The Arabidopsis SUMO E3 ligase AtMMS21 dissociates the E2Fa/DPa complex in. Huan Hu, Qian Wu, Guohui Lu, Panglian Xu, Chengwei Yang *
|
|
|
- Dwayne Stone
- 5 years ago
- Views:
Transcription
1 Plant Cell Advance Publication. Published on August 4, 2016, doi: /tpc The Arabidopsis SUMO E3 ligase AtMMS21 dissociates the E2Fa/DPa complex in cell cycle regulation Yiyang Liu 1, Jianbin Lai 1, Mengyuan Yu 1, Feige Wang, Juanjuan Zhang, Jieming Jiang, Huan Hu, Qian Wu, Guohui Lu, Panglian Xu, Chengwei Yang * Guangdong Provincial Key Laboratory of Biotechnology for Plant Development, School of Life Science, South China Normal University, Guangzhou , China These authors contributed equally to this work. * To whom correspondence should be addressed. Yangchw@scnu.edu.cn Short title: AtMMS21 regulates the E2Fa/Dpa complex The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors ( is Chengwei Yang (Yangchw@scnu.edu.cn) Word count: 5868 (Abstract: 158; Introduction: 876; Results: 2073; Discussion: 1292; Methods: 1469) Word count for Figure legends: 1241 Reference number: 62 Figure number: 7 (All published in color) Supplemental figure number: 5 Supplemental data set: American Society of Plant Biologists. All Rights Reserved
2 SYNOPSIS The Arabidopsis SUMO E3 ligase AtMMS21 dissociates the E2Fa/DPa complex by physical competition and SUMOylation in the G1/S cell cycle transition. ABSTRACT Development requires the proper execution and regulation of the cell cycle, via precise, conserved mechanisms. Critically, the E2F/DP complex controls the expression of essential genes during cell cycle transitions. Here, we discovered the molecular function of the Arabidopsis thaliana SUMO E3 ligase METHYL METHANESULFONATE SENSITIVITY GENE21 (AtMMS21) in regulating the cell cycle via the E2Fa/DPa pathway. DPa was identified as an AtMMS21-interacting protein and AtMMS21 competes with E2Fa for interaction with DPa. Moreover, DPa is a substrate for SUMOylation mediated by AtMMS21, and this SUMOylation enhances the dissociation of the E2Fa/DPa complex. AtMMS21 also affects the subcellular localization of E2Fa/DPa. The E2Fa/DPa target genes are upregulated in the root of mms21-1 and mms21-1 mutants showed increased endoreplication. Overexpression of DPa affected the root development of mms21-1 and overexpression of AtMMS21 completely recovered the abnormal phenotypes of 35S:E2Fa-DPa plants. Our results suggest that AtMMS21 dissociates the E2Fa/DPa complex via competition and SUMOylation in the regulation of plant cell cycle Key words: Arabidopsis, AtMMS21; Cell cycle; E2Fa/DPa; SUMOylation INTRODUCTION Cell division and differentiation are precisely controlled by the cell cycle machinery, which monitors critical checkpoints, including the G1/S and G2/M transitions (Hartwell and Kastan, 1994; Dewitte and Murray, 2003). Models of the cell cycle suggest that processes such as mitosis and endoreduplication predominantly rely on checkpoint regulation by cyclin-dependent kinases (CDKs) (Gutierrez, 2009; Satyanarayana and Kaldis, 2009). During the G1/S transition, CDKs phosphorylate 2
3 retinoblastoma protein (Rb) to suppress its inhibition of the E2F/DP transcription factor complex, pushing the cell into S phase (Weinberg, 1995; Boniotti and Gutierrez, 2001; Nakagami et al., 2002). The E2F family plays a major role in cell cycle regulation by controlling gene expression during the checkpoint (Müller and Helin, 2000). E2F binds with its partner DP, which provides a second DNA-binding site, stabilizing the association of E2F with DNA (Bandara et al., 1993; Helin et al., 1993; Krek et al., 1993). The E2F/DP gene family is conserved in higher eukaryotes. In Arabidopsis thaliana, the homologous proteins E2Fa, E2Fb, or E2Fc form heterodimers with DPa or DPb via their dimerization domains (Magyar et al., 2000; Ramirez-Parra and Gutierrez, 2000). The transactivation function of E2F is controlled by its nuclear localization, which is mediated by the interaction between specific E2F and DP partners (Kosugi and Ohashi, 2002). E2Fa controls the G1/S transition by inducing the transcription of genes required for cell cycle progression and DNA replication (Vandepoele et al., 2005). Overexpression of E2Fa and DPa in Arabidopsis induces plant cells to undergo either ectopic cell division or enhanced DNA endoreduplication (De Veylder et al., 2002). Moreover, overexpression of dominant negative CDKB1;1 enhances the endoreplication phenotypes but represses the extra cell division in E2Fa-DPa-overexpressing plants (Boudolf et al., 2004), suggesting a potential linkage between the G1/S and G2/M checkpoints. The cell cycle is controlled by a network of posttranslational protein modifications. For instance, CDKs establish a complex phosphorylation network (Malumbres, 2014), whereas cyclins are generally regulated by ubiquitination-mediated degradation (Glotzer et al., 1991). SUMOylation, which transfers the small ubiquitin-like modifier (SUMO) onto protein substrates, is a critical modification of cell cycle regulation (Dasso, 2008). Depletion of the SUMO-conjugating enzyme UBC9 results in a block of the G2/M transition in yeast and failure of embryonic development in mice (Al-Khodairy et al., 1995; Nacerddine et al., 2005). Conversely, the SUMO proteases Ulp1 and Ulp2, which remove SUMO from substrates, are also essential for cell cycle progression (Li and Hochstrasser, 2000; Taylor et al., 2002). Multiple key regulators of the cell cycle are subject to SUMOylation. For instance, in human cells, SUMO 3
4 attaches to Rb to repress its inhibition of the activity of the E2F/DP complex (Ledl et al., 2005; Li et al., 2006), whereas the SUMO E3 ligase PIASy increases prb-dependent repression via recruitment of E2F-responsive promoters (Man et al., 2006). CDK/Cyclin complexes are also potential targets of SUMOylation. The stability of CDK6, which is critically involved in the G1/S transition, is mediated by SUMO modification in glioblastoma cells (Bellail et al., 2014). Conjugation of SUMO with Cyclin D1 is critical for the nuclear localization of Cyclin D1 in the oncogene-induced senescence signaling pathway (Wang et al., 2011). Recently, a large-scale quantitative proteomics study provided evidence that SUMOylation affects all aspects of cell cycle progression (Schimmel et al., 2014). Similar to ubiquitination, SUMOylation occurs through a cascade that includes a heterodimeric activating enzyme (E1), a conjugating enzyme (E2), and (usually) a SUMO ligase (E3) (Wilkinson and Henley, 2010). SUMO E3 enzymes stimulate substrate SUMOylation under physiological conditions. Multiple SUMO ligases, including a group of Siz/PIAS-family proteins with an SP-RING domain, have been implicated in various cellular pathways in different species. For instance, the five vertebrate PIAS proteins are involved in many processes, including gene expression and signal transduction (Palvimo, 2007), and another member, MMS21, is critical for genome maintenance (Stephan et al., 2011; Jacome et al., 2015). Recent studies have shown that SUMOylation in plants regulates stress tolerance and development via targeting protein components in related signaling pathways (Saracco et al., 2007; Elrouby and Coupland, 2010; Miller et al., 2010). For instance, SUMOylation targets DELLAs in the gibberellin pathway (Conti et al., 2014) and CESTA in the brassinosteroid pathway (Khan et al., 2014). The Arabidopsis SUMO ligase SIZ1 participates in responses to stresses such as drought and freezing (Catala et al., 2007; Miura et al., 2007). The Sugimoto group and our group previously reported that depletion of AtMMS21 (also named HPY2), encoding a SUMO E3 enzyme with an SP-RING domain, impairs stem cell niche maintenance during root development in Arabidopsis (Huang et al., 2009; Ishida et al., 2009). Further dissection of the function of AtMMS21 by our group revealed that AtMMS21 is a conserved component of the 4
5 SMC5/6 complex and is involved in drought tolerance, DNA damage response, and meiosis (Zhang et al., 2010; Xu et al., 2013; Liu et al., 2014; Yuan et al., 2014). In the absence of AtMMS21, the levels of several G2/M phase transition regulators, such as CYCB1, CDKB1, and CDKB2, are reduced, and the endoreplication level is dramatically increased (Ishida et al., 2009). However, the molecular mechanism underlying how AtMMS21 participates in cell cycle control remains unclear. In this study, we found that AtMMS21 dissociates the E2Fa/DPa complex in plants, identifying a regulatory mechanism for cell cycle progression RESULTS Identification of cell cycle components interacting with AtMMS21 We previously demonstrated that the mms21-1 mutant displays severe defects in root and leaf development (Huang et al., 2009) (Figure 1 A). To uncover the role of AtMMS21 in cell cycle regulation, we examined the ploidy level of the mms21-1 mutant by flow cytometry analysis. In the cotyledons and first pairs of true leaves of 13-d-old plants, mms21-1 had fewer nuclei with 2C DNA contents and more nuclei with higher DNA contents, compared with the wild type (Figure 1 B), consistent with the results from hpy2-1, another AtMMS21 mutant allele (Ishida et al., 2009). This phenotype suggests that endoreplication is enhanced in the absence of AtMMS21, implying the important role of AtMMS21 in plant cell cycle regulation. To elucidate the molecular function of AtMMS21 in cell cycle progression, we next used yeast two-hybrid screens to identify cell cycle-related components that interact with AtMMS21. We tested eight critical regulators, CDKA, CYCB1;1, RBR, E2Fa, E2Fb, E2Fc, DPa and DPb, in the two-hybrid assay to examine their interactions with AtMMS21. Most of these regulators did not interact with AtMMS21, except for DPa, which forms heterodimers with E2F members in the regulation of checkpoint transition DPa interacts with AtMMS21 We used an independent yeast two-hybrid experiment to confirm that DPa interacts with AtMMS21 (Figure 2 A) and identify the functional domains required for their 5
6 interaction in yeast. DPa contains a DNA-binding domain at the N terminus and a dimerization domain at the C terminus (Magyar et al., 2000). Deletion of the C-terminal region of DPa abolished the interaction with AtMMS21, whereas deletion of its N-terminal domain did not affect this interaction. The C-terminal fragment without the dimerization domain did not interact with AtMMS21, implying that binding to AtMMS21 requires the dimerization domain of DPa (Figure 2 B). To determine the functional region of AtMMS21 that interacts with DPa, we investigated two truncated forms of AtMMS21, finding that the N terminus of AtMMS21 specifically interacted with DPa in yeast (Figure 2 C). We also observed that the rice homologues OsDP and OsMMS21 interacted in yeast (Figure 2 D), suggesting that the DPa-MMS21 interaction is conserved in different plant species. The physical interaction between DPa and AtMMS21 was further confirmed by an in vitro pull-down assay. Compared with the control sample, FLAG-tagged DPa protein was pulled down by GST-AtMMS21, indicating that DPa directly associates with AtMMS21 (Figure 2 E). To further explore the association between DPa and AtMMS21 in plant cells, CFP-fused AtMMS21 and YFP-fused DPa were co-expressed in Arabidopsis protoplasts and confocal microscopy revealed that the CFP and YFP signals predominantly overlapped (Figure 2 F). In addition, the bimolecular fluorescence complementation data indicated that DPa and AtMMS21 interacted with each other in cytoplasm and nucleus (Supplemental Figure 1). Direct evidence for this interaction in vivo was provided by a co-immunoprecipitation assay using transgenic plants expressing FLAG-tagged DPa and MYC-tagged AtMMS21, revealing that DPa-FLAG was specifically immunoprecipitated with MYC-AtMMS21 (Figure 2 G). Taken together, these results supported the conclusion that DPa interacts with AtMMS AtMMS21 interferes with the interaction between E2Fa and DPa Heterodimer formation between DPa and E2Fa requires the DPa dimerization domain (Magyar et al., 2000) and this dimerization domain interacted with AtMMS21 in our yeast two-hybrid assay. Therefore, we next tested whether the AtMMS21-DPa interaction interferes with the DPa-E2Fa interaction in a yeast three-hybrid assay. A 6
7 prey construct expressing E2Fa was transformed into yeast with a bait construct expressing DPa with or without AtMMS21. The relative activity was reduced in the presence of AtMMS21 (Figure 3 A), indicating that AtMMS21 attenuates the E2Fa-DPa interaction in yeast. To confirm the effect of AtMMS21 on the E2Fa/DPa complex in plant cells, YFP-E2Fa was expressed in protoplasts from wild-type or transgenic plants producing DPa-FLAG or both DPa-FLAG and MYC-AtMMS21. YFP-E2Fa was specifically immunoprecipitated with DPa-FLAG, but this interaction was abolished by AtMMS21 in planta (Figure 3 B). These data collectively support the conclusion that AtMMS21 interferes with the E2Fa-DPa interaction SUMOylation of DPa mediated by AtMMS21 dissociates the E2Fa/DPa complex Because AtMMS21 is a SUMO E3 ligase, we were interested in determining whether DPa is a substrate for SUMOylation and if so, whether AtMMS21 affects its SUMOylation. First, FLAG-tagged DPa was expressed in a reconstituted E. coli system with Arabidopsis SUMOylation machinery proteins (Okada et al., 2009). In the presence of E1, E2, and AtSUMO1, a DPa-FLAG signal with higher molecular weight was detected in the immunoblot, suggesting that DPa is a potential substrate for SUMOylation. The GPS-SUMO1.0 software (Zhao et al., 2014) predicted lysine 146 of DPa to be a target residue. However, mutating K146 did not affect SUMO conjugation of DPa (data not shown). When we mutated two other lysines (K139, K140) adjacent to the predicted site, along with K146, the SUMOylation of DPa was almost abolished (Figure 3 C), suggesting that the three lysines are major target sites for DPa SUMOylation. We purified the SUMOylation components and used them to determine the effect of AtMMS21 on SUMO modification of DPa. The SUMOylation of DPa was enhanced with increasing AtMMS21 level, indicating that AtMMS21 facilitates the attachment of SUMO to DPa (Figure 3 D). Our structure prediction revealed that the SUMOylation sites on DPa (K139, 140, 146) reside at the edge of its dimerization domain, which interacts with E2Fa (Figure 3 E), suggesting that SUMOylation may affect the DPa-E2Fa interaction. Based on 7
8 previous studies in mammalian cells that examined the function of SUMOylation by fusing SUMO with its target protein (Ross et al., 2002), we fused AtSUMO1(ΔGG) to the N-terminal truncated version of DPa (DPa-C) at the position adjacent to its SUMOylation sites to mimic the SUMO-conjugated form. The results of yeast two-hybrid analysis indicated that the interaction between E2Fa and SUMO-fused DPa-C was much weaker than that between E2Fa and the sample without SUMO fusion (Figure 3 F), suggesting that SUMOylation of DPa interferes with its interaction with E2Fa AtMMS21 affects the translocation of E2Fa/DPa Since we determined that AtMMS21 interferes with the interaction between DPa and E2Fa, we next examined the consequences of this interference in the cell. Previous studies have revealed that when E2Fa or DPa is expressed separately, the protein localizes in the cytoplasm and nucleus; however, co-expression of both proteins drives complete nuclear localization (Kosugi and Ohashi, 2002). We therefore examined the effect of AtMMS21 on the translocation of the E2Fa/DPa complex in protoplasts. Consistent with the previous data, we observed DPa-GFP in the nucleus and cytoplasm in most of the cells. By contrast, we detected DPa-GFP only in the nucleus in approximately 80% of the cells co-expressing CFP-E2Fa. Interestingly, when AtMMS21 was also overexpressed in the cells, the percentage of protoplasts with complete nuclear localization of DPa-GFP dramatically decreased, even in the presence of CFP-E2Fa (Figure 4 A). We also examined the subcellular localization of E2Fa in protoplasts from transgenic plants. In wild-type protoplasts, the YFP-E2Fa signal was detected throughout the cell. By contrast, YFP-E2Fa localized to the nucleus in approximately 80% of cells from plants constitutively overexpressing DPa. However, the strict nucleus location of YFP-E2Fa was only observed in approximately 30% of cells from plants co-overexpressing DPa and AtMMS21 (Figure 4 B), suggesting that AtMMS21 dramatically disrupted the translocation of E2Fa/DPa, possibly by interfering with the DPa-E2Fa interaction. Because E2Fb also forms heterodimers with DPa and regulates 8
9 cell cycle progression (Kosugi and Ohashi, 2002), we also performed a similar experiment to detect whether AtMMS21 affects the subcellular localization of E2Fb/DPa. Interestingly, the effect of AtMMS21 on the translocation of E2Fb was not significant (Supplemental Figure 2), implying the specificity of E2Fa in this regulation process. Taken together, these results suggest that AtMMS21 impairs the translocation of E2Fa/DPa from the cytoplasm to the nucleus Overexpression of DPa has effects on the root development of mms21-1 The data from our biochemistry and cell biology experiments suggest that AtMMS21 affects the E2Fa/DPa complex. To test the hypothesis, we compared the expression levels of ETG1, ORC2, MCM3, and PCNA1 (De Veylder et al., 2002; Egelkrout et al., 2002; Stevens et al., 2002; Diaz-Trivino et al., 2005; Takahashi et al., 2008), which are target genes of E2Fa/DPa, in the roots of wild-type and mms21-1 seedlings. The result indicated that the E2Fa target genes were upregulated in the mms21-1 roots (Figure 5 A), providing evidence for the functional interaction between AtMMS21 and E2Fa/DPa. Next, we used a genetic approach to explore the functional interaction among these proteins in plants. The physiological functions of the E2Fa/DPa complex can be studied by overexpression in Arabidopsis (De Veylder et al., 2002; Kosugi and Ohashi, 2003; Boudolf et al., 2004). To dissect the effect of AtMMS21 on the in vivo function of DPa, the DPa overexpression construct was introduced into the mms21-1 mutant by a genetic cross. The morphology of control 35S:DPa plants was identical to that of wild-type plants (Supplemental Figure 3), consistent with the results from the Inze group (De Veylder et al., 2002), suggesting that overexpressing DPa alone does not affect the development of wild-type plants. Surprisingly, overexpressing DPa in mms21-1 severely enhanced the mutant defect in root development at various growth stages (Figure 5 B). Analysis of the root meristem region provided an additional clue about the effect of DPa on root development. The meristem cell number was lower in mms21-1 plants than in wild-type or 35S:DPa plants. However, overexpressing DPa in mms21-1 obviously 9
10 reduced the meristem cell number of this mutant and even completely destroyed the meristem region in some plants (Figure 5 C), suggesting that overexpressing DPa in the AtMMS21 mutant enhances cell differentiation. To uncover the reason for the effects of 35S:DPa in mms21-1, the expression of the E2Fa-target genes was measured in the protoplasts generated from mms21-1 or 35S:DPa mms21-1 with or without RNA interference for E2Fa. In mms21-1, the RNA levels of target genes were significantly increased in the presence of 35S:DPa. However, this increase was dramatically suppressed when E2Fa was knockdown (Figure 5 D), providing evidence that the abnormal cell differentiation is dependent on the E2Fa/DPa complex AtMMS21 interferes with the function of the E2Fa/DPa complex in plants Previous studies have demonstrated that co-expression of DPa and E2Fa results in early arrested development and extra endoreduplication in Arabidopsis (De Veylder et al., 2002), providing a perfect system for us to detect the effect of AtMMS21 on the in vivo function of this complex. We crossed plants homozygous for 35S:E2Fa with plants homozygous for 35S:DPa and heterozygous for 35S:AtMMS21 (Supplemental Figure 4). Half of the offspring had short roots and curled cotyledons and half of the plants developed normally, like wild-type seedlings (Figure 6 A; Supplemental Figure 4). PCR analysis of individual plants confirmed that plants with the arrested development phenotype contained both the 35S:E2Fa and 35S:DPa constructs, whereas the plants with normal phenotypes contained three constructs, 35S:E2Fa, 35S:DPa, and 35S:AtMMS21 (data not shown). The transcript levels of E2Fa and DPa did not change in the presence of AtMMS21 (Supplemental Figure 4), and overexpressing AtMMS21 had no obvious effect on plant development (Supplemental Figure 3), strongly suggesting that the phenotype difference resulted from the interaction among these proteins. The analysis of root length indicated that overexpressing AtMMS21 completely rescued defective root development in seedlings co-expressing E2Fa and DPa (Figures 6 A, B). The abnormal leaf development and the extensive endoreduplication in cotyledons in the 35S:E2Fa-DPa plants were similar 10
11 with the previous results from the Inze group (De Veylder et al., 2002). However, these abnormal phenotypes were returned to normal by overexpression of AtMMS21 (Figures 6 C, D). Additionally, the results from hypocotyl and root cap indicated that abnormal cell division occurred in the E2Fa-DPa transgenic plants (De Veylder et al., 2002; Wildwater et al., 2005) was suppressed by the overexpression of AtMMS21 (Supplemental Figure 5). We found that the expression of E2Fa-target genes increased in plants overexpressing E2Fa and DPa but returned to nearly normal levels in the presence of AtMMS21 overexpression (Figure 6 E). These results strongly suggest that AtMMS21 has important effects on the function of the E2Fa/DPa complex in vivo. We also evaluated the contribution of physical competition or SUMOylation to the function of E2Fa-DPa in planta. Given the SP-RING domain is required for the SUMO ligase activity of AtMMS21, the Cys-to-Ser (C178S) and His-to-Ala (H180A) mutations in this domain destroy its SUMO E3 activity (Ishida et al., 2009). When we introduced the mutated version of AtMMS21 with C178S and H180A into 35S:E2Fa-DPa plants, the defective root development of these plants was only partially rescued (Figure 6 F), suggesting that SUMOylation and physical competition both contribute to the effect of AtMMS21 on the E2Fa/DPa complex DISCUSSION Plant development relies largely on the balance between cell proliferation and differentiation. The G1/S transition is a crucial crossroad at the interface between cell proliferation and differentiation (De Veylder et al., 2003). At the G1/S transition, CDKA/CYCD complexes phosphorylate RBR to dissociate E2F/DP transcription factors from RBR repression and to activate S-phase gene expression (Gutierrez, 2009). DPa forms heterodimers with E2Fa or E2Fb to enhance E2F/DPa translocation to the nucleus (Kosugi and Ohashi, 2002). Our data indicated that AtMMS21 interacts with DPa and significantly suppresses the translocation of E2Fa/DPa but not the E2Fb/DPa complex. This specificity may be a result of the difference in the structures for protein interaction. A previous study indicated that the expression of CDKB1:1 is upregulated in E2Fa-DPa overproducing plants (Boudolf et al., 2004), suggesting that the 11
12 E2Fa/DPa complex is also involved in the G2/M transition. The levels of CDKBs at the G2/M transition are reduced (Ishida et al., 2009), while the expression levels of ETG1, ORC2, MCM3, and PCNA1 are increased in the absence of AtMMS21, suggesting that the regulation of E2Fa/DPa by AtMMS21a is critical for the G1/S transition. These results also imply that AtMMS21 may have other potential targets to control the level of CDKB during the G2/M transition. The enhancement of endoreplication in the AtMMS21 mutant may result from stimulating the G1/S transition and blocking the G2/M transition. In a future study, it would be interesting to test whether overexpression of CDKB or CYCB in mms21-1 is able to overcome the defect in the cell cycle progression. Additionally, because E2Fa stimulates cell proliferation or endoreplication in different types of tissues (Magyar et al., 2012), the interaction between DPa and AtMMS21 may affect different types of cell cycle switches in specific tissues. Our data show that the production of excess AtMMS21 reduces the interaction affinity between DPa and E2Fa in plant cells. Because AtMMS21 functions as a SUMO ligase, this interference may result from physical competition or SUMOylation. On the one hand, the dimerization domain of DPa interacts with E2Fa (Ramirez-Parra and Gutierrez, 2000), and our results indicate that this domain is also required for the interaction with AtMMS21; therefore, these proteins may physically compete, supported by our yeast three-hybrid assays. On the other hand, DPa is a substrate for SUMOylation, and AtMMS21 enhances this reaction. Interestingly, the SUMOylation sites on DPa are adjacent to its dimerization domain. Our SUMO fusion experiment revealed that attachment of SUMO, a 10 kd polypeptide, may interfere with the protein interaction around the modified sites and also has important effects on the E2Fa/DPa complex. Indeed, human Rb, a crucial factor that inhibits the E2F/DP complex, undergoes SUMOylation, which stimulates the release of the E2F/DP complex (Ledl et al., 2005; Li et al., 2006), suggesting that SUMOylation is critical to this pathway at the G1/S transition in various species. The finding that mutated AtMMS21 without SUMO ligase activity only partially rescued the abnormal development of plants overproducing DPa and E2Fa provides evidence that both physical competition and 12
13 SUMOylation contribute to the dissociation of the E2Fa/DPa complex (Figure 7 A). The interaction between Arabidopsis E2F and DP regulates their nuclear translocation and transactivation (Kosugi and Ohashi, 2002), suggesting that AtMMS21 may affect the E2Fa/DPa translocation and the expression of target genes by biochemical interference. A recent study showing that human MMS21 regulates SUMOylation and nuclear-to-cytoplasmic translocation of sknac-smyd1 in myogenesis revealed that SUMOylation has a conserved function in the translocation of protein complexes (Berkholz et al., 2014). Given that transcription occurs in the nucleus, the impairment of translocation would reduce the level of E2Fa/DPa in the nucleus, thereby reducing target gene expression. The previous study showed that overexpression of a dominant negative form of CDKB1;1 enhances the endoreplication in the plants coexpressing E2Fa/DPa, possibly by stimulating G1/S and blocking G2/M transition (Boudolf et al., 2004). Similarly, the E2Fa-target genes are upregulated in the roots of mms21-1, suggesting that DPa and E2Fa may form a more stable complex with higher transcriptional activity and stimulate the G1/S transition in the absence of AtMMS21, but at the same time, the G2/M transition is blocked because the levels of CDKBs and CYCBs are reduced in this mutant (Ishida et al., 2009), resulting in enhanced differentiation (Figure 7 B). When DPa is overexpressed in the wild-type plants, the excess DPa may have no significant effect on endogenous E2Fa/DPa activity. However, when DPa is overexpressed in mms21-1 plants, the excess DPa may escape from the interaction with AtMMS21 and enhance the interaction with endogenous E2Fa, resulting in a more severe differentiation phenotype than that of the mms21-1 mutant. The real time PCR result in Figure 5 D showed that 35S:DPa dramatically increases the expression of E2Fa/DPa target genes in mms21-1, and these effects are suppressed by interfering with E2Fa expression, supporting the idea that the phenotype is dependent on the higher activity of the E2Fa/DPa complex. As a model, in mms21-1, the G1/S transition is stimulated but the G2/M transition is suppressed. When DPa is overexpressed in mms21-1, the G1/S transition is more active because the excess DPa is not inhibited by AtMMS21 but the G2/M transition is still blocked due to downregulation of CDKB/CYCB in the absence 13
14 of AtMMS21 (Ishida et al., 2009), resulting in enhanced differentiation. Furthermore, in the 35S:E2Fa-DPa plants, there may not be sufficient levels of endogenous AtMMS21 to completely dissociate the excess E2Fa/DPa complex, resulting in abnormal cell division or endoreplication. When AtMMS21 is also overexpressed in the plants, the E2Fa/DPa complex is dissociated completely, recovering the abnormal phenotype to normal. Previous studies showed that E2Fa/DPa controls both division and endoreplication in different tissues (De Veylder et al., 2002; Boudolf et al., 2004). Differentiation is enhanced in cotyledons and some root regions in 35S:DPa-E2Fa, these phenotypes are completely similar with that in mms21-1. It is interesting that the root stem cell region in mms21-1 is overdifferentiated (Huang et al., 2009; Ishida et al., 2009), while the number of root stem cells in 35S:E2Fa-DPa is increased (Wildwater et al., 2005). It is possible that, in the stem cell region of 35S:DPa-E2Fa, the G1/S transition is stimulated but the G2/M transition is not inhibited in the presence of AtMMS21, resulting in enhanced cell division. These data support our model that the regulation of E2Fa/DPa by AtMMS21 is important for the G1/S transition. The data from our current work show that DPa is an important target for AtMMS21 in cell cycle control. However, numerous proteins are targets of SUMOylation, but cells have only a few SUMO E3 ligases; therefore, many factors in different pathways may share one SUMO ligase. For instance, AtSIZ1, another SUMO ligase in Arabidopsis, has been reported to regulate more than ten substrates in different signaling pathways (Wilkinson and Henley, 2010). That is why the developmental phenotypes of mms21-1 and 35S:E2Fa-DPa differ slightly. However, the cell cycle related phenotypes, such as the endoreplication levels in cotyledons, the root developmental defect, and the expression levels of E2Fa-target genes are similar in these two types of plants, providing evidence for their associated functions in cell cycle regulation. It will be interesting to identify other targets for AtMMS21 in different cellular processes in the future. Given that MMS21, E2F, and DP are conserved among various species, it is possible that the mechanism we discovered in Arabidopsis is also applicable to other organisms. For example, our data provide evidence for an interaction between rice 14
15 MMS21 and DP proteins, implying that this interaction is conserved, at least in plants. A previous study in human cells indicated that hmms21 is also involved in the G1/S transition (Ni et al., 2012). Therefore, in the future study, it will be interesting to determine whether the regulatory mechanism mediated by the MMS21-DP interaction is also conserved in mammalian cells METHODS Plant materials and growth conditions The Arabidopsis thaliana Columbia-0 (WT) and mms21-1 (CS848340) were described previously (Huang et al., 2009). The seeds of the transgenic 35S:E2Fa and 35S:DPa lines (De Veylder et al., 2002) were kindly provided by the Inze Laboratory. Seeds were surface sterilized for 2 min in 75% ethanol followed by 5 min in 1% NaClO solution, rinsed five times with sterile water, plated on Murashige and Skoog (MS) medium with 1.5% Suc and 0.8% agar, and then stratified at 4 C in the dark for 2 d. Plants were grown under long-day conditions (16 h of light/8 h of dark, 80 μe s -1 m -2 light intensity) at 22 C Generation of transgenic plants For the 35S:MYC-AtMMS21 construct, the coding sequence of AtMMS21 was obtained by PCR amplification and cloned into the PBA002-MYC vector at the BamHI/SpeI sites. To generate the 35S:MYC-AtMMS21mut construct, site-directed mutagenesis was performed using the MutanBest kit (Takara). For the 35S:DPa-FLAG construct, DPa was amplified and fused with a FLAG tag at its C terminus in the vector ppzpy122, then the fragment containing 35S:DPa-FLAG was subcloned into pcambia1302 at the HindIII/SpeI sites. For the E2Fa-RNAi construct, the fragment containing the inverted repeat sequences of E2Fa spliced by an intron was cloned into the pcang-myc vector by BamHI and SpeI. The constructs were transformed into Agrobacterium EHA105, which was then used to transform A. thaliana (Columbia) by the floral-dip method (Clough and Bent, 1998). Homozygous lines of transgenic plants were used in this study. The 15
16 S:MYC-AtMMS21 construct was introduced into homozygous 35S:DPa plants (De Veylder et al. 2002) for phenotype analysis or 35S:DPa-FLAG plants for biochemistry analysis Yeast two-hybrid and three-hybrid assays Yeast two-hybrid assays were performed according to the manufacturer s instructions for the Matchmaker GAL4-based two-hybrid system 3 (Clontech). AtMMS21 was cloned into the pgbkt7 vector. The coding sequences of CDKA1, CYCB1;1, E2Fa, E2Fb, E2Fc, RBR, DPa, and DPb were cloned into the pgadt7 vector. For the interaction domain characterization, the truncated products were generated as described in the figure legends. For the SUMO1-DPa-C fusion construct, SUMO-1(ΔGG, amino acids 1 91) was fused to N terminus of DPa-C (amino acids ) by overlap PCR and cloned into pgbkt7. The bait and prey constructs were transformed into the yeast strain AH109 (Clontech). Protein protein interactions were tested by stringent (SD/ Leu/ Trp/ His) selection supplied with 3-amino-1,2,4-triazole and β-galactosidase activity measurement according to the manufacturer s protocol (Clontech). Yeast three-hybrid system (Clontech, Cambridge, USA) was applied to analyze the effect of AtMMS21 on the E2FA DPa interaction. E2Fa was cloned into pgadt7 and DPa was cloned into the three-hybrid vector pbridge (Clontech, Cambridge, USA) fused with GAL4. AtMMS21 was cloned under the control of a MET25 promoter in the same pbridge vector. The bait and prey pair was co-transformed into the yeast strain Y2HGold. A quantitative analysis of α-galactosidase activity was performed using p-nitrophenylα-d-galactopyranoside (PNP-α-Gal, Sigma-Aldrich, St Louis, MO, USA) according to the Yeast Protocols Handbook (Clontech) In vitro pull down assay GST or GST-AtMMS21 recombinant proteins were incubated with glutathione sepharose (GE Healthcare) in a binding buffer (50 mm Tris, ph 7.4, 120 mm NaCl, 5% Glycerol, 0.5% NP-40, 1 mm PMSF, 1 mm β-mercaptoethanol), for 2 h at 4 C, then 16
17 were collected and mixed with supernatant containing His 6 -DPA-FLAG and incubated at room temperature for 60 min. After rinsed for five times with the washing buffer (50 mm Tris ph 7.4, 120 mm NaCl, 5% Glycerol, 0.5% NP-40), the bound proteins were boiled in SDS sample buffer and subjected to SDS PAGE and immunoblotting Coimmunoprecipitation The regular coimmunoprecipitation assay was performed using transgenic plants harboring both 35S:DPa-FLAG and 35S:DPa-FLAG-MYC-AtMMS21. Proteins were extracted from the leaves of 3-week-old transgenic plants in extraction buffer (50 mm Tris-HCl, ph 7.4, 150 mm NaCl, 2 mm MgCl 2, 20% glycerol, and 0.1% Nonidet P-40) containing protease inhibitor cocktail (Roche). After centrifugation at 13,000 g for 12 min, the supernatant was incubated with anti-myc antibody (Clontech) at 4 C overnight. Then, 50 μl of protein A agarose beads (Sigma) was added. After 3 h of incubation at 4 C, the beads were centrifuged and washed four times with washing buffer (50 mm Tris-HCl, ph 7.4, 150 mm NaCl, 2 mm MgCl 2, 10% glycerol, and 0.1% Nonidet P-40). Proteins were eluted with SDS sample buffer and analyzed by immunoblotting. For the interference assay, the YFP-E2Fa construct was transformed into Arabidopsis protoplasts (Yoo et al., 2007) from the indicated transgenic plants. After 60 h, protoplasts were harvested for immunoprecipitation following the co-ip protocol described above using FLAG M2 Affinity Gel (Sigma) SUMOylation assay The SUMOylation assay in E. coli was performed as previously described (Okada et al. 2009). DPa fused with a FLAG tag was cloned into pcdfduet-1(novagen) and expressed in bacteria carrying pet28-sae1a-his-atsae2 (E1) (Budhiraja et al., 2009) and pacycduet-1-sce1-sumo1 (GG) (E2 and SUMO). The transformed cells were cultured in LB medium to OD 600 of 0.5 and induced by 0.5 mm IPTG. After incubation for 12 hours at 25 C, cells were harvested and used for immunoblotting. To detect the effect of AtMMS21 on the SUMOylation of DPa, the Arabidopsis 17
18 SUMO E1 (SAE1a-His 6 -AtSAE2), E2 (His 6 -SCE1), His 6 -SUMO1 (GG), His 6 -DPa-FLAG, and GST-AtMMS21 was expressed in E.coil BL21(DE3) and purified by Ni-NTA Agarose (QIAGEN) or Glutathione Sepharose 4B (GE healthcare). For the in vitro SUMOylation assay, His 6 -SAE2/SAE1a (500 ng), His 6 -SCE1 (0 or 100 ng), His 6 -SUMO1(GG) (4 μg), substrate protein His 6 -DPa-FLAG (1 μg), GST-AtMMS21 (0, 90 ng or 900 ng) and SUMO reaction buffer (50 mm Tris, ph 7.4, 100 mm NaCl, 5% glycerol, 5 mm ATP, 5 mm MgCl 2 ) were mixed together in a total reaction volume of 25 ml and incubated at 30 C for 3 hours. After boiling in SDS sample buffer, the samples were then analyzed by immunoblotting Subcellular localization detection For the DPA localization analysis, E2Fa was cloned and fused with CFP under a 35S promoter in a modified pbluescript SK vector. DPa was amplified and fused with GFP in the pcambia1302 vector to generate DPa-GFP. Next, AtMMS21 was cloned into the XhoI site of pcambia1302-dpa by replacing the DNA fragment encoding Hygromycin resistance, resulting in a plasmid expressing both DPa-GFP and AtMMS21. The constructs were cotransformed into Arabidopsis protoplasts as described previously (Yoo et al., 2007), and only the protoplasts expressing with both GFP and CFP fluorescence were used for analysis. For E2Fa or E2Fb localization analysis, E2Fa or E2Fb was cloned into the modified pbluescript SK vector containing 35S:YFP. The plasmid YFP-E2Fa or YFP-E2Fb was transformed into the protoplasts from transgenic plants overexpressing DPa-FLAG or DPa-FLAG/MYC-AtMMS21. The fluorescent GFP or YFP signals in transformed protoplasts were examined by confocal microscopy (Leica LSM710) Root length analysis and microscopy The primary roots of plants incubated vertically on MS medium were photographed using a Canon camera, and the lengths of the primary roots at indicated day were determined using Digimizer 3.2 software. For confocal laser imaging of roots, roots were counterstained with 10 μg/ml propidium iodide (PI, Sigma) for 1 min, and 18
19 mounted in water for confocal microscopy. For hypocotyl observation, the tissues were placed overnight in ethanol and subsequently washed in water, then mounted in chloroacetaldehyde:water:glycerol (8:3:1) solution for 10 to 20 min before microscopy. Confocal images were taken using a Zeiss LSM 710 laser scanning microscope with the following excitation/emission wavelengths: 561 nm /591 to 635 nm for PI, 488 nm /505 to 530 nm for GFP, 514 nm /530 to 600 nm for YFP, and 458 nm /475 to 525 nm for CFP Flow cytometric analysis Plants were chopped with a razor blade in 400 µl nuclei extraction buffer (Partec Cystain UV Precise P), the suspernatent was filtered over a 30 µm mesh, and 1.6 ml staining buffer (Partec Cystain UV Precise P) was added. The nuclei were analyzed by CyFlow Cube 8 with CyView software (Partec, Germany). At least 8000 nuclei were scored in triplicates for each sample Gene expression analysis Total RNA was extracted using the Plant RNAprep Pure Kit (TIANGEN) with DNase I treatment following the manufacturer s instructions. Reverse transcription was performed using a PrimeScript RT reagent kit (Takara). After the RT reaction, the complementary DNA template was subjected to PCR using SYBR Premix Ex Taq (Takara) in a Bio-Rad CFX 96 system (C1000 Thermal Cycler) and detected by Bio-Rad CFX Manager software (Bio-Rad, Hercules, CA). RT-qPCR was performed with three replicates and UBQ10 as a reference gene. For the measurement of gene expression in protoplasts, the transformation was performed as described (Yoo et al., 2007). The protoplasts were collected for RNA extraction after 60 hours Accession Numbers Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AtMMS21 (At3g15150), SAE1 (At3g57870), SAE2 (At2g21470), SCE1 (AT3G57870), SUMO1 19
20 (At4G26840), CDKA1;1 (At3g48750), CYCB1;1 (At4g37490), E2Fa (At2g36010), E2Fb (At5g22220), E2Fc (At1g47870), DPa (At5g02470), DPb (At5g03415), RBR (At3g12280), ETG1 (At2g40550), ORC2 (At2g37560), MCM3 (At5g46280), PCNA1 (At1g07370), UBQ10 (At4g05320), OsDP (LOC_Os01g48700), OsMMS21 (LOC_Os05g48880) SUPPLEMENTAL DATA Supplemental Figure 1. Detection of DPa-AtMMS21 interaction by bimolecular fluorescence complementation. Supplemental Figure 2. Subcellular distribution of YFP-fused E2Fb in protoplasts. Supplemental Figure 3. The phenotypes of WT, 35S:DPa, 35S:E2Fa, and 35S:AtMMS21 seedlings. Supplemental Figure 4. The generation and identification of plants carrying 35S:E2Fa-DPa-AtMMS21. Supplemental Figure 5. The phenotypes of hypocotyl and root cap of the 35S:E2Fa-DPa-AtMMS21 plants. Supplemental Data Set 1. Primers used in this study ACKNOWLEDGMENTS This work was supported by the National Natural Science Foundation of China ( , U ), Major Project from the Education Department of Guangdong Province (2014), the Natural Science Foundation of Guangdong (S ), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2010). We thank Prof. Lieven De Veylder (Ghent University) and the Arabidopsis Biological Resource Center for the seeds, and thank Prof. Bachmair for the SUMO E1 plasmid used in this study AUTHOR CONTRIBUTIONS Y.L., J.L. and C.Y. conceived the project and designed the research; Y.L., J.L., M.Y., F.W., J.Z., J.J., H.H., Q.W., G.L. and P.X. performed the research; Y.L., J.L., M.Y. and 20
21 601 C.Y. analyzed the data; J.L. and C.Y. wrote the article REFERENCES Al-Khodairy, F., Enoch, T., Hagan, I.M., and Carr, A.M. (1995). The Schizosaccharomyces pombe hus5 gene encodes a ubiquitin conjugating enzyme required for normal mitosis. Journal of Cell Science 108: Bandara, L., Buck, V., Zamanian, M., Johnston, L., and La Thangue, N. (1993). Functional synergy between DP-1 and E2F-1 in the cell cycle-regulating transcription factor DRTF1/E2F. The EMBO journal 12: Bellail, A.C., Olson, J.J., and Hao, C. (2014). SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. Nature communications 5: Berkholz, J., Michalick, L., and Munz, B. (2014). The E3 SUMO ligase Nse2 regulates sumoylation and nuclear-to-cytoplasmic translocation of sknac Smyd1 in myogenesis. Journal of cell science 127: Boniotti, M.B., and Gutierrez, C. (2001). A cell-cycle-regulated kinase activity phosphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. The Plant Journal 28: Boudolf, V., Vlieghe, K., Beemster, G.T., Magyar, Z., Acosta, J.A.T., Maes, S., Van Der Schueren, E., Inzé, D., and De Veylder, L. (2004). The plant-specific cyclin-dependent kinase CDKB1; 1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. The Plant Cell 16: Budhiraja, R., Hermkes, R., Müller, S., Schmidt, J., Colby, T., Panigrahi, K., Coupland, G., and Bachmair, A. (2009). Substrates related to chromatin and to RNA-dependent processes are modified by Arabidopsis SUMO isoforms that differ in a conserved residue with influence on desumoylation. Plant physiology 149: Catala, R., Ouyang, J., Abreu, I.A., Hu, Y., Seo, H., Zhang, X., and Chua, N.-H. (2007). The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and 21
22 drought responses. The Plant Cell 19: Clough, S.J., and Bent, A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16: Conti, L., Nelis, S., Zhang, C., Woodcock, A., Swarup, R., Galbiati, M., Tonelli, C., Napier, R., Hedden, P., Bennett, M., and Sadanandom, A. (2014). Small Ubiquitin-like Modifier protein SUMO enables plants to control growth independently of the phytohormone gibberellin. Developmental cell 28: Dasso, M. (2008). Emerging roles of the SUMO pathway in mitosis. Cell Division 3: 5. De Veylder, L., Joubès, J., and Inzé, D. (2003). Plant cell cycle transitions. Current opinion in plant biology 6: De Veylder, L., Beeckman, T., Beemster, G.T., de Almeida Engler, J., Ormenese, S., Maes, S., Naudts, M., Van Der Schueren, E., Jacqmard, A., Engler, G., and Inzé D. (2002). Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa DPa transcription factor. The EMBO journal 21: Dewitte, W., and Murray, J.A. (2003). The plant cell cycle. Annual Review of Plant Biology 54: Diaz-Trivino, S., del Mar Castellano, M., de la Paz Sanchez, M., Ramirez-Parra, E., Desvoyes, B., Gutierrez, C. (2005). The genes encoding Arabidopsis ORC subunits are E2F targets and the two ORC1 genes are differently expressed in proliferating and endoreplicating cells. Nucleic Acids Research 33: Egelkrout, E.M., Mariconti, L., Settlage, S.B., Cella, R., Robertson, D., and Hanley-Bowdoin, L. (2002). Two E2F elements regulate the proliferating cell nuclear antigen promoter differently during leaf development. The Plant Cell 14: Elrouby, N., and Coupland, G. (2010). Proteome-wide screens for small ubiquitin-like modifier (SUMO) substrates identify Arabidopsis proteins implicated in diverse biological processes. Proceedings of the National 22
23 Academy of Sciences 107: Glotzer, M., Murray, A.W., and Kirschner, M.W. (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349: Gutierrez, C. (2009). The Arabidopsis cell division cycle. The Arabidopsis book/american Society of Plant Biologists 7. Hartwell, L.H., and Kastan, M.B. (1994). Cell cycle control and cancer. Science 266: Helin, K., Wu, C.-L., Fattaey, A.R., Lees, J.A., Dynlacht, B.D., Ngwu, C., and Harlow, E. (1993). Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes & Development 7: Huang, L., Yang, S., Zhang, S., Liu, M., Lai, J., Qi, Y., Shi, S., Wang, J., Wang, Y., and Xie, Q., and Yang, C. (2009). The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root. The Plant Journal 60: Ishida, T., Fujiwara, S., Miura, K., Stacey, N., Yoshimura, M., Schneider, K., Adachi, S., Minamisawa, K., Umeda, M., and Sugimoto, K. (2009). SUMO E3 ligase HIGH PLOIDY2 regulates endocycle onset and meristem maintenance in Arabidopsis. The Plant Cell 21: Jacome, A., Gutierrez-Martinez, P., Schiavoni, F., Tenaglia, E., Martinez, P., Rodríguez-Acebes, S., Lecona, E., Murga, M., Méndez, J., Blasco, M.A., Fernandez-Capetillo, O. (2015). NSMCE2 suppresses cancer and aging in mice independently of its SUMO ligase activity. The EMBO journal 34: Khan, M., Rozhon, W., Unterholzner, S.J., Chen, T., Eremina, M., Wurzinger, B., Bachmair, A., Teige, M., Sieberer, T., and Isono, E. (2014). Interplay between phosphorylation and SUMOylation events determines CESTA protein fate in brassinosteroid signalling. Nature communications 5: Kosugi, S., and Ohashi, Y. (2002). Interaction of the Arabidopsis E2F and DP proteins confers their concomitant nuclear translocation and transactivation. Plant 23
24 physiology 128: Kosugi, S., and Ohashi, Y. (2003). Constitutive E2F expression in tobacco plants exhibits altered cell cycle control and morphological change in a cell type-specific manner. Plant physiology 132: Krek, W., Livingston, D.M., and Shirodkar, S. (1993). Binding to DNA and the Retinoblastoma Gene Product Promoted by Complex Formation of Different E2F Family Members. Science 262: Ledl, A., Schmidt, D., and Müller, S. (2005). Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 24: Li, S.-J., and Hochstrasser, M. (2000). The Yeast ULP2 (SMT4) Gene Encodes a Novel Protease Specific for the Ubiquitin-Like Smt3 Protein. Molecular and Cellular Biology 20: Li, T., Santockyte, R., Shen, R.-F., Tekle, E., Wang, G., Yang, D.C., and Chock, P.B. (2006). Expression of SUMO-2/3 induced senescence through p53-and prb-mediated pathways. Journal of Biological Chemistry 281: Liu, M., Shi, S., Zhang, S., Xu, P., Lai, J., Liu, Y., Yuan, D., Wang, Y., Du, J., and Yang, C. (2014). SUMO E3 ligase AtMMS21 is required for normal meiosis and gametophyte development in Arabidopsis. BMC plant biology 14: 153. Müller, H., and Helin, K. (2000). The E2F transcription factors: key regulators of cell proliferation. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1470: M1-M12. Magyar, Z., Atanassova, A., De Veylder, L., Rombauts, S., and Inzé, D. (2000). Characterization of two distinct DP-related genes from Arabidopsis thaliana. FEBS letters 486: Magyar, Z., Horváth, B., Khan, S., Mohammed, B., Henriques, R., De Veylder, L., Bakó, L., Scheres, B., and Bögre, L. (2012). Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR bound and RBR free complexes. The EMBO journal 31: Malumbres, M. (2014). Cyclin-dependent kinases. Genome Biology 15:
25 Man, J.-H., Li, H.-Y., Zhang, P.-J., Zhou, T., He, K., Pan, X., Liang, B., Li, A.-L., Zhao, J., Gong, W.-L., Jin, B.F., Xia, Q., Yu, M., Shen, B.F., and Zhang, X.M. (2006). PIAS3 induction of PRB sumoylation represses PRB transactivation by destabilizing its retention in the nucleus. Nucleic acids research 34: Miller, M.J., Barrett-Wilt, G.A., Hua, Z., and Vierstra, R.D. (2010). Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proceedings of the National Academy of Sciences 107: Miura, K., Jin, J.B., Lee, J., Yoo, C.Y., Stirm, V., Miura, T., Ashworth, E.N., Bressan, R.A., Yun, D.-J., and Hasegawa, P.M. (2007). SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. The Plant Cell 19: Nacerddine, K., Lehembre, F., Bhaumik, M., Artus, J., Cohen-Tannoudji, M., Babinet, C., Pandolfi, P.P., and Dejean, A. (2005). The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Developmental cell 9: Nakagami, H., Kawamura, K., Sugisaka, K., Sekine, M., and Shinmyo, A. (2002). Phosphorylation of retinoblastoma-related protein by the cyclin D/cyclin-dependent kinase complex is activated at the G1/S-phase transition in tobacco. The Plant Cell 14: Ni, H.-J., Chang, Y.-N., Kao, P.-H., Chai, S.-P., Hsieh, Y.-H., Wang, D.-H., and Fong, J.C. (2012). Depletion of SUMO ligase hmms21 impairs G1 to S transition in MCF-7 breast cancer cells. Biochimica et Biophysica Acta (BBA)-General Subjects 1820: Okada, S., Nagabuchi, M., Takamura, Y., Nakagawa, T., Shinmyozu, K., Nakayama, J.-i., and Tanaka, K. (2009). Reconstitution of Arabidopsis thaliana SUMO pathways in E. coli: functional evaluation of SUMO machinery proteins and mapping of SUMOylation sites by mass spectrometry. Plant and cell physiology 50:
26 Palvimo, J. (2007). PIAS proteins as regulators of small ubiquitin-related modifier (SUMO) modifications and transcription. Biochemical Society Transactions 35: Ramirez-Parra, E., and Gutierrez, C. (2000). Characterization of wheat DP, a heterodimerization partner of the plant E2F transcription factor which stimulates E2F DNA binding. FEBS letters 486: Ross, S., Best, J.L., Zon, L.I., and Gill, G. (2002). SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Molecular cell 10: Saracco, S.A., Miller, M.J., Kurepa, J., and Vierstra, R.D. (2007). Genetic analysis of SUMOylation in Arabidopsis: conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant physiology 145: Satyanarayana, A., and Kaldis, P. (2009). Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28: Schimmel, J., Eifler, K., Sigurðsson, J.O., Cuijpers, S.A., Hendriks, I.A., Verlaan-de Vries, M., Kelstrup, C.D., Francavilla, C., Medema, R.H., Olsen, J.V., and Vertegaal, A.C. (2014). Uncovering SUMOylation dynamics during cell-cycle progression reveals FoxM1 as a key mitotic SUMO target protein. Molecular cell 53: Stephan, A.K., Kliszczak, M., and Morrison, C.G. (2011). The Nse2/Mms21 SUMO ligase of the Smc5/6 complex in the maintenance of genome stability. FEBS letters 585: Stevens, R., Mariconti, L., Rossignol, P., Perennes, C., Cella, R., and Bergounioux, C. (2002). Two E2F sites in the Arabidopsis MCM3 promoter have different roles in cell cycle activation and meristematic expression. Journal of Biological Chemistry 277: Takahashi, N., Lammens, T., Boudolf, V., Maes, S., Yoshizumi, T., De Jaeger, G., Witters, E., Inze, D., and De Veylder, L. (2008). The DNA replication checkpoint aids survival of plants deficient in the novel replisome factor ETG1. 26
27 The EMBO journal 27: Taylor, D.L., Ho, J.C., Oliver, A., and Watts, F.Z. (2002). Cell-cycle-dependent localisation of Ulp1, a Schizosaccharomyces pombe Pmt3 (SUMO)-specific protease. Journal of cell science 115: Vandepoele, K., Vlieghe, K., Florquin, K., Hennig, L., Beemster, G.T., Gruissem, W., Van de Peer, Y., Inzé, D., and De Veylder, L. (2005). Genome-wide identification of potential plant E2F target genes. Plant physiology 139: Wang, X.D., Lapi, E., Sullivan, A., Ratnayaka, I., Goldin, R., Hay, R., and Lu, X. (2011). SUMO-modified nuclear cyclin D1 bypasses Ras-induced senescence. Cell Death & Differentiation 18: Weinberg, R.A. (1995). The retinoblastoma protein and cell cycle control. Cell 81: Wildwater, M., Campilho, A., Perez-Perez, J.M., Heidstra, R., Blilou, I., Korthout, H., Chatterjee, J., Mariconti, L., Gruissem, W., and Scheres, B. (2005). The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 123: Wilkinson, K., and Henley, J. (2010). Mechanisms, regulation and consequences of protein SUMOylation. The Biochemical journal 428: Xu, P., Yuan, D., Liu, M., Li, C., Liu, Y., Zhang, S., Yao, N., and Yang, C. (2013). AtMMS21, an SMC5/6 complex subunit, is involved in stem cell niche maintenance and DNA damage responses in Arabidopsis roots. Plant physiology 161: Yoo, S.-D., Cho, Y.-H., and Sheen, J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature protocols 2: Yuan, D., Lai, J., Xu, P., Zhang, S., Zhang, J., Li, C., Wang, Y., Du, J., Liu, Y., and Yang, C. (2014). AtMMS21 regulates DNA damage response and homologous recombination repair in Arabidopsis. DNA repair 21: Zhang, S., Qi, Y., and Yang, C. (2010). Arabidopsis SUMO E3 ligase AtMMS21 27
28 regulates root meristem development. Plant signaling & behavior 5: Zhao, Q., Xie, Y., Zheng, Y., Jiang, S., Liu, W., Mu, W., Liu, Z., Zhao, Y., Xue, Y., and Ren, J. (2014). GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic acids research 42: W325-W FIGURE LEGENDS Figure 1. The phenotype of mms21-1. (A) The phenotypes of 1-week-old (left panel) and 3-week-old (right panel) wild-type and mms21-1 plants. Bar=1 cm. (B) The endoreplication levels in wild-type and mms21-1 plants were quantitatively analyzed by flow cytometry. The cotyledons or the first pair of true leaves (leaf 1-2) of 13-day-old plants were collected for analysis. The data are means ± SD from at least three biological replicates
29 Figure 2. DPa interacts with AtMMS21. (A) The interaction between DPa and AtMMS21 was confirmed in a yeast two-hybrid assay. (B) Identification of the domain on DPa required for its interaction with AtMMS21 by yeast two-hybrid analysis. The DNA binding domain (red) and dimerization domain (blue) of DPa and the SP-RING domain (purple) of AtMMS21 are indicated. (C) Identification of the domain on AtMMS21 required for its interaction with DPa by yeast two-hybrid analysis. (D) The interaction between rice DP and MMS21 in a yeast two-hybrid assay. (E) The interaction between DPa and AtMMS21 in an in vitro pull-down assay. The DPa-FLAG proteins were incubated with immobilized GST or GST-AtMMS21, and proteins immunoprecipitated with glutathione sepharose were detected using anti-flag antibody. The amounts of GST and GST-AtMMS21 are shown in the bottom panel. (F) The subcellular colocalization of DPa and AtMMS21. CFP-AtMMS21 and YFP-DPa were co-expressed in protoplasts. After 48 h, the CFP and YFP signals were detected and merged. Bar=10 µm. (G) The interaction between DPa and AtMMS21 in an in vivo co-immunoprecipitation assay. Total protein extracts from transgenic plants carrying 35S:DPa-FLAG alone or both 35S:DPa-FLAG and 35S:MYC-AtMMS21 were immunoprecipitated with the immobilized anti-myc antibody. The proteins from crude lysates (left) and immunoprecipitated proteins (right) were detected using anti-flag or anti-myc antibody Figure 3. AtMMS21 enhances the SUMOylation of DPa and interferes with the interaction between E2Fa and DPa. (A) The effects of AtMMS21 on the interaction between DPa and E2Fa in a yeast three-hybrid assay. Yeast cells were transformed with pbridge-bd-dpa-promet-atmms21 or pbridge-bd-dpa with pgadt7-e2fa and cultured on SD-L-W-M medium. The relative α-galactosidase activities are shown. The activity from the sample with BD-DPa and AD-E2Fa was set to 1. The data are means ± SD from three independent experiments. *** p<0.001, Student s t test. (B) The effects of AtMMS21 on the DPa E2Fa interaction in plant cells. YFP-E2Fa was expressed in protoplasts from wild-type or transgenic plants overexpressing DPa-FLAG alone or both DPa-FLAG and MYC-AtMMS21. The interaction was detected by 29
30 immunoprecipitation on anti-flag resin. The total lysates (input) and the immunoprecipitated proteins (IP) were detected with an antibody recognizing YFP. The expression levels of DPa-FLAG and MYC-AtMMS21 were also detected using the indicated antibodies. (C) The SUMO conjugation of DPa was detected in a reconstituted SUMOylation system in E. coli. In the presence of E1 and SUMO1, the unconjugated and SUMO-conjugated WT or the mutant with K139/140/146R substitutions of FLAG-tagged DPa with or without E2 were detected with anti-flag antibody. (D) AtMMS21 mediates the in vitro SUMOylation of DPa. Affinity-purified DPa-FLAG was used as a substrate in an in vitro SUMOylation assay. The SUMOylation levels of DPa in different samples were detected in an immunoblot using anti-flag antibody. * indicates an unspecific signal. (E) The positions of SUMO attachment sites on the DPa protein. The three-dimensional structure of the C terminus of Arabidopsis DPa was predicted using Swiss Model. The potential SUMOylation sites are indicated. (F) The effect of SUMO on the interaction between DPa and E2Fa. SUMO1 was fused to DPa-C (144 aa to 292 aa) in BD vector and used in a yeast two-hybrid assay with AD-E2Fa to detect their interaction. BD-DPa-C without SUMO was used as a control Figure 4. AtMMS21 affects the translocation of E2Fa/DPa. (A) Subcellular distribution of GFP-fused DPa proteins in protoplasts. Plasmids carrying 35S:DPa-GFP alone, or both 35S:DPa-GFP and 35S:AtMMS21, were transfected into wild-type protoplasts with or without CFP-E2Fa. When co-expressed with CFP-E2Fa, only the protoplasts with both CFP and GFP signals were used for analysis. Representative GFP signals from the majority of protoplasts from DPa-GFP alone, a combination of DPa-GFP and CFP-E2Fa, and a combination of DPa-GFP, AtMMS21, and CFP-E2Fa are shown. The autofluorescence from chloroplasts and bright field (BF) signals were also detected and merged. Bar=10 µm. Statistical data from means ± SD from three independent biological replicates (n>100) are shown in the right panel. (B) Subcellular distribution of YFP-fused E2Fa in protoplasts. YFP-E2Fa was transiently expressed in protoplasts from wild-type and transgenic plants carrying 35S:DPa alone 30
31 or both 35S:DPa and 35S:AtMMS21. Representative YFP signals from the majority of the indicated protoplasts are shown. Bar=10 µm. Statistical data from means ± SD from three independent biological replicates (n>100) are shown in the right panel. *** p<0.001, Student s t test Figure 5. Overexpression of DPa affects root development in mms21-1. (A) The relative expression levels of E2Fa target genes in the roots of 4-day-old seedlings were analyzed by real-time PCR. The expression level in WT was set to 1. The data are means ± SD from triplicate experiments. *p<0.05, ** p<0.01, Student s t test. (B) The phenotypes of mms21-1 and mms S:DPa plants. The photograph was taken 15 days after germination. Bar= 1 cm. Statistical data of root length shown in the right panel are means ± SD from at least 30 seedlings in three biological independent experiments. ** p<0.01, Student s t test. (C) Representative root apical meristems from WT, 35S:DPa, mms21-1, and mms S:DPa plants. Roots from 5-d-old seedlings were stained with PI. The meristem region is indicated by arrowheads; quiescent center is indicated by the lower arrowhead. Bar=100 µm. (D) The relative expression levels of E2Fa target genes in the protoplasts of mms21-1 and mms S:DPa with or without RNA interference for E2Fa. The protoplasts were collected 60 hours after transformation and used for RNA extraction for real time PCR. The expression level in mms21-1 with empty vector was set to 1. The data are means ± SD from three experiments. *p<0.05, ** p<0.01, Student s t test Figure 6. AtMMS21 interferes with the function of the E2Fa/DPa complex in plants. (A) The phenotypes of WT, 35S: E2Fa-DPa, and three independent lines (#1, #2, #3) from verified 35S:DPa-E2Fa-AtMMS21 plants. The photograph was taken 7 days after germination. Bar =1 cm. (B) Statistical data for root lengths of the indicated plants. The results are means ± SD from at least 35 seedlings per line at 7 DAG in three independent experiments. *** p<0.001, Student s t test. (C) The phenotypes of 3-week-old seedlings. (D) The endoreplication levels in the indicated plants were quantitatively analyzed by flow cytometry. The cotyledons of eight-day-old seedlings 31
32 were used for analysis. The results are means ± SD from of at least three biological replicates. (E) The relative expression levels of E2Fa target genes in the indicated plants were analyzed by real-time PCR. The expression level in WT was set to 1. The data are means ± SD from triplicate experiments. ** p<0.01, *** p<0.001, Student s t test. (F) The effect of mutated AtMMS21 on the function of E2Fa/DPa. The root length of two independent lines (#1, #2) of 35S:E2Fa-DPa-AtMMS21mut was compared with that of WT, 35S:E2Fa-DPa, or 35S:E2Fa-DPa-AtMMS21. The photograph was taken at 7 DAG, and the statistical data are means ± SD from at least 20 seedlings. Bar= 1 cm. *** p<0.001, Student s t test Figure 7. Predicted model of the role of AtMMS21 in plant cell cycle regulation. (A) The effect of physical competition and SUMOylation on the E2Fa/DPa complex. (B) The role of AtMMS21 in cell cycle checkpoint regulation
33 Figure 1. The phenotype of mms21-1. (A) The phenotypes of 1-week-old (left panel) and 3-week-old (right panel) wild-type and mms21-1 plants. Bar=1 cm. (B) The endoreplication levels in wild-type and mms21-1 plants were quantitatively analyzed by flow cytometry. The cotyledons or the first pair of true leaves (leaf 1-2) of 13-day-old plants were collected for analysis. The data are means ± SD from at least three biological replicates.
34 Figure 2. DPa interacts with AtMMS21. (A) The interaction between DPa and AtMMS21 was confirmed in a yeast two-hybrid assay. (B) Identification of the domain on DPa required for its interaction with AtMMS21 by yeast two-hybrid analysis. The DNA binding domain (red) and dimerization domain (blue) of DPa and the SP-RING domain (purple) of AtMMS21 are indicated. (C) Identification of the domain on AtMMS21 required for its interaction with DPa by yeast two-hybrid analysis. (D) The interaction between rice DP and MMS21 in a yeast two-hybrid assay. (E) The interaction between DPa and AtMMS21 in an in vitro pull-down assay. The DPa-FLAG proteins were incubated with immobilized GST or GST-AtMMS21, and proteins immunoprecipitated with glutathione sepharose were detected using anti-flag antibody. The amounts of GST and GST-AtMMS21 are shown in the bottom panel. (F) The subcellular colocalization of DPa and AtMMS21. CFP-AtMMS21 and YFP-DPa were co-expressed in protoplasts. After 48 h, the CFP and YFP signals were detected and merged. Bar=10 µm. (G) The interaction between DPa and AtMMS21 in an in vivo co-immunoprecipitation assay. Total protein extracts from transgenic plants carrying 35S:DPa-FLAG alone or both 35S:DPa-FLAG and 35S:MYC-AtMMS21 were immunoprecipitated with the immobilized anti-myc antibody. The proteins from crude lysates (left) and immunoprecipitated proteins (right) were detected using anti-flag or anti-myc antibody.
35 Figure 3. AtMMS21 enhances the SUMOylation of DPa and interferes with the interaction between E2Fa and DPa. (A) The effects of AtMMS21 on the interaction between DPa and E2Fa in a yeast three-hybrid assay. Yeast cells were transformed with pbridge-bd-dpa-promet-atmms21 or pbridge-bd-dpa with pgadt7-e2fa and cultured on SD-L-W-M medium. The relative α-galactosidase activities are shown. The activity from the sample with BD-DPa and AD-E2Fa was set to 1. The data are means ± SD from three independent experiments. *** p<0.001, Student s t test. (B) The effects of AtMMS21 on the DPa E2Fa interaction in plant cells. YFP-E2Fa was expressed in protoplasts from wild-type or transgenic plants overexpressing DPa-FLAG alone or both DPa-FLAG and MYC-AtMMS21. The interaction was detected by immunoprecipitation on anti-flag resin. The total lysates (input) and the immunoprecipitated proteins (IP) were detected with an antibody recognizing YFP. The expression levels of DPa-FLAG and MYC-AtMMS21 were also detected using the indicated antibodies. (C) The SUMO conjugation of DPa was detected in a reconstituted SUMOylation system in E. coli. In the presence of E1 and SUMO1, the unconjugated and SUMO-conjugated WT or the mutant with K139/140/146R substitutions of FLAG-tagged DPa with or without E2 were detected with anti-flag antibody. (D) AtMMS21 mediates the in vitro SUMOylation of DPa. Affinity-purified DPa-FLAG was used as a substrate in an in vitro SUMOylation assay. The
36 SUMOylation levels of DPa in different samples were detected in an immunoblot using anti-flag antibody. * indicates an unspecific signal. (E) The positions of SUMO attachment sites on the DPa protein. The three-dimensional structure of the C terminus of Arabidopsis DPa was predicted using Swiss Model. The potential SUMOylation sites are indicated. (F) The effect of SUMO on the interaction between DPa and E2Fa. SUMO1 was fused to DPa-C (144 aa to 292 aa) in BD vector and used in a yeast two-hybrid assay with AD-E2Fa to detect their interaction. BD-DPa-C without SUMO was used as a control.
37 Figure 4. AtMMS21 affects the translocation of E2Fa/DPa. (A) Subcellular distribution of GFP-fused DPa proteins in protoplasts. Plasmids carrying 35S:DPa-GFP alone, or both 35S:DPa-GFP and 35S:AtMMS21, were transfected into wild-type protoplasts with or without CFP-E2Fa. When co-expressed with CFP-E2Fa, only the protoplasts with both CFP and GFP signals were used for analysis. Representative GFP signals from the majority of protoplasts from DPa-GFP alone, a combination of DPa-GFP and CFP-E2Fa, and a combination of DPa-GFP, AtMMS21, and CFP-E2Fa are shown. The autofluorescence from chloroplasts and bright field (BF) signals were also detected and merged. Bar=10 µm. Statistical data from means ± SD from three independent biological replicates (n>100) are shown in the right panel. (B) Subcellular distribution of YFP-fused E2Fa in protoplasts. YFP-E2Fa was transiently expressed in protoplasts from wild-type and transgenic plants carrying 35S:DPa alone or both 35S:DPa and 35S:AtMMS21. Representative YFP signals from the majority of the indicated protoplasts are shown. Bar=10 µm. Statistical data from means ± SD from three independent biological replicates (n>100) are shown in the right panel. *** p<0.001, Student s t test.
38 Figure 5. Overexpression of DPa affects root development in mms21-1. (A) The relative expression levels of E2Fa target genes in the roots of 4-day-old seedlings were analyzed by real-time PCR. The expression level in WT was set to 1. The data are means ± SD from triplicate experiments. *p<0.05, ** p<0.01, Student s t test. (B) The phenotypes of mms21-1 and mms S:DPa plants. The photograph was taken 15 days after germination. Bar= 1 cm. Statistical data of root length shown in the right panel are means ± SD from at least 30 seedlings in three biological independent experiments. ** p<0.01, Student s t test. (C) Representative root apical meristems from WT, 35S:DPa, mms21-1, and mms S:DPa plants. Roots from 5-d-old seedlings were stained with PI. The meristem region is indicated by arrowheads; quiescent center is indicated by the lower arrowhead. Bar=100 µm. (D)
39 The relative expression levels of E2Fa target genes in the protoplasts of mms21-1 and mms S:DPa with or without RNA interference for E2Fa. The protoplasts were collected 60 hours after transformation and used for RNA extraction for real time PCR. The expression level in mms21-1 with empty vector was set to 1. The data are means ± SD from three experiments. *p<0.05, ** p<0.01, Student s t test.
40 Figure 6. AtMMS21 interferes with the function of the E2Fa/DPa complex in plants. (A) The phenotypes of WT, 35S: E2Fa-DPa, and three independent lines (#1, #2, #3) from verified 35S:DPa-E2Fa-AtMMS21 plants. The photograph was taken 7 days after germination. Bar =1 cm. (B) Statistical data for root lengths of the indicated plants. The results are means ± SD from at least 35 seedlings per line at 7 DAG in three independent experiments. *** p<0.001, Student s t test. (C) The phenotypes of 3-week-old seedlings. (D) The endoreplication levels in the indicated plants were quantitatively analyzed by flow cytometry. The cotyledons of eight-day-old seedlings were used for analysis. The results are means ± SD from of at least three biological replicates. (E) The relative expression levels of E2Fa target genes in the indicated plants were analyzed by real-time PCR. The expression level in WT was set to 1. The data are means ± SD from triplicate experiments. ** p<0.01, *** p<0.001, Student s t test. (F) The effect of mutated AtMMS21 on the function of E2Fa/DPa. The root
41 length of two independent lines (#1, #2) of 35S:E2Fa-DPa-AtMMS21mut was compared with that of WT, 35S:E2Fa-DPa, or 35S:E2Fa-DPa-AtMMS21. The photograph was taken at 7 DAG, and the statistical data are means ± SD from at least 20 seedlings. Bar= 1 cm. *** p<0.001, Student s t test.
42 Figure 7. Predicted model of the role of AtMMS21 in plant cell cycle regulation. (A) The effect of physical competition and SUMOylation on the E2Fa/DPa complex. (B) The role of AtMMS21 in cell cycle checkpoint regulation.
Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter
 Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter 9/10/2008 1 Learning Objectives Explain similarities and differences between fungal, mammalian and plant cell cycles Explain
Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter 9/10/2008 1 Learning Objectives Explain similarities and differences between fungal, mammalian and plant cell cycles Explain
Juan C. del Pozo, a,b Sara Diaz-Trivino, a Nerea Cisneros, a and Crisanto Gutierrez a,1
 The Plant Cell, Vol. 18, 2224 2235, September 2006, www.plantcell.org ª 2006 American Society of Plant Biologists The Balance between Cell Division and Endoreplication Depends on E2FC-DPB, Transcription
The Plant Cell, Vol. 18, 2224 2235, September 2006, www.plantcell.org ª 2006 American Society of Plant Biologists The Balance between Cell Division and Endoreplication Depends on E2FC-DPB, Transcription
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Plant Molecular and Cellular Biology Lecture 8: Mechanisms of Cell Cycle Control and DNA Synthesis Gary Peter
 Plant Molecular and Cellular Biology Lecture 8: Mechanisms of Cell Cycle Control and DNA Synthesis Gary Peter 9/10/2008 1 Learning Objectives Explain why a cell cycle was selected for during evolution
Plant Molecular and Cellular Biology Lecture 8: Mechanisms of Cell Cycle Control and DNA Synthesis Gary Peter 9/10/2008 1 Learning Objectives Explain why a cell cycle was selected for during evolution
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
The DP-E2F-like Gene DEL1 Controls the Endocycle in Arabidopsis thaliana
 Current Biology, Vol. 15, 59 63, January 11, 2005, 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.12.038 The DP-E2F-like Gene DEL1 Controls the Endocycle in Arabidopsis thaliana Kobe Vlieghe,
Current Biology, Vol. 15, 59 63, January 11, 2005, 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.12.038 The DP-E2F-like Gene DEL1 Controls the Endocycle in Arabidopsis thaliana Kobe Vlieghe,
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Principles of Genetics
 Principles of Genetics Snustad, D ISBN-13: 9780470903599 Table of Contents C H A P T E R 1 The Science of Genetics 1 An Invitation 2 Three Great Milestones in Genetics 2 DNA as the Genetic Material 6 Genetics
Principles of Genetics Snustad, D ISBN-13: 9780470903599 Table of Contents C H A P T E R 1 The Science of Genetics 1 An Invitation 2 Three Great Milestones in Genetics 2 DNA as the Genetic Material 6 Genetics
JMJ14-HA. Col. Col. jmj14-1. jmj14-1 JMJ14ΔFYR-HA. Methylene Blue. Methylene Blue
 Fig. S1 JMJ14 JMJ14 JMJ14ΔFYR Methylene Blue Col jmj14-1 JMJ14-HA Methylene Blue Col jmj14-1 JMJ14ΔFYR-HA Fig. S1. The expression level of JMJ14 and truncated JMJ14 with FYR (FYRN + FYRC) domain deletion
Fig. S1 JMJ14 JMJ14 JMJ14ΔFYR Methylene Blue Col jmj14-1 JMJ14-HA Methylene Blue Col jmj14-1 JMJ14ΔFYR-HA Fig. S1. The expression level of JMJ14 and truncated JMJ14 with FYR (FYRN + FYRC) domain deletion
Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang Chen, Qiang Dong, Jiangqi Wen, Kirankumar S. Mysore and Jian Zhao
 New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells
 Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Cell Cycle Regulation by Chlamydomonas Cyclin-Dependent Protein Kinases
 Plant Cell Advance Publication. Published on February 5, 2018, doi:10.1105/tpc.18.00103 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 IN BRIEF Cell Cycle Regulation by Chlamydomonas
Plant Cell Advance Publication. Published on February 5, 2018, doi:10.1105/tpc.18.00103 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 IN BRIEF Cell Cycle Regulation by Chlamydomonas
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
2. Yeast two-hybrid system
 2. Yeast two-hybrid system I. Process workflow a. Mating of haploid two-hybrid strains on YPD plates b. Replica-plating of diploids on selective plates c. Two-hydrid experiment plating on selective plates
2. Yeast two-hybrid system I. Process workflow a. Mating of haploid two-hybrid strains on YPD plates b. Replica-plating of diploids on selective plates c. Two-hydrid experiment plating on selective plates
TheA-TypeCyclinCYCA2;3IsaKeyRegulatorofPloidyLevels in Arabidopsis Endoreduplication W OA
 The Plant Cell, Vol. 18, 382 396, February 2006, www.plantcell.org ª 2006 American Society of Plant Biologists TheA-TypeCyclinCYCA2;3IsaKeyRegulatorofPloidyLevels in Arabidopsis Endoreduplication W OA
The Plant Cell, Vol. 18, 382 396, February 2006, www.plantcell.org ª 2006 American Society of Plant Biologists TheA-TypeCyclinCYCA2;3IsaKeyRegulatorofPloidyLevels in Arabidopsis Endoreduplication W OA
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
Cell Cycle Control in the Fission Yeast Schizosaccharomyces pombe
 Journal of Generul Microbiology ( 1989, 131, 2 123-2 127. Printed in Great Britain 2123 Cell Cycle Control in the Fission Yeast Schizosaccharomyces pombe The Tenth Fleming Lecture ByPAUL NURSE Imperial
Journal of Generul Microbiology ( 1989, 131, 2 123-2 127. Printed in Great Britain 2123 Cell Cycle Control in the Fission Yeast Schizosaccharomyces pombe The Tenth Fleming Lecture ByPAUL NURSE Imperial
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
RNA Synthesis and Processing
 RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011) /tpc
 Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011). 10.1105/tpc.110.080788 Supplemental Figure S1. Phylogenetic tree of MYC2-related proteins from Arabidopsis and other plants. Phenogram representation
Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011). 10.1105/tpc.110.080788 Supplemental Figure S1. Phylogenetic tree of MYC2-related proteins from Arabidopsis and other plants. Phenogram representation
Genetic Characterization and Functional Analysis of the GID1 Gibberellin Receptors in Arabidopsis W
 The Plant Cell, Vol. 18, 3399 3414, December 2006, www.plantcell.org ª 2006 American Society of Plant Biologists Genetic Characterization and Functional Analysis of the GID1 Gibberellin Receptors in Arabidopsis
The Plant Cell, Vol. 18, 3399 3414, December 2006, www.plantcell.org ª 2006 American Society of Plant Biologists Genetic Characterization and Functional Analysis of the GID1 Gibberellin Receptors in Arabidopsis
Three different fusions led to three basic ideas: 1) If one fuses a cell in mitosis with a cell in any other stage of the cell cycle, the chromosomes
 Section Notes The cell division cycle presents an interesting system to study because growth and division must be carefully coordinated. For many cells it is important that it reaches the correct size
Section Notes The cell division cycle presents an interesting system to study because growth and division must be carefully coordinated. For many cells it is important that it reaches the correct size
CELL CYCLE AND DIFFERENTIATION
 CELL CYCLE AND DIFFERENTIATION Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada d.purnomosari@ugm.ac.id WHAT IS CELL CYCLE? 09/12/14 d.purnomosari@ugm.ac.id
CELL CYCLE AND DIFFERENTIATION Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada d.purnomosari@ugm.ac.id WHAT IS CELL CYCLE? 09/12/14 d.purnomosari@ugm.ac.id
Quiz answers. Allele. BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 17: The Quiz (and back to Eukaryotic DNA)
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 17: The Quiz (and back to Eukaryotic DNA) http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Quiz answers Kinase: An enzyme
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 17: The Quiz (and back to Eukaryotic DNA) http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Quiz answers Kinase: An enzyme
Eukaryotic Gene Expression
 Eukaryotic Gene Expression Lectures 22-23 Several Features Distinguish Eukaryotic Processes From Mechanisms in Bacteria 123 Eukaryotic Gene Expression Several Features Distinguish Eukaryotic Processes
Eukaryotic Gene Expression Lectures 22-23 Several Features Distinguish Eukaryotic Processes From Mechanisms in Bacteria 123 Eukaryotic Gene Expression Several Features Distinguish Eukaryotic Processes
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING. AnitaHajdu. Thesis of the Ph.D.
 THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Dr. Fred Cross, Rockefeller (KITP Bio Networks 3/26/2003) 1
 Outline Cell growth as the driver for cell cycle (in microbes): coordination of growth and division A basic principle organizing cell cycle control: why cyclin-dependent kinase activity must oscillate
Outline Cell growth as the driver for cell cycle (in microbes): coordination of growth and division A basic principle organizing cell cycle control: why cyclin-dependent kinase activity must oscillate
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
National Institute of Biological Sciences, Beijing, Zhongguancun Life Science Park, Beijing , P.R. China b
 This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
GACE Biology Assessment Test I (026) Curriculum Crosswalk
 Subarea I. Cell Biology: Cell Structure and Function (50%) Objective 1: Understands the basic biochemistry and metabolism of living organisms A. Understands the chemical structures and properties of biologically
Subarea I. Cell Biology: Cell Structure and Function (50%) Objective 1: Understands the basic biochemistry and metabolism of living organisms A. Understands the chemical structures and properties of biologically
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
SUPPLEMENTARY INFORMATION
 PRC2 represses dedifferentiation of mature somatic cells in Arabidopsis Momoko Ikeuchi 1 *, Akira Iwase 1 *, Bart Rymen 1, Hirofumi Harashima 1, Michitaro Shibata 1, Mariko Ohnuma 1, Christian Breuer 1,
PRC2 represses dedifferentiation of mature somatic cells in Arabidopsis Momoko Ikeuchi 1 *, Akira Iwase 1 *, Bart Rymen 1, Hirofumi Harashima 1, Michitaro Shibata 1, Mariko Ohnuma 1, Christian Breuer 1,
AP Biology Unit 6 Practice Test 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8
 AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition
 Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplemental Data. Gao et al. (2012). Plant Cell /tpc
 Supplemental Figure 1. Plant EMP Proteins. (A) The Accession numbers of the 12 EMP members from Arabidopsis. (B) Phylogenetic analysis of EMP proteins from Arabidopsis, human and yeast using the Mac Vector
Supplemental Figure 1. Plant EMP Proteins. (A) The Accession numbers of the 12 EMP members from Arabidopsis. (B) Phylogenetic analysis of EMP proteins from Arabidopsis, human and yeast using the Mac Vector
Epigenetics and Flowering Any potentially stable and heritable change in gene expression that occurs without a change in DNA sequence
 Epigenetics and Flowering Any potentially stable and heritable change in gene expression that occurs without a change in DNA sequence www.plantcell.org/cgi/doi/10.1105/tpc.110.tt0110 Epigenetics Usually
Epigenetics and Flowering Any potentially stable and heritable change in gene expression that occurs without a change in DNA sequence www.plantcell.org/cgi/doi/10.1105/tpc.110.tt0110 Epigenetics Usually
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
The geneticist s questions
 The geneticist s questions a) What is consequence of reduced gene function? 1) gene knockout (deletion, RNAi) b) What is the consequence of increased gene function? 2) gene overexpression c) What does
The geneticist s questions a) What is consequence of reduced gene function? 1) gene knockout (deletion, RNAi) b) What is the consequence of increased gene function? 2) gene overexpression c) What does
Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus:
 m Eukaryotic mrna processing Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus: Cap structure a modified guanine base is added to the 5 end. Poly-A tail
m Eukaryotic mrna processing Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus: Cap structure a modified guanine base is added to the 5 end. Poly-A tail
Honors Biology Reading Guide Chapter 11
 Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
16 CONTROL OF GENE EXPRESSION
 16 CONTROL OF GENE EXPRESSION Chapter Outline 16.1 REGULATION OF GENE EXPRESSION IN PROKARYOTES The operon is the unit of transcription in prokaryotes The lac operon for lactose metabolism is transcribed
16 CONTROL OF GENE EXPRESSION Chapter Outline 16.1 REGULATION OF GENE EXPRESSION IN PROKARYOTES The operon is the unit of transcription in prokaryotes The lac operon for lactose metabolism is transcribed
Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm.
 Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm Paolo A. Sabelli a,1, Yan Liu a,2, Ricardo A. Dante a,3, Lucina E. Lizarraga
Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm Paolo A. Sabelli a,1, Yan Liu a,2, Ricardo A. Dante a,3, Lucina E. Lizarraga
Regulation of Gene Expression
 Chapter 18 Regulation of Gene Expression Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Chapter 18 Regulation of Gene Expression Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Last time: Obtaining information from a cloned gene
 Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
** * * * Col-0 cau1 CAU1. Actin2 CAS. Actin2. Supplemental Figure 1. CAU1 affects calcium accumulation.
 Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Chapter 15 Active Reading Guide Regulation of Gene Expression
 Name: AP Biology Mr. Croft Chapter 15 Active Reading Guide Regulation of Gene Expression The overview for Chapter 15 introduces the idea that while all cells of an organism have all genes in the genome,
Name: AP Biology Mr. Croft Chapter 15 Active Reading Guide Regulation of Gene Expression The overview for Chapter 15 introduces the idea that while all cells of an organism have all genes in the genome,
A redundant function for the N-terminal tail of Ndc80 in kinetochore-microtubule interaction in Saccharomyces cerevisiae.
 Genetics: Published Articles Ahead of Print, published on July 30, 2012 as 10.1534/genetics.112.143818 A redundant function for the N-terminal tail of Ndc80 in kinetochore-microtubule interaction in Saccharomyces
Genetics: Published Articles Ahead of Print, published on July 30, 2012 as 10.1534/genetics.112.143818 A redundant function for the N-terminal tail of Ndc80 in kinetochore-microtubule interaction in Saccharomyces
Role of an Atypical E2F Transcription Factor in the Control of Arabidopsis Cell Growth and Differentiation
 The Plant Cell, Vol. 16, 2350 2363, September 2004, www.plantcell.org ª 2004 American Society of Plant Biologists Role of an Atypical E2F Transcription Factor in the Control of Arabidopsis Cell Growth
The Plant Cell, Vol. 16, 2350 2363, September 2004, www.plantcell.org ª 2004 American Society of Plant Biologists Role of an Atypical E2F Transcription Factor in the Control of Arabidopsis Cell Growth
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
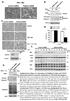 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Types of biological networks. I. Intra-cellurar networks
 Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Purification of yeast CKM. (a) Silver-stained SDS-PAGE analysis of CKM purified through a TAP-tag engineered into the Cdk8 C-terminus. (b) Kinase activity
Supplementary Figures Supplementary Figure 1. Purification of yeast CKM. (a) Silver-stained SDS-PAGE analysis of CKM purified through a TAP-tag engineered into the Cdk8 C-terminus. (b) Kinase activity
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds
 HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON
 PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root
 The Plant Journal (2009) 60, 666 678 doi: 10.1111/j.1365-313X.2009.03992.x The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root Lixia Huang 1,, Songguang
The Plant Journal (2009) 60, 666 678 doi: 10.1111/j.1365-313X.2009.03992.x The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root Lixia Huang 1,, Songguang
Reading Assignments. A. Systems of Cell Division. Lecture Series 5 Cell Cycle & Cell Division
 Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division
 Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Prof. Fahd M. Nasr. Lebanese university Faculty of sciences I Department of Natural Sciences.
 Prof. Fahd M. Nasr Lebanese university Faculty of sciences I Department of Natural Sciences fnasr@ul.edu.lb B3206 Microbial Genetics Eukaryotic M. G. The yeast Saccharomyces cerevisiae as a genetic model
Prof. Fahd M. Nasr Lebanese university Faculty of sciences I Department of Natural Sciences fnasr@ul.edu.lb B3206 Microbial Genetics Eukaryotic M. G. The yeast Saccharomyces cerevisiae as a genetic model
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supporting online material
 Supporting online material Materials and Methods Target proteins All predicted ORFs in the E. coli genome (1) were downloaded from the Colibri data base (2) (http://genolist.pasteur.fr/colibri/). 737 proteins
Supporting online material Materials and Methods Target proteins All predicted ORFs in the E. coli genome (1) were downloaded from the Colibri data base (2) (http://genolist.pasteur.fr/colibri/). 737 proteins
Development 143: doi: /dev : Supplementary information
 Supplementary Materials and Methods Plant materials The mutants and transgenic plants used in the present study were as follows: E361 (from Alex Webb s laboratory); tmm-1, ptmm::tmm-gfp and flp-1 (from
Supplementary Materials and Methods Plant materials The mutants and transgenic plants used in the present study were as follows: E361 (from Alex Webb s laboratory); tmm-1, ptmm::tmm-gfp and flp-1 (from
Lecture Series 5 Cell Cycle & Cell Division
 Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Division Read Chapter 19 pages 651-663 663 only (Benefits of Sex & Meiosis sections these are in Chapter
Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Division Read Chapter 19 pages 651-663 663 only (Benefits of Sex & Meiosis sections these are in Chapter
3.a.2- Cell Cycle and Meiosis
 Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. 3.a.2- Cell Cycle and Meiosis EU 3.A: Heritable information provides for continuity of life.
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. 3.a.2- Cell Cycle and Meiosis EU 3.A: Heritable information provides for continuity of life.
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular iology of the Cell Figure S1 Krüger et al. Arabidopsis Plasmodium H. sapiens* 1) Xenopus* 1) Drosophila C.elegans S.cerevisae S.pombe L.major T.cruzi T.brucei DCP5 CITH
Supplemental Materials Molecular iology of the Cell Figure S1 Krüger et al. Arabidopsis Plasmodium H. sapiens* 1) Xenopus* 1) Drosophila C.elegans S.cerevisae S.pombe L.major T.cruzi T.brucei DCP5 CITH
BIOLOGY. Chapter 10 CELL REPRODUCTION PowerPoint Image Slideshow
 BIOLOGY Chapter 10 CELL REPRODUCTION PowerPoint Image Slideshow FIGURE 10.1 A sea urchin begins life as a single cell that (a) divides to form two cells, visible by scanning electron microscopy. After
BIOLOGY Chapter 10 CELL REPRODUCTION PowerPoint Image Slideshow FIGURE 10.1 A sea urchin begins life as a single cell that (a) divides to form two cells, visible by scanning electron microscopy. After
A simple model for the eukaryotic cell cycle. Andrea Ciliberto
 A simple model for the eukaryotic cell cycle Andrea Ciliberto The cell division cycle G1 cell division Start S (DNA Replication) Finish M (mitosis) G2/M G2 Kohn, Mol. Biol. Cell., 1999 How did we get to
A simple model for the eukaryotic cell cycle Andrea Ciliberto The cell division cycle G1 cell division Start S (DNA Replication) Finish M (mitosis) G2/M G2 Kohn, Mol. Biol. Cell., 1999 How did we get to
Data Sheet. Azide Cy5 RNA T7 Transcription Kit
 Cat. No. Size 1. Description PP-501-Cy5 10 reactions à 40 µl For in vitro use only Quality guaranteed for 12 months Store all components at -20 C. Avoid freeze and thaw cycles. DBCO-Sulfo-Cy5 must be stored
Cat. No. Size 1. Description PP-501-Cy5 10 reactions à 40 µl For in vitro use only Quality guaranteed for 12 months Store all components at -20 C. Avoid freeze and thaw cycles. DBCO-Sulfo-Cy5 must be stored
Molecular Biology (9)
 Molecular Biology (9) Translation Mamoun Ahram, PhD Second semester, 2017-2018 1 Resources This lecture Cooper, Ch. 8 (297-319) 2 General information Protein synthesis involves interactions between three
Molecular Biology (9) Translation Mamoun Ahram, PhD Second semester, 2017-2018 1 Resources This lecture Cooper, Ch. 8 (297-319) 2 General information Protein synthesis involves interactions between three
Welcome to Class 21!
 Welcome to Class 21! Introductory Biochemistry! Lecture 21: Outline and Objectives l Regulation of Gene Expression in Prokaryotes! l transcriptional regulation! l principles! l lac operon! l trp attenuation!
Welcome to Class 21! Introductory Biochemistry! Lecture 21: Outline and Objectives l Regulation of Gene Expression in Prokaryotes! l transcriptional regulation! l principles! l lac operon! l trp attenuation!
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Prokaryotic Regulation
 Prokaryotic Regulation Control of transcription initiation can be: Positive control increases transcription when activators bind DNA Negative control reduces transcription when repressors bind to DNA regulatory
Prokaryotic Regulation Control of transcription initiation can be: Positive control increases transcription when activators bind DNA Negative control reduces transcription when repressors bind to DNA regulatory
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Transport between cytosol and nucleus
 of 60 3 Gated trans Lectures 9-15 MBLG 2071 The n GATED TRANSPORT transport between cytoplasm and nucleus (bidirectional) controlled by the nuclear pore complex active transport for macro molecules e.g.
of 60 3 Gated trans Lectures 9-15 MBLG 2071 The n GATED TRANSPORT transport between cytoplasm and nucleus (bidirectional) controlled by the nuclear pore complex active transport for macro molecules e.g.
Growth and development of Arabidopsis thaliana under single-wavelength red
 1 Supplementary Information 2 3 4 Growth and development of Arabidopsis thaliana under single-wavelength red and blue laser light 5 6 7 8 Authors Amanda Ooi 1 *, Aloysius Wong 1 *, Tien Khee Ng 2, Claudius
1 Supplementary Information 2 3 4 Growth and development of Arabidopsis thaliana under single-wavelength red and blue laser light 5 6 7 8 Authors Amanda Ooi 1 *, Aloysius Wong 1 *, Tien Khee Ng 2, Claudius
REVIEW SESSION. Wednesday, September 15 5:30 PM SHANTZ 242 E
 REVIEW SESSION Wednesday, September 15 5:30 PM SHANTZ 242 E Gene Regulation Gene Regulation Gene expression can be turned on, turned off, turned up or turned down! For example, as test time approaches,
REVIEW SESSION Wednesday, September 15 5:30 PM SHANTZ 242 E Gene Regulation Gene Regulation Gene expression can be turned on, turned off, turned up or turned down! For example, as test time approaches,
Review (V1): Phases of Cell Cycle
 Review (V1): Phases of Cell Cycle The cell cycle consists of 4 distinct phases: - G 1 phase, - S phase (synthesis), - G 2 phase - and M phase (mitosis). Interphase: combines G 1, S, and G 2 Activation
Review (V1): Phases of Cell Cycle The cell cycle consists of 4 distinct phases: - G 1 phase, - S phase (synthesis), - G 2 phase - and M phase (mitosis). Interphase: combines G 1, S, and G 2 Activation
Phenol-Chloroform reagents. Selection guide. OH ; MW : High quality reagents for use in nucleic acid purification.
 Phenol-Chloroform reagents Extraction with phenol and phenol/chloroform mixtures is a universal method for purification of DNA and RNA. Proteins and restriction enzymes are removed by phenol and chloroform
Phenol-Chloroform reagents Extraction with phenol and phenol/chloroform mixtures is a universal method for purification of DNA and RNA. Proteins and restriction enzymes are removed by phenol and chloroform
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
Optimization of the heme biosynthesis pathway for the production of. 5-aminolevulinic acid in Escherichia coli
 Supplementary Information Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli Junli Zhang 1,2,3, Zhen Kang 1,2,3, Jian Chen 2,3 & Guocheng Du 2,4
Supplementary Information Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli Junli Zhang 1,2,3, Zhen Kang 1,2,3, Jian Chen 2,3 & Guocheng Du 2,4
Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport
 Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Regulation of gene expression. Premedical - Biology
 Regulation of gene expression Premedical - Biology Regulation of gene expression in prokaryotic cell Operon units system of negative feedback positive and negative regulation in eukaryotic cell - at any
Regulation of gene expression Premedical - Biology Regulation of gene expression in prokaryotic cell Operon units system of negative feedback positive and negative regulation in eukaryotic cell - at any
Plant transformation
 Plant transformation Objectives: 1. What is plant transformation? 2. What is Agrobacterium? How and why does it transform plant cells? 3. How is Agrobacterium used as a tool in molecular genetics? References:
Plant transformation Objectives: 1. What is plant transformation? 2. What is Agrobacterium? How and why does it transform plant cells? 3. How is Agrobacterium used as a tool in molecular genetics? References:
Supplemental Figure 1. Phenotype of ProRGA:RGAd17 plants under long day
 Supplemental Figure 1. Phenotype of ProRGA:RGAd17 plants under long day conditions. Photo was taken when the wild type plant started to bolt. Scale bar represents 1 cm. Supplemental Figure 2. Flowering
Supplemental Figure 1. Phenotype of ProRGA:RGAd17 plants under long day conditions. Photo was taken when the wild type plant started to bolt. Scale bar represents 1 cm. Supplemental Figure 2. Flowering
Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells
 Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells By: Patrick Rutledge 1 Dr. Jennifer Lorang 2,3, Dr. Marc Curtis 2,3, Dr. Thomas Wolpert 2,3 BioResource Research 1, Botany and
Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells By: Patrick Rutledge 1 Dr. Jennifer Lorang 2,3, Dr. Marc Curtis 2,3, Dr. Thomas Wolpert 2,3 BioResource Research 1, Botany and
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Name: SBI 4U. Gene Expression Quiz. Overall Expectation:
 Gene Expression Quiz Overall Expectation: - Demonstrate an understanding of concepts related to molecular genetics, and how genetic modification is applied in industry and agriculture Specific Expectation(s):
Gene Expression Quiz Overall Expectation: - Demonstrate an understanding of concepts related to molecular genetics, and how genetic modification is applied in industry and agriculture Specific Expectation(s):
