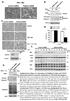
Appoptosin interacts with mitochondrial outer-membrane. fusion proteins and regulates mitochondrial morphology
|
|
|
- Cory Newman
- 5 years ago
- Views:
Transcription
1 2016. Published by The Company of Biologists Ltd. Appoptosin interacts with mitochondrial outer-membrane fusion proteins and regulates mitochondrial morphology Cuilin Zhang 1, Zhun Shi 1, Lingzhi Zhang 1, Zehua Zhou 1, Xiaoyuan Zheng 1, Guiying Liu 1, Guojun Bu 1, Paul E Fraser 2, Huaxi Xu 1,3, Yun-wu Zhang 1,* 1 Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, Fujian , China. 2 Tanz Centre for Research in Neurodegenerative Diseases and Department of Medical Biophysics, University of Toronto, Toronto, Ontario M5T 2S8, Canada. 3 Degenerative Disease Research Program, Sanford-Burnham-Prebys Medical Discovery Institute, La Jolla, CA 92037, USA. *Author for correspondence (yunzhang@xmu.edu.cn). JCS Advance Online Article. Posted on 26 January 2016
2 ABSTRACT Mitochondrial morphology is regulated by fusion and fission machinery. Impaired mitochondria dynamics cause various diseases including Alzheimer s disease (AD). Appoptosin is a mitochondrial carrier protein located in the mitochondrial inner membrane. Appoptosin overexpression causes reactive oxygen species (ROS) overproduction and caspase-dependent apoptosis, while appoptosin downregulation abolishes -amyloid-induced mitochondrial fragmentation and neuronal death in AD. Herein, we found that appoptosin overexpression resulted in mitochondrial fragmentation in a manner independent of its carrier function, ROS production, or caspase activation. Although appoptosin did not affect levels of mitochondrial outer-membrane fusion (MFN1 and MFN2), inner-membrane fusion (OPA1) and fission (DRP1 and FIS1) proteins, appoptosin interacted with MFN1 and MFN2, as well as the mitochondrial ubiquitin ligase MITOL but not OPA1, FIS1 or DRP1. Appoptosin overexpression impaired the interaction between MFN1 and MFN2 and mitochondrial fusion. On the other hand, co-expression of MFN1, MITOL and a dominant negative form of DRP1, DRP1 K38A partially rescued appoptosin-induced mitochondrial fragmentation and apoptosis, whereas co-expression of FIS1 aggravated appoptosin-induced apoptosis. Together, our results demonstrate that appoptosin can interact with mitochondrial outer-membrane fusion proteins and regulates mitochondrial morphology. KEY WORDS: Appoptosin, Apoptosis, Mitochondrial dynamics, MFN1, MFN2, MITOL
3 INTRODUCTION Mitochondria are dynamic organelles, whose shapes continually change from filament to fragment (vice versa) by the balance between the rates of mitochondrial fusion and fission. Morphology variations in the mitochondria are physiologically important for cell homeostasis. Mitochondrial fusion is necessary for the fidelity of oxidative phosphorylation, the complementation of damaged mitochondria and the repairing of small amounts of mitochondrial damage. While mitochondrial fission is required for new mitochondria formation, it is also involved in mitochondrial mobility, mitophagy, cell mitosis and apoptosis (Youle and van der Bliek, 2012). Mitochondrial fusion is separated by two events as the outer membrane fusion and the inner membrane fusion. In mammalian cells, two GTPases MFN1 and MFN2, which anchor on the outer membrane of mitochondria, are responsible for the outer membrane fusion (Chen et al., 2003; Koshiba et al., 2004). However, compared to MFN2, MFN1 is relatively specific in regulating mitochondrial fusion (Shen et al., 2007). MFN1-harboring mitochondria have higher tethering efficiency than those with MFN2 and purified MFN1 also possesses higher GTPase activity than MFN2 (Ishihara et al., 2004). In contrast, MFN2 is more tissue specific in expression pattern than MFN1 (Liesa et al., 2009). In addition to promoting mitochondrial fusion (Chen et al., 2003; Neuspiel et al., 2005), MFN2 is also reported to be involved in various signaling cascades and acts as a pro-apoptotic and anti-proliferative protein (Guo et al., 2007; Papanicolaou et al., 2011; Shen et al., 2007; Wang et al., 2015). Mitochondrial inner membrane fusion is mediated by the inner membrane or intra-mitochondrial space located protein OPA1 (Alavi and Fuhrmann, 2013). However, there are eight OPA1 isoforms generated from alternative splicing and processing during maturation, making the mechanism of inner-membrane fusion much more complicated (Westermann, 2010). Mitochondrial fission is primarily mediated by FIS1 and DRP1 in mammalian cells. During the fission process, mitochondrial outer-membrane protein FIS1 recruits the GTPase DRP1 from the cytosol to the cytosolic face of mitochondria, where oligomeric DRP1 assembles into spiral structure and severs mitochondria membranes following GTP hydrolysis (Westermann, 2010).
4 Recently, MITOL (also known as MARCH V), which belongs to the MARCH (membrane associated RING-CH E3 ubiquitin ligase) family, has been illustrated to be an important regulator of mitochondrial morphology. MITOL is a mitochondrial ubiquitin ligase and has been reported to interact with DRP1, FIS1, MFN2 (Nakamura et al., 2006) and acetylated MFN1 (Park et al., 2014). MITOL ubiquitinates FIS1 and DRP1 for their degradation. Overexpression of MITOL can also promote the formation of long tubular mitochondria in a manner that is dependent on MFN2 activity (Nakamura et al., 2006), and reverse phenotypes caused by FIS1 overexpression (Yonashiro et al., 2006). On the other hand, MITOL is also reported to be a regulator of subcellular trafficking of DRP1 and therefore is required for DRP1-dependent mitochondrial fission (Karbowski et al., 2007). Disruption of mitochondria homeostasis has been found in and proposed as the causes of diseases such as cancer, cardiovascular disease, diabetes and neurodegenerative diseases (Archer, 2013; Jheng et al., 2012). Alzheimer s disease (AD) is a most common neurodegenerative disease and is primarily caused by accumulation/deposition of neurotoxic -amyloid (A ) peptides in vulnerable brain regions (Jiang et al., 2014; Lemere, 2013). Mitochondrial morphology was found to be abnormal in degenerating dendrites of AD patient brains (Saraiva et al., 1985). More recently, mitochondria were found to be redistributed away from axons in the pyramidal neurons of AD brain, accompanied by decreased levels of DRP1, OPA1, MFN1 and MFN2, and increased levels of FIS1 (Wang et al., 2009). Another research group also confirmed the change of FIS1, OPA1, MFN1 and MFN2, except that they found increased levels of DRP1 in AD brain instead (Manczak et al., 2011). Moreover, overproduction of A as well as A treatment can cause mitochondrial fragmentation through affecting these mitochondrial morphology-regulating proteins (Manczak et al., 2010; Wang et al., 2008). These results demonstrate that mitochondria dynamics is perturbed in AD. However, the underlying mechanism has yet to be fully elucidated. We recently identified a novel pro-apoptotic protein appoptosin (also known as SLC25A38) that belongs to the mitochondria carrier family (MCP) and resides in the mitochondrial inner membrane. The physiological function of appoptosin is to transport glycine/δ-aminolevulinic acid between mitochondria and the cytosol to initiate heme
5 biosynthesis (Guernsey et al., 2009; Kannengiesser et al., 2011). We find that overexpression of appoptosin results in reactive oxygen species (ROS) overproduction and caspase-dependent apoptosis. Importantly, the levels of appoptosin are upregulated in neurons acutely treated with A and in brain samples of AD patients, whereas downregulation of appoptosin abrogates mitochondrial fragmentation and caspase activation caused by insults of glutamate and A in neurons (Zhang et al., 2012). These results suggest that appoptosin is a crucial regulator of A -induced neuronal injury and mitochondrial dysfunction in AD. In the present study, we found that overexpression of appoptosin directly induced mitochondrial fragmentation in an ROS overproduction- and caspase activation-independent manner. In addition, we showed that appoptosin interacted with MFN1, MFN2 and MITOL but not OPA1, FIS1 or DRP1. Overexpression of appoptosin decreased the heterotypic interaction of MFN1-MFN2 and reduced mitochondrial fusion activity. Co-expression of MFN1, MITOL and a dominant negative form of DRP1, DRP1 K38A, rescued appoptosin-induced apoptosis, whereas co-expression of FIS1 aggravated appoptosin-induced apoptosis. Finally, we found that SLC25A26, another MCP member closely related to SLC25A38, could interact with MITOL but not MFN1 or MFN2, and the expression of SLC25A26 did not affect mitochondrial morphology. Together, these results suggest that appoptosin is a unique MCP that can interact with mitochondrial outer-membrane fusion proteins and regulate mitochondrial morphology. RESULTS Overexpression of appoptosin induces mitochondrial fragmentation We previously found that downregulation of appoptosin prevented mitochondrial fragmentation and apoptosis induced by Aβ and glutamate treatments in neuronal cells (Zhang et al., 2012). These results imply that appoptosin may modulate mitochondrial morphology. To prove this, we overexpressed appoptosin in Hela cells (Fig. 1A) and neurons (Fig. 1B). We found that overexpressed myc-appoptosin co-localized well with the mitochondrial marker MitoTracker Red and this is consistent with the suggested mitochondrial inner membrane localization of appoptosin. Notably, we also found that mitochondria in myc-appoptosin overexpressing cells had dot-like small punctum
6 morphology in contrast to the long filamentous morphology in pcmv-myc transfected cells (Fig. 1A,B). These results suggest that overexpression of appoptosin leads to dramatic mitochondrial fragmentation. Interestingly, we found that mitochondrial morphology was not affected (supplementary material Fig. S1A,B) when appoptosin was downregulated (supplementary material Fig. S1C), implying that only an excess of appoptosin levels can interfere with mitochondrial morphology. Appoptosin-induced mitochondrial fragmentation is not dependent on its carrier function, ROS generation or caspase activity Appoptosin functions as a carrier of glycine and δ-aminolevulinic acid to transport them across the mitochondrial inner membrane for initiating heme synthesis. Since overexpression of appoptosin induces caspase-dependent cell apoptosis through promoting heme synthesis and ROS generation (Zhang et al., 2012), oxidative stress might be one of the causes for appoptosin-induced mitochondrial fragmentation. Actually, oxidative stress has been suggested to cause mitochondrial fragmentation by increasing the mitochondrial translocation of DRP1 (Iqbal and Hood, 2014; Wu et al., 2011). Herein, after transfection with appoptosin in Hela cells, we treated cells with an ROS scavenger N-acetyl-L-cysteine (NAC) (Fig. 2A), an anti-oxidant resveratrol (RSV) (Fig. 2B), a caspase inhibitor Z-VAD (Fig. 2C) and a heme-synthesis inhibitor succinylacetone (SA) (Fig. 2D). However, we found that none of these chemicals could block appoptosin-induced mitochondrial fragmentation (Fig. 2A-E), suggesting that this process is not dependent on ROS generation, caspase activity or the carrier function of appoptosin. Appoptosin does not affect levels of proteins involved in mitochondrial fusion and fission Mitochondrial morphology is maintained by the balance between fusion and fission machinery. To determine the mechanism underlying appoptosin-induced mitochondrial fragmentation, we first studied whether appoptosin may affect proteins involved in mitochondrial morphology regulation. However, we found that over-expression of appoptosin did not affect protein levels of DRP1, FIS1, MFN1, MFN2 or OPA1 (Fig. 3A,B). Since translocation of DRP1 from the cytosol to mitochondria is a requisite event in DRP1-dependent mitochondrial fragmentation, we studied whether appoptosin may affect
7 DRP1 translocation. We transfected Hela cells with pcmv-myc or myc-appoptosin and then separated cytosolic and mitochondrial fractions. Western blot results showed that overexpression of appoptosin did not alter DRP1 content in either cytosolic or mitochondrial fractions (Fig. 3C,D), suggesting that the translocation of DRP1 is not required for appoptosin-induced mitochondrial fragmentation. Appoptosin interacts with MFN1, MFN2 and MITOL Next, we studied whether appoptosin may interact with these mitochondrial morphology-regulating proteins. We co-expressed myc-appoptosin with HA-tagged major regulators of mitochondrial morphology in HEK 293T cells and carried out co-immunoprecipitation study. We found that an anti-myc antibody immunoprecipitated appoptosin, as well as HA-tagged MFN1 (Fig. 4C) and MFN2 (Fig. 4D) but not DRP1 (Fig. 4A), FIS1 (Fig. 4B) or OPA1 (Fig. 4E). On the other hand, although an anti-ha antibody immunoprecipitated all HA-tagged proteins (Fig. 4A-E), it only immunoprecipitated appoptosin in HA-MFN1 (Fig. 4C) and HA-MFN2 (Fig. 4D) expressing cells. In addition, we found that endogenous appoptosin could also be pulled down by anti-mfn1 (Fig. 4G) and anti-mfn2 (Fig. 4H) antibodies. These results indicate that appoptosin can interact with MFN1 and MFN2 but not DRP1, FIS1 or OPA1. MITOL is reported to be an important regulator of mitochondrial morphology in recent years. Herein, in cells co-expressing myc-appoptosin and HA-MITOL, we found that both myc and HA antibodies immunoprecipitated appoptosin and MITOL (Fig. 4F), indicating that appoptosin also interacts with MITOL. Since overexpression of appoptosin promotes ROS production and apoptosis, we studied whether interactions between appoptosin and MFN1/MFN2/MITOL were mediated by ROS production and apoptosis. However, appoptosin still interacted with MFN1/MFN2/MITOL upon treatments with NAC and Z-VAD (supplementary material Fig. S2A,B), implying that ROS levels and apoptosis do not contribute to such interactions. Overexpression of appoptosin reduces MFN1/MFN2 hetero-oligomer formation and mitochondrial fusion It has been demonstrated that MFN1 and MFN2 regulates fusion of mitochondrial outer-membrane by forming homo- or hetero-oligomers in cis or in trans (Detmer and Chan,
8 2007). We tested whether overexpression of appoptosin could affect the formation of MFN1 and MFN2 homo-oligomers and MFN1/MFN2 hetero-oligomers. As shown in Fig. 5A, overexpression of appoptosin reduced the hetero-interaction of MFN1-MFN2 but not homo-interaction of MFN1-MFN1 and MFN2-MFN2. To measure mitochondrial fusion activity, we generated mitochondrial targeted GFP (mtgfp) and mitochondrial targeted RFP (mtrfp) plasmids by fusing GFP and RFP with COX8, respectively. When cells expressing mtgfp were mixed with cells expressing mtrfp and induced for fusion by polyethylene glycol (PEG) for 7 h, we found that 100% fused cells contained fully fused mitochondria in controls, as demonstrated by colocalization of red and green fluorescent signals (Fig. 5B). However, only about 33% fused cells contained fully fused mitochondria and 67% fused cells contained partially fused mitochondria in appoptosin overexpressing cells (Fig. 5B), indicating decreased fusion efficiency upon appoptosin overexpression. Appoptosin-mediated mitochondrial fragmentation is alleviated by overexpression of MFN1 and MITOL MFN1 and MFN2 regulates fusion of mitochondrial outer membrane, whereas FIS1 and DRP1 regulates mitochondrial fission (Knott et al., 2008; Westermann, 2010). We first ascertained the effects of these proteins on mitochondrial morphology. As shown in Fig. 6A, transient overexpression of DRP1 had no effect on mitochondrial morphology and this was consistent with previous studies (Pitts et al., 1999; Smirnova et al., 1998). DRP1 K38A is a dominant negative mutant of DRP1 and its overexpression has been shown to inhibit mitochondrial fission and block cell death (Frank et al., 2001; James et al., 2003). We found that transient overexpression of DRP1 K38A led mitochondria to fuse into inter-connected network. On the other hand, transient overexpression of FIS1 resulted in a fragmentation of mitochondria into dot like puncta (Gomes and Scorrano, 2008). Conversely, mitochondria in MFN1- and MFN2-expressing cells hyper-fused into grape-like clusters. Mitochondria in MITOL expressing cells appeared to be inter-connected and elongated filaments, and this was consistent with previous reports (Nakamura et al., 2006; Yonashiro et al., 2006). To investigate whether appoptosin-induced mitochondria fragmentation can be affected by factors involved in mitochondria fusion, we co-expressed myc-appoptosin and
9 HA-MFN1 or HA-MITOL in Hela cells. We found that overexpression of MFN1 reversed appoptosin-induced mitochondria fragmentation by turning mitochondria into highly fused morphology (Fig. 6B-C). Overexpression of MITOL also dramatically rescued appoptosin-induced mitochondrial fragmentation (Fig. 6B-C). In addition, we found that co-expression of DRP1 K38A, which was reported to block mitochondrial fission and promote mitochondrial fusion (Frank et al., 2001; James et al., 2003), also markedly ameliorated appoptosin-induced mitochondrial fragmentation (Fig. 6B-C). Appoptosin-induced apoptosis is inhibited by DRP1 K38A, MFN1 and MITOL, and aggravated by FIS1 Since mitochondrial fission is directly involved in the initiation of apoptosis (Frank et al., 2001; Karbowski et al., 2002) and overexpression of appoptosin induces apoptosis, we studied whether these mitochondria morphology regulators may affect appoptosin-induced apoptosis. As expected, overexpression of appoptosin alone induced dramatic PARP cleavage (indicative of apoptosis) in Hela cells (Fig. 7A-E). In addition, we found that overexpression of FIS1 alone induced marked PARP cleavage and that co-expression of FIS1 with appoptosin further enhanced appoptosin-induced PARP cleavage (Fig. 7A, F). In contrast, overexpression of DRP1 K38A (Fig. 7B, F), MFN1 (Fig. 7C, F), or MITOL (Fig. 7E, F) alone had no notable effect on PARP cleavage but their co-expression significantly reduced appoptosin-induced PARP cleavage. On the other hand, although MFN2 is a mitochondrial fusion protein, its overexpression alone resulted in marked PARP cleavage (Fig. 7D, F). PARP cleavage resulted from co-expression of MFN2 with appoptosin was comparable to that from overexpression of MFN2 alone but more intensive than that from overexpression of appoptosion alone (Fig. 7D, F). However, since co-expression of MFN2 seemed to interfere with appoptosin overexpression (Fig. 7D), the exact role of MFN2 in affecting appoptosin-induced apoptosis requires further determination. Moreover, we studied whether co-expression of mitochondrial fusion/fission proteins affects appoptosin-promoted ROS production. However, co-expression of none of these proteins affected ROS production in cells overexpressing appoptosin (supplementary material Fig. S3), even though co-expression of MFN1, MITOL and DRP1 K38A attenuated mitochondrial fragmentation (Fig. 6) and apoptosis (Fig. 7). This may suggest that
10 mitochondrial fragmentation is not necessarily required for increased ROS production. SLC25A26 does not affect mitochondrial morphology Another mitochondrial carrier family member, SLC25A26 is a close analogue to appoptosin and identified as mitochondrial S-adenosylmethionine transporter (Agrimi et al., 2004; Haitina et al., 2006). In order to study whether the mitochondrial morphology-regulating effect of appoptosin applies to other mitochondrial carrier family members, we transiently overexpressed SLC25A26 in Hela cells. However, we found that overexpression of SLC25A26 had no obvious effect on mitochondrial morphology (Fig. 8A-B). We also analyzed and found that SLC25A26 could interact with MITOL but not MFN1 or MFN2 (Fig. 8C, D). DISCUSSION Mitochondrial morphology is regulated by a series of proteins involved in mitochondrial fusion and fission. Dysregulation of these proteins may impair mitochondria homeostasis and lead to the pathogenesis of various diseases, including AD. However, the detailed mechanism underlying mitochondrial morphology regulation is still obscure and requires further elucidation. We previously found that the MCP member appoptosin can mediate A neurotoxicity in AD and a reduction of appoptosin may abolish A -induced mitochondrial fragmentation. Herein, we further showed that overexpression of appoptosin directly resulted in mitochondrial fragmentation. However, although overexpression of appoptosin can cause ROS overproduction and caspase activation, we found that neither reduction of ROS nor inhibition of caspase activity affected appoptosin-induced mitochondrial fragmentation. These results confirm that mitochondrial fragmentation is an early event during cell death (Karbowski et al., 2002). Although levels of mitochondrial fission (DRP1 and FIS1) and fusion (OPA1, MFN1 and MFN2) proteins have been shown to be altered in AD brain (Manczak et al., 2011; Wang et al., 2009), we found that overexpression of appoptosin did not affect their protein levels. Rather, we found that appoptosin interacted with MFN1 and MFN2, as well as the mitochondrial ubiquitin ligase MITOL, but not DRP1, FIS1 or OPA1. In addition, we found that overexpression of appoptosin decreased the hetero-interaction between MFN1 and
11 MFN2 and caused the reduction of mitochondrial fusion activity. Since the fusion activity of MFN1-MFN2 heterotypic oligomers is comparable to those of homo-oligomers of MFN1 and MFN2 (Detmer and Chan, 2007), overexpression of appoptosin may interfere with the fusion machinery through impairing the interaction between MFN1 and MFN2, and thus leading to mitochondrial fragmentation. Interestingly, significant efforts have been devoted to studying the fission machinery deficits in AD, with a focus on DRP1 and findings showing that excessive ROS increases DRP1 activity (Iqbal and Hood, 2014; Wu et al., 2011), that A interacts with DRP1 and increases its activity (Manczak et al., 2011), that S-nitrosylation of DRP1 enhances its activity (Cho et al., 2009), and that phosphorylation of DRP1 at specific sites promotes its activity (Chang and Blackstone, 2007; Cribbs and Strack, 2007; Merrill et al., 2011; Meuer et al., 2007; Rambold et al., 2011; Rehman et al., 2012; Taguchi et al., 2007). In contrast, research on the fusion machinery in AD is limited. Hence our findings suggest that a defect in the fusion machinery may be equally important in AD, which deserves further scrutiny. Appoptosin overexpression-induced mitochondrial fragmentation and apoptosis were at least partially reversed by co-expression with MFN1, MITOL, and the dominant negative form of DRP1, DRP1 K38A. These results further support the idea of targeting mitochondria dynamics for AD therapy. Interestingly, although appoptosin also interacted with the mitochondrial fusion protein MFN2, co-expression with MFN2 has a stronger effect on inducing apoptosis than appoptosin overexpression alone. This is possibly because that MFN2 is also involved in various signaling cascades and acts as a pro-apoptotic and anti-proliferative protein (Guo et al., 2007; Papanicolaou et al., 2011; Shen et al., 2007; Wang et al., 2015). Indeed, herein we also found that overexpression of MFN2 alone resulted in apoptosis. Appoptosin belongs to the MCP family, whose members are primarily located in the inner membrane of mitochondria and shuttle metabolites, nucleotides, and cofactors between cytoplasm and mitochondrial matrix (Palmieri, 1994). However, to our knowledge we are the first to show that a MCP member (i.e. appoptosin) can regulate mitochondrial morphology. To study whether other MCP members may also regulate mitochondrial morphology, we investigated SLC25A26, another MCP member closely related to
12 appoptosin. However, although we found that SLC25A26 interacted with MITOL, overexpression of SLC25A26 had no effect on mitochondrial morphology. Since SLC25A26 does not interact with MFN1 or MFN2, it is possible that interaction with MFN1 and/or MFN2 is a prerequisite for appoptosin-induced mitochondrial fragmentation. To address this, we have generated several appoptosin deletion constructs according to its mitochondrial carrier domains (supplementary material Fig. S4A,B). However, although these appoptosin deletion mutants do not interact with MFN1/MFN2 (data not shown), overexpression of them also resulted in mitochondrial fragmentation and apoptosis (supplementary material Fig. S4C-E). Since we noticed that these overexpressed appoptosin deletion mutants were localized allover the cell body rather than specifically in mitochondria, we speculate that these deletion mutants may induce mitochondrial fragmentation and cell death through a different mechanism (possibly by overwhelming the Ubiquitin-Proteasome system) than that of full-length appoptosin. This deserves further scrutiny. Together, our study identifies a novel function of the pro-apoptotic appoptosin in regulating mitochondrial morphology, which may strengthen our understanding on the interplay between mitochondrial morphology and apoptosis, as well as AD pathologies. MATERIALS AND METHODS Cells, plasmids, antibodies and reagents Maintenance of Hela cells (Wang et al., 2006), HEK 293T cells and primary neurons derived from mouse at embryonic day 17.5 (Zhang et al., 2012) has been described previously. Hela cells and HEK 293T cells were originally from ATCC. The myc-appoptosin expression plasmid has also been described previously (Zhang et al., 2012). MFN1, MFN2, DRP1, FIS1, MITOL and DRP1 K38A expressing plasmids were constructed using pcmv-ha or pegfp-c1 as backbone. Mitochondria targeted GFP (mtgfp) and mitochondria targeted RFP (mtrfp) were generated by fusing GFP or RFP to mitochondria localized COX8, following the previously reported procedure(partikian et al., 1998). Anti-appoptosin antibody and rabbit anti-ha antibody were from Abcam (ab133614), UK; anti-myc antibody (9E10, SC-40)) was from Santa Cruz Biotechnology, USA; mouse
13 anti-ha antibody was from Abmart (26D11), China; anti-mfn1 ( AP) and anti-fis1 ( AP) antibodies were from Proteintech, USA; anti-mfn2 (WH M3-100UG) antibody was from Sigma Aldrich, USA; anti-actin (#4970), anti-cleaved PARP (#5625), anti-cox IV (#4850), anti-drp1 (#8570) was from Cell Signaling, USA. All the primary antibodies were used at 1:1000 dilution. Normal secondary antibodies were from Pierce Biotechnology (Thermo Fisher Scientific, USA) and fluorescence-conjugated secondary antibodies were from Life Technologies, USA. RSV was from Bio Basic Inc, Canada; NAC, Z-VAD, 2, 7-dichlorodihydrofluorescein diacetate (CM-H2DCFDA), propidium iodide (PI), 4, 6-diamidino-2-phenylindole (DAPI) were from Sigma Aldrich, USA; MitoTracker Red was from Life Technologies, USA; protease and phosphatase inhibitor cocktails were from Roche, Switzerland. Plasmids transfection Plasmids were transfected into cells by using Turbofect (Thermo Fisher Scientific, USA) and following the manufacturer s instruction. PEG cell fusion assay Hela cells were first transfected with (i) pcmv-myc & mtgfp, (ii) pcmv-myc & mtrfp, (iii) myc-appoptosin & mtgfp, or (iv) myc-appoptosin & mtrfp. Then cells from (i) were mixed with those from (ii), and cells from (iii) were mixed with those from (iv). After co-culture on cover slips for 24 h, cells were washed with PBS once, treated with prewarmed 40% PEG 1500 for 5 min, washed with PBS for 5 times and cultured in cycloheximide-containing medium for 7 h. Cells were then fixed by 4% paraformaldehyde, counterstained with DAPI and observed under microscopy. Mitochondria isolation Fractionation of mitochondria and the cytosol was carried out by using a Cell Mitochondria Isolation Kit (Beyotime, China), following the manufacturer s instruction. Briefly, cells were pelleted by centrifugation at 600 g for 5 min at 4, re-suspended in mitochondria isolation solution with protease inhibitor cocktail, and homogenized on ice using a tight-fitting pestle attached to a homogenizer. The homogenate was then centrifuged at different speeds to isolate intact cells, mitochondria and cytosol fractions.
14 Immunofluorescence microscopy MitoTracker Red was added into cell media 30 min before fixation. Cells were then fixed in 4% paraformaldehyde saline for 15 min at room temperature, permeabilized, immunostained with indicated antibodies, incubated with fluorescence-conjugated secondary antibodies, counterstained with DAPI and visualized under a two photon microscope (FV10-MEP, Olympus, Japan). Statistics Results were expressed as means±sem using GraphPad Prism 5 software. Unpaired t test was used to assess statistical significance in two groups. Acknowledgements We thank all members at the Facility Core of College of Medicine, Xiamen University for their technical assistance. Competing interests The authors declare no competing or financial interests. Author contributions C.Z., P.E.F., H.X. and Y.w.Z. conceived and designed the experiments; C.Z., Z.S., L.Z., Z.Z., X.Z. and G.L. performed the experiments; C.Z., G.B., H.X. and Y.w.Z. analyzed and interpreted the data; C.Z. and Y.w.Z. wrote the paper. Funding This work was supported in part by grants from National Natural Science Foundation of China (Nos , , , and U ), Canadian Institutes of Health China-Canada AD Initiative (TAD ), National Institutes of Health (R01AG021173, R01AG038710, R01AG044420, and R01NS046673), Fujian Provincial Department of Science and Technology (2015Y4008), and Xiamen University President Fund ( ).
15 REFERENCES Agrimi, G., Di Noia, M. A., Marobbio, C. M., Fiermonte, G., Lasorsa, F. M. and Palmieri, F. (2004). Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem J 379, Alavi, M. V. and Fuhrmann, N. (2013). Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics. Mol Neurodegener 8, 32. Archer, S. L. (2013). Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med 369, Chang, C. R. and Blackstone, C. (2007). Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282, Chen, H., Detmer, S. A., Ewald, A. J., Griffin, E. E., Fraser, S. E. and Chan, D. C. (2003). Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, Cho, D. H., Nakamura, T., Fang, J., Cieplak, P., Godzik, A., Gu, Z. and Lipton, S. A. (2009). S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324, Cribbs, J. T. and Strack, S. (2007). Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8, Detmer, S. A. and Chan, D. C. (2007). Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol 176, Frank, S., Gaume, B., Bergmann-Leitner, E. S., Leitner, W. W., Robert, E. G., Catez, F., Smith, C. L. and Youle, R. J. (2001). The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1, Gomes, L. C. and Scorrano, L. (2008). High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta 1777, Guernsey, D. L., Jiang, H., Campagna, D. R., Evans, S. C., Ferguson, M., Kellogg, M. D., Lachance, M., Matsuoka, M., Nightingale, M., Rideout, A. et al. (2009). Mutations in
16 mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat Genet 41, Guo, X., Chen, K. H., Guo, Y., Liao, H., Tang, J. and Xiao, R. P. (2007). Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res 101, Haitina, T., Lindblom, J., Renstrom, T. and Fredriksson, R. (2006). Fourteen novel human members of mitochondrial solute carrier family 25 (SLC25) widely expressed in the central nervous system. Genomics 88, Iqbal, S. and Hood, D. A. (2014). Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am J Physiol Cell Physiol 306, C Ishihara, N., Eura, Y. and Mihara, K. (2004). Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117, James, D. I., Parone, P. A., Mattenberger, Y. and Martinou, J. C. (2003). hfis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278, Jheng, H. F., Tsai, P. J., Guo, S. M., Kuo, L. H., Chang, C. S., Su, I. J., Chang, C. R. and Tsai, Y. S. (2012). Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32, Jiang, S., Li, Y., Zhang, X., Bu, G., Xu, H. and Zhang, Y. W. (2014). Trafficking regulation of proteins in Alzheimer's disease. Mol Neurodegener 9, 6. Kannengiesser, C., Sanchez, M., Sweeney, M., Hetet, G., Kerr, B., Moran, E., Fuster Soler, J. L., Maloum, K., Matthes, T., Oudot, C. et al. (2011). Missense SLC25A38 variations play an important role in autosomal recessive inherited sideroblastic anemia. Haematologica 96, Karbowski, M., Lee, Y. J., Gaume, B., Jeong, S. Y., Frank, S., Nechushtan, A., Santel, A., Fuller, M., Smith, C. L. and Youle, R. J. (2002). Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159, Karbowski, M., Neutzner, A. and Youle, R. J. (2007). The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 178, Knott, A. B., Perkins, G., Schwarzenbacher, R. and Bossy-Wetzel, E. (2008). Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9, Koshiba, T., Detmer, S. A., Kaiser, J. T., Chen, H., McCaffery, J. M. and Chan, D. C.
17 (2004). Structural basis of mitochondrial tethering by mitofusin complexes. Science 305, Lemere, C. A. (2013). Immunotherapy for Alzheimer's disease: hoops and hurdles. Mol Neurodegener 8, 36. Liesa, M., Palacin, M. and Zorzano, A. (2009). Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89, Manczak, M., Calkins, M. J. and Reddy, P. H. (2011). Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Human Molecular Genetics 20, Manczak, M., Mao, P., Calkins, M. J., Cornea, A., Reddy, A. P., Murphy, M. P., Szeto, H. H., Park, B. and Reddy, P. H. (2010). Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis 20 Suppl 2, S Merrill, R. A., Dagda, R. K., Dickey, A. S., Cribbs, J. T., Green, S. H., Usachev, Y. M. and Strack, S. (2011). Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS Biol 9, e Meuer, K., Suppanz, I. E., Lingor, P., Planchamp, V., Goricke, B., Fichtner, L., Braus, G. H., Dietz, G. P., Jakobs, S., Bahr, M. et al. (2007). Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ 14, Nakamura, N., Kimura, Y., Tokuda, M., Honda, S. and Hirose, S. (2006). MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep 7, Neuspiel, M., Zunino, R., Gangaraju, S., Rippstein, P. and McBride, H. (2005). Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem 280, Palmieri, F. (1994). Mitochondrial carrier proteins. FEBS Lett 346, Papanicolaou, K. N., Khairallah, R. J., Ngoh, G. A., Chikando, A., Luptak, I., O'Shea, K. M., Riley, D. D., Lugus, J. J., Colucci, W. S., Lederer, W. J. et al. (2011). Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31, Park, Y. Y., Nguyen, O. T., Kang, H. and Cho, H. (2014). MARCH5-mediated quality control on acetylated Mfn1 facilitates mitochondrial homeostasis and cell survival. Cell Death Dis 5,
18 e1172. Partikian, A., Olveczky, B., Swaminathan, R., Li, Y. and Verkman, A. S. (1998). Rapid diffusion of green fluorescent protein in the mitochondrial matrix. J Cell Biol 140, Pitts, K. R., Yoon, Y., Krueger, E. W. and McNiven, M. A. (1999). The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell 10, Rambold, A. S., Kostelecky, B., Elia, N. and Lippincott-Schwartz, J. (2011). Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 108, Rehman, J., Zhang, H. J., Toth, P. T., Zhang, Y., Marsboom, G., Hong, Z., Salgia, R., Husain, A. N., Wietholt, C. and Archer, S. L. (2012). Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. Faseb J 26, Saraiva, A. A., Borges, M. M., Madeira, M. D., Tavares, M. A. and Paula-Barbosa, M. M. (1985). Mitochondrial abnormalities in cortical dendrites from patients with Alzheimer's disease. J Submicrosc Cytol 17, Shen, T., Zheng, M., Cao, C., Chen, C., Tang, J., Zhang, W., Cheng, H., Chen, K. H. and Xiao, R. P. (2007). Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem 282, Smirnova, E., Shurland, D. L., Ryazantsev, S. N. and van der Bliek, A. M. (1998). A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143, Taguchi, N., Ishihara, N., Jofuku, A., Oka, T. and Mihara, K. (2007). Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282, Wang, R., Zhang, Y. W., Zhang, X., Liu, R., Zhang, X., Hong, S., Xia, K., Xia, J., Zhang, Z. and Xu, H. (2006). Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. Faseb J 20, Wang, W., Xie, Q., Zhou, X., Yao, J., Zhu, X., Huang, P., Zhang, L., Wei, J., Xie, H., Zhou, L. et al. (2015). Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett 358, Wang, X., Su, B., Lee, H. G., Li, X., Perry, G., Smith, M. A. and Zhu, X. (2009). Impaired
19 balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 29, Wang, X., Su, B., Siedlak, S. L., Moreira, P. I., Fujioka, H., Wang, Y., Casadesus, G. and Zhu, X. (2008). Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105, Westermann, B. (2010). Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11, Wu, S., Zhou, F., Zhang, Z. and Xing, D. (2011). Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. Febs J 278, Yonashiro, R., Ishido, S., Kyo, S., Fukuda, T., Goto, E., Matsuki, Y., Ohmura-Hoshino, M., Sada, K., Hotta, H., Yamamura, H. et al. (2006). A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. Embo J 25, Youle, R. J. and van der Bliek, A. M. (2012). Mitochondrial fission, fusion, and stress. Science 337, Zhang, H., Zhang, Y. W., Chen, Y., Huang, X., Zhou, F., Wang, W., Xian, B., Zhang, X., Masliah, E., Chen, Q. et al. (2012). Appoptosin is a novel pro-apoptotic protein and mediates cell death in neurodegeneration. Journal of Neuroscience 32,
20 FIGURES Fig. 1. Overexpression of appoptosin induces mitochondrial fragmentation. (A) Hela cells and (B) mouse primary neurons were transfected with pcmv-myc (as control) and myc-appoptosin (appop) for 24 h. MitoTracker Red was added into medium 30 min before fixation to stain mitochondria. After immunostaining with an anti-myc antibody (to indicate appoptosin), cells were counterstained with DAPI and observed under a confocol microscope. The number of cells with different types of mitochondria morphology (normal and fragmented) were quantitatively analyzed (n>100). Signals for myc-appoptosin, mitochondria and nuclei were shown in green, red and blue, respectively. Scale bars in A: 5 μm for zoom in images, and 10 μm for the others. Scale bars in B: 50 μm for zoom in images, and 100 μm for the others.
21 Fig. 2. Appoptosin-induced mitochondrial fragmentation is not dependent on ROS generation or caspase activation. Hela cells were transfected with pcmv-myc or myc-appoptosin (appop). Four hours later, the media were refreshed and the cells were treated with (A) 1 mm NAC, (B) 50 μm RSV, (C) 50 μm Z-VAD, or (D) 1 μm SA for additional 20 h. After staining with MitoTracker Red, fixation, immunostaining with an anti-myc antibody (to indicate appoptosin) and staining with DAPI, cells were observed under a confocol microscope. Signals for myc-appoptosin, mitochondria and nuclei were shown in green, red and blue, respectively. (E) The number of cells with different types of mitochondria in (A-D) were counted and the percent of cells with fragmented mitochondria were compared (n>100). Scale bars: 5 μm for zoom in images, and 10 μm for the others.
22 Fig. 3. Appoptosin does not affect the levels of mitochondrial fusion and fission proteins and the mitochondrial translocation of DRP1. (A) Hela cells were transfected with pcmv-myc (-) and myc-appoptosin (+) for 24 h before subjected to Western blot analysis. (B) Protein levels of DRP1, FIS1, MFN1, MFN2 and OPA1 in (A) were quantified by densitometry and normalized to those of -actin for comparison, n=3. (C) Cells were transfected with myc-appoptosin (+) or control (-) for 24 h. Mitochondria (mito) and the cytosol (cyto) were then separated and subjected to Western blot analysis. (D) Protein levels of DRP1 in (C) were quantified by densitometry and normalized to those of tubulin or COX-IV for comparison, n=3. ns: not significant.
23 Fig. 4. Appoptosin interacts with MFN1, MFN2 and MITOL. Myc-appoptosin was co-transfected with (A) HA-DRP1, (B) HA-FIS1, (C) HA-MFN1, (D) HA-MFN2, (E) HA-OPA1 or (F) HA-MITOL in HEK 293T cells for 24 h. Cell lysates were subjected to co-immunoprecipitation (co-ip) with mouse IgG (migg), mouse anti-myc antibody or mouse anti-ha antibody and then Western blot analysis. (G) HEK 293T cell lysates were subjected to co-ip with rabbit IgG (RIgG) or rabbit anti-mfn1 antibody and then Western blot analysis. (H) HEK 293T cell lysates were subjected to co-ip with migg or mouse anti-mfn2 antibody and then Western blot analysis.
24 Fig. 5. Overexpression of appoptosin impairs MFN1-MFN2 interaction and mitochondrial fusion. (A) HA-MFN1 & myc-mfn1, HA-MFN1 & myc-mfn2, or HA-MFN2 & myc-mfn2 were co-expressed in 293T cells. After equal splitting, cells were transfected with control or appoptosin plasmids for 24 h. Cell lysates were subjected to co-immunoprecipitation (IP) with the mouse anti-myc antibody 9E10 and then Western blot analysis. (B) After co-culture of Hela cells expressing pcmv-myc/mtgfp with those expressing pcmv-myc/mtrfp (left panels), or cells expressing myc-appoptosin/mtgfp with those expressing myc-appoptosin/mtrfp (right panels), cells were treated with 40% PEG 1500 for 5 min, and then incubated in cycloheximide-containing medium for 7 h. Cells were then fixed, counterstained with DAPI and observed under microscopy. Bottom panels were zoom in images of upper panels. Scale bars: 20 μm for upper panels and 5 μm for zoom in images. The number of fusing cell clusters with different mitochondrial fusion status (full or partial) were counted for comparison, n 100, **p<0.01.
25 Fig. 6. Appoptosin-induced mitochondrial fragmentation is alleviated by promoting mitochondrial fusion. (A) Hela cells transfected with pcmv-ha, HA-DRP1, HA-FIS1, HA-DRP1 K38A, HA-MFN1, HA-MFN2 and HA-MITOL were treated with MitoTracker Red, immuno-fluorescence stained and observed under a confocal microscope. Mitochondria and HA signals were shown in red and green, respectively. (B) Myc-appoptosin was co-transfected with HA-MFN1, HA-MITOL or HA-DRP1 K38A into Hela cells for 24 h. Cells were subjected to immuno-fluorescence staining and counterstaining with DAPI. Myc-appoptosin, HA and DAPI signals were shown in green, red and blue, respectively. (C) Quantitative analysis of numbers of transfected cells with normal, fragmented or aggregated/fused mitochondria in (B), n 100. Scale bars: 5 μm for zoom in images, and 10 μm for the other ones. *p<0.05; **p<0.01.
26 Fig. 7. Appoptosin-induced apoptosis is aggravated by FIS1 and alleviated by DRP1 K38A, MFN1 and MITOL. Myc-appoptosin was co-transfected with (A) HA-FIS1, (B) HA-DRP1 K38A, (C) HA-MFN1, (D) HA-MFN2, or (E) HA-MITOL in Hela cells for 24 h. Cell lysates were subjected to Western-Blot analysis to study levels of cleaved PARP (c-parp). (F) Densitometry quantification analysis of (A-E), n=3, ns: not significant; *p<0.05; **p<0.01; ***p<0.001.
27 Fig. 8. SLC25A26 does not affect mitochondrial morphology. (A) Hela cells were transfected with pcmv-myc, myc-appoptosin (appop) or myc-slc25a26 (25A26) for 24 h, and then treated with MitoTracker Red and immuno-fluorescene stained for observation under a confocal microscope. (B) Quantitative analysis of numbers of transfected cells with fragmented mitochondria in (A), n=80, ns: not significant; **p<0.01. (C) Myc-SLC25A26 was co-transfected with HA-MFN1 or HA-MFN2 in HEK 293T cells for 24 h. Cell lysates were subjected to co-ip with migg, mouse anti-myc antibody or mouse anti-ha antibody, and then Western blot analysis. (D) Myc-appoptosin was co-transfected with HA-MITOL in HEK 293T cells for 24 h. Cell lysates were subjected to co-ip with migg, mouse anti-myc antibody or mouse anti-ha antibody, and then Western blot analysis.
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supporting Information
 Supporting Information Wang et al. 10.1073/pnas.0804871105 SI Materials and Methods Cell Culture and Transfection. Human neuroblastoma M17 cells were grown as described before (1). Transfection was performed
Supporting Information Wang et al. 10.1073/pnas.0804871105 SI Materials and Methods Cell Culture and Transfection. Human neuroblastoma M17 cells were grown as described before (1). Transfection was performed
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
What are mitochondria?
 What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
Mitochondrial dynamics in ischemia and reperfusion
 Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
09/30/2017. Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center
 09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
Mitofusin 1 is degraded at G 2 /M phase through ubiquitylation by MARCH5
 Park and Cho Cell Division 2012, 7:25 SHORT REPORT Open Access Mitofusin 1 is degraded at G 2 /M phase through ubiquitylation by MARCH5 Yong-Yea Park 1,2 and Hyeseong Cho 1,2* Abstract Background: Mitochondria
Park and Cho Cell Division 2012, 7:25 SHORT REPORT Open Access Mitofusin 1 is degraded at G 2 /M phase through ubiquitylation by MARCH5 Yong-Yea Park 1,2 and Hyeseong Cho 1,2* Abstract Background: Mitochondria
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
Mitochondria associate with the cytoskeleton. Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008)
 Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1
 Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with
 Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1
 Research Article 619 Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1 Yong-Yea Park 1,2, Seungmin Lee 1,2, Mariusz Karbowski
Research Article 619 Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1 Yong-Yea Park 1,2, Seungmin Lee 1,2, Mariusz Karbowski
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX.
 Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Mitochondrie et muscle lisse. Vladimir Veksler
 Mitochondrie et muscle lisse Vladimir Veksler Main mitochonrial functions Energy production ATP consumption rate in smooth and striated muscle smooth muscle striated (cardiac) muscle at rest 30 µmol/min/g
Mitochondrie et muscle lisse Vladimir Veksler Main mitochonrial functions Energy production ATP consumption rate in smooth and striated muscle smooth muscle striated (cardiac) muscle at rest 30 µmol/min/g
SUPPLEMENTARY INFORMATION
 Cell viability rate 0.8 0.6 0 0.05 0.1 0.2 0.3 0.4 0.5 0.7 1 Exposure duration (s) Supplementary Figure 1. Femtosecond laser could disrupt and turn off GFP without photons at 473 nm and keep cells alive.
Cell viability rate 0.8 0.6 0 0.05 0.1 0.2 0.3 0.4 0.5 0.7 1 Exposure duration (s) Supplementary Figure 1. Femtosecond laser could disrupt and turn off GFP without photons at 473 nm and keep cells alive.
Nitric Oxide Links Mitochondrial Fission to Alzheimer's Disease
 Nitric Oxide Links Mitochondrial Fission to Alzheimer's Disease Benedikt Westermann* Institut für Zellbiologie and Bayreuther Zentrum für Molekulare Biowissenschaften, Universität Bayreuth, 95440 Bayreuth,
Nitric Oxide Links Mitochondrial Fission to Alzheimer's Disease Benedikt Westermann* Institut für Zellbiologie and Bayreuther Zentrum für Molekulare Biowissenschaften, Universität Bayreuth, 95440 Bayreuth,
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer s Disease
 9090 The Journal of Neuroscience, July 15, 2009 29(28):9090 9103 Cellular/Molecular Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer s Disease Xinglong Wang, 1 Bo Su, 1 Hyoung-gon Lee,
9090 The Journal of Neuroscience, July 15, 2009 29(28):9090 9103 Cellular/Molecular Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer s Disease Xinglong Wang, 1 Bo Su, 1 Hyoung-gon Lee,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
Role of Mitochondrial Remodeling in Programmed Cell Death in
 Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Plant mitochondrial dynamics
 Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity
 Research Article 6535 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity Naotada Ishihara, Yuka Eura and Katsuyoshi Mihara* Department of Molecular Biology, Graduate
Research Article 6535 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity Naotada Ishihara, Yuka Eura and Katsuyoshi Mihara* Department of Molecular Biology, Graduate
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr
 The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
Mitochondrial Quality Control
 Mitochondrial Quality Control You are here Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 938 Charleston, May 20, 2015 biology4kids.com Cryo-electron microscopy Fluorescent microscopy Yeast Human Semi-autonomous,
Mitochondrial Quality Control You are here Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 938 Charleston, May 20, 2015 biology4kids.com Cryo-electron microscopy Fluorescent microscopy Yeast Human Semi-autonomous,
Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis
 Molecular Biology of the Cell Vol. 15, 5001 5011, November 2004 Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis Yang-ja Lee,* Seon-Yong Jeong,* Mariusz
Molecular Biology of the Cell Vol. 15, 5001 5011, November 2004 Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis Yang-ja Lee,* Seon-Yong Jeong,* Mariusz
Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health
 I R E V I E W S E Review in Advance first posted online on August 29, 2012. (Changes may still occur before final publication online and in print.) N C A D V A N Fusion and Fission: Interlinked Processes
I R E V I E W S E Review in Advance first posted online on August 29, 2012. (Changes may still occur before final publication online and in print.) N C A D V A N Fusion and Fission: Interlinked Processes
MITOCHONDRIAL BIOGENESIS AND REDOX REGULATION
 MITOCHONDRIAL BIOGENESIS AND REDOX Claude A. Piantadosi, MD Professor of Medicine and Pathology Duke University Medical Center Durham, N.C. USA Objectives Provide an overview of the physiological and pathological
MITOCHONDRIAL BIOGENESIS AND REDOX Claude A. Piantadosi, MD Professor of Medicine and Pathology Duke University Medical Center Durham, N.C. USA Objectives Provide an overview of the physiological and pathological
Supplemental Figures S1 S5
 Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells Ines Witte (1), Sebastian Altenhöfer (1), Petra Wilgenbus (1), Julianna Amort (1), Albrecht M. Clement
Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells Ines Witte (1), Sebastian Altenhöfer (1), Petra Wilgenbus (1), Julianna Amort (1), Albrecht M. Clement
Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission fusion proteins
 Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission fusion proteins Shengnan Wu, Feifan Zhou, Zhenzhen Zhang and Da Xing MOE Key Laboratory
Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission fusion proteins Shengnan Wu, Feifan Zhou, Zhenzhen Zhang and Da Xing MOE Key Laboratory
Mitochondrial Quality Control
 Mitochondrial Quality Control Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 998 Stockholm, May 16, 2017 Respiration (ATP generation) Cofactors & key metabolites synthesis Ion homeostasis Lipid homeostasis
Mitochondrial Quality Control Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 998 Stockholm, May 16, 2017 Respiration (ATP generation) Cofactors & key metabolites synthesis Ion homeostasis Lipid homeostasis
Mitochondrial Quality Control
 Mitochondrial Quality Control Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 998 Lincoln, June 14, 2016 Respiration (ATP generation) Cofactors & key metabolites synthesis Ion homeostasis Lipid homeostasis
Mitochondrial Quality Control Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 998 Lincoln, June 14, 2016 Respiration (ATP generation) Cofactors & key metabolites synthesis Ion homeostasis Lipid homeostasis
FSC-W FSC-H CD4 CD62-L
 Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Inactivation of MARCH5 Prevents Mitochondrial Fragmentation and Interferes with Cell Death in a Neuronal Cell Model
 Inactivation of MARCH5 Prevents Mitochondrial Fragmentation and Interferes with Cell Death in a Neuronal Cell Model Lei Fang 1, Charles Hemion 1, David Goldblum 2, Peter Meyer 1,2, Selim Orgül 2, Stephan
Inactivation of MARCH5 Prevents Mitochondrial Fragmentation and Interferes with Cell Death in a Neuronal Cell Model Lei Fang 1, Charles Hemion 1, David Goldblum 2, Peter Meyer 1,2, Selim Orgül 2, Stephan
Biological Process Term Enrichment
 Biological Process Term Enrichment cellular protein localization cellular macromolecule localization intracellular protein transport intracellular transport generation of precursor metabolites and energy
Biological Process Term Enrichment cellular protein localization cellular macromolecule localization intracellular protein transport intracellular transport generation of precursor metabolites and energy
Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant
 9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
M i t o c h o n d r i a
 M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage
 Scientific Report Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage Kuan Zhang, Huihui Li & Zhiyin Song* Abstract Mitochondrial inner membrane fusion depends
Scientific Report Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage Kuan Zhang, Huihui Li & Zhiyin Song* Abstract Mitochondrial inner membrane fusion depends
Gene Control Mechanisms at Transcription and Translation Levels
 Gene Control Mechanisms at Transcription and Translation Levels Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 9
Gene Control Mechanisms at Transcription and Translation Levels Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 9
The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells
 Research Article 3049 The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells Daniel Tondera*, Frank Czauderna, Katharina Paulick, Rolf Schwarzer, Jörg Kaufmann and Ansgar
Research Article 3049 The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells Daniel Tondera*, Frank Czauderna, Katharina Paulick, Rolf Schwarzer, Jörg Kaufmann and Ansgar
Mitochondrial kiss-and-run : interplay between mitochondrial motility and fusion fission dynamics
 The EMBO Journal (2009) 28, 3074 3089 & 2009 European Molecular Biology Organization All Rights Reserved 0261-4189/09 www.embojournal.org : interplay between mitochondrial motility and fusion fission dynamics
The EMBO Journal (2009) 28, 3074 3089 & 2009 European Molecular Biology Organization All Rights Reserved 0261-4189/09 www.embojournal.org : interplay between mitochondrial motility and fusion fission dynamics
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells
 Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
SENP3-mediated desumoylation of dynamin-related protein 1 promotes cell death following ischaemia
 The EMBO Journal (2013) 32, 1514 1528 www.embojournal.org SENP3-mediated desumoylation of dynamin-related protein 1 promotes cell death following ischaemia THE EMBO JOURNAL Chun Guo, Keri L Hildick, Jia
The EMBO Journal (2013) 32, 1514 1528 www.embojournal.org SENP3-mediated desumoylation of dynamin-related protein 1 promotes cell death following ischaemia THE EMBO JOURNAL Chun Guo, Keri L Hildick, Jia
Mitochondrial Membrane Potential by Object Spot Counting
 A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins
 Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins Xia-Chun Li, Huazhong University of Science and Technology Yu Hu, Huazhong University
Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins Xia-Chun Li, Huazhong University of Science and Technology Yu Hu, Huazhong University
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb97 P ( μm, hours) 1 2 4 P DMSO Figure S1 unningham et al. E 97 65 27 MFN1 GFP-Parkin Opa1 ctin GPDH HEK293 GFP-Parkin 19 115 97 65 27 Mitochondrial Fraction SH-SY5Y GFP-Parkin Mito DMSO Mito
DOI:.38/ncb97 P ( μm, hours) 1 2 4 P DMSO Figure S1 unningham et al. E 97 65 27 MFN1 GFP-Parkin Opa1 ctin GPDH HEK293 GFP-Parkin 19 115 97 65 27 Mitochondrial Fraction SH-SY5Y GFP-Parkin Mito DMSO Mito
Cancer Letters 323 (2012) Contents lists available at SciVerse ScienceDirect. Cancer Letters. journal homepage:
 Cancer Letters 323 (212) 62 68 Contents lists available at SciVerse ScienceDirect Cancer Letters journal homepage: www.elsevier.com/locate/canlet Mitofusin 1 inhibits an apoptosis-associated amino-terminal
Cancer Letters 323 (212) 62 68 Contents lists available at SciVerse ScienceDirect Cancer Letters journal homepage: www.elsevier.com/locate/canlet Mitofusin 1 inhibits an apoptosis-associated amino-terminal
Youngnam N. Jin 1, Yanxun V. Yu 2, Soner Gundemir 3, Chulman Jo 3, Mei Cui 4, Kim Tieu 4, Gail V. W. Johnson 1,3 * Abstract.
 Impaired Mitochondrial Dynamics and Nrf2 Signaling Contribute to Compromised Responses to Oxidative Stress in Striatal Cells Expressing Full-Length Mutant Huntingtin Youngnam N. Jin 1, Yanxun V. Yu 2,
Impaired Mitochondrial Dynamics and Nrf2 Signaling Contribute to Compromised Responses to Oxidative Stress in Striatal Cells Expressing Full-Length Mutant Huntingtin Youngnam N. Jin 1, Yanxun V. Yu 2,
Toxicological Targets. Russell L. Carr Department of Basic Sciences College of Veterinary Medicine
 Toxicological Targets Russell L. Carr Department of Basic Sciences College of Veterinary Medicine Toxicology Definitions = study of poisons Poison = any agent capable of producing a deleterious response
Toxicological Targets Russell L. Carr Department of Basic Sciences College of Veterinary Medicine Toxicology Definitions = study of poisons Poison = any agent capable of producing a deleterious response
Way to impose membrane curvature
 Way to impose membrane curvature Sar1 reticulons? clathrin and other vesicle coats dynamin BAR domains McMahon, 2005 The ER is continuous with the nuclear envelope and contains tubules and sheets Gia Voeltz
Way to impose membrane curvature Sar1 reticulons? clathrin and other vesicle coats dynamin BAR domains McMahon, 2005 The ER is continuous with the nuclear envelope and contains tubules and sheets Gia Voeltz
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain
 SUPPLEMENTAL INFORMATION Supplemental Figure Legends Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain mitochondrial NAD +. A) Extracellular tryptophan, even at 5 µm,
SUPPLEMENTAL INFORMATION Supplemental Figure Legends Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain mitochondrial NAD +. A) Extracellular tryptophan, even at 5 µm,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease
 British Journal of Pharmacology DOI:10.1111/bph.12516 www.brjpharmacol.org Themed Issue: Mitochondrial Pharmacology: Energy, Injury & Beyond REVIEW Mitochondrial fusion and fission proteins: novel therapeutic
British Journal of Pharmacology DOI:10.1111/bph.12516 www.brjpharmacol.org Themed Issue: Mitochondrial Pharmacology: Energy, Injury & Beyond REVIEW Mitochondrial fusion and fission proteins: novel therapeutic
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation
 2252 Research Article The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation Jian Zhao 1, *, Tong Liu 1, Shao-Bo Jin 2, Nikolay Tomilin
2252 Research Article The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation Jian Zhao 1, *, Tong Liu 1, Shao-Bo Jin 2, Nikolay Tomilin
Drosophila Apoptosis and the Regulation of the Caspase Cascade
 Drosophila Apoptosis and the Regulation of the Caspase Cascade Kate Stafford March 18, 2005 Abstract The caspase cascade in Drosophila is controlled primarily by DIAP1 (Drosophila inhibitor of apoptosis),
Drosophila Apoptosis and the Regulation of the Caspase Cascade Kate Stafford March 18, 2005 Abstract The caspase cascade in Drosophila is controlled primarily by DIAP1 (Drosophila inhibitor of apoptosis),
Molecular and Pathogenesis Study of Cystinosis (Period: Jul Jun. 09)
 Final Report to the Cystinosis Research Foundation: Molecular and Pathogenesis Study of Cystinosis (Period: Jul. 07 - Jun. 09) Taosheng Huang, MD, PhD Mentor; Sha Tang, PhD Research Fellow Hypothesis:
Final Report to the Cystinosis Research Foundation: Molecular and Pathogenesis Study of Cystinosis (Period: Jul. 07 - Jun. 09) Taosheng Huang, MD, PhD Mentor; Sha Tang, PhD Research Fellow Hypothesis:
Physiological and Pathological Significance of Dynamin-Related Protein 1 (Drp1)-Dependent Mitochondrial Fission in the Nervous System
 http://dx.doi.org/10.5607/en.2013.22.3.149 Exp Neurobiol. 2013 Sep;22(3):149-157. pissn 1226-2560 eissn 2093-8144 Review Article Physiological and Pathological Significance of Dynamin-Related Protein 1
http://dx.doi.org/10.5607/en.2013.22.3.149 Exp Neurobiol. 2013 Sep;22(3):149-157. pissn 1226-2560 eissn 2093-8144 Review Article Physiological and Pathological Significance of Dynamin-Related Protein 1
Types of biological networks. I. Intra-cellurar networks
 Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014
 Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014 2 Abstract In this experiment, we used immunofluorescence to isolate certain organelles and see where they
Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014 2 Abstract In this experiment, we used immunofluorescence to isolate certain organelles and see where they
Review Article The Emerging Role of Proteolysis in Mitochondrial Quality Control and the Etiology of Parkinson s Disease
 son s Disease Volume 2012, Article ID 382175, 16 pages doi:10.1155/2012/382175 Review Article The Emerging Role of Proteolysis in Mitochondrial Quality Control and the Etiology of son s Disease Riya Shanbhag,
son s Disease Volume 2012, Article ID 382175, 16 pages doi:10.1155/2012/382175 Review Article The Emerging Role of Proteolysis in Mitochondrial Quality Control and the Etiology of son s Disease Riya Shanbhag,
The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development. Kyle Pemberton
 The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development Kyle Pemberton Acknowledgement Dr. Xu Lab Members Brittany Mersman Nicki Patel Pallavi Mhaskar Jason Cocjin Committee
The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development Kyle Pemberton Acknowledgement Dr. Xu Lab Members Brittany Mersman Nicki Patel Pallavi Mhaskar Jason Cocjin Committee
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
GO ID GO term Number of members GO: translation 225 GO: nucleosome 50 GO: calcium ion binding 76 GO: structural
 GO ID GO term Number of members GO:0006412 translation 225 GO:0000786 nucleosome 50 GO:0005509 calcium ion binding 76 GO:0003735 structural constituent of ribosome 170 GO:0019861 flagellum 23 GO:0005840
GO ID GO term Number of members GO:0006412 translation 225 GO:0000786 nucleosome 50 GO:0005509 calcium ion binding 76 GO:0003735 structural constituent of ribosome 170 GO:0019861 flagellum 23 GO:0005840
The University of Jordan. Accreditation & Quality Assurance Center. COURSE Syllabus
 The University of Jordan Accreditation & Quality Assurance Center COURSE Syllabus 1 Course title Principles of Genetics and molecular biology 2 Course number 0501217 3 Credit hours (theory, practical)
The University of Jordan Accreditation & Quality Assurance Center COURSE Syllabus 1 Course title Principles of Genetics and molecular biology 2 Course number 0501217 3 Credit hours (theory, practical)
Yeast Genome-wide Screens to Ascertain the Genetic Landscape for Barth Syndrome. Christopher R. McMaster, PhD Dalhousie University
 Yeast Genome-wide Screens to Ascertain the Genetic Landscape for Barth Syndrome Christopher R. McMaster, PhD Dalhousie University Using Systematic Genetics to Identify Modifies Genes that Affect Fitness
Yeast Genome-wide Screens to Ascertain the Genetic Landscape for Barth Syndrome Christopher R. McMaster, PhD Dalhousie University Using Systematic Genetics to Identify Modifies Genes that Affect Fitness
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
REGULATION OF MITOCHONDRIAL DIVISION BY THE DRP1 RECEPTORS. Thesis by. Oliver Calvin Losόn
 REGULATION OF MITOCHONDRIAL DIVISION BY THE DRP1 RECEPTORS Thesis by Oliver Calvin Losόn In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY
REGULATION OF MITOCHONDRIAL DIVISION BY THE DRP1 RECEPTORS Thesis by Oliver Calvin Losόn In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY
S1 Gene ontology (GO) analysis of the network alignment results
 1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
Utah School of Medicine, Salt Lake City, UT Tel.: ; Fax: ;
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 25, pp. 17312 17320, June 23, 2006 2006 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Dimeric Dnm1-G385D Interacts
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 25, pp. 17312 17320, June 23, 2006 2006 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Dimeric Dnm1-G385D Interacts
Effects of the BH3-only protein human Noxa on mitochondrial dynamics
 FEBS Letters 583 (2009) 2349 2354 journal homepage: www.febsletters.org Effects of the BH3-only protein human Noxa on mitochondrial dynamics Ha-Na Woo a,c,e, Young-Woo Seo b, Ae Ran Moon c, Seon-Yong Jeong
FEBS Letters 583 (2009) 2349 2354 journal homepage: www.febsletters.org Effects of the BH3-only protein human Noxa on mitochondrial dynamics Ha-Na Woo a,c,e, Young-Woo Seo b, Ae Ran Moon c, Seon-Yong Jeong
A homo-dimer of annexin V protects against ischemia reperfusion injury in lung transplantation
 A homo-dimer of annexin V protects against ischemia reperfusion injury in lung transplantation K Hashimoto, H Kim, H Oishi, M Chen, I Iskender, J Sakamoto, A Ohsumi, Z Guan, DM Hwang, TK Waddell, M Cypel,
A homo-dimer of annexin V protects against ischemia reperfusion injury in lung transplantation K Hashimoto, H Kim, H Oishi, M Chen, I Iskender, J Sakamoto, A Ohsumi, Z Guan, DM Hwang, TK Waddell, M Cypel,
NucView TM 488 Caspase-3 Assay Kit for Live Cells
 NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells
 Molecular Biology of the Cell Vol. 19, 2402 2412, June 2008 The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells Shilpa Gandre-Babbe and Alexander
Molecular Biology of the Cell Vol. 19, 2402 2412, June 2008 The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells Shilpa Gandre-Babbe and Alexander
Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer
 Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer Nexcelom Bioscience LLC. 360 Merrimack Street, Building 9 Lawrence, MA 01843 T: 978.327.5340 F: 978.327.5341
Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer Nexcelom Bioscience LLC. 360 Merrimack Street, Building 9 Lawrence, MA 01843 T: 978.327.5340 F: 978.327.5341
Programmed Cell Death
 Programmed Cell Death Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada d.purnomosari@ugm.ac.id What is apoptosis? a normal component of the development
Programmed Cell Death Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada d.purnomosari@ugm.ac.id What is apoptosis? a normal component of the development
Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization
 Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression
 Traffic 2010; 11: 827 842 2010 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2010.01055.x The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression Danming Tang, Hebao Yuan and Yanzhuang
Traffic 2010; 11: 827 842 2010 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2010.01055.x The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression Danming Tang, Hebao Yuan and Yanzhuang
CELB40060 Membrane Trafficking in Animal Cells. Prof. Jeremy C. Simpson. Lecture 2 COPII and export from the ER
 CELB40060 Membrane Trafficking in Animal Cells Prof. Jeremy C. Simpson Lecture 2 COPII and export from the ER Today s lecture... The COPII coat - localisation and subunits Formation of the COPII coat at
CELB40060 Membrane Trafficking in Animal Cells Prof. Jeremy C. Simpson Lecture 2 COPII and export from the ER Today s lecture... The COPII coat - localisation and subunits Formation of the COPII coat at
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology
 Research Article 1201 Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology Diana Stojanovski 1, Olga S. Koutsopoulos 1, Koji Okamoto 2 and Michael T. Ryan 1, * 1 Department
Research Article 1201 Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology Diana Stojanovski 1, Olga S. Koutsopoulos 1, Koji Okamoto 2 and Michael T. Ryan 1, * 1 Department
Department Curriculum and Assessment Outline
 Department: Science Year Group: 10 Teaching, learning and assessment during the course: Combined Science 1 2 B1 Key concepts in Biology B2 Cells and control What are the structure and function of cells.
Department: Science Year Group: 10 Teaching, learning and assessment during the course: Combined Science 1 2 B1 Key concepts in Biology B2 Cells and control What are the structure and function of cells.
Bio-Plex Pro RBM Apoptosis Assays
 Acute Phase Apoptosis Cancer Cardiovascular Disease Cytokines Chemokines, Growth Factors Diabetes Gene Expression Genotyping Immunoglobulin Isotyping MicroRNA Expression Bio-Plex Pro RBM Apoptosis Assays
Acute Phase Apoptosis Cancer Cardiovascular Disease Cytokines Chemokines, Growth Factors Diabetes Gene Expression Genotyping Immunoglobulin Isotyping MicroRNA Expression Bio-Plex Pro RBM Apoptosis Assays
Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy
 3112 Research Article Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy David A. Zeevi 1, *, Shaya Lev 2,3, *, Ayala
3112 Research Article Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy David A. Zeevi 1, *, Shaya Lev 2,3, *, Ayala
Cardiolipin Remodeling by ALCAT1 Regulates Dilated Cardiomyopathy Through Oxidative Stress and Mitophagy. Yuguang (Roger) Shi
 Cardiolipin Remodeling by ALCAT1 Regulates Dilated Cardiomyopathy Through xidative Stress and Mitophagy Yuguang (Roger) Shi yus11@psu.eud Cardiolipin Biosynthetic and Remodeling Pathways Phosphatidic Acid
Cardiolipin Remodeling by ALCAT1 Regulates Dilated Cardiomyopathy Through xidative Stress and Mitophagy Yuguang (Roger) Shi yus11@psu.eud Cardiolipin Biosynthetic and Remodeling Pathways Phosphatidic Acid
Antioxidant Assay Kit
 Antioxidant Assay Kit Catalog Number KA1622 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Antioxidant Assay Kit Catalog Number KA1622 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
