...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
|
|
|
- Denis McCarthy
- 5 years ago
- Views:
Transcription
1 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up atoms) pb Superflud Helum (Many partcles n quantum ground state) Superconductor (Many pared electrons n same quantum state) Lecture 17 November 16, / 21
2 Open systems How to deal wth partcle exchange? Extra partcles N N + dn Energy ganed by addng a partcle? du = TdS PdV + µdn wth µ = ( U ) N S,V Mathematcally µdn equvalent to ncludng an extra type of work. Lecture 17 November 16, / 21
3 Chemcal Potental µ and thermodynamc potentals Defntons unchanged F = U TS H = U + PV, G = H TS = F + PV Therefore dg = SdT + VdP + µdn etc. µ = Also, for many speces µ = ( U N ) ( U ) ( F ) ( G ) N = S,V N = T,V N T,P S,V,N j = ( F N ) µ s useful wth ANY boundary condton T,V,N j = ( G N )P,T,N j Lecture 17 November 16, / 21
4 Chemcal potental and Gbbs Free Energy Consder the two ways to wrte the free energy of αn partcles: αg(p, T, N) = G(P, T, αn) Take the dervatve of both sdes wth respect to α: ( ) G(P, T, αn) G = α P,T ( ) G(P, T, αn) = N (αn) = N ( ) G(P, T, αn) α N ( ) G(P, T, N) = N N P,T P,T P,T G = Nµ for a pure substance Lecture 17 November 16, / 21
5 State functons n terms of µ G = Nµ for a pure substance The chemcal potental for one speces s the specfc Gbbs free energy! µ = G(T, P, N)/N we fnd that: dµ = sdt + vdp µ can be wrtten as a functon of P and T only for a pure substance. s = ( µ/ T ) p,n v = ( µ/ p) T,N ; for a pure substance Lecture 17 November 16, / 21
6 Contrbuton to µ Be careful wth extensve ( N) and ntensve quattes. dg = N(vdP sdt ) + µdn d(g/n) = dg = dµ = (vdp sdt ) The fnal term vanshed for large N! µ s typcally ncreased by rasng the pressure, or lowerng the temperature. These are sngle-phase relatons: G s also reduced by spontaneous chemcal reactons. Lecture 17 November 16, / 21
7 A closed system equlbrates Closed system, two parts, A and B, ntally out of equlbrum. Va Ua Na Vb Ub Nb du A + du B = 0, dn A + dn B = 0, dv A + dv B = 0. Second law: ds A + ds B 0 ds(u, V, N) = du T + P T dv µ T dn Whch, when appled to the two-part system gves, ( 1 1 ) ( PA du A + P ) ( B µa dv A µ ) B dn A 0 T A T B T A T B T A T B Hold that thought... Lecture 17 November 16, / 21
8 Flow of heat and partcles From prevous slde... Va Ua Na Vb Ub Nb ( 1 1 ) ( PA du A + P ) ( B µa dv A µ ) B dn A 0 T A T B T A T B T A T B At equlbrum T A = T B, P A = P B and µ A = µ B. energy flows from hot to cold untl T A = T B. volume moves from hgh to low pressure (for equal T) partcles flow from hgh to low chemcal potental (for equal T). Partcles (mass) flow along gradents of the chemcal potental. Lecture 17 November 16, / 21
9 Phase separaton n Planets Chemcal potental ncludes gravty: µ = u Ts + Pv + mg r h. Heavy atoms fall to the bottom: can be drawn up f soluble (u) Lecture 17 November 16, / 21
10 Chemstry Do I really have to do all these? Lecture 17 November 16, / 21
11 Chemstry of an solated system Consder Isolated system : du = 0, dv = 0, S(U, V, N 1, N 2...) TdS = du + PdV µ 1 dn 1 µ 2 dn 2 µ 3 dn 3... Equlbrum (maxmum entropy): ds = 0 µ dn = 0 solated system at equlbrum Lecture 17 November 16, / 21
12 Thermodynamcs n Chemstry Molecules react to form other molecules. A chemcal potental can P be defned for each. du = TdS PdV + P µ dn dg = SdT + VdP + µ dn Total Gbbs, G s also the sum of the chemcal potentals X X G= µ N = dg = N dµ + µ dn Equatng these expressons for dg yelds the Gbbs-Duhem relaton: X N dµ = S dt + V dp Ths gves balance of concentraton of components Lecture 17 November 16, / 21
13 Chemcal equlbrum of a reactng system Closed system n equlbrum wth a T, P reservor. Amounts of each component N are all nternal degrees of freedom. Mnmse G (set dg = 0) dg = µ dn = 0 closed system at equlbrum, fxed T The dn are constraned by the reacton equaton b dn = 0 e.g. H O 2 H 2 O leads to dn H dn O 2 dn H2 O = 0 Combnng mnmsaton of G and constrant: chemcal process equlbrum: b µ = 0 e.g. µ H µ O 2 = µ H2 O Reacton contnues untl chemcal potentals are equal. Lecture 17 November 16, / 21
14 Chemcal Potental for sngle component deal gas dg = VdP SdT + µdn For constant N, ntegratng g from T=0, P=P 0, we get dµ = dg = ( ) d(g/n) dt P,N ( ) d(g/n) dt + dp T,N dp = (S/N)dT + (V /N)dP = c P dt + RT P dp µ = c P T + RT ln(p/p 0 ) Where we used the Thrd Law for S(T=0)=0. Lecture 17 November 16, / 21
15 Statng the obvous Non-nteractng objects n the same system can be treated as ndependent deal gases, thanks to... Dalton s Law Total pressure s the sum of partal pressures P = p Raoult s Law Partal pressure s proportonal to concentraton p V = N RT e.g. dlute chemcals n soluton, photons n a cavty etc. Lecture 17 November 16, / 21
16 Example: Solublty Surroundngs, ncl. external chemcal potental T 0, P 0, µ (0), Epecfc enthalpy of soluton δh (bndng to solvent). Fnd concentraton, c, of n the system at equlbrum? Equlbrum system T = T 0, P 0 = p N/N, µ = µ (0) µ (0) Usng Raoult s Law (p = P 0 N /N = c P 0 ) = µ = δh + RT ln p /P 0 Rearrangng: c = exp(µ (0) δh)/rt If nsoluble: δh µ (0) there s stll some n soluton, If soluble, δh < µ (0) ( s soluble ) the concentraton s larger than 1, (not an deal gas) Lecture 17 November 16, / 21
17 Reacton between deal gases at fxed T, N Dlute soluton Ideal Gas = Ideal Soluton. Ideal gas mxture: P = p Raoult s Law: p V = N RT For sothermal reacton dg = V dp SdT + µ dn dg dµ = RT dp p Get change n µ wth pressure by ntegratng from reference state µ 0, µ = µ 0 + RT ln[p /p 0 ] Substtutng nto equlbrum condton b µ = 0 Lecture 17 November 16, / 21
18 Equlbrum constant...from prevous b (µ 0 + RT ln[p /p 0 ]) = 0 Each reacton has an Equlbrum constant (whch depends on T). [ ] ln(k) ln (p /p 0 ) b = b µ 0 RT = K(T ) (p /p 0 ) b = exp[ b µ 0 ] reference state) RT If we consder T=0, Gbbs/µ s the same as Enthalpy At T=0, b µ s just the enthalpy of reacton. K(T) looks lke the Boltzmann factor exp E/kT. Lecture 17 November 16, / 21
19 Measurng K(T) wthout a chemcalpotentalometer Dfferentatng wrt T and usng s = µ/ T P ( (ln K) ) T p = 1 RT 2 b (µ 0 + Ts 0 ) = b H 0 RT 2 b H 0 s just the molar enthalpy of reacton, whch s readly measurable. Smlar to the Gbbs-Helmholtz Equaton (handn queston). Lecture 17 November 16, / 21
20 Chemstry made easy Need only measure Chemcal Potental µ for each component (N measurements), not K for every possble reacton (N! measurements) Lecture 17 November 16, / 21
21 The Chemcal Potental s... The extra energy from addng a partcle du = TdS PdV + µdn The specfc Gbbs µ = g for a pure substance. The quantty whch drves partcle flow. The quantty whch defnes chemcal equlbrum All of the above! Lecture 17 November 16, / 21
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
a for save as PDF Chemistry 163B Introduction to Multicomponent Systems and Partial Molar Quantities
 a for save as PDF Chemstry 163B Introducton to Multcomponent Systems and Partal Molar Quanttes 1 the problem of partal mmolar quanttes mx: 10 moles ethanol C 2 H 5 OH (580 ml) wth 1 mole water H 2 O (18
a for save as PDF Chemstry 163B Introducton to Multcomponent Systems and Partal Molar Quanttes 1 the problem of partal mmolar quanttes mx: 10 moles ethanol C 2 H 5 OH (580 ml) wth 1 mole water H 2 O (18
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
NAME and Section No.
 Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Chapter 5. Simple Mixtures Fall Semester Physical Chemistry 1 (CHM2201)
 Chapter 5. Simple Mixtures 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The thermodynamic description of mixtures 5.1 Partial molar quantities 5.2 The thermodynamic of Mixing 5.3 The chemical
Chapter 5. Simple Mixtures 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The thermodynamic description of mixtures 5.1 Partial molar quantities 5.2 The thermodynamic of Mixing 5.3 The chemical
Chapter 18, Part 1. Fundamentals of Atmospheric Modeling
 Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Effect of adding an ideal inert gas, M
 Effect of adding an ideal inert gas, M Add gas M If there is no change in volume, then the partial pressures of each of the ideal gas components remains unchanged by the addition of M. If the reaction
Effect of adding an ideal inert gas, M Add gas M If there is no change in volume, then the partial pressures of each of the ideal gas components remains unchanged by the addition of M. If the reaction
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
TP A SOLUTION. For an ideal monatomic gas U=3/2nRT, Since the process is at constant pressure Q = C. giving ) =1000/(5/2*8.31*10)
 T A SOLUTION For an deal monatomc gas U/nRT, Snce the process s at constant pressure Q C pn T gvng a: n Q /( 5 / R T ) /(5/*8.*) C V / R and C / R + R 5 / R. U U / nr T (/ ) R T ( Q / 5 / R T ) Q / 5 Q
T A SOLUTION For an deal monatomc gas U/nRT, Snce the process s at constant pressure Q C pn T gvng a: n Q /( 5 / R T ) /(5/*8.*) C V / R and C / R + R 5 / R. U U / nr T (/ ) R T ( Q / 5 / R T ) Q / 5 Q
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Osmotic pressure and protein binding
 Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Name: SID: Discussion Session:
 Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
V T for n & P = constant
 Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
3) Thermodynamic equation to characterize interfaces
 3) Thermodynamc equaton to characterze nterfaces 3.1) Gbbs Model Realty: rapd contnuous change of chemcal and thermodynamc propertes Replaced by model (constant propertes up to the surface) uv bulk uv
3) Thermodynamc equaton to characterze nterfaces 3.1) Gbbs Model Realty: rapd contnuous change of chemcal and thermodynamc propertes Replaced by model (constant propertes up to the surface) uv bulk uv
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
between standard Gibbs free energies of formation for products and reactants, ΔG! R = ν i ΔG f,i, we
 hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chapter 3. Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc.
 Chapter 3 Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc. Concepts Energy functions F and G Chemical potential, µ Partial Molar properties
Chapter 3 Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc. Concepts Energy functions F and G Chemical potential, µ Partial Molar properties
Lecture 2 Grand Canonical Ensemble GCE
 Lecture 2 Grand Canoncal Ensemble GCE 2.1 hermodynamc Functons Contnung on from last day we also note that thus, dω = df dµ µd = Sd P dv dµ (2.1) P = V = S = From the expresson for the entropy, we therefore
Lecture 2 Grand Canoncal Ensemble GCE 2.1 hermodynamc Functons Contnung on from last day we also note that thus, dω = df dµ µd = Sd P dv dµ (2.1) P = V = S = From the expresson for the entropy, we therefore
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Solution Thermodynamics
 CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Chemistry 163B Free Energy and Equilibrium E&R ( ch 6)
 Chemstry 163B Free Energy and Equlbrum E&R ( ch 6) 1 ΔG reacton and equlbrum (frst pass) 1. ΔG < spontaneous ( natural, rreversble) ΔG = equlbrum (reversble) ΔG > spontaneous n reverse drecton. ΔG = ΔHΔS
Chemstry 163B Free Energy and Equlbrum E&R ( ch 6) 1 ΔG reacton and equlbrum (frst pass) 1. ΔG < spontaneous ( natural, rreversble) ΔG = equlbrum (reversble) ΔG > spontaneous n reverse drecton. ΔG = ΔHΔS
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
Introduction to Interfacial Segregation. Xiaozhe Zhang 10/02/2015
 Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Chapter 5 rd Law of Thermodynamics
 Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Envr 210, Chapter 3, Intermolecular forces and partitioning Free energies and equilibrium partitioning chemical potential fugacity activity coef.
 Envr 20, Chapter 3, Intermolecular forces and parttonng Free energes and equlbrum parttonng chemcal potental fugacty actvty coef. phase transfer- actvty coef and fugactes more on free energes and equlbrum
Envr 20, Chapter 3, Intermolecular forces and parttonng Free energes and equlbrum parttonng chemcal potental fugacty actvty coef. phase transfer- actvty coef and fugactes more on free energes and equlbrum
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
4) It is a state function because enthalpy(h), entropy(s) and temperature (T) are state functions.
 Chemical Thermodynamics S.Y.BSc. Concept of Gibb s free energy and Helmholtz free energy a) Gibb s free energy: 1) It was introduced by J.Willard Gibb s to account for the work of expansion due to volume
Chemical Thermodynamics S.Y.BSc. Concept of Gibb s free energy and Helmholtz free energy a) Gibb s free energy: 1) It was introduced by J.Willard Gibb s to account for the work of expansion due to volume
Homework Chapter 21 Solutions!!
 Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Some properties of the Helmholtz free energy
 Some properties of the Helmholtz free energy Energy slope is T U(S, ) From the properties of U vs S, it is clear that the Helmholtz free energy is always algebraically less than the internal energy U.
Some properties of the Helmholtz free energy Energy slope is T U(S, ) From the properties of U vs S, it is clear that the Helmholtz free energy is always algebraically less than the internal energy U.
Physical Chemistry I for Biochemists. Chem340. Lecture 16 (2/18/11)
 hyscal Chemstry I or Bochemsts Chem34 Lecture 16 (/18/11) Yoshtaka Ish Ch4.6, Ch5.1-5.5 & HW5 4.6 Derental Scannng Calormetry (Derental hermal Analyss) sample = C p, s d s + dh uson = ( s )Kdt, [1] where
hyscal Chemstry I or Bochemsts Chem34 Lecture 16 (/18/11) Yoshtaka Ish Ch4.6, Ch5.1-5.5 & HW5 4.6 Derental Scannng Calormetry (Derental hermal Analyss) sample = C p, s d s + dh uson = ( s )Kdt, [1] where
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
Non-Ideality Through Fugacity and Activity
 Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Chemistry 163B Introduction to Multicomponent Systems and Partial Molar Quantities
 Chemstry 163 Itroducto to Multcompoet Systems ad Partal Molar Quattes 1 the problem of partal mmolar quattes mx: 10 moles ethaol C H 5 OH (580 ml) wth 1 mole water H O (18 ml) get (580+18)=598 ml of soluto?
Chemstry 163 Itroducto to Multcompoet Systems ad Partal Molar Quattes 1 the problem of partal mmolar quattes mx: 10 moles ethaol C H 5 OH (580 ml) wth 1 mole water H O (18 ml) get (580+18)=598 ml of soluto?
,..., k N. , k 2. ,..., k i. The derivative with respect to temperature T is calculated by using the chain rule: & ( (5) dj j dt = "J j. k i.
 Suppleentary Materal Dervaton of Eq. 1a. Assue j s a functon of the rate constants for the N coponent reactons: j j (k 1,,..., k,..., k N ( The dervatve wth respect to teperature T s calculated by usng
Suppleentary Materal Dervaton of Eq. 1a. Assue j s a functon of the rate constants for the N coponent reactons: j j (k 1,,..., k,..., k N ( The dervatve wth respect to teperature T s calculated by usng
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
Problem Points Score Total 100
 Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
The Euler Equation. Using the additive property of the internal energy U, we can derive a useful thermodynamic relation the Euler equation.
 The Euler Equation Using the additive property of the internal energy U, we can derive a useful thermodynamic relation the Euler equation. Let us differentiate this extensivity condition with respect to
The Euler Equation Using the additive property of the internal energy U, we can derive a useful thermodynamic relation the Euler equation. Let us differentiate this extensivity condition with respect to
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Using Spectrophotometric Methods to Determine an Equilibrium Constant Prelab
 Usng Spectrophotometrc Methods to Determne an Equlbrum Constant Prelab 1. What s the purpose of ths experment? 2. Wll the absorbance of the ulbrum mxture (at 447 nm) ncrease or decrease as Fe soluton s
Usng Spectrophotometrc Methods to Determne an Equlbrum Constant Prelab 1. What s the purpose of ths experment? 2. Wll the absorbance of the ulbrum mxture (at 447 nm) ncrease or decrease as Fe soluton s
MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7
 2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
PHYS 705: Classical Mechanics. Newtonian Mechanics
 1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Poisson brackets and canonical transformations
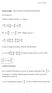 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Equilibrium Statistical Mechanics
 Equlbrum Statstcal Mechancs Ref. Davdson, Statstcal Mechancs McGraw-Hll, 96 We wsh to study plasmas n equlbrum for at least two reasons: (a) Many tmes real plasmas are near true equlbrum, at least locally
Equlbrum Statstcal Mechancs Ref. Davdson, Statstcal Mechancs McGraw-Hll, 96 We wsh to study plasmas n equlbrum for at least two reasons: (a) Many tmes real plasmas are near true equlbrum, at least locally
CHEMISTRY Midterm #2 answer key October 25, 2005
 CHEMISTRY 123-01 Mdterm #2 answer key October 25, 2005 Statstcs: Average: 70 pts (70%); Hghest: 97 pts (97%); Lowest: 33 pts (33%) Number of students performng at or above average: 62 (63%) Number of students
CHEMISTRY 123-01 Mdterm #2 answer key October 25, 2005 Statstcs: Average: 70 pts (70%); Hghest: 97 pts (97%); Lowest: 33 pts (33%) Number of students performng at or above average: 62 (63%) Number of students
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
2. Introduction to Thermodynamics
 . Introducton to hermodynamcs.a..b..c..d..e..f..g..h. Introductory Remarks State Varables and Exact Dfferentals Some Mechancal Equatons of State he Laws of hermodynamcs Fundamental Equaton of hermodynamcs
. Introducton to hermodynamcs.a..b..c..d..e..f..g..h. Introductory Remarks State Varables and Exact Dfferentals Some Mechancal Equatons of State he Laws of hermodynamcs Fundamental Equaton of hermodynamcs
Chemical Reaction Engineering
 Lecture 19 hemcal Reacton Engneerng (RE) s the feld that studes the rates and mechansms of chemcal reactons and the desgn of the reactors n whch they take place. Web Lecture 19 lass Lecture 17 uesday 3/19/2013
Lecture 19 hemcal Reacton Engneerng (RE) s the feld that studes the rates and mechansms of chemcal reactons and the desgn of the reactors n whch they take place. Web Lecture 19 lass Lecture 17 uesday 3/19/2013
Lecture Note 3. Eshelby s Inclusion II
 ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
Outlet temperature of a WGS reactor (Stage I) for the conversion of CO, applied for the abatement of CO to a fixed value.
 Department of Energy Poltecnco d Mlano Va Lambruschn 56 MILAN Exercses of Fundamentals of Chemcal Processes Prof. Ganpero Gropp Exercse utlet temperature of a WGS reactor (Stage I for the converson of
Department of Energy Poltecnco d Mlano Va Lambruschn 56 MILAN Exercses of Fundamentals of Chemcal Processes Prof. Ganpero Gropp Exercse utlet temperature of a WGS reactor (Stage I for the converson of
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Thermodynamic condition for equilibrium between two phases a and b is G a = G b, so that during an equilibrium phase change, G ab = G a G b = 0.
 CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
The ChemSep Book. Harry A. Kooijman Consultant. Ross Taylor Clarkson University, Potsdam, New York University of Twente, Enschede, The Netherlands
 The ChemSep Book Harry A. Koojman Consultant Ross Taylor Clarkson Unversty, Potsdam, New York Unversty of Twente, Enschede, The Netherlands Lbr Books on Demand www.bod.de Copyrght c 2000 by H.A. Koojman
The ChemSep Book Harry A. Koojman Consultant Ross Taylor Clarkson Unversty, Potsdam, New York Unversty of Twente, Enschede, The Netherlands Lbr Books on Demand www.bod.de Copyrght c 2000 by H.A. Koojman
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
Electrochemistry Thermodynamics
 CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
Chapter One Mixture of Ideal Gases
 herodynacs II AA Chapter One Mxture of Ideal Gases. Coposton of a Gas Mxture: Mass and Mole Fractons o deterne the propertes of a xture, we need to now the coposton of the xture as well as the propertes
herodynacs II AA Chapter One Mxture of Ideal Gases. Coposton of a Gas Mxture: Mass and Mole Fractons o deterne the propertes of a xture, we need to now the coposton of the xture as well as the propertes
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Lagrange Multipliers. A Somewhat Silly Example. Monday, 25 September 2013
 Lagrange Multplers Monday, 5 September 013 Sometmes t s convenent to use redundant coordnates, and to effect the varaton of the acton consstent wth the constrants va the method of Lagrange undetermned
Lagrange Multplers Monday, 5 September 013 Sometmes t s convenent to use redundant coordnates, and to effect the varaton of the acton consstent wth the constrants va the method of Lagrange undetermned
Lecture 3 Examples and Problems
 Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
The Standard Gibbs Energy Change, G
 The Standard Gibbs Energy Change, G S univ = S surr + S sys S univ = H sys + S sys T S univ = H sys TS sys G sys = H sys TS sys Spontaneous reaction: S univ >0 G sys < 0 More observations on G and Gº I.
The Standard Gibbs Energy Change, G S univ = S surr + S sys S univ = H sys + S sys T S univ = H sys TS sys G sys = H sys TS sys Spontaneous reaction: S univ >0 G sys < 0 More observations on G and Gº I.
More on phase diagram, chemical potential, and mixing
 More on phase diagram, chemical potential, and mixing Narayanan Kurur Department of Chemistry IIT Delhi 13 July 2013 Melting point changes with P ( ) Gα P T = V α V > 0 = G α when P Intersection point
More on phase diagram, chemical potential, and mixing Narayanan Kurur Department of Chemistry IIT Delhi 13 July 2013 Melting point changes with P ( ) Gα P T = V α V > 0 = G α when P Intersection point
y i x P vap 10 A T SOLUTION TO HOMEWORK #7 #Problem
 SOLUTION TO HOMEWORK #7 #roblem 1 10.1-1 a. In order to solve ths problem, we need to know what happens at the bubble pont; at ths pont, the frst bubble s formed, so we can assume that all of the number
SOLUTION TO HOMEWORK #7 #roblem 1 10.1-1 a. In order to solve ths problem, we need to know what happens at the bubble pont; at ths pont, the frst bubble s formed, so we can assume that all of the number
CHEMICAL ENGINEERING
 Postal Correspondence GATE & PSUs -MT To Buy Postal Correspondence Packages call at 0-9990657855 1 TABLE OF CONTENT S. No. Ttle Page no. 1. Introducton 3 2. Dffuson 10 3. Dryng and Humdfcaton 24 4. Absorpton
Postal Correspondence GATE & PSUs -MT To Buy Postal Correspondence Packages call at 0-9990657855 1 TABLE OF CONTENT S. No. Ttle Page no. 1. Introducton 3 2. Dffuson 10 3. Dryng and Humdfcaton 24 4. Absorpton
Conservation of Angular Momentum = "Spin"
 Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
II. Equilibrium Thermodynamics. Lecture 8: The Nernst Equation
 II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2013
 Lecture 8/8/3 Unversty o Washngton Departent o Chestry Chestry 45/456 Suer Quarter 3 A. The Gbbs-Duhe Equaton Fro Lecture 7 and ro the dscusson n sectons A and B o ths lecture, t s clear that the actvty
Lecture 8/8/3 Unversty o Washngton Departent o Chestry Chestry 45/456 Suer Quarter 3 A. The Gbbs-Duhe Equaton Fro Lecture 7 and ro the dscusson n sectons A and B o ths lecture, t s clear that the actvty
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Mass Transfer Processes
 Mass Transfer Processes S. Majd Hassanzadeh Department of Earth Scences Faculty of Geoscences Utrecht Unversty Outlne: 1. Measures of Concentraton 2. Volatlzaton and Dssoluton 3. Adsorpton Processes 4.
Mass Transfer Processes S. Majd Hassanzadeh Department of Earth Scences Faculty of Geoscences Utrecht Unversty Outlne: 1. Measures of Concentraton 2. Volatlzaton and Dssoluton 3. Adsorpton Processes 4.
Be true to your work, your word, and your friend.
 Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Transfer Functions. Convenient representation of a linear, dynamic model. A transfer function (TF) relates one input and one output: ( ) system
 Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
