Matter. Energy- which is a property of matter!! Matter: anything that takes up space and has mass
|
|
|
- Nathaniel George
- 5 years ago
- Views:
Transcription
1
2 Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!!
3 Matter is made up of moving particles! Instead of using the word moving, we use the kinetic because it is a science word for movement!
4 We have 1 main Theory for this unit! And we call it : The Kinetic Theory of Matter! The theory states that All matter is made of tiny constantly moving particles. This is because all matter is made of moving particles. The motion of the particles controls what the matter looks like!
5 Matter looks like 4 different things, and we call them States of Matter
6 Here are the 4 States of Matter we will discuss this unit 1.Solid 2.Liquid 3.Gas 4.Plasma
7 Kinetic Theory of Matter Matter will become any or sometimes all of these States because they are controlled by temperature
8 Temperature A physical property of matter that determines how much heat energy an object can contain. An example of this energy is how hot or cold an object can become.
9
10 Lets begin with Solids Definite shape and volume Slow vibration of particles The bonded particles vibrate within the substance but lack enough energy to break free
11 There are 2 types of solids: 1. Crystalline 2. Amorphous
12 Solids Crystals: a solid whose particles are arranged in repeating geometric patterns
13 Solids Amorphous solids Particles do not have a repeating pattern
14 State of Matter: Liquids No definite shape: takes the shape of its container Definite volume Medium vibration of particles The particles have enough energy to break the bonds holding them together, not enough energy to escape from each other
15 Liquids The particles of a liquid can move pass one another in a process called flow or pour (flow can be any direction while pour is under the influence of gravity)
16 There is 1 main principal you need to remember about liquids: * Archimedes principle: The buoyancy force on an object in a fluid is equal to the weight of the fluid displaced by the object Buoyancy is the ability of a fluid to exert an upward force on an object immersed in it Bill Nye: Buoyancy
17 State of Matter: Gases No definite shape (takes the shape of its container) or volume (the particles can expand and contract to fill the space available) Fast vibration of particles The particles have enough energy to separate completely from one another
18 State of Matter: Plasma Is a super heated gas of positive & negative particles When the molecules are traveling so fast, they begin to break apart and electrons become stripped from their atoms. This is called ionization The fastest movement of particles & is the MOST common form in the universe!
19
20 Pressure: is a force that can influence all states of matter! Is caused by particles colliding with their environment or each other It is measured in units of Pascal (Pa): Equal to 1 newton per square meter (1 N = force needed to move 1 kg 1m/sec/sec)
21 Solid Liquid Gas
22
23 All matter changes into a new state, but there are names for each change and we call them a change Of phase.
24 Plasma Ionization deionization
25 The phase from. Liquid to Gas: Evaporation Takes place below the boiling point of the liquid
26 The phase from. Solid to Liquid: Melting Requires energy to break the physical bonds holding the particles together, this energy is called heat of fusion
27 The phase from. Liquid to Gas: Vaporization Taking in Energy Takes place above the boiling point of the liquid
28 In order for the phase from Liquid to Gas to occur energy is needed to make the particles separate, we call this heat of vaporization
29 The phase from. Gas to Liquid: Condensation Releases energy (heat of vaporization) The same amount of energy is required to change from a liquid to a gas
30 The phase from. Liquid to Solid: Solidification (freezing) Releases energy (heat of fusion), the same amount of energy required to change from a solid to a liquid (the heat of fusion in reverse)
31 The phase from. Solid to Gas or Gas to Solid: Sublimation A solid changes directly to a gas without going through the liquid state A gas changes directly to a solid without going through the liquid state
32 Heat of vaporization Energy to overcome attractive forces of cohesion and/or adhesion which holds the particles close together thus allowing them to completely separate from each other forming a gas
33 The behavior of attraction between molecules is determined by a change in temperature. Molecules are held together by: 1.Cohesion 2.Adhesion
34 Cohesion is the attraction between the same kind of particles Adhesion is the attraction between different kinds of particles
35 fun facts!! BEC (Bose-Einstein Condensate): State (phase) of matter obtained when atoms are super-cooled until they condense into super-atoms which then behave like waves Slowest vibration of particles (within a few billionths of a degree above absolute zero)
Do Now Monday, January 23, 201
 Do Now Monday, January 23, 201 What do you recall about states of matter? Write your answer using complete sentences. 3.5 minutes Do Now Check By the end of the day today, IWBAT Describe the various states
Do Now Monday, January 23, 201 What do you recall about states of matter? Write your answer using complete sentences. 3.5 minutes Do Now Check By the end of the day today, IWBAT Describe the various states
Chapter 2. States of Matter
 Chapter 2 States of Matter 2-1 Matter Matter Matter Anything that takes up space and has mass. Is air matter? Yes. It takes up space and has mass. It has atoms. All matter is made up of atoms. ( Dalton
Chapter 2 States of Matter 2-1 Matter Matter Matter Anything that takes up space and has mass. Is air matter? Yes. It takes up space and has mass. It has atoms. All matter is made up of atoms. ( Dalton
CHAPTER 4 - STATES OF MATTER. Mr. Polard Physical Science Ingomar Middle School
 CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
Chapter 14 9/21/15. Solids, Liquids & Gasses. Essential Questions! Kinetic Theory! Gas State! Gas State!
 Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Solids, Liquids, and Gases. Chapter 14
 Solids, Liquids, and Gases Chapter 14 Matter & Thermal Energy Matter can exist as a solid, a liquid, a gas or a plasma. The Molecular Kinetic Theory of Matter explains their differences and how they can
Solids, Liquids, and Gases Chapter 14 Matter & Thermal Energy Matter can exist as a solid, a liquid, a gas or a plasma. The Molecular Kinetic Theory of Matter explains their differences and how they can
Unit 1 Lesson 6 Changes of State. Copyright Houghton Mifflin Harcourt Publishing Company
 The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
Section 1 Matter and Energy
 CHAPTER OUTLINE Section 1 Matter and Energy Key Idea questions > What makes up matter? > What is the difference between a solid, a liquid, and a gas? > What kind of energy do all particles of matter have?
CHAPTER OUTLINE Section 1 Matter and Energy Key Idea questions > What makes up matter? > What is the difference between a solid, a liquid, and a gas? > What kind of energy do all particles of matter have?
THE PHASES OF MATTER. Solid: holds its shape and does not flow. The molecules in a solid vibrate in place, but on average, don t move very far.
 THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
Liquids & Solids: Section 12.3
 Liquids & Solids: Section 12.3 MAIN IDEA: The particles in and have a range of motion and are not easily. Why is it more difficult to pour syrup that is stored in the refrigerator than in the cabinet?
Liquids & Solids: Section 12.3 MAIN IDEA: The particles in and have a range of motion and are not easily. Why is it more difficult to pour syrup that is stored in the refrigerator than in the cabinet?
Chapter 8. Chapter 8. Preview. Bellringer. Chapter 8. Particles of Matter. Objectives. Chapter 8. Particles of Matter, continued
 States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
Chapter 3. Preview. Section 1 Three States of Matter. Section 2 Behavior of Gases. Section 3 Changes of State. States of Matter.
 States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
Unit 4: The Nature of Matter
 16 16 Table of Contents Unit 4: The Nature of Matter Chapter 16: Solids, Liquids, and Gases 16.1: Kinetic Theory 16.2: Properties and Fluids 16.3: Behavior of Gases 16.1 Kinetic Theory Kinetic Theory kinetic
16 16 Table of Contents Unit 4: The Nature of Matter Chapter 16: Solids, Liquids, and Gases 16.1: Kinetic Theory 16.2: Properties and Fluids 16.3: Behavior of Gases 16.1 Kinetic Theory Kinetic Theory kinetic
Solids (cont.) Describe the movement of particles in a solid and the forces between them.
 Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
CHEMISTRY Matter and Change. Chapter 12: States of Matter
 CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
Matter and Thermal Energy
 Section States of Matter Can you identify the states of matter present in the photo shown? Kinetic Theory The kinetic theory is an explanation of how particles in matter behave. Kinetic Theory The three
Section States of Matter Can you identify the states of matter present in the photo shown? Kinetic Theory The kinetic theory is an explanation of how particles in matter behave. Kinetic Theory The three
Unit 4: Gas Laws. Matter and Phase Changes
 Unit 4: Gas Laws Matter and Phase Changes ENERGY and matter What is 에너지 A fundamental property of the universe that cannot be easily defined. Energy No one knows what energy is, only what it does or has
Unit 4: Gas Laws Matter and Phase Changes ENERGY and matter What is 에너지 A fundamental property of the universe that cannot be easily defined. Energy No one knows what energy is, only what it does or has
Term Info Picture. Anything that has mass and takes up space; everything is made of matter.
 Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
4 Discuss and evaluate the 5th state of matter. 3 - Differentiate among the four states of matter in terms of energy,
 Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Name Date Class THE NATURE OF GASES
 13.1 THE NATURE OF GASES Section Review Objectives Describe the assumptions of the kinetic theory as it applies to gases Interpret gas pressure in terms of kinetic theory Define the relationship between
13.1 THE NATURE OF GASES Section Review Objectives Describe the assumptions of the kinetic theory as it applies to gases Interpret gas pressure in terms of kinetic theory Define the relationship between
Chapter: States of Matter
 Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
Chapter 7.1. States of Matter
 Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Chapter 3. States of Matter
 Chapter 3 States of Matter 1. Solid 2. Liquid 3. Gas States of Matter Two More (discuss later) Plasma Bose-Einstein condensate States of Matter Solid (definite shape and volume) Particles are tightly packed
Chapter 3 States of Matter 1. Solid 2. Liquid 3. Gas States of Matter Two More (discuss later) Plasma Bose-Einstein condensate States of Matter Solid (definite shape and volume) Particles are tightly packed
Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion.
 Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Most substances can be in three states: solid, liquid, and gas.
 States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
Kinetic Theory of Matter
 1 Temperature and Thermal Energy Kinetic Theory of Matter The motion of the particles in matter is described by kinetic theory of matter. Matter is composed of particles that are atoms, molecules, or ions
1 Temperature and Thermal Energy Kinetic Theory of Matter The motion of the particles in matter is described by kinetic theory of matter. Matter is composed of particles that are atoms, molecules, or ions
STATES OF MATTER. Chapter 3
 STATES OF MATTER Chapter 3 Labs done so far for ch. 3 sections 1 and 2: 1. Distilled wood and related read of temperatures with plateaus for substances produced 2. Distilling solution X (BP/CP evaporation/condensation)
STATES OF MATTER Chapter 3 Labs done so far for ch. 3 sections 1 and 2: 1. Distilled wood and related read of temperatures with plateaus for substances produced 2. Distilling solution X (BP/CP evaporation/condensation)
There are three phases of matter: Solid, liquid and gas
 FLUIDS: Gases and Liquids Chapter 4 of text There are three phases of matter: Solid, liquid and gas Solids: Have form, constituents ( atoms and molecules) are in fixed positions (though they can vibrate
FLUIDS: Gases and Liquids Chapter 4 of text There are three phases of matter: Solid, liquid and gas Solids: Have form, constituents ( atoms and molecules) are in fixed positions (though they can vibrate
IES LAURETUM SCIENCE NAME.
 IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
Chapter 10 States of Matter
 Chapter 10 States of Matter 1 Section 10.1 The Nature of Gases Objectives: Describe the assumptions of the kinetic theory as it applies to gases. Interpret gas pressure in terms of kinetic theory. Define
Chapter 10 States of Matter 1 Section 10.1 The Nature of Gases Objectives: Describe the assumptions of the kinetic theory as it applies to gases. Interpret gas pressure in terms of kinetic theory. Define
CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS
 CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Particle nature of Matter *** 2. States of Matter **** 3. Interchange in states of Matter
CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Particle nature of Matter *** 2. States of Matter **** 3. Interchange in states of Matter
Chapter 3 Phases of Matter Physical Science
 Chapter 3 Phases of Matter Physical Science CH 3- States of Matter 1 What makes up matter? What is the difference between a solid, a liquid, and a gas? What kind of energy do all particles of matter have?
Chapter 3 Phases of Matter Physical Science CH 3- States of Matter 1 What makes up matter? What is the difference between a solid, a liquid, and a gas? What kind of energy do all particles of matter have?
The States of Matter
 The States of Matter AZ State Standards Concept 1: Structure and Properties of Matter Understand physical, chemical, and atomic properties of matter. PO 1. Describe substances based on their physical properties.
The States of Matter AZ State Standards Concept 1: Structure and Properties of Matter Understand physical, chemical, and atomic properties of matter. PO 1. Describe substances based on their physical properties.
Matter & Energy. Objectives: properties and structures of the different states of matter.
 Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Study Guide for Chapters 2, 3, and 10
 Study Guide for Chapters 2, 3, and 10 1. What is matter? Where can it be found? Anything that has mass and takes up space. 2. What units are used to measure volume? Liters and meters cubed 3. How would
Study Guide for Chapters 2, 3, and 10 1. What is matter? Where can it be found? Anything that has mass and takes up space. 2. What units are used to measure volume? Liters and meters cubed 3. How would
Chapter 13 - States of Matter. Section 13.1 The nature of Gases
 Chapter 13 - States of Matter Section 13.1 The nature of Gases Kinetic energy and gases Kinetic energy: the energy an object has because of its motion Kinetic theory: all matter is made if particles in
Chapter 13 - States of Matter Section 13.1 The nature of Gases Kinetic energy and gases Kinetic energy: the energy an object has because of its motion Kinetic theory: all matter is made if particles in
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
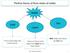 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
CBSE Class 9 Science Revision Notes CHAPTER 1 Matter in our Surroundings
 CBSE Class 9 Science Revision Notes CHAPTER 1 Matter in our Surroundings Anything that occupies space and has mass and is felt by senses is called matter. According to indian ancient philosphor, matter
CBSE Class 9 Science Revision Notes CHAPTER 1 Matter in our Surroundings Anything that occupies space and has mass and is felt by senses is called matter. According to indian ancient philosphor, matter
When liquid water crystallizes it has six sides. Create a snowflake with six sides.
 When liquid water crystallizes it has six sides. Create a snowflake with six sides. Purpose: To create a supersaturated solution and observe the crystal lattice of borax snowflakes. The state of matter
When liquid water crystallizes it has six sides. Create a snowflake with six sides. Purpose: To create a supersaturated solution and observe the crystal lattice of borax snowflakes. The state of matter
Science 8 Chapter 7 Section 1
 Science 8 Chapter 7 Section 1 Describing Fluids (pp. 268-277) What is a fluid? Fluid: any thing that flows; a liquid or a gas While it would seem that some solids flow (sugar, salt, etc), they are not
Science 8 Chapter 7 Section 1 Describing Fluids (pp. 268-277) What is a fluid? Fluid: any thing that flows; a liquid or a gas While it would seem that some solids flow (sugar, salt, etc), they are not
Name Date Class STATES OF MATTER. Match the correct state of matter with each description of water by writing a letter on each line.
 10 STATES OF MATTER SECTION 10.1 THE NATURE OF GASES (pages 267 272) This section describes how the kinetic theory applies to gases. It defines gas pressure and explains how temperature is related to the
10 STATES OF MATTER SECTION 10.1 THE NATURE OF GASES (pages 267 272) This section describes how the kinetic theory applies to gases. It defines gas pressure and explains how temperature is related to the
Chemistry Joke. Once you ve seen 6.02 x You ve seen a mole!
 States of Matter Chemistry Joke Once you ve seen 6.02 x 10 23 atoms You ve seen a mole! Kinetic Theory Kinetic Theory explains the states of matter based on the concept that the particles in all forms
States of Matter Chemistry Joke Once you ve seen 6.02 x 10 23 atoms You ve seen a mole! Kinetic Theory Kinetic Theory explains the states of matter based on the concept that the particles in all forms
Section 16.3 Phase Changes
 Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Chapter 10: States of Matter. Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding
 Chapter 10: States of Matter Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding Pressure standard pressure the pressure exerted at sea level in dry air
Chapter 10: States of Matter Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding Pressure standard pressure the pressure exerted at sea level in dry air
Matter changes phase when energy is added or removed
 Section 12.4 Phase Changes Explain how the addition and removal of energy can cause a phase change. Interpret a phase diagram. Matter changes phase when energy is added or removed Energy Changes Accompanying
Section 12.4 Phase Changes Explain how the addition and removal of energy can cause a phase change. Interpret a phase diagram. Matter changes phase when energy is added or removed Energy Changes Accompanying
Name Date Class STATES OF MATTER
 13 STATES OF MATTER Chapter Test A A. Matching Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A Column B 1. amorphous
13 STATES OF MATTER Chapter Test A A. Matching Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A Column B 1. amorphous
IGCSE Double Award Extended Coordinated Science
 IGCSE Double Award Extended Coordinated Science Physics 5 - Thermal Properties of Matter Thermal Expansion You need to know thermal expansions for solids, liquids, and gases, and their applications. Thermal
IGCSE Double Award Extended Coordinated Science Physics 5 - Thermal Properties of Matter Thermal Expansion You need to know thermal expansions for solids, liquids, and gases, and their applications. Thermal
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES
 THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
Changing States of Matter By Cindy Grigg
 By Cindy Grigg 1 On Earth, almost all matter exists in just three states. Matter is usually a solid, a liquid, or a gas. Plasma, the fourth state of matter, is rare on Earth. It sometimes can be found
By Cindy Grigg 1 On Earth, almost all matter exists in just three states. Matter is usually a solid, a liquid, or a gas. Plasma, the fourth state of matter, is rare on Earth. It sometimes can be found
Gases and States of Matter: Unit 8
 Gases and States of Matter: Unit 8 States of Matter There are three states (also called phases) of matter. The picture represents the same chemical substance, just in different states. There are three
Gases and States of Matter: Unit 8 States of Matter There are three states (also called phases) of matter. The picture represents the same chemical substance, just in different states. There are three
Physical Science Exam 3 Study Guide. Dr. Karoline Rostamiani. Chapter 3
 Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Quiz Review Topical Questions
 Quiz Review Topical Questions Kinetic Theory of Matter Expansion and Contraction Solids, Liquids, Gases States of Matter Phase Changes Distillation Water Properties Kinetic Theory 1. The kinetic theory
Quiz Review Topical Questions Kinetic Theory of Matter Expansion and Contraction Solids, Liquids, Gases States of Matter Phase Changes Distillation Water Properties Kinetic Theory 1. The kinetic theory
Matter and Its Properties. Unit 2
 Matter and Its Properties Unit 2 Lesson 1: Physical & Chemical Properties & Changes Unit 2: Matter and Its Properties Section 1: Physical Properties & Change Lesson 1: Physical & Chemical Properties &
Matter and Its Properties Unit 2 Lesson 1: Physical & Chemical Properties & Changes Unit 2: Matter and Its Properties Section 1: Physical Properties & Change Lesson 1: Physical & Chemical Properties &
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Name Date Class STATES OF MATTER. SECTION 13.1 THE NATURE OF GASES (pages )
 Name Date Class 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains
Name Date Class 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
Gases, Liquids and Solids
 Chapter 5 Gases, Liquids and Solids The States of Matter Gases Pressure Forces between one molecule and another are called intermolecular forces. Intermolecular forces hold molecules together and kinetic
Chapter 5 Gases, Liquids and Solids The States of Matter Gases Pressure Forces between one molecule and another are called intermolecular forces. Intermolecular forces hold molecules together and kinetic
Matter & Energy. Kinetic Theory of Matter. Kinetic Theory of Matter. Kinetic Theory of Matter. Kinetic Theory of Matter. Temperature.
 Matter & Energy 1) All matter is made up of atoms and molecules that act as tiny particles. 1 2 2) These tiny particles are always in motion. State of matter depends on its molecular motion as measured
Matter & Energy 1) All matter is made up of atoms and molecules that act as tiny particles. 1 2 2) These tiny particles are always in motion. State of matter depends on its molecular motion as measured
States of Matter. What physical changes and energy changes occur as matter goes from one state to another?
 Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Chemistry Day 5. Friday, August 31 st Tuesday, September 4 th, 2018
 Chemistry Day 5 Friday, August 31 st Tuesday, September 4 th, 2018 Do-Now Title: BrainPOP: States of Matter 1. Write down today s FLT 2. List two examples of gases 3. List two examples of things that are
Chemistry Day 5 Friday, August 31 st Tuesday, September 4 th, 2018 Do-Now Title: BrainPOP: States of Matter 1. Write down today s FLT 2. List two examples of gases 3. List two examples of things that are
Chemistry B11 Chapter 6 Gases, Liquids, and Solids
 Chapter 6 Gases, Liquids, and Solids States of matter: the physical state of matter depends on a balance between the kinetic energy of particles, which tends to keep them apart, and the attractive forces
Chapter 6 Gases, Liquids, and Solids States of matter: the physical state of matter depends on a balance between the kinetic energy of particles, which tends to keep them apart, and the attractive forces
STATES OF MATTER. The Four States of Ma/er. Four States. Solid Liquid Gas Plasma
 STATES OF MATTER The Four States of Ma/er Solid Liquid Gas Plasma Four States STATES OF MATTER Ø What makes a substance a par:cular state of ma
STATES OF MATTER The Four States of Ma/er Solid Liquid Gas Plasma Four States STATES OF MATTER Ø What makes a substance a par:cular state of ma
The OTHER TWO states of matter
 ` The OTHER TWO states of matter LIQUIDS A decrease in the average kinetic energy of gas particles causes the temperature to decrease. As it cools, the particles tend to move more slowly if they slow down
` The OTHER TWO states of matter LIQUIDS A decrease in the average kinetic energy of gas particles causes the temperature to decrease. As it cools, the particles tend to move more slowly if they slow down
CIE Physics IGCSE. Topic 2: Thermal Physics
 CIE Physics IGCSE Topic 2: Thermal Physics Summary Notes Simple kinetic molecular model of matter Molecular model Solids Molecules close together in regular pattern Strong intermolecular forces of attraction
CIE Physics IGCSE Topic 2: Thermal Physics Summary Notes Simple kinetic molecular model of matter Molecular model Solids Molecules close together in regular pattern Strong intermolecular forces of attraction
Thermal Physics. Temperature (Definition #1): a measure of the average random kinetic energy of all the particles of a system Units: o C, K
 Thermal Physics Internal Energy: total potential energy and random kinetic energy of the molecules of a substance Symbol: U Units: J Internal Kinetic Energy: arises from random translational, vibrational,
Thermal Physics Internal Energy: total potential energy and random kinetic energy of the molecules of a substance Symbol: U Units: J Internal Kinetic Energy: arises from random translational, vibrational,
Matter, Atoms & Molecules
 Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
NAME DATE CLASS TEST DATE:
 1 TEST DATE: 2 Vocabulary Chapter 8 Solids, liquids, and gases Condensation Crystals Evaporation Heat of fusion Heat of vaporization Kinetic theory of matter Plasma States of matter Thermal expansion Chapter
1 TEST DATE: 2 Vocabulary Chapter 8 Solids, liquids, and gases Condensation Crystals Evaporation Heat of fusion Heat of vaporization Kinetic theory of matter Plasma States of matter Thermal expansion Chapter
CHAPTER 10. States of Matter
 CHAPTER 10 States of Matter Kinetic Molecular Theory Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
CHAPTER 10 States of Matter Kinetic Molecular Theory Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
CHAPTER 10. Kinetic Molecular Theory. Five Assumptions of the KMT. Atmospheric Pressure
 Kinetic Molecular Theory CHAPTER 10 States of Matter Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
Kinetic Molecular Theory CHAPTER 10 States of Matter Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
Kinetic Theory. States of Matter. Thermal Energy. Four States of Matter. Kinetic Energy. Solid. Liquid. Definition: How particles in matter behave
 Kinetic Theory Definition: How particles in matter behave States of Matter All Matter is composed of small particles. Particles are in constant random motion. Particles collide with each other and walls
Kinetic Theory Definition: How particles in matter behave States of Matter All Matter is composed of small particles. Particles are in constant random motion. Particles collide with each other and walls
OUTLINE. States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry
 UNIT 6 GASES OUTLINE States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry STATES OF MATTER Remember that all matter exists in three physical states: Solid Liquid
UNIT 6 GASES OUTLINE States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry STATES OF MATTER Remember that all matter exists in three physical states: Solid Liquid
ch 12 acad.notebook January 12, 2016 Ch 12 States of Matter (solids, liquids, gases, plasma, Bose Einstein condensate)
 Ch 12 States of Matter (solids, liquids, gases, plasma, Bose Einstein condensate) BIG IDEA The kinetic molecular theory explains the different properties of solids, liquids and gases. I CAN: 1) use the
Ch 12 States of Matter (solids, liquids, gases, plasma, Bose Einstein condensate) BIG IDEA The kinetic molecular theory explains the different properties of solids, liquids and gases. I CAN: 1) use the
S8P1C. STATES OF MATTER E.Q. WHAT ARE THE STATES OF MATTER? WHAT ARE THE CHARACTERISTICS OF EACH STATE OF MATTER?
 S8P1C. STATES OF MATTER E.Q. WHAT ARE THE STATES OF MATTER? WHAT ARE THE CHARACTERISTICS OF EACH STATE OF MATTER? ATOMIC MODEL PROJECT BE CREATIVE!!! MIXTURES VS. SUBSTANCES TASK DUE BY FRIDAY, NOVEMBER
S8P1C. STATES OF MATTER E.Q. WHAT ARE THE STATES OF MATTER? WHAT ARE THE CHARACTERISTICS OF EACH STATE OF MATTER? ATOMIC MODEL PROJECT BE CREATIVE!!! MIXTURES VS. SUBSTANCES TASK DUE BY FRIDAY, NOVEMBER
Chapter 22 States of matter. Section 1 matter Section 2 Changes of State
 Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
When liquid water crystallizes it has six sides. Create a snowflake with six sides.
 When liquid water crystallizes it has six sides. Create a snowflake with six sides. Purpose: To create a supersaturated solution and observe the crystal lattice of borax snowflakes. The state of matter
When liquid water crystallizes it has six sides. Create a snowflake with six sides. Purpose: To create a supersaturated solution and observe the crystal lattice of borax snowflakes. The state of matter
Chapter 13 States of Matter Forces of Attraction 13.3 Liquids and Solids 13.4 Phase Changes
 Chapter 13 States of Matter 13.2 Forces of Attraction 13.3 Liquids and Solids 13.4 Phase Changes I. Forces of Attraction (13.2) Intramolecular forces? (forces within) Covalent Bonds, Ionic Bonds, and metallic
Chapter 13 States of Matter 13.2 Forces of Attraction 13.3 Liquids and Solids 13.4 Phase Changes I. Forces of Attraction (13.2) Intramolecular forces? (forces within) Covalent Bonds, Ionic Bonds, and metallic
Name: Class: Date: Figure 3-1
 Name: Class: Date: Chapter 3 test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A gas has a. a definite volume but no definite shape. b. a definite shape
Name: Class: Date: Chapter 3 test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A gas has a. a definite volume but no definite shape. b. a definite shape
CHEM. Ch. 12 Notes ~ STATES OF MATTER
 CHEM. Ch. 12 Notes ~ STATES OF MATTER NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 12.1 topics States of Matter: SOLID, LIQUID, GAS, PLASMA I. Kinetic Theory
CHEM. Ch. 12 Notes ~ STATES OF MATTER NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 12.1 topics States of Matter: SOLID, LIQUID, GAS, PLASMA I. Kinetic Theory
Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Chapter 11 THE NATURE OF GASES States of Matter Describe the motion of gas particles according to the kinetic theory Interpret gas pressure in terms of kinetic theory Key Terms: 1. kinetic energy 2. gas
Chapter 11 THE NATURE OF GASES States of Matter Describe the motion of gas particles according to the kinetic theory Interpret gas pressure in terms of kinetic theory Key Terms: 1. kinetic energy 2. gas
Chapter: Heat and States
 Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Bell Ringer. What are the two types of mixtures? What is an element? What is a compound?
 Bell Ringer What are the two types of mixtures? What is an element? What is a compound? MATTER Solids, Liquids, & Gases States of Matter & Kinetic Molecular Theory Kinetic Molecular Theory KMT Tiny, constantly
Bell Ringer What are the two types of mixtures? What is an element? What is a compound? MATTER Solids, Liquids, & Gases States of Matter & Kinetic Molecular Theory Kinetic Molecular Theory KMT Tiny, constantly
The physical state of a substance can be changed by increasing or decreasing its temperature.
 Chemistry Lecture #63: Changes of State The physical state of a substance can be changed by increasing or decreasing its temperature. For example, a solid substance can be converted into a liquid by heating
Chemistry Lecture #63: Changes of State The physical state of a substance can be changed by increasing or decreasing its temperature. For example, a solid substance can be converted into a liquid by heating
States of Matter Unit
 Learning Target Notes Section 1: Matter and Energy What makes up matter? Matter is made of atoms and molecules that are in constant motion. Kinetic Theory of Matter A. Particles that make up matter are
Learning Target Notes Section 1: Matter and Energy What makes up matter? Matter is made of atoms and molecules that are in constant motion. Kinetic Theory of Matter A. Particles that make up matter are
Matter: Properties & Change
 Matter: Properties & Change Essential Vocabulary 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are all alike, but are different from the atoms of other elements. 6.P.2.2
Matter: Properties & Change Essential Vocabulary 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are all alike, but are different from the atoms of other elements. 6.P.2.2
Pg , Syllabus
 Pg. 169 171, 173-175 Syllabus 5.7 5.14 www.cgrahamphysics.com What do you remember? End www.cgrahamphysics.com How do particles move? 3 of 30 Boardworks Ltd 2012 4 of 30 Boardworks Ltd 2012 States of matter
Pg. 169 171, 173-175 Syllabus 5.7 5.14 www.cgrahamphysics.com What do you remember? End www.cgrahamphysics.com How do particles move? 3 of 30 Boardworks Ltd 2012 4 of 30 Boardworks Ltd 2012 States of matter
2. What is meant by Chemical State?. 3. Changing states of matter is about changing,,, and other.
 Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Physical Science Chapter 5 Cont3. Temperature & Heat
 Physical Science Chapter 5 Cont3 Temperature & Heat What are we going to study? Heat Transfer Phases of Matter The Kinetic Theory of Gases Thermodynamics Specific Heat (Capacity) Specific Heat Latent Heat
Physical Science Chapter 5 Cont3 Temperature & Heat What are we going to study? Heat Transfer Phases of Matter The Kinetic Theory of Gases Thermodynamics Specific Heat (Capacity) Specific Heat Latent Heat
Kinetic Theory of Matter. Matter & Energy
 Kinetic Theory of Matter Matter & Energy 1 Kinetic Theory of Matter All matter is made up of atoms and molecules that act as tiny particles. 2 Kinetic Theory of Matter These tiny particles are always in
Kinetic Theory of Matter Matter & Energy 1 Kinetic Theory of Matter All matter is made up of atoms and molecules that act as tiny particles. 2 Kinetic Theory of Matter These tiny particles are always in
2. THE STATES OF MATTER
 2. THE STATES OF MATTER 2.1. THE THREE STATES OF MATTER Every substance can take on several distinct forms called phases or states of aggregation of matter. Four states of matter are observable in everyday
2. THE STATES OF MATTER 2.1. THE THREE STATES OF MATTER Every substance can take on several distinct forms called phases or states of aggregation of matter. Four states of matter are observable in everyday
States of Matter: Study Guide
 Name: nswer KEY States of Matter: Study Guide Period: Date: 1. Describe the volume, shape and molecular arrangement in the following states of matter: Solid Volume Shape Molecular rrangement Definite Definite
Name: nswer KEY States of Matter: Study Guide Period: Date: 1. Describe the volume, shape and molecular arrangement in the following states of matter: Solid Volume Shape Molecular rrangement Definite Definite
CHM Solids, Liquids, and Phase Changes (r15) Charles Taylor 1/9
 CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
Chapter 11. Freedom of Motion. Comparisons of the States of Matter. Liquids, Solids, and Intermolecular Forces
 Liquids, Solids, and Intermolecular Forces Chapter 11 Comparisons of the States of Matter The solid and liquid states have a much higher density than the gas state The solid and liquid states have similar
Liquids, Solids, and Intermolecular Forces Chapter 11 Comparisons of the States of Matter The solid and liquid states have a much higher density than the gas state The solid and liquid states have similar
Chapter 11: Liquids, Solids, and Intermolecular Forces. Mrs. Brayfield
 Chapter 11: Liquids, Solids, and Intermolecular Forces Mrs. Brayfield 11.1: Intermolecular Forces Intermolecular forces are attractive forces that exist between all molecules and atoms The state of matter
Chapter 11: Liquids, Solids, and Intermolecular Forces Mrs. Brayfield 11.1: Intermolecular Forces Intermolecular forces are attractive forces that exist between all molecules and atoms The state of matter
Kinetic Theory (Kinetikos - Moving ) Based on the idea that particles of matter are always in motion
 Chapter 10 Kinetic Theory (Kinetikos - Moving ) Based on the idea that particles of matter are always in motion The motion has consequences Behavior of Gases Physical Properties of Gases Ideal Gas an imaginary
Chapter 10 Kinetic Theory (Kinetikos - Moving ) Based on the idea that particles of matter are always in motion The motion has consequences Behavior of Gases Physical Properties of Gases Ideal Gas an imaginary
Chemistry A: States of Matter Packet Name: Hour: Page!1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Unit 4 - Energy & Heat SOL PS.6,7
 Unit 4 - Energy & Heat SOL PS.6,7 Bill Nye - Energy I. PS.6a - Potential & Kinetic Energy A. Energy is the ability to do work or cause change. B. Energy is either Potential or Kinetic. a. Potential Energy
Unit 4 - Energy & Heat SOL PS.6,7 Bill Nye - Energy I. PS.6a - Potential & Kinetic Energy A. Energy is the ability to do work or cause change. B. Energy is either Potential or Kinetic. a. Potential Energy
STATES OF MATTER STATES OF MATTER. The Four States of Matter 3/5/2015. Solid. Liquid Commonly found on Gas Earth Plasma
 Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) STATES OF MATTER The Four States of Matter Solid } Liquid Commonly found on Gas Earth Plasma STATES OF MATTER Based upon particle
Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) STATES OF MATTER The Four States of Matter Solid } Liquid Commonly found on Gas Earth Plasma STATES OF MATTER Based upon particle
STATES OF MATTER STATES OF MATTER. The Four States of Matter 3/5/2015
 The Four States of Matter Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) Solid } Liquid Commonly found on Gas Earth Plasma Based upon particle arrangement Based upon energy of
The Four States of Matter Unit 10: States of Matter Lesson 10.1: States and Their Changes (Review) Solid } Liquid Commonly found on Gas Earth Plasma Based upon particle arrangement Based upon energy of
Matter In Our Surroundings
 Matter In Our Surroundings Introduction Matter is a substance which occupies space and has weight. Like the air we breath, the food we eat, stones, clouds, etc even a small drop of water or a particle
Matter In Our Surroundings Introduction Matter is a substance which occupies space and has weight. Like the air we breath, the food we eat, stones, clouds, etc even a small drop of water or a particle
