Phase Space N p t 2 2 t 1 Phase space p, p, p,..., p, r, r, r,..., r N N 1 N r
|
|
|
- Shannon Atkins
- 6 years ago
- Views:
Transcription
1 Phase Space p t 2 2 t1 1 Phase space p,..., 1, p2, p3,..., p, r1, r1, r3 r r
2 Phase Orbt If thehamltonan of the system s denoted by H(q,p), the moton of phase pont can be along the phase orbt and s determned by the canoncal equaton of moton p H q q H p (=1 1,2...s) (1.1) 1) P Phase Orbt Constant energy surface H(q,p)=E (1.2) H ( q, p) E Therefore the phase orbt must le on a surface of constant energy (ergodc surface). 2
3 - space and -space Let us defne - space as phase space of one partcle (atom or molecule). The macrosystem phase space (-space) s equal to the sum of - spaces. The set of possble mcrostates can be presented by contnues set of phase ponts. Every pont can move by tself along t s own phase orbt. The overall pcture of ths movement possesses certan nterestng features, whch are best apprecated n terms of what we call a densty functon (q,p;t). Ths functon s defned n such a way that at any tme t, the number of representatve ponts n the volume element (d 3 q d 3 p) around the pont (q,p) of the phase space s gven by the product (q,p;t) d 3 q d 3 p. Clearly, the densty functon (q,p;t) symbolzes the manner n whch the members of the ensemble are dstrbuted over varous possble mcrostates at varous nstants of tme. 3
4 Functon of Statstcal Dstrbuton Let us suppose that the probablty of system detecton n the volume ddpdqdp 1... dp s dq 1... dq s near pont (p,q) equal dw (p,q)= (q,p)d. The functon of statstcal dstrbuton (densty functon) of the system over mcrostates n the case of nonequlbrum systems s also depends on tme. The statstcal average of a gven dynamcal physcal quantty f(p,q) s equal f f ( p, q) ( q, ( q, p ; t ) d p; t) d 3 3 qd qd 3 p 3 p (1.3) The rght phase portrat of the system can be descrbed by the set of ponts dstrbuted n phase space wth the densty. Ths number can be consdered as the descrpton of great (number of ponts) number of systems each of whch has the same structure as the system under observaton copes of such system at partcular tme, whch are by themselves exstng n admssble mcrostates 4
5 Statstcal Ensemble The number of macroscopcally dentcal systems dtbtd dstrbuted along admssble mcrostates wth densty defned as statstcal ensemble. A statstcal ensembles are defned and named by the dstrbuton functon whch characterzes t. The statstcal average value have the same meanng as the ensemble average value. An ensemble s sad to be statonary f does not dependd explctly l on tme,.e. at all tmes (1.4) t 0 Clearly, for such an ensemble the average value <f> of any physcal quantty f(p,q) wll be ndependent of tme. aturally, then, a statonary ensemble qualfes to represent a system n equlbrum. To determne the crcumstances under whch h Eq. (1.4) can hld hold, wehave to make arather study of the movement of the representatve ponts n the phase space. 5
6 Lovlle s theorem and ts consequences Consder an arbtrary "volume" " n the relevant regon of the phase space and let the "surface enclosng ths volume ncreases wth tme s gven by t d (1.5) where d d(d 3 q d 3 p). On the other hand, the net rate at whch h the representatve ponts flow out of the volume (across the boundng surface ) sgvenby σ ρ( ν n )dσ (1.6) here v s the vector of the representatve ponts n the regon of the surface element d, whle s the (outward) nˆ unt vector normal to ths element. By the dvergence theorem, (1.6) can bewrtten as 6
7 Statstcs of Multpartcle Systems n Thermodynamc Equlbrum The macroscopc thermodynamc parameters, X = (V,P,T, ), are macroscopcally observable quanttes that are, n prncple, functons of the canoncal varables,.e. f f( p1, p2,..., ps, q1, q2,..., qs), 1,2,..., n, n s ( f, f,..., f ) ( V, P, T,...) X 1 2 n X X ( p, p,..., p, q, q,..., q ) 1 2 s 1 2 s However, the specfcaton of all the macroparameters X does not determne a unque mcrostate, p p ( X), q q ( X) Consequently, on the bass of macroscopc measurements, one can make only statstcal statements about the values of the mcroscopc varables.
8 Statstcal Descrpton of Mechancal Systems Statstcal descrpton of mechancal systems s utlzed for mult-partcle problems, where ndvdual solutons for all the consttutve atoms are not affordable, or necessary. Statstcal descrpton can be used to reproduce averaged macroscopc parameters and propertes of the system. Comparson of objectves es of the determnstc and statstcal approaches: Determnstc partcle dynamcs Statstcal mechancs Provdes the phase vector, as a functon of tme Q(t), based on the vector of ntal condtons Q(0) Provdes the tme-dependent probablty densty to observe the phase vector Q, w(q,t), based on the ntal value w(q,0)
9 Statstcal Descrpton of Mechancal Systems From the contemporary pont of vew, statstcal mechancs can be regarded as a herarchcal multscale method, whch elmnates the atomstc degrees of freedom, whle establshng a determnstc mappng from the atomc to macroscale varables, and a probablstc mappng from the macroscale to the atomc varables: Mcrostates Macrostates (p,q) X k k determnstc conformty probablstc conformty
10 Dstrbuton Functon Though the specfcaton of a macrostate X cannot determne the mcrostate (p,q) = (p 1,p 2,,p s ; q 1,q 2,,q s ), a probablty densty w of all the mcrostates can be found, w p, p,..., p ; q, q,..., q ; t 1 2 s 1 2 s or abbrevated: wpqt (,, ) The probablty of fndng the system n a gven phase volume G: The normalzaton condton: WGt (, ) wpqtdpdq (,, ) ( pq, ) G w( p, q, t) dpdq 1
11 Statstcal Ensemble Wthn the statstcal descrpton, the moton of one sngle system wth gven ntal condtons s not consdered; thus, p(t), q(t) are not sought. Instead, the moton of a whole set of phase ponts, representng the collecton of possble states of the gven system. Such a set of phase ponts s called a phase space ensemble. If each pont n the phase space s consdered as a random quantty wth a partcular probablty ascrbed to every possble state (.e. a probablty densty w(p,q,t) s ntroduced n the phase space), the relevant phase space ensemble s called a statstcal ensemble. p t = t 2 : G 2 G volume n the phase space, occuped by the statstcal ensemble. t = t 1 : G 1 q
12 Statstcal Averagng Statstcal average (expectaton) of an arbtrary physcal quantty F(p,q), s gven most generally by the ensemble average, F() t F( p, q) w( p, q,) t dpdq ( pq, ) The root-mean-square fluctuaton (standard devaton): ( F) F F 2 The curve representng the real moton (the expermental curve) wll mostly proceed wthn the band of wdth 2Δ(F) F True value F F ( F ) For some standard equlbrum systems, thermodynamc parameters can be obtaned, usng a sngle phase space ntegral. Ths approach s dscussed below. t
13 Ergodc Hypothess and the Tme Average Evaluaton of the ensemble average (prevous slde) requres the knowledge of the dstrbuton functon w for a system of nterest. Alternatvely, the statstcal average can be obtaned by utlzng the ergodc hypothess n the form, Here, the rght-hand hand sde s the tme average (n practce, tme t s chosen fnte, though as large as possble) F 1 t F F p (), q () d, t t 0 Ths approach requres F as a functon of the generalzed coordnates. Some examples Internal energy: U H( p, q) 2Ek Temperature: T k U du Change of entropy: S U T vl Gas dffuson constant: t D 3 B T 2 2 T 1 1 F C T V dt Here, E k mean knetc energy per degree of freedom v mean velocty of molecules l mean free path of gas partcles
14 Law of Moton of a Statstcal Ensemble A statstcal ensemble s descrbed by the probablty densty n phase space, w(p,q,t). It s mportant to know how to fnd w(p,q,t) at an arbtrary tme t, when the ntal functon w(p,q,0) q0) at the tme t = 0sgven gven. In other words, the equaton of moton satsfed by the functon w(p,q,t) s needed. p w(p,q,t 1 ) w(p,q,t qt 2 ) w(p,q,0) Γ 2 Γ 1 Γ 0 q The moton of of an ensemble n phase space may be consdered as the moton of a phase space flud n analogy to the moton of an ordnary flud n a 3D space. Louvlle s theorem clams that Due to Louvlle s theorem, the followng equaton of moton holds 2s w H w H w [ H, w], [ H, w] (Posson bracket) t 1 q p p q
15 Equlbrum Statstcal Ensemble: Ergodc Hypothess For a system n a state of thermodynamc equlbrum the probablty densty n phase space must not depend explctly on tme, w t Thus, the equaton of moton for an equlbrum statstcal ensemble reads 0 [ H, w] 0 A drect soluton of ths equaton s not tractable. Therefore, the ergodc hypothess (n a more general form) s utlzed: the probablty densty n phase space at equlbrum depends only on the total energy: w ( p, q ) H ( p, q, a ) otes: the Hamltonan gves the total energy requred; the Hamltonan may depend on the values of external parameters a = (a 1, a 2, ), besdes the phase vector X. Ths dstrbuton functon satsfes the equlbrum equaton of moton, because [ H, ( H )] 0 Exercse: Check the above equalty.
16 Ensemble Method Ensemble? : Infnte number of mental replca of the system of nterest Large Reservor (const.t) All the ensemble members have the Same,V.T Energes can be exchanged but molecules cannot. Current = 20 but nfnty
17 Two postulates Long tme average = Ensemble average at nfnty t tme E1 E2 E3 E4 E5 In an ensemble, the systems of enembles are dstrbuted unformly (equal probablty or frequency) Ergodc Hypothess Prncple of equal a pror probablty
18 Averagng Method Probablty of observng partcular quantum state P n Ensemble average of a dynamc property n E E P Tme average and ensemble average U lm E t lm n E P
19 Calculaton of Probablty n Ensemble Several methods are avalable Method of Undetermned multpler : :
20 Maxmzaton of Weght Most probable dstrbuton Weght W! n! n! n! ! n!
21 Ensembles Mcro canoncal ensemble: E,V, Canoncal ensemble: T,V, Constant pressure ensemble: T,P, Grand canoncal ensemble: T,V,μ 21
22 Canoncal Ensemble: The canoncal ensemble occurs when a system wth fxed V and and the system s at constant temperature (connected to an nfnte heat bath). In the canoncal ensemble, the probablty of each mcrostate s proportonal to exp (- βem).
23 E 1 E EE 2 1 Systems 1 and 2 are weakly coupled such that they can exchange energy. What wll be E 1? E, EE E EE BA: each confguraton s equally probable; but the number of states that gve an energy E 1 s not know. 23
24 E, E E E E E E E E E E E ln, ln ln ln E 1, E E1 Energy s conserved! 0 de E 1 =-de 2 1, V 1 1 ln E E 2 1 ln 1 E 1 0 E 1 1 E 1,V 1 2,V 2 Ths can be seen as ln 1 E 1 ln 2 EE 1 an equlbrum E1 E2 condton, V, V ln E E V, 24
25 Entropy and number of confguratons Conjecture: S ln 25
26 S k ln E B Wth k B = J/K In thermodynamcs, the absolute (Kelvn) temperature scale was defned such that S E V, 1 T But we defne: n 1 de TdS-pdV d ln EE E V, 26
27 And ths gves the statstcal defnton of temperature: k B E 1 ln E T In short: V, Entropy and temperature are both related to the fact that we can COUT states. Basc assumpton: 1. leads to an equlbrum condton: equal temperatures 2. leads to a maxmum of entropy 3. leads to the thrd law of thermodynamcs 27
28 umber of confguratons How large s? For macroscopc systems, super-astronomcally large. For nstance, for a glass of water at room temperature: Macroscopc devatons from the second law of thermodynamcs are not forbdden, but they are extremely unlkely. 28
29 Canoncal ensemble 1/k B T Consder a small system that can exchange heat wth a bg rese ln E E E E E E E E ln ln ln Hence, the probablty to fnd E : P E E E E E k T E E exp E k T B EE exp E k T j expe k T P E B j j B j B Boltzmann dstrbuton 29
30 Averagng Method Probablty of observng partcular quantum state P n Ensemble average of a dynamc property n E E P Tme average and ensemble average U lm E t lm n E P
31 Thermodynamcs What s the average age energy e of the system? E Compare: EP E FT 11 T E E exp E exp E j j ln e p E ln Q ln exp VT,, Hence: F ln Q VT,, kt B 31
32 Canoncal Partton Functon Q e j E j
33 The Boltzmann Dstrbuton Task : Fnd the domnatng confguraton for gven and total energy E. Fnd Max. W whch satsfes ; n dn 0 E t En E 0 dn
34 Method of Undetermned Maxmum weght, W Multplers Recall the method to fnd mn, max of a functon d ln W 0 lnw dn 0 Method of undetermned multpler : Constrants should be multpled by a constant and added to the man varaton equaton.
35 Method of undetermned multplers Method of undetermned multplers ln ln E dn dn dn dn W W d 0 ln dn E dn W 0 ln E W 0 E dn
36 n n W ln ln ln j j j n n n n n W ) ln ( ln ln 1 ln 1 ln 1 ln ln n n n j j j j j j n n n n n n n 1 ln 1 ln ) ln ( j j j j j n n n n n lnw n n n W ln 1) (ln 1) (ln ln
37 ln 0 E n E e n E j j e e n E j j j e 1 j e E j Boltzmann Dstrbuton E E j e e n P (Probablty functon for energy dstrbuton) j
38 Canoncal Partton Functon Boltzmann Dstrbuton P n e e E E j e Q Canoncal Partton Functon j E Q e j E j
39 Canoncal Dstrbuton: Prelmnary Issues One mportant prelmnary ssue related to the use of Gbbs canoncal dstrbuton s the addtvty of the Hamltonan of a mechancal system. Structure of the Hamltonan of an atomc system: kn H E 1 2 U 1 2 ( p, p,..., p ) ( r, r,..., r ) p ( ) (, ) (,, )... 2 W1 r W2 r rj W3 r rj rk 2m, j, j, k j Here, knetc energy and the one-body bd potental tlare addtve,.e. they can be expanded nto the components, each correspondng to one partcle n the system: kn kn kn E ( p 1, p 2,..., p ) E ( p 1) E ( p 2)... W1( r ) W1( r1) W1( r2)... Two-body and hgher order potentals are non-addtve (functon Q 2 does not exst), W ( r ) W r r W ( r, r )... Q ( r)... Q ( r )..., 2 j j 2 j 2 2 j, j
40 Canoncal Dstrbuton: Prelmnary Issues Thus, f the nter-partcle nteracton s neglgble, W W... E W kn the system s descrbed by an addtve Hamltonan, p H h, h W ( ) 2 1 r 2m Here, H s the total Hamltonan, and h s the one-partcle Hamltonan. For the statstcal descrpton, t s suffcent that ths requrement holds for the averaged quanttes only. The mult-body components, W >1, cannot be completely excluded from the physcal consderaton, as they are responsble for heat transfer and establshng the thermodynamc equlbrum between consttutve parts of the total system. A mcromodel wth small averaged contrbutons to the total energy due to partcle-partcle nteractons s called the deal gas. Example: partcles n a crcular cavty. Statstcally averaged value W 2 s small: 3 partcles: 5 partcles: tot tot W E W E
41 Canoncal Dstrbuton Suppose that system under nvestgaton Σ 1 s n thermal contact and thermal equlbrum wth a much larger system Σ 2 that serve as the thermostat,or heat or heat bath at the temperature T. From the mcroscopc pont of vew, both Σ 1 and Σ 2 are mechancal systems whose states are descrbed by the phase vectors (sets of canoncal varables X 1 and X 2 ). The entre system Σ 1 +Σ 2 s adabatcally solated, and therefore the mcrocanoncal dstrbuton s applcable to Σ 1 +Σ 2, 1 w( p1, q1; p2, q2) E H( p1, q1; p2, q2) ( E) Assume 1 and 2 are number of partcles n Σ 1 and Σ 2 respectvely. Provded that 1 << 2, the Gbbs canoncal dstrbuton apples to Σ 1 : Thermostat T Σ 1 2 Σ 1 Σ H( p, q) 1 kt wpq (, ) e ( pq, ) ( p1, q1) Z
42 CanoncalDstrbuton: Partton Functon The normalzaton factor Z for the canoncal dstrbuton called the ntegral over states or partton functon s computed as H( pqa,, ) 1 kt Z e dpdq 3 (2 )! ( pq, ) H ( pr, ) 1 kt e d d d 1... d (2 )! p p r r ( pr, ) Σ 2 Thermostat T 1 Σ 1 2 Before the normalzaton, ths ntegral represents the statstcally t t averaged phase volume occuped by the canoncal ensemble. The total energy for the canoncal ensemble s not fxed and n prncple t may The total energy for the canoncal ensemble s not fxed, and, n prncple, t may occur arbtrary n the range from to (for the nfntely large thermostat, 2 ).
43 Partton Functon and Thermodynamc Propertes The partton functon Z s the major computatonal characterstc of the canoncal ensemble. The knowledge of Z allows computng thermodynamc parameters of the closed sothermal system (a V, external parameter): Free energy: (relates to mechancal work) Entropy (varety of mcrostates) Pressure Internal energy FTV (, ) ktlnz F S kln ZT ln Z T T V F P kt ln Z VT V 2 U FTSkT ln Z T These are the major results n terms of practcal calculatons over canoncal ensembles. Class exercse: check the last three above formulas wth the the method of thermodynamc potentals, usng the frst formula for the free energy.
44 Thermodynamc Propertes and Canoncal Ensemble Internal Energy U Q U E, V ( qs) E P, V E e E 1 Q ( qs) E 1 Q ln Q Q e E, V
45 Thermodynamc Propertes and Canoncal Ensemble Pressure at state Small Adabatc expanson of system dx ( w ) ( de P ) PdV F dx E V F dx PdV w dv de F E V
46 Thermo recall (2) Frst law of thermodynamcs de TdS pdv Helmholtz Free energy: F ETS df SdT pdv FT 1 F F F F T 1T T 1T T F TS E 46
47 We have assume quantum mechancs (dscrete states) but we are nterested n the classcal lmt exp 2 1 p d d exp 3 h! p 2 m 1 3 h Volume of phase space (partcle n a box) E r U r 1 Partcles are ndstngushable! Integraton over the momenta can be carred out for most system p p m dp exp dp exp 2 m 2 m 47
48 Defne de Brogle wave length: h 2 m Partton functon: 1 Q, V, T d exp 3! r U r 48
49 Example: deal gas 1 Q, V, T d expu r 3! r 1 V d 1 3 3! r! Free energy: Pressur e: V F ln 3! 3 ln ln ln 3 ln V F P V T V Energy: E F k T B 49
50 Ideal gas (2) Chemcal potental: = F T,V, j F ln 3 ln V ln 3 ln 1 IG 0 ln 50
51 Ensembles Mcro canoncal ensemble: E,V, Canoncal ensemble: T,V, Constant pressure ensemble: T,P, Grand canoncal ensemble: T,V,μ 51
52 Summary: Canoncal ensemble (,V,T) Partton functon: 1 Q, V, T d exp 3! r U r Probablty to fnd a partcular confguraton P Free energy exp U F ln Q VT,, 52
53 Thermodynamc Propertes and Canoncal Ensemble Pressure at quantum state P P Pressure PP E 1 E P Pe Q Q V ln Q E e V, Q V 1 ln Q P lnv Probablty 1 E E e Equaton of State n Statstcal Mechancs
54 Thermodynamc Propertes and Canoncal Ensemble w q du Entropy rev rev PdE E dp P E d du w q du ) ( rev w PdV dv E P de P rev dp Q PdP E dp w dv dv V d ) ln ln ( 1 PdP Q ln 1 ) (
55 Thermodynamc Propertes and Canoncal Ensemble Entropy q q PdE rev rev 1 d ( ln PdP q P ln P ) TdS d( U ln Q) TdS rev S S S k ln Q U / T S0 k ln Q U / T k P ln P k ln P The only functon that lnks heat (path ntegral) and state property s TEMPERATURE. 1/ kt
56 Summary of Thermodynamc Propertes n Canoncal Ensemble U S ln Q kt ( ) lnt ln Q kln Q ( ) lnt V, V, ln Q ln Q H kt( ) V, ( ) T, All thermodynamc propertes lnt lnv Can be obtaned from A kt ln Q PARTITIO FUCTIO G ln Q ) lnv ktln Q ( T, ln Q kt T, V, j
57 Ensembles Mcro canoncal ensemble: E,V, Canoncal ensemble: T,V, Constant pressure ensemble: T,P, Grand canoncal ensemble: T,V,μ 57
58 Constant pressure smulatons: V, E, E E V V 1/k B T PT,P,T ensemble p/k B T Consder a small system that can exchange volume and energy wth a bg reservor ln ln ln V V, E E ln V, E E V E V V E EE, V V E pv ln EV kt kt, B B Hence, the probablty to fnd E,V : P E, V EE, exp E V V pv jk, jk, E E V V j, k exp Ej pv k exp E pv 58
59 Thermo recall (4) Frst law of thermodynamcs Hence and d E T d S p d V + d 1 S = EV, T S V T, p T 59
60 ,P,T,, ensemble (2) In the classcal lmt, the partton functon becomes 1 3,, exp d exp Q P T dv PV U r! r The probablty to fnd a partcular confguraton: r, V r, exp P V PV U r 60
61 Grand canoncal smulatons:, E, E E 1/k B T μ,v,t VT ensemble -μ/k B T Consder a small system that can exchange partcles and energy wth a bg reservor ln ln ln, E E ln, E E E E EE, E ln E kt kt, B B Hence, the probablty to fnd E, : P E, EE, exp E jk, jk, E E j, k exp Ej k k exp E 61
62 Thermo recall (5) Frst law of thermodynamcs Hence and d E T d S p d V + d 1 S = EV, T S TV, T 62
63 μ,v,t, ensemble (2) In the classcal lmt, the partton functon becomes exp Q, V, T d expur 3! r 1 The probablty to fnd a partcular confguraton:, r exp, r P U r 63
64 Classcal Statstcal Mechancs It s not easy to derve all the partton functons usng quantum mechancs Classcal mechancs can be used wth neglgble error when energy dfference between energy levels (E) aresmaller thank kt. However, vbraton and electronc states cannot be treated wth classcal mechancs. (The energyspacngs areorder of kt)
65 Phase Space Recall Hamltonan ofewtonanmechancs H ( r H, p ) KE(knetc energy) PE(potental energy) p ( r, p ) U ( r1, r2,..., r 2m H p r H r p Instead of takng replca of systems (ensemble members), use abstract phase p space compose of momentum space and poston space (6) Average of nfnte phase space )
66 Ensemble Average U 1 lm E( ) d lm 0 np ( ) E( ) d P ( d ) Fracton of Ensemble members n ths range (d) d) Usng smlar technque used for Boltzmann dstrbuton b P ( ) d exp( H / kt ) d... exp( H / kt ) d
67 Canoncal Partton Functon Phase Integral T... exp( H / kt ) d Canoncal Partton Functon Q... c exp( H / kt ) d Match between Quantum and Classcal Mechancs c lm... exp( E / kt )... exp( H / kt) d!h T ) For rgorous dervaton see Hll, Chap.6 c 1 F
68 Canoncal Partton Functon n Classcal Mechancs Q 1... exp( H / kt ) d! h F
69 Example : Translatonal Partton Functon for an Ideal Gas H ( r H ( r, p, p 3 2 p H 2m ) KE(knetc energy) PE(potental energy) p ) U ( r, r2,..., r 2m 1 ) o potental energy, 3 dmensonal space. Q 1... exp( 3! h 1! h 3 exp( 1 2 mkt 2! h 3 / 2 p 2m V 2 p 2m ) dp 3 ) dp... dp V 0 1 dr dr 1 2 dr dr... dr 3 1
70 Sem Classcal Partton Functon The energy of a molecule s dstrbuted n dfferent modes Vbraton, Rotaton (Internal : depends only on T) Translaton (External : depends on T and V) Assumpton 1 Hamltonan operator can be separated nto two parts (nternal + center of mass moton) H op H CM op H nt op Q exp( E CM E kt nt ) exp( E kt CM ) exp( E kt nt ) Q Q CM (, V, T ) Qnt (, T )
71 Sem Classcal Partton Functon Internal parts are densty ndependent and most of the components have the same value wth deal gases. Q nt (,, T ) Qnt (,0, T ) For solds and polymerc molecules, ths assumpton s not vald any more.
72 Sem Classcal Partton Functon Assumpton 2 For T > 50K, classcal approxmaton can be used for translatonal part.
73 H CM Q! 3 p 2 x p 2 y 2m p 2 z U ( r, r,..., r p x y z 3... exp( ) dp... 3! h 2mkT Z 1 p 2 p ) ( U / kt) dr 3 1/ 2 2 h 2 mkt Z... ( U / kt) dr1 dr2... dr 3 Confguraton Integral Q 1 3 nt Q! Z For non central forces (orentaton effect) 1 Z d... ( U / kt) dr dr... dr d... d 1
74 Canoncal Ensembles After adopton of the ergodc hypothess, t then remans to determne the actual form of the functon φ(h). Ths functon depends on the type of the thermodynamc system under consderaton,.e. on the character of the nteracton between the system and the external bodes. We wll consder canoncal ensembles of two types of systems: 1) Adabatcally solated systems that have no contact wth the surroundngs and have a specfed energy E. The correspondng statstcal ensemble s referred to as the mcrocanoncal ensemble, and the dstrbuton functon mcrocanoncal dstrbuton. 2) Closed sothermal systems that are n contact and thermal equlbrum wth an external thermostat of a gven temperature T. The correspondng statstcal ensemble s referred to as the canoncal ensemble,, and the dstrbuton functon Gbbs canoncal dstrbuton. Both systems do not exchange partcles wth the envronment.
75 Mcrocanoncal Dstrbuton For an adabatcally solated system wth constant t external parameters, a, the total t energy cannot vary. Therefore, only such mcrostates X can occur, for whch H ( p, q, a) E constant Ths mples (δ Drac s delta functon) and fnally: wpq (, ) E H( pqa,, ) E, a 1 wpq (, ) E H( pqa,, ) ( Ea, ) where Ω s the normalzaton factor, pq ( Ea, ) EH ( pqa,, ) dpdq pq ( pq, ) (P,T,V, ) Wthn the mcrocanoncal ensemble, all the energetcally allowed mcrostates have an equal probablty to occur.
76 Mcrocanoncal Dstrbuton: Integral Over States The normalzaton o factor Ω s gven by ( Ea, ) ( Ea, ) E a Phase volume where Γ s the ntegral over states, or phase ntegral: ( E, a ) 1 f (2 )! 1 (2 ) f! H ( p, q, a) E dpdq d (, ) 1 1 E H( pq, ) d p... d p d q... d q ( pq, ) 0, x 0 1, x 0 ( x), - Planck's constant, j - number of DOF per partcle Γ(E,a) represents the normalzed phase volume, enclosed wthn the hypersurface of gven energy determned by the equaton H(X,a) = E. Phase ntegral Γ s a dmensonless quantty. Thus the normalzaton factor Ω shows the rate at whch the phase volume vares due to a change of total energy at fxed external parameters.
77 Mcrocanoncal Dstrbuton: Integral Over States The ntegral over states s a major calculaton characterstc of the mcrocanoncal ensemble. The knowledge of Γ allows computng thermodynamc parameters of the closed adabatc system: 1 S 1 S k ln, T, P E ( E, V) V E (These are the major results n terms of practcal calculatons over mcrocanoncal ensembles.)
78 Summary: mcro canoncal ensemble (,V,E) Partton functon: 1 3,, d d, Q V E H E h! p r p r Probablty to fnd a partcular confguraton Free energy P 1 S ln Q V,, E 78
79 Mcrocanoncal Ensemble: Illustratve Examples We wll consder one-dmensonal llustratve examples of computng the phase ntegral, entropy and temperature for mcrocanoncal ensembles: Sprng-mass harmonc oscllator Pendulum (non-harmonc oscllator) We wll use the Hamltonan equatons of moton to get the phase space trajectory, and dthen evaluate the phase ntegral.
80 Harmonc Oscllator: Hamltonan Hamltonan: general form H T( p) U( x) Knetc energy T 2 p 2m k m Potental energy U kx 2 2 x Potental energy s a quadratc functon of the coordnate (dsplacement form the equlbrum poston) The total Hamltonan H ( p, x) p kx 2m Parameters: m 10 kg, k /m
81 Harmonc Oscllator: Equatons of Moton and Soluton Hamltonan and equatons moton: Parameters: m10 kg, k 2510 /m 2 2 p kx H H p H ( x, p) x, p x, p kx 2m 2 p x m Intal condtons (m, m/s): x(0) 0.2, x (0) 0 x (0) 0.4, x (0) 0 x(0) 0.6, x (0) 0
82 Harmonc Oscllator: Total Energy Total energy: E H( x, p) Const (at any x( t), p( t)) E 1 x(0) 0.2 x (0) 0 E 2 E 3 x(0) 0.4 x (0) 0 x(0) 0.6 x (0) 0 E J, E J, E J
83 Phase ntegral: Harmonc Oscllator: Phase Integral 1 ( E) A( E), A( E) E H( x, p) dxdp E H(, j ) 2 2 ( x, p) x p x p 0 j0 2 x / step for x, 2 p / step for p, number of ntegraton steps x max p max A 1 A 2 A For the harmonc oscllator, phase volume grows lnearly wth the ncrease of total energy
84 Harmonc Oscllator: Entropy and Temperature 23 Entropy: S kln, k J/K 22 S J/K 22 S2 22 S J/K J/K 1 S Temperature: T E We perturb the ntal condtons (on 0.1% or less) and compute new values The temperature s computed then, as 1 1 S S 2 kn T ( benchmark: T E ) E E k T T T K K K E and S.
85 Pendulum: Total Energy Total energy: 2 p E H(, p) mglcos Const (at any ( t), p( t)) 2 2ml E 1 (0) 0.3 (0) 0 E 2 (0) 1.8 (0) 0 E 3 (0) 3.12 (0) 0 p l - angular momentum E J, E J, E J
86 Phase ntegral: Pendulum: Phase Integral 1 ( E) A( E), A( E) E H(, p) ddp E H(, j ) 2 2 (, p ) p p 0 j0 2 x / step for, 2 p / step for p, number of steps max p max A 1 A 2 A For the pendulum, phase volume grows O-lnearly wth the ncrease of total energy at large ampltudes
87 Pendulum: Entropy and Temperature Entropy: S kln, 23 k J/K 22 S J/K 22 S2 22 S J/K J/K 1 S Temperature: T E We perturb the ntal condtons (on 0.1% or less) and compute new values The temperature s computed then, as 1 S S 2 kn T ( benchmark: T E ) E E k T 1 T T K 6.3K K 96.0 K E and S.
88 Summary of the Statstcal Method: Mcrocanoncal Dstrbuton 1. Analyze the physcal model; justfy applcablty of the mcrocanoncal dstrbuton. 2. Model lndvdual d partcles and dboundares. 3. Model nteracton between partcles and between partcles and boundares. 4. Set up ntal condtons and solve for the determnstc trajectores (MD). 5. Compute two values of the total energy and the phase ntegral for the orgnal and perturbed ntal t condtons. 6. Usng the method of thermodynamc parameters, compute entropy, temperature and other thermodynamc parameters. If possble compare the obtaned value of temperature wth benchmark values. 1 S 1 S kln, T, P E ( E, V ) V E 7. If requred, accomplsh an extended analyss of macroscopc propertes (e.g. functons T(E), S(E), S(T), etc.) by repeatng the steps 4-7.
89 Free Energy and Isothermal Processes Free energy, also Hl Helmholtz hlt potental tls of mportance for the descrpton of sothermal processes. It s defned as the dfference between nternal energy and the product of temperature and entropy. F U TS Snce free energy s a thermodynamc potental, the functon F(T,V,, ) ) guarantees the full knowledge of all thermodynamc quanttes. Physcal content of free energy: the change of the free energy df of a system at constant temperature, represents the work accomplshed by, or over, the system. Indeed, df du TdS SdT du TdS W df SdT W W Isothermal processes tend to a mnmum of free energy,.e. due to the defnton, smultaneously to a mnmum of nternal energy and maxmum of entropy.
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
12. The Hamilton-Jacobi Equation Michael Fowler
 1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Property calculation I
 .0, 3.0, 0.333,.00 Introducton to Modelng and Smulaton Sprng 0 Part I Contnuum and partcle methods Property calculaton I Lecture 3 Markus J. Buehler Laboratory for Atomstc and Molecular Mechancs Department
.0, 3.0, 0.333,.00 Introducton to Modelng and Smulaton Sprng 0 Part I Contnuum and partcle methods Property calculaton I Lecture 3 Markus J. Buehler Laboratory for Atomstc and Molecular Mechancs Department
10. Canonical Transformations Michael Fowler
 10. Canoncal Transformatons Mchael Fowler Pont Transformatons It s clear that Lagrange s equatons are correct for any reasonable choce of parameters labelng the system confguraton. Let s call our frst
10. Canoncal Transformatons Mchael Fowler Pont Transformatons It s clear that Lagrange s equatons are correct for any reasonable choce of parameters labelng the system confguraton. Let s call our frst
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Mechanics Physics 151
 Mechancs Physcs 151 Lecture 3 Lagrange s Equatons (Goldsten Chapter 1) Hamlton s Prncple (Chapter 2) What We Dd Last Tme! Dscussed mult-partcle systems! Internal and external forces! Laws of acton and
Mechancs Physcs 151 Lecture 3 Lagrange s Equatons (Goldsten Chapter 1) Hamlton s Prncple (Chapter 2) What We Dd Last Tme! Dscussed mult-partcle systems! Internal and external forces! Laws of acton and
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
CHAPTER 6. LAGRANGE S EQUATIONS (Analytical Mechanics)
 CHAPTER 6 LAGRANGE S EQUATIONS (Analytcal Mechancs) 1 Ex. 1: Consder a partcle movng on a fxed horzontal surface. r P Let, be the poston and F be the total force on the partcle. The FBD s: -mgk F 1 x O
CHAPTER 6 LAGRANGE S EQUATIONS (Analytcal Mechancs) 1 Ex. 1: Consder a partcle movng on a fxed horzontal surface. r P Let, be the poston and F be the total force on the partcle. The FBD s: -mgk F 1 x O
THEOREMS OF QUANTUM MECHANICS
 THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
THEOREMS OF QUANTUM MECHANICS In order to develop methods to treat many-electron systems (atoms & molecules), many of the theorems of quantum mechancs are useful. Useful Notaton The matrx element A mn
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
More metrics on cartesian products
 More metrcs on cartesan products If (X, d ) are metrc spaces for 1 n, then n Secton II4 of the lecture notes we defned three metrcs on X whose underlyng topologes are the product topology The purpose of
More metrcs on cartesan products If (X, d ) are metrc spaces for 1 n, then n Secton II4 of the lecture notes we defned three metrcs on X whose underlyng topologes are the product topology The purpose of
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
ESCI 341 Atmospheric Thermodynamics Lesson 10 The Physical Meaning of Entropy
 ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Canonical transformations
 Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Poisson brackets and canonical transformations
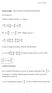 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Module 1 : The equation of continuity. Lecture 1: Equation of Continuity
 1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
coordinates. Then, the position vectors are described by
 Revewng, what we have dscussed so far: Generalzed coordnates Any number of varables (say, n) suffcent to specfy the confguraton of the system at each nstant to tme (need not be the mnmum number). In general,
Revewng, what we have dscussed so far: Generalzed coordnates Any number of varables (say, n) suffcent to specfy the confguraton of the system at each nstant to tme (need not be the mnmum number). In general,
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Notes on Analytical Dynamics
 Notes on Analytcal Dynamcs Jan Peters & Mchael Mstry October 7, 004 Newtonan Mechancs Basc Asssumptons and Newtons Laws Lonely pontmasses wth postve mass Newtons st: Constant velocty v n an nertal frame
Notes on Analytcal Dynamcs Jan Peters & Mchael Mstry October 7, 004 Newtonan Mechancs Basc Asssumptons and Newtons Laws Lonely pontmasses wth postve mass Newtons st: Constant velocty v n an nertal frame
Monte Carlo method II
 Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
Course MP3 Lecture 5 14/11/2006 Monte Carlo method II How to put some real physcs nto the Monte Carlo method Dr James Ellott 5.1 Monte Carlo method revsted In lecture 4, we ntroduced the Monte Carlo (MC)
Thermodynamics and Kinetics of Solids 33. III. Statistical Thermodynamics. Â N i = N (5.3) N i. i =0. Â e i = E (5.4) has a maximum.
 hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
x = , so that calculated
 Stat 4, secton Sngle Factor ANOVA notes by Tm Plachowsk n chapter 8 we conducted hypothess tests n whch we compared a sngle sample s mean or proporton to some hypotheszed value Chapter 9 expanded ths to
Stat 4, secton Sngle Factor ANOVA notes by Tm Plachowsk n chapter 8 we conducted hypothess tests n whch we compared a sngle sample s mean or proporton to some hypotheszed value Chapter 9 expanded ths to
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Chapter 1. Probability
 Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
5.62 Physical Chemistry II Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
PHYS 705: Classical Mechanics. Newtonian Mechanics
 1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
Lecture 5: Ideal monatomic gas
 Lecture 5: Ideal monatomc gas Statstcal mechancs of the perfect gas Ams: Key new concepts and methods: Countng states Waves n a box. Demonstraton that β / kt Heat, work and Entropy n statstcal mechancs
Lecture 5: Ideal monatomc gas Statstcal mechancs of the perfect gas Ams: Key new concepts and methods: Countng states Waves n a box. Demonstraton that β / kt Heat, work and Entropy n statstcal mechancs
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
Snce h( q^; q) = hq ~ and h( p^ ; p) = hp, one can wrte ~ h hq hp = hq ~hp ~ (7) the uncertanty relaton for an arbtrary state. The states that mnmze t
 8.5: Many-body phenomena n condensed matter and atomc physcs Last moded: September, 003 Lecture. Squeezed States In ths lecture we shall contnue the dscusson of coherent states, focusng on ther propertes
8.5: Many-body phenomena n condensed matter and atomc physcs Last moded: September, 003 Lecture. Squeezed States In ths lecture we shall contnue the dscusson of coherent states, focusng on ther propertes
5.04, Principles of Inorganic Chemistry II MIT Department of Chemistry Lecture 32: Vibrational Spectroscopy and the IR
 5.0, Prncples of Inorganc Chemstry II MIT Department of Chemstry Lecture 3: Vbratonal Spectroscopy and the IR Vbratonal spectroscopy s confned to the 00-5000 cm - spectral regon. The absorpton of a photon
5.0, Prncples of Inorganc Chemstry II MIT Department of Chemstry Lecture 3: Vbratonal Spectroscopy and the IR Vbratonal spectroscopy s confned to the 00-5000 cm - spectral regon. The absorpton of a photon
Lecture 3: Boltzmann distribution
 Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Lecture 3: Boltzmann dstrbuton Statstcal mechancs: concepts Ams: Dervaton of Boltzmann dstrbuton: Basc postulate of statstcal mechancs. All states equally lkely. Equal a-pror probablty: Statstcal vew of
Physics 141. Lecture 14. Frank L. H. Wolfs Department of Physics and Astronomy, University of Rochester, Lecture 14, Page 1
 Physcs 141. Lecture 14. Frank L. H. Wolfs Department of Physcs and Astronomy, Unversty of Rochester, Lecture 14, Page 1 Physcs 141. Lecture 14. Course Informaton: Lab report # 3. Exam # 2. Mult-Partcle
Physcs 141. Lecture 14. Frank L. H. Wolfs Department of Physcs and Astronomy, Unversty of Rochester, Lecture 14, Page 1 Physcs 141. Lecture 14. Course Informaton: Lab report # 3. Exam # 2. Mult-Partcle
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Week3, Chapter 4. Position and Displacement. Motion in Two Dimensions. Instantaneous Velocity. Average Velocity
 Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Density matrix. c α (t)φ α (q)
 Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
χ x B E (c) Figure 2.1.1: (a) a material particle in a body, (b) a place in space, (c) a configuration of the body
 Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
Quantum Statistical Mechanics
 Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
Chapter 8 Quantum Statstcal Mechancs 8.1 Mcrostates For a collecton of N partcles the classcal mcrostate of the system s unquely specfed by a vector (q, p) (q 1...q N, p 1...p 3N )(q 1...q 3N,p 1...p 3N
EPR Paradox and the Physical Meaning of an Experiment in Quantum Mechanics. Vesselin C. Noninski
 EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
PHYS 705: Classical Mechanics. Canonical Transformation II
 1 PHYS 705: Classcal Mechancs Canoncal Transformaton II Example: Harmonc Oscllator f ( x) x m 0 x U( x) x mx x LT U m Defne or L p p mx x x m mx x H px L px p m p x m m H p 1 x m p m 1 m H x p m x m m
1 PHYS 705: Classcal Mechancs Canoncal Transformaton II Example: Harmonc Oscllator f ( x) x m 0 x U( x) x mx x LT U m Defne or L p p mx x x m mx x H px L px p m p x m m H p 1 x m p m 1 m H x p m x m m
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
PHYS 705: Classical Mechanics. Hamilton-Jacobi Equation
 1 PHYS 705: Classcal Mechancs Hamlton-Jacob Equaton Hamlton-Jacob Equaton There s also a very elegant relaton between the Hamltonan Formulaton of Mechancs and Quantum Mechancs. To do that, we need to derve
1 PHYS 705: Classcal Mechancs Hamlton-Jacob Equaton Hamlton-Jacob Equaton There s also a very elegant relaton between the Hamltonan Formulaton of Mechancs and Quantum Mechancs. To do that, we need to derve
Convergence of random processes
 DS-GA 12 Lecture notes 6 Fall 216 Convergence of random processes 1 Introducton In these notes we study convergence of dscrete random processes. Ths allows to characterze phenomena such as the law of large
DS-GA 12 Lecture notes 6 Fall 216 Convergence of random processes 1 Introducton In these notes we study convergence of dscrete random processes. Ths allows to characterze phenomena such as the law of large
Joint Statistical Meetings - Biopharmaceutical Section
 Iteratve Ch-Square Test for Equvalence of Multple Treatment Groups Te-Hua Ng*, U.S. Food and Drug Admnstraton 1401 Rockvlle Pke, #200S, HFM-217, Rockvlle, MD 20852-1448 Key Words: Equvalence Testng; Actve
Iteratve Ch-Square Test for Equvalence of Multple Treatment Groups Te-Hua Ng*, U.S. Food and Drug Admnstraton 1401 Rockvlle Pke, #200S, HFM-217, Rockvlle, MD 20852-1448 Key Words: Equvalence Testng; Actve
k t+1 + c t A t k t, t=0
 Macro II (UC3M, MA/PhD Econ) Professor: Matthas Kredler Fnal Exam 6 May 208 You have 50 mnutes to complete the exam There are 80 ponts n total The exam has 4 pages If somethng n the queston s unclear,
Macro II (UC3M, MA/PhD Econ) Professor: Matthas Kredler Fnal Exam 6 May 208 You have 50 mnutes to complete the exam There are 80 ponts n total The exam has 4 pages If somethng n the queston s unclear,
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
3.1 Expectation of Functions of Several Random Variables. )' be a k-dimensional discrete or continuous random vector, with joint PMF p (, E X E X1 E X
 Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Statstcs 1: Probablty Theory II 37 3 EPECTATION OF SEVERAL RANDOM VARIABLES As n Probablty Theory I, the nterest n most stuatons les not on the actual dstrbuton of a random vector, but rather on a number
Problem Points Score Total 100
 Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
4 Analysis of Variance (ANOVA) 5 ANOVA. 5.1 Introduction. 5.2 Fixed Effects ANOVA
 4 Analyss of Varance (ANOVA) 5 ANOVA 51 Introducton ANOVA ANOVA s a way to estmate and test the means of multple populatons We wll start wth one-way ANOVA If the populatons ncluded n the study are selected
4 Analyss of Varance (ANOVA) 5 ANOVA 51 Introducton ANOVA ANOVA s a way to estmate and test the means of multple populatons We wll start wth one-way ANOVA If the populatons ncluded n the study are selected
8.592J: Solutions for Assignment 7 Spring 2005
 8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
Week 9 Chapter 10 Section 1-5
 Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
14 The Postulates of Quantum mechanics
 14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
The equation of motion of a dynamical system is given by a set of differential equations. That is (1)
 Dynamcal Systems Many engneerng and natural systems are dynamcal systems. For example a pendulum s a dynamcal system. State l The state of the dynamcal system specfes t condtons. For a pendulum n the absence
Dynamcal Systems Many engneerng and natural systems are dynamcal systems. For example a pendulum s a dynamcal system. State l The state of the dynamcal system specfes t condtons. For a pendulum n the absence
Chap.5 Statistical Thermodynamics
 Chap5 Statstcal Thermodynamcs () Free expanson & adabatc from macroscopc: Δ S dq T R adabatc Q, free expanson W rrev rrev ΔU, deal gas ΔT If reversble & sothermal QR + WR ΔU 因 Uf(T) RT QR WR ( Pd ) Pd
Chap5 Statstcal Thermodynamcs () Free expanson & adabatc from macroscopc: Δ S dq T R adabatc Q, free expanson W rrev rrev ΔU, deal gas ΔT If reversble & sothermal QR + WR ΔU 因 Uf(T) RT QR WR ( Pd ) Pd
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Physics 207: Lecture 20. Today s Agenda Homework for Monday
 Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Mathematical Preparations
 1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
APPENDIX A Some Linear Algebra
 APPENDIX A Some Lnear Algebra The collecton of m, n matrces A.1 Matrces a 1,1,..., a 1,n A = a m,1,..., a m,n wth real elements a,j s denoted by R m,n. If n = 1 then A s called a column vector. Smlarly,
APPENDIX A Some Lnear Algebra The collecton of m, n matrces A.1 Matrces a 1,1,..., a 1,n A = a m,1,..., a m,n wth real elements a,j s denoted by R m,n. If n = 1 then A s called a column vector. Smlarly,
Lecture 12: Discrete Laplacian
 Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Fermi Statistics and Fermi Surface. Sommerfeld Theory. 2.1 Fermi Statistics and Fermi Surface
 erm Statstcs and erm Surface.1 erm Statstcs and erm Surface Snce Drude model, t too a quarter of a century for a breathrough to occur. That arose from the development of quantum mechancs and recognton
erm Statstcs and erm Surface.1 erm Statstcs and erm Surface Snce Drude model, t too a quarter of a century for a breathrough to occur. That arose from the development of quantum mechancs and recognton
Probability Theory. The nth coefficient of the Taylor series of f(k), expanded around k = 0, gives the nth moment of x as ( ik) n n!
 8333: Statstcal Mechancs I Problem Set # 3 Solutons Fall 3 Characterstc Functons: Probablty Theory The characterstc functon s defned by fk ep k = ep kpd The nth coeffcent of the Taylor seres of fk epanded
8333: Statstcal Mechancs I Problem Set # 3 Solutons Fall 3 Characterstc Functons: Probablty Theory The characterstc functon s defned by fk ep k = ep kpd The nth coeffcent of the Taylor seres of fk epanded
Lecture 14: Forces and Stresses
 The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructions
 THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructons by George Hardgrove Chemstry Department St. Olaf College Northfeld, MN 55057 hardgrov@lars.acc.stolaf.edu Copyrght George
THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructons by George Hardgrove Chemstry Department St. Olaf College Northfeld, MN 55057 hardgrov@lars.acc.stolaf.edu Copyrght George
Irregular vibrations in multi-mass discrete-continuous systems torsionally deformed
 (2) 4 48 Irregular vbratons n mult-mass dscrete-contnuous systems torsonally deformed Abstract In the paper rregular vbratons of dscrete-contnuous systems consstng of an arbtrary number rgd bodes connected
(2) 4 48 Irregular vbratons n mult-mass dscrete-contnuous systems torsonally deformed Abstract In the paper rregular vbratons of dscrete-contnuous systems consstng of an arbtrary number rgd bodes connected
Transfer Functions. Convenient representation of a linear, dynamic model. A transfer function (TF) relates one input and one output: ( ) system
 Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Transfer Functons Convenent representaton of a lnear, dynamc model. A transfer functon (TF) relates one nput and one output: x t X s y t system Y s The followng termnology s used: x y nput output forcng
Fermi-Dirac statistics
 UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
The non-negativity of probabilities and the collapse of state
 The non-negatvty of probabltes and the collapse of state Slobodan Prvanovć Insttute of Physcs, P.O. Box 57, 11080 Belgrade, Serba Abstract The dynamcal equaton, beng the combnaton of Schrödnger and Louvlle
The non-negatvty of probabltes and the collapse of state Slobodan Prvanovć Insttute of Physcs, P.O. Box 57, 11080 Belgrade, Serba Abstract The dynamcal equaton, beng the combnaton of Schrödnger and Louvlle
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
A how to guide to second quantization method.
 Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Kernel Methods and SVMs Extension
 Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
