The City School. Prep Section Worksheets for Intervention Classes (1 st Term) PAF Chapter SCIENCE
|
|
|
- Jocelin Cunningham
- 5 years ago
- Views:
Transcription
1 The City School PAF Chapter Prep Section Worksheets for Intervention Classes (1 st Term) SCIENCE
2 FROM CELLS TO ORGANISMS Q1. Encircle the best answer from the given options. 1. Size of an animal cell is a. Same as size of a plant cell b. Smaller than plant cell c. Bigger than plant cell 2. The thread like structure present in the nucleus a) cytoplasm b) chromosomes c) chloroplast 3. They are made up of proteins called DNA a) Chloroplast b) Genes c) Genes and chromosomes 4. The characteristics of an organism are passed down from one generation to the next by a) genes b) chromosome c) nucleus 5. Function of nucleus is a. Cell repair b. Control all functions of cell c. Both of the above Q2. (a) - Differentiate between an animal cell and a plant cell. Part of cell Animal cell Plant cell Size of cell cytoplasm vacuole Shape of cell (b) Complete the table. Part of a microscope Stage mirror Stage clips Objective lens Its function The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 2 of 12
3 Q3. Fill in the blanks with suitable words. a) A unicellular organism is so simple that its single cell can perform all the b) In a multi cellular organism all the cells are specialized for different functions, this is called division of c) Cells have different to carry out different functions. d) A nerve cell is also called e) Xylem tissue and phloem tissue together make tissue or f) A plant has two systems in it, system and system. g) The largest human cell in terms of volume is cell, which has a diameter similar to that of a strand of hair. h) Sponges are very simple animals whose cells are not arranged into i) A jellyfish has tissues but does not have Q4. Draw and label an animal and a plant cell. The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 3 of 12
4 Elements and Compounds Q1. Fill in the blanks. 1. A reaction is a process in which new substances are formed. 2. Compounds (e.g. water) can be broken down into simpler substances by passing an electric current through it, in a chemical process called 3. are the building blocks of all matter, including living and non-living things. 4. The most abundant element in our universe is, followed by Helium. 5. is the most abundant element by mass in the Earth s crust and in the human body. 6. Every element is represented by an internationally recognized. 7. In the periodic table, elements are placed according to their properties into columns called and rows called 8. The Russian scientist was the first to arrange elements in the periodic table. 9. A can be used to describe a chemical reaction. 10. is a very light and inert (inactive) gas used to fill air-ships and balloons. Q2. Write the constituent elements of the following compounds. 1. Water 2. Sand (silicon dioxide) 3. Iron sulphide 4. Sodium chloride 5. Magnesium oxide Q3. Which of the following word equations show the formation of compound and which shows the break-down of compound? 1. Mercuric oxide mercury + oxygen 2. Potassium + chlorine potassium chloride 3. Sodium hydroxide sodium + hydrogen + oxygen Q4. Give three examples of the following: 1. Elements that are represented by only 1 letter. 2. Elements that are represented by the first two letters of their English names. 3. Elements that are represented by the first letter and one other letter in their names. The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 4 of 12
5 Q5. Are the following statements True or False? Correct the statement if it is false. 1. Chlorine is a greenish yellow, poisonous gas. ( ) 2. In the periodic table, non-metals are found in between metals and metalloids. ( ) 3. The early Greeks believed that there were two elements: Earth and fire. ( ) 4. The properties of compounds are different from those of their constituent elements. ( ) 5. Copper has shiny appearance that is why it s used to make electrical wires. ( ) 6. Going across a period from left to right, elements change from being metallic to non-metallic in character. ( ) Q6. Write down the names of the elements against their chemical symbols. 1. Ca 2. I 3. Zn 4. N 5. Al Q7. Write down the chemical symbols of the following elements. 1. Copper (cuprum) 2. Tungsten (wolfram) 3. Sulphur 4. Chlorine 5. Helium The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 5 of 12
6 Elements, Compounds Q1: Read the following information on elements, compounds. Fill in the blanks where necessary. Elements: A pure substance containing only one kind of. An element is always uniform all the way through (homogeneous). An element be separated into simpler materials. (except during nuclear reactions). Over 100 existing elements are listed and classified on the. Compounds: A pure substance containing two or more kinds of. The atoms are combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. A compound is always homogeneous (uniform). Compounds be separated by physical means. Separating a compound requires a chemical reaction. The properties of a compound are usually different than the properties of the elements it contains. Q2: Give the names and symbols of 5 metals and 5 non-metals. Q3: Lithium and Sodium are in group 1 Find another element in group 1. Q4: Sodium and magnesium are in period 3. Find another element in period 3. Q5: What do you think a group is? Q6: What do you think a period is? Q7:From the following list of substances, underline the ones that are elements. silver carbon dioxide sodium chromium water hydrogen carbon nitrogen oxygen gold sugar salt air sulphur magnesium nickel The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 6 of 12
7 Mixtures: Two or more or NOT chemically combined. No reaction between substances. Mixtures can be uniform (called ) and are known as solutions. Mixtures can also be non-uniform (called ). Mixtures can be separated into their components by chemical or physical means. The properties of a mixture are similar to the properties of its components. Part 2: Classify each of the following as elements (E), compounds (C) or Mixtures (M). Write the letter X if it is none of these. Diamond Sugar (C6H12O6) Milk Iron (Fe) Air Sulfuric Acid (H2SO4) Gasoline Electricity Krypton (K) Bismuth (Bi) Uranium (U) Popcorn Water (H2O) Alcohol (CH3OH) Pail of Garbage A dog Ammonia (NH3) Salt (NaCl) Energy Gold (Au) Wood Bronze Ink Pizza Dry Ice (CO2) Baking Soda (NaHCO3) Titanium (Ti) Concrete Part 3: Match each diagram with its correct description. Diagrams will be used once. A B C D E 1. Pure Element only one type of atom present. 2. Mixture of two elements two types of uncombined atoms present. 3. Pure compound only one type of compound present. 4. Mixture of two compounds two types of compounds present. 5. Mixture of a compound and an element. The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 7 of 12
8 Part 4: Column A lists a substance. In Column B, list whether the substance is an element (E), a compound (C), a Heterogeneous Mixture (HM), or a Solution (S). (Remember a solution is a homogeneous mixture.) In Column C, list TWO physical properties of the substance. Column A Column B Column C 1. Summer Sausage 2. Steam 3. Salt Water 4. Pencil lead (Pb) 5. Dirt 6. Pepsi 7. Silver (Ag) 8. Toothpaste (Na2HPO4) 9. A burrito 10. Italian Dressing 11. Chicken Soup 12. Lemonade The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 8 of 12
9 FORCES AND THEIR EFFECTS: 1) The diagram shows four forces acting on a plane in flight. (a) Which arrow represents air resistance? Give the letter.... (b) (i) When the plane is flying at a constant height, which two forces must be balanced? Give the letters.... and... (ii) When the plane is flying at a constant speed in the direction shown, which two forces must be balanced? Give the letters.... and... (c) (i) Just before take-off, the plane is speeding up along the ground. Which statement is true? Tick the correct box. Force B is zero. Force B is greater than force D. Force D is equal to force B. Force D is greater than force B. The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 9 of 12
10 (ii) Which statement is true about the plane just as it leaves the ground? Tick the correct box. Force C is zero. Force C is greater than force A. Force A is equal to force C. Force A is greater than force C. 2. Anil sits on a mat at the top of a helter-skelter and then slides down a chute around the outside. A chute B mat (a (i) Name two of the forces acting on Anil as he slides from point A to point B The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 10 of 12
11 THE SOLAR SYSTEM AND BEYOND 1) Path an object follows as it revolves around another object. a) revolution b) rotation c) Orbit d) ellipse 2) The Earth ROTATES on its axis once every hours. a) 23 b) 27 c) 24 d) 28 3) Movement of one object in orbit around another object. The Earth goes around the sun. a) Revolution b) rotation c) spin d) axis 4) The phase of the moon that follows the waning crescent is called. a) full moon b) new moon c) third quarter 5) For a solar eclipse to occur, a) the sun must be directly between Earth and the moon b) the moon must be directly between Earth and the sun c) the moon must be directly behind Earth d) Earth must be directly between the sun and the moon Q2.The diagram represents a lunar eclipse. a) True b) False The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 11 of 12
12 Q3. The diagram represents a solar eclipse. a. True b. False 5. Question: Pluto is the smallest planet. a. True b. False 6. Question: Earth is the fourth planet from the sun. a. True b. False 7. Question: Match the following-write the answer in the final column a) A. Saturn 1. Farthest planet from the sun PLUTO b) B. Mars 2. Called the red planet c) C. Pluto 3. Is a large star d) D. Sun 4. Has rings around it Fill in the Blank Mercury Saturn Pluto Neptune Uranus Earth Mars Saturn Venus 1. is the closest plant to the sun. 2. is the largest planet. 1 points Fill in the Blank (1 point each) Planet, Rotation, energy, orbit, energy, crater, star, moonlight, solar moon, season, constellation spinning round and round. 2. -largest object you can see in the night sky ball of hot gases 4. - light and heat from the sun. 5. -light of the moon 6. -path around an object large ball of rock and gas that follows a path around the sun hole, valley, or indention on a planet or moon made by a large rock. 9. stars that form a star picture time of year that has a certain kind of weather 21. Discussion: How does the earth move? Explain your answer. The City School /PAF Chapter / Prep Section / Worksheet for Intervention Class /Mathematics /Class 7 Page 12 of 12
PAF Chapter Prep Section Science Class 7 Worksheets for Intervention Classes
 The City School PAF Chapter Prep Section Science Class 7 Worksheets for Intervention Classes From cells to organisms Q1. Encircle the best answer from the given options. 1. Size of an animal cell is a.
The City School PAF Chapter Prep Section Science Class 7 Worksheets for Intervention Classes From cells to organisms Q1. Encircle the best answer from the given options. 1. Size of an animal cell is a.
The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20]
![The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20] The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20]](/thumbs/89/99385122.jpg) The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20] TOPICS: DICHOTOMOUS KEY, ELEMENTS AND COMPOUNDS, MIXTURES Q-1:
The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20] TOPICS: DICHOTOMOUS KEY, ELEMENTS AND COMPOUNDS, MIXTURES Q-1:
PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key)
 The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
The City School PAF Chapter
 The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 Candidate Name: Index Number: Section: Branch/Campus: Date: INSTRUCTIONS: Write your name, index number, section, branch/campus
The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 Candidate Name: Index Number: Section: Branch/Campus: Date: INSTRUCTIONS: Write your name, index number, section, branch/campus
Unit B Vocabulary. Word Definition Sentence. 12 Trade-off. 15 Atom. 15 Atomic Mass. 15 Element. Family of Elements. Periodic Table of Elements
 Unit B Vocabulary Word Definition Sentence 12 Trade-off 15 Atom 15 Atomic Mass 15 Element 15 Family of Elements 15 Periodic Table of Elements 16 Chemical Formula Unit B Vocabulary 16 Compound 16 Molecule
Unit B Vocabulary Word Definition Sentence 12 Trade-off 15 Atom 15 Atomic Mass 15 Element 15 Family of Elements 15 Periodic Table of Elements 16 Chemical Formula Unit B Vocabulary 16 Compound 16 Molecule
Grade 9 End semester exam Revision sheet Answer key. Kingdom of Bahrain Ministry of Education Ahlia School -ABCD
 Grade 9 End semester exam Revision sheet Answer key Question 1: Directions: Put a check mark in the column that each type of matter describes. 1. Oxygen Substances Element Compound Mixtures 2. Granite
Grade 9 End semester exam Revision sheet Answer key Question 1: Directions: Put a check mark in the column that each type of matter describes. 1. Oxygen Substances Element Compound Mixtures 2. Granite
IES LAURETUM SCIENCE NAME. MIXTURES, ELEMENTS AND COMPOUNDS
 IES LAURETUM SCIENCE NAME. MIXTURES, ELEMENTS AND COMPOUNDS 1 CONTENTS 1. INTRODUCING MIXTURES 2. CLASSIFYING MIXTURES 3. SEPARATING MIXTURES 4. WHAT ARE SOLUTIONS? 5. PURE SUBSTANCES: ELEMENTS AND COMPOUNDS
IES LAURETUM SCIENCE NAME. MIXTURES, ELEMENTS AND COMPOUNDS 1 CONTENTS 1. INTRODUCING MIXTURES 2. CLASSIFYING MIXTURES 3. SEPARATING MIXTURES 4. WHAT ARE SOLUTIONS? 5. PURE SUBSTANCES: ELEMENTS AND COMPOUNDS
Mixtures, Elements, and Compounds
 Mixtures, Elements, and Compounds Chapter 3 (plus K4 & K5) (Big 11 & 12) Matter: Building Blocks of the Universe Atoms and the Periodic Table Section 3-1 Classes of Matter It is important to classify,
Mixtures, Elements, and Compounds Chapter 3 (plus K4 & K5) (Big 11 & 12) Matter: Building Blocks of the Universe Atoms and the Periodic Table Section 3-1 Classes of Matter It is important to classify,
Science Grade 5 Chapter 5: Comparing Kinds of Matter Lesson2: Elements
 Element: is a material that cannot be broken down into anything simpler by chemical reactions. o There are 118 elements o Most elements are solids, some are gasses and few are liquid at room temperature
Element: is a material that cannot be broken down into anything simpler by chemical reactions. o There are 118 elements o Most elements are solids, some are gasses and few are liquid at room temperature
Periodic Table of Elements
 Periodic Table of Elements chlorine nitrogen helium gold oxygen silver mercury hydrogen neodymium sodium niobium carbon Elements Science has come along way since Aristotle s theory of Air, Water, Fire,
Periodic Table of Elements chlorine nitrogen helium gold oxygen silver mercury hydrogen neodymium sodium niobium carbon Elements Science has come along way since Aristotle s theory of Air, Water, Fire,
Chapter 15 & 16 Science Review (PATTERNS IN THE SKY, OUR SOLAR SYSTEM)
 Chapter 15 & 16 Science Review (PATTERNS IN THE SKY, OUR SOLAR SYSTEM) The Milky Way the galaxy that contains our solar system Our solar system is a speck in the Milky Way galaxy Pluto is now considered
Chapter 15 & 16 Science Review (PATTERNS IN THE SKY, OUR SOLAR SYSTEM) The Milky Way the galaxy that contains our solar system Our solar system is a speck in the Milky Way galaxy Pluto is now considered
Matter Review Packet
 Matter Review Packet 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up the compound
Matter Review Packet 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up the compound
2 THE NATURE OF MATTER
 2 THE NATURE OF MATTER I. Tick ( ) the most appropriate answer. 1. Gases have (a) infinite free surfaces (b) two free surfaces (c) one free surface (d) no free surfaces 2. Solids have (a) definite volume,
2 THE NATURE OF MATTER I. Tick ( ) the most appropriate answer. 1. Gases have (a) infinite free surfaces (b) two free surfaces (c) one free surface (d) no free surfaces 2. Solids have (a) definite volume,
CHEMISTRY ELEMENTS, COMPOUNDS & MIXTURES
 CHEMISTRY ELEMENTS, COMPOUNDS & MIXTURES Lesson Intentions In this lesson we will classify substances as Elements, Compounds, Mixtures Key Words 1. Compounds 2. Mixtures 3. Elementary 4. Symbols 5. Reaction
CHEMISTRY ELEMENTS, COMPOUNDS & MIXTURES Lesson Intentions In this lesson we will classify substances as Elements, Compounds, Mixtures Key Words 1. Compounds 2. Mixtures 3. Elementary 4. Symbols 5. Reaction
The Fundamental Ideas in Chemistry
 The Fundamental Ideas in Chemistry Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C1 The Fundamental Ideas in Chemistry Silver Level Question Paper Time
The Fundamental Ideas in Chemistry Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C1 The Fundamental Ideas in Chemistry Silver Level Question Paper Time
3/1/2010. created by Ms Janelle Tay\2010. Learning Objectives
 1 Learning Objectives Define what elements, compounds and mixtures are. Give the names and symbols of common elements. State how elements are classified. State what the building block of an element is.
1 Learning Objectives Define what elements, compounds and mixtures are. Give the names and symbols of common elements. State how elements are classified. State what the building block of an element is.
Ch. 3 Answer Key. O can be broken down to form two atoms of H and 1 atom of O. Hydrogen and oxygen are elements.
 Ch. 3 Answer Key 1. The Greeks believed that all matter is made of elements. We currently believe the same thing. However, the Greeks believed that there were 4 elements: earth, water, air and fire. Instead,
Ch. 3 Answer Key 1. The Greeks believed that all matter is made of elements. We currently believe the same thing. However, the Greeks believed that there were 4 elements: earth, water, air and fire. Instead,
MATTER & ENERGY STUDY GUIDE. 9 Weeks Test Date: Parent Signature (BONUS!):
 Name: Pd: MATTER & ENERGY STUDY GUIDE 9 Weeks Test Date: Parent Signature (BONUS!): 6.5A MATTER Matter is anything that has mass and takes up space. Give EXAMPLES and NON-EXAMPLES of matter. (42) EXAMPLES
Name: Pd: MATTER & ENERGY STUDY GUIDE 9 Weeks Test Date: Parent Signature (BONUS!): 6.5A MATTER Matter is anything that has mass and takes up space. Give EXAMPLES and NON-EXAMPLES of matter. (42) EXAMPLES
Unit 2 Lesson 1 What Objects Are Part of the Solar System? Copyright Houghton Mifflin Harcourt Publishing Company
 Unit 2 Lesson 1 What Objects Are Part of the Solar System? Florida Benchmarks SC.5.E.5.2 Recognize the major common characteristics of all planets and compare/contrast the properties of inner and outer
Unit 2 Lesson 1 What Objects Are Part of the Solar System? Florida Benchmarks SC.5.E.5.2 Recognize the major common characteristics of all planets and compare/contrast the properties of inner and outer
CHEM 1305: Introductory Chemistry
 CHEM 1305: Introductory Chemistry Properties of Matter From Chapter 2 and 3 Textbook Introductory Chemistry: Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Physical States of Matter
CHEM 1305: Introductory Chemistry Properties of Matter From Chapter 2 and 3 Textbook Introductory Chemistry: Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Physical States of Matter
SPI Use data to draw conclusions about the major components of the universe.
 SPI 0607.6.1 - Use data to draw conclusions about the major components of the universe. o Stars are huge, hot, brilliant balls of gas trillions of kilometers away. A Galaxy is a collection of billions
SPI 0607.6.1 - Use data to draw conclusions about the major components of the universe. o Stars are huge, hot, brilliant balls of gas trillions of kilometers away. A Galaxy is a collection of billions
Lesson 1.2 Classifying Matter
 Lesson 1.2 Classifying Matter Vocabulary element atom chemical bond mixture molecule compound chemical formula What is Matter Made Of? What is matter? Why is one kind of matter different from another kind
Lesson 1.2 Classifying Matter Vocabulary element atom chemical bond mixture molecule compound chemical formula What is Matter Made Of? What is matter? Why is one kind of matter different from another kind
Year 8 Chemistry Knowledge Organiser Topic 1: Periodic Table
 KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
Chemistry Unit 1: Section1 - Elements, Compounds, & Mixtures
 Chemistry Unit 1: Section1 - Elements, Compounds, & Mixtures PURE SUBSTANCES A pure substance is called an element. An element is a pure substance because it cannot be separated into any other substances.
Chemistry Unit 1: Section1 - Elements, Compounds, & Mixtures PURE SUBSTANCES A pure substance is called an element. An element is a pure substance because it cannot be separated into any other substances.
Atoms and Elements Review
 Atoms and Elements Review YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS: ALCHEMY ELEMENT ATOM SUBATOMIC DEMOCRITUS DALTON THOMSON RUTHERFORD BOHR ELECTRON NEUTRAL PROTON NEUTRON ORBIT
Atoms and Elements Review YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS: ALCHEMY ELEMENT ATOM SUBATOMIC DEMOCRITUS DALTON THOMSON RUTHERFORD BOHR ELECTRON NEUTRAL PROTON NEUTRON ORBIT
Atoms and Elements Class Notes and Class Work
 Atoms and Elements Class Notes and Class Work Introduction to Matter Property: Characteristics matter has. Law: A rule nature seems to follow. It s been observed regularly. Theory: Tries to explain the
Atoms and Elements Class Notes and Class Work Introduction to Matter Property: Characteristics matter has. Law: A rule nature seems to follow. It s been observed regularly. Theory: Tries to explain the
Science In Action 9 - Unit 1 Matter and Chemical Change Summary of Key Concepts and Review Questions Booklet
 10. Matter can be described and organized by its physical and chemical properties Key Concepts Workplace Hazardous Materials Information System (WHMIS) and safety substances and their properties. Recognition
10. Matter can be described and organized by its physical and chemical properties Key Concepts Workplace Hazardous Materials Information System (WHMIS) and safety substances and their properties. Recognition
ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED. Jan 12-13, 2014
 ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED Jan 12-13, 2014 WHAT ARE ELEMENTS? Elements are pure substances Made of only one kind of material Has definite properties, and Is the same
ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED Jan 12-13, 2014 WHAT ARE ELEMENTS? Elements are pure substances Made of only one kind of material Has definite properties, and Is the same
Unit 12 Lesson 1 What Objects Are Part of the Solar System?
 Unit 12 Lesson 1 What Objects Are Part of the Solar System? The Solar System Earth, other planets, and the moon are part of a solar system. A solar system is made up of a star and the planets and other
Unit 12 Lesson 1 What Objects Are Part of the Solar System? The Solar System Earth, other planets, and the moon are part of a solar system. A solar system is made up of a star and the planets and other
Worksheet on Elements, Compounds and Mixtures
 Worksheet on Elements, Compounds and Mixtures Name : ( ) Date: 05042007 Class : Instruction: Answer all questions 1-9 below by filling the correct answer in the given bracket. 1. How many atoms are present
Worksheet on Elements, Compounds and Mixtures Name : ( ) Date: 05042007 Class : Instruction: Answer all questions 1-9 below by filling the correct answer in the given bracket. 1. How many atoms are present
ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED
 ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas
ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas
ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED
 ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas
ELEMENTS, COMPOUNDS AND MIXTURES AND HOW THEY ARE REPRESENTED 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas
Classification of Matter. Elements, Compounds, Mixtures
 Classification of Matter Elements, Compounds, Mixtures Introducing Little Miss Element Hi! I am Little Miss Element I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a
Classification of Matter Elements, Compounds, Mixtures Introducing Little Miss Element Hi! I am Little Miss Element I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a
-discovered set of patterns that applied to all elements published 1st periodic table. -wrote properties of each on note cards (density, color)
 Dmitri Mendeleev -discovered set of patterns that applied to all elements -1869 published 1st periodic table -total of 63 elements discovered -wrote properties of each on note cards (density, color) -noticed
Dmitri Mendeleev -discovered set of patterns that applied to all elements -1869 published 1st periodic table -total of 63 elements discovered -wrote properties of each on note cards (density, color) -noticed
Unit 3: Physical Science Classifying Matter in our Daily Lives
 Science 7 Unit 3: Physical Science Classifying Matter in our Daily Lives Name Period Purpose: I understand the relationship between atoms, molecules, elements, compounds, and mixtures and can also provide
Science 7 Unit 3: Physical Science Classifying Matter in our Daily Lives Name Period Purpose: I understand the relationship between atoms, molecules, elements, compounds, and mixtures and can also provide
composition of matter, and the changes that matter undergoes. Examples of Uses of Chemistry in Everyday Life
 Name Matter and Change: Unit Objective Study Guide Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, all of the work leading up to the final
Name Matter and Change: Unit Objective Study Guide Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, all of the work leading up to the final
The Sun s center is much hotter than the surface. The Sun looks large and bright in the sky. Other stars look much smaller.
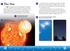 The Sun A star is a huge ball of hot, glowing gases. The Sun is a star. The width of the Sun is equal to the width of 100 Earths placed side by side. The Sun is extremely hot. The surface of the Sun has
The Sun A star is a huge ball of hot, glowing gases. The Sun is a star. The width of the Sun is equal to the width of 100 Earths placed side by side. The Sun is extremely hot. The surface of the Sun has
Year 10 Revision. Atomic Structure C minutes. 75 marks. Page 1 of 28
 Year 0 Revision Atomic Structure C.-5 75 minutes 75 marks Page of 28 Q. A substance made of only one type of atom is called an element. The chemical symbols and positions of six elements in the periodic
Year 0 Revision Atomic Structure C.-5 75 minutes 75 marks Page of 28 Q. A substance made of only one type of atom is called an element. The chemical symbols and positions of six elements in the periodic
Scientist used to believe that matter was made up of four elements (air, earth, fire and water).
 Scientist used to believe that matter was made up of four elements (air, earth, fire and water). We now know that all matter in the universe is made of slightly more than 100 different substances called
Scientist used to believe that matter was made up of four elements (air, earth, fire and water). We now know that all matter in the universe is made of slightly more than 100 different substances called
CHEMISTRY NOTES. Elements and the periodic table. name of the element. A. Element 1. Definition a substance made of one kind of atom
 CHEMISTRY NOTES Elements and the periodic table A. Element 1. Definition a substance made of one kind of atom a. Atom smallest particle of an element Nucleus Protons = Positive charge Neutrons = No charge
CHEMISTRY NOTES Elements and the periodic table A. Element 1. Definition a substance made of one kind of atom a. Atom smallest particle of an element Nucleus Protons = Positive charge Neutrons = No charge
Chemistry Test 1 Study Guide
 Name: Date: Chemistry Test 1 Study Guide Due Date: Test Date: Important Topics: Please review the concepts on this study guide as well as any other notes/worksheets from this unit. I. Aim # 22 Chemistry-
Name: Date: Chemistry Test 1 Study Guide Due Date: Test Date: Important Topics: Please review the concepts on this study guide as well as any other notes/worksheets from this unit. I. Aim # 22 Chemistry-
Name Date. Matter: Pure Substances and Mixtures
 Name Date_ Matter: Pure Substances and Mixtures Part I: Directions: Classify each of the materials below. In the center column, state whether the material is a pure substance or a mixture. If the material
Name Date_ Matter: Pure Substances and Mixtures Part I: Directions: Classify each of the materials below. In the center column, state whether the material is a pure substance or a mixture. If the material
Selected Topics Starry, Starry Night. Exploring the Universe of Science 1
 Selected Topics Starry, Starry Night 2015 Exploring the Universe of Science 1 Revolution & Rotation Key concepts: What is the difference between revolution vs. rotation? Describe the basis for day, month,
Selected Topics Starry, Starry Night 2015 Exploring the Universe of Science 1 Revolution & Rotation Key concepts: What is the difference between revolution vs. rotation? Describe the basis for day, month,
Name: Chemistry Unit Review Science 9
 Name: Chemistry Unit Review Science 9 Do not forget to study for notes, assignments and quizzes! 1. Classify each of the following as a physical or a chemical change. a) Garbage rotting d) Digesting food
Name: Chemistry Unit Review Science 9 Do not forget to study for notes, assignments and quizzes! 1. Classify each of the following as a physical or a chemical change. a) Garbage rotting d) Digesting food
Chemistry Chapter 1 Test Review
 Chemistry Chapter 1 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A chemical can be defined as a. a toxic substance. b. an unnatural additive
Chemistry Chapter 1 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A chemical can be defined as a. a toxic substance. b. an unnatural additive
3.1 Classification of Matter. Copyright 2009 by Pearson Education, Inc.
 Chapter 3 Atoms and Elements 3.1 Classification of Matter Copyright 2009 by Pearson Education, Inc. 1 Matter Matter is the stuff that makes up all things. Copyright 2009 by Pearson Education, Inc. 2 Pure
Chapter 3 Atoms and Elements 3.1 Classification of Matter Copyright 2009 by Pearson Education, Inc. 1 Matter Matter is the stuff that makes up all things. Copyright 2009 by Pearson Education, Inc. 2 Pure
2 nd Term Worksheet [ ] Subject Chemistry Class VII Name : Sec. :
![2 nd Term Worksheet [ ] Subject Chemistry Class VII Name : Sec. : 2 nd Term Worksheet [ ] Subject Chemistry Class VII Name : Sec. :](/thumbs/84/89274725.jpg) 1 chem (vii) 2 nd Term Worksheet [2018 19] Subject Chemistry Class VII Name : Sec. : Chapter 4 [Atomic Structure] Check Point: [62] [A] Answer the following questions: 1. What do you understand by the
1 chem (vii) 2 nd Term Worksheet [2018 19] Subject Chemistry Class VII Name : Sec. : Chapter 4 [Atomic Structure] Check Point: [62] [A] Answer the following questions: 1. What do you understand by the
CLASS COPY Structure and Properties of Matter Parts of the atom
 CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
WHAT IS CHEMISTRY? Chapter Preview Questions
 WHAT IS CHEMISTRY? 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron atoms. d. iron salts. 1 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron
WHAT IS CHEMISTRY? 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron atoms. d. iron salts. 1 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron
Atoms and Elements Review KEY
 Atoms and Elements Review KEY YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS: ELEMENT ATOM WHMIS HHPS SDS PURE MIXTURE COMPOUND MOLECULE DIATOMIC HETEROGENEOUS HOMOGENEOUS METALS NON-METALS
Atoms and Elements Review KEY YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS: ELEMENT ATOM WHMIS HHPS SDS PURE MIXTURE COMPOUND MOLECULE DIATOMIC HETEROGENEOUS HOMOGENEOUS METALS NON-METALS
Pure Substances and Mixtures Picture Vocabulary. 8P1A Pure Substances and Mixtures
 Pure Substances and Mixtures Picture Vocabulary 8P1A Pure Substances and Mixtures T.I.P Chart for pure substances & mixtures terms Make a TIP chart to use for terms about pure substances & mixtures. Write
Pure Substances and Mixtures Picture Vocabulary 8P1A Pure Substances and Mixtures T.I.P Chart for pure substances & mixtures terms Make a TIP chart to use for terms about pure substances & mixtures. Write
2.3 Elements and Compounds > Chapter 2 Matter and Change. 2.3 Elements and Compounds. 2.1 Properties of Matter 2.2 Mixtures. 2.4 Chemical Reactions
 Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Nozha Directorate of Education Form : 4 th primary. Ismailia Road Branch
 Governorate Department : science Nozha Directorate of Education Form : 4 th primary Nozha Language Schools Revision sheet Ismailia Road Branch Question on Lesson ( 1 ) I ) Complete the following statements
Governorate Department : science Nozha Directorate of Education Form : 4 th primary Nozha Language Schools Revision sheet Ismailia Road Branch Question on Lesson ( 1 ) I ) Complete the following statements
A Matter of Fact. Mixtures, Elements and Compounds
 A Matter of Fact Mixtures, Elements and Compounds Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter
A Matter of Fact Mixtures, Elements and Compounds Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter
Chapter 2 Atoms and the Periodic Table
 Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Section 1: Elements Pages 56-59
 Study Guide Chapter 3 Elements, Compounds, and Mixtures Section 1: Elements Pages 56-59 1. Which of the following processes is NOT a physical or chemical change? a. crushing b. weighing c. melting d. passing
Study Guide Chapter 3 Elements, Compounds, and Mixtures Section 1: Elements Pages 56-59 1. Which of the following processes is NOT a physical or chemical change? a. crushing b. weighing c. melting d. passing
Astronomy 3. Earth Movements Seasons The Moon Eclipses Tides Planets Asteroids, Meteors, Comets
 Astronomy 3 Earth Movements Seasons The Moon Eclipses Tides Planets Asteroids, Meteors, Comets Earth s Movements Orbit- the path in which an object travels around another object in space Revolution the
Astronomy 3 Earth Movements Seasons The Moon Eclipses Tides Planets Asteroids, Meteors, Comets Earth s Movements Orbit- the path in which an object travels around another object in space Revolution the
Lesson 1 The Structure of the Solar System
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:
 ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
Unit 6: Chemistry Test 1 Study Guide
 Name: Due Date: Unit 6: Chemistry Test 1 Study Guide Date: Test Date: Unit 6 Important Topics: Please review the concepts on this study guide as well as any other notes/worksheets from this unit. I. Aim
Name: Due Date: Unit 6: Chemistry Test 1 Study Guide Date: Test Date: Unit 6 Important Topics: Please review the concepts on this study guide as well as any other notes/worksheets from this unit. I. Aim
1UNIT. The Universe. What do you remember? Key language. Content objectives
 1UNIT The Universe What do you remember? What are the points of light in this photo? What is the difference between a star and a planet? a moon and a comet? Content objectives In this unit, you will Learn
1UNIT The Universe What do you remember? What are the points of light in this photo? What is the difference between a star and a planet? a moon and a comet? Content objectives In this unit, you will Learn
Chapter 3-1. proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small
 Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Which of the following planets are all made up of gas? When a planets orbit around the Sun looks like an oval, it s called a(n)
 When a planets orbit around the Sun looks like an oval, it s called a(n) - ellipse - circle - axis - rotation Which of the following planets are all made up of gas? - Venus, Mars, Saturn and Pluto - Jupiter,
When a planets orbit around the Sun looks like an oval, it s called a(n) - ellipse - circle - axis - rotation Which of the following planets are all made up of gas? - Venus, Mars, Saturn and Pluto - Jupiter,
Complete this study guide to receive 5 bonus points on your test. Only study guides that are complete will receive the bonus.
 CHEMISTRY AND PERIODIC TABLE STUDY GUIDE Assigned: Thursday, 09 10 14 Due: Thursday, 09 18 14 Test Day: Friday, 09 19 14 Complete this study guide to receive 5 bonus points on your test. Only study guides
CHEMISTRY AND PERIODIC TABLE STUDY GUIDE Assigned: Thursday, 09 10 14 Due: Thursday, 09 18 14 Test Day: Friday, 09 19 14 Complete this study guide to receive 5 bonus points on your test. Only study guides
Elements and Reactivity Revision Notes
 Elements and Reactivity Revision Notes Elements There are just over 100 elements in the Periodic Table. Elements are made up of one type of atom. Every element has a name, atomic number and symbol. Element
Elements and Reactivity Revision Notes Elements There are just over 100 elements in the Periodic Table. Elements are made up of one type of atom. Every element has a name, atomic number and symbol. Element
Fundamentals of General, Organic & Biological Chemistry 4 th Edition. Matter and Life
 Fundamentals of General, Organic & Biological Chemistry 4 th Edition Chapter One Matter and Life Mohammed Hashmat Ali Southeast Missouri State University 2003 Prentice Hall, Inc. 1.1 Chemistry: The Central
Fundamentals of General, Organic & Biological Chemistry 4 th Edition Chapter One Matter and Life Mohammed Hashmat Ali Southeast Missouri State University 2003 Prentice Hall, Inc. 1.1 Chemistry: The Central
Motion of the planets
 Our Solar system Motion of the planets Our solar system is made up of the sun and the 9 planets that revolve around the sun Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus, Neptune & Pluto (maybe?)
Our Solar system Motion of the planets Our solar system is made up of the sun and the 9 planets that revolve around the sun Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus, Neptune & Pluto (maybe?)
Unit 6 Lesson 4 What Are the Planets in Our Solar System? Copyright Houghton Mifflin Harcourt Publishing Company
 Unit 6 Lesson 4 What Are the Planets in Our Solar System? What other objects are near Earth in this part of space? Earth and millions of other objects make up our solar system. In Our Corner of Space A
Unit 6 Lesson 4 What Are the Planets in Our Solar System? What other objects are near Earth in this part of space? Earth and millions of other objects make up our solar system. In Our Corner of Space A
TEKS Cluster: Space. identify and compare the physical characteristics of the Sun, Earth, and Moon
 5.8 Earth and space. The student knows that there are recognizable patterns in the natural world and among the Sun, Earth, and Moon system. 5.8(C) 5.8(D) demonstrate that Earth rotates on its axis once
5.8 Earth and space. The student knows that there are recognizable patterns in the natural world and among the Sun, Earth, and Moon system. 5.8(C) 5.8(D) demonstrate that Earth rotates on its axis once
The Particulate Nature of Matter
 Matter Objectives Learn about the composition of matter. Learn the difference between elements and compounds. Learn to distinguish between physical and chemical properties and changes. Learn to distinguish
Matter Objectives Learn about the composition of matter. Learn the difference between elements and compounds. Learn to distinguish between physical and chemical properties and changes. Learn to distinguish
1 Elements. TAKE A LOOK 2. Identify Look at the illustration, and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 5 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 5 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
1. Read the following article and fill in the vocabulary crossword. An Atom Apart: by Leslie Cargile
 Grade 6 Science Summer Work International School of Arts and Sciences ISAS 2012 2013 Name: 1. Read the following article and fill in the vocabulary crossword. An Atom Apart: by Leslie Cargile Have you
Grade 6 Science Summer Work International School of Arts and Sciences ISAS 2012 2013 Name: 1. Read the following article and fill in the vocabulary crossword. An Atom Apart: by Leslie Cargile Have you
WHAT IS CHEMISTRY? [pg. vii]
![WHAT IS CHEMISTRY? [pg. vii] WHAT IS CHEMISTRY? [pg. vii]](/thumbs/94/119800358.jpg) CH 11 T1 INTRODUCING MATTER & ATOMIC THEORY 1 You have mastered this topic when you can: 1) define the terms CHEMISTRY, ELEMENT, ATOM, COMPOUND, MOLECULE, ION, MATTER, MASS, WEIGHT and INERTIA. 2) define
CH 11 T1 INTRODUCING MATTER & ATOMIC THEORY 1 You have mastered this topic when you can: 1) define the terms CHEMISTRY, ELEMENT, ATOM, COMPOUND, MOLECULE, ION, MATTER, MASS, WEIGHT and INERTIA. 2) define
What s in Our Solar System?
 The Planets What s in Our Solar System? Our Solar System consists of a central star (the Sun), the main eight planets orbiting the sun, the dwarf planets, moons, asteroids, comets, meteors, interplanetary
The Planets What s in Our Solar System? Our Solar System consists of a central star (the Sun), the main eight planets orbiting the sun, the dwarf planets, moons, asteroids, comets, meteors, interplanetary
...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1]
![...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1] ...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1]](/thumbs/76/74051985.jpg) High Demand Questions QUESTIONSHEET 1 The boxes represent particles of different gases. One box shows the particles of elements in group 0 (group 8). A B C D What name is given to group 0 (8) elements?
High Demand Questions QUESTIONSHEET 1 The boxes represent particles of different gases. One box shows the particles of elements in group 0 (group 8). A B C D What name is given to group 0 (8) elements?
Matter and Energy. Section 2.1 Chapter 2. Representations of Matter: Models and Symbols. Goal 1. Goal 2
 Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
A. The moon B. The sun C. Jupiter D. Earth A. 1 B. 2 C. 3 D. 4. Sky Science Unit Review Konrad. Here is a selection of PAT style questions.
 Sky Science Unit Review Konrad Here is a selection of PAT style questions. Use the following information to answer the next question 1. 2. The source of light that allows astronimors to see Jupitor through
Sky Science Unit Review Konrad Here is a selection of PAT style questions. Use the following information to answer the next question 1. 2. The source of light that allows astronimors to see Jupitor through
Lego Molecules. Standards 1.b. Students know all matter is made of atoms, which may combine to form molecules.
 Science Stars: 5 th grade Lesson Plan Lego Molecules Standards 1.b. Students know all matter is made of atoms, which may combine to form molecules. d. Students know that each element is made of one kind
Science Stars: 5 th grade Lesson Plan Lego Molecules Standards 1.b. Students know all matter is made of atoms, which may combine to form molecules. d. Students know that each element is made of one kind
Qualitative Chemistry Unit 2. Matter A Central Idea in Chemistry
 Qualitative Chemistry Unit 2 Matter A Central Idea in Chemistry Unit Warm-Up 1. What do chemists study? 2. How do atoms differ from molecules? 3. Describe a chemical change (chemical reaction) you have
Qualitative Chemistry Unit 2 Matter A Central Idea in Chemistry Unit Warm-Up 1. What do chemists study? 2. How do atoms differ from molecules? 3. Describe a chemical change (chemical reaction) you have
CLASS PERIOD STUDENT NAME SOLAR SYSTEM PROJECT 2.2 P THE INNER & OUTER PLANETS
 STUDENT NAME CLASS PERIOD 2.2 P SOLAR SYSTEM PROJECT THE INNER & OUTER PLANETS ROCKY 59 DAYS 88 DAYS NO THIN ZERO MERCURY WHAT MAKES MERCURY UNIQUE OR DIFFERENT FROM THE OTHERS? IT IS THE SMALLEST PLANET.
STUDENT NAME CLASS PERIOD 2.2 P SOLAR SYSTEM PROJECT THE INNER & OUTER PLANETS ROCKY 59 DAYS 88 DAYS NO THIN ZERO MERCURY WHAT MAKES MERCURY UNIQUE OR DIFFERENT FROM THE OTHERS? IT IS THE SMALLEST PLANET.
Unit 1: The Earth in the Universe
 Unit 1: The Earth in the Universe 1. The Universe 1.1. First ideas about the Universe 1.2. Components and origin 1.3. Sizes and distances 2. The Solar System 3. The planet Earth 3.1. Movements of the Earth
Unit 1: The Earth in the Universe 1. The Universe 1.1. First ideas about the Universe 1.2. Components and origin 1.3. Sizes and distances 2. The Solar System 3. The planet Earth 3.1. Movements of the Earth
Name Date Hour Table. Semester One Review #1-11 Directions: Mark the correct answer on each of the following questions.
 Semester One Review #1-11 Directions: Mark the correct answer on each of the following questions. 1. Which of the following are subatomic particles? A. negative Electrons, neutral nuclei, negative elements
Semester One Review #1-11 Directions: Mark the correct answer on each of the following questions. 1. Which of the following are subatomic particles? A. negative Electrons, neutral nuclei, negative elements
5. The mass of oxygen required to completely convert 4.0 grams of hydrogen to water is 1) 8.0 grams; 2) 2.0 grams; 3) 32 grams; 4) 16 grams.
 CHEMISTRY TEST NAME: MASS AND VOLUME DATE: EQUATION RELATIONSHIPS Directions: For each of the following questions, choose the number that best answers the question and place it on your answer sheet. Directions:
CHEMISTRY TEST NAME: MASS AND VOLUME DATE: EQUATION RELATIONSHIPS Directions: For each of the following questions, choose the number that best answers the question and place it on your answer sheet. Directions:
SNC1P - Chemistry Test Review
 SNC1P - Chemistry Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is an example of a physical property? a. solubility
SNC1P - Chemistry Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is an example of a physical property? a. solubility
Classification of Matter
 Classification of Matter All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that
Classification of Matter All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that
Physical Science QUIZ-1. Unit Q10 Q11 Q12 Q13 Q14 Q15 Q16 Q17. Total. Teacher s Use Only. Student s Name. Max Score. Question Number.
 Physical Science QUIZ-1 Unit Teacher s Use Only Student s Name Date 2016-2017 Academic Year- Term Question Number Max Score Point Scored Duration Grade minutes G Q1 Q2 Q3 Instructions Fill in your student
Physical Science QUIZ-1 Unit Teacher s Use Only Student s Name Date 2016-2017 Academic Year- Term Question Number Max Score Point Scored Duration Grade minutes G Q1 Q2 Q3 Instructions Fill in your student
Elements. Boiling Point. Help you identify a specific element
 Section 1 * Lab * 2 wksts * Quiz Pure substance Only one type of particle Called Atoms Metals Nonmetals Elements Categories Boiling Point Help you identify a specific element Melting Point Characteristic
Section 1 * Lab * 2 wksts * Quiz Pure substance Only one type of particle Called Atoms Metals Nonmetals Elements Categories Boiling Point Help you identify a specific element Melting Point Characteristic
Mercury Named after: Mercury, the fast-footed Roman messenger of the gods. Mean Distance from the Sun: 57,909,175 km (35,983,093.1 miles) or 0.
 Mercury Named after: Mercury, the fast-footed Roman messenger of the gods. Mean Distance from the Sun: 57,909,175 km (35,983,093.1 miles) or 0.387 astronomical units Diameter: 4,879.4 km (3,031.92 miles)
Mercury Named after: Mercury, the fast-footed Roman messenger of the gods. Mean Distance from the Sun: 57,909,175 km (35,983,093.1 miles) or 0.387 astronomical units Diameter: 4,879.4 km (3,031.92 miles)
Look around the room and list several examples of matter. Also list some examples that are not matter.
 Look around the room and list several examples of matter. Also list some examples that are not matter. As a result of what we do in class today, you will be able to: Explain the relationship between matter,
Look around the room and list several examples of matter. Also list some examples that are not matter. As a result of what we do in class today, you will be able to: Explain the relationship between matter,
Worksheet #1: Atomic Spectra Answer the following questions using your Unit 3 notes.
 Worksheet #1: Atomic Spectra 1. How did Bohr expand on Rutherford s model of the atom? 2. Compare the energy of an electron in the ground state and an electron in the excited state. 3. When an electron
Worksheet #1: Atomic Spectra 1. How did Bohr expand on Rutherford s model of the atom? 2. Compare the energy of an electron in the ground state and an electron in the excited state. 3. When an electron
UNIT 2: Matter and its changes. Mrs. Turner
 UNIT 2: Matter and its changes Mrs. Turner Preassessment Take out a sheet of paper and number it from 1-25. Write down your answers to plug them into your clickers. Don t worry about not knowing an answer
UNIT 2: Matter and its changes Mrs. Turner Preassessment Take out a sheet of paper and number it from 1-25. Write down your answers to plug them into your clickers. Don t worry about not knowing an answer
Chemistry. The building blocks of matter Made of protons, neutrons and electrons. Pure substances that cannot be separated.
 Chemistry CHEMISTRY NOTES Atom- Element- Compound- Molecule- The building blocks of matter Made of protons, neutrons and electrons. Pure substances that cannot be separated. Ex: Gold 2 or more elements
Chemistry CHEMISTRY NOTES Atom- Element- Compound- Molecule- The building blocks of matter Made of protons, neutrons and electrons. Pure substances that cannot be separated. Ex: Gold 2 or more elements
Full file at
 16 Chapter 2: Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element. a.
16 Chapter 2: Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element. a.
Molecules, Compounds, and Mixtures
 Molecules, Compounds, and Mixtures REMEMBER... Atoms are the basic building blocks of all the matter around us. An element is a pure substance that cannot be broken down into smaller parts. Each type of
Molecules, Compounds, and Mixtures REMEMBER... Atoms are the basic building blocks of all the matter around us. An element is a pure substance that cannot be broken down into smaller parts. Each type of
THE SUN, THE MOON AND OUR SOLAR SYSTEM TEACHER NOTES TO SHARE WITH STUDENTS
 THE SUN, THE MOON AND OUR SOLAR SYSTEM TEACHER NOTES TO SHARE WITH STUDENTS The Sun is the biggest feature in our solar system. It is the largest object and contains approximately 98% of the total solar
THE SUN, THE MOON AND OUR SOLAR SYSTEM TEACHER NOTES TO SHARE WITH STUDENTS The Sun is the biggest feature in our solar system. It is the largest object and contains approximately 98% of the total solar
Section 3.1 Matter, Elements, & Atoms. 8 th Grade Earth & Space Science - Class Notes
 Section 3.1 Matter, Elements, & Atoms 8 th Grade Earth & Space Science - Class Notes What is Matter? Matter is anything that has volume and mass. Everything in the world is made up of matter. On Earth,
Section 3.1 Matter, Elements, & Atoms 8 th Grade Earth & Space Science - Class Notes What is Matter? Matter is anything that has volume and mass. Everything in the world is made up of matter. On Earth,
Atomic Structure and The Periodic Table. Unit 3
 Atomic Structure and The Periodic Table Unit 3 Lesson 1: Atoms Unit 5: Atomic Structure & The Periodic Table Atoms How small can things get? If you break a stone wall into smaller and smaller pieces, you
Atomic Structure and The Periodic Table Unit 3 Lesson 1: Atoms Unit 5: Atomic Structure & The Periodic Table Atoms How small can things get? If you break a stone wall into smaller and smaller pieces, you
1. The Sun is the largest and brightest object in the universe. 2. The period that the Earth takes to revolve once around the Sun is approximately a
 PLEASE ANSWER YOUR QUESTIONS ON THIS PROVIDED QUESTION PAPER. EACH QUESTION IS FOLLOWED BY ANSWERS MARKED A AND B, OR A, B, C AND D. ONLY ONE ANSWER IS CORRECT. CHOOSE THE MOST CORRECT ANSWER AND CIRCLE
PLEASE ANSWER YOUR QUESTIONS ON THIS PROVIDED QUESTION PAPER. EACH QUESTION IS FOLLOWED BY ANSWERS MARKED A AND B, OR A, B, C AND D. ONLY ONE ANSWER IS CORRECT. CHOOSE THE MOST CORRECT ANSWER AND CIRCLE
ACP Chemistry (821) - Mid-Year Review
 ACP Chemistry (821) - Mid-Year Review *Be sure you understand the concepts involved in each question. Do not simply memorize facts!* 1. What is chemistry? Chapter 1: Chemistry 2. What is the difference
ACP Chemistry (821) - Mid-Year Review *Be sure you understand the concepts involved in each question. Do not simply memorize facts!* 1. What is chemistry? Chapter 1: Chemistry 2. What is the difference
The Outer Planets (pages )
 The Outer Planets (pages 720 727) Gas Giants and Pluto (page 721) Key Concept: The first four outer planets Jupiter, Saturn, Uranus, and Neptune are much larger and more massive than Earth, and they do
The Outer Planets (pages 720 727) Gas Giants and Pluto (page 721) Key Concept: The first four outer planets Jupiter, Saturn, Uranus, and Neptune are much larger and more massive than Earth, and they do
