TEACHER NOTES: ICE CUBE POSTER
|
|
|
- Jennifer Reed
- 5 years ago
- Views:
Transcription
1 TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure changes of state in terms of the particle model. TASK: Draw a poster that explains why an ice cube melts (and evaporates) when left out of the freezer. SUGGESTED APPROACH: Please read the introduction to this book to get the most out of this task. It is suitable for a homework task or class activity. In class, use a starter activity as the stimulus to the task; introduce the task and ACE Learning Ladder, and allow minutes to complete it. Starter suggestions: matching key words melting, evaporating, condensing, solidification, freezing to pictures of these events, e.g. melting ice cube. Match words and phrases describing the behaviour of solids, liquids and gases to the correct state. Allow learners to use secondary resources such as class notes, textbooks and library books to develop their poster. In the plenary, peer or self assess using the ACE Learning Ladder. RESOURCES: A4 plain paper, pencils, pens, rulers. PRIOR LEARNING EXPERIENCE: Before students attempt this task, they must be familiar with: properties of solids, liquids and gases changes of state between the three states of matter particle theory, arrangement and behaviour of particles in the three states of matter. CHEMISTRY ACE TASKS: TEACHER NOTES
2 TASK SHEET: Some students were watching an ice cube in a beaker as it slowly melted. They were wondering why it melts. When they inspected the beaker the next lesson, the water was gone. Draw a poster that explains why an ice cube melts when left out of the freezer and what happens to the water when it is left in a beaker for a while. Use a particle model to help explain your ideas. KEY WORDS boiling, compressible, conservation of mass, density, energy, evaporating, fixed, forces between particles, freezing, gas, liquid, melting, moving randomly, particles, solid, solidification, states of matter, temperature, vibrating CHEMISTRY ACE TASKS: TASK SHEET
3 ACE LEARNING LADDER: ACE LEARNING LADDER Assessment check Advanced Confident Establishing What you could include: You will have drawn a detailed poster explaining why an ice cube melts, drawing on detailed scientific knowledge and understanding. You might: Draw a detailed particle diagram for the water particles in each state, showing that water particles are molecules. Explain why energy is required for the ice to melt or evaporate and where this comes from. Use the idea of melting points and boiling points to describe the changes. Compare the melting and evaporating of an ice cube to observations that would be expected from other substances undergoing the same processes. Use a range of appropriate scientific words, symbols and units accurately. You will have drawn a poster explaining why an ice cube melts, drawing on scientific knowledge and understanding. You might: Draw a particle diagram for the water particles in each state. Explain the differences in movement and energy of the particles at each state. Explain what has to happen to the particles to be able to melt or evaporate. Describe whether the melting and evaporating of an ice cube is a physical or chemical change. Use a range of appropriate scientific words, symbols and units. You will have drawn a simple poster explaining why an ice cube melts, drawing on some scientific knowledge and understanding. You might: Draw a simple particle diagram for the water particles in each state, with help. State how the particles are arranged in each state, what their movement is like and how much energy they have. Describe what happens when the ice cube melts and when it evaporates, in terms of what would be observed. State if melting and evaporating are a physical or chemical change. Use some appropriate scientific words, symbols and units. CHEMISTRY ACE TASKS: ACE LEARNING LADDER
4 SUPPORT SHEET 1: ESTABLISHING TO CONFIDENT Correctly identify the state of matter shown in the diagrams below. Complete the sentences about each before starting the task, by choosing which sentence finishers correctly describe the state shown in each diagram. This represents a The particles have high levels of energy medium levels of energy low levels of energy. They can move about past one another cannot move about can move about completely freely. The particles are arranged in a regular pattern randomly and apart from one another randomly but in contact with one another. This represents a The particles have high levels of energy medium levels of energy low levels of energy. They can move about past one another cannot move about can move about completely freely. The particles are arranged in a regular pattern randomly and apart from one another randomly but in contact with one another. CHEMISTRY ACE TASKS: SUPPORT SHEET 1
5 SUPPORT SHEET 1: ESTABLISHING TO CONFIDENT This represents a The particles have high levels of energy medium levels of energy low levels of energy. They can move about past one another cannot move about can move about completely freely. The particles are arranged in a regular pattern randomly and apart from one another randomly but in contact with one another. CHEMISTRY ACE TASKS: SUPPORT SHEET 1
6 SUPPORT SHEET 2: CONFIDENT TO ADVANCED Use the spaces below to plan particle diagrams for each state of matter. What explanations will you need to include to describe what each state is like? How could you describe what happens to particles within substances moving between each of the states shown? CHEMISTRY ACE TASKS: SUPPORT SHEET 2
7 SUPPORT SHEET 3: ADVANCED EXTEND AND STRETCH Water has the chemical formula H 2 O. It exists as molecules. What does this formula tell us about what water is made from? Use the spaces below to plan particle diagrams for each state of matter, which clearly show water is a molecule. CHEMISTRY ACE TASKS: SUPPORT SHEET 3
6-3 Particle model of matter Physics
 6-3 Particle model of matter Physics.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Physics.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Trilogy
 6-3 Particle model of matter Trilogy.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Trilogy.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter
 AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter Test (Levels 4 9) Time allowed: 50 minutes Question Links to Student Progress Sheet Score Total Marks Available Score Estimated Grade
AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter Test (Levels 4 9) Time allowed: 50 minutes Question Links to Student Progress Sheet Score Total Marks Available Score Estimated Grade
INTRODUCTION TO LESSON CLUSTER 7
 INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
Table of Contents. Diagnostic Pre-test... 5 Lesson 1: What Is an Atom? Lesson 5: Gases. Lesson 6: Melting and Freezing. Lesson 2: What Are Molecules?
 Table of Contents Diagnostic Pre-test................. 5 Lesson 1: What Is an Atom? What Can You See?................ 11 What Is Matter Made Of?........... 12 Describing the Atom............... 13 What
Table of Contents Diagnostic Pre-test................. 5 Lesson 1: What Is an Atom? What Can You See?................ 11 What Is Matter Made Of?........... 12 Describing the Atom............... 13 What
Chapter 22 States of matter. Section 1 matter Section 2 Changes of State
 Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
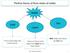 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Term Info Picture. Anything that has mass and takes up space; everything is made of matter.
 Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
Unit 1 Lesson 6 Changes of State. Copyright Houghton Mifflin Harcourt Publishing Company
 The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
INPUT~ Explore It! Station Directions: This is one of the four INPUT stations. They may be completed in any order.
 INPUT~ Explore It! Station Directions: This is one of the four INPUT stations. They may be completed in any order. One member of the group will read the task cards in order. The group will be responsible
INPUT~ Explore It! Station Directions: This is one of the four INPUT stations. They may be completed in any order. One member of the group will read the task cards in order. The group will be responsible
Science Notebook Layout DONʼT COPY UNDERLINED TEXT Mr. Needhamʼs web page:
 20 Rating cocoas 10/ See teacher for data or copy from someone in your group Analysis of cocoa 10/4 21 What made test unfair? Bias: influence in an unfair way. How could bian have influenced that outcome
20 Rating cocoas 10/ See teacher for data or copy from someone in your group Analysis of cocoa 10/4 21 What made test unfair? Bias: influence in an unfair way. How could bian have influenced that outcome
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
! Name: STUDENT JOURNAL Week 13 Radioactivity & Physical Changes
 ! Name: Period: STUDENT JOURNAL Week 13 Radioactivity & Physical Changes Overarching Goal for the Week: Distinguish between the types of physical changes and the change in particle motion Learning Objectives:
! Name: Period: STUDENT JOURNAL Week 13 Radioactivity & Physical Changes Overarching Goal for the Week: Distinguish between the types of physical changes and the change in particle motion Learning Objectives:
Chemistry A: States of Matter Packet Name: Hour: Page!1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Lockerbie Academy. S1 Science. Elements, Compounds and Mixtures Homework Booklet
 Lockerbie Academy S Science Elements, Compounds and Mixtures Homework Booklet Homework From Lesson - Research and Design Researching Use the internet or books from a library to research the element below
Lockerbie Academy S Science Elements, Compounds and Mixtures Homework Booklet Homework From Lesson - Research and Design Researching Use the internet or books from a library to research the element below
Name: Block: Date: Student Notes. OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter.
 Name: Block: Date: LCPS Core Experience Heat Transfer Student Notes OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter. LINK 1. Particles in
Name: Block: Date: LCPS Core Experience Heat Transfer Student Notes OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter. LINK 1. Particles in
6th Grade: Great Salt Lake is Salty
 Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
Topic review. hi.com.au Science Resource Centre. Using scientific language 1. You should be familiar with the words in the following list.
 Topic review Using scientific language 1. You should be familiar with the words in the following list. condense solid boiling matter gas diffusion melting solidify particles substance liquid property compression
Topic review Using scientific language 1. You should be familiar with the words in the following list. condense solid boiling matter gas diffusion melting solidify particles substance liquid property compression
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
W X gas liquid solid Y Z. C X and Y D Y and Z X Y Z. C Z to X D Z to Y
 1 In which changes do the particles move further apart? W X gas liquid solid Y Z W and X W and Z X and Y Y and Z 2 iagrams X, Y and Z represent the three states of matter. X Y Z Which change occurs during
1 In which changes do the particles move further apart? W X gas liquid solid Y Z W and X W and Z X and Y Y and Z 2 iagrams X, Y and Z represent the three states of matter. X Y Z Which change occurs during
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company
 Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
Lesson 02: Physical Properties of Matter. 01 Matter
 Chemistry 11, Physical Properties, Unit 02 1 Lesson 02: Physical Properties of Matter 01 Matter Almost everything in the universe is made of matter matter has volume matter has mass matter is made up of
Chemistry 11, Physical Properties, Unit 02 1 Lesson 02: Physical Properties of Matter 01 Matter Almost everything in the universe is made of matter matter has volume matter has mass matter is made up of
Chemistry A: States of Matter Packet Name: Hour: Page 1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Kinetic Theory of Matter
 1 Temperature and Thermal Energy Kinetic Theory of Matter The motion of the particles in matter is described by kinetic theory of matter. Matter is composed of particles that are atoms, molecules, or ions
1 Temperature and Thermal Energy Kinetic Theory of Matter The motion of the particles in matter is described by kinetic theory of matter. Matter is composed of particles that are atoms, molecules, or ions
Solids (cont.) Describe the movement of particles in a solid and the forces between them.
 Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
P6 Molecules and matter. Student Book answers. P6.1 Density. Question Answer Marks Guidance. 1 a m 3 (= 0.80 m 0.60 m 0.
 P6. Density a 0.024 m 3 (= 0.80 m 0.60 m 0.05 m) b = 2500 kg/m 3 2 a 36 g 48 g = 88 g 2 b =. g/cm 3 3 a i 0.000 40 m 3 (= 0.0 m 0.080 m 0.05 m) 3 a ii = 9 000 kg/m 3 3 b v = = 7.9 0 8 m 3 thickness t =
P6. Density a 0.024 m 3 (= 0.80 m 0.60 m 0.05 m) b = 2500 kg/m 3 2 a 36 g 48 g = 88 g 2 b =. g/cm 3 3 a i 0.000 40 m 3 (= 0.0 m 0.080 m 0.05 m) 3 a ii = 9 000 kg/m 3 3 b v = = 7.9 0 8 m 3 thickness t =
The Can Demonstration
 The Can Demonstration With your table, make a prediction as to what will happen to the can. Discuss why you think that the can imploded. What are some reasons? https://www.youtube.com/watch?v=xg5niowf_zw&t=28s
The Can Demonstration With your table, make a prediction as to what will happen to the can. Discuss why you think that the can imploded. What are some reasons? https://www.youtube.com/watch?v=xg5niowf_zw&t=28s
What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
Properties of Matter
 Grade 7 Science, Quarter 2, Unit 2.1 Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Identify different substances using data about characteristic
Grade 7 Science, Quarter 2, Unit 2.1 Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Identify different substances using data about characteristic
Chapter Review USING VOCABULARY UNDERSTANDING CONCEPTS. Skills Worksheet
 Skills Worksheet Chapter Review USING VOCABULARY 1. Academic Vocabulary Which of the following words means a set of steps or events? a. reaction b. process c. principle d. role For each pair of terms,
Skills Worksheet Chapter Review USING VOCABULARY 1. Academic Vocabulary Which of the following words means a set of steps or events? a. reaction b. process c. principle d. role For each pair of terms,
THE PHASES OF MATTER. Solid: holds its shape and does not flow. The molecules in a solid vibrate in place, but on average, don t move very far.
 THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
SG 4 Elements and Chemical Bonds 5 States of Matter
 Name Date Period SG 4 Elements and Chemical Bonds 5 States of Matter 4.1 Electrons and Energy Levels Directions: On the line before each definition, write the term that matches it correctly. Each term
Name Date Period SG 4 Elements and Chemical Bonds 5 States of Matter 4.1 Electrons and Energy Levels Directions: On the line before each definition, write the term that matches it correctly. Each term
Foundations of Chemistry
 Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
States of Matter. What physical changes and energy changes occur as matter goes from one state to another?
 Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Chapter 3. Preview. Section 1 Three States of Matter. Section 2 Behavior of Gases. Section 3 Changes of State. States of Matter.
 States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
Lesson 2 Changes in State
 Lesson 2 Changes in State Student Labs and Activities Page Launch Lab 25 Content Vocabulary 26 Lesson Outline 27 MiniLab 29 Content Practice A 30 Content Practice B 31 Language Arts Support 32 School to
Lesson 2 Changes in State Student Labs and Activities Page Launch Lab 25 Content Vocabulary 26 Lesson Outline 27 MiniLab 29 Content Practice A 30 Content Practice B 31 Language Arts Support 32 School to
SOLIDS, LIQUIDS AND GASES
 CHEMIS TRY CONTENTS 17 SOLIDS, LIQUIDS AND GASES 17 Solids, Liquids and Gases 147 18 Solutions and Crystallisation 155 19 Separating Mixtures 162 20 Elements, Compounds and Mixtures 171 All materials exist
CHEMIS TRY CONTENTS 17 SOLIDS, LIQUIDS AND GASES 17 Solids, Liquids and Gases 147 18 Solutions and Crystallisation 155 19 Separating Mixtures 162 20 Elements, Compounds and Mixtures 171 All materials exist
Two students investigated the change of state of stearic acid from liquid to solid.
 Two students investigated the change of state of stearic acid from liquid to solid. They measured how the temperature of stearic acid changed over 5 minutes as it changed from liquid to solid. Figure shows
Two students investigated the change of state of stearic acid from liquid to solid. They measured how the temperature of stearic acid changed over 5 minutes as it changed from liquid to solid. Figure shows
Solids, Liquids, and Gases
 Date Class _ Solids, Liquids, and Gases Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. _ 1. The surface of water can act like a sort of skin due to a property
Date Class _ Solids, Liquids, and Gases Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. _ 1. The surface of water can act like a sort of skin due to a property
States of Matter. Chemistry The Four States of Matter
 States of Matter Chemistry The Four States of Matter 1 What is matter? Any substance that has mass and takes up space. Brian Pop Video http://glencoe.mcgrawhill.com/sites/dl/free/0078600472/164155/0004468
States of Matter Chemistry The Four States of Matter 1 What is matter? Any substance that has mass and takes up space. Brian Pop Video http://glencoe.mcgrawhill.com/sites/dl/free/0078600472/164155/0004468
2. What is meant by Chemical State?. 3. Changing states of matter is about changing,,, and other.
 Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Section 2: Changes of State (p. 68) 20 HOLT SCIENCE AND TECHNOLOGY
 Plasmas (p. 67) 24. More than 99 percent of the known matter in the universe is in the plasma state. 25. Plasmas are made up of particles that have broken apart. 26. Plasmas have a definite shape and volume.
Plasmas (p. 67) 24. More than 99 percent of the known matter in the universe is in the plasma state. 25. Plasmas are made up of particles that have broken apart. 26. Plasmas have a definite shape and volume.
IES LAURETUM SCIENCE NAME.
 IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
OBJECTIVES: By the end of class, students will be able to DO NOW
 7 th Grade Science Unit: Matter and Periodic Table Name: Date: Tuesday, December 6, 2016 Lesson: Matter 7 Changes of State OBJECTIVES: By the end of class, students will be able to SWBAT describe the process
7 th Grade Science Unit: Matter and Periodic Table Name: Date: Tuesday, December 6, 2016 Lesson: Matter 7 Changes of State OBJECTIVES: By the end of class, students will be able to SWBAT describe the process
4. Every CHANGE in matter includes a change in, which is conserved in a chemical reaction and. TRANSFORMED from one form to another.
 Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
States of Matter 1 of 21 Boardworks Ltd 2016
 States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
Task E. Make the following points:
 Using models to teach the particle theory Make the following points: 20 minutes Research has shown that there are differences in the ways teachers use models to explain particles, even within a single
Using models to teach the particle theory Make the following points: 20 minutes Research has shown that there are differences in the ways teachers use models to explain particles, even within a single
Conserving Water in the Desert
 Conserving Water in the Desert Lesson 2: What s the Matter? Water s Changing States Enduring Understanding Water is the only natural substance that is found in three different physical states - liquid,
Conserving Water in the Desert Lesson 2: What s the Matter? Water s Changing States Enduring Understanding Water is the only natural substance that is found in three different physical states - liquid,
Physical Science Exam 3 Study Guide. Dr. Karoline Rostamiani. Chapter 3
 Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Name Class Date. What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
Chemistry #3 Notebook States of Matter
 Name Hour Test Date Group # Chemistry #3 Notebook States of Matter LEARNING TARGETS I CAN model the motion and arrangement of particles in typical solids, liquids and gasses. I CAN describe how the motion
Name Hour Test Date Group # Chemistry #3 Notebook States of Matter LEARNING TARGETS I CAN model the motion and arrangement of particles in typical solids, liquids and gasses. I CAN describe how the motion
Chapter 8. Chapter 8. Preview. Bellringer. Chapter 8. Particles of Matter. Objectives. Chapter 8. Particles of Matter, continued
 States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
C1a The particulate nature of matter
 C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
Name: New Document 1. Class: Date: 83 minutes. Time: 82 marks. Marks: Comments:
 New Document Name: Class: Date: Time: 83 minutes Marks: 82 marks Comments: Q. Solid, liquid and gas are three different states of matter. (a) Describe the difference between the solid and gas states, in
New Document Name: Class: Date: Time: 83 minutes Marks: 82 marks Comments: Q. Solid, liquid and gas are three different states of matter. (a) Describe the difference between the solid and gas states, in
Rashid School for Boys. Year 7 Science. Particles. Name: Form:
 Rashid School for Boys Year Science Particles Name: Form: 1 By the end of this topic.. Unit Particles Level 3 I know that ice melts when it gets too warm and that liquid water turns into solid water (ice)
Rashid School for Boys Year Science Particles Name: Form: 1 By the end of this topic.. Unit Particles Level 3 I know that ice melts when it gets too warm and that liquid water turns into solid water (ice)
Notes: Phases of Matter and Phase Changes
 Name: Date: IP 670 Notes: Phases of Matter and Phase Changes There are four main phases of matter: We are only going to talk about the first three today. Solids Liquids Gases Molecular Molecules Wiggle
Name: Date: IP 670 Notes: Phases of Matter and Phase Changes There are four main phases of matter: We are only going to talk about the first three today. Solids Liquids Gases Molecular Molecules Wiggle
States of Matter. Changes in State
 CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
Year 7 Science. 7C1: The Particle Model. PPA Challenge
 Year 7 Science 7C1: The Particle Model PPA Challenge Name: Form: Task Sheet 1 (Bronze Challenge): The Particle Model Use the words in the box to label the diagram below. This particle diagram shows the
Year 7 Science 7C1: The Particle Model PPA Challenge Name: Form: Task Sheet 1 (Bronze Challenge): The Particle Model Use the words in the box to label the diagram below. This particle diagram shows the
Properties and Structure of Matter
 Properties and Structure of Matter Chapter 10 You can use a spider map to organize the main ideas and supporting details of a topic such as properties of matter. Look at the example shown below. The central
Properties and Structure of Matter Chapter 10 You can use a spider map to organize the main ideas and supporting details of a topic such as properties of matter. Look at the example shown below. The central
4.3.1 Changes of state and the particle model Density of materials. ρ = m. Content. Key opportunities for skills development
 4.3 Particle model of matter The particle model is widely used to predict the behaviour of solids, liquids and gases and this has many applications in everyday life. It helps us to explain a wide range
4.3 Particle model of matter The particle model is widely used to predict the behaviour of solids, liquids and gases and this has many applications in everyday life. It helps us to explain a wide range
Characteristic Properties of Matter
 Grade 8 Science, Quarter 2, Unit 2.1 Characteristic Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Measure the mass and volume of regular and
Grade 8 Science, Quarter 2, Unit 2.1 Characteristic Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Measure the mass and volume of regular and
Sit with your group from yesterday. You have 5 minutes to finish your poster and be ready to present your property of water to the class.
 To get out: Yellow Packet To pick up: Poster and markers Sit with your group from yesterday. You have 5 minutes to finish your poster and be ready to present your property of water to the class. Homework:
To get out: Yellow Packet To pick up: Poster and markers Sit with your group from yesterday. You have 5 minutes to finish your poster and be ready to present your property of water to the class. Homework:
Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4
 Cumulative Test 1 Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4 1. Why can you change ice into water but not into glass? 2. Why can't you see air? 3. Describe the ways in which ice, liquid water, and
Cumulative Test 1 Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4 1. Why can you change ice into water but not into glass? 2. Why can't you see air? 3. Describe the ways in which ice, liquid water, and
LEVEL ZERO VOICE CATALYST (10 minutes, individual work):
 LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Predict the properties of a metal. 2. Predict the properties of a nonmetal. 3. Which is more reactive? Ca or Cs? 4. How many electrons does N
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Predict the properties of a metal. 2. Predict the properties of a nonmetal. 3. Which is more reactive? Ca or Cs? 4. How many electrons does N
PROJECT 2. Steamy Story Comic Strip
 Evaluation A successful performance will correctly represent the atomic structure of your chosen element. You should creatively express through movement, dance, and facial and bodily expression the electrical
Evaluation A successful performance will correctly represent the atomic structure of your chosen element. You should creatively express through movement, dance, and facial and bodily expression the electrical
Thermal Effects. IGCSE Physics
 Thermal Effects IGCSE Physics Starter What is the difference between heat and temperature? What unit is thermal energy measured in? And what does it depend on? In which direction does heat flow? Heat (Thermal
Thermal Effects IGCSE Physics Starter What is the difference between heat and temperature? What unit is thermal energy measured in? And what does it depend on? In which direction does heat flow? Heat (Thermal
Part A (Level 1) A Matching (3 marks, 1 mark each) B True or false questions (7 marks, 1 mark each) Name: ( ) Time and Marks Class: Date:
 S1 Science Test Unit Name: ( ) Time and Marks Class: Date: Part A: 35 min / 100 marks Parts A & B: 45 min / 120 marks Note: 1 Attempt ALL questions. 2 Write your answers in the spaces provided on the Answer
S1 Science Test Unit Name: ( ) Time and Marks Class: Date: Part A: 35 min / 100 marks Parts A & B: 45 min / 120 marks Note: 1 Attempt ALL questions. 2 Write your answers in the spaces provided on the Answer
Lesson Plan: Stearic Acid
 Lesson Plan: Stearic Acid Created by: In this lesson, students investigate how stearic acid undergoes a 2014 AACT Middle School phase change from solid to liquid and back from liquid to solid. Content
Lesson Plan: Stearic Acid Created by: In this lesson, students investigate how stearic acid undergoes a 2014 AACT Middle School phase change from solid to liquid and back from liquid to solid. Content
NAME DATE CLASS TEST DATE:
 1 TEST DATE: 2 Vocabulary Chapter 8 Solids, liquids, and gases Condensation Crystals Evaporation Heat of fusion Heat of vaporization Kinetic theory of matter Plasma States of matter Thermal expansion Chapter
1 TEST DATE: 2 Vocabulary Chapter 8 Solids, liquids, and gases Condensation Crystals Evaporation Heat of fusion Heat of vaporization Kinetic theory of matter Plasma States of matter Thermal expansion Chapter
Chapter 14 9/21/15. Solids, Liquids & Gasses. Essential Questions! Kinetic Theory! Gas State! Gas State!
 Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
The Changing Phases of Matter
 The Changing Phases of Matter Teacher's Guide Editors: Brian A. Jerome, Ph.D. Stephanie Zak Jerome Assistant Editor: Louise Marrier Graphics: Lyndsey Canfield Dean Ladago Fred Thodal Lucy Rouse www.visuallearningco.com
The Changing Phases of Matter Teacher's Guide Editors: Brian A. Jerome, Ph.D. Stephanie Zak Jerome Assistant Editor: Louise Marrier Graphics: Lyndsey Canfield Dean Ladago Fred Thodal Lucy Rouse www.visuallearningco.com
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Developing an understanding of particle theory
 The National Strategies Secondary Developing an understanding of particle theory 1 For pupils to understand the particle theory properly we need to: teach a simple model challenge pupils to use the model
The National Strategies Secondary Developing an understanding of particle theory 1 For pupils to understand the particle theory properly we need to: teach a simple model challenge pupils to use the model
KINETIC PARTICLE THEORY
 KINETIC PARTICLE THEORY IMPORTANT DEFINITIONS: The mixing process in gases or solutions due to the random motion of particles is called Diffusion. The process by which a liquid changes into a vapour at
KINETIC PARTICLE THEORY IMPORTANT DEFINITIONS: The mixing process in gases or solutions due to the random motion of particles is called Diffusion. The process by which a liquid changes into a vapour at
5.4 The Kinetic Molecular Theory and Changes of State
 5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
WARM-UP. 1. What are the four states of matter? 2. What is melting point? 3. How does water change from a liquid to a gas? 4. Define viscosity.
 WARM-UP 1. What are the four states of matter? 2. What is melting point? 3. How does water change from a liquid to a gas? 4. Define viscosity. STATES OF MATTER: WEB QUEST With your lab partner, you will
WARM-UP 1. What are the four states of matter? 2. What is melting point? 3. How does water change from a liquid to a gas? 4. Define viscosity. STATES OF MATTER: WEB QUEST With your lab partner, you will
Lesson Plan Book-stacking Activity
 T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
MINI quiz Review: 1) All matter is made up of. 2) All particles in a substance are the same. 3) There is between the particles.
 MINI quiz Review: 1) All matter is made up of. 2) All particles in a substance are the same. 3) There is between the particles. 4) The particles are always. 5) The particles in a substance are to one another.
MINI quiz Review: 1) All matter is made up of. 2) All particles in a substance are the same. 3) There is between the particles. 4) The particles are always. 5) The particles in a substance are to one another.
States of Matter: Solid, Liquid, and Gas
 Movie Special Effects Activity 2 States of Matter: Solid, Liquid, and Gas GOALS In this activity you will: Create an animation to illustrate the behavior of particles in different phases of matter, and
Movie Special Effects Activity 2 States of Matter: Solid, Liquid, and Gas GOALS In this activity you will: Create an animation to illustrate the behavior of particles in different phases of matter, and
Density: The property that compares an object s mass to its volume. Mass is the measure of the amount of matter that makes up an object.
 Science Chapter 6: Matter Study Guide Lesson One: Properties of Matter A property is a characteristic of an object. You can identify properties of matter using your senses. Color, Size, Shape, Texture,
Science Chapter 6: Matter Study Guide Lesson One: Properties of Matter A property is a characteristic of an object. You can identify properties of matter using your senses. Color, Size, Shape, Texture,
4 Discuss and evaluate the 5th state of matter. 3 - Differentiate among the four states of matter in terms of energy,
 Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
3.3 Phase Changes Charactaristics of Phase Changes phase change
 A large iceberg contains enough fresh water to supply millions of people with water for a year. As it moves into warmer areas, the ice changes to liquid water and eventually disappears. What happens when
A large iceberg contains enough fresh water to supply millions of people with water for a year. As it moves into warmer areas, the ice changes to liquid water and eventually disappears. What happens when
NAME: SCORE: /100 NUMBER: MATTER WEBQUEST
 NAME: SCORE: /100 NUMBER: MATTER WEBQUEST Directions: Complete the following tasks by going to the websites and answering the questions. Use your time wisely as you will be graded on your use of class
NAME: SCORE: /100 NUMBER: MATTER WEBQUEST Directions: Complete the following tasks by going to the websites and answering the questions. Use your time wisely as you will be graded on your use of class
GRADE 8: Materials 1. UNIT 8M.1 7 hours. Atoms and molecules. Resources. About this unit. Previous learning. Expectations
 GRADE 8: Materials 1 Atoms and molecules UNIT 8M.1 7 hours About this unit This is the first of four units on materials for Grade 8. This unit builds on all the units in Grade 7, providing a theoretical
GRADE 8: Materials 1 Atoms and molecules UNIT 8M.1 7 hours About this unit This is the first of four units on materials for Grade 8. This unit builds on all the units in Grade 7, providing a theoretical
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4923060500* CO-ORDINATED SCIENCES 0654/61 Paper 6 Alternative to Practical October/November 2014
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4923060500* CO-ORDINATED SCIENCES 0654/61 Paper 6 Alternative to Practical October/November 2014
States of Matter: A Solid Lesson where Liquids Can be a Gas!
 TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
KS3 Science. Heat and Energy
 KS3 Science Heat and Energy Heat and Energy Key Words Write a definition for each of the key words listed below Key words States of matter Melt Freeze Evaporate Condense Heat Conduction Convention Radiation
KS3 Science Heat and Energy Heat and Energy Key Words Write a definition for each of the key words listed below Key words States of matter Melt Freeze Evaporate Condense Heat Conduction Convention Radiation
Physics. Practical 5: Density. Practical Objective. Content Objective. Apparatus. Your teacher may watch to see if you can:
 The density of a substance is the mass of a unit volume of that substance. Almost all substances are most dense when they are solids and least dense when they are gases. The arrangement of particles can
The density of a substance is the mass of a unit volume of that substance. Almost all substances are most dense when they are solids and least dense when they are gases. The arrangement of particles can
Solids, liquids and gases
 Solids, liquids and gases Duration 60 minutes Lesson overview Students share what they know about the three states of matter solid, liquid and gas and consider some of their scientific properties. They
Solids, liquids and gases Duration 60 minutes Lesson overview Students share what they know about the three states of matter solid, liquid and gas and consider some of their scientific properties. They
Name... Class... Date... Specific heat capacity and specific latent heat
 Specific heat capacity and specific latent heat Specification references: P3.2.2 Temperature changes in a system and specific heat capacity P3.2.3 Changes of heat and specific latent heat Aims This is
Specific heat capacity and specific latent heat Specification references: P3.2.2 Temperature changes in a system and specific heat capacity P3.2.3 Changes of heat and specific latent heat Aims This is
Name: Regents Chemistry: Mr. Palermo. Student Version. Notes: Unit 6A Heat
 Name: Regents Chemistry: Mr. Palermo Student Version Notes: Unit 6A Heat Name: KEY IDEAS Heat is a transfer of energy (usually thermal energy) from a body of higher temperature to a body of lower temperature.
Name: Regents Chemistry: Mr. Palermo Student Version Notes: Unit 6A Heat Name: KEY IDEAS Heat is a transfer of energy (usually thermal energy) from a body of higher temperature to a body of lower temperature.
Calorimetry. A calorimeter is a device in which this energy transfer takes place
 Calorimetry One technique for measuring specific heat involves heating a material, adding it to a sample of water, and recording the final temperature This technique is known as calorimetry A calorimeter
Calorimetry One technique for measuring specific heat involves heating a material, adding it to a sample of water, and recording the final temperature This technique is known as calorimetry A calorimeter
States of matter. Book page , Syllabus /09/2016
 States of matter Book page 169 171, 173-175 Syllabus 5.7 5.14 05/09/2016 cgrahamphysics.com 2015 What is my state of matter? sand Decaffeinated coffee Glass Supercritical fluids Supercritical fluids Coldest
States of matter Book page 169 171, 173-175 Syllabus 5.7 5.14 05/09/2016 cgrahamphysics.com 2015 What is my state of matter? sand Decaffeinated coffee Glass Supercritical fluids Supercritical fluids Coldest
Introductory chemistry
 1 Introductory chemistry Science Anchors Science anchors are ongoing engaging tasks that students can work on independently. They are curriculum based, clearly defined and differentiated for students.
1 Introductory chemistry Science Anchors Science anchors are ongoing engaging tasks that students can work on independently. They are curriculum based, clearly defined and differentiated for students.
3. Watch video All About Solids, Liquids, & Gases. Watch first eight minutes.
 Structure and Transformation of Matter Original (2008) Lesson Plan I can classify matter into different categories. I can describe the differences between solid, liquid, and gas. Lesson 1: Search for Matter
Structure and Transformation of Matter Original (2008) Lesson Plan I can classify matter into different categories. I can describe the differences between solid, liquid, and gas. Lesson 1: Search for Matter
1 Three States of Matter
 CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
This activity has been used in an introductory chemistry course (prep chemistry or GOB course) Learning Goals: Prerequisite knowledge
 This activity has been used in an introductory chemistry course (prep chemistry or GOB course) Learning Goals: Name phase changes Identify phase changes at molecular (particulate) level Name intermolecular
This activity has been used in an introductory chemistry course (prep chemistry or GOB course) Learning Goals: Name phase changes Identify phase changes at molecular (particulate) level Name intermolecular
