UNIT 2 The Particulate Nature of Matter
|
|
|
- Reginald Wheeler
- 5 years ago
- Views:
Transcription
1 UNIT 2 The Particulate Nature of Matter Take a moment to think about all of the different properties which can be exhibited by matter. The list seems endless. Matter can be found in a variety of forms, including everything from people to plants to rocks to water to air, and so on. The number of different properties associated with these forms of matter is even greater. The universe is filled with a seemingly unlimited amount of diversity. And yet, we have already found that it is possible to classify all of the different forms of matter which we normally observe into just three categories or phases: solids, liquids, and gases. In Unit 2 we shall go a step further. We shall consider whether or not there is one single scientific model which can both describe all three of the phases of matter, and which can also explain the variety of properties which are exhibited by these phases. Such a model, if it could be found, would be very powerful. Not surprisingly, the search for this model has intrigued scientists for thousands of years.
2 PARTICLES (A Model for Everything) Is there a single scientific model for matter which can explain the great diversity of matter which you see around you? You are probably somewhat familiar with one model which was proposed in ancient Greece. Aristotle (and others) argued that the properties of matter could be explained by assuming that all matter was made up of varying amounts of just five basic elements: earth, water, fire, wind, and quintessence. Earth and water were said to have the property of "gravity" because they naturally fall to the ground when you let go of them. Fire and wind were said to have "levity" because they tend to go up instead of down. The sun, moon, and stars neither went up nor fell down, but seemed to move endlessly in perfect circles. Thus, they were assumed to be made wholly from the fifth, perfect element, quintessence. Based on this model of matter, if something fell to the ground when it was released (as a rock does), then it was concluded that the object was made mostly out of earth and water. On the other hand, birds were thought to be made at least partly out of fire and wind, since they had the ability to fly. Paper was said to be made out of a combination of earth and fire, hence it was fairly light. When paper burned, the fire was released, and all that was left was the ashes (earth). Thus, as must be true for all scientific models, this model had the ability to explain, at least partly, the observed properties of matter. Another model for matter grew out of the religion and philosophy of Taoism. The adherents of this religion desired to explain everything in terms of creation and annihilation cycles. Based on this, they postulated that the five basic elements for all of matter were wood, dirt, fire, water, and metal. The creation cycle is shown below, and the explanation was as follows: Water creates wood, because as you water a tree it grows and produces wood. Wood creates fire when it burns. Fire creates dirt, because after wood is burned by fire the ashes (dirt) is what is left. Dirt must create metal, because metal is found by mining for it in the ground. And finally, metal can create water, because metal left outside overnight will be found to have condensation (water) on it in the morning. wood water metal fire dirt The annihilation cycle relied on equally imaginative reasoning and is shown below. metal wood dirt fire water Just for fun, you might want to try to come up with an explanation for this cycle. (A hint to get you started: They said that water annihilated fire because water extinguishes fire when it is poured onto it.) In terms of its ability to explain or predict the properties of matter, this model was about as good as the Greek model discussed earlier. There is yet another model of matter that comes to us from antiquity. Democritus of ancient Greece proposed that all matter consisted of tiny particles (called atoms). There were a finite number of different kinds of atoms, and the properties of a substance were said to depend on what kinds of atoms made up the substance. Unfortunately for Democritus, this model seemed ridiculous to almost everyone else at that time, including Aristotle. And no wonder! How could a solid object (like a table) hold together if it were made out of little particles? And certainly liquids, such as water, did not look as if they were made out of anything solid. And if air were made out of particles, wouldn't these particles just fall to the ground like sand? Alas, the atomic model of matter died a quick death. UNIT 2 PARTICLES II-1
3 Of course, today the atomic (particulate) model of matter is considered to be the best theory we have for matter. By making a few adjustments to Democritus' model, we obtain a theory which can give explanations for the properties of all the matter around us. This model has become an indispensable way for scientists to look at the world. Before a more complete description of the particulate nature of matter (i.e. the model that says everything is made out of little particles called atoms) is developed here, a few observations about elementary school students and their understanding of the model should be made. Elementary Students and Atoms In general, students today do not find the atomic model of matter any more reasonable to accept than did people in Democritus' time. This is so for several reasons. First, individual atoms are too small to be seen or felt. Yet, this concept is introduced while students are still concrete thinkers. Thus, students are asked to imagine things of which they have no sensory experience. Second, there is no activity which you can do with students which will prove that atoms exist. At best, you can only infer that they might exist. (See the Brownian Motion Activity below.) And finally, the most obvious properties of many substances, such as liquids, appear to be most easily explained by not using a particle model. Despite all this, it is still being recommended that a simplified model of the particulate nature of matter be introduced and utilized in the later elementary grades due to its universal applicability in physical science. This is especially true as students learn about scientific models, for the atomic model of matter is the best way to explain a wide diversity of physical phenomena they will experience. The important thing to remember is that the atomic model should be introduced only in order to explain other phenomena. It is not worth studying if you simply want to memorize the names of the various parts of an atom and how they combine to form molecules. Brownian Motion Atoms and molecules are too small to be seen with optical microscopes. However, it is possible to infer the presence of atoms and molecules by observing their effects on larger objects. In this activity you will observe the small, jerky motions (Brownian Motion) of droplets of butterfat in milk. The jerky motions are caused by collisions between molecules in the milk and the butterfat droplets. As you make your observations, keep in mind that you will not see individual atoms or molecules moving. Instead, you must infer their presence based on your observations. PROCEDURE A) Use 1% milk, or dilute a small quantity of 2% milk by one-half. Put one drop of the 1% milk on a microscope slide, and cover it with a cover slip. B) View the liquid at a magnification of about 400X using maximum contrast. It might be advantageous to have the room lights off.) C) Look for the random "jiggling" of the butterfat droplets. Do the larger droplets move more, or less, than the smaller droplets? Is this as expected? The observation of Brownian motion is considered historically important in the affirmation of the existence of molecules and their motion. Most elementary students do not see such an obvious connection. Many students suspect that the observed motion results from the movement of live creatures. UNIT 2 PARTICULATE NATURE OF MATTER II-2
4 Basic Particle Model Content Overview Below is a summary of the main components of a particulate model for matter. As this unit continues, some of these ideas will be extended. The summary below is meant as a starting point for further investigation. 1. All matter is made up of tiny indestructible particles called atoms. There is nothing else contained within matter except for these atoms. (Atoms themselves are composed of particles called protons, neutrons, and electrons, but these are usually bound tightly together in the atoms. The elementary school teacher should probably know what these terms refer to and also what is meant by the term nucleus [the center of the atom containing the protons and neutrons], but these words should be of little or no concern to the lower elementary student) 2. Atoms are very small. They can not be seen, even if the most powerful optical microscope in the world is used. More than a trillion ( 1,000,000,000,000 ) atoms could fit on top of the period at the end of this sentence without any of them overlapping. If atoms were lined up end to end it would take over a million of them to reach a length of 1 mm. 3. There are about 100 different kinds of atoms. 4. Two or more atoms can be bound together in a semipermanent way to form a Molecule. Most substances we encounter in everyday life are made of molecules, although a few substances (helium, for example) are composed of individual atoms. There are millions of different molecules that can be formed from atoms. When the bonds between atoms are created or destroyed to make a new molecule, we say that a chemical reaction has taken place. 5. Atoms and molecules, whether they are making up a solid, liquid, or a gas, are always moving. 6. The atoms in solids and liquids are so close together that they are effectively touching each other. (See diagram below.) The positions where the atoms are not touching is empty space. The atoms making up a solid can not move around too far. They simply remain in one location and vibrate back and forth. The atoms or molecules in a liquid are free to move around and change positions. 7. The particles making up a gas are far apart, in general, but they are constantly colliding with each other as they move around. II-3
5 Here are simple models for solids, liquids, and gases that can involve students in a classroom: Solids: The students stand together (say, within one arm s length away from each other rather than actually touching) and imagine that one foot is glued to the floor. The students can wiggle and jiggle, but that one foot must not move. It is clear from this model why a solid retains its shape. Liquids: The individual students are now free to move about as well as wiggle and jiggle, provided they stay within one arm s length of other students. Note that the shape of the group may change as the students move about, just as the shape of a liquid can deform. (Although the particles collide with each other in a real liquid, some constraints obviously need to be placed on the students). Gases: In this phase the individual students are free to move about anywhere within the classroom. (They will quickly enough fill the entire container! ). II-4
Copyright 2008 NSTA. All rights reserved. For more information, go to Pennies
 Pennies A shiny new penny is made up of atoms. Put an X next to all the things on the list that describe the atoms that make up the shiny new penny. hard soft I N G O D L I B E R T Y W E T R U S T 2 0
Pennies A shiny new penny is made up of atoms. Put an X next to all the things on the list that describe the atoms that make up the shiny new penny. hard soft I N G O D L I B E R T Y W E T R U S T 2 0
Atomic Theory. Introducing the Atomic Theory:
 Atomic Theory Chemistry is the science of matter. Matter is made up of things called atoms, elements, and molecules. But have you ever wondered if atoms and molecules are real? Would you be surprised to
Atomic Theory Chemistry is the science of matter. Matter is made up of things called atoms, elements, and molecules. But have you ever wondered if atoms and molecules are real? Would you be surprised to
Physical Science and Nature of Science Assessment Probes
 Physical Science and Nature of Science Assessment Probes Concept Matrix...6 Pennies...7 2 Is It a Solid?...25 3 Thermometer...33. 4 Floating Balloon... 39 5 Hot and Cold Balloons...45 6 Mirror on the Wall...5
Physical Science and Nature of Science Assessment Probes Concept Matrix...6 Pennies...7 2 Is It a Solid?...25 3 Thermometer...33. 4 Floating Balloon... 39 5 Hot and Cold Balloons...45 6 Mirror on the Wall...5
What is Matter? Three states of matter
 What is Matter? Matter is what people often call stuff. In fact, stuff sounds almost like the German word for matter, stoff. All objects and materials we can touch are made of matter, and all matter takes
What is Matter? Matter is what people often call stuff. In fact, stuff sounds almost like the German word for matter, stoff. All objects and materials we can touch are made of matter, and all matter takes
Vocabulary atom atomos Dalton's atomic theory law of constant composition law of definite proportions law of multiple proportions matter.
 1.3 Early Atomic Theory Lesson Objectives The student will: define matter and explain how it is composed of building blocks known as atoms. give a short history of how the concept of the atom developed.
1.3 Early Atomic Theory Lesson Objectives The student will: define matter and explain how it is composed of building blocks known as atoms. give a short history of how the concept of the atom developed.
Matter, Force, Energy, Motion, and the Nature of Science (NOS)
 Matter, Force, Energy, Motion, and the Nature of Science (NOS) Elementary SCIEnCE Dr. Suzanne Donnelly Longwood University donnellysm@longwood.edu Welcome! Introductions What will we be doing this week?
Matter, Force, Energy, Motion, and the Nature of Science (NOS) Elementary SCIEnCE Dr. Suzanne Donnelly Longwood University donnellysm@longwood.edu Welcome! Introductions What will we be doing this week?
5. Which word describes the tone of
 Name: Date: WEEK 36 1 Read the text and then answer the questions. What do you have in common with a pencil, a star, and a bird? The answer is matter. Everything in the universe, including the planets,
Name: Date: WEEK 36 1 Read the text and then answer the questions. What do you have in common with a pencil, a star, and a bird? The answer is matter. Everything in the universe, including the planets,
Introductory Quantum Chemistry Prof. K. L. Sebastian Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore
 Introductory Quantum Chemistry Prof. K. L. Sebastian Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore Lecture - 4 Postulates Part 1 (Refer Slide Time: 00:59) So, I
Introductory Quantum Chemistry Prof. K. L. Sebastian Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore Lecture - 4 Postulates Part 1 (Refer Slide Time: 00:59) So, I
Classification of Matter. Chapter 10 Classification of Matter
 Chapter 10 Classification of Matter Grade 7 Classification of Matter Matter is anything that has mass and occupies space. We can classify matter based on whether it s solid, liquid, or gas. 2 1 Understanding
Chapter 10 Classification of Matter Grade 7 Classification of Matter Matter is anything that has mass and occupies space. We can classify matter based on whether it s solid, liquid, or gas. 2 1 Understanding
Atoms, Elements, and the Periodic Table
 chapter 00 3 3 Atoms, Elements, and the Periodic Table section 1 Structure of Matter Before You Read Take a deep breath. What fills your lungs? Can you see it or hold it in your hand? What You ll Learn
chapter 00 3 3 Atoms, Elements, and the Periodic Table section 1 Structure of Matter Before You Read Take a deep breath. What fills your lungs? Can you see it or hold it in your hand? What You ll Learn
Chapter 7.1. States of Matter
 Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
Unit 2: Essentials of Chemistry. Chapter 1-2, 4-5
 Unit 2: Essentials of Chemistry Chapter 1-2, 4-5 Objectives 8 explain the nature of science including the use of the validity of the scientific method and the difference between a hypothesis, theory and
Unit 2: Essentials of Chemistry Chapter 1-2, 4-5 Objectives 8 explain the nature of science including the use of the validity of the scientific method and the difference between a hypothesis, theory and
Chapter 7 Notes. Matter is made of tiny particles in constant motion
 Chapter 7 Notes Section 7.1 Matter is made of tiny particles in constant motion Atomic Theory Greek philosophers (430 BC ) Democritus and Leucippus proposed that matter is made of tiny particles called
Chapter 7 Notes Section 7.1 Matter is made of tiny particles in constant motion Atomic Theory Greek philosophers (430 BC ) Democritus and Leucippus proposed that matter is made of tiny particles called
5th Grade. Slide 1 / 67. Slide 2 / 67. Slide 3 / 67. Matter and Its Interactions. Table of Contents: Matter and Its Interactions
 Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Chemistry Day 7. Friday, September 7 th Monday, September 10 th, 2018
 Chemistry Day 7 Friday, September 7 th Monday, September 10 th, 2018 Do-Now Title: Ch. 13/14 Notes C 1. Write down today s FLT 2. List the three states of matter and list an example for each 3. List two
Chemistry Day 7 Friday, September 7 th Monday, September 10 th, 2018 Do-Now Title: Ch. 13/14 Notes C 1. Write down today s FLT 2. List the three states of matter and list an example for each 3. List two
Atoms. Grade Level: 4 6. Teacher Guidelines pages 1 2 Instructional Pages pages 3 5 Activity Pages pages 6 7 Homework Page page 8 Answer Key page 9
 Atoms Grade Level: 4 6 Teacher Guidelines pages 1 2 Instructional Pages pages 3 5 Activity Pages pages 6 7 Homework Page page 8 Answer Key page 9 Classroom Procedure: 1. Display the different items collected
Atoms Grade Level: 4 6 Teacher Guidelines pages 1 2 Instructional Pages pages 3 5 Activity Pages pages 6 7 Homework Page page 8 Answer Key page 9 Classroom Procedure: 1. Display the different items collected
Time to develop a model
 ATOMIC THEORY ONCE UPON A TIME People have been fascinated with matter for a long time. What is matter? What is all this stuff around us made of? Can it be broken down? Are there different types of matter?
ATOMIC THEORY ONCE UPON A TIME People have been fascinated with matter for a long time. What is matter? What is all this stuff around us made of? Can it be broken down? Are there different types of matter?
HIGH SCHOOL SCIENCE. Physical Science 9: Atomic Structure
 HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
PHYSICS 107. Lecture 1: The Puzzle of Motion. In American universities there are three main types of physics courses for nonspecialists.
 PHYSICS 107 Lecture 1: The Puzzle of Motion About this course In American universities there are three main types of physics courses for nonspecialists. The first kind teaches about the physics of everyday
PHYSICS 107 Lecture 1: The Puzzle of Motion About this course In American universities there are three main types of physics courses for nonspecialists. The first kind teaches about the physics of everyday
Solids, liquids and gases
 Solids, liquids and gases Duration 60 minutes Lesson overview Students share what they know about the three states of matter solid, liquid and gas and consider some of their scientific properties. They
Solids, liquids and gases Duration 60 minutes Lesson overview Students share what they know about the three states of matter solid, liquid and gas and consider some of their scientific properties. They
Particle Theory of Matter. By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that:
 Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Leonie Boshoff-Mostert Edited by Anne Starace
 GASES, LIQUIDS AND SOLIDS: Condensation Leonie Boshoff-Mostert Edited by Anne Starace Abstract Matter is sorted into three groups: solids, liquids and gases. Solids, liquids and gases each have characteristic
GASES, LIQUIDS AND SOLIDS: Condensation Leonie Boshoff-Mostert Edited by Anne Starace Abstract Matter is sorted into three groups: solids, liquids and gases. Solids, liquids and gases each have characteristic
Atomic Pudding Models of the Atom
 Atomic Pudding Models of the Atom Think About It The drawing depicts a very tiny sample of gold taken from a gold ring. The spheres in the cube of gold are so small that they cannot be seen. What are the
Atomic Pudding Models of the Atom Think About It The drawing depicts a very tiny sample of gold taken from a gold ring. The spheres in the cube of gold are so small that they cannot be seen. What are the
The Particulate Nature of Matter
 Matter Objectives Learn about the composition of matter. Learn the difference between elements and compounds. Learn to distinguish between physical and chemical properties and changes. Learn to distinguish
Matter Objectives Learn about the composition of matter. Learn the difference between elements and compounds. Learn to distinguish between physical and chemical properties and changes. Learn to distinguish
Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, University of California, Santa Barbara
 Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, 2007-08 University of California, Santa Barbara [Remember to get answers from a wide variety of students in the audience.
Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, 2007-08 University of California, Santa Barbara [Remember to get answers from a wide variety of students in the audience.
that when friction is present, a is needed to keep an object moving. 21. State Newton s first law of motion.
 Chapter 3 Newton s First Law of Motion Inertia Exercises 31 Aristotle on Motion (pages 29 30) Fill in the blanks with the correct terms 1 Aristotle divided motion into two types: and 2 Natural motion on
Chapter 3 Newton s First Law of Motion Inertia Exercises 31 Aristotle on Motion (pages 29 30) Fill in the blanks with the correct terms 1 Aristotle divided motion into two types: and 2 Natural motion on
Talk Science Professional Development
 Talk Science Professional Development Transcript for Grade 5 Scientist Case: The Water to Ice Investigations 1. The Water to Ice Investigations Through the Eyes of a Scientist We met Dr. Hugh Gallagher
Talk Science Professional Development Transcript for Grade 5 Scientist Case: The Water to Ice Investigations 1. The Water to Ice Investigations Through the Eyes of a Scientist We met Dr. Hugh Gallagher
Name: Packet Due Date: Tuesday, 9/18. Science
 Name: Packet Due Date: Tuesday, 9/18 Science Module 2 Chapter 1 Phase Change Describing Phase Change at Two Scales What happened to the liquid in Titan s Lake? (NGSS Performance Expectations: MS-PS1-1;
Name: Packet Due Date: Tuesday, 9/18 Science Module 2 Chapter 1 Phase Change Describing Phase Change at Two Scales What happened to the liquid in Titan s Lake? (NGSS Performance Expectations: MS-PS1-1;
Matter and Energy: What are atoms?
 Matter and Energy: What are atoms? By Encyclopaedia Britannica, adapted by Newsela staff on 03.31.17 Word Count 518 Level MAX An illustration of an atom. The nucleus, containing neutrons and protons, is
Matter and Energy: What are atoms? By Encyclopaedia Britannica, adapted by Newsela staff on 03.31.17 Word Count 518 Level MAX An illustration of an atom. The nucleus, containing neutrons and protons, is
Gases: Properties and Behaviour
 SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
A most elegant philosophy about the Theory Of Everything
 A most elegant philosophy about the Theory Of Everything Author: Harry Theunissen (pseudonym) Email: htheunissen61@hotmail.com Abstract: Given a simple set of assumptions, this paper gives an elegant explanation
A most elegant philosophy about the Theory Of Everything Author: Harry Theunissen (pseudonym) Email: htheunissen61@hotmail.com Abstract: Given a simple set of assumptions, this paper gives an elegant explanation
The History of Astronomy
 The History of Astronomy http://www.phys.uu.nl/~vgent/babylon/babybibl_intro.htm http://mason.gmu.edu/~jmartin6/howe/images/pythagoras.jpg http://www.russellcottrell.com/greek/aristarchus.htm http://www.mesopotamia.co.uk/astronomer/homemain.html
The History of Astronomy http://www.phys.uu.nl/~vgent/babylon/babybibl_intro.htm http://mason.gmu.edu/~jmartin6/howe/images/pythagoras.jpg http://www.russellcottrell.com/greek/aristarchus.htm http://www.mesopotamia.co.uk/astronomer/homemain.html
Matter, Atoms & Molecules
 Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Characteristic Properties of Matter
 Grade 8 Science, Quarter 2, Unit 2.1 Characteristic Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Measure the mass and volume of regular and
Grade 8 Science, Quarter 2, Unit 2.1 Characteristic Properties of Matter Overview Number of instructional days: 15 (1 day = 50 minutes) Content to be learned Measure the mass and volume of regular and
Is It Matter? salt. rocks. Mars. baby powder. Jupiter. milk. steam. air. rotten apples. light. heat. dust. sound waves. love. water. cells.
 Is It Matter? Listed below is a list of things that are considered matter and things that are not considered matter. Put an X next to each of the things that you consider to be matter. rocks salt baby
Is It Matter? Listed below is a list of things that are considered matter and things that are not considered matter. Put an X next to each of the things that you consider to be matter. rocks salt baby
What Is The Matter? Matter Concept Map
 What Is The Matter? Matter Concept Map Phases of Matter Solid Liquid Gas Use the selections below to complete the concept map for the phases of matter. Write the letter of each characteristic in the appropriate
What Is The Matter? Matter Concept Map Phases of Matter Solid Liquid Gas Use the selections below to complete the concept map for the phases of matter. Write the letter of each characteristic in the appropriate
Heating and Cooling Explained By The Particle Model. Notes: Part 2/4
 Heating and Cooling Explained By The Particle Model Notes: Part 2/4 Particles are the building blocks of all things. What are Particles? Some people call them molecules. Particles are NOT alive. How many
Heating and Cooling Explained By The Particle Model Notes: Part 2/4 Particles are the building blocks of all things. What are Particles? Some people call them molecules. Particles are NOT alive. How many
Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years.
 Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years. Chemistry lies at the roots of civilization! Chemical reactions are
Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years. Chemistry lies at the roots of civilization! Chemical reactions are
Second Grade: Unit 2: Properties of Matter. Matter solid liquid gas property
 Second Grade: Unit 2: Properties of Matter Matter solid liquid gas property Background: The universe is made of only two entities: matter and energy. Examples of energy are light, heat, and sound. Everything
Second Grade: Unit 2: Properties of Matter Matter solid liquid gas property Background: The universe is made of only two entities: matter and energy. Examples of energy are light, heat, and sound. Everything
Sub atomic Mass in a.m.u. Relative Position in the
 IDEAS ABOUT ATOMS In chapter one we looked briefly at the ideas of the Ancient Greeks about atoms. You will remember that the main idea involved tiny particles of matter that could not be broken down.
IDEAS ABOUT ATOMS In chapter one we looked briefly at the ideas of the Ancient Greeks about atoms. You will remember that the main idea involved tiny particles of matter that could not be broken down.
Hot and Cold Balloons
 Hot and Cold Balloons Moira filled a balloon with air. She tightly tied the balloon so no air could get in or out of the balloon. She kept the balloon in a warm room. An hour later she put the balloon
Hot and Cold Balloons Moira filled a balloon with air. She tightly tied the balloon so no air could get in or out of the balloon. She kept the balloon in a warm room. An hour later she put the balloon
The Story of the Atom. A history of atomic theory over many years
 The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
Matter: Properties and Change
 Matter: Properties and Change 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are
Matter: Properties and Change 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are
Name Class Date. Energy Energy
 CHAPTER 13 3 What Is Energy? SECTION Work and Energy KEY IDEAS As you read this section, keep these questions in mind: How are energy and work related? Why is potential energy called energy of position?
CHAPTER 13 3 What Is Energy? SECTION Work and Energy KEY IDEAS As you read this section, keep these questions in mind: How are energy and work related? Why is potential energy called energy of position?
5 th Grade Lesson Plan: Matter and Chemical Reactions
 5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
GRADE 5. Units of Study: Using Variables in the Inquiry Process Astronomy: Earth, Sun, Moon, Planets (Solar System) and Beyond Elements and Compounds
 GRADE 5 Course Overview In fifth grade, students use the inquiry process more independently throughout the year with teacher support, as needed. Students practice designing, conducting, evaluating, and
GRADE 5 Course Overview In fifth grade, students use the inquiry process more independently throughout the year with teacher support, as needed. Students practice designing, conducting, evaluating, and
Matter: Properties and Change
 Matter: Properties and Change Date: 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element
Matter: Properties and Change Date: 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element
Chapter Preview. Improving Comprehension
 Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
Do Now Monday, January 23, 201
 Do Now Monday, January 23, 201 What do you recall about states of matter? Write your answer using complete sentences. 3.5 minutes Do Now Check By the end of the day today, IWBAT Describe the various states
Do Now Monday, January 23, 201 What do you recall about states of matter? Write your answer using complete sentences. 3.5 minutes Do Now Check By the end of the day today, IWBAT Describe the various states
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
What's Up, Earth? Header Insert Image 1 here, right justified to wrap. Grade Level. 3rd. Time Required: 60 minutes
 What's Up, Earth? Header Insert Image 1 here, right justified to wrap Image 1 ADA Description:? Caption:? Image file path:? Source/Rights: Copyright? Grade Level 3rd Time Required: 60 minutes Group Size:
What's Up, Earth? Header Insert Image 1 here, right justified to wrap Image 1 ADA Description:? Caption:? Image file path:? Source/Rights: Copyright? Grade Level 3rd Time Required: 60 minutes Group Size:
What are atoms? -:-.L'::'
 What are atoms? -:-.L'::' What is the smallest particle of dust? thing you can think of? A single grain of sand? A Now try to imagine something so small that you would need millions of them to make one
What are atoms? -:-.L'::' What is the smallest particle of dust? thing you can think of? A single grain of sand? A Now try to imagine something so small that you would need millions of them to make one
Unit 3: Physical Science Seedlings in a Jar
 Science 7 Unit 3: Physical Science Seedlings in a Jar Name Period Introduction: Systems Analysis with seeds Today we will create a closed jar containing 5 bean seeds, air, and a moist paper towel. Nothing
Science 7 Unit 3: Physical Science Seedlings in a Jar Name Period Introduction: Systems Analysis with seeds Today we will create a closed jar containing 5 bean seeds, air, and a moist paper towel. Nothing
Learning About Our Solar System
 Learning About Our Solar System By debbie Routh COPYRIGHT 2004 Mark Twain Media, Inc. ISBN 978-1-58037-876-5 Printing No. 404007-EB Mark Twain Media, Inc., Publishers Distributed by Carson-Dellosa Publishing
Learning About Our Solar System By debbie Routh COPYRIGHT 2004 Mark Twain Media, Inc. ISBN 978-1-58037-876-5 Printing No. 404007-EB Mark Twain Media, Inc., Publishers Distributed by Carson-Dellosa Publishing
Stieff Research Group - COPYRIGHT 2009
 Introduction Chemistry is the study of matter and energy. Chemists are the scientists who often find explanations to everyday events by examining particles of matter at the submicroscopic level. Although
Introduction Chemistry is the study of matter and energy. Chemists are the scientists who often find explanations to everyday events by examining particles of matter at the submicroscopic level. Although
SNC1D CHEMISTRY 2/8/2013. ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P ) Atomic Theory. Atomic Theory
 SNC1D CHEMISTRY ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P.168-175) Atomic Theory Thousands of years ago Greek philosophers were asking themselves questions like, If you take a gold bar and cut it
SNC1D CHEMISTRY ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P.168-175) Atomic Theory Thousands of years ago Greek philosophers were asking themselves questions like, If you take a gold bar and cut it
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:
 ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
PLASMA: IT MATTERS (MODIFIED FOR ADEED)
 PLASMA: IT MATTERS (MODIFIED FOR ADEED) Overview: Many people know about the three states of matter: solid, liquid, and gas. The fourth state, plasma, is less understood. Plasma plays a significant role
PLASMA: IT MATTERS (MODIFIED FOR ADEED) Overview: Many people know about the three states of matter: solid, liquid, and gas. The fourth state, plasma, is less understood. Plasma plays a significant role
Chapter 2 Energy, Force, and Motion Lesson 6 Describing Motion C, D; 8.2C, D; 8.4A; 8.6B
 Table of Contents Texas Essential Knowledge and Skills Correlation Chart....... 7 Chapter 1 Matter..................................... 11 Lesson 1 Atoms and Elements.......................... 12 6.5A*,
Table of Contents Texas Essential Knowledge and Skills Correlation Chart....... 7 Chapter 1 Matter..................................... 11 Lesson 1 Atoms and Elements.......................... 12 6.5A*,
Ch(3)Matter & Change. John Dalton
 Ch(3)Matter & Change John Dalton What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter. The Particle Theory of Matter 1.
Ch(3)Matter & Change John Dalton What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter. The Particle Theory of Matter 1.
Atomic Structure and The Periodic Table. Unit 3
 Atomic Structure and The Periodic Table Unit 3 Lesson 1: Atoms Unit 5: Atomic Structure & The Periodic Table Atoms How small can things get? If you break a stone wall into smaller and smaller pieces, you
Atomic Structure and The Periodic Table Unit 3 Lesson 1: Atoms Unit 5: Atomic Structure & The Periodic Table Atoms How small can things get? If you break a stone wall into smaller and smaller pieces, you
What Do You Think? Investigate GOALS
 Activity 3 Atoms and Their Masses GOALS In this activity you will: Explore the idea of atoms by trying to isolate a single atom. Measure how many times greater the mass of a copper atom is than a magnesium
Activity 3 Atoms and Their Masses GOALS In this activity you will: Explore the idea of atoms by trying to isolate a single atom. Measure how many times greater the mass of a copper atom is than a magnesium
This is a brief overview of some of the very basic and classic laws of physics and how magnetic energy theory fits into those laws.
 This is a brief overview of some of the very basic and classic laws of physics and how magnetic energy theory fits into those laws. There are changes coming to our planet and solar system and after these
This is a brief overview of some of the very basic and classic laws of physics and how magnetic energy theory fits into those laws. There are changes coming to our planet and solar system and after these
OBJECTIVES: By the end of class, students will be able to DO NOW
 7 th Grade Science Unit: Matter and Periodic Table Lesson: Matter 9 States of Matter Demonstrations Name: Date: Thursday, December 8, 2016 OBJECTIVES: By the end of class, students will be able to SWBAT
7 th Grade Science Unit: Matter and Periodic Table Lesson: Matter 9 States of Matter Demonstrations Name: Date: Thursday, December 8, 2016 OBJECTIVES: By the end of class, students will be able to SWBAT
Lesson 1 Matter and Its Properties
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
An Introduction to Electricity and Circuits
 An Introduction to Electricity and Circuits Materials prepared by Daniel Duke 4 th Sept 2013. This document may be copied and edited freely with attribution. This course has been designed to introduce
An Introduction to Electricity and Circuits Materials prepared by Daniel Duke 4 th Sept 2013. This document may be copied and edited freely with attribution. This course has been designed to introduce
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
The Physical Properties And Physical Changes of Substances
 The Physical Properties And Physical Changes of Substances A. Definitions In Science 1. Science is the observation, identification, description, experimental investigation, and theoretical explanation
The Physical Properties And Physical Changes of Substances A. Definitions In Science 1. Science is the observation, identification, description, experimental investigation, and theoretical explanation
What Do You Think? Investigate GOALS
 Fun with the Periodic Table Activity 4 Are Atoms Indivisible? Do not try this at home. It may ruin your TV. GOALS In this activity you will: Observe or learn the behavior of a cathode ray in the presence
Fun with the Periodic Table Activity 4 Are Atoms Indivisible? Do not try this at home. It may ruin your TV. GOALS In this activity you will: Observe or learn the behavior of a cathode ray in the presence
Conceptual Physics Matter Atoms
 Conceptual Physics Matter Atoms Lana Sheridan De Anza College July 20, 2017 Last time projectile motion orbits escape speed heliocentric model Kepler s laws review Overview matter atoms elements atomic
Conceptual Physics Matter Atoms Lana Sheridan De Anza College July 20, 2017 Last time projectile motion orbits escape speed heliocentric model Kepler s laws review Overview matter atoms elements atomic
4.2 A Scientific View of Energy Kinetic Energy
 4. A Universe of Matter and Energy 4.1 Matter and Energy in Everyday Life The eternal mystery of the world is its comprehensibility. The fact that it is comprehensible is a miracle. Albert Einstein (1879
4. A Universe of Matter and Energy 4.1 Matter and Energy in Everyday Life The eternal mystery of the world is its comprehensibility. The fact that it is comprehensible is a miracle. Albert Einstein (1879
The conservation of matter and mass
 The conservation of matter and mass Critical teaching ideas - Science Continuum F to 10 Level: Working towards level 10 Student everyday experiences For many students the idea that matter is conserved
The conservation of matter and mass Critical teaching ideas - Science Continuum F to 10 Level: Working towards level 10 Student everyday experiences For many students the idea that matter is conserved
2 THE SCIENTIFIC REVOLUTION IN ENGLAND AND EUROPE, Lesson Title: The Scientific Revolution in England and Europe,
 2 THE SCIENTIFIC REVOLUTION IN ENGLAND AND EUROPE, 1500-1700 FOR TEACHERS Lesson Title: The Scientific Revolution in England and Europe, 1500-1700 Area of Learning: states of affairs; change Aims: Pupils
2 THE SCIENTIFIC REVOLUTION IN ENGLAND AND EUROPE, 1500-1700 FOR TEACHERS Lesson Title: The Scientific Revolution in England and Europe, 1500-1700 Area of Learning: states of affairs; change Aims: Pupils
Putting the World in a Box
 Putting the World in a Box OBJECTIVES The student will construct models of the particulate level in chemistry and describe the models limitations. The student will use particulate level models to explain
Putting the World in a Box OBJECTIVES The student will construct models of the particulate level in chemistry and describe the models limitations. The student will use particulate level models to explain
Forces and Newton s Laws
 chapter 3 section 1 Forces Forces and Newton s Laws What You ll Learn how force and motion are related what friction is between objects the difference between mass and weight Before You Read When you hit
chapter 3 section 1 Forces Forces and Newton s Laws What You ll Learn how force and motion are related what friction is between objects the difference between mass and weight Before You Read When you hit
Stars and Galaxies. Evolution of Stars
 Stars and Galaxies Evolution of Stars What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
Stars and Galaxies Evolution of Stars What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
Hot Sync. Materials Needed Today
 Chapter 4 Lesson 1 Materials Needed Today Please take these materials out of your backpack. Pencil Blank sheet of paper for notes. Hot Sync Wednesday 1/8/14 Answer the following questions in complete sentences
Chapter 4 Lesson 1 Materials Needed Today Please take these materials out of your backpack. Pencil Blank sheet of paper for notes. Hot Sync Wednesday 1/8/14 Answer the following questions in complete sentences
Conceptual Understandings for K-2 Teachers
 AFK12SE/NGSS Strand Disciplinary Core Ideas PS1: Matter and Its Interactions How can one explain the structure, properties, and interactions of matter? PS1. A: Structure and Properties of Matter How do
AFK12SE/NGSS Strand Disciplinary Core Ideas PS1: Matter and Its Interactions How can one explain the structure, properties, and interactions of matter? PS1. A: Structure and Properties of Matter How do
Matter and Energy: What is matter?
 Matter and Energy: What is matter? By Encyclopaedia Britannica, adapted by Newsela staff on 03.30.17 Word Count 544 Level 830L This photo of John Muir Glacier in Alaska shows two of the three main states
Matter and Energy: What is matter? By Encyclopaedia Britannica, adapted by Newsela staff on 03.30.17 Word Count 544 Level 830L This photo of John Muir Glacier in Alaska shows two of the three main states
Agenda. Chapter 10, Problem 26. All matter is made of atoms. Atomic Structure 4/8/14. What is the structure of matter? Atomic Terminology
 Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
 Physics The study of the energy, matter, and forces in the Universe Why do stars move in the sky? How can heat be changed into electricity? What is the difference between an atom of one substance and an
Physics The study of the energy, matter, and forces in the Universe Why do stars move in the sky? How can heat be changed into electricity? What is the difference between an atom of one substance and an
Unsaved Test, Version: 1 1
 Name: Key Concepts Select the term that best completes the statement. A. beam balance B. graduated cylinder C. scale D. matter E. mass F. newton G. volume H. height I. weight J. kilogram 1. Anything that
Name: Key Concepts Select the term that best completes the statement. A. beam balance B. graduated cylinder C. scale D. matter E. mass F. newton G. volume H. height I. weight J. kilogram 1. Anything that
Lesson Plan: Plate Tectonics
 Lesson Plan: Plate Tectonics Lesson Plan: Plate Tectonics Lesson Plan Content: This lesson plan and slide presentation is to be used in conjunction with: 1 x plate tectonics teacher briefing 1 x plate
Lesson Plan: Plate Tectonics Lesson Plan: Plate Tectonics Lesson Plan Content: This lesson plan and slide presentation is to be used in conjunction with: 1 x plate tectonics teacher briefing 1 x plate
States of Matter. Solids, Liquids, and Gases
 States of Matter Solids, Liquids, and Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
States of Matter Solids, Liquids, and Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
SCH3U1 This presentation and more can be found at
 SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
The SI unit for Energy is the joule, usually abbreviated J. One joule is equal to one kilogram meter squared per second squared:
 Chapter 2 Energy Energy is an extremely loaded term. It is used in everyday parlance to mean a number of different things, many of which bear at most a passing resemblance to the term as used in physical
Chapter 2 Energy Energy is an extremely loaded term. It is used in everyday parlance to mean a number of different things, many of which bear at most a passing resemblance to the term as used in physical
The Kinetic Theory. 1. All matter is composed of many tiny, discrete particles, usually molecules or atoms.
 The Kinetic Theory The Kinetic Theory describes the motion of particles within a gas, liquid, or solid. As with all theories, this is an attempt to explain the observed behavior of each phase; solid, liquid,
The Kinetic Theory The Kinetic Theory describes the motion of particles within a gas, liquid, or solid. As with all theories, this is an attempt to explain the observed behavior of each phase; solid, liquid,
Leonie Boshoff-Mostert Edited by Anne Starace
 GASES, LIQUIDS AND SOLIDS Density Leonie Boshoff-Mostert Edited by Anne Starace Abstract Matter is sorted into three groups: solids, liquids and gases. Solids, liquids and gases each have characteristic
GASES, LIQUIDS AND SOLIDS Density Leonie Boshoff-Mostert Edited by Anne Starace Abstract Matter is sorted into three groups: solids, liquids and gases. Solids, liquids and gases each have characteristic
States of Matter in Food
 Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
How tightly can I pack wiggling students into a classroom?
 How tightly can I pack wiggling students into a classroom? A bronze sculpture of a cougar is sitting in the sun. In the morning, when the temperature is 20 degrees C, the tips of the ears are 11 cm apart.
How tightly can I pack wiggling students into a classroom? A bronze sculpture of a cougar is sitting in the sun. In the morning, when the temperature is 20 degrees C, the tips of the ears are 11 cm apart.
Binder. Notes: DO NOW
 CONTENT OBJECTIVE: SWBAT EXPLAIN HOW THE MODEL OF THE ATOM HAS EVOLVED. Binder HW for checking: 2.1 HW SKILL OBJECTIVES: SWBAT STOP AND THINK ALOUD TO MAKE SURE OF THEIR UNDERSTANDING SWBAT HIGHLIGHT FOR
CONTENT OBJECTIVE: SWBAT EXPLAIN HOW THE MODEL OF THE ATOM HAS EVOLVED. Binder HW for checking: 2.1 HW SKILL OBJECTIVES: SWBAT STOP AND THINK ALOUD TO MAKE SURE OF THEIR UNDERSTANDING SWBAT HIGHLIGHT FOR
Chapter 1: Useful definitions
 Chapter 1: Useful definitions 1.1. Cross-sections (review) The Nuclear and Radiochemistry class listed as a prerequisite is a good place to start. The understanding of a cross-section being fundamentai
Chapter 1: Useful definitions 1.1. Cross-sections (review) The Nuclear and Radiochemistry class listed as a prerequisite is a good place to start. The understanding of a cross-section being fundamentai
3 Newton s First Law of Motion Inertia. Forces cause changes in motion.
 Forces cause changes in motion. A ball at rest in the middle of a flat field is in equilibrium. No net force acts on it. If you saw it begin to move across the ground, you d look for forces that don t
Forces cause changes in motion. A ball at rest in the middle of a flat field is in equilibrium. No net force acts on it. If you saw it begin to move across the ground, you d look for forces that don t
BEFORE YOU READ. Forces and Motion Gravity and Motion STUDY TIP. After you read this section, you should be able to answer these questions:
 CHAPTER 2 1 SECTION Forces and Motion Gravity and Motion BEFORE YOU READ After you read this section, you should be able to answer these questions: How does gravity affect objects? How does air resistance
CHAPTER 2 1 SECTION Forces and Motion Gravity and Motion BEFORE YOU READ After you read this section, you should be able to answer these questions: How does gravity affect objects? How does air resistance
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
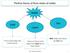 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
1 Development of the Atomic Theory
 CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
PHY101: Major Concepts in Physics I. Photo: J. M. Schwarz
 PHY101: Major Concepts in Physics I Photo: J. M. Schwarz Announcements We will be talking about the laws of thermodynamics today, which will help get you ready for next week s lab on the Stirling engine.
PHY101: Major Concepts in Physics I Photo: J. M. Schwarz Announcements We will be talking about the laws of thermodynamics today, which will help get you ready for next week s lab on the Stirling engine.
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.1 Introduction to Atoms
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
