Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission fusion proteins
|
|
|
- Ruby Richard
- 5 years ago
- Views:
Transcription
1 Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission fusion proteins Shengnan Wu, Feifan Zhou, Zhenzhen Zhang and Da Xing MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou, China Keywords dynamin-related protein 1 (Drp1); fission; high-fluence low-power laser irradiation (HF-LPLI); mitofusin 2 (Mfn2); oxidative stress Correspondence Da Xing, MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou , China Fax: Tel: xingda@scnu.edu.cn (Received 14 October 2010, revised 1 December 2010, accepted 10 January 2011) doi: /j x Mitochondria are dynamic organelles that undergo continual fusion and fission to maintain their morphology and functions, but the mechanism involved is still not clear. Here, we investigated the effect of mitochondrial oxidative stress triggered by high-fluence low-power laser irradiation (HF- LPLI) on mitochondrial dynamics in human lung adenocarcinoma cells (ASTC-a-1) and African green monkey SV40-transformed kidney fibroblast cells (COS-7). Upon HF-LPLI-triggered oxidative stress, mitochondria displayed a fragmented structure, which was abolished by exposure to dehydroascorbic acid, a reactive oxygen species scavenger, indicating that oxidative stress can induce mitochondrial fragmentation. Further study revealed that HF-LPLI caused mitochondrial fragmentation by inhibiting fusion and enhancing fission. Mitochondrial translocation of the profission protein dynamin-related protein 1 (Drp1) was observed following HF-LPLI, demonstrating apoptosis-related activation of Drp1. Notably, overexpression of Drp1 increased mitochondrial fragmentation and promoted HF-LPLIinduced apoptosis through promoting cytochrome c release and caspase-9 activation, whereas overexpression of mitofusin 2 (Mfn2), a profusion protein, caused the opposite effects. Also, neither Drp1 overexpression nor Mfn2 overexpression affected mitochondrial reactive oxygen species generation, mitochondrial depolarization, or Bax activation. We conclude that mitochondrial oxidative stress mediated through Drp1 and Mfn2 causes an imbalance in mitochondrial fission fusion, resulting in mitochondrial fragmentation, which contributes to mitochondrial and cell dysfunction. Introduction Mitochondria are dynamic organelles that frequently move, undergo fission and fuse with one another to maintain their structure and functions [1]. Aside from influencing mitochondrial morphology and the degree of connectivity of mitochondrial networks, mitochondrial fission fusion can contribute to the repair of Abbreviations CFP, cyan fluorescent protein; COX IV, cytochrome c oxidase subunit IV; DCF, 2,7-dichlorofluorescein; Drp1, dynamin-related protein; FCM, flow cytometry; FITC, fluorescein isothiocyanate; FRAP, fluorescence recovery after photobleaching; FRET, Förster resonance energy transfer; HF-LPLI, high-fluence low-power laser irradiation; H2DCFDA, dichlorodihydrofluorescein diacetate; Mfn2, mitofusin 2; MitoTracker, MitoTracker Deeper Red 633; MMP, mitochondrial membrane potential; PI, propidium iodide; RNAi, RNA interference; ROS, reactive oxygen species; shrna, short hairpin RNA; STS, staurosporine; TMRM, tetramethylrhodamine methyl ester; YFP, yellow fluorescent protein. FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 941
2 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. defective mitochondria, the proper segregation of mitochondria into daughter cells during cell division, the efficiency of oxidative phosphorylation, and intramitochondrial calcium signal propagation [1]. Imbalanced fission fusion is involved in various pathological processes [2,3], including neuronal injury [4 6], cellular senescence [7], ischemia reperfusion [8], and muscle atrophy [9]. During apoptosis, mitochondria often fragment into smaller units, and it remains unresolved whether this event has a significant impact on the rate of cell death, or merely accompanies apoptosis as an epiphenomenon [10]. Many apoptosis stimuli, such as staurosporine (STS), have been reported to caus mitochondria to fragment during the apoptotic process [10]. Recent experiments have demonstrated that mitochondrial morphology is an important determinant of mitochondrial function [11]. Mitochondrial shape depends on the balance between fission and fusion, and is controlled by multiple proteins that mediate remodeling of the outer and inner mitochondrial membranes [12]. Many of the gene products mediating the fission and fusion processes have been identified in yeast screens, and most are conserved in mammals, including the fission mediators dynamin-related protein (Drp1) (Dnm1 in yeast) and Fis1, the fusion mediators mitofusin 1 and mitofusin 2 (Mfn2) (Fzo1 in yeast), and optic atrophy 1 (Mgm1 in yeast) [12]. Unbalanced fusion leads to mitochondrial elongation, and unbalanced fission leads to excessive mitochondrial fragmentation, both of which impair mitochondrial function [12]. However, it is still unclear how extracellular stimuli modulate intracellular signaling processes to control mitochondrial dynamics and morphology. Previous studies have demonstrated that high-fluence low-power laser irradiation (HF-LPLI) can induce mitochondrial oxidative stress in human lung adenocarcinoma cells (ASTC-a-1) and SV40-transformed African green monkey kidney fibroblast cells (COS-7) cells through selectively exciting the endogenous photoacceptor (cytochrome c oxidase) by laser irradiation (632.8 nm) [13,14]. The oxidative stress causes mitochondrial permeability transition pores to open for a long time, causes mitochondrial depolarization, cytochrome c release, and caspase-3 activation, and finally results in cell apoptosis [13 15]. However, whether HF-LPLI-induced mitochondrial oxidative stress can induce mitochondrial morphological changes is still not clear. Thus, in the present study, we investigated the regulatory pathways involved in mitochondrial dynamics following HF-LPLI, using fluorescent imaging, western blot and flow cytometry techniques in ASTC-a-1 cells and COS-7 cells. Results Mitochondrial oxidative stress caused by HF-LPLI Dichlorodihydrofluorescein diacetate (H2DCFDA) is a reactive oxygen species (ROS)-sensitive probe that can be used to detect ROS production in living cells. It passively diffuses into cells, where its acetate groups are cleaved by intracellular esterases, releasing the corresponding dichlorodihydrofluorescein derivative. Dichlorodihydrofluorescein oxidation yields a fluorescent adduct, 2,7-dichlorofluorescein (DCF), that is trapped inside the cell. Thus, we used DCF to label ROS and monitor the changes in ROS in ASTC-a-1 cells under various treatments. The ROS level correlates positively with the DCF fluorescence emission intensity. As is known, the recording laser used in confocal microscopy, such as 488 nm for DCF, can probably cause an artefactual ROS signal through photosensitization and consequent in situ photo-oxidation of the dye. Therefore, we tried to increase the recording interval time and decrease the power intensity of the recording laser to avoid this problem in control studies, and then applied the same set of experimental parameters in the following studies (Fig. 1A, upper panel). As shown in Fig. 1A (upper panel), cells treated with HF-LPLI showed a significant increase in DCF fluorescence immediately after the treatment, as opposed to the poor increase observed in control cells. As shown in Fig. 1B, for quantitative analysis of the DCF signal, we normalized the initial fluorescence intensity of each term as 100 a.u., and then made a comparison between different experimental groups, as the ability to take up H2DCFDA varies slightly between cells, even in the same cell line. Quantitative analysis of DCF fluorescence emission intensities (Fig. 1B) gave similar results as those in Fig. 1A. Also, the highest DCF signal caused by HF-LPLI was found in mitochondria, as clearly shown by overlapping of the spatial mappings of fluorescence from mitochondria and the ROSspecific probes MitoTracker Deeper Red 633 (Mito- Tracker) and DCF, respectively (Fig. 1A, lower panel). In addition, dehydroascorbic acid (vitamin C, a ROS scavenger) pretreatment totally inhibited ROS generation caused by HF-LPLI (Fig. 1A,B). These data demonstrate that HF-LPLI triggers mitochondrial oxidative stress. Mitochondrial fragmentation through oxidative stress caused by HF-LPLI Mitochondrial shapes were examined in two independent cell lines, ASTC-a-1 cells and COS-7 cells. Cells 942 FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
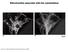
3 S. Wu et al. Mitochondrial fragmentation caused by HF-LPLI A Fig. 1. HF-LPLI causes mitochondrial ROS generation. (A) Representative sequential images of ASTC-a-1 cells stained with H2DCFDA under various treatments (upper panel). The increase in DCF fluorescence represents the generation of ROS. HF-LPLI caused intracellular ROS generation. Vitamin C (Vit C) pretreatment totally inhibited HF-LPLI-induced ROS generation. Representative images are shown of cells doubly stained with DCF (green emission) and MitoTracker (red emission) to label ROS and mitochondria, respectively, 15 min after HF-LPLI treatment (lower panel). ROS were mainly generated in mitochondria after HF-LPLI treatment. Scale bar: 10 lm. (B) Quantitative analysis of relative DCF fluorescence emission intensities (directly related to ROS generation) from ASTC-a-1 cells after various treatments. Control groups received no treatment. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05, Student s t-test). B were transiently transfected with pdsred-mit to localize mitochondria, and the morphological changes of mitochondria in positively transfected cells were monitored by confocal microscopy. Most control cells ( 98%) showed normal, short, tubular mitochondria (Fig. 2A,B). By contrast, under HF-LPLI treatment at a fluence of 120 JÆcm )2, only 25% cells displayed the normal tubular mitochondria seen in control cells, and 75% of the HF-LPLI-treated cells had mitochondria with a fragmented, punctiform morphology (Fig. 2A,B). These data demonstrate that HF-LPLI induces mitochondrial fragmentation by triggering oxidative stress. We also investigated the correlation between the laser fluence of HF-LPLI and the severity of mitochondrial fragmentation in the two cell lines. STS was used as a positive control to induce mitochondrial fragmentation (Fig. 2B). Cells were irradiated at various laser fluences in the range from 60 to 240 JÆcm )2. A significant positive correlation was found between laser fluence and the percentage of cells with fragmented mitochondria (Fig. 2B). Moreover, HF-LPLIinduced mitochondrial fragmentation was completely prevented by vitamin C pretreatment (Fig. 2A,B), demonstrating that the changes were mediated by oxidative stress caused by HF-LPLI. HF-LPLI inhibits mitochondrial fusion Given the alterations in mitochondrial morphology under HF-LPLI treatment, it is likely that an impaired fission fusion balance is involved. To measure the FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 943
4 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. A B Fig. 2. HF-LPLI causes mitochondrial fragmentation through ROS generation (A) ASTC-a-1 cells and COS-7 cells were transiently transfected with pdsred-mit, and, 48 h after transfection, cells expressing DsRed-mit were subjected to various treatments. Scale bars: 10 lm. Representative confocal microscopic images show two types of mitochondrial morphology under normal conditions and HF-LPLI treatment (120 JÆcm )2 ): normal tubular and fragmented mitochondria, respectively. Vitamin C (Vit C) pretreatment totally prevented mitochondrial fragmentation caused by HF-LPLI. (B) ASTCa-1 cells and COS-7 cells expressing DsRedmit were treated with HF-LPLI at a fluence of JÆcm )2 with or without vitamin C. Quantitative analysis of the percentage of cells with fragmented mitochondria reveals that HF-LPLI increases fragmentation of mitochondria, and that the fragmentation correlates with the laser fluence and is prevented by vitamin C pretreatment. STS was used as a positive control to identify mitochondrial fragmentation. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05, Student s t-test). occurrence of mitochondrial fission fusion events under HF-LPLI treatment (120 JÆcm )2 ), ASTC-a-1 cells were labeled with the mitochondria-targeted fluorescent probe MitoTracker. Cells with similar shapes were chosen and monitored by time-lapse confocal microscopic imaging. Mitochondrial behavior in the entire cell was monitored for the following 25 min, with or without HF-LPLI treatment (Fig. 3). It took 20 min for the mitochondria to complete the fission fusion cycle under normal conditions (Fig. 3, upper panel). However, we did not observe mitochondrial fusion caused by HF-LPLI even with a longer period of treatment (25 min) (Fig. 3, lower panel). Because the two-dimensional picture did not convincingly demonstrate the mitochondrial fission fusion events, we obtained the three-dimensional picture with the use of Z-stack software and confocal microscopy to confirm the occurrence of fission fusion events in each mitochondrial morphological study (data not shown). These data demonstrated that HF-LPLI inhibits or delays mitochondrial fusion. Recruitment of Drp1 to mitochondria caused by HF-LPLI We explored the involvement of Drp1 in HF-LPLI-induced apoptosis at a fluence of 120 JÆcm )2 in ASTC-a-1 cells. Both control cells and HF-LPLI-treated cells were labeled with MitoTracker, and endogenous Drp1 in the cells was detected by immunofluorescence. Images were obtained by confocal microscopy. As shown in Fig. 4A, HF-LPLI resulted in an obvious increase in the mitochondrial accumulation of Drp1. Vitamin C pretreatment totally prevented HF-LPLI-induced mitochondrial 944 FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
5 S. Wu et al. Mitochondrial fragmentation caused by HF-LPLI Fig. 3. HF-LPLI inhibits mitochondrial fusion. ASTC-a-1 cells were stained with MitoTracker to localize mitochondria. Mitochondrial behavior in the cells was monitored for 25 min. Active fission and fusion (filled arrowhead) of individual mitochondria could be observed in the control cell (upper panel). Abnormal fission of individual mitochondria could be observed in HF-LPLI (120 JÆcm )2 )-treated cells (lower panel). Data are representative confocal microscopic images of at least five independent experiments. Scale bars: 10 lm. accumulation of Drp1 (Fig. 4A). Also, cells were transiently cotransfected with pyfp-drp1 and pdsred-mit, and 48 h after transfection, cells coexpressing the two plasmids were subjected to HF-LPLI treatment. Yellow fluorescent protein (YFP)-Drp1 was mainly localized in the cytoplasm, but a small amount was associated with mitochondria (Fig. 4B, upper panel). The subcellular locations of YFP-Drp1 were changed from partial association with mitochondria to complete recruitment to mitochondria in response to HF-LPLI (Fig. 4B, lower panel). We also assessed the cycling properties of YFP- Drp1 at 150 min after HF-LPLI treatment, using fluorescence recovery after photobleaching (FRAP). As expected, we observed almost complete inhibition of the fluorescence recovery of YFP-Drp1 in cells treated with HF-LPLI (Fig. 4C), indicating the stable association of Drp1 with mitochondria induced by HF-LPLI. Western blotting analysis of the levels of Drp1 in the mitochondrial and cytosolic fractions also demonstrated the recruitment of Drp1 to mitochondria under HF-LPLI treatment (Fig. 4D). These data demonstrate that Drp1 is activated and probably involved in HF-LPLI-induced mitochondrial fragmentation. Effects of Drp1 Mfn2 overexpression on mitochondrial dynamics in cells under normal conditions To visualize mitochondria, ASTC-a-1 cells were transiently cotransfected with pdsred-mit and pyfp-drp1 YFP-Mfn2. The efficiency of transient transfection was demonstrated by flow cytometry (FCM) analysis (Fig. 5A). Forty-eight hours after transfection, the positively transfected cells were evaluated by confocal microscopy (Fig. 5B). At steady state, most control cells (i.e. only pdsred-mit positively transfected cells) ( 99%) showed normal, short, tubular mitochondria (Fig. 5B,C). Conversely, among Drp1-overexpressing cells, 9% displayed normal mitochondria as seen in control cells (Fig. 5B,C), but 90% had mitochondria with a fragmented, punctiform structure (Fig. 5B,C). Also, Mfn2-overexpressing cells showed a large population ( 92%) of mitochondria with an elongated, net-like structure (Fig. 5B,C). These data demonstrate that Drp1 overexpression causes mitochondrial fission and Mfn2 overexpression causes mitochondrial elongation in ASTC-a-1 cells. Effects of Drp1 and Mfn2 on mitochondrial dysfunction under HF-LPLI treatment Because mitochondrial morphology is critical for mitochondrial function, we investigated the effect of Drp1 Mfn2 overexpression on mitochondrial dysfunction under HF-LPLI treatment ( JÆcm )2 ). ASTC-a-1 cells were transiently transfected with pdrp1 Mfn2, and 48 h after transfection the transfectants were selected by growth in medium containing G418 for the next 24 h. Then, overexpression was confirmed by western blot analysis (Fig. 6A). ROS levels (as indicated by the DCF fluorescent signal) were significantly increased in HF-LPLI-treated cells as compared with control cells (Fig. 6B). Notably, transient overexpression of either Drp1 or Mfn2 did not change the ROS levels in HF-LPLI-treated cells. These data demonstrate that neither Drp1 nor Mfn2 overexpression affects mitochondrial oxidative stress caused by HF-LPLI. FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 945
6 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. A B C D Fig. 4. HF-LPLI causes recruitment of Drp1 to mitochondria. (A) Mitochondria in ASTC-a-1 cells were stained with MitoTracker (red emission), and endogenous Drp1 was detected by immunofluorescence with antibody against Drp1 (green emission) 170 min after HF-LPLI treatment (120 JÆcm )2 ). The merged image clearly shows the recruitment of Drp1 to mitochondria in response to HF-LPLI treatment. Vitamin C (Vit C) pretreatment totally prevented mitochondrial translocation of Drp1 induced by HF-LPLI. Data are representative confocal microscopic images of at least five independent experiments. Scale bars: 10 lm. (B) ASTC-a-1 cells were transiently cotransfected with pyfp-drp1 and pdsred-mit. Forty-eight hours after transfection, cells coexpressing YFP-Drp1 and DsRed-mit were treated with HF-LPLI at a fluence of 120 JÆcm )2. The control group received no treatment. Representative confocal microscopic images reveal increased association of YFP-Drp1 with mitochondria in response to HF-LPLI (n = 5). Scale bars: 10 lm. (C) ASTC-a-1 cells were transiently transfected with pyfp-drp1. Fortyeight hours after transfection, relative fluorescence emission intensities of YFP-Drp1 recorded during photobleaching protocols were plotted as a function of time. YFP-Drp1 cycling between cytosol and mitochondria completely ceased after HF-LPLI treatment. Data represent the mean ± standard error of the mean of at least five independent experiments. (D) Western blot analysis was employed to study the translocation of Drp1 to mitochondria in response to HF-LPLI treatment. The levels of Drp1 in the cytosolic fraction decreased by 150 min post-hf- LPLI treatment. Drp1 was activated by HF-LPLI. We also measured mitochondrial membrane potential (MMP) with the fluorescent dye tetramethylrhodamine methyl ester (TMRM), and the level of the decrease in MMP in HF-LPLI-treated cells was not changed by either Drp1 or Mfn2 overexpression (Fig. 6C,D). Also, vitamin C pretreatment totally prevented the change in MMP caused by HF-LPLI (Fig. 6D). These data demonstrate that neither Drp1 nor Mfn2 overexpression affects mitochondrial depolarization caused by HF-LPLI. 946 FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
7 S. Wu et al. Mitochondrial fragmentation caused by HF-LPLI A B C Fig. 5. Effects of Drp1 and Mfn2 on mitochondrial morphology (A) ASTC-a-1 cells were transiently cotransfected with pyfp-drp1 YFP-Mfn2 and pdsred-mit. The transfection efficiencies of YFP-Drp1 and YFP-Mfn2 were detected by FCM 48 h after transfection. Mitochondrial morphology was monitored in cells coexpressing YFP-Drp1 YFP-Mfn2 and DsRed-mit. Representative confocal microscopic images (B) and quantification analysis (C) reveal that transient overexpression of Drp1 promotes mitochondrial fragmentation, whereas overexpression of Mfn2 causes elongated mitochondrial morphology. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05, Student s t-test). Scale bars: 10 lm. FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 947
8 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. A B C D E F G H Control ASTC-a-1 Vit C HF-LPLI + Vit C PI-A PI-A Q1 Q2 Q1 Q2 PI-A Q1 Q Q3 Q Q3 Q Q3 Q4 PI FITC-A FITC-A FITC-A HF-LPLI HF-LPLI + Mfn2 HF-LPLI + Drp1 HF-LPLI + z-vad-fmk I PI-A Q1 Q2 Q3 Q4 PI-A Q1 Q2 Q3 Q PI-A Q1 Q2 Q3 Q FITC-A FITC-A FITC-A FITC-A Annexin V-FITC PI-A Q1 Q2 Q3 Q To explore the role of Drp1 in mitochondrial proapoptotic functions under HF-LPLI treatment, we knocked down Drp1 expression with the short hairpinactivated gene silencing system. To generate ASTC-a-1 cell lines that stably suppress the endogenous gene for Drp1, we transferred plasmids containing Drp1 short 948 FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
9 S. Wu et al. Mitochondrial fragmentation caused by HF-LPLI Fig. 6. Effects of Drp1 and Mfn2 on HF-LPLI-induced mitochondrial functional changes. ASTC-a-1 cells were transiently transfected with pdrp1 Mfn2. Forty-eight hours after transfection, the transfectants were selected by growth in medium containing G418 for the next 24 h. Transfection efficiencies were examined by western blot analysis with antibody against Drp1 Mfn2 (A). Relative DCF (B) and TMRM (C) fluorescence emission intensities in cells after HF-LPLI treatment were measured as described in Experimental procedures. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05, Student s t-test). (D) Quantitative analysis of TMRM fluorescence emission intensities over time after various treatments. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05, Student s t-test). Neither Drp1 nor Mfn2 overexpression had an effect on mitochondrial depolarization and ROS generation caused by HF-LPLI (120 JÆcm )2 ). Vitamin C (Vit C) pretreatment totally prevented mitochondrial depolarization caused by HF-LPLI. (E) Both normal cells and Drp1 RNAi cells were treated with HF-LPLI (120 JÆcm )2 ), fractionated into cytosol and mitochondria, and analyzed for the distribution of Bax and Drp1 by western blot analysis. The fractionation quality was verified by the distribution of specific subcellular markers: COX IV for mitochondria and b-actin for cytosol. Drp1 knockdown did not affect translocation of Bax to mitochondria following HF-LPLI treatment. (F) Immunoblotting of ASTC-a-1 cells treated with HF-LPLI with or without Drp1 Mfn2 overexpression was performed to detect the level of cytochrome c, with COX IV and b-actin as markers of the amounts of mitochondrial and cytosolic proteins in cells, respectively. Cells without any treatment were set as control groups. (G) Spectrofluorometric analysis of caspase-9 activation after HF-LPLI treatment (120 JÆcm )2 ) in living cells. The cells were excited at the wavelength of CFP (434 ± 5 nm), resulting in a CFP emission peak (476 nm) and FRET emission peak (528 nm) caused by FRET from CFP. The fluorescence emission spectra were obtained with a luminescence spectrometer. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05 versus control group; #P < 0.05 versus the indicated group; Student s t-test). Cytochrome c release and caspase-9 activation were both enhanced by Drp1 overexpression, but inhibited by Mfn2 overexpression. (H) Representative cell death analysis by FCM based on annexin V FITC PI double staining was performed in cells under various treatments. (I) Quantitative analysis of the FCM data shown in (H). Drp1 overexpression promotes cell apoptosis caused by HF-LPLI, whereas Mfn2 overexpression inhibits the apoptosis. Vitamin C pretreatment totally prevented the apoptosis caused by HF-LPLI. Z-VAD-fmk pretreatment also inhibited the apoptosis caused by HF-LPLI. Data represent the mean ± standard error of the mean of at least five independent experiments (*P < 0.05 versus control group; Student s t-test). hairpin RNA (shrna) into cells with G418 as a selective marker. Selection of the transfected cells with nearly complete depletion of Drp1 required 1 week of growth in the presence of G418. Bax activation was monitored under HF-LPLI treatment by western blot analysis (Fig. 6E). In control cells, Bax was detected mainly in the cytosolic fraction (Fig. 6E). Following HF-LPLI treatment, Bax levels decreased in the cytosolic fraction and increased in the mitochondrial fraction, suggesting activation of Bax caused by HF-LPLI. However, the levels of Bax in the two sections were not affected by Drp1 RNA interference (RNAi) (Fig. 6E), demonstrating that Drp1 is not required for Bax activation in response to HF-LPLI. Cytochrome c release was also monitored under HF- LPLI treatment, by western blot analysis. The results are shown in Fig. 6F. In control cells, cytochrome c was detected mainly in the mitochondrial fraction (Fig. 6F). Following HF-LPLI treatment, cytochrome c levels decreased in the mitochondrial fraction (Fig. 6F). Drp1 overexpression accelerated the release of cytochrome c to the cytosol, whereas Mfn2 overexpression had an opposite effect (Fig. 6F). To examine the effect of Drp1 Mfn2 on caspase-9 activation following HF-LPLI treatment, spectrofluorometric analysis, a technique for monitoring the overall profile of Fo rster resonance energy transfer (FRET) fluorescence emission from a group of cells, was applied to measure the activation of caspase-9. ASTCa-1 cells stably expressing SCAT9 were transiently transfected with pdrp1 Mfn2. Forty-eight hours after transfection, the samples were treated with HF-LPLI at a fluence of 120 JÆcm )2. Six hours later, all groups were investigated with a luminescence spectrometer for fluorescence emission spectra. The FRET cyan fluorescent protein (CFP) ratio is inversely proportional to the caspase-9 activity. Under HF-LPLI treatment, caspase-9 activity in the cells overexpressing Drp1 was much lower than that in non-overexpressing cells, whereas the activity of caspase-9 in Mfn2-overexpressing cells was higher than that in non-overexpressing cells (Fig. 6G). These data suggest that Drp1 overexpression promotes caspase-9 activation caused by HF-LPLI, whereas Mfn2 overexpression inhibits this activation. Analysis of the results of FCM based on annexin V fluorescein isothiocyanate (FITC) propidium iodide (PI) double staining was used to determine the role of Drp1 Mfn2 overexpression in cell apoptosis 6 h after HF-LPLI treatment. The data in Fig. 6H,I show that cell apoptosis caused by HF-LPLI was significantly promoted by Drp1 overexpression, whereas Mfn2 overexpression had the opposite effect. Vitamin C pretreatment totally prevented the apoptosis (Fig. 6H,I), demonstrating that ROS generation is a determinant of HF-LPLI-induced apoptosis. Also, HF-LPLI-induced apoptosis could be inhibited by the caspase inhibitor z-vad-fmk (Fig. 6H,I), demonstrating that the apoptosis was caspase-dependent. Experiments performed in COS-7 cells gave similar results (Fig. 6I). FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 949
10 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. Discussion Here, we have presented a method to expose mitochondria in ASTC-a-1 cells and COS-7 cells to incremental doses of ROS by photoexcitation of endogenous photoacceptor (cytochrome c oxidase) in the mitochondrial respiratory chain [16 18] by HF-LPLI, the result that has been clearly identified in our earlier studies [13,14]. This method of localized excitation caused the generation of triggering ROS (presumably singlet oxygen) inside mitochondria (Fig. 1), enabling us to demonstrate the induction of mitochondrial fragmentation caused by mitochondrial oxidative stress in living cells, as the ROS scavenger prevented the fragmentation (Fig. 2). In this study, we have shown that HF-LPLI results in perturbed mitochondrial dynamics and causes mitochondrial fragmentation that impacts on mitochondrial function and cell function. It is likely that HF-LPLI affects mitochondrial dynamics through the differential modulation of mitochondrial fission and fusion proteins, causing an impaired mitochondrial fission fusion balance, because manipulation of Drp1 and Mfn2 expression changed the effects of HF-LPLI. One major observation of this study was that HF- LPLI-induced oxidative stress caused mitochondrial fragmentation in ASTC-a-1 cells and COS-7 cells, as shown by confocal microscopic imaging (Fig. 2). Because mitochondrial morphology is tightly controlled by the balance between mitochondrial fission and fusion [12], we hypothesize that HF-LPLI-induced mitochondrial fragmentation is caused by enhanced fission, reduced fusion, or both. In support of this notion, using the mitochondria-targeted fluorescent probe MitoTracker, we were able to demonstrate that mitochondria in HF-LPLI-treated cells were nearly unable to fuse as compared with control mitochondria (Fig. 3). At the molecular level, we found that HF- LPLI-triggered ROS resulted in Drp1 activation, as indicated by a significantly increased association with mitochondria (Fig. 4). Although the ROS originate predominantly from mitochondria, owing to photoexcitation of cytochrome c oxidase, they may diffuse out to the cytosol, as shown in Fig. 1A. Also, the added vitamin C can be easily taken up by the cytosol, whereas it can be taken up only slowly by mitochondria. Therefore, the inhibition of Drp1 mitochondrial translocation by vitamin C may occur primarily through changes in cytosolic ROS. The activation of Drp1 may contribute to the increased fission rate in our models, as it has been reported that a cell line with an endogenous loss of activity mutation in Drp1 displays resistance to hydrogen peroxide-induced cell death [19]. The change in Drp1 activity caused by HF- LPLI is highly likely to be involved in a mechanism of calcineurin-dependent translocation of the Drp1 to mitochondria in dysfunction-induced fragmentation [20,21]. In detail, when mitochondrial depolarization is associated with a sustained cytosolic Ca 2+ rise, it activates the cytosolic phosphatase calcineurin, which normally interacts with Drp1 [20]. Calcineurin-dependent dephosphorylation of Drp1, and in particular of its conserved Ser637, regulates its translocation to mitochondria, as substantiated by site-directed mutagenesis [20]. Our results support this point of view, because ROS generation in response to HF-LPLI can induce obvious and severe mitochondrial depolarization [13,14], and increased Ca 2+ levels are also seen after HF-LPLI (data not shown). On the other hand, opinion on the relationship between apoptosis and fission is divided about whether this phenomenon of fragmentation is simply a consequence of apoptosis or plays a more active role in the process. Some investigators have suggested that mitochondrial fission may promote cytochrome c release and therefore act to drive caspase activation during apoptosis [22,23]. However, other data suggest that apoptosis-associated mitochondrial fission is a consequence rather than a cause of apoptosis, and reflects events involving some hitherto unrecognized connection between members of the Bcl-2 family and the mitochondrial morphogenesis machinery [24 27]. Therefore, it is probable that oxidative stress is impacting directly on cell death without affecting mitochondrial fragmentation. It is known that changes in mitochondrial morphology often affect mitochondrial function [28,29]. In this regard, it is important to note that aspects of mitochondrial dysfunction following HF-LPLI, i.e. increased ROS levels, reduced MMP, Bax activation, and cytochrome c release (Fig. 6B F), were all observed in the cells characterized by mitochondrial fragmentation (Fig. 2). Therefore, it is likely that mitochondrial fragmentation contributes to HF-LPLIinduced mitochondrial dysfunction. In support of this, Drp1 overexpression accelerated cytochrome c release and caspase-9 activation, and thus promoted cell apoptosis, under HF-LPLI treatment, whereas Mfn2 overexpression inhibited these processes (Fig. 6F I). It has been reported that, upon apoptotic stimulation, Drp1 is recruited to the mitochondrial outer membrane [22,30 32], where it colocalizes with Bax and Mfn2 at fission sites [24,33]. Drp1 function is required for apoptotic mitochondrial fission, as expression of a dominant negative mutant (Drp1K38A) or downregulation of Drp1 by RNAi delays mitochondrial fragmentation, cytochrome c release, caspase activation, 950 FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
11 S. Wu et al. Mitochondrial fragmentation caused by HF-LPLI and cell death [22,30,34,35]. In the present study, Drp1 knockdown had no effect on Bax activation by HF- LPLI (Fig. 6E). The data suggest that the modulation of cytochrome c release by Drp1 is a downstream event of Bax activation. On the other hand, Drp1 Mfn2 overexpression modulated the release of cytochrome c (Fig. 6F), whereas it had no effect on HF-LPLI-induced generation of ROS and collapse of MMP (Fig. 6B D), indicating that apoptosis-associated fission was a relatively downstream event and quite close to mitochondrial outer membrane permeabilization. It is known that HF- LPLI triggers mitochondrial ROS generation through excitation of the endogenous photoacceptor cytochrome c oxidase in the mitochondrial respiratory chain [13,14]. The process may not be affected by Drp1 Mfn2, as there has been no report indicating a correlation between Drp1 Mfn2 and the complexes in the respiratory chain. Previously, we have demonstrated that HF-LPLI-induced MMP is caused by the ROS-induced opening of mitochondrial permeability transition pores, but not the direct permeabilization of the outer mitochondrial membrane [14]. This may explain why Drp1 Mfn2 can modulate cytochrome c release but not mitochondrial depolarization. Nevertheless, given the presence of HF-LPLI-triggered ROS in mitochondria and the mitochondrial locations of other mitochondrial dynamic-regulated proteins, the possibility that HF-LPLI may also induce mitochondrial dysfunction through other pathways cannot be ruled out. Taken together, the findings presented here suggest that abnormal mitochondrial dynamics are involved in mitochondrial dysfunction after HF-LPLI stimulation. The data indicate a balance tipped towards mitochondrial fission that facilitates HF-LPLI-induced apoptosis. Given the study on mitochondrial fission performed in ASTC-a-1 cells and COS-7 cells, it is very likely that abnormal mitochondrial dynamics may be a common pathway leading to cellular dysfunction that is critical to apoptosis in various cells. Experimental procedures Cell culture and transfection ASTC-a-1 cells and COS-7 cells were grown on 22-mm culture glasses, in DMEM (Life Technologies, Grand Island, NY, USA) supplemented with 15% fetal bovine serum (Gibco, Grand Island, NY, USA), 50 unitsæml )1 penicillin, and 50 lgæml )1 streptomycin, in 5% CO 2 95% air at 37 C in a humidified incubator. In all experiments, 70 85% confluent cultures were used. Downregulation of Drp1 levels in ASTC-a-1 cells was achieved by RNAi, using a vector-based shrna approach, with the target sequence for Drp1 (a gift from J.-C. Martinou, University of Geneva, Switzerland). The shrna sequences were transfected into cells with Lipofectamine 2000 reagent (Invitrogen Life Technologies, Grand Island, NY, USA), according to the manufacturer s protocol. Forty-eight hours after transfection, the cells were washed once with NaCl Tris and grown in fresh medium supplemented with 800 lgæml )1 G418 (Sigma-Aldrich, St Louis, MO, USA) for another 24 h to select the transfectants. The cultures were then washed with NaCl P i, and incubated in fresh growth medium in order to start the experiment. Chemicals The following fluorescent probes were used in our experiments: MitoTracker (100 nm) was used to label mitochondria; H2DCFDA (10 lm) was used to detect the generation of ROS; and TMRM (100 nm) was used to monitor MMP. All of these probes were purchased from Molecular Probes (Eugene, OR, USA). The optimal incubation time for each probe was determined experimentally. STS (1 lm) was purchased from Sigma-Aldrich (St Louis, MO, USA). zvad-fmk (20 lm) was purchased from BD PharMingen (San Diego, CA, USA). Dehydroascorbic acid, an oxidized form of ascorbic acid (vitamin C; Sigma- Aldrich, St Louis, MO, USA) (100, 200 and 400 lm for 60, 120 and 240 JÆcm )2 HF-LPLI treatments, respectively), was added to the cell culture medium 30 min before laser irradiation in order to scavenge ROS. Monoclonal antibodies against cytochrome c and cytochrome c oxidase subunit IV (COX IV) were purchased from BD PharMingen. Monoclonal antibodies against Drp1, Mfn2 and b-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibodies against Bax were purchased from Cell Signaling Technology (Beverly, MA, USA). Mitochondrial and cytosolic fractions were obtained with the Mitochondria Isolation Kit (Cat: KGA 827; KeyGEN, Nanjing, China). HF-LPLI treatment for single-cell analysis For irradiation of cells, a 633-nm He Ne laser inside a confocal laser scanning microscope (LSM510-ConfoCor2) (Zeiss, Jena, Germany) was used in HF-LPLI treatment. Laser irradiation was performed through the objective lens of the microscope. In this setup, only the cells under observation were irradiated by the laser. A minitype culture chamber with a CO 2 supply (Tempcontrol 37-2 digital; Zeiss) was used in order to keep cells under normal culture conditions (37 C, 5% CO 2 ) during irradiation. Under the HF-LPLI treatment, the cells in selected area were irradiated for 10 min with a laser fluence of 60, 120 or 240 JÆcm )2. FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 951
12 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. Detection of ROS generation and MMP decrease Cells grown on 96-well plates were labeled with H2DCFDA or TMRM and then irradiated with an He Ne laser (635 nm, semiconductor laser, NL-FBA ; nlight Photonics Corporation, Vancouver, WA, USA) at fluence of 60, 120 or 240 JÆcm )2 in the dark. The interval wells were filled with ink in order to minimize the scattered or reflected light. Immediately after irradiation, fluorescence emission intensity was recorded at the indicated time with an Infinite 2000 plate reader (Tecan, Mo nnedorf, Switzerland). Immunofluorescence microscopy Cells grown on 22-mm culture glasses were first labeled with MitoTracker, and then fixed for 10 min in 4% paraformaldehyde; this was followed by permeabilization with 0.15% Triton X-100 in NaCl P i for 15 min. The cells were then incubated for 1 h in blocking buffer (2% BSA in NaCl P i ), and overnight with a rabbit monoclonal antibody against Drp1 (dilution range 1 : 50 to 1 : 500) antibody. Cells were washed three times for 10 min each in blocking buffer, and then incubated for 2 h with secondary antibody [Alexa Fluor 488 dye-conjugated goat anti-(rabbit IgG)] (argon ion laser, excitation 488 nm, emission bandpass nm) (Molecular Probes, Eugene, OR, USA). Images were acquired with a LSM510-ConfoCor2 microscope (Zeiss) through a 40 oil fluorescence objective (LSM510-ConfoCor2; Zeiss, Jena, Germany). Imaging analysis of living cells In order to obtain images of single cells, the confocal microscope was used. Cell images before and after laser irradiation were acquired with a Plan-Neofluar 100 NA1.3, oil-immersed objective lens. Cells were maintained at 37 C and 5% CO 2 during imaging, in a minitype culture chamber with a CO 2 supply. The following specific settings were used for light excitation and emissions. Mito- Tracker: He Ne laser; excitation, 633 nm; emission (long pass), 650 nm. DCF: argon ion laser; excitation, 488 nm; emission (bandpass), nm. TMRM: He Ne laser; excitation, 543 nm, emission (bandpass), nm. DsRed: argon ion laser; excitation, 488 nm; emission (long pass), 560 nm. YFP: argon ion laser; excitation, 514 nm; emission (bandpass), nm. For intracellular measurements, the desired areas were chosen in the confocal image. Quantitative analysis of mitochondrial shape changes was performed by evaluating which cells displayed fragmented mitochondria after addition of the inducer at the indicated time. Organelles were classified as fragmented when 50% of the total cellular mitochondria displayed a major axis of < 2 lm in ASTC-a-1 cells and COS-7 cells [36]. FRAP FRAP analysis was performed with an LSM510-ConfoCor2 confocal laser-scanning microscope. Briefly, ASTC-a-1 cells coexpressing YFP-Drp1 and DsRed-mit were treated without or with HF-LPLI (120 JÆcm )2 ) for 10 min. Then, each mitochondrial region of interest was photobleached by scanning for 50 s with an argon laser at the highest power. Decrease in fluorescence in the region after photobleaching and recovery of fluorescence in the region after photobleaching were then analyzed by confocal microscopy with low laser power at the indicated times after photobleaching. For all of the images, the noise levels were reduced by line scan averaging. FCM For FCM analysis, an annexin V FITC Apoptosis Detection Kit (annexin V FITC conjugate, PI dyes, and binding buffer) (BD PharMingen) was used. FCM was performed on a FACScanto II flow cytometer (Becton Dickinson, Mountain View, CA, USA) with excitation at 488 nm. Fluorescent emission of FITC was measured at bandpass nm, and that of DNA PI complexes at nm. Cell debris was excluded from analysis by an appropriate forward light scatter threshold setting. Compensation was used wherever necessary. The number of events considered in FCM was per independent experiment. Western blot analysis Cells were harvested in 300 ll of lysis buffer (50 mm Tris HCl, ph 8.0, 150 mm NaCl, 50 mm b-glycerophosphate, 1% Triton X-100, 100 mm phenylmethanesulfonyl fluoride). The resulting lysates were resolved by 4 2% SDS PAGE, and transferred to pure nitrocellulose blotting membranes (BioTrace NT; Pall Life Science, Pensacola, FL, USA). The membranes were blocked in 10 mm Tris HCl (ph 7.4), 150 mm NaCl and 0.1% Tween-20 containing 5% nonfat milk, and then probed with different antibodies (Drp1, 1 : 1000; Mfn2, 1 : 1000; COX IV, 1 : 1000; b-actin, 1 : 2000; Bax, 1 : 1000; cytochrome c, 1 : 1000). Proteins were detected with an Odyssey two-color infrared imaging system (LI-COR; Lincoln, NE, USA). Mitochondrial isolation was performed with a mitochondrial isolation kit (Cat: KGA 827; KeyGEN). Spectrofluorometric analysis ASTC-a-1 cells stably expressing SCAT9, a FRET reporter of caspase-9 activity [15], were transiently transfected with pdrp1 Mfn2, cultured for 48 h, and then treated with HF-LPLI (120 JÆcm )2 ). The cells were transferred into a 96-well flat-bottomed microplate. The microplate was then 952 FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
13 S. Wu et al. Mitochondrial fragmentation caused by HF-LPLI placed inside the sample chamber of a luminescence spectrometer (LS55; PerkinElmer, Wellesley, MA, USA), and the fluorescence emission spectra were then acquired. The step length of the scanning spectra was 2 nm. The excitation wavelength of SCAT9 was 434 ± 5 nm, and the emission fluorescence channel was bandpass nm. Statistics Unless otherwise indicated, data were analyzed with oneway ANOVA. All results shown were from at least five independent experiments. Acknowledgements We thank Y. Gotoh (Institute of Molecular and Cellular Bioscience, University of Tokyo) for kindly providing pdsred-mit, J.-C. Martinou (University of Geneva) for kindly providing pshrna Drp1, R. J. Youle (National Institutes of Health) for kindly providing pyfp Mfn2 and pyfp Drp1, and M. Miura (Riken Brain Science Institute) for kindly providing pscat9. This research is supported by the National Basic Research Program of China (2010CB732602; 2011CB910402), the Program for Changjiang Scholars and Innovative Research Team in University (IRT0829), and the National Natural Science Foundation of China ( ; ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. References 1 Chen H & Chan DC (2005) Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14, Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125, Liesa M, Palacin M & Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89, Wang X, Su B, Lee H, Li X, Perry G, Smith MA & Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer s disease. J Neurosci 29, Cho D, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z & Lipton SA (2009) S-nitrosylation of Drp1 mediates b-amyloid-related mitochondrial fission and neuronal injury. Science 324, Van Laar VS & Berman SB (2009) Mitochondrial dynamics in Parkinson s disease. Exp Neurol 218, Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ & Cho H (2010) Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci 123, Ong S, Subrayan S, Lim SY, Yellon DM, Davidson SM & Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia reperfusion injury. Circulation 121, Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Piccolo PD, Foretz M et al. (2010) Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29, Karbowski M & Youle RJ (2003) Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ 10, McBride HM, Neuspiel M & Wasiak S (2006) Mitochondria: more than just a power house. Curr Biol 16, Westermann B (2008) Molecular machinery of mitochondrial fusion and fission. J Biol Chem 283, Wu S, Xing D, Wang F, Chen T & Chen W (2007) Mechanistic study of apoptosis induced by high fluence low-power laser irradiation using fluorescence imaging techniques. J Biomed Opt 12, Wu S, Xing D, Gao X & Chen WR (2008) High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol 218, Chu J, Wu S & Xing D (2010) Survivin mediates selfprotection through ROS cdc25c CDK1 signaling pathway during tumor cell apoptosis induced by high fluence low-power laser irradiation. Cancer Lett 297, Karu T (1999) Primary and secondary mechanisms of action of visible to near-ir radiation on cells. J Photochem Photobiol B 49, Karu T, Pyatibrat L & Kalendo G (2004) Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci 3, Karu T, Pyatibrat L, Kolyakov S & Afanasyeva N (2005) Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B 81, Tanaka A, Kobayashi S & Fujiki Y (2006) Peroxisome division is impaired in a CHO cell mutant with an inactivating point-mutation in dynamin-like protein 1 gene. Exp Cell Res 312, Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P & Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105, Cho SG, Du Q, Huang S & Dong Z (2010) Drp1 dephosphorylation in ATP depletion-induced FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS 953
14 Mitochondrial fragmentation caused by HF-LPLI S. Wu et al. mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol 299, Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL & Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1, Youle RJ & Karbowski M (2005) Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6, Karbowski M, Norris KL, Cleland MM, Jeong SY & Youle RJ (2006) Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, Arnoult D, Grodet A, Lee YJ, Estaquier J & Blackstone C (2005) Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem 280, Delivani P, Adrain C, Taylor RC, Duriez PJ & Martin SJ (2006) Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell 21, Sheridan C, Delivani P, Cullen SP & Martin SJ (2008) Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome c release. Mol Cell 31, Chen H, Chomyn A & Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280, Chen F, Detmer SA, Ewald AJ, Griffin EE, Fraser SE & Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, Breckenridge DG, Stojanovic M, Marcellus RC & Shore GC (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160, Arnoult A, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M & Blackstone C (2005) Bax Bak-dependent release of DDP TIMM8a promotes Drp1-mediated mitochondrial fission and apoptosis during programmed cell death. Curr Biol 15, Wasiak S, Zunino R & McBride HM (2007) Bax Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177, Karbowski M, Lee YG, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL & Youle RJ (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159, Germain M, Mathai JP, McBride HM & Shore GC (2005) Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 24, Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J et al. (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25, Cereghetti GM, Costal V & Scorrano L (2010) Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ 17, FEBS Journal 278 (2011) ª 2011 The Authors Journal compilation ª 2011 FEBS
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplemental Figures S1 S5
 Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells Ines Witte (1), Sebastian Altenhöfer (1), Petra Wilgenbus (1), Julianna Amort (1), Albrecht M. Clement
Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells Ines Witte (1), Sebastian Altenhöfer (1), Petra Wilgenbus (1), Julianna Amort (1), Albrecht M. Clement
Supporting Information
 Supporting Information Wang et al. 10.1073/pnas.0804871105 SI Materials and Methods Cell Culture and Transfection. Human neuroblastoma M17 cells were grown as described before (1). Transfection was performed
Supporting Information Wang et al. 10.1073/pnas.0804871105 SI Materials and Methods Cell Culture and Transfection. Human neuroblastoma M17 cells were grown as described before (1). Transfection was performed
Mitochondrial dynamics in ischemia and reperfusion
 Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis
 Molecular Biology of the Cell Vol. 15, 5001 5011, November 2004 Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis Yang-ja Lee,* Seon-Yong Jeong,* Mariusz
Molecular Biology of the Cell Vol. 15, 5001 5011, November 2004 Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis Yang-ja Lee,* Seon-Yong Jeong,* Mariusz
Role of Mitochondrial Remodeling in Programmed Cell Death in
 Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer
 Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer Nexcelom Bioscience LLC. 360 Merrimack Street, Building 9 Lawrence, MA 01843 T: 978.327.5340 F: 978.327.5341
Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer Nexcelom Bioscience LLC. 360 Merrimack Street, Building 9 Lawrence, MA 01843 T: 978.327.5340 F: 978.327.5341
SUPPLEMENTARY INFORMATION
 Cell viability rate 0.8 0.6 0 0.05 0.1 0.2 0.3 0.4 0.5 0.7 1 Exposure duration (s) Supplementary Figure 1. Femtosecond laser could disrupt and turn off GFP without photons at 473 nm and keep cells alive.
Cell viability rate 0.8 0.6 0 0.05 0.1 0.2 0.3 0.4 0.5 0.7 1 Exposure duration (s) Supplementary Figure 1. Femtosecond laser could disrupt and turn off GFP without photons at 473 nm and keep cells alive.
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with
 Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Apoptosis in Mammalian Cells
 Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
RayBio CaspGLOW TM Fluorescein Active Caspase-3 Staining Kit
 RayBio CaspGLOW TM Fluorescein Active Caspase-3 Staining Kit User Manual Version 1.0 May 10 th, 2015 RayBio Caspase-3 Fluorometric Assay Kit Protocol (Cat#: 68FLS-Casp3-S) RayBiotech, Inc. We Provide You
RayBio CaspGLOW TM Fluorescein Active Caspase-3 Staining Kit User Manual Version 1.0 May 10 th, 2015 RayBio Caspase-3 Fluorometric Assay Kit Protocol (Cat#: 68FLS-Casp3-S) RayBiotech, Inc. We Provide You
NucView TM 488 Caspase-3 Assay Kit for Live Cells
 NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
Mitochondrial Membrane Potential by Object Spot Counting
 A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy
 Electronic Supplementary Material Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy Minhuan Lan 1,, Shaojing Zhao 1,, Zhenyu
Electronic Supplementary Material Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy Minhuan Lan 1,, Shaojing Zhao 1,, Zhenyu
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
Apoptosis Detection Using the BD Accuri C6 Flow Cytometer
 Apoptosis Detection Using the BD Accuri C6 Flow Cytometer Stacey Roys, Marketing Applications Specialist, BD Biosciences Cyndy Lane, Senior Product Manager, BD Biosciences 23-14519-00 Outline What is apoptosis?
Apoptosis Detection Using the BD Accuri C6 Flow Cytometer Stacey Roys, Marketing Applications Specialist, BD Biosciences Cyndy Lane, Senior Product Manager, BD Biosciences 23-14519-00 Outline What is apoptosis?
In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes
 Supporting information for In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes Yuting Zhou, Jianmin Song, Lei Wang*, Xuting Xue, Xiaoman Liu, Hui Xie*, and Xin Huang* MIIT Key Laboratory
Supporting information for In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes Yuting Zhou, Jianmin Song, Lei Wang*, Xuting Xue, Xiaoman Liu, Hui Xie*, and Xin Huang* MIIT Key Laboratory
Caspase 8 (active) Red Staining Kit
 ab65618 Caspase 8 (active) Red Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of activated Caspase 8 in living cells This product is for research use only and is not
ab65618 Caspase 8 (active) Red Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of activated Caspase 8 in living cells This product is for research use only and is not
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant
 9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
Mitochondria associate with the cytoskeleton. Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008)
 Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Caspase 3 (active) Red Staining Kit
 ab65617 Caspase 3 (active) Red Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of activated Caspase 3 in living cells. This product is for research use only and is not
ab65617 Caspase 3 (active) Red Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of activated Caspase 3 in living cells. This product is for research use only and is not
What are mitochondria?
 What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
For the rapid, sensitive and accurate detection of active Caspase 9 in living cells
 ab65615 Caspase 9 (active) FITC Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of active Caspase 9 in living cells This product is for research use only and is not intended
ab65615 Caspase 9 (active) FITC Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of active Caspase 9 in living cells This product is for research use only and is not intended
protein biology cell imaging Automated imaging and high-content analysis
 protein biology cell imaging Automated imaging and high-content analysis Automated imaging and high-content analysis Thermo Fisher Scientific offers a complete portfolio of imaging platforms, reagents
protein biology cell imaging Automated imaging and high-content analysis Automated imaging and high-content analysis Thermo Fisher Scientific offers a complete portfolio of imaging platforms, reagents
Transporters and Membrane Motors Nov 15, 2007
 BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
Mitofusin 1 is degraded at G 2 /M phase through ubiquitylation by MARCH5
 Park and Cho Cell Division 2012, 7:25 SHORT REPORT Open Access Mitofusin 1 is degraded at G 2 /M phase through ubiquitylation by MARCH5 Yong-Yea Park 1,2 and Hyeseong Cho 1,2* Abstract Background: Mitochondria
Park and Cho Cell Division 2012, 7:25 SHORT REPORT Open Access Mitofusin 1 is degraded at G 2 /M phase through ubiquitylation by MARCH5 Yong-Yea Park 1,2 and Hyeseong Cho 1,2* Abstract Background: Mitochondria
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX.
 Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1
 Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
09/30/2017. Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center
 09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
Biochimica et Biophysica Acta
 Biochimica et Biophysica Acta 1832 (2013) 453 474 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis PSAP induces a unique
Biochimica et Biophysica Acta 1832 (2013) 453 474 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis PSAP induces a unique
 http://cwp.embo.org/w09-13/index.html Roads to Ruin is s o t p o ap healthy cell autophagic cell death ne cr os is Thanks to Seamus Martin! Evolution of apoptosis signalling cascades Inhibitor Adopted
http://cwp.embo.org/w09-13/index.html Roads to Ruin is s o t p o ap healthy cell autophagic cell death ne cr os is Thanks to Seamus Martin! Evolution of apoptosis signalling cascades Inhibitor Adopted
Effects of the BH3-only protein human Noxa on mitochondrial dynamics
 FEBS Letters 583 (2009) 2349 2354 journal homepage: www.febsletters.org Effects of the BH3-only protein human Noxa on mitochondrial dynamics Ha-Na Woo a,c,e, Young-Woo Seo b, Ae Ran Moon c, Seon-Yong Jeong
FEBS Letters 583 (2009) 2349 2354 journal homepage: www.febsletters.org Effects of the BH3-only protein human Noxa on mitochondrial dynamics Ha-Na Woo a,c,e, Young-Woo Seo b, Ae Ran Moon c, Seon-Yong Jeong
Caspase 3 (active) Red Staining Kit
 ab65617 Caspase 3 (active) Red Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of activated Caspase 3 in living cells. This product is for research use only and is not
ab65617 Caspase 3 (active) Red Staining Kit Instructions for Use For the rapid, sensitive and accurate detection of activated Caspase 3 in living cells. This product is for research use only and is not
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Carboxyl-terminal modulator protein induces apoptosis by regulating mitochondrial function in lung cancer cells
 INTERNATIONAL JOURNAL OF ONCOLOGY 40: 1515-1524, 2012 Carboxyl-terminal modulator protein induces apoptosis by regulating mitochondrial function in lung cancer cells SOON-KYUNG HWANG 1,2*, ARASH MINAI-TEHRANI
INTERNATIONAL JOURNAL OF ONCOLOGY 40: 1515-1524, 2012 Carboxyl-terminal modulator protein induces apoptosis by regulating mitochondrial function in lung cancer cells SOON-KYUNG HWANG 1,2*, ARASH MINAI-TEHRANI
Reconstructing Mitochondrial Evolution?? Morphological Diversity. Mitochondrial Diversity??? What is your definition of a mitochondrion??
 Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial
Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer s Disease
 9090 The Journal of Neuroscience, July 15, 2009 29(28):9090 9103 Cellular/Molecular Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer s Disease Xinglong Wang, 1 Bo Su, 1 Hyoung-gon Lee,
9090 The Journal of Neuroscience, July 15, 2009 29(28):9090 9103 Cellular/Molecular Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer s Disease Xinglong Wang, 1 Bo Su, 1 Hyoung-gon Lee,
SENP3-mediated desumoylation of dynamin-related protein 1 promotes cell death following ischaemia
 The EMBO Journal (2013) 32, 1514 1528 www.embojournal.org SENP3-mediated desumoylation of dynamin-related protein 1 promotes cell death following ischaemia THE EMBO JOURNAL Chun Guo, Keri L Hildick, Jia
The EMBO Journal (2013) 32, 1514 1528 www.embojournal.org SENP3-mediated desumoylation of dynamin-related protein 1 promotes cell death following ischaemia THE EMBO JOURNAL Chun Guo, Keri L Hildick, Jia
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells
 Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1
 Research Article 619 Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1 Yong-Yea Park 1,2, Seungmin Lee 1,2, Mariusz Karbowski
Research Article 619 Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1 Yong-Yea Park 1,2, Seungmin Lee 1,2, Mariusz Karbowski
Waithe et al Supplementary Figures
 Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Apoptosis: Death Comes for the Cell
 Apoptosis: Death Comes for the Cell Joe W. Ramos joeramos@hawaii.edu From Ingmar Bergman s The Seventh Seal 1 2 Mutations in proteins that regulate cell proliferation, survival and death can contribute
Apoptosis: Death Comes for the Cell Joe W. Ramos joeramos@hawaii.edu From Ingmar Bergman s The Seventh Seal 1 2 Mutations in proteins that regulate cell proliferation, survival and death can contribute
Roles of the mammalian mitochondrial fission and fusion mediators. Fis1, Drp1 and Opa1 in apoptosis
 Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1 and Opa1 in apoptosis Yang-ja Lee 1#, Seon-Yong Jeong 1#, Mariusz Karbowski 1, Carolyn L Smith 2, Richard J Youle 1* 1 Biochemistry
Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1 and Opa1 in apoptosis Yang-ja Lee 1#, Seon-Yong Jeong 1#, Mariusz Karbowski 1, Carolyn L Smith 2, Richard J Youle 1* 1 Biochemistry
Intracellular GSH Assay Kit
 ab112132 Intracellular GSH Assay Kit Instructions for Use For detecting Intracellular GSH in cells by using our proprietary green fluorescence probe This product is for research use only and is not intended
ab112132 Intracellular GSH Assay Kit Instructions for Use For detecting Intracellular GSH in cells by using our proprietary green fluorescence probe This product is for research use only and is not intended
High photostability and enhanced fluorescence of gold nanoclusters by silver doping-supporting information
 High photostability and enhanced fluorescence of gold nanoclusters by silver doping-supporting information Size measurements Figure S1 P2 FTIR measurements Figure S2 P2 XPS measurements Figure S3 P3 Photo-physical
High photostability and enhanced fluorescence of gold nanoclusters by silver doping-supporting information Size measurements Figure S1 P2 FTIR measurements Figure S2 P2 XPS measurements Figure S3 P3 Photo-physical
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
Protein kinase C- activation promotes recovery of mitochondrial function and cell survival following oxidant injury in renal cells
 Am J Physiol Renal Physiol 303: F515 F526, 2012. First published June 6, 2012; doi:10.1152/ajprenal.00072.2012. Protein kinase C- activation promotes recovery of mitochondrial function and cell survival
Am J Physiol Renal Physiol 303: F515 F526, 2012. First published June 6, 2012; doi:10.1152/ajprenal.00072.2012. Protein kinase C- activation promotes recovery of mitochondrial function and cell survival
Cytochrome c, cytochrome b 5 and electron transfer
 Cytochrome c, cytochrome b 5 and electron transfer Covalent bonds Cytochrome c is a major player in membrane associated electron transport systems in bacteria and mitochondria. Cytochrome c has two axial
Cytochrome c, cytochrome b 5 and electron transfer Covalent bonds Cytochrome c is a major player in membrane associated electron transport systems in bacteria and mitochondria. Cytochrome c has two axial
Name: TF: Section Time: LS1a ICE 5. Practice ICE Version B
 Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health
 I R E V I E W S E Review in Advance first posted online on August 29, 2012. (Changes may still occur before final publication online and in print.) N C A D V A N Fusion and Fission: Interlinked Processes
I R E V I E W S E Review in Advance first posted online on August 29, 2012. (Changes may still occur before final publication online and in print.) N C A D V A N Fusion and Fission: Interlinked Processes
Hybrid Gold Superstructures: Synthesis and. Specific Cell Surface Protein Imaging Applications
 Supporting Information Hybrid Gold Nanocube@Silica@Graphene-Quantum-Dot Superstructures: Synthesis and Specific Cell Surface Protein Imaging Applications Liu Deng, Ling Liu, Chengzhou Zhu, Dan Li and Shaojun
Supporting Information Hybrid Gold Nanocube@Silica@Graphene-Quantum-Dot Superstructures: Synthesis and Specific Cell Surface Protein Imaging Applications Liu Deng, Ling Liu, Chengzhou Zhu, Dan Li and Shaojun
FSC-W FSC-H CD4 CD62-L
 Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
MITOHEALTH Nordic Centre of Excellence Workshop in: Mitochondrial function and metabolic diseases
 MITOHEALTH Nordic Centre of Excellence Workshop in: Mitochondrial function and metabolic diseases Quantitative multi-parameter microscopy of mitochondrial function in living cells Werner J.H. Koopman,
MITOHEALTH Nordic Centre of Excellence Workshop in: Mitochondrial function and metabolic diseases Quantitative multi-parameter microscopy of mitochondrial function in living cells Werner J.H. Koopman,
SUPPLEMENTARY INFORMATION 1. MATERIALS AND METHODS
 SUPPLEMENTARY INFORMATION 1. MATERIALS AND METHODS Citrate/phosphate buffer preparation Buffer solutions were prepared with potassium gluconate (10 mm), sodium gluconate (40 mm), sodium phosphate (10 mm),
SUPPLEMENTARY INFORMATION 1. MATERIALS AND METHODS Citrate/phosphate buffer preparation Buffer solutions were prepared with potassium gluconate (10 mm), sodium gluconate (40 mm), sodium phosphate (10 mm),
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Endoplasmic reticulum BIK initiates DRP1- regulated remodelling of mitochondrial cristae during apoptosis
 The EMBO Journal (25) 24, 1546 1556 & 25 European Molecular Biology Organization All Rights Reserved 261-4189/5 www.embojournal.org Endoplasmic reticulum BIK initiates DRP1- regulated remodelling of mitochondrial
The EMBO Journal (25) 24, 1546 1556 & 25 European Molecular Biology Organization All Rights Reserved 261-4189/5 www.embojournal.org Endoplasmic reticulum BIK initiates DRP1- regulated remodelling of mitochondrial
Fluorescence Workshop UMN Physics June 8-10, 2006 Basic Spectroscopic Principles Joachim Mueller
 Fluorescence Workshop UMN Physics June 8-10, 2006 Basic Spectroscopic Principles Joachim Mueller Fluorescence, Light, Absorption, Jablonski Diagram, and Beer-Law First stab at a definition: What is fluorescence?
Fluorescence Workshop UMN Physics June 8-10, 2006 Basic Spectroscopic Principles Joachim Mueller Fluorescence, Light, Absorption, Jablonski Diagram, and Beer-Law First stab at a definition: What is fluorescence?
Plant mitochondrial dynamics
 Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Toxicological Targets. Russell L. Carr Department of Basic Sciences College of Veterinary Medicine
 Toxicological Targets Russell L. Carr Department of Basic Sciences College of Veterinary Medicine Toxicology Definitions = study of poisons Poison = any agent capable of producing a deleterious response
Toxicological Targets Russell L. Carr Department of Basic Sciences College of Veterinary Medicine Toxicology Definitions = study of poisons Poison = any agent capable of producing a deleterious response
Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization
 Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2018 A Series of Two-photon Absorption Pyridinium Sulfonate Inner Salts Targeting
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2018 A Series of Two-photon Absorption Pyridinium Sulfonate Inner Salts Targeting
Chlamydia trachomatis Infection Inhibits Both Bax and Bak Activation Induced by Staurosporine
 INFECTION AND IMMUNITY, Sept. 2004, p. 5470 5474 Vol. 72, No. 9 0019-9567/04/$08.00 0 DOI: 10.1128/IAI.72.9.5470 5474.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Chlamydia
INFECTION AND IMMUNITY, Sept. 2004, p. 5470 5474 Vol. 72, No. 9 0019-9567/04/$08.00 0 DOI: 10.1128/IAI.72.9.5470 5474.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Chlamydia
Ratiometric Fluorescence Imaging of Cellular Glutathione
 Supporting Information for: Ratiometric Fluorescence Imaging of Cellular Glutathione Gun-Joong Kim, Kiwon Lee, Hyockman Kwon, and Hae-Jo Kim, * Department of Chemistry, and Department of Bioscience and
Supporting Information for: Ratiometric Fluorescence Imaging of Cellular Glutathione Gun-Joong Kim, Kiwon Lee, Hyockman Kwon, and Hae-Jo Kim, * Department of Chemistry, and Department of Bioscience and
Co-localization Analysis of Bak-Drp1 on Mitochondria Using Super-resolution Microscopy
 Co-localization Analysis of Bak-Drp1 on Mitochondria Using Super-resolution Microscopy Danzi Song University of Tokyo Research Internship Program Ozawa Laboratory Abstract The mechanistic connection between
Co-localization Analysis of Bak-Drp1 on Mitochondria Using Super-resolution Microscopy Danzi Song University of Tokyo Research Internship Program Ozawa Laboratory Abstract The mechanistic connection between
M i t o c h o n d r i a
 M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
Functions and dysfunctions of mitochondrial dynamics
 Functions and dysfunctions of mitochondrial dynamics Scott A. Detmer and David C. Chan Abstract Recent findings have sparked renewed appreciation for the remarkably dynamic nature of mitochondria. These
Functions and dysfunctions of mitochondrial dynamics Scott A. Detmer and David C. Chan Abstract Recent findings have sparked renewed appreciation for the remarkably dynamic nature of mitochondria. These
Technical Bulletin. Caspase 9 Fluorescein (FLICA) Assay. Catalog Number CS0300 Storage Temperature 2-8 C
 1 Caspase 9 Fluorescein (FLICA) Assay Catalog Number CS0300 Storage Temperature 2-8 C Technical Bulletin Product Description Caspases are detected by immunoprecipitation, immunoblotting with caspase specific
1 Caspase 9 Fluorescein (FLICA) Assay Catalog Number CS0300 Storage Temperature 2-8 C Technical Bulletin Product Description Caspases are detected by immunoprecipitation, immunoblotting with caspase specific
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
Plant Molecular and Cellular Biology Lecture 8: Mechanisms of Cell Cycle Control and DNA Synthesis Gary Peter
 Plant Molecular and Cellular Biology Lecture 8: Mechanisms of Cell Cycle Control and DNA Synthesis Gary Peter 9/10/2008 1 Learning Objectives Explain why a cell cycle was selected for during evolution
Plant Molecular and Cellular Biology Lecture 8: Mechanisms of Cell Cycle Control and DNA Synthesis Gary Peter 9/10/2008 1 Learning Objectives Explain why a cell cycle was selected for during evolution
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Death-associated Protein 3 Localizes to the Mitochondria and Is Involved in the Process of Mitochondrial Fragmentation during Cell Death*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 35, Issue of August 27, pp. 36732 36738, 2004 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Death-associated
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 35, Issue of August 27, pp. 36732 36738, 2004 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Death-associated
Death Ligand- Death Receptor. Annexin V. Fas PI MKK7 PIP3. pro-caspase-3. Granzyme B. Caspase Activation. Bid. Bcl-2 Caspase-3 Caspase-12
 International Corporation life. science. discovery. { Apoptosis Apoptosis Kits International Corporation Apoptosis Signal Cascade and Detection Kits Annexin V Kits MEBCYTO Apoptosis Kit Annexin V Apoptosis
International Corporation life. science. discovery. { Apoptosis Apoptosis Kits International Corporation Apoptosis Signal Cascade and Detection Kits Annexin V Kits MEBCYTO Apoptosis Kit Annexin V Apoptosis
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
Regulation of mitochondrial permeability transition pore by PINK1
 Regulation of mitochondrial permeability transition pore by PINK1 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Giaime,
Regulation of mitochondrial permeability transition pore by PINK1 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Giaime,
Report. Bcl-2 Proteins EGL-1 and CED-9 Do Not Regulate Mitochondrial Fission or Fusion in Caenorhabditis elegans
 Current Biology 19, 768 773, May 12, 2009 ª2009 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.03.022 Bcl-2 Proteins EGL-1 and CED-9 Do Not Regulate Mitochondrial Fission or Fusion in Caenorhabditis
Current Biology 19, 768 773, May 12, 2009 ª2009 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.03.022 Bcl-2 Proteins EGL-1 and CED-9 Do Not Regulate Mitochondrial Fission or Fusion in Caenorhabditis
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Delivery. Delivery Processes. Delivery Processes: Distribution. Ultimate Toxicant
 Delivery Ultimate Toxicant The chemical species that reacts with the endogenous target. Toxicity depends on the concentration (dose) of the ultimate toxicant at the target site Delivery Processes Absorption
Delivery Ultimate Toxicant The chemical species that reacts with the endogenous target. Toxicity depends on the concentration (dose) of the ultimate toxicant at the target site Delivery Processes Absorption
Dual-Wavelength Lasing from Organic Dye Encapsulated Metal-Organic Framework Microcrystals
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2019 Electronic Supplementary Information Dual-Wavelength Lasing from Organic Dye Encapsulated Metal-Organic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2019 Electronic Supplementary Information Dual-Wavelength Lasing from Organic Dye Encapsulated Metal-Organic
Lecture 7 Cell Biolog y ٢٢٢ ١
 Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
12/5/2014. The cell cycle and cell death. The cell cycle: cells duplicate their contents and divide
 The cell cycle and cell death The cell cycle: cells duplicate their contents and divide 1 The cell cycle may be divided into 4 phases Eucaryotic cell division: Mitosis (nuclear division) Cytokinesis (cell
The cell cycle and cell death The cell cycle: cells duplicate their contents and divide 1 The cell cycle may be divided into 4 phases Eucaryotic cell division: Mitosis (nuclear division) Cytokinesis (cell
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr
 The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
Bax/Bak-Dependent Release of DDP/TIMM8a Promotes Drp1-Mediated Mitochondrial Fission and Mitoptosis during Programmed Cell Death
 Current Biology, Vol. 15, 2112 2118, December 6, 2005, ª2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.10.041 Bax/Bak-Dependent Release of DDP/TIMM8a Promotes Drp1-Mediated Mitochondrial
Current Biology, Vol. 15, 2112 2118, December 6, 2005, ª2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.10.041 Bax/Bak-Dependent Release of DDP/TIMM8a Promotes Drp1-Mediated Mitochondrial
Technical Bulletin. Caspase 13 Fluorescein (FLICA) Assay. Catalog Number CS0320 Storage Temperature 2-8 C
 Caspase 13 Fluorescein (FLICA) Assay Catalog Number CS0320 Storage Temperature 2-8 C Technical Bulletin Product Description Caspases are detected by immunoprecipitation, immunoblotting with caspase specific
Caspase 13 Fluorescein (FLICA) Assay Catalog Number CS0320 Storage Temperature 2-8 C Technical Bulletin Product Description Caspases are detected by immunoprecipitation, immunoblotting with caspase specific
Protease Inhibitor Cocktail A (1 tablet / 7 10 ml, Roche Cat# ) Protease inhibitor Cocktail B (0.5ml per 250ml, Calbiochem Cat# )
 Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
DNA DAMAGE-INDUCED APOPTOSIS: INHIBITION BY CALMODULIN ANTAGONIST, FAS RECEPTOR ANTIBODY AND CASPASE INHIBITORS
 DNA DAMAGE-INDUCED APOPTOSIS: INHIBITION BY CALMODULIN ANTAGONIST, FAS RECEPTOR ANTIBODY AND CASPASE INHIBITORS R. Ray, B. J. Benton, M. E. Burke, T. Rockwood, K. R. Bhat, D. R. Anderson, J. P. Petrali,
DNA DAMAGE-INDUCED APOPTOSIS: INHIBITION BY CALMODULIN ANTAGONIST, FAS RECEPTOR ANTIBODY AND CASPASE INHIBITORS R. Ray, B. J. Benton, M. E. Burke, T. Rockwood, K. R. Bhat, D. R. Anderson, J. P. Petrali,
CHAPTER 3. Cell Structure and Genetic Control. Chapter 3 Outline
 CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization
 Article Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization Ann Cassidy-Stone, 1 Jerry E. Chipuk, 2,7 Elena Ingerman,
Article Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization Ann Cassidy-Stone, 1 Jerry E. Chipuk, 2,7 Elena Ingerman,
