Chap.5 Statistical Thermodynamics
|
|
|
- Kelly McCarthy
- 6 years ago
- Views:
Transcription
1 Chap5 Statstcal Thermodynamcs () Free expanson & adabatc from macroscopc: Δ S dq T R adabatc Q, free expanson W rrev rrev ΔU, deal gas ΔT If reversble & sothermal QR + WR ΔU 因 Uf(T) RT QR WR ( Pd ) Pd d ΔS dq T R T from mcroscopc: statstcal thermodynamc: d( RT ln ) R ln R ln > RT ln *defnton: Boltzmann & Gbbs thermodynamc probablty, Ω: n! Ω The no of dfferent ways that a (n!)(n!)(n!) unque macro-confguraton can be acheved!, where n: total no of partcles n : partcle no at state Edted by Prof Yung-Jung Hsu
2 S KlnΩ From mcroscopc (Boltzmann hypothess), where K: Boltzmann constant R / o Ω Δ S S - S Kln Ω!! Ω ntal, Ω fnal!*! ( /)!*( /)! Ωfanl ()! ΔS Kln Kln K[ln(!) - ln(!)] Ω ntal (!) sterlng s formula: lnx! XlnX - X (by 泰勒展開式 ) ΔS K[ln - - ( ln - )] K ln Kln Rln > ( /) () Entropy of mxng From macroscopc vewpont: S M -R X ln X From mcroscopc vewpont: At ntal state: pure substance A & pure substance B! For A: ΩA SA K ln Ω A!! For B: ΩB SB K lnω B! for ntal state S S + S A B Edted by Prof Yung-Jung Hsu
3 At fnal state: deal soluton A-B! Ω A -B S K lnω (!)(!) the entropy change of mxng f A-B Δ S ΔS K ln - ΔS! (!)( f Kln ΔS ΔSM K[ln!-!-ln!] K[( + ) ln - ln - ln] R[X lnx - X lnx ] A A B B!)! (!)(!) (3) The thrd law for a perfectly ordered crystal of the compound AB at K: *hgh-ordered arrangement of atoms no randomness at all ( 無亂度 ) the locaton of atoms on the lattce ponts s fxed only a sngle mcroscopc state exsts to represent the mcrostate Ω( 在特定巨觀狀態下, 所能存在之微觀狀態之數目 ) By Boltzmann hypothess: S K ln Ω (TK & perfect crystallnty) the concept of 3 rd Law for crystals wth mperfectons at K: Crystal defect: vacances, ntersttals, dslocatons Compostonal defect: non-stochometry( 非當量 ), sotopes *random arrangement of atoms 有亂度 (S>) some randomness exsts on the placement of atoms a few mcrostates may exst Ω>, S> 3 Edted by Prof Yung-Jung Hsu
4 (4) Thermodynamc probablty and probablty Defnton: P The probablty that the system wll be n mcrostate 在特定巨觀狀態下, 微觀狀態 所出現之機率 Ω The total number of mcrostate exsted n a specfc macroconfguraton 在特定巨觀狀態下, 所能存在之微觀狀態數目 Correlaton: Assume two black & two whte balls are arranged n a lne, how many confguratons wll exst?! 4! Ω 6( 有 6 種排法 )!!!! b w Assumpton: for a unque confguraton, each arrangement s equally possble to exst In the current example, the probablty of each arrangement s /6 As a result, we realze P Ω & P 3 Gbbs Hypothess: For a closed system wth constant T and energy (canoncal ensemble), the energy could be expressed as: S -K P ln P, where P s the probablty that the system wll be n mcrostate 4 at equlbrum state: Macroscopc states may possbly consst of many mcrostates, then whch one wll be observed when the system s at equlbrum? From macroscopc vewpont: energetc consderaton From mcroscopc vewpont: possblty consderaton The macroscopc state we observed at equlbrum s the one wth the most possble mcrostates! 4 Edted by Prof Yung-Jung Hsu
5 5 Boltzmann dstrbuton (entropy calculaton for energy level) a knetc molecular theory by Maxwell: ( 分子動力學 ) - mv f(v) α v *exp( ) The probablty of a molecule havng energy E s proportonal -E to e b defnton: Consder partcles are put nto m boxes, havng dfferent energy levels, and there are no lmt on the number of partcles exstng at a specfc energy level Combned wth Maxwell s hypothess The probablty for a partcular partcle to be found n the energy E s gven by: E exp( ) P, E exp( ) The probablty for a partcle beng n a partcular state at energy E s proportonal to -E e Boltzmann dstrbuton c Ideal gas molecular dstrbuton: Consder a column wth specfc volume contans gas molecules: By Boltzmann dstrbuton: P P P E + E exp P P ote that mgh exp mgh exp exp( E ), P E exp( ) 5 Edted by Prof Yung-Jung Hsu
6 If the gas s deal, the pressure at the top & bottom wll be proportonal to & o, respectvely Pressure, mgh Mgh exp exp Barometrc equaton Pressure, RT d Degeneracy 簡倂態 Consder an energy E level; there may be a number of dstngushable states g, each havng the same energy We need to modfy Boltzmann dstrbuton The probablty that a partcle wll be n a specfc energy level possessng g dstngushable states should be modfed accordngly: P E g * exp( ) E, g * exp( ) e Thermodynamc propertes: (no degeneracy) Consder a system wth energy levels n equlbrum: From mcroscopc vewpont: to maxmze the entropy S S -K P ln P P, : number of partcles found at th energy level P, : total number of partcles E E, E: total energy of system E < E > P E, <E>: average energy for a partcle n system 6 Edted by Prof Yung-Jung Hsu
7 By maxmzng the entropy term assocated wth other relatons, we can obtan the expresson of S for a system contanng one partcle: < E > S Kln +, <E>: average energy nternal energy T E exp( < ) E E > P E E E exp( ) U U ln S Kln + ln F (A) U-TS, 將上式帶入 F -ln f Dstngush ablty of partcle 可識別性 Consder four partcles are put n two boxes, there are fve macro confguratons For mcrostate (): If the four partcles are total dfferent (dstngushable), there wll be four way of arrangements Eg atom n sold state If the four partcles are dentcal (ndstngushable), there wll be one way to reach mcrostate () Eg atom n gas or lqud state On the bass of ths consderaton, the Dstngush ablty s needed to be checked when a system contans many partcles ow, consder a system contans two partcles; the grand partton functon s gven by Φ 7 Edted by Prof Yung-Jung Hsu
8 Take partcles nto account, we can obtan Φ, f these partcles are dentcal and ndstngushable, the grand partton functon should be modfed to ensure we do not over-count the number of mcrostates Φ (partcles are dentcal, for gases)! For one partcle exstng n system: ln S Kln + F -ln ln U For partcles exstng n system: Sold Gas ln ln S Kln + S K[ln( ) + ] + F -ln Φ ln U F -(ln( ) + ) Φ! ln U 6 Other approach * Before 9, Boltzmann dstrbuton: P E g *exp( ) E g *exp( ) o lmtaton on the number of partcles exstng at E Wthout takng care of the over-count ssue (usually assumng dstngushable partcles) * After the development of quantum mechancs at 9 8 Edted by Prof Yung-Jung Hsu
9 Bose-Ensten dstrbuton: g E - μ exp( ) - especally vald for 輻射能量之分佈 o lmtaton on the number of partcles exstng at E Consder the partcles are ndstngushable * Ferm-Drac dstrbuton: g E - μ exp( ) + especally vald for 金屬與半導體中電子之分佈 Subject to Paul Excluson Prncple Consder the partcles are ndstngushable * At hgh temp & low chemcal potental (low densty of partcles), both B-E & F-D become M-B model E - μ E - μ E (exp( ) >>, exp( ) exp( )) 9 Edted by Prof Yung-Jung Hsu
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Thermodynamics and Kinetics of Solids 33. III. Statistical Thermodynamics. Â N i = N (5.3) N i. i =0. Â e i = E (5.4) has a maximum.
 hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
At zero K: All atoms frozen at fixed positions on a periodic lattice.
 September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
#64. ΔS for Isothermal Mixing of Ideal Gases
 #64 Carnot Heat Engne ΔS for Isothermal Mxng of Ideal Gases ds = S dt + S T V V S = P V T T V PV = nrt, P T ds = v T = nr V dv V nr V V = nrln V V = - nrln V V ΔS ΔS ΔS for Isothermal Mxng for Ideal Gases
#64 Carnot Heat Engne ΔS for Isothermal Mxng of Ideal Gases ds = S dt + S T V V S = P V T T V PV = nrt, P T ds = v T = nr V dv V nr V V = nrln V V = - nrln V V ΔS ΔS ΔS for Isothermal Mxng for Ideal Gases
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Lecture 3: Boltzmann distribution
 Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
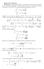 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
Chapter 1. Probability
 Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
V T for n & P = constant
 Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
ESCI 341 Atmospheric Thermodynamics Lesson 10 The Physical Meaning of Entropy
 ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Quantum Statistical Mechanics
 Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
5.62 Physical Chemistry II Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
Monte Carlo method II
 Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
CHAPTER 2. Energy Bands and Carrier Concentration in Thermal Equilibrium
 CHAPTER 2 Energy Bands and Carrier Concentration in Thermal Equilibrium 光電特性 Ge 被 Si 取代, 因為 Si 有較低漏電流 Figure 2.1. Typical range of conductivities for insulators, semiconductors, and conductors. Figure
CHAPTER 2 Energy Bands and Carrier Concentration in Thermal Equilibrium 光電特性 Ge 被 Si 取代, 因為 Si 有較低漏電流 Figure 2.1. Typical range of conductivities for insulators, semiconductors, and conductors. Figure
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
Statistical mechanics canonical ensemble
 canoncal ensemble system n thermal equlbrum wth bath of system probablty of mcro state T = k/ bath system p = 1 Z e E Z = e E average energy of system he = p E = what about? P E E e P e E = @ ln Z @ replcate
canoncal ensemble system n thermal equlbrum wth bath of system probablty of mcro state T = k/ bath system p = 1 Z e E Z = e E average energy of system he = p E = what about? P E E e P e E = @ ln Z @ replcate
Property calculation I
 .0, 3.0, 0.333,.00 Introducton to Modelng and Smulaton Sprng 0 Part I Contnuum and partcle methods Property calculaton I Lecture 3 Markus J. Buehler Laboratory for Atomstc and Molecular Mechancs Department
.0, 3.0, 0.333,.00 Introducton to Modelng and Smulaton Sprng 0 Part I Contnuum and partcle methods Property calculaton I Lecture 3 Markus J. Buehler Laboratory for Atomstc and Molecular Mechancs Department
Chapters 18 & 19: Themodynamics review. All macroscopic (i.e., human scale) quantities must ultimately be explained on the microscopic scale.
 Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
Lecture 2 Grand Canonical Ensemble GCE
 Lecture 2 Grand Canoncal Ensemble GCE 2.1 hermodynamc Functons Contnung on from last day we also note that thus, dω = df dµ µd = Sd P dv dµ (2.1) P = V = S = From the expresson for the entropy, we therefore
Lecture 2 Grand Canoncal Ensemble GCE 2.1 hermodynamc Functons Contnung on from last day we also note that thus, dω = df dµ µd = Sd P dv dµ (2.1) P = V = S = From the expresson for the entropy, we therefore
Physics 115. Molecular motion and temperature Phase equilibrium, evaporation
 Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Name: SID: Discussion Session:
 Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Phase Space N p t 2 2 t 1 Phase space p, p, p,..., p, r, r, r,..., r N N 1 N r
 Phase Space p t 2 2 t1 1 Phase space p,..., 1, p2, p3,..., p, r1, r1, r3 r r Phase Orbt If thehamltonan of the system s denoted by H(q,p), the moton of phase pont can be along the phase orbt and s determned
Phase Space p t 2 2 t1 1 Phase space p,..., 1, p2, p3,..., p, r1, r1, r3 r r Phase Orbt If thehamltonan of the system s denoted by H(q,p), the moton of phase pont can be along the phase orbt and s determned
Lecture 5: Ideal monatomic gas
 Lecture 5: Ideal monatomc gas Statstcal mechancs of the perfect gas Ams: Key new concepts and methods: Countng states Waves n a box. Demonstraton that β / kt Heat, work and Entropy n statstcal mechancs
Lecture 5: Ideal monatomc gas Statstcal mechancs of the perfect gas Ams: Key new concepts and methods: Countng states Waves n a box. Demonstraton that β / kt Heat, work and Entropy n statstcal mechancs
Using T.O.M to Estimate Parameter of distributions that have not Single Exponential Family
 IOSR Journal of Mathematcs IOSR-JM) ISSN: 2278-5728. Volume 3, Issue 3 Sep-Oct. 202), PP 44-48 www.osrjournals.org Usng T.O.M to Estmate Parameter of dstrbutons that have not Sngle Exponental Famly Jubran
IOSR Journal of Mathematcs IOSR-JM) ISSN: 2278-5728. Volume 3, Issue 3 Sep-Oct. 202), PP 44-48 www.osrjournals.org Usng T.O.M to Estmate Parameter of dstrbutons that have not Sngle Exponental Famly Jubran
Thermodynamics and Gases
 hermodynamcs and Gases Last tme Knetc heory o Gases or smple (monatomc) gases Atomc nature o matter Demonstrate deal gas law Atomc knetc energy nternal energy Mean ree path and velocty dstrbutons From
hermodynamcs and Gases Last tme Knetc heory o Gases or smple (monatomc) gases Atomc nature o matter Demonstrate deal gas law Atomc knetc energy nternal energy Mean ree path and velocty dstrbutons From
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
1. Connection between QM and Thermodynamics
 Calculaton of hermodynamc and Knetc Propertes 1. Connecton between QM and hermodynamcs We have focused to ths pont on the many approaches and detals of calculatng the nternal tronc energy of a sngle molecule,
Calculaton of hermodynamc and Knetc Propertes 1. Connecton between QM and hermodynamcs We have focused to ths pont on the many approaches and detals of calculatng the nternal tronc energy of a sngle molecule,
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Study of the possibility of eliminating the Gibbs paradox within the framework of classical thermodynamics *
 tudy of the possblty of elnatng the Gbbs paradox wthn the fraework of classcal therodynacs * V. Ihnatovych Departent of Phlosophy, Natonal echncal Unversty of Ukrane Kyv Polytechnc Insttute, Kyv, Ukrane
tudy of the possblty of elnatng the Gbbs paradox wthn the fraework of classcal therodynacs * V. Ihnatovych Departent of Phlosophy, Natonal echncal Unversty of Ukrane Kyv Polytechnc Insttute, Kyv, Ukrane
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Non-Ideality Through Fugacity and Activity
 Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
χ x B E (c) Figure 2.1.1: (a) a material particle in a body, (b) a place in space, (c) a configuration of the body
 Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Solution Thermodynamics
 CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
NAME and Section No.
 Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
The Geometry of Logit and Probit
 The Geometry of Logt and Probt Ths short note s meant as a supplement to Chapters and 3 of Spatal Models of Parlamentary Votng and the notaton and reference to fgures n the text below s to those two chapters.
The Geometry of Logt and Probt Ths short note s meant as a supplement to Chapters and 3 of Spatal Models of Parlamentary Votng and the notaton and reference to fgures n the text below s to those two chapters.
Chapter 5 rd Law of Thermodynamics
 Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Chapter 6. Series-Parallel Circuits ISU EE. C.Y. Lee
 Chapter 6 Series-Parallel Circuits Objectives Identify series-parallel relationships Analyze series-parallel circuits Determine the loading effect of a voltmeter on a circuit Analyze a Wheatstone bridge
Chapter 6 Series-Parallel Circuits Objectives Identify series-parallel relationships Analyze series-parallel circuits Determine the loading effect of a voltmeter on a circuit Analyze a Wheatstone bridge
Topics on Statistical Mechanics TCMM Lecture Notes
 Topcs on Statstcal Mechancs TCMM Lecture otes Fernando Fernandes Department of Chemstry and Bochemstry, Faculty of Scences, Unversty of Lsbon, Portugal Emal: fslva@fc.ul.pt A short revew of basc concepts
Topcs on Statstcal Mechancs TCMM Lecture otes Fernando Fernandes Department of Chemstry and Bochemstry, Faculty of Scences, Unversty of Lsbon, Portugal Emal: fslva@fc.ul.pt A short revew of basc concepts
Equilibrium Statistical Mechanics
 Equlbrum Statstcal Mechancs Ref. Davdson, Statstcal Mechancs McGraw-Hll, 96 We wsh to study plasmas n equlbrum for at least two reasons: (a) Many tmes real plasmas are near true equlbrum, at least locally
Equlbrum Statstcal Mechancs Ref. Davdson, Statstcal Mechancs McGraw-Hll, 96 We wsh to study plasmas n equlbrum for at least two reasons: (a) Many tmes real plasmas are near true equlbrum, at least locally
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
Markov Chain Monte Carlo (MCMC), Gibbs Sampling, Metropolis Algorithms, and Simulated Annealing Bioinformatics Course Supplement
 Markov Chan Monte Carlo MCMC, Gbbs Samplng, Metropols Algorthms, and Smulated Annealng 2001 Bonformatcs Course Supplement SNU Bontellgence Lab http://bsnuackr/ Outlne! Markov Chan Monte Carlo MCMC! Metropols-Hastngs
Markov Chan Monte Carlo MCMC, Gbbs Samplng, Metropols Algorthms, and Smulated Annealng 2001 Bonformatcs Course Supplement SNU Bontellgence Lab http://bsnuackr/ Outlne! Markov Chan Monte Carlo MCMC! Metropols-Hastngs
PROPERTIES OF FRACTIONAL EXCLUSION STATISTICS IN INTERACTING PARTICLE SYSTEMS
 PROPERTIES OF FRACTIONAL EXCLUSION STATISTICS IN INTERACTING PARTICLE SYSTEMS DRAGOŞ-VICTOR ANGHEL Department of Theoretcal Physcs, Hora Hulube Natonal Insttute for Physcs and Nuclear Engneerng (IFIN-HH),
PROPERTIES OF FRACTIONAL EXCLUSION STATISTICS IN INTERACTING PARTICLE SYSTEMS DRAGOŞ-VICTOR ANGHEL Department of Theoretcal Physcs, Hora Hulube Natonal Insttute for Physcs and Nuclear Engneerng (IFIN-HH),
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
THERMAL DISTRIBUTION IN THE HCL SPECTRUM OBJECTIVE
 ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
PES 2130 Fall 2014, Spendier Lecture 7/Page 1
 PES 2130 Fall 2014, Spender Lecture 7/Page 1 Lecture today: Chapter 20 (ncluded n exam 1) 1) Entropy 2) Second Law o hermodynamcs 3) Statstcal Vew o Entropy Announcements: Next week Wednesday Exam 1! -
PES 2130 Fall 2014, Spender Lecture 7/Page 1 Lecture today: Chapter 20 (ncluded n exam 1) 1) Entropy 2) Second Law o hermodynamcs 3) Statstcal Vew o Entropy Announcements: Next week Wednesday Exam 1! -
Introduction to Density Functional Theory. Jeremie Zaffran 2 nd year-msc. (Nanochemistry)
 Introducton to Densty Functonal Theory Jereme Zaffran nd year-msc. (anochemstry) A- Hartree appromatons Born- Oppenhemer appromaton H H H e The goal of computatonal chemstry H e??? Let s remnd H e T e
Introducton to Densty Functonal Theory Jereme Zaffran nd year-msc. (anochemstry) A- Hartree appromatons Born- Oppenhemer appromaton H H H e The goal of computatonal chemstry H e??? Let s remnd H e T e
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
Physics 114 Exam 2 Fall 2014 Solutions. Name:
 Physcs 114 Exam Fall 014 Name: For gradng purposes (do not wrte here): Queston 1. 1... 3. 3. Problem Answer each of the followng questons. Ponts for each queston are ndcated n red. Unless otherwse ndcated,
Physcs 114 Exam Fall 014 Name: For gradng purposes (do not wrte here): Queston 1. 1... 3. 3. Problem Answer each of the followng questons. Ponts for each queston are ndcated n red. Unless otherwse ndcated,
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
ERRATA For the book A farewell to Entropy, Statistical Thermodynamics Based on Information
 ERRAA For the book A farewell to Entropy, Statstcal hermodynamcs Based on Informaton Chapter : Page, lnes 4-6 of paragraph should be revsed to: hs defnton s vald wthout any reference to the atomc consttuency
ERRAA For the book A farewell to Entropy, Statstcal hermodynamcs Based on Informaton Chapter : Page, lnes 4-6 of paragraph should be revsed to: hs defnton s vald wthout any reference to the atomc consttuency
Equation of State Modeling of Phase Equilibrium in the Low-Density Polyethylene Process
 Equaton of State Modelng of Phase Equlbrum n the Low-Densty Polyethylene Process H. Orbey, C. P. Boks, and C. C. Chen Ind. Eng. Chem. Res. 1998, 37, 4481-4491 Yong Soo Km Thermodynamcs & Propertes Lab.
Equaton of State Modelng of Phase Equlbrum n the Low-Densty Polyethylene Process H. Orbey, C. P. Boks, and C. C. Chen Ind. Eng. Chem. Res. 1998, 37, 4481-4491 Yong Soo Km Thermodynamcs & Propertes Lab.
Process Modeling. Improving or understanding chemical process operation is a major objective for developing a dynamic process model
 Process Modelng Improvng or understandng chemcal process operaton s a major objectve for developng a dynamc process model Balance equatons Steady-state balance equatons mass or energy mass or energy enterng
Process Modelng Improvng or understandng chemcal process operaton s a major objectve for developng a dynamc process model Balance equatons Steady-state balance equatons mass or energy mass or energy enterng
Fermi-Dirac statistics
 UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
Composite Hypotheses testing
 Composte ypotheses testng In many hypothess testng problems there are many possble dstrbutons that can occur under each of the hypotheses. The output of the source s a set of parameters (ponts n a parameter
Composte ypotheses testng In many hypothess testng problems there are many possble dstrbutons that can occur under each of the hypotheses. The output of the source s a set of parameters (ponts n a parameter
0.1 Required Classical Mechanics
 0.1 Requred Classcal Mechancs The subject of statstcal mechancs deals wth macroscopc (large szed systems consstng of 10 23 partcles) systems. As a result, they have a huge number of degrees of freedom.
0.1 Requred Classcal Mechancs The subject of statstcal mechancs deals wth macroscopc (large szed systems consstng of 10 23 partcles) systems. As a result, they have a huge number of degrees of freedom.
Exercises of Fundamentals of Chemical Processes
 Department of Energ Poltecnco d Mlano a Lambruschn 4 2056 MILANO Exercses of undamentals of Chemcal Processes Prof. Ganpero Gropp Exercse 7 ) Estmaton of the composton of the streams at the ext of an sothermal
Department of Energ Poltecnco d Mlano a Lambruschn 4 2056 MILANO Exercses of undamentals of Chemcal Processes Prof. Ganpero Gropp Exercse 7 ) Estmaton of the composton of the streams at the ext of an sothermal
Assignment 4. Adsorption Isotherms
 Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
1. Mean-Field Theory. 2. Bjerrum length
 1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS. Version 2 ECE IIT, Kharagpur
 Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
原子模型 Atomic Model 有了正確的原子模型, 才會發明了雷射
 原子模型 Atomic Model 有了正確的原子模型, 才會發明了雷射 原子結構中的電子是如何被發現的? ( 1856 1940 ) 可以參考美國物理學會 ( American Institute of Physics ) 網站 For in-depth information, check out the American Institute of Physics' History Center
原子模型 Atomic Model 有了正確的原子模型, 才會發明了雷射 原子結構中的電子是如何被發現的? ( 1856 1940 ) 可以參考美國物理學會 ( American Institute of Physics ) 網站 For in-depth information, check out the American Institute of Physics' History Center
= z 20 z n. (k 20) + 4 z k = 4
 Problem Set #7 solutons 7.2.. (a Fnd the coeffcent of z k n (z + z 5 + z 6 + z 7 + 5, k 20. We use the known seres expanson ( n+l ( z l l z n below: (z + z 5 + z 6 + z 7 + 5 (z 5 ( + z + z 2 + z + 5 5
Problem Set #7 solutons 7.2.. (a Fnd the coeffcent of z k n (z + z 5 + z 6 + z 7 + 5, k 20. We use the known seres expanson ( n+l ( z l l z n below: (z + z 5 + z 6 + z 7 + 5 (z 5 ( + z + z 2 + z + 5 5
Einstein-Podolsky-Rosen Paradox
 H 45 Quantum Measurement and Spn Wnter 003 Ensten-odolsky-Rosen aradox The Ensten-odolsky-Rosen aradox s a gedanken experment desgned to show that quantum mechancs s an ncomplete descrpton of realty. The
H 45 Quantum Measurement and Spn Wnter 003 Ensten-odolsky-Rosen aradox The Ensten-odolsky-Rosen aradox s a gedanken experment desgned to show that quantum mechancs s an ncomplete descrpton of realty. The
Why a particular process occurs? Is it due to decrease of energy? Model study 1: An adiabatic ( 絕熱 ) system insulation
 17 Spontaneity, Entropy ( 熵 ) and Free Energy ( 自由能 ) Question: Why a particular process occurs? Is it due to decrease of energy? Model study 1: An adiabatic ( 絕熱 ) system insulation ideal gas vacuum q
17 Spontaneity, Entropy ( 熵 ) and Free Energy ( 自由能 ) Question: Why a particular process occurs? Is it due to decrease of energy? Model study 1: An adiabatic ( 絕熱 ) system insulation ideal gas vacuum q
y i x P vap 10 A T SOLUTION TO HOMEWORK #7 #Problem
 SOLUTION TO HOMEWORK #7 #roblem 1 10.1-1 a. In order to solve ths problem, we need to know what happens at the bubble pont; at ths pont, the frst bubble s formed, so we can assume that all of the number
SOLUTION TO HOMEWORK #7 #roblem 1 10.1-1 a. In order to solve ths problem, we need to know what happens at the bubble pont; at ths pont, the frst bubble s formed, so we can assume that all of the number
Physics for Scientists & Engineers 2
 Equpotental Surfaces and Lnes Physcs for Scentsts & Engneers 2 Sprng Semester 2005 Lecture 9 January 25, 2005 Physcs for Scentsts&Engneers 2 1 When an electrc feld s present, the electrc potental has a
Equpotental Surfaces and Lnes Physcs for Scentsts & Engneers 2 Sprng Semester 2005 Lecture 9 January 25, 2005 Physcs for Scentsts&Engneers 2 1 When an electrc feld s present, the electrc potental has a
