Bioenergetics and high-energy compounds
|
|
|
- Cori Rodgers
- 6 years ago
- Views:
Transcription
1 Bioenergetics and high-energy compounds Tomáš Kučera Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital 2017
2 Bioenergetics how organisms gain, convert, store and utilize energy
3 Gibbs free energy G = H TS G = H T S = Q p T S G decrease in a biological process represents its maximum recoverable work. equilibrium: G = 0 spontaneous (exergonic) process: G < 0 (it can do work) endergonic process: G > 0
4 Gibbs free energy one of the thermodynamic potentials no information on the rate it is given by the mechanism (im-)possibility of a process given only by the initial and final states a catalyst (enzyme) can accelerate equilibrium attainment, not change its state possibility of coupling depends on temperature: equilibrium: T = H S H S G = H T S + Both enthalpically favored (exothermic) and entropically favored. Spontaneous (exergonic) at all temperatures. Enthalpically favored but entropically opposed. Spontaneous only at temperatures below T = H S. + + Enthalpically opposed (endothermic) but entropically favored. Spontaneous only at temperatures above T = H S. + Both enthalpically and entropically opposed. Unspontaneous (endergonic) at all temperatures. Rewritten from Voet, D., Voet, J. G.: Biochemistry, John Wiley & Sons, Inc., 2011 (4th edition)
5 Chemical equilibria Reaction a A + b B c C + d D G = G 0 + RT ln [C]c [D] d [A] a [B] b ( G 0 = standard G change of the reaction) constant term depends only on the reaction variable term depends on temperature and concentrations of reactants and products Equilibrium G = 0 [C] c [D] d K eq = [A] a [B] b G 0 = RT ln K eq = e G 0 RT G 0 and K eq directly related 10-fold change in K eq changes G 0 by 5.7 kj mol 1
6 Free energy changes G 0 = G 0 f (products) G 0 f (reactants) G 0 f = G 0 of formation Compound G 0 f (kj mol 1 ) acetaldehyde acetate acetyl-coa a cis-aconitate C 2 (g) C 2 (aq) HC citrate dihydroxyacetone ethanol fructose fructose-6-phosphate fructose-1,6-bisphosphate fumarate α-d-glucose glucose-6-phosphate Compound G 0 f (kj mol 1 ) glyceraldehyde-3-phosphate H H 2 (g) 0.0 H 2 (l) isocitrate α-ketoglutarate lactate l-malate H oxaloacetate phosphoenolpyruvate phosphoglycerate phosphoglycerate pyruvate succinate succinyl-coa a a for formation from free elements + free CoA Rewritten after Voet, D., Voet, J. G.: Biochemistry, John Wiley & Sons, Inc., 2011 (4th edition)
7 Free energy changes standard state activity 1 mol l 1 25 C 1 bar biochemical standard state water activity = 1 ph = 7 substances undergoing acid-base dissociation: c = total c of all species at ph = 7
8 Coupled reactions A + B C + D G 1 D + E F + G G 2 A + B + E C + F + G G 3 = G 1 + G 2 < 0 Glucose phosphorylation: Glc + ATP Glc-6- P + ADP endergonic reaction: glucose + P glucose-6- P G 0 = 13.8 kj mol 1 exergonic reaction: ATP + H 2 ADP + P G 0 = 30.5 kj mol 1 coupled reaction: glucose + ATP glucose-6- P + ADP G 0 = 16.7 kj mol 1
9 Redox potential also oxidation-reduction (reduction) potential expresses the substance s readiness to accept electrons ox + n e red (half-cell) Voet, D., Voet, J. G.: Biochemistry, John Wiley & Sons, Inc., 2011 (4th edition) A ox + B red n e A red + B ox ernst equation G = G 0 + RT ln [A red][b ox ] [A ox ][B red ] G = nf E E = E 0 RT nf ln [red] [ox] E = E 0 RT nf ln [A red][b ox ] [A ox ][B red ]
10 Redox potential E as an energy scale Reduced form xidized form E 0 (V) ΔG 0 acetaldehyde acetate -0,60 values higher H2 2H + -0,42 (reductant) isocitrate 2-oxoglutarate + C2-0,38 glutathione-sh glutathione-ss -0,34 ADH + H + AD + -0,32 glyceraldehyde-3-phosphate + H3P4 1,3-bisphosphoglycerate -0,28 FADH2 FAD -0,20 lactate pyruvate -0,19 malate oxalacetate -0,17 cytochrome b (Fe 2+ ) cytochrome b (Fe 3+ ) 0,00 succinate fumarate +0,03 dihydroubiquinone ubiquinone +0,10 cytochrome c (Fe 2+ ) cytochrome c (Fe 3+ ) +0,26 +ne ne H ,29 + values H2 ½ 2 +0,82 (oxidant) lower exergonic reaction endergonic reaction Voet, D., Voet, J. G.: Biochemistry, John Wiley & Sons, Inc., 2011 (4th edition) The actual direction of the reaction depends also on the [red]/[ox] ratio (and/or other factors)
11 Redox potential E 0 = 0 V for standard hydrogen half-reaction (electrode) H + at ph0, 25 C, 1 bar in equilibrium with Pt-black electrode saturated with H 2 ph = 7 E 0 = 0.421V
12 High-energy compounds contain high-energy bond hydrolyzed to drive endergonic reactions ATP a central role (universal energy currency of the cell) 3 phosphoryl groups bound by one phosphoester and two phosphoanhydride bonds H 2 P γ phosphoanhydride bonds P β phosphoester bond P α H H H H H H adenosine AMP ADP ATP Redrawn according to Voet, D., Voet, J. G.: Biochemistry, John Wiley & Sons, Inc., 2011 (4th edition)
13 ATP R 1 P + R 2 H R 1 H + R 2 P phosphoryl transfer reaction enormous metabolic significance ATP + H 2 ADP + P G 0 = 30.5 kj mol 1 ATP + H 2 AMP + P P G 0 = 45.6 kj mol 1 P P + H 2 2 P G 0 = 19.2 kj mol 1 kinetic stability, thermodynamic instability (high G 0 ) cell energy charge (usually ) [ATP] [ADP] [ATP] + [ADP] + [AMP] adenylate kinase: ATP + AMP 2 ADP ATP is formed using more exergonic reactions
14 Coupled reactions A B G 0 = 16.7 kj mol 1 [B] [A] = K eq = e G RT 0 = A + ATP + H 2 B + ADP + P + H + at equilibrium: K eq = [B] [A] [ADP][ P ] = [ATP] G 0 = 13.8 kj mol 1 [B] [A] = K [ATP] eq [ADP][ P ] = = the equilibrium B/A ratio is 10 8 times higher! n ATP molecules hydrolyzed the ratio is 10 8n times higher!
15 ATP consumption low-energy phosphorylated compounds TP interconversions formation of CTP, GTP, UTP, datp, dctp, dgtp, dttp nucleoside diphosphate kinase ATP + DP ADP + TP processes based on protein conformational changes protein folding active transport movements
16 ATP ATP formation substrate-level phosphorylation oxidative phosphorylation (photophosphorylation) adenylate kinase reaction phosphagens ATP turnover average adult resting person about 3 mol h 1 (1.5 kg h 1 ), i.e. about 40 kg d 1 strenuous activity up to 0.5 kg min 1
17 High-energy bonds no high-energy bond exists! phosphoanhydrides resonance stabilization higher solvation energy of the hydrolysis products electrostatic repulsion P H P H H P P H + P + R + P Redrawn from Berg, J. M., Tymoczko, Gatto, G. J. Jr., J. L., Stryer, L.: Biochemistry, W. H. Freeman and Company, 2012 (8th edition) other anhydrides phosphosulphates, acylphosphates carbamoylphosphate phosphoguanidines (phosphagens) phosphocreatine, phosphoarginine enol phosphates
18 High-energy compounds there are no high-energy compounds as well! H 2 P P P H H H H H H adenosine triphosphate (ATP) P P H H H H H H adenosine diphosphate (ADP) H 2 H 2C C P acetylphosphate (acylphosphates) phosphocreatine (phosphamides) H 2C P C C P phosphoenolpyruvate (enolphosphates) acetylcoenzyme A (thioesters) H C H + 2 CH 3 CH 2 CoA S CCH 3 C
19 Energy metabolism scheme amino acids fatty acids β-oxidation sugars glycolysis pyruvate alternative pathways ADH AD + ADH AD + fermentative AD + regeneration lactate ethanol propionate butyrate butanol formate H2 C2 acetate 2,3-butandiol succinate oxidative decarboxylation citric acid cycle Ac~S CoA Calvin cycle C 2 ADH AD + ADPH ADP + ADP respiratory chain 2 photosynthetic electron transport chain hν ADP ATP oxidative phosphorylation H 2 photophosphorylation ATP
20 The End konec the end Thank you for your attention!
21 Gibbs free energy depends on temperature: equilibrium: T = H S G = H T S + Both enthalpically favored (exothermic) and entropically favored. Spontaneous (exergonic) at all temperatures. Enthalpically favored but entropically opposed. Spontaneous only at temperatures below T = H S. + + Enthalpically opposed (endothermic) but entropically favored. Spontaneous only at temperatures above T = H S. + Both enthalpically and entropically opposed. Unspontaneous (endergonic) at all temperatures.
22 Free energy changes Compound G 0 f (kj mol 1 ) acetaldehyde acetate acetyl-coa a cis-aconitate C 2 (g) C 2 (aq) HC citrate dihydroxyacetone ethanol fructose fructose-6-phosphate fructose-1,6-bisphosphate fumarate α-d-glucose glucose-6-phosphate Com glyc H + H 2 H 2 isoc α-k lact l-m H oxa pho 2-ph 3-ph pyr suc suc a for f
23 Compound G 0 f (kj mol 1 ) glyceraldehyde-3-phosphate H H 2 (g) 0.0 H 2 (l) isocitrate α-ketoglutarate lactate l-malate H oxaloacetate phosphoenolpyruvate phosphoglycerate phosphoglycerate pyruvate succinate succinyl-coa a a for formation from free elements + free CoA.: Biochemistry, John Wiley & Sons, Inc., 2011 (4th edition)
24 High-energy compounds hydride bonds H 2 phosphoanhydride bonds phosphoester bond P γ P β P α H H H ADP ATP H H H adenosine AMP
25 High-energy bonds electrostatic repulsion P H P H H P H + P other anhydrides phosphosulphates, acylphosphates carbamoylphosphate phosphoguanidines (phosphagens) phos phosphoarginine
26 R P + + P edrawn from Berg, J. M., Tymoczko, Gatto,. J. Jr., J. L., Stryer, L.: Biochemistry, W. H. eeman and Company, 2012 (8th edition)
27
28 Reduced form xidized form E 0 (V) ΔG 0 acetaldehyde acetate -0,60 values higher H 2 2H + -0,42 (reductant) isocitrate 2-oxoglutarate + C 2-0,38 glutathione-sh glutathione-ss -0,34 ADH + H + AD + -0,32 glyceraldehyde-3-phosphate + H 3P 4 1,3-bisphosphoglycerate -0,28 FADH 2 FAD -0,20 lactate pyruvate -0,19 malate oxalacetate -0,17 cytochrome b (Fe 2+ ) cytochrome b (Fe 3+ ) 0,00 succinate fumarate +0,03 dihydroubiquinone ubiquinone +0,10 cytochrome c (Fe 2+ ) cytochrome c (Fe 3+ ) +0,26 +ne ne H ,29 + values H 2 ½ 2 +0,82 (oxidant) lower exergonic reaction endergonic reaction
29 amino acids fatty acids β-oxidation sugars glycolysis pyruvate alternative pathways ADH AD + ADH AD + fermentative AD + regeneration lactate ethanol propionate butyrate butanol formate H2 C2 acetate 2,3-butandiol succinate oxidative decarboxylation citric acid cycle Ac~S CoA Calvin cycle C 2 ADH AD + ADPH ADP + ADP respiratory chain 2 photosynthetic electron transport chain hν ADP ATP oxidative phosphorylation H 2 photophosphorylation ATP
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Energy in Chemical and Biochemical Reactions
 Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
Free Energy. because H is negative doesn't mean that G will be negative and just because S is positive doesn't mean that G will be negative.
 Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Department of Chemistry and Biochemistry University of Lethbridge. Biochemistry II. Bioenergetics
 Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
BIOLOGICAL SCIENCE. Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge. FIFTH EDITION Freeman Quillin Allison
 BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially
 Bioenergetics Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to
Bioenergetics Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to
CHEM-E3215 Advanced Biochemistry
 CHEM-E3215 Advanced Biochemistry 30. Jan. 2018 Prof. Silvan Scheller Lecture 10 Energy conservation general (some calculations) Energy conservation in anaerobes: e.g. methanogensis Life close to the thermodynamic
CHEM-E3215 Advanced Biochemistry 30. Jan. 2018 Prof. Silvan Scheller Lecture 10 Energy conservation general (some calculations) Energy conservation in anaerobes: e.g. methanogensis Life close to the thermodynamic
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
CHAPTER 15 Metabolism: Basic Concepts and Design
 CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
BIS Office Hours
 BIS103-001 001 ffice ours TUE (2-3 pm) Rebecca Shipman WED (9:30-10:30 am) TUE (12-1 pm) Stephen Abreu TUR (12-1 pm) FRI (9-11 am) Steffen Abel Lecture 2 Topics Finish discussion of thermodynamics (ΔG,
BIS103-001 001 ffice ours TUE (2-3 pm) Rebecca Shipman WED (9:30-10:30 am) TUE (12-1 pm) Stephen Abreu TUR (12-1 pm) FRI (9-11 am) Steffen Abel Lecture 2 Topics Finish discussion of thermodynamics (ΔG,
Activity: Identifying forms of energy
 Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Lecture 10. Proton Gradient-dependent ATP Synthesis. Oxidative. Photo-Phosphorylation
 Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lecture 2: Biological Thermodynamics [PDF] Key Concepts
![Lecture 2: Biological Thermodynamics [PDF] Key Concepts Lecture 2: Biological Thermodynamics [PDF] Key Concepts](/thumbs/76/73872045.jpg) Lecture 2: Biological Thermodynamics [PDF] Reading: Berg, Tymoczko & Stryer: pp. 11-14; pp. 208-210 problems in textbook: chapter 1, pp. 23-24, #4; and thermodynamics practice problems [PDF] Updated on:
Lecture 2: Biological Thermodynamics [PDF] Reading: Berg, Tymoczko & Stryer: pp. 11-14; pp. 208-210 problems in textbook: chapter 1, pp. 23-24, #4; and thermodynamics practice problems [PDF] Updated on:
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Outer Glycolysis mitochondrial membrane Glucose ATP
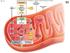 Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Enzymes I. Dr. Mamoun Ahram Summer semester,
 Enzymes I Dr. Mamoun Ahram Summer semester, 2017-2018 Resources Mark's Basic Medical Biochemistry Other resources NCBI Bookshelf: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=books The Medical Biochemistry
Enzymes I Dr. Mamoun Ahram Summer semester, 2017-2018 Resources Mark's Basic Medical Biochemistry Other resources NCBI Bookshelf: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=books The Medical Biochemistry
Thermodynamics of Biological Systems ...
 Chapter 3 Thermodynamics of Biological Systems........................ Chapter utline v Thermodynamic concepts Systems Isolated systems cannot exchange matter or energy with surroundings Closed systems
Chapter 3 Thermodynamics of Biological Systems........................ Chapter utline v Thermodynamic concepts Systems Isolated systems cannot exchange matter or energy with surroundings Closed systems
This is an example of cellular respiration, which can be used to make beer and wine using different metabolic pathways For these reasons we call this
 Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
- BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU
 - BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU Bioenergetics the field of biochemistry concerned with the transfer and use of energy by biological system BIOLOGICAL IMPORTANCE: Suitable fuel
- BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU Bioenergetics the field of biochemistry concerned with the transfer and use of energy by biological system BIOLOGICAL IMPORTANCE: Suitable fuel
Biochemistry 3300 Problems (and Solutions) Metabolism I
 (1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes
(1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes
Change to Office Hours this Friday and next Monday. Tomorrow (Abel): 8:30 10:30 am. Monday (Katrina): Cancelled (05/04)
 Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová
 Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Review Questions - Lecture 5: Metabolism, Part 1
 Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Be sure to understand:
 Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
3.1 Metabolism and Energy
 3.1 Metabolism and Energy Metabolism All of the chemical reactions in a cell To transform matter and energy Step-by-step sequences metabolic pathways Metabolic Pathways Anabolic reactions Build large molecules
3.1 Metabolism and Energy Metabolism All of the chemical reactions in a cell To transform matter and energy Step-by-step sequences metabolic pathways Metabolic Pathways Anabolic reactions Build large molecules
Metabolism and enzymes
 Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Kuntarti. PDF Created with deskpdf PDF Writer - Trial ::
 Kuntarti Principles of Bioenergetics Definitions the study of energy transformations in living organism (Albert L Lehninger) the study of the balance between energy intake in the form of food and energy
Kuntarti Principles of Bioenergetics Definitions the study of energy transformations in living organism (Albert L Lehninger) the study of the balance between energy intake in the form of food and energy
Applications of Free Energy. NC State University
 Chemistry 433 Lecture 15 Applications of Free Energy NC State University Thermodynamics of glycolysis Reaction kj/mol D-glucose + ATP D-glucose-6-phosphate + ADP ΔG o = -16.7 D-glucose-6-phosphate p D-fructose-6-phosphate
Chemistry 433 Lecture 15 Applications of Free Energy NC State University Thermodynamics of glycolysis Reaction kj/mol D-glucose + ATP D-glucose-6-phosphate + ADP ΔG o = -16.7 D-glucose-6-phosphate p D-fructose-6-phosphate
Overview of Metabolism and Bioenergetics!
 verview of Metabolism and Bioenergetics! Wichit Suthammarak Department of Biochemistry, Faculty of Medicine Siriraj Hospital -- July 30 th, 2014! Metabolism Chemical transformation! Cell or organism! A
verview of Metabolism and Bioenergetics! Wichit Suthammarak Department of Biochemistry, Faculty of Medicine Siriraj Hospital -- July 30 th, 2014! Metabolism Chemical transformation! Cell or organism! A
Chapter 13 Principles of Bioenergetics
 Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
Biochemical Pathways
 Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Microbiology II Microbial physiology I Energetics
 Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses
Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS
 Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS IN THE GRAND SCHEME The purpose of this workshop is to get the Learning Communities to begin thinking about why certain chemical
Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS IN THE GRAND SCHEME The purpose of this workshop is to get the Learning Communities to begin thinking about why certain chemical
I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI.
 Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Lecture 7: Enzymes and Energetics
 Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
Chapter 6- An Introduction to Metabolism*
 Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
1 THE LAWS OF THERMODYNAMICS CONCEPT OF CHEMICAL EQUILIBRIUM
 1 THE LAWS OF THERMODYNAMICS CONCEPT OF CHEMICAL EQUILIBRIUM 1.1. THE LAWS OF THERMODYNAMICS Living organisms are centres of numerous transformations during which energy is converted from one form to another.
1 THE LAWS OF THERMODYNAMICS CONCEPT OF CHEMICAL EQUILIBRIUM 1.1. THE LAWS OF THERMODYNAMICS Living organisms are centres of numerous transformations during which energy is converted from one form to another.
Energy Transformation, Cellular Energy & Enzymes (Outline)
 Energy Transformation, Cellular Energy & Enzymes (Outline) Energy conversions and recycling of matter in the ecosystem. Forms of energy: potential and kinetic energy The two laws of thermodynamic and definitions
Energy Transformation, Cellular Energy & Enzymes (Outline) Energy conversions and recycling of matter in the ecosystem. Forms of energy: potential and kinetic energy The two laws of thermodynamic and definitions
Outline. Metabolism: Energy and Enzymes. Forms of Energy. Chapter 6
 Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 107 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Chapter 6: Energy Flow in the Life of a Cell
 Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Flow of Energy. Flow of Energy. Energy and Metabolism. Chapter 6
 Energy and Metabolism Chapter 6 Flow of Energy Energy: the capacity to do work -kinetic energy: the energy of motion -potential energy: stored energy Energy can take many forms: mechanical electric current
Energy and Metabolism Chapter 6 Flow of Energy Energy: the capacity to do work -kinetic energy: the energy of motion -potential energy: stored energy Energy can take many forms: mechanical electric current
Metabolism and Enzymes
 Energy Basics Metabolism and Enzymes Chapter 5 Pgs. 77 86 Chapter 8 Pgs. 142 162 Energy is the capacity to cause change, and is required to do work. Very difficult to define quantity. Two types of energy:
Energy Basics Metabolism and Enzymes Chapter 5 Pgs. 77 86 Chapter 8 Pgs. 142 162 Energy is the capacity to cause change, and is required to do work. Very difficult to define quantity. Two types of energy:
CHAPTER 8. An Introduction to Metabolism
 CHAPTER 8 An Introduction to Metabolism WHAT YOU NEED TO KNOW: Examples of endergonic and exergonic reactions. The key role of ATP in energy coupling. That enzymes work by lowering the energy of activation.
CHAPTER 8 An Introduction to Metabolism WHAT YOU NEED TO KNOW: Examples of endergonic and exergonic reactions. The key role of ATP in energy coupling. That enzymes work by lowering the energy of activation.
An Introduction to Metabolism
 Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
AN INTRODUCTION TO METABOLISM. Metabolism, Energy, and Life
 AN INTRODUCTION TO METABOLISM Metabolism, Energy, and Life 1. The chemistry of life is organized into metabolic pathways 2. Organisms transform energy 3. The energy transformations of life are subject
AN INTRODUCTION TO METABOLISM Metabolism, Energy, and Life 1. The chemistry of life is organized into metabolic pathways 2. Organisms transform energy 3. The energy transformations of life are subject
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Chapter 8: An Introduction to Metabolism. 1. Energy & Chemical Reactions 2. ATP 3. Enzymes & Metabolic Pathways
 Chapter 8: An Introduction to Metabolism 1. Energy & Chemical Reactions 2. ATP 3. Enzymes & Metabolic Pathways 1. Energy & Chemical Reactions 2 Basic Forms of Energy Kinetic Energy (KE) energy in motion
Chapter 8: An Introduction to Metabolism 1. Energy & Chemical Reactions 2. ATP 3. Enzymes & Metabolic Pathways 1. Energy & Chemical Reactions 2 Basic Forms of Energy Kinetic Energy (KE) energy in motion
Lecture 21 - Introduction to Metabolism: Bioenergetics
 Lecture 21 - Introduction to Metabolism: Bioenergetics Key Concepts Energy conversion in biological systems Metabolic redox reactions Review of thermodynamic principles and coupled reactions The adenylate
Lecture 21 - Introduction to Metabolism: Bioenergetics Key Concepts Energy conversion in biological systems Metabolic redox reactions Review of thermodynamic principles and coupled reactions The adenylate
An Introduction to Metabolism
 An Introduction to Metabolism Chapter 8 Objectives Distinguish between the following pairs of terms: catabolic and anabolic pathways; kinetic and potential energy; open and closed systems; exergonic and
An Introduction to Metabolism Chapter 8 Objectives Distinguish between the following pairs of terms: catabolic and anabolic pathways; kinetic and potential energy; open and closed systems; exergonic and
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
2.A.2- Capture and Storage of Free Energy
 2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
Chapter 2 Energy in Biology Demand and Use
 Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Energy Transformation and Metabolism (Outline)
 Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Chapter 8 Notes. An Introduction to Metabolism
 Chapter 8 Notes An Introduction to Metabolism Describe how allosteric regulators may inhibit or stimulate the activity of an enzyme. Objectives Distinguish between the following pairs of terms: catabolic
Chapter 8 Notes An Introduction to Metabolism Describe how allosteric regulators may inhibit or stimulate the activity of an enzyme. Objectives Distinguish between the following pairs of terms: catabolic
Cell Energy Notes ATP THE ENDOSYMBIOTIC THEORY. CELL ENERGY Cells usable source of is called ATP stands for. Name Per
 Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Thermodynamics is the study of energy and its effects on matter
 00Note Set 3 1 THE ENERGETICS OF LIFE Thermodynamics and Bioenergetics: Thermodynamics is the study of energy and its effects on matter Bioenergetics is the quantitative analysis of how organisms gain
00Note Set 3 1 THE ENERGETICS OF LIFE Thermodynamics and Bioenergetics: Thermodynamics is the study of energy and its effects on matter Bioenergetics is the quantitative analysis of how organisms gain
Welcome to Class 8! Introductory Biochemistry! Announcements / Reminders! Midterm TA led Review Sessions!
 Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
The altercation described above is responsible for the formation of what critical biological structure?
 LAUGH to release tension and get points!!!! Extra Bonus +2 The altercation described above is responsible for the formation of what critical biological structure? This hydrophobic interaction is the basis
LAUGH to release tension and get points!!!! Extra Bonus +2 The altercation described above is responsible for the formation of what critical biological structure? This hydrophobic interaction is the basis
Energy. Energy & Laws of Thermodynamics. Energy - Outline. Energy - the capacity to do work
 http://www.biotopics.co.uk/jmolapplet/atpjdisplay.htm - utline Flow of in living organism otential energy and kinetic energy Laws of Thermodynamics and energy transformations Biochemical pathways and energy
http://www.biotopics.co.uk/jmolapplet/atpjdisplay.htm - utline Flow of in living organism otential energy and kinetic energy Laws of Thermodynamics and energy transformations Biochemical pathways and energy
Energy for biological processes
 1 Energy transfer When you have finished revising this topic, you should: be able to explain the difference between catabolic and anabolic reactions be able to describe the part played by in cell metabolism
1 Energy transfer When you have finished revising this topic, you should: be able to explain the difference between catabolic and anabolic reactions be able to describe the part played by in cell metabolism
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
f) Adding an enzyme does not change the Gibbs free energy. It only increases the rate of the reaction by lowering the activation energy.
 Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Lecture #14. Chapter 17 Free Energy and Equilibrium Constants
 Lecture #14 Chapter 17 Free Energy and Equilibrium Constants Josiah W. Gibbs 1839-1903 Gibbs Free Energy (G) The maximum energy released by a system occurring at constant temperature and pressure that
Lecture #14 Chapter 17 Free Energy and Equilibrium Constants Josiah W. Gibbs 1839-1903 Gibbs Free Energy (G) The maximum energy released by a system occurring at constant temperature and pressure that
Lecture 3: Thermodynamics
 3 LAWS OF THERMODYNAMICS Lecture 3: Thermodynamics Matter and energy are conserved Margaret A. Daugherty Fall 2004 Entropy always increases Absolute zero is unattainable System and Surroundings 1st Law
3 LAWS OF THERMODYNAMICS Lecture 3: Thermodynamics Matter and energy are conserved Margaret A. Daugherty Fall 2004 Entropy always increases Absolute zero is unattainable System and Surroundings 1st Law
Unit 3: Cellular Energetics Guided Reading Questions (50 pts total)
 AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
Chapter 9 in Chang Text
 Section 8.0: CHEMICAL EQUILIBRIUM Chapter 9 in Chang Text ..the direction of spontaneous change at constant T and P is towards lower values of Gibbs Energy (G). this also applies to chemical reactions
Section 8.0: CHEMICAL EQUILIBRIUM Chapter 9 in Chang Text ..the direction of spontaneous change at constant T and P is towards lower values of Gibbs Energy (G). this also applies to chemical reactions
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
Chapter 8. Biology. Energy Processing. Metabolism & ATP. Slide 1 / 142 Slide 2 / 142. Slide 3 / 142. Slide 4 / 142. Slide 5 / 142.
 Slide 1 / 142 Slide 2 / 142 Biology Energy Processing www.njctl.org Acetyl Co-A aerobic anabolic pathway anaerobic synthase Calvin Cycle catabolic pathway cellular respiration chlorophyll citric acid cycle
Slide 1 / 142 Slide 2 / 142 Biology Energy Processing www.njctl.org Acetyl Co-A aerobic anabolic pathway anaerobic synthase Calvin Cycle catabolic pathway cellular respiration chlorophyll citric acid cycle
1 of 8 Class notes lectures 6a, b, c
 1 of 8 Class notes lectures 6a, b, c Last time: 1) entropy calculations 2) Gibb s Free energy Today: Field Trip READ CHAPT 15.11 before coming to field trip. Be prepared to ask questions and take notes.
1 of 8 Class notes lectures 6a, b, c Last time: 1) entropy calculations 2) Gibb s Free energy Today: Field Trip READ CHAPT 15.11 before coming to field trip. Be prepared to ask questions and take notes.
*The entropy of a system may decrease, but the entropy of the system plus its surroundings must always increase
 AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
An Introduction to Metabolism
 An Introduction to Metabolism The living cell is a microscopic factory where life s giant processes can be performed: -sugars to amino acids to proteins and vise versa -reactions to dismantle polymers
An Introduction to Metabolism The living cell is a microscopic factory where life s giant processes can be performed: -sugars to amino acids to proteins and vise versa -reactions to dismantle polymers
All organisms require a constant expenditure of energy to maintain the living state - "LIFE".
 CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
MitoSeminar II: Some calculations in bioenergetics
 MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
An Introduction to Metabolism. Chapter 8
 An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
An Introduction to Metabolism
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism
Objectives INTRODUCTION TO METABOLISM. Metabolism. Catabolic Pathways. Anabolic Pathways 3/6/2011. How to Read a Chemical Equation
 Objectives INTRODUCTION TO METABOLISM. Chapter 8 Metabolism, Energy, and Life Explain the role of catabolic and anabolic pathways in cell metabolism Distinguish between kinetic and potential energy Distinguish
Objectives INTRODUCTION TO METABOLISM. Chapter 8 Metabolism, Energy, and Life Explain the role of catabolic and anabolic pathways in cell metabolism Distinguish between kinetic and potential energy Distinguish
Bio102 Problems Photosynthesis
 Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
