Microbiology II Microbial physiology I Energetics
|
|
|
- Cornelia Davidson
- 6 years ago
- Views:
Transcription
1 Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses Transport Movement ΔG<0 exergonic Isothermal, irreversible conditions Heat Work ΔG>0 endergonic Christopher Bräsen
2 Lecture Plan Mikrobielle Physiologie I Energetik Bräsen Mikrobielle Physiologie II Einige Prinzipien und Mechanismen im zentralen Kohlenstoffmetabolismus Bräsen Keine Vorlesung Bräsen Mikrobielle Physiologie III Nitrat Atmung Bräsen Mikrobielle Physiologie IV Acetogenese und der Acetyl CoA/Kohlenmonoxid Dehydrogenase Weg Bräsen Mikrobielle Physiologie V Anaerobe Nahrungskette und Methanogenese Bräsen Mikrobielle Physiologie VI Sulfate Reduktion Bräsen Antibiotika (Penicillium notatum) Meckenstock Mikroorganismen in der Umwelt (Geobacter metallireducens) Meckenstock Mikrobielles Wachstum (Elusimicrobium minutum) Meckenstock Mikrobielle Fortbewegung (Thioploca) Meckenstock Viren (T4) Meckenstock Geschichte der Mikrobiologie Meckenstock Wrap up/ausweichtermin Meckenstock/Bräsen
3 Textbooks Fuchs Allgemeine Mikrobiologie (Thieme) Brock Mikrobiologie (Pearson) Campbell/Reece Biologie (Pearson) Stryer Biochemie (Spektrum) Nelson/Cox Lehninger: Principles in Biochemistry (W.H. Freeman and Company)
4 Questions 1 Which are the two basal mechanisms of ATP synthesis and what are their characteristics? In which two parts can the energy metabolism be devided? How much energy is required to synthesize ATP from ADP and Pi considering cellular concentrations of reactants? How much energy is approximately gained from one H + flowing back into the cell (e.g. E. coli)? How can ATP yields be estimated if the energy yielding catabolic reaction is known (overall efficiency of energy metabolism ~60%)? What are energy rich intermediates? Give three examples. Name the sites of SSP in glycolysis? Whichenzymecatalysestheonly oxidation reaction in this pathway? How can ΔG values be calculated from Redoxpotentials, give the equation? What is the frequently used cosubstrate in dehydrogenase catalyzed reactions?
5 Energy transformation Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses Transport Movement ΔG<0 exergonic Isothermal, irreversible conditions Heat Work ΔG>0 endergonic Catabolism and Anabolism are coupled through the adenylate system
6 Energetics as 1 + bs 2 cp 1 + dp 2 ΔG? ΔG 0 = [cδg f0 (P1) + dδg f0 (P2)] [aδg f0 (S1) + bδg f0 (S2)] In biological systems with protons as reactants: ΔG 0 (ph 7 ([10 7 M] instead of ph 0 [10 0 =1 M]) ΔG f 0 (H + ) = 0 ΔG f 0 (H + ) = 40 kj/mol ΔG = ΔG 0 + RT x ln [P 1] c [P 2 ] d [S 1 ] a [S 2 ] b ΔG = ΔG 0 + 5,7 kj/mol x lg [P 1 ] c [P 2 ] d [S 1 ] a [S 2 ] b
7 Example: Aerobic Glucose oxidation ΔG f0 [kj/mol) C 6 H 12 O 6 + O 2 CO 2
8 Example: Aerobic Glucose oxidation ΔG f0 [kj/mol) C 6 H 12 O 6 + O 2 6 CO 2
9 Example: Aerobic Glucose oxidation ΔG f0 [kj/mol) C 6 H 12 O O 2 6 CO H 2 O
10 Gibbs free energies of formation In Brock Mikrobiologie, 13. Auflage, Anhang
11 Gibbs free energies of formation In Brock Mikrobiologie, 13. Auflage, Anhang
12 Energetics
13 Example: Aerobic Glucose oxidation ΔG f0 [kj/mol) C 6 H 12 O O 2 6 CO H 2 O ΔG 0 = [6 ( 394) CO ( 237) H2O] [( 917) glucose + 6 (0) O 2 ] = [ ] = 2869 kj/mol How much ATP can be synthesized from this catabolic reaction?
14 ATP Why energy rich (=high ΔG of hydrolysis, high group transfer potential)? ATP Resonance stabilization of products of hydrolysis exceeds resonance stabilization of the compound itself Electrostatic repulsion between negatively charged phosphate oxygen atoms favors separation of the phosphates Stabilization through hydratation: Water can bind more efficiently to ADP and Pi than to the Anhydride bond in ATP
15 Energetics of ATP synthetsis How much energy is required for ATP synthesis? ADP 3 + P i 2 ATP 4 + H 2 O Standard conditions: ΔG 0 = +32 kj/mol Cellular conditions: ~10 mm ATP, ~1 mm ADP, ~10 mm P i ΔG = ΔG 0 + RT x ln [ATP]/[ADP][Pi] ΔG = +32 kj/mol kj/mol x lg 10 3 = +49 kj/mol
16 Constants and Units
17 Energy transformation Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses Transport Movement ΔG<0 exergonic Isothermal, irreversible conditions Heat Work ΔG>0 endergonic Catabolism and Anabolism are coupled through the adenylate system
18 Energetics of ATP synthetsis How much energy is required for ATP synthesis? ADP 3 + P i 2 ATP 4 + H 2 O Standard conditions: ΔG 0 = +32 kj/mol Cellular conditions: ~10 mm ATP, ~1 mm ADP, ~10 mm P i ΔG = ΔG 0 + RT x ln [ATP]/[ADP][Pi] ΔG = +32 kj/mol kj/mol x lg 10 3 = +49 kj/mol Regarding the irreversibility of the whole metabolism: Efficiency ~60% ~ kj/mol
19 Example: Aerobic Glucose oxidation ΔG f0 [kj/mol) C 6 H 12 O O 2 6 CO H 2 O ΔG 0 = [6 ( 394) CO ( 237) H2O] [( 917) glucose + 6 (0) O 2 ] = [ ] = 2869 kj/mol How much ATP can be synthesized from this catabolic reaction? 2869/ ATP
20 Mechanisms of ATP synthesis Most energy yielding catabolic rections are redox reactions as 1 (red.) + bs 2 (ox.) cp 1 (ox.) + dp 2 (red.) Oxidative part Reductive part Substrat 1 red. Substrat 2 ox. ATP synthesis via Substrate level phosphorylation (SSP) ATP n x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) Produkt 1 ox. Produkt 2 red. (given: thermodynamic and mechanistic feasability (?))
21 Example: Aerobic Glucose oxidation Most energy yielding catabolic rections are redox reactions Glucose (red.) + 6 O 2 (ox.) 6 CO 2 (ox.) + 6H 2 O (red.) Oxidative part Reductive part Electron donating half reaction Electron accepting half reaction Glucose = S1 red. 6 O 2 = S2 ox. ATP synthesis via Substrate level phosphorylation (SSP) ATP n x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) 6 CO 2 = P1 ox. 12 H 2 O = P2 red.
22 Energy metabolism Oxidative part Reductive part Substrat 1 red. Substrat 2 ox. ATP synthesis via Substrate level phosphorylation (SSP) ATP n x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) Produkt 1 ox. Produkt 2 red. Cytoplasm (dehydrogenases, kinases) energy rich phosphoryl compounds natp/s1 Energy quantum 1 ATP = 50 kj/mol Membrane bound (ET proteins, ATP synthase) Electrochemical proton potential (ΔµH + =nh + /S2) Energy quantum 1H + =18 kj/mol (=1/3 ATP)
23 Substrate level phosphorylation The energy of a highly exergonic reaction (often oxidation of carbonyl to carboxyl groups) is used to form energy rich intermediates (phosphorylated intermediates), e.g. GAP 1,3BPG 3 PG, pyruvate (acetyl CoA acetyl~p) ( acetate)
24 Substrate level phosphorylation The energy of a highly exergonic reaction is used to form energy rich intermediates often through oxidation of carbonyl to carboxyl groups, e.g. GAP 1,3BPG 3 PG, pyruvate acetyl CoA acetyl~p acetate Energy rich intermediates are: Phosphate anhydrides (1,3 Bisphosphoglycerate, Acetyl P) Enolester (PEP) Thioesters Transfer phosphorylgroups to ADP
25 Substrate level phosphorylation The energy of a highly exergonic reaction is used to form energy rich intermediates often through oxidation of carbonyl to carboxyl groups, e.g. GAP 1,3BPG pyruvate acetyl CoA) Energy rich intermediates are: Phosphate anhydrides (1,3 Bisphosphoglycerate, Acetyl P) Enolester (PEP) Thioesters Transfer phosphorylgroups to ADP
26 Glyceraldehyde 3 phosphate oxidation ΔG ~ 50 kj/mol Glyceraldehyde 3 phosphate 3 Phosphoglycerate
27 Glyceraldehyde 3 phosphate Dehydrogenase
28 1,3 bisphosphoglycerate as energy rich intermediate 1,3 Bisphosphoglycerate = Carboxyl/Phosphoanhydride bond!
29 Substrate level phosphorylation Why energy rich (=high ΔG of hydrolysis, high group transfer potential)? Phosphoenolpyruvate (PEP), involved in ATP synthesis in glycolysis, has a very strongly negative ΔG of Pi hydrolysis (~ 52 kj/mol). Removal of Pi from ester linkage in PEP is spontaneous because the enol spontaneously converts to a ketone (Keto enol tautomerie, ~ 30 kj/mol).
30 Pyruvate Kinase
31 Pyruvate Kinase
32 Substrate level phosphorylation Why energy rich (=high ΔG of hydrolysis, high group transfer potential)? Thioester (e.g. Acetyl CoA) Resonance stabilization of products of hydrolysis exceeds resonance stabilization of the compound itself Stabilization through hydratation The thioester is more energy rich compared to the oxygen ester because the higher resonance stabilization of the latter
33 Substrate level phosphorylation Why energy rich (=high ΔG of hydrolysis, high group transfer potential)? Thioester (e.g. Acetyl CoA) Resonance stabilization of products of hydrolysis exceeds resonance stabilization of the compound itself Stabilization through hydratation The thioester is more energy rich compared to the oxygen ester because the higher resonance stabilization of the latter P i ADP acetyl CoA acetyl~p acetate HS CoA ATP
34 Energy metabolism Oxidative part Reductive part Substrat 1 red. Substrat 2 ox. ATP synthesis via Substrate level phosphorylation (SSP) ATP n x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) Produkt 1 ox. Produkt 2 red. Cytoplasm (dehydrogenases, kinases) energy rich phosphoryl compounds natp/s1 Energy quantum 1 ATP = 50 kj/mol Membrane bound (ET proteins, ATP synthase) Electrochemical proton potential (ΔµH + =nh + /S2) Energy quantum 1H + =18 kj/mol (=1/3 ATP)
35 NAD + /NADH + H + Hydride ion transfer! NADH formation can be followed by observing the appearance of the absorbance at 340 nm (molar extinction coefficient 340 = 6,200 M 1 cm 1 ).
36 NAD(P) + /NAD(P)H + H + Dehydrogenase catalysed reactions: + Hydride anion ion transfer! Water soluble electron carrier Freely diffusable NADP in anabolism NAD in catabolism R O H +H 2 O + H + H + C OH GAP 3 Phosphglycerate Aldehyde H Aldehydehydrate Carboxylic acid + H + H + Malate Oxaloacetate Isocitrate Oxalosuccinate Alcohol Aldehyde FAD (Quinone) + H + H + Succinate Fumarate Single C C bond Double C=C bond
37 Energy metabolism Oxidative part Reductive part Substrat 1 red. Substrat 2 ox. ATP synthesis via Substrate level phosphorylation (SSP) ATP n x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) Produkt 1 ox. Produkt 2 red. Cytoplasm (dehydrogenases, kinases) energy rich phosphoryl compounds natp/s1 Energy quantum 1 ATP = 50 kj/mol Membrane bound (ET proteins, ATP synthase) Electrochemical proton potential (ΔµH + =nh + /S2) Energy quantum 1H + =18 kj/mol (=1/3 ATP)
38 Electron transport phosphorylation In this simple representation of the chemiosmotic theory applied to mitochondria, electrons from NADH and other oxidizable substrates pass through a chain of carriers arranged asymmetrically in the inner membrane. Electron flow is accompanied by proton transfer across the membrane, producing both a chemical gradient (ΔpH ) and an electrical gradient (Δψ). The inner mitochondrial membrane is impermeable to protons; protons can reenter the matrix only through proton specific channels (F o ). The proton motive force that drives protons back into the matrix provides the energy for ATP synthesis, catalyzed by the F 1 complex associated with F o. Electron transport chain Σ 10H + /2e ~3ATP
39 Electron transport phosphorylation At the cytoplasmic membrane impermeable to protons Coupled to the formation of an electrochemical proton potential = energy rich intermediate Electron transport chain Σ 10H + /2e ~3ATP
40 Electron transport phosphorylation Electrochemical proton potential ΔµH + gradient [H + ] o > [H + ] i ΔpH, acidic outside alkaline inside ΔΨ, electrogenic 3 4 H + /ATP ΔG = +50 kj ΔG= n F ΔE 50 kj = 0.13 V (0.17 V) 4 x 96.5 ( 3) ΔG = ΔµH + = ~17 kj/mol Brock Mikrobiologie Pearson 2013
41 Electrochemical proton potential ΔµH + gradient [H + ] o > [H + ] i ΔpH, acidic outside alkaline inside ΔΨ, electrogenic ΔG = ΔµH + = F ΔΨ RT ΔpH (i o) = 96.5 x 0.15V x 0.5 = 17.4 kj/mol E. coli: ΔpH ~ 0.5 ΔΨ ~ 0.15V ΔG/F = ΔµH + /F = ΔΨ RT/F ΔpH (i o) = Δp [V] proton motive force Δp = ΔΨ + Z ΔpH (i o) Z = 2.3 RT/F = V The poten al difference of at least 0.13 V 0.17 V corresponds to a ph difference of ~2.2 3 Brock Mikrobiologie Pearson 2013
42 Electrochemical proton potential ΔµH + gradient [H + ] o > [H + ] i ΔpH, acidic outside alkaline inside ΔΨ, electrogenic ΔG = ΔµH + = F ΔΨ RT ΔpH (i o) E. coli: ΔpH ~ 0.5 ΔΨ ~ 0.15V ΔG = ΔµH + = 96.5 kj/mol V 0.15 V kj/mol 0.5 (i o) ΔG = ΔµH + = ~18 kj/mol Brock Mikrobiologie Pearson 2013
43 Electron transport chain Σ 10H + /2e ~3 ATP
44 Example: Aerobic Glucose oxidation Oxidative part Reductive part Glucose 6 O 2 ATP synthesis via Substrate level phosphorylation (SSP) ATP 12 x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) 6 CO 2 12 H 2 O Cytoplasm (dehydrogenases, kinases) energy rich phosphoryl compounds natp/s1 Energy quantum 1 ATP = 50 kj/mol Membrane bound (ET proteins, ATP synthase) Electrochemical proton potential (ΔµH + =nh + /S2) Energy quantum 1H + =18 kj/mol (=1/3 ATP)
45 Example: Aerobic Glucose oxidation Most energy yielding catabolic rections are redox reactions as 1 (red.) + bs 2 (ox.) cp 1 (ox.) + dp 2 (red.) Oxidative part Reductive part Electron donating half reaction Electron accepting half reaction Glucose = S1 red. 6 O 2 = S2 ox. ATP synthesis via Substrate level phosphorylation (SSP) ATP n x 2[H] ATP ATP synthesis via Electron transport phosphorylation (ETP) 6 CO 2 = P1 ox. 12 H 2 O = P2 red.
46 Redox potentials The redox potenial indicate the tendency of a redox couple to donate electrons to (=reduce) or to accept electrons from (=oxidize) the standard hydrogen electrode. E 0 (2 H + /H 2 ) = 0 mv (under Standard conditions 25 C, 1 M ) E 0 (X ox /X red ) < 0 tends to reduce E 0 (X ox /X red ) > 0 tends to oxidize (H + /H 2 ) E 0 (2 H + /H 2 ) = 420 mv (under Standard conditions 25 C, 1 M, ph 7) E E 0.. E V E 0, lg..
47 ΔG and redox potentials ΔG 0 = n F ΔE 0 ΔE 0 =(E 0 [Akzeptor] E 0 [Donor])
48 Example: Aerobic Glucose oxidation as 1 (red.) + bs 2 (ox.) cp 1 (ox.) + dp 2 (red.) Oxidative part Reductive part Electron donating half reaction Electron accepting half reaction Glucose = S1 red. 6 O 2 = S2 ox. ΔG 0 = n F ΔE 0 ΔE 0 =(E 0 [Akzeptor] E 0 [Donor]) ATP (SSP) n x 2[H] ATP (ETP) 6 CO 2 = P1 ox. 12 H 2 O = P2 red. 430 mv +820 mv ΔG 0 = 24 x 96.5 kj/mol V x ( ( 0.43)) = 2895 kj/mol
49 Example: Aerobic Glucose oxidation Oxidative part Reductive part How much energy is gained from oxidative and reductive part, respectively? Electron donating half reaction Glucose = S1 red. Electron accepting half reaction 6 O 2 = S2 ox. Can be estimated if the electron carrier (incl. E 0 ) is known. ATP n x 2[H] ATP Assumption: NAD + /NADH ( 0.32 V) is the sole electron carrier (as a proxy). 6 CO 2 = P1 ox. 12 H 2 O = P2 red. 430 mv +820 mv
50 Example: Aerobic Glucose oxidation First half reaction: 430 mv 320 mv Glucose (red.) + 12 NAD + (ox.) + 6 H 2 O 6 CO 2(ox.) + 12 NADH (red.) + 12 H + How energy is gained from oxidative and reductive part, respectively? Can be estimated if the electron carrier (incl. E 0 ) is known. Assumption: NAD + /NADH ( 0.32 V) is the sole electron carrier (as a proxy). ΔG 0 = n F ΔE 0 ΔE 0 =(E 0 [Akzeptor] E 0 [Donor]) E 0 (CO 2 /glucose) = 430 mv E 0 (NAD+/NADH) = 320 mv ΔG 0 = 24 x 96.5 kj/mol V x ( 0.32 V ( 0.43 V)) = 255 kj/mol 4 ATP via SSP
51 Example: Aerobic Glucose oxidation Second half reaction: 320 mv +820 mv 12 NADH (red.) + 12 H O 2 (ox.) 12 NAD + (ox.) + 12 H 2O (red.) How energy is gained from oxidative and reductive part, respectively? Can be estimated if the electron carrier (incl. E 0 ) is known. Assumption: NAD + /NADH ( 0.32 V) is the sole electron carrier (as a proxy). ΔG 0 = n F ΔE 0 ΔE 0 =(E 0 [Akzeptor] E 0 [Donor]) E 0 (O 2 /H 2 O) = +820mV E 0 (NAD+/NADH) = 320 mv ΔG 0 = 24 x 96.5 kj/mol V x (0.82 V ( 0.32 V)) = 2640 kj/mol 34 ATP via ETP
52 Aerobic glucose degradation Oxidative part Glucose Σ 4 ATP (SLP) + 10 NADH + 2 UQH 2 Transport Σ ~38 ATP Embden Meyerhof pathway Entner Doudoroff pathway Glucose 2 Pyruvate 4 [H] 1 2 ATP (SSP) Reductive part 6 O 2 Pyruvate dehydrogenase complex 2 Acteyl CoA 4 [H] 2 CO 2 24 [H] 10 NADH 100 H + 2 UQH 2 12 H + Respiratory chain ~34 ATP (ETP) Citric acid cycle 16 [H] 2 ATP (SSP) 12 H 2 O 4 CO 2
53 Questions 1 Which are the two basal mechanisms of ATP synthesis and what are their characteristics? In which two parts can the energy metabolism be devided? How much energy is required to synthesize ATP from ADP and Pi considering cellular concentrations of reactants? How much energy is approximately gained from one H + flowing back into the cell (e.g. E. coli)? How can ATP yields be estimated if the energy yielding catabolic reaction is known (overall efficiency of energy metabolism ~60%)? What are energy rich intermediates? Give three examples. Name the sites of SSP in glycolysis? Whichenzymecatalysestheonly oxidation reaction in this pathway? How can ΔG values be calculated from Redoxpotentials, give the equation? What is the frequently used cosubstrate in dehydrogenase catalyzed reactions?
54 Exercize: Anaerobic Glucose oxidation ( O 2, without exogenous electron acceptor) ΔG f0 [kj/mol) C 6 H 12 O 6 C 3 H 5 O 3 (Lactate) Give the complete reaction equation. Calculate the standard free energy (ph 7). How many ATP can maximally be synthesized? Compare and discuss in the light of the aerobic degradation.
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Lecture 10. Proton Gradient-dependent ATP Synthesis. Oxidative. Photo-Phosphorylation
 Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
CHAPTER 15 Metabolism: Basic Concepts and Design
 CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
Change to Office Hours this Friday and next Monday. Tomorrow (Abel): 8:30 10:30 am. Monday (Katrina): Cancelled (05/04)
 Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Department of Chemistry and Biochemistry University of Lethbridge. Biochemistry II. Bioenergetics
 Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Bioenergetics and high-energy compounds
 Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová
 Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
Review Questions - Lecture 5: Metabolism, Part 1
 Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
Chapter 2 Energy in Biology Demand and Use
 Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
Biochemistry 3300 Problems (and Solutions) Metabolism I
 (1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes
(1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes
Biochemical Pathways
 Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
MitoSeminar II: Some calculations in bioenergetics
 MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
All organisms require a constant expenditure of energy to maintain the living state - "LIFE".
 CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Outer Glycolysis mitochondrial membrane Glucose ATP
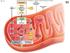 Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
State state describe
 Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Be sure to understand:
 Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Biology Reading Assignment: Chapter 9 in textbook
 Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
Energy Exchanges Exam: What to Study
 Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Energy in Chemical and Biochemical Reactions
 Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Metabolism Review. A. Top 10
 A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
Microbiology II. Fermentations Acetogenesis and the CODH/ACS pathway Syntrophy. Christopher Bräsen. Methyl branch. Carbonyl branch
 Microbiology II Fermentations Acetogenesis and the CODH/ACS pathway Syntrophy Methyl branch ΔµH + Carbonyl branch CODH/ACS enzyme complex Phosphotransacetylase Acetyl P Christopher Bräsen Acetate Kinase
Microbiology II Fermentations Acetogenesis and the CODH/ACS pathway Syntrophy Methyl branch ΔµH + Carbonyl branch CODH/ACS enzyme complex Phosphotransacetylase Acetyl P Christopher Bräsen Acetate Kinase
f) Adding an enzyme does not change the Gibbs free energy. It only increases the rate of the reaction by lowering the activation energy.
 Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Respiration and Photosynthesis
 Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Photosynthesis and Cellular Respiration
 Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
CP Biology Unit 5 Cell Energy Study Guide. Electron Carriers Electron Transport Chain Fermentation Glycolysis Krebs cycle Light-Dependent Reactions
 Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Courtesy of Elsevier. Used with permission.
 Chemistry 5.07 2013 Problem Set 5 Answers Problem 1 Succinate dehydrogenase (SDH) is a heterotetramer enzyme complex that catalyzes the oxidation of succinate to fumarate with concomitant reduction of
Chemistry 5.07 2013 Problem Set 5 Answers Problem 1 Succinate dehydrogenase (SDH) is a heterotetramer enzyme complex that catalyzes the oxidation of succinate to fumarate with concomitant reduction of
Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially
 Bioenergetics Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to
Bioenergetics Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to
Biology Reading Assignments:
 Biology 205 5.13.08 Reading Assignments: Chapter 3 Energy, Catalysis and Biosynthesis pgs. 83-94; 106-116 (Note the various roles of nucleotide based carrier molecules); work questions 3-2 and 3-3 Chapter
Biology 205 5.13.08 Reading Assignments: Chapter 3 Energy, Catalysis and Biosynthesis pgs. 83-94; 106-116 (Note the various roles of nucleotide based carrier molecules); work questions 3-2 and 3-3 Chapter
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Aerobic Cellular Respiration
 Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Chapter 5. The Chloroplast. 5.1 Matter and Energy Pathways in Living Systems. Photosynthesis & Cellular Respiration
 Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 107 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
ATP. Division Ave. High School AP Biology. Cellular Respiration Stage 4: Electron Transport Chain. Cellular respiration. The point is to make ATP!
 ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
Welcome to Class 8! Introductory Biochemistry! Announcements / Reminders! Midterm TA led Review Sessions!
 Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Photosynthesis and Cellular Respiration Practice Test Name
 Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
CHEM-E3215 Advanced Biochemistry
 CHEM-E3215 Advanced Biochemistry 30. Jan. 2018 Prof. Silvan Scheller Lecture 10 Energy conservation general (some calculations) Energy conservation in anaerobes: e.g. methanogensis Life close to the thermodynamic
CHEM-E3215 Advanced Biochemistry 30. Jan. 2018 Prof. Silvan Scheller Lecture 10 Energy conservation general (some calculations) Energy conservation in anaerobes: e.g. methanogensis Life close to the thermodynamic
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Bio102 Problems Photosynthesis
 Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
BCH 4054 Spring 2001 Chapter 21 Lecture Notes
 BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
Cell Energy Notes ATP THE ENDOSYMBIOTIC THEORY. CELL ENERGY Cells usable source of is called ATP stands for. Name Per
 Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Energy Metabolism exergonic reaction endergonic reaction Energy of activation
 Metabolism Energy Living things require energy to grow and reproduce Most energy used originates from the sun Plants capture 2% of solar energy Some captured energy is lost as metabolic heat All energy
Metabolism Energy Living things require energy to grow and reproduce Most energy used originates from the sun Plants capture 2% of solar energy Some captured energy is lost as metabolic heat All energy
Advanced Cell Biology. Lecture 8
 Advanced Cell Biology. Lecture 8 Alexey Shipunov Minot State University January 28, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 8 January 28, 2013 1 / 33 Outline Questions and answers Energy and
Advanced Cell Biology. Lecture 8 Alexey Shipunov Minot State University January 28, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 8 January 28, 2013 1 / 33 Outline Questions and answers Energy and
I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI.
 Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
AP Biology Cellular Respiration
 AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS
 Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS IN THE GRAND SCHEME The purpose of this workshop is to get the Learning Communities to begin thinking about why certain chemical
Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS IN THE GRAND SCHEME The purpose of this workshop is to get the Learning Communities to begin thinking about why certain chemical
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
Cellular Energy. How Organisms Obtain Energy Section 2: Photosynthesis Section 3: Cellular Respiration. Click on a lesson name to select.
 Section 1: How Organisms Obtain Energy Section 2: Photosynthesis Section 3: Cellular Respiration Click on a lesson name to select. Section 1 How Organisms Obtain Energy Transformation of Energy Energy
Section 1: How Organisms Obtain Energy Section 2: Photosynthesis Section 3: Cellular Respiration Click on a lesson name to select. Section 1 How Organisms Obtain Energy Transformation of Energy Energy
Cellular respiration ATP. Cellular Respiration Stage 4: Electron Transport Chain. AP Biology. The point is to make ATP! What s the point?
 ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
Photosynthesis and cellular respirations
 The Introduction of Biology Defining of life Basic chemistry, the chemistry of organic molecules Classification of living things History of cells and Cells structures and functions Photosynthesis and cellular
The Introduction of Biology Defining of life Basic chemistry, the chemistry of organic molecules Classification of living things History of cells and Cells structures and functions Photosynthesis and cellular
Outline. Metabolism: Energy and Enzymes. Forms of Energy. Chapter 6
 Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Chapter 13 Principles of Bioenergetics
 Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
This is an example of cellular respiration, which can be used to make beer and wine using different metabolic pathways For these reasons we call this
 Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 5: Photosynthesis: The Energy of Life pg : Pathways of Photosynthesis pg
 UNIT 2: Metabolic Processes Chapter 5: Photosynthesis: The Energy of Life pg. 210-240 5.2: Pathways of Photosynthesis pg. 220-228 Light Dependent Reactions Photosystem II and I are the two light capturing
UNIT 2: Metabolic Processes Chapter 5: Photosynthesis: The Energy of Life pg. 210-240 5.2: Pathways of Photosynthesis pg. 220-228 Light Dependent Reactions Photosystem II and I are the two light capturing
Lecture 7: Enzymes and Energetics
 Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Activity: Identifying forms of energy
 Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Name Date Class. Photosynthesis and Respiration
 Concept Mapping Photosynthesis and Respiration Complete the Venn diagram about photosynthesis and respiration. These terms may be used more than once: absorbs, Calvin cycle, chlorophyll, CO 2, H 2 O, Krebs
Concept Mapping Photosynthesis and Respiration Complete the Venn diagram about photosynthesis and respiration. These terms may be used more than once: absorbs, Calvin cycle, chlorophyll, CO 2, H 2 O, Krebs
*The entropy of a system may decrease, but the entropy of the system plus its surroundings must always increase
 AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
Oxidative Phosphorylation versus. Photophosphorylation
 Photosynthesis Oxidative Phosphorylation versus Photophosphorylation Oxidative Phosphorylation Electrons from the reduced cofactors NADH and FADH 2 are passed to proteins in the respiratory chain. In eukaryotes,
Photosynthesis Oxidative Phosphorylation versus Photophosphorylation Oxidative Phosphorylation Electrons from the reduced cofactors NADH and FADH 2 are passed to proteins in the respiratory chain. In eukaryotes,
Section A: The Principles of Energy Harvest
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
Photosynthesis and Cellular Respiration Note-taking Guide
 Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
of catabolic processes, like glycolysis and the Krebs cycle, as hydride ions (H ). This free energy is used to regenerate ATP in the matrix.
 Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely
Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely
2.A.2- Capture and Storage of Free Energy
 2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
Unit 3: Cellular Energetics Guided Reading Questions (50 pts total)
 AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
Lecture 9: Photosynthesis
 Lecture 9: Photosynthesis I. Characteristics of Light A. Light is composed of particles that travel as waves 1. Comprises a small part of the electromagnetic spectrum B. Radiation varies in wavelength
Lecture 9: Photosynthesis I. Characteristics of Light A. Light is composed of particles that travel as waves 1. Comprises a small part of the electromagnetic spectrum B. Radiation varies in wavelength
AP Biology Day 16. Monday, September 26, 2016
 AP Biology Day 16 Monday, September 26, 2016 CW/HW Assignments 1. Ch. 9 Guided Reading 2. Ch. 9 Video Cornell Notes (2) PLANNER 1. Ch. 9 Video Cornell Notes (weebly) 2. Study & schedule test retake! unit
AP Biology Day 16 Monday, September 26, 2016 CW/HW Assignments 1. Ch. 9 Guided Reading 2. Ch. 9 Video Cornell Notes (2) PLANNER 1. Ch. 9 Video Cornell Notes (weebly) 2. Study & schedule test retake! unit
METABOLISM. What is metabolism? Categories of metabolic reactions. Total of all chemical reactions occurring within the body
 METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
- BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU
 - BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU Bioenergetics the field of biochemistry concerned with the transfer and use of energy by biological system BIOLOGICAL IMPORTANCE: Suitable fuel
- BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU Bioenergetics the field of biochemistry concerned with the transfer and use of energy by biological system BIOLOGICAL IMPORTANCE: Suitable fuel
Supplementary thermodynamics as applied to biosystems
 Supplementary thermodynamics as applied to biosystems Glucose is transferred to glucose-6-phosphate, abbreviated here to G6P. The reaction may be written Glucose + phosphate G6P + H 2 O G o = 13.8kJ/mol
Supplementary thermodynamics as applied to biosystems Glucose is transferred to glucose-6-phosphate, abbreviated here to G6P. The reaction may be written Glucose + phosphate G6P + H 2 O G o = 13.8kJ/mol
Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain *
 OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Unit 1C Practice Exam (v.2: KEY)
 Unit 1C Practice Exam (v.2: KEY) 1. Which of the following statements concerning photosynthetic pigments (chlorophylls a and b, carotenes, and xanthophylls) is correct? (PT1-12) a. The R f values obtained
Unit 1C Practice Exam (v.2: KEY) 1. Which of the following statements concerning photosynthetic pigments (chlorophylls a and b, carotenes, and xanthophylls) is correct? (PT1-12) a. The R f values obtained
