Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
|
|
|
- Lindsey Woods
- 6 years ago
- Views:
Transcription
1 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids, membranes, organelles..! Photosynthetic autotrophs use the energy of sunlight to convert lowg C 2 and H 2 into energyrich complex sugar molecules. 6C 2 + 6H 2 à (CH 2 ) This reaction has a large positive ΔH and a large negative ΔS. The products have more enthalpy and are more ordered than the reactants. Heterotrophs extract the chemical potential energy stored in sugars and other organic compounds and release C 2 and H 2. (CH 2 ) à 6C 2 + 6H 2 1
2 This reaction has a large negative ΔH and a large positive ΔS. The products have lost energy and are less ordered. The G is released slowly in many steps using several metabolic pathways. For any (bio)chemical reaction recall that ΔG = RT ln(k eq ) where ΔG = G P G S R = Gas Constant = 8.31 J / mol. K. T = Temp in K. Under standard conditions: ΔG o = RT ln(k eq ) o refers to 25 o C, 55 M H 2, [reactant] = 1M Biochemists prefer ph 7 to ph 0 where [H + ] = 1M ΔG 'o = RT ln(k ' eq) where ' refers to ph = 7. RT ln(k ' eq) If K' eq = 19 at 25 o C then, = (8.315 J/mol K)(298 K)(ln19) = 7,296 J/mol = 7.3 kj/mol If reactants and products are present at 1M we can predict the direction of the reaction: K ' eq ΔG 'o Direction > 1 negative Forward < 1 positive Reverse = 1 0 2
3 When ΔG 'o is negative, the products contain less G than the reactants. ΔG 'o < 0 ΔG 'o > 0 R P G P R Reaction! When ΔG 'o is positive the products contain more G than the reactants. For a chemical reaction at equilibrium, the rates of the forward and reverse reactions are equal and no net change is occurring but no work can be done and ΔG = 0. For this reaction, Glc1P phosphoglucomutase Glc6P K ' eq = [Glc6P] = 19 ΔG'o [Glc1P] = 7.3 kj/mol When [Glc6P] = [Glc1P] = 1 M, the reaction will spontaneously convert Glc1P into Glc6P until equilibrium is established. Glc1P Glc6P 3
4 What if we add 1 mm to 100 mm? The reaction will convert Glc6P into Glc1P to reestablish equilibrium. Glc1P Glc6P So the actual ΔG under nonstandard conditions is positive. The actual ΔG under nonstandard conditions for: A + B C + D is ΔG = ΔG 'o + RT ln [C][D] [A][B] where the concentrations are actual. Notes: 1. Both ΔG and ΔG 'o are theoretical maxima. Some G is always lost as heat. 2. Even if ΔG 'o is positive, the reaction can go forward if ΔG is negative. i.e. if the second term is negative and bigger than ΔG 'o. 3. ΔG s of sequential reactions are additive because ΔG is pathindependent. For example, ΔG'o Glc1P + H 2 Glc + P i kj Glc + P i Glc6P + H kj Glc1P Glc6P 7.3 kj! 4
5 The G released in 1 reaction can be used to drive a 2 nd reaction as long as the two share a common intermediate. E.g. Glc Nearly all of the G stored in carbohydrates, lipids, and AA is temporarily stored in ATP, the cell s energy currency, for use in building cell constituents and doing cellular work such as muscle contraction and active transport of molecules against concentration gradients. So ATP is the common intermediate between catabolism and anabolism. ATP is not an energy storage molecule. Catabolism ADP + P i ATP Anabolism The synthesis of ATP is a highly endergonic reaction and the hydrolysis of ATP is a highly exergonic reaction. ATP + H 2 ADP + P i ΔG 'o = 30 kj / mole How does ATP store chemical potential energy or why does hydrolysis release this energy? There are 4 parts to the answer: 1. Relief of charge repulsion. ne molecule with 4 negative charges is converted into 2 molecules with 2 negative charges each. 5
6 P P P H 1 H Rib Adenine P H + H P P Rib Adenine [ ]3 P H H + + P P Rib Adenine 2. There are more resonance forms of ADP + P i than of ATP, so there is an entropy increase due to hydrolysis. P P P P 3. At the usual ph of cells, ADP ionizes and LeChatelier s Principle pulls the reaction forward. H +! 4. Solvation of ADP + P i > the solvation of ATP which lowers the G of the products relative to the reactants. 6
7 Notes: 1. In cells, Mg ATP 2 and Mg ADP are present. Hydrolysis of Mg ATP 2 has a different ΔG 'o than ATP In cells, [ATP] ~ 2.25 mm; [ADP] ~ 0.25 mm; [P i ] ~ 1.65 mm These concentrations are far from standard. So actual ΔG = 50 to 65 kj / mole. ther compounds have large ΔG of hydrolysis for reasons similar to ATP: 1. 1,3 bisphosphoglycerate P C C HCH + H 2 + P HCH i CH 2 P CH 2 P 1,3 bisphosphoglycerate 3phosphoglycerate ΔG'o = 49 kj/mol 7
8 2. C C P CH 2 Phosphoenolpyruvate + H 2 C C + P i CH 3 Pyruvate ΔG'o = 62 kj/mol There is more than enough G released in the above two reactions to form ATP from ADP + P i.! 1. So cells use highg compounds to make ATP. This is called: Substrate Level Phosphorylation. Two other processes for regenerating ATP are: 2. Photophosphorylation in chloroplasts. 3. xidative phosphorylation in mitochondria. How are reactions coupled? For e.g. how can ATP hydrolysis be used to convert Glu + NH 3 à Gln, G an endergonic process? Glu C NH 2 Glu C! 8
9 1. The phosphate from ATP is transferred to an enzyme or to a substrate which becomes activated. In this case, the G of Glu is raised by phosphorylation. ATP Glu C P NH 3 P i G Glu C NH 2 ADP Glu C 2. The reaction is completed by displacement of the P i by NH 3 +, which is a downhill Greleasing reaction. If ATP has so much G, why does it not spontaneously decompose? 9
10 The reaction is spontaneous, but very slow because it has a very high activation energy ΔG. So enzymes lower the activation energy and allow the G release. ther nucleoside triphosphates are energetically equivalent to ATP and are also used by cells. e.g. GTP These are usually made by the following reactions catalysed by nucleoside diphosphate kinases: ATP + NDP ADP + NTP ΔG' o = 0 The following reactions release about the same amount of G as ATP hydrolysis and also can be used as energy currency : ADP + H 2 à AMP + P i ATP + H 2 à AMP + PP i ΔG' o ~ 33 kj/mole ΔG' o ~ 33 kj/mole 10
11 P H 2 P + P H + H P PP i is inorganic pyrophosphate. ΔG' o ~ 29 kj/mole! Another type of energy storage is provided by thioester bonds. By comparison, oxygen esters are resonance stabilized, and thus store less G than thioesters. E.g. Coenzyme A 0 CH 3 C S CoA G H H CoA SH + CH 3 C H H δ CH 3 C CH 3 C CH 3 CH 3 δ + + CH H 3 H kj/mol 11
12 Electron flow can also do work. 1. Cu 2+ has a higher electron affinity than Zn 2+, so when Zn is placed in a copper sulphate solution, electrons flow spontaneously from the Zn to the Cu 2+ : Zn Zn e 1 Cu e 1 Cu The Zn metal erodes and Zn 2+ goes into the solution. Cu deposits on the Zn. Cu 2+ S 2 4 is blue. As Cu 2+ is converted into Cu, the blue colour of the solution fades. The zinc is oxidized (loses electrons) and the copper is reduced (gains electrons). verall, the Redox reaction is: Cu +2 + Zn Cu + Zn +2 By separating the two half reactions, and permitting the electrons to flow through a conductor, the G released can be used to do work.! 12
13 The electrons fall down the G hill from Zn to Cu. G e 1 Zn e 1 Cu 2. A second type of electron flow involves 2. C 6 H C 2 + H 2 + 2,840 kj / mole! 2 has a higher electron affinity than glucose, so electrons flow from glucose to 2 releasing G. The C is oxidized and the is reduced. In 2, bonding e 1 are shared equally by the 2. In C 2 and H 2 the electronegative pulls e 1 away from C and H. In C 2, the C s have lost a share of the e 1 they had in glucose. In living cells, this oxidation is a multistep process involving glycolysis, tricarboxylic acid cycle, and mitochondrial respiration. At various points, e 1 are transferred to electron carriers, and then to 2. 13
14 The electron flow is used to do the work of making ATP. 1. A watersoluble, diffusible electron carrier is: NAD + Nicotinamide Adenine Dinucleotide. Nicotinamide is also known as Niacin and Vitamin B6. NAD + = Adenine1'Ribose5'PP5'Ribose1'Nicotinamide Electrons are transferred in the form of H, a hydride ion. For e.g., the enzyme malate dehydrogenase removes electrons from Lmalate producing oxaloacetate and reducing NAD + to NADH. H C CH NAD + + NADH + H C C CH 2 C CH 2 C LMalate xaloacetate! Malate is dehydrogenated and oxidized. It loses 2 electrons in the form of 2H. 14
15 NAD + accepts H and is reduced. A proton is released. ver 200 dehydrogenase enzymes are known that use NAD Two watersoluble, covalentlybound electron carriers are: FMN, Flavine Mononucleotide and FAD, Flavine Adenine Dinucleotide FAD + 2H à FADH 2 Here, 1 or 2 H are transferre yellow H 3 C H 3 C red/blue intermediates N N CH 2 R 2H + N + NH 2e Flavin + Ribitol = Riboflavin = Vit B 2 colourless FMN = Riboflavin + phosphate H 3 C H 3 C H N NH N N CH 2 H R! 15
16 The relative affinity of an atom or molecule for electrons can be measured. The reference point is a solution containing: H 2 at 101 kpa + 1 M H + (ph = 0) 1/2 H 2 H + + e If the reference cell and test cell both contain this solution, no electrons will flow so the electromotive force, emf, will be 0 Volts. If 1 M Fe 3+ + Fe 2+ are in one cell, Fe 3+ will draw electrons from H 2 and the emf will be Volts. This is called the standard reduction potential, E o. Fe 3+ + e Fe 2+ 1/2 H 2 H + + e The net reaction is: 1/2 H 2 + Fe 3+ H + + Fe 2+ 16
17 If the iron is replaced by 1 M NADH + NAD +, e will flow from the test cell to the reference cell. H + will draw e from NADH: NADH NAD + + H + + 2e 2H + + 2e H 2 E o = emf = 0.32 Volts For biochemists, the test cells are always kept at ph 7 and reported as: E 'o A Table of E 'o values, where the half reactions are written as reductions, can be used to predict the directions of Redox reactions. 17
18 The more positive is the E 'o (bottom of the Table) the stronger is the oxidizing agent. i.e. The reactions go as written in the Table. In combining pairs of half reactions the more positive E 'o reactions are written as reductions (e on the left) and the more negative E 'o are written as oxidations (e on the right). e.g. Acetaldehyde / NAD + From the Table: Acetaldehyde + 2H + + 2e Ethanol E 'o = V NAD + + 2e + H + NADH E 'o = 0.32 V So the coupled reaction is: NADH NAD + + 2e + H + Acetaldehyde + 2H + + 2e Ethanol Acetaldehyde + H + + NADH Ethanol + NAD + ΔE 'o = V 0.32 V = V (acceptor donor) 18
19 Another way to do this is to reverse the sign of the E 'o value for the reaction that is written as an oxidation, and then add the E 'o values. NADH NAD + + 2e + H + E 'o = Acetaldehyde + 2H + + 2e Ethanol E 'o = Acetaldehyde + H + + NADH Ethanol + NAD + ΔE 'o = V + ( V) = V ΔE 'o = V + ( V) = V 19
20 For any pair of 1/2 reactions the ΔG can be calculated from the difference in emf: ΔG 'o = nfδe 'o n = # of moles of e transferred F = Faraday constant = kj / Volt mole ΔG 'o = 2 (96.5) (0.123) = 23.7 kj / mole Under nonstandard conditions: E = E 'o + RT ln [electron acceptor] nf [electron donor] Remember, ΔE 'o = E 'o acceptor E 'o donor 20
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
Department of Chemistry and Biochemistry University of Lethbridge. Biochemistry II. Bioenergetics
 Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
CHAPTER 15 Metabolism: Basic Concepts and Design
 CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
This is an example of cellular respiration, which can be used to make beer and wine using different metabolic pathways For these reasons we call this
 Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially
 Bioenergetics Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to
Bioenergetics Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Chapter 13 Principles of Bioenergetics
 Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
Lecture 7: Enzymes and Energetics
 Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Activity: Identifying forms of energy
 Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Welcome to Class 8! Introductory Biochemistry! Announcements / Reminders! Midterm TA led Review Sessions!
 Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
Biochemical Pathways
 Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Overview of Metabolism and Bioenergetics!
 verview of Metabolism and Bioenergetics! Wichit Suthammarak Department of Biochemistry, Faculty of Medicine Siriraj Hospital -- July 30 th, 2014! Metabolism Chemical transformation! Cell or organism! A
verview of Metabolism and Bioenergetics! Wichit Suthammarak Department of Biochemistry, Faculty of Medicine Siriraj Hospital -- July 30 th, 2014! Metabolism Chemical transformation! Cell or organism! A
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
AHL Topic 8 IB Biology Miss Werba
 CELL RESPIRATION & PHOTOSYNTHESIS AHL Topic 8 IB Biology Miss Werba TOPIC 8 CELL RESPIRATION & PHOTOSYNTHESIS 8.1 CELL RESPIRATION 1. STATE that oxidation involves the loss of electrons from an element,
CELL RESPIRATION & PHOTOSYNTHESIS AHL Topic 8 IB Biology Miss Werba TOPIC 8 CELL RESPIRATION & PHOTOSYNTHESIS 8.1 CELL RESPIRATION 1. STATE that oxidation involves the loss of electrons from an element,
Section A: The Principles of Energy Harvest
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
Be sure to understand:
 Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Bioenergetics and high-energy compounds
 Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Lecture 21 - Introduction to Metabolism: Bioenergetics
 Lecture 21 - Introduction to Metabolism: Bioenergetics Key Concepts Energy conversion in biological systems Metabolic redox reactions Review of thermodynamic principles and coupled reactions The adenylate
Lecture 21 - Introduction to Metabolism: Bioenergetics Key Concepts Energy conversion in biological systems Metabolic redox reactions Review of thermodynamic principles and coupled reactions The adenylate
I. Enzymes as Catalysts Chapter 4
 8/29/11 I. Enzymes as Catalysts Chapter 4 Enzymes and Energy Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Enzymes Activation Energy A class
8/29/11 I. Enzymes as Catalysts Chapter 4 Enzymes and Energy Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Enzymes Activation Energy A class
Energy in Chemical and Biochemical Reactions
 Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI.
 Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Outline. Metabolism: Energy and Enzymes. Forms of Energy. Chapter 6
 Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
Respiration and Photosynthesis
 Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Chapter 6. Ground Rules Of Metabolism
 Chapter 6 Ground Rules Of Metabolism Alcohol Dehydrogenase An enzyme Breaks down ethanol and other toxic alcohols Allows humans to drink Metabolism Is the totality of an organism s chemical reactions Arises
Chapter 6 Ground Rules Of Metabolism Alcohol Dehydrogenase An enzyme Breaks down ethanol and other toxic alcohols Allows humans to drink Metabolism Is the totality of an organism s chemical reactions Arises
Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS
 Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS IN THE GRAND SCHEME The purpose of this workshop is to get the Learning Communities to begin thinking about why certain chemical
Dr. Mallery Biology 150 Workshop Fall Semester ENERGY and METABOLISM ANSWERS IN THE GRAND SCHEME The purpose of this workshop is to get the Learning Communities to begin thinking about why certain chemical
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
Cell Energy: The Big Picture. So, What Exactly is ATP. Adenosine Triphosphate. Your turn to Practice converting ATP to ADP:
 Understanding How Living Things Obtain and Use Energy. Cell Energy: The Big Picture Most Autotrophs produce food (sugar) using light energy during Photosynthesis. Then, both Autotrophs and Heterotroph
Understanding How Living Things Obtain and Use Energy. Cell Energy: The Big Picture Most Autotrophs produce food (sugar) using light energy during Photosynthesis. Then, both Autotrophs and Heterotroph
*The entropy of a system may decrease, but the entropy of the system plus its surroundings must always increase
 AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
MitoSeminar II: Some calculations in bioenergetics
 MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and
AN INTRODUCTION TO METABOLISM. Metabolism, Energy, and Life
 AN INTRODUCTION TO METABOLISM Metabolism, Energy, and Life 1. The chemistry of life is organized into metabolic pathways 2. Organisms transform energy 3. The energy transformations of life are subject
AN INTRODUCTION TO METABOLISM Metabolism, Energy, and Life 1. The chemistry of life is organized into metabolic pathways 2. Organisms transform energy 3. The energy transformations of life are subject
CP Biology Unit 5 Cell Energy Study Guide. Electron Carriers Electron Transport Chain Fermentation Glycolysis Krebs cycle Light-Dependent Reactions
 Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Lecture 10. Proton Gradient-dependent ATP Synthesis. Oxidative. Photo-Phosphorylation
 Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
Metabolism and Enzymes
 Energy Basics Metabolism and Enzymes Chapter 5 Pgs. 77 86 Chapter 8 Pgs. 142 162 Energy is the capacity to cause change, and is required to do work. Very difficult to define quantity. Two types of energy:
Energy Basics Metabolism and Enzymes Chapter 5 Pgs. 77 86 Chapter 8 Pgs. 142 162 Energy is the capacity to cause change, and is required to do work. Very difficult to define quantity. Two types of energy:
(kilo ) or heat energy (kilo ) C. Organisms carry out conversions between potential energy and kinetic energy 1. Potential energy is energy;
 I. Biological work requires energy A. Energy is the to do work B. Energy is expressed in units of work (kilo ) or heat energy (kilo ) C. Organisms carry out conversions between potential energy and kinetic
I. Biological work requires energy A. Energy is the to do work B. Energy is expressed in units of work (kilo ) or heat energy (kilo ) C. Organisms carry out conversions between potential energy and kinetic
Cell Energy Notes ATP THE ENDOSYMBIOTIC THEORY. CELL ENERGY Cells usable source of is called ATP stands for. Name Per
 Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Photosynthesis and Cellular Respiration
 Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
Cellular Respiration. Mitochondria Rule! Mr. Kurt Kristensen
 Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
of catabolic processes, like glycolysis and the Krebs cycle, as hydride ions (H ). This free energy is used to regenerate ATP in the matrix.
 Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely
Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Metabolism and enzymes
 Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Chapter 5. Energy Flow in the Life of a Cell
 Chapter 5 Energy Flow in the Life of a Cell Including some materials from lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc.. Review
Chapter 5 Energy Flow in the Life of a Cell Including some materials from lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc.. Review
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Energy Exchanges Exam: What to Study
 Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Biology Slide 1 of 20
 Biology 1 of 20 8-1 Energy and Life 2 of 20 8-1 Energy and Life Autotrophs and Heterotrophs Where do plants get the energy they need to produce food? Living things need energy to survive. This energy comes
Biology 1 of 20 8-1 Energy and Life 2 of 20 8-1 Energy and Life Autotrophs and Heterotrophs Where do plants get the energy they need to produce food? Living things need energy to survive. This energy comes
BIOLOGICAL SCIENCE. Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge. FIFTH EDITION Freeman Quillin Allison
 BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
How Cells Work. Learning Objectives
 How Cells Work Chapter 5 Learning Objectives 1. Physics tells us that in any energy transformation: a) energy is neither created nor destroyed, and b) there is always some energy lost in an unusable form
How Cells Work Chapter 5 Learning Objectives 1. Physics tells us that in any energy transformation: a) energy is neither created nor destroyed, and b) there is always some energy lost in an unusable form
Chapter 6- An Introduction to Metabolism*
 Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
3.1 Metabolism and Energy
 3.1 Metabolism and Energy Metabolism All of the chemical reactions in a cell To transform matter and energy Step-by-step sequences metabolic pathways Metabolic Pathways Anabolic reactions Build large molecules
3.1 Metabolism and Energy Metabolism All of the chemical reactions in a cell To transform matter and energy Step-by-step sequences metabolic pathways Metabolic Pathways Anabolic reactions Build large molecules
Flow of Energy. Flow of Energy. Energy and Metabolism. Chapter 6
 Energy and Metabolism Chapter 6 Flow of Energy Energy: the capacity to do work -kinetic energy: the energy of motion -potential energy: stored energy Energy can take many forms: mechanical electric current
Energy and Metabolism Chapter 6 Flow of Energy Energy: the capacity to do work -kinetic energy: the energy of motion -potential energy: stored energy Energy can take many forms: mechanical electric current
Chapter 5. Table of Contents. Section 1 Energy and Living Things. Section 2 Photosynthesis. Section 3 Cellular Respiration
 Photosynthesis and Cellular Respiration Table of Contents Section 1 Energy and Living Things Section 2 Photosynthesis Section 3 Cellular Respiration Section 1 Energy and Living Things Objectives Analyze
Photosynthesis and Cellular Respiration Table of Contents Section 1 Energy and Living Things Section 2 Photosynthesis Section 3 Cellular Respiration Section 1 Energy and Living Things Objectives Analyze
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
ATP ATP. The energy needs of life. Living economy. Where do we get the energy from? 9/11/2015. Making energy! Organisms are endergonic systems
 Making energy! ATP The energy needs of life rganisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active transport temperature regulation 2007-2008
Making energy! ATP The energy needs of life rganisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active transport temperature regulation 2007-2008
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 107 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
An Introduction to Metabolism
 Chapter 8 An Introduction to Metabolism Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 8 1. An organism s metabolism transforms matter and energy, subject to the laws of
Chapter 8 An Introduction to Metabolism Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 8 1. An organism s metabolism transforms matter and energy, subject to the laws of
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Biology Reading Assignments:
 Biology 205 5.13.08 Reading Assignments: Chapter 3 Energy, Catalysis and Biosynthesis pgs. 83-94; 106-116 (Note the various roles of nucleotide based carrier molecules); work questions 3-2 and 3-3 Chapter
Biology 205 5.13.08 Reading Assignments: Chapter 3 Energy, Catalysis and Biosynthesis pgs. 83-94; 106-116 (Note the various roles of nucleotide based carrier molecules); work questions 3-2 and 3-3 Chapter
- BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU
 - BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU Bioenergetics the field of biochemistry concerned with the transfer and use of energy by biological system BIOLOGICAL IMPORTANCE: Suitable fuel
- BIOENERGETICS - DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU Bioenergetics the field of biochemistry concerned with the transfer and use of energy by biological system BIOLOGICAL IMPORTANCE: Suitable fuel
Cellular Respiration: Harvesting Chemical Energy
 Lecture 13 9/30/05 I. General Principles Cellular Respiration: arvesting Chemical Energy Chapter 9 Lecture utline 1. Regulation of Enzymes: competitive, allosteric, phosphorylation 2. Equilibrium 3. Digestion
Lecture 13 9/30/05 I. General Principles Cellular Respiration: arvesting Chemical Energy Chapter 9 Lecture utline 1. Regulation of Enzymes: competitive, allosteric, phosphorylation 2. Equilibrium 3. Digestion
BIS Office Hours
 BIS103-001 001 ffice ours TUE (2-3 pm) Rebecca Shipman WED (9:30-10:30 am) TUE (12-1 pm) Stephen Abreu TUR (12-1 pm) FRI (9-11 am) Steffen Abel Lecture 2 Topics Finish discussion of thermodynamics (ΔG,
BIS103-001 001 ffice ours TUE (2-3 pm) Rebecca Shipman WED (9:30-10:30 am) TUE (12-1 pm) Stephen Abreu TUR (12-1 pm) FRI (9-11 am) Steffen Abel Lecture 2 Topics Finish discussion of thermodynamics (ΔG,
Biology Reading Assignment: Chapter 9 in textbook
 Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
CHAPTER 8. An Introduction to Metabolism
 CHAPTER 8 An Introduction to Metabolism WHAT YOU NEED TO KNOW: Examples of endergonic and exergonic reactions. The key role of ATP in energy coupling. That enzymes work by lowering the energy of activation.
CHAPTER 8 An Introduction to Metabolism WHAT YOU NEED TO KNOW: Examples of endergonic and exergonic reactions. The key role of ATP in energy coupling. That enzymes work by lowering the energy of activation.
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
Advanced Cell Biology. Lecture 8
 Advanced Cell Biology. Lecture 8 Alexey Shipunov Minot State University January 28, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 8 January 28, 2013 1 / 33 Outline Questions and answers Energy and
Advanced Cell Biology. Lecture 8 Alexey Shipunov Minot State University January 28, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 8 January 28, 2013 1 / 33 Outline Questions and answers Energy and
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Key Concepts 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 8.2 The free-energy change of a reaction tells us
Chapter 8: An Introduction to Metabolism Key Concepts 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 8.2 The free-energy change of a reaction tells us
Review Questions - Lecture 5: Metabolism, Part 1
 Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Chapter 8 Metabolism: Energy, Enzymes, and Regulation
 Chapter 8 Metabolism: Energy, Enzymes, and Regulation Energy: Capacity to do work or cause a particular change. Thus, all physical and chemical processes are the result of the application or movement of
Chapter 8 Metabolism: Energy, Enzymes, and Regulation Energy: Capacity to do work or cause a particular change. Thus, all physical and chemical processes are the result of the application or movement of
Lecture 2: Biological Thermodynamics [PDF] Key Concepts
![Lecture 2: Biological Thermodynamics [PDF] Key Concepts Lecture 2: Biological Thermodynamics [PDF] Key Concepts](/thumbs/76/73872045.jpg) Lecture 2: Biological Thermodynamics [PDF] Reading: Berg, Tymoczko & Stryer: pp. 11-14; pp. 208-210 problems in textbook: chapter 1, pp. 23-24, #4; and thermodynamics practice problems [PDF] Updated on:
Lecture 2: Biological Thermodynamics [PDF] Reading: Berg, Tymoczko & Stryer: pp. 11-14; pp. 208-210 problems in textbook: chapter 1, pp. 23-24, #4; and thermodynamics practice problems [PDF] Updated on:
METABOLISM. What is metabolism? Categories of metabolic reactions. Total of all chemical reactions occurring within the body
 METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
An Introduction to Metabolism
 Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Cellular Respiration. The mechanism of creating cellular energy. Thursday, 11 October, 12
 Cellular Respiration The mechanism of creating cellular energy What do we know?? What do we know?? Grade 5 - Food --> Energy What do we know?? Grade 5 - Food --> Energy Grade 10 - glu. + O2 --> CO2 + H20
Cellular Respiration The mechanism of creating cellular energy What do we know?? What do we know?? Grade 5 - Food --> Energy What do we know?? Grade 5 - Food --> Energy Grade 10 - glu. + O2 --> CO2 + H20
Chapter 6: Energy Flow in the Life of a Cell
 Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The capacity to do work Types of Energy: 1) Potential Energy = Stored energy Positional (stored in location of object) Chemical (stored
Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The capacity to do work Types of Energy: 1) Potential Energy = Stored energy Positional (stored in location of object) Chemical (stored
Making energy! ATP. The point is to make ATP!
 Making energy! ATP The point is to make ATP! 2008-2009 The energy needs of life Organisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active
Making energy! ATP The point is to make ATP! 2008-2009 The energy needs of life Organisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
AP Biology Day 16. Monday, September 26, 2016
 AP Biology Day 16 Monday, September 26, 2016 CW/HW Assignments 1. Ch. 9 Guided Reading 2. Ch. 9 Video Cornell Notes (2) PLANNER 1. Ch. 9 Video Cornell Notes (weebly) 2. Study & schedule test retake! unit
AP Biology Day 16 Monday, September 26, 2016 CW/HW Assignments 1. Ch. 9 Guided Reading 2. Ch. 9 Video Cornell Notes (2) PLANNER 1. Ch. 9 Video Cornell Notes (weebly) 2. Study & schedule test retake! unit
Big Idea #2. Energy. Types of Potential Energy. Kinetic Energy. Chemical Potential Energy. Metabolism
 Big Idea #2 Biological Systems utilize free energy and molecular building blocks to grow, to reproduce and to maintain dynamic homeostasis Life runs on chemical reactions rearranging atoms transforming
Big Idea #2 Biological Systems utilize free energy and molecular building blocks to grow, to reproduce and to maintain dynamic homeostasis Life runs on chemical reactions rearranging atoms transforming
Biochemistry 3300 Problems (and Solutions) Metabolism I
 (1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes
(1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
Bio102 Problems Photosynthesis
 Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Chapter 6: Energy Flow in the Life of a Cell
 Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Outer Glycolysis mitochondrial membrane Glucose ATP
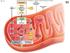 Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
