Task E. Make the following points:
|
|
|
- Aldous Williamson
- 6 years ago
- Views:
Transcription
1 Using models to teach the particle theory Make the following points: 20 minutes Research has shown that there are differences in the ways teachers use models to explain particles, even within a single department. The purpose of this session is to explore some of the differences about the models that are used in teaching science at Key Stage 3 and point out that there is often no right or wrong answer, but that we use different models for different purposes. This session also attempts to make clear that whilst this is so, some models are better than others and that it would be helpful if teachers within a department adopted a consistent approach. Slide 3.6 Task E 10 minutes Show participants Slide 3.6 to introduce them to Task E. Ask them to draw quickly (allow no more than 1 minute) a particle picture to represent a solid, liquid and gas. Then ask them to compare notes and discuss any differences. Allow 2-3 minutes for this. Task E (part 1): Pictures of particles Draw a particle picture to represent: A solid A liquid A gas Slide 3.6 Slides 3.7/3.8 Next show participants Slide 3.7, and then Slide Misconceptions in Key Stage 3 science notes for course tutors Crown copyright 2002
2 Solid, liquid and gas picture 1 Solid Slide 3.7 Liquid Gas Solid, liquid and gas picture 2 Solid Slide 3.8 Liquid Gas Say that: Particle theory will probably be new to pupils at Key Stage 3. It is not in the programme of study at Key Stage 2, although primary teachers may have referred to it. Many textbooks tend to show solids, liquids and gases as in the first example. However, others suggest that many of the particles representing a liquid should be touching as in the second example. 24 Misconceptions in Key Stage 3 science notes for course tutors Crown copyright 2002
3 Reasons suggested for this picture are that when solids melt to form liquids their volumes are about the same, and that you cannot easily compress solids or liquids. In air (a mixture of gases) the particles are about nine diameters apart; hence gases are relatively easy to compress. Additional guidance The marking scheme for the end of the Key Stage 3 science tests allocates marks for identifying that 50% of particles should be touching in a liquid, but there is no standard set or expressed for this. Handout 3.9 Handout 3.10 Whilst this may be a useful picture to explain the solids, liquids and gases, it doesn t work with other phenomena such as expansion of solids. The particletouching model is not a correct model; rather, it is a good enough model that can be used to explain phenomena such as the comparative noncompressibility of liquids and solids. Use Handouts 3.9 and 3.10 to illustrate how a good enough model can be used in teaching particles.
4 A good enough version of the billiard ball model to explain a range of physical phenomena Handout 3.9 Basic premises All matter consists of tiny particles. Types of particle vary in their volume. Types of particle vary in their mass. A crystalline solid has a set shape and a fixed volume that is independent of that of its container. To represent this the particles in the particle model: are arranged in rows and sheets; are close together; are held together tightly; have energy and vibrate around a point; cannot easily change places. A liquid has a fixed volume and takes up the shape of its container up to the level of the surface of the liquid. To represent this the particles in the particle model: have no set pattern; are also close together; are not so tightly held together as in a solid; have more energy and move randomly; can change places. A gas takes up the whole volume of its container. If that container is open, it will diffuse into the air. To represent this the particles in the particle model: are not arranged in any way; are far apart; are very weakly held together; have much more energy and move very rapidly in all directions; constantly change places in no pattern. Note: the distance between particles in air is about nine diameters. 29 Misconceptions in Key Stage 3 science notes for course tutors Crown copyright 2002
5 Developing a sequence of good enough particle models Handout 3.10 Scientific idea Matter made of particles that are moving, very small and broadly of similar size Particles are of different size/mass When particles interact there are forces acting between them Particles are of different types. There are a set number of atoms and all other particles are made from these The types of particle interactions: A + B AB AB A + B AB + C AC + B AB + CD AC + BD Teaching model Billiard ball (Handout 3.9) Computer animations Use marbles, ball bearings, beads, etc. to illustrate Use magnetic marbles, or sticky and non-sticky balls to illustrate forces Use commercially produced models such as Molymod or Lego to illustrate different types Use Molymod type or Lego to model each reaction type and model reactions whilst they take place Can be used to explain Changes of state, solidifying, melting, evaporating Density, viscosity, movement of substances through cell membranes, Brownian motion Dissolving Elements, compounds and chemical change Patterns in chemical change: combination disassociation displacement (special case of recombination) recombination 30 Misconceptions in Key Stage 3 science notes for course tutors Crown copyright 2002
Developing an understanding of particle theory
 The National Strategies Secondary Developing an understanding of particle theory 1 For pupils to understand the particle theory properly we need to: teach a simple model challenge pupils to use the model
The National Strategies Secondary Developing an understanding of particle theory 1 For pupils to understand the particle theory properly we need to: teach a simple model challenge pupils to use the model
Chapter 6 Kinetic Particle Theory
 Chapter 6 Kinetic Particle Theory To understand that matter is made up of small particles which are in constant and random motion. To describe simple model of solids, liquids and gases, in terms of the
Chapter 6 Kinetic Particle Theory To understand that matter is made up of small particles which are in constant and random motion. To describe simple model of solids, liquids and gases, in terms of the
INTRODUCTION TO LESSON CLUSTER 7
 INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
TEACHER NOTES: ICE CUBE POSTER
 TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
Some possible models and analogies for teaching about elements and compounds. Additional guidance
 Some possible models and analogies for teaching about elements and compounds Remind participants that using ideas of particles to clarify the differences between elements, compounds and mixtures is an
Some possible models and analogies for teaching about elements and compounds Remind participants that using ideas of particles to clarify the differences between elements, compounds and mixtures is an
Evaluation : Misconceptions in Key Stage 3 science
 Key Stage 3 National Strategy science Evaluation : Misconceptions in Key Stage 3 science For completion by teachers What were the most successful aspects of today s sessions? What changes would you suggest
Key Stage 3 National Strategy science Evaluation : Misconceptions in Key Stage 3 science For completion by teachers What were the most successful aspects of today s sessions? What changes would you suggest
KINETIC PARTICLE THEORY
 KINETIC PARTICLE THEORY IMPORTANT DEFINITIONS: The mixing process in gases or solutions due to the random motion of particles is called Diffusion. The process by which a liquid changes into a vapour at
KINETIC PARTICLE THEORY IMPORTANT DEFINITIONS: The mixing process in gases or solutions due to the random motion of particles is called Diffusion. The process by which a liquid changes into a vapour at
States of Matter. Chemistry The Four States of Matter
 States of Matter Chemistry The Four States of Matter 1 What is matter? Any substance that has mass and takes up space. Brian Pop Video http://glencoe.mcgrawhill.com/sites/dl/free/0078600472/164155/0004468
States of Matter Chemistry The Four States of Matter 1 What is matter? Any substance that has mass and takes up space. Brian Pop Video http://glencoe.mcgrawhill.com/sites/dl/free/0078600472/164155/0004468
IES LAURETUM SCIENCE NAME.
 IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
Chapter 7: Kinetic Molecular Theory. 7.1 States of Matter
 Chapter 7: Kinetic Molecular Theory 7.1 States of Matter 7.1 KMT and Changes in State Matter: anything with mass and volume Mass: quantity of matter that a substance or object contains (g or kg) Volume:
Chapter 7: Kinetic Molecular Theory 7.1 States of Matter 7.1 KMT and Changes in State Matter: anything with mass and volume Mass: quantity of matter that a substance or object contains (g or kg) Volume:
Term Info Picture. Anything that has mass and takes up space; everything is made of matter.
 Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
States of Matter. Chemistry The Four States of Matter. Chumbler - Properties of Matter 1
 Chemistry The Four States of Matter Chumbler - Properties of Matter 1 The Four States of Matter Solid Liquid Gas Plasma Four States Chumbler - Properties of Matter 2 The Four States of Matter Basis of
Chemistry The Four States of Matter Chumbler - Properties of Matter 1 The Four States of Matter Solid Liquid Gas Plasma Four States Chumbler - Properties of Matter 2 The Four States of Matter Basis of
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
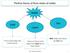 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
C1a The particulate nature of matter
 C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
States of Matter. Essential Question: How does the movement of atoms and molecules relate to matter s different phases?
 States of Matter Essential Question: How does the movement of atoms and molecules relate to matter s different phases? These notes come from pages 60 to 73 in your Physical Science Textbook All Matter
States of Matter Essential Question: How does the movement of atoms and molecules relate to matter s different phases? These notes come from pages 60 to 73 in your Physical Science Textbook All Matter
Table of Contents. Diagnostic Pre-test... 5 Lesson 1: What Is an Atom? Lesson 5: Gases. Lesson 6: Melting and Freezing. Lesson 2: What Are Molecules?
 Table of Contents Diagnostic Pre-test................. 5 Lesson 1: What Is an Atom? What Can You See?................ 11 What Is Matter Made Of?........... 12 Describing the Atom............... 13 What
Table of Contents Diagnostic Pre-test................. 5 Lesson 1: What Is an Atom? What Can You See?................ 11 What Is Matter Made Of?........... 12 Describing the Atom............... 13 What
6-3 Particle model of matter Physics
 6-3 Particle model of matter Physics.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Physics.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
States of Matter 1 of 21 Boardworks Ltd 2016
 States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
The lower the energy of a substance, the interaction between its atoms and molecules.
 PHYSICAL STATES OF MATTER Kinetic Molecular Theory To understand the different states in which matter can exist, we need to understand something called the Kinetic Molecular Theory of Matter. Kinetic Molecular
PHYSICAL STATES OF MATTER Kinetic Molecular Theory To understand the different states in which matter can exist, we need to understand something called the Kinetic Molecular Theory of Matter. Kinetic Molecular
Properties and Structure of Matter
 Properties and Structure of Matter Chapter 10 You can use a spider map to organize the main ideas and supporting details of a topic such as properties of matter. Look at the example shown below. The central
Properties and Structure of Matter Chapter 10 You can use a spider map to organize the main ideas and supporting details of a topic such as properties of matter. Look at the example shown below. The central
Chapter 7.1. States of Matter
 Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Foundations of Chemistry
 Foundations of Chemistry Physical Properties Physical Properties As you read in Lesson 1, the arrangement of atoms determines whether matter is a substance or a mixture. The arrangement of atoms also determines
Foundations of Chemistry Physical Properties Physical Properties As you read in Lesson 1, the arrangement of atoms determines whether matter is a substance or a mixture. The arrangement of atoms also determines
Lesson 02: Physical Properties of Matter. 01 Matter
 Chemistry 11, Physical Properties, Unit 02 1 Lesson 02: Physical Properties of Matter 01 Matter Almost everything in the universe is made of matter matter has volume matter has mass matter is made up of
Chemistry 11, Physical Properties, Unit 02 1 Lesson 02: Physical Properties of Matter 01 Matter Almost everything in the universe is made of matter matter has volume matter has mass matter is made up of
SOLIDS, LIQUIDS AND GASES
 CHEMIS TRY CONTENTS 17 SOLIDS, LIQUIDS AND GASES 17 Solids, Liquids and Gases 147 18 Solutions and Crystallisation 155 19 Separating Mixtures 162 20 Elements, Compounds and Mixtures 171 All materials exist
CHEMIS TRY CONTENTS 17 SOLIDS, LIQUIDS AND GASES 17 Solids, Liquids and Gases 147 18 Solutions and Crystallisation 155 19 Separating Mixtures 162 20 Elements, Compounds and Mixtures 171 All materials exist
Solids (cont.) Describe the movement of particles in a solid and the forces between them.
 Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Chapter 22 States of matter. Section 1 matter Section 2 Changes of State
 Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Physical Science Exam 3 Study Guide. Dr. Karoline Rostamiani. Chapter 3
 Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
1 Three States of Matter
 CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
6-3 Particle model of matter Trilogy
 6-3 Particle model of matter Trilogy.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
6-3 Particle model of matter Trilogy.0 A teacher uses a tray filled with table tennis balls to model how particles are arranged in materials, as shown in Figure Figure. Initially the balls are arranged
1. 2. Differentiate states of matter. Arrangement of Particles
 Lesson 2 Predict three facts that will be discussed in Lesson 2 after reading the headings. Record your predictions in your Science Journal. Definition: Define physical property, and give two examples.
Lesson 2 Predict three facts that will be discussed in Lesson 2 after reading the headings. Record your predictions in your Science Journal. Definition: Define physical property, and give two examples.
Ch(3)Matter & Change. John Dalton
 Ch(3)Matter & Change John Dalton What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter. The Particle Theory of Matter 1.
Ch(3)Matter & Change John Dalton What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter. The Particle Theory of Matter 1.
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Molecular mass is the sum of the atomic masses of all of the atoms in the molecule
 PERIODIC TABLE IA 1 PERIODIC TABLE IA 2 V. MATTER-Matter is anything that occupies space and has mass. Symbols and Formulas Symbols represent individual atoms of an element: H O Cl Br Ag Formulas represent
PERIODIC TABLE IA 1 PERIODIC TABLE IA 2 V. MATTER-Matter is anything that occupies space and has mass. Symbols and Formulas Symbols represent individual atoms of an element: H O Cl Br Ag Formulas represent
ENERGY IN CHEMISTRY. R. Ashby Duplication by permission only.
 CH 11 TOPIC 28 CHANGING STATES OF MATTER 1 You have mastered this topic when you can: 1) define or describe: ENERGY, POTENTIAL ENERGY, KINETIC ENERGY & KINETIC MOLECULAR THEORY 2) define or describe HEAT
CH 11 TOPIC 28 CHANGING STATES OF MATTER 1 You have mastered this topic when you can: 1) define or describe: ENERGY, POTENTIAL ENERGY, KINETIC ENERGY & KINETIC MOLECULAR THEORY 2) define or describe HEAT
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company
 Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
The Next Generation Science Standards (NGSS) CHAPTER 2, LESSON 1 HEAT, TEMPERATURE, AND CONDUCTION MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES
 THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
Gases: Properties and Behaviour
 SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
Matter & Energy. Objectives: properties and structures of the different states of matter.
 Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Chem 1075 Chapter 13 Liquids and Solids Lecture Outline
 Chem 1075 Chapter 13 Liquids and Solids Lecture Outline Slide 2-3 Properties of Liquids Unlike gases, liquids respond dramatically to temperature and pressure changes. We can study the liquid state and
Chem 1075 Chapter 13 Liquids and Solids Lecture Outline Slide 2-3 Properties of Liquids Unlike gases, liquids respond dramatically to temperature and pressure changes. We can study the liquid state and
Matter Lesson 2. Learning Goal 3: I can describe the differences between physical and chemical changes of matter.
 Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Copyright 2015 Edmentum - All rights reserved. During which of the following phase changes is there a gain in energy? I.
 Study Island Copyright 2015 Edmentum - All rights reserved. Generation Date: 03/16/2015 Generated By: Kristina Brown 1. Examine the phase-change diagram below. During which of the following phase changes
Study Island Copyright 2015 Edmentum - All rights reserved. Generation Date: 03/16/2015 Generated By: Kristina Brown 1. Examine the phase-change diagram below. During which of the following phase changes
5.4 The Kinetic Molecular Theory and Changes of State
 5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
LAB 11 Molecular Geometry Objectives
 LAB 11 Molecular Geometry Objectives At the end of this activity you should be able to: Write Lewis structures for molecules. Classify bonds as nonpolar covalent, polar covalent, or ionic based on electronegativity
LAB 11 Molecular Geometry Objectives At the end of this activity you should be able to: Write Lewis structures for molecules. Classify bonds as nonpolar covalent, polar covalent, or ionic based on electronegativity
Year 7 Science. 7C1: The Particle Model. PPA Challenge
 Year 7 Science 7C1: The Particle Model PPA Challenge Name: Form: Task Sheet 1 (Bronze Challenge): The Particle Model Use the words in the box to label the diagram below. This particle diagram shows the
Year 7 Science 7C1: The Particle Model PPA Challenge Name: Form: Task Sheet 1 (Bronze Challenge): The Particle Model Use the words in the box to label the diagram below. This particle diagram shows the
Rashid School for Boys. Year 7 Science. Particles. Name: Form:
 Rashid School for Boys Year Science Particles Name: Form: 1 By the end of this topic.. Unit Particles Level 3 I know that ice melts when it gets too warm and that liquid water turns into solid water (ice)
Rashid School for Boys Year Science Particles Name: Form: 1 By the end of this topic.. Unit Particles Level 3 I know that ice melts when it gets too warm and that liquid water turns into solid water (ice)
States of Matter. Solids, Liquids, and Gases
 States of Matter Solids, Liquids, and Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
States of Matter Solids, Liquids, and Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
Kinetic Theory of Matter notes 2012
 Kinetic Theory of Matter notes 2012 Kinetic Theory of Matter 3 parts: 1) All matter is made up of and that act as tiny 2) These tiny particles are always in. State of matter depends on its molecular motion
Kinetic Theory of Matter notes 2012 Kinetic Theory of Matter 3 parts: 1) All matter is made up of and that act as tiny 2) These tiny particles are always in. State of matter depends on its molecular motion
Matter & Energy. Kinetic Theory of Matter. Kinetic Theory of Matter. Kinetic Theory of Matter. Kinetic Theory of Matter. Temperature.
 Matter & Energy 1) All matter is made up of atoms and molecules that act as tiny particles. 1 2 2) These tiny particles are always in motion. State of matter depends on its molecular motion as measured
Matter & Energy 1) All matter is made up of atoms and molecules that act as tiny particles. 1 2 2) These tiny particles are always in motion. State of matter depends on its molecular motion as measured
What is Matter? How can matter be classified? Every sample of matter is either an element, a compound, or a mixture.
 Matter Section 1 What is Matter? How can matter be classified? Every sample of matter is either an element, a compound, or a mixture. matter: anything that has mass and takes up space Matter Section 1
Matter Section 1 What is Matter? How can matter be classified? Every sample of matter is either an element, a compound, or a mixture. matter: anything that has mass and takes up space Matter Section 1
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4
 Cumulative Test 1 Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4 1. Why can you change ice into water but not into glass? 2. Why can't you see air? 3. Describe the ways in which ice, liquid water, and
Cumulative Test 1 Name Date CUMULATIVE TEST FOR LESSON CLUSTERS 1-4 1. Why can you change ice into water but not into glass? 2. Why can't you see air? 3. Describe the ways in which ice, liquid water, and
Matter A Review. Has mass Takes up space. Chemistry is the study of MATTER!
 Matter A Review Has mass Takes up space ex. Chemistry is the study of MATTER! Topic 4.D - Classifying Unit 1 Organizing Matter 2011 Classifications of Matter Matter Anything that has mass and takes up
Matter A Review Has mass Takes up space ex. Chemistry is the study of MATTER! Topic 4.D - Classifying Unit 1 Organizing Matter 2011 Classifications of Matter Matter Anything that has mass and takes up
Mixture Examples. Classifications of Matter. Matter A Review. Topic 4.D - Classifying. Mixtures. Types of Mixtures 9/4/2011. Has mass Takes up space
 Matter A Review Has mass Takes up space ex. Chemistry is the study of MATTER! Topic 4.D - Classifying Unit 1 Organizing Matter 2011 Classifications of Matter Mixtures Contain more than one kind of matter
Matter A Review Has mass Takes up space ex. Chemistry is the study of MATTER! Topic 4.D - Classifying Unit 1 Organizing Matter 2011 Classifications of Matter Mixtures Contain more than one kind of matter
Matter. Energy- which is a property of matter!! Matter: anything that takes up space and has mass
 Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!! Matter is made up of moving particles! Instead of
Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!! Matter is made up of moving particles! Instead of
Unit 1 Lesson 6 Changes of State. Copyright Houghton Mifflin Harcourt Publishing Company
 The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
Chapter 8. Chapter 8. Preview. Bellringer. Chapter 8. Particles of Matter. Objectives. Chapter 8. Particles of Matter, continued
 States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
2. What is meant by Chemical State?. 3. Changing states of matter is about changing,,, and other.
 Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Lesson 9: States of Matter
 Lesson 9: States of Matter Do Now 6O, 6S 11.8.18 Take out HW 6.14 to be checked. Copy info into CJ keep CJ out and open on desk throughout class. On Do Now Page #5, copy and answer: 1. If you use a magnet
Lesson 9: States of Matter Do Now 6O, 6S 11.8.18 Take out HW 6.14 to be checked. Copy info into CJ keep CJ out and open on desk throughout class. On Do Now Page #5, copy and answer: 1. If you use a magnet
Matter, Atoms & Molecules
 Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Chapter 3. Preview. Section 1 Three States of Matter. Section 2 Behavior of Gases. Section 3 Changes of State. States of Matter.
 States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter. The Solid State. Particles are tightly packed, very close together (strong cohesive forces) Low kinetic energy (energy of motion)
 States of Matter The Solid State Particles are tightly packed, very close together (strong cohesive forces) Low kinetic energy (energy of motion) Fixed shape and volume Crystalline or amorphous structure
States of Matter The Solid State Particles are tightly packed, very close together (strong cohesive forces) Low kinetic energy (energy of motion) Fixed shape and volume Crystalline or amorphous structure
6th Grade: Great Salt Lake is Salty
 Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
Curriculum written by Megan Black in partnership with The Great Salt Lake Institute at Westminster College. 6th Grade: Great Salt Lake is Salty Lesson Description: In this lesson students will compare
DEMONSTRATION 4.1 MOLECULES HITTING EACH OTHER
 DEMONSTRATION 4.1 MOLECULES HITTING EACH OTHER Directions for doing the demonstration are in the Science Book Teacher's Guide. 1. Students should include these ideas: The air moving out of the hair dryer
DEMONSTRATION 4.1 MOLECULES HITTING EACH OTHER Directions for doing the demonstration are in the Science Book Teacher's Guide. 1. Students should include these ideas: The air moving out of the hair dryer
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
States of Matter. Changes in State
 CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key)
 The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
Matter: Properties and Change
 Matter: Properties and Change 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are
Matter: Properties and Change 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are
Subject Link to prior learning: Term Duration (approx.) Module
 . Cells and organisation Cells as the fundamental unit of living organisms, including how to observe, interpret and record cell structure using a light microscope. The functions of the cell wall, cell
. Cells and organisation Cells as the fundamental unit of living organisms, including how to observe, interpret and record cell structure using a light microscope. The functions of the cell wall, cell
CHAPTER 4 - STATES OF MATTER. Mr. Polard Physical Science Ingomar Middle School
 CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
Two students investigated the change of state of stearic acid from liquid to solid.
 Two students investigated the change of state of stearic acid from liquid to solid. They measured how the temperature of stearic acid changed over 5 minutes as it changed from liquid to solid. Figure shows
Two students investigated the change of state of stearic acid from liquid to solid. They measured how the temperature of stearic acid changed over 5 minutes as it changed from liquid to solid. Figure shows
Classification of Matter. States of Matter Physical and Chemical Properties Physical and Chemical Changes
 1 Classification of Matter States of Matter Physical and Chemical Properties Physical and Chemical Changes 2 Classification of Matter Matter is anything that has mass and occupies space. We can classify
1 Classification of Matter States of Matter Physical and Chemical Properties Physical and Chemical Changes 2 Classification of Matter Matter is anything that has mass and occupies space. We can classify
States of Matter. What physical changes and energy changes occur as matter goes from one state to another?
 Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
CHEMISTRY Matter and Change. Chapter 12: States of Matter
 CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
Chapter Preview. Improving Comprehension
 Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
THE GASEOUS STATE OF MATTER
 THE GASEOUS STATE OF MATTER The gaseous state of matter is a form of matter in which the particles are in a high state of energy, which causes them to vibrate rapidly, experiencing a strong repulsion among
THE GASEOUS STATE OF MATTER The gaseous state of matter is a form of matter in which the particles are in a high state of energy, which causes them to vibrate rapidly, experiencing a strong repulsion among
Unit 7G Particle model of solids, liquids and gases. About the unit. Expectations. Science Year 7. Where the unit fits in
 Science Year 7 Unit 7G Particle model of solids, liquids and gases About the unit In this unit pupils: learn how the particle model can be used to explain differences between solids, liquids and gases
Science Year 7 Unit 7G Particle model of solids, liquids and gases About the unit In this unit pupils: learn how the particle model can be used to explain differences between solids, liquids and gases
Characteristic Properties of Matter
 Grade 8 Science, Quarter 2, Unit 2.1 Characteristic Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Measure the mass and volume of regular and
Grade 8 Science, Quarter 2, Unit 2.1 Characteristic Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Measure the mass and volume of regular and
Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible
 Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Name Date Class STATES OF MATTER
 13 STATES OF MATTER Chapter Test A A. Matching Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A Column B 1. amorphous
13 STATES OF MATTER Chapter Test A A. Matching Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A Column B 1. amorphous
Diffusion, Brownian Motion, Solids/Liquids/Gases
 iffusion, rownian Motion, Solids/Liquids/Gases Question Paper 2 Level IGSE Subject hemistry (0620/0971) Exam oard ambridge International Examinations (IE) Topic The particulate nature of matter Sub-Topic
iffusion, rownian Motion, Solids/Liquids/Gases Question Paper 2 Level IGSE Subject hemistry (0620/0971) Exam oard ambridge International Examinations (IE) Topic The particulate nature of matter Sub-Topic
Self-Check. change in pressure. 6. I can explain how the volume of a gas is changed by a change in temperature S. Coates
 Self-Check YES NO 1. I can describe how atoms move in a solid, liquid, and gas 2. I can describe the speed/energy of the atoms in a solid, liquid, and gas. 3. I can explain how the distance between atoms
Self-Check YES NO 1. I can describe how atoms move in a solid, liquid, and gas 2. I can describe the speed/energy of the atoms in a solid, liquid, and gas. 3. I can explain how the distance between atoms
The Singapore Copyright Act applies to the use of this document.
 Title Use of analogy in teaching the particulate theory of matter Author(s) Boo Hong Kwen & Toh Kok Aun Source Teaching and Learning, 17(2),79-85 Published by Institute of Education (Singapore) This document
Title Use of analogy in teaching the particulate theory of matter Author(s) Boo Hong Kwen & Toh Kok Aun Source Teaching and Learning, 17(2),79-85 Published by Institute of Education (Singapore) This document
Study Guide for Chapters 2, 3, and 10
 Study Guide for Chapters 2, 3, and 10 1. What is matter? Where can it be found? Anything that has mass and takes up space. 2. What units are used to measure volume? Liters and meters cubed 3. How would
Study Guide for Chapters 2, 3, and 10 1. What is matter? Where can it be found? Anything that has mass and takes up space. 2. What units are used to measure volume? Liters and meters cubed 3. How would
Topic review. hi.com.au Science Resource Centre. Using scientific language 1. You should be familiar with the words in the following list.
 Topic review Using scientific language 1. You should be familiar with the words in the following list. condense solid boiling matter gas diffusion melting solidify particles substance liquid property compression
Topic review Using scientific language 1. You should be familiar with the words in the following list. condense solid boiling matter gas diffusion melting solidify particles substance liquid property compression
Its Properties. Its Changes
 Chapter Introduction Lesson 1 Lesson 2 Chapter Wrap-Up Matter and Its Properties Matter and Its Changes What gives a substance its unique identity? What do you think? Before you begin, decide if you agree
Chapter Introduction Lesson 1 Lesson 2 Chapter Wrap-Up Matter and Its Properties Matter and Its Changes What gives a substance its unique identity? What do you think? Before you begin, decide if you agree
Matter: Properties and Change
 Matter: Properties and Change Date: 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element
Matter: Properties and Change Date: 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 1, LESSON 1 MOLECULES MATTER MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance
The Next Generation Science Standards (NGSS) CHAPTER 1, LESSON 1 MOLECULES MATTER MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance
W X gas liquid solid Y Z. C X and Y D Y and Z X Y Z. C Z to X D Z to Y
 1 In which changes do the particles move further apart? W X gas liquid solid Y Z W and X W and Z X and Y Y and Z 2 iagrams X, Y and Z represent the three states of matter. X Y Z Which change occurs during
1 In which changes do the particles move further apart? W X gas liquid solid Y Z W and X W and Z X and Y Y and Z 2 iagrams X, Y and Z represent the three states of matter. X Y Z Which change occurs during
Elements, compounds or mixtures? (1)
 Elements, compounds or mixtures? (1) n science, it is important to know the difference between elements, compounds and mixtures. Try to explain what you think each of these words means: 1. An element is
Elements, compounds or mixtures? (1) n science, it is important to know the difference between elements, compounds and mixtures. Try to explain what you think each of these words means: 1. An element is
Lesson 1 Solids, Liquids, and Gases
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
PROPERTIES OF MATTER
 PROPERTIES OF MATTER LAW OF CONSERVATION OF MATTER Matter cannot be created nor destroyed, it can only be changed from one form to another. Matter and energy are interchangeable according to E=mc 2 (E=amount
PROPERTIES OF MATTER LAW OF CONSERVATION OF MATTER Matter cannot be created nor destroyed, it can only be changed from one form to another. Matter and energy are interchangeable according to E=mc 2 (E=amount
Draw the Lewis structures of all 7 diatomic elements
 Warm up Draw the Lewis structures of all 7 diatomic elements States of Matter - Part 1 - Gasses Definitions kinetic-molecular theory particles of matter are always in motion ideal gas hypothetical gas
Warm up Draw the Lewis structures of all 7 diatomic elements States of Matter - Part 1 - Gasses Definitions kinetic-molecular theory particles of matter are always in motion ideal gas hypothetical gas
Station 1 Water is a polar molecule and has a very unique structure
 Station 1 Water is a polar molecule and has a very unique structure A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is
Station 1 Water is a polar molecule and has a very unique structure A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is
Chemistry A: States of Matter Packet Name: Hour: Page 1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
NAME: NAME: Date: TOE INVESTIGATION 3 POGIL
 NAME: NAME: Date: Goal for the Lesson: TOE INVESTIGATION 3 POGIL Use the concept of energy transformation to describe how the energy of a pendulum changes during each swing. Use your knowledge of kinetic
NAME: NAME: Date: Goal for the Lesson: TOE INVESTIGATION 3 POGIL Use the concept of energy transformation to describe how the energy of a pendulum changes during each swing. Use your knowledge of kinetic
NAME: ACTIVITY SHEETS PHYSICS AND CHEMISTRY (SECONDARY 3 rd YEAR)
 NAME: ACTIVITY SHEETS PHYSICS AND CHEMISTRY (SECONDARY 3 rd YEAR) ACTIVITY 1: Matter Lesson 2 THE PARTICULATE NATURE OF MATTER 1-What is matter? 2-What is a particle (corpuscle)? Set some examples 3-What
NAME: ACTIVITY SHEETS PHYSICS AND CHEMISTRY (SECONDARY 3 rd YEAR) ACTIVITY 1: Matter Lesson 2 THE PARTICULATE NATURE OF MATTER 1-What is matter? 2-What is a particle (corpuscle)? Set some examples 3-What
Physical Properties of Matter & What is in Mr. Skoldberg s Car?
 Physical Properties of Matter & What is in Mr. Skoldberg s Car? Name: Date: Background: Matter is anything that has mass and takes up space. Physical properties can be measured or observed using your senses
Physical Properties of Matter & What is in Mr. Skoldberg s Car? Name: Date: Background: Matter is anything that has mass and takes up space. Physical properties can be measured or observed using your senses
Objective Students will gain an understanding of how the properties of a solid material can affect how it interacts with water.
 OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
