The Atypical Cadherin Fat Directly Regulates Mitochondrial Function and Metabolic State
|
|
|
- Nickolas Holmes
- 6 years ago
- Views:
Transcription
1 The Atypical Cadherin Fat Directly Regulates Mitochondrial Function and Metabolic State Anson Sing, 1,2,5 Yonit Tsatskis, 1,5 Lacramioara Fabian, 3 Ian Hester, 1 Robyn Rosenfeld, 1,2 Mauro Serricchio, 4 Norman Yau, 1,2 Maïlis Bietenhader, 4 Riya Shanbhag, 4 Andrea Jurisicova, 1 Julie A. Brill, 2,3 G. Angus McQuibban, 4, * and Helen McNeill 1,2, * 1 Lunenfeld-Tanenbaum Research Institute, Mt. Sinai Hospital, Toronto, ON M5G 1X5, Canada 2 Department of Molecular Genetics, University of Toronto, Toronto, ON M5S 1A8, Canada 3 Cell Biology Program, The Hospital for Sick Children, Toronto, ON M5G OA4, Canada 4 Department of Biochemistry, University of Toronto, Toronto, ON M5S 1A8, Canada 5 Co-first author *Correspondence: angus.mcquibban@utoronto.ca (G.A.M.), mcneill@lunenfeld.ca (H.M.) SUMMARY Fat (Ft) cadherins are enormous cell adhesion molecules that function at the cell surface to regulate the tumor-suppressive Hippo signaling pathway and planar cell polarity (PCP) tissue organization. Mutations in Ft cadherins are found in a variety of tumors, and it is presumed that this is due to defects in either Hippo signaling or PCP. Here, we show Drosophila Ft functions in mitochondria to directly regulate mitochondrial electron transport chain integrity and promote oxidative phosphorylation. Proteolytic cleavage releases a soluble 68 kda fragment (Ft mito ) that is imported into mitochondria. Ft mito binds directly to NADH dehydrogenase ubiquinone flavoprotein 2 (Ndufv2), a core component of complex I, stabilizing the holoenzyme. Loss of Ft leads to loss of complex I activity, increases in reactive oxygen species, and a switch to aerobic glycolysis. Defects in mitochondrial activity in ft mutants are independent of Hippo and PCP signaling and are reminiscent of the Warburg effect. INTRODUCTION Coordinate regulation of tissue growth and tissue organization is critical to normal development, and dysregulation can promote tumor growth and metastasis. Fat (Ft) cadherins are enormous cell adhesion molecules (>500 kda, Figure 1A) that control both tissue growth and tissue organization and are thus good candidates for genes involved in tumor growth and metastasis. Consistent with this hypothesis, mutations in Ft4 are found in a wide spectrum of human cancers (Morris et al., 2013). Genetic studies classified ft as a Drosophila tumor suppressor gene (Bryant et al., 1988) and indicated that Ft regulates tissue growth via the conserved tumor-suppressive Hippo signaling pathway (reviewed by Staley and Irvine, 2012; Thomas and Strutt, 2012). However, direct biochemical links between Ft and the Hippo pathway remain unclear. Ft binds to another large cadherin, Dachsous (Ds), and together, they regulate a form of tissue organization known as planar cell polarity (PCP), as well as the Hippo pathway (Figures 1B and 1C). Structure-function studies indicate that Hippo and PCP regulation are genetically separable and map to distinct regions of the Ft cytoplasmic domain (Figure 1A and Figure S1A available online) (Bossuyt et al., 2014; Matakatsu and Blair, 2012; Pan et al., 2013; Zhao et al., 2013). There are eight regions of high sequence conservation within the cytoplasmic domain. Surprisingly, some of the most highly conserved regions have little or no function in either Hippo pathway activity or PCP (Figures 1A and 1D). Some aspects of ft function still remain poorly understood. For example, the Hippo-dependent tissue overgrowth of ft mutants (Figure 1C) is only seen after an extended larval period (Bryant et al., 1988), and at early stages, ft mutants have greatly reduced overall growth (Figure 1E). The basis of this early defect in larval growth is unknown. Mitochondria are critical for cellular growth and proliferation, providing ATP via oxidative phosphorylation (OXPHOS), generating reactive oxygen species (ROS), controlling cytosolic calcium levels, and providing biosynthetic intermediate metabolites needed for growth (Nunnari and Suomalainen, 2012). The mitochondrial electron transport chain (ETC) consists of large protein complexes (CI CIV) that, via sequential redox reactions, pump protons across the mitochondrial membrane to generate a gradient used by CV to generate ATP. CI is the largest of the ETC complexes, a >900 kda machine composed of subunits, that provides an entry point for electrons in the ETC. Mutations in CI can lead to loss of OXPHOS and a switch to glycolysis as an energy source (Efremov and Sazanov, 2011). A switch in metabolism from OXPHOS to glycolysis in the presence of oxygen is characteristic of many tumors and is known as the Warburg effect (reviewed in Lunt and Vander Heiden, 2011; Wallace, 2012). Mutations in CI can result in increased levels of ROS (Ishikawa et al., 2008; Hirst, 2013). Although high levels of ROS are toxic, at Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1293
2 (legend on next page) 1294 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
3 physiologic levels, ROS function as signaling molecules with important roles in stem cell maintenance, cell fate determination, cell-cycle control, and tumor proliferation (Hamanaka and Chandel, 2010; Owusu-Ansah and Banerjee, 2009; Owusu-Ansah et al., 2008b). We reasoned that identifying proteins that directly bind to the conserved cytoplasmic domain of Ft could provide new insight into how Ft regulates tissue growth and tissue organization, and possibly illuminate why Ft cadherins are mutated in human cancers. We screened for proteins that bind Ft and then conducted in vivo RNAi screens in Drosophila to identify Ft-binding proteins relevant to Ft function. Surprisingly, we find that Ft binds nicotinamide adenine dinucleotide (NADH) dehydrogenase ubiquinone flavoprotein 2 (Ndufv2), a core component of CI, and CG1746, a core component of CV, the F 0 ATP synthase. We show that the intracellular domain of Ft is cleaved to release a cytosolic fragment (Ft mito ) that is imported into mitochondria in cell lines and in Drosophila larvae. Loss of Ft or Ndufv2 leads to increased ROS levels and upregulation of the ROS target c-jun N-terminal kinase (JNK). ft mutants lose CI activity, increase glycolysis, and have aberrant mitochondrial cristae. Blue native PAGE (BN-PAGE) analysis of mitochondrial ETC complexes demonstrates that Ft mito is a component of CI and that loss of ft results in defects in CI and CV assembly. These defects are independent of PCP and Hippo signaling, with phenotypic consequences reminiscent of the Warburg effect. Taken together, our data indicate that Ft-dependent regulation of mitochondrial activity is critical for tissue growth, and imply that loss of Ft may facilitate metabolic changes seen in cancer cells. RESULTS Mitochondrial Proteins Bind to the Cytoplasmic Domain of Ft and Function in Ft-Dependent Processes We previously conducted yeast two-hybrid (Y2H) screens to identify proteins that bind the cytoplasmic domain of Ft (Fanto et al., 2003) and identified 58 Ft-binding proteins (Table S1). To examine the biological relevance of these Ft-binding proteins in vivo, we expressed double-stranded RNA (dsrna) under control of the galactose-4 (GAL4)/upstream activating sequence (UAS) system to knock down their expression (Dietzl et al., 2007; Ni et al., 2009). We examined at least two independent RNAi lines for each gene and assayed PCP and growth in the Drosophila eye and wing. In the fly eye, PCP is evident in the polarized arrangement of ommatidia (Figure 1B). Hippo-dependent growth defects can also be assayed based on interommatidial proliferation. Analysis of semithin sections from >200 eyes identified clear PCP defects upon knockdown of three Ft-binding proteins: Ndufv2, CG1746, and mitochondrial-processing protease (Mpp) (Figure 1F; Table S1). Unexpectedly, these are all mitochondrial proteins. Because PCP defects associated with knockdown of Ndufv2 were strikingly similar to defects observed upon loss of ft (compare Figures 1B, ii, and 1F, i), with clean dorsal-ventral inversions in polarity in the absence of apoptosis or degeneration, we focused on understanding the function of Ndufv2 in Ft-dependent signaling. eygal4 > Ndufv2 dsrna induced PCP defects in the eye that had no disruptions in photoreceptor morphology, apical-basal polarity, or specification. Knockdown of Ndufv2 in the wing, using engal4, resulted in a swirling pattern of hairs, indicating that Ndufv2 is required for PCP in the wing (Figure S1B). To confirm that knockdown of Ndufv2 disrupts PCP, three independently derived RNAi lines were examined for their effects on PCP in the Drosophila eye. All three lines produced PCP defects when driven with eygal4 (Figure S1C). eygal4 drives expression early in eye development, at the time ft acts in establishing PCP (Strutt and Strutt, 2002). Importantly, UAS-Ndufv2 expression rescues PCP defects induced by RNAi-mediated knockdown of Ndufv2 (Figure 1F, iv). Ndufv2 is a highly conserved member of CI, which provides an entry point for electrons in the ETC in eukaryotes (Brandt, 2006). Ndufv2 is also called the 24 kda subunit in mammals (Hirst, 2013). Ndufv2 has a highly conserved iron-sulfur (Fe-S) cluster that has been hypothesized to act as an antioxidant to prevent the generation of ROS. We tested if the Fe-S cluster of Ndufv2 is essential for normal PCP by mutating two conserved cysteine residues that coordinate binding of the Fe-S cluster, generating UAS-Ndufv2DFeS. UAS-Ndufv2DFeS was well expressed (Figure S4A) but was unable to rescue the PCP defects induced by knockdown of Ndufv2 (Figure S1D). Thus, the Fe-S cluster of the Ndufv2 is needed for normal PCP. Because no previous links have been made between mitochondria and PCP, the discovery that Ndufv2 binds Ft and that loss of Ndufv2 causes PCP defects was surprising. To confirm the Y2H interaction, and to determine if the Ft and Ndufv2 Figure 1. Mitochondrial Proteins Regulate PCP and Bind Ft (A) The intracellular domain (ICD) of Ft contains eight highly conserved regions (red boxes); structure-function studies identified domains important for PCP regulation (blue lines) or Hippo pathway control (red lines). Dashed lines indicate weak requirements. Schematic on right illustrates PCP organization of ommatidia in adult fly eyes. Black circles indicate rhabdomeres of ommatidia. (B) Adult Drosophila eye sections reveal organized PCP of ommatidia in controls (i ; black arrows), whereas fat mutants display polarity inversions (ii; red arrows). (C) Wing imaginal discs from extended third-instar ft larvae show dramatic overgrowth compared to controls. (D) Regions 1 and 2 (see 1A schema) are highly conserved in evolution. DmFat, D. melanogaster; AgFat, Anopheles gambiae; TcFat, Tribolium castaneum; DpFat, Daphnia pulex; BfFat, Branchiostoma floridae; MmFat4, M. musculus; HsFat4, H. sapiens. (E) ft fd larvae are delayed in growth compared to age-matched WT control larvae. (F) Knockdown of Ndufv2 (i), CG1746 (ii), and Mpp (iii) using eygal4 driver results in disrupted PCP similar to loss of ft. PCP in Ndufv2 knockdown eyes is rescued with a WT Ndufv2 transgene (iv; 0 out of 165 [0%] inverted ommatidia versus 35 out of 194 [18%] for Ndufv2 knockdown alone). Open circles indicate disrupted ommatidia where polarity was not scored. (G) In-vitro-GST pull-down using purified proteins indicates interaction between highly conserved Ft regions 1 and 2 with Ndufv2. Ponceau S serves as loading control. See also Figures S1 and S2C and Table S1. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1295
4 A B Figure 2. ft and Ndufv2 Regulate PCP and Hippo Pathway Targets (A and B) fj-lacz is upregulated in both ft fd clones (A) and clones expressing Ndufv2 dsrna (B) in the larval eye. (C and D) Hippo targets Crb (C) and Ex (D) are upregulated in the posterior compartments of larval wing discs expressing Ndufv2 dsrna driven with engal4. Engrailed (En) marks the posterior compartment. (E) Ex is also upregulated in Ndufv2 knockdown clones in the wing. (F) Bantam-GFP, a Hippo sensor, is reduced in Ndufv2 knockdown tissue. ft clones marked by lack of GFP, Ndufv2 knockdown clones positive for GFP. See also Figure S2. C D E F interaction was direct, we used glutathione S-transferase (GST) pull-down assays with purified fragments of the Ft cytoplasmic domain and His-tagged Ndufv2 (Figures 1G and S1E). These data indicate that the N-terminal region of Ndufv2 binds directly to the highly conserved regions 1 and 2 of Ft (Figure S2C). Strikingly, deletion of four amino acids of region 2 abrogates interaction with Ndufv2. We found that region 1 also contributes to binding stability between Ft and Ndufv2 but is not sufficient to confer binding in this assay (Figure 1G; data not shown). These data indicate that the highly conserved region 2 is critical for direct binding between Ft and Ndufv2 in vitro. Loss of the CI Core Component Ndufv2 Leads to Activation of Hippo and PCP Target Genes in Larval Wings and Eyes Because we found that Ndufv2 binds directly to Ft, and that loss of Ndufv2 alters PCP, we examined if Ndufv2 knockdown affects Ft pathway target expression. A well-characterized PCP target of Ft is four-jointed (fj). Loss of ft leads to upregulation of fj in mitotic clones, as assessed by fj-lacz (Yang et al., 2002; Figure 2A). To test whether Ndufv2 affects fj expression, we generated clones of cells expressing Ndufv2 dsrna in eye discs from flies containing a fj-lacz reporter. fj-lacz expression was clearly increased in Ndufv2 dsrna clones, indicating that Ndufv2 regulates expression of the Ft-PCP target fj in vivo (Figure 2B). Activation of the Hippo pathway can be observed in vivo by monitoring expression of Yki targets such as Crumbs (Crb), Bantam, and Expanded (Ex). Expression of Ndufv2 dsrna in the posterior compartment of wing discs with engal4 resulted in marked 1296 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
5 upregulation of Crb (Figure 2C) and Ex (Figure 2D). Clones of cells expressing Ndufv2 dsrna also showed upregulation of Ex (Figure 2E). Bantam-GFP, which is inhibited by Yki, was repressed by depletion of Ndufv2 (Figure 2F). Activated caspase staining and 5-ethynyl-2 0 -deoxyuridine (EdU) incorporation revealed that strong knockdown of Ndufv2 leads to both increased apoptosis and increased proliferation, indicating disruption of growth regulation (Figures S2A and S2B). These data show that loss of Ndufv2 alters expression of Hippo targets. Loss of Ft Leads to Defects in Mitochondrial Morphogenesis and Cristae Architecture Because the cytoplasmic domain of Ft binds mitochondrial proteins, we wondered if mitochondrial structure is affected in ft mutants. We examined mitochondria in developing Drosophila sperm because they undergo dramatic remodeling during spermatid differentiation (Fuller, 1993), allowing us to closely examine mitochondrial morphogenesis and ultrastructure. All the mitochondria in each early round spermatid aggregate and fuse to form a spherical mitochondrial derivative called the Nebenkern. During elongation, the Nebenkern splits and extends along the developing flagellum (Figures 3A, i iv). Analysis of germline clones of ft mutants revealed defects in Nebenkern morphology. In early round spermatids, Nebenkerne are lobate and broken, with vacuolated areas (Figure 3A, v). In elongating spermatids, Nebenkerne are aberrant, with areas of uneven condensation (Figure 3A, vi). Similar mitochondrial defects were seen in ft larval testes (Figures 3A, v and vi, insets). Thus, ft is necessary for normal mitochondrial morphogenesis during sperm development. Expression of Ndufv2 dsrna resulted in similar Nebenkern defects (Figures 3A, vii and viii), highlighting the requirement for both ft and Ndufv2 in mitochondrial morphogenesis. We used transmission electron microscopy to analyze mitochondrial ultrastructure of ft mutants in maturing sperm and surrounding cyst cells (Figure 3B). Defects in mitochondrial morphogenesis and ultrastructure were observed in ft mutant spermatids (Figure 3B, iii). Mitochondria in wild-type (WT) somatic cyst cells (which surround developing sperm) are elongated and exhibited regularly spaced, flattened cristae (Figure 3B, ii). In contrast, mitochondria in ft mutant cyst cells were round and with swollen and irregular cristae (Figures 3B, iv, and 3C). Similar defects in cristae morphology were seen in eye and wing discs obtained from ft fd and ft alb larvae (Figure S3A), indicating that this is not a tissue- or allele-specific effect. These dramatic alterations in mitochondrial architecture in ft mutants support a role for Ft in mitochondria and raised the possibility that loss of Ft affects mitochondrial function. Loss of Ft or Ndufv2 Leads to Increased ROS We asked if ROS, a by-product of OXPHOS, is perturbed in ft and Ndufv2 mutants because structural analysis of CI suggested that the Ndufv2 Fe-S cluster may have a role in ROS regulation (Sazanov and Hinchliffe, 2006), and our rescue analysis indicates a critical role for the Fe-S cluster in PCP. We measured ROS levels by staining with dihydroethidium (DHE), which becomes more fluorescent at higher superoxide (O 2 ) levels. We first examined the effect of reduction of Ndufv2 on ROS levels using dsrna in Drosophila S2 cells and found that loss of Ndufv2 leads to increased levels of ROS in tissue culture cells (Figures S3B and S3C). Reduction of Ndufv2 in the posterior compartment of the wing, by expressing dsrna driven by engal4, also leads to dramatically increased ROS levels in vivo (Figure S3D). Staining of control discs revealed that under normal conditions, a gradient of ROS exists in the eye disc, with expression that is high at the equator and low at the poles (Figures 3D and S3E). Interestingly, this gradient of ROS is similar to the proposed Ft activity gradient (Yang et al., 2002). To test if loss of ft affected ROS levels, we generated mitotic clones of ft mutant tissue in eye discs. We found that ft mutant clones have increased DHE staining, indicating that loss of ft leads to increased ROS levels (Figure 3E). Loss of ft also leads to increases in expression of a reporter of oxidative stress, glutathione S-transferase D1 (GSTD1)-GFP (Sykiotis and Bohmann, 2008) (Figure 3F), confirming that loss of ft leads to increases in ROS in vivo. Overexpression of yki, or loss of the core Hippo kinase wts, does not lead to increased ROS, indicating that the increased ROS is not due to loss of Hippo regulation by ft (Figures 3G and 3H; data not shown). Loss of Ft or Ndufv2 Leads to Activation of the ROS Target JNK ROS activate the JNK pathway (Hamanaka and Chandel, 2010; Owusu-Ansah and Banerjee, 2009; Owusu-Ansah et al., 2008b), which has been suggested to be a regulator of PCP (reviewed in McNeill, 2010). puckered (puc) is a target of the JNK pathway, and its expression reflects JNK activity. Expression of Ndufv2 dsrna using engal4 led to strong activation of puc-lacz (Figure 3I), consistent with increased ROS leading to activation of JNK signaling. Strikingly, ft clones also display strong increases in puc-lacz expression (Figure 3J), indicating that loss of ft leads to activation of JNK signaling. Thus, the ROS target JNK is upregulated in Ndufv2- and ft-depleted tissue. The Cytoplasmic Domain of Ft Contains Multiple Mitochondrial-Targeting Sequences Ft functions at the cell surface in cadherin-mediated adhesion (Ma et al., 2003), whereas Ndufv2 is a core component of mitochondrial CI. Our data indicate that Ft binds directly to Ndufv2 in vitro; however, in vivo, Ft and Ndufv2 could potentially interact either at the cell surface or in mitochondria. We generated antibodies to Ndufv2 and confirmed their specificity (Figure S4A). Costaining for the mitochondrial protein CVa demonstrated that Ndufv2 localized to mitochondria in S2 cells (data not shown) and in Drosophila discs (Figure S4B). We did not detect any labeling of Ndufv2 at the cell surface in cultured cells or Drosophila imaginal discs (Figures 4A and S4B; data not shown). We hypothesized that Ft might interact with Ndufv2 inside mitochondria. Ft is posttranslationally processed to generate a 450 kda extracellular fragment and a 110 kda transmembrane fragment (Figure 4E) (Feng and Irvine, 2009; Sopko Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1297
6 A E F G H B I J C D (legend on next page) 1298 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
7 et al., 2009). In addition, a more labile cytoplasmic fragment of 68 kda is generated (Figure S4D). In silico analysis of the cytoplasmic domain of Ft with MitoProt II detected two mitochondrial-targeting sequences (MTSs) and their corresponding Mpp cleavage sites (Figure S5). To test if these putative MTSs can direct mitochondrial localization, we generated constructs expressing Ft-MTS fragments tagged with a FLAG epitope (Figure S6A). Immunofluorescence and subcellular fractionation of COS (CV-1 [simian] in Origin, and carrying the SV40 genetic material) cells and S2 cells demonstrated that fragments of Ft containing the predicted MTSs coupled to FLAG localize to mitochondria (Figures 4B and S6B S6D). Our studies also indicate that there is an additional MTS signal, located after predicted MTS1 and MTS2, that is not identified by in silico analysis (Figures S6B S6D). This is not surprising because MitoProt II does not recognize all mitochondrial-targeting motifs. Together, these in silico and in vitro data indicate that the cytoplasmic domain of Ft contains multiple regions that could direct mitochondrial localization. The Ft Cytoplasmic Domain Is Cleaved, Releasing a 68 kda Fragment that Is Imported into Mitochondria To determine if Ft can be detected in mitochondria, we first used cultured cell overexpression experiments. Because a 110 kda transmembrane form of Ft (FtDECD) can rescue most defects of ft mutants (Matakatsu and Blair, 2006), we transfected Drosophila S2 cells with FtDECD and immunostained with antibodies to the FLAG epitope, Ndufv2, and CVa. A portion of Flag-tagged Ft colocalized with Ndufv2 and CVa in S2 cells, indicating mitochondrial localization (Figure 4A). Interestingly, expression of the entire cytosolic domain of Ft (Ft -IC, 74 kda) was not sufficient to confer mitochondrial localization (Figures S6B and S6D), suggesting that processing at the plasma membrane facilitates production of a Ft fragment that is competent to enter mitochondria. Consistent with this, soluble Ft-IC cannot rescue ft mutants, whereas a membrane-tethered Ft-IC can (Bossuyt et al., 2014; Matakatsu and Blair, 2012; Pan et al., 2013; Zhao et al., 2013). To determine if endogenous Ft localizes to mitochondria, we examined a Drosophila cell line, D11, that expresses full-length Ft (Ft-FL). Staining of D11 cells and Drosophila tissue showed colocalization of Ft staining with the mitochondrial marker CVa (Figures 4C and 4D). In addition, cell fractionation experiments revealed that an 68 kda fragment of the endogenous Ft intracellular domain is enriched in mitochondrial fractions from D11 cells (Figure 4F). To determine if the 68 kda fragment of Ft is located within mitochondria, we conducted protease protection assays on intact mitochondria isolated from D11 cells. The 68 kda fragment is resistant to protease digestion (Figure 4G) in intact organelles. In contrast, the 110 kda fragment was digested by protease treatment, suggesting that the transmembrane form of Ft is associated with mitochondria but is not imported into mitochondria. Importantly, addition of Triton X-100 to permeabilize mitochondrial membranes resulted in digestion of the 68 kda fragment, verifying that this fragment of Ft is capable of digestion by Proteinase K but is protected from proteolysis until membranes are solubilized. Taken together, these data indicate that a 68 kda Ft fragment is imported into mitochondria. We refer to this 68 kda Ft fragment as Ft mito. Ft Is Processed into Ft mito In Vivo during Drosophila Development To determine if Ft processing occurs in vivo, we isolated eye discs from WT and ft larvae and conducted fractionation and protease digestion experiments (Figures 4H and S4C). In total larval extracts, the 110 kda form of Ft was the most abundant form. Isolation of mitochondrial fractions of WT larvae revealed enrichment of the endogenous 68 kda form of Ft. Both the 110 and 68 kda fragments were missing in immunoblots of mitochondrial extracts obtained from ft larvae (Figure S4C), confirming that the 68 kda fragment represents mitochondrial Ft (Ft mito ). As in cultured cells, Ft mito is resistant to protease digestion in larval extracts, confirming its localization inside mitochondria. Interestingly, Ft mito appears as a doublet in disc extracts (Figures 4H and S4C), suggesting that additional processing may occur in vivo. Discs, testes, and fat body (Figures 4D and Figure 3. Loss of ft or Ndufv2 Results in Defective Mitochondria and Perturbations in ROS and JNK Pathway Activation (A) Schematics of spermatid differentiation (i and ii). n, nucleus. (i) Spermatids in onion stage with fused mitochondria that form the Nebenkern. (ii) Elongated spermatid cysts. (iii viii) Overlays of phase-contrast and epifluorescence micrographs of live preparations stained with Hoechst to reveal DNA (blue) show defects in Nebenkern morphology in ft fd mutant clones (GFP negative) in early spermatids (v). In elongated spermatid cysts, aberrant clumping occurs along the Nebenkern (vi). Insets show spermatids from ft fd larval testes. (vii and viii) Early and elongating spermatids expressing Ndufv2 dsrna show defects in Nebenkern morphology. Inset shows high-magnification detail of Nebenkern defect. Arrowheads indicate nuclei. Arrows indicate Nebenkerne. Scale bars, 20 mm. (B) Transmission electron micrographs of early elongated spermatids and cyst cell mitochondria. (i) WT spermatids show axonemes (arrowhead) and Nebenkerne (arrow) of comparable size. (ii) WT cyst cell mitochondria are elongated and show regularly spaced, flattened cristae. (iii) In ft fd mutants, the two halves of each Nebenkern show large variation in size (arrow). Scale bar, 500 nm. (iv) In ft fd mutants, mitochondria (arrows) appear round or oval and have swollen cristae. Insets (iii and iv) show mitochondria from WT and ft mutant cyst cells at high magnification. Scale bar, 500 nm. (C) Percentage of defective mitochondria is quantified. *p = , Student s t test. WT, n = 39. ft fd, n = 34. (D) DHE staining of WT eye discs reveals a ROS gradient that is strong at the equator and weaker toward the poles. (E and F) ROS are upregulated in ft fd mitotic clones (marked by lack of GFP [E] or b-gal [F] staining) as detected by DHE (E) and GSTD1-GFP (F). (G and H) Overexpression of Yki S168A -GFP driven with engal4 (G) or wts e1 clones (clone marked by lack of GFP in H) do not show changes in ROS as detected by DHE. (I and J) The JNK pathway activity reporter puc-lacz is upregulated in Ndufv2-depleted tissue (I, Ndufv2 dsrna driven with engal4) as well as in ft fd clones (J, GFP negative). See also Figure S3 and Table S3. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1299
8 A B C D E F G H I J (legend on next page) 1300 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
9 S3F; data not shown) display clear colocalization of Ft and mitochondrial markers; however, interestingly, the staining was often unevenly distributed, suggesting that there may be spatial constraints. Protease protection assays of OptiPrep gradient fractions from whole-larval extracts confirm that Ft is localized in mitochondria, where the protease-protected band appears as a single 68 kda form (Figures 4I and 4J). Ds binding to the extracellular domain of Ft is important for the regulation of Hippo and PCP signaling. We tested if loss of ds altered cleavage or import of Ft into mitochondria. We found that Ft mito is clearly detectable in ds mutant larval discs (Figures 4H and S4C). Thus, mitochondrial localization of Ft occurs in the absence of Ds binding. Loss of Ft Leads to a Loss of CI Activity in Drosophila Larvae Our in vivo data indicate that Ft is cleaved and imported into mitochondria, where it can bind Ndufv2. Loss of ft or knockdown of Ndufv2 leads to increased ROS, as does loss of other components of CI. These data suggested that Ft mito might regulate CI activity. We tested if CI activity was affected in ft mutants using larval mitochondrial extracts. Significantly, we find that loss of ft leads to a dramatic loss of CI activity (Figure 5A). Consistent with the loss of CI activity, ft mutant larvae have reduced growth, similar to that of mutants that have lost CI, due to deletion of a CI assembly factor, dcia (Figure 5B). The loss of CI activity led us to ask if there was an alteration in metabolism in ft mutants. We measured lactate levels to see if there was a switch from OXPHOS to glycolysis in the absence of Ft. Strikingly, ft mutants have significantly increased levels of lactate, reflecting increased glycolysis (Figure 5C). This increase in lactate was detectable both in age-matched and in size-matched larvae (which are much older due to the delayed growth of ft mutants). Loss of Ft Causes a Loss of Assembled CI Holoenzyme We wondered if Ft regulates the stability of Ndufv2; however, we found only mild changes in Ndufv2 total levels in SDS-PAGE analysis of ft larvae (Figure 5D). We hypothesized that Ft might affect the assembly or stability of the CI holoenzyme, which would result in the loss of CI activity. CI forms a complex of >900 kda that can be resolved on nondenaturing, BN-PAGE. BN-PAGE analysis of mitochondria from WT larvae shows a clear band for fully assembled CI at 980 kda, as well as complex V (CV) monomers at 800 kda and CV dimers at 1,100 kda (Figures 5E and S4F). As previously published, loss of a well-characterized CI assembly factor (dcia30) results in loss of CI, with no effects on the assembly of CV monomer (CV1) or CV dimer (CV2) (Figure 5E and 5G; Cho et al., 2012). Strikingly, BN-PAGE analysis of mitochondria isolated from ft mutants shows loss of assembled CI, suggesting that Ft is needed for assembly or stability of CI (Figure 5E). Western blot analysis with antibodies to CI components Ndufv2 and Ndufs3 confirmed loss of assembled CI in ft fd mutants (Figures 5G and S4E). Overexposure of gels reveals that there is some CI present but at drastically reduced levels. In-gel assays confirm that CI activity is dramatically reduced in ft mutants (Figure 5F). There is also a consistent, though modest, increase in CII activity and mobility shifts in CII and CV activity. Western blot analysis demonstrates that a null ft allele, ft fd, shows a dramatic loss of CI, whereas the hypomorphic ft allele, ft alb, does not significantly alter CI assembly and/or stability (Figure 6A). Importantly, western blotting for Ndufv2 reveals that defects in CI can be rescued by expression of Ft-FL on a bacterial artificial chromosome (BAC) transgene. Impaired CI assembly provides an explanation for the reduced CI activity seen in ft fd mutants. Interestingly, ft fd mutants show an increase in complex-associated cytochrome c (CytoC), possibly reflecting metabolic compensation (Figures 6A 6C). Mutations of Hippo pathway components, wts and ds, do not affect CI levels but do show an increase in CytoC, possibly due to regulation of mitochondrial dynamics (Nagaraj et al., 2012). We hypothesized that the 68 kda Ft mito might be a component of mitochondrial complexes. Immunoblotting of larval mitochondrial extracts separated on BN-PAGEs with affinity-purified antibodies to Ft revealed that Ft mito is part of the CI holoenzyme (Figures 5G and S4E). Importantly, the Ft signal is lost in CI assembly mutants, which also lose Ndufv2 staining as well as other CI components. No signal was detected in CV complexes. Together, these data indicate that Ft mito is incorporated into CI and is needed for CI assembly or stability in vivo. Loss of Ft Leads to Defects in CV Assembly Immunoblotting with antibodies to CVa reveals normally assembled CV1 and CV2 in WT larvae and CI assembly mutants (Figures 5G and 6A). Strikingly, however, CV is incorrectly assembled in both ft fd and ft alb mutants, with reduction of CV dimers, and the appearance of a novel prominent band at Figure 4. Ft Is Cleaved and Imported into Mitochondria Where It Colocalizes with Ndufv2 (A) Ndufv2 and Ft colocalize in mitochondria of S2 cells transfected with FtDECD-FLAG (Ft lacking the ECD). (B) A Ft fragment containing in-silico-predicted MTS1 is targeted to mitochondria in COS cells. (C and D) Endogenous Ft colocalizes with mitochondrial marker CVa in D11 cells (C) and larval tissues (D). (E) Schematic of Ft cleavage products. The 68 kda Ft mito fragment is targeted to mitochondria, indicated by red arrows in (F) (H), whereas black arrowheads indicate the 110 kda fragment. (F) Ft mito is specifically enriched in mitochondrial fractions from D11 cells, along with Porin, but not a-tubulin. Asterisk denotes a 50 kda Ft fragment found in the total and heavy membrane fractions. T, total; C, cytoplasmic; H, heavy membranes; M, mitochondrial fraction. (G) In D11 cells, Ft mito is protected from protease digestion by Proteinase K, as is CVa, whereas the 110 kda fragment is degraded. (H) Ft mito,cva, and Porin are protected from protease digestion in WT and ds larval discs. (I) Subcellular fractionation of WT larval extracts produces a fraction containing Ft and NdufS3, but not ER marker Calnexin, and diminished levels of the peroxisome marker Pex5 (red box). (J) Ft mito in fraction 6 is protected from digestion in low protease concentrations but is degraded either with the addition of detergent or with excess protease. See also Figures S3A, S4, S5, and S6. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1301
10 A B C D E F G Figure 5. Ft Is Necessary for Mitochondrial CI and CV Stability and Function (A) CI activity is severely reduced in ft mutant larvae. Values represent the mean ± SD of three independent experiments. Student s t test was used to calculate significance: *p < (B) The delayed growth phenotype of ft fd larvae resembles that of dcia mutants. Both are significantly smaller than age-matched WT control larvae. (C) Lactate levels are increased in extracts from both age- and size-matched ft fd larvae compared to controls. Values represent the mean ± SD of three independent experiments. Student s t test was used to calculate statistical significance: *p < 0.05; **p < (D) Immunoblots against lysates from WT versus ft fd larval eye/brain complexes show a slight decrease in Ndufv2, increase in CytoC, and no change in PDH, CVa, and Porin protein levels. a-actin serves as a loading control Cell 158, , September 11, 2014 ª2014 Elsevier Inc. (legend continued on next page)
11 500 kda. Normal assembly of CV in ft mutants can be rescued by expression of Ft-FL provided by a BAC transgene (Figure 6A). These data suggest that Ft mito is also needed for proper assembly and/or stability of CV. Our Y2H screens had indicated that Ft interacts with CG1746, a component of CV, and our RNAi screens had demonstrated that loss of CG1746 leads to PCP defects (Figure 1F; Table S1). GST pull-down assays confirmed that CG1746 binds directly to the cytoplasmic domain of Ft (Figure S7D). We do not detect Ft mito on assembled CV in BN-PAGE analysis, suggesting that this interaction may be weak or transient (Figure 5G). However, the obvious defects in CV assembly in ft mutants suggest that the Ft-CG1746 interaction is biologically relevant. Importantly, ft-dependent defects in CV are not secondary to reduced CI because loss of CI (through mutation of a CI assembly factor, dcia30) does not lead to accumulation of the aberrant 500 kda CV band (Figures 5G and 6B). Because CV dimerization is essential for cristae formation, one interesting possibility is that the cristae morphology defects in ft mutants may be due to loss of interaction between CG1746 and Ft. The Ndufv2-Binding Region of Ft Is Necessary for Normal Larval Growth and Mitochondrial Function We reasoned that if Ft binding to Ndufv2 is essential for mitochondrial complex assembly and OXPHOS, blocking this interaction should result in CI and CV defects. We have here defined region 2 as the domain that binds Ndufv2. Previous studies have shown that BAC transgenes expressing a Ft-FL lacking region 2 (FLD2) (Figure 6D) can fully rescue Hippo and PCP signaling (Pan et al., 2013), implying that this region does not function in Hippo or PCP signaling. In contrast, rescuing ft mutants with a BAC transgene-expressing Ft with a deletion of region 8 (FLD8) rescues Hippo pathway defects, but not PCP defects, implying that this region is essential for PCP activity. We tested if BAC transgenes containing Ft-FL can rescue the early growth defects of ft mutant larva. Full-length ft can rescue this early growth defect, confirming that the growth delay is due to Ft loss (Figures 6E and 6F). Significantly, FLD2 fails to rescue larval growth and lactic acid production (Figure 6G). BN-PAGE analysis shows that Ft-FL and FLD8 can rescue CI and CV assembly, whereas FLD2 fails to rescue CI or CV assembly (Figure 6H). Thus, the region of Ft that binds Ndufv2 is essential for mitochondrial complex stability and mitochondrial ETC activity. DISCUSSION Here, we show an unexpected and direct role for Ft in regulating mitochondrial morphology and metabolism. We find that Ft controls ROS production and promotes OXPHOS activity during Drosophila development. Ft is cleaved, generating a 68 kda fragment that is imported into mitochondria, where it binds Ndufv2, promotes CI and CV assembly and/or stability, and enhances the activity of the ETC. To our knowledge, this is the first example of a cell surface protein that functions to directly stabilize the mitochondrial ETC and promote OXPHOS. Ft functions at the cell surface to activate the Hippo kinase pathway. Activation of the Hippo kinase pathway represses transcription of Yki target genes that promote cell proliferation. The proteolytic cleavage that generates Ft mito will inactivate the ability of Ft to inhibit cell proliferation because Ft can only function to repress proliferation when at the plasma membrane. Release of Ft mito allows it to enter mitochondria, where it binds Ndufv2 and stabilizes CI and CV, promoting OXPHOS. We propose that cleavage of Ft can function as a switch mechanism to coordinate cell cycle and metabolism (Figure 7). Altering the levels of Ft mito could allow an organism to directly adjust metabolic rates in accordance with changing energy requirements. In support of this rheostat model, we find that defects in ETC assembly are rescued in a dose-dependent manner by one and two copies of the Ft transgene (Figure 6I). We do not know the identity of the protease that cleaves Ft, releasing Ft mito. We hypothesize that regulation of this cleavage would provide a potent mechanism for regulating metabolism. Because CV is a major source of cellular ATP, we measured ATP levels in ft mutant larvae. Loss of ft leads to relatively minor effects on total ATP levels (Figure S7A). Defects in OXPHOS can be compensated for by increased glycolysis. Analysis of ft mutant larva showed greatly increased levels of lactic acid (Figure 5C), consistent with a shift in metabolism of ft mutants from OXPHOS to glycolysis. This is intriguing because ft is a classic Drosophila tumor suppressor gene, and a hallmark of cancer cells in mammals is a shift from oxidative metabolism to aerobic glycolysis, known as the Warburg effect. ft mutants show a long delay before discs begin to overgrow. Our data suggest that the mitochondrial defects in ft mutants are responsible for the initial delay in growth. We propose that the switch to glycolysis eventually permits the overgrowth characteristic of Hippo pathway mutants. Mitochondria Provide Feedback Signals that Regulate Hippo Signaling and PCP Knockdown of multiple mitochondrial proteins results in defects in PCP (Table S2). To our knowledge, this is the first time that perturbation of mitochondria has been shown to affect PCP. We do not yet understand how mitochondria impact PCP. ATP levels are not a likely effector because we find that loss of 5 0 AMP-activated protein kinase (AMPK), a key regulator of cellular ATP levels (Figure S7B), does not alter PCP, and ATP levels are not significantly decreased in ft mutant larvae. Our observation that ROS levels are present in a gradient in the eye disc during (E) BN-PAGE of crude mitochondrial isolates from larvae extracts reveals CI deficiencies in ft fd and dcia ex80. Wedges indicate increasing amounts of extract. Arrow and asterisk denote CI; arrowheads indicate CV bands. (F) Clear-native-PAGE (bottom; for loading control) and in-gel activity assays (top) reveal strong decreases in CI activity, increase in CII activity, and a shift in CIV mobility. CV activity is perturbed. (G) Immunoblot of BN-PAGE confirms the absence of CI in ft fd and dcia ex80, and Ft staining indicates that Ft associates with CI. Assembly of CV is also disrupted in ft fd mutants, with a CVa-containing fragment of 500 kda. See also Figure S7. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1303
12 A B C D E F G H I Figure 6. Ft-Conserved Region 2 Is Necessary for Mitochondrial Function (A) The hypomorphic allele ft alb shows defects in CV assembly, whereas CI is unaffected as shown by Ndufv2 levels. Expression of two copies of a full-length ft BAC transgene (ft FL ) rescues all of these defects in a ft fd background Cell 158, , September 11, 2014 ª2014 Elsevier Inc. (legend continued on next page)
13 Figure 7. Model of Ft Processing and Function at the Cell Surface and in Stabilizing the ETC in Mitochondria Ft is processed to a 450 and 110 kda receptor at the cell membrane where it coordinates PCP and restricts growth via the Hippo pathway. Ft is further cleaved and transported to mitochondria, relieving repression of growth by the Hippo pathway. Once inside mitochondria, Ft interacts with CI and CV to promote OXPHOS. In cells lacking Ft, both PCP and Hippo pathways are dysregulated, leading to PCP defects and unrestricted growth. CI and CV are destabilized, leading to release of ROS and impaired OXPHOS, resulting in a metabolic shift to glycolysis and higher levels of lactate. development raises the possibility that ROS may have an instructive role in PCP. However, ROS scavengers did not strongly randomize PCP, and ROS induction disrupted PCP but also caused photoreceptor defects (Table S3). More studies are needed to define a direct role for ROS in PCP. We found that loss of CI affects Hippo pathway activity, indicating that mitochondria signal back to growth control. Previous studies have elucidated mechanisms by which the Hippo pathway affects mitochondrial function, by inducing transcription of mitochondrial biogenesis genes (Nagaraj et al., 2012; Ohsawa et al., 2012). Activation of Yki leads to decreased production of ROS, increased transcription of mitochondrial fusion genes, and increased mitochondrial function. Our finding that loss of mitochondrial CI leads to increased ROS and increased activation of Yki target genes suggests that cells can sense mitochondrial disruption and send signals back to regulate Hippo pathway-dependent recovery mechanisms, a form of mitochondrial retrograde signaling. Ft Cadherin Mitochondrial-Binding Motifs and Targeting Sequences Are Ancient and Conserved The cytoplasmic domain of Ft has several regions that are evolutionarily conserved. Heretofore, the function of some of the most highly conserved residues of Ft has been unclear. Ft binding to CI and stabilization of CI depend on these highly conserved residues (Figures 6 and S7C), suggesting that the urbilaterian ancestor had a form of Ft mito and that an ancient function of Ft is to regulate mitochondrial activity. Consistent with this model, mammalian Ft4 has two high-confidence MTSs, similar to Ft (Figure S5), supporting the conservation of a mitochondrial role for Ft4. The role of Ft in PCP (Saburi et al., 2008, 2012), and possibly Hippo activity (Cappello et al., 2013), is conserved in mammals. This deep conservation of CI-binding sequences suggests conservation of the mitochondrial functions of Ft cadherins. The finding that Ft mito promotes CI levels raises many questions for future studies. Although we can detect Ft mito comigrating with CI on BN-PAGEs and can demonstrate direct binding of Ft mito to the CI component Ndufv2, we do not yet know the stoichiometry of Ft mito in CI complexes. Ft mito may function to stabilize already assembled CI or, instead, may promote the assembly of new CI complexes. Because we have shown that loss of Ft leads to increased ROS and that ROS is normally expressed in a gradient that reflects predicted Ft activity, another attractive possibility is that Ft mito might be present in a subset of CI molecules reflecting perhaps the spatial environment of the mitochondrion. Also, Ft mito is not ubiquitously distributed within mitochondria, which may hint at a specialized role for Ft mito. Structural studies are needed to discern exactly how Ft mito interacts with mitochondrial OXPHOS complexes to control mitochondrial activity. Interestingly, our Y2H screens also revealed that Ft binds to CG1746, a component of CV. This suggests that there may be multiple ways in which Ft mito may impact mitochondrial function. (B) Complex-associated CytoC is upregulated in both ft fd and dcia ex80. (C) The ft fd/x13 transheterozygote phenocopies ft fd in both CI and CV defects. wts and ds mutants retain intact CI and CV but upregulate CytoC. (D) Schematic of FL, FLD2, and FLD8 rescue constructs. (E and F) FL and FLD8 rescue the delay in growth of ft fd larvae, whereas FLD2 does not. (G) Lactate levels are elevated in FLD2-expressing ft fd. Levels normalized to FL-rescued lactate content. One-sample Student s t test was used to test significance: m 0 = 1; n = 4; p = (H) CI and CV defects in ft fd larvae are partially rescued by FLD8, but not FLD2. (I) The rescue of CI and CV deficiencies in ft fd larvae is ft FL dosage dependent. See also Figure S7. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1305
14 Ft Function in Mitochondria Is Separable from Hippo and PCP Effects Previous studies indicated that the Hippo and PCP functions of Ft map to distinct regions of the Ft cytoplasmic domain. We show here that the regulation of CI by Ft is separable from its roles in PCP and Hippo signaling. Although loss of ft leads to increased ROS, loss of wts or overexpression of yki does not increase ROS. In addition, BN-PAGE analysis demonstrates that CI and CV levels are not altered in wts and ds mutants (Figure 6C), further indicating that the mitochondrial effects of Ft to promote CI and CV levels are independent of Hippo signaling. Importantly, in the context of Ft-FL expressed under the control of its endogenous promoter, deletion of regions 1 and 2 has no detectable effect on PCP or Hippo activity. Interestingly, in experiments in which truncated versions of Ft are overexpressed via the UAS/GAL4 system, these regions do have weak PCP and Hippo activity. Because we show here that loss of mitochondrial integrity can lead to PCP and Hippo defects, we suggest that the weak Hippo and PCP effects seen in overexpression experiments may be in part due to disruption of the mitochondrial functions of Ft. To our knowledge, this is the first demonstration of direct regulation of mitochondrial complex stability by a cell surface protein, and our study provides a novel mechanism to understand how cell surface signals can directly affect mitochondrial activity and cellular metabolism. Cleavage of the cytoplasmic domain of Ft promotes CI, thereby increasing OXPHOS activity, and inactivates the function of Ft as an inhibitor of Yki, thus driving cell proliferation. This dual function of Ft provides a powerful mechanism to regulate tissue growth. In cancer cells, we speculate that the loss of Ft cadherins in mitochondria might provide an initial impediment to proliferation, due to defects in ETC activity; however, the switch to glycolysis can support tumor growth. In addition, loss of Ft leads to increased ROS production, which can promote DNA damage, stimulate stem cell proliferation, and induce signaling pathways that enhance cancer cell growth. These effects of Ft on mitochondrial function, as well as the alterations in PCP and Hippo signaling, are critical aspects that must be considered when investigating the mechanisms underlying diseases caused by loss of Ft cadherins. EXPERIMENTAL PROCEDURES Drosophila Strains and Genetics For a list of stocks used, statistical analyses, and detailed experimental conditions, see the Extended Experimental Procedures. GST Pull-Downs GST pull-downs were performed as described by Sambrook and Russell (2006). His-tagged Ndufv2 or CG1746 recombinant proteins were expressed in bacteria and batch purified using nickel beads. GST-tagged Ft was similarly expressed in bacteria and incubated with glutathione-conjugated beads. Eluted Ndufv2 or CG1746 was then added to assay for direct binding. Drosophila Testis Preps and Electron Microscopy Adult and larval testes were dissected in TIB (testes isolation buffer) (Casal et al., 1990) with Hoechst (8.3 mg/ml) (Sigma-Aldrich), and live squashed preparations of male germ cells were made as previously described by Regan and Fuller (1990). Germline clones were made using the hsflp-frt system. Two-day-old larvae were heat shocked for 1 2 hr at 37 C for 2 3 consecutive days. For RNAi experiments, Vienna Drosophila RNAi Center (VDRC) line #22194 was crossed with BamGal4. Electron microscopy was performed as described by Fabian et al. (2010). ROS Staining with DHE ROS levels were monitored using the superoxide indicator DHE, as described by Owusu-Ansah et al. (2008a). Imaginal discs were dissected in S2 media, stained in 30 mm DHE for 5 min, then fixed in 4% paraformaldehyde for 5 min. Cell Fractionation and Mitochondrial Protection Assays Mitochondria from D11 cells and eye/brain complexes were isolated using the QIAGEN Qproteome Mitochondria Isolation Kit (37612). Whole-larval extracts were fractionated using OptiPrep density gradient media (Sigma-Aldrich; D1556) using a 20%/25%/30%/40% gradient (Bayat et al., 2012) and spun in a swinging bucket rotor. Fractions were collected after the spin was completed, and purified mitochondria were harvested from the boundary between the 20% and 25% gradients. Protection of purified mitochondrial protein was ascertained by the addition of Proteinase K at 4 C for 10 min alone or with 1% Triton X-100. The NADH Activity Assay Mitochondria were purified from Drosophila larvae and NADH: ubiquinone oxidoreductase activity was monitored as a decrease in absorbance at 600 nm in a colorimetric CI activity assay as described in Cho et al. (2012). Lactate Assay Larvae were homogenized in 100 ml of PBS with 30 strokes of a Kontes pestle. Samples were heat shocked at 60 C for 15 min and centrifuged at 16,000 3 g for 5 min; 5 10 ml of the supernatant was used for lactate determination using a Lactate Assay Kit (BioVision Technologies; K ). All lactate readings were normalized to protein content. Mitochondrial Isolation and BN-PAGE Mitochondria were purified from larvae by differential centrifugation using mitochondrial isolation medium (MIM; 250 mm sucrose, 10 mm Tris/HCl [ph 7.4], 0.15 mm MgCl 2, with protease inhibitor). Mitochondria were resuspended in 13 NativePAGE Sample Buffer (Invitrogen) with 1% digitonin and protease inhibitors, and incubated for 15 min on ice. Samples were centrifuged at 16,200 3 g for 30 min at 4 C, and supernatant was resuspended with G250 sample additive and NativePAGE Sample Buffer. Mitochondria were then visualized with the Novex NativePAGE Bis Tris Gel System (Invitrogen) using 3% 12% Bis Tris NativePAGEs as previously described by Cho et al. (2012). In-Gel Functional Assay Mitochondria from Drosophila larvae were isolated as described by Walker et al. (2006), resuspended in lysis buffer (50 mm Bis Tris [ph 7], 750 mm 6-aminocaproic acid, 2% [w/v] digitonin, and protease inhibitor cocktail), cleared by centrifugation at 16,000 3 g at 4 C, and supplemented with glycerol to a final concentration of 4%. A total of 40 mg of mitochondrial protein was resolved on a 4% 13% native gel using a cathode buffer containing 0.05% (w/v) deoxycholate and 0.01% (w/v) n-dodecyl b-d-maltoside. In-gel activity assays were performed as described by Wittig et al. (2007). Briefly, CI activity was measured in a NADH:nitrotetrazolium blue (NTB) reductase assay. Gel strips from clear-native gels above were incubated in assay buffer (2.5 mg/ml NADH and 0.1 mg/ml NTB in 5 mm Tris/HCl [ph 7.4]) for 5 min, then fixed in 50% methanol and 10% acetic acid. Similarly, assays for CII and CIV activity were carried out in CII assay buffer (200 mm sodium succinate, 200 mm phenazine methosulfate, and 0.1 mg/ml NTB in 5 mm Tris/HCl [ph 7.4]) or CIV assay buffer (0.5 mg/ml diaminobenzidine and 50 mm horse heart CytoC in 50 mm sodium phosphate [ph 7.2]), incubated for min, then fixed. CV activity was monitored by following ATP hydrolysis activity. Gel strips were preincubated in 35 mm Tris/HCl, 270 mm glycine (ph 8.3), then transferred to assay buffer (35 mm Tris/HCl, 270 mm glycine, 14 mm MgSO 4, 0.2% Pb(NO 3 ) 2, 1306 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
15 and 8 mm ATP [ph 8.3]). Gel strips were then fixed in 50% methanol for 30 min to stop the reaction. SUPPLEMENTAL INFORMATION Supplemental Information includes Extended Experimental Procedures, seven figures, and three tables and can be found with this article online at doi.org/ /j.cell AUTHOR CONTRIBUTIONS A.S. and R.R. conducted RNAi screens. A.S. and Y.T. carried out genetic analyses. I.H. performed GST-pull-down experiments. L.F. conducted testis analysis. M.B. and R.R. carried out colocalization experiments. H.M. performed in silico analysis. Y.T. and N.Y. performed fractionation. Y.T. performed protease protection and BN-PAGE experiments. M.S. and R.S. performed metabolic and in-gel assays. H.M. wrote the manuscript with input from all other coauthors. ACKNOWLEDGMENTS We thank G. Halder, U. Banerjee, S. Blair, D. Bohmann, K. Irvine, M. Simon, Y. Heng, D. Holmyard, K. Conway, K. Hales, and D. Walker for flies, advice, imaging, antibodies, and other critical reagents. M.S. was supported by PBBEP3_ from the Swiss National Science Foundation. This work was supported by funding from the CIHR to J.A.B. (MOP ), G.A.M. (MOP-79408), and H.M. (MOP and MOP-97933). Received: March 10, 2014 Revised: June 9, 2014 Accepted: July 10, 2014 Published: September 11, 2014 REFERENCES Bayat, V., Thiffault, I., Jaiswal, M., Tétreault, M., Donti, T., Sasarman, F., Bernard, G., Demers-Lamarche, J., Dicaire, M.J., Mathieu, J., et al. (2012). Mutations in the mitochondrial methionyl-trna synthetase cause a neurodegenerative phenotype in flies and a recessive ataxia (ARSAL) in humans. PLoS Biol. 10, e Bossuyt, W., Chen, C.L., Chen, Q., Sudol, M., McNeill, H., Pan, D., Kopp, A., and Halder, G. (2014). An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene 33, Brandt, U. (2006). Energy converting NADH:quinone oxidoreductase (complex I). Annu. Rev. Biochem. 75, Bryant, P.J., Huettner, B., Held, L.I., Jr., Ryerse, J., and Szidonya, J. (1988). Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev. Biol. 129, Cappello, S., Gray, M.J., Badouel, C., Lange, S., Einsiedler, M., Srour, M., Chitayat, D., Hamdan, F.F., Jenkins, Z.A., Morgan, T., et al. (2013). Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat. Genet. 45, Casal, J., González, C., and Ripoll, P. (1990). Spindles and centrosomes during male meiosis in Drosophila melanogaster. Eur. J. Cell Biol. 51, Cho, J., Hur, J.H., Graniel, J., Benzer, S., and Walker, D.W. (2012). Expression of yeast NDI1 rescues a Drosophila complex I assembly defect. PLoS One 7, e Dietzl, G., Chen, D., Schnorrer, F., Su, K.C., Barinova, Y., Fellner, M., Gasser, B., Kinsey, K., Oppel, S., Scheiblauer, S., et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, Efremov, R.G., and Sazanov, L.A. (2011). Respiratory complex I: steam engine of the cell? Curr. Opin. Struct. Biol. 21, Fabian, L., Wei, H.C., Rollins, J., Noguchi, T., Blankenship, J.T., Bellamkonda, K., Polevoy, G., Gervais, L., Guichet, A., Fuller, M.T., and Brill, J.A. (2010). Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in Drosophila. Mol. Biol. Cell 21, Fanto, M., Clayton, L., Meredith, J., Hardiman, K., Charroux, B., Kerridge, S., and McNeill, H. (2003). The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development 130, Feng, Y., and Irvine, K.D. (2009). Processing and phosphorylation of the Fat receptor. Proc. Natl. Acad. Sci. USA 106, Fuller, M. (1993). Spermatogenesis. In The Development of Drosophila, A. Martinez-Arias and M. Bates, eds. (NY: Cold Spring Harbor Press), pp Hamanaka, R.B., and Chandel, N.S. (2010). Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 35, Hirst, J. (2013). Mitochondrial complex I. Annu. Rev. Biochem. 82, Ishikawa, K., Takenaga, K., Akimoto, M., Koshikawa, N., Yamaguchi, A., Imanishi, H., Nakada, K., Honma, Y., and Hayashi, J. (2008). ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, Lunt, S.Y., and Vander Heiden, M.G. (2011). Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, Ma, D., Yang, C.H., McNeill, H., Simon, M.A., and Axelrod, J.D. (2003). Fidelity in planar cell polarity signalling. Nature 421, Matakatsu, H., and Blair, S.S. (2006). Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development 133, Matakatsu, H., and Blair, S.S. (2012). Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development 139, McNeill, H. (2010). Planar cell polarity: keeping hairs straight is not so simple. Cold Spring Harb. Perspect. Biol. 2, a Morris, L.G., Ramaswami, D., and Chan, T.A. (2013). The FAT epidemic: a gene family frequently mutated across multiple human cancer types. Cell Cycle 12, Nagaraj, R., Gururaja-Rao, S., Jones, K.T., Slattery, M., Negre, N., Braas, D., Christofk, H., White, K.P., Mann, R., and Banerjee, U. (2012). Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes Dev. 26, Ni, J.Q., Liu, L.P., Binari, R., Hardy, R., Shim, H.S., Cavallaro, A., Booker, M., Pfeiffer, B.D., Markstein, M., Wang, H., et al. (2009). A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 182, Nunnari, J., and Suomalainen, A. (2012). Mitochondria: in sickness and in health. Cell 148, Ohsawa, S., Sato, Y., Enomoto, M., Nakamura, M., Betsumiya, A., and Igaki, T. (2012). Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature 490, Owusu-Ansah, E., and Banerjee, U. (2009). Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, Owusu-Ansah, E., Yavari, A., and Banerjee, U. (2008a). A protocol for in vivo detection of reactive oxygen species. Protoc. Exch. Published online February 27, Owusu-Ansah, E., Yavari, A., Mandal, S., and Banerjee, U. (2008b). Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 40, Pan, G., Feng, Y., Ambegaonkar, A.A., Sun, G., Huff, M., Rauskolb, C., and Irvine, K.D. (2013). Signal transduction by the Fat cytoplasmic domain. Development 140, Regan, C.L., and Fuller, M.T. (1990). Interacting genes that affect microtubule function in Drosophila melanogaster: two classes of mutation revert the failure to complement between haync2 and mutations in tubulin genes. Genetics 125, Cell 158, , September 11, 2014 ª2014 Elsevier Inc. 1307
16 Saburi, S., Hester, I., Fischer, E., Pontoglio, M., Eremina, V., Gessler, M., Quaggin, S.E., Harrison, R., Mount, R., and McNeill, H. (2008). Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 40, Saburi, S., Hester, I., Goodrich, L., and McNeill, H. (2012). Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development 139, Sambrook, J., and Russell, D.W. (2006). Detection of protein-protein interactions using the GST fusion protein pulldown technique. CSH Protoc. 2006, pdb.prot3757. Sazanov, L.A., and Hinchliffe, P. (2006). Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311, Sopko, R., Silva, E., Clayton, L., Gardano, L., Barrios-Rodiles, M., Wrana, J., Varelas, X., Arbouzova, N.I., Shaw, S., Saburi, S., et al. (2009). Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr. Biol. 19, Staley, B.K., and Irvine, K.D. (2012). Hippo signaling in Drosophila: recent advances and insights. Dev. Dyn. 241, Strutt, H., and Strutt, D. (2002). Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev. Cell 3, Sykiotis, G.P., and Bohmann, D. (2008). Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, Thomas, C., and Strutt, D. (2012). The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Dev. Dyn. 241, Walker, D.W., Hájek, P., Muffat, J., Knoepfle, D., Cornelison, S., Attardi, G., and Benzer, S. (2006). Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc. Natl. Acad. Sci. USA 103, Wallace, D.C. (2012). Mitochondria and cancer. Nat. Rev. Cancer 12, Wittig, I., Karas, M., and Schägger, H. (2007). High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol. Cell. Proteomics 6, Yang, C.H., Axelrod, J.D., and Simon, M.A. (2002). Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108, Zhao, X., Yang, C.H., and Simon, M.A. (2013). The Drosophila Cadherin Fat regulates tissue size and planar cell polarity through different domains. PLoS One 8, e Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
17 Supplemental Information EXTENDED EXPERIMENTAL PROCEDURES Fly Stocks All WT flies are yw unless otherwise noted. Mutant alleles of ft are described in FlyBase ( briefly, ft fd and ft x13 are null alleles; the molecular nature of the hypomorphic allele, ft alb, is unknown. RNAi stocks for expression of dsrna under control of GAL4/UAS were obtained from VDRC (Dietzl et al., 2007) and the NIG-fly stock center (see Table S1 for a full list of RNAi lines). CG7719 served as the control RNAi. RNAi lines were driven using eygal4, GMR-Gal4, engal4, bamgal4 or yw, UAS-dicer2/Y hshid; eygal4, GMR-Gal4/CyO (generously provided by Claude Desplan). For Ndufv2 knockdown, VDRC line #22194 was used unless otherwise specified. GSTD1-GFP was provided by Dirk Bohmann. UAS-Ndufv2 transgenic flies were generated by site-specific (fc31-mediated) P-element transformation using an Ndufv2 cassette amplified from cdna. UAS-Ndufv2DFeS transgenic flies were generated by site-directed mutagenesis of C169S and C173S in the above Ndufv2 cassette, and was targeted to the same attp landing site used for the UAS-Ndufv2 transgene (injections done by BestGene Inc. attp site on ch. 3 at 86Fb used strain #24749). ft and ampka 3 clones were generated using FLP-FRT-mediated recombination (Xu and Rubin, 1993) with hsflp; ft fd FRT40A/Ubi-GFP FRT40A, orampka 3, FRT101/ubiGFP, FRT101; hsflp/+. Ndufv2 RNAi clones were generated with w; act > y+ > Gal4, UAS-GFP/UAS-Ndufv2 RNAi ; MKRS, hsflp/+. P{A92}pucE69/TM6B,Se was obtained from Utpal Bannerjee and is available from DGRC-Kyoto (stock #109029). The fj-lacz reporter was previously described (Sopko et al., 2009). Complex I assembly mutant (dcia30 ex80 /TM6) was provided by David Walker and described previously (Cho et al., 2012). ft fd mutants were rescued with ft fd /CyO, GFP; P[acman]- ft /TM6B (expressing full-length Ft - FL, or versions lacking region 2 - FLD2 or region 8 - FLD8) flies obtained from Kenneth Irvine as previously described (Pan et al., 2013). All other fly stocks were obtained from the Bloomington Drosophila Stock Center. Antibodies The following antibodies were used: mouse M2 anti-flag (Sigma), mouse anti-cva (MitoSciences), mouse anti-porin (MitoSciences), mouse anti-pdh (MitoSciences), mouse anti-ndufs3 (MitoSciences), mouse anti-b-gal (Promega), mouse anti-lamin (DSHB), anti-a-tubulin (DSHB), mouse anti-actin (Millipore, clone C4), rabbit anti-cytochrome C (Boster Immunoleader), rat anti- Crumbs (gift from Ulrich Tepass), guinea pig anti-expanded (gift from Rick Fehon), anti-activated Caspase3 (Cell Signaling #9661S), rabbit anti-calnexin (Abcam), rabbit anti-pex5 (Sigma SAB ), anti-his (Sigma SAB ) anti-ndufv2 (raised in rat against full-length GST-tagged Ndufv2), anti-fat (raised in rat against a GST-tagged intracellular fragment of Fat consisting of residues ), horseradish peroxidase-conjugated anti-rat and mouse IgG secondary antibodies (Jackson Labs), and Alexa Fluor or Cy3-conjugated anti-rat and mouse IgG secondary antibodies (Invitrogen or Jackson Labs). Tangential Eye Sections Adult heads were embedded in Durcupan resin. Tangential eye sections were obtained as previously described (Tomlinson and Ready, 1987). Drosophila Testis Preps Adult and larval testes were dissected in TIB (testes isolation buffer) (Casal et al., 1990) with Hoechst (1:2000 or 8.3 mg/ml) (Sigma, St. Louis, MO) and live squashed preparations of Drosophila male germ cells were made as previously described (Regan and Fuller, 1990). Preparations were examined on an epifluorescence microscope (Zeiss Axioplan 2, Axiocam black and white camera, Axiovision software (Carl Zeiss)). Germline clones were made using the hsflp-frt system. Two day-old larvae were heat shocked for 1h at 37 C (in an incubator) or for 2h at 37 C (in a water bath), for 2-3 consecutive days. For RNAi experiments, VDRC line #22194 line was crossed with BamGal4, as described (Chen and McKearin, 2003). Electron Microscopy Adult and 3rd instar larval testes (WT and ft mutants) were dissected in 0.1M phosphate buffer (PB) (ph = 7.4) on ice and immediately subjected to high-pressure freezing (Leica HPM100). Samples were then subjected to freeze substitution (Leica AFS2) in 1% osmium tetroxide and 0.1% uranyl acetate in HPLC grade acetone. Samples were washed several times in acetone and embedded in a Quetol-Spurr resin mixture. Alternatively, testes were fixed immediately after dissection in a freshly prepared solution of 2.5% glutaraldehyde + 2% paraformaldehyde in PB. Samples were further prepared following a previously described protocol (Fabian et al., 2010) and embedded in Quetol-Spurr resin. 100nm sections were cut on an RMC MT6000 ultramicrotome, stained with 2% aqueous uranyl acetate and 0.2% lead citrate and viewed in a JEOL JTE transmission electron microscope (JEOL, Peabody, MA; The Hospital for Sick Children Electron Microscopy Facility). Images were captured with AMTv542 software (Advanced Microscopy Techniques, Danvers, MA). Imaginal eye and wing discs were dissected as above, fixed overnight in fresh Trumps fixative (1% glutaraldehyde, 4% paraformaldehyde, 0.005% CaCl 2 ), and postfixed with 1% OsO4 in phosphate buffer for 60 min. Samples were dehydrated in acetone and infiltrated with 1:2, 1:1, 2:1 resin:acetone mixture, followed by 100% resin. Discs were flat embedded (EMS Standard flat embedding mold). 80 nm sections were cut, stained and viewed as above. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. S1
18 Cell Culture and Transfection S2 and D11 cells were cultured in M3 media supplemented with 10% FBS. D11 culture media was supplemented with 10 mg/ml of insulin. S2 cells were transfected using FuGENE 6 (Roche) or Effectene Transfection Kit (QIAGEN). DHE Stain Dihydroethidium staining was performed as previously described (Owusu-Ansah et al., 2008a). Imaginal discs were fixed with 2% PFA for 5 0. EdU Incorporation EdU incorporation assay was performed using the Click-iT EdU AlexaFluor594 Imaging kit (Invitrogen Cat#10084) as per the manufacturer s protocol. Immunoblotting Cell lysis and immunoprecipitation with Protein-G Mag SepharoseTM Xtra (GE Healthcare) were performed according to the manufacturer s instructions. Samples were electrophoresed on 12.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes, blocked in 5% skim milk powder in Tris-buffered saline overnight and subsequently incubated with primary and secondary antibodies. Chemiluminescence detection was performed on a Versadoc imager (Bio-Rad). Immunofluorescence To prepare samples for immunofluorescence, cells or late 3rd instar larval eye discs were washed with PBS, fixed with 4% PFA, washed again with PBS, permeabilized in 0.1% Triton X-100 and then blocked one hour in goat serum before incubation with primary and secondary antibodies in 5% (imaginal discs) or 10% (cells) goat serum. Imaginal discs were imaged on a Nikon D-Eclipse C1 confocal microscope. Cells were imaged using a Zeiss AxioVision fluorescence microscope. Cell Fractionation and Mitochondrial Protection Assay Mitochondria from D11 cells or eye/brain complexes from WT, ds UAO71 and ft alb /ft fd larvae were isolated using the QIAGEN Qproteome mitochondria isolation kit (#37612). Whole larval extracts were fractionated using OptiPrep density gradient media (Sigma D1556) using a 20%/25%/30%/40% gradient (Bayat et al., 2012), and spun in a swinging bucket rotor at xg for 18 hr at 4 C using an Optima Max ultracentrifuge (Beckman Coulter). Fractions were collected after the spin was completed and purified mitochondria harvested from the boundary between the 20% and 25% gradients. Aliquots of mitochondria were treated with 5, 6 or 10 mg/ml proteinase K at 4 C for 10 min. If needed, 1% Triton X-100 was included during the proteinase K digestion. After digestion, proteinase K was quenched by addition of 1X Complete Protease Inhibitor (Roche Applied Science) followed by incubation at 4 C for 10 min. Mitochondrial In-Gel Activity Assays Mitochondria from Drosophila larvae were isolated as described (Walker et al., 2006), resuspended in lysis buffer (50 mm Bis-Tris, ph 7, 750 mm 6-aminocaproic acid, 2% (w/v) digitonin, protease inhibitor cocktail), cleared by centrifugation at x g at 4 C and supplemented with glycerol to a final concentration of 4%. 40 mg of mitochondrial protein was resolved on a 4% 13% native gel using a cathode buffer containing 0.05% (w/v) deoxycholate and 0.01% (w/v) n-dodecyl b-d-maltoside. In-gel activity assays were performed as described (Wittig et al., 2007). Briefly, complex I activity was measured in a NADH:NTB reductase assay. Gel strips from clear-native gels above were incubated in assay buffer (2.5 mg/ml NADH, 0.1 mg/ml NTB in 5mM Tris/HCl, ph 7.4) for 5 min, then fixed in 50% methanol, 10% acetic acid. Similarly, assays for complex II and IV activity were carried out in complex II assay buffer (200 mm sodium succinate, 200 mm phenazine methosulfate, 0.1 mg/ml NTB in 5mM Tris/HCl, ph 7.4) or complex IV assay buffer (0.5 mg/ml diaminobenzidine, 50 mm horse heart cytochrome c in 50 mm sodium phosphate, ph 7.2), incubated for min then fixed. Complex V activity was monitored by following ATP hydrolysis activity. Gel strips were preincubated in 35 mm Tris, 270 mm glycine, ph 8.3, then transferred to assay buffer (35 mm Tris, 270 mm glycine, 14 mm MgSO 4, 0.2% Pb(NO 3 ) 2, 8 mm ATP, ph 8.3). Gel strips were then fixed in 50% methanol for 30 min to stop the reaction. NADH: Ubiquinone Oxidoreductase Activity Assay Mitochondria purified from 5 female larvae were resuspended in 100 ml of mitochondrial isolation medium (MIM, 250 mm sucrose, 10 mm Tris (ph 7.4), 0.15 mm MgCl 2 ), and 5 ml were used in a colorimetric complex I activity assay as previously described (Cho et al., 2012). NADH: ubiquinone oxidoreductase activity was monitored as a decrease in absorbance at 600 nm, for 1 hr, with no inhibitor and in the presence of rotenone (2 um). The measured activities were normalized to citrate synthase activity. Mitochondrial Isolation and BN-PAGE Mitochondria for BN-PAGE were purified from larvae by differential centrifugation using mitochondrial isolation medium (MIM, 250 mm sucrose, 10 mm Tris (ph 7.4), 0.15 mm MgCl 2, with protease inhibitor). Mitochondria were resuspended in 1x Native PAGE Sample buffer (Invitrogen) with 1% digitonin and protease inhibitors, and incubated for 15 min on ice. Samples were S2 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
19 centrifuged at x g for 30min at 4 C and supernatant was resuspended with G250 sample additive and Native PAGE Sample Buffer (Invitrogen). Mitochondria were then visualized with the Novex NativePAGE Bis Tris Gel system (Invitrogen) using 3% 12% Bis-Tris Native PAGE gels as previously described (Cho et al., 2012). For further immunodetection, samples were transferred to PVDF membranes with transfer buffer containing 20% methanol and no SDS. Membranes were blocked in 5% skim milk powder in Tris-buffered saline for 1hr and incubated with primary and secondary antibodies. Chemiluminescence detection was performed on a Versadoc imager (Bio-Rad). Lactate Assay 30 yw larvae and the equivalent number of ft fd larvae were homogenized in 100 ml of PBS with 30 strokes of a Kontes pestle. For protein determination, 5 ml were taken for a BCA assay (BCA Protein Assay Kit, Pierce, 23227). The remaining samples were heat shocked at 60 C for 15 min and centrifuged at g for 5 min; 5-10 ml of the supernatant were used for lactate determination using a Lactate Assay Kit (Biovision, K , Milpitas, CA, USA). All lactate readings were normalized to protein content. ROS Determination 3x10 6 cells were collected and incubated with or without 5 mm Antimycin A at 25 C for 2.5 hr. 5 mm MitoSOX Red was added, and incubated at 25 C for 30 min then washed with media. Fluorescence was read at 510 nm excitation, 580 nm emission. Protein Purification and GST Pull-Down Protein purification and GST pull-downs were performed as previously described (Sambrook and Russell, 2006). Briefly, bacterial cells were transformed and induced with IPTG to allow expression of recombinant proteins. Cells were sonicated and centrifuged, and supernatant was incubated with Nickel or Glutathione beads at 4 C. Supernatant was removed after spin and protein was eluted off Nickel beads with 250mM Imidazole. Eluted protein was incubated with Glutathione-conjugated protein, washed and beads boiled before loading on SDS-PAGE for visualization with anti-his antibody. ATP Assay Lysates for ATP assays were prepared from whole larvae as previously described (Monserrate et al., 2012). Lysates were diluted 1:100 and ATP concentration was determined using the CellTiter-Glo kit (Promega product#g7571) as per manufacturer s protocol. SUPPLEMENTAL REFERENCES Chen, D., and McKearin, D.M. (2003). A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130, Monserrate, J.P., Chen, M.Y., and Brachmann, C.B. (2012). Drosophila larvae lacking the bcl-2 gene, buffy, are sensitive to nutrient stress, maintain increased basal target of rapamycin (Tor) signaling and exhibit characteristics of altered basal energy metabolism. BMC Biol. 10, 63. Tomlinson, A., and Ready, D.F. (1987). Cell fate in the Drosophila ommatidium. Dev. Biol. 123, Xu, T., and Rubin, G.M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, Cell 158, , September 11, 2014 ª2014 Elsevier Inc. S3
20 Figure S1. Reduced Levels of Ndufv2 Lead to PCP Defects, Related to Figure 1 (A) The intracellular domain (ICD) of Ft contains 8 highly conserved regions (red boxes). Recent structure-function studies: (I) Matakatsu and Blair, 2012; (II) Zhao et al., 2013; (III) Bossuyt et al., 2014; (IV) Pan et al., 2013; have identified domains that are important for PCP regulation (blue lines) or Hpo pathway control (red lines) or both (PH, purple). Domains are labeled with nomenclature used in each study. Dashed lines indicate weak requirements for Ft. (B) Wing hair polarity is disturbed in Ndufv2 knockdown but not in UAS-GFP control. PCP defects are observed in a region of the wing distal to the posterior crossvein. UAS-GFP and Ndufv2 dsrna were driven with engal4. (C) Tangential sections of adult eyes with diminished Ndufv2 expression. eygal4 driven Ndufv2 dsrna from the VDRC (lines and ) and the NIG (line 5703R-1) results in PCP defects. The NIG line also displays defects in ommatidial morphology as denoted by the black open circles; these are not scored for polarity. Yellow lines demarcate the endogenous equator. (D) Expression of UAS-Ndufv2DFeS driven by eygal4 is unable to completely rescue PCP defects induced by dsrna against Ndufv2 (compare with Figure 1F). (E) Purified His-Ndufv2 and His-Atro-Cterm (positive control) copurify with GST-FtIC but not with GST alone. S4 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
21 Figure S2. Depletion of Ndufv2 Results in Increased Cell Death and Increased Proliferation, Related to Figures 1 and 2 (A and B) Expression of Ndufv2 dsrna in posterior compartments of wing discs (marked by aen) causes increased apoptosis, as shown by acaspase staining (A), and increased proliferation, as shown by EdU incorporation (B). (C) His-tagged full-length, DMTS and N-term fragments of Ndufv2 bind to Ft-ICD and N-2 fragments of Ft in GST-pull-down analysis. Truncated Ndufv2 containing only the Fe-S binding domain does not bind Ft. Schematic of Ndufv2, domains and truncations used are below. Ft-binding region is marked with a red bar, and binding affinities to Ft marked to the right. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. S5
22 Figure S3. ft Affects Mitochondrial Morphology, and Ndufv2 Affects ROS Levels, Related to Figures 3 and 4 (A) Transmission electron micrographs of wild-type mitochondria show regularly spaced, flattened cristae. In ft alb and ft fd mutants, mitochondria have swollen and irregular cristae. Scale bars, 200 nm. (B) Lysates of S2 cells left untreated or treated with dsrna against Ndufv2 were probed using Ndufv2 antibody. Ndufv2 protein levels are appreciably reduced upon dsrna treatment. (C) S2 cells treated with dsrna against Ndufv2 produce more ROS as detected by DHE fluorescence. Error bars represent standard error. (D) ROS are appreciably increased in the posterior compartment of 3rd instar larval wing discs expressing Ndufv2 dsrna driven with engal4 as compared to control (en>gfp). The effect is stronger in the VDRC RNAi lines than in the NIG RNAi line. ROS detected by DHE. Posterior right. (E) ROS (as indicated by GSTD1-GFP) in early 3rd instar eye discs are highest at the posterior equatorial region. (F) Structural Illumination Microscopy (SIM) of Drosophila tissue shows partial costaining of Ft and CVa. S6 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
23 Figure S4. Specificity of Anti-Ndufv2 Antibodies, Expression Levels of Ndufv2 Transgenes, and Sequential Processing of Ft, Related to Figures 4 and 5 (A) engal4-driven expression of Ndufv2 dsrna in the posterior compartment of the wing imaginal disc (as marked by aen in red) demonstrates both efficacy of Ndufv2 knockdown (by VDRC line 22194) as well as specificity of Ndufv2 antibody. UAS-Ndufv2 and UAS-Ndufv2DFeS transgenes are ectopically expressed by engal4 at similar levels. (B) Ndufv2 colocalizes with CVa in wild-type wing discs. Boxed region shown in higher magnification in lower panels. (C) Ft mito is enriched in vivo in mitochondrial fractions from wild-type and ds - larval discs, but is absent in ft alb/fd disc extracts. *NS nonspecific fragment detected by Ft antibody. (D) Heat shock induced expression of a full-length HA-tagged ft transgene in Drosophila embryos reveals multiple forms of Ft: aha (left) or aft (right) antibodies detect 560, 110 and 68kDa bands. atubulin (lower left) serves as a loading control. (E) BN-PAGE western blot analysis shows loss of both Ndufv2 and Ft in ft fd crude mitochondrial isolates from whole larvae. (F) BN-PAGE of crude mitochondrial extracts from whole larvae. Relative positions of mitochondrial complexes are labeled to the right (full view of gel from Figure 5E). An asterisk denotes the CI position in WT mitochondria. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. S7
24 (legend on next page) S8 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
25 Figure S5. Predicted Mitochondrial Targeting Sequences in the Cytosolic Domain of Ft, Related to Figure 4 Peptide sequence for the intracellular fragment of (A) D. melanogaster Ft and (D) M. musculus Ft4 contain predicted mitochondrial targeting sequences (MTS). The two targeting sequences with highest probability of mitochondrial import are marked in bold. Red asterisks denote predicted cleavage sites for Mpp in (A) and for PMPC in (D). Sample readouts from MitoProt II are shown in (B), (C), (E) and (F). Cell 158, , September 11, 2014 ª2014 Elsevier Inc. S9
26 Figure S6. Ft Contains Multiple Mitochondrial-Targeting Sequences that Direct Mitochondrial Import in Transfected Cell Assays, Related to Figure 4 (A) Schematic and summary of Ft intracellular fragments used in mitochondrial localization assays. (B) Ft fragments a (not shown), b (not shown), c, d and e partially colocalize with MitoGFP in COS cells, whereas f, g, and IC do not, as detected by immunofluorescence (IF). (C) FtIC runs as a discrete 74kDa band, whereas FtDECD is processed into 110 and 68kDa forms. (D) Immunoblots of protease protection assays in S2 cells validate the IF data for IC, a, d and e. Notably the IC fragment is not detected in the mitochondrial fraction, whereas truncated fragments are protected in the matrix. T total extract, M mitochondrial fraction. S10 Cell 158, , September 11, 2014 ª2014 Elsevier Inc.
27 Figure S7. Alignment of the Intracellular Domain of Ft from Various Organisms Reveals Regions of High Conservation that Can Interact with Mitochondrial Proteins, Related to Figures 5 and 6 (A) ATP levels in whole larval extracts from ft fd mutants show no reproducible difference from wild-type control. Error bars represent standard deviation, n = 6 samples of > 10 larvae each. (B) Mitotic clones of ampka 3 (marked by lack of GFP, clone border marked by dashed line) generated in the pupal retina following starvation display normal polarity as determined by polarity markers Arm (red) and apkc (blue). (C) Regions of the Ft intracellular domain have been shown to function in processes including PCP and Hippo (see Figure S1A). Regions 1 and 2, demarcated by red boxes, are highly conserved among urbilaterian ancestors and found in this study to bind the mitochondrial Ndufv2 and CG1746 proteins. DmFat D. melanogaster, AgFat Anopheles gambiae, TcFat Tribolium castaneum, DpFat Daphnia pulex, BfFat Branchiostoma floridae, MmFat4 M. musculus and HsFat4 H. sapiens. (D) In vitro GST-pull-down analysis using purified proteins indicates interaction between regions 1 and 2 of Ft with CG1746 (see Figure 1G for schematic of Ft fragments). Ponceau S staining indicates loading. Cell 158, , September 11, 2014 ª2014 Elsevier Inc. S11
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Role of Mitochondrial Remodeling in Programmed Cell Death in
 Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Reconstructing Mitochondrial Evolution?? Morphological Diversity. Mitochondrial Diversity??? What is your definition of a mitochondrion??
 Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
The Atypical Cadherin Fat Directly Regulates Mitochondrial Function and Metabolic State
 The Atypical Cadherin Fat Directly Regulates Mitochondrial Function and Metabolic State by Anson David Sing A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy
The Atypical Cadherin Fat Directly Regulates Mitochondrial Function and Metabolic State by Anson David Sing A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplemental material
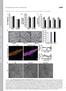 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
TCA Cycle. Voet Biochemistry 3e John Wiley & Sons, Inc.
 TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
RNA Synthesis and Processing
 RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
Scale in the biological world
 Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Aerobic Cellular Respiration
 Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
Gene regulation I Biochemistry 302. Bob Kelm February 25, 2005
 Gene regulation I Biochemistry 302 Bob Kelm February 25, 2005 Principles of gene regulation (cellular versus molecular level) Extracellular signals Chemical (e.g. hormones, growth factors) Environmental
Gene regulation I Biochemistry 302 Bob Kelm February 25, 2005 Principles of gene regulation (cellular versus molecular level) Extracellular signals Chemical (e.g. hormones, growth factors) Environmental
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Importance of Protein sorting. A clue from plastid development
 Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
MITOCHONDRIAL LAB. We are alive because we make a lot of ATP and ATP makes (nonspontaneous) chemical reactions take place
 MITOCHONDRIAL LAB We are alive because we make a lot of ATP and ATP makes (nonspontaneous) chemical reactions take place We make about 95% of our ATP in the mitochondria We will isolate mitochondria, and
MITOCHONDRIAL LAB We are alive because we make a lot of ATP and ATP makes (nonspontaneous) chemical reactions take place We make about 95% of our ATP in the mitochondria We will isolate mitochondria, and
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
GO ID GO term Number of members GO: translation 225 GO: nucleosome 50 GO: calcium ion binding 76 GO: structural
 GO ID GO term Number of members GO:0006412 translation 225 GO:0000786 nucleosome 50 GO:0005509 calcium ion binding 76 GO:0003735 structural constituent of ribosome 170 GO:0019861 flagellum 23 GO:0005840
GO ID GO term Number of members GO:0006412 translation 225 GO:0000786 nucleosome 50 GO:0005509 calcium ion binding 76 GO:0003735 structural constituent of ribosome 170 GO:0019861 flagellum 23 GO:0005840
Types of biological networks. I. Intra-cellurar networks
 Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Chapter 6: A Tour of the Cell
 Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
Honors Biology Reading Guide Chapter 11
 Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins
 RESEARCH ARTICLE 2315 Development 133, 2315-2324 (2006) doi:10.1242/dev.02401 Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins Hitoshi Matakatsu and Seth S. Blair*
RESEARCH ARTICLE 2315 Development 133, 2315-2324 (2006) doi:10.1242/dev.02401 Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins Hitoshi Matakatsu and Seth S. Blair*
CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON
 PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
Biological Process Term Enrichment
 Biological Process Term Enrichment cellular protein localization cellular macromolecule localization intracellular protein transport intracellular transport generation of precursor metabolites and energy
Biological Process Term Enrichment cellular protein localization cellular macromolecule localization intracellular protein transport intracellular transport generation of precursor metabolites and energy
M i t o c h o n d r i a
 M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Supplementary Information
 Supplementary Information Supplementary Figure 1. JAK/STAT in early wing development (a-f) Wing primordia of second instar larvae of the indicated genotypes labeled to visualize expression of upd mrna
Supplementary Information Supplementary Figure 1. JAK/STAT in early wing development (a-f) Wing primordia of second instar larvae of the indicated genotypes labeled to visualize expression of upd mrna
Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization
 Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport
 Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 m
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression
 Traffic 2010; 11: 827 842 2010 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2010.01055.x The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression Danming Tang, Hebao Yuan and Yanzhuang
Traffic 2010; 11: 827 842 2010 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2010.01055.x The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression Danming Tang, Hebao Yuan and Yanzhuang
7.06 Cell Biology EXAM #3 KEY
 7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Regulation of Gene Expression in Bacteria and Their Viruses
 11 Regulation of Gene Expression in Bacteria and Their Viruses WORKING WITH THE FIGURES 1. Compare the structure of IPTG shown in Figure 11-7 with the structure of galactose shown in Figure 11-5. Why is
11 Regulation of Gene Expression in Bacteria and Their Viruses WORKING WITH THE FIGURES 1. Compare the structure of IPTG shown in Figure 11-7 with the structure of galactose shown in Figure 11-5. Why is
7.013 Problem Set
 7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes
 Article Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes Tom Bender, Gabrielle Pena & Jean-Claude Martinou * Abstract At the pyruvate branch point, the fermentative
Article Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes Tom Bender, Gabrielle Pena & Jean-Claude Martinou * Abstract At the pyruvate branch point, the fermentative
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
Exam 1 ID#: October 4, 2007
 Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Lecture 7 Cell Biolog y ٢٢٢ ١
 Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
Honors Biology Fall Final Exam Study Guide
 Honors Biology Fall Final Exam Study Guide Helpful Information: Exam has 100 multiple choice questions. Be ready with pencils and a four-function calculator on the day of the test. Review ALL vocabulary,
Honors Biology Fall Final Exam Study Guide Helpful Information: Exam has 100 multiple choice questions. Be ready with pencils and a four-function calculator on the day of the test. Review ALL vocabulary,
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
The geneticist s questions. Deleting yeast genes. Functional genomics. From Wikipedia, the free encyclopedia
 From Wikipedia, the free encyclopedia Functional genomics..is a field of molecular biology that attempts to make use of the vast wealth of data produced by genomic projects (such as genome sequencing projects)
From Wikipedia, the free encyclopedia Functional genomics..is a field of molecular biology that attempts to make use of the vast wealth of data produced by genomic projects (such as genome sequencing projects)
Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
 Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry 1. The study of cells has been limited by their small size, and so they were
Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry 1. The study of cells has been limited by their small size, and so they were
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10244 a O07391_MYCAV/127-243 NLPC_HAEIN/80-181 SPR_SHIFL/79-183 P74160_SYNY3/112-245 O24914_HELPY/301-437 Q51835_PORGI/68-178 DPP6_BACSH/163-263 YKFC_BACSU/185-292 YDHO_ECOLI/153-263
doi:10.1038/nature10244 a O07391_MYCAV/127-243 NLPC_HAEIN/80-181 SPR_SHIFL/79-183 P74160_SYNY3/112-245 O24914_HELPY/301-437 Q51835_PORGI/68-178 DPP6_BACSH/163-263 YKFC_BACSU/185-292 YDHO_ECOLI/153-263
Full-length GlpG sequence was generated by PCR from E. coli genomic DNA. (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
 Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
Supplementary Methods Protein expression and purification Full-length GlpG sequence was generated by PCR from E. coli genomic DNA (with two sequence variations, D51E/L52V, from the gene bank entry aac28166),
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Initiation of translation in eukaryotic cells:connecting the head and tail
 Initiation of translation in eukaryotic cells:connecting the head and tail GCCRCCAUGG 1: Multiple initiation factors with distinct biochemical roles (linking, tethering, recruiting, and scanning) 2: 5
Initiation of translation in eukaryotic cells:connecting the head and tail GCCRCCAUGG 1: Multiple initiation factors with distinct biochemical roles (linking, tethering, recruiting, and scanning) 2: 5
Biophysics 490M Project
 Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
S1 Gene ontology (GO) analysis of the network alignment results
 1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
09/30/2017. Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center
 09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Chapter 6: A Tour of the Cell
 AP Biology Reading Guide Fred and Theresa Holtzclaw Chapter 6: A Tour of the Cell Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
AP Biology Reading Guide Fred and Theresa Holtzclaw Chapter 6: A Tour of the Cell Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells
 Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Life Sciences 1a: Section 3B. The cell division cycle Objectives Understand the challenges to producing genetically identical daughter cells Understand how a simple biochemical oscillator can drive the
Regulation of gene expression. Premedical - Biology
 Regulation of gene expression Premedical - Biology Regulation of gene expression in prokaryotic cell Operon units system of negative feedback positive and negative regulation in eukaryotic cell - at any
Regulation of gene expression Premedical - Biology Regulation of gene expression in prokaryotic cell Operon units system of negative feedback positive and negative regulation in eukaryotic cell - at any
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Richik N. Ghosh, Linnette Grove, and Oleg Lapets ASSAY and Drug Development Technologies 2004, 2:
 1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
Photosynthesis. Chapter 10. Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece
 Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Three types of RNA polymerase in eukaryotic nuclei
 Three types of RNA polymerase in eukaryotic nuclei Type Location RNA synthesized Effect of α-amanitin I Nucleolus Pre-rRNA for 18,.8 and 8S rrnas Insensitive II Nucleoplasm Pre-mRNA, some snrnas Sensitive
Three types of RNA polymerase in eukaryotic nuclei Type Location RNA synthesized Effect of α-amanitin I Nucleolus Pre-rRNA for 18,.8 and 8S rrnas Insensitive II Nucleoplasm Pre-mRNA, some snrnas Sensitive
Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain
 SUPPLEMENTAL INFORMATION Supplemental Figure Legends Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain mitochondrial NAD +. A) Extracellular tryptophan, even at 5 µm,
SUPPLEMENTAL INFORMATION Supplemental Figure Legends Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain mitochondrial NAD +. A) Extracellular tryptophan, even at 5 µm,
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Written Exam 15 December Course name: Introduction to Systems Biology Course no
 Technical University of Denmark Written Exam 15 December 2008 Course name: Introduction to Systems Biology Course no. 27041 Aids allowed: Open book exam Provide your answers and calculations on separate
Technical University of Denmark Written Exam 15 December 2008 Course name: Introduction to Systems Biology Course no. 27041 Aids allowed: Open book exam Provide your answers and calculations on separate
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
Rex-Family Repressor/NADH Complex
 Kasey Royer Michelle Lukosi Rex-Family Repressor/NADH Complex Part A The biological sensing protein that we selected is the Rex-family repressor/nadh complex. We chose this sensor because it is a calcium
Kasey Royer Michelle Lukosi Rex-Family Repressor/NADH Complex Part A The biological sensing protein that we selected is the Rex-family repressor/nadh complex. We chose this sensor because it is a calcium
An intracellular phase transition drives nucleolar assembly and size control
 An intracellular phase transition drives nucleolar assembly and size control Steph Weber Department of Biology McGill University Membranes spatially organize eukaryotic cells Cells also contain membraneless
An intracellular phase transition drives nucleolar assembly and size control Steph Weber Department of Biology McGill University Membranes spatially organize eukaryotic cells Cells also contain membraneless
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016
 Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Mitochondria Mitochondria were first seen by kollicker in 1850 in muscles and called them sarcosomes. Flemming (1882) described these organelles as
 Mitochondria Mitochondria were first seen by kollicker in 1850 in muscles and called them sarcosomes. Flemming (1882) described these organelles as filia Altmann (1890) observed these structures and named
Mitochondria Mitochondria were first seen by kollicker in 1850 in muscles and called them sarcosomes. Flemming (1882) described these organelles as filia Altmann (1890) observed these structures and named
Two Frizzled Planar Cell Polarity Signals in the Drosophila Wing Are Differentially Organized by the Fat/Dachsous Pathway
 Two Frizzled Planar Cell Polarity Signals in the Drosophila Wing Are Differentially Organized by the Fat/Dachsous Pathway Justin Hogan, Meagan Valentine, Chris Cox, Kristy Doyle, Simon Collier* Department
Two Frizzled Planar Cell Polarity Signals in the Drosophila Wing Are Differentially Organized by the Fat/Dachsous Pathway Justin Hogan, Meagan Valentine, Chris Cox, Kristy Doyle, Simon Collier* Department
Bypass and interaction suppressors; pathway analysis
 Bypass and interaction suppressors; pathway analysis The isolation of extragenic suppressors is a powerful tool for identifying genes that encode proteins that function in the same process as a gene of
Bypass and interaction suppressors; pathway analysis The isolation of extragenic suppressors is a powerful tool for identifying genes that encode proteins that function in the same process as a gene of
