STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
|
|
|
- Posy Wilcox
- 6 years ago
- Views:
Transcription
1 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle, or even lvng cells obey certan mcroscopc laws wth specfed nterpartcle nteractons, what are the observable propertes of a macroscopc system contanng a large number of such partcles? Examples of mcroscopc and macroscopc varables are gven below for /6th mole of an n- component monoatomc gas (approx. 0 3 molecules obeyng classcal mechancs. Mcroscopc varables 3x0 3 postons (x,y,z 3x0 3 veloctes (x,y,z Macroscopc varables n+ ndependent thermodynamc varables - e.g. for n,,t PHASE SPACE The multdmensonal space defned by the mcroscopc varables of a system. n the example above, t would be a 6x0 3 -dmensonal space, wth ndependent varables (r,p (r,r,r 3,...,r ;p,p,p 3,...,p where bold symbols ndcate vectors, r s the poston and p the momentum (p mu. Momenta are used rather than veloctes, because the classcal and, especally, the quantum equatons of change are more elegantly wrtten n terms of momenta. The evoluton of such a system n tme s descrbed by ewton's laws: dr du u ; ( r, r, r3..., r m dt dt r where U s the total potental energy of the system, the gradent of whch s mnus the force. For a much smpler system, a one-dmensonal harmonc oscllator, phase space s two dmensonal, wth coordnates the poston and the momentum varables. ESEMBLES An ensemble s a collecton of all mcrostates of a system, consstent wth the constrants wth whch we characterze a system macroscopcally. For example, a collecton of all possble states of Some materal n ths secton s derved from Chap. 3, D. Chandler, "ntroducton to modern statstcal mechancs", Oxford Unversty Press, 987.
2 CHE 54 Ensembles the 0 3 molecules of gas n the contaner of volume wth a gven total energy U s a statstcal mechancal ensemble. For the one-dmensonal harmonc oscllator wth a gven energy, the phase space s a crcular trajectory n poston and momentum space. ERGODC HYPOTHESS Expermental measurements on any macroscopc system are performed by observng a system for a fnte perod of tme, durng whch the system samples a very large number of possble mcrostates. n order to connect the measured propertes wth the propertes calculated from statstcal mechancs, we have to assume that: For suffcently long tmes, a macroscopc system wll evolve through (or wll come arbtrarly close to all mcroscopc states consstent wth the macroscopc constrants we mpose n order to control the system. n other words, expermental measurements (performed by tme averages and ensemble averages are equvalent. The "ergodc hypothess" s more than just a hypothess. t s a general property of almost all real systems composed of a large number of partcles. The ergodc hypothess s equvalent to the postulate of classcal thermodynamcs that there exst stable equlbrum states fully characterzed by n+ ndependent macroscopc varables. ote that the ergodc hypothess does not state anythng about the relatve probabltes of observng gven states - t just states that all states wll eventually be observed. A general property F wll thus obey the followng rule: Fobserved Σ P v F v <F> probablty of value of property ensemble average fndng the system F n mcrostate v n mcrostate v MCROCAOCAL ESEMBLE: COSTAT U,, Basc Postulate of Statstcal Mechancs: For an solated system at constant U, and all mcroscopc states of a system are equally lkely at thermodynamc equlbrum. f Ω(,U s number of mcrostates wth energy U, then the probablty of mcrostate v, accordng to the postulate above, s P v /Ω (,U The concept of densty of states s really of quantum mechancal orgn. However, t can be ntroduced wthout resort to quantum mechancs by assumng that a gven contaner s parttoned
3 CHE 54 Ensembles 3 nto many small compartments, and the veloctes of molecules are dscretzed n terms of the possble drectons and magntudes. Example: Balls n a box Let us consder a very smple example, namely a box wth 0 slots, each contanng a ball that can be at the bottom of the slot (wth energy 0, or at one of two hgher levels, wth energes + and +, respectvely. The number of mcrostates for ths system depends on the overall energy of the box. There are: 0 state wth energy 0; 0 states wth energy +; states wth energy + and so on. As you can see, the number of mcrostates for even a smple system ncreases rapdly wth the energy of the system. Defnton of Entropy Let us defne a quantty S, such that: S k B lnω(,u where k B s a constant (to be later dentfed as Boltzmann's constant, k B R/ A.380x0-3 J/K, where A s Avogadro's umber, A 6.03x0 3 mol -. S has the followng propertes:. S s extensve: f we have two ndependent subsystems, A and B, then SA+B k B ln(ωa+b k B ln(ω A Ω B k B lnω A + k B lnω B S A +S B The reason for ths s that, for ndependent subsystems, each mcrostate of system A can be combned wth a mcrostate of system B to gve a mcrostate of the combned system. For mxng two fluds, we only get the above expresson f we assume that the partcles n the systems are ndstngushable. f partcles were dstngushable addtonal "states" would be avalable to the combned system resultng from the possblty of exchangng the postons and momenta of partcles n dfferent regons. Although the ndstngushablty of partcles s really of quantum mechancal orgn, t was ntroduced ad hoc by Gbbs before the development of quantum mechancs, precsely to make statstcal-mechancal entropy an extensve property.. S s maxmzed at equlbrum: For a system wth nternal constrants (e.g. nternal rgd walls or barrers to energy transfer, the number of possble mcrostates s always less than the number of mcrostates after the constrants are removed. S (,U > S (,U; nternal constrants
4 CHE 54 Ensembles 4 To see ths second property, consder the box wth partcles of the example above, and thnk of any constrant to the system at a gven energy (say U+. An example of a "constrant" would be to have that the frst fve slots have exactly unt of energy. The number of mcrostates n ths case s (5x55, less than the 55 states avalable to the unconstraned system. n concluson, S has the same propertes as the entropy. Statement ( above s the mcroscopc statement of the Second Law of thermodynamcs. From the Fundamental Equaton of thermodynamcs, du Td S Pd + μ d d S du + T P T d μ T d [] The second form of the fundamental equaton (the "Entropy Representaton" s the most useful for Statstcal Mechancs. Snce (from equaton S T ln Ω k T B β [] The symbol β s commonly used n statstcal mechancs to denote the nverse temperature. Example (cont.: Balls n a box n the example above, we can now defne the temperature of the box from equaton. ntally, as the energy ncreases, the temperature ncreases, as expected. However, somethng strange happens to the system at energes greater than +0 - can you guess what that s? What s the sgn of the temperature for energes > +0? s ths physcally reasonable? CAOCAL ESEMBLE: COSTAT,T Let us frst obtan the equlbrum condton for two systems, and, that are placed n thermal contact, so that they can exchange energy. The number of mcrostates avalable to the combned system must be a maxmum at equlbrum, snce the combned system s under condtons of constant and U dscussed prevously. Mathematcally, the condton for equlbrum s that
5 CHE 54 Ensembles 5 S total s maxmum δs total 0 δs + δs S S 0 δu + δu 0 Snce the total system s solated, δu + δu 0. Combnng wth the above condton, we obtan S S T T n other words, systems that can exchange energy much have the same temperature at equlbrum. ow, let us assume that one of the two systems s much larger than the other, so that t effectvely acts as a "constant-temperature bath." The total system (small system + bath s agan consdered under U condtons. ow consder the small system at a gven mcrostate ν wth energy U ν. The energy of the bath s U B U - U ν. The bath can be n any of Ω(U B Ω(U - U ν mcrostates. Snce the probablty of the total system beng n any partcular combnaton of mcrostates wth a gven total energy s the same, the probablty of fndng the small system n state ν s P ν Ω (U - U ν exp (ln(ω (U - U ν We can expand lnω around Ω(U gven that U ν s much smaller than U: ln Ω ln ν ν + ( Ω( U U ln( Ω( U U hgher ordr terms and substtutng back n the expresson for P ν usng lnω/ β /k B T, P ν exp ( - βu ν [3] Ths s a very mportant result. n words, we are fndng that the probablty of each mcrostate n the canoncal ensemble (constant, T s proportonal to the exponental of the energy dvded by the temperature. n order to fnd the absolute probablty of each mcrostate, we need to make sure that the sum of all the probabltes s one. The normalzaton constant for ths s called the "canoncal partton functon," Q. Q(, T exp( β [4] all mcrostates U The summaton over mcrostates s performed over all energes and partcle postons. For the smple example of the box wth partcles we have been followng, the partton functon at a gven nverse temperature β would be: Q(β + 0exp(β + 55exp(β +
6 CHE 54 Ensembles 6 Where the frst term n the summaton comes from the sngle energy state wth U 0, the second from the 0 states wth U + and so on. For ths smple system, both volume and number of partcles are fxed, so they do not appear n the summaton - however, n the general case the partton functon would be a functon of both and. Once the partton functon s defned, the probablty of each mcrostate can now be wrtten explctly: P ν exp( βu ν Q [5] Therefore, n the canoncal ensemble, a general property F s gven by all mcrostates F exp( βu < F > [6] exp( βu all mcrostates t s possble to relate dervatves of lnq to thermodynamc propertes. For example, ln Q ( Q / β β Q U exp( βu ν ν, exp( βu ν < U > One can also calculate the averaged squared fluctuaton of energy n the canoncal ensemble: Pν U ν ( Pν ν ( δu ( U < U > < U > < U > U ( Q / β Q ( Q / β Q ln Q β < U > β usng the defnton of the heat capacty, C < U >, we get T, ( δ U k T [7] B C Ths s a remarkable result! We have obtaned a relatonshp between the thermodynamc parameters of a system and the sze of the spontaneous fluctuatons. t s nterestng to note that the heat capacty of the system, C, s an extensve varable (grows lnearly wth the sze of the system. Ths mples that the relatve magntude of the spontaneous fluctuatons grows as:
7 CHE 54 Ensembles 7 / ( δu / B < U > ( k T C < U > / [8] For a macroscopc system, O(0 3, ths s a very small number: the energy of an deal gas system at equlbrum wth a room-temperature bath s constant to roughly part n 0. However, for small systems typcally used n smulatons, 00-,000, so that typcal fluctuatons are 0%-3%. Q can be dentfed wth a famlar thermodynamc functon. To do ths, let us wrte k B ln Q k B ln β exp( U ν [9] ν As just shown, the relatve fluctuatons n energy for a macroscopc system are very small. We can approxmate the sum n equaton 9 by summng just the domnant terms. All of these wll have U ν <U>. There are Ω(<U> such terms (snce ths s the number of mcrostates at that U, and thus k B lnq k B ln(ω (<U> exp(-β<u> k B lnω (<U> - <U>/ T S - U / T - A / T [0] Ths should have been expected. n the mcrocanoncal (const. U ensemble the mportant functon Ω s such that k B lnω S. Recall that S s the functon maxmzed at const. U. n the canoncal (T ensemble, the thermodynamc functon beng mnmzed s A, or equvalently -A / T s maxmzed. We see a smlar relatonshp of -A / T wth k B lnq. The approxmaton s exact at the "thermodynamc lmt" (for an nfnte system. The table below summarzes the connectons between "classcal" and "statstcal" thermodynamc propertes dscussed thus far. Const. and U Const., and T Classcal Statstcal Classcal Statstcal Entropy, S s maxmzed at equl. k B lnω S Helmholtz Energy, A s mnmzed at equl. k B lnq -A / T Example (Chandler 3.4 Consder a system of dstngushable ndependent partcles, each of whch can exst n one of two states separated by energy ε. We can specfy the state of the system, ν, by lstng ν ( j n, n,..., n j,..., n, n 0 or The total energy of the system for a gven state s
8 CHE 54 Ensembles 8 Frst, we can obtan thermodynamc propertes of ths model from the mcrocanoncal ensemble. The number of mcrostates wth energy mε s: So that (usng Strlng s approxmaton, : β lnω E lnω ε m ( ( ln( m++ lnm ε ( mln( m+ mlnm ε m ε ln m m ε ln m βε ln m Equvalently,, whch gves E 0 at T0 (only ground state s populated and Eε/ as T (all states are equally lkely. The same result could have been obtaned n the canoncal ensemble: The exponentals factor nto an uncoupled product: The nternal energy E s ensemble., exactly the same as the result n the mcrocanoncal
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
ChE 503 A. Z. Panagiotopoulos 1
 ChE 503 A. Z. Panagiotopoulos 1 STATISTICAL MECHANICAL ENSEMLES 1 MICROSCOPIC AND MACROSCOPIC ARIALES The central question in Statistical Mechanics can be phrased as follows: If particles (atoms, molecules,
ChE 503 A. Z. Panagiotopoulos 1 STATISTICAL MECHANICAL ENSEMLES 1 MICROSCOPIC AND MACROSCOPIC ARIALES The central question in Statistical Mechanics can be phrased as follows: If particles (atoms, molecules,
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
ESCI 341 Atmospheric Thermodynamics Lesson 10 The Physical Meaning of Entropy
 ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
Week3, Chapter 4. Position and Displacement. Motion in Two Dimensions. Instantaneous Velocity. Average Velocity
 Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
Lecture 3: Boltzmann distribution
 Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
5.62 Physical Chemistry II Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
NAME and Section No.
 Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Mathematical Preparations
 1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Module 1 : The equation of continuity. Lecture 1: Equation of Continuity
 1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
II.D Many Random Variables
 II.D Many Random Varables Wth more than one random varable, the set of outcomes s an -dmensonal space, S x = { < x 1, x 2,, x < }. For example, descrbng the locaton and velocty of a gas partcle requres
II.D Many Random Varables Wth more than one random varable, the set of outcomes s an -dmensonal space, S x = { < x 1, x 2,, x < }. For example, descrbng the locaton and velocty of a gas partcle requres
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture Notes on Linear Regression
 Lecture Notes on Lnear Regresson Feng L fl@sdueducn Shandong Unversty, Chna Lnear Regresson Problem In regresson problem, we am at predct a contnuous target value gven an nput feature vector We assume
Lecture Notes on Lnear Regresson Feng L fl@sdueducn Shandong Unversty, Chna Lnear Regresson Problem In regresson problem, we am at predct a contnuous target value gven an nput feature vector We assume
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Lecture 12: Discrete Laplacian
 Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Chap.5 Statistical Thermodynamics
 Chap5 Statstcal Thermodynamcs () Free expanson & adabatc from macroscopc: Δ S dq T R adabatc Q, free expanson W rrev rrev ΔU, deal gas ΔT If reversble & sothermal QR + WR ΔU 因 Uf(T) RT QR WR ( Pd ) Pd
Chap5 Statstcal Thermodynamcs () Free expanson & adabatc from macroscopc: Δ S dq T R adabatc Q, free expanson W rrev rrev ΔU, deal gas ΔT If reversble & sothermal QR + WR ΔU 因 Uf(T) RT QR WR ( Pd ) Pd
Inner Product. Euclidean Space. Orthonormal Basis. Orthogonal
 Inner Product Defnton 1 () A Eucldean space s a fnte-dmensonal vector space over the reals R, wth an nner product,. Defnton 2 (Inner Product) An nner product, on a real vector space X s a symmetrc, blnear,
Inner Product Defnton 1 () A Eucldean space s a fnte-dmensonal vector space over the reals R, wth an nner product,. Defnton 2 (Inner Product) An nner product, on a real vector space X s a symmetrc, blnear,
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
PHYS 705: Classical Mechanics. Canonical Transformation II
 1 PHYS 705: Classcal Mechancs Canoncal Transformaton II Example: Harmonc Oscllator f ( x) x m 0 x U( x) x mx x LT U m Defne or L p p mx x x m mx x H px L px p m p x m m H p 1 x m p m 1 m H x p m x m m
1 PHYS 705: Classcal Mechancs Canoncal Transformaton II Example: Harmonc Oscllator f ( x) x m 0 x U( x) x mx x LT U m Defne or L p p mx x x m mx x H px L px p m p x m m H p 1 x m p m 1 m H x p m x m m
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
APPENDIX A Some Linear Algebra
 APPENDIX A Some Lnear Algebra The collecton of m, n matrces A.1 Matrces a 1,1,..., a 1,n A = a m,1,..., a m,n wth real elements a,j s denoted by R m,n. If n = 1 then A s called a column vector. Smlarly,
APPENDIX A Some Lnear Algebra The collecton of m, n matrces A.1 Matrces a 1,1,..., a 1,n A = a m,1,..., a m,n wth real elements a,j s denoted by R m,n. If n = 1 then A s called a column vector. Smlarly,
EPR Paradox and the Physical Meaning of an Experiment in Quantum Mechanics. Vesselin C. Noninski
 EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
Quantum Statistical Mechanics
 Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Density matrix. c α (t)φ α (q)
 Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
12. The Hamilton-Jacobi Equation Michael Fowler
 1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
3.1 Expectation of Functions of Several Random Variables. )' be a k-dimensional discrete or continuous random vector, with joint PMF p (, E X E X1 E X
 Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
ANSWERS. Problem 1. and the moment generating function (mgf) by. defined for any real t. Use this to show that E( U) var( U)
 Econ 413 Exam 13 H ANSWERS Settet er nndelt 9 deloppgaver, A,B,C, som alle anbefales å telle lkt for å gøre det ltt lettere å stå. Svar er gtt . Unfortunately, there s a prntng error n the hnt of
Econ 413 Exam 13 H ANSWERS Settet er nndelt 9 deloppgaver, A,B,C, som alle anbefales å telle lkt for å gøre det ltt lettere å stå. Svar er gtt . Unfortunately, there s a prntng error n the hnt of
where the sums are over the partcle labels. In general H = p2 2m + V s(r ) V j = V nt (jr, r j j) (5) where V s s the sngle-partcle potental and V nt
 Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
Mechanics Physics 151
 Mechancs Physcs 5 Lecture 0 Canoncal Transformatons (Chapter 9) What We Dd Last Tme Hamlton s Prncple n the Hamltonan formalsm Dervaton was smple δi δ p H(, p, t) = 0 Adonal end-pont constrants δ t ( )
Mechancs Physcs 5 Lecture 0 Canoncal Transformatons (Chapter 9) What We Dd Last Tme Hamlton s Prncple n the Hamltonan formalsm Dervaton was smple δi δ p H(, p, t) = 0 Adonal end-pont constrants δ t ( )
THEOREMS OF QUANTUM MECHANICS
 THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
MATH 829: Introduction to Data Mining and Analysis The EM algorithm (part 2)
 1/16 MATH 829: Introducton to Data Mnng and Analyss The EM algorthm (part 2) Domnque Gullot Departments of Mathematcal Scences Unversty of Delaware Aprl 20, 2016 Recall 2/16 We are gven ndependent observatons
1/16 MATH 829: Introducton to Data Mnng and Analyss The EM algorthm (part 2) Domnque Gullot Departments of Mathematcal Scences Unversty of Delaware Aprl 20, 2016 Recall 2/16 We are gven ndependent observatons
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture Note 3. Eshelby s Inclusion II
 ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
x = , so that calculated
 Stat 4, secton Sngle Factor ANOVA notes by Tm Plachowsk n chapter 8 we conducted hypothess tests n whch we compared a sngle sample s mean or proporton to some hypotheszed value Chapter 9 expanded ths to
Stat 4, secton Sngle Factor ANOVA notes by Tm Plachowsk n chapter 8 we conducted hypothess tests n whch we compared a sngle sample s mean or proporton to some hypotheszed value Chapter 9 expanded ths to
Poisson brackets and canonical transformations
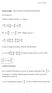 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
CHAPTER 6. LAGRANGE S EQUATIONS (Analytical Mechanics)
 CHAPTER 6 LAGRANGE S EQUATIONS (Analytcal Mechancs) 1 Ex. 1: Consder a partcle movng on a fxed horzontal surface. r P Let, be the poston and F be the total force on the partcle. The FBD s: -mgk F 1 x O
CHAPTER 6 LAGRANGE S EQUATIONS (Analytcal Mechancs) 1 Ex. 1: Consder a partcle movng on a fxed horzontal surface. r P Let, be the poston and F be the total force on the partcle. The FBD s: -mgk F 1 x O
At zero K: All atoms frozen at fixed positions on a periodic lattice.
 September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
Winter 2008 CS567 Stochastic Linear/Integer Programming Guest Lecturer: Xu, Huan
 Wnter 2008 CS567 Stochastc Lnear/Integer Programmng Guest Lecturer: Xu, Huan Class 2: More Modelng Examples 1 Capacty Expanson Capacty expanson models optmal choces of the tmng and levels of nvestments
Wnter 2008 CS567 Stochastc Lnear/Integer Programmng Guest Lecturer: Xu, Huan Class 2: More Modelng Examples 1 Capacty Expanson Capacty expanson models optmal choces of the tmng and levels of nvestments
Complete subgraphs in multipartite graphs
 Complete subgraphs n multpartte graphs FLORIAN PFENDER Unverstät Rostock, Insttut für Mathematk D-18057 Rostock, Germany Floran.Pfender@un-rostock.de Abstract Turán s Theorem states that every graph G
Complete subgraphs n multpartte graphs FLORIAN PFENDER Unverstät Rostock, Insttut für Mathematk D-18057 Rostock, Germany Floran.Pfender@un-rostock.de Abstract Turán s Theorem states that every graph G
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Foundations of Arithmetic
 Foundatons of Arthmetc Notaton We shall denote the sum and product of numbers n the usual notaton as a 2 + a 2 + a 3 + + a = a, a 1 a 2 a 3 a = a The notaton a b means a dvdes b,.e. ac = b where c s an
Foundatons of Arthmetc Notaton We shall denote the sum and product of numbers n the usual notaton as a 2 + a 2 + a 3 + + a = a, a 1 a 2 a 3 a = a The notaton a b means a dvdes b,.e. ac = b where c s an
A how to guide to second quantization method.
 Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
n α j x j = 0 j=1 has a nontrivial solution. Here A is the n k matrix whose jth column is the vector for all t j=0
 MODULE 2 Topcs: Lnear ndependence, bass and dmenson We have seen that f n a set of vectors one vector s a lnear combnaton of the remanng vectors n the set then the span of the set s unchanged f that vector
MODULE 2 Topcs: Lnear ndependence, bass and dmenson We have seen that f n a set of vectors one vector s a lnear combnaton of the remanng vectors n the set then the span of the set s unchanged f that vector
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
The Multiple Classical Linear Regression Model (CLRM): Specification and Assumptions. 1. Introduction
 ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
Using T.O.M to Estimate Parameter of distributions that have not Single Exponential Family
 IOSR Journal of Mathematcs IOSR-JM) ISSN: 2278-5728. Volume 3, Issue 3 Sep-Oct. 202), PP 44-48 www.osrjournals.org Usng T.O.M to Estmate Parameter of dstrbutons that have not Sngle Exponental Famly Jubran
IOSR Journal of Mathematcs IOSR-JM) ISSN: 2278-5728. Volume 3, Issue 3 Sep-Oct. 202), PP 44-48 www.osrjournals.org Usng T.O.M to Estmate Parameter of dstrbutons that have not Sngle Exponental Famly Jubran
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS. Version 2 ECE IIT, Kharagpur
 Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
Einstein-Podolsky-Rosen Paradox
 H 45 Quantum Measurement and Spn Wnter 003 Ensten-odolsky-Rosen aradox The Ensten-odolsky-Rosen aradox s a gedanken experment desgned to show that quantum mechancs s an ncomplete descrpton of realty. The
H 45 Quantum Measurement and Spn Wnter 003 Ensten-odolsky-Rosen aradox The Ensten-odolsky-Rosen aradox s a gedanken experment desgned to show that quantum mechancs s an ncomplete descrpton of realty. The
8.6 The Complex Number System
 8.6 The Complex Number System Earler n the chapter, we mentoned that we cannot have a negatve under a square root, snce the square of any postve or negatve number s always postve. In ths secton we want
8.6 The Complex Number System Earler n the chapter, we mentoned that we cannot have a negatve under a square root, snce the square of any postve or negatve number s always postve. In ths secton we want
Linear Approximation with Regularization and Moving Least Squares
 Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
2E Pattern Recognition Solutions to Introduction to Pattern Recognition, Chapter 2: Bayesian pattern classification
 E395 - Pattern Recognton Solutons to Introducton to Pattern Recognton, Chapter : Bayesan pattern classfcaton Preface Ths document s a soluton manual for selected exercses from Introducton to Pattern Recognton
E395 - Pattern Recognton Solutons to Introducton to Pattern Recognton, Chapter : Bayesan pattern classfcaton Preface Ths document s a soluton manual for selected exercses from Introducton to Pattern Recognton
Part II Statistical Mechanics, Lent 2005
 Part II Statstcal Mechancs, Lent 25 Prof. R.R. Horgan February 14, 25 1 Statstcal Mechancs 1.1 Introducton The classcal and quantum dynamcs of smple systems wth few degrees of freedom s well understood
Part II Statstcal Mechancs, Lent 25 Prof. R.R. Horgan February 14, 25 1 Statstcal Mechancs 1.1 Introducton The classcal and quantum dynamcs of smple systems wth few degrees of freedom s well understood
From Biot-Savart Law to Divergence of B (1)
 From Bot-Savart Law to Dvergence of B (1) Let s prove that Bot-Savart gves us B (r ) = 0 for an arbtrary current densty. Frst take the dvergence of both sdes of Bot-Savart. The dervatve s wth respect to
From Bot-Savart Law to Dvergence of B (1) Let s prove that Bot-Savart gves us B (r ) = 0 for an arbtrary current densty. Frst take the dvergence of both sdes of Bot-Savart. The dervatve s wth respect to
Chapter 5. Solution of System of Linear Equations. Module No. 6. Solution of Inconsistent and Ill Conditioned Systems
 Numercal Analyss by Dr. Anta Pal Assstant Professor Department of Mathematcs Natonal Insttute of Technology Durgapur Durgapur-713209 emal: anta.bue@gmal.com 1 . Chapter 5 Soluton of System of Lnear Equatons
Numercal Analyss by Dr. Anta Pal Assstant Professor Department of Mathematcs Natonal Insttute of Technology Durgapur Durgapur-713209 emal: anta.bue@gmal.com 1 . Chapter 5 Soluton of System of Lnear Equatons
Lecture 2 Grand Canonical Ensemble GCE
 Lecture 2 Grand Canoncal Ensemble GCE 2.1 hermodynamc Functons Contnung on from last day we also note that thus, dω = df dµ µd = Sd P dv dµ (2.1) P = V = S = From the expresson for the entropy, we therefore
Lecture 2 Grand Canoncal Ensemble GCE 2.1 hermodynamc Functons Contnung on from last day we also note that thus, dω = df dµ µd = Sd P dv dµ (2.1) P = V = S = From the expresson for the entropy, we therefore
Rigid body simulation
 Rgd bod smulaton Rgd bod smulaton Once we consder an object wth spacal etent, partcle sstem smulaton s no longer suffcent Problems Problems Unconstraned sstem rotatonal moton torques and angular momentum
Rgd bod smulaton Rgd bod smulaton Once we consder an object wth spacal etent, partcle sstem smulaton s no longer suffcent Problems Problems Unconstraned sstem rotatonal moton torques and angular momentum
Monte Carlo method II
 Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
Errors for Linear Systems
 Errors for Lnear Systems When we solve a lnear system Ax b we often do not know A and b exactly, but have only approxmatons  and ˆb avalable. Then the best thng we can do s to solve ˆx ˆb exactly whch
Errors for Lnear Systems When we solve a lnear system Ax b we often do not know A and b exactly, but have only approxmatons  and ˆb avalable. Then the best thng we can do s to solve ˆx ˆb exactly whch
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
coordinates. Then, the position vectors are described by
 Revewng, what we have dscussed so far: Generalzed coordnates Any number of varables (say, n) suffcent to specfy the confguraton of the system at each nstant to tme (need not be the mnmum number). In general,
Revewng, what we have dscussed so far: Generalzed coordnates Any number of varables (say, n) suffcent to specfy the confguraton of the system at each nstant to tme (need not be the mnmum number). In general,
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
14 The Postulates of Quantum mechanics
 14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
Problem Points Score Total 100
 Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
8.592J: Solutions for Assignment 7 Spring 2005
 8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Lagrangian Field Theory
 Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Week 8: Chapter 9. Linear Momentum. Newton Law and Momentum. Linear Momentum, cont. Conservation of Linear Momentum. Conservation of Momentum, 2
 Lnear omentum Week 8: Chapter 9 Lnear omentum and Collsons The lnear momentum of a partcle, or an object that can be modeled as a partcle, of mass m movng wth a velocty v s defned to be the product of
Lnear omentum Week 8: Chapter 9 Lnear omentum and Collsons The lnear momentum of a partcle, or an object that can be modeled as a partcle, of mass m movng wth a velocty v s defned to be the product of
CIS526: Machine Learning Lecture 3 (Sept 16, 2003) Linear Regression. Preparation help: Xiaoying Huang. x 1 θ 1 output... θ M x M
 CIS56: achne Learnng Lecture 3 (Sept 6, 003) Preparaton help: Xaoyng Huang Lnear Regresson Lnear regresson can be represented by a functonal form: f(; θ) = θ 0 0 +θ + + θ = θ = 0 ote: 0 s a dummy attrbute
CIS56: achne Learnng Lecture 3 (Sept 6, 003) Preparaton help: Xaoyng Huang Lnear Regresson Lnear regresson can be represented by a functonal form: f(; θ) = θ 0 0 +θ + + θ = θ = 0 ote: 0 s a dummy attrbute
