PES 2130 Fall 2014, Spendier Lecture 7/Page 1
|
|
|
- Patrick Chase
- 5 years ago
- Views:
Transcription
1 PES 2130 Fall 2014, Spender Lecture 7/Page 1 Lecture today: Chapter 20 (ncluded n exam 1) 1) Entropy 2) Second Law o hermodynamcs 3) Statstcal Vew o Entropy Announcements: Next week Wednesday Exam 1! - and out: what wll not be covered on exam - Equaton Sheet ueston regardng W2: Monatomc vs datomc Indeed hydrogen s a datomc gas. hs s because monatomc hydrogen s very reactve and unstable. Monatomc hydrogen wll react wth almost any other chemcal. ydrogen gas = 2 Oxygen s smlar. he most stable s datomc oxygen O 2. Next s tratomc (ozone) O 3 and the least stable (very unstable) s monoatomc. Monoatomc oxygen s very shortly lvng speces combnng easly (due to very hgh electronegatvty) wth large number o elements ncludng another oxygen atom. Example o monatomc gases: helum, neon, argon, krypton, and radon Last lecture: Reversble and Irreversble Processes (Fundamentals o Physcs book) Any tme there s heat low through a nte temperature drop, t s an rreversble process. I you want a reversble process, heat only should low when the temperatures are nntesmally derent (whch would take orever). eat Engnes he ecency o a heat engne s dened as the rato o the work done to the amount o heat brought n rom the hot reservor: W L For a cyclc process, C 1 eat Pump (Rergerators) Devce that does work n order to move heat rom cold to hot. Rergerators are nothng more than engnes run n reverse: they take heat out o a cold reservor, and use work to put more heat nto the hot reservor. Coecent o Perormance: L K For a cyclc process, W he Carnot Cycle Carnot Engne Ecency L L C 1 1 K C L L
2 PES 2130 Fall 2014, Spender Lecture 7/Page 2 DEMO: Strlng engne he Strlng engne (heat cycle) wll run when t s placed on a dsh o crushed ce or on a mug o hot water. he workng volume may be lled wth helum whch makes the engne run aster. Strlng Engne: Comparson wth the Carnot cycle shows that each engne has sothermal heat transers at temperatures and L. owever, the two sotherms o the Strlng engne cycle are connected, not by adabatc processes as or the Carnot engne but by constant-volume processes. o ncrease the temperature o a gas at constant volume reversbly rom L to (process da) requres a transer o energy as heat to the workng substance rom a thermal reservor whose temperature can be vared smoothly between those lmts. Also, a reverse transer s requred n process bc. hus, reversble heat transers (and correspondng entropy changes) occur n all our o the processes that orm the cycle o a Strlng engne, not just two processes as n a Carnot engne. Now we are ready to make the connecton to entropy! Entropy Any method nvolvng the noton o entropy, the very exstence o whch depends on the second law o thermodynamcs, wll doubtless seem too many ar-etched, and may repel begnners as obscure and dcult o comprehenson. - Wllard Gbbs, Graphcal Methods n the hermodynamcs o Fluds (1873) Let us start our dscusson o entropy by lookng at one o the last steps n our dervaton o the Carnot ecency: L L L L 0 Now, we dene a varable S, such that: d ds ( s constant)
3 PES 2130 Fall 2014, Spender Lecture 7/Page 3 and snce 1 ds d d S S S, where s the total energy transerred as heat durng the process hen, we can see that over the entre Carnot cycle, L 0 L the varable S, whch we now reer to as the entropy, returns to the same value. In other words the change n entropy adds to zero over the entre cycle. he net entropy change per cycle: S SL S 0 (In a Carnot engne there are two reversble energy transers as heat, and thus two changes n the entropy o the workng substance - one at temperature and one at L.) Entropy as a State Varable Now, perhaps that s just some artact o the Carnot cycle, but, usng calculus, we can show that over any cycle, the entropy returns to ts orgnal value (see proo n book). hereore, entropy s a state varable! It can descrbe the state o a system (lke the temperature, volume, pressure, E nt, etc.). A state uncton only depends on a state and not the path the system takes (unlke W and ). hs means that we want to calculate the entropy o an rreversble process we can use: Srr, gas S S Srev, gas d In other words, we use a correspondng reversble path to do the ntegraton. he 2nd Law o hermodynamcs All heat engnes have a maxmum ecency that s much less than 100% because o the second law o thermodynamcs. No engne can exceed Carnot ecency, because heat does not low spontaneously rom cold to hot. No process s possble whose sole result s the transer o heat rom a body o lower temperature to a body o hgher temperature. Rudol Clausus (1854) No process s possble n whch the sole result s the absorpton o heat rom a reservor and ts complete converson nto work. Lord Kelvn (1851) In other words, snce objects and ther envronment wll always reach a thermal equlbrum eventually, you can't keep gettng work out o the system! "here s no such thng as a perpetual moton machne!"
4 PES 2130 Fall 2014, Spender Lecture 7/Page 4 Statements above are concerned wth work and are consdered the rst statements o the second law beore entropy was dened. Entropy - Measures the amount o dsorder n a system. Let s examne now an nntesmal sothermal expanson: snce d E nt = 0 ( = 0, b/c o constant temperature) d = dw = pdv = nr dv V dv d ds V nr Now, t turns out that the ncremental ractonal volume ncrease (or added heat) s drectly related to the ncrease n dsorder o the gas. Snce there s more space to occupy, there are more ways whch the molecules can exst where the gas has the same state. he second law o thermodynamcs can be stated n terms o entropy: No process s possble n whch the total entropy o an solated system decreases. S 0 he term solated system here s crucal. he entropy o some object can decrease, t s n contact wth another object whose entropy ncreases just as much or more. It s certanly possble or somethng to have more order than t dd at some pont n the past, but t just means that somethng else, n contact wth t now has less order. rreversble process: S rr 0 reversble process: S rev 0 We just talked about entropy beng a state varable and that we want to calculate the entropy o an rreversble process we can use a correspondng reversble path to do the ntegraton: Srr, gas S S Srev, gas d But doesn't ths volate the 2nd law o thermodynamcs? No, snce we have not consdered the whole system, only the gas. he second law apples to the system as a whole, not just one component. We would also need to calculate the changes n entropy o the surroundngs whch can be dcult.
5 PES 2130 Fall 2014, Spender Lecture 7/Page 5 Example: A 250g potato at 293K s thrown nto a 2.00 L pot o water at 373K. a) What s the nal equlbrum temperature o the potato-water system? b) What s the change n entropy o ths system, assumng t s solated rom the surroundngs? Specc heat o potato: c p = 3430 J kg -1 K -1 Specc heat o water: c w = 4187 J kg -1 K -1
6 PES 2130 Fall 2014, Spender Lecture 7/Page 6 A statstcal Vew o Entropy and the Arrow o me You can slde a book across a table. It stops due to energy lost rom rcton. But, have you ever seen a book start sldng takng energy rom the thermal energy o the table? You could start out wth all o the ar n ths room n one hal and vacuum n the other hal, separated by a partton. hen remove the partton and the ar would ll the room. But, you wouldn t expect or a room lled wth ar to spontaneously separate nto hal lled and hal empty. Nether o these scenaros s prohbted by the conservaton laws or by the rst law o thermodynamcs. Why don t many thngs that happen spontaneously n nature also happen n reverse? Macroscopc and Mcroscopc States In order to understand ths apparent drecton n tme we need to understand how to nd a partcular macro-state gven the statstcs o the mcro-states whch make t up. As example, let s look at a smpler problem: a con toss. Let s say we have one con. For smplcty, t s motonless and can only be n one place. Its state, whch we now specy as ts macro-state s dened as whether t s a heads or a tals. ow many ways can you make each macro-state?.e. what s the number o possble mcro-states (called "multplcty" n your textbook). Only one way or each state - one mcro-state or each way. So, we toss a sngle con n the ar, what s the probablty that t wll land n each macro-state? 1 or 1? P 1 # mcrostates mcrostates mcrostates mcrostates mcrostates
7 PES 2130 Fall 2014, Spender Lecture 7/Page 7 P 1 # mcrostates mcrostates 11 2 Now, lets do the same wth two cons. here are 3 derent macro-states, but or each macro-state, there s a derent number o ways to make t. For one tal and one head, there are 2 ways to make t. So, we toss 2 cons n the ar, what s the probablty that they wll come up all heads? ( ) ( ) What s the probablty that they wll come up 1 head and 1 tal? ( ) ( ) Notce that the total number o mcro-states = 2 N = where N s the number o cons. mcrostates So, or 100 cons, there are mcro-states and the possblty o throwng 100 heads s 1 n 1,267,650,600,228,229,401,496,703,205,376! Now, the number o molecules n ths room s approxmately 1 x Consder the macro-states as beng (heads) a molecule s n one hal o the room and (tals) t s n the other hal. What s the probablty that on a random samplng o the molecules poston, we nd them all n one hal o the room? So, ths tendency towards dsorder s just a consequence o the probablty o ndng a partcular macro-state gven the statstcs o the mcro-states whch make t up! hs s the underlyng nature o entropy, and the underlyng nature o the drecton o tme. Calculatng Entropy
8 PES 2130 Fall 2014, Spender Lecture 7/Page 8 One can calculate the entropy, rst expressed by Ludwg Boltzmann ( ) an Austran physcst and phlosopher), as: S k w, B ln where k B =1.38 x JK -1, s the Boltzmann constant and w s the number o all mcrostates avalable to the system. Or, snce only the derence n entropes are ever used n calculatons: S S S k ln w k ln w w SkB ln w B B
Chapter 5 rd Law of Thermodynamics
 Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamics and Gases
 hermodynamcs and Gases Last tme Knetc heory o Gases or smple (monatomc) gases Atomc nature o matter Demonstrate deal gas law Atomc knetc energy nternal energy Mean ree path and velocty dstrbutons From
hermodynamcs and Gases Last tme Knetc heory o Gases or smple (monatomc) gases Atomc nature o matter Demonstrate deal gas law Atomc knetc energy nternal energy Mean ree path and velocty dstrbutons From
General Formulas applicable to ALL processes in an Ideal Gas:
 Calormetrc calculatons: dq mcd or dq ncd ( specc heat) Q ml ( latent heat) General Formulas applcable to ALL processes n an Ideal Gas: P nr du dq dw dw Pd du nc d C R ( monoatomc) C C R P Specc Processes:
Calormetrc calculatons: dq mcd or dq ncd ( specc heat) Q ml ( latent heat) General Formulas applcable to ALL processes n an Ideal Gas: P nr du dq dw dw Pd du nc d C R ( monoatomc) C C R P Specc Processes:
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
TEST 5 (phy 240) 2. Show that the volume coefficient of thermal expansion for an ideal gas at constant pressure is temperature dependent and given by
 ES 5 (phy 40). a) Wrte the zeroth law o thermodynamcs. b) What s thermal conductvty? c) Identyng all es, draw schematcally a P dagram o the arnot cycle. d) What s the ecency o an engne and what s the coecent
ES 5 (phy 40). a) Wrte the zeroth law o thermodynamcs. b) What s thermal conductvty? c) Identyng all es, draw schematcally a P dagram o the arnot cycle. d) What s the ecency o an engne and what s the coecent
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Chapters 18 & 19: Themodynamics review. All macroscopic (i.e., human scale) quantities must ultimately be explained on the microscopic scale.
 Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
Spring Force and Power
 Lecture 13 Chapter 9 Sprng Force and Power Yeah, energy s better than orces. What s net? Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi IN THIS CHAPTER, you wll learn how to solve problems
Lecture 13 Chapter 9 Sprng Force and Power Yeah, energy s better than orces. What s net? Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi IN THIS CHAPTER, you wll learn how to solve problems
Force = F Piston area = A
 CHAPTER III Ths chapter s an mportant transton between the propertes o pure substances and the most mportant chapter whch s: the rst law o thermodynamcs In ths chapter, we wll ntroduce the notons o heat,
CHAPTER III Ths chapter s an mportant transton between the propertes o pure substances and the most mportant chapter whch s: the rst law o thermodynamcs In ths chapter, we wll ntroduce the notons o heat,
PHYS 1443 Section 004 Lecture #12 Thursday, Oct. 2, 2014
 PHYS 1443 Secton 004 Lecture #1 Thursday, Oct., 014 Work-Knetc Energy Theorem Work under rcton Potental Energy and the Conservatve Force Gravtatonal Potental Energy Elastc Potental Energy Conservaton o
PHYS 1443 Secton 004 Lecture #1 Thursday, Oct., 014 Work-Knetc Energy Theorem Work under rcton Potental Energy and the Conservatve Force Gravtatonal Potental Energy Elastc Potental Energy Conservaton o
PHYS 1441 Section 002 Lecture #15
 PHYS 1441 Secton 00 Lecture #15 Monday, March 18, 013 Work wth rcton Potental Energy Gravtatonal Potental Energy Elastc Potental Energy Mechancal Energy Conservaton Announcements Mdterm comprehensve exam
PHYS 1441 Secton 00 Lecture #15 Monday, March 18, 013 Work wth rcton Potental Energy Gravtatonal Potental Energy Elastc Potental Energy Mechancal Energy Conservaton Announcements Mdterm comprehensve exam
Chapter 6 Second Law of Thermodynamics
 Capter 6 Second Law o Termodynamcs Te rst law o termodynamcs s an energy conservaton statement. It determnes weter or not a process can take place energetcally. It does not tell n wc drecton te process
Capter 6 Second Law o Termodynamcs Te rst law o termodynamcs s an energy conservaton statement. It determnes weter or not a process can take place energetcally. It does not tell n wc drecton te process
Physical Chemistry I for Biochemists. Chem340. Lecture 16 (2/18/11)
 hyscal Chemstry I or Bochemsts Chem34 Lecture 16 (/18/11) Yoshtaka Ish Ch4.6, Ch5.1-5.5 & HW5 4.6 Derental Scannng Calormetry (Derental hermal Analyss) sample = C p, s d s + dh uson = ( s )Kdt, [1] where
hyscal Chemstry I or Bochemsts Chem34 Lecture 16 (/18/11) Yoshtaka Ish Ch4.6, Ch5.1-5.5 & HW5 4.6 Derental Scannng Calormetry (Derental hermal Analyss) sample = C p, s d s + dh uson = ( s )Kdt, [1] where
3-1 Introduction: 3-2 Spontaneous (Natural) Process:
 - Introducton: * Reversble & Irreversble processes * Degree of rreversblty * Entropy S a state functon * Reversble heat engne Carnot cycle (Engne) * Crteron for Eulbrum SU,=Smax - Spontaneous (Natural)
- Introducton: * Reversble & Irreversble processes * Degree of rreversblty * Entropy S a state functon * Reversble heat engne Carnot cycle (Engne) * Crteron for Eulbrum SU,=Smax - Spontaneous (Natural)
Physical Chemistry I for Biochemists. Lecture 18 (2/23/11) Announcement
 Physcal Chestry I or Bochests Che34 Lecture 18 (2/23/11) Yoshtaka Ish Ch5.8-5.11 & HW6 Revew o Ch. 5 or Quz 2 Announceent Quz 2 has a slar orat wth Quz1. e s the sae. ~2 ns. Answer or HW5 wll be uploaded
Physcal Chestry I or Bochests Che34 Lecture 18 (2/23/11) Yoshtaka Ish Ch5.8-5.11 & HW6 Revew o Ch. 5 or Quz 2 Announceent Quz 2 has a slar orat wth Quz1. e s the sae. ~2 ns. Answer or HW5 wll be uploaded
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
CHAPTER 8 Potential Energy and Conservation of Energy
 CHAPTER 8 Potental Energy and Conservaton o Energy One orm o energy can be converted nto another orm o energy. Conservatve and non-conservatve orces Physcs 1 Knetc energy: Potental energy: Energy assocated
CHAPTER 8 Potental Energy and Conservaton o Energy One orm o energy can be converted nto another orm o energy. Conservatve and non-conservatve orces Physcs 1 Knetc energy: Potental energy: Energy assocated
Chapter 21 - The Kinetic Theory of Gases
 hapter 1 - he Knetc heory o Gases 1. Δv 8.sn 4. 8.sn 4. m s F Nm. 1 kg.94 N Δ t. s F A 1.7 N m 1.7 a N mv 1.6 Use the equaton descrbng the knetc-theory account or pressure:. hen mv Kav where N nna NA N
hapter 1 - he Knetc heory o Gases 1. Δv 8.sn 4. 8.sn 4. m s F Nm. 1 kg.94 N Δ t. s F A 1.7 N m 1.7 a N mv 1.6 Use the equaton descrbng the knetc-theory account or pressure:. hen mv Kav where N nna NA N
#64. ΔS for Isothermal Mixing of Ideal Gases
 #64 Carnot Heat Engne ΔS for Isothermal Mxng of Ideal Gases ds = S dt + S T V V S = P V T T V PV = nrt, P T ds = v T = nr V dv V nr V V = nrln V V = - nrln V V ΔS ΔS ΔS for Isothermal Mxng for Ideal Gases
#64 Carnot Heat Engne ΔS for Isothermal Mxng of Ideal Gases ds = S dt + S T V V S = P V T T V PV = nrt, P T ds = v T = nr V dv V nr V V = nrln V V = - nrln V V ΔS ΔS ΔS for Isothermal Mxng for Ideal Gases
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
ESCI 341 Atmospheric Thermodynamics Lesson 10 The Physical Meaning of Entropy
 ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
Lecture 3 Examples and Problems
 Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
Work is the change in energy of a system (neglecting heat transfer). To examine what could
 Work Work s the change n energy o a system (neglectng heat transer). To eamne what could cause work, let s look at the dmensons o energy: L ML E M L F L so T T dmensonally energy s equal to a orce tmes
Work Work s the change n energy o a system (neglectng heat transer). To eamne what could cause work, let s look at the dmensons o energy: L ML E M L F L so T T dmensonally energy s equal to a orce tmes
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Physics 207 Lecture 27
 hyscs 07 Lecture 7 hyscs 07, Lecture 7, Dec. 6 Agenda: h. 0, st Law o Thermodynamcs, h. st Law o thermodynamcs ( U Q + W du dq + dw ) Work done by an deal gas n a ston Introducton to thermodynamc cycles
hyscs 07 Lecture 7 hyscs 07, Lecture 7, Dec. 6 Agenda: h. 0, st Law o Thermodynamcs, h. st Law o thermodynamcs ( U Q + W du dq + dw ) Work done by an deal gas n a ston Introducton to thermodynamc cycles
Chapter 3 and Chapter 4
 Chapter 3 and Chapter 4 Chapter 3 Energy 3. Introducton:Work Work W s energy transerred to or rom an object by means o a orce actng on the object. Energy transerred to the object s postve work, and energy
Chapter 3 and Chapter 4 Chapter 3 Energy 3. Introducton:Work Work W s energy transerred to or rom an object by means o a orce actng on the object. Energy transerred to the object s postve work, and energy
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Physics 115. Molecular motion and temperature Phase equilibrium, evaporation
 Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
PHYS 1441 Section 002 Lecture #16
 PHYS 1441 Secton 00 Lecture #16 Monday, Mar. 4, 008 Potental Energy Conservatve and Non-conservatve Forces Conservaton o Mechancal Energy Power Today s homework s homework #8, due 9pm, Monday, Mar. 31!!
PHYS 1441 Secton 00 Lecture #16 Monday, Mar. 4, 008 Potental Energy Conservatve and Non-conservatve Forces Conservaton o Mechancal Energy Power Today s homework s homework #8, due 9pm, Monday, Mar. 31!!
Lecture 16. Chapter 11. Energy Dissipation Linear Momentum. Physics I. Department of Physics and Applied Physics
 Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Chapter 1. Probability
 Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
University Physics AI No. 10 The First Law of Thermodynamics
 Unversty hyscs I No he Frst Law o hermodynamcs lass Number Name Ihoose the orrect nswer Whch o the ollowng processes must volate the rst law o thermodynamcs? (here may be more than one answer!) (,B,D )
Unversty hyscs I No he Frst Law o hermodynamcs lass Number Name Ihoose the orrect nswer Whch o the ollowng processes must volate the rst law o thermodynamcs? (here may be more than one answer!) (,B,D )
Name: SID: Discussion Session:
 Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Period & Frequency. Work and Energy. Methods of Energy Transfer: Energy. Work-KE Theorem 3/4/16. Ranking: Which has the greatest kinetic energy?
 Perod & Frequency Perod (T): Tme to complete one ull rotaton Frequency (): Number o rotatons completed per second. = 1/T, T = 1/ v = πr/t Work and Energy Work: W = F!d (pcks out parallel components) F
Perod & Frequency Perod (T): Tme to complete one ull rotaton Frequency (): Number o rotatons completed per second. = 1/T, T = 1/ v = πr/t Work and Energy Work: W = F!d (pcks out parallel components) F
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Order (Boltzmann 1875) Information (Shannon 1948) Entropy (Clausius 1855) dq T Cycle. 2 nd LAW. 5. ENTROPY & 2 nd LAW OF THERMODYNAMICS.
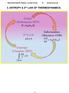 5. ENROPY & nd LAW OF HERMODYNAMIC. Order (Boltzmann 875) ln k B e nd LAW 0 Inormaton (hannon 948) Q bt k B ln e Entropy (Clausus 855) dq Cycle 0 4 5. he Clausus Inequalty. wo emprcal statements o the
5. ENROPY & nd LAW OF HERMODYNAMIC. Order (Boltzmann 875) ln k B e nd LAW 0 Inormaton (hannon 948) Q bt k B ln e Entropy (Clausus 855) dq Cycle 0 4 5. he Clausus Inequalty. wo emprcal statements o the
STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Chapter 20 The First Law of Thermodynamics
 Chapter he Frst aw o hermodynamcs. developng the concept o heat. etendng our concept o work to thermal processes 3. ntroducng the rst law o thermodynamcs. Heat and Internal Energy Internal energy: s the
Chapter he Frst aw o hermodynamcs. developng the concept o heat. etendng our concept o work to thermal processes 3. ntroducng the rst law o thermodynamcs. Heat and Internal Energy Internal energy: s the
Chapter 7. Potential Energy and Conservation of Energy
 Chapter 7 Potental Energy and Conservaton o Energy 1 Forms o Energy There are many orms o energy, but they can all be put nto two categores Knetc Knetc energy s energy o moton Potental Potental energy
Chapter 7 Potental Energy and Conservaton o Energy 1 Forms o Energy There are many orms o energy, but they can all be put nto two categores Knetc Knetc energy s energy o moton Potental Potental energy
Shuai Dong. Isaac Newton. Gottfried Leibniz
 Computatonal pyscs Sua Dong Isaac Newton Gottred Lebnz Numercal calculus poston dervatve ntegral v velocty dervatve ntegral a acceleraton Numercal calculus Numercal derentaton Numercal ntegraton Roots
Computatonal pyscs Sua Dong Isaac Newton Gottred Lebnz Numercal calculus poston dervatve ntegral v velocty dervatve ntegral a acceleraton Numercal calculus Numercal derentaton Numercal ntegraton Roots
V T for n & P = constant
 Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Physics 2A Chapters 6 - Work & Energy Fall 2017
 Physcs A Chapters 6 - Work & Energy Fall 017 These notes are eght pages. A quck summary: The work-energy theorem s a combnaton o Chap and Chap 4 equatons. Work s dened as the product o the orce actng on
Physcs A Chapters 6 - Work & Energy Fall 017 These notes are eght pages. A quck summary: The work-energy theorem s a combnaton o Chap and Chap 4 equatons. Work s dened as the product o the orce actng on
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Problem Points Score Total 100
 Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Thermodynamic constraints on fluctuation phenomena
 hermodynamc constrants on luctuaton phenomena O J E Maroney he Centre or me and he School o Physcs Unversty o Sydney NSW 2006 Australa and Permeter Insttute or heoretcal Physcs 3 Carolne St N, Waterloo,
hermodynamc constrants on luctuaton phenomena O J E Maroney he Centre or me and he School o Physcs Unversty o Sydney NSW 2006 Australa and Permeter Insttute or heoretcal Physcs 3 Carolne St N, Waterloo,
ESCI 341 Atmospheric Thermodynamics Lesson 6 Thermodynamic Processes
 ESCI 341 Atmosherc Thermodynamcs Lesson 6 Thermodynamc Processes Reerences: An Introducton to Atmosherc Thermodynamcs, Tsons Introducton to Theoretcal Meteorology, Hess Physcal Chemstry (4 th edton), Lene
ESCI 341 Atmosherc Thermodynamcs Lesson 6 Thermodynamc Processes Reerences: An Introducton to Atmosherc Thermodynamcs, Tsons Introducton to Theoretcal Meteorology, Hess Physcal Chemstry (4 th edton), Lene
A Tale of Friction Basic Rollercoaster Physics. Fahrenheit Rollercoaster, Hershey, PA max height = 121 ft max speed = 58 mph
 A Tale o Frcton Basc Rollercoaster Physcs Fahrenhet Rollercoaster, Hershey, PA max heght = 11 t max speed = 58 mph PLAY PLAY PLAY PLAY Rotatonal Movement Knematcs Smlar to how lnear velocty s dened, angular
A Tale o Frcton Basc Rollercoaster Physcs Fahrenhet Rollercoaster, Hershey, PA max heght = 11 t max speed = 58 mph PLAY PLAY PLAY PLAY Rotatonal Movement Knematcs Smlar to how lnear velocty s dened, angular
Chapter 07: Kinetic Energy and Work
 Chapter 07: Knetc Energy and Work Conservaton o Energy s one o Nature s undamental laws that s not volated. Energy can take on derent orms n a gven system. Ths chapter we wll dscuss work and knetc energy.
Chapter 07: Knetc Energy and Work Conservaton o Energy s one o Nature s undamental laws that s not volated. Energy can take on derent orms n a gven system. Ths chapter we wll dscuss work and knetc energy.
Physics 2A Chapter 3 HW Solutions
 Phscs A Chapter 3 HW Solutons Chapter 3 Conceptual Queston: 4, 6, 8, Problems: 5,, 8, 7, 3, 44, 46, 69, 70, 73 Q3.4. Reason: (a) C = A+ B onl A and B are n the same drecton. Sze does not matter. (b) C
Phscs A Chapter 3 HW Solutons Chapter 3 Conceptual Queston: 4, 6, 8, Problems: 5,, 8, 7, 3, 44, 46, 69, 70, 73 Q3.4. Reason: (a) C = A+ B onl A and B are n the same drecton. Sze does not matter. (b) C
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Conservation of Energy
 Lecture 3 Chapter 8 Physcs I 0.3.03 Conservaton o Energy Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Lecture Capture: http://echo360.uml.edu/danylov03/physcsall.html 95.4, Fall 03,
Lecture 3 Chapter 8 Physcs I 0.3.03 Conservaton o Energy Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Lecture Capture: http://echo360.uml.edu/danylov03/physcsall.html 95.4, Fall 03,
Physics for Scientists and Engineers. Chapter 9 Impulse and Momentum
 Physcs or Scentsts and Engneers Chapter 9 Impulse and Momentum Sprng, 008 Ho Jung Pak Lnear Momentum Lnear momentum o an object o mass m movng wth a velocty v s dened to be p mv Momentum and lnear momentum
Physcs or Scentsts and Engneers Chapter 9 Impulse and Momentum Sprng, 008 Ho Jung Pak Lnear Momentum Lnear momentum o an object o mass m movng wth a velocty v s dened to be p mv Momentum and lnear momentum
Lecture 2 Solution of Nonlinear Equations ( Root Finding Problems )
 Lecture Soluton o Nonlnear Equatons Root Fndng Problems Dentons Classcaton o Methods Analytcal Solutons Graphcal Methods Numercal Methods Bracketng Methods Open Methods Convergence Notatons Root Fndng
Lecture Soluton o Nonlnear Equatons Root Fndng Problems Dentons Classcaton o Methods Analytcal Solutons Graphcal Methods Numercal Methods Bracketng Methods Open Methods Convergence Notatons Root Fndng
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chapter 8: Potential Energy and The Conservation of Total Energy
 Chapter 8: Potental Energy and The Conservaton o Total Energy Work and knetc energy are energes o moton. K K K mv r v v F dr Potental energy s an energy that depends on locaton. -Dmenson F x d U( x) dx
Chapter 8: Potental Energy and The Conservaton o Total Energy Work and knetc energy are energes o moton. K K K mv r v v F dr Potental energy s an energy that depends on locaton. -Dmenson F x d U( x) dx
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Finite Difference Method
 7/0/07 Instructor r. Ramond Rump (9) 747 698 rcrump@utep.edu EE 337 Computatonal Electromagnetcs (CEM) Lecture #0 Fnte erence Method Lecture 0 These notes ma contan coprghted materal obtaned under ar use
7/0/07 Instructor r. Ramond Rump (9) 747 698 rcrump@utep.edu EE 337 Computatonal Electromagnetcs (CEM) Lecture #0 Fnte erence Method Lecture 0 These notes ma contan coprghted materal obtaned under ar use
Chapter 7: Conservation of Energy
 Lecture 7: Conservaton o nergy Chapter 7: Conservaton o nergy Introucton I the quantty o a subject oes not change wth tme, t means that the quantty s conserve. The quantty o that subject remans constant
Lecture 7: Conservaton o nergy Chapter 7: Conservaton o nergy Introucton I the quantty o a subject oes not change wth tme, t means that the quantty s conserve. The quantty o that subject remans constant
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Momentum. Momentum. Impulse. Momentum and Collisions
 Momentum Momentum and Collsons From Newton s laws: orce must be present to change an object s elocty (speed and/or drecton) Wsh to consder eects o collsons and correspondng change n elocty Gol ball ntally
Momentum Momentum and Collsons From Newton s laws: orce must be present to change an object s elocty (speed and/or drecton) Wsh to consder eects o collsons and correspondng change n elocty Gol ball ntally
Randomness and Computation
 Randomness and Computaton or, Randomzed Algorthms Mary Cryan School of Informatcs Unversty of Ednburgh RC 208/9) Lecture 0 slde Balls n Bns m balls, n bns, and balls thrown unformly at random nto bns usually
Randomness and Computaton or, Randomzed Algorthms Mary Cryan School of Informatcs Unversty of Ednburgh RC 208/9) Lecture 0 slde Balls n Bns m balls, n bns, and balls thrown unformly at random nto bns usually
11/19/2013. PHY 113 C General Physics I 11 AM 12:15 PM MWF Olin 101
 PHY 113 C General Pyss I 11 AM 12:15 PM MWF Oln 101 Plan or Leture 23: Capter 22: Heat engnes 1. ermodynam yles; work and eat eeny 2. Carnot yle 3. Otto yle; desel yle 4. Bre omments on entropy 11/19/2013
PHY 113 C General Pyss I 11 AM 12:15 PM MWF Oln 101 Plan or Leture 23: Capter 22: Heat engnes 1. ermodynam yles; work and eat eeny 2. Carnot yle 3. Otto yle; desel yle 4. Bre omments on entropy 11/19/2013
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
ONE-DIMENSIONAL COLLISIONS
 Purpose Theory ONE-DIMENSIONAL COLLISIONS a. To very the law o conservaton o lnear momentum n one-dmensonal collsons. b. To study conservaton o energy and lnear momentum n both elastc and nelastc onedmensonal
Purpose Theory ONE-DIMENSIONAL COLLISIONS a. To very the law o conservaton o lnear momentum n one-dmensonal collsons. b. To study conservaton o energy and lnear momentum n both elastc and nelastc onedmensonal
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
Problem Free Expansion of Ideal Gas
 Problem 4.3 Free Expanon o Ideal Ga In general: ds ds du P dv P dv NR V dn Snce U o deal ga ndependent on olume (du=), and N = cont n the proce: dv In a ere o nntemal ree expanon, entropy change by: S
Problem 4.3 Free Expanon o Ideal Ga In general: ds ds du P dv P dv NR V dn Snce U o deal ga ndependent on olume (du=), and N = cont n the proce: dv In a ere o nntemal ree expanon, entropy change by: S
Lecture 10 Support Vector Machines II
 Lecture 10 Support Vector Machnes II 22 February 2016 Taylor B. Arnold Yale Statstcs STAT 365/665 1/28 Notes: Problem 3 s posted and due ths upcomng Frday There was an early bug n the fake-test data; fxed
Lecture 10 Support Vector Machnes II 22 February 2016 Taylor B. Arnold Yale Statstcs STAT 365/665 1/28 Notes: Problem 3 s posted and due ths upcomng Frday There was an early bug n the fake-test data; fxed
Physics 207, Lecture 13, Oct. 15. Energy
 Physcs 07 Lecture 3 Physcs 07, Lecture 3, Oct. 5 Goals: Chapter 0 Understand the relatonshp between moton and energy Dene Potental Energy n a Hooke s Law sprng Deelop and explot conseraton o energy prncple
Physcs 07 Lecture 3 Physcs 07, Lecture 3, Oct. 5 Goals: Chapter 0 Understand the relatonshp between moton and energy Dene Potental Energy n a Hooke s Law sprng Deelop and explot conseraton o energy prncple
Homework Chapter 21 Solutions!!
 Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Page 1. Clicker Question 9: Physics 131: Lecture 15. Today s Agenda. Clicker Question 9: Energy. Energy is Conserved.
 Physcs 3: Lecture 5 Today s Agenda Intro to Conseraton o Energy Intro to some derent knds o energy Knetc Potental Denton o Mechancal Energy Conseraton o Mechancal Energy Conserate orces Examples Pendulum
Physcs 3: Lecture 5 Today s Agenda Intro to Conseraton o Energy Intro to some derent knds o energy Knetc Potental Denton o Mechancal Energy Conseraton o Mechancal Energy Conserate orces Examples Pendulum
: Numerical Analysis Topic 2: Solution of Nonlinear Equations Lectures 5-11:
 764: Numercal Analyss Topc : Soluton o Nonlnear Equatons Lectures 5-: UIN Malang Read Chapters 5 and 6 o the tetbook 764_Topc Lecture 5 Soluton o Nonlnear Equatons Root Fndng Problems Dentons Classcaton
764: Numercal Analyss Topc : Soluton o Nonlnear Equatons Lectures 5-: UIN Malang Read Chapters 5 and 6 o the tetbook 764_Topc Lecture 5 Soluton o Nonlnear Equatons Root Fndng Problems Dentons Classcaton
Physics 4C. Chapter 19: Conceptual Questions: 6, 8, 10 Problems: 3, 13, 24, 31, 35, 48, 53, 63, 65, 78, 87
 Physcs 4C Solutons to Chater 9 HW Chater 9: Concetual Questons: 6, 8, 0 Problems:,, 4,,, 48,, 6, 6, 78, 87 Queston 9-6 (a) 0 (b) 0 (c) negate (d) oste Queston 9-8 (a) 0 (b) 0 (c) negate (d) oste Queston
Physcs 4C Solutons to Chater 9 HW Chater 9: Concetual Questons: 6, 8, 0 Problems:,, 4,,, 48,, 6, 6, 78, 87 Queston 9-6 (a) 0 (b) 0 (c) negate (d) oste Queston 9-8 (a) 0 (b) 0 (c) negate (d) oste Queston
General Tips on How to Do Well in Physics Exams. 1. Establish a good habit in keeping track of your steps. For example, when you use the equation
 General Tps on How to Do Well n Physcs Exams 1. Establsh a good habt n keepng track o your steps. For example when you use the equaton 1 1 1 + = d d to solve or d o you should rst rewrte t as 1 1 1 = d
General Tps on How to Do Well n Physcs Exams 1. Establsh a good habt n keepng track o your steps. For example when you use the equaton 1 1 1 + = d d to solve or d o you should rst rewrte t as 1 1 1 = d
v c motion is neither created nor destroyed, but transferred via interactions. Fri. Wed (.18,.19) Introducing Potential Energy RE 6.
 r. 6.5-.7 (.) Rest Mass,ork by Changng orces Columba Rep 3pm, here RE 6.b (last day to drop) ed. 6.8-.9(.8,.9) Introducng Potental Energy RE 6.c Tues. H6: Ch 6 Pr s 58,59, 99(a-c), 05(a-c) moton s nether
r. 6.5-.7 (.) Rest Mass,ork by Changng orces Columba Rep 3pm, here RE 6.b (last day to drop) ed. 6.8-.9(.8,.9) Introducng Potental Energy RE 6.c Tues. H6: Ch 6 Pr s 58,59, 99(a-c), 05(a-c) moton s nether
Temperature. Chapter Temperature Scales
 Chapter 12 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum Entropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 8 we dscussed the
Chapter 12 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum Entropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 8 we dscussed the
PHYS 705: Classical Mechanics. Newtonian Mechanics
 1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
Lecture 10: Euler s Equations for Multivariable
 Lecture 0: Euler s Equatons for Multvarable Problems Let s say we re tryng to mnmze an ntegral of the form: {,,,,,, ; } J f y y y y y y d We can start by wrtng each of the y s as we dd before: y (, ) (
Lecture 0: Euler s Equatons for Multvarable Problems Let s say we re tryng to mnmze an ntegral of the form: {,,,,,, ; } J f y y y y y y d We can start by wrtng each of the y s as we dd before: y (, ) (
OPTIMISATION. Introduction Single Variable Unconstrained Optimisation Multivariable Unconstrained Optimisation Linear Programming
 OPTIMIATION Introducton ngle Varable Unconstraned Optmsaton Multvarable Unconstraned Optmsaton Lnear Programmng Chapter Optmsaton /. Introducton In an engneerng analss, sometmes etremtes, ether mnmum or
OPTIMIATION Introducton ngle Varable Unconstraned Optmsaton Multvarable Unconstraned Optmsaton Lnear Programmng Chapter Optmsaton /. Introducton In an engneerng analss, sometmes etremtes, ether mnmum or
INFORMATION OF EVENTS WITH DISCRETE OUTCOMES: METHODS OF PHYSICS
 CHAPER 6 INFORMAION OF EVENS WIH DISCREE OUCOMES: MEHODS OF PHYSICS ORIENAION It s mportant to understand the manner n whch the concept of nformaton s appled n physcs, because t s closely algned wth the
CHAPER 6 INFORMAION OF EVENS WIH DISCREE OUCOMES: MEHODS OF PHYSICS ORIENAION It s mportant to understand the manner n whch the concept of nformaton s appled n physcs, because t s closely algned wth the
You will analyze the motion of the block at different moments using the law of conservation of energy.
 Physcs 00A Homework 7 Chapter 8 Where s the Energy? In ths problem, we wll consder the ollowng stuaton as depcted n the dagram: A block o mass m sldes at a speed v along a horzontal smooth table. It next
Physcs 00A Homework 7 Chapter 8 Where s the Energy? In ths problem, we wll consder the ollowng stuaton as depcted n the dagram: A block o mass m sldes at a speed v along a horzontal smooth table. It next
NAME and Section No.
 Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
= r. / cisely It was not isothermal, nor exactly adia- ! If / l/l /! i i \ i LjSj?
 376 Lea & Burke Physcs; The Nature of Thngs 19.44 J»(Pa) At constant V A \ LjSj?! '/! If / l/l /!! j j J [ ^ 1 I X j j> 1 ' : / J! 60 100 T('K) 200 Constant V lnes are mapped onto straght lnes on the P-T
376 Lea & Burke Physcs; The Nature of Thngs 19.44 J»(Pa) At constant V A \ LjSj?! '/! If / l/l /!! j j J [ ^ 1 I X j j> 1 ' : / J! 60 100 T('K) 200 Constant V lnes are mapped onto straght lnes on the P-T
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
Energy and Energy Transfer
 Energy and Energy Transer Chapter 7 Scalar Product (Dot) Work Done by a Constant Force F s constant over the dsplacement r 1 Denton o the scalar (dot) product o vectors Scalar product o unt vectors = 1
Energy and Energy Transer Chapter 7 Scalar Product (Dot) Work Done by a Constant Force F s constant over the dsplacement r 1 Denton o the scalar (dot) product o vectors Scalar product o unt vectors = 1
Complex Variables. Chapter 18 Integration in the Complex Plane. March 12, 2013 Lecturer: Shih-Yuan Chen
 omplex Varables hapter 8 Integraton n the omplex Plane March, Lecturer: Shh-Yuan hen Except where otherwse noted, content s lcensed under a BY-N-SA. TW Lcense. ontents ontour ntegrals auchy-goursat theorem
omplex Varables hapter 8 Integraton n the omplex Plane March, Lecturer: Shh-Yuan hen Except where otherwse noted, content s lcensed under a BY-N-SA. TW Lcense. ontents ontour ntegrals auchy-goursat theorem
