C- and D-class MADS-Box Genes from Phalaenopsis equestris (Orchidaceae) Display Functions in Gynostemium and Ovule Development
|
|
|
- Ginger Wilkerson
- 6 years ago
- Views:
Transcription
1 C- and D-class MADS-Box Genes from Phalaenopsis equestris (Orchidaceae) Display Functions in Gynostemium and Ovule Development You-Yi Chen 1, Pei-Fang Lee 2, Yu-Yun Hsiao 3, Wan-Lin Wu 1, Zhao-Jun Pan 3, Yung-I. Lee 4, Ke-Wei Liu 5,6, Li-Jun Chen 5, Zhong-Jian Liu 5,6,7, * and Wen-Chieh Tsai 1, * 1 Institute of Tropical Plant Sciences, National Cheng Kung University, Tainan 701, Taiwan 2 Department of Biotechnology, Fooyin University, Kaohsiung 831, Taiwan 3 Department of Life Sciences, National Cheng Kung University, Tainan 701, Taiwan 4 Botany Department, National Museum of Natural Science, Taichung 404, Taiwan 5 Shenzhen Key Laboratory for Orchid Conservation and Utilization, The National Orchid Conservation Center of China and The Orchid Conservation & Research Center of Shenzhen, Shenzhen , PR China 6 The Center for Biotechnology and BioMedicine, Graduate School at Shenzhen, Tsinghua University, Shenzhen , PR China 7 College of Forestry, South China Agricultural University, Guangzhou , PR China *Corresponding authors: s, tsaiwc@mail.ncku.edu.tw, liuzj@sinicaorchid.org; Fax: (Received December 23, 2011; Accepted March 29, 2012) Regular Paper Gynostemium and ovule development in orchid are unique developmental processes in the plant kingdom. Characterization of C- and D-class MADS-box genes could help reveal the molecular mechanisms underlying gynostemium and ovule development in orchids. In this study, we isolated and characterized a C- and a D-class gene, PeMADS1 and PeMADS7, respectively, from Phalaenopsis equestris. These two genes showed parallel spatial and temporal expression profiles, which suggests their cooperation in gynostemium and ovule development. Furthermore, only PeMADS1 was ectopically expressed in the petals of the gylp (gynostemium-like petal) mutant, whose petals were transformed into gynostemium-like structures. Protein protein interaction analyses revealed that neither PeMADS1 and PeMADS7 could form a homodimer or a heterodimer. An E-class protein was needed to bridge the interaction between these two proteins. A complementation test revealed that PeMADS1 could rescue the phenotype of the AG mutant. Overexpression of PeMADS7 in Arabidopsis caused typical phenotypes of the D-class gene family. Together, these results indicated that both C-class PeMADS1 and D-class PeMADS7 play important roles in orchid gynostemium and ovule development. Keywords: C-class MADS-box gene D-class MADS-box gene Gynostemium Orchid Ovule Phalaenopsis equestris. Abbreviations: CaMV, Cauliflower mosaic virus; DAP, days after pollination; EST, expressed sequence tag; ORF, open reading frame; RACE, rapid amplification of cdna ends; RT PCR, reverse transcription PCR; SEM, scanning electron microscopy; UTR, untranslated region. Accession numbers: PeMADS1, AF234617; PeMADS7, JN Introduction In plants, MADS-box-containing transcriptional regulators have central roles in floral development (Weigel and Meyerowitz 1994, Münster et al. 1997, Theissen et al. 2000). In the ABCDE model, the unique identity of different floral organs in each whorl is determined by the combined interaction of five classes of homeotic genes, A-, B-, C-, D- and E-class genes (Weigel and Meyerowitz 1994, Zahn et al. 2005). The A and E genes control sepal development; B, C and E genes specify petal identity and regulate stamen formation; C and E genes have functions in carpel morphogenesis; and D and E genes are involved in ovule development. These gene products constitute multimeric regulatory complexes specifying organ identity by specifically recognizing the cis-regulatory elements of their target genes (Egea-Cortines et al. 1999, Honma and Goto 2001, Theissen and Saedler 2001, Ferrario et al. 2003). The family of Orchidaceae is the largest family of flowering plants, with the number of species exceeding 25,000 (Atwood 1986). The family has developed elaborate mechanisms for radial adaptation and represents a highly advanced and terminal line of floral evolution in the monocotyledons. Several factors promoting orchid species richness have been speculated upon. These factors include specific interaction between the orchid flower and pollinator (Cozzolino and Widmer 2005), sequential and rapid interplay between drift and natural selection (Tremblay et al. 2005), obligate interaction with mycorrhiza (Otero and Flanagan 2006), and Plant Cell Physiol. 53(6): (2012) doi: /pcp/pcs048, available online at The Author Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please journals.permissions@oup.com 1053
2 Y.-Y. Chen et al. Crassulacean acid metabolism and epiphytism (Silvera et al. 2009). In addition to the success of their ecological manipulations, orchids have several unique reproductive strategies that contribute to their success. These reproductive strategies include a fine, delicate gynostemium and labellum, which makes the orchid flower a precise instrument for animal pollination and not self-pollination, mature pollen grains packaged as pollinia, pollination-regulated ovary/ovule development, synchronized timing of micro- and mega-gametogenesis for effective fertilization, and the release of thousands or millions of immature embryos (seeds without endosperm) in mature pods (Yu and Goh 2001, Hsiao et al. 2011b). Thus, the Orchidaceae are rich subjects for exploring developmental biology to define the highly specialized structures in orchid reproduction. However, although orchids have unique floral morphology and adaptive reproduction strategies, the relationship between the function of genes and morphological structures remains poorly understood. In the ABCDE model, B-class genes were first investigated for their roles in perianth development in several orchid species (Tsai et al. 2004, Tsai et al. 2005, Kim et al. 2007, Tsai et al. 2008c, Chang et al. 2010, Mondragon-Palomino and Theissen 2011). Duplicated four-clade B-class genes with differential expression patterns in orchid floral organs, as well as divergent protein behaviors of the encoded B-class genes, support the unique evolutionary routes of B-class genes associated with unique labellum innovation in orchid (Mondragon-Palomino and Theissen 2008, Tsai et al. 2008a, Pan et al. 2011). In addition to the specialized labellum, the gynostemium, fused by the male and female reproductive organs, is a key innovation of the floral organ in orchid. Another unique developmental phenomenon in orchids is the initiation of ovule development triggered by pollination (Tsai et al. 2008b). Recently, C/D-class genes were investigated for their functions in gynostemium and ovule development (Hsiao et al. 2011b). Orchid C-class genes have been identified from Phalaenopsis, Dendrobium, Oncidium and Cymbidium (Skipper et al. 2006, Song et al. 2006, Xu et al. 2006, Hsu et al. 2010, Wang et al. 2011). Spatiotemporal expression analyses suggest that C- and D-class genes act redundantly in floral development in Phalaenopsis Hatsuyuki and Oncidium Gower Ramsey (Song et al. 2006, Hsu et al. 2010). In Dendrobium thyrsiflorum, both C-class DthyrAG1 and D-class DthyrAG2 are involved in various aspects of flower development, and DthyrAG2 has a more prominent role than DthyrAG1 in late ovule development (Skipper et al. 2006). Notably, Cymbidium ensifolium showed duplication of C-class genes followed by subfunctionalization (Wang et al. 2011). However, the exact function of these C/D-class genes needs further investigation. In this study, we isolated two C/D-class genes, PeMADS1 and PeMADS7, from Phalaenopsis equestris and characterized their functions in gynostemium and ovule development. These results open the way to understanding the molecular mechanisms of orchid gynostemium and ovule morphogenesis. Results Flower organs and post-pollination syndrome in P. equestris Phalaenopsis equestris has small-sized flowers, and the entire flower is usually oval shaped or sometimes star shaped (Fig. 1A, B). Each flower has two main petals and three sepals behind them. The median petal is highly modified to an enlarged petal, called a lip or labellum. The male and female reproductive parts are fused in a structure, the gynostemium or column, located in the center of the flower. The pollen grains stick together to form the pollinia at the upper tip of the gynostemium under the anther cap. The deposited pollen are accepted in the stigmatic cavity underlying the rostellum. After pollination, the stigma closes and then swells and persists on the fruit. The ovary starts to develop and gradually enlarges in diameter and length (Fig. 1C). We used scanning electron micrography (SEM) to study ovule development. Before pollination, the ovary showed no ovules or ovule initials along the placental ridge (Fig. 2A). At 4 days after pollination (DAP), the placenta showed no significant changes (Fig. 2B). At about 16 DAP, the placental protuberances differentiated from a single epidermal layer of the placenta (Fig. 2C). At about DAP, the placental ridges continued to elongate and branch to form thousands Fig. 1 Structure of a Phalaenopsis equestris flower. (A) Front view showing flower organs. Inset: column showing the stigmatic cavity. (B) Back view of the same flower showing the inferior ovary. (C) Morphological changes in the ovary at various days after pollination. Bar = 1 cm. 1054
3 C and D function in orchids The 1,009 bp PeMADS1 full-length cdna encodes a 239 amino acid protein, and the 1,031 bp PeMADS7 full-length cdna encodes a 235 amino acid protein. Multiple sequence alignment with other C- and D-class proteins of plants demonstrated that both proteins had a typical MIKC-type domain structure (Fig. 3). In addition, both PeMADS1 and PeMADS7 contain the AG motif I and II at their C-terminus (Fig. 3) which are synapomorphies for the AG subfamily of seed plants (Kramer et al. 2004). Furthermore, we identified a nine amino acid MD motif located downstream of the AG motif II and specific to monocot D-class proteins (Yun et al. 2004) in the PeMADS7 protein. These results suggest that PeMADS1 and PeMADS7 belong to the C- and D-class MADS-box genes, respectively. Fig. 2 Scanning electron micrography of the developing ovule of Phalaenopsis. (A) 0 DAP ovary; (B) 4 DAP ovary; (C) 16 DAP ovules; (D) 32 DAP ovules; (E) 40 DAP ovules; (F) 48 DAP ovules; (G) 56 DAP ovules; (H) 64 DAP ovules. DAP, days after pollination. of finger-like ovule primordia (Fig. 2D, E). At 48 DAP, the inner integument appeared as a collar-like growth near the tip of the primordia, and the outer integument was initiated shortly thereafter (Fig. 2F). At 56 DAP, the outer and inner integuments had extended and nearly enclosed the tip of the nucellus (Fig. 2G). At 64 DAP, the two integuments completely enclosed the nucellus (Fig. 2H). Identification and sequence analyses of C- and D-class MADS-box genes from P. equestris Search of the expressed sequence tag (EST) database for orchid flowers (Tsai et al. 2006, Fu et al. 2011, Hsiao et al. 2011a) revealed one sequence with high similarity to C-class MADS-box genes and another with high similarity to D-class genes. Both sequences were partial cdnas with incomplete open reading frames (ORFs). To clone these two genes, we obtained full-length sequences of the cdnas by rapid amplification of cdna ends (RACE). We determined the nucleotide sequences of the 10 cloned RACE products for the individual MADS-box genes and assembled them with the C- and D-class ESTs, respectively. We identified the complete sequences of the C- and D-class full-length cdna in P. equestris and called them PeMADS1 and PeMADS7, repectively. Phylogenetic relationship of PeMADS with other C/D-class MADS-box genes To determine the phylogenetic relationships among PeMADS1, PeMADS7 and other AGAMOUS-like genes, we reconstructed the phylogeny of known C/D-class MADS-box genes using the conceptual amino acid sequences of the respective genes. Phylogenetic analysis revealed that PeMADS1 was assigned to the C-class group and closely related to PhalAG1 derived from Phalaenopsis Hatsuyuki. PeMADS7 was assigned to the D-class group and was closely related to PhalAG2 of Phalaenopsis (Fig. 4). Genomic organization of PeMADS genes To understand the genome organization of PeMADS1 and PeMADS7 in the P. equestris genome, we performed Southern blot analysis. DNA gel blots containing 30 mg of P. equestris genomic DNA digested with BglII, EcoRI and HindIII for PeMADS1, and DraI, EcoRI and HindIII for PeMADS7 were hybridized with probes derived from the 3 0 -specific region of the PeMADS genes (Supplementary Fig. S1). DNA blots revealed one band for PeMADS1 with BglII and EcoRI digestion and two bands with HindIII (Supplementary Fig. S1, lanes 1 3). However, one band was detected for PeMADS7 with DraI, EcoRI and HindIII digestion (Supplementary Fig. S1, lanes 4 6). Therefore, both PeMADS1 and PeMADS7 are represented as single-copy genes in the P. equestris genome. However, we cannot exclude that more than one C- or D-class gene exists in the P. equestris genome because we did not use class-specific probes. The two bands for PeMADS1 with HindIII digestion might result from the genomic region detected by the PeMADS1 probe containing a HindIII recognition site. In addition, the probes used in the Southern blots were gene specific for differentiating between PeMADS1 and PeMADS7 because of no common bands in the same digestion. Expression profiles of the two PeMADS genes We characterized the gene expression profiles of PeMADS1 and PeMADS7 by reverse transcription PCR (RT PCR). Both PeMADS1 and PeMADS7 transcripts were absent in the 1055
4 Y.-Y. Chen et al. Fig. 3 Alignment of the deduced amino acid sequences of PeMADS genes and other C- and D-class genes generated by use of PILEUP and displayed by PRETTYBOX. Consensus is denoted by a black box; similarity is denoted by gray; differences are indicated by white; gaps in the alignment are indicated by full stops; and positions not occupied by an amino acid are indicated by a. The MADS-, I-, K- and C-domains are indicated between double-headed arrows. The AG motifs I and II are indicated by a black line. The MD motif is highlighted by a black box. AVAG2 (BAD83772) is from Asparagus virgatus; CeMADS1 (ADP00515) is from Cymbidium ensifolium; LMADS2 (AAS01766) is from Lilium longiflorum; DcOAG1 (AAZ95250) and DcOAG2 (AAZ95251) are from Dendrobium crumenatum; and OSMADS3 (Q40704) is from Oryza sativa. vegetative tissues pedicels, leaves and roots (Fig. 5A). PeMADS1 mrna was detected in all developmental stages of flower buds. However, PeMADS7 mrna was detected from only stage 2 to 4 mature flower buds (Fig. 5A). The the fact that expression of PeMADS7 induced in stage 2 was significantly reduced in stage 3 and 4 flower buds suggests that PeMADS7 has an essential function in stage 2 developing flower bud. Spatial expression analyses revealed both PeMADS1 and PeMADS7 transcripts only in the gynostemium and absent in sepals, petals and lip (Fig. 5A). These results suggest the important roles of these two PeMADS genes in gynostemium development. Because C- and D-class MADS-box genes are also involved in ovule development, and pollination is a key regulatory event in orchid ovule initiation, we determined the temporal mrna expression patterns of PeMADS1 and PeMADS7 in developing ovule triggered by pollination. The mrna expression of both PeMADS1 and PeMADS7 was low in the undeveloped ovary (Figs. 1C, 5B). After pollination, the expression of both genes was gradually increased up to 16 DAP (Fig. 5B). At 32 DAP, the expression of PeMADS1 was decreased and then increased up to 56 DAP (Fig. 5B). The expression of PeMADS7 was slightly decreased at 32 DAP and then increased to a peak at 64 DAP (Fig. 5B). Therefore, pollination may have triggered the mrna accumulation of PeMADS1 and PeMADS7, and their function may be associated with ovule development. The decreased expression of both genes at 32 DAP might reflect the developmental change of the placental ridges (Fig. 2D). The expression of both genes could not be detected at 80 DAP or in dry seeds, protocorms and seedlings (Fig. 5C). Thus, PeMADS1 and PeMADS7 may not be involved in seed, protocorm or seedling development. In situ localization of PeMADS transcripts The detailed spatial and temporal expression patterns of PeMADS1 and PeMADS7 were further investigated by in situ hybridization with antisense RNA probes. Both PeMADS1 and PeMADS7 share highly similar mrna expression patterns (Fig. 6). At the early stage of floral developmental, transcripts of PeMADS1 were detected at the floral primordium (Fig. 6A). However, PeMADS7 transcripts were barely detected in the floral primodium (Fig. 6L). At the later stage, the two genes had a similar distribution in the rostellum and stigmatic surface 1056
5 C and D function in orchids of the gynostemium (Fig. 6B, M). In addition, PeMADS1 transcripts could be detected in the pollinium (Fig. 6B). During ovule development, the mrna expression for both PeMADS genes was detected in the placenta before pollination (Fig. 6C, N). After pollination, expression was observed in the ovule primordial and developing ovules (Fig. 6D, E, F for PeMADS1; Fig. 6O, P, Q for PeMADS7). At later stages, the expression of both was detected in nucellus tissue, integument and embryo sac (Fig. 6G, H for PeMADS1; Fig. 6R, S for PeMADS7). At 80 DAP, neither gene was expressed (Fig. 6I, T). The negative control was sense RNA used as a probe (Fig. 6J, U). Fig. 4 Phylogenetic analysis of C- and D MADS-box lineages and gymnosperm AG-like genes. Published plant MADS-box protein sequences were retrieved from GenBank: PLENA, AAB25101 (Antirrhinum majus); FARINELLI, CAB42988 (A. majus); FBP6, CAA48635 (Petunia hybrida); FBP7, CAA57311 (P. hybrida); FBP11, CAA57445 (P. hybrida); pmads3, CAA51417 (P. hybrida); NAG1, AAA17033 (Nicotiana tabacum); TAG1, AAA34197 (Solanum lycopersicon); TAG11, AAA34197 (S. lycopersicon); DcMADS4, CAC81071 (Daucus carota); GAGA1, CAA08800 (Gerbera hybrida); GAGA2, CAA08801 (G. hybrida); AGAMOUS, CAA37642 (Arabidopsis thaliana); SHP1, AAA32730 (A. thaliana); SHP2, AAA32735 (A. thaliana); STK, AAC49080 (A. thaliana); MdMADS10, CAA04324 (Malus domestica); MdMADS14, CAC80857 (M. domestica); GhMADS2, AAN15183 (Gossypium hirsutum); STAG1, AAD45814 (Fragaria ananassa); VvMADS1, AAK58564 (Vitis vinifera); VvMADS5, AAM21345 (V. vinifera); SLM1, CAA56655 (Silene latifolia); MpMADS2, BAB70737 (Magnolia precossimina); McAG, AAO20104 (Momordica charantia); DcOAG1, AAZ95250 (Dendrobium crumenatum); DcOAG2, AAZ95251 (D. crumenatum); DthyrAG1, AAY86364 (D. thyrsiflorum); DthyrAG, AAY86365 (D. thyrsiflorum); PhalAG1, BAE80120 (Phalaenopsis hybrid cultivar); PhalAG2, BAE80121 (Phalaenopsis hybrid cultivar); ApMADS2, BAC66963 (Agapanthus praecox); LMADS2, AAS01766 (L. longiflorum); HvAG1, AAL93196 (Hordeum vulgare); HvAG2, AAL93197 (H. vulgare); WAG, BAC22939 (Triticum aestivum); OsMADS3, AAA99964 (O. sativa); OsMADS58, BAE54300 (O. sativa); ZAG1, AAA02933 (Zea mays); DAL2, CAA55867 (Picia abies); CeMADS1, GU (Cymbidium ensifolium); CeMADS2, GU (C. ensifolium). Bootstrap values from 1,000 replicates are indicated on most major nodes. PeMADS1 and PeMADS7 are highlighted by open boxes. The monophyletic floral homeotic gene groups are labeled by black lines at the right margin. Ectopic expression of PeMADS1 associated with gynostemium-like petal formation To explore further the roles of PeMADS1 and PeMADS7 in regulating gynostemium formation in Phalaenopsis, we examined the mrna expression of the two genes in the gylp (gynostemium-like petal) mutant derived from somaclonal variation (Fig. 7). The wild-type flower of the Phalaenopsis hybrid CD1 shows three yellow sepals, two yellow petals, one red lip and one yellow gynostemiun (Fig. 7A). However, the gylp flower has conversions of petals to gynostemium-like structures. The gynostemium-like petals show an irregular shape and surface, with two sterile pollinium-like structures at the distal margin (Fig. 7B). SEM results revealed the adaxial cells of the wild-type petal epidermis with a round-shield shape and a smooth surface (Fig. 7C, D). However, the adaxial cells of the gylp petal epidermis are conical with cuticular striations (Fig. 7E, F), which is similar to the gynostemium cells of the wild-type and mutant plant (Fig. 7G, H for the wild type; Fig. 7I, J for the mutant). Results of expression analysis showed that PeMADS1 but not PeMADS7 was ectopically expressed in the petals in the gylp mutant (Fig. 7K, L). These results suggest that PeMADS1 has a function in gynostemium morphogenesis. Interaction behaviors of C-, D- and E-class PeMADS proteins To investigate whether PeMADS1 and PeMAD7 are involved in gynostemium and ovule development based on a biochemical interaction, we performed yeast two-hybrid analysis with colony-lift filter assays. The coding parts of PeMADS1 and PeMADS7 cdnas were separately cloned into the binding domain vector pgbkt7 and the activation domain vector pgadt7. The results showed that PeMADS1 and PeMADS7 were not able to interact with each other (Fig. 8A). In addition, none of the proteins could form homodimers. E-class proteins can act as cofactors with ABCD homeotic genes in specifying different floral whorls (Malcomber and Kellogg 2005), so we wondered whether the orchid SEP protein PeMADS8 (P. equestris MADS8, Supplementary Fig. S2) could interact with C-class PeMADS1 and D-class PeMADS7 protein. The results showed that PeMADS8 interacted with PeMADS1 and PeMADS7 (Fig. 8A). These results raised the possibility that 1057
6 Y.-Y. Chen et al. Fig. 5 Analyses of spatial and temporal expression patterns of PeMADS1 and PeMADS7 in P. equestris by RT PCR. (A) Expression patterns of PeMADS1 and PeMADS7 in various organs and developmental stages of floral bud. Se, sepals; Pe, petals; Li, lip; Gy, gynostemium; P, pedicel; F, floral stalk; L, leaf; R, root; B1, stage 1 flower bud (0 1 mm); B2, stage 2 flower bud (1 2 mm); B3, stage 3 flower bud (2 5 mm); B4, stage 4 flower bud (5 10 mm). (B) Expression pattern analyses of PeMADS1 and PeMADS7 in various developmental stages of ovule by RT PCR (right panel). Real-time quantitative RT PCR analysis of the mrna expression of PeMADS1 and PeMADS7 (left panel), performed in triplicate. DAP, days after pollination. (C) Expression patterns of PeMADS1 and PeMADS7 in various developmental stages of ovule and embryo. PeMADS8 could be a bridge protein between PeMADS1 and PeMADS7 to form a multimeric complex. To test this, we performed yeast three-hybrid experiments by cloning PeMADS8 into the bridge site of the pbridge vector. Yeast strain AH109 could grow on selective medium only when all three proteins were expressed (Fig. 8B), which suggests that an interaction between PeMADS1 and PeMADS7 could be mediated by the presence of PeMADS8. Functional analyses of the two PeMADS genes using transgenic Arabidopsis To investigate the role of the putative function of C- and D-class PeMADS genes, we constructed transgenic Arabidopsis plants expressing PeMADS1 and PeMADS7 under the control of the Cauliflower mosaic virus (CaMV) 35S promoter by Agrobacterium-mediated transformation. We obtained 15 independent 35S::PeMADS1 transgenic plants, and all plants were phenotypically indistinguishable from wild-type plants. For further examination of the function of PeMADS1, a complementation test was performed and it revealed that PeMADS1 can rescue the ag-4 mutant. Floral organs of the wild-type flower are organized in four whorls, with typically four sepals in the first whorl, four petals in the second whorl, six stamens in the third whorl, and two fused carpels in the fourth whorl (Fig. 9C). Because the ag-4 mutant fails to specify carpels and lacks floral meristem determinancy, the mutant flower shows an internal flower in place of the fourth-whorl carpels (Sleburth et al. 1995; Fig. 9A, D). Ecotopic expression of PeMADS1 in ag-4 plants dramatically influenced the flower phenotype. The flowers of 35S- PeMADS1 ag-4 plants showed two types of carpeloid organs in the fourth whorl: one type showed ovule-like structures along the margins of the fourth whorl organs (Fig. 9E, G), and the other type showed two unfused carpeloid organs with stigmatic papillae (Fig. 9F, H). The 35S-PeMADS1 ag-4 plants showing homeotic conversion of sepals to carpeloid organs in the fourth whorl, suggesting that PeMADS1 could partially complement the ag-4 mutant. For functional analysis of D-class PeMADS7, out of 13 independent PeMADS7 transgenic lines obtained, eight lines showed identical novel phenotypes. As shown in Fig. 10, transgenic plants showed alternations in both vegetative and reproductive tissues. Compared with wild-type plants, the 35S::PeMADS7 transgenic Arabidopsis plants were extremely early flowering (even before production of the fourth rosette leaf; Fig. 10A, B, D). The height of all these lines was reduced (data not shown). The rosette leaves of transgenic plants had upwardly curled profiles and were smaller than wild-type plants (Fig. 10A, B). The flower buds from 35S::PeMADS7 opened prematurely, and the stigma extended more severely (Fig. 10C). The sepals and petals were reduced in size (Fig. 10E, F, G; Table 1). However, we observed no homeotic conversion of floral organs. Of note, perianth abscission was inhibited and seed sterility was increased in bent siliques in 1058
7 C and D function in orchids Fig. 6 In situ localization of PeMADS1 and PeMADS7 in developing floral buds and ovules. Sections were hybridized with the antisense 3 0 -specific PeMADS1 (A I) or PeMADS7 (L T) RNA probes or sense RNA probes (J, PeMADS1, bar = 200 mm; U, PeMADS7, bar = 10 mm). (A and L) Flower primordium. Bar = 50 mm. (B and M) Developing flower buds. Bar = 200 mm. (C and N) Ovary tissue before pollination. Bar = 10 mm. (D and O) Placenta with ovule primordial at 4 DAP. Bar = 10 mm. (E and P) Placenta with ovule primordial at 16 DAP. Bar = 10 mm. (F and Q) Placenta with developing ovule at 32 DAP. Bar = 10 mm. (G and R) Developing ovule at 48 DAP. Bar = 10 mm. (H and S) Developing ovule at 64 DAP. Bar = 20 mm. (I and T) Developing seed at 80 DAP. Bar = 20 mm. DAP, day after pollination; Se, sepal; Pe, petal; Li, lip; Ac, anther cap; Ro, rostellum; Ss, stigmatic surface; P, placenta; Op, ovule primordium; O, developing ovule; Es, embryonic sac; I, integument. N, nucellus. transgenic 35S::PeMADS7 (Fig. 10H, I; Table 1). In wild-type Arabidopsis, pollination and fertilization are followed by abscission of the organs in the perianth. The sepals and petals are typically shed in a turgid state shortly after anthesis (Bleecker and Patterson 1997). Flowers of transgenic plants ectopically expressing PeMADS7 lasted up to maturation of seeds as compared with only 1 d for wild-type flowers (Fig. 10H). Each inflorescence of the transgenic plants had more blooming flowers than those of wild-type plants because flowers of transgenic plants had extended longevity (Fig. 10J, K). Discussion Comparing the developmental mechanisms of reproductive organs in different angiosperm groups should allow the identification of the molecular differences that underlie the diverse morphology of reproductive organs in flowering plants. A gynostemium, the fused reproductive organs of the androecium and gynoecium, is normally considered peculiar to orchids. The development of the gynostemium, which involves whorls 3 and 4, is of interest for elucidating the roles of C-class genes in orchids. In most orchid flowers, ovule development is precisely and completely triggered by pollination, and thus orchids offer a unique system to study D-class genes involved in ovule initiation and continued development of orchids. In addition, the developmental process between floral and ovule development differs considerably in orchids as compared with other plant species, where ovule development occurs simultaneously with perianth development. To investigate the roles of C/D-class genes in regulation of gynostemium and ovule development and the discontinuous developmental process between floral and ovule development in orchids, we identified PeMADS1 and PeMADS7 as belonging to the angiosperm C- and D-class genes, respectively, which was supported by sequence alignment and phylogenetic analysis (Figs. 3, 4). Two highly conserved motifs specific to C/D proteins, AG motifs I and II, were found in the C-terminal regions of PeMADS1 and PeMADS7 (Fig. 3). In addition, we found a unique MD motif specific to D-class proteins of monocots (Yun et al. 2004) in the extension of the AG motifs I and II of PeMADS7 (Fig. 3). The presence of these specific conserved sequences in the C-terminal regions of the two proteins confirms their identities as C- or D-class MADS-box genes. 1059
8 Y.-Y. Chen et al. Fig. 7 Flower morphology of (A) wild-type Phalaenopsis hybrida CD1 and its somaclonal variant (B) the gylp mutant. Arrowhead, pollinium-like structure. SEM of (C) cell morphology of wild-type petal epidermis and (D) an enlarged picture; (E) cell morphology of gylp mutant gynostemium-like petal epidermis and (F) an enlarged picture; (G) cell morphology of wild-type gynostemium epidermis and (H) an enlarged picture; (I) cell morphology of gylp mutant gynostemium epidermis and (J) enlarged picture. (K) Expression patterns of PeMADS1 and PeMADS7 in Phalaenopsis hybrida CD1 and its somaclonal variant the gylp mutant by RT PCR. Se, sepals; Pe, petals; Li, lip; Gy, gynostemium; Gylp, gynostemium-like petals. (L) Real-time quantitative RT PCR analysis of the expression of PeMADS1 and PeMADS7 in Phalaenopsis hybrida CD1 (left panel) and the gylp mutant (right panel), performed in triplicate. 1060
9 C and D function in orchids Fig. 8 Protein protein interaction behavior among PeMADS1, PeMADS7 and an orchid SEPALLATA-like PeMADS8 protein by the yeast two- and three-hybrid system. (A) Yeast-two hybrid assays. PeMADS1, PeMADS7 and the SEPALLATA-like (E-class) gene PeMADS8 were cloned into the binding domain vector pgbkt7 and the activation domain vector pgadt7. Activation of ADE2, HIS3 and lacz is indicated by growth on selection medium lacking adenine, histidine, leucine and tryptophan ( 4) and by the blue color, respectively. The yeast strain AH109 transformed with vectors pgadt7 + pgbkt7 and PeMADS4 + PeMADS6 were used as negative and positive controls, respectively. (B) Yeast three-hybrid assay of PeMADS8 used as a bridge protein between PeMADS1 and PeMADS7. The yeast strain AH109 was co-transformed with both pgadt7 vector containing PeMADS1 or PeMADS7 and pbridge vector containing the PeMADS1 and PeMADS7 fusion with the GAL4 DNA-binding domain and PeMADS8 as bridge protein. Spatial and temporal expression analysis showed that both PeMADS1 and PeMADS7 were expressed during flower and ovule development in P. equestris, except that PeMADS7 was not expressed at the flower primordium during very early flower development. The results were consistent with the C- and D-class gene expression patterns in Dendrobium and Oncidium (Skipper et al. 2006, Hsu et al. 2010). However, detection of transcripts of C- and D-class genes in the labellum was reported in Phalaenopsis (Song et al. 2006). These inconsistent observations may be explained by cross-hybridization of probes to the unexpected targets in the labellum. Thus, C/D-class genes may not be required for establishing labellum identity. Interestingly, we found that PeMADS1 and PeMADS7 were co-expressed in the placenta before ovule initiation, which is controlled by pollination in orchids, so constitutive expression of C- and D-class genes in ovule initiation may not be sufficient to trigger ovule development. Thus, extra factors may be needed for the regulation of ovule initiation. Recently, the PI/GLO-like PeMADS6 in P. equestris (Tsai et al. 2005) and OrcPI and OrcPI2 in Orchis italica (Salemme et al. 2011) were suggested as negative regulators in orchid ovule development. The identification of positive and/or negative regulators and understanding their regulation critical for ovule initiation in orchids will advance our knowledge of the molecular mechanism underlying ovule development of angiosperms. A previous study showed that loss of function of C-class gene in orchids causes homeotic mutation of flowers, with stamens replaced by petals and carpels replaced by a reiteration of the sequence sepals petals petals (Wang et al. 2011). This type of mutation is a phenocopy of C-function mutants in Arabidopsis (Bowman et al. 1989, Bowman et al. 1991), Antirrhinum (Davies et al. 1999) and Ipomoea nil (Nitasaka 2003). In rice, a loss-of-function assay of the C-class gene OSMADS3 showed that all stamens were homeotically transformed into lodicule-like organs, as well as increased lodicule number in whorl 2 and carpel number in whorl 4 (Yamaguchi et al. 2006). However, RNA-silenced lines of another C-class gene, OSMADS58, produced florets that reiterated a set of floral organs consisting of lodicules, stamens/ectopic lodicules and carpel-like organs (Yamaguchi et al. 2006). These two C-class genes may have been partially subfunctionalized during rice evolution (Yamaguchi et al. 2006). In this study, we provide evidence that ectopic expression of PeMADS1 in petals is a vital factor for transforming the petal into a gynostemium-like structure. However, according to the ABC model, ectopic expression of the C-class gene in petals could transform it into a stamen, because B and C specify stamen identity. Thus, the gynostemium may be more homologous to stamens than carpels. Actually, B-class genes such as AP3/DEF-like PeMADS4 and PI/GLO-like PeMADS6 were found to be significantly expressed in the gynostemium (Tsai et al. 2004). According to the floral quartet model, C-class gene products and SEPALATTA (SEP, E-class gene) proteins assemble into quaternary complexes, which specify carpel identity (Theissen and Saedler 2001, Zahn et al. 2005). Otherwise, ovule identity is controlled by the C-, D- and E-class protein complexes (Zahn et al. 2005). Interaction analyses of the Arabidopsis C-class protein AG, SHATTERPROOF1 (SHP1), SHP2 and D-class SEEDSTICK (STK) by yeast two-hybrid assay revealed no interaction among them. Interaction between C- and D-class proteins could be performed by the E-class SEP3 protein as a mediator (Favaro et al. 2003). Similar results also show no 1061
10 Y.-Y. Chen et al. Fig. 9 Ectopic expression of PeMADS1 in the ag-4 mutant. (A) Inflorescence of an ag-4 mutant. (B) Inflorescence of a transgenic ag-4 mutant plant ectopically expressing PeMADS1. (C) Wild-type flower. (D) ag-4 mutant flower. (E) ag-4 mutant ectopically expressing PeMADS1 produced an ectopic flower in the center of flowers. (F) Enlarged view of E showing that the ectopic flower has the first-whorl flower organ with an ovule-like structure. (G) The ag-4 mutant ectopically expressing PeMADS1 produced a carpel-like structure at the center of flowers. (H) Enlarged view of G showing clearly visible stigmatic papillae. Fig. 10 Phenotype analysis of transgenic Arabidopsis overexpressing PeMADS7. (A) Fourteen-day-old wild-type Arabidopsis. (B) Fourteen-day-old 35S::PeMADS7 transgenic Arabidopsis flowered early and produced curled rosette leaves (arrow); fb, flower buds. (C) Enlarged region of the white circle in (B). Inset: dissected flower bud from inflorescence. (D) A 20-day-old 35S::PeMADS7 transgenic Arabidopsis plant (right) flowered earlier than the wild-type plant (left). At this stage, wild-type plants did not flower and produced only rosette leaves. (E) A 35S::PeMADS7 mature flower (right) showed reduced size of sepals and petals as compared with the wild-type flower (left). (F) 35S::PeMADS7 flower sepals (right) were smaller than wild-type flower sepals (left). (G) 35S::PeMADS7 flower petals (right) were smaller than wild-type flower petals (left). (H) Phenotypes of silique and flower longevity in wild-type and 35S::PeMADS7 transgenic plants. Upper panel: wild-type plant. Lower panel: 35S::PeMADS7 transgenic plant. (I) Seed sterility is reduced in a 35S::PeMADS7 transgenic plant. Upper panel: wild-type plant. Lower panel: 35S::PeMADS7 transgenic plant. (J) Inflorescence of a wild-type plant. (K) Inflorescence of a 35S::PeMADS7 transgenic plant. 1062
11 C and D function in orchids Table 1 Comparison of sepal length, petal length and seed weight between wild-type and transgenic Phalaenopsis equestris plants Plants Wild-type plant 35S::PeMADS7 Sepal length a (mm) 1.95 ± ± 0.19 Petal length b (mm) 3.17 ± ± ,0000-seed weight (g) a Values are obtained from 200 sepals from wild-type and 35S::PeMADS7 plants. b Values are obtained from 200 petals from wild-type and 35S::PeMADS7 plants. interaction between C- and D-class proteins in wheat. Their interaction should be bridged by the E-class protein (Yamada et al. 2009). Our results are consistent with those of previous reports suggesting conserved protein behaviors between C- and D-class proteins and among C-, D- and E-class proteins in eudicots and monocots. We also observed that the E-class PeMADS8 has an overlapping expression pattern with PeMADS1 and PeMADS7 in the gynostemium and ovules (data not shown). PeMADS1 PeMADS8 PeMADS7 protein complexes may play crucial roles in Phalaenopsis gynostemium and ovule development. Ectopic expression of many AG homologous genes in eudicots, such as NAG1 from tobacco (Kempin et al. 1993), TAG1 from tomato (Pnueli et al. 1994, Busi et al. 2003), CUM1 from cucumber (Kater etal. 1998), CaMADS1 from hazelnut (Rigola et al. 2001), VvMADS1 from grapevine (Boss et al. 2001), MASAKO C1 and D1 from rose (Kitahara et al. 2004), and TrAG and TrSHP from Taihangia rupestris (Lü et al. 2007), resulted in phenotypic alterations mimicking those of ectopic expression of AG in Arabidopsis (Mizukami and Ma 1992). The conserved C-function of gymnosperm AGAMOUS homologs, such as SAG1 from Picea mariana and DAL2 from Picea abies, was shown by ectopic expression in Arabidopsis (Rutledge et al. 1998, Tandre et al. 1998). Moreover, functional studies showed that DcOAG1, an AG ortholog from D. crumenatum, could replace the functions of AG in Arabidopsis (Xu et al. 2006). These shared phenotypes of transgenic Arabidopsis include early flowering, curly leaves, loss of inflorescence determinancy and homeotic transformation in the first and the second flower whorls. However, transgenic Arabidopsis constitutively expressing OMADS4 (Oncidium) and CeMADS1 (Cymbidium) showed only early flowering and a terminal flower phenotype, respectively (Hsu et al. 2010, Wang et al. 2011). These differences might be due to the diverse C-class gene functions in orchids. In this study, since no sepal-to-carpel and petal-to-stamen phenotypes were obtained by overexpressing PeMADS1 in wild-type Arabidopsis, the Phalaenopsis C-class gene might not function in whorl 1 and 2 of Arabidopsis flowers. This is probably due to the fact that Phalaenopsis C-class proteins could not interact with the class B and SEP proteins in Arabidopsis. However, overexpression of PeMADS1 could restore the female organ with carpelloid structures in the ag-4 mutant. Conserved functions of orchid C-class genes for specifying carpel identity could occur in whorl 4 of Arabidopsis flowers, which indicates that some kind of competence of whorl 4 is needed for PeMADS1 function to specify carpel identity. Studies of Petunia have revealed that ectopic expression of D-class FBP7 or FBP11 induces the formation of ovules on sepals and petals (Colombo et al. 1995). However, ectopic expression of FBP7 or FBP11 in Arabidopsis failed to induce ectopic ovule formation (Favaro et al. 2002). Consistently, transgenic Arabidopsis phenotypes of overexpressed D-class genes, such as petunia FBP7 or FBP11, rice OsMADS13, lily LMADS2, lisianthus EgMADS1, cotton GbAGL1 and Oncidium OMADS2, include early flowering and curly leav (Favaro et al. 2002, Tzeng et al. 2002, Liu et al. 2010, Hsu et al. 2010). Interestingly, LMADS2 and EgMADS1 also show homeotic conversion of sepals into carpel-like structures and petals into stamen-like structures (Tzeng et al. 2002). In our study, transgenic phenotypes of overexpressed PeMADS7 agreed with reports of the overexpression of FBP7, FBP11 or OsMADS13 in Arabidopsis resulting in phenotypes of early flowering, curly leaf, reduced size of sepals, and petals without homeotic conversions. In addition to these effects, PeMADS7 transgenic plants also showed bent siliques and increased seed sterility, which indicates that the function of PeMADS7 is related to ovule development in orchids. In conclusion, both PeMADS1 and PeMADS7 play important roles in orchid gynostemium and ovule development. The similarity of expression patterns and differences compared with ectopic expression in Arabidopsis suggest that functional conservation and diversification of these two genes probably occurs when they perform functions in orchid gynostemium and ovule development. Identification and functional analyses of upstream and downstream candidates of these two genes will provide more knowledge about the evolution of orchid gynostemium and ovule development. Materials and Methods Plant materials Phalaenopsis mutants with gynostemium-like petals were provided by OX Flowers Farm. All plants were grown in a greenhouse at the National Cheng Kung University under natural light (photosynthetic photon flux density 90 mmol m 2 s 1 ) and controlled temperature (23 27 C). Scanning electron microscopy Samples were fixed in FAA [18 : 1 : 1 of ethanol (50%), glacial acetic acid and formalin). After dehydration in an alcohol acetone series, samples were critical-point-dried, sputtercoated with platinum and observed by SEM (Hitachi S-4200, Tokyo) with an accelerating voltage of 15 kv. Photographs were taken with use of Verichrome pan film (Kodak). RNA preparation For RNA extraction, samples were collected and immersed in liquid nitrogen, and stored at 80 C until RNA extraction. 1063
12 Y.-Y. Chen et al. Total RNA was extracted following the method described by Tsai et al. (2004) RACE of PeMADS cdna ends We used 5 0 RACE to obtain the full-length cdna by use of the SMART RACE cdna amplification kit (Clontech). First-strand cdnas were synthesized from 400 ng of total RNA of stage 4 flower buds following the manufacturer s protocol. The cdna containing the 5 0 end for PeMADS clones was obtained by PCR amplification with a 5 0 -specific universal primer (Clontech) and a 3 0 gene-specific primer for different PeMADS genes. The gene-specific primers for PeMADS1 and PeMADS7 were 5 0 -GG AATCAAATGGAGGCATCACTTCATAC-3 0 and 5 0 -GCTCTTTC GTTATCTGCTATCTTGGCC-3 0, respectively. The thermal cycling was initial denaturation at 94 C for 5 s, then 25 cycles at 94 C for 30 s, 65 C for 30 s, 72 C for 2 min and a final extension at 72 C for 5 min. RACE products were reamplified with the nested universal primer provided in the RACE kit and a PeMADS gene-specific nested primer. The gene-specific nested primers for PeMADS1 and PeMADS7 were 5 0 -CTGCATGTAGTC AATCTCAGCATGCAGC-3 0 and 5 0 -GCCTCTTTCAAGTCGGTT TTCAAGTTGC-3 0. The PCR protocol consisted of initial denaturation at 94 C for 5 min, then 30 cycles at 94 C for 30 s, 60 C for 30 s, 72 C for 2 min and a final extension at 72 C for 5 min. The PCR products were cloned into pgem-t Easy vector (Promega) and sequenced on both strands from 10 positive clones. Sequence data analysis Raw DNA sequence data were edited by using Sequencher V. 4.1 (GeneCode) to remove vector and poly(a) sequences, and poor quality data. Computer-processed sequences were checked manually, compared with electropherograms and edited further to improve the quality and reliability of data. Phylogenetic analysis The 43 genes used in the phylogenetic analysis were downloaded from GenBank, and sequence alignment was done by Clustal W (Thompson et al. 1994). Non-conserved I- and C-regions were excluded from the alignment. Phylogenetic trees were constructed by the Neighbor Joining method and evaluated by bootstrap values estimated by 1,000 replicate runs. Isolation of genomic DNA and Southern blot analysis Genomic DNA was isolated from Phalaenopsis leaves as described (Tsai et al. 2004). Samples of the genomic DNA were digested with restriction enzymes BglII, EcoRI and HindIII for PeMADS1 hybridization. Genomic DNA was digested with restriction enzymes DraI, EcoRI and HindIII for PeMADS7 hybridization, then resolved on 0.8% agarose gels, and transferred to a Hybond-N + nylon membrane (Amersham Pharmacia Biotech) by a vacuum transfer system (Amersham Pharmacia Biotech). The partial C-terminal region and 3 0 -untranslated region (UTR) sequences were selected as the probes. A PeMADS1-specific probe (303 bp) was generated by PCR with the PeMADS1-specific internal primer pair 5 0 -AGCAC AGCAGCAGCATCAGCATATGAG-3 0 and 5 0 -GGAACATTGAT CAGTTGGAAGCTGTGG-3 0. A PeMADS7-specific probe (305 bp) was amplified with the PeMADS7-specific internal primer pair 5 0 -TGAGAGCATTCCAAGCTTCGAC-3 0 and 5 0 -GC TGCATTATATATGTCATTTGCTCC-3 0. Southern blots were hybridized with the a- 32 P-labeled probes. Pre-hybridization and hybridization were performed following the standard protocols (Sambrook et al., 2001). After hybridization, the filter was rinsed with 1 SSC, 0.1% SDS, then washed with 1 SSC, 0.1% SDS at 65 C for 1 h, then washed again with 0.1 SSC, 0.1% SDS at 65 C for 20 min. RT PCR analyses RNA samples were treated with RQ1 DNase (Promega) to remove remnant DNA, and synthesis of first-strand cdna was carried out by using the Superscript II kit (Invitrogen). Gene-specific primers were 5 0 -CTTCAAGCATGGAGCCGA AGGA-3 0 and 5 0 -GGAACATTGATCAGTTGGAAGCTGTGG-3 0 for PeMADS1, and 5 0 -GAGATCATGGGGAGGGGAAAAATTG AGATC-3 0 and 5 0 -GCTGCATTATATATGTCATTTGCTCC-3 0 for PeMADS7. The actin gene of Phalaenopsis was used as an internal control with the gene-specific primers 5 0 -GGCTAACA GAGAGAAGATGACC-3 0 and 5 0 -AATAGACCCTCCAATCCA GAC-3 0. The PCR protocol was initial denaturation at 94 C for 3 min, then 30 cycles of amplification (94 C for 30 s, 55 C for 30 s, 72 C for 1 min) and 72 C for 3 min. The amplified products were separated on an agarose gel and photographed. Real-time quantitative RT PCR RNA samples were treated with RQ1 DNase (Promega) to remove remnant DNA, then underwent synthesis of first-strand cdna by use of the Superscript II kit (Invitrogen). The primers for real-time RT PCR were designed by using Primer Express (Applied Biosystems). Gene-specific primers were 5 0 -TGATGCC TCCATTTGATTCCA-3 0 and 5 0 -ATAACGGTCATTGGGATC CATT-3 0 for PeMADS1; 5 0 -GCATCTCATTACTCACACCATCA AG-3 0 and 5 0 -TGATCGGCTTTTGTCTCATAGC-3 0 for PeMADS7; and 5 0 -CCGGATCAGCAAAGGTTGA-3 0 and 5 0 -AA GATTTGCATCCCTCCCC-3 0 for ubiqutin, used as an internal quantification control. Each real-time RT PCR contained 5 ng of cdna, 20 mm primers and 12.5 ml of SYBR GREEN PCR Master Mix (Applied Biosystems), and water was added to 25 ml. Real-time PCR involved use of the ABI 7500 Real-Time PCR Instrument (Applied Biosystems). The PCR protocol consisted of initial denaturation at 94 C for 10 min, then 40 cycles at 94 C for 15 s, 60 C for 1 min and a dissociation stage, 95 C for 15 s, 60 C for 1 min, 95 C for 15 s. For each real-time RT PCR, each sample was analyzed in triplicate. Data analysis involved use of sequencing detection software (Version 1.2.2; Applied Biosystems). 1064
13 C and D function in orchids In situ hybridization Samples were fixed in 4% (v/v) paraformaldehyde and 0.5% (v/v) glutaraldehyde for 24 h at 4 C, then dehydrated through an ethanol series, embedded in Histoplast and sectioned at 8 mm by use of the Shandon Finesse 325 rotary microtome (Thermo). Digoxigenin-labeled sense and antisense RNA probes containing a partial C-terminal region and 3 0 -UTR as described previously were synthesized following the manufacturer s instructions (Roche Applied Science). Hybridization and immunological detection of the signals with alkaline phosphatase were as described (Boehringer Mannheim). Yeast two- and three-hybrid assays Yeast two-hybrid assays were performed by using the MATCHMAKER system (Clontech). An XhoI fragment containing the full-length coding region of PeMADS1 and EcoRI fragments containing the full-length coding region of PeMADS7 and PeMADS8 were generated by PCR amplification and cloned into the binding domain vector pgbkt7 and the activation domain vector pgadt7. Primers for cloning the full-length coding region were 5 0 -CTCGAGTTATGGAGCC GAAGGA-3 0 and 5 0 -CTCGAGTTTCACCCAAGTTGCA-3 0 for PeMADS1; 5 0 -GAATTCATGGGGAGGGGAAAAATTGAG ATC-3 0 and 5 0 -GAATTCGACTAGTAGTTTTTGTAAAGC TCGC-3 0 for PeMADS7; and 5 0 -GAATTCATGGGAAGAGGGA GAGTGGAGC-3 0 and 5 0 -GAATTCGGGGGAAAGCCAGAAAT TTGTA-3 0 for PeMADS8. For yeast three-hybrid assays, we used the prey vector (pgadt7) and adaptor vector (pbridge) provided with the MATCHMAKER system (Clontech). An XhoI fragment containing PeMADS1 and an EcoRI fragment containing PeMADS7 were cloned into the pgadt7 vector. A NotI fragment containing PeMADS8 was cloned into the bridge site of the pbridge vector, and both PeMADS1 and PeMADS7 were cloned into the DNA-binding domain, respectively. Proper fusion of the constructs was confirmed by sequence determination. Yeast strain AH109 was used for transformation and involved the lithium acetate and colony-lift filter assay methods (Gietz et al. 1992). The transformants were screened on selection medium lacking adenine, histidine, leucine and tryptophan ( 4) according to the manufacturer s instructions (Clontech). Construction of transformed fusions An SmaI fragment containing the full-length cdna of PeMADS1 and an XbaI fragment containing the full-length cdna of PeMADS7 were cloned into the binary vector pbi121 (Clontech) under the control of a CaMV 35S promoter. The sense constructs were verified by PCR and then introduced into Agrobacterium tumefaciens GV3101 for plant transformation. The primer pairs for the full-length PeMADS1 cdna were 5 0 -CCCGGGTATGGAGCCGAAGGAGAAGATGGGGAGGG-3 0 and 5 0 -CCCGGGCCATCACCCAAGTTGCAGAGCTGTTTGC-3 0, and for PeMADS TCTAGACTTTGCTGTTTCTCGCCCT CCG-3 0 and 5 0 -TCTAGAGCTGCATTATATATGTCATTTGCT CC-3 0. Plant transformation The floral dip method was used for Arabidopsis transformation (Clough and Bent 1998). To select transformed Arabidopsis, seeds (T 0 ) were screened on media supplemented with 50 mgml 1 kanamycin (Sigma-Aldrich). After 2 weeks selection, the kanamycin-resistant seedlings (T 1 ) were transferred to soil and grown as previously described. Kanamycin segregation in the T 1 generation was analyzed by 2 test. The homozygous, kanamycin-resistant T 2 generation was used to confirm the integration fragment by PCR for each construct. Transformed lines with a segregation ratio of 3 : 1 were collected for further analysis. Complementation test To generate 35S-PeMADS1 ag-4 plants, pistils of transgenic 35S-PeMADS1 homologous plants were pollinated with pollen grains from ag-4 homozygous mutants, and all plants in the F 1 generation were kanamycin resistant. In the F 2 generation, approximately 75% of the plants were kanamycin resistant. For identifying ag-4 homozygous plants carrying 35S-PeMADS1, a point mutation in the 3 0 splice site from AG to AA in the fifth intron of AG was verified by genomic PCR and sequencing. Supplementary data Supplementary data are available at PCP online. Funding This work was supported by the National Science Council, Taiwan [grant Nos. NSC B MY3 and NSC B MY3 to W.C.T.]. Acknowledgments We thank Dr. Elliot M. Meyerowitz (Division of Biology, California Institute of Technology, Pasadena, CA, USA) for the gift of ag-4 seeds. We also thank Dr. Michel Delseny for critical reading of the manuscript and helpful discussion. References Atwood, J.T. (1986) The size of Orchidaceae and the systematic distribution of epiphytic orchids. Selbyana 9: Bleecker, A.B. and Patterson, S.E. (1997) Last exit: senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 9: Boss, P.K., Vivier, M., Matsumoto, S., Dry, I.B. and Thomas, M.R. (2001) A cdna from grapevine (Vitis vinifera L.), which shows homology to 1065
Duplicated C-Class MADS-Box Genes Reveal Distinct Roles in Gynostemium Development in Cymbidium ensifolium (Orchidaceae)
 Duplicated C-Class MADS-Box Genes Reveal Distinct Roles in Gynostemium Development in Cymbidium ensifolium (Orchidaceae) Shih-Yu Wang 1, Pei-Fang Lee 2,9, Yung-I Lee 3,9, Yu-Yun Hsiao 4, You-Yi Chen 5,
Duplicated C-Class MADS-Box Genes Reveal Distinct Roles in Gynostemium Development in Cymbidium ensifolium (Orchidaceae) Shih-Yu Wang 1, Pei-Fang Lee 2,9, Yung-I Lee 3,9, Yu-Yun Hsiao 4, You-Yi Chen 5,
The Plant Cell, November. 2017, American Society of Plant Biologists. All rights reserved
 The Genetics of Floral Development Teaching Guide Overview The development of flowers in angiosperm plants provided a critical evolutionary advantage, allowing more options for pollen dispersal and seed
The Genetics of Floral Development Teaching Guide Overview The development of flowers in angiosperm plants provided a critical evolutionary advantage, allowing more options for pollen dispersal and seed
Redefining C and D in the Petunia ABC W
 The Plant Cell, Vol. 24: 2305 2317, June 2012, www.plantcell.org ã 2012 American Society of Plant Biologists. All rights reserved. RESEARCH ARTICLES Redefining C and D in the Petunia ABC W Klaas Heijmans,
The Plant Cell, Vol. 24: 2305 2317, June 2012, www.plantcell.org ã 2012 American Society of Plant Biologists. All rights reserved. RESEARCH ARTICLES Redefining C and D in the Petunia ABC W Klaas Heijmans,
Shanhua Lü a,b, Xiaoqiu Du a, Wenliang Lu a, Kang Chong a, and Zheng Meng a,
 EVOLUTION & DEVELOPMENT 9:1, 92 104 (2007) Two AGAMOUS-like MADS-box genes from Taihangia rupestris (Rosaceae) reveal independent trajectories in the evolution of class C and class D floral homeotic functions
EVOLUTION & DEVELOPMENT 9:1, 92 104 (2007) Two AGAMOUS-like MADS-box genes from Taihangia rupestris (Rosaceae) reveal independent trajectories in the evolution of class C and class D floral homeotic functions
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Ectopic Expression of Carpel-Specific MADS Box Genes from Lily and Lisianthus Causes Similar Homeotic Conversion of Sepal and Petal in Arabidopsis 1
 Ectopic Expression of Carpel-Specific MADS Box Genes from Lily and Lisianthus Causes Similar Homeotic Conversion of Sepal and Petal in Arabidopsis 1 Tsai-Yu Tzeng 2, Hsing-Yu Chen 2, and Chang-Hsien Yang*
Ectopic Expression of Carpel-Specific MADS Box Genes from Lily and Lisianthus Causes Similar Homeotic Conversion of Sepal and Petal in Arabidopsis 1 Tsai-Yu Tzeng 2, Hsing-Yu Chen 2, and Chang-Hsien Yang*
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development
 A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
The MADS Box Gene FBP2 Is Required for SEPALLATA Function in Petunia
 The Plant Cell, Vol. 15, 914 925, April 2003, www.plantcell.org 2003 American Society of Plant Biologists The MADS Box Gene FBP2 Is Required for SEPALLATA Function in Petunia Silvia Ferrario, a Richard
The Plant Cell, Vol. 15, 914 925, April 2003, www.plantcell.org 2003 American Society of Plant Biologists The MADS Box Gene FBP2 Is Required for SEPALLATA Function in Petunia Silvia Ferrario, a Richard
Isolation of a differentially spliced C-type flower specific AG-like MADS-box gene from Crocus sativus and characterization of its expression
 BIOLOGIA PLANTARUM 49 (4): 499-504, 2005 Isolation of a differentially spliced C-type flower specific AG-like MADS-box gene from Crocus sativus and characterization of its expression A.S. TSAFTARIS*, **,1,
BIOLOGIA PLANTARUM 49 (4): 499-504, 2005 Isolation of a differentially spliced C-type flower specific AG-like MADS-box gene from Crocus sativus and characterization of its expression A.S. TSAFTARIS*, **,1,
Molecular Genetics of. Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS
 Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Cloning and expression analysis of an E-class MADS-box gene from Populus deltoides
 African Journal of Biotechnology Vol. 8 (19), pp. 4789-4796, 5 October, 2009 Available online at http://www.academicjournals.org/ajb ISSN 1684 5315 2009 Academic Journals Full Length Research Paper Cloning
African Journal of Biotechnology Vol. 8 (19), pp. 4789-4796, 5 October, 2009 Available online at http://www.academicjournals.org/ajb ISSN 1684 5315 2009 Academic Journals Full Length Research Paper Cloning
Flower development of Phalaenopsis orchid involves functionally divergent SEPALLATA-like genes
 Research Flower development of Phalaenopsis orchid involves functionally divergent SEPALLATA-like genes Zhao-Jun Pan 1, You-Yi Chen 2, Jian-Syun Du 1, Yun-Yu Chen 3, Mei-Chu Chung 4, Wen-Chieh Tsai 2,5,
Research Flower development of Phalaenopsis orchid involves functionally divergent SEPALLATA-like genes Zhao-Jun Pan 1, You-Yi Chen 2, Jian-Syun Du 1, Yun-Yu Chen 3, Mei-Chu Chung 4, Wen-Chieh Tsai 2,5,
Supplemental Data. Perea-Resa et al. Plant Cell. (2012) /tpc
 Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Supplementary Figure S1. Amino acid alignment of selected monocot FT-like and TFL-like sequences. Sequences were aligned using ClustalW and analyzed
 Supplementary Figure S1. Amino acid alignment of selected monocot FT-like and TFL-like sequences. Sequences were aligned using ClustalW and analyzed using the Geneious software. Accession numbers of the
Supplementary Figure S1. Amino acid alignment of selected monocot FT-like and TFL-like sequences. Sequences were aligned using ClustalW and analyzed using the Geneious software. Accession numbers of the
The Orchid MADS-Box Genes Controlling Floral Morphogenesis
 Review Article Special Issue: Evolution of MADS-box genes in Monocots TheScientificWorldJOURNAL (2006) 1, 109 120 TSW Development and Embryology ISSN 1537-744X; DOI 10.1100/tswde.2006.321 The Orchid MADS-Box
Review Article Special Issue: Evolution of MADS-box genes in Monocots TheScientificWorldJOURNAL (2006) 1, 109 120 TSW Development and Embryology ISSN 1537-744X; DOI 10.1100/tswde.2006.321 The Orchid MADS-Box
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Supplemental Data. Yang et al. (2012). Plant Cell /tpc
 Supplemental Figure 1. Mature flowers of P. heterotricha. (A) An inflorescence of P. heterotricha showing the front view of a zygomorphic flower characterized by two small dorsal petals and only two fertile
Supplemental Figure 1. Mature flowers of P. heterotricha. (A) An inflorescence of P. heterotricha showing the front view of a zygomorphic flower characterized by two small dorsal petals and only two fertile
75 Development of floral organ identity: stories from the MADS house Günter Theißen Recent studies on AGAMOUS-LIKE2-, DEFICIENS- and GLOBOSA-like MADS
 75 Development of floral organ identity: stories from the MADS house Günter Theißen Recent studies on AGAMOUS-LIKE2-, DEFICIENS- and GLOBOSA-like MADS-box genes in diverse seed plant species have provided
75 Development of floral organ identity: stories from the MADS house Günter Theißen Recent studies on AGAMOUS-LIKE2-, DEFICIENS- and GLOBOSA-like MADS-box genes in diverse seed plant species have provided
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Lilium longiflorum and Molecular Floral Development: the ABCDE Model
 Lilium longiflorum and Molecular Floral Development: the ABCDE Model V.A. Benedito Laboratório de Melhoramento de Plantas CENA/USP Av. Centenario, 303 13400-961 Piracicaba-SP Brazil G.C. Angenent, J.M.
Lilium longiflorum and Molecular Floral Development: the ABCDE Model V.A. Benedito Laboratório de Melhoramento de Plantas CENA/USP Av. Centenario, 303 13400-961 Piracicaba-SP Brazil G.C. Angenent, J.M.
New Phytologist. II. Phylogeny and subfunctionalization within the AGAMOUS subfamily
 Review MADS reloaded: evolution of the AGAMOUS subfamily genes Author for correspondence: Martin M. Kater Tel: +39 02 50315050 Email: martin.kater@unimi.it Ludovico Dreni and Martin M. Kater Department
Review MADS reloaded: evolution of the AGAMOUS subfamily genes Author for correspondence: Martin M. Kater Tel: +39 02 50315050 Email: martin.kater@unimi.it Ludovico Dreni and Martin M. Kater Department
Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla
 Plant Biotechnology Journal (2015), pp. 1 15 doi: 10.1111/pbi.12383 Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla Choun-Sea Lin 1, *, Chen-Tran Hsu 1, De-Chih Liao
Plant Biotechnology Journal (2015), pp. 1 15 doi: 10.1111/pbi.12383 Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla Choun-Sea Lin 1, *, Chen-Tran Hsu 1, De-Chih Liao
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Regulatory Change in YABBY-like Transcription Factor Led to Evolution of Extreme Fruit Size during Tomato Domestication
 SUPPORTING ONLINE MATERIALS Regulatory Change in YABBY-like Transcription Factor Led to Evolution of Extreme Fruit Size during Tomato Domestication Bin Cong, Luz Barrero, & Steven Tanksley 1 SUPPORTING
SUPPORTING ONLINE MATERIALS Regulatory Change in YABBY-like Transcription Factor Led to Evolution of Extreme Fruit Size during Tomato Domestication Bin Cong, Luz Barrero, & Steven Tanksley 1 SUPPORTING
Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla
 Plant Biotechnology Journal (2016) 14, pp. 284 298 doi: 10.1111/pbi.12383 Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla Choun-Sea Lin 1, *, Chen-Tran Hsu 1, De-Chih
Plant Biotechnology Journal (2016) 14, pp. 284 298 doi: 10.1111/pbi.12383 Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla Choun-Sea Lin 1, *, Chen-Tran Hsu 1, De-Chih
Contents. Foreword. ORCHID BIOTECHNOLOGY II World Scientific Publishing Co. Pte. Ltd.
 Foreword Preface v vii 1. Molecular Phylogeny and Biogeography of Phalaenopsis Species 1 1.1 Introduction... 1 1.2 Biogeographical Pattern of Genus Phalaenopsis.. 6 1.3 Molecular Phylogeny and Biogeography
Foreword Preface v vii 1. Molecular Phylogeny and Biogeography of Phalaenopsis Species 1 1.1 Introduction... 1 1.2 Biogeographical Pattern of Genus Phalaenopsis.. 6 1.3 Molecular Phylogeny and Biogeography
Plant Structure, Growth, and Development
 Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Evolution and Development of Flower Diversity. Kelsey Galimba Di Stilio Lab Department of Biology University of Washington
 Evolution and Development of Flower Diversity Kelsey Galimba Di Stilio Lab Department of Biology University of Washington Lecture Outline Evolution of Angiosperms! The first flower! Morphological Diversity
Evolution and Development of Flower Diversity Kelsey Galimba Di Stilio Lab Department of Biology University of Washington Lecture Outline Evolution of Angiosperms! The first flower! Morphological Diversity
Reproductive Development
 Plant Reproduction Chapter 42 Angiosperms represent an evolutionary innovation with their production of flowers and fruits Plants go through developmental changes leading to reproductive maturity by adding
Plant Reproduction Chapter 42 Angiosperms represent an evolutionary innovation with their production of flowers and fruits Plants go through developmental changes leading to reproductive maturity by adding
RNAi Suppression of AGAMOUS-like Genes Causes Field Sterility in Populus
 RNAi Suppression of AGAMOUS-like Genes Causes Field Sterility in Populus Haiwei Lu and Steven H. Strauss Oregon State University Forest Tree Workshop PAG XXVI, San Diego, CA, 2018 The containment issue
RNAi Suppression of AGAMOUS-like Genes Causes Field Sterility in Populus Haiwei Lu and Steven H. Strauss Oregon State University Forest Tree Workshop PAG XXVI, San Diego, CA, 2018 The containment issue
Flowers Seeds Pollination Germination
 * Flowers Seeds Pollination Germination *In order for plants to be successful in many different environments they must be able to reproduce themselves. *The reproductive patterns of plants reflect the
* Flowers Seeds Pollination Germination *In order for plants to be successful in many different environments they must be able to reproduce themselves. *The reproductive patterns of plants reflect the
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
Effect of Acetosyringone on Agrobacterium-mediated Transformation of Eustoma grandiflorum Leaf Disks
 JARQ 51 (4), 351-355 (2017) https://www.jircas.go.jp Improvement in Agrobacterium-mediated Transformation of Eustoma grandiflorum by Acetosyringone Effect of Acetosyringone on Agrobacterium-mediated Transformation
JARQ 51 (4), 351-355 (2017) https://www.jircas.go.jp Improvement in Agrobacterium-mediated Transformation of Eustoma grandiflorum by Acetosyringone Effect of Acetosyringone on Agrobacterium-mediated Transformation
Ectopic expression of LLAG1, an AGAMOUS homologue from lily (Lilium longi orum Thunb.) causes oral homeotic modi cations in Arabidopsis
 Journal of Experimental Botany, Vol. 55, No. 401, pp. 1391±1399, June 2004 DOI: 10.1093/jxb/erh156 Advance Access publication 21 May, 2004 RESEARCH PAPER Ectopic expression of LLAG1, an AGAMOUS homologue
Journal of Experimental Botany, Vol. 55, No. 401, pp. 1391±1399, June 2004 DOI: 10.1093/jxb/erh156 Advance Access publication 21 May, 2004 RESEARCH PAPER Ectopic expression of LLAG1, an AGAMOUS homologue
Development 143: doi: /dev : Supplementary information
 Supplementary Materials and Methods Plant materials The mutants and transgenic plants used in the present study were as follows: E361 (from Alex Webb s laboratory); tmm-1, ptmm::tmm-gfp and flp-1 (from
Supplementary Materials and Methods Plant materials The mutants and transgenic plants used in the present study were as follows: E361 (from Alex Webb s laboratory); tmm-1, ptmm::tmm-gfp and flp-1 (from
. Supplementary Information
 . Supplementary Information Supplementary Figure S1. Mature embryo sac observations. Supplementary Figure S2. STT observations. Supplementary Figure S3. Comparison of the PTB1 cdna with that of the mutant.
. Supplementary Information Supplementary Figure S1. Mature embryo sac observations. Supplementary Figure S2. STT observations. Supplementary Figure S3. Comparison of the PTB1 cdna with that of the mutant.
The mode of development in animals and plants is different
 The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
State Key Laboratory of Crop Stress Biology in Arid Areas, Northwest A&F University, Yangling, Shaanxi, China
 Molecular cloning and expression of the male sterility-related CtYABBY1 gene in flowering Chinese cabbage (Brassica campestris L. ssp chinensis var. parachinensis) X.L. Zhang 1,2,3 and L.G. Zhang 1,2,3
Molecular cloning and expression of the male sterility-related CtYABBY1 gene in flowering Chinese cabbage (Brassica campestris L. ssp chinensis var. parachinensis) X.L. Zhang 1,2,3 and L.G. Zhang 1,2,3
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4, 116,000 120M Open access books available International authors and editors Downloads Our authors
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4, 116,000 120M Open access books available International authors and editors Downloads Our authors
Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang Chen, Qiang Dong, Jiangqi Wen, Kirankumar S. Mysore and Jian Zhao
 New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Genome-wide Identification of Lineage Specific Genes in Arabidopsis, Oryza and Populus
 Genome-wide Identification of Lineage Specific Genes in Arabidopsis, Oryza and Populus Xiaohan Yang Sara Jawdy Timothy Tschaplinski Gerald Tuskan Environmental Sciences Division Oak Ridge National Laboratory
Genome-wide Identification of Lineage Specific Genes in Arabidopsis, Oryza and Populus Xiaohan Yang Sara Jawdy Timothy Tschaplinski Gerald Tuskan Environmental Sciences Division Oak Ridge National Laboratory
Molecular Evolution of MADS-box Genes in Cotton (Gossypium L.)
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2007 Molecular Evolution of MADS-box Genes in Cotton (Gossypium L.) Wusheng Liu
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2007 Molecular Evolution of MADS-box Genes in Cotton (Gossypium L.) Wusheng Liu
Nature Genetics: doi: /ng Supplementary Figure 1. ssp mutant phenotypes in a functional SP background.
 Supplementary Figure 1 ssp mutant phenotypes in a functional SP background. (a,b) Statistical comparisons of primary and sympodial shoot flowering times as determined by mean values for leaf number on
Supplementary Figure 1 ssp mutant phenotypes in a functional SP background. (a,b) Statistical comparisons of primary and sympodial shoot flowering times as determined by mean values for leaf number on
673 Comparative Genomics of Angiosperm MADS Box Genes Yale University, New Haven, CT. 674 The evolution of plant architecture in Brassicaceae
 673 Comparative Genomics of Angiosperm MADS Box Genes Vivian F. Irish Yale University, New Haven, CT. MADS box genes encode key transcriptional regulators that have been implicated in the control of various
673 Comparative Genomics of Angiosperm MADS Box Genes Vivian F. Irish Yale University, New Haven, CT. MADS box genes encode key transcriptional regulators that have been implicated in the control of various
DOWNLOAD PDF ANALYSIS OF EXPRESSION OF PHALAENOPSIS FLORAL ESTS WEN-CHIEH TSAI. [ET AL.]
![DOWNLOAD PDF ANALYSIS OF EXPRESSION OF PHALAENOPSIS FLORAL ESTS WEN-CHIEH TSAI. [ET AL.] DOWNLOAD PDF ANALYSIS OF EXPRESSION OF PHALAENOPSIS FLORAL ESTS WEN-CHIEH TSAI. [ET AL.]](/thumbs/93/111554121.jpg) Chapter 1 : Publications Authored by Wen-Chieh Tsai PubFacts The most highly transcribed genes in Phalaenopsis floral buds are those coding for RNA-dependent RNA polymerase of Cymbidium mosaic virus, followed
Chapter 1 : Publications Authored by Wen-Chieh Tsai PubFacts The most highly transcribed genes in Phalaenopsis floral buds are those coding for RNA-dependent RNA polymerase of Cymbidium mosaic virus, followed
*Modifications in reproduction were key adaptations enabling plants to spread into a variety of terrestrial habitats.
 Plant Reproduction *Modifications in reproduction were key adaptations enabling plants to spread into a variety of terrestrial habitats. Reproduction In Plants Plant reproduction is the production of new
Plant Reproduction *Modifications in reproduction were key adaptations enabling plants to spread into a variety of terrestrial habitats. Reproduction In Plants Plant reproduction is the production of new
Anatomy of Plants Student Notes
 Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Unit 11: Plants Guided Reading Questions (75 pts total)
 Name: AP Biology Biology, Campbell and Reece, 7th Edition Adapted from chapter reading guides originally created by Lynn Miriello Unit 11: Plants Guided Reading Questions (75 pts total) Chapter 29 Plant
Name: AP Biology Biology, Campbell and Reece, 7th Edition Adapted from chapter reading guides originally created by Lynn Miriello Unit 11: Plants Guided Reading Questions (75 pts total) Chapter 29 Plant
Structures of Seed Plants
 CHAPTER 12 SECTION 4 Introduction to Plants Structures of Seed Plants BEFORE YOU READ After you read this section, you should be able to answer these questions: What are the functions of roots and stems?
CHAPTER 12 SECTION 4 Introduction to Plants Structures of Seed Plants BEFORE YOU READ After you read this section, you should be able to answer these questions: What are the functions of roots and stems?
The MIK region rather than the C-terminal domain of
 The MIK region rather than the C-terminal domain of Blackwell Publishing Ltd AP3-like class B floral homeotic proteins determines functional specificity in the development and evolution of petals Kunmei
The MIK region rather than the C-terminal domain of Blackwell Publishing Ltd AP3-like class B floral homeotic proteins determines functional specificity in the development and evolution of petals Kunmei
To B or Not to B a Flower: The Role of DEFICIENS and GLOBOSA Orthologs in the Evolution of the Angiosperms
 Journal of Heredity 2005:96(3):1 16 doi:10.1093/jhered/esi033 ª 2005 The American Genetic Association To B or Not to B a Flower: The Role of DEFICIENS and GLOBOSA Orthologs in the Evolution of the Angiosperms
Journal of Heredity 2005:96(3):1 16 doi:10.1093/jhered/esi033 ª 2005 The American Genetic Association To B or Not to B a Flower: The Role of DEFICIENS and GLOBOSA Orthologs in the Evolution of the Angiosperms
Plant Growth and Development Part I. Levels of Organization
 Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules 1
Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules 1
Genetic transformation of table grape via organogenesis and field evaluation of DefH9-iaaM transgenic plants
 Genetic transformation of table grape via organogenesis and field evaluation of DefH9-iaaM transgenic plants Mezzetti B., Silvestroni O., Costantini E. Dipartimento di Scienze Ambientali e delle Produzioni
Genetic transformation of table grape via organogenesis and field evaluation of DefH9-iaaM transgenic plants Mezzetti B., Silvestroni O., Costantini E. Dipartimento di Scienze Ambientali e delle Produzioni
Supplemental Figure 1. Comparison of Tiller Bud Formation between the Wild Type and d27. (A) and (B) Longitudinal sections of shoot apex in wild-type
 A B 2 3 3 2 1 1 Supplemental Figure 1. Comparison of Tiller Bud Formation between the Wild Type and d27. (A) and (B) Longitudinal sections of shoot apex in wild-type (A) and d27 (B) seedlings at the four
A B 2 3 3 2 1 1 Supplemental Figure 1. Comparison of Tiller Bud Formation between the Wild Type and d27. (A) and (B) Longitudinal sections of shoot apex in wild-type (A) and d27 (B) seedlings at the four
Edward M. Golenberg Wayne State University Detroit, MI
 Edward M. Golenberg Wayne State University Detroit, MI Targeting Phragmites Success As Invasive Species Phragmites displays multiple life history parameters High seed output and small seed size Rapid growth
Edward M. Golenberg Wayne State University Detroit, MI Targeting Phragmites Success As Invasive Species Phragmites displays multiple life history parameters High seed output and small seed size Rapid growth
Worksheet for Morgan/Carter Laboratory #16 Plant Diversity II: Seed Plants
 Worksheet for Morgan/Carter Laboratory #16 Plant Diversity II: Seed Plants BE SURE TO CAREFULLY READ THE INTRODUCTION PRIOR TO ANSWERING THE QUESTIONS!!! You will need to refer to your text book to answer
Worksheet for Morgan/Carter Laboratory #16 Plant Diversity II: Seed Plants BE SURE TO CAREFULLY READ THE INTRODUCTION PRIOR TO ANSWERING THE QUESTIONS!!! You will need to refer to your text book to answer
Sporic life cycles involve 2 types of multicellular bodies:
 Chapter 3- Human Manipulation of Plants Sporic life cycles involve 2 types of multicellular bodies: -a diploid, spore-producing sporophyte -a haploid, gamete-producing gametophyte Sexual Reproduction in
Chapter 3- Human Manipulation of Plants Sporic life cycles involve 2 types of multicellular bodies: -a diploid, spore-producing sporophyte -a haploid, gamete-producing gametophyte Sexual Reproduction in
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Analysis of the Petunia TM6 MADS Box Gene Reveals Functional Divergence within the DEF/AP3 Lineage W
 This article is published in The Plant Cell Online, The Plant Cell Preview Section, which publishes manuscripts accepted for publication after they have been edited and the authors have corrected proofs,
This article is published in The Plant Cell Online, The Plant Cell Preview Section, which publishes manuscripts accepted for publication after they have been edited and the authors have corrected proofs,
Chapter 23: Plant Diversity and Life Cycles
 Chapter 23: Plant Diversity and Life Cycles Section 1: Introduction to Plants Cuticle: a waxy or fatty and watertight layer on the external wall of epidermal cells Spore: a reproductive cell or multicellular
Chapter 23: Plant Diversity and Life Cycles Section 1: Introduction to Plants Cuticle: a waxy or fatty and watertight layer on the external wall of epidermal cells Spore: a reproductive cell or multicellular
BIOLOGY 317 Spring First Hourly Exam 4/20/12
 Name: Lab sect. (TA name/time): BIOLOGY 317 Spring 2012 First Hourly Exam 4/20/12 1) (24 pts) Match the letter of the family given on the right with the characteristics for a plant described on the left.
Name: Lab sect. (TA name/time): BIOLOGY 317 Spring 2012 First Hourly Exam 4/20/12 1) (24 pts) Match the letter of the family given on the right with the characteristics for a plant described on the left.
Kingdom Plantae. Biology : A Brief Survey of Plants. Jun 22 7:09 PM
 Kingdom Plantae Biology 2201 6.1 6.2 : A Brief Survey of Plants The study of plants is called botany. Plants are believed to have evolved from green algae. The main plant (land) characteristics are as
Kingdom Plantae Biology 2201 6.1 6.2 : A Brief Survey of Plants The study of plants is called botany. Plants are believed to have evolved from green algae. The main plant (land) characteristics are as
BIOLOGY 366 PLANT SYSTEMATICS EXAM 1 SPRING POINTS TOTAL (LECTURE 60, LAB PRACTICAL 40)
 BIOLOGY 366 PLANT SYSTEMATICS EXAM 1 SPRING 2013 100 POINTS TOTAL (LECTURE 60, LAB PRACTICAL 40) SECTION 1 (Short answer; 40 points total): Be as precise as possible in your answers. 1. Name two synapomorphies
BIOLOGY 366 PLANT SYSTEMATICS EXAM 1 SPRING 2013 100 POINTS TOTAL (LECTURE 60, LAB PRACTICAL 40) SECTION 1 (Short answer; 40 points total): Be as precise as possible in your answers. 1. Name two synapomorphies
Embryo Development. Embryo Development. Embryo Development. Embryo Development (Cont.) Vegetative Plant Development
 Vegetative Plant Development Chapter 37 Embryo Development Begins once the egg cell is fertilized -The growing pollen tube enters angiosperm embryo sac and releases two sperm cells -One sperm fertilizes
Vegetative Plant Development Chapter 37 Embryo Development Begins once the egg cell is fertilized -The growing pollen tube enters angiosperm embryo sac and releases two sperm cells -One sperm fertilizes
Computational identification and analysis of MADS box genes in Camellia sinensis
 www.bioinformation.net Hypothesis Volume 11(3) Computational identification and analysis of MADS box genes in Camellia sinensis Madhurjya Gogoi*, Sangeeta Borchetia & Tanoy Bandyopadhyay Department of
www.bioinformation.net Hypothesis Volume 11(3) Computational identification and analysis of MADS box genes in Camellia sinensis Madhurjya Gogoi*, Sangeeta Borchetia & Tanoy Bandyopadhyay Department of
BIO10 Plant Lecture Notes ch. 17. Plant Kingdom
 Plant Kingdom Characteristics of the Plant Kingdom; eukaryotic, multicellular, sexually reproducing organisms autotroph feed themselves by photosynthesis Facts about members of this kingdom the dominant
Plant Kingdom Characteristics of the Plant Kingdom; eukaryotic, multicellular, sexually reproducing organisms autotroph feed themselves by photosynthesis Facts about members of this kingdom the dominant
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
By Jonathan I. Watkinson. Virginia Polytechnic Institute and State University. Doctor of Philosophy Horticulture
 Characterization of two genes, trehalose-6-phosphate synthase/phosphatase and nucleotide binding protein, shown to be differentially regulated in roots of Cypripedium parviflorum var. pubescens grown with
Characterization of two genes, trehalose-6-phosphate synthase/phosphatase and nucleotide binding protein, shown to be differentially regulated in roots of Cypripedium parviflorum var. pubescens grown with
Basic Principles of Plant Science EXAMINING PLANT STRUCTURES AND FUNCTIONS
 Basic Principles of Plant Science EXAMINING PLANT STRUCTURES AND FUNCTIONS Cellular Structure of Plants Cells are the structural basis of all living organisms. A cell is a tiny structure that forms the
Basic Principles of Plant Science EXAMINING PLANT STRUCTURES AND FUNCTIONS Cellular Structure of Plants Cells are the structural basis of all living organisms. A cell is a tiny structure that forms the
Curriculum vitae Xigang Liu
 Curriculum vitae Xigang Liu 1, EDUCATION: 09/1993-07/1997 B.S. Major: Biology. College of Life Sciences, Hebei Normal University Academic Degree Paper: RAPD analysis of Taigu genic male-sterile wheat and
Curriculum vitae Xigang Liu 1, EDUCATION: 09/1993-07/1997 B.S. Major: Biology. College of Life Sciences, Hebei Normal University Academic Degree Paper: RAPD analysis of Taigu genic male-sterile wheat and
Levels of Organization
 Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Plant
Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Plant
Tepal formation and expression pattern of B-class paleoap3-like MADS-box genes in crocus (Crocus sativus L.)
 Plant Science 170 (2006) 238 246 www.elsevier.com/locate/plantsci Tepal formation and expression pattern of B-class paleoap3-like MADS-box genes in crocus (Crocus sativus L.) Athanasios S. Tsaftaris a,b,
Plant Science 170 (2006) 238 246 www.elsevier.com/locate/plantsci Tepal formation and expression pattern of B-class paleoap3-like MADS-box genes in crocus (Crocus sativus L.) Athanasios S. Tsaftaris a,b,
Slide 1 / 86. Angiosperms: The Flowering Plants
 Slide 1 / 86 Angiosperms: The Flowering Plants Slide 2 / 86 Brief Phylogeny of Plants Monocot Dicot This presentation will focus on angiosperms Angiosperm Gymnosperm Seeded Plants Non-Seeded plants Vascular
Slide 1 / 86 Angiosperms: The Flowering Plants Slide 2 / 86 Brief Phylogeny of Plants Monocot Dicot This presentation will focus on angiosperms Angiosperm Gymnosperm Seeded Plants Non-Seeded plants Vascular
Eukaryotic vs. Prokaryotic genes
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 18: Eukaryotic genes http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Eukaryotic vs. Prokaryotic genes Like in prokaryotes,
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 18: Eukaryotic genes http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Eukaryotic vs. Prokaryotic genes Like in prokaryotes,
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplemental Data. Hou et al. (2016). Plant Cell /tpc
 Supplemental Data. Hou et al. (216). Plant Cell 1.115/tpc.16.295 A Distance to 1 st nt of start codon Distance to 1 st nt of stop codon B Normalized PARE abundance 8 14 nt 17 nt Frame1 Arabidopsis inflorescence
Supplemental Data. Hou et al. (216). Plant Cell 1.115/tpc.16.295 A Distance to 1 st nt of start codon Distance to 1 st nt of stop codon B Normalized PARE abundance 8 14 nt 17 nt Frame1 Arabidopsis inflorescence
BOTANY, PLANT PHYSIOLOGY AND PLANT GROWTH Lesson 6: PLANT PARTS AND FUNCTIONS Part 4 - Flowers and Fruit
 BOTANY, PLANT PHYSIOLOGY AND PLANT GROWTH Lesson 6: PLANT PARTS AND FUNCTIONS Part 4 - Flowers and Fruit Script to Narrate the PowerPoint, 06PowerPointFlowers and Fruit.ppt It is not permitted to export
BOTANY, PLANT PHYSIOLOGY AND PLANT GROWTH Lesson 6: PLANT PARTS AND FUNCTIONS Part 4 - Flowers and Fruit Script to Narrate the PowerPoint, 06PowerPointFlowers and Fruit.ppt It is not permitted to export
Chapter III- The Flower
 Worksheet Class 8-Flower, Pollination and Fertilization, Ecosystem. Chapter III- The Flower 1.Name the following. a.a flower in which both male and female reproductive organs are lacking. b.the groups
Worksheet Class 8-Flower, Pollination and Fertilization, Ecosystem. Chapter III- The Flower 1.Name the following. a.a flower in which both male and female reproductive organs are lacking. b.the groups
Life Science Chapter 11 SEED PLANTS PART 2
 Life Science Chapter 11 SEED PLANTS PART 2 Advanced Seed Producing Advanced Seed Producing Vascular Plants Class: Gymnospermae Class: Angiospermae» Subclass: Monocotyledoneae» Subclass: Dicotyledoneae
Life Science Chapter 11 SEED PLANTS PART 2 Advanced Seed Producing Advanced Seed Producing Vascular Plants Class: Gymnospermae Class: Angiospermae» Subclass: Monocotyledoneae» Subclass: Dicotyledoneae
Anatomy of Flowering Plants
 Dry Lab BIOLOGY Anatomy of Flowering Plants Investigation Manual ANATOMY OF FLOWERING PLANTS Table of Contents 2 Overview 2 Outcomes 2 Time Requirements 3 Background 6 Safety 6 Materials 7 Activity 1 10
Dry Lab BIOLOGY Anatomy of Flowering Plants Investigation Manual ANATOMY OF FLOWERING PLANTS Table of Contents 2 Overview 2 Outcomes 2 Time Requirements 3 Background 6 Safety 6 Materials 7 Activity 1 10
"PRINCIPLES OF PHYLOGENETICS: ECOLOGY AND EVOLUTION" Integrative Biology 200B Spring 2011
 "PRINCIPLES OF PHYLOGENETICS: ECOLOGY AND EVOLUTION" Integrative Biology 200B Spring 2011 Evolution and development ("evo-devo") The last frontier in our understanding of biological forms is an understanding
"PRINCIPLES OF PHYLOGENETICS: ECOLOGY AND EVOLUTION" Integrative Biology 200B Spring 2011 Evolution and development ("evo-devo") The last frontier in our understanding of biological forms is an understanding
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Introduction to Botany. Lecture 35
 Introduction to Botany. Lecture 35 Alexey Shipunov Minot State University December 1st, 2010 Outline Phylogeny of angiosperms so far 1 Phylogeny of angiosperms so far 2 Outline Phylogeny of angiosperms
Introduction to Botany. Lecture 35 Alexey Shipunov Minot State University December 1st, 2010 Outline Phylogeny of angiosperms so far 1 Phylogeny of angiosperms so far 2 Outline Phylogeny of angiosperms
IGCSE Double Award Extended Coordinated Science
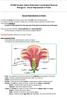 IGCSE Double Award Extended Coordinated Science Biology 8.2 - Sexual Reproduction in Plants Sexual Reproduction in Plants In a plant, the flower is the sexual organ, and it has both male and females parts.
IGCSE Double Award Extended Coordinated Science Biology 8.2 - Sexual Reproduction in Plants Sexual Reproduction in Plants In a plant, the flower is the sexual organ, and it has both male and females parts.
Analysis of Transgenic Tobacco with Overexpression of Arabidopsis WUSCHEL Gene
 Acta Botanica Sinica 2004, 46 (2): 224 229 http://www.chineseplantscience.com Analysis of Transgenic Tobacco with Overexpression of Arabidopsis WUSCHEL Gene LI Jun-Hua 1, 2, XU Yun-Yuan 1, CHONG Kang 1*,
Acta Botanica Sinica 2004, 46 (2): 224 229 http://www.chineseplantscience.com Analysis of Transgenic Tobacco with Overexpression of Arabidopsis WUSCHEL Gene LI Jun-Hua 1, 2, XU Yun-Yuan 1, CHONG Kang 1*,
The Break-through in the Reduction of Juvenile Phase in Apple Using Transgenic Approaches
 The Break-through in the Reduction of Juvenile Phase in Apple Using Transgenic Approaches N. Kotoda, M. Wada, T. Masuda and J. Soejima Department of Apple Research, National Institute of Fruit Tree Science,
The Break-through in the Reduction of Juvenile Phase in Apple Using Transgenic Approaches N. Kotoda, M. Wada, T. Masuda and J. Soejima Department of Apple Research, National Institute of Fruit Tree Science,
PLANT GROWTH. IB Topic 9.3 & 9.4 Urry text ref: Ch 28 & 31
 PLANT GROWTH IB Topic 9.3 & 9.4 Urry text ref: Ch 28 & 31 INDETERMINATE GROWTH = throughout life meristems like stem cells in humans Shoot tip (shoot apical meristem and young leaves) lateral Axillary
PLANT GROWTH IB Topic 9.3 & 9.4 Urry text ref: Ch 28 & 31 INDETERMINATE GROWTH = throughout life meristems like stem cells in humans Shoot tip (shoot apical meristem and young leaves) lateral Axillary
Liu, Yang (2012) The characterization of a novel abscission-related gene in Arabidopsis thaliana. PhD thesis, University of Nottingham.
 Liu, Yang (2012) The characterization of a novel abscission-related gene in Arabidopsis thaliana. PhD thesis, University of Nottingham. Access from the University of Nottingham repository: http://eprints.nottingham.ac.uk/12529/3/thesis_part_2_final.pdf
Liu, Yang (2012) The characterization of a novel abscission-related gene in Arabidopsis thaliana. PhD thesis, University of Nottingham. Access from the University of Nottingham repository: http://eprints.nottingham.ac.uk/12529/3/thesis_part_2_final.pdf
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Ms.Sastry, AP Biology Unit 4/Chp 26 to 34/Diversity 1 Chapter in class follow along lecture notes
 Ms.Sastry, AP Biology Unit 4/Chp 26 to 34/Diversity 1 Chapter 26 34 in class follow along lecture notes Chp 26 Origin of life: 1) When did earth form? 2) What is the order of evolution of life forms on
Ms.Sastry, AP Biology Unit 4/Chp 26 to 34/Diversity 1 Chapter 26 34 in class follow along lecture notes Chp 26 Origin of life: 1) When did earth form? 2) What is the order of evolution of life forms on
Name Section Lab 4 Flowers, Pollination and Fruit
 Name Section Lab 4 Flowers, Pollination and Fruit Flowers are designed on plants for sexual reproduction. They contain organs that produce gametes (sex cells), which, after fertilization, lead to the formation
Name Section Lab 4 Flowers, Pollination and Fruit Flowers are designed on plants for sexual reproduction. They contain organs that produce gametes (sex cells), which, after fertilization, lead to the formation
2. Yeast two-hybrid system
 2. Yeast two-hybrid system I. Process workflow a. Mating of haploid two-hybrid strains on YPD plates b. Replica-plating of diploids on selective plates c. Two-hydrid experiment plating on selective plates
2. Yeast two-hybrid system I. Process workflow a. Mating of haploid two-hybrid strains on YPD plates b. Replica-plating of diploids on selective plates c. Two-hydrid experiment plating on selective plates
Unit 7: Plant Evolution, Structure and Function
 Time: 7 Days (some time spent working over breaks on this topic) and then an exam 16% of the AP Exam is on this material. Topics Covered: Reproduction, growth, and development Structural, physiological,
Time: 7 Days (some time spent working over breaks on this topic) and then an exam 16% of the AP Exam is on this material. Topics Covered: Reproduction, growth, and development Structural, physiological,
B-Function Expression in the Flower Center Underlies the Homeotic Phenotype of Lacandonia schismatica (Triuridaceae) C W OA
 The Plant Cell, Vol. 22: 3543 3559, November 2010, www.plantcell.org ã 2010 American Society of Plant Biologists B-Function Expression in the Flower Center Underlies the Homeotic Phenotype of Lacandonia
The Plant Cell, Vol. 22: 3543 3559, November 2010, www.plantcell.org ã 2010 American Society of Plant Biologists B-Function Expression in the Flower Center Underlies the Homeotic Phenotype of Lacandonia
Heterosis and inbreeding depression of epigenetic Arabidopsis hybrids
 Heterosis and inbreeding depression of epigenetic Arabidopsis hybrids Plant growth conditions The soil was a 1:1 v/v mixture of loamy soil and organic compost. Initial soil water content was determined
Heterosis and inbreeding depression of epigenetic Arabidopsis hybrids Plant growth conditions The soil was a 1:1 v/v mixture of loamy soil and organic compost. Initial soil water content was determined
Objectives. To identify plant structures and functions. To describe the structure of plant cells. To explain the process of reproduction in plants.
 1 Objectives To identify plant structures and functions. To describe the structure of plant cells. To explain the process of reproduction in plants. 2 Main Menu Plant Cell Biology Plant Structures Roots
1 Objectives To identify plant structures and functions. To describe the structure of plant cells. To explain the process of reproduction in plants. 2 Main Menu Plant Cell Biology Plant Structures Roots
