Research. Summary. Introduction
|
|
|
- Arabella Lewis
- 5 years ago
- Views:
Transcription
1 Research A role for LATERAL ORGAN BOUNDARIES-DOMAIN 16 during the interaction Arabidopsis Meloidogyne spp. provides a molecular link between lateral root and root-knot nematode feeding site development Javier Cabrera 1, Fernando E. Dıaz-Manzano 1, Marıa Sanchez 1, Marie-No elle Rosso 2, Teresa Melillo 3, Tatsuaki Goh 4, Hidehiro Fukaki 4, Susana Cabello 5, Julia Hofmann 5, Carmen Fenoll 1 and Carolina Escobar 1 1 Facultad de Ciencias Ambientales y Bioquımica, Universidad de Castilla-La Mancha, Av. Carlos III s/n, E Toledo, Spain; 2 INRA, Aix-Marseille Universite, UMR 1163, Biotechnologie des Champignons Filamenteux, F Marseille, France; 3 Istituto per la Protezione de lle Piante, CNR, Bari, Italy; 4 Department of Biology, Graduate School of Science, Kobe University, 1-1 Rokkodai, Kobe , Japan; 5 Division of Plant Protection, Department of Crop Sciences, University of Natural Resources and Applied Life Sciences, Konrad Lorenz Str. 24, Tulln a. d. Donau A-3430, Austria Author for correspondence: Carolina Escobar Tel: ext carolina.escobar@uclm.es Received: 10 February 2014 Accepted: 21 March 2014 doi: /nph Key words: Arabidopsis, auxins, giant cells (GCs), Lateral Organ Boundaries-Domain 16 (LBD16), lateral root, root-knot nematodes (RKNs), xylem pole pericycle (XPP). Summary Plant endoparasitic nematodes induce the formation of their feeding cells by injecting effectors from the esophageal glands into root cells. Although vascular cylinder cells seem to be involved in the formation of root-knot nematode (RKN) feeding structures, molecular evidence is scarce. We address the role during gall development of LATERAL ORGAN BOUNDARIES-DOMAIN 16 (LBD16), a key component of the auxin pathway leading to the divisions in the xylem pole pericycle (XPP) for lateral root (LR) formation. Arabidopsis T-DNA tagged J0192 and J0121 XPP marker lines, LBD16 and DR5::GUS promoter lines, and isolated J0192 protoplasts were assayed for nematode-dependent gene expression. Infection tests in LBD16 knock-out lines were used for functional analysis. J0192 and J0121 lines were activated in early developing galls and giant cells (GCs), resembling the pattern of the G2/M-transition specific Pro CycB1;1 :CycB1;1(NT)-GUS line. LBD16 was regulated by auxins in galls as in LRs, and induced by RKN secretions. LBD16 loss of function mutants and a transgenic line with defective XPP cells showed a significantly reduced infection rate. The results show that genes expressed in the dividing XPP, particularly LBD16, are important for gall formation, as they are for LR development. Introduction Plant parasitic nematodes represent a major threat to agriculture (Bird et al., 2009) and the need to develop nematode resistance is intensifying with the banning of pesticides (Atkinson et al., 2012). Nematodes induce the formation of sophisticated feeding structures inside the root that operate as physiological sinks to supply nutrients to the nematode (Perry & Moens, 2011). Among the most damaging groups of endoparasites are the rootknot nematodes (RKNs; Meloidogyne spp.) and the cyst nematodes (CNs; Heterodera spp. and Globodera spp.). Juveniles are attracted by root chemical signals that modify nematode behavior and gene expression, penetrate the root and establish in the vascular cylinder, transforming selected root cells into their feeding sites (Perry & Moens, 2011; Teillet et al., 2013). In Arabidopsis, females of Heterodera schachtii usually develop in syncytia from procambial or pericycle cells, whereas males develop in syncytia from pericycle cells (Golinowski et al., 1996; Sobczak et al., 1997). From these initial cells, the syncytium incorporates neighboring cells that fuse via dissolution of their cell walls along plasmodesmata (Sobczak & Golinowski, 2009). Based on histological observations, it is believed that giant cells (GCs) induced by RKNs are most commonly formed from parenchymatic cells within the stele that surround the nematode head and that the primary effector molecules stimulating GC formation are esophageal gland secretions (Berg et al., 2009). Among these, Mi16D10, which interacts with nuclear plant targets, such as SCARECROW-like transcription factors, may function in the extensive transcriptional reprogramming that accompanies GC ontogenesis (Quentin et al., 2013). Selected cells undergo mitosis with aborted cytokinesis, becoming enlarged, multinucleated, endoreduplicated cells that feed the nematode (Caillaud et al., 632
2 Phytologist Research ; Gheysen & Mitchum, 2009; Almeida-Engler & Favery, 2011). Concurrently, root pericycle and cortical cells proliferate around the nematode, increasing the girth of the root and forming a gall (Berg et al., 2009). There are parallelisms between lateral root (LR) development, RKN gall formation and the establishment of Rhizobium symbionts, based on similar expression patterns of genes encoding transcription factors and cell cycle regulators (Mathesius, 2003). This, together with the common activation patterns of tagged lines in LRs and galls (Barthels et al., 1997) and the pericycle cell divisions that accompany nematode establishment, suggests a relationship between LR emergence and RKN feeding site formation. However, clear molecular evidence is still lacking. Arabidopsis LRs originate exclusively from pericycle founder cells located opposite the xylem poles (xylem pole pericycle (XPP) cells). Members of the LATERAL ORGAN BOUNDARIES- DOMAIN (LBD) transcription factor family have been shown to play a crucial role in LR development (Okushima et al., 2007; Bell et al., 2012), being highly conserved across plant species. The transcription of LBD16, LBD18 and LBD29 is activated by AUXIN RESPONSE FACTOR 7(ARF7) and ARF19 in the auxin signaling cascade, leading to the emergence of a new LR (Okushima et al., 2007; Lee et al., 2009). The localized activity of LBD16 and its related LBDs is involved in the symmetry breaking of LR founder cells for LR initiation (Goh et al., 2012). Interestingly, LBD16, LBD17, LBD18 and LBD29 have been identified as important regulators that function during callus induction, thereby establishing a molecular link between auxin signaling and plant regeneration programs (Fan et al., 2012). Auxin is a key regulatory factor that causes changes in gene expression upon nematode infection (Goverse & Bird, 2011). Direct evidence for this role of auxin is the finding that promoters containing ARF binding sites, such as the promoter of an auxin responsive gene from the Gretchen Hagen 3 family of soybean (GH3) and the synthetic auxin-inducible promoter, DR5, are activated at early stages of gall and syncytium development (Hutangura et al., 1999; Karczmarek et al., 2004). Additionally, Meloidogyne incognita infection did not progress in the tomato (Solanum lycopersicum) auxin-insensitive mutant diageotropica, dgt (Richardson & Price, 1982). However, consistent and solid findings based on genetic analysis are only available for CNs, in which a range of Arabidopsis mutants in auxin transport, homeostasis and responses have been tested (e.g. mutations at the AXR2 locus, PIN-FORMED 1 and PIN-FORMED 2/auxin efflux carrier component 2: axr2, pin1-1 and pin2/eir1-1, respectively Goverse et al., 2000). To date, a direct molecular relationship between LR development, gall and GC development and auxin signaling has not yet been described. This work shows that the promoter of LBD16, which encodes a transcription factor involved in LR initiation, is active from very early stages of gall development, regulated by auxins in galls and induced by nematode secretions. XPP marker lines (J0192 and J0121) were also activated in galls with a pattern resembling that of the G2-M transition marker Pro CycB1;1 :CycB1;1(NT)- GUS in XPP dividing cells. The activation pattern of the auxin sensor DR5 paralleled that of LBD16 only during the early infection stages. Furthermore, functional studies indicate that LBD16 is a key molecular player in XPP cells during GC and gall development. Materials and Methods Nematode populations Meloidogyne javanica (MIK; Portillo et al., 2009) and M. arenaria (obtained from Dr M. F. Andres, CSIC, Spain) were multiplied in vitro on cucumber (Cucumis sativus cv Hoffmanns Giganta) grown in 0.3% Gamborg medium (Gamborg et al., 1968) with 3% sucrose. Heterodera schachtii population (from Dr J. Hofmann, BOKU, Austria) in mustard seedlings (Sinapsis alba cv Albatros). Egg hatching was stimulated in sterile water for RKNs and in sterile 3 mm ZnCl 2 for CNs. Plant material, growth conditions and nematode inoculation Arabidopsis thaliana (L.) Heynh Columbia-0 (Col-0) was the background of all the transgenic lines. For expression analysis, we used J0121, J0192 (Laplaze et al., 2005), DR5::GUS (Ulmasov et al., 1997) and two different versions of plbd16::gus carrying either a 1500-bp or a 2500-bp promoter sequence (Laplaze et al., 2005; Okushima et al., 2007). Col-0, lbd16-1, 35S:LBD16- SRDX (Okushima et al., 2007), plbd16:lbd16-srdx (Goh et al., 2012), J0121 and J0121>>DTA (Laplaze et al., 2005) were used for the infection tests. The T-DNA insertion mutant lbd16-1 (SALK_095791) was genotyped by PCR to identify homozygous plants (Supporting Information Fig. S1). The seeds were surface-sterilized and sown in modified Gamborg B5 medium (Escobar et al., 2003). For expression analysis, three independent experiments were performed with plants per line each. The plates were inoculated 12 d after germination with freshly hatched M. javanica, M. arenaria (Escobar et al., 2003) or H. schachtii (Golinowski et al., 1996) juveniles and carefully examined every 12 h (Portillo et al., 2013). Galls or syncytia were hand-dissected for microscopy inspection at selected developmental time-points. For functional analysis, three independent infection tests were performed with a minimum of 60 individual plants per line per experiment (Fig. S2). The plates were inoculated with 7 10 M. javanica, M. arenaria or H. schachtii juveniles per main root. A thin temperate (30 37 C) film of 0.3% agarose in Gamborg medium was placed over the roots to facilitate nematode penetration. Plants were vertically grown to avoid early appearance of LRs. The plates were systematically examined under the stereomicroscope at 10 d post infection (dpi) to determine the number of infections per plant. To estimate GC size in Col-0, J0121, 35S::LBD16-SRDX and J0121>>DTA, galls were hand-dissected at 15 dpi, fixed and embedded (Barcala et al., 2010). The sections were stained with 1% toluidine blue in 1% borax solution (TAAB). The TrakEM2 (Cardona et al., 2012) plug-in for FIJI (Schindelin et al., 2012) was used to measure GC areas. Two representative galls from each
3 634 Research Phytologist genotype were entirely sectioned into 2-lm sections. The 10 sections in which the GCs showed the greatest expansion in each gall were selected to quantify the area occupied by the GCs (< 100 GCs sections scored) as well as to obtain the mean area and standard errors among the sections as in Portillo et al. (2013). Statistical analysis of the infection parameters and GC areas was performed using ANOVA and the Schefee test using the SPSS package (IBM, Armonk, NY, USA) with P < Meloidogyne incognita secretions and protoplast isolation Stylet secretions were collected from freshly hatched J2s of M. incognita after induction in resorcinol as described by Rosso et al. (1999). The secreted proteins were separated by ion exchange chromatography on a High Q anion exchange resin (Bio-Rad, Melville, NY, USA) in Bis-Tris (20 mm; ph 6). The fraction eluted with 0.4 M NaCl, dialyzed with Bis-Tris 20 mm, ph 6, and concentrated by ultrafiltration using YM50- and YM3-kDa membranes, was tested in protoplasts prepared from J0192 Arabidopsis leaves (Fig. S8d). Mesophyll protoplasts were isolated from Arabidopsis J0192 and DR5::GFP using a modified method described by Sheen (2002). Leaves from 4-wk-old plants grown in vitro were collected, cut into mm 2 strips, transferred to 0.5 M mannitol and plasmolyzed for 1 h. They were transferred to a cell wall digestion solution containing 0.8% Cellulase R-10 (Serva, Heidelberg, Germany) and 0.2% Macerozyme (Serva) in 0.4 M mannitol. Protoplasts were collected, washed and incubated in Murashige and Skoog medium supplemented with 4.4 g l 1 vitamins (Sigma), 20 g l 1 sucrose, 0.6 g l 1 MES, 0.05 mg l 1 kinetin and 40 mg l 1 ampicillin with different concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) or in a medium lacking auxin but containing nematode secretions (5 7 ll). As a negative control, the same medium without auxins or secretions was used. The fluorescence background was assessed with Col-0 plants. Protoplasts were maintained in a growth chamber at 25 C in darkness. Three to four independent experiments with intact protoplasts per treatment in each experiment were scored for the percentage of GFP-expressing protoplasts. This ranged from 0% in all the control samples to 25 50% in the auxin- and secretion-treated samples in each independent experiment. The micrographs shown are representative of the intact protoplast after each treatment. Histological analysis of GUS and GFP expression For GUS staining, hand-dissected galls were prefixed in 0.5% glutaraldehyde for 5 min under moderate vacuum and incubated for 5 8 h in a solution containing 5 mm EDTA (ph 8), 0.05% Triton X-100, 0.05 mm K 3 Fe(CN) 6, 0.05 mm K 4 Fe(CN) 6 and 1mgml 1 X-GlcA in 50 mm sodium phosphate buffer. Galls were photographed under a Leica Mz125 stereomicroscope (Leica Microsystems, Wetzlar, Germany) or a Nikon eclipse 90i microscope (Nikon Corp., Tokyo, Japan). For tissue localization of GUS expression, galls were fixed, sectioned at 2 lm and processed as in Barcala et al. (2010). A Leica TCS SP2 confocal laser scanning microscope was used for the detection of GFP expression. Most galls were also handsectioned and immediately stained with 0.5 lgml 1 propidium iodide (PI) in phosphate-buffered saline (PBS) for 5 min, or freshly embedded in 5% low melting agarose and subsequently sectioned. Vibroslices were stained with PI under the same conditions, and immediately observed under the confocal microscope. The emission spectra were set to 515, and 617 nm for GFP, chloroplast autofluorescence and PI, respectively. A longpass 500-nm dichroic beam splitter was used. In silico analysis of GC and gall transcriptomes The genes that were up-regulated in 3-dpi GCs and gall transcriptomes (Barcala et al., 2010) were manually compared with the sets of enriched genes in each root cell type described by Brady et al. (2007). To identify the transcripts enriched in GCs and galls, the v 2 test was used with a significance level of P < The abundance of each group in the Arabidopsis genome was used as the background. Results GCs and galls show transcriptional similarities with undifferentiated root cell types It has been established that the CN H. schachtii induces syncytium formation from root procambial or pericycle cells (Golinowski et al., 1996; Sobczak et al., 1997). However, the root cell types contributing to gall and/or GC formation are still a matter of debate. To determine if specific root cell types shared molecular determinants with early-developing galls and GCs, we performed pairwise comparisons between the up-regulated genes in RKN feeding structures at 3 dpi (Barcala et al., 2010), and genes characteristically expressed in each of the 16 root cell types available from Brady et al. (2007). Only the transcripts that were enriched in the lateral root primordium (LRP) and quiescent center cells were overrepresented in both galls and GCs after v 2 analyses (P < 0.05; see the Materials and Methods section; Fig. 1; Table S1). Transcripts characteristic of developing xylem were overrepresented specifically in GCs, whereas transcripts from the endodermis were enriched in galls but not in GCs (Fig. 1; Table S1). These results suggest that the commonly induced genes in both galls and GCs show transcriptional profile similarities to those observed in undifferentiated root cell types, such as those from the LRP and the quiescent center. Two marker lines for LRP initial cells are activated in GCs and galls Two Arabidopsis GAL4-GFP enhancer trap lines, J0192 and J0121 (Laplaze et al., 2005), were inoculated with M. javanica. Line J0192 is an early and very specific marker line for XPP cells that divide to form a new LRP before its emergence, whereas J0121 is specific for two to three root pericycle cell files adjacent to the xylem poles along the entire root starting at the elongation
4 Phytologist Research 635 Fig. 1 Schematic representation of root cell types with transcriptomic similarity to developing galls and giant cells (GCs) induced by Meloidogyne javanica. Specific Arabidopsis root cell transcriptomes (Brady et al., 2007) were compared with the up-regulated genes in 3-d post infection (dpi) GCs and gall transcriptomes (Barcala et al., 2010). Overrepresented gene overlaps were identified by v 2 analysis (P < 0.05). A color code is indicated for the overrepresented root cell transcriptomes in galls and GCs. The table outlines the root marker cell lines used, showing v 2 and P-values; P < 0.05 indicates overrepresentation or enrichment. pse, protophloem sieve elements; mse, metaphloem sieve element; CC, companion cells. zone (Laplaze et al., 2005). In the J0192>>GFP line, GFP expression is driven by the promoter region of LBD16 (At2g42430; Laplaze et al., 2005). We monitored the time-courses of J0192>>GFP and J0121>>GFP expression in young galls (Fig. 2). We also performed in vivo monitoring by following the same galls along the consecutive infection stages (Fig. S3). Both lines were activated as early as 1 dpi (Fig. S3). The J0192>>GFP line showed GFP expression in the center of the gall at 2 dpi (Fig. 2a) and by 4 and also 6 dpi, the entire vascular cylinder inside the gall was expressing GFP (Fig. 2c,e; all channels for the confocal images in Fig. 2 are shown in Fig. S4). Semithin sections of 7-dpi galls from the GAL4-GUS enhancer trap line J0192>>GUS also showed a strong staining inside the vascular cylinder, according to GFP expression, extended from the pericycle layer inwards, including the GCs (Fig. 2g). Fig. 2 Two independent pericycle markers are active in Arabidopsis galls and giant cells (GCs) formed by Meloidogyne javanica at very early developmental stages. Confocal images of galls stained with propidium iodide (red fluorescence) showing GFP (green) at 2, 4 and 6 d post infection (dpi) from lines J0192>>GFP and J0121>>GFP (a, c, e and b, d, f, respectively) are presented. (g, h) Semithin sections of J0192>>GUS (g) and J0121>>GUS at 6 dpi (h). Asterisks, GCs; N, nematode. Bars, 100 lm. A closer inspection of hand-dissected infected roots during the migration-establishment phase (12 24 h post inoculation (hpi)) revealed a pair of contiguous pericycle cells expressing GFP near the nematode head (Fig. 3a, a1, a2), which closely resembled the induction observed during LRP emergence in the pericycle (Fig. 3e). J0192 was frequently activated in XPP cells on both
5 636 Research Phytologist Fig. 3 Xylem pericycle pole (XPP) cells in Arabidopsis galls induced by Meloidogyne javanica express molecular markers of lateral root (LR) initials and the G2/M cell cycle phase. (a, a1, a2, b, b1, c, c1, c2, d, d1) Confocal images of galls stained with propidium iodide (red fluorescence) showing GFP (green) from line J0192>>GFP, which marks the identity of LR initials. (a, a1) Galls at h post infection (hpi). (a2) Detailed view of the XPP, xylem (X) and nematode (N) head with a close-up image of the overlap with the transmission image of (a1) as indicated. (b, b1, c, c1, c2, d, d1) Galls at hpi (b, b1), hpi (c, c1, c2) and hpi (d, d1). (e h) Confocal images of lateral root primordia (LRPs) at different stages from uninfected roots of line J0192>>GFP. (i k) Transmission images of galls infected at 12, and 72 hpi from line ProCycB1;1:CycB1;1(NT)-GUS, which marks cells in G2/M. White arrows indicate the activated cells at the XPPs and the direction of dividing cells relative to the root vascular cylinder. Bars: 100 lm, except for enlarged photos (a1, a2, b1, b1, c2 and d1), 25 lm. sides of the vascular cylinder inside the gall (Fig. 3b, b1), contrary to the induction in only one side of the XPP during LR formation (Fig. 3e,f). As the galls developed and more cells formed, the GFP signal increased toward the vascular cylinder (Fig. 3c, c1), whereas in the LRP divisions occurred toward the cortex (Fig. 3e h). The labeled vascular cylinder cells exhibited abnormal irregular division planes (see close-up image in Fig. 3c1) and the number of labeled cell layers increased during gall development until they had filled the entire vascular cylinder as early as hpi (Fig. 3d, d1). The restricted induction of J0192, a well-defined marker line for LRP initials, inside the galls at early infection stages seems to correlate with the results from the in silico analysis. Similar results were obtained for the J0121>>GFP line. At 1 dpi, GFP expression in pericycle cells inside the gall was similar to that of the adjacent root segments (Fig. S3a, a1). At 2 dpi, when the gall began to swell, additional GFP spread toward the vascular cylinder, whereas the adjacent root portions maintained the expression in two to three files of XPP cells (Fig. 2b). GFP expression increased toward the center of the gall during the infection (4 and 6 dpi; Fig. 2d,f). Accordingly, staining of J0121>>GUS galls at 6 dpi showed a strong signal in all the cells inside the gall, including the GCs (Fig. 2h), similar to J0192>>GUS. The Pro CycB1;1 :CycB1;1(NT)-GUS reporter line (Colon- Carmona et al., 1999) allows the detection of cells undergoing the G2/M transition, which are actively engaged in cell division. CycB1;1(NT)-GUS expression was observed at both sides of the XPP during nematode migration and establishment (12 24 hpi; Fig. 3i). At hpi, cells in the XPP were also clearly stained at both sides of the vascular cylinder (Fig. 3j) until a strong GUS stain was eventually detected in all the vascular cylinder cells inside the gall (72 hpi; Fig. 3k). Therefore, the temporal pattern of divisions in the gall resembled the expression pattern observed
6 Phytologist Research 637 (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) (n) (o) Fig. 4 LATERAL ORGAN BOUNDARIES-DOMAIN 16 (LBD16)::GUS and the synthetic auxin-inducible promoter, DR5::GUS activation patterns show strong similarities only at early infection stages of Meloidogyne javanica in Arabidopsis. Galls from LBD16::GUS showed a strong stain in the center of the gall at 3 and 7 d post infection (dpi) (a, b) that decreased at 13 and disappeared at 21 dpi (c, d). At the early stages of infection (3 and 7 dpi), the activation patterns of DR5::GUS were similar (e, f). At medium-to-late stages of infection (13 and 21 dpi), a clear signal was still present in DR5::GUS (g, h). Semithin sections of galls from LBD16::GUS at 4 dpi (i) and from DR5::GUS at 4 dpi (j) showed GUS staining in giant cells (GCs) and in the small dividing cells inside the vascular cylinder. A comparative graph of the positive galls in both lines (k) shows that the percentage of LBD16::GUS-positive galls dropped to 8% at 15 dpi and eventually to 0% at 21 dpi, whereas the GUS staining for DR5 expression was maintained in over 80% of the galls until 29 dpi (dark blue bars, % positive galls for DR5; light blue bars, % positive galls for LBD16). Noninfected control roots for LBD16::GUS and DR5::GUS lines showed a clear GUS signal in lateral root primordials (LRPs) (m, o), and only a positive signal in the root tip of DR5::GUS, but not of LBD16::GUS (n, l). Asterisks, GCs. Bars: (a h) 200 lm; (i, j) 50 lm; (l, m o) 500 lm. in the J0192>>GFP and J0192>>GUS lines, which began at both sides of the XPP in the gall and eventually filled up the entire vascular cylinder inside the gall. LBD16, a key regulator in the auxin signaling pathway leading to LR formation, is expressed in galls during early/ middle gall development In the J0192>>GFP line, GFP expression is driven by the promoter region of LBD16 (At2g42430; Laplaze et al., 2005), encoding a transcription factor expressed specifically during the first stages of LRP formation and induced by auxins (Okushima et al., 2007; Lee et al., 2009). A transgenic line containing 2500 bp of the LBD16 promoter region fused to GUS (plbd16::gus; Okushima et al., 2007) was infected with M. javanica, and its expression pattern was compared with that of the auxin sensor synthetic promoter DR5 (Ulmasov et al., 1997; Fig. 4). It showed strong expression at 3 dpi in the center of the gall (Fig. 4a), similar to DR5::GUS (Fig. 4e). The expression was strong at 7 dpi in both marker lines (Fig. 4b,f). However, at dpi the intensity of the GUS stain in the plbd16::gus galls decreased, and some of the galls no longer expressed GUS (Fig. 4c,k); yet, DR5-driven GUS expression remained strong (Fig. 4g,k). In completely expanded 21-dpi galls of the plbd16::gus line, we seldom observed any GUS expression (Fig. 4d), but most 21-dpi galls in DR5::GUS remained GUS-positive (Fig. 4h). A comparative graph of the GUS-positive galls in both lines (Fig. 4k) shows that at 2 dpi all galls tested from both lines showed GUS staining, but the percentage of LBD16::GUS-positive galls dropped to 9% at 15 dpi, whereas DR5 expression was maintained until 29 dpi (80%). A clear signal was observed in all cell types inside the vascular cylinder from the pericycle inward in stained plbd16::gus gall sections, including the GCs at 4 dpi (Fig. 4i), similar to the J0192>>GUS line (Figs 2, 4) and the DR5::GUS line at 4 dpi (Fig. 4j). Uninfected control plants of the plbd16::gus line only expressed GUS in the LRP (Fig. 4l,m), and DR5::GUS line and those of the DR5::GUS line only expressed GUS in the LRP and the root tip (Fig. 4n, o). Thus, plbd16::gus and DR5::GUS were expressed in
7 638 Research Phytologist M. javanica galls at early infection stages in the same gall tissues and cells; however, at middle/late infection stages, they showed opposite patterns. Additionally, the plbd16 activation pattern observed after nematode infection validated the results obtained with the enhancer trap line J0192 (Figs 2, 3). A shorter, 1500-bp version of the LBD16 promoter mimicked the expression pattern obtained for the 2500-bp promoter in galls (Fig. S5; Laplaze et al., 2005). However, the intensity of the GUS stain was weaker than that observed with the 2500-bp promoter under the same experimental conditions. We found that only one putative ARF binding element was present in the 1500-bp promoter version (Fig. S5e; top), whereas up to three ARF elements were present in the 2500-bp promoter (Fig. S5e; bottom), which could explain the higher intensity of the signal. Our results obtained with the DR5::GUS line and M. javanica are somewhat contradictory to those of Karczmarek et al. (2004) with M. incognita, who showed that DR5 was active at very early stages (18 24 hpi) around the nematode head but decreased at 3 5 dpi and eventually disappeared from 10 dpi onwards. However, Absmanner et al. (2013) showed DR5 signal until late infection stages. In our case, GUS signal was also maintained until late infection stages (> 29 dpi; Figs 4, S6). To rule out the possibility that GUS protein stability could influence our results, transgenic lines carrying DR5::GFP were inoculated because GFP has a shorter half-life than GUS (de Ruijter et al., 2003). Strong GFP fluorescence was detected at 11, 15, 21 and 28 dpi in the center of the galls, which matches the GUS staining pattern obtained with the DR5::GUS line inside the vascular cylinder including the GCs (Fig. S7). These results confirm that the galls formed by M. javanica in Arabidopsis contained auxins as late as 29 dpi, when the gall is completely developed. LBD16 is regulated by auxins during gall development LBD16 is a key regulator in the auxin signaling pathway leading to the formation of new LRs (Okushima et al., 2007). To further investigate its role in galls, 3-dpi galls from plbd16::gus plants were incubated for 4 d on medium containing 300 lm a-(phenyl ethyl-2-one)-indole-3-acetic acid (PEO-IAA), an auxin Fig. 5 LATERAL ORGAN BOUNDARIES-DOMAIN 16 (LBD16)is regulated by auxins and responds to nematode secretions. Two day post infection (dpi) LBD16::GUS (2500-bp version) Arabidopsis galls infected with Meloidogyne javanica were transferred to plates containing either 300 lm a-(phenyl ethyl-2-one)-indole-3-acetic acid (PEO-IAA) (Hayashi et al., 2008) in dimethyl sulfoxide (DMSO) or only DMSO, which was used as a control. GUS activity was examined 4 d later. (a, b) A clear signal was detected in both the lateral root primordial (LRP) (a) and the galls (b) in the DMSO treatment. (a1, b1) PEO-IAA treatment led to the disappearance of GUS signal in the LRP (a1) and in the center of the gall (b1). (c, c1, d, d1, e, e1) Representative protoplasts of the J0192>>GFP line incubated with buffer alone (c, c1), nematode (Meloidogyne incognita) secretions (d, d1) or the synthetic auxin 2,4-D (e, e1). Right panels are confocal images showing GFP (green) and chloroplast autofluorescence (red). The left panels show the corresponding transmission images. Bars: (a, a1, b, b1) 200 lm; (c, c1, d, d1, e, e1) 20 lm. For the protoplast analysis, three to four independent experiments were performed per treatment and a total of protoplasts counted in each of the replicates per treatment. The proportion of active protoplasts was variable (25 50%) with either auxins or nematode secretions, but no GFP was ever detected in buffer-treated controls. For an overview image of protoplast with the different treatments, see Supporting Information Fig. S8.
8 Phytologist Research 639 (a) (b) Fig. 6 LATERAL ORGAN BOUNDARIES- DOMAIN 16 (LBD16) function is crucial for Arabidopsis gall development. (a) Meloidogyne javanica gall formation in the loss-of-function lbd16-1 mutant l showed a significant reduction in the nematode infection rate compared with Columbia (Col- 0). (b, c) Similar results were obtained with three independent lines of 35S::LBD16-SRDX (b) and plbd16::lbd16-srdx (c). (d) A significant reduction in the infection rate was also observed in two independent 35S:: LBD16-SRDX lines tested with Meloidogyne arenaria. Statistical analysis was performed with three independent experiments per line and at least 60 plants per experiment using ANOVA and the Schefee test; significant differences with Col-0: *, P < 0.05; values are means SE. (c) (d) antagonist that inhibits the auxin signaling pathway by binding to the SCF TIR1/AFBs ubiquitin ligase complex (Hayashi et al., 2008). The expression of the LBD16 promoter in the young LRP (Fig. 5a, a1) and in 6-dpi galls (Fig. 5b, b1) was inhibited compared with the control, indicating that its expression in galls is at least partially regulated by auxins. LBD16 is activated by nematode secretions in protoplasts Freshly isolated leaf protoplasts from the line J0192>>GFP were incubated with buffer, auxin or fractionated secretions extracted from Meloidogyne incognita juveniles. Representative protoplasts for each treatment are shown in Fig. 5 (see Fig. S8 for an overview and the Materials and Methods section). GFP signal was clearly detected in protoplasts incubated with secretions in an auxin-free medium (Fig. 5d, d1), whereas no signal was ever detected in the control with only buffer (Fig. 5c, c1). Protoplasts incubated in a medium containing a synthetic auxin (2,4-dichlorophenoxyacetic acid; Fig. 5e, e1) also showed clear signal. The auxin inducibility of LBD16 is in accordance with previous reports (Okushima et al., 2007; Lee et al., 2009) and with our results in planta (Fig. 4). These data provide a possible explanation for LBD16 induction in the pericycle cells when the nematode is inside the root vascular cylinder, and are compatible with the presence of an auxin maximum in the gall. significant reduction in the number of infections per root compared with Col-0 (Fig. 6a; 36%; P < 0.05). Three independent 35S::LBD16-SRDX lines (Fig. 6b) containing the LBD16 coding sequence fused to the transcriptional repressor domain SRDX driven by the 35S promoter also showed a strong reduction (42 52%). The same protein fusion driven by the native LBD16 promoter, which is active in the LR initial cells at the XPP, showed a reduction in the infection rate that was slightly lower than that in lines with the 35S promoter, but significant (22%; Fig. 6c; P < 0.05). Those successfully established nematodes in 35S::LBD16-SRDX lines formed smaller galls with consistently less expanded GCs than in controls at 14 dpi (three- or four-fold reduction in the mean total area occupied by the GCs; Fig. 7; P < 0.05; see the Materials and Methods section). Therefore, the function of LBD16 is crucial for proper gall formation and GC development. plbd16::gus and DR5::GUS plants infected with M. arenaria mimicked the activation pattern shown during infection with M. javanica (Fig. S6). The LBD16-SRDX lines most severely affected by infection with M. javanica (Fig. 6) also showed a significant decrease in the infection rate with M. arenaria (almost 54% compared with the wild type; Fig. 6d; P < 0.05). Therefore, LBD16 function is conserved in galls formed by both RKN species, in accordance with their similar expression patterns. The role of LBD16 in the LR initial cells at the XPP is crucial for proper gall formation and GC development Meloidogyne javanica infection tests with different LBD16 loss-offunction lines were carried out (Figs 6, S5). lbd16-1, a homozygous insertion mutant line that was characterized for LR formation (SALK_095791; Okushima et al., 2007), exhibited a LBD16 is not active in the feeding sites of CNs and does not show a clear functional role during syncytium development Syncytia induced by CNs are known to develop from pericycle cells (Golinowski et al., 1996; Sobczak et al., 1997) and auxins have also been shown to play a role during CN infection
9 640 Research Phytologist (a) (c) (b) (d) Fig. 7 LATERAL ORGAN BOUNDARIES- DOMAIN 16 (LBD16) function is crucial for the development of giant cells (GCs) induced by Meloidogyne javanica in Arabidopsis. (a, b) Toluidine staining of semithin (2-lm) sections of galls from Columbia (Col-0) (a) and the 35S::LBD16-SRDX line (b) shown at the same magnification. Asterisks, giant cells (GCs); N, nematode. Bars, 50 lm. (c) Graph of the average GC area from representative galls in both lines; N = 20 sections of 2 lm from each of the lines tested. (d) Gall width. Bars represent the average width of galls per line, as indicated, from three independent experiments (N = 15). Significant differences: *, P < 0.05; values are means SE. (Goverse & Bird, 2011). Therefore, we analyzed the activation pattern of LBD16 and its putative function during infection with H. schachtii and syncytium establishment. The 2500-bp LBD16:: GUS line did not stain positively for GUS inside the feeding cells at any of the stages of syncytium development (Fig. 8a), nor during syncytium expansion (6 dpi; Fig. 8b), or in a well-established syncytium (11 dpi; Fig. 8c). However, this line showed clear GUS staining in the LRP in the same roots, as in the noninfected control roots (see blue signal in Fig. 4m) (Fig. 8a,b arrows). In agreement with this result, three independent 35S::LBD16-SRDX lines showed no differences in the infection rate compared with the wild type (Fig. 8d). Thus, the lines with the most severe phenotype during RKN infection (Fig. 6) did not show any significant effect on CNs, suggesting that molecular components other than LBD16, either those from the pericyle or those involved in LR formation, may be important for syncytium formation. Genetic ablation experiments reinforce the importance of molecular components in the XPP for gall and GC development To further investigate the importance of molecular components in the XPP during gall formation, the J0121>>GFP line was used in genetic ablation experiments using the diphtheria toxin chain A (DTA) gene. F1 plants from crosses between two homozygous lines, J0121>>GFP and UAS-DTA (J0121>>DTA), were assayed for infection with M. javanica. These F1 plants are unable to form LRs, confirming that the XPP cells are necessary for LRP development (Laplaze et al., 2005). The results showed a drastic decrease (> 80%) in gall formation in the J0121>>DTA plants (Fig. 9b), and 70% of the plants failed to host any infection (Fig. 9c) as compared with the J0121>>GFP control line. Moreover, a major reduction in galls and GC size (> 50%) was observed in the few (a) (c) Fig. 8 LATERAL ORGAN BOUNDARIES-DOMAIN 16 (LBD16) expression and functional analysis do not support a role for LBD16 in syncytium development in Arabidopsis. (a c) The LBD16::GUS line showed no signal in syncytia formed by Heterodera schachtii at 3, 6 or 11 d post infection (dpi; arrows). Only the lateral root primordial (LRP) in (a) and (b) showed GUS staining (arrows). LR, lateral root; N, nematode; Sy, syncytia. (d) Infection tests of three independent 35S:LBD16-SRDX lines showed no significant effect on the infection rate (number of syncytia per main root and length) relative to Columbia (Col-0). This result was based on scores of syncytia formed over the length of the main root, and values are means SE. Statistical analysis was performed with three independent experiments per line; at least 50 plants per experiment were tested. ANOVA and the Schefee test were performed (P < 0.05). Bars, 200 lm. galls formed in the J0121>>DTA line compared with the control (Figs 7d, 9d f; see the Materials and Methods section). These results confirmed the importance of molecular components in the XPP, where LBD16 is activated, during nematode establishment in gall and GC development. (b) (d)
10 Phytologist Research 641 (a) (c) Fig. 9 Genetic ablation of xylem pericycle pole (XPP) cells interferes with gall and giant cell (GC) development induced by Meloidogyne javanica in Arabidopsis. F1 plants from crosses between two homozygous lines, J0121>>GFP and a UAS- DTA line (J0121>>DTA), which express the diphtheria toxin chain A in the domain of the tagged LATERAL ORGAN BOUNDARIES- DOMAIN 16 (LBD16) gene, were used. (a) Seedlings of the J0121>>GFP control line and the J0121>>DTA line were inoculated 5 d after germination when they had identical root phenotypes. (b) Percentage of galls per main root in control J0121>>GFP and J0121>>DTA lines. A significant reduction in infection (> 80%) was observed. Statistical analysis was performed with three independent experiments per line (with a total of at least 217 plants per line) using ANOVA and the Schefee test; significant differences: *, P < 0.05; values are means SE. (c) The percentage of plants with more than one gall was also considerably lower in J0121>>DTA compared with J0121>>GFP. (d, e) Semithin sections (4 lm) from 14 d post infection (dpi) galls stained with toluidine blue in control J0121>>GFP (d) and ablated J0121>>DTA (e) lines; asterisks, GCs; N, nematode. Bars, 50 lm. (f) In a comparison of the two lines, the average GC area showed a significant reduction of > 50% in the J0121>>DTA line compared with the control J0121>>GFP (P < 0.05); N = 20 sections of 2 lm from each of the lines tested; significant differences: *, P < 0.05; values are means SE. (b) (d) (e) (f) Discussion Plant endoparasitic nematodes transform host root cells into their feeding sites (Gheysen & Mitchum, 2009). Heterodera schachtii nematodes select a single procambial or pericycle cell for initiation of their syncytia in Arabidopsis roots (Golinowski et al., 1996; Sobczak et al., 1997). No such certainty exists for the ontogeny of RKN galls and GCs, although root pericycle and cortical cells proliferate around the nematode, contributing to the formation of the galls (Berg et al., 2009). Division of pericycle cells is necessary for LR formation, where LBD16 participates in the auxin signaling cascade leading to the division of specific XPP cells to form the new organ (Goh et al., 2012). We address the expression pattern and the functional role of LBD16 in galls. Our results confirm the importance of specific gene expression in the XPP for nematode feeding site formation, and identify one likely molecular component: the transcription factor, LBD16, involved in both LR formation and gall development following auxin responses. Galls and GCs show transcriptional similarities to undifferentiated root cell types We found that the transcriptomes of 3-dpi galls and GCs were enriched in genes characteristic of two root cell types: LR initials and quiescent center cells (Fig. 1). These genes have varied functions, such as cell cycle regulation and cytoskeleton or cell wall remodeling (Table S1). Some encode transcription factors, such as HSFB4, which are characteristic of the quiescent center
11 642 Research Phytologist transcriptome (Table S1). Other genes related to cell wall relaxation, such as the expansin AtEXPA6 induced in galls and GCs in transcriptomic analyses and by q-pcr (Barcala et al., 2010), are also characteristic of the LRP (Table S1). The resistance to M. incognita of the auxin-insensitive tomato mutant dgt (Richardson & Price, 1982), which lacks LRs, as well as the similarities in the gene expression of plant transcription factors (e.g. Medicago truncatula homologues to PHANTASTICA and Class I knotted, Mt-phan and Mt-knox-1, respectively) and cell cycle regulators during the development of galls, LRs and nodules, point to a correlation between these processes (Goverse et al., 2000; Mathesius, 2003; Moreno-Risueno et al., 2010). Additionally, 39 out of 103 promoter tag lines displaying a distinct response to nematode infection also exhibited activity at LR initiation sites (Barthels et al., 1997). Furthermore, the characteristic genes expressed in LR initials were overrepresented in GCs and galls in a comparative in silico transcriptomic analysis based on statistics (Fig. 1). Taken together, these data reinforce the similarity between the two processes. Molecular confirmation of this parallel is inferred from the analysis described in the next sections. The specific activation of two XPP marker lines during RKN infection connects LR initial cell identity with gall and GC formation Two XPP marker lines, J0121 and J0192, showed strong and distinct GFP expression in the galls formed by M. javanica. In J0192, a marker induced specifically in those XPP cells competent to become LR founder cells, the pericycle on both sides of the vascular cylinder often started to express GFP when the nematode reached the vascular cylinder during migration and establishment (Figs 2, 3, S3, S4). Yet in LRs, the J0192>>GFP signal was distributed in XPP cell rows only on one side of the vascular cylinder and in an alternate manner (Fig. 3; Moreno-Risueno et al., 2010). In both marker lines, the signal progressed inwards in galls and outwards in LRs, and its position(s) with respect to the vascular cylinder (frequently on two sides in galls and only on one side in LRs) differed between the two processes (Laplaze et al., 2005; Figs 2, 3). Abnormal irregular division planes were frequently observed inside the labeled cells of the gall vascular cylinder (close-up in Fig. 3). By contrast, in LRs anticlinal divisions occurred initially, followed by periclinal divisions that formed the LRP (Fig. 3; Lavenus et al., 2013). Eventually, the entire vascular cylinder inside the gall was labeled, including the GCs (Fig. 2). The infection tests performed with the LBD16 mutant lines (the gene that drives GFP expression in J0192) confirm a role for LBD16 in the dividing XPP during gall development (Figs 6, 7). A significant reduction (P < 0.05) of at least 20% in gall formation relative to the wild-type controls was observed in the three independent approaches used to investigate its role. In addition, the GUS expression pattern of the marker line Pro CycB1;1 :CycB1;1 (NT)-GUS, active only during the G2/M transition, mimicked that of J0192 in XPP cells during early nematode establishment and in most cells inside the vascular cylinder of the gall as the infection progressed (Figs 2, 3). These results suggest that the founder cells contained in the XPP that divide to form a new LR (Peret et al., 2009) also divide during early gall formation, and are consistent with our in silico analysis, which indicated enrichment in LR initial cell genes in gall and GC transcriptomes (Fig. 1). Thus, we confirmed that there is a high molecular similarity between gall formation and LR formation, except for the subtle differences already mentioned. The importance of XPP-specific genes during infection was also shown in the genetic ablation experiments using the DTA gene (which inhibits protein synthesis) driven by the enhancer trap line J0121. The J0121>>DTA line showed a clear reduction in infection rates, and most of the plants were unable to host any galls (70%). Additionally, the development of galls and GCs was clearly impaired (Figs 7, 9). However, other transduction pathways or redundant molecular components may also be involved in gall and GC development because some galls were still formed when XPP cells were severely compromised. A novel view of cell reprogramming has been reported by Sugimoto et al. (2010), showing that calli form from the differentiation of pericycle-like cells with meristematic features (large nuclei, small vacuoles and dense cytoplasm) present in the organ. This occurs through the ectopic activation of an LR developmental program that produced root meristem-like tissue. Whether gall development is also related to this process of callus formation through the activation of an LR initiation-like program remains to be elucidated. Interestingly, LBD transcription factors are key regulators in the callus induction process (Fan et al., 2012) as their ectopic expression triggers spontaneous callus formation but their suppression inhibits this process (Fan et al., 2012). Moreover, the formation of a highly organized LR or unorganized callus depends on LBD abundance (Fan et al., 2012). Our findings on the importance of LBD16 in early gall development are consistent with its role in LR and callus development. LBD16 is specifically expressed in the LRP only during the early stages of development and is regulated by auxins (Laplaze et al., 2005). In plants infected with M. javanica and M. arenaria, LBD16 expression was detected very early after infection, 1 dpi up to dpi (Figs 2 4, S1, S2, S6) and was regulated by auxins in galls, as shown by its inhibition by PEO-IAA, similar to LRP (Fig. 5; Okushima et al., 2007; Lee et al., 2009). Although its expression at early stages appeared to correlate with the presence of auxins in the same cell types, as shown by DR5::GUS, at later stages the mere presence of auxins in the gall was not sufficient to activate LBD16 expression (Figs 4, S6). Additionally, the presence of auxin-response elements ARF binding sites in the LBD16 promoter sequence correlated with the intensity of the GUS signal (Fig. S5). However, both promoter versions were active only during the early stages of infection. These results may indicate the necessity for a threshold level of auxins in the gall to allow LBD16 expression, which would mimic the scenario that occurs during the first divisions of LR development. However, the absence of GUS signal in the LBD16::GUS syncytia (where DR5 was highly activated; Karczmarek et al., 2004) suggests that other signals apart from auxins could be contributing to early activation and/or late repression of LBD16 in galls and GCs, and that different molecular components in pericycle cells may participate in the formation of syncytia as compared with galls.
12 Phytologist Research 643 The involvement of auxins in nematode feeding site development is broadly accepted (Goverse & Bird, 2011). Here, we show that the auxin sensor DR5 (fused to either GUS or GFP) is active until the late stages (45 dpi) of M. javanica and M. arenaria infection (Figs 4, S6). This suggests a putative role for auxins not only during the initial infection stages (nematode establishment) but also in feeding site maintenance. Some genes induced in nematode feeding sites until late infection stages have auxin response cis elements in their promoters (Wang et al., 2007; Karczmarek et al., 2008; Wieczorek et al., 2008; Swiecicka et al., 2009). Most of the relevant studies related to the role of auxin in nematode feeding site formation were performed using CNs and were focused on alterations in auxin transport (Goverse & Bird, 2011). However, auxin transduction pathways that trigger the activation of nematode-induced genes have not been well studied. One of the few exceptions is a member of the transcription factor family WRKY (WRKY23). The response of WRKY23 to auxins is controlled by the Aux/IAA protein SOLITARY-ROOT (SLR/ IAA14) (Grunewald et al., 2008). It is induced by both RKN and CN, and loss-of-function mutants were more resistant to infection by CNs (Grunewald et al., 2009). However, other plant- or nematode-derived signals could participate in its activation (Grunewald et al., 2009). This would partially agree with the activation of LBD16 by nematode secretions (see next section). However, contrary to what was observed for WRKY23, LBD16 is not activated by CNs, and CN infection was not affected in LBD16 loss-of-function lines (Fig. 8). This result agrees with transcriptional data from microaspirated H. schachtii syncytia, showing that LBD16 was down-regulated (Szakasits et al., 2009). As mentioned in the introduction, the pericycle is a key cell type for the formation of the syncytium, reinforcing the idea that molecular processes occurring in the pericycle during cyst nematode infection are not mediated by the same molecular components as in galls, for example LBD16. Nematode secretions activate LBD16 in protoplasts We investigated the possibility that nematode secretions could trigger changes in pericycle cells by inducing the expression of LBD16, as XPP cells near the nematode head were frequently activated (Fig. 3). We demonstrated that, similarly to exogenous auxins, secretions from M. incognita juveniles were able to induce LBD16 expression in Arabidopsis leaf protoplasts (Figs 5, S8). Therefore, the presence of the nematode itself could cause a local increase in auxin concentrations in the XPP. Auxin compounds have been identified in nematode secretions (De Meutter et al., 2005; Goverse & Bird, 2011). Alternatively, infective nematodes may manipulate auxin homeostasis in an indirect way by releasing enzymes that interfere with local active auxin concentrations in plants. In this respect, functional analysis of a CN gene encoding a secreted chorismate mutase, an enzyme involved in the conversion of the auxin precursor tryptophan, suggests that nematodes may be able to redirect the biosynthesis of auxins in planta (Doyle & Lambert, 2003; Goverse & Bird, 2011). Whether LBD16 induction by nematode secretions is triggered directly by auxins or is caused by the activation of signaling cascades leading to LBD16 expression through other molecular components has yet to be elucidated. However, the activation of individual protoplasts suggests that nematode secretions function in a cell-autonomous manner to activate LBD16 (Figs 5, S8). In this context, it is possible that nematode secretions directly or indirectly activate XPP cells to start proliferation, as they appear on both sides of the vascular cylinder (Fig. 3). Support for this comes from the irregular cell divisions observed in developing galls of the J0192>>GFP line (Fig. 3). One of the best characterized secreted peptides from RKNs, 16D10, showed a high similarity with the C-terminal conserved motif of the plant CLAVATA3/ESR (CLE) protein family (Huang et al., 2006; Teillet et al., 2013). Overexpression of CLE peptides in Arabidopsis caused strikingly irregular anticlinal divisions (symmetric and asymmetric) in the pericycle (Meng et al., 2012). Additionally, the establishment of asymmetry in Arabidopsis LR founder cells is regulated by LBD16 and other LBD proteins (Goh et al., 2012). Thus, nematodes might use similar mechanisms for gall formation. In conclusion, we have demonstrated the important role in gall and GC formation of a molecular component specific for the XPP LR initial cells, that is, LBD16. As we have shown that RKN feeding sites share a clear common transducer with LR formation, this supports the view that RKN may at least partially hijack plant transduction pathways leading to LR formation. LBD16 showed a specific function during RKN establishment and not during the establishment of CNs, was regulated by auxins in galls and was induced by nematode secretions. Finally, we described peculiarities of cell reprogramming during gall formation similar to those occurring during LR and callus formation, where LBDs play a major role in the control of both developmental programs. Acknowledgements We thank Lucıa Mu~noz for her technical assistance, Ana Belen Yuste for Fig. S3 and Dr Laplaze for the J0192, J0121>>DTA and J0121 seeds. We also thank Dr Janice de Almeida and Dr Gilbert Engler for their confocal microscopy training. This work was supported by grants from the Spanish Government to C.E. (AGL ) and C.F. (CSD ) and the Castilla-La Mancha Government to C.F. and C.E. (PCI ). References Absmanner B, Stadler R, Hammes UZ Phloem development in nematode-induced feeding sites: the implications of auxin and cytokinin. Frontiers in Plant Science 4: 241. Almeida-Engler J, Favery B The plant cytoskeleton remodelling in nematode induced feeding sites. In: Jones J, Gheysen G, Fenoll C, eds. Genomics and molecular genetics of plant nematode interactions. Dordrecht, the Netherlands: Springer, Atkinson HJ, Lilley CJ, Urwin PE Strategies for transgenic nematode control in developed and developing world crops. Current Opinion in Biotechnology 23: Barcala M, Garcıa A, Cabrera J, Casson S, Lindsey K, Favery B, Garcıa-Casado G, Solano R, Fenoll C, Escobar C Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant Journal 61:
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter
 Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter 9/10/2008 1 Learning Objectives Explain similarities and differences between fungal, mammalian and plant cell cycles Explain
Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter 9/10/2008 1 Learning Objectives Explain similarities and differences between fungal, mammalian and plant cell cycles Explain
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
SUPPLEMENTARY INFORMATION
 PRC2 represses dedifferentiation of mature somatic cells in Arabidopsis Momoko Ikeuchi 1 *, Akira Iwase 1 *, Bart Rymen 1, Hirofumi Harashima 1, Michitaro Shibata 1, Mariko Ohnuma 1, Christian Breuer 1,
PRC2 represses dedifferentiation of mature somatic cells in Arabidopsis Momoko Ikeuchi 1 *, Akira Iwase 1 *, Bart Rymen 1, Hirofumi Harashima 1, Michitaro Shibata 1, Mariko Ohnuma 1, Christian Breuer 1,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Department of Biology, Indiana University, Bloomington, Indiana 47405
 The Plant Cell, Vol. 20: 3258 3272, December 2008, www.plantcell.org ã 2008 American Society of Plant Biologists Phosphate Availability Alters Lateral Root Development in Arabidopsis by Modulating Auxin
The Plant Cell, Vol. 20: 3258 3272, December 2008, www.plantcell.org ã 2008 American Society of Plant Biologists Phosphate Availability Alters Lateral Root Development in Arabidopsis by Modulating Auxin
Lecture 4: Radial Patterning and Intercellular Communication.
 Lecture 4: Radial Patterning and Intercellular Communication. Summary: Description of the structure of plasmodesmata, and the demonstration of selective movement of solutes and large molecules between
Lecture 4: Radial Patterning and Intercellular Communication. Summary: Description of the structure of plasmodesmata, and the demonstration of selective movement of solutes and large molecules between
DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Other cellular processes (17%)
 Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Ginkgo leaf. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts.
 Ginkgo leaf Figure 22-30 Ginkgo tree. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts. The vein pattern is dichotomous: Divided
Ginkgo leaf Figure 22-30 Ginkgo tree. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts. The vein pattern is dichotomous: Divided
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Ron et al SUPPLEMENTAL DATA
 Ron et al SUPPLEMENTAL DATA Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model Mily Ron, Kaisa Kajala,
Ron et al SUPPLEMENTAL DATA Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model Mily Ron, Kaisa Kajala,
Auxin minimum defines a developmental window for lateral root initiation
 Research New Phytologist Auxin minimum defines a developmental window for lateral root initiation Joseph G. Dubrovsky 1, Selene Napsucialy-Mendivil 1,Jér^o:me Duclercq 2,3, Yan Cheng 4, Svetlana Shishkova
Research New Phytologist Auxin minimum defines a developmental window for lateral root initiation Joseph G. Dubrovsky 1, Selene Napsucialy-Mendivil 1,Jér^o:me Duclercq 2,3, Yan Cheng 4, Svetlana Shishkova
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Reproduction, Seeds and Propagation
 Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Host-Pathogen Interaction. PN Sharma Department of Plant Pathology CSK HPKV, Palampur
 Host-Pathogen Interaction PN Sharma Department of Plant Pathology CSK HPKV, Palampur-176062 PATHOGEN DEFENCE IN PLANTS A BIOLOGICAL AND MOLECULAR VIEW Two types of plant resistance response to potential
Host-Pathogen Interaction PN Sharma Department of Plant Pathology CSK HPKV, Palampur-176062 PATHOGEN DEFENCE IN PLANTS A BIOLOGICAL AND MOLECULAR VIEW Two types of plant resistance response to potential
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
1982 Engineering durable root-knot nematode resistance in crops by RNAi silencing of a root-knot nematode parasitism gene
 1982 Engineering durable root-knot nematode resistance in crops by RNAi silencing of a root-knot nematode parasitism gene Dr. Richard S. Hussey, Department of Plant Pathology, University of Georgia, Athens,
1982 Engineering durable root-knot nematode resistance in crops by RNAi silencing of a root-knot nematode parasitism gene Dr. Richard S. Hussey, Department of Plant Pathology, University of Georgia, Athens,
Auxin is not asymmetrically distributed in initiating Arabidopsis leaves. *Author for correspondence: Marcus G Heisler
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Auxin is not asymmetrically distributed in initiating Arabidopsis leaves Neha Bhatia 1 and Marcus G. Heisler 1* Affiliations
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Auxin is not asymmetrically distributed in initiating Arabidopsis leaves Neha Bhatia 1 and Marcus G. Heisler 1* Affiliations
Major Plant Hormones 1.Auxins 2.Cytokinins 3.Gibberelins 4.Ethylene 5.Abscisic acid
 Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
POTASSIUM IN PLANT GROWTH AND YIELD. by Ismail Cakmak Sabanci University Istanbul, Turkey
 POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
Outline. Leaf Development. Leaf Structure - Morphology. Leaf Structure - Morphology
 Outline 1. Leaf Structure: Morphology & Anatomy 2. Leaf Development A. Anatomy B. Sector analysis C. Leaf Development Leaf Structure - Morphology Leaf Structure - Morphology 1 Leaf Structure - Morphology
Outline 1. Leaf Structure: Morphology & Anatomy 2. Leaf Development A. Anatomy B. Sector analysis C. Leaf Development Leaf Structure - Morphology Leaf Structure - Morphology 1 Leaf Structure - Morphology
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
RNA-seq to study rice defense responses upon parasitic nematode infections. Tina Kyndt
 RNA-seq to study rice defense responses upon parasitic nematode infections Tina Kyndt 1 Rice (Oryza sativa L.) Most important staple food for at least half of the human population Monocot model plant Paddy
RNA-seq to study rice defense responses upon parasitic nematode infections Tina Kyndt 1 Rice (Oryza sativa L.) Most important staple food for at least half of the human population Monocot model plant Paddy
Useful Propagation Terms. Propagation The application of specific biological principles and concepts in the multiplication of plants.
 Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
Eukaryotic Gene Expression
 Eukaryotic Gene Expression Lectures 22-23 Several Features Distinguish Eukaryotic Processes From Mechanisms in Bacteria 123 Eukaryotic Gene Expression Several Features Distinguish Eukaryotic Processes
Eukaryotic Gene Expression Lectures 22-23 Several Features Distinguish Eukaryotic Processes From Mechanisms in Bacteria 123 Eukaryotic Gene Expression Several Features Distinguish Eukaryotic Processes
10/4/2017. Chapter 39
 Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
Topic 14. The Root System. II. Anatomy of an Actively Growing Root Tip
 Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
C MPETENC EN I C ES LECT EC UR U E R
 LECTURE 7: SUGAR TRANSPORT COMPETENCIES Students, after mastering the materials of Plant Physiology course, should be able to: 1. To explain the pathway of sugar transport in plants 2. To explain the mechanism
LECTURE 7: SUGAR TRANSPORT COMPETENCIES Students, after mastering the materials of Plant Physiology course, should be able to: 1. To explain the pathway of sugar transport in plants 2. To explain the mechanism
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds
 HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells
 Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells By: Patrick Rutledge 1 Dr. Jennifer Lorang 2,3, Dr. Marc Curtis 2,3, Dr. Thomas Wolpert 2,3 BioResource Research 1, Botany and
Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells By: Patrick Rutledge 1 Dr. Jennifer Lorang 2,3, Dr. Marc Curtis 2,3, Dr. Thomas Wolpert 2,3 BioResource Research 1, Botany and
Cell type-specific auxin responses in the Arabidopsis root. Dominant expression patterns in cell type-specific auxin responses
 A map of cell type-specific auxin responses Bargmann et al. Molecular Systems Biology 2013 Supplementary Information Table of Content Supplementary Materials and methods p2-3 Supplementary Figures p4-15
A map of cell type-specific auxin responses Bargmann et al. Molecular Systems Biology 2013 Supplementary Information Table of Content Supplementary Materials and methods p2-3 Supplementary Figures p4-15
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA
 Himber et al. Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA silencing SUPPLEMENTAL MATERIAL Supplemental material 1. Short-range movement of GFP silencing affects a nearly
Himber et al. Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA silencing SUPPLEMENTAL MATERIAL Supplemental material 1. Short-range movement of GFP silencing affects a nearly
ENDODERMIS & POLARITY
 https://en.wikipedia.org/wiki/casparian_strip ENDODERMIS & POLARITY Niloufar Pirayesh 13.01.2016 PCDU SEMINAR 2 What is Endodermis? It helps with Regulate the movement of water ions and hormones. (in and
https://en.wikipedia.org/wiki/casparian_strip ENDODERMIS & POLARITY Niloufar Pirayesh 13.01.2016 PCDU SEMINAR 2 What is Endodermis? It helps with Regulate the movement of water ions and hormones. (in and
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I
 BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
Figure 18.1 Blue-light stimulated phototropism Blue light Inhibits seedling hypocotyl elongation
 Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Plant Structure, Growth, and Development
 Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
1. (a) Why are the two kinds of self-incompatibiltiy (SI) mechanisms called sporophytic and gametophytic?
 Bio 328 -Spring 2005 NAME: Test #1 Please provide succinct answers in the space provided under each question. Unless otherwise noted in the margin the value of each question is 3 points. 1. (a) Why are
Bio 328 -Spring 2005 NAME: Test #1 Please provide succinct answers in the space provided under each question. Unless otherwise noted in the margin the value of each question is 3 points. 1. (a) Why are
Research. Summary. Introduction
 Research Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: a functional role for gene repression Mary Portillo 1, *, Javier
Research Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: a functional role for gene repression Mary Portillo 1, *, Javier
Bio 100 Guide 27.
 Bio 100 Guide 27 http://www.offthemarkcartoons.com/cartoons/1994-11-09.gif http://www.cneccc.edu.hk/subjects/bio/album/chapter20/images/plant_growth.jpg http://pgjennielove.files.wordpress.com/2008/06/apical_meristem.png
Bio 100 Guide 27 http://www.offthemarkcartoons.com/cartoons/1994-11-09.gif http://www.cneccc.edu.hk/subjects/bio/album/chapter20/images/plant_growth.jpg http://pgjennielove.files.wordpress.com/2008/06/apical_meristem.png
Redirection of auxin flow in Arabidopsis thaliana roots after infection by root-knot nematodes
 Journal of Experimental Botany, Vol. 67, No. 15 pp. 4559 4570, 2016 doi:10.1093/jxb/erw230 Advance Access publication 15 June 2016 This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html
Journal of Experimental Botany, Vol. 67, No. 15 pp. 4559 4570, 2016 doi:10.1093/jxb/erw230 Advance Access publication 15 June 2016 This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html
BOTANY LAB #1 MITOSIS AND PLANT TISSUES
 Mitosis and cytokinesis in plants BOTANY LAB #1 MITOSIS AND PLANT TISSUES In plants the formation of new cells takes place in specialized regions of meristematic tissue. Meristematic tissues contain immature,
Mitosis and cytokinesis in plants BOTANY LAB #1 MITOSIS AND PLANT TISSUES In plants the formation of new cells takes place in specialized regions of meristematic tissue. Meristematic tissues contain immature,
** * * * Col-0 cau1 CAU1. Actin2 CAS. Actin2. Supplemental Figure 1. CAU1 affects calcium accumulation.
 Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Lab Exercise 4: Primary Growth and Tissues in Stems
 Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON
 PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
Lecture-6. The physiological basis of adventitious root formation in cutting and layering. Learning objective
 Lecture-6 The physiological basis of adventitious root formation in cutting and layering Learning objective Introduction To know about the physiological, anatomical and biochemical basis of root formation
Lecture-6 The physiological basis of adventitious root formation in cutting and layering Learning objective Introduction To know about the physiological, anatomical and biochemical basis of root formation
Supplemental material
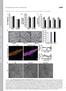 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
Maria V. Yamburenko, Yan O. Zubo, Radomíra Vanková, Victor V. Kusnetsov, Olga N. Kulaeva, Thomas Börner
 ABA represses the transcription of chloroplast genes Maria V. Yamburenko, Yan O. Zubo, Radomíra Vanková, Victor V. Kusnetsov, Olga N. Kulaeva, Thomas Börner Supplementary data Supplementary tables Table
ABA represses the transcription of chloroplast genes Maria V. Yamburenko, Yan O. Zubo, Radomíra Vanková, Victor V. Kusnetsov, Olga N. Kulaeva, Thomas Börner Supplementary data Supplementary tables Table
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
CONTROL OF GROWTH BY HORMONES
 CONTROL OF GROWTH BY HORMONES Growth and organogenesis are controlled......by genes (independent of environment): e.g., number of primary vascular bundles, general shape of a leaf or flower...by genes
CONTROL OF GROWTH BY HORMONES Growth and organogenesis are controlled......by genes (independent of environment): e.g., number of primary vascular bundles, general shape of a leaf or flower...by genes
RESEARCH ARTICLE Advancements in Life Sciences International Quarterly Journal of Biological Sciences
 www.-journal.com/ ISSN 2310-5380/ May 2016 RESEARCH ARTICLE Advancements in Life Sciences International Quarterly Journal of Biological Sciences ARTICL E INFO Date Received: 05/01/2016; Date Revised: 06/05/2016;
www.-journal.com/ ISSN 2310-5380/ May 2016 RESEARCH ARTICLE Advancements in Life Sciences International Quarterly Journal of Biological Sciences ARTICL E INFO Date Received: 05/01/2016; Date Revised: 06/05/2016;
The Microscopic Observation of Mitosis in Plant and Animal Cells
 The Microscopic Observation of Mitosis in Plant and Animal Cells Prelab Assignment Before coming to lab, read carefully the introduction and the procedures for each part of the experiment, and then answer
The Microscopic Observation of Mitosis in Plant and Animal Cells Prelab Assignment Before coming to lab, read carefully the introduction and the procedures for each part of the experiment, and then answer
CELL CYCLE AND DIFFERENTIATION
 CELL CYCLE AND DIFFERENTIATION Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada d.purnomosari@ugm.ac.id WHAT IS CELL CYCLE? 09/12/14 d.purnomosari@ugm.ac.id
CELL CYCLE AND DIFFERENTIATION Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada d.purnomosari@ugm.ac.id WHAT IS CELL CYCLE? 09/12/14 d.purnomosari@ugm.ac.id
Is that artificial turf or real grass? Its thicker than Bermuda!
 Is that artificial turf or real grass? Its thicker than Bermuda! 1 Using Plant Growth Regulators Growth regulators DO NOT interfere with plant respiration, photosynthesis, or other internal plant functions
Is that artificial turf or real grass? Its thicker than Bermuda! 1 Using Plant Growth Regulators Growth regulators DO NOT interfere with plant respiration, photosynthesis, or other internal plant functions
Arabidopsis ASA1 Is Important for Jasmonate-Mediated Regulation of Auxin Biosynthesis and Transport during Lateral Root Formation W OA
 The Plant Cell, Vol. 21: 1495 1511, May 2009, www.plantcell.org ã 2009 American Society of Plant Biologists Arabidopsis ASA1 Is Important for Jasmonate-Mediated Regulation of Auxin Biosynthesis and Transport
The Plant Cell, Vol. 21: 1495 1511, May 2009, www.plantcell.org ã 2009 American Society of Plant Biologists Arabidopsis ASA1 Is Important for Jasmonate-Mediated Regulation of Auxin Biosynthesis and Transport
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
KINETIC ANALYSIS OF AUXIN-INDUCED TRANSCRIPTION FACTOR NETWORKS CONTROLLING LATERAL ROOT DEVELOPMENT IN ARABIDOPSIS THALIANA STACEY REAVIS LUNDY
 KINETIC ANALYSIS OF AUXIN-INDUCED TRANSCRIPTION FACTOR NETWORKS CONTROLLING LATERAL ROOT DEVELOPMENT IN ARABIDOPSIS THALIANA BY STACEY REAVIS LUNDY A Thesis Submitted to the Graduate Faculty of WAKE FOREST
KINETIC ANALYSIS OF AUXIN-INDUCED TRANSCRIPTION FACTOR NETWORKS CONTROLLING LATERAL ROOT DEVELOPMENT IN ARABIDOPSIS THALIANA BY STACEY REAVIS LUNDY A Thesis Submitted to the Graduate Faculty of WAKE FOREST
PLANT HORMONES-Introduction
 PLANT HORMONES-Introduction By convention hormone are said to be a substances whose site of synthesis and site of action are different; the two events are separated by space and time. Hormones are known
PLANT HORMONES-Introduction By convention hormone are said to be a substances whose site of synthesis and site of action are different; the two events are separated by space and time. Hormones are known
Three different fusions led to three basic ideas: 1) If one fuses a cell in mitosis with a cell in any other stage of the cell cycle, the chromosomes
 Section Notes The cell division cycle presents an interesting system to study because growth and division must be carefully coordinated. For many cells it is important that it reaches the correct size
Section Notes The cell division cycle presents an interesting system to study because growth and division must be carefully coordinated. For many cells it is important that it reaches the correct size
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON
 (17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
Plant Tissues and Organs. Topic 13 Plant Science Subtopics , ,
 Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
3.B.1 Gene Regulation. Gene regulation results in differential gene expression, leading to cell specialization.
 3.B.1 Gene Regulation Gene regulation results in differential gene expression, leading to cell specialization. We will focus on gene regulation in prokaryotes first. Gene regulation accounts for some of
3.B.1 Gene Regulation Gene regulation results in differential gene expression, leading to cell specialization. We will focus on gene regulation in prokaryotes first. Gene regulation accounts for some of
Plant Propagation PLS 3221/5222
 Plant Propagation PLS 3221/5222 Dr. Sandra Wilson Dr. Mack Thetford Chapter 2 Introduction to the Biology of Plant Propagation -A review- 1 5. Plant Hormones and Plant development Phytohormones Nt Naturally
Plant Propagation PLS 3221/5222 Dr. Sandra Wilson Dr. Mack Thetford Chapter 2 Introduction to the Biology of Plant Propagation -A review- 1 5. Plant Hormones and Plant development Phytohormones Nt Naturally
Plants are sessile. 10d-17/giraffe-grazing.jpg
 Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Supplementary Information for. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria
 Supplementary Information for Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria Isabella Santi 1 *, Neeraj Dhar 1, Djenet Bousbaine 1, Yuichi Wakamoto, John D.
Supplementary Information for Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria Isabella Santi 1 *, Neeraj Dhar 1, Djenet Bousbaine 1, Yuichi Wakamoto, John D.
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
The mode of development in animals and plants is different
 The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
The Science of Plants in Agriculture Pl.Sci 102. Getting to Know Plants
 The Science of Plants in Agriculture Pl.Sci 102 Getting to Know Plants Growth and Development of Plants Growth and Development of Plants Why it s important to have knowledge about plant development. What
The Science of Plants in Agriculture Pl.Sci 102 Getting to Know Plants Growth and Development of Plants Growth and Development of Plants Why it s important to have knowledge about plant development. What
Class XI Chapter 6 Anatomy of Flowering Plants Biology
 Class XI Chapter 6 Anatomy of Flowering Plants Biology Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark
Class XI Chapter 6 Anatomy of Flowering Plants Biology Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark
DEVELOPMENTAL GENETICS OF ARABIDOPSIS THALIANA
 DEVELOPMENTAL GENETICS OF ARABIDOPSIS THALIANA CHASE BALLARD LINDA EAN HECTOR LOPEZ DR. JOANNA WERNER-FRACZEK IN COLLABORATION WITH DR. PATRICIA SPRINGER S LAB AT UCR AND ROBERT KOBLE PURPOSE OF RESEARCH
DEVELOPMENTAL GENETICS OF ARABIDOPSIS THALIANA CHASE BALLARD LINDA EAN HECTOR LOPEZ DR. JOANNA WERNER-FRACZEK IN COLLABORATION WITH DR. PATRICIA SPRINGER S LAB AT UCR AND ROBERT KOBLE PURPOSE OF RESEARCH
AP Biology Unit 6 Practice Test 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8
 AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Gene expression in prokaryotic and eukaryotic cells, Plasmids: types, maintenance and functions. Mitesh Shrestha
 Gene expression in prokaryotic and eukaryotic cells, Plasmids: types, maintenance and functions. Mitesh Shrestha Plasmids 1. Extrachromosomal DNA, usually circular-parasite 2. Usually encode ancillary
Gene expression in prokaryotic and eukaryotic cells, Plasmids: types, maintenance and functions. Mitesh Shrestha Plasmids 1. Extrachromosomal DNA, usually circular-parasite 2. Usually encode ancillary
Lecture 19. A Sieve Plate with large Sieve Pores. Secondary Phloem. Secondary phloem (cont d)
 Lecture 19 Secondary phloem (cont d) Secondary Phloem in Tilia americana (American Basswood) Secondary Phloem of Tilia Stained with Toluidine Blue & viewed with Crossed Polarizers. Secondary Phloem A Sieve
Lecture 19 Secondary phloem (cont d) Secondary Phloem in Tilia americana (American Basswood) Secondary Phloem of Tilia Stained with Toluidine Blue & viewed with Crossed Polarizers. Secondary Phloem A Sieve
Questions for Biology IIB (SS 2006) Wilhelm Gruissem
 Questions for Biology IIB (SS 2006) Plant biology Wilhelm Gruissem The questions for my part of Biology IIB, Plant Biology, are provided for self-study and as material for the exam. Please note that the
Questions for Biology IIB (SS 2006) Plant biology Wilhelm Gruissem The questions for my part of Biology IIB, Plant Biology, are provided for self-study and as material for the exam. Please note that the
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang Chen, Qiang Dong, Jiangqi Wen, Kirankumar S. Mysore and Jian Zhao
 New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
Ethylene is critical to the maintenance of primary root growth and Fe. homeostasis under Fe stress in Arabidopsis
 Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis Guangjie Li, Weifeng Xu, Herbert J. Kronzucker, Weiming Shi * Supplementary Data Supplementary
Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis Guangjie Li, Weifeng Xu, Herbert J. Kronzucker, Weiming Shi * Supplementary Data Supplementary
Plant Structure and Function
 Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
IB Bio: Plant Biology. Topic 9
 IB Bio: Plant Biology Topic 9 9.1: Transport in xylem How and why does water move up a plant? How do plants conserve water? 9.2: Transport in phloem How and why and where does food move in a plant? 9.3:
IB Bio: Plant Biology Topic 9 9.1: Transport in xylem How and why does water move up a plant? How do plants conserve water? 9.2: Transport in phloem How and why and where does food move in a plant? 9.3:
Last time: Obtaining information from a cloned gene
 Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
