The Singapore Copyright Act applies to the use of this document.
|
|
|
- Nelson Fisher
- 5 years ago
- Views:
Transcription
1 Title Use of analogy in teaching the particulate theory of matter Author(s) Boo Hong Kwen & Toh Kok Aun Source Teaching and Learning, 17(2),79-85 Published by Institute of Education (Singapore) This document may be used for private study or research purpose only. This document or any part of it may not be duplicated and/or distributed without permission of the copyright owner. The Singapore Copyright Act applies to the use of this document.
2 se of Analogy in Teaching The ParticULate Theory of Matter Boo Hong Kwen & Toh Kok Aun Over the past two decades, there has been considerable interest among science educators concerning the use of analogies in teaching science concepts, theories and models (Treagust et al. 1989; Duit 1991; Clement 1993). In the history of science, analogies have been tools of discovery and invention. For instance, Kepler developed his concepts of planetary motion from the workings of a clock (Bronowski 1973) and Huygens utilised movement of water waves to conceptualise the nature of light (Duit 1991). As suggested by Duit (1991), the term "analogy" refers to comparisons of structures between two domains; a domain familiar or known to the learner and a new domain. Treagust et al. (1992) further suggest that "an analogy is a relation between parts of the structures of two conceptual domains and may be viewed as a comparison statement on the grounds that these structures bear some resemblance to one another". In the context of science teaching, Treagust (1993) offers an operational definition of analogy as "a process of identifying similarities between two concepts. One concept, which is familiar, is referred to as the analog, and the other concept, which is unfamiliar, is called the target". The following are some advantages of analogies:
3 80 Teaching & Learning They are valuable tools in conceptual change learning. They provide visualisation and understanding of the abstract by pointing to similarities in the real world. They may incite pupils' interest and hence have a motivational effect. They force the teacher to take into consideration pupils' prior knowledge and may reveal misconceptions in previously taught topics. However, an analogy is a double-edged sword in that uncritical use of it may lead to misconceptions. This is because there is no such thing as a perfect analogy, that is, it is almost always impossible to have a perfect one-to-one fit between the analog and the target. Some pupils may only remember the analog and not the target, while others may focus on irrelevant aspects of the analog to form incorrect ideas about the target (Cosgrove and Osborne 1985; Glynn 1989; Duit 1991). One way of addressing problems associated with uncritical use of analogies is to adopt the six-step approach suggested by Harrison and Treagust (1994) which is based on a modification of Glynn's (1991) TWA (Teaching-With-Analogy) model. Although the six steps below are numbered from 1 to 6, the numbering does not necessarily reflect the order in which the steps are to be carried out. Each step is important but the order in which the steps are employed depends on the individual teacher's style, the particular target concept, the analogy being used and the lesson structure that the teacher has in mind. The six steps are as follows: 1. Introduce the target concept to be learned. 2. Cue the pupils' memory of the analog. 3. Identify the relevant features of the analog. 4. Map out the similarities between the analog and the target. 5. Indicate where the analogy breaks down.
4 Use of Analogy in Teaching Draw conclusions about the target concept. In the next section, an illustration of the use of this six-step approach in teaching the particulate theory of matter is given. This section illustrates the use of six-step approach in using analogy to teach the particulate theory of matter, which is crucial to understanding the way in which particles are relatedin the three states of matter; namely, the solid, liquid and gaseous states. Thus, if one were to adopt the above six-step approach, one could begin by introducing the relevant features of the particulate theory. This will include the following ideas concerning the target concept: Matter can exist in three physical states; namely, solid, liquid and gaseous. Matter is made up of particles which are in constant motion, resulting in their possession of kinetic energy which tends to keep them well spaced out in any substance. Solids have fixed shapes and volumes because particles in solids vibrate about fixed positions. Liquids have fixed volumes but no fixed shapes because the particles in liquids are able to slide over each other. In solids and liquids, particles are close enough to each other for the attractive forces between them to be important. In gases, there are no significant forces of attraction among the particles comprising the gas. This means that they are moving independently of each other in all directions and at great speeds. Because of their great speeds, a gas will rapidly spread out to fill any container into which it is placed and cannot be said to have a shape of its own. Having introduced the target concept to be learned, the next step could involve cueing pupils' memory of the analog. The teacher could say,
5 82 Teaching & Learning "Let's use this class as an analogy to make the three states of matter more real to us. Right now, as I am talking to you and explaining the particulate theory of matter, each of you are expected to stay at your own seats. You could be nodding your heads, moving yourpens, taking notes, raising your harzd and so forth, but you are not allowed to leave your seats. However, in the next half of this lesson, when you are doing your practical work, you are free to move around this laboratory, bzrt none of you can leave this room except under special circumstances. When the bell rings at the end of the school day, you are completely free to move. In other words, you would be leaving this room and heading in different directions for home." The next step could then involve identifying relevant features of the analog. Here, the teacher could hold before the pupils, a piece of ice cube and say, "This ice cube is made up of tiny particles called molecules. We are going to imagine that each pupil in this class is like a molecule of water in this ice cube. Remember, however, that the number of molecules in even a tiny piece of ice will contain many times more molecules than there are pupils in this class." (Step 5 - Here the teacher gives an instance of where the analogy breaks down.) Having identified relevant features of the analog, the teacher could then proceed to map out the similarities between the analog and the target. The teacher could say, "Solids have fixed shapes and volumes. This led scientists to think that each particle (molecule) has a fixed position. Thus, the particles in the solid state would be similar to a class of pupils like you are right now -sitting in your fixed positions, listening to meand taking notes. Each pupil has afixed position just like each molecule in water has a fixed position. Each molecule of water can vibrate about fixed positions but cannot move from one position to another. Similarly, you are not allowed to get up from your seats but you can move your arms, legs, necks and talk. In other words, you are like particles in the solid state vibrating about fixed positions." "When ice absorbs heat, it melts to form liquid water. Liquids have fixed volumes and they take the shapes of the container they are in (i.e. liquids have no fixed shapes) because the particles in the liquid are free to move within a fixed volume. This is somezuhat similar to this class when each of you is carrying out practical work. In this 'liquid' state, each of you is free to move anywhere within this room
6 Use of Analogy in Teaching 83 ('the container') but you cannot leave the room except under special 8, circumstances. "When liquid water absorbs heat, it evaporates to form water vapour, a gas. Gases have neither fixed volume nor shape. They completely fill their container and spread out to all parts of the enclosing vessel. This is like each of you at the end of the school day when you are no longer held close to each other (no attractive forces whatsoever between the particles in a gas) and you spread randomly throughout the container (in this case, the whole of Singaporeand perhaps, even Johore Bahru)." "Now, coming back to the case of the liquid state. When each of you is carrying out the practical work, think about the special circumstances such as when I send some of you on different errands. In this case, the pupils who leave the room are like molecules of water which occasionally gain sufficient energy to evaporate and leave the liquid surface. When pupils who are sent out on errands return to the class, they are like molecules which evaporate and condense back into liquid water." Having mapped out the similarities between the analog and the target, the teacher could proceed to address the limitations of the analogy or to indicate where the analogy breaks down. Such limitations could include the following: Molecules are very, very small and there would be many more water molecules in the ice cube than there are people on our planet. Particles in the solid and liquid states are closely packed, whereas pupils in class are not. Particles in the solid state vibrate continuously but pupils' movements are variable. Particles cannot think and hence, have no control over their movements whereas pupils do think and have control over their own movements. In the "solid and "liquid cases, the teacher in the class who is in control cannot be compared to anything in the target. In the gaseous state, particles have high instantaneous velocities
7 84 Teaching & Learning and are involved in collisions with other gas particles or with the walls of their container. Pupils after school are not likely to have high instantaneous velocities and are not likely to collide with each other (not even when they are playing vigorous games such as soccer, basketball or netball after school). The final step involved would be for the teacher to draw conclusions about the target concept. Here, the teacher could point out that, whilst it is impossible to visualise the behaviour of particles in the three different states, the analogy drawn on the movement of pupils in the class demonstrates that particles in the solid state vibrate about fixed positions resulting in fixed shape and volume; particles in the liquid state are mobile within a pre-determined volume resulting in fixed volume but variable shape; and particles in the gaseous state move independently of each other, resulting in variable volume and shape. Given the abstract nature of many scientific concepts, the above modified Teaching-With-Analogy approach should be included as part of the repertoire of approaches held by a Science teacher. The use of analogies in teaching is in line with well-known theories of learning such as Ausubel's which highlight the importance of prior knowledge of the learner and the importance of providing linkages between prior knowledge of the learner and the new knowledge to be acquired. The systematic use of the approach which ensures congruence between the way the teacher and the pupils visualise the analog and the target should address concerns about pupils' misconceptions through lack of good understanding of the analogy and through over-extension of irrelevant features of the analog to the target.
8 Use of Analogy in Teaching 85 REFERENCES Bronowski, J. (1973). The Ascent of Man. London: British broadcasting corporation. Clement, J. (1993). Using bridging analogies and anchoring intuitions to deal with students' preconceptions in physics. Journal of Research in Science Teaching, 30 (10): Cosgrove, M. & Osborne, R. (1985). A teaching sequence on electric current. In R. Osborne & P. Freyberg (eds.), Learning in Science: The Implications of Children's Science. Auckland: Heinemann. Duit, R. (1991). On the role of analogies, similes and metaphors in learning science. Science Education, 75: Glynn, S. (1989). The teaching with analogies (TWA) model: Explaining concepts in expository texts. In K.D. Muth (ed.), Children's Comprehension of Narrative and Expository Text: Research into Practice. Newark DE: International Reading Association. Glynn, S. (1991). Explaining science concepts: A teaching-with-analogies model. In S. Glynn, R. Yeany & B. Britton (eds.) The Psychology of Learning Science. Hillsdale, N.J.: Erlbaum Associates. Harrision, A. & Treagust, D. (1994). The three states of matter are like students at school. Atistvalian Science Teachers fournal, 40 (2): Treagust, D., Duit, R., Lindauer, I. & Joslin, P. (1989). Teachers' use of analogies in their regular routines. Research in Science Education, 19: Treagust, D., Duit, R., Joslin, P. & Lindauer, I. (1992). Science teachers' use of analogies: Observations from classroom practice. International Journal of Science Education, 14(4): Treagust, D. (1993). The evolution of an approach for using analogies in teaching & learning science. Research in Science Education, 23:
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
TEACHER NOTES: ICE CUBE POSTER
 TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
States of Matter. What physical changes and energy changes occur as matter goes from one state to another?
 Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Hot and Cold Balloons
 Hot and Cold Balloons Moira filled a balloon with air. She tightly tied the balloon so no air could get in or out of the balloon. She kept the balloon in a warm room. An hour later she put the balloon
Hot and Cold Balloons Moira filled a balloon with air. She tightly tied the balloon so no air could get in or out of the balloon. She kept the balloon in a warm room. An hour later she put the balloon
Physical Science Exam 3 Study Guide. Dr. Karoline Rostamiani. Chapter 3
 Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
INTRODUCTION TO LESSON CLUSTER 7
 INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
UNIT 2 The Particulate Nature of Matter
 UNIT 2 The Particulate Nature of Matter Take a moment to think about all of the different properties which can be exhibited by matter. The list seems endless. Matter can be found in a variety of forms,
UNIT 2 The Particulate Nature of Matter Take a moment to think about all of the different properties which can be exhibited by matter. The list seems endless. Matter can be found in a variety of forms,
Chapter Preview. Improving Comprehension
 Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
Notes: Phases of Matter and Phase Changes
 Name: Date: IP 670 Notes: Phases of Matter and Phase Changes There are four main phases of matter: We are only going to talk about the first three today. Solids Liquids Gases Molecular Molecules Wiggle
Name: Date: IP 670 Notes: Phases of Matter and Phase Changes There are four main phases of matter: We are only going to talk about the first three today. Solids Liquids Gases Molecular Molecules Wiggle
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
Chapter 3. States of Matter
 Chapter 3 States of Matter 1. Solid 2. Liquid 3. Gas States of Matter Two More (discuss later) Plasma Bose-Einstein condensate States of Matter Solid (definite shape and volume) Particles are tightly packed
Chapter 3 States of Matter 1. Solid 2. Liquid 3. Gas States of Matter Two More (discuss later) Plasma Bose-Einstein condensate States of Matter Solid (definite shape and volume) Particles are tightly packed
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
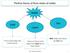 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Gases: Properties and Behaviour
 SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
The Singapore Copyright Act applies to the use of this document.
 Title The development of a two-tier multiple choice diagnostic instrument to identify secondary three and four students (14-17 years old) alternative conceptions in chemical bonding Author(s) Tan Kim Chwee,
Title The development of a two-tier multiple choice diagnostic instrument to identify secondary three and four students (14-17 years old) alternative conceptions in chemical bonding Author(s) Tan Kim Chwee,
Developing an understanding of particle theory
 The National Strategies Secondary Developing an understanding of particle theory 1 For pupils to understand the particle theory properly we need to: teach a simple model challenge pupils to use the model
The National Strategies Secondary Developing an understanding of particle theory 1 For pupils to understand the particle theory properly we need to: teach a simple model challenge pupils to use the model
6-3 Particle model of matter Trilogy
 6-3 Particle model of matter Trilogy.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Trilogy.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
Task E. Make the following points:
 Using models to teach the particle theory Make the following points: 20 minutes Research has shown that there are differences in the ways teachers use models to explain particles, even within a single
Using models to teach the particle theory Make the following points: 20 minutes Research has shown that there are differences in the ways teachers use models to explain particles, even within a single
CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS
 CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Particle nature of Matter *** 2. States of Matter **** 3. Interchange in states of Matter
CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Particle nature of Matter *** 2. States of Matter **** 3. Interchange in states of Matter
ENERGY IN CHEMISTRY. R. Ashby Duplication by permission only.
 CH 11 TOPIC 28 CHANGING STATES OF MATTER 1 You have mastered this topic when you can: 1) define or describe: ENERGY, POTENTIAL ENERGY, KINETIC ENERGY & KINETIC MOLECULAR THEORY 2) define or describe HEAT
CH 11 TOPIC 28 CHANGING STATES OF MATTER 1 You have mastered this topic when you can: 1) define or describe: ENERGY, POTENTIAL ENERGY, KINETIC ENERGY & KINETIC MOLECULAR THEORY 2) define or describe HEAT
Solids, liquids and gases
 Solids, liquids and gases Duration 60 minutes Lesson overview Students share what they know about the three states of matter solid, liquid and gas and consider some of their scientific properties. They
Solids, liquids and gases Duration 60 minutes Lesson overview Students share what they know about the three states of matter solid, liquid and gas and consider some of their scientific properties. They
Chemistry A: States of Matter Packet Name: Hour: Page!1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
4 Discuss and evaluate the 5th state of matter. 3 - Differentiate among the four states of matter in terms of energy,
 Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
CHM Solids, Liquids, and Phase Changes (r15) Charles Taylor 1/9
 CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
Chapter 7.1. States of Matter
 Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
6-3 Particle model of matter Physics
 6-3 Particle model of matter Physics.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Physics.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
Copyright 2008 NSTA. All rights reserved. For more information, go to Pennies
 Pennies A shiny new penny is made up of atoms. Put an X next to all the things on the list that describe the atoms that make up the shiny new penny. hard soft I N G O D L I B E R T Y W E T R U S T 2 0
Pennies A shiny new penny is made up of atoms. Put an X next to all the things on the list that describe the atoms that make up the shiny new penny. hard soft I N G O D L I B E R T Y W E T R U S T 2 0
Solids (cont.) Describe the movement of particles in a solid and the forces between them.
 Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
INTRODUCTION TO LESSON CLUSTER 8 Explaining Evaporation and Boiling
 INTRODUCTION TO LESSON CLUSTER 8 Explaining Evaporation and Boiling A. Lesson Cluster Goals and Lesson Objectives Goals: Students should be able to explain evaporation and boiling, both in macroscopic
INTRODUCTION TO LESSON CLUSTER 8 Explaining Evaporation and Boiling A. Lesson Cluster Goals and Lesson Objectives Goals: Students should be able to explain evaporation and boiling, both in macroscopic
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES
 THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
Key concept (age 11-14) BCL1.4: Diffusion and the cell membrane
 Biology> Big idea BCL: The cellular basis of life > Topic BCL1: Cells Key concept (age 11-14) BCL1.4: Diffusion and the cell membrane What s the big idea? A big idea in biology is that organisms are made
Biology> Big idea BCL: The cellular basis of life > Topic BCL1: Cells Key concept (age 11-14) BCL1.4: Diffusion and the cell membrane What s the big idea? A big idea in biology is that organisms are made
SCIENCE STUDENT BOOK. 11th Grade Unit 3
 SCIENCE STUDENT BOOK 11th Grade Unit 3 Unit 3 GASES AND MOLES SCIENCE 1103 GASES AND MOLES INTRODUCTION 3 1. KINETIC MOLECULAR THEORY 5 EVIDENCE 5 CHARACTERISTICS 7 SELF TEST 1 14 2. BOYLE S LAW 16 EXPERIMENTAL
SCIENCE STUDENT BOOK 11th Grade Unit 3 Unit 3 GASES AND MOLES SCIENCE 1103 GASES AND MOLES INTRODUCTION 3 1. KINETIC MOLECULAR THEORY 5 EVIDENCE 5 CHARACTERISTICS 7 SELF TEST 1 14 2. BOYLE S LAW 16 EXPERIMENTAL
THE GASEOUS STATE OF MATTER
 THE GASEOUS STATE OF MATTER The gaseous state of matter is a form of matter in which the particles are in a high state of energy, which causes them to vibrate rapidly, experiencing a strong repulsion among
THE GASEOUS STATE OF MATTER The gaseous state of matter is a form of matter in which the particles are in a high state of energy, which causes them to vibrate rapidly, experiencing a strong repulsion among
Chapter 10 States of Matter
 Chapter 10 States of Matter 1 Section 10.1 The Nature of Gases Objectives: Describe the assumptions of the kinetic theory as it applies to gases. Interpret gas pressure in terms of kinetic theory. Define
Chapter 10 States of Matter 1 Section 10.1 The Nature of Gases Objectives: Describe the assumptions of the kinetic theory as it applies to gases. Interpret gas pressure in terms of kinetic theory. Define
States of matter. 22 Science Alive for VELS Level 5
 States of matter E verything around you is made of matter. Anything that has mass and takes up space is matter. The air we breathe, the water we drink and the food we eat are all different types of matter.
States of matter E verything around you is made of matter. Anything that has mass and takes up space is matter. The air we breathe, the water we drink and the food we eat are all different types of matter.
The Singapore Copyright Act applies to the use of this document.
 Title The use of venn diagrams in chemistry teaching Author(s) Tan, Jason Source Teaching and Learning, 7(2)71-75 Published by Institute of Education (Singapore) This document may be used for private study
Title The use of venn diagrams in chemistry teaching Author(s) Tan, Jason Source Teaching and Learning, 7(2)71-75 Published by Institute of Education (Singapore) This document may be used for private study
Chemistry A: States of Matter Packet Name: Hour: Page 1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Matter, Atoms & Molecules
 Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
KINETIC PARTICLE THEORY
 KINETIC PARTICLE THEORY IMPORTANT DEFINITIONS: The mixing process in gases or solutions due to the random motion of particles is called Diffusion. The process by which a liquid changes into a vapour at
KINETIC PARTICLE THEORY IMPORTANT DEFINITIONS: The mixing process in gases or solutions due to the random motion of particles is called Diffusion. The process by which a liquid changes into a vapour at
Chemistry Day 5. Friday, August 31 st Tuesday, September 4 th, 2018
 Chemistry Day 5 Friday, August 31 st Tuesday, September 4 th, 2018 Do-Now Title: BrainPOP: States of Matter 1. Write down today s FLT 2. List two examples of gases 3. List two examples of things that are
Chemistry Day 5 Friday, August 31 st Tuesday, September 4 th, 2018 Do-Now Title: BrainPOP: States of Matter 1. Write down today s FLT 2. List two examples of gases 3. List two examples of things that are
Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion.
 Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Copyright 2015 Edmentum - All rights reserved. During which of the following phase changes is there a gain in energy? I.
 Study Island Copyright 2015 Edmentum - All rights reserved. Generation Date: 03/16/2015 Generated By: Kristina Brown 1. Examine the phase-change diagram below. During which of the following phase changes
Study Island Copyright 2015 Edmentum - All rights reserved. Generation Date: 03/16/2015 Generated By: Kristina Brown 1. Examine the phase-change diagram below. During which of the following phase changes
THE CORPUSCULAR NATURE OF MATTER AND ITS PHYSICAL STATES
 THE CORPUSCULAR NATURE OF MATTER AND ITS PHYSICAL STATES In this unit we are going to study the matter from a microscopic point of view using the kinetic theory. We will understand the properties of the
THE CORPUSCULAR NATURE OF MATTER AND ITS PHYSICAL STATES In this unit we are going to study the matter from a microscopic point of view using the kinetic theory. We will understand the properties of the
Chapter: Heat and States
 Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Physical Science and Nature of Science Assessment Probes
 Physical Science and Nature of Science Assessment Probes Concept Matrix...6 Pennies...7 2 Is It a Solid?...25 3 Thermometer...33. 4 Floating Balloon... 39 5 Hot and Cold Balloons...45 6 Mirror on the Wall...5
Physical Science and Nature of Science Assessment Probes Concept Matrix...6 Pennies...7 2 Is It a Solid?...25 3 Thermometer...33. 4 Floating Balloon... 39 5 Hot and Cold Balloons...45 6 Mirror on the Wall...5
Solid Liquid Gas 1. Solids have a fixed volume and a definite shape.
 1 MATTER:- Anything or everything which occupies space and has mass is called matter. This word is used to cover all the substances and the material from which the universe is made. For example, the air
1 MATTER:- Anything or everything which occupies space and has mass is called matter. This word is used to cover all the substances and the material from which the universe is made. For example, the air
States of Matter. Changes in State
 CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
heat By cillian bryan and scott doyle
 heat By cillian bryan and scott doyle What is heat Heat energy is the result of the movement of tiny particles called atoms molecules or ions in solids, liquids and gases. Heat energy can be transferred
heat By cillian bryan and scott doyle What is heat Heat energy is the result of the movement of tiny particles called atoms molecules or ions in solids, liquids and gases. Heat energy can be transferred
Properties of Matter BEFORE READING BUILD BACKGROUND PREVIEW TEXT AND GRAPHICS
 READING FOCUS SKILLS: Main Idea and Details, Cause and Effect VOCABULARY: density, gas, liquid, mass, matter, mixture, solid, solubility, solution, state of matter, suspension, volume EXTENSIONS: Writing,
READING FOCUS SKILLS: Main Idea and Details, Cause and Effect VOCABULARY: density, gas, liquid, mass, matter, mixture, solid, solubility, solution, state of matter, suspension, volume EXTENSIONS: Writing,
What Is The Matter? Matter Concept Map
 What Is The Matter? Matter Concept Map Phases of Matter Solid Liquid Gas Use the selections below to complete the concept map for the phases of matter. Write the letter of each characteristic in the appropriate
What Is The Matter? Matter Concept Map Phases of Matter Solid Liquid Gas Use the selections below to complete the concept map for the phases of matter. Write the letter of each characteristic in the appropriate
CBSE Class 9 Science Revision Notes CHAPTER 1 Matter in our Surroundings
 CBSE Class 9 Science Revision Notes CHAPTER 1 Matter in our Surroundings Anything that occupies space and has mass and is felt by senses is called matter. According to indian ancient philosphor, matter
CBSE Class 9 Science Revision Notes CHAPTER 1 Matter in our Surroundings Anything that occupies space and has mass and is felt by senses is called matter. According to indian ancient philosphor, matter
5.4 The Kinetic Molecular Theory and Changes of State
 5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
Matter is made of atoms and molecules
 Name Per Talking to the Text Atoms and Molecules pt.2 Author Says (important ideas, vocabulary) Matter is made of atoms and molecules We have already used the term atom and molecule a couple of times.
Name Per Talking to the Text Atoms and Molecules pt.2 Author Says (important ideas, vocabulary) Matter is made of atoms and molecules We have already used the term atom and molecule a couple of times.
States of Matter in Food
 Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
OUTLINE. States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry
 UNIT 6 GASES OUTLINE States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry STATES OF MATTER Remember that all matter exists in three physical states: Solid Liquid
UNIT 6 GASES OUTLINE States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry STATES OF MATTER Remember that all matter exists in three physical states: Solid Liquid
Chapter: States of Matter
 Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
MS-PS1 Matter and Its Interactions
 MS-PS1 Matter and Its Interactions Key Ideas and Common Student Misconceptions This document compiles Key Ideas and developed by AAAS Project 2061 and the National Science Foundation. For additional information
MS-PS1 Matter and Its Interactions Key Ideas and Common Student Misconceptions This document compiles Key Ideas and developed by AAAS Project 2061 and the National Science Foundation. For additional information
DOWNLOAD PDF SOLIDS, LIQUIDS, AND GASES AT HOME
 Chapter 1 : What are some examples of gases around the house? Yahoo Answers Naming examples of solids, liquids, and gases is a common homework assignment because it makes you think about phase changes
Chapter 1 : What are some examples of gases around the house? Yahoo Answers Naming examples of solids, liquids, and gases is a common homework assignment because it makes you think about phase changes
Knowing Your Audience:
 Knowing Your Audience: Astronomy Misconceptions by the Public Ka Chun Yu Curator of Space Science Denver Museum of Nature & Science 2001 Colorado Blvd. Denver, CO 80205 (DMNS) 1 / 36 Introduction What
Knowing Your Audience: Astronomy Misconceptions by the Public Ka Chun Yu Curator of Space Science Denver Museum of Nature & Science 2001 Colorado Blvd. Denver, CO 80205 (DMNS) 1 / 36 Introduction What
SCH 3UI Unit 08 Outline: Kinetic Molecular Theory and the Gas Laws. The States of Matter Characteristics of. Solids, Liquids and Gases
 SCH 3UI Unit 08 Outline: Kinetic Molecular Theory and the Gas Laws Lesson Topics Covered Handouts to Print 1 Note: The States of Matter solids, liquids and gases state and the polarity of molecules the
SCH 3UI Unit 08 Outline: Kinetic Molecular Theory and the Gas Laws Lesson Topics Covered Handouts to Print 1 Note: The States of Matter solids, liquids and gases state and the polarity of molecules the
Foundations of Chemistry
 Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
Matter. Energy- which is a property of matter!! Matter: anything that takes up space and has mass
 Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!! Matter is made up of moving particles! Instead of
Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!! Matter is made up of moving particles! Instead of
Matter & Energy. Objectives: properties and structures of the different states of matter.
 Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4
 Cumulative Test 1 Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4 1. Why can you change ice into water but not into glass? 2. Why can't you see air? 3. Describe the ways in which ice, liquid water, and
Cumulative Test 1 Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4 1. Why can you change ice into water but not into glass? 2. Why can't you see air? 3. Describe the ways in which ice, liquid water, and
Matter and Its Changes
 Matter and Its Changes Matter: anything that has mass and occupies space (has volume) anything that has inertia and requires a force to start or stop it from moving Observation: gathering information using
Matter and Its Changes Matter: anything that has mass and occupies space (has volume) anything that has inertia and requires a force to start or stop it from moving Observation: gathering information using
Perfect Guide. Notes. 2nd Edition. Ryan Bong
 Perfect Guide `O Level PHYSICS PHYSICS Notes 2nd Edition Ryan Bong 72 Preface PERFECT GUIDE TO O LEVEL PHYSICS NOTES (2nd Edition) is a study aid for the G.C.E. O Level Physics examination. With comprehensive
Perfect Guide `O Level PHYSICS PHYSICS Notes 2nd Edition Ryan Bong 72 Preface PERFECT GUIDE TO O LEVEL PHYSICS NOTES (2nd Edition) is a study aid for the G.C.E. O Level Physics examination. With comprehensive
Chapter 8. Chapter 8. Preview. Bellringer. Chapter 8. Particles of Matter. Objectives. Chapter 8. Particles of Matter, continued
 States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
Matter and Its Properties. Unit 2
 Matter and Its Properties Unit 2 Lesson 1: Physical & Chemical Properties & Changes Unit 2: Matter and Its Properties Section 1: Physical Properties & Change Lesson 1: Physical & Chemical Properties &
Matter and Its Properties Unit 2 Lesson 1: Physical & Chemical Properties & Changes Unit 2: Matter and Its Properties Section 1: Physical Properties & Change Lesson 1: Physical & Chemical Properties &
IES LAURETUM SCIENCE NAME.
 IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
Core Chemistry UNIT 1: Matter & Energy Section 1: The Law of Conservation of Mass Section 2: States of Matter & Intro to Thermodynamics
 Core Chemistry UNIT 1: Matter & Energy Section 1: The Law of Conservation of Mass Section 2: States of Matter & Intro to Thermodynamics UNIT 1 Synapsis In our first unit we will explore matter & energy
Core Chemistry UNIT 1: Matter & Energy Section 1: The Law of Conservation of Mass Section 2: States of Matter & Intro to Thermodynamics UNIT 1 Synapsis In our first unit we will explore matter & energy
Term Info Picture. Anything that has mass and takes up space; everything is made of matter.
 Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
SPH3U1 Lesson 03 Energy
 THERMAL ENERGY AND LATENT HEAT LEARNING GOALS Students will learn: Heat changes the amount of thermal energy in an object Temperature is a measure of the average thermal energy in an object Heat capacity
THERMAL ENERGY AND LATENT HEAT LEARNING GOALS Students will learn: Heat changes the amount of thermal energy in an object Temperature is a measure of the average thermal energy in an object Heat capacity
States of Matter 1 of 21 Boardworks Ltd 2016
 States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
The Physical Properties And Physical Changes of Substances
 The Physical Properties And Physical Changes of Substances A. Definitions In Science 1. Science is the observation, identification, description, experimental investigation, and theoretical explanation
The Physical Properties And Physical Changes of Substances A. Definitions In Science 1. Science is the observation, identification, description, experimental investigation, and theoretical explanation
Chapter 3. Preview. Section 1 Three States of Matter. Section 2 Behavior of Gases. Section 3 Changes of State. States of Matter.
 States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
Properties of Matter
 Grade 7 Science, Quarter 1, Unit 1.1 Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Identify different substances using data about characteristic
Grade 7 Science, Quarter 1, Unit 1.1 Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Identify different substances using data about characteristic
PSTs mental models about role and distribution of ozone layer and ozone layer depletion
 PSTs mental models about role and distribution of ozone layer and ozone layer depletion 1. Subject and Problem In the last two hundred years, human activities have a harmful effect on Earth. Several environmental
PSTs mental models about role and distribution of ozone layer and ozone layer depletion 1. Subject and Problem In the last two hundred years, human activities have a harmful effect on Earth. Several environmental
What's Up, Earth? Header Insert Image 1 here, right justified to wrap. Grade Level. 3rd. Time Required: 60 minutes
 What's Up, Earth? Header Insert Image 1 here, right justified to wrap Image 1 ADA Description:? Caption:? Image file path:? Source/Rights: Copyright? Grade Level 3rd Time Required: 60 minutes Group Size:
What's Up, Earth? Header Insert Image 1 here, right justified to wrap Image 1 ADA Description:? Caption:? Image file path:? Source/Rights: Copyright? Grade Level 3rd Time Required: 60 minutes Group Size:
Creative Classroom Lessons
 Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA SCIENCE FOR TECHNICIANS OUTCOME 3 - ENERGY TUTORIAL 2 HEAT
 EDEXCEL NATIONAL CERTIFICATE/DIPLOMA SCIENCE FOR TECHNICIANS OUTCOME 3 - ENERGY TUTORIAL 2 HEAT 3. Energy Mechanical work, energy and power: work - energy relationship, gravitational potential energy,
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA SCIENCE FOR TECHNICIANS OUTCOME 3 - ENERGY TUTORIAL 2 HEAT 3. Energy Mechanical work, energy and power: work - energy relationship, gravitational potential energy,
LESSON 6: Dew Drops ESTIMATED TIME Setup: 5 10 minutes Procedure: minutes
 LESSON 6: Dew Drops ESTIMATED TIME Setup: 5 10 minutes Procedure: 15 20 minutes DESCRIPTION Use jars of hot and cold water to demonstrate how water changes states. OBJECTIVE This lesson demonstrates the
LESSON 6: Dew Drops ESTIMATED TIME Setup: 5 10 minutes Procedure: 15 20 minutes DESCRIPTION Use jars of hot and cold water to demonstrate how water changes states. OBJECTIVE This lesson demonstrates the
Kinetic Theory of Matter notes 2012
 Kinetic Theory of Matter notes 2012 Kinetic Theory of Matter 3 parts: 1) All matter is made up of and that act as tiny 2) These tiny particles are always in. State of matter depends on its molecular motion
Kinetic Theory of Matter notes 2012 Kinetic Theory of Matter 3 parts: 1) All matter is made up of and that act as tiny 2) These tiny particles are always in. State of matter depends on its molecular motion
2 Changes of State KEY IDEAS READING TOOLBOX ADDING AND REMOVING ENERGY. States of Matter. As you read this section, keep these questions in mind:
 CHAPTER 3 States of Matter 2 Changes of State SECTION KEY IDEAS As you read this section, keep these questions in mind: What happens when a substance changes from one state of matter to another? What happens
CHAPTER 3 States of Matter 2 Changes of State SECTION KEY IDEAS As you read this section, keep these questions in mind: What happens when a substance changes from one state of matter to another? What happens
Name: New Document 1. Class: Date: 83 minutes. Time: 82 marks. Marks: Comments:
 New Document Name: Class: Date: Time: 83 minutes Marks: 82 marks Comments: Q. Solid, liquid and gas are three different states of matter. (a) Describe the difference between the solid and gas states, in
New Document Name: Class: Date: Time: 83 minutes Marks: 82 marks Comments: Q. Solid, liquid and gas are three different states of matter. (a) Describe the difference between the solid and gas states, in
Its Properties. Its Changes
 Chapter Introduction Lesson 1 Lesson 2 Chapter Wrap-Up Matter and Its Properties Matter and Its Changes What gives a substance its unique identity? What do you think? Before you begin, decide if you agree
Chapter Introduction Lesson 1 Lesson 2 Chapter Wrap-Up Matter and Its Properties Matter and Its Changes What gives a substance its unique identity? What do you think? Before you begin, decide if you agree
Activity Template. Drexel-SDP GK-12 ACTIVITY
 Activity Template Drexel-SDP GK-12 ACTIVITY Subject Area(s): Sound Associated Unit: None Associated Lesson: None Activity Title: Density and Pitch, is there a relationship? Grade Level: 8 (7-9) Activity
Activity Template Drexel-SDP GK-12 ACTIVITY Subject Area(s): Sound Associated Unit: None Associated Lesson: None Activity Title: Density and Pitch, is there a relationship? Grade Level: 8 (7-9) Activity
Learning Goals and Assessments in IQWST
 Learning Goals and Assessments in IQWST Joseph Krajcik Michigan State University Workshop on Developing Assessments to Meet the Goals of the 2012 Framework for K-12 Science Education September 13, 2012
Learning Goals and Assessments in IQWST Joseph Krajcik Michigan State University Workshop on Developing Assessments to Meet the Goals of the 2012 Framework for K-12 Science Education September 13, 2012
C1a The particulate nature of matter
 C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
Rashid School for Boys. Year 7 Science. Particles. Name: Form:
 Rashid School for Boys Year Science Particles Name: Form: 1 By the end of this topic.. Unit Particles Level 3 I know that ice melts when it gets too warm and that liquid water turns into solid water (ice)
Rashid School for Boys Year Science Particles Name: Form: 1 By the end of this topic.. Unit Particles Level 3 I know that ice melts when it gets too warm and that liquid water turns into solid water (ice)
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Grade Level 3rd Melting, Freezing, and More!: Phase Transitions Steven Scroggins, Ailey Crow, Tom Holcombe, and Terence Choy California
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Grade Level 3rd Melting, Freezing, and More!: Phase Transitions Steven Scroggins, Ailey Crow, Tom Holcombe, and Terence Choy California
5th Grade. Slide 1 / 67. Slide 2 / 67. Slide 3 / 67. Matter and Its Interactions. Table of Contents: Matter and Its Interactions
 Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Post-Show HOT AND COLD. Gases. Liquids. Solids. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Chapter 3 Phases of Matter Physical Science
 Chapter 3 Phases of Matter Physical Science CH 3- States of Matter 1 What makes up matter? What is the difference between a solid, a liquid, and a gas? What kind of energy do all particles of matter have?
Chapter 3 Phases of Matter Physical Science CH 3- States of Matter 1 What makes up matter? What is the difference between a solid, a liquid, and a gas? What kind of energy do all particles of matter have?
Standards Alignment... 5 Safe Science... 9 Scientific Inquiry... 11
 Standards Alignment... 5 Safe Science... 9 Scientific Inquiry... 11 Properties of Matter Let Me Count the Ways... 15 A Bear Eggs-pedition...25 Color Match...33 Going Nuts...39 Soup-er Floaters and Sinkers...55
Standards Alignment... 5 Safe Science... 9 Scientific Inquiry... 11 Properties of Matter Let Me Count the Ways... 15 A Bear Eggs-pedition...25 Color Match...33 Going Nuts...39 Soup-er Floaters and Sinkers...55
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
LESSON PLAN-1 T1-Session
 LESSON PLAN-1 T1-Session 2015-2016 For the month of March Class : IX Subject : Chemistry Theme: Matter in our surroundings Periods: (10) Theory: (7) Practical:(3) Objectives (Concepts & Skills) Basic Concept
LESSON PLAN-1 T1-Session 2015-2016 For the month of March Class : IX Subject : Chemistry Theme: Matter in our surroundings Periods: (10) Theory: (7) Practical:(3) Objectives (Concepts & Skills) Basic Concept
Satish Chandra. Unit I, REAL GASES. Lecture Notes Dated: Dec 08-14, Vander-Waals Gas
 Vander-Waals Gas Lecture Notes Dated: Dec 08-14, 01 Many equations have been proposed which describe the pvt relations of real gases more accurately than does the equation of state of an ideal gas. Some
Vander-Waals Gas Lecture Notes Dated: Dec 08-14, 01 Many equations have been proposed which describe the pvt relations of real gases more accurately than does the equation of state of an ideal gas. Some
