of catabolic processes, like glycolysis and the Krebs cycle, as hydride ions (H ). This free energy is used to regenerate ATP in the matrix.
|
|
|
- Martha Eaton
- 5 years ago
- Views:
Transcription
1 Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely responsible for the production of ATP in chemotrophs (and even serve this purpose in phototrophs when light is limited). The first bilayer, the outer membrane, defines the boundaries of the organelle; it is about 70% protein by mass. It is very porous but even so, contains a transmembrane channel called porin. This protein allows the passage of molecules up to about 10,000 daltons or so to travel back and forth across the outer membrane; the outer membrane is fairly porous. Inside of the outer membrane is another membrane called the inner membrane. The inner membrane has a higher protein content, almost no cholesterol, and a higher proportion of unsaturated lipids. Almost nothing can travel across the inner membrane without the assistance of transport proteins (except water); its virtually impermeable. It is loaded with transport proteins and enzymes for metabolism. In between the outer and inner membrane is a space called the intermembrane space. Because the outer membrane is so porous, the intermembrane space and the cytoplasm are virtually homogeneous. Inside the inner membrane is an area called the matrix. The TCA Cycle takes place in the matrix on soluble and membrane bound enzymes. Recall that the primary product of the Krebs Cycle, with respect to energy, is NADH. NADH carries the free energy produced as a result - of catabolic processes, like glycolysis and the Krebs cycle, as hydride ions (H ). This free energy is used to regenerate ATP in the matrix. II. OXIDATION AND REDUCTION The regeneration of ATP depends upon the transfer of free energy in the form of hydride ions; high, energy electrons. NAD and FAD pick up the electrons in catabolic reactions of glycolysis and the TCA cycle. They then carry the electrons where they are used to drive endergonic processes. In a chemical reaction, if one substance loses electrons and another substance gains electrons, that chemical reaction is called an oxidation-reduction (red-ox) reaction: H 2(g) ½O 2(g) -> H2O(l) This is a red-ox reaction because electrons are transferred. The oxidation state of hydrogen goes from 0 to 1, its oxidations state increases; hydrogen is oxidized. The oxidation state of oxygen goes from 0 to -2, its oxidation state is reduced; oxygen is reduced. Since hydrogen is reduced and oxygen is oxidized, electrons are transferred from hydrogen to oxygen (electrons are transferred from the oxidized substance to the reduced substance). Likewise, NADH carries electrons to endergonic processes: NADH(aq) X (aq) -> NAD (aq) XH(aq) NADH loses electrons so it is oxidized. The electron acceptor, X, gets the electrons so it is reduced. NADH is a reducing agent. Reduction Potentials The ability of one substance to reduce another substance (transfer electrons to another
2 substance) is called its reduction potential,. For example, the reaction for the transfer of 2 electrons from NADH to oxygen and hydrogen ion is: NADH(aq) H (aq) ½O (aq) -> NAD (aq) H O(l) 2 2 the reduction potential for the process,, is 1.136V The relation between the standard free energy of a process and its reduction potential is given by: G = -n where n is the number of electrons transferred, is Faraday s constant, ~96.5kJ/V Notice that large positive reduction potentials lead to large negative standard free energy changes. So you see where the energy of the electrons is used; its freed by the electron transfer. The standard free energy change for the transfer of 2e- from NADH to oxygen is about -219kJ per mole of NADH; quite a bit of free energy. So what does the reduction potential tell you: the larger the reduction potential, the greater the probability that free energy will be generated when electrons are transferred to the acceptor (the greater the likely hood that the transfer will be spontaneous). As with other state functions, reversing the reaction reverses the reduction potential. Consider the same reaction reversed: NAD (aq) H O(l) -> NADH(aq) H (aq) ½O (aq) 2 2 = V The standard free energy change for the transfer of 2e- from water to NADH is about 219kJ per mole of water. This reaction has almost no chance of being spontaneous: - G = RTln([NADH][H ][O ] /[NAD ]) ½ 2 at normal body temperature, the ratio of product to reactant would have to be less than ~0.919 for the transfer to be spontaneous Dinucleotides in Metabolism There are primarily two dinucleotide coenzymes involved in the regeneration of ATP: NAD and FAD. The role of each is the same: Catabolism of organic material results in the release of large amount of free energy. This free energy is carried by electrons. The electrons are carried by the dinucleotides as hydride ions. NAD 2H 2e -> NADH H - - FAD 2H 2e -> FADH shows how NAD is reduced to NADH when it catches electrons. shows how FAD is reduced to FADH when it catches electrons. 2 2 Neither of these is a favorable reaction (they both have negative reduction potentials), but the free energy discharge resulting from catabolism drives the transfer of electrons to these carriers (one of the things accomplished by catabolism is loading these molecules up with electrons). The electrons they carry are the fuel that drives the re-attachment of phosphate to ADP (ATP synthesis/regeneration). So note, if loading the molecules with electrons requires free energy, unloading the electrons results in the release of free energy! III. OXIDATIVE PHOSPHORYLATION The primary product of the Krebs Cycle (and thus the catabolism of monosaccharides and other such carbon compounds), is NADH. The standard reduction potential for NAD is -0.32V. This means that this redox pair will clearly favor the oxidation of NADH as opposed to the reduction of NAD ; NADH carries high energy electrons that it wants to dump somewhere. When the electrons are dumped, they are accompanied with a good deal of free energy. That free energy is used to produce ATP from ADP; to attach phosphate groups to ADP molecules. ATP synthesis is called oxidative phosphorylation because electrons carries are oxidized so that ADP may be phosphorylated. So, what exactly do those electrons transferred do?
3 Electron Transport and Hydrogen Pumping First recall that the inner membrane is virtually impermeable to everything except water and that the intermembrane space is virtually the same as the cytoplasm because the outer membrane is so porus. Electron carriers deliver electrons to membrane proteins of the mitochondrial inner membrane. These proteins are electron transport machines; they transfer electrons to other electron transport machines until the electrons eventually end up transferred to oxygen and hydrogen ion (and water is made). Remember, these are high energy electrons, they carry a boatload of free energy. When they are transferred, the free energy is used to cause the electron transport proteins to pump hydrogen from inside the matrix into the intermembrane space. Why is energy needed for this? One, the innermembrane is not permeable to H. And two, even if it was, pumping H out creates an imbalance of H. The free energy is needed to push H out into i) a larger H concentration, and ii) a positive environment. Part of the free energy of the electrons is used to create a concentration/charge imbalance (gradient) of H. Each time electrons are transferred to a new electron transporter, several hydrogen ions are pumped out of the matrix. This is done about three times; one pair of electrons can supply the energy to expel about 10 H. The end result of this is to create a situation where there s a ton of hydrogen ion and positive charge outside an area that has less hydrogen ion and a more negative charge. Consequently the hydrogen ion outside of the matrix is extremely attracted to the matrix, however, the membrane is impermeable to hydrogen ion, so that attraction is potential energy! Part of the free energy from the electron transfer is converted into potential energy of attraction. Although the inner membrane is impermeable to H, there is a way for the H to return; a protein complex called ATP synthetase. So you got all these hydrogen ions outside the matrix with all that energy of attraction to the inside of the matrix. If they find a way in, there ll be a whole lot of free energy released on their re-entry. And that s just what ATP synthetase uses. It takes the free energy of the H returning into the matrix and uses it to attach phosphate to ADP! ATP synthesis is coupled to a type of osmosis, which is driven by electron transport.
4 Mechanics of Electron Transfer Each time something picks up electrons, it is reduced. Most of these such transfers occur using iron-sulfur (Fe-S) centers, cytochromes, copper ion, flavoproteins, and coenzyme Q. For instance, if electrons are transferred to an Fe-S center: Fe e -> Fe 3-2 the change in oxidation states causes conformational changes that result in H expulsion from the matrix. Osmosis without ATP Synthesis If H re-enters the mitochondria, somehow, without ATP synthesis, the free energy is released as thermal energy; heat. In fact, that is a mechanism many organisms use to keep warm. Hydrogen ion enters the matrix through special membrane proteins not linked to ATP synthesis. IV. USING ATP ATP/ADP Transport The ATP produced in the mitochondria is transported into the cytoplasm by ATP/ADP transport proteins. These membrane proteins transport an ADP into the matrix only when there is an ATP to transport out (once in the intermembrane space, each gets to or from the cytoplasm through porin in the outer membrane). Once in the cytoplasm, the ATP is distributed and used as needed. Quantitative Aspects of ATP Hydrolysis Whenever an there is a need for free energy, ATP is hydrolyzed according to the reaction: ATP(aq) H2O(l) ADP(aq) Pi(aq) 2 G ~ -30.5kJ/mol, 37 C, ph=7.0, [Mg ]~5mM 2 Normal conditions for a typical human red blood cell is 37 C, ph=7.0, [Mg ]~5mM, [ATP]~2.25mM, [ADP]~0.25mM, [P i]~1.65mm. The free energy change ( G) for the hydrolysis of 4 ATP under such conditions is: G = -5.27x10 kj/mol (about 53,000J of energy released for every mole of ATP hydrolyzed). The act of hoisting a one-pound weight in the air (about 5 feet) requires the expenditure of about 2 2 PE = gmh = 9.8m /s (0.455kg)(1.524m) ~ 6.8J The amount of ATP you d have to hydrolyze to complete that task is 6.8J/x = 53,000/1mol -4 x ~ 1.3x10 moles of ATP (about 130 mol of ATP) Each glucose completing glycolysis, the Kreb Cycle, and oxidative phosphorylation regenerates about 31 molecules of ATP. So you d need about 4 moles of glucose to raise a 1.0 pound weight 5 feet in the air. A typical candy bar contains about 100,000 moles of glucose (equivalents), so only about one-twenty-five thousandth of a candy bar would be required for that task.
5 SIDE NOTE A device for measuring reduction potential is a potentiometer. This device will measure the flow of electrons from one process to another. Two solutions, A and B, are connected by a wire and an agar bridge. The agar bridge will allow the passage of small ions and the wire will allow the flow of electrons. As shown, solution A is connected to the wiring on the left of a voltmeter (the side with the negative numbers) and solution B is connected to the wiring on the right side of the voltmeter (the side with the positive numbers). The direction of the electron flow will be opposite the needle deflection. For instance, if electrons flow from A to B (left to right), the needle will deflect left of zero toward the negative numbers (solution A is oxidized, solution B is reduced). If electrons flow from B to A (right to left), the needle will deflect right of zero toward the positive numbers (solution A is reduced, solution B is oxidized). In these devices, there will usually be a standard reaction on the positive side. A typical standard is a solution of 1M H (ph=0) and 1.0 atm H 2 called a hydrogen reference half-cell. Any solution connected to the reference cell that registers a negative voltage is sending electrons to the reference cell (hydrogen ion is reduced to molecular hydrogen gas). On the other hand, if a positive voltage is registered, the reference is sending electrons to the other solution, causing one of its components to be reduced. Solutions with a voltage less than zero are better at causing reduction (they undergo oxidation yielding electrons that reduce hydrogen ion; they re better reducing agents). Solutions with a voltage greater than zero are better at causing oxidation (they undergo reduction consuming electrons from the oxidation of molecular hydrogen gas; they re better oxidizing agents). The voltage registered is the reduction potential.
6 Spontaneity and Reduction Potential The spontaneity of a process is judged by its standard free energy change, G. A negative standard free energy change means spontaneity is not difficult to achieve. On the other hand, a positive standard free energy change means spontaneity will be difficult to achieve. As you should know, the relation between the standard free energy change, and spontaneity (the free energy change) is: G = G RTln([P]/[R]) where [P]=product and [R]=reactant; if P/R > 1 you re adding to G ; if P/R < 1, you re subtracting from G Adding to G makes G more positive; less spontaneous. Subtracting from G makes G more negative; more spontaneous. Reduction potential is a gauge of how easy it would be for some entity to accept electrons; to be reduced. It is related to the standard free energy change: G = -n ' where n is the number of electrons transferred, is Faraday s constant, ~96.5kJ/V, and ' is the reduction potential If the reduction potential is negative, G will be positive and the only way the transfer will be spontaneous is if P/R < 1 and the absolute value of RTln([P]/[R]) > G. If the reduction potential is positive, G will be negative and the transfer will be spontaneous as long as the absolute value of RTln([P]/[R]) < G. CASE 1 - NAD is reduced when it picks up electrons: NAD 2H 2e -> NADH H For this half-reaction (we don t show where the electrons come from, only where they re going, so it s a half-reaction); ' = -0.32V. The reduction potential is negative, so the standard free energy change will be positive and the transfer of electrons to NAD will not tend to take place spontaneously. - Reverse the reaction and we have NADH being oxidized: NADH H -> NAD 2H 2e For this half-reaction (we don t show where the electrons are going, only where they come from, so it s a half-reaction); ' = 0.32V. The reduction potential is positive so the standard free energy change will be negative and the transfer of electrons to NAD will tend to take place spontaneously. What does the reduction potential tell us about this reaction? That its easier to oxidized NADH than to reduce NAD! CASE 2 Consider a reaction that is not a half-reaction (we know the electrons come from NADH and go to oxygen); the reduction of oxygen to water using NADH: NADH H ½O 2 -> NAD H2O ' = 1.14V This reaction should tend toward spontaneous; the reduction potential is positive so the standard free energy change should be negative. CASE 3 If you know the reduction potentials for different reactions, you can determine the reduction potential for the reactions combined the same way we use Hess s Law. You just have to decide which way you want the electrons transferred. - FAD 2H 2e -> FADH2 ' = V 3-2 Fe e -> Fe ' = 0.771V 2 Suppose we want to transfer electrons from Fe to FAD. We d have to rearrange the iron reaction, then add the resulting reactions to get the reduction potential: - FAD 2H 2e -> FADH2 ' = V 2 3-2Fe -> 2Fe 2e ' = V =================================================== 2 3 FAD 2H 2Fe -> FADH 2Fe ' = V 2 The reduction potential for this reaction is a large negative number. There is almost no chance that iron(ii) can transfer electrons to FAD without a good deal of energy input. There is very little chance it ll happen spontaneously.
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
All organisms require a constant expenditure of energy to maintain the living state - "LIFE".
 CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
Energy Exchanges Exam: What to Study
 Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
ATP. Division Ave. High School AP Biology. Cellular Respiration Stage 4: Electron Transport Chain. Cellular respiration. The point is to make ATP!
 ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
Cellular respiration ATP. Cellular Respiration Stage 4: Electron Transport Chain. AP Biology. The point is to make ATP! What s the point?
 ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
ETC/CHEMIOSIS. By: Leslie, Kelsey, Morgan
 ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
BIOLOGY 111. CHAPTER 7: Vital Harvest: Deriving Energy From Food
 BIOLOGY 111 CHAPTER 7: Vital Harvest: Deriving Energy From Food Deriving Energy from Food: What is the best carbohydrate source (for energy) in our food? Glucose! Where is the energy stored in glucose?
BIOLOGY 111 CHAPTER 7: Vital Harvest: Deriving Energy From Food Deriving Energy from Food: What is the best carbohydrate source (for energy) in our food? Glucose! Where is the energy stored in glucose?
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
BCH 4054 Spring 2001 Chapter 21 Lecture Notes
 BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
CHAPTER 15 Metabolism: Basic Concepts and Design
 CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
Cellular Respiration. The mechanism of creating cellular energy. Thursday, 11 October, 12
 Cellular Respiration The mechanism of creating cellular energy What do we know?? What do we know?? Grade 5 - Food --> Energy What do we know?? Grade 5 - Food --> Energy Grade 10 - glu. + O2 --> CO2 + H20
Cellular Respiration The mechanism of creating cellular energy What do we know?? What do we know?? Grade 5 - Food --> Energy What do we know?? Grade 5 - Food --> Energy Grade 10 - glu. + O2 --> CO2 + H20
MITOCW watch?v=vykadbjib8a
 MITOCW watch?v=vykadbjib8a The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
MITOCW watch?v=vykadbjib8a The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain *
 OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Cellular Energetics. Photosynthesis, Cellular Respiration and Fermentation
 Cellular Energetics Photosynthesis, Cellular Respiration and Fermentation TEKS B.4 Science concepts. The student knows that cells are the basic structures of all living things with specialized parts that
Cellular Energetics Photosynthesis, Cellular Respiration and Fermentation TEKS B.4 Science concepts. The student knows that cells are the basic structures of all living things with specialized parts that
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
AP Biology Cellular Respiration
 AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
Division Ave. High School AP Biology
 Overview 10 reactions u convert () to pyruvate (3C) u produces: 4 & NADH u consumes: u net: & NADH C-C-C-C-C-C fructose-1,6bp P-C-C-C-C-C-C-P DHAP P-C-C-C G3P C-C-C-P H P i P i pyruvate C-C-C 4 4 NAD +
Overview 10 reactions u convert () to pyruvate (3C) u produces: 4 & NADH u consumes: u net: & NADH C-C-C-C-C-C fructose-1,6bp P-C-C-C-C-C-C-P DHAP P-C-C-C G3P C-C-C-P H P i P i pyruvate C-C-C 4 4 NAD +
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
Electrochemistry & Redox. Voltaic Cells. Electrochemical Cells
 Electrochemistry & Redox An oxidation-reduction (redox) reaction involves the transfer of electrons from the reducing agent to the oxidising agent. OXIDATION - is the LOSS of electrons REDUCTION - is the
Electrochemistry & Redox An oxidation-reduction (redox) reaction involves the transfer of electrons from the reducing agent to the oxidising agent. OXIDATION - is the LOSS of electrons REDUCTION - is the
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Cellular respiration. How do living things stay alive? Cellular Respiration Burning. Photosynthesis. Cellular Respiration
 How do living things stay alive? Cellular Respiration Burning Happens in ALL living things inside cells and has the main goal of producing ATP the fuel of life It does not matter whether the organisms
How do living things stay alive? Cellular Respiration Burning Happens in ALL living things inside cells and has the main goal of producing ATP the fuel of life It does not matter whether the organisms
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
Activity: Identifying forms of energy
 Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Photosynthesis and Cellular Respiration Practice Test Name
 Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
State state describe
 Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Aerobic Cellular Respiration
 Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
TCA Cycle. Voet Biochemistry 3e John Wiley & Sons, Inc.
 TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
Respiration and Photosynthesis
 Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
GR QUIZ WITH ANS KEY Cellular Processes. Part I: Multiple Choice. 1. In leaf cell, the synthesis of ATP occurs in which of the following?
 GR QUIZ WITH ANS KEY Cellular Processes Part I: Multiple Choice 1. In leaf cell, the synthesis of ATP occurs in which of the following? I. Ribosomes II. Mitochondria III. Chloroplasts A. I only B. II only
GR QUIZ WITH ANS KEY Cellular Processes Part I: Multiple Choice 1. In leaf cell, the synthesis of ATP occurs in which of the following? I. Ribosomes II. Mitochondria III. Chloroplasts A. I only B. II only
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
AHL Topic 8 IB Biology Miss Werba
 CELL RESPIRATION & PHOTOSYNTHESIS AHL Topic 8 IB Biology Miss Werba TOPIC 8 CELL RESPIRATION & PHOTOSYNTHESIS 8.1 CELL RESPIRATION 1. STATE that oxidation involves the loss of electrons from an element,
CELL RESPIRATION & PHOTOSYNTHESIS AHL Topic 8 IB Biology Miss Werba TOPIC 8 CELL RESPIRATION & PHOTOSYNTHESIS 8.1 CELL RESPIRATION 1. STATE that oxidation involves the loss of electrons from an element,
Cellular Respiration. Mitochondria Rule! Mr. Kurt Kristensen
 Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
f) Adding an enzyme does not change the Gibbs free energy. It only increases the rate of the reaction by lowering the activation energy.
 Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
1. How is a partially charged battery like ADP?
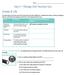 Name The chart below shows key terms from the lesson with their definitions. Complete the chart by writing a strategy to help you remember the meaning of each term. One has been done for you. Term Definition
Name The chart below shows key terms from the lesson with their definitions. Complete the chart by writing a strategy to help you remember the meaning of each term. One has been done for you. Term Definition
This is an example of cellular respiration, which can be used to make beer and wine using different metabolic pathways For these reasons we call this
 Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
4 GETTING READY TO LEARN Preview Key Concepts 4.1 Chemical Energy and ATP All cells need chemical energy.
 CHAPTER 4 Cells and Energy GETTING READY TO LEARN Preview Key Concepts 4.1 Chemical Energy and ATP All cells need chemical energy. 4.2 Overview of Photosynthesis The overall process of photosynthesis produces
CHAPTER 4 Cells and Energy GETTING READY TO LEARN Preview Key Concepts 4.1 Chemical Energy and ATP All cells need chemical energy. 4.2 Overview of Photosynthesis The overall process of photosynthesis produces
MitoSeminar II: Some calculations in bioenergetics
 MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
Biology A: Chapter 5 Annotating Notes
 Name: Pd: Biology A: Chapter 5 Annotating Notes -As you read your textbook, please fill out these notes. -Read each paragraph state the big/main idea on the left side. - On the right side you should take
Name: Pd: Biology A: Chapter 5 Annotating Notes -As you read your textbook, please fill out these notes. -Read each paragraph state the big/main idea on the left side. - On the right side you should take
Section A: The Principles of Energy Harvest
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
Outline. Metabolism: Energy and Enzymes. Forms of Energy. Chapter 6
 Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Lecture 7: Enzymes and Energetics
 Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
METABOLISM. What is metabolism? Categories of metabolic reactions. Total of all chemical reactions occurring within the body
 METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
How Cells Work. Learning Objectives
 How Cells Work Chapter 5 Learning Objectives 1. Physics tells us that in any energy transformation: a) energy is neither created nor destroyed, and b) there is always some energy lost in an unusable form
How Cells Work Chapter 5 Learning Objectives 1. Physics tells us that in any energy transformation: a) energy is neither created nor destroyed, and b) there is always some energy lost in an unusable form
Be sure to understand:
 Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
BIOLOGICAL SCIENCE. Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge. FIFTH EDITION Freeman Quillin Allison
 BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
ΔG o' = ηf ΔΕ o' = (#e ( V mol) ΔΕ acceptor
 Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
AP Biology Day 16. Monday, September 26, 2016
 AP Biology Day 16 Monday, September 26, 2016 CW/HW Assignments 1. Ch. 9 Guided Reading 2. Ch. 9 Video Cornell Notes (2) PLANNER 1. Ch. 9 Video Cornell Notes (weebly) 2. Study & schedule test retake! unit
AP Biology Day 16 Monday, September 26, 2016 CW/HW Assignments 1. Ch. 9 Guided Reading 2. Ch. 9 Video Cornell Notes (2) PLANNER 1. Ch. 9 Video Cornell Notes (weebly) 2. Study & schedule test retake! unit
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
Cell Energy Notes ATP THE ENDOSYMBIOTIC THEORY. CELL ENERGY Cells usable source of is called ATP stands for. Name Per
 Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
Bio102 Problems Photosynthesis
 Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Metabolism Review. A. Top 10
 A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Photosynthesis and Cellular Respiration
 Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
Photosynthesis. Chapter 10. Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece
 Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Energy in the World of Life
 Cellular Energy Energy in the World of Life Sustaining life s organization requires ongoing energy inputs Assembly of the molecules of life starts with energy input into living cells Energy Conversion
Cellular Energy Energy in the World of Life Sustaining life s organization requires ongoing energy inputs Assembly of the molecules of life starts with energy input into living cells Energy Conversion
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
Life Requires FREE ENERGY!
 Life Requires FREE ENERGY! Ok, so Growth, reproduction and homeostasis of living systems requires free energy To be alive/stay living, you need to use energy. Duh But really, why is energy so important?
Life Requires FREE ENERGY! Ok, so Growth, reproduction and homeostasis of living systems requires free energy To be alive/stay living, you need to use energy. Duh But really, why is energy so important?
Metabolism, Energy and Life - 1
 Metabolism, Energy and Life - 1 Thousands of chemical reactions occur in our cells and tissues to keep us alive (and hopefully healthy). Monomers are assembled into the macromolecules we need for cell
Metabolism, Energy and Life - 1 Thousands of chemical reactions occur in our cells and tissues to keep us alive (and hopefully healthy). Monomers are assembled into the macromolecules we need for cell
Chapter 5. Table of Contents. Section 1 Energy and Living Things. Section 2 Photosynthesis. Section 3 Cellular Respiration
 Photosynthesis and Cellular Respiration Table of Contents Section 1 Energy and Living Things Section 2 Photosynthesis Section 3 Cellular Respiration Section 1 Energy and Living Things Objectives Analyze
Photosynthesis and Cellular Respiration Table of Contents Section 1 Energy and Living Things Section 2 Photosynthesis Section 3 Cellular Respiration Section 1 Energy and Living Things Objectives Analyze
Cellular Energy: Photosythesis
 Cellular Energy: hotosythesis Cellular respiration and photosynthesis are chemical reactions that provide kinetic and potential energy for cells Sunlight energy hotosynthesis in chloroplasts Glucose +
Cellular Energy: hotosythesis Cellular respiration and photosynthesis are chemical reactions that provide kinetic and potential energy for cells Sunlight energy hotosynthesis in chloroplasts Glucose +
Energy for biological processes
 1 Energy transfer When you have finished revising this topic, you should: be able to explain the difference between catabolic and anabolic reactions be able to describe the part played by in cell metabolism
1 Energy transfer When you have finished revising this topic, you should: be able to explain the difference between catabolic and anabolic reactions be able to describe the part played by in cell metabolism
Unit 3: Cellular Energetics Guided Reading Questions (50 pts total)
 AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
PHOTOSYNTHESIS STARTS WITH
 Name Date Period PHOTOSYNTHESIS STARTS WITH 1. Molecules that collect light energy are called _P. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best. 3. _C is the main light absorbing
Name Date Period PHOTOSYNTHESIS STARTS WITH 1. Molecules that collect light energy are called _P. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best. 3. _C is the main light absorbing
Photosynthesis and Cellular Respiration Note-taking Guide
 Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
Table 4.3. Thermo. of Metabolism pp : Like Problem S1. Phosphorylations involving ATP. 361 Lec 21 Fri., 6oct17
 Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Phosphorylations involving ATP 361 Lec 21 Fri., 6oct17 1 Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Oxidations and Reductions
Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Phosphorylations involving ATP 361 Lec 21 Fri., 6oct17 1 Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Oxidations and Reductions
CP Biology Unit 5 Cell Energy Study Guide. Electron Carriers Electron Transport Chain Fermentation Glycolysis Krebs cycle Light-Dependent Reactions
 Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Forms of stored energy in cells
 Forms of stored energy in cells Electrochemical gradients Covalent bonds (ATP) Reducing power (NADH) During photosynthesis, respiration and glycolysis these forms of energy are converted from one to another
Forms of stored energy in cells Electrochemical gradients Covalent bonds (ATP) Reducing power (NADH) During photosynthesis, respiration and glycolysis these forms of energy are converted from one to another
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová
 Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Unit 5 Cellular Energy
 Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 5: Photosynthesis: The Energy of Life pg : Pathways of Photosynthesis pg
 UNIT 2: Metabolic Processes Chapter 5: Photosynthesis: The Energy of Life pg. 210-240 5.2: Pathways of Photosynthesis pg. 220-228 Light Dependent Reactions Photosystem II and I are the two light capturing
UNIT 2: Metabolic Processes Chapter 5: Photosynthesis: The Energy of Life pg. 210-240 5.2: Pathways of Photosynthesis pg. 220-228 Light Dependent Reactions Photosystem II and I are the two light capturing
TRANSPORT ACROSS MEMBRANE
 TRANSPORT ACROSS MEMBRANE The plasma membrane functions to isolate the inside of the cell from its environment, but isolation is not complete. A large number of molecules constantly transit between the
TRANSPORT ACROSS MEMBRANE The plasma membrane functions to isolate the inside of the cell from its environment, but isolation is not complete. A large number of molecules constantly transit between the
The SUN Project Tray as Mitochondrion
 The SUN Project Tray as Mitochondrion Ann Batiza, Ph.D. and Mary Gruhl, Ph.D, MATRIX Outer membrane (brown tray) Carbon compound INTERMEMBRANE SPACE Inner membrane (gray tray) Proton Electron ATP ADP P
The SUN Project Tray as Mitochondrion Ann Batiza, Ph.D. and Mary Gruhl, Ph.D, MATRIX Outer membrane (brown tray) Carbon compound INTERMEMBRANE SPACE Inner membrane (gray tray) Proton Electron ATP ADP P
2.A.2- Capture and Storage of Free Energy
 2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
Chapter 5. The Chloroplast. 5.1 Matter and Energy Pathways in Living Systems. Photosynthesis & Cellular Respiration
 Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
4.1 Chemical Energy and ATP. KEY CONCEPT All cells need chemical energy.
 4.1 Chemical Energy and ATP KEY CONCEPT All cells need chemical energy. 4.1 Chemical Energy and ATP The chemical energy used for most cell processes is carried by ATP. Molecules in food store chemical
4.1 Chemical Energy and ATP KEY CONCEPT All cells need chemical energy. 4.1 Chemical Energy and ATP The chemical energy used for most cell processes is carried by ATP. Molecules in food store chemical
I. Enzymes as Catalysts Chapter 4
 8/29/11 I. Enzymes as Catalysts Chapter 4 Enzymes and Energy Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Enzymes Activation Energy A class
8/29/11 I. Enzymes as Catalysts Chapter 4 Enzymes and Energy Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Enzymes Activation Energy A class
Chapter 4: Energy From the sun to you in two easy steps
 Chapter 4: Energy From the sun to you in two easy steps Lectures by Mark Manteuffel, St. Louis Community College Learning Objectives Understand and be able to explain the following: How energy flows from
Chapter 4: Energy From the sun to you in two easy steps Lectures by Mark Manteuffel, St. Louis Community College Learning Objectives Understand and be able to explain the following: How energy flows from
MITOCHONDRIAL LAB. We are alive because we make a lot of ATP and ATP makes (nonspontaneous) chemical reactions take place
 MITOCHONDRIAL LAB We are alive because we make a lot of ATP and ATP makes (nonspontaneous) chemical reactions take place We make about 95% of our ATP in the mitochondria We will isolate mitochondria, and
MITOCHONDRIAL LAB We are alive because we make a lot of ATP and ATP makes (nonspontaneous) chemical reactions take place We make about 95% of our ATP in the mitochondria We will isolate mitochondria, and
Photosynthesis and Cellular Respiration Note-taking Guide
 Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reectlons, light-dependent, Calvin cycle, thylakoid, oxygen, light-harvesting, two, chloroplasts, photosynthesis,
Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reectlons, light-dependent, Calvin cycle, thylakoid, oxygen, light-harvesting, two, chloroplasts, photosynthesis,
