A, S125A MC23 S127A, T128A + MC24 S131A, E132A, S133A + MC25 E144A, N145A 2 MC26 V146, L148 + MC27 S150G, P151G + MC28 S155, V156 + MC29 R166K + MC30 D168N + MC31 Q171A, Q172A + MC32 V174A, E175A + Apoptosis Protection a a Apoptosis protection data for previously generated mutants were as reported in (Garvey et al., 2002a). The mutation sites for the previously generated mutants were determined by resequencing of the mutant plasmids and are different in M8 (previously reported as R95A, Y96A, and R97A), M9 (previously reported as E105A, E106A, and D108A), and M18 (previously reported as E111A, L112A, and R113A). mutants of MC159 to form ternary complexes with Fas and FADD. In contrast to the requirement for the DED2 hydrophobic patch in caspase-8 recruitment, both single (L117G, F118G) and double (L117G and F118G) hydrophobic-patch mutants of MC159 had essentially no effect on its ability to form a ternary complex with
8 Crystal Structure of Viral FLIP MC Figure 4. The Hydrophobic Patch of Caspase-8, but Not MC159, Is Involved in FADD Interaction, and MC159 Does Not Compete with Caspase-8 Recruitment (A) Immunoprecipitation of wild-type and hydrophobic patch mutants of caspase-8 (C360S) and FADD is shown. (B) Ternary complex formation of hydrophobic patch mutants of MC159 with Fas and FADD. Contaminating bands are indicated by an asterisk. Please note that the last two lanes were cut and pasted from another gel. (C) MC159 does not compete with caspase-8 recruitment. Human lymphoma H9 cells were transfected with GFP-fusion constructs for expressing GFP (Vct), MC159:GFP, and cflip(s):gfp as indicated. The DISC was induced by anti-fas stimulation as indicated, and its components were detected from the immunoprecipitates and cell lysates by probing against caspase-8 (upper panels) and GFP (lower panels). Please note that the last lane of the lysates was cut and pasted due to misloading in sample lanes. Data are representative of two independent experiments. (D) Caspase-8 recruitment in the presence of increasing amounts of MC150 transfection is shown. Fas and FADD (Figure 4B). This suggests that the hydrophobic patch is not required for MC159 recruitment. To elucidate whether MC159 competes with caspase- 8 for recruitment to FADD, we performed a DISC assay in H9 cells with either GFP-MC159 or GFP-cFLIP(S) transfection. Upon stimulation by anti-fas antibody, the cell lysates were immunoprecipitated, resolved by sodium dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) and probed with either anti-caspase-8 or anti- GFP antibodies. Whereas cflip(s) transfection strongly inhibited caspase-8 recruitment, MC159 transfection did not attenuate caspase-8 recruitment to the DISC (Figure 4C). In fact, a detailed titration on the transfection of GFP-MC159 fusion plasmid indicated that MC159 might have subtly enhanced caspase-8 recruitment (Figure 4D). These and the hydrophobic patch mutation data suggest that caspase-8 and MC159 apparently utilize different structural features for FADD interaction. An Extensive Surface of MC159 and the Charge Triad Region of FADD Are Both Required for Ternary Complex Formation Among the previously generated MC159 mutants, most are on buried residues and therefore may cause structural perturbations. Only M2 (H23A, E24A, and D25A), M3 (H33A, D34A), M6 (R69A, D71A), and M16 (E18A, E19A, and D21A) contain surface-exposed residues and are defective in protection against apoptosis (Table 2). These residues may directly participate in FADD interaction. To thoroughly map the molecular determinants of MC159 recruitment, we generated 32 additional MC159 mutants on surface-exposed residues, most of which contain charged or large (either hydrophobic or hydrophilic) side chains. We characterized them with the in vitro assay on ternary complex formation. Nine out of the 32 mutants were defective in ternary complex formation (Table 2). Mapping of MC159 mutations that were defective in ternary complex formation (Figure 3A) showed the involvement of an extensive region of the structure in its recruitment (Figure 5A). This suggests that each MC159 may contact at least two adjacent FADD molecules. On one side of the structure, a region near the charge triad in DED1, which comprises mutants M2 (H23A, E24A, and D25A), M6 (R69A and D71A), M16 (E18A, E19A, and D21A), and MC5 (T40A, T41A, T43A, and Q44A), is important (Figure 5B). For residues within the charge triad in this region, even the very conserved substitutions E24Q, R69K, and D71N abolished ternary complex formation. On the same side of the structure, the charge triad in DED2 does not seem to be as important, as E111Q, R166K, and D168N mutations at worst weakened ternary complex formation. Therefore, on this side of the MC159 structure, it appears only DED1 is important for its recruitment. Most of the remaining mutations map to the opposite face of MC159 (Figure 5C). They comprise mutants M3 (H33A and D34A), MC9 (T56A and L57A), MC14 (L79A and S80A), MC16 (T94A and R95A), MC22 (C120A, N121A, L122A, N123A, P124A, and S125A), and MC25 (E144A and N145A). Residues from both DED1 and DED2 participate in interactions at this side. The importance of the charge triad of MC159 in FADD interaction suggests that the same charge triad region of FADD may be reciprocally important for interaction with MC159. To test this hypothesis, we performed site-directed mutagenesis on the charge triad region of FADD, R72E, and D74K and tested the ability of these FADD mutants to form a ternary complex with MC159. As predicted, mutants R72E and D74K completely abolished and weakened ternary complex formation, respectively (data not shown), suggesting that the charge triad region of FADD is involved in MC159 recruitment (Figure 5D).
9 Molecular Cell 946 Figure 5. Interaction between MC159 and FADD and a Proposed Model for the Inhibition of Caspase Activation by MC159 (A) Mapping of surface mutations of MC159 that are defective in FADD interaction onto the stereo ribbon diagram of its structure. Residues E18, E19, and D21, magenta; H23, E24, and D25, red; H33 and D34, cyan; T40, T41, T43, and Q44: dark blue; T56 and L57, green; R69 and D71, yellow; L79 and S80, blue; T94 and R95, gold; C120, N121, L122, N123, P124, and S125, yellow; E145 and N145, red. (B and C) Mapping of the same mutations onto the molecular surface of MC159 is shown. Residue numbers are shown. (B) is shown in the same orientation as in (A), and (C) is related to (A) by a 180º rotation about the vertical axis. (D) Mapping of FADD mutations R72E and D74K, within the charge triad, onto its surface. The location of the conserved hydrophobic patch (F25 and L26) is also shown. (E) A proposed model for the inhibition of caspase activation at the DISC by MC159 via disruption of FADD self-association. The pentagons represent monomeric Fas/FADD/caspase-8 complexes, and filled yellow circles represent MC159 molecules. The Fas/FADD/ caspase-8 complexes likely interact with each other via both trimeric associations, as dictated by trimeric FasL, and dimeric associations from FADD self-association. Schematically, the blue and red faces of the pentagons mediate trimeric associations, and the face adjacent to the blue mediates dimeric associations. These two types of associations could mediate the infinite oligomerization of DISC during signaling. MC159 likely interacts with at least two neighboring FADD molecules and disrupts DISC oligomerization by competing with the same face used for the dimeric FADD self-association. Discussion Cooperative Ternary Complex Assembly in Caspase-8 and MC159 Recruitment By using recombinant proteins, we showed that Fas DD, FADD, and MC159 are cooperatively assembled. Similarly, the ternary complex of Fas DD, FADD, and caspase-8 are also cooperatively assembled (data not shown). These molecular assembly processes likely recapitulate the early event in Fas signaling in vivo, the formation of the signaling protein oligomeric transduction structures (SPOTS) (Siegel et al., 2004). Like the ternary complex formation, assembly of SPOTS is absolutely dependent on Fas DD and FADD and is enhanced by caspase-8, regardless of its catalytic activity (Siegel et al., 2004). Because it is detrimental to activate caspases in the absence of distinct signaling events, this cooperative assembly appears to act as a safety measure in preventing accidental DISC formation. Other oligomeric death-inducing complexes such as complex II formed by TRADD, FADD, and caspase-8 (Micheau and Tschopp, 2003) and PIDDosome formed by PIDD, RAIDD, and caspase-2 (Tinel and Tschopp, 2004) likely obey the same assembly mechanism. MC159 as a Template for Tandem DED Structures Unlike beads on a string, DED1 and DED2 are rigidly associated to form a single compact structure in which each domain performs its unique structural role in the context of the tandem DEDs and is not interchangeable in position with the other. This integral structure of tandem DEDs explains the previous observation that both DEDs of MC159 are required for its antiapoptotic function (Garvey et al., 2002a). On an isolated domain level, DED1 and DED2 differ significantly. Whereas DED2 appears to be a bona fide DED and closely resembles the DED structures in FADD (Eberstadt et al., 1998) and PEA-15 (Hill et al., 2002), DED1 appears to be equally distant to these known DED structures as to other structures in the DD superfamily such as DDs, PYDs, and CARDs. The association between DED1 and DED2 in tandem DEDs reveals a mode of interaction between DEDs that differs significantly from observed CARD/ CARD and DD/DD interactions. Structural analyses reveal two distinctive surface features in DED structures. The first feature is the conserved hydrophobic patches. In DED1, this conserved hydrophobic patch is used to support the DED1/DED2 interface and is buried at this interface. In contrast, the conserved DED2 hydrophobic patch is surface exposed and available for interactions. The second distinctive surface feature is the conserved hydrogen-bonded charge triad, which we refer to here as the E/D-RxDL motif. It is a signature of DEDs and is not present in DDs, CARDs, or PYDs. This region of the DED surface also appears to be a hot spot for protein-protein interactions. Molecular Mechanism for the Recruitment of Caspase-8, and Possibly Caspase-10, cflips and Herpesvirus vflips: the Conserved Hydrophobic Patch The first structure of a tandem DED-containing protein provides a template for understanding the molecular basis of procaspase recruitment and FLIP inhibition during
10 Crystal Structure of Viral FLIP MC DR signaling. The conserved hydrophobic patch on DEDs was first identified from the NMR structure of FADD, which comprises two central large hydrophobic residues, F25 and L26 (Eberstadt et al., 1998). It was shown to be important for the interaction of FADD with caspase-8. Here, we show that the conserved DED2 hydrophobic patch of caspase-8 is important for its recruitment to FADD. We speculate that the use of the hydrophobic patch for DISC recruitment is likely to be true for caspase-10, cflip, and herpesvirus vflips as well. In these tandem DED-containing proteins, the Phe residue in the hydrophobic patch is strictly conserved as Phe or Tyr, and the Leu residue is strictly conserved as Leu or Ile (Figure 3A) (Tibbetts et al., 2003). Therefore, cflip would compete directly with caspase-8 for DISC recruitment, as shown here (Figure 4C). In addition, herpesvirus vflips would also likely inhibit caspase activation by direct competition with caspase-8 for DISC recruitment. However, this scenario is not true for MC159. As we ve shown here, mutations of the DED2 hydrophobic patch residues did not affect MC159 recruitment. The lack of involvement of the hydrophobic patch in MC159 recruitment may be rationalized by its less-conserved substitutions at this site; the conserved Phe is replaced by a Leu and the conserved Leu is replaced by a Phe. In FADD, it appears that the Phe position has to be an aromatic residue as changes to Tyr and Trp retained its ability to interact with caspase-8, whereas a change to Val abolished the interaction (Eberstadt et al., 1998). It is likely then that the Phe to Leu substitution in MC159 also abolishes its interaction with the hydrophobic patch of FADD. The same Phe to Leu change is also observed in MC160, another vflip-like protein from MCV (Shisler and Moss, 2001). The only other DED-containing protein that does not have the conserved hydrophobic patch is PEA-15, a protein not involved in death receptor signaling and regulation. Molecular Mechanism for MC159 Recruitment and Inhibition of Caspase Activation: the Charge Triad and the Disruption of FADD Self-Association In contrast to the involvement of the hydrophobic patch, we have shown here that MC159 uses an extensive surface encompassing the charge triad for interaction with FADD. This is consistent with the previous observation that the RxDL motifs of MC159 play important roles in MC159 function (Garvey et al., 2002a). The extensive nature of the interaction surface suggests that each MC159 contacts at least two adjacent FADD molecules. Reciprocally, we showed that the charge triad of FADD is important for interaction with MC159. However, because MC159 does not compete with caspase-8 for recruitment, how does it inhibit caspase activation and apoptosis? To answer this question, we have to analyze the basic requirement for DISC assembly. Because FasL is intrinsically trimeric, the assembled DISC should at least be trimeric. In addition, because FADD self-associates, higher order of aggregation is generated. If we assume that FADD self-associates via a dimeric interaction, this dimerization of trimers would provide a mode of infinite aggregation (Figure 5E) into variably sized SPOTS that are observable under a light microscope. This existence of the dimeric symmetry would be consistent with the requirement for dimerization in caspase-8 activation (Boatright et al., 2003; Donepudi et al., 2003). In addition, the formation of higher-order oligomers is essential for caspase activation as trimeric soluble FasL is rather inefficient in inducing caspase activation whereas hexameric FasL is much more efficient (Holler et al., 2003). Interestingly, we have recently shown that FADD also self-associates via the RxDL motif in the charge triad region (Muppidi et al., 2005). Therefore, MC159 would compete with and disrupt FADD self-association. In the absence of FADD self-association, higher-order oligomerization of DISC would be abolished and, therefore, caspase activation would be inhibited (Figure 5E). This model of MC159 function elegantly explains the molecular basis for its antiapoptotic activity. It is supported by the observation that MC159 reduces the size of SPOTS during Fas signaling (Siegel et al., 2004). Similarly, it blocks the formation of FADD death effector filaments, which are self-oligomerized FADD molecules upon overexpression, and inhibits caspase activation and apoptosis (Garvey et al., 2002b; Siegel et al., 1998). Therefore, unlike cflips and herpesvirus vflips, MC159 uses a noncompetitive mechanism for inhibiting caspase activation at the DISC level. Experimental Procedures Protein Expression and Purification MC159 from Molluscum contagiosum virus (residues and residues 1 187) was expressed as His-tagged proteins in E. coli by using 20ºC induction and was purified by Ni-affinity and gel filtration chromatography. Human Fas DD, human FADD, human FADD DD, and human FADD DED proteins, either His-tagged or with no tags, were expressed similarly by using low temperature inductions. FADD and FADD DED constructs both contain the F25Y mutation that retains FADD function but improves its solution behavior (Eberstadt et al., 1998). Crystallization and Structure Determination of MC159 Selenomethionine-substituted full-length MC159 was crystallized with the hanging drop vapor diffusion method under mm sodium and potassium phosphate at ph 5.0. The crystals were highly variable; upon screening many crystals, a single-wavelength anomalous diffraction data set was collected at 3.8 Å resolution with the SGX ID beam line at the Advanced Photon Source (Table 1). Diffraction data were processed and scaled by using HKL2000 software (Otwinowski and Minor, 1997). The crystal belongs to space group P222 1 with cell dimensions of a = 84.0 Å, b = Å, and c = Å. Selenium sites were determined with direct method calculations in SnB (Weeks and Miller, 1999) and fed into SOLVE (Terwilliger, 2004)for phase determination. In combination with 4-fold noncrystallographic symmetry averaging in RESOLVE (Terwilliger, 2004), an interpretable map was obtained. Facilitated by the known selenium sites as markers for methionine residues, all four molecules in the crystallographic asymmetric unit were traced and built (residues 2 187) by using O (Jones et al., 1991). The structure revealed that a large segment of the C-terminal tail (residues ) is disordered in the crystal. This C-terminal tail is not important for the function of MC159 (Garvey et al., 2002a). Another construct (residues 1 187), made based on the ordered part of the molecule, gave soluble proteins and was crystallized with the hanging-drop vapor-diffusion method under M sodium acetate in 0.1 M imidazole at ph 6.5. The crystals belong to space group P and diffracted beyond 1.2 Å resolution at the X4A beamline of the National Synchrotron Light Source (NSLS). The structure was determined by molecular replacement calculations in COMO (Jogl et al., 2001) by using the partially refined model from the initial crystal form. All crystallographic refinement calculations were performed in CNS (Brunger et al., 1998), and the structures were analyzed and
11 Molecular Cell 948 displayed with Setor (Evans, 1993), Molscript/Raster3D (Kraulis, 1991; Merritt and Murphy, 1994), and Grasp software (Nicholls et al., 1991). Mutagenesis and His-Tag Pull-Down Mutagenesis of MC159 was performed with the QuikChange Site- Directed Mutagenesis Kit (Stratagene). Wild-type and mutant Histagged MC159 proteins were first purified with Ni-affinity to assess their expression levels. Then, cell lysates containing estimated equivalent quantities of MC159 proteins were mixed with those of nontagged Fas DD and FADD. The mixtures were incubated at room temperature for 2 hours, and His-tag pull-down experiments were performed to determine whether they form ternary complexes with Fas and FADD. Immunoprecipitation and Western Blot Human embryonic kidney tumor line 293T cells were seeded at 10 6 / well in complete Dulbecco s modified Eagle s medium (DMEM) in six well plates and cultured overnight. The cells were transfected with 3 mg of plasmid DNA by using Fugene 6 (Roche) reagent according to the manufacturer s instructions. The cells were cultured for 24 to w48 hours, harvested, and lysed on ice by 0.5 ml of the TNTG lysis buffer (30 mm Tris-HCl [ph 8.0], 150 mm NaCl, 1% Triton X-100, and 10% glycerol) with 13 protease inhibitor cocktail (Roche) for 30 minutes. The lysates were centrifuged at 10,000 3 g at 8ºC for 10 minutes. The protein-normalized lysates were subjected to immunoprecipitation by adding 10 mg/ml appropriate antibody, 30 ml of protein G-agarose (Roche), and rotating for more than 2 hr in the cold room. The precipitates were washed five times with cold TNTG lysis buffer and then subjected to 4% to w20% Tris/Glycine-SDS PAGE under reduced and denaturing conditions. The resolved protein bands were transferred onto nitrocellulose membranes and probed with 1 mg/ml of appropriate primary antibodies in PBS-T (phosphatebuffered saline with 0.1% Tween-20) at room temperature for 2 hr and then with appropriate HRP-conjugated secondary antibodies for 1 hr. The blots were then developed by the SuperSignaling system (Pierce) according to the manufacturer s instructions. For DISC assay, human lymphoma H9 cells were transfected with up to 20 mg of plasmid by electroporation with the BTX Electro Cell Manipulator 600. The transfected cells were cultured in 10 ml of complete RPMI medium for 36 hr, and viable cells were purified by gradient centrifugation. Each of transfected cells was subjected to mock or anti-fas (Apo-1.3, 1 mg/ml) stimulation for 10 min at 37ºC. The stimulated cells were washed twice with cold PBS followed by the immunoprecipitation and Western blotting protocols described above. Supplemental Data Supplemental Data including one figure and two tables are available online with this article at 20/6/939/DC1/. Acknowledgments We thank Dr. Stephen R. Wasserman for the use of SGX-CAT at Sector 31 of the Advanced Photon Source, which was constructed and operated by Structural GenomiX, Inc.; Randy Abramowitz and John Schwanof for use of the X4A beamline at NSLS; Dr. Jeffrey Cohen for providing MC159 mutants; and Samantha Jayawickrama for some of the mutagenesis work. J.K.Y is a postdoctoral fellow of the Serono Foundation. L.X.Z., F.Y.W., and M.J.L. are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. This work was supported by NIH grant R01 AI to H.W. Received: September 11, 2005 Revised: October 18, 2005 Accepted: October 19, 2005 Published: December 21, 2005 References Bertin, J., Armstrong, R.C., Ottilie, S., Martin, D.A., Wang, Y., Banks, S., Wang, G.H., Senkevich, T.G., Alnemri, E.S., Moss, B., et al. (1997). Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA 94, Boatright, K.M., Renatus, M., Scott, F.L., Sperandio, S., Shin, H., Pedersen, I.M., Ricci, J.E., Edris, W.A., Sutherlin, D.P., Green, D.R., and Salvesen, G.S. (2003). A unified model for apical caspase activation. Mol. Cell 11, Boldin, M.P., Goncharov, T.M., Goltsev, Y.V., and Wallach, D. (1996). Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85, Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. (1998). Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D54, Chinnaiyan, A.M., O Rourke, K., Tewari, M., and Dixit, V.M. (1995). FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, Chou, J.J., Matsuo, H., Duan, H., and Wagner, G. (1998). Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 recruitment. Cell 94, Donepudi, M., Mac Sweeney, A., Briand, C., and Grutter, M.G. (2003). Insights into the regulatory mechanism for caspase-8 activation. Mol. Cell 11, Eberstadt, M., Huang, B., Chen, Z., Meadows, R.P., Ng, S.-C., Zheng, L., Lenardo, M.J., and Fesik, S.W. (1998). NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature 392, Evans, S.V. (1993). SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J. Mol. Graph. 11, French, L.E., and Tschopp, J. (2003). Protein-based therapeutic approaches targeting death receptors. Cell Death Differ. 10, Garvey, T.L., Bertin, J., Siegel, R., Lenardo, M., and Cohen, J. (2002a). The death effector domains (DEDs) of the molluscum contagiosum virus MC159 v-flip protein are not functionally interchangeable with each other or with the DEDs of caspase-8. Virology 300, Garvey, T.L., Bertin, J., Siegel, R.M., Wang, G.H., Lenardo, M.J., and Cohen, J.I. (2002b). Binding of FADD and caspase-8 to molluscum contagiosum virus MC159 v-flip is not sufficient for its antiapoptotic function. J. Virol. 76, Goltsev, Y.V., Kovalenko, A.V., Arnold, E., Varfolomeev, E.E., Brodianskii, V.M., and Wallach, D. (1997). CASH, a novel caspase homologue with death effector domains. J. Biol. Chem. 272, Han, D.K., Chaudhary, P.M., Wright, M.E., Friedman, C., Trask, B.J., Riedel, R.T., Baskin, D.G., Schwartz, S.M., and Hood, L. (1997). MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc. Natl. Acad. Sci. USA 94, Hill, J.M., Vaidyanathan, H., Ramos, J.W., Ginsberg, M.H., and Werner, M.H. (2002). Recognition of ERK MAP kinase by PEA-15 reveals a common docking site within the death domain and death effector domain. EMBO J. 21, Hiller, S., Kohl, A., Fiorito, F., Herrmann, T., Wider, G., Tschopp, J., Grutter, M.G., and Wuthrich, K. (2003). NMR structure of the apoptosis- and inflammation-related NALP1 pyrin domain. Structure (Camb) 11, Holler, N., Tardivel, A., Kovacsovics-Bankowski, M., Hertig, S., Gaide, O., Martinon, F., Tinel, A., Deperthes, D., Calderara, S., Schulthess, T., et al. (2003). Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol. Cell. Biol. 23, Holm, L., and Sander, C. (1995). Dali: a network tool for protein structure comparison. Trends Biochem. Sci. 20, Hu, S., Vincenz, C., Buller, M., and Dixit, V.M. (1997a). A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J. Biol. Chem. 272,
12 Crystal Structure of Viral FLIP MC Hu, S., Vincenz, C., Ni, J., Gentz, R., and Dixit, V.M. (1997b). I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95- induced apoptosis. J. Biol. Chem. 272, Huang, B., Eberstadt, M., Olejniczak, E.T., Meadows, R.P., and Fesik, S.W. (1996). NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384, Inohara, N., Koseki, T., Hu, Y., Chen, S., and Nunez, G. (1997). CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc. Natl. Acad. Sci. USA 94, Irmler, M., Thome, M., Hahne, M., Schneider, P., Hofmann, K., Steiner, V., Bodmer, J.L., Schroter, M., Burns, K., Mattmann, C., et al. (1997). Inhibition of death receptor signals by cellular FLIP. Nature 388, Itoh, N., and Nagata, S. (1993). A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J. Biol. Chem. 268, Jogl, G., Tao, X., Xu, Y., and Tong, L. (2001). COMO: a program for combined molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 57, Jones, T.A., Zou, J.-Y., Cowan, S.W., and Kjeldgaard, M. (1991). Improved methods for building models in electron density maps and the location of errors in those models. Acta Crystallgr. A A47, Kischkel, F.C., Hellbardt, S., Behrmann, I., Germer, M., Pawlita, M., Krammer, P.H., and Peter, M.E. (1995). Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14, Kohl, A., and Grutter, M.G. (2004). Fire and death: the pyrin domain joins the death-domain superfamily. C. R. Biol. 327, Kraulis, P.J. (1991). MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, Locksley, R.M., Killeen, N., and Lenardo, M.J. (2001). The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, Medema, J.P., Scaffidi, C., Kischkel, F.C., Shevchenko, A., Mann, M., Krammer, P.H., and Peter, M.E. (1997). FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16, Merritt, E.A., and Murphy, M.E. (1994). Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 50, Micheau, O., and Tschopp, J. (2003). Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, Muppidi, J.R., Lobito, A.A., Ramaswamy, M., Yang, J.K., Wang, L., Wu, H., and Siegel, R.M. (2005). Homotypic FADD interactions through a conserved RXDLL motif are required for death receptorinduced apoptosis. Cell Death Diff., in press. Muzio, M., Chinnaiyan, A.M., Kischkel, F.C., O Rourke, K., Shevchenko, A., Ni, J., Scaffidi, C., Bretz, J.D., Zhang, M., Gentz, R., et al. (1996). FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85, Nicholls, A., Sharp, K.A., and Honig, B. (1991). Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, Otwinowski, Z., and Minor, W. (1997). Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, Peter, M.E., and Krammer, P.H. (2003). The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10, Qin, H., Srinivasula, S.M., Wu, G., Fernandes-Alnemri, T., Alnemri, E.S., and Shi, Y. (1999). Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399, Rasper, D.M., Vaillancourt, J.P., Hadano, S., Houtzager, V.M., Seiden, I., Keen, S.L., Tawa, P., Xanthoudakis, S., Nasir, J., Martindale, D., et al. (1998). Cell death attenuation by Usurpin, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 5, Reed, J.C., Doctor, K.S., and Godzik, A. (2004). The domains of apoptosis: a genomics perspective. Sci. STKE 29, re9. Salvesen, G.S. (2002). Caspases and apoptosis. Essays Biochem. 38, Salvesen, G.S., and Dixit, V.M. (1999). Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. USA 96, Searles, R.P., Bergquam, E.P., Axthelm, M.K., and Wong, S.W. (1999). Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi s sarcoma-associated herpesvirus/ human herpesvirus 8. J. Virol. 73, Shi, Y. (2004). Caspase activation: revisiting the induced proximity model. Cell 117, Shisler, J.L., and Moss, B. (2001). Molluscum contagiosum virus inhibitors of apoptosis: The MC159 v-flip protein blocks Fas-induced activation of procaspases and degradation of the related MC160 protein. Virology 282, Shu, H.B., Halpin, D.R., and Goeddel, D.V. (1997). Casper is a FADDand caspase-related inducer of apoptosis. Immunity 6, Siegel, R.M., Martin, D.A., Zheng, L., Ng, S.Y., Bertin, J., Cohen, J., and Lenardo, M.J. (1998). Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J. Cell Biol. 141, Siegel, R.M., Muppidi, J.R., Sarker, M., Lobito, A., Jen, M., Martin, D., Straus, S.E., and Lenardo, M.J. (2004). SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J. Cell Biol. 167, Srinivasula, S.M., Ahmad, M., Ottilie, S., Bullrich, F., Banks, S., Wang, Y., Fernandes-Alnemri, T., Croce, C.M., Litwack, G., Tomaselli, K.J., et al. (1997). FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J. Biol. Chem. 272, Tartaglia, L.A., Ayres, T.M., Wong, G.H., and Goeddel, D.V. (1993). A novel domain within the 55 kd TNF receptor signals cell death. Cell 74, Terwilliger, T. (2004). SOLVE and RESOLVE: automated structure solution, density modification and model building. J. Synchrotron Radiat. 11, Thome, M., and Tschopp, J. (2001). Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 1, Thome, M., Schneider, P., Hofmann, K., Fickenscher, H., Meinl, E., Neipel, F., Mattmann, C., Burns, K., Bodmer, J.L., Schroter, M., et al. (1997). Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386, Tibbetts, M.D., Zheng, L., and Lenardo, M.J. (2003). The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 4, Tinel, A., and Tschopp, J. (2004). The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304, Wajant, H. (2002). The Fas signaling pathway: more than a paradigm. Science 296, Weeks, C.M., and Miller, R. (1999). Optimizing shake-and-bake for proteins. Acta Crystallogr. D Biol. Crystallogr. 55, Xiao, T., Towb, P., Wasserman, S.A., and Sprang, S.R. (1999). Threedimensional structure of a complex between the death domains of Pelle and Tube. Cell 99, Accession Numbers The coordinates have been deposited in the RCSB Protein Data Bank with the PDB codes of 2BBZ for the 3.8 Å resolution MC159 structure and 2BBR for the 1.2 Å resolution MC159 structure.
13 Supplemental Data Crystal Structure of MC159 Reveals Molecular Mechanism of DISC Assembly and FLIP Inhibition Jin Kuk Yang, Liwei Wang, Lixin Zheng, Fengyi Wan, Misonara Ahmed, Michael J. Lenardo, and Hao Wu Table S1. Structural Alignment and Homology Search Structural Alignment Name Distance cutoff # Residues Aligned RMSD DED1 DED2 3.0Å Å FADD DED 3.0Å Å PEA-15 DED 3.0Å Å DED2 FADD DED 3.0Å Å PEA-15 DED 3.0Å Å Structural Homology Search with DALI, Top 8 Matches DED1 DED2 Name Z score # Residues Aligned RMSD FADD DED Å Pelle DD Å Nalp1 PYD Å p75 DD Å Asc PYD Å Iceberg CARD Å TNF-R1 DD Å P84 DD Å FADD DED Å PEA-15 DED Å Asc PYD Å Caspase-9 CARD Å Iceberg CARD Å Pelle DD Å Nalp1 PYD Å p75 DD Å
14 Table S2. Charge Triad Defined by the E/D-RxDL Motif in DED1 and DED2 (in parenthesis) Residue Atom Residue Atom Distance (Å) R69 (R166) Nε D71 (D168) Oδ1 2.9 (2.9) R69 (R166) Nη2 D71 (D168) Oδ2 2.9 (2.9) R69 (R166) Nη1 E24 (E111) Oε1 3.3 (3.3) R69 (R166) Nη1 E24 (E111) Oε2 2.9 (2.8) Figure S1. Characterization of MC159 Mutants Using His-tag Pull-Down The definitions of the mutants are shown in Table 2. MC159 mutants M2 and M3 are of full-length MC159 (residues 1-241) while the remaining mutants are of truncated MC159 (residues 1 187). Contaminants are labeled by an asterisk.
Crystal Structure of RAIDD Death Domain Implicates Potential Mechanism of PIDDosome Assembly
 doi:10.1016/j.jmb.2005.12.082 J. Mol. Biol. (2006) 357, 358 364 COMMUNICATION Crystal Structure of RAIDD Death Domain Implicates Potential Mechanism of PIDDosome Assembly Hyun Ho Park and Hao Wu* Department
doi:10.1016/j.jmb.2005.12.082 J. Mol. Biol. (2006) 357, 358 364 COMMUNICATION Crystal Structure of RAIDD Death Domain Implicates Potential Mechanism of PIDDosome Assembly Hyun Ho Park and Hao Wu* Department
Supplemental Data. Structure of the Rb C-Terminal Domain. Bound to E2F1-DP1: A Mechanism. for Phosphorylation-Induced E2F Release
 Supplemental Data Structure of the Rb C-Terminal Domain Bound to E2F1-DP1: A Mechanism for Phosphorylation-Induced E2F Release Seth M. Rubin, Anne-Laure Gall, Ning Zheng, and Nikola P. Pavletich Section
Supplemental Data Structure of the Rb C-Terminal Domain Bound to E2F1-DP1: A Mechanism for Phosphorylation-Induced E2F Release Seth M. Rubin, Anne-Laure Gall, Ning Zheng, and Nikola P. Pavletich Section
Supplementary materials. Crystal structure of the carboxyltransferase domain. of acetyl coenzyme A carboxylase. Department of Biological Sciences
 Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supporting Information
 Supporting Information Structural Basis of the Antiproliferative Activity of Largazole, a Depsipeptide Inhibitor of the Histone Deacetylases Kathryn E. Cole 1, Daniel P. Dowling 1,2, Matthew A. Boone 3,
Supporting Information Structural Basis of the Antiproliferative Activity of Largazole, a Depsipeptide Inhibitor of the Histone Deacetylases Kathryn E. Cole 1, Daniel P. Dowling 1,2, Matthew A. Boone 3,
Death Domain Assembly Mechanism Revealed by Crystal Structure of
 Death Domain Assembly Mechanism Revealed by Crystal Structure of the Oligomeric PIDDosome Core Complex Hyun Ho Park, 1 Emmanuelle Logette, 2 Stefan Raunser, 3 Solange Cuenin, 2 Thomas Walz, 3 Jurg Tschopp,
Death Domain Assembly Mechanism Revealed by Crystal Structure of the Oligomeric PIDDosome Core Complex Hyun Ho Park, 1 Emmanuelle Logette, 2 Stefan Raunser, 3 Solange Cuenin, 2 Thomas Walz, 3 Jurg Tschopp,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
The Death Domain Superfamily in Intracellular Signaling of Apoptosis and Inflammation
 Annu. Rev. Immunol. 2007. 25:561 86 First published online as a Review in Advance on January 2, 2007 The Annual Review of Immunology is online at immunol.annualreviews.org This article s doi: 10.1146/annurev.immunol.25.022106.141656
Annu. Rev. Immunol. 2007. 25:561 86 First published online as a Review in Advance on January 2, 2007 The Annual Review of Immunology is online at immunol.annualreviews.org This article s doi: 10.1146/annurev.immunol.25.022106.141656
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Any protein that can be labelled by both procedures must be a transmembrane protein.
 1. What kind of experimental evidence would indicate that a protein crosses from one side of the membrane to the other? Regions of polypeptide part exposed on the outside of the membrane can be probed
1. What kind of experimental evidence would indicate that a protein crosses from one side of the membrane to the other? Regions of polypeptide part exposed on the outside of the membrane can be probed
Supplementary Information. The protease GtgE from Salmonella exclusively targets. inactive Rab GTPases
 Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Supplementary Information The protease GtgE from Salmonella exclusively targets inactive Rab GTPases Table of Contents Supplementary Figures... 2 Supplementary Figure 1... 2 Supplementary Figure 2... 3
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
Supplementary Figure 1 Crystallization. a, Crystallization constructs of the ET B receptor are shown, with all of the modifications to the human wild-type the ET B receptor indicated. Residues interacting
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Supplementary Table 1: Data collection, phasing and refinement statistics ChbC/Ta 6 Br 12 Native ChbC Data collection Space group P4 3 2 1 2 P4 3 2 1 2 Cell dimensions a, c (Å) 132.75, 453.57 132.81, 452.95
Nature Structural & Molecular Biology doi: /nsmb Supplementary Figure 1. CRBN binding assay with thalidomide enantiomers.
 Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
ml. ph 7.5 ph 6.5 ph 5.5 ph 4.5. β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS
 a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
a UV28 absorption (mau) 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex + GDP β 2 AR-Gs complex + GTPγS β 2 AR-Gs complex dissociated complex excess nucleotides b 9 8 7 5 3 β 2 AR-Gs complex β 2 AR-Gs complex
Expanded View Figures
 The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
The EMBO Journal Structure of a Dm peptide bound to the OT module Tobias Raisch et al Expanded View Figures A Hs Dm 262 297 685 8 HEAT HEAT MIF4G 9BD 1SHD 761 91 193 169 1152 1317 16 1376 1467 HEAT HEAT
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN
 THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
THE CRYSTAL STRUCTURE OF THE SGT1-SKP1 COMPLEX: THE LINK BETWEEN HSP90 AND BOTH SCF E3 UBIQUITIN LIGASES AND KINETOCHORES Oliver Willhoft, Richard Kerr, Dipali Patel, Wenjuan Zhang, Caezar Al-Jassar, Tina
Secondary Structure. Bioch/BIMS 503 Lecture 2. Structure and Function of Proteins. Further Reading. Φ, Ψ angles alone determine protein structure
 Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
SUPPLEMENTARY INFORMATION
 Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplementary Results DNA binding property of the SRA domain was examined by an electrophoresis mobility shift assay (EMSA) using synthesized 12-bp oligonucleotide duplexes containing unmodified, hemi-methylated,
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Exam I Answer Key: Summer 2006, Semester C
 1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
doi:1.138/nature1737 Supplementary Table 1 variant Description FSEC - 2B12 a FSEC - 6A1 a K d (leucine) c Leucine uptake e K (wild-type like) K (Y18F) K (TS) K (TSY) K288A mutant, lipid facing side chain
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
Cks1 CDK1 CDK1 CDK1 CKS1. are ice- lobe. conserved. conserved
 Cks1 d CKS1 Supplementary Figure 1 The -Cks1 crystal lattice. (a) Schematic of the - Cks1 crystal lattice. -Cks1 crystallizes in a lattice that contains c 4 copies of the t - Cks1 dimer in the crystallographic
Cks1 d CKS1 Supplementary Figure 1 The -Cks1 crystal lattice. (a) Schematic of the - Cks1 crystal lattice. -Cks1 crystallizes in a lattice that contains c 4 copies of the t - Cks1 dimer in the crystallographic
Structural characterization of NiV N 0 P in solution and in crystal.
 Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
Supplementary Figure 1 Structural characterization of NiV N 0 P in solution and in crystal. (a) SAXS analysis of the N 32-383 0 -P 50 complex. The Guinier plot for complex concentrations of 0.55, 1.1,
Acta Crystallographica Section D
 Supporting information Acta Crystallographica Section D Volume 70 (2014) Supporting information for article: Structural characterization of the virulence factor Nuclease A from Streptococcus agalactiae
Supporting information Acta Crystallographica Section D Volume 70 (2014) Supporting information for article: Structural characterization of the virulence factor Nuclease A from Streptococcus agalactiae
schematic diagram; EGF binding, dimerization, phosphorylation, Grb2 binding, etc.
 Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R)
 Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary figure 1. Comparison of unbound ogm-csf and ogm-csf as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine
 Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
Supplementary figure 1. Comparison of unbound and as captured in the GIF:GM-CSF complex. Alignment of two copies of unbound ovine GM-CSF (slate) with bound GM-CSF in the GIF:GM-CSF complex (GIF: green,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
doi:10.1038/nature11054 Supplementary Fig. 1 Sequence alignment of Na v Rh with NaChBac, Na v Ab, and eukaryotic Na v and Ca v homologs. Secondary structural elements of Na v Rh are indicated above the
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
SUPPLEMENTARY INFORMATION
 Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
Supplementary materials Figure S1 Fusion protein of Sulfolobus solfataricus SRP54 and a signal peptide. a, Expression vector for the fusion protein. The signal peptide of yeast dipeptidyl aminopeptidase
SUPPLEMENTARY INFORMATION
 Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Fig. 1 Influences of crystal lattice contacts on Pol η structures. a. The dominant lattice contact between two hpol η molecules (silver and gold) in the type 1 crystals. b. A close-up view of the hydrophobic
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Introduction to Comparative Protein Modeling. Chapter 4 Part I
 Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
doi:10.1038/nature12045 Supplementary Table 1 Data collection and refinement statistics. Native Pt-SAD X-ray source SSRF BL17U SPring-8 BL41XU Wavelength (Å) 0.97947 1.07171 Space group P2 1 2 1 2 1 P2
Supplementary Figure 3 a. Structural comparison between the two determined structures for the IL 23:MA12 complex. The overall RMSD between the two
 Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase Cwc27
 Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Acta Cryst. (2014). D70, doi:10.1107/s1399004714021695 Supporting information Volume 70 (2014) Supporting information for article: Structure and evolution of the spliceosomal peptidyl-prolyl cistrans isomerase
Supporting Information
 Supporting Information Structural Analysis of the Binding of Type I, I 1/2, and II Inhibitors to Eph Tyrosine Kinases Jing Dong, *1 Hongtao Zhao, 1 Ting Zhou, 1 Dimitrios Spiliotopoulos, 1 Chitra Rajendran,
Supporting Information Structural Analysis of the Binding of Type I, I 1/2, and II Inhibitors to Eph Tyrosine Kinases Jing Dong, *1 Hongtao Zhao, 1 Ting Zhou, 1 Dimitrios Spiliotopoulos, 1 Chitra Rajendran,
Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets
 Supporting information Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets Wan-Na Chen, Christoph Nitsche, Kala Bharath Pilla, Bim Graham, Thomas
Supporting information Sensitive NMR Approach for Determining the Binding Mode of Tightly Binding Ligand Molecules to Protein Targets Wan-Na Chen, Christoph Nitsche, Kala Bharath Pilla, Bim Graham, Thomas
SUPPLEMENTARY INFORMATION
 Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Supplementary Table 1: Amplitudes of three current levels. Level 0 (pa) Level 1 (pa) Level 2 (pa) TrkA- TrkH WT 200 K 0.01 ± 0.01 9.5 ± 0.01 18.7 ± 0.03 200 Na * 0.001 ± 0.01 3.9 ± 0.01 12.5 ± 0.03 200
Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps
 Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
Cell Reports Supplemental Information Structure and Function of Neisseria gonorrhoeae MtrF Illuminates a Class of Antimetabolite Efflux Pumps Chih-Chia Su, Jani Reddy Bolla, Nitin Kumar, Abhijith Radhakrishnan,
According to the manufacture s direction (Pierce), RNA and DNA
 Supplementary method Electrophoretic Mobility-shift assay (EMSA) According to the manufacture s direction (Pierce), RNA and DNA oligonuleotides were firstly labeled by biotin. TAVb (1pM) was incubated
Supplementary method Electrophoretic Mobility-shift assay (EMSA) According to the manufacture s direction (Pierce), RNA and DNA oligonuleotides were firstly labeled by biotin. TAVb (1pM) was incubated
Direct Binding of FADD to the TRAIL receptor DR5 is regulated by. the Death Effector Domain of FADD
 JBC Papers in Press. Published on June 1, 2004 as Manuscript M401680200 Direct Binding of FADD to the TRAIL receptor DR5 is regulated by the Death Effector Domain of FADD Lance R. Thomas 1, Adrianna Henson
JBC Papers in Press. Published on June 1, 2004 as Manuscript M401680200 Direct Binding of FADD to the TRAIL receptor DR5 is regulated by the Death Effector Domain of FADD Lance R. Thomas 1, Adrianna Henson
Packing of Secondary Structures
 7.88 Lecture Notes - 4 7.24/7.88J/5.48J The Protein Folding and Human Disease Professor Gossard Retrieving, Viewing Protein Structures from the Protein Data Base Helix helix packing Packing of Secondary
7.88 Lecture Notes - 4 7.24/7.88J/5.48J The Protein Folding and Human Disease Professor Gossard Retrieving, Viewing Protein Structures from the Protein Data Base Helix helix packing Packing of Secondary
Types of biological networks. I. Intra-cellurar networks
 Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
Signal Transduction Phosphorylation Protein kinases. Misfolding diseases. Protein Engineering Lysozyme variants
 Signal Transduction Phosphorylation Protein kinases Misfolding diseases Protein Engineering Lysozyme variants Cells and Signals Regulation The cell must be able to respond to stimuli Cellular activities
Signal Transduction Phosphorylation Protein kinases Misfolding diseases Protein Engineering Lysozyme variants Cells and Signals Regulation The cell must be able to respond to stimuli Cellular activities
Nature Structural and Molecular Biology: doi: /nsmb.2938
 Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
Supplementary Figure 1 Characterization of designed leucine-rich-repeat proteins. (a) Water-mediate hydrogen-bond network is frequently visible in the convex region of LRR crystal structures. Examples
FW 1 CDR 1 FW 2 CDR 2
 Supplementary Figure 1 Supplementary Figure 1: Interface of the E9:Fas structure. The two interfaces formed by V H and V L of E9 with Fas are shown in stereo. The Fas receptor is represented as a surface
Supplementary Figure 1 Supplementary Figure 1: Interface of the E9:Fas structure. The two interfaces formed by V H and V L of E9 with Fas are shown in stereo. The Fas receptor is represented as a surface
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6
 Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
Supporting Information
 Supporting Information Allosteric communication disrupted by small molecule binding to the Imidazole glycerol phosphate synthase protein-protein interface. Ivan Rivalta*,#, George P. Lisi #, Ning-Shiuan
Supporting Information Allosteric communication disrupted by small molecule binding to the Imidazole glycerol phosphate synthase protein-protein interface. Ivan Rivalta*,#, George P. Lisi #, Ning-Shiuan
Structural Perspectives on Drug Resistance
 Structural Perspectives on Drug Resistance Irene Weber Departments of Biology and Chemistry Molecular Basis of Disease Program Georgia State University Atlanta, GA, USA What have we learned from 20 years
Structural Perspectives on Drug Resistance Irene Weber Departments of Biology and Chemistry Molecular Basis of Disease Program Georgia State University Atlanta, GA, USA What have we learned from 20 years
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Supplementary Figure 1 Identification of the ScDcp2 minimal region interacting with both ScDcp1 and the ScEdc3 LSm domain. Pull-down experiment of untagged ScEdc3 LSm with various ScDcp1-Dcp2-His 6 fragments.
Apoptosis in Mammalian Cells
 Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
NMR study of complexes between low molecular mass inhibitors and the West Nile virus NS2B-NS3 protease
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
Biochemistry Quiz Review 1I. 1. Of the 20 standard amino acids, only is not optically active. The reason is that its side chain.
 Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
Detailed description of overall and active site architecture of PPDC- 3dThDP, PPDC-2HE3dThDP, PPDC-3dThDP-PPA and PPDC- 3dThDP-POVA
 Online Supplemental Results Detailed description of overall and active site architecture of PPDC- 3dThDP, PPDC-2HE3dThDP, PPDC-3dThDP-PPA and PPDC- 3dThDP-POVA Structure solution and overall architecture
Online Supplemental Results Detailed description of overall and active site architecture of PPDC- 3dThDP, PPDC-2HE3dThDP, PPDC-3dThDP-PPA and PPDC- 3dThDP-POVA Structure solution and overall architecture
Chapter 6. The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR
 The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition
 Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary information
 Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
Supplementary information The structural basis of modularity in ECF-type ABC transporters Guus B. Erkens 1,2, Ronnie P-A. Berntsson 1,2, Faizah Fulyani 1,2, Maria Majsnerowska 1,2, Andreja Vujičić-Žagar
Structure and RNA-binding properties. of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex
 Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Structure and RNA-binding properties of the Not1 Not2 Not5 module of the yeast Ccr4 Not complex Varun Bhaskar 1, Vladimir Roudko 2,3, Jerome Basquin 1, Kundan Sharma 4, Henning Urlaub 4, Bertrand Seraphin
Supporting Information
 Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supporting Information Ottmann et al. 10.1073/pnas.0907587106 Fig. S1. Primary structure alignment of SBT3 with C5 peptidase from Streptococcus pyogenes. The Matchmaker tool in UCSF Chimera (http:// www.cgl.ucsf.edu/chimera)
Supplementary Information. Structural basis for precursor protein-directed ribosomal peptide macrocyclization
 Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
Supplementary Information Structural basis for precursor protein-directed ribosomal peptide macrocyclization Kunhua Li 1,3, Heather L. Condurso 1,3, Gengnan Li 1, Yousong Ding 2 and Steven D. Bruner 1*
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
doi:10.1038/nature10955 Supplementary Figures Supplementary Figure 1. Electron-density maps and crystallographic dimer structures of the motor domain. (a f) Stereo views of the final electron-density maps
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Protease Inhibitor Cocktail A (1 tablet / 7 10 ml, Roche Cat# ) Protease inhibitor Cocktail B (0.5ml per 250ml, Calbiochem Cat# )
 Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Macromolecular X-ray Crystallography
 Protein Structural Models for CHEM 641 Fall 07 Brian Bahnson Department of Chemistry & Biochemistry University of Delaware Macromolecular X-ray Crystallography Purified Protein X-ray Diffraction Data collection
Protein Structural Models for CHEM 641 Fall 07 Brian Bahnson Department of Chemistry & Biochemistry University of Delaware Macromolecular X-ray Crystallography Purified Protein X-ray Diffraction Data collection
Bacterial protease uses distinct thermodynamic signatures for substrate recognition
 Bacterial protease uses distinct thermodynamic signatures for substrate recognition Gustavo Arruda Bezerra, Yuko Ohara-Nemoto, Irina Cornaciu, Sofiya Fedosyuk, Guillaume Hoffmann, Adam Round, José A. Márquez,
Bacterial protease uses distinct thermodynamic signatures for substrate recognition Gustavo Arruda Bezerra, Yuko Ohara-Nemoto, Irina Cornaciu, Sofiya Fedosyuk, Guillaume Hoffmann, Adam Round, José A. Márquez,
CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 10717 10722, September 1997 Cell Biology CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis NAOHIRO INOHARA, TAKEYOSHI
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 10717 10722, September 1997 Cell Biology CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis NAOHIRO INOHARA, TAKEYOSHI
Supplementary information for:
 SUPPLEMETARY IFRMATI Supplementary information for: Structure of a β 1 -adrenergic G protein-coupled receptor Tony Warne, Maria J. Serrano-Vega, Jillian G. Baker#, Rouslan Moukhametzianov, Patricia C.
SUPPLEMETARY IFRMATI Supplementary information for: Structure of a β 1 -adrenergic G protein-coupled receptor Tony Warne, Maria J. Serrano-Vega, Jillian G. Baker#, Rouslan Moukhametzianov, Patricia C.
Supplemental Information
 Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1. Definition and assessment of ciap1 constructs.
 Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations
Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations
RNA Polymerase I Contains a TFIIF-Related DNA-Binding Subcomplex
 Molecular Cell, Volume 39 Supplemental Information RNA Polymerase I Contains a TFIIFRelated DNABinding Subcomplex Sebastian R. Geiger, Kristina Lorenzen, Amelie Schreieck, Patrizia Hanecker, Dirk Kostrewa,
Molecular Cell, Volume 39 Supplemental Information RNA Polymerase I Contains a TFIIFRelated DNABinding Subcomplex Sebastian R. Geiger, Kristina Lorenzen, Amelie Schreieck, Patrizia Hanecker, Dirk Kostrewa,
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr
 The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
SUPPLEMENTARY INFORMATION
 www.nature.com/nature 1 Figure S1 Sequence alignment. a Structure based alignment of the plgic of E. chrysanthemi (ELIC), the acetylcholine binding protein from the snail Lymnea stagnalis (AchBP, PDB code
www.nature.com/nature 1 Figure S1 Sequence alignment. a Structure based alignment of the plgic of E. chrysanthemi (ELIC), the acetylcholine binding protein from the snail Lymnea stagnalis (AchBP, PDB code
Gene regulation I Biochemistry 302. Bob Kelm February 25, 2005
 Gene regulation I Biochemistry 302 Bob Kelm February 25, 2005 Principles of gene regulation (cellular versus molecular level) Extracellular signals Chemical (e.g. hormones, growth factors) Environmental
Gene regulation I Biochemistry 302 Bob Kelm February 25, 2005 Principles of gene regulation (cellular versus molecular level) Extracellular signals Chemical (e.g. hormones, growth factors) Environmental
The Potassium Ion Channel: Rahmat Muhammad
 The Potassium Ion Channel: 1952-1998 1998 Rahmat Muhammad Ions: Cell volume regulation Electrical impulse formation (e.g. sodium, potassium) Lipid membrane: the dielectric barrier Pro: compartmentalization
The Potassium Ion Channel: 1952-1998 1998 Rahmat Muhammad Ions: Cell volume regulation Electrical impulse formation (e.g. sodium, potassium) Lipid membrane: the dielectric barrier Pro: compartmentalization
SEPTEMBER 10, 2010 VOLUME 285 NUMBER 37 JOURNAL OF BIOLOGICAL CHEMISTRY 28749
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 285, NO. 37, pp. 28749 28763, September 10, 2010 2010 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Bcl-2 and Bax Interact
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 285, NO. 37, pp. 28749 28763, September 10, 2010 2010 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Bcl-2 and Bax Interact
SI Text S1 Solution Scattering Data Collection and Analysis. SI references
 SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
Supplementary Information
 Supplementary Information An engineered protein antagonist of K-Ras/B-Raf interaction Monique J. Kauke, 1,2 Michael W. Traxlmayr 1,2, Jillian A. Parker 3, Jonathan D. Kiefer 4, Ryan Knihtila 3, John McGee
Supplementary Information An engineered protein antagonist of K-Ras/B-Raf interaction Monique J. Kauke, 1,2 Michael W. Traxlmayr 1,2, Jillian A. Parker 3, Jonathan D. Kiefer 4, Ryan Knihtila 3, John McGee
Protein Dynamics. The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron.
 Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
SOCS3 binds specific receptor JAK complexes to control cytokine signaling by direct kinase inhibition SUPPLEMENTARY INFORMATION
 SOCS3 binds specific receptor JAK complexes to control cytokine signaling by direct kinase inhibition Nadia J. Kershaw 1,2, James M. Murphy 1,2, Nicholas P.D. Liau 1,2, Leila N. Varghese 1,2, Artem Laktyushin
SOCS3 binds specific receptor JAK complexes to control cytokine signaling by direct kinase inhibition Nadia J. Kershaw 1,2, James M. Murphy 1,2, Nicholas P.D. Liau 1,2, Leila N. Varghese 1,2, Artem Laktyushin
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
1. Amino Acids and Peptides Structures and Properties
 1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
Advanced Certificate in Principles in Protein Structure. You will be given a start time with your exam instructions
 BIRKBECK COLLEGE (University of London) Advanced Certificate in Principles in Protein Structure MSc Structural Molecular Biology Date: Thursday, 1st September 2011 Time: 3 hours You will be given a start
BIRKBECK COLLEGE (University of London) Advanced Certificate in Principles in Protein Structure MSc Structural Molecular Biology Date: Thursday, 1st September 2011 Time: 3 hours You will be given a start
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
SUPPLEMENTARY INFORMATION doi:10.1038/nature11539 Supplementary Figure 1 Schematic representation of plant (A) and mammalian (B) P 2B -ATPase domain organization. Actuator (A-), nucleotide binding (N-),
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
RayBio Rat TNF-alpha ELISA Kit (For Lysates)
 RayBio Rat TNF-alpha ELISA Kit (For Lysates) Catalog #: ELR-TNFa-CL User Manual Last revised April 15, 2016 Caution: Extraordinarily useful information enclosed ISO 13485 Certified 3607 Parkway Lane, Suite
RayBio Rat TNF-alpha ELISA Kit (For Lysates) Catalog #: ELR-TNFa-CL User Manual Last revised April 15, 2016 Caution: Extraordinarily useful information enclosed ISO 13485 Certified 3607 Parkway Lane, Suite
Table 1. Kinetic data obtained from SPR analysis of domain 11 mutants interacting with IGF-II. Kinetic parameters K D 1.
 Kinetics and Thermodynamics of the Insulin-like Growth Factor II (IGF-II) Interaction with IGF-II/Mannose 6-phosphate Receptor and the function of CD and AB Loop Solvent-exposed Residues. Research Team:
Kinetics and Thermodynamics of the Insulin-like Growth Factor II (IGF-II) Interaction with IGF-II/Mannose 6-phosphate Receptor and the function of CD and AB Loop Solvent-exposed Residues. Research Team:
Crystal Structure of Fibroblast Growth Factor 9 (FGF9) Reveals Regions. Implicated in Dimerization and Autoinhibition
 JBC Papers in Press. Published on November 1, 2000 as Manuscript M006502200 Crystal Structure of Fibroblast Growth Factor 9 (FGF9) Reveals Regions Implicated in Dimerization and Autoinhibition 1 Copyright
JBC Papers in Press. Published on November 1, 2000 as Manuscript M006502200 Crystal Structure of Fibroblast Growth Factor 9 (FGF9) Reveals Regions Implicated in Dimerization and Autoinhibition 1 Copyright
Structure of the α-helix
 Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
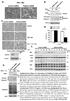 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Biochemistry 3100 Sample Problems Binding proteins, Kinetics & Catalysis
 (1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
(1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
The Structure of FADD and Its Mode of Interaction with Procaspase-8
 Molecular Cell 22, 599 610, June 9, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.molcel.2006.04.018 The Structure of FADD and Its Mode of Interaction with Procaspase-8 Paul E. Carrington, 1,2 Cristinel Sandu,
Molecular Cell 22, 599 610, June 9, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.molcel.2006.04.018 The Structure of FADD and Its Mode of Interaction with Procaspase-8 Paul E. Carrington, 1,2 Cristinel Sandu,
