Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential β-catenin phosphorylation
|
|
|
- Raymond Spencer
- 5 years ago
- Views:
Transcription
1 2718 Research Article Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential β-catenin phosphorylation Monica D. David 1, *, Andrée Yeramian 1, *, Mireia Duñach 2, Marta Llovera 1, Carles Cantí 1, Antonio García de Herreros 3, Joan X. Comella 1, and Judit Herreros 1, 1 Laboratori d Investigació, Hospital Universitari Arnau de Vilanova, Departament de Ciències Mèdiques Bàsiques, Universitat de Lleida, IRBLleida, Spain 2 Departament de Bioquímica i Biologia Molecular, Universitat Autònoma de Barcelona, Spain 3 Programa de Recerca en Càncer, IMIM-Hospital del Mar, Parc de Recerca Biomèdica de Barcelona, Spain *These authors contributed equally to this work Present address: Institut de Neurociències, Universitat Autònoma de Barcelona, Spain Author for correspondence ( j.herreros@cmb.udl.cat) Accepted 26 May , Published by The Company of Biologists 2008 doi: /jcs Summary Tyrosine phosphorylation of β-catenin, a component of adhesion complexes and of the Wnt pathway, affects cell adhesion, migration and gene transcription. By reducing β-catenin availability using shrna-mediated gene silencing or expression of intracellular N-cadherin, we show that β-catenin is required for axon growth downstream of brain-derived neurotrophic factor (BDNF) signalling and hepatocyte growth factor (HGF) signalling. We demonstrate that the receptor tyrosine kinases (RTKs) Trk and Met interact with and phosphorylate β-catenin. Stimulation of Trk receptors by neurotrophins (NTs) results in phosphorylation of β-catenin at residue Y654, and increased axon growth and branching. Conversely, pharmacological inhibition of Trk or expression of a Y654F mutant blocks these effects. β-catenin phosphorylated at Y654 colocalizes with the cytoskeleton at growth cones. However, HGF, which also increases axon growth and branching, induces β-catenin phosphorylation at Y142 and a nuclear localization. Interestingly, dominant-negative N-TCF4 abolishes the effects of HGF in axon growth and branching, but not that of NTs. We conclude that NT- and HGF-signalling differentially phosphorylate β-catenin, targeting this protein to distinct compartments to regulate axon morphogenesis by TCF4- transcription-dependent and -independent mechanisms. These results place β-catenin downstream of growth-factor RTK signalling in axon differentiation. Supplementary material available online at Key words: β-catenin, Neurotrophins, Hepatocyte growth factor, Axon growth, Trk, Met Introduction The establishment of proper neuronal connections is crucial to brain physiology, and requires the outgrowth and arborization of axons and dendrites before synaptic contacts are stabilized. Both extracellular cues and an intrinsic cell programme promote neuronal polarity and axon growth (Arimura and Kaibuchi, 2007). A variety of extracellular signals play a major role in neuronal differentiation and synapse formation (Arimura and Kaibuchi, 2007; Ciani and Salinas, 2005; Dalva et al., 2007; Huang and Reichardt, 2001; Takeichi, 2007). Neurotrophins (NTs) and their Trk receptors are among the best-known regulators of neuronal development and function (Chao, 2003; Huang and Reichardt, 2001; Huang and Reichardt, 2003; Zampieri and Chao, 2006). TrkB (Ntrk2) and TrkC (Ntrk3) are expressed in the hippocampus, and their ligands BDNF, NT-3 and NT-4 regulate neurite outgrowth and branching in hippocampal neurons (Collazo et al., 1992; Labelle and Leclerc, 2000; Lindholm et al., 1996; Martinez et al., 1998; Vicario-Abejon et al., 1998). Hepatocyte growth factor (HGF) and its receptor tyrosine kinase (RTK) Met are also expressed in the hippocampus, in which HGF is a neurotrophic factor for hippocampal neurons (Honda et al., 1995; Korhonen et al., 2000). Furthermore, Wnt factors regulate neuronal differentiation through different signalling pathways (Ciani and Salinas, 2005). Despite the abundance of molecules controlling neuronal morphogenesis, the intracellular mechanisms that integrate these signals to define the morphology of the neuritic arbor are poorly understood. β-catenin is a cytoplasmic protein that plays a crucial role in Ca 2+ -dependent cell-cell adhesion and Wnt signalling (Lilien and Balsamo, 2005; Nelson and Nusse, 2004). As a component of the adhesion complex, β-catenin binds to the intracellular domain of cadherins and to α-catenin, which in turn can bind to actin (Drees et al., 2005; Yamada et al., 2005). In the canonical Wnt pathway, Wnt proteins inhibit glycogen synthase kinase-3β (GSK-3β), which otherwise targets β-catenin for degradation, resulting in β-catenin nuclear translocation and activation of T-cellfactor lymphoid-enhancing-factor (TCF/LEF)-driven gene transcription (Ciani and Salinas, 2005; Logan and Nusse, 2004; Nelson and Nusse, 2004). The regulation of cell adhesion underlies important processes, such as neurite extension, synaptogenesis, cell migration and epithelialmesenchymal transition, in development and metastasis (Brembeck et al., 2006; Dalva et al., 2007; Nelson and Nusse, 2004; Takeichi, 2007; Thiery, 2002). Tyrosine phosphorylation of β-catenin by RTKs is known to alter cell adhesion (Nelson and Nusse, 2004; Lilien and Balsamo, 2005). For example, the epidermal growth factor (EGF) receptor (EGFR) associates with β-catenin in the core region, which
2 β-catenin phosphorylation in axon growth 2719 Fig. 1. β-catenin interacts with TrkA in PC12 6/15 cells, and with TrkB and TrkC in hippocampal neurons. (A) Control (Myc) or β-catenin immunoprecipitation from PC12 6/15 cells starved or stimulated with NGF (100 ng/ml) for different times (as indicated) shows that TrkA (detected with anti-pan-trk antibodies: 140-kDa and 110-kDa forms) co-immunoprecipitates with β- catenin, independent of its phosphorylation state. (B) Control (GFP) or pan-trk immunoprecipitation from hippocampal neurons untreated or treated with BDNF or NT-3 (50 ng/ml; 5 minutes) shows that TrkB and TrkC co-immunoprecipitate with β-catenin. contains residue Y654, and induces β-catenin phosphorylation (Hoschuetzky et al., 1994; Takahashi et al., 1997), resulting in decreased affinity for cadherin (Hoschuetzky et al., 1994; Nelson and Nusse, 2004; Roura et al., 1999; Takahashi et al., 1997). Similarly, in epithelial cells, phosphorylation of Y142 by the non-rtks Fer and Fyn or by the RTK Met weakens the binding of β-catenin to α- catenin (Brembeck et al., 2004; Lilien and Balsamo, 2005; Nelson and Nusse, 2004; Piedra et al., 2001). Activation of β-catenin signalling by Met and phosphorylation of β-catenin Y142 leads to its nuclear translocation and to increased TCF-mediated gene transcription (Brembeck et al., 2004; Danilkovitch-Miagkova et al., 2001). In addition, Fer interacts with cadherin indirectly and phosphorylates PTP1B (PTPN1), regulating the binding of PTP1B to cadherin and the continuous dephosphorylation of β-catenin Y654 (Balsamo et al., 1998; Balsamo et al., 1996; Piedra et al., 2003; Xu et al., 2002; Xu et al., 2004). Together, the available data indicate that the tyrosine-phosphorylation state of β-catenin, resulting from the balance of phosphatase and kinase activities, regulates cell migration by decreasing adhesion and activating β-catenin signalling in epithelial tissue. In the nervous system, cadherin and β-catenin regulate axon extension (Riehl et al., 1996), dendritogenesis (Yu and Malenka, 2003), synaptic assembly and plasticity (Bamji, 2005; Bamji et al., 2006; Tai et al., 2007; Yu and Malenka, 2003). Interestingly, tyrosine phosphorylation of β-catenin also regulates synapse formation and function (Bamji et al., 2006; Murase et al., 2002), and the growthcone response to the guidance cue Drosophila Slit (Rhee et al., 2007; Rhee et al., 2002). Here, we investigated the relationship between β-catenin and growth-factor RTK signalling in axon morphogenesis. We found that β-catenin is required for axon extension. Stimulation of the neuronal RTKs Trk and Met by NTs and HGF results in β-catenin tyrosine phosphorylation. β-catenin phosphorylation at Y654 by BDNF- or NT-3-signalling increases axon growth and branching in hippocampal neurons, whereas expression of the mutant Y654F or Trk inhibition abolishes the NT effects. We also demonstrate that HGF stimulates axon growth and branching by phosphorylating β-catenin at Y142. Finally, we present evidence suggesting that the regulation of axon morphogenesis induced by NTs and the phosphorylation of β-catenin at Y645 occur independently of TCF4. By contrast, Met activation results in phospho-y142 (Y142-P) β-catenin nuclear localization and TCF4-mediated gene transcription to promote axon growth and branching. Our results identify a crosstalk between β-catenin- and growth-factor-signalling networks during axon morphogenesis. Results Trk interacts with β-catenin β-catenin has been shown to associate with and to be a substrate of EGFR (Hoschuetzky et al., 1994; Takahashi et al., 1997). Here we explored whether it interacts with other RTKs, such as Trk, in neuronal cell lines and primary neurons. PC12 6/15 cells, a clone of PC12 cells stably expressing the non-neuronal form of human TrkA (Hempstead et al., 1992; Llovera et al., 2004) were treated with nerve growth factor (NGF). TrkA and β-catenin coimmunoprecipitated from PC12 6/15 lysates independently of NGF stimulation (Fig. 1A). To investigate whether this association extended to other Trk receptors, we used hippocampal neurons, which express TrkB and TrkC (Martinez et al., 1998). Using antipan-trk antibodies we immunoprecipitated TrkB and TrkC from untreated and BDNF- or NT-3-treated neurons. β-catenin coimmunoprecipitated with TrkB and TrkC from hippocampal neuron lysates (Fig. 1B). Thus, β-catenin interacts with Trk receptors in PC12 6/15 cells and developing neurons. Trk phosphorylates β-catenin at Y654 The interaction of Trk receptors with β-catenin raises the possibility that β-catenin could be phosphorylated by these RTKs. In in-vitro-phosphorylation assays, recombinant TrkA kinase or TrkA immunoprecipitated from PC12 6/15 cells was incubated with recombinant wild-type (WT) or mutant β-catenin in the presence or absence of the Trk-kinase inhibitor K252a. Following the addition of ATP, TrkA kinase produced a robust tyrosine phosphorylation of WT GST-β-catenin (Fig. 2A). Preincubation of TrkA kinase with K252a reduced TrkA kinase activation to basal levels and blocked GST-β-catenin phosphorylation (Fig. 2A). Interestingly, phosphorylation of mutant Y654F GST-β-catenin by TrkA kinase was ~45% lower than that of WT GST-β-catenin, whereas phosphorylation of Y142F GST-β-catenin was ~15% lower than phosphorylation of the WT protein (Fig. 2A). TrkA immunoprecipitated from PC12 6/15 cells also induced β-catenin tyrosine phosphorylation upon addition of ATP, and the amount of this phosphorylation was decreased by 30% by addition of K252a (Fig. 2B). Furthermore, Y654F β-catenin phosphorylation was significantly lower (~40%) than that of WT β-catenin, whereas the residual phosphorylation of Y654F β-catenin was not inhibited by K252a (Fig. 2B). Together, these kinase assays indicate that TrkA phosphorylates β-catenin in vitro at Y654. To study further the role of Trk in β-catenin phosphorylation, β-catenin immunoprecipitation was performed on lysates from PC12 6/15 cells and hippocampal neurons in the presence or absence of pervanadate (to inhibit tyrosine phosphatases) for subsequent western blot analysis using phosphospecific antibodies. Y654-P β-catenin was detected in both PC12 6/15 and hippocampal cell lysates, and levels of Y654-P β-catenin increased following NGF stimulation of PC12 6/15 cells and BDNF or NT-3 stimulation of hippocampal neurons, correlating with Trk phosphorylation (Fig. 2C,D). Stimulation with BDNF (50 ng/ml) for 10 minutes
3 (16) Fig. 2. Trk phosphorylates β-catenin at Y654. (A) In vitro kinase assay using recombinant TrkA kinase and WT or mutant GST-β-catenin. Anti-phospho-Tyr (anti-tyr-p) western blot shows phosphorylated TrkA kinase and GST β-catenin. WT GST β-catenin is phosphorylated by TrkA kinase upon addition of ATP, whereas K252a inhibits TrkA-induced β-catenin phosphorylation. Phosphorylation of mutant Y654F and Y142F GST β-catenin by TrkA kinase is ~45% and 15% lower than that of WT GST β-catenin, respectively. The panel below shows the anti-β-catenin western blot for the GST β-catenin input in the kinase assay (0.8 μg). (B) In vitro kinase assay using TrkA immunoprecipitated from PC12 6/15 cells, and recombinant WT and Y654F β-catenin. Anti-Tyr-P western blot shows TrkA (140-kDa and 110-kDa bands) and the tyrosine phosphorylation of β-catenin. Phosphorylation of WT β-catenin is decreased by preincubation with K252a (100 nm), whereas phosphorylation of Y654F β-catenin is lower than that of WT β-catenin and is not inhibited by K252a. In the first lane, no recombinant β-catenin was added to control for phosphorylation of any endogenous β-catenin that might have been immunoprecipitated with TrkA. Quantification corresponds to % of the increase in the intensity of Tyr-P β-catenin relative to ATP divided by total β-catenin. (C) Control (Myc) or β-catenin immunoprecipitation from PC12 6/15 cells untreated or treated with NGF (100 ng/ml) and pervanadate for the last 10 minutes of stimulation. Phosphorylation of β-catenin Y654 increases after NGF treatment in parallel with TrkA stimulation as detected by anti-tyr-p western blot. (D) Control (His) or β-catenin immunoprecipitation from untreated hippocampal neurons or those treated for 10 minutes with 50 ng/ml BDNF or NT-3 in the presence of pervanadate. Upon NT stimulation, phosphorylation of β-catenin at Y654 increases parallel with phosphorylation of TrkB and/or TrkC detected by anti-phospho-tyr western blot. (E) Control or β-catenin immunoprecipitation from hippocampal neurons untreated or treated for 10 minutes with 50 ng/ml BDNF with or without pervanadate. Level of Y654-P β-catenin increases with BDNF treatment both with and without pervanadate, whereas phosphorylation at Y142 is maintained at the basal level, increasing only slightly with pervanadate. TrkB and N-cadherin co-immunoprecipitate with β-catenin, and N-cadherin association with β-catenin shows a small decrease after pervanadate treatment. (F) β-catenin immunoprecipitation from untreated neurons or those treated with 50 ng/ml BDNF for the indicated times in the presence of pervanadate for the last 10 minutes of stimulation. The level of Y654-P β-catenin peaks at 1 hour and is reduced after overnight (o/n) stimulation. Quantifications in C-F correspond to % of the increase in the intensity of the phosphospecific β-catenin (Y654-P or Y142-P) band relative to the control normalized to total β-catenin. induced the phosphorylation of Y654, which peaked at 1 hour and was reduced after overnight treatment (Fig. 2E,F). By contrast, phosphorylation of Y142 was maintained at basal levels (Fig. 2E), indicating that NT signalling specifically phosphorylates β-catenin Y654. In addition to TrkB, N-cadherin also co-immuno precipitated with β-catenin (Fig. 2E), suggesting that β-catenin, N-cadherin and Trk interact in a large complex. Altogether, these findings demonstrate that Trk receptors phosphorylate β-catenin Y654 in PC12 6/15 cells and in primary neurons. Met interacts with β-catenin and phosphorylates β-catenin Y142 HGF and Met, its RTK, are both expressed in the hippocampus (Honda et al., 1995; Korhonen et al., 2000). This localization led us to examine whether Met, similar to Trk, interacts with and phosphorylates β-catenin. We found that Met and β-catenin coimmunoprecipitate from hippocampal-neuron lysates and that this association decreases upon HGF treatment with or without pervanadate by 87.6±3.3% and 85.6±5.1%, respectively, compared
4 β-catenin phosphorylation in axon growth 2721 Fig. 3. Met phosphorylates β-catenin at Y142. (A) In vitro kinase assay with recombinant Met kinase and WT or mutant GST β-catenin. Anti-phospho-Tyr (anti-tyr-p) western blot shows phosphorylated Met kinase upon addition of ATP and its basal activation ( ATP condition). Anti-Y142-P-β-catenin western blot (top) indicates that WT and Y654F GST β-catenin are phosphorylated by Met kinase at Y142, whereas phosphorylation of Y142F GST β-catenin is as low as in basal condition ( ATP). (B) Control (His) and β-catenin immunoprecipitation from untreated hippocampal neurons or those treated with 50 ng/ml HGF for 10 minutes with or without pervanadate. The level of Y142-P β-catenin increases with HGF treatments, whereas phosphorylation at Y654 is not stimulated by HGF signalling. Met and α-catenin coimmunoprecipitate with β-catenin, and the recovery of both proteins decreases upon phosphorylation of β-catenin at Y142. (C) β-catenin immunoprecipitation from untreated neurons or those treated with 50 ng/ml HGF for the indicated times. The level of Y142-P β-catenin peaks at 10 minutes, is maintained at similar levels up to 1 hour and starts decaying slightly after overnight (o/n) stimulation. Quantifications in B and C correspond to % of the increase in the intensity of the phosphospecific β- catenin band relative to the control normalized to total β-catenin. with control (n 3) (Fig. 3B). This interaction suggested that β- catenin is phosphorylated by Met. Using antibodies specific for Y142-P β-catenin, recombinant Met kinase was found to induce the phosphorylation of WT GST-β-catenin at Y142 upon addition of ATP. This decreased to basal levels when using mutant Y142F GST-β-catenin (Fig. 3A). By contrast, phosphorylation of Y654F GST-β-catenin was similar to that of the WT protein. Furthermore, prior treatment with HGF (50 ng/ml, 10 minutes) with or without pervanadate induced the phosphorylation of β-catenin Y142 in β- catenin immunoprecipitates isolated from hippocampal neurons (Fig. 3B). Following a peak phosphorylation at 10 minutes, Y142- P was maintained up to 1 hour and started to decay slightly after overnight stimulation with HGF (Fig. 3C). In contrast to BDNFand NT-3-signalling, HGF treatment did not stimulate Y654 phosphorylation (Fig. 3B), indicating that NT- and HGF-signalling phosphorylate different tyrosine residues. Interestingly, α-catenin (Fig. 3B) and N-cadherin (data not shown) co-immunoprecipitated with β-catenin, and the phosphorylation of β-catenin at Y142 resulted in decreased amounts of α-catenin in the β-catenin immunoprecipitates (88±5% and 77±3.4% lower in HGF and HGF+pervanadate, respectively, than in control; n 3; Fig. 3B). Together, these findings indicate that Met interacts with β-catenin that is engaged in the adhesion complex and phosphorylates Y142, resulting in the detachment of α-catenin from β-catenin. β-catenin is involved in axon growth in hippocampal neurons Next we investigated the possible role of β-catenin and its interactions with Trk and Met in axon growth because both types of molecules have been implicated in axon morphogenesis (Honda et al., 1995; Huang and Reichardt, 2001; Huang and Reichardt, 2003; Riehl et al., 1996). First, we analyzed the involvement of β- catenin in axon growth in the absence of neurotrophic-factor signalling. In order to do this, we designed scrambled and β-catenin shrna vectors for lentiviral infection of hippocampal neurons. Quantification of western blots of neurons expressing β-catenin shrna#1 and shrna#2 indicated a decrease of total levels of β- catenin by about 25% and 20%, respectively, at 3 days of infection compared with neurons expressing scrambled shrna (Fig. 4A). Anti-β-catenin immunostaining indicates reduced β-catenin immunoreactivity, especially along the axon of GFP-positive neurons expressing β-catenin shrna#1 (Fig. 4A). We measured axon length in neurons expressing scrambled or β-catenin shrnas. In these experiments, axon length was measured at 3 days in vitro (DIV) because this was the earliest time point at which we observed a significant reduction in the levels of β-catenin and axon length was still easily measured. Neurons expressing β-catenin shrnas exhibited significantly shorter axons than neurons expressing scrambled shrna (Fig. 4B,C). Furthermore, stimulation of TrkB and Met by BDNF and HGF, respectively, increased axon length in neurons expressing scrambled shrna (Fig. 4C). However, in neurons expressing β-catenin shrna#1 and shrna#2, BDNF or HGF treatment could not stimulate axon growth (Fig. 4C). These results demonstrate that β-catenin is required for axon growth downstream of BDNF- and HGF-signalling. To further test the requirement of β-catenin in axon growth, we also expressed the intracellular domain of N-cadherin, which was previously used to study dendritogenesis (Yu and Malenka, 2003). Intracellular N-cadherin sequesters β-catenin, inhibiting β-catenin degradation and its transactivation capacity (Sadot et al., 1998). For these experiments, neurons were co-transfected with enhanced yellow fluorescent protein (EYFP) and β-catenin or intracellular N-cadherin (1:4 ratio) (Yu and Malenka, 2003), and stimulated with either BDNF or HGF before axon length was measured. BDNF and HGF promoted axon growth in neurons expressing β-catenin (see below) but these effects were inhibited in neurons that express intracellular N-cadherin (Fig. 4D). Together with our results on β- catenin knockdown, these findings demonstrate that decreasing the availability of β-catenin inhibits the axon growth induced by BDNFand HGF-signalling. NTs regulate axon growth and branching by phosphorylating β-catenin Y654 We then looked at the physiological consequences of β-catenin phosphorylation by Trk by studying axon growth induced by NTs in neurons co-expressing WT or mutant β-catenin and EYFP. Neurons expressing EYFP alone or together with β-catenin did not show axon-length values that were significantly different (data not shown). Neurons were treated with BDNF, NT-3 or both and axon length was measured at 2 DIV. Stimulation with BDNF, NT-3 or
5 (16) Fig. 4. β-catenin is involved in axon growth downstream of BDNF- and HGF-signalling. (A) Western blot from hippocampal neurons infected with lentiviraldriven scrambled shrna or β-catenin shrna#1 or #2 shows the decrease in β-catenin expression following expression of β-catenin shrnas compared with scrambled shrna (scr) or non-infected (ni) neurons. Actin was used as a loading control. Plot represents quantification of β-catenin corrected against the loading control (n 4 experiments). Panels show confocal images for anti-β-catenin and anti-gfp (indicating transduced neurons) immunostainings in neurons expressing scrambled shrna or β-catenin shrna#1. Note the lower levels of β-catenin especially along the axon in β-catenin-shrna#1-expressing neurons. Arrows indicate colocalization between GFP and β-catenin immunostainings in neurons expressing scrambled shrna that is reduced in neurons expressing β-catenin shrna#1. Scale bar: 20 μm. (B) Hippocampal neurons expressing scrambled shrna or β-catenin shrna#1 fixed and immunostained against GFP (driven by the lentiviral vector) at 3 DIV show the decrease in axon length obtained by expressing β-catenin shrna#1. Scale bar: 40 μm. (C) Quantification of axon length of neurons expressing scrambled or β-catenin shrna, untreated or treated with BDNF or HGF (normalized to untreated neurons expressing scrambled shrna). Neurons expressing β-catenin shrna#1 or #2 display axons that are shorter than controls. Stimulation with BDNF or HGF (50 ng/ml) promotes axon growth in neurons expressing scrambled shrna, but not in neurons expressing β-catenin shrna#1 or #2 (n=4-8 experiments). **P 0.01, ***P when compared with the untreated neurons expressing scrambled shrna; #, P 0.05; ##, P 0.01 when compared with the BDNF- or HGF-treated neurons expressing scrambled shrna. (D) Quantification of axon length in neurons expressing intracellular N-cadherin or β-catenin, either untreated or stimulated with BDNF or HGF (normalized to the respective untreated neurons). Neurons expressing β-catenin display axons longer than controls upon BDNF or HGF (50 ng/ml) stimulation. By contrast, in neurons expressing intracellular N-cadherin, BDNF or HGF cannot stimulate axon growth (n=4 experiments). *P 0.05, ***P when compared with the corresponding untreated control; #, P 0.05, ##, P 0.01 when compared with the BDNF- or HGF-stimulated β-catenin-expressing neurons. both increased axon length in neurons expressing EYFP alone by 31%, 18% and 39%, respectively (Fig. 5A,B). Neurons overexpressing β-catenin treated with BDNF, NT-3 or both exhibited axons 44%, 20% and 29% longer, respectively, than untreated neurons (Fig. 5A,B). Thus, NT treatment increases axon growth in a similar manner in neurons overexpressing β-catenin to those that are not. BDNF together with NT-3 did not increase axon length significantly more than BDNF or NT-3 alone, suggesting that signalling by only one NT was already maximal. Neurons expressing β-catenin treated with BDNF together with the pharmacological inhibitor of Trk activity, K252a, displayed axons with a length similar to that of controls (Fig. 5A,B). We found that, in addition to increasing axon length, BDNF and NT-3 also increased axonal branching (measured as total axonal branch tip number, TABTN) by 35% and 37%, respectively, compared with control neurons. Furthermore, addition of K252a together with BDNF reduced
6 β-catenin phosphorylation in axon growth 2723 Fig. 5. β-catenin phosphorylation at Y654 regulates axon growth and branching induced by NTs. (A) Hippocampal neurons transfected with EYFP alone (control) or with EYFP and WT or mutant (Y654F or Y142F) β-catenin. BDNF and NT-3 (50 ng/ml) induce axon growth and branching in control neurons and in neurons overexpressing WT or Y142F β-catenin, but NT-induced axon growth is inhibited in neurons expressing the mutation Y654F. Treatment with BDNF plus K252a results in axons with lengths and branching values similar to those of untreated neurons and of Y654F-expressing cells. Scale bar: 70 μm. (B) Quantification of axon length normalized to the respective control. BDNF and NT-3 increase axon length in control EYFP neurons and in neurons expressing WT or Y142F β- catenin, but not in neurons expressing the Y654F mutant. Treatment with BDNF together with NT-3 (B+NT3) does not result in a significant further increase. Treatment with K252a (100 nm) and BDNF (B+K252a) abolishes the increase in axon length induced by BDNF. (C) Quantification of axon branching (TABTN) shows that BDNF and NT-3 increase branching of axons expressing WT and Y142F β-catenin, but expression of Y654F β-catenin abolishes the branching induced by NTs. K252a (100 nm) together with BDNF abolishes BDNF-promoted branching (*P 0.05, **P 0.01, ***P 0.001; n=3-8 experiments). branching to values similar to the control (Fig. 5A,C). K252a has recently been reported to inhibit Met (Morotti et al., 2002) as well as Trk. However, because BDNF binds to TrkB and K252a inhibited the effects of BDNF, our results strongly suggest that Trk receptors mediate the effects of BDNF in axon growth and branching. We also examined the role of β-catenin phosphorylation at Y654 in axon growth and branching by using mutant Y654F β-catenin, which is not phosphorylatable at Y654. Untreated neurons expressing Y654F β-catenin exhibited axons of similar length to those of neurons expressing WT β-catenin (data not shown). Interestingly, we found that neurons expressing Y654F β-catenin treated with BDNF or NT-3 exhibited axon lengths similar to those of untreated neurons (Fig. 5A,B). We therefore concluded that expression of the Y654F β-catenin mutant blocks the NT-induced stimulation of axon growth. Expressing Y654F β-catenin also abolished the NT-induced axon branching (Fig. 5A,C). Conversely,
7 (16) Fig. 6. β-catenin phosphorylation at Y142 regulates axon growth induced by HGF. (A) Hippocampal neurons transfected with EYFP alone (control) or with EYFP and WT or mutant (Y654F or Y142F) β-catenin. HGF (50 ng/ml) induces axon growth in neurons expressing WT or Y654F β-catenin, but HGF-induced axon growth is inhibited in neurons expressing the mutation Y142F. Scale bar: 40 μm. (B) Quantification of axon length normalized to the respective control shows that HGF increases axon length in neurons expressing WT or Y654F β-catenin, but not in neurons expressing Y142F. (C) Quantification of axon branching shows that HGF treatment induces axon branching that is blocked by the Y142F mutation (*P 0.05, **P 0.01; n=4-7 experiments). expression of the Y142F β-catenin mutant did not affect the length or branching of the axon that was induced by NTs (Fig. 5). Altogether, these results demonstrate that phosphorylation at β- catenin Y654 (and not at Y142) is required for the axon extension and branching promoted by NTs. HGF regulates axon growth and branching by phosphorylating β-catenin Y142 Treatment with HGF also increased axon growth of hippocampal neurons expressing EYFP alone (18%) or EYFP and WT β-catenin (35%, compared with untreated neurons; Fig. 6A,B). Remarkably, upon HGF treatment, neurons expressing Y142F β-catenin showed no increase in axon length compared with control cells (Fig. 6A,B). By contrast, neurons expressing Y654F β-catenin treated with HGF exhibited axons longer than untreated neurons (41% longer compared with control; Fig. 6A,B). Treatment with BDNF plus HGF did not increase axon length further than BDNF or HGF alone (data not shown). Moreover, HGF signalling increased axon branching in neurons expressing EYFP, EYFP and WT β-catenin or EYFP and Y654F β-catenin (Fig. 6A,C). However, the axon branching induced by HGF was blocked by expressing Y142F β-catenin. These results demonstrate that phosphorylation of Y142 is required for the regulation of axon growth and branching induced by HGF. Dendrite length and branching of EYFP-expressing neurons was not stimulated by BDNF or HGF treatments at 2 DIV, suggesting that dendrite morphogenesis is not regulated by tyrosine phosphorylation of β-catenin at this stage of the hippocampal-neuron development (Table 1). In summary, our data indicate that β-catenin is specifically phosphorylated at two different sites, Y654 and Y142, by NT- and HGF-signalling to regulate axon growth and branching. Y654-P β-catenin colocalizes with the neuronal cytoskeleton, whereas Y142-P is found in the nucleus To begin to elucidate the downstream events resulting from β- catenin tyrosine phosphorylation at Y654 and Y142, we examined the subcellular localization of the phosphorylated forms, Y654-P and Y142-P, in hippocampal neurons. β-catenin Y654-P was present in the cytoplasm, along the axon, and at neurite tips and Table 1. Dendrite morphogenesis is not regulated by tyrosine phosphorylation of β-catenin in 2-DIV hippocampal neurons N.S., not significant Treatment Average ± s.e.m. Significance (n) Fold increase of dendrite length compared with control Control 1±0.12 N.S (11) BDNF 1.16±0.13 N.S. (9) HGF 1.00±0.07 N.S. (6) Dendrite branching (TDBTN) compared with control Control 3.37±0.29 N.S. (11) BDNF 3.51±0.48 N.S. (9) HGF 3.49±0.79 N.S. (6)
8 β-catenin phosphorylation in axon growth 2725 Fig. 7. Y654-P β-catenin colocalizes with the neuronal cytoskeleton and Y142-P β-catenin is found in the nucleus. (A) Double immunostaining for endogenous β-catenin and F-actin (phalloidin) in hippocampal neurons shows that β-catenin and F-actin partially colocalize (arrows) at neurite tips and lamella (inset). (B) Confocal image of hippocampal neurons fixed using detergent fixation shows the double immunostaining for Y654-P β-catenin and βiii-tubulin that colocalize at enlarged growth cones where microtubules unbundle and along the axon (arrows). Y654-P β-catenin immunostaining can also be observed along filopodial-like processes and axonal-spread areas devoid of βiii-tubulin (arrowheads). (C) Confocal image of neurons fixed by detergent fixation shows the double immunostaining for Y654-P β-catenin and F-actin (phalloidin). Similar to anti-β-catenin antibodies, anti-y654-pβ-catenin antibodies stain the cell body and neurite tips, where Y654-P β-catenin and F-actin colocalize (arrows). (D) β-catenin and Y142-P β-catenin colocalize within the cytoplasm of neurons (arrows), whereas colocalization of Hoestch and anti-y142-p-β-catenin immunostaining shows that Y142-P β-catenin is found in the nucleus (arrowheads). (E) AntiY654-P-β-catenin antibodies stain the cell membrane at cell contacts and the cytoplasm, but the nucleus (stained by Hoestch) is negative. Scale bars: 15 μm (A), 20 μm (B,C) and 45 μm (D,E). growth cones, where it colocalized with actin and βiii-tubulin (Fig. 7A-C). By contrast, β-catenin Y142-P localized to the cytoplasm and nucleus of neurons (Fig. 7D). Anti-Met and anti-β-catenin immunostainings also colocalized along neurites, at tips and in the cytoplasm (supplementary material Fig. S1). However, staining of anti-met and Hoestch did not significantly colocalize (supplementary material Fig. S1), suggesting that Met does not translocate to the nucleus to maintain Y142 phosphorylation. Thus, phosphorylation at Y654 and Y142 targets β-catenin to different subcellular compartments, suggesting that these two phosphorylated forms of β-catenin involve distinct mechanisms to regulate axon morphogenesis. indicating that N-TCF4 blocks β-catenin nuclear signalling and that, in hippocampal neurons, Wnt3a acts through the canonical pathway to increase axon growth. Interestingly, HGF-induced axon growth and branching was also prevented in neurons expressing N-TCF4, whereas the effects induced by BDNF and NT-3 were not altered (Fig. 8). These results indicate that HGF signals through TCF4 in axon growth and branching. In summary, our results indicate that HGF signalling phosphorylates β-catenin at Y142, inducing its nuclear translocation and TCF4-mediated gene transcription to regulate axon morphogenesis. By contrast, NT signalling promotes axon growth and branching by phosphorylating β-catenin Y654 independently of TCF4 function. Dominant-negative N-TCF4 blocks the effect of HGF in axon growth Discussion In canonical Wnt signalling, nuclear β-catenin binds to the promoter of TCF/LEF to regulate gene transcription (Logan and Nusse, 2004). Consequently, TCF factors are likely candidates in the response activated by nuclear β-catenin. Because Y142-P β-catenin was observed in the nucleus, we investigated whether dominant-negative N-TCF4, which cannot bind β-catenin (Roose et al., 1999), was able to modify the effects of NTs or HGF on axon growth and branching. As a positive control, we treated N-TCF4-expressing hippocampal neurons with Wnt3a, which signals through the canonical Wnt pathway in dorsal-root-ganglia neurite outgrowth (Lu et al., 2004). Wnt3a-treated neurons overexpressing EYFP alone or together with WT β-catenin exhibited axons 47% or 46% longer than controls, respectively. Expression of N-TCF4 abolished the stimulation of axon growth that was induced by Wnt3a (Fig. 8B), Brain function relies on the establishment of the correct pattern of neuronal connections During development, neurons extend their axons under the influence of many signalling molecules (Ayala et al., 2007; Ciani and Salinas, 2005; Dalva et al., 2007; Huang and Reichardt, 2001), which results in the unique morphology of axon arbors and the formation of synapses. β-catenin is a component of the celladhesion complex and of the canonical Wnt pathway, which plays a prominent role in development, synapse formation and disease (Ciani and Salinas, 2005; Clevers, 2006; Goda, 2002; Logan and Nusse, 2004; Moon et al., 2004; Salinas and Price, 2005). In neurons, β-catenin tyrosine phosphorylation regulates synapse formation and function (Bamji et al., 2006; Murase et al., 2002). Here, we have shown that differential β-catenin tyrosine phosphorylation by growth factor (NTs and HGF) signalling
9 (16) Fig. 8. N-TCF4 abolishes the effect of HGF in axon growth and branching. (A) Hippocampal neurons transfected with EYFP and N-TCF4, untreated or treated with BDNF, NT-3 and HGF (50 ng/ml). N-TCF4 abolishes the axon growth induced by HGF, but not by BDNF or NT-3. Scale bar: 40 μm. (B) Quantification of axon length in neurons expressing EYFP or EYFP plus N-TCF4 normalized to the respective untreated control shows that HGF and Wnt3a (used as positive control; 50 ng/ml) do not increase axon length in neurons expressing N-TCF4, whereas NTs similarly stimulate axon growth in N-TCF4-expressing or control neurons. (C) Quantification of axon branching in neurons expressing EYFP alone or EYFP and N-TCF4 shows that HGF-induced branching is inhibited by N-TCF4 expression, whereas the BDNF- and NT-3-induced effects are not affected (n=5-7 experiments). *P 0.05, **P 0.01, ***P 0.001, when compared with the corresponding untreated controls. #, P 0.05; ##, P 0.01; ###, P 0.001, when compared with the corresponding stimulated control. regulates axon morphogenesis. First, by expressing lentiviraldriven β-catenin shrnas or sequestering β-catenin with intracellular N-cadherin, we demonstrated the requirement for β- catenin in axon outgrowth downstream of growth-factor signalling. Second, we showed that the neuronal RTKs Trk and Met phosphorylate β-catenin. We identified Y654 and Y142 as the sites that are phosphorylated upon NT and HGF stimulation, respectively. We also demonstrated the key role that the phosphorylations of Y654 and Y142 play in the regulation of axon growth and branching that are elicited by either family of growth factors. Our data indicate that, although NT- and HGF-signalling converge on β-catenin, the pathways that control the growth and arborization of the axon diverge downstream of β-catenin Y654 and Y142 phosphorylations. The NT-phosphorylated form, Y654- P β-catenin, is found at growth cones, whereas Y142-P β-catenin translocates to the nucleus. Furthermore, the transcription factor TCF4, a central player of canonical Wnt signalling, is needed for HGF- but not for NT-signalling during axon growth and branching. Therefore, the signalling cascades that are activated by growth factors during axon morphogenesis share components of the Wnt pathway. We have concluded that HGF and NTs, by activating their RTKs, phosphorylate β-catenin at different sites, thus involving transcription-dependent and -independent mechanisms that result in the fine tuning of axon morphogenesis. In this paper we presented evidence for the interaction of β-catenin with Trk and Met, and demonstrate for the first time the involvement of β-catenin phosphorylation downstream of both RTKs in the regulation of axon growth and branching. Previous studies illustrated the relationship between β-catenin phosphorylation and RTKs such as EGFR, Met and platelet-derived growth factor (PDGF) receptor in epithelial and tumoral cell migration (Brembeck et al., 2004; Hiscox and Jiang, 1999; Hoschuetzky et al., 1994; Theisen et al., 2007). We have shown that β-catenin is associated with TrkA, TrkB and TrkC in PC12 6/15 cells and hippocampal neurons. The interaction of β-catenin with Trk was independent of ligand stimulation, as also described for EGFR (Hoschuetzky et al., 1994). In addition, β-catenin is a direct substrate of TrkA kinase in vitro. Substitution of Y654 by a phenylalanine (Y654F) results in a significant reduction of β-catenin phosphorylation by TrkA, whereas a Y142F mutation affected β-catenin phosphorylation to a much lesser extent. These experiments demonstrate that TrkA phosphorylates Y654 in vitro. Using TrkA immunoprecipitated from PC12 6/15 cells, the residual tyrosine phosphorylation of Y654F β- catenin was not prevented by a Trk inhibitor. TrkB was reported to associate with the non-rtk Fyn (Iwasaki et al., 1998; Pereira and Chao, 2007), which phosphorylates β-catenin at Y142 (Piedra et al., 2003). Our results also suggest that TrkA or a kinase that co-immunoprecipitates with TrkA, possibly Fyn, phosphorylates Y654F β- catenin in vitro. More importantly, β-catenin is phosphorylated at Y654 following activation of all three Trk receptors in PC12 6/15 cells and primary neurons. Phosphorylation of Y142 was not induced by BDNF, suggesting that NT signalling specifically targets β- catenin Y654 in a cellular context. Moreover, we have shown that β-catenin also associates with neuronal Met. β-catenin was phosphorylated by Met at Y142 in vitro and in neurons following HGF stimulation. By contrast, phosphorylation of mutant Y654F was similar to that of WT β-catenin and HGF treatment did not stimulate phosphorylation of Y654 in neurons, indicating a selectivity of HGF-Met signalling for Y142. Finally, we observed a decreased association of Met and β-catenin upon Y142 phosphorylation. Together, these findings extend to a neuronal context our knowledge about HGF signalling in non-neural cells (Brembeck et al., 2004; Monga et al., 2002; Rasola et al., 2007). BDNF, NT-3 and HGF all induce axon growth and branching in hippocampal neurons (Korhonen et al., 2000; Labelle and Leclerc, 2000; Vicario-Abejon et al., 1998). By expressing β-catenin single point mutants, we demonstrate that phosphorylation of β-catenin Y654 is required for the regulation of axon growth and branching induced by BDNF or NT-3. Conversely, phosphorylation of β- catenin Y142 is required for HGF signalling in axon morphogenesis. The effects of BDNF and HGF on axon growth were not additive, suggesting maximal signalling by each growth factor alone. Our results demonstrate that different growth factors target β-catenin to partially stimulate the growth and arborization of the axon, thus
10 β-catenin phosphorylation in axon growth 2727 Fig. 9. Model for the role of β-catenin tyrosine phosphorylation in axon growth and branching. Adhesion complexes are assembled at the plasma membrane of the cell body, axon and growth cone. β-catenin residue Y654 is found at the region that binds to N-cadherin, whereas β-catenin Y142 is in the α-catenin-binding region (Aberle et al., 1996; Lilien and Balsamo, 2005). α-catenin also binds to actin (Drees et al., 2005; Yamada et al., 2005). Trk and Met associate with a pool of β- catenin that is bound to N-cadherin and α-catenin. β-catenin phosphorylation by HGF-Met signalling at β-catenin Y142 targets β-catenin to the nucleus, where it activates TCF4-mediated transcription to increase axon growth and branching. The detachment of Y142-P β-catenin from the adhesion complex might imply dynamic regulation of Y654 phosphorylation, perhaps by Src-family tyrosine kinases (Roura et al., 1999), and its dephosphorylation before nuclear translocation of Y142-P β-catenin (according to the lack of nuclear immunostaining for Y654-P β-catenin). β-catenin phosphorylation by NT-Trk signalling at Y654 detaches β- catenin from N-cadherin (Bamji et al., 2006; Huber and Weis, 2001; Lilien and Balsamo, 2005; Roura et al., 1999), and Y654-P β-catenin associates with actin and microtubules at the growth cone, possibly regulating the cytoskeleton to promote axon growth and branching. Note that, whereas we demonstrate that β-catenin αcatenin dissociates upon phosphorylation of β-catenin Y142, the depicted β-catenin dissociation from N-cadherin upon Y654 phosphorylation is based on the references above. For simplicity, Met has been located at the cell body and Trk at the growth-cone plasma membrane. N, N-cadherin; β, β-catenin; α, α-catenin;, F-actin;.., microtubules. contributing to the complex network of pathways that direct this process. In agreement with this conclusion, regulating the availability of β-catenin by either shrna-mediated β-catenin silencing or by sequestering it by expressing intracellular N- cadherin (Yu and Malenka, 2003) affected axon growth both in the presence and absence of exogenous growth factors. The ability of intracellular N-cadherin to block β-catenin transactivation (Sadot et al., 1998) and to place β-catenin away from the RTK could explain the inhibition of the axon growth that is otherwise promoted by BDNF and HGF. Together, these results indicate that β-catenin is required for axon growth downstream of growth-factor signalling. Dendritic morphogenesis was not stimulated by BDNF or HGF signalling, suggesting that it is not regulated by tyrosine phosphorylation of β-catenin at the initial stages of the hippocampalneuron development that were analyzed. These results do not rule out a possible role of β-catenin phosphorylation on the maturation of the dendritic tree, in agreement with the implication of β-catenin in this later process (Yu and Malenka, 2003). What are the mechanisms by which NT- and HGF-signalling regulate axon extension and branching? Tyrosine phosphorylation of β-catenin was proposed to regulate cell migration and gene transcription by detaching β-catenin from the cadherin-catenins complex (Huber and Weis, 2001; Lilien and Balsamo, 2005; Nelson and Nusse, 2004; Roura et al., 1999). In keeping with this motion, Bamji et al. (Bamji et al., 2006) showed that tyrosine phosphorylation of β-catenin upon BDNF treatment reduces β- catenin affinity for N-cadherin and mobilizes synaptic vesicles during synapse formation. Likewise, Murase et al. (Murase et al., 2002) reported that depolarization, which mimics the effect of a tyrosine-kinase inhibitor on β-catenin redistribution to dendritic spines, increases β-catenin and cadherin association in mature hippocampal cultures. By contrast, only upon pervanadate treatment, we were able to observe β-catenin detachment from N-cadherin in our younger neurons (Ozawa and Kemler, 1998) (Fig. 2E and data not shown). This result indicates that it is necessary to inhibit tyrosine phosphatases, which are working in consonance with tyrosine kinases (Balsamo et al., 1998; Kypta et al., 1996; Nelson and Nusse, 2004), to detect the detachment of β-catenin from N- cadherin in 2-DIV hippocampal neurons. This result also suggests that a phosphatase that is developmentally regulated dephosphorylates Y654 β-catenin. In addition, β-catenin and cadherins change their location before and after synapse formation, showing a synaptic distribution as neurons mature (Bamji et al., 2006; Bamji et al., 2003; Benson and Tanaka, 1998; Uchida et al., 1996). Thus, the dynamic localization of cadherin-catenin complexes might result in the immunoprecipitation of adhesion complexes predominantly assembled at local pools (i.e. synapses) in older neurons, where the phosphorylation and detachment of β- catenin could mainly take place. Y654-P and Y142-P β-catenin were found in different subcellular locations in hippocampal neurons. Y654-P β-catenin is present at neurite tips and growth cones, where it colocalizes with cytoskeletal components. This observation suggests that NTs, by phosphorylating Y654 β-catenin, stimulate axon growth and branching by locally regulating the cytoskeleton. Guidance cues regulate growth-cone motility and behaviour by eliciting local synthesis of proteins (Piper and Holt, 2004), and by extensively affecting the actin and microtubule cytoskeleton (Kalil and Dent, 2005). Although pathways activated by NTs may involve signalling to the nucleus (Chao, 2003; Huang and Reichardt, 2001; Sole et al., 2004), NTs can also transduce their signals locally and affect the cytoskeleton to regulate motility, neuronal morphology and axon growth (Chen et al., 2006; Gehler et al., 2004; Miyamoto et al., 2006; Ye et al., 2003; Zhou et al., 2004). NGF induces growth of dorsal-root-ganglia neuron axons through inactivation of GSK3β and regulation of the microtubule-plus-end-binding protein APC at neurite tips (Arevalo
11 (16) and Chao, 2005; Zhou et al., 2004). BDNF increases length and number of filopodia, a structure considered the first step in the formation of axonal branches (Gallo and Letourneau, 2004), by activating actin depolymerizing factor (ADF)/cofilin (Chen et al., 2006; Gehler et al., 2004) and decreasing RhoA signalling (Yamashita et al., 1999). Indeed, members of the Rho family of small GTPases are key regulators of neuronal morphogenesis (Govek et al., 2005; Luo, 2000). Furthermore, the HGF- or BDNFinduced motile phenotypes and neurite outgrowth are mediated by Rho GTPases (Bosse et al., 2007; Pante et al., 2005; Royal et al., 2000; Zhou et al., 2007). Constitutively active RhoA reduces dendrite branching, suggesting that interactions with the actin cytoskeleton are crucial for the effects of β-catenin in dendritic morphogenesis (Yu and Malenka, 2003). Future work should help to clarify the mechanisms mediating β-catenin phosphorylation and the growth cone in response to NTs in axon morphogenesis. Phosphorylated Y142 β-catenin was reported to decrease the interaction of α-catenin β-catenin and to increase the interaction with BCL9-2/Legless, allowing nuclear translocation of β-catenin and transcription of its target genes (Brembeck et al., 2004). We found that, in neurons, α-catenin β-catenin dissociate in response to the HGF-induced β-catenin phosphorylation at Y142. The α- catenin β-catenin dissociation was observed on β-catenin immunocomplexes also containing Met, thus suggesting that the RTK interacts with and phosphorylates a pool of β-catenin bound to the adhesion complex. Y142-P β-catenin, contrary to Y654-P, was observed in the nucleus in neurons. Consistent with this finding, we have shown that HGF-induced axon growth and branching requires TCF4 transcriptional activation. This dependence on TCF4 function is also shared by Wnt3a signalling in axon growth and for the Robo Abl Cables N-cadherin β-catenin regulation of axon guidance mediated by β-catenin Y489 phosphorylation (Rhee et al., 2007). By contrast, activity-induced dendritic arborization does not require TCF-transcriptional activation but rather availability of the cadherin-catenin complex (Yu and Malenka, 2003). By contrast, phosphorylation of Y654 stimulates the association of β-catenin to the basal transcription factor TATA-binding protein and increases TCF4-mediated transcription in non-neural cell lines (Hecht et al., 1999; Piedra et al., 2001). Furthermore, it has been suggested that Bcr-Abl phosphorylation of β-catenin Y86 and/or Y654 regulates nuclear β-catenin signalling in chronic myeloid leukaemia (Coluccia et al., 2007). Our results in developing neurons, however, indicate that NTs modulate axon growth and branching by phosphorylating β-catenin Y654 independently of TCF4 function, possibly by affecting the cytoskeleton. Because β-catenin-null mice die during early development, the function of β-catenin in the embryonic period has not been tested in vivo. Using conditional-knockout animals, a role of β-catenin on dendrite development of postnatally born hippocampal dentategyrus neurons was identified (Gao et al., 2007). β-catenin conditional-knockout animals also showed a mild phenotype in mood- and anxiety-related behavioural models (Gould et al., 2008). However, axon morphogenesis of hippocampal neurons has not been analyzed during the embryonic development of these animals. Expression of β-catenin deletion mutants in retinal ganglion cells of live Xenopus tadpoles (Elul et al., 2003) resulted in altered axonal arborization, suggesting that interactions with the N- and C-terminal domains of β-catenin (containing either Y142 or Y654) are required to shape these axons in vivo. Investigating the impact of β-catenin phosphorylation in axon morphogenesis during mouse development will require the generation of Y654F- and Y142F-knockin mice. Our results place β-catenin at a convergence point of both the NT-Trk and HGF-Met signalling pathways in the regulation of axon growth and branching. Thus, our findings illustrate the complex networks operating in axon morphogenesis, because stimulation of different RTKs results in the activation of TCF4-transcriptiondependent and -independent mechanisms downstream of β-catenin (Fig. 9). It would be interesting to investigate which are the TCF4 target genes responsible for the axon growth and branching that is promoted by HGF. In summary, this work highlights the important role played by β-catenin in integrating trophic signals that emanate from RTKs during axon morphogenesis. Materials and Methods Materials BDNF and NT-3 were obtained from Alomone Labs, NGF from Sigma, HGF and K252a from Calbiochem, and Wnt3a from R&D Systems. Antibodies were purchased from the following companies: Met from Santa Cruz, Y654-P and Y142-P from Abcam, βiii-tubulin from Covance, phospho-tyrosine (Tyr-P; 4G10), TrkB and TrkC from Upstate Biotechnology, β-actin from Sigma, β-catenin from BD Transduction Laboratories, N-cadherin from Zymed, α-catenin from the Hybridoma Bank, and EYFP and GFP from Acris Antibodies. TrkA immunoprecipitation and in vitro kinase assays TrkA was isolated from a PC12 clone overexpressing human TrkA (PC12 6/15) following stimulation with NGF 100 ng/ml (7 minutes at 37 C). Cells were lysed in RIPA buffer (50 mm Tris-HCl, ph 7.5, 150 mm NaCl, 5 mm EDTA ph 8, 1% NP- 40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mm DTT) containing Complete protease inhibitor (Roche), 40 mm β-glycerophosphate, 1 mm sodium ortovanadate and 25 mm sodium fluoride. Cell lysates were incubated with anti-pan-trk antibodies (Martin- Zanca et al., 1989) followed by Protein-G Sepharose (Sigma). GST β-catenin recombinant proteins (Roura et al., 1999) were produced in BL21 bacteria and cleaved from GST with thrombin where necessary (8 U to cleave 1 mg protein, 80 minutes, 30 C). GST β-catenin (0.8 μg; 6.7 pmols) phosphorylation by recombinant TrkA and Met kinases (Cell Signalling) was performed following the manufacturer s instructions (15 minutes, 30 C). β-catenin (1.5 μg; 17 pmols) phosphorylation by immunoprecipitated TrkA was assayed (20 minutes, 30 C) in kinase buffer [50 mm HEPES, ph 7.4, 10 mm MgCl2, 5 mm MnCl2, 0.5 mm EDTA, 1 mm DTT, 0.1% NP-40, 1 mm sodium ortovanadate with or without 100 nm K252a (preincubated with the kinase 5 minutes, RT) and 0.1 mm ATP]. Samples were analyzed by western blot. Hippocampal cultures and transfection Rat primary hippocampal neurons were isolated from 18- to 19-day embryos and cultured in DMEM medium supplemented with N2 and B27. Neurons were plated on poly-d-lysine-coated glass coverslips either at cells/mm 2 for transfection or biochemical experiments or 70 cells/mm 2 for immunostaining following lentiviral infection. WT, Y654F and Y142F β-catenin cdnas were obtained and cloned into pcdna3.1 as described (Piedra et al., 2003). Xenopus intracellular N-cadherin (containing part of the fifth extracellular domain, the transmembrane and the cytoplasmic domains) was kindly provided by X. Yu (Yu and Malenka, 2003). Cells were co-transfected at 1 DIV with 1.2 μg of β-catenin or cadherin constructs and 0.3 μg of EYFP DNA using Lipofectamine 2000 (Invitrogen) (Espinet et al., 2000). After 3 hours the medium was changed and supplemented with appropriate growth factors, Wnt3a (all at 50 ng/ml) and K252a (100 nm; preincubated 20 minutes before BDNF addition) when necessary. Neurons were fixed at 2 DIV and immunostained with anti-gfp antibodies. Immunofluorescence and measurements For phosphospecific β-catenin immunostaining, neurons were treated with pervanadate (1 mm, 15 minutes, 37 C) before fixation. Neurons were fixed with 4% paraformaldehyde (PFA) for 20 minutes at room temperature (RT). For cytoskeletal staining, detergent fixation (3% formaldehyde, 0.2% glutaraldehyde, 0.2% Triton X100, 10 mm EGTA ph 7.2) was used (10 minutes, 37 C) where indicated. Binding of TRITC or FITC-phalloidin (0.2 μg/ml in PBS, 1 hour at RT; Sigma) to localize actin filaments was performed in cells permeabilized with 0.2% Triton X-100 in PBS without blocking. Cells were washed with phosphate-buffered saline (PBS), and blocked and permeabilized in PBS containing 5% foetal calf serum, 5% horse serum, 0.2% glycine and 0.1% Triton X-100. Secondary antibodies were Alexa Fluor 488 and Alexa Fluor 594 (Molecular Probes). Micrographs were obtained using an inverted Olympus IX70 microscope (10, 0.3 NA and 20, 0.4 NA) equipped with epifluorescence optics and a camera (Olympus OM-4 Ti). Images were acquired using DPM Manager Software. Alternatively, images were obtained using an Olympus confocal IX70 microscope and the FluoView 500 Software, and processed using MacBiophotonics ImageJ software ( Axon length was
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
5- Semaphorin-Plexin-Neuropilin
 5- Semaphorin-Plexin-Neuropilin 1 SEMAPHORINS-PLEXINS-NEUROPILINS ligands receptors co-receptors semaphorins and their receptors are known signals for: -axon guidance -cell migration -morphogenesis -immune
5- Semaphorin-Plexin-Neuropilin 1 SEMAPHORINS-PLEXINS-NEUROPILINS ligands receptors co-receptors semaphorins and their receptors are known signals for: -axon guidance -cell migration -morphogenesis -immune
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Neurite initiation. Neurite formation begins with a bud that sprouts from the cell body. One or several neurites can sprout at a time.
 Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Bio 127 Section I Introduction to Developmental Biology. Cell Cell Communication in Development. Developmental Activities Coordinated in this Way
 Bio 127 Section I Introduction to Developmental Biology Cell Cell Communication in Development Gilbert 9e Chapter 3 It has to be EXTREMELY well coordinated for the single celled fertilized ovum to develop
Bio 127 Section I Introduction to Developmental Biology Cell Cell Communication in Development Gilbert 9e Chapter 3 It has to be EXTREMELY well coordinated for the single celled fertilized ovum to develop
Mechanisms of Human Health and Disease. Developmental Biology
 Mechanisms of Human Health and Developmental Biology Joe Schultz joe.schultz@nationwidechildrens.org D6 1 Dev Bio: Mysteries How do fertilized eggs become adults? How do adults make more adults? Why and
Mechanisms of Human Health and Developmental Biology Joe Schultz joe.schultz@nationwidechildrens.org D6 1 Dev Bio: Mysteries How do fertilized eggs become adults? How do adults make more adults? Why and
Isoform-Specific Dephosphorylation of Dynamin1 by Calcineurin Couples Neurotrophin Receptor Endocytosis to Axonal Growth
 Article Isoform-Specific Dephosphorylation of Dynamin1 by Calcineurin Couples Neurotrophin Receptor Endocytosis to Axonal Growth Daniel Bodmer, 1,2 Maria Ascaño, 1,2 and Rejji Kuruvilla 1, * 1 Department
Article Isoform-Specific Dephosphorylation of Dynamin1 by Calcineurin Couples Neurotrophin Receptor Endocytosis to Axonal Growth Daniel Bodmer, 1,2 Maria Ascaño, 1,2 and Rejji Kuruvilla 1, * 1 Department
Axon Guidance. Multiple decision points along a growing axon s trajectory Different types of axon guidance cues:
 Axon Guidance Multiple decision points along a growing axon s trajectory Different types of axon guidance cues: Contact mediated - requires direct contact by growth cone Long range - growth cone responds
Axon Guidance Multiple decision points along a growing axon s trajectory Different types of axon guidance cues: Contact mediated - requires direct contact by growth cone Long range - growth cone responds
Hierarchical Organization of Guidance Receptors: Silencing of Netrin Attraction by Slit Through a Robo/DCC Receptor Complex
 Hierarchical Organization of Guidance Receptors: Silencing of Netrin Attraction by Slit Through a Robo/DCC Receptor Complex Elke Stein and Marc Tessier-Lavigne* Axonal growth cones that cross the nervous
Hierarchical Organization of Guidance Receptors: Silencing of Netrin Attraction by Slit Through a Robo/DCC Receptor Complex Elke Stein and Marc Tessier-Lavigne* Axonal growth cones that cross the nervous
COMPUTER SIMULATION OF DIFFERENTIAL KINETICS OF MAPK ACTIVATION UPON EGF RECEPTOR OVEREXPRESSION
 COMPUTER SIMULATION OF DIFFERENTIAL KINETICS OF MAPK ACTIVATION UPON EGF RECEPTOR OVEREXPRESSION I. Aksan 1, M. Sen 2, M. K. Araz 3, and M. L. Kurnaz 3 1 School of Biological Sciences, University of Manchester,
COMPUTER SIMULATION OF DIFFERENTIAL KINETICS OF MAPK ACTIVATION UPON EGF RECEPTOR OVEREXPRESSION I. Aksan 1, M. Sen 2, M. K. Araz 3, and M. L. Kurnaz 3 1 School of Biological Sciences, University of Manchester,
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Selective Targeting of ER Exit Sites Supports Axon Development
 Traffic 2009; 10: 1669 1684 2009 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2009.00974.x Selective Targeting of ER Exit Sites Supports Axon Development Meir Aridor 1 and Kenneth N. Fish 2, 1 Department
Traffic 2009; 10: 1669 1684 2009 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2009.00974.x Selective Targeting of ER Exit Sites Supports Axon Development Meir Aridor 1 and Kenneth N. Fish 2, 1 Department
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Advanced Higher Biology. Unit 1- Cells and Proteins 2c) Membrane Proteins
 Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Critical Role for Kalirin in Nerve Growth Factor Signaling through TrkA
 MOLECULAR AND CELLULAR BIOLOGY, June 2005, p. 5106 5118 Vol. 25, No. 12 0270-7306/05/$08.00 0 doi:10.1128/mcb.25.12.5106 5118.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
MOLECULAR AND CELLULAR BIOLOGY, June 2005, p. 5106 5118 Vol. 25, No. 12 0270-7306/05/$08.00 0 doi:10.1128/mcb.25.12.5106 5118.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
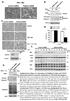 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Formation of the Cortex
 Formation of the Cortex Neuronal Birthdating with 3 H-thymidine 3H-thymidine is incorporated into the DNA during the S-phase (replication of DNA). It marks all mitotic cells Quantitative technique. (you
Formation of the Cortex Neuronal Birthdating with 3 H-thymidine 3H-thymidine is incorporated into the DNA during the S-phase (replication of DNA). It marks all mitotic cells Quantitative technique. (you
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
ROLE OF RACK1 IN AXONAL OUTGROWTH OF DEVELOPING NEURONS
 ROLE OF RACK1 IN AXONAL OUTGROWTH OF DEVELOPING NEURONS A thesis submitted to the Kent State University Honors College In partial fulfillment of the requirements For University Honors by Joel Serre May
ROLE OF RACK1 IN AXONAL OUTGROWTH OF DEVELOPING NEURONS A thesis submitted to the Kent State University Honors College In partial fulfillment of the requirements For University Honors by Joel Serre May
Supplemental table S7.
 Supplemental table S7. GO terms significantly enriched in significantly up-regulated genes of the microarray. K: number of genes from the input cluster in the given category. F: number of total genes in
Supplemental table S7. GO terms significantly enriched in significantly up-regulated genes of the microarray. K: number of genes from the input cluster in the given category. F: number of total genes in
Cell Adhesion and Signaling
 Cell Adhesion and Signaling mchuang@ntu.edu.tw Institute of Anatomy and Cell Biology 1 Transactivation NATURE REVIEWS CANCER VOLUME 7 FEBRUARY 2007 85 2 Functions of Cell Adhesion cell cycle proliferation
Cell Adhesion and Signaling mchuang@ntu.edu.tw Institute of Anatomy and Cell Biology 1 Transactivation NATURE REVIEWS CANCER VOLUME 7 FEBRUARY 2007 85 2 Functions of Cell Adhesion cell cycle proliferation
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
Zool 3200: Cell Biology Exam 5 4/27/15
 Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
Graduate Institute t of fanatomy and Cell Biology
 Cell Adhesion 黃敏銓 mchuang@ntu.edu.tw Graduate Institute t of fanatomy and Cell Biology 1 Cell-Cell Adhesion and Cell-Matrix Adhesion actin filaments adhesion belt (cadherins) cadherin Ig CAMs integrin
Cell Adhesion 黃敏銓 mchuang@ntu.edu.tw Graduate Institute t of fanatomy and Cell Biology 1 Cell-Cell Adhesion and Cell-Matrix Adhesion actin filaments adhesion belt (cadherins) cadherin Ig CAMs integrin
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
 DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition
 Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Axon guidance I. Paul Garrity March 15, /9.013
 Axon guidance I Paul Garrity March 15, 2004 7.68/9.013 Neuronal Wiring: Functional Framework of the Nervous System Stretch reflex circuit Early theories of axonogenesis Schwann: many neurons link to form
Axon guidance I Paul Garrity March 15, 2004 7.68/9.013 Neuronal Wiring: Functional Framework of the Nervous System Stretch reflex circuit Early theories of axonogenesis Schwann: many neurons link to form
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Reading. Lecture VI. Making Connections 9/17/12. Bio 3411 Lecture VI. Making Connections. Bio 3411 Monday September 17, 2012
 Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
CELL-CELL COMMUNICATION
 CELL-CELL COMMUNICATION paracrine & juxtacrine signalling autocrine & intracrine signalling methods to study cell-cell communication: attraction & repulsion chemotaxis & chemokinesis substrate preference
CELL-CELL COMMUNICATION paracrine & juxtacrine signalling autocrine & intracrine signalling methods to study cell-cell communication: attraction & repulsion chemotaxis & chemokinesis substrate preference
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Bio 3411, Fall 2006, Lecture 19-Cell Death.
 Types of Cell Death Questions : Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can
Types of Cell Death Questions : Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figures
 Supplementary Figures Supplementary Fig. S1: Normal development and organization of the embryonic ventral nerve cord in Platynereis. (A) Life cycle of Platynereis dumerilii. (B-F) Axonal scaffolds and
Supplementary Figures Supplementary Fig. S1: Normal development and organization of the embryonic ventral nerve cord in Platynereis. (A) Life cycle of Platynereis dumerilii. (B-F) Axonal scaffolds and
ADAM FAMILY. ephrin A INTERAZIONE. Eph ADESIONE? PROTEOLISI ENDOCITOSI B A RISULTATO REPULSIONE. reverse. forward
 ADAM FAMILY - a family of membrane-anchored metalloproteases that are known as A Disintegrin And Metalloprotease proteins and are key components in protein ectodomain shedding Eph A INTERAZIONE B ephrin
ADAM FAMILY - a family of membrane-anchored metalloproteases that are known as A Disintegrin And Metalloprotease proteins and are key components in protein ectodomain shedding Eph A INTERAZIONE B ephrin
Richik N. Ghosh, Linnette Grove, and Oleg Lapets ASSAY and Drug Development Technologies 2004, 2:
 1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
THE PROBLEMS OF DEVELOPMENT. Cell differentiation. Cell determination
 We emphasize these points from Kandel in Bi/CNS 150 Bi/CNS/NB 150: Neuroscience Read Lecture Lecture Friday, October 2, 2015 Development 1: pp 5-10 Introduction Brains evolved All higher animals have brains
We emphasize these points from Kandel in Bi/CNS 150 Bi/CNS/NB 150: Neuroscience Read Lecture Lecture Friday, October 2, 2015 Development 1: pp 5-10 Introduction Brains evolved All higher animals have brains
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development June 23, 2009 Concepts Cell-Cell Communication Cells develop in the context of their environment, including: - their immediate
Biology 4361 - Developmental Biology Cell-Cell Communication in Development June 23, 2009 Concepts Cell-Cell Communication Cells develop in the context of their environment, including: - their immediate
Characterization of an NGF P-TrkA Retrograde-Signaling Complex and Age-Dependent Regulation of TrkA Phosphorylation in Sympathetic Neurons
 The Journal of Neuroscience, October 1, 1999, 19(19):8207 8218 Characterization of an NGF P-TrkA Retrograde-Signaling Complex and Age-Dependent Regulation of TrkA Phosphorylation in Sympathetic Neurons
The Journal of Neuroscience, October 1, 1999, 19(19):8207 8218 Characterization of an NGF P-TrkA Retrograde-Signaling Complex and Age-Dependent Regulation of TrkA Phosphorylation in Sympathetic Neurons
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
FSC-W FSC-H CD4 CD62-L
 Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Inactivation of Ras by p120gap via Focal Adhesion Kinase Dephosphorylation Mediates RGMa-Induced Growth Cone Collapse
 The Journal of Neuroscience, May 20, 2009 29(20):6649 6662 6649 Cellular/Molecular Inactivation of Ras by p120gap via Focal Adhesion Kinase Dephosphorylation Mediates RGMa-Induced Growth Cone Collapse
The Journal of Neuroscience, May 20, 2009 29(20):6649 6662 6649 Cellular/Molecular Inactivation of Ras by p120gap via Focal Adhesion Kinase Dephosphorylation Mediates RGMa-Induced Growth Cone Collapse
Signaling to the Nucleus by an L-type Calcium Channel- Calmodulin Complex Through the MAP Kinase Pathway
 Signaling to the Nucleus by an L-type Calcium Channel- Calmodulin Complex Through the MAP Kinase Pathway Ricardo E. Dolmetsch, Urvi Pajvani, Katherine Fife, James M. Spotts, Michael E. Greenberg Science
Signaling to the Nucleus by an L-type Calcium Channel- Calmodulin Complex Through the MAP Kinase Pathway Ricardo E. Dolmetsch, Urvi Pajvani, Katherine Fife, James M. Spotts, Michael E. Greenberg Science
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A)
 Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its
 Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,800 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,800 116,000 120M Open access books available International authors and editors Downloads Our
Type VI Adenylyl Cyclase Regulates Neurite Extension by Binding to Snapin and Snap25
 MOLECULAR AND CELLULAR BIOLOGY, Dec. 2011, p. 4874 4886 Vol. 31, No. 24 0270-7306/11/$12.00 doi:10.1128/mcb.05593-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Type VI Adenylyl
MOLECULAR AND CELLULAR BIOLOGY, Dec. 2011, p. 4874 4886 Vol. 31, No. 24 0270-7306/11/$12.00 doi:10.1128/mcb.05593-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Type VI Adenylyl
Mathematical Modeling and Analysis of Crosstalk between MAPK Pathway and Smad-Dependent TGF-β Signal Transduction
 Processes 2014, 2, 570-595; doi:10.3390/pr2030570 Article OPEN ACCESS processes ISSN 2227-9717 www.mdpi.com/journal/processes Mathematical Modeling and Analysis of Crosstalk between MAPK Pathway and Smad-Dependent
Processes 2014, 2, 570-595; doi:10.3390/pr2030570 Article OPEN ACCESS processes ISSN 2227-9717 www.mdpi.com/journal/processes Mathematical Modeling and Analysis of Crosstalk between MAPK Pathway and Smad-Dependent
A Loss-of-Function Screen for Phosphatases that Regulate Neurite Outgrowth Identifies PTPN12 as a Negative Regulator of TrkB Tyrosine Phosphorylation
 Downloaded from orbit.dtu.dk on: Dec 29, 2018 A Loss-of-Function Screen for Phosphatases that Regulate Neurite Outgrowth Identifies PTPN12 as a Negative Regulator of TrkB Tyrosine Phosphorylation Ambjørn,
Downloaded from orbit.dtu.dk on: Dec 29, 2018 A Loss-of-Function Screen for Phosphatases that Regulate Neurite Outgrowth Identifies PTPN12 as a Negative Regulator of TrkB Tyrosine Phosphorylation Ambjørn,
7.06 Spring 2004 PS 6 KEY 1 of 14
 7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
7.013 Spring 2005 Problem Set 4
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 4 FRIDAY April 8th,
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 4 FRIDAY April 8th,
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016
 Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
LKB1/STRAD Promotes Axon Initiation During Neuronal Polarization
 LKB1/STRAD Promotes Axon Initiation During Neuronal Polarization Maya Shelly, 1 Laura Cancedda, 1 Sarah Heilshorn, 1,2 Germán Sumbre, 1 and Mu-ming Poo 1, * 1 Division of Neurobiology, Department of Molecular
LKB1/STRAD Promotes Axon Initiation During Neuronal Polarization Maya Shelly, 1 Laura Cancedda, 1 Sarah Heilshorn, 1,2 Germán Sumbre, 1 and Mu-ming Poo 1, * 1 Division of Neurobiology, Department of Molecular
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX.
 Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
7.013 Problem Set
 7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
Supplementary Figure 1. Confirmation of REF52-hE2F1p::4NLS-d4Venus reporter cells and characterization of E2F dynamics. (a) Alignment of E2F dynamics
 Supplementary Figure 1. Confirmation of REF52-hE2F1p::4NLS-d4Venus reporter cells and characterization of E2F dynamics. (a) Alignment of E2F dynamics trajectories to endogenous E2F1 mrna expression. Gray
Supplementary Figure 1. Confirmation of REF52-hE2F1p::4NLS-d4Venus reporter cells and characterization of E2F dynamics. (a) Alignment of E2F dynamics trajectories to endogenous E2F1 mrna expression. Gray
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Waithe et al Supplementary Figures
 Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
S1 Gene ontology (GO) analysis of the network alignment results
 1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Trophic Factors. Trophic Factors. History 2. History Growth Factors. Giles Plant
 217 - Growth Factors Giles Plant Role in: Growth and Trophic Factors Soluble/diffusible factors - polypeptides Proliferation Differentiation (ie Cancer) Survival (degenerative diseases) Innervation Maintenance
217 - Growth Factors Giles Plant Role in: Growth and Trophic Factors Soluble/diffusible factors - polypeptides Proliferation Differentiation (ie Cancer) Survival (degenerative diseases) Innervation Maintenance
Cytokines regulate interactions between cells of the hemapoietic system
 Cytokines regulate interactions between cells of the hemapoietic system Some well-known cytokines: Erythropoietin (Epo) G-CSF Thrombopoietin IL-2 INF thrombopoietin Abbas et al. Cellular & Molecular Immunology
Cytokines regulate interactions between cells of the hemapoietic system Some well-known cytokines: Erythropoietin (Epo) G-CSF Thrombopoietin IL-2 INF thrombopoietin Abbas et al. Cellular & Molecular Immunology
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
RANK. Alternative names. Discovery. Structure. William J. Boyle* SUMMARY BACKGROUND
 RANK William J. Boyle* Department of Cell Biology, Amgen, Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799, USA * corresponding author tel: 805-447-4304, fax: 805-447-1982, e-mail: bboyle@amgen.com
RANK William J. Boyle* Department of Cell Biology, Amgen, Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799, USA * corresponding author tel: 805-447-4304, fax: 805-447-1982, e-mail: bboyle@amgen.com
Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance
 Published Online: 22 November, 2004 Supp Info: http://doi.org/10.1083/jcb.200405053 Downloaded from jcb.rupress.org on April 8, 2018 JCB: ARTICLE Phosphorylation of DCC by Fyn mediates Netrin-1 signaling
Published Online: 22 November, 2004 Supp Info: http://doi.org/10.1083/jcb.200405053 Downloaded from jcb.rupress.org on April 8, 2018 JCB: ARTICLE Phosphorylation of DCC by Fyn mediates Netrin-1 signaling
6 Mechanotransduction
 6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
AMACHER, Jeanine Investigating the Mechanism of Endosomal Trafficking in Neurotrophic Signaling Activity
 Investigating the Mechanism of Endosomal Trafficking in Neurotrophic Signaling Activity Extensive neuronal networks coordinate crucial processes within the human body. One of the key players in neuronal
Investigating the Mechanism of Endosomal Trafficking in Neurotrophic Signaling Activity Extensive neuronal networks coordinate crucial processes within the human body. One of the key players in neuronal
Lipniacki 2004 Ground Truth
 Abstract Lipniacki 2004 Ground Truth The two-feedback-loop regulatory module of nuclear factor kb (NF-kB) signaling pathway is modeled by means of ordinary differential equations. signaling pathway: https://en.wikipedia.org/wiki/signaling_pathway
Abstract Lipniacki 2004 Ground Truth The two-feedback-loop regulatory module of nuclear factor kb (NF-kB) signaling pathway is modeled by means of ordinary differential equations. signaling pathway: https://en.wikipedia.org/wiki/signaling_pathway
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Activation of ADF/Cofilin Mediates Attractive Growth Cone Turning Toward Nerve Growth Factor and Netrin-1
 Activation of ADF/Cofilin Mediates Attractive Growth Cone Turning Toward Nerve Growth Factor and Netrin-1 Bonnie M. Marsick, 1,2 Kevin C. Flynn, 3 * Miguel Santiago-Medina, 4 James R. Bamburg, 3,5 Paul
Activation of ADF/Cofilin Mediates Attractive Growth Cone Turning Toward Nerve Growth Factor and Netrin-1 Bonnie M. Marsick, 1,2 Kevin C. Flynn, 3 * Miguel Santiago-Medina, 4 James R. Bamburg, 3,5 Paul
The majority of cells in the nervous system arise during the embryonic and early post
 Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Massive loss of neurons in embryos occurs during normal development (!)
 Types of Cell Death Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can spread) Apoptosis
Types of Cell Death Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can spread) Apoptosis
AP Biology Gene Regulation and Development Review
 AP Biology Gene Regulation and Development Review 1. What does the regulatory gene code for? 2. Is the repressor by default active/inactive? 3. What changes the repressor activity? 4. What does repressor
AP Biology Gene Regulation and Development Review 1. What does the regulatory gene code for? 2. Is the repressor by default active/inactive? 3. What changes the repressor activity? 4. What does repressor
Cell Biology Review. The key components of cells that concern us are as follows: 1. Nucleus
 Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Molecular Cell Biology 5068 In Class Exam 1 September 30, Please print your name:
 Molecular Cell Biology 5068 In Class Exam 1 September 30, 2014 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 1 September 30, 2014 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Systems theory of Smad signalling
 Systems theory of Smad signalling D.C. Clarke, M.D. Betterton and X. Liu Abstract: Transforming growth factor-b (TGFb) signalling is an important regulator of cellular growth and differentiation. The principal
Systems theory of Smad signalling D.C. Clarke, M.D. Betterton and X. Liu Abstract: Transforming growth factor-b (TGFb) signalling is an important regulator of cellular growth and differentiation. The principal
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
Mesoderm Induction CBT, 2018 Hand-out CBT March 2018
 Mesoderm Induction CBT, 2018 Hand-out CBT March 2018 Introduction 3. Books This module is based on the following books: - 'Principles of Developement', Lewis Wolpert, et al., fifth edition, 2015 - 'Developmental
Mesoderm Induction CBT, 2018 Hand-out CBT March 2018 Introduction 3. Books This module is based on the following books: - 'Principles of Developement', Lewis Wolpert, et al., fifth edition, 2015 - 'Developmental
