Equilibrium Statistical Mechanics
|
|
|
- Alexia Byrd
- 5 years ago
- Views:
Transcription
1 Equlbrum Statstcal Mechancs Ref. Davdson, Statstcal Mechancs McGraw-Hll, 96 We wsh to study plasmas n equlbrum for at least two reasons: (a) Many tmes real plasmas are near true equlbrum, at least locally and n regard to some of the propertes. (b) For stuatons outsde equlbrum, where rates are mportant, f the rate of one process s known by measurement or mechanstc calculaton, the rate of ts nverse process can be deduced from equlbrum propertes (mcro reversblty). Statstcal Mechancs bulds on the quantum mechancal notons of orbtal, energy state and excluson prncple, to analyze ensembles of many partcles and deduce ther macroscopc propertes. In the end, Statstcal Mechancs deduces the laws of Thermodynamcs from those of partcle physcs. In addton, t provdes specfc formulae for calculatng thermodynamc quanttes, whch can only be defned and nterrelated by Thermodynamcs alone. Instead of solvng n detal the mult-partcle equatons of moton and then takng the pertnent average, Statstcal Mechancs uses only a few results from Quantum theory and brdges the transton to the macroscopc world by means of a few smple postulates of a statstcal nature. The most mportant of these s the equ-probablty of mcro-states, whch we wll see soon. The plausblty of ths postulate arses from a property of classcal dynamcal systems, namely, that many-degree of freedom systems evolve n tme n a quas-random manner, such that over the long run they spend equal tme n any dynamcal state compatble wth the constrants. A smlar stuaton occurs n nteractng mult scale quantum systems, where perturbatons of sngle-partcle states ensure over the long run that all possble mult-partcle states are occuped the same fracton of the tme. Despte ths plausble foundaton, t s startlng to see the power and quantty of the deductons whch can be obtaned from such smple statements. Ther ablty to re-create the well establshed emprcal scence of thermodynamcs s the best check of the valdty of the statstcal postulates. On the other hand, ther rgorous logcal justfcaton s a dffcult and subtle task whch has kept mathematcans busy for over a century. Defntons for Quantum Statstcal Mechancs: Sngle-partcle orbtal: Set of values of all quantum numbers for a sngle partcle n a gven volume and feld. Many-partcle orbtal: Set of values of all quantum numbers for a gven number of partcles n a gven volume and feld. Mcrostate: Same thng as a many-partcle orbtal. Energy level: Physcally allowed value of -partcle energy. Degeneracy of an energy level: Number of sngle-partcle orbtals whch all have the same energy. More precsely, n any (small) volume n 6-D phase space (x, xw)
2 Energy dstrbuton: Specfcaton of the number of partcles whch have each energy level. Macrostate: Same as energy dstrbuton. Statstcal weght of a macro state: Number of mcro states n t,.e., number of ways n whch the gven number of partcles can occupy the avalable orbtals for a gven energy dstrbuton. System energy: Sum total of the partcle energes. Ferm-Drac statstcs: Specfcatons that no more than one partcle can exst per sngle- partcle orbtal. Also called Paul excluson prncple. Bose-Ensten Statstcs: Specfcaton that any number of partcles may have the same quantum numbers,.e. the same sngle-partcle orbtal. Postulates of Quantum Statstcal Mechancs. All mcro states correspondng to prescrbed system energy, number of partcles, volume and feld, are equally probable.. Partcles are ndstngushable (but mcrostates are not).. The macroscopcally observed value of a quantty Φ can be calculated by equal-weght averagng over all accessble mcrostates. As the number of partcles ncreases, one macrostate becomes much more probable (more mcro states n t) than any other; thus, an alternatve way of calculatng Φ as to assume that only the most probable macrostate exsts. Example : Three energy levels: t o = t = t = Doubly degenerate: g o = g = g = Ferm-Drac statstcs (excluson) 4 partcles Level orbtal orbtal Level orbtal orbtal Level orbtal orbtal E=, W= E=, W=4 E=4, W= E=4, W=4 E=5, W=4 E=6,W= N N N W =, E = (!!!!!!! W = 4, E = (!!!!!!! W =, E = 4 W = 4, E = 4 W = 4, E = 5 W =, E = 6 (!!!!!!!
3 Example : Three energy levels: t o = t = t = Doubly degenerate: g o = g = g = Bose-Ensten statstcs (no excluson prncple) 4 partcles Level orbtal orbtal 4 4 Level orbtal orbtal Level orbtal orbtal E=, W=5 E=, W=8 E=, W=8 E=, W= E=, W=9 etc N N N 4 E =, W = 5 E =, W = 8 E =, W = 8 E =, W = E =, W = 9 E = 4, W = 9 E =, W = 8 macrostate E = 4, W = E = 5, W = E = 6, W = 8 4 E = 4, W = 5 E = 5, W = 8 E = 6, W = 9 E = 7, W = 8 4 E = 8, W = 5 ( 5!!! = 5 = 5) 4!!!!!! ( 4!!! = 4 = 8)!!!!!!! (!! = = )!!!!!!! (!! = = 9)!!!!!! Quantum statstcs of ndependent partcles: Gven an external feld, suppose we have N partcles wth a total energy E prescrbed. We can (from Quantum mechancs) fnd the -partcle energy levels, t, each contanng g orbtals (degeneracy = g ). A macrostate s a specfcaton of the number of partcles N per energy level, wthn n the constrants, N = N levels E = N t levels These are two knds of Q.M. systems of many partcles: (a) Those for whch no two partcles can share an orbtal (Ferm-Drac statstcs) (b) Those where any number of partcles can occupy the same orbtal (Bose-Ensten statstcs)
4 Electrons, protons and neutrons (and many atoms, as long as they hold fractonal spn) obey F.D.; photons (and many atoms and even speces composed by fermons, as long as ther spn s nteger) obey B.E.. Let us calculate the statstcal weght of a macrostate n both cases. For F.D., the number of ways of selectng N orbtals for occupancy out of the g > N n level t s, g g! = N N!(g N )! Then, for any gven choce n level t, there are a smlar number of choces n other levels, and altogether, g! W F.D. = Π N!(g N )! (and g N, of course) For B.E. statstcs, n each level of t the number of ways of dstrbutng N partcles over g orbtals wthout restrcton as to how many per orbtal s the number of combnatons wth repetton of N objects taken from a group of g ; that number s, N + g (N + g )! = N N!(g )! Ths can be demonstrated wth a smple example havng N = 4 g =. Pck one realzaton: X X- XX X XX-X X Box Box Rearrange: XX XX ; X XXX Number of (objects+parttons)=n + (g ) Number of ways to scramble these enttes = (N + (g ))! But objects (N ) can be separately rearranged wthout affectng the count, so dvde by N!. Also, parttons can be rearranged, so dvde by (g )!: W = (N + g )! N!(g )! Altogether then, (N + g )! W B.E. = Π N!(g )! The most probable macrostate s that set of numbers N whch maxmzes W (or better ln W ) subject to gven N, E. Usng Lagrange multplers, and the approxmaton (Strlng s), N N ln(π) ln N N! πn ln N! = + + N ln N N N ln N N (for large N) e 4
5 For B.E. statstcs: φ = ln W αn βe = (N +g ) ln(n +g ) (N +g ) N ln N +N (g ) ln(g )+g αn βt N φ = ln(n + g ) + ln N + α βt = N g g ln + = α + βt ln + = α + βt N N snce both g and N are large numbers. Then we have, N g = α B.E. e e +βe For F.D. statstcs: φ = [g ln g g N ln N + N (g N ) ln(g N ) + g N αn βt N ] φ N = ln N + + ln(g N ) + α βt = g ln = α + βt N Therefore we have (and s easy to see that N < g ), N = g e α e +βe + F.D The classcal lmt or Dlute systems are those for whch g» N, so that only a few of the orbtals n each level are actually occuped. Then, regardless of the knd of statstcs (F.D. or B.E.), only sngle occupancy of orbtals s probable, and one should get a common lmt α+βe from the above two cases. Snce g» N t must be true that e», or α + βt s a postve, large number. Then, α βe N g e e both F.D. and B.E. Ths s called corrected Boltzmann statstcs snce t s smlar (but not dentcal) to the result n classcal mechancs (worked out by Boltzmann), where partcles are dstngushable. Manly due to ther large translatonal degeneracy, gases are n ths category most of the tme. It s shown n Quantum Mechancs that the number of possble states for a gven energy s (L/λ DB ), where L s the box sze (or the potental well sze), and λ DB = h s the mv DeBrogle wavelength. For a plasma at K, λ DB (electrons) 5Å, so the degeneracy s large ndeed. 5
6 Sgnfcance of α, β, W In general, a Lagrange multpler s the senstvty of the maxmzed functon to the correspondng constrant. To see ths, consder, Maxmze f(x ) =,,... n Subject to g j (x ) = c j j =,,...m < n φ = f(x ) λ j (g j c j ) j For maxmzaton, we mpose, φ f g j = λ j = x x x j g j = c j and calculate the correspondng x = x M and λ = λ M. M Suppose we now perturb ( one ) of the c j s, say c n, and solve agan. The maxmum f = f(x ) wll change by dc n, or df = f x x M dc n c j =n g j x g ( j x ) λ j dc n = λ j dc n x j c n c j =n x j c n c j =n X X- X But, snce g = c j, g j c n = δ jn, so df = dc n j λ j δ jn = λ n dc n f.e., λ n = q.e.d. c n c j =n g ( j cn ) cj n Suppose now the constrants c j are allowed to vary along a certan drecton n ther own m-space,.e., dc dc dc m = = = = dt ν ν ν m where dt s an arbtrary parameter. and we want to maxmze f also wth respect to such changes. Clearly, we frst must assume that for any set of c j s the x s are chosen such as to maxmze f for those fxed c j s. Then the change of f MAX due to the dc j s s f MAX df MAX = dc j = dt λ j ν j j ) c j c =j and snce dt s arbtrary, we must have the lnear relaton among the λ s: ν j λ j = j j 6
7 (Note ths s the equaton of the plane normal to dc j /ν j = dt n m-space.) Applyng ths to our maxmzaton of ln W wth fxed N (multpler α), E (multpler β) and V, we calculate, ln W α = N V,E ln W β = E Now, turnng to W, t s an aggregate system property whch s maxmum when the system n equlbrum (maxmum lkelhood), at fxed N, E. From classcal thermodynamcs, the same property s possessed by the Entropy, S of the system, so that we must have S = f(w ), wth f a monotancally ncreasng functon. Now, f we consder two non-nteractng together, we know that S = S + S. But also, snce ther probabltes are ndependent, W = W W, or ln W = ln W + ln W. Hence f must be lnear, and gnorng any constant shft, S = k ln W (k stll undetermned). S S Hence α = ; β = k N k E V,E Now, thermodynamcally, E Where µ s by defnton µ = N S,V V,N S µ S = ; = N T E T V,E V,N de = T ds pdv + µdn (chemcal potental), from here, µ and so α = β = (any statstcs) kt kt Ths relates α and β to known thermodynamc functons (µ, T ). We stll need to dentfy the constant k. V,N Dlute Systems The Partton Functon. The followng apples only to corrected Boltzmann statstcs,.e., α βe µ N = g e = g e kt We can now relate α, β (.e., µ, T ) to the actual constrants N, E: α βe N = N = e g e βe The group Q(β) = g e = e α βe E = t = e g N t e βe can be calculated a pror, once the quantum orbtals mechancs problem of one partcle n the gven volume and feld has been solved. It s called 7
8 the Partton Functon, and t plays an mportant role n chemcal equlbrum and other statstcal mechancs dervatons. In terms of Q, N e βe N = e α Q(β) and then = g N Q(β) therefore, we can wrte, Also, snce, then, whch can be wrtten as, µ = kt ln Q N E = e α g t e βe α Q = e β E N = Q Q β = ln Q β E = NkT ln Q T Systems wth non-nteractng degrees of freedom. In many dlute systems, translaton, rotaton, vbraton, exctaton, etc. nteract very weakly wth each other, so that the quantum mechancs problems can be solved separately, leadng to ndependent sets of translatonal, rotatonal, etc. energy levels and degeneraces, and to a total energy, Then, t = t tr. + t rot. + t vb. + texc. + Q = g e βe = e βe = e βe levels orbtals tr. rot. vb. exc. = e βe.tr. e βe.rot. tr. rot. and so we can calculate separately the varous partton functons, and then multply them, Also then, snce µ = kt ln Q/N Q = Q tr. Q rot. Q vb. Q exc. Q tr. µ = µ tr. + µ rot. + = kt ln kt ln Q rot. N X X- X all others have no N E E tr. + E rot. E tr. ln Q tr. and = ln(q tr. Q rot ) = =, etc. N β N N β 8
9 α βe Entropy of a dlute system. We have S = k ln W, ln W = lm (ln W F D ) and N = g e. N <<g [ ] ln W F D = g ln g g J N ln N + ; N (g N ) ln(g N ) + g J ; N ( ln(g N ) = ln g + ln N ) ln g N g N «g g [ ] N ( N ) ln W = mmmm g ln g N ln N (g N ) ln g = N ln N ++ln g g g «( S = k N ln N ) = k N ( + α + βt ) = k[n( + α) + βe] g or Hence, an alternatve defnton of the chemcal potental s G µ S ( = k µ ) N + E kt T µn = E + NkT T S The Gbbs free energy. By defnton, the Gbbs free energy G s G E + P V T S Now, then, and snce, Then, dg = de + P dv + V dp T ds SdT de = T ds P dv + µdn dg = SdT + V dp + µdn N T,P Ths has the advantage that (T, P ) are ntensve varables. We can buld up the G of a system by addng new molecules (dn) whle mantanng the same T and P. Snce µ s also ntensve, µ = µ(t, P ), so µ s constant durng ths buldng up, and we obtan smply, G = µn Notce we cannot go from µ = ( E/ N) V,S to E = µn, snce when we keep V, S constant and vary N, p wll also vary, hence µ wll too. Equaton of state of dlute systems. We have calculated for a dlute system, µn = E + NkT T S Snce µn = G, we have, G = E + NkT T S 9
10 But G n general s E + P V T S, so we conclude, P V = NkT Now, by comparng to the deal gas law, R P V = N T we dentfy Boltzmann s constant, R 8.4J/(mol. K) J k B = = =.8 6. (Molecules/mol.) K N A In retrospect, the reason we arrve at P V = NkT s that we are assumng non-nteractve systems, where the energy levels and g s are calculated for each partcle as f t were alone n the box. Statstcal mechancs can also be appled to more complex systems, of course, but the methods are somewhat more dffcult (canoncal and grand-canoncal ensemble, as opposed to out mcro-canoncal ensemble). N A Mult-component systems. Suppose there are speces A, B, C, non-reactng for now. The total W s the product of W = W A W B W C and then ln W = ln W A + ln W B + ln W C. There are number constrants and one E constrant: N A = Formng agan, N A B N j j Nk C k A B C j j k k j k N B = N C = E = t N A + t N B + t N C φ = ln W A + ln W B + ln W C α A N A α B N B α C N C βe A and we dfferentate relatve to each N, each N B C j, and each N k. We then get for each speces a F.D. or B.E. (or corrected Boltzmann) dstrbuton wth a dfferent α for each, but wth a common β. Followng the same steps as before, we can agan relate these to the µ s and the T : µ A µ B µ C α A = α B = α C = β = kt kt kt kt and can also prove that G = N A µ A + N B µ B + N C µ C P V = (N A + N B + N C )kt
11 and (for dlute systems) Q A Q B Q C µ A = kt ln, µ B = kt ln, µ C = kt ln N A N B N C Reactng Systems. Suppose now that A, B, C can nterconvert accordng to the reacton, ν A A + ν B B ν C C Ths means that N A, N B, N C are not fxed, but that, f they change accordng to ths reacton, dn A dn B dn C = = (a drecton n N A, N B, N C space) ν A ν B ν C But we have proven before that for the object functon (ln W or S n our case) stll to be maxmum, there must exst a relatonshp among the multplers of the form, or, n terms of the µ = kt α, ν A α A + ν B α B = ν C α C ν A µ A + ν B µ B = ν C µ C st form of the law of mass acton In terms of the N s and Q s, Q A Q B Q C Q A Q B Q C ν A ln + ν B ln = ν C ln = N A N B N C N A N B N C or, In terms of P s, N ν A N ν B ν A ν B ν C Q ν A Q ν B = nd form of the law of mass acton A B A B N ν C Q ν C C C ν A ν B ν A ν B n A n B q A q B N j Q j ν C = ν C wth n j =, q j = n q V V C C kt kt kt P A = N A P B = N B P C = N C V V V kt Q A ν A kt Q B ν B P ν A P ν B V V A B = rd form of the law of mass acton P ν C C kt Q C V ν C The mportance of ths one s that the RHS wll be shown to depend on T only. It s called the Equlbrum constant K P (T ) for the reacton. The zero of energy. For a sngle speces, or for non-reactng speces, the t s can be measured from arbtrary levels; a shft t ' = t t merely makes a new p.f. Q ' = t E /kt Q and a new ' µ = µ t. However, when speces can nterconvert, they generally lberate or absorb
12 defnte amounts of energy n the process. If A + B + ΔE C then n the state n whch t A = and t B =, we must say that C has an energy ΔE, not arbtrary anymore. In all, we can assgn arbtrary zero levels of energy only to a set of non-nterconvertble atoms or partcles. The zeros of all the others then follow from ther energes of formaton. In chemcal thermodynamcs, the conventon s to assgn zero enthalpy to the pure speces at 98 K, atm, n the natural state (O, H, C (graphte),...). Then, for nstance, snce O + O O + 59Kcal, the enthalpy per mole of O at 98 K, atm s, The Translatonal Partton Functon 59 kcal ( per atom, J). mole 6 Consder a sngle partcle n a rectangular box, wth sdes L x, L y, L z. The frst task s to solve the Schrödnger equaton n order to obtan the allowable energes and quantum numbers (hence the degeneraces). The general Schrödnger equaton s (wth I = h/π), I ψ T p I = H (ψ T ) ; H = + V ; p = \ () t m Assume separaton of the tme dependence: Substtute and dvde by ψ T : ψ T (t, x) = Π(t)ψ(x) () I dπ = H (ψ) = t (4) π dt ψ where t s the (so far arbtrary) separaton constant. Ths gves the two equatons, and so, (E/ )t Ce dπ t + π = (4a) dt I H (ψ) = tψ Substtute n (6), dvde by ψ: I X xx Y yy Z zz t = (8) m X Y Z (4b) π = Ce, ψ (E/ )t T = ψ(x) (5) For our case, there s no potental energy nsde the box: V (x) = for ( < x, y, z < L x,y,z ), so (4b) reduces to, I \ ψ = tψ m or, I \ ψ + tψ = (6) m Assume next a soluton that s also separable n (x, y, z): ψ(x, y, z) = X(x)Y (y)z(z) (7)
13 Each of the terms X xx, etc. must be separately a constant, and we therefore obtan X equatons, wth, m X xx + t x X = I m Y yy + t y Y = (9) I m Z zz + t z Z = I t x + t y + t z = t () The partcle s confned by the box, and so we must have ψ = at each wall. Ths gves the boundary condtons: X() = X(Lx ) = Y () = Y (L y ) = () Z() = Z(Lz ) = The solutons that are zero at x = y = z = are, mt x mt y mt z X = A x sn x ; Y = A y sn y ; Z = A z sn z () I I I and mposng zero value at x = L x, y = L y, z = L z gves the condtons, mt x L x = n x π (n x =,,,...) I mt y = π =,,,...) () I L y n y (n y mt z L z = n z π (n z =,,,...) I Where the numbers (n x, n y, n z ) dentfy a quantum state, and are the quantum numbers for ths problem. Fnally, mposng t x + t y + t z = t gves, ( ) π I n n x y nz t = + + (4) L L L m x y z whch gves all the possble energy levels of the partcle. Clearly, there are many possble combnatons of n x, n y, n z gvng the same energy t (degenerate states), and the degeneracy wll ncrease wth the sze of the box. We can now calculate the translatonal partton functon as, Q tr. = e E/kT (5) n x n y n z In ths form, snce the summaton ncludes all the quantum numbers, the degeneracy factors are not needed. In detal, π n n x y n + + z mkt L L L Q tr. x y z = e (6) n x n y n z
14 π The summaton can be approxmated as an ntegral, provded «. Recallng that mkt L the DeBrogle wavelength for a partcle wth average thermal energy s, h πi πi λ DB = = = (7) p m kt mkt m DB We see that the condton to go to a contnuum vew n the (n x, n y, n z ) space s <<, or L a matter wave much shorter than the contaner sze. Notce ths s a weaker condton than that we already assumed for a dlute (Boltzmann) system: λ DB «n / (dstance between partcles), and so the contnuum approxmaton s well justfed. We then wrte, = ˆ ˆ ˆ n π n x y n + + z mkt Lx Ly Lz Q tr. dn x dn y dn z e (8) ˆ π ˆ π n ˆ x mkt mkt π n y z L n L mkt e x dn e y x dny e L z dn z Change varables to n x = mkt Lx ξ, n y = mkt Ly η, n z = mkt Lz ζ and use π π π e ξ π dξ =, etc. We obtan, ( ) Q tr. L x L y L z π = (mkt ) / (πi) λ Note that πi = h, and L x L y L z = V (the box volume). So, Q tr. / πmkt = V (9) h Ths can be wrtten n terms of λ DB as Q tr. = (π/) / V/λ DB, whch gves Q tr. an nterpretaton: (roughly) the number of DeBrogle boxes that ft nto the volume. Notce Q tr. s proportonal to volume. The other peces, Q rot, Q vb, etc. are ndependent of V, and so Q V altogether, and q = V Q = q(t ). Ths proves that the equlbrum constant K P, as derved prevously, s ndeed only a functon of temperature. 4
15 MIT OpenCourseWare Ionzed Gases Fall 4 For nformaton about ctng these materals or our Terms of Use, vst:
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
5.62 Physical Chemistry II Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
THERMAL DISTRIBUTION IN THE HCL SPECTRUM OBJECTIVE
 ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Thermodynamics and Kinetics of Solids 33. III. Statistical Thermodynamics. Â N i = N (5.3) N i. i =0. Â e i = E (5.4) has a maximum.
 hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Quantum Statistical Mechanics
 Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Fermi-Dirac statistics
 UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
PHYS 705: Classical Mechanics. Newtonian Mechanics
 1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Lecture 3: Boltzmann distribution
 Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Monte Carlo method II
 Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
ESCI 341 Atmospheric Thermodynamics Lesson 10 The Physical Meaning of Entropy
 ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
At zero K: All atoms frozen at fixed positions on a periodic lattice.
 September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
Canonical transformations
 Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
THE CHINESE REMAINDER THEOREM. We should thank the Chinese for their wonderful remainder theorem. Glenn Stevens
 THE CHINESE REMAINDER THEOREM KEITH CONRAD We should thank the Chnese for ther wonderful remander theorem. Glenn Stevens 1. Introducton The Chnese remander theorem says we can unquely solve any par of
THE CHINESE REMAINDER THEOREM KEITH CONRAD We should thank the Chnese for ther wonderful remander theorem. Glenn Stevens 1. Introducton The Chnese remander theorem says we can unquely solve any par of
14 The Postulates of Quantum mechanics
 14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
PHYS 705: Classical Mechanics. Canonical Transformation II
 1 PHYS 705: Classcal Mechancs Canoncal Transformaton II Example: Harmonc Oscllator f ( x) x m 0 x U( x) x mx x LT U m Defne or L p p mx x x m mx x H px L px p m p x m m H p 1 x m p m 1 m H x p m x m m
1 PHYS 705: Classcal Mechancs Canoncal Transformaton II Example: Harmonc Oscllator f ( x) x m 0 x U( x) x mx x LT U m Defne or L p p mx x x m mx x H px L px p m p x m m H p 1 x m p m 1 m H x p m x m m
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Linear Approximation with Regularization and Moving Least Squares
 Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
Physics 115. Molecular motion and temperature Phase equilibrium, evaporation
 Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
ANSWERS. Problem 1. and the moment generating function (mgf) by. defined for any real t. Use this to show that E( U) var( U)
 Econ 413 Exam 13 H ANSWERS Settet er nndelt 9 deloppgaver, A,B,C, som alle anbefales å telle lkt for å gøre det ltt lettere å stå. Svar er gtt . Unfortunately, there s a prntng error n the hnt of
Econ 413 Exam 13 H ANSWERS Settet er nndelt 9 deloppgaver, A,B,C, som alle anbefales å telle lkt for å gøre det ltt lettere å stå. Svar er gtt . Unfortunately, there s a prntng error n the hnt of
1 (1 + ( )) = 1 8 ( ) = (c) Carrying out the Taylor expansion, in this case, the series truncates at second order:
 68A Solutons to Exercses March 05 (a) Usng a Taylor expanson, and notng that n 0 for all n >, ( + ) ( + ( ) + ) We can t nvert / because there s no Taylor expanson around 0 Lets try to calculate the nverse
68A Solutons to Exercses March 05 (a) Usng a Taylor expanson, and notng that n 0 for all n >, ( + ) ( + ( ) + ) We can t nvert / because there s no Taylor expanson around 0 Lets try to calculate the nverse
Einstein-Podolsky-Rosen Paradox
 H 45 Quantum Measurement and Spn Wnter 003 Ensten-odolsky-Rosen aradox The Ensten-odolsky-Rosen aradox s a gedanken experment desgned to show that quantum mechancs s an ncomplete descrpton of realty. The
H 45 Quantum Measurement and Spn Wnter 003 Ensten-odolsky-Rosen aradox The Ensten-odolsky-Rosen aradox s a gedanken experment desgned to show that quantum mechancs s an ncomplete descrpton of realty. The
Density matrix. c α (t)φ α (q)
 Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
CHAPTER 14 GENERAL PERTURBATION THEORY
 CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
Poisson brackets and canonical transformations
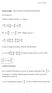 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
12. The Hamilton-Jacobi Equation Michael Fowler
 1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
5.04, Principles of Inorganic Chemistry II MIT Department of Chemistry Lecture 32: Vibrational Spectroscopy and the IR
 5.0, Prncples of Inorganc Chemstry II MIT Department of Chemstry Lecture 3: Vbratonal Spectroscopy and the IR Vbratonal spectroscopy s confned to the 00-5000 cm - spectral regon. The absorpton of a photon
5.0, Prncples of Inorganc Chemstry II MIT Department of Chemstry Lecture 3: Vbratonal Spectroscopy and the IR Vbratonal spectroscopy s confned to the 00-5000 cm - spectral regon. The absorpton of a photon
Advanced Quantum Mechanics
 Advanced Quantum Mechancs Rajdeep Sensarma! sensarma@theory.tfr.res.n ecture #9 QM of Relatvstc Partcles Recap of ast Class Scalar Felds and orentz nvarant actons Complex Scalar Feld and Charge conjugaton
Advanced Quantum Mechancs Rajdeep Sensarma! sensarma@theory.tfr.res.n ecture #9 QM of Relatvstc Partcles Recap of ast Class Scalar Felds and orentz nvarant actons Complex Scalar Feld and Charge conjugaton
1 Derivation of Rate Equations from Single-Cell Conductance (Hodgkin-Huxley-like) Equations
 Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Problem Set 9 Solutions
 Desgn and Analyss of Algorthms May 4, 2015 Massachusetts Insttute of Technology 6.046J/18.410J Profs. Erk Demane, Srn Devadas, and Nancy Lynch Problem Set 9 Solutons Problem Set 9 Solutons Ths problem
Desgn and Analyss of Algorthms May 4, 2015 Massachusetts Insttute of Technology 6.046J/18.410J Profs. Erk Demane, Srn Devadas, and Nancy Lynch Problem Set 9 Solutons Problem Set 9 Solutons Ths problem
Lecture 20: Noether s Theorem
 Lecture 20: Noether s Theorem In our revew of Newtonan Mechancs, we were remnded that some quanttes (energy, lnear momentum, and angular momentum) are conserved That s, they are constant f no external
Lecture 20: Noether s Theorem In our revew of Newtonan Mechancs, we were remnded that some quanttes (energy, lnear momentum, and angular momentum) are conserved That s, they are constant f no external
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
PART I: MULTIPLE CHOICE (32 questions, each multiple choice question has a 2-point value, 64 points total).
 CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
A how to guide to second quantization method.
 Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
0.1 Required Classical Mechanics
 0.1 Requred Classcal Mechancs The subject of statstcal mechancs deals wth macroscopc (large szed systems consstng of 10 23 partcles) systems. As a result, they have a huge number of degrees of freedom.
0.1 Requred Classcal Mechancs The subject of statstcal mechancs deals wth macroscopc (large szed systems consstng of 10 23 partcles) systems. As a result, they have a huge number of degrees of freedom.
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Fermi Statistics and Fermi Surface. Sommerfeld Theory. 2.1 Fermi Statistics and Fermi Surface
 erm Statstcs and erm Surface.1 erm Statstcs and erm Surface Snce Drude model, t too a quarter of a century for a breathrough to occur. That arose from the development of quantum mechancs and recognton
erm Statstcs and erm Surface.1 erm Statstcs and erm Surface Snce Drude model, t too a quarter of a century for a breathrough to occur. That arose from the development of quantum mechancs and recognton
10.34 Numerical Methods Applied to Chemical Engineering Fall Homework #3: Systems of Nonlinear Equations and Optimization
 10.34 Numercal Methods Appled to Chemcal Engneerng Fall 2015 Homework #3: Systems of Nonlnear Equatons and Optmzaton Problem 1 (30 ponts). A (homogeneous) azeotrope s a composton of a multcomponent mxture
10.34 Numercal Methods Appled to Chemcal Engneerng Fall 2015 Homework #3: Systems of Nonlnear Equatons and Optmzaton Problem 1 (30 ponts). A (homogeneous) azeotrope s a composton of a multcomponent mxture
Snce h( q^; q) = hq ~ and h( p^ ; p) = hp, one can wrte ~ h hq hp = hq ~hp ~ (7) the uncertanty relaton for an arbtrary state. The states that mnmze t
 8.5: Many-body phenomena n condensed matter and atomc physcs Last moded: September, 003 Lecture. Squeezed States In ths lecture we shall contnue the dscusson of coherent states, focusng on ther propertes
8.5: Many-body phenomena n condensed matter and atomc physcs Last moded: September, 003 Lecture. Squeezed States In ths lecture we shall contnue the dscusson of coherent states, focusng on ther propertes
where the sums are over the partcle labels. In general H = p2 2m + V s(r ) V j = V nt (jr, r j j) (5) where V s s the sngle-partcle potental and V nt
 Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Lecture 4 Hypothesis Testing
 Lecture 4 Hypothess Testng We may wsh to test pror hypotheses about the coeffcents we estmate. We can use the estmates to test whether the data rejects our hypothess. An example mght be that we wsh to
Lecture 4 Hypothess Testng We may wsh to test pror hypotheses about the coeffcents we estmate. We can use the estmates to test whether the data rejects our hypothess. An example mght be that we wsh to
PHYS 705: Classical Mechanics. Hamilton-Jacobi Equation
 1 PHYS 705: Classcal Mechancs Hamlton-Jacob Equaton Hamlton-Jacob Equaton There s also a very elegant relaton between the Hamltonan Formulaton of Mechancs and Quantum Mechancs. To do that, we need to derve
1 PHYS 705: Classcal Mechancs Hamlton-Jacob Equaton Hamlton-Jacob Equaton There s also a very elegant relaton between the Hamltonan Formulaton of Mechancs and Quantum Mechancs. To do that, we need to derve
10.34 Fall 2015 Metropolis Monte Carlo Algorithm
 10.34 Fall 2015 Metropols Monte Carlo Algorthm The Metropols Monte Carlo method s very useful for calculatng manydmensonal ntegraton. For e.g. n statstcal mechancs n order to calculate the prospertes of
10.34 Fall 2015 Metropols Monte Carlo Algorthm The Metropols Monte Carlo method s very useful for calculatng manydmensonal ntegraton. For e.g. n statstcal mechancs n order to calculate the prospertes of
PROPERTIES OF FRACTIONAL EXCLUSION STATISTICS IN INTERACTING PARTICLE SYSTEMS
 PROPERTIES OF FRACTIONAL EXCLUSION STATISTICS IN INTERACTING PARTICLE SYSTEMS DRAGOŞ-VICTOR ANGHEL Department of Theoretcal Physcs, Hora Hulube Natonal Insttute for Physcs and Nuclear Engneerng (IFIN-HH),
PROPERTIES OF FRACTIONAL EXCLUSION STATISTICS IN INTERACTING PARTICLE SYSTEMS DRAGOŞ-VICTOR ANGHEL Department of Theoretcal Physcs, Hora Hulube Natonal Insttute for Physcs and Nuclear Engneerng (IFIN-HH),
Integrals and Invariants of Euler-Lagrange Equations
 Lecture 16 Integrals and Invarants of Euler-Lagrange Equatons ME 256 at the Indan Insttute of Scence, Bengaluru Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng,
Lecture 16 Integrals and Invarants of Euler-Lagrange Equatons ME 256 at the Indan Insttute of Scence, Bengaluru Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng,
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Lagrangian Field Theory
 Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Psychology 282 Lecture #24 Outline Regression Diagnostics: Outliers
 Psychology 282 Lecture #24 Outlne Regresson Dagnostcs: Outlers In an earler lecture we studed the statstcal assumptons underlyng the regresson model, ncludng the followng ponts: Formal statement of assumptons.
Psychology 282 Lecture #24 Outlne Regresson Dagnostcs: Outlers In an earler lecture we studed the statstcal assumptons underlyng the regresson model, ncludng the followng ponts: Formal statement of assumptons.
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Lecture 12: Discrete Laplacian
 Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
THEOREMS OF QUANTUM MECHANICS
 THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
763622S ADVANCED QUANTUM MECHANICS Solution Set 1 Spring c n a n. c n 2 = 1.
 7636S ADVANCED QUANTUM MECHANICS Soluton Set 1 Sprng 013 1 Warm-up Show that the egenvalues of a Hermtan operator  are real and that the egenkets correspondng to dfferent egenvalues are orthogonal (b)
7636S ADVANCED QUANTUM MECHANICS Soluton Set 1 Sprng 013 1 Warm-up Show that the egenvalues of a Hermtan operator  are real and that the egenkets correspondng to dfferent egenvalues are orthogonal (b)
LINEAR REGRESSION ANALYSIS. MODULE IX Lecture Multicollinearity
 LINEAR REGRESSION ANALYSIS MODULE IX Lecture - 30 Multcollnearty Dr. Shalabh Department of Mathematcs and Statstcs Indan Insttute of Technology Kanpur 2 Remedes for multcollnearty Varous technques have
LINEAR REGRESSION ANALYSIS MODULE IX Lecture - 30 Multcollnearty Dr. Shalabh Department of Mathematcs and Statstcs Indan Insttute of Technology Kanpur 2 Remedes for multcollnearty Varous technques have
C/CS/Phy191 Problem Set 3 Solutions Out: Oct 1, 2008., where ( 00. ), so the overall state of the system is ) ( ( ( ( 00 ± 11 ), Φ ± = 1
 C/CS/Phy9 Problem Set 3 Solutons Out: Oct, 8 Suppose you have two qubts n some arbtrary entangled state ψ You apply the teleportaton protocol to each of the qubts separately What s the resultng state obtaned
C/CS/Phy9 Problem Set 3 Solutons Out: Oct, 8 Suppose you have two qubts n some arbtrary entangled state ψ You apply the teleportaton protocol to each of the qubts separately What s the resultng state obtaned
Lecture 10 Support Vector Machines II
 Lecture 10 Support Vector Machnes II 22 February 2016 Taylor B. Arnold Yale Statstcs STAT 365/665 1/28 Notes: Problem 3 s posted and due ths upcomng Frday There was an early bug n the fake-test data; fxed
Lecture 10 Support Vector Machnes II 22 February 2016 Taylor B. Arnold Yale Statstcs STAT 365/665 1/28 Notes: Problem 3 s posted and due ths upcomng Frday There was an early bug n the fake-test data; fxed
10. Canonical Transformations Michael Fowler
 10. Canoncal Transformatons Mchael Fowler Pont Transformatons It s clear that Lagrange s equatons are correct for any reasonable choce of parameters labelng the system confguraton. Let s call our frst
10. Canoncal Transformatons Mchael Fowler Pont Transformatons It s clear that Lagrange s equatons are correct for any reasonable choce of parameters labelng the system confguraton. Let s call our frst
Mechanics Physics 151
 Mechancs Physcs 151 Lecture 3 Lagrange s Equatons (Goldsten Chapter 1) Hamlton s Prncple (Chapter 2) What We Dd Last Tme! Dscussed mult-partcle systems! Internal and external forces! Laws of acton and
Mechancs Physcs 151 Lecture 3 Lagrange s Equatons (Goldsten Chapter 1) Hamlton s Prncple (Chapter 2) What We Dd Last Tme! Dscussed mult-partcle systems! Internal and external forces! Laws of acton and
Chapter 13: Multiple Regression
 Chapter 13: Multple Regresson 13.1 Developng the multple-regresson Model The general model can be descrbed as: It smplfes for two ndependent varables: The sample ft parameter b 0, b 1, and b are used to
Chapter 13: Multple Regresson 13.1 Developng the multple-regresson Model The general model can be descrbed as: It smplfes for two ndependent varables: The sample ft parameter b 0, b 1, and b are used to
LECTURE 9 CANONICAL CORRELATION ANALYSIS
 LECURE 9 CANONICAL CORRELAION ANALYSIS Introducton he concept of canoncal correlaton arses when we want to quantfy the assocatons between two sets of varables. For example, suppose that the frst set of
LECURE 9 CANONICAL CORRELAION ANALYSIS Introducton he concept of canoncal correlaton arses when we want to quantfy the assocatons between two sets of varables. For example, suppose that the frst set of
3.1 Expectation of Functions of Several Random Variables. )' be a k-dimensional discrete or continuous random vector, with joint PMF p (, E X E X1 E X
 Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 6.265/15.070J Fall 2013 Lecture 12 10/21/2013. Martingale Concentration Inequalities and Applications
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY 6.65/15.070J Fall 013 Lecture 1 10/1/013 Martngale Concentraton Inequaltes and Applcatons Content. 1. Exponental concentraton for martngales wth bounded ncrements.
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 6.65/15.070J Fall 013 Lecture 1 10/1/013 Martngale Concentraton Inequaltes and Applcatons Content. 1. Exponental concentraton for martngales wth bounded ncrements.
