Solution Thermodynamics
|
|
|
- Gyles Sparks
- 5 years ago
- Views:
Transcription
1 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD January 7, 001
2 Soluton hermodynamcs by S.. Howard hs treatment of soluton thermodynamcs descrbes common soluton parameters nvolvng volume, enthalpy, entropy and bbs energy. Furthermore, t contans the natural constrants placed on solutons by mathematcal consderatons. Soluton terms are ether ntegral or partal propertes. Integral propertes descrbe the propertes of the entre soluton whle partal s used to descrbe the propertes of any one component n the soluton. Before formal consderatons begn, t s useful to consder an example stuaton where there s a need for partal molar quanttes. Such a case s easly llustrated by the followng stuaton. he reacton between go and SO to form sold Forsterte s go (1) + SO (1) = g SO 4(s). (1) At equlbrum there s one lqud phase consstng of a soluton of go and SO and one sold phase consstng of pure g SO 4. If one needed to know what temperature-pressure loc are requred to mantan a fxed equlbrum dstrbuton of the consttuents n ths reacton, one would use the Clausus-Clapeyron equaton dp d ln ΔH = ΔV () he use of ths equaton requres knowng the volume and enthalpy changes for the reacton. he volume change s ΔV = Volume of 1 mole of pure, sold Forsterte Volume of moles of - go n the lqud soluton - of go and SO Volume of 1 mole of SO n the lqud soluton of go and SO he enthalpy change may be wrtten n a correspondng way. It s of nterest to note that t s possble to measure each of the partal molar volumes to obtan ΔV but t s not possble to ever measure the absolute partal molar enthalpes. However, t s a farly straghtforward mater to measure the change n the enthalpes, ΔH. A second process that llustrates the need for soluton propertes s the reacton that descrbes equlbrum n the Betterton Process where Pb s deznced usng PbCl. PbCl (1) + Zn (1) = ZnCl (1) + Pb (1) (3) At equlbrum there et two lqud phases: a lqud metallc phase of Pb and Zn, and a molten salt phase of ZnCl and PbCl. he volume change for the reacton n ths case s ΔV = Volume of 1 mole of ZnCl n the molten salt phase of equlbrum composton. Volume of 1 mole of PbCl n the + molten salt - phase of equlbrum composton Volume of 1 mole of Pb n the molten metallc phase of equlbrum composton. - Volume of 1 mole of Zn n the molten metallc phase of equlbrum composton. S.. Howard 001
3 Soluton hermodynamcs by S.. Howard 3 Absolute Values he volume that one mole of a consttuent occupes n soluton rarely equals the volume of the consttuent n the pure state because the nteracton forces n soluton are dfferent from those n the pure component. he volume of one mole of n soluton s denoted by the symbol V and may be determned expermentally by the methods descrbed below. easurement and Defnton of Absolute Partal olar Volume here are three methods of measurng V. 1. he absolute partal molar volume s equal to the ncrease n the total volume V of soluton when one mole of speces s added to a very large quantty of the ntal soluton. V = Δ V (4) Snce V vares wth composton, a large quantty of soluton s specfed so that the ntal soluton s concentraton s essentally unaffected by the addton of one mole of.. he second method for measurng V s only a mnor modfcaton of the frst method. he only dfference s that rather than addng one mole of to a large quantty of soluton, a smaller quantty of s added. Consequently, the volume change s for the smaller quantty of and to obtan the volume change per mole one must dvde by Δn whch s the moles of added to the soluton. ΔV V = Δn (5) hs procedure becomes more precse as Δn 0 because the change n soluton composton becomes smaller. herefore, the best value of V s n the lmt. V = n Lmo ΔV Δn V = n, P, n j (6) he subscrpts,p, and n j ndcate that V s measured whle, P, and the number of moles of other soluton components reman fxed. Snce V s defned as a partal dervatve t s easy to see why V s called the partal molar volume of speces n soluton. 3. he defnton n Eq. [6] mples another method whch may be used to determne the partal molar volume, V. If one starts wth n j moles of component j at temperature and pressure P and adds to t speces n several ncrements, a curve lke that shown n Fgure1 s obtaned. Accordng to Eq. [6], the slope of the lne n Fgure 1 equals the partal molar volume of speces at the composton selected. For example, the partal molar volume of at n = 0.06 s 38.9cm 3 mole. S.. Howard 001
4 Soluton hermodynamcs by S.. Howard 4 Fgure 1. Partal olar Volume of, V, from Volume vs. n bbs-duhem Equaton he bbs-duhem Equaton descrbes a constrant on the behavor of the partal molar quanttes n a soluton. he followng dervaton s n terms of volume but apples to H, S, and, as well. Constant temperature and pressure are assumed throughout the development. he total volume V of a bnary soluton composed of n 1 moles of component 1 and n moles of component s whch may be dvded by (n 1 + n ) to gve the ntegral molar volume where X 1 = the mole fracton of component 1 X = the mole fracton of component. V = n V 1 + n V (7) V = X 1 V 1 + X V (8) Consderaton of Fgure 1 shows that a bnary soluton s volume s dependent upon the number of moles of each component. hat s, V = f(n 1, n ) at constant and P. he total dfferental of V gves V dv = n 1, P, n j V dn 1 + n, P However, the followng expresson for dv s obtaned from Eq. [7]: Subtractng Eq. [9] from Eq. [10] gves, n j dn = V 1 dn 1 + V dn (9) dv = n 1 dv 1 + n dv + V 1 dn 1 + V dn (10) S.. Howard 001
5 Soluton hermodynamcs by S.. Howard 5 Dvdng by (n 1 + n ) gves n 1 dv 1 + n dv = 0 (11) X 1 dv 1 + X dv = 0 (1) hs mportant result s called the bbs-duhem Equaton. It shows that change n one partal molar volume s related to change n the partal molar volume of the other component. he Slope-Intercept ethod wo valuable relatonshps are obtaned by dfferentatng Eq. [8] wth respect to X and combnng the result wth Eq. [1] to gve V x = V - V 1 (13) Note that dx 1 = -dx snce X 1 + X = 1. Elmnaton of V 1 or V wth Eq. [8] yelds V 1 =V- V X (14) x V V = V + (1 - X ) x (15) hese two equatons are the bass of the angent-intercept ethod of graphcally determnng partal molar volumes from a plot of V versus X. As ndcated on Fgure, V 1 equals the ntercept at pure component 1 and V equals the tangent ntercept at pure component. V V 3 cm gmole X V V x V (1 X ) X V V 1 0 X 1 ole Fracton of X Fgure. he angent Intercept ethod of Fndng Partal olar Volumes S.. Howard 001
6 Soluton hermodynamcs by S.. Howard 6 Summary of Absolute Quanttes As stated prevously, the above development s drectly applcable to the thermodynamc propertes H, S, and. In general, the partal molar quantty s defned as Q Q = n, P,n j (16) he ntegral molar quantty for a bnary soluton s related to the partal molar quanttes as and the bbs-duhem Equaton requres that Q = X 1 Q I + X Q (17) X 1 Q I + X Q = 0 he angent-intercept ethod may be used to obtan the partal molar quanttes Q from the ntegral molar quantty Q. he quantty Q can be V, H, S, or. Absolute values are determnable for V and S, but not for H and. Snce only changes n H and are requred for thermochemcal calculatons, there s no need for the absolute values of H and. However, the changes n H and must be related to some standard (or reference) state. When ths s done the changes n H and are called the relatve enthalpy and relatve bbs energy. he next secton consders these relatve values. Relatve Values (or of xng ) o assgn numercal values to the thermodynamc soluton functons of enthalpy and bbs energy t s necessary to relate them to a reference state. Snce absolute heat energy cannot be measured, all tabulated energy and enthalpy data equals the change from some reference state. For solds and lquds ths state s the pure component at the pressure and temperature of nterest. he dfference between the partal molar volume n soluton V 1 and the molar volume of the pure speces s V called the relatve partal molar volume and s denoted by the symbol V In general, V V - V (18) Q Q - Q (19) he parameters on the left sde of Eqs. [18] and [19] are also called partal molar quanttes of mxng, because the term mxng mples a quantty relatve to the reference state whch s the pure component. he dscusson of relatve quanttes began by ctng the necessty of relatng the enthalpy and free energy quanttes to a reference state so that the change from the reference state could be measured. ethods of measurng these changes for H and are presented below. S.. Howard 001
7 Soluton hermodynamcs by S.. Howard 7 easurement of Relatve Partal olar Enthalpy and bbs Free Energy he partal molar heat of mxng of speces can be measured calormetrcally by droppng a small amount of lqud component at temperature nto a soluton at temperature. he transfer of heat requred to return the soluton to the orgnal temperature equals the heat of mxng for the small quantty Δ n. herefore, H ΔH = Lm n o Δ Δn, P, n other H = n, P, n other (0) Of course, the quantty Δ n. must be small enough so as not to change the soluton s composton, because H =f(x ). he partal free energy of mxng may be determned by measurng the partal pressure of speces above the soluton and above pure provdng deal behavor of the gas prevals. = = R ln a P = R ln o P (1) Here o P s the pressure of the pure component. A second method of measurng s by a galvanc cell. If the galvanc cell shown n Fgure 3 creates the potental E, then the partal molar bbs free energy of mxng s = nfe () where n = the number of equvalents for the cell Joules F = Faraday s constant: 96,55 volt * equvalent he relatve partal molar entropy of speces may be calculated from the equaton = H S (3) E, volts Pure Ionc Conductor wth + ons Soluton wth Fgure 3. alvanc Cell for Determnng Actvtes of Component S.. Howard 001
8 Soluton hermodynamcs by S.. Howard 8 Relatve Integral olar Quanttes he ntegral quanttes refer to one mole or soluton. Lke partal quanttes, ntegral quanttes are both absolute and relatve. For example, the absolute volume of one mole of a bnary soluton (two components) s gven by equaton V x1 V1 + xv = (4) and n general Q x1 Q1 + xq = (5) he relatve ntegral molar quantty Q may also be called the ntegral molar quantty of mxng. he angent-intercept ethod and Relatve Quanttes Relatve partal molar quanttes may be obtaned from the relatve ntegral molar quanttes by the angent-intercept ethod as llustrated n Fgure. he same procedure used to derve Eqs. [13] and [14] s followed to obtan Q Q = 1 Q x (6) x ( ) Q = Q + 1 x bbs-duhem Equaton Lkewse, the bbs-duhem Equaton for relatve quanttes has the general form 1 1 = Q (7) x X Q + X Q 0 (8) Excess Values he excess quanttes are defned merely for convenence. hey are smply the dfference between the actual value of the relatve quantty and the value of the relatve quantty were the soluton deal. Q, deal = Q Q (9) An deal soluton s a soluton n whch the nteracton among the atoms and molecules of unlke components s the same as the nteractons among lke atoms and molecules. In such case S deal 1 V = 0 (30) deal 1 H = 0 (31) deal 1 = R ln X (3) 1 deal = R ln X (33) herefore, S.. Howard 001
9 Soluton hermodynamcs by S.. Howard 9 sx V = V (34) H = H (35) a = R ln a R ln X = R ln = R ln γ X (36) S = S + R ln X (37) For the ntegral excess molar quanttes S V = V (38) m H = H (39) = S + R( X 1 ln X 1 + X ln X ) (40) ( X ln X + X X ) = R (41) 1 1 ln As wth all other partal quanttes, the bbs-duhem Equaton s vald. 1 1 = X Q + X Q 0 (4) Lkewse, the angent-intercept ethod may be used to obtan excess partal molar quanttes from the correspondng excess ntegral molar quantty. Summary he four thermodynamc quanttes V, H, S, and may refer to ether one mole of a soluton component (partal molar quantty) or to one mole of soluton (ntegral molar quantty). hese quanttes may be reported as the absolute value (except for H and ), relatve value, or excess value. he bbs-duhem Equaton apples to any of the partal molar quanttes and has the general form X Q + X Q 0 (43) 1 1 = where Q1 = any partal molar quantty for component 1 Q = any correspondng partal molar quantty for component Lkewse, the angent-intercept ethod may be used to fnd any partal molar quantty from the correspondng ntegral molar quantty Q Q 1 = Q X (44) X ( ) Q = Q + 1 X Q X (45) Any ntegral molar quantty may be found from the correspondng partal molar quanttes accordng to the followng summaton Q = X Q + X Q... (46) S.. Howard 001
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
between standard Gibbs free energies of formation for products and reactants, ΔG! R = ν i ΔG f,i, we
 hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
I wish to publish my paper on The International Journal of Thermophysics. A Practical Method to Calculate Partial Properties from Equation of State
 I wsh to publsh my paper on The Internatonal Journal of Thermophyscs. Ttle: A Practcal Method to Calculate Partal Propertes from Equaton of State Authors: Ryo Akasaka (correspondng author) 1 and Takehro
I wsh to publsh my paper on The Internatonal Journal of Thermophyscs. Ttle: A Practcal Method to Calculate Partal Propertes from Equaton of State Authors: Ryo Akasaka (correspondng author) 1 and Takehro
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Solution Thermodynamics
 CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
Kernel Methods and SVMs Extension
 Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
Module 2. Random Processes. Version 2 ECE IIT, Kharagpur
 Module Random Processes Lesson 6 Functons of Random Varables After readng ths lesson, ou wll learn about cdf of functon of a random varable. Formula for determnng the pdf of a random varable. Let, X be
Module Random Processes Lesson 6 Functons of Random Varables After readng ths lesson, ou wll learn about cdf of functon of a random varable. Formula for determnng the pdf of a random varable. Let, X be
Transfer Functions. Convenient representation of a linear, dynamic model. A transfer function (TF) relates one input and one output: ( ) system
 Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Process Modeling. Improving or understanding chemical process operation is a major objective for developing a dynamic process model
 Process Modelng Improvng or understandng chemcal process operaton s a major objectve for developng a dynamc process model Balance equatons Steady-state balance equatons mass or energy mass or energy enterng
Process Modelng Improvng or understandng chemcal process operaton s a major objectve for developng a dynamc process model Balance equatons Steady-state balance equatons mass or energy mass or energy enterng
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
Assignment 4. Adsorption Isotherms
 Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
(1) The saturation vapor pressure as a function of temperature, often given by the Antoine equation:
 CE304, Sprng 2004 Lecture 22 Lecture 22: Topcs n Phase Equlbra, part : For the remander of the course, we wll return to the subject of vapor/lqud equlbrum and ntroduce other phase equlbrum calculatons
CE304, Sprng 2004 Lecture 22 Lecture 22: Topcs n Phase Equlbra, part : For the remander of the course, we wll return to the subject of vapor/lqud equlbrum and ntroduce other phase equlbrum calculatons
Foundations of Arithmetic
 Foundatons of Arthmetc Notaton We shall denote the sum and product of numbers n the usual notaton as a 2 + a 2 + a 3 + + a = a, a 1 a 2 a 3 a = a The notaton a b means a dvdes b,.e. ac = b where c s an
Foundatons of Arthmetc Notaton We shall denote the sum and product of numbers n the usual notaton as a 2 + a 2 + a 3 + + a = a, a 1 a 2 a 3 a = a The notaton a b means a dvdes b,.e. ac = b where c s an
a for save as PDF Chemistry 163B Introduction to Multicomponent Systems and Partial Molar Quantities
 a for save as PDF Chemstry 163B Introducton to Multcomponent Systems and Partal Molar Quanttes 1 the problem of partal mmolar quanttes mx: 10 moles ethanol C 2 H 5 OH (580 ml) wth 1 mole water H 2 O (18
a for save as PDF Chemstry 163B Introducton to Multcomponent Systems and Partal Molar Quanttes 1 the problem of partal mmolar quanttes mx: 10 moles ethanol C 2 H 5 OH (580 ml) wth 1 mole water H 2 O (18
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
10.34 Numerical Methods Applied to Chemical Engineering Fall Homework #3: Systems of Nonlinear Equations and Optimization
 10.34 Numercal Methods Appled to Chemcal Engneerng Fall 2015 Homework #3: Systems of Nonlnear Equatons and Optmzaton Problem 1 (30 ponts). A (homogeneous) azeotrope s a composton of a multcomponent mxture
10.34 Numercal Methods Appled to Chemcal Engneerng Fall 2015 Homework #3: Systems of Nonlnear Equatons and Optmzaton Problem 1 (30 ponts). A (homogeneous) azeotrope s a composton of a multcomponent mxture
ON A DETERMINATION OF THE INITIAL FUNCTIONS FROM THE OBSERVED VALUES OF THE BOUNDARY FUNCTIONS FOR THE SECOND-ORDER HYPERBOLIC EQUATION
 Advanced Mathematcal Models & Applcatons Vol.3, No.3, 2018, pp.215-222 ON A DETERMINATION OF THE INITIAL FUNCTIONS FROM THE OBSERVED VALUES OF THE BOUNDARY FUNCTIONS FOR THE SECOND-ORDER HYPERBOLIC EUATION
Advanced Mathematcal Models & Applcatons Vol.3, No.3, 2018, pp.215-222 ON A DETERMINATION OF THE INITIAL FUNCTIONS FROM THE OBSERVED VALUES OF THE BOUNDARY FUNCTIONS FOR THE SECOND-ORDER HYPERBOLIC EUATION
The Multiple Classical Linear Regression Model (CLRM): Specification and Assumptions. 1. Introduction
 ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
This column is a continuation of our previous column
 Comparson of Goodness of Ft Statstcs for Lnear Regresson, Part II The authors contnue ther dscusson of the correlaton coeffcent n developng a calbraton for quanttatve analyss. Jerome Workman Jr. and Howard
Comparson of Goodness of Ft Statstcs for Lnear Regresson, Part II The authors contnue ther dscusson of the correlaton coeffcent n developng a calbraton for quanttatve analyss. Jerome Workman Jr. and Howard
Linear Regression Analysis: Terminology and Notation
 ECON 35* -- Secton : Basc Concepts of Regresson Analyss (Page ) Lnear Regresson Analyss: Termnology and Notaton Consder the generc verson of the smple (two-varable) lnear regresson model. It s represented
ECON 35* -- Secton : Basc Concepts of Regresson Analyss (Page ) Lnear Regresson Analyss: Termnology and Notaton Consder the generc verson of the smple (two-varable) lnear regresson model. It s represented
Lecture 12: Discrete Laplacian
 Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Structure and Drive Paul A. Jensen Copyright July 20, 2003
 Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
The ChemSep Book. Harry A. Kooijman Consultant. Ross Taylor Clarkson University, Potsdam, New York University of Twente, Enschede, The Netherlands
 The ChemSep Book Harry A. Koojman Consultant Ross Taylor Clarkson Unversty, Potsdam, New York Unversty of Twente, Enschede, The Netherlands Lbr Books on Demand www.bod.de Copyrght c 2000 by H.A. Koojman
The ChemSep Book Harry A. Koojman Consultant Ross Taylor Clarkson Unversty, Potsdam, New York Unversty of Twente, Enschede, The Netherlands Lbr Books on Demand www.bod.de Copyrght c 2000 by H.A. Koojman
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
ACTM State Calculus Competition Saturday April 30, 2011
 ACTM State Calculus Competton Saturday Aprl 30, 2011 ACTM State Calculus Competton Sprng 2011 Page 1 Instructons: For questons 1 through 25, mark the best answer choce on the answer sheet provde Afterward
ACTM State Calculus Competton Saturday Aprl 30, 2011 ACTM State Calculus Competton Sprng 2011 Page 1 Instructons: For questons 1 through 25, mark the best answer choce on the answer sheet provde Afterward
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
More metrics on cartesian products
 More metrcs on cartesan products If (X, d ) are metrc spaces for 1 n, then n Secton II4 of the lecture notes we defned three metrcs on X whose underlyng topologes are the product topology The purpose of
More metrcs on cartesan products If (X, d ) are metrc spaces for 1 n, then n Secton II4 of the lecture notes we defned three metrcs on X whose underlyng topologes are the product topology The purpose of
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Electrical double layer: revisit based on boundary conditions
 Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
EEE 241: Linear Systems
 EEE : Lnear Systems Summary #: Backpropagaton BACKPROPAGATION The perceptron rule as well as the Wdrow Hoff learnng were desgned to tran sngle layer networks. They suffer from the same dsadvantage: they
EEE : Lnear Systems Summary #: Backpropagaton BACKPROPAGATION The perceptron rule as well as the Wdrow Hoff learnng were desgned to tran sngle layer networks. They suffer from the same dsadvantage: they
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Module 3: The Whole-Process Perspective for Thermochemical Hydrogen
 "Thermodynamc Analyss of Processes for Hydrogen Generaton by Decomposton of Water" by John P. O'Connell Department of Chemcal Engneerng Unversty of Vrgna Charlottesvlle, VA 2294-4741 A Set of Energy Educaton
"Thermodynamc Analyss of Processes for Hydrogen Generaton by Decomposton of Water" by John P. O'Connell Department of Chemcal Engneerng Unversty of Vrgna Charlottesvlle, VA 2294-4741 A Set of Energy Educaton
DUE: WEDS FEB 21ST 2018
 HOMEWORK # 1: FINITE DIFFERENCES IN ONE DIMENSION DUE: WEDS FEB 21ST 2018 1. Theory Beam bendng s a classcal engneerng analyss. The tradtonal soluton technque makes smplfyng assumptons such as a constant
HOMEWORK # 1: FINITE DIFFERENCES IN ONE DIMENSION DUE: WEDS FEB 21ST 2018 1. Theory Beam bendng s a classcal engneerng analyss. The tradtonal soluton technque makes smplfyng assumptons such as a constant
Global Sensitivity. Tuesday 20 th February, 2018
 Global Senstvty Tuesday 2 th February, 28 ) Local Senstvty Most senstvty analyses [] are based on local estmates of senstvty, typcally by expandng the response n a Taylor seres about some specfc values
Global Senstvty Tuesday 2 th February, 28 ) Local Senstvty Most senstvty analyses [] are based on local estmates of senstvty, typcally by expandng the response n a Taylor seres about some specfc values
,..., k N. , k 2. ,..., k i. The derivative with respect to temperature T is calculated by using the chain rule: & ( (5) dj j dt = "J j. k i.
 Suppleentary Materal Dervaton of Eq. 1a. Assue j s a functon of the rate constants for the N coponent reactons: j j (k 1,,..., k,..., k N ( The dervatve wth respect to teperature T s calculated by usng
Suppleentary Materal Dervaton of Eq. 1a. Assue j s a functon of the rate constants for the N coponent reactons: j j (k 1,,..., k,..., k N ( The dervatve wth respect to teperature T s calculated by usng
In this section is given an overview of the common elasticity models.
 Secton 4.1 4.1 Elastc Solds In ths secton s gven an overvew of the common elastcty models. 4.1.1 The Lnear Elastc Sold The classcal Lnear Elastc model, or Hooean model, has the followng lnear relatonshp
Secton 4.1 4.1 Elastc Solds In ths secton s gven an overvew of the common elastcty models. 4.1.1 The Lnear Elastc Sold The classcal Lnear Elastc model, or Hooean model, has the followng lnear relatonshp
MA 323 Geometric Modelling Course Notes: Day 13 Bezier Curves & Bernstein Polynomials
 MA 323 Geometrc Modellng Course Notes: Day 13 Bezer Curves & Bernsten Polynomals Davd L. Fnn Over the past few days, we have looked at de Casteljau s algorthm for generatng a polynomal curve, and we have
MA 323 Geometrc Modellng Course Notes: Day 13 Bezer Curves & Bernsten Polynomals Davd L. Fnn Over the past few days, we have looked at de Casteljau s algorthm for generatng a polynomal curve, and we have
Osmotic pressure and protein binding
 Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Advanced Circuits Topics - Part 1 by Dr. Colton (Fall 2017)
 Advanced rcuts Topcs - Part by Dr. olton (Fall 07) Part : Some thngs you should already know from Physcs 0 and 45 These are all thngs that you should have learned n Physcs 0 and/or 45. Ths secton s organzed
Advanced rcuts Topcs - Part by Dr. olton (Fall 07) Part : Some thngs you should already know from Physcs 0 and 45 These are all thngs that you should have learned n Physcs 0 and/or 45. Ths secton s organzed
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
Chapter One Mixture of Ideal Gases
 herodynacs II AA Chapter One Mxture of Ideal Gases. Coposton of a Gas Mxture: Mass and Mole Fractons o deterne the propertes of a xture, we need to now the coposton of the xture as well as the propertes
herodynacs II AA Chapter One Mxture of Ideal Gases. Coposton of a Gas Mxture: Mass and Mole Fractons o deterne the propertes of a xture, we need to now the coposton of the xture as well as the propertes
PETE 310 Lectures # 24 & 25 Chapter 12 Gas Liquid Equilibrium
 ETE 30 Lectures # 24 & 25 Chapter 2 Gas Lqud Equlbrum Thermal Equlbrum Object A hgh T, Object B low T Intal contact tme Intermedate tme. Later tme Mechancal Equlbrum ressure essels Vale Closed Vale Open
ETE 30 Lectures # 24 & 25 Chapter 2 Gas Lqud Equlbrum Thermal Equlbrum Object A hgh T, Object B low T Intal contact tme Intermedate tme. Later tme Mechancal Equlbrum ressure essels Vale Closed Vale Open
Outlet temperature of a WGS reactor (Stage I) for the conversion of CO, applied for the abatement of CO to a fixed value.
 Department of Energy Poltecnco d Mlano Va Lambruschn 56 MILAN Exercses of Fundamentals of Chemcal Processes Prof. Ganpero Gropp Exercse utlet temperature of a WGS reactor (Stage I for the converson of
Department of Energy Poltecnco d Mlano Va Lambruschn 56 MILAN Exercses of Fundamentals of Chemcal Processes Prof. Ganpero Gropp Exercse utlet temperature of a WGS reactor (Stage I for the converson of
Module 9. Lecture 6. Duality in Assignment Problems
 Module 9 1 Lecture 6 Dualty n Assgnment Problems In ths lecture we attempt to answer few other mportant questons posed n earler lecture for (AP) and see how some of them can be explaned through the concept
Module 9 1 Lecture 6 Dualty n Assgnment Problems In ths lecture we attempt to answer few other mportant questons posed n earler lecture for (AP) and see how some of them can be explaned through the concept
8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS
 SECTION 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS 493 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS All the vector spaces you have studed thus far n the text are real vector spaces because the scalars
SECTION 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS 493 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS All the vector spaces you have studed thus far n the text are real vector spaces because the scalars
Inductance Calculation for Conductors of Arbitrary Shape
 CRYO/02/028 Aprl 5, 2002 Inductance Calculaton for Conductors of Arbtrary Shape L. Bottura Dstrbuton: Internal Summary In ths note we descrbe a method for the numercal calculaton of nductances among conductors
CRYO/02/028 Aprl 5, 2002 Inductance Calculaton for Conductors of Arbtrary Shape L. Bottura Dstrbuton: Internal Summary In ths note we descrbe a method for the numercal calculaton of nductances among conductors
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
Formulas for the Determinant
 page 224 224 CHAPTER 3 Determnants e t te t e 2t 38 A = e t 2te t e 2t e t te t 2e 2t 39 If 123 A = 345, 456 compute the matrx product A adj(a) What can you conclude about det(a)? For Problems 40 43, use
page 224 224 CHAPTER 3 Determnants e t te t e 2t 38 A = e t 2te t e 2t e t te t 2e 2t 39 If 123 A = 345, 456 compute the matrx product A adj(a) What can you conclude about det(a)? For Problems 40 43, use
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
y i x P vap 10 A T SOLUTION TO HOMEWORK #7 #Problem
 SOLUTION TO HOMEWORK #7 #roblem 1 10.1-1 a. In order to solve ths problem, we need to know what happens at the bubble pont; at ths pont, the frst bubble s formed, so we can assume that all of the number
SOLUTION TO HOMEWORK #7 #roblem 1 10.1-1 a. In order to solve ths problem, we need to know what happens at the bubble pont; at ths pont, the frst bubble s formed, so we can assume that all of the number
PART I: MULTIPLE CHOICE (32 questions, each multiple choice question has a 2-point value, 64 points total).
 CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
COMPOSITE BEAM WITH WEAK SHEAR CONNECTION SUBJECTED TO THERMAL LOAD
 COMPOSITE BEAM WITH WEAK SHEAR CONNECTION SUBJECTED TO THERMAL LOAD Ákos Jósef Lengyel, István Ecsed Assstant Lecturer, Professor of Mechancs, Insttute of Appled Mechancs, Unversty of Mskolc, Mskolc-Egyetemváros,
COMPOSITE BEAM WITH WEAK SHEAR CONNECTION SUBJECTED TO THERMAL LOAD Ákos Jósef Lengyel, István Ecsed Assstant Lecturer, Professor of Mechancs, Insttute of Appled Mechancs, Unversty of Mskolc, Mskolc-Egyetemváros,
Name: SID: Discussion Session:
 Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Linear Approximation with Regularization and Moving Least Squares
 Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
CSci 6974 and ECSE 6966 Math. Tech. for Vision, Graphics and Robotics Lecture 21, April 17, 2006 Estimating A Plane Homography
 CSc 6974 and ECSE 6966 Math. Tech. for Vson, Graphcs and Robotcs Lecture 21, Aprl 17, 2006 Estmatng A Plane Homography Overvew We contnue wth a dscusson of the major ssues, usng estmaton of plane projectve
CSc 6974 and ECSE 6966 Math. Tech. for Vson, Graphcs and Robotcs Lecture 21, Aprl 17, 2006 Estmatng A Plane Homography Overvew We contnue wth a dscusson of the major ssues, usng estmaton of plane projectve
McCabe-Thiele Diagrams for Binary Distillation
 McCabe-Thele Dagrams for Bnary Dstllaton Tore Haug-Warberg Dept. of Chemcal Engneerng August 31st, 2005 F V 1 V 2 L 1 V n L n 1 V n+1 L n V N L N 1 L N L 0 VN+1 Q < 0 D Q > 0 B FIGURE 1: Smplfed pcture
McCabe-Thele Dagrams for Bnary Dstllaton Tore Haug-Warberg Dept. of Chemcal Engneerng August 31st, 2005 F V 1 V 2 L 1 V n L n 1 V n+1 L n V N L N 1 L N L 0 VN+1 Q < 0 D Q > 0 B FIGURE 1: Smplfed pcture
3.1 Expectation of Functions of Several Random Variables. )' be a k-dimensional discrete or continuous random vector, with joint PMF p (, E X E X1 E X
 Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Poisson brackets and canonical transformations
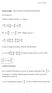 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Difference Equations
 Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Chapter 5 rd Law of Thermodynamics
 Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS. Version 2 ECE IIT, Kharagpur
 Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
How Strong Are Weak Patents? Joseph Farrell and Carl Shapiro. Supplementary Material Licensing Probabilistic Patents to Cournot Oligopolists *
 How Strong Are Weak Patents? Joseph Farrell and Carl Shapro Supplementary Materal Lcensng Probablstc Patents to Cournot Olgopolsts * September 007 We study here the specal case n whch downstream competton
How Strong Are Weak Patents? Joseph Farrell and Carl Shapro Supplementary Materal Lcensng Probablstc Patents to Cournot Olgopolsts * September 007 We study here the specal case n whch downstream competton
Estimation of the composition of the liquid and vapor streams exiting a flash unit with a supercritical component
 Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
1 Derivation of Rate Equations from Single-Cell Conductance (Hodgkin-Huxley-like) Equations
 Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
The Geometry of Logit and Probit
 The Geometry of Logt and Probt Ths short note s meant as a supplement to Chapters and 3 of Spatal Models of Parlamentary Votng and the notaton and reference to fgures n the text below s to those two chapters.
The Geometry of Logt and Probt Ths short note s meant as a supplement to Chapters and 3 of Spatal Models of Parlamentary Votng and the notaton and reference to fgures n the text below s to those two chapters.
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Week3, Chapter 4. Position and Displacement. Motion in Two Dimensions. Instantaneous Velocity. Average Velocity
 Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Modelli Clamfim Equazione del Calore Lezione ottobre 2014
 CLAMFIM Bologna Modell 1 @ Clamfm Equazone del Calore Lezone 17 15 ottobre 2014 professor Danele Rtell danele.rtell@unbo.t 1/24? Convoluton The convoluton of two functons g(t) and f(t) s the functon (g
CLAMFIM Bologna Modell 1 @ Clamfm Equazone del Calore Lezone 17 15 ottobre 2014 professor Danele Rtell danele.rtell@unbo.t 1/24? Convoluton The convoluton of two functons g(t) and f(t) s the functon (g
χ x B E (c) Figure 2.1.1: (a) a material particle in a body, (b) a place in space, (c) a configuration of the body
 Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Assortment Optimization under MNL
 Assortment Optmzaton under MNL Haotan Song Aprl 30, 2017 1 Introducton The assortment optmzaton problem ams to fnd the revenue-maxmzng assortment of products to offer when the prces of products are fxed.
Assortment Optmzaton under MNL Haotan Song Aprl 30, 2017 1 Introducton The assortment optmzaton problem ams to fnd the revenue-maxmzng assortment of products to offer when the prces of products are fxed.
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Expectation-Maximization Algorithm
 The Expectaton-Maxmaton Algorthm Charles Elan elan@cs.ucsd.edu November 16, 2007 Ths chapter explans the EM algorthm at multple levels of generalty. Secton 1 gves the standard hgh-level verson of the algorthm.
The Expectaton-Maxmaton Algorthm Charles Elan elan@cs.ucsd.edu November 16, 2007 Ths chapter explans the EM algorthm at multple levels of generalty. Secton 1 gves the standard hgh-level verson of the algorthm.
Indeterminate pin-jointed frames (trusses)
 Indetermnate pn-jonted frames (trusses) Calculaton of member forces usng force method I. Statcal determnacy. The degree of freedom of any truss can be derved as: w= k d a =, where k s the number of all
Indetermnate pn-jonted frames (trusses) Calculaton of member forces usng force method I. Statcal determnacy. The degree of freedom of any truss can be derved as: w= k d a =, where k s the number of all
CHARACTERISTICS OF COMPLEX SEPARATION SCHEMES AND AN ERROR OF SEPARATION PRODUCTS OUTPUT DETERMINATION
 Górnctwo Geonżynera Rok 0 Zeszyt / 006 Igor Konstantnovch Mladetskj * Petr Ivanovch Plov * Ekaterna Nkolaevna Kobets * Tasya Igorevna Markova * CHARACTERISTICS OF COMPLEX SEPARATION SCHEMES AND AN ERROR
Górnctwo Geonżynera Rok 0 Zeszyt / 006 Igor Konstantnovch Mladetskj * Petr Ivanovch Plov * Ekaterna Nkolaevna Kobets * Tasya Igorevna Markova * CHARACTERISTICS OF COMPLEX SEPARATION SCHEMES AND AN ERROR
Numerical Heat and Mass Transfer
 Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
2 Finite difference basics
 Numersche Methoden 1, WS 11/12 B.J.P. Kaus 2 Fnte dfference bascs Consder the one- The bascs of the fnte dfference method are best understood wth an example. dmensonal transent heat conducton equaton T
Numersche Methoden 1, WS 11/12 B.J.P. Kaus 2 Fnte dfference bascs Consder the one- The bascs of the fnte dfference method are best understood wth an example. dmensonal transent heat conducton equaton T
APPENDIX 2 FITTING A STRAIGHT LINE TO OBSERVATIONS
 Unversty of Oulu Student Laboratory n Physcs Laboratory Exercses n Physcs 1 1 APPEDIX FITTIG A STRAIGHT LIE TO OBSERVATIOS In the physcal measurements we often make a seres of measurements of the dependent
Unversty of Oulu Student Laboratory n Physcs Laboratory Exercses n Physcs 1 1 APPEDIX FITTIG A STRAIGHT LIE TO OBSERVATIOS In the physcal measurements we often make a seres of measurements of the dependent
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
V. Electrostatics. Lecture 25: Diffuse double layer structure
 V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
Non-Ideality Through Fugacity and Activity
 Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
LNG CARGO TRANSFER CALCULATION METHODS AND ROUNDING-OFFS
 CARGO TRANSFER CALCULATION METHODS AND ROUNDING-OFFS CONTENTS 1. Method for determnng transferred energy durng cargo transfer. Calculatng the transferred energy.1 Calculatng the gross transferred energy.1.1
CARGO TRANSFER CALCULATION METHODS AND ROUNDING-OFFS CONTENTS 1. Method for determnng transferred energy durng cargo transfer. Calculatng the transferred energy.1 Calculatng the gross transferred energy.1.1
