Neuromuscular synaptogenesis in wild-type and mutant zebrafish
|
|
|
- Jack Perkins
- 5 years ago
- Views:
Transcription
1 Developmental Biology 285 (2005) Neuromuscular synaptogenesis in wild-type and mutant zebrafish Jessica A. Panzer a, Sarah M. Gibbs a, Roland Dosch b,1, Daniel Wagner b,2, Mary C. Mullins b, Michael Granato b, Rita J. Balice-Gordon a, * a Department of Neuroscience, University of Pennsylvania School of Medicine, 215 Stemmler Hall, 3610 Hamilton Walk, Philadelphia, PA , USA b Department of Cell and Developmental Biology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA Received for publication 2 February 2005, revised 20 June 2005, accepted 21 June 2005 Available online 15 August 2005 Abstract Genetic screens for synaptogenesis mutants have been performed in many organisms, but few if any have simultaneously screened for defects in pre- and postsynaptic specializations. Here, we report the results of a small-scale genetic screen, the first in vertebrates, for defects in synaptogenesis. Using zebrafish as a model system, we identified seven mutants that affect different aspects of neuromuscular synapse formation. Many of these mutant phenotypes have not been previously reported in zebrafish and are distinct from those described in other organisms. Characterization of mutant and wild-type zebrafish, from the time that motor axons first arrive at target muscles through adulthood, has provided the new information about the cellular events that occur during neuromuscular synaptogenesis. These include insights into the formation and dispersal of prepatterned AChR clusters, the relationship between motor axon elongation and synapse size, and the development of precise appositions between presynaptic clusters of synaptic vesicles in nerve terminals and postsynaptic receptor clusters. In addition, we show that the mechanisms underlying synapse formation within the myotomal muscle itself are largely independent of those that underlie synapse formation at myotendinous junctions and that the outgrowth of secondary motor axons requires at least one cue not necessary for the outgrowth of primary motor axons, while other cues are required for both. One-third of the mutants identified in this screen did not have impaired motility, suggesting that many genes involved in neuromuscular synaptogenesis were missed in large scale motility-based screens. Identification of the underlying genetic defects in these mutants will extend our understanding of the cellular and molecular mechanisms that underlie the formation and function of neuromuscular and other synapses. D 2005 Elsevier Inc. All rights reserved. Keywords: Motor neuron; Muscle fiber; Nerve terminal; Acetylcholine receptors; Neuromuscular junction; Axon outgrowth Introduction The specificity of synaptic connections that is essential for nervous system function arises during development through a series of events, including axon outgrowth, pathway selection, target selection, synaptogenesis, and synapse elimination. One of the systems widely used to * Corresponding author. Fax: address: rbaliceg@mail.med.upenn.edu (R.J. Balice-Gordon). 1 Current address: Universite de Geneve, Switzerland. 2 Current address: Department of Biochemistry and Cell Biology, Rice University, Houston, TX, USA. study these events is the neuromuscular synapse between spinal motor neurons and skeletal muscle fibers (Goda and Davis, 2003; Sanes and Lichtman, 1999). During vertebrate embryonic development, as motor neurons extend axons into target muscles, prepatterned clusters of non-synaptic acetylcholine receptors (AChRs) are distributed throughout the central region of the muscle (Lin et al., 2001; Yang et al., 2001). Motor neuron growth cones release agrin, which activates a muscle specific kinase, MuSK, resulting in the stabilization of synaptic AChR clusters and other postsynaptic modifications (Sanes and Lichtman, 2001). Despite this general understanding, many underlying cellular and molecular events remain to be elucidated, including the interactions /$ - see front matter D 2005 Elsevier Inc. All rights reserved. doi: /j.ydbio
2 J.A. Panzer et al. / Developmental Biology 285 (2005) between motor axon growth cones and prepatterned AChR clusters, the cell cell signaling that mediates the differentiation of presynaptic nerve terminals and postsynaptic specializations, and how synapse number, size, and location are regulated. Zebrafish are a useful model system in which to study these events. The development of spinal motor neurons and myotomal muscle targets has been described in detail (Devoto et al., 1996; Eisen, 1998; Westerfield and Eisen, 1988; Zeller et al., 2002) and is similar to other vertebrates, including mammals. The optical transparency and rapid development of embryos, which facilitates cellular analysis, and the ability to perform mutagenesis screens are powerful advantages that have revealed genetic, molecular, and cellular mechanisms underlying many aspects of neural development. Previous zebrafish mutagenesis screens did not focus on mutants that affect synaptogenesis, in part because the immunostaining-based assays and high resolution required to identify mutants are generally not well suited to high-throughput screening. The large scale Tübingen screen identified more than 160 mutants with impaired locomotion (Granato et al., 1996; Haffter et al., 1996). Analyses of the mutants identified in the Tübingen, as well as other screens, have revealed that several have defects in the function of postsynaptic proteins (Granato et al., 1996; Haffter et al., 1996). These mutants include nic1, which lacks the a subunit of AChRs (Westerfield et al., 1990); sofa potato, which lacks functional AChRs due to a point mutation in the y subunit (Ono et al., 2001, 2004); twister, which has a gain-of-function mutation in the a subunit of the AChR receptor and which displays pre- and postsynaptic defects due to increased neuromuscular activity (Lefebvre et al., 2004); twitch once, which lacks rapsyn and thus also lacks clustered AChRs (Ono et al., 2002); unplugged, which encodes a MuSK homolog and in which axon pathfinding is aberrant but synaptogenesis is largely unaffected (Zhang et al., 2004); and the two acetylcholinesterase mutants ache (Behra et al., 2002) and zieharmonika (Downes and Granato, 2004), which also show reduced clustering of AChRs. While mutagenesis screens focusing on synaptogenesis have also been performed in Drosophila and C. elegans (Kopczynski et al., 1996; Kraut et al., 2001; Aberle et al., 2002; Jin, 2002), to the best of our knowledge, no screens employing simultaneous visualization of presynaptic terminals and postsynaptic neurotransmitter receptor clusters have been performed in any organism. Here, we report seven mutants with defects in neuromuscular synaptogenesis that were identified in a smallscale mutagenesis screen (Dosch et al., 2004; Wagner et al., 2004) employing an immunostaining-based assay for presynaptic motor axons and terminals as well as postsynaptic AChR clusters. Characterization of wild-type and mutant embryos has provided several new insights into the earliest cellular and molecular events that underlie neuromuscular synaptogenesis. Methods Mutagenesis screen Wild-type Tübingen (TÜ) and AB male zebrafish were mutagenized with 3 or 3.3 mm ethylnitrosourea (ENU) as described previously. Briefly, mutagenized TÜ and AB males were then crossed to TÜ and AB wild-type females, respectively, and the F1 progeny raised to adults. F1 TÜ females were then crossed to F1 AB males (and vice-versa) to generate F2 families. Since the AB and TÜ strains are polymorphic to each other, this step introduces a chromosomal mapping cross which allows F3 embryos to be used for chromosomal mapping (Dosch et al., 2004; Wagner et al., 2004; Birely et al., 2005). One to 13 pairs of fish per F2 family were crossed, and the F3 embryos were raised to 48 hpf, at which time embryos per cross were fixed and immunostained as described below. F2 families in which ca. 25% of F2 pair-wise crosses generated clutches in which ca. 25% of embryos exhibited abnormalities in the number, size, pattern, or colocalization of synaptic components were scored as potentially containing a mutation of interest and maintained. The F3 offspring of these families were then raised, and 5 to 30 F3 progeny per family were crossed, and the resulting embryos rescreened for the mutant phenotype. Only those families in which a phenotype was seen in both the F2 and F3 generations were further characterized. For subsequent analyses, mutants were sorted on the basis of external phenotypes or motility, except in the case of p37er which was sorted after staining for AChRs as described below. Mutants were maintained as heterozygous lines, and homozygous mutant embryos were obtained by crosses between identified carriers. No phenotype was observed in heterozygote carriers of these mutations. Embryos were raised at 28.5-C in E3 medium (in mm: 5 NaCl, 0.17 KCl, 0.33 CaCl 2, 0.33 MgSO 4 ). Immunostaining Embryos were anesthetized and fixed in 4% paraformaldehyde, 1% dimethyl sulfoxide in phosphate-buffered saline (PBS); after fixation and rinsing in PBS, embryos were incubated in 1 mg/ml collagenase (Sigma) in PBS for 8 75 min depending on embryo age. At 120 hpf, the skin was removed from the tail with fine forceps, and larvae were incubated in collagenase for up to 15 min. After collagenase treatment, embryos were rinsed in PBS and incubated in 10 Ag/ml fluorescently conjugated a-bungarotoxin (abtx; Molecular Probes, Eugene, OR) diluted in blocking solution (0.1% sodium azide, 2% BSA, 0.5% Triton X-100 in PBS). After rinsing, embryos were incubated overnight at 4-C in primary antibody diluted in blocking solution: SV2 (1:50 1:100, Developmental Studies Hybridoma Bank (DSHB)), Zn5 (1:250 1:500, DSHB), F59, F310, or MF20 (1:50 1:500, gift of Dr. Frank Stockdale). In some cases, staining
3 342 J.A. Panzer et al. / Developmental Biology 285 (2005) with multiple primary antibodies was done sequentially. In zebrafish, the SV2 antibody has been used to label presynaptic terminals (cf. Niell et al., 2004; Jonz and Nurse, 2003; Jontes et al., 2004). Comparison of SV2 immunostaining in transgenic embryos expressing VAMP-GFP under control of the a-actin promoter (Niell et al., 2004; Y. Song, J. Panzer and R. Balice-Gordon, unpublished results) shows that SV2 staining reveals synaptic vesicles in motor axons and nascent terminals (Supplementary Data- Fig. 1). In embryos younger than 48 hpf, clusters of synaptic vesicles were often distributed along motor axons, including within fine filopodia, in addition to those localized to nerve terminals, as has been observed in developing neurons from other organisms (Supplementary Data-Fig. 1; Lupa and Hall, 1989; Sabo and McAllister, 2003). At all ages examined, SV2 staining of motor axons was similar or identical to that observed with Znp1 (Eisen et al., 1989), but nerve terminal staining was more robust with SV2; thus, SV2 was used for most experiments. SV2 staining of the spinal cord was sometimes also observed, but this was variable across age and experiment in wild types as well as in each mutant line, probably due to variability in the embryo permeabilization steps in the immunostaining protocol. After rinsing, embryos were incubated overnight at 4-C in the appropriate fluorescently conjugated secondary antibody (Jackson Labs) diluted in blocking solution. Embryos were rinsed with PBS, placed in VectaShield (Vector Lab., Inc.), mounted onto glass slides, and coverslips were sealed with nail polish. Embryos were visualized using epifluorescence or confocal microscopy at times magnification (Leica TCS 4D system). Each figure panel is a single plane projection of a z-stack of Am-thick planes. Because of the rostral-to-caudal gradient in embryonic development, the timing of events is illustrated and was quantified for the same myotomal segments, centered around the caudal yolk extension, except where indicated. Quantification of motor axon branching, neuromuscular synapse number, size, colocalization of presynaptic nerve terminals and postsynaptic AChR clusters, motor neuron number and muscle fiber number, size, and presence of cross-striations was performed using interactive software (MetaMorph, Universal Imaging, Inc.). Immunostained structures were counted as neuromuscular synapses when presynaptic SV2- stained vesicle clusters (green) were apposed to postsynaptic rhodamine alphabtx (RaBTX)-stained AChR clusters (red) with at least 50% colocalization (yellow). Secondary motor neuron number was counted at 48 hpf in one spinal cord hemisegment per embryo centered on the caudal yolk extension, using Zn5, which stains secondary motor neuron cell bodies in addition to their axons. In situ hybridization Islet-1 and islet-2 riboprobes (gift of Dr. S. Ekker) were synthesized using a DIG-RNA labeling kit (Roche) according to the manufacturer s directions. Embryos (24 28 hpf) were fixed and washed as above then dehydrated in methanol and frozen at 20-C for at least 30 min. Following freezing, embryos were rehydrated and washed in PBT (0.2% BSA, 0.2% Tween-20 in PBS), treated with proteinase K (10 Ag/ml in PBT) for 5 10 min, and then fixed for 20 min. Following washing with PBT, embryos were prehybridized for 2 5 h at 65-C in hybridization buffer (50% formamide, 5 SSC, 50 Ag/ml heparin, 5 mm EDTA, 0.5 mg/ml ribosomal RNA (Sigma), 0.1% Tween- 20, adjusted to ph 6.0 using 1 M citric acid) and then allowed to hybridize overnight at 65-C in ng of islet-1 or islet-2 riboprobe diluted in hybridization buffer. The following day, a series of washes were performed in hybridization buffer, SSC, and PBT, followed by a 2 3 h incubation in blocking solution (2% lamb serum in PBT). Embryos were then incubated overnight at 4-C in alkaline phosphatase conjugated anti-digoxigenin antibody (Roche, diluted 1:4000 in blocking solution). The following day, the embryos were washed with PBT, then with AP buffer (100 mm NaCl, 50 mm MgCl 2, 100 mm Tris ph 9.5, 0.1% Tween-20), followed by incubation in NBT/BCIP (Roche, diluted 1:50 in AP buffer) until satisfactory signal developed (3 12 h). Embryos were then washed in PBT, PBS, then cleared in 80% glycerol, and mounted onto glass slides. Embryos were visualized using transmitted light, and images were taken at several focal planes using a color CCD camera (Hamamatsu, Inc.). In order to show each motor neuron in focus, each figure panel is a composite of images taken at 2 4 focal planes. Segments centered around the caudal yolk extension were counted in each embryo. Islet-1 positive primary motor neurons were counted at 26 hpf because, at later times, secondary motor neurons that are being generated around this time are also positive for Islet-1. Islet-2 positive primary motor neurons were counted at 24 hpf because, at later times, there are many more Islet-2 positive secondary motor neurons, and primary and secondary motor neurons are difficult to distinguish. TUNEL plus immunostaining Zebrafish embryos were fixed, washed with PBS, dehydrated in methanol, and frozen for at least 30 min at 20-C. Embryos were rehydrated, washed with PBST (0.1% Tween-20 in PBS), incubated in collagenase for min, and washed several times in PBS. Embryos were incubated in equilibration buffer (Chemicon) for 1 h and then incubated at 37-C in 30% Tdt enzyme in 70% reaction buffer (Chemicon). The TUNEL labeling reaction was stopped via washing with Stop/Wash buffer (Chemicon). Embryos were blocked in 10% fetal bovine serum (FBS) in PBST and incubated overnight in fluoresceinconjugated anti-digoxigenin (diluted ca. 1:1 in 10% FBS in PBST, Chemicon) and Zn5 (1:250, DSHB) at 4-C. The following day, embryos were washed with FBS/PBST and
4 J.A. Panzer et al. / Developmental Biology 285 (2005) incubated in fluorescently conjugated secondary antibody (Jackson Labs) overnight at 4-C. Embryos were washed with PBS, mounted, and imaged as described above for immunostaining. Behavioral assays Gross motility was assessed via head and/or tail tap with a fine plastic probe, and elicited movements observed under a stereomicroscope (Leica MZ12.5). For some mutants, response to head tap with a fine tungsten probe was recorded using high-speed video microscopy (800 frames/ s; MotionPro 2000, Redlake). Digitally recorded images were analyzed using interactive software (MetaMorph). Mapping and complementation testing F3 embryos were collected, sorted by external phenotype, and frozen in MeOH. For p37er, which lacks an external phenotype, mutants were identified by labeling AChR receptor clusters in live 120 hpf larvae by incubating larvae at 4-C for 1 h in 10 Ag/ml RaBTX 10% DMSO diluted in E3. DNA was extracted by incubating dried embryos in 0.2 mg/ml proteinase K diluted in lysis buffer (50 mm Tris, ph 8.5, 100 mm NaCl, 50 mm EDTA, 1% SDS) at 60-C for at least 3 h. For bulk segregant analysis, DNA from mutants and normal siblings was pooled, and PCR was performed using a set of 240 simple sequence length polymorphisms (SSLP) evenly distributed over the zebrafish genome (Johnson et al., 1996; Knapik et al., 1996). After linkage to one or several markers was established, PCR using DNA from single mutant and normal sibling embryos was used to confirm linkage and identify individual recombinant mutant embryos. Testing for non-complementation was performed by scoring the progeny of several crosses of heterozygote carriers by immunostaining or other assays. Results Neuromuscular synaptogenesis in wild-type zebrafish After exiting a spinal cord segment at hpf, the axon of each primary motor neuron (MiP, CaP, and RoP) grows along the common pathway, lateral to the notochord, to the choice point, pauses for several hours, and grows across the medial surface of one myotomal muscle along a cell-type-specific trajectory (Figs. 1A C). Axons subsequently grow along the lateral myosepta and branch extensively to innervate more lateral muscle fibers (Eisen et al., 1986; Myers et al., 1986). Approximately 5 h later, the axons of secondary motor axons (ca per hemisegment) begin to extend into the periphery along the pathways pioneered by primary motor axons (Mos et al., 1988; Myers et al., 1986; Westerfield et al., 1986). Between hpf, as primary motor axons exit a spinal cord segment and extend towards the choice point (Figs. 1D G, asterisks), prepatterned AChR clusters are present on most if not all medially located muscle fibers, well in advance of motor axon growth cones (Fig. 1D, caudal-most segments; Fig. 1F, brackets). Each muscle fiber has one large diffuse cluster located in the middle of the myotome (mean 1.04 T 0.1; n = 8 fish, 24 somites analyzed; Figs. 1D G). The large prepatterned AChR clusters in the region of the future endplate band are morphologically similar to those observed in mammals during early stages of embryonic development (Feng et al., 2000; Lin et al., 2001; Lupa and Hall, 1989; Yang et al., 2001). However, in zebrafish embryos, prepatterned AChR clusters are present in myotomal muscles well before primary motor axons exit the spinal cord. Thus, prepatterned AChR clusters are present in myotomal muscles prior to any motor innervation. The prepatterned AChR clusters are initially large, elongated, and diffuse (Fig. 1G, bracket). Immunostaining for presynaptic terminals shows that the clusters located on muscle pioneer fibers at the choice point are among the first to be innervated and remain large and elongated throughout subsequent development (Fig. 1E, asterisk). As motor axons approach the choice point, the diffuse prepatterned AChR clusters on muscle fibers dorsal and ventral to the choice point disappear as far as muscle fibers away from the growing nerve front (Fig. 1D, rostral-most segments; Figs. 1E, F). As this occurs, small punctate non-synaptic AChR clusters appear in this region (Fig. 1F, clusters at bottom of brackets). These observations show that prepatterned AChR clusters are transient during the earliest stages of neuromuscular synaptogenesis. Furthermore, our results suggest that prepatterned AChR clusters are dispersed independently of direct contact with motor axon growth cones. Between 20 and 28 hpf, each primary motor axon pauses at the choice point and then extends into its appropriate territory in the myotome (Eisen et al., 1986). Neuromuscular synapses are first formed on muscle fibers at or near the choice point (Figs. 1E G, asterisks; Liu and Westerfield, 1992), and synapses are then formed on the medial surface of the myotome on muscle fibers that are dorsally and ventrally adjacent to the muscle pioneer fibers located at the choice point (Fig. 1H). These synapses are ca. 2-fold smaller than those formed on muscle pioneer fibers (Fig. 1I). AChRs are clustered beneath outgrowing motor axons but are not yet precisely colocalized with clusters of synaptic vesicles in nascent presynaptic terminals (Figs. 1H, I), as they are at later times (cf. Figs. 1J O). Few non-synaptic AChR clusters are present on myotomal muscle fibers at this time. However, diffuse unclustered AChRs are present at the as yet uninnervated myotendinous junctions that separate adjacent myotomes (Fig. 1H, arrowhead). By 48 hpf, motor axons in wild-type embryos have extended branches into and formed synapses with laterally located muscle fibers and have turned laterally at the edge of
5 344 J.A. Panzer et al. / Developmental Biology 285 (2005) Fig. 1. Neuromuscular synaptogenesis in wild-type zebrafish. Top: summary of neuromuscular synaptogenesis in wild-type and mutant zebrafish embryos. (A) Primary motor neuron axons CaP (pink), MiP (blue), and RoP (green) exit the spinal cord through the ventral root and extend along the common pathway to the choice point (asterisk) located at the horizontal myoseptum as shown in the left myotomal segment. At the choice point, axons pause and then proceed to innervate a cell-appropriate territory. At later time points, MiP retracts its ventral axon, the dorsal MiP and ventral CaP axons reach the edge of the myotome and turn laterally, and RoP elaborates an axonal arbor laterally near the horizontal myoseptum as shown in the right myotomal segment. Beginning ca. 5 h after primary motor axons pioneer the common pathway, secondary motor axons (dark blue) exit the spinal cord and extend into the periphery as shown in the right myotomal segment. Each mutant phenotype reported here is indicated at the stage where the defect is particularly prominent, although each mutant has defects that are present at several of these stages. (B) Cross-section at 24 hpf illustrating the medial location of the common pathway, choice point, the initial medial projections of MiP and CaP, and the lateral turn of these axons at the edge of the myotome (SC, spinal cord; NC, notochord). (C) The mature pattern of muscle fiber innervation consists of several neuromuscular synapses distributed along the length of each fiber. Muscle fibers are multiple innervated by one primary motor neuron (pink) and several secondary motor neurons (dark blue). (D O) Neuromuscular synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). Except where indicated, all images are oriented so that rostral is to the left and dorsal is at the top. SV2 staining of the spinal cord (cf. panel J) was sometimes also observed, but this was variable across age and experiment. (D) At 22 hpf, primary motor axons in rostral segments have reached the choice point, while, in caudal segments, motor axons are just exiting the spinal cord (sc). Scale bar for panel D = 10 Am. (E G) Higher magnification view of boxed regions in panel D; the edge of the spinal cord is indicated with dashed line. (E) As synapses form on muscle pioneer fibers at the choice point, prepatterned AChR clusters have completely disappeared. Asterisks indicate the choice point. Scale bar in panel E for panels E G = 10 Am. (F) Elongated diffuse prepatterned AChR clusters disappear and small punctate non-synaptic AChR clusters appear in this region (brackets). (G) Elongated diffuse prepatterned AChR clusters are present along the medial surface of the myotome (brackets), well in advance of outgrowing motor axons. Dashed lines indicate the edges of muscle fibers, demonstrating that there is one large diffuse AChR cluster per muscle fiber at this stage. (H, I) At 24 hpf in wild-type zebrafish, motor axons have formed synapses en passant along the medial surface of the myotome. Large synapses are formed at the choice point (asterisks); arrowhead in panel H indicates unclustered AChRs at myosepta. Scale bar for panels H, J, L, and N = 10 Am. (I) Higher magnification view of boxed region in panel H; in panels I, K, M, and O, the boxed region in the panel above is rotated so that the upper left of the box is to the left. AChR clusters and synaptic vesicle clusters along motor axons beyond the choice point are not yet precisely colocalized (note lack of yellow). Scale bar for panels I, K, M, and O = 10 Am. (J, K) At 48 hpf, wild-type motor axons have turned laterally (J, arrowhead) and grow along the lateral myosepta, and axon branches are present in all muscle fiber layers. Synapses form along all axon branches (K), and AChR clusters and synaptic vesicle clusters along motor axon branches are well colocalized (note yellow). (L) By 72 hpf, motor axons have innervated the full length of the lateral myosepta, and motor axon branching is more elaborate than observed at earlier ages. (M) Numerous colocalized AChR clusters and synaptic vesicle clusters have formed along motor axon branches in all muscle layers. (N, O) By 120 hpf in wild-type embryos, motor axon branching increases, and some synapses (arrowhead) possess a morphology similar to that observed at adult neuromuscular synapses (cf. Fig. 2).
6 J.A. Panzer et al. / Developmental Biology 285 (2005) Fig. 2. Neuromuscular synapses in adult zebrafish. (A C) Presynaptic vesicle clusters and axons are labeled with antibodies against SV2 and neurofilament (green, motor axons/terminals) and with abtx (red, postsynaptic AChRs). (D) Presynaptic vesicle clusters are labeled with antibodies against SV2 (green, presynaptic vesicles) and with abtx (red, postsynaptic AChRs). Scale bar in panel A for panels A and C = 10 Am. Scale bar in panel B for panels B and D = 10 Am. (A, B) Neuromuscular synapses on laterally located slow muscle fibers are punctate, and each muscle fiber possesses multiple neuromuscular synapses (A, edges of muscle fibers are marked with dashed lines). Non-specific staining of muscle nuclei is also apparent. (C, D) On fast muscle fibers, each synaptic area consists of two parallel rows of morphologically distinct synapses. In one row, synapses are punctate (D, arrowhead), and in the other, they are more continuous (D, arrow). Each fast muscle fiber possesses multiple neuromuscular synapses (C, edges of muscle fibers are marked with dashed lines). Multiple axons can be seen innervating a single synaptic area (C, arrows). the myotome (Fig. 1J, arrowhead) and have grown along and innervated the myosepta (Fig. 1J; Liu and Westerfield, 1992). In contrast to earlier developmental stages, presynaptic terminals are almost always precisely apposed to AChR clusters (Fig. 1K). Because neuromuscular synaptogenesis is well underway but the number and pattern of synapses remains relatively simple, the mutagenesis screen described below was performed at this age. By 72 hpf, motor axons innervate the full extent of the lateral myosepta in wild-type embryos, and axon branching within muscle layers and synapse number has increased dramatically compared to earlier stages (Fig. 1L; Downes and Granato, 2004). From hpf, the number of synapses increases further, and synapses become more or less evenly distributed throughout the myotome (Figs. 1L, N). Synapses are present throughout all muscle fiber layers Fig. 3. Mutants in neuromuscular synaptogenesis at 48 hpf. (A H) Neuromuscular synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). Scale bar in panel A for panels A H = 10 Am. (A) Wild-type neuromuscular synapses at 48 hpf. Neuromuscular innervation in (B) stumpy p37er (sty p37er ), (C) bossu (bos), (D) slytherin (srn), (E) millhouse (mlh), (F) xavier (xav), (G) buzz lightyear (lyr), and (H) where s waldo (wdo) mutant embryos at 48 hpf. Note that in mlh the size of the somites is reduced, likely reflecting reduced muscle fiber number and diameter and that, in srn, somites are slightly shorter in length (Table 2). Somite size and shape in the other mutant lines are similar between mutants, normal siblings, and wild-type zebrafish.
7 346 J.A. Panzer et al. / Developmental Biology 285 (2005) and at the myosepta (Figs. 1L, N). By 120 hpf, postsynaptic AChR clusters are located in a cup-like primary fold in the muscle fiber membrane with a presynaptic terminal located at the center of each AChR cluster (Fig. 1O, arrowhead). In adult zebrafish, motor neuron innervation is distributed along the length of individual muscle fibers, and most muscle fibers are innervated by one primary motor neuron and one or several secondary motor neurons (Mos et al., 1988; Westerfield et al., 1986). However, neuromuscular synaptic morphology as revealed by immunostaining in adults has not been well described. On most slow muscle fibers, synapses are punctate and are similar to those present at 120 hpf (Figs. 2A, B). On most fast muscle fibers, each synaptic area consists of two parallel rows of morphologically distinct synapses (Figs. 2C, D). In one row, synapses are small, round, and punctate (Fig. 2D, arrowhead), and in the second row, synapses are more continuous and branched (Fig. 2D, arrow). Multiple axons appear to innervate a single synaptic site (Fig. 2C, arrows). Identification of mutants in neuromuscular synaptogenesis Because of the relative simplicity of the pattern of neuromuscular synapses at 48 hpf, embryos were screened at this age using immunostaining as described above for defects in neuromuscular synapse formation. A total of 111 genomes were screened using this approach. Seven mutants (stumpy p37er (sty p37er ), bossu (bos), slytherin (srn), milhouse (mlh), xavier (xav), buzz lightyear (lyr), and where s waldo (wdo)) were identified with defects in different aspects of neuromuscular synaptogenesis (Fig. 3, Table 1). We also determined whether the neuromuscular synaptic phenotypes observed in these mutants were secondary to, or occurred in parallel with, changes in motor neuron or muscle fiber number using in situ hybridization or immunostaining for cell-type-specific markers. For the majority of the mutants identified in this screen, motor neuron and muscle fiber number were unaffected (Table 2). Taken together, these analyses suggest that the synaptic defects in most mutants are unlikely to be due to general defects in the development of presynaptic motor neurons or postsynaptic muscle fibers. As described below, mapping of many of these mutations to linkage groups and complementation testing with known mutants with some similar phenotypes suggested that all but one of these mutants, sty p37er, is novel. Since it was not possible to perform complementation testing with the large number of motility mutants Table 1 Neuromuscular phenotypes in mutant zebrafish Mutant a Neuromuscular synaptic phenotype Motility b External phenotype milhouse p2fa Reduced size of neuromuscular synapses (Fig. 4) Progressive paralysis Reduced body size 23.3% (262/1126) bossu p31era 23.4% AChR clusters reduced in size (Fig. 5) Normal amplitude C-bend, reduced amplitude tail bends (486/2080) xavier p6di Reduced number of neuromuscular synapses Progressive paralysis 23.8% formed within myotome (Fig. 6) (406/1706) buzz lightyear p13er 25.9% (517/2000) where s waldo p106re 27.0% (112/415) slytherin p31erb 25.4% (332/1305) stumpy p37er 27.6% (232/840) Reduced number of neuromuscular synapses formed along myosepta (Fig. 6) Reduced neuromuscular synapses within dorsal myotome, abnormalities in secondary motor axon outgrowth (Fig. 7) Increased number and size of neuromuscular synapses (Fig. 8) Neuromuscular synapses reduced in myotome, increased at myosepta, AChR cluster size increased in adults (Fig. 9) Reduced amplitude C-bends, reduced amplitude tail bends Unaffected Multiple C-bends, prolonged contractions, high amplitude tail flips Unaffected Slightly smaller head, small pectoral fins Slightly reduced overall body size None None Slightly shortened body, slight compression of notochord a Allele designation and percent of mutant offspring arising from heterozygous crosses (number of mutants observed/total number of offspring). Numbers did not differ significantly from expected Mendelian ratios (Chi-square test). b Motility was assessed by response to head tap observed under a stereomicroscope. Representative mutants for each phenotype (excluding stumpy p37er and wdo) were filmed using high-speed video microscopy. None
8 J.A. Panzer et al. / Developmental Biology 285 (2005) Table 2 Motor neuron and muscle fiber number in mutant zebrafish Mutant Primary motor neuron number Secondary motor neurons Muscle fibers Islet-1 a Islet-2 b Number c % TUNEL + d Number e Morphology sty p37er 1.9 T 0.03 (43) f m 1.5 T 0.02 (18) m 40 T 1.5 (6) m 0 m 11.4 T 0.3 (7) Normal ns 1.5 T 0.02 (32) ns 40 T 1.0 (5) ns 0.3 ns 11.5 T 0.2 (10) bos m 1.9 T 0.02 (9) m 1.5 T 0.03 (19) m 32 T 0.7* (14) m 0.2 m 12.0 T 0.1 (7) Gaps between some ns 1.8 T 0.06 (7) ns 1.5 T 0.02 (20) ns 38 T 0.9 (14) ns 0.3 ns 12.1 T 0.2 (7) slow muscle fibers srn m 2.7 T 0.05* (33) m 1.7 T 0.03* (16) m 37 T 1.9 (6) m 1.1 m 11.6 T 0.2 (15) Normal ns 2.0 T 0.03 (50) ns 1.4 T 0.03 (36) ns 42 T 2.2 (5) ns 0.2 ns 11.4 T 0.2 (14) mlh m 1.7 T 0.07* (25) 1.3 T 0.03 (30) nd nd m 10.1 T 0.2* (6) Thin, wavy ns 1.9 T 0.05 (38) ns 11.5 T 0.2 (9) xav 2.1 T 0.04 (23) 1.5 T 0.02 (41) m 36 T 1.0 (7) m 1.2 m 10.9 T 0.1* (13) Normal ns 38 T 0.8 (10) ns 0.2 ns 11.7 T 0.2 (10) lyr 1.9 T 0.02 (66) 1.3 T 0.01 (106) m 44 T 1.2 (6) m 0 m 12.1 T 0.1 (11) Normal ns 44 T 1.3 (7) ns 0.5 ns 11.9 T 0.1 (10) wdo nd nd m 34 T 1.4 (8) m 0.4 m 12.1 T 0.1 (6) Normal ns 36 T 1.9 (5) ns 0.3 ns 12.3 T 0.1 (8) All values are presented as mean T SEM (number of embryos examined). Asterisk indicates significant difference between mutants (m) and normal siblings (ns) (Student s t test, P < 0.05). nd = not determined. a Mean number of islet-1 positive cell bodies in the ventral spinal cord per hemisegment at 26 hpf. In wild type embryos, RoP and MiP, located in the ventral spinal cord at the rostral somite boundary, express islet-1 (Appel et al., 1995; Appel and Eisen, 1998; Lewis and Eisen, 2004). Primary motor neuron number was counted in 6 10 hemisegments per embryo. Because wdo mutants display no defects in primary motor neuron synaptogenesis, primary motor neuron number was not analyzed in this mutant. b Mean number of islet-2 positive cell bodies in the ventral spinal cord per hemisegment at 24 hpf. In wild type embryos, CaP, located in ventral spinal cord at the center of the somite, expresses islet-2 (Appel and Eisen, 1998; Appel et al., 1995; Lewis and Eisen, 2004). Occasionally, a transient primary motor neuron, VaP, also expresses islet-2 (data not shown). Primary motor neuron number was counted in hemisegments per embryo. c Mean number of Zn5 positive cell bodies in the ventral spinal cord per hemisegment at 48 (sty, bos, sln, mlh, lyr) or72(wdo) hpf. Motor neuron number was counted from z-stacks of confocal images taken of two hemisegments centered at the caudal end of the yolk extension for each embryo. Since motor neurons in mlh mutant embryos do not stain with the Zn5 antibody at any stage, secondary motor neuron number was not assessed in this mutant. d Mean percent of Zn5 positive cell bodies that are also TUNEL + in the ventral spinal cord per hemisegment. The number of embryos examined is the same as in the preceding column. e Mean number of MF20 positive muscle fibers in the most lateral fast muscle fiber layer per hemisegment at 48 (sty, bos, sln, mlh, lyr) or72(wdo) hpf. Muscle fibers were counted in two ventral and two dorsal hemisegments per embryo. f Mean number of neurons per hemisegment. When mutants and normal sibling were evaluated separately, data are presented separately for m and ns. In some cases, a mixed population of embryos (25% mutant, 75% normal) was examined; data are presented as the mean of the entire population. Number of motor neurons per hemisegment was distributed normally for all mixed populations (mean = median). identified in the Tübingen screen (Granato et al., 1996), we cannot rule out that some of our mutants with motility defects may be alleles of previously identified motility mutants whose neuromuscular phenotype has not been assessed. Below, we describe how the mutants identified in this screen inform on the events that occur during normal neuromuscular synaptogenesis. Initial synapse formation at the choice point is disrupted in mutant milhouse In mlh mutant embryos, motor axon outgrowth to the choice point occurs as in normal siblings. Prepatterned AChR clusters in mlh mutants and normal siblings are similar in number and size (Figs. 4A, C, caudal-most segments, arrows) and are similarly dispersed in advance of the growing nerve front (Figs. 4A, C, rostral-most segments). However, the first neuromuscular synapses formed on the muscle pioneer fibers at the choice point are ca. 3-fold smaller than in normal siblings (Fig. 4E) and lack close apposition of presynaptic terminals with AChR clusters (Figs. 4B, D). Similarly, as axons grow along the medial surface of the myotome, fewer neuromuscular synapses are formed compared to normal siblings. In mlh mutant embryos, a small decrease in the number of primary motor neurons expressing islet-1 and a decrease in the number and diameter of slow and fast muscle fibers were observed (Table 2). Thus, while some aspects of the neuromuscular synaptic phenotype in mlh may be secondary to alterations in pre- or postsynaptic cells, the phenotype of mutant mlh suggests that dispersion of prepatterned AChR clusters is not dependent on the normal formation of the first large neuromuscular synapses on muscle pioneer fibers. AChR cluster formation is disrupted in mutant bossu As primary motor axons extend beyond the choice point in wild-type embryos at 25 hpf, AChRs form discrete clusters beneath outgrowing motor axons (Figs. 5A, B BVV). In bos mutants, motor axon outgrowth and the number and size of synaptic vesicle clusters are similar to those in normal siblings (Figs. 5C, D DVV, F). However, in bos mutants, postsynaptic AChR clusters are smaller than those in normal siblings, and these microclusters are only diffusely aligned with motor axons compared to the more
9 348 J.A. Panzer et al. / Developmental Biology 285 (2005) Fig. 4. Initial synapse formation at the choice point is disrupted in mutant millhouse. (A D) Neuromuscular synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). Scale bar in panel A for panels A and C = 10 Am. Scale bar in panel B for panels B and D = 10 Am. (A B) In wild-type embryos at 24 hpf, motor axons in rostral segments have extended beyond the choice point, while, in caudal segments, motor axons have not reached the choice point and prepatterned AChR clusters have not yet been dispersed (arrows). (B) Higher magnification view of boxed region in panel A. Note the large synapses at the choice point (asterisk). (C D) In mlh mutants, motor axons reach the choice point and begin to extend beyond it, but initial synapse formation is impaired. (D) Higher magnification view of boxed region in panel C. AChR clusters on muscle pioneer fibers at the choice point (asterisk) are small and not precisely colocalized with presynaptic vesicle clusters that are also reduced in size. (C) Despite impaired neuromuscular synaptogenesis, prepatterned AChR clusters (arrow) form and are dispersed normally (note the lack of non-synaptic AChR clusters in rostral segments). (E) At the choice point, synaptic vesicle clusters, AChR clusters, and neuromuscular synapses are significantly reduced in size in mlh mutant embryos (asterisk indicates P < 0.05, Student s t test; n = 5 mutants and 5 normal siblings examined). precise alignment of large receptor clusters in normal siblings (Fig. 5E). Although primary motor neuron and muscle fiber number are unaffected in bos mutants, a small but significant decrease in secondary motor neuron number was seen in bos mutant embryos (Table 2) at 48 hpf. Mapping using SSLPs placed the bos mutation on LG 20 (57 recombinants/884 meioses for SSLP marker z8976). Two previously identified zebrafish mutants with some phenotypic similarities to bos, parachute (pac) and shortfin Fig. 5. AChR cluster formation is disrupted in mutant bossu. (A D) Neuromuscular synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). (BV, DV) SV2 staining of synaptic vesicles. (BVV, DVV) abtx staining of AChRs. Except where indicated, all images are oriented so that rostral is to the left and dorsal is at the top. Scale bar in panel A for panels A and C = 10 Am. Scale bar in panel B for panels B BVVand D DVV= 10Am. (A BVV) In wild-type embryos at 25 hpf, AChRs form discrete clusters beneath outgrowing motor axons (BVV). (C DVV) Inbos mutants at 25 hpf, motor axon outgrowth and the number of synaptic vesicle clusters are similar to that observed in normal siblings. However, AChRs fail to form discrete clusters, but rather form small microclusters (DVV) that are only diffusely aligned with motor axons (D). Scale bars = 10 Am. (E F) Quantification of neuromuscular synaptogenesis in bos mutant embryos at 25 hpf. (E) Size of synaptic vesicle (SV2+) and AChR clusters at 25 hpf. AChR cluster size is significantly reduced in bos mutant embryos (asterisk indicates P < 0.05; n = 10 mutants and 10 normal siblings examined). (F) The number of SV2+ puncta and AChR clusters along motor axons are unaffected in mutant embryos.
10 J.A. Panzer et al. / Developmental Biology 285 (2005) Fig. 6. Synapse formation on myotomal muscle fibers or along the myosepta are selectively disrupted in mutants xavier and buzz lightyear. (A C) Synapses are labeled with SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). Scale bar in panel A for panels A C = 10 Am. (A) Wild-type neuromuscular synapses at 48 hpf. Numerous synapses are present on motor axon branches within the myotome, and motor axons have extended along lateral myosepta (arrow). (B) In xav mutant embryos, motor axon branches are short and synapse formation within the myotome is decreased, although the distance that motor axons have grown out along the lateral myosepta is the same as in normal siblings (arrow). (C) In lyr, motor axon branching is slightly reduced, but synapses are greatly reduced along the myoseptal boundaries (arrows). (D) Measurement of the distance that motor axons have extended along the lateral myosepta. Asterisk indicates statistically significant difference between mutants and normal siblings (Student s t test, P < 0.05; n = 12 mutant myosepta, 9 wild-type myosepta examined for each mutant line). (E) Number of synaptic vesicle (SV2+) clusters and AChR clusters per 100 Am 2 of myotome. Asterisks indicate significant difference between mutants and normal siblings (Student s t test, P < 0.05; xav: n = 6 mutants, 4 normal siblings; lyr n = 9 mutants and 9 normal siblings examined). Fig. 7. Secondary motor axon outgrowth and synapse formation are disrupted in mutant where s waldo. (A B) Synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). (A) Wild-type neuromuscular synapse at 72 hpf. (B) In wdo mutant embryos at 72 hpf, a small reduction in the number of dorsal synapses is present in ca. 1/2 of myotomal segments (bracket). Scale bar in panel A for panels A C = 10 Am. Scale bar in panel D for panels D F = 10 Am. (C) Quantification of the percentage of myotomes with abnormal dorsal nerve projections. Asterisk indicates significant difference between mutants and normal siblings (Student s t test, P < 0.05; n = 10 mutants, 10 normal siblings examined). (D F) Secondary motor axons are labeled with antibodies against Zn5. (D) At 72 hpf in wild-type embryos, secondary motor axons have grown out along the pathways pioneered by primary motor neurons along ventral (v), dorsal (d), and mediolateral (ml) pathways. (E, F) In wdo mutant embryos, the dorsal secondary motor nerve fails to grow out in most segments (E, arrows), and secondary motor axons exit ectopically from the spinal cord (F, arrows).
11 350 J.A. Panzer et al. / Developmental Biology 285 (2005) (sof), map to LG 20. Testing for non-complementation with pac (0 mutant progeny in 5 crosses; n = 263 embryos examined) and sof (0 mutant progeny in 3 sof/sof bos/+ crosses; n = 280 progeny examined) demonstrates that the mutation in bos is not allelic with either of these genes. Synapse formation on myotomal muscle fibers or along the myosepta is selectively disrupted in mutants xavier and buzz lightyear ectopically (Fig. 7F, arrows), and secondary motor nerves often grow in aberrant directions. Because primary motor axon outgrowth appears unaffected in wdo, these phenotypes suggest that secondary motor axon outgrowth requires cues that are not necessary for the outgrowth of primary motor axons. In mutant xav, motor axon outgrowth and extension along the lateral myosepta are similar to that in normal siblings (Figs. 6A, B, arrows; D). Motor axon branches, however, fail to extend from the medial nerve into lateral muscle fiber layers, resulting in a dramatic decrease in the number of intramuscular synapses (Fig. 6E). While xav mutant embryos demonstrate a small reduction in the number of fast muscle fibers (Table 2), motor axon branching and intramuscular synapse number remain dramatically reduced at later developmental stages in xav mutants (data not shown). The xav mutation maps to LG 14 (12 recombinants/168 meioses for SSLP marker z7108). No previously identified zebrafish mutants that have been mapped to LG 14 share the xav phenotype. In mutant lyr, while motor axon branching and synaptogenesis within the myotome are reduced (Fig. 6E), there is a dramatic reduction in motor axon extension and synaptogenesis along the myosepta (Figs. 6C, arrows; D). At later stages, the number, size, and alignment of presynaptic vesicle clusters and postsynaptic AChR clusters appear largely normal in lyr mutant embryos (data not shown). The contrasting phenotypes observed in xav and lyr mutant embryos suggest that the cues modulating innervation of the myotendinous junctions and those modulating motor axon branch and synapse formation within the myotomal muscle itself are largely distinct. Secondary motor axon outgrowth and synapse formation are disrupted in mutant where s waldo In mutant wdo, overall primary motor axon outgrowth and branching are similar to that in normal siblings (Figs. 7A, B). At 72 hpf, a small reduction in the number of neuromuscular synapses in the dorsal region of ca. 1/2 of myotome segments is observed (Fig. 7B, bracket). In addition, at 72 hpf, a severe defect in secondary motor axon outgrowth is apparent (Fig. 7C). In wdo normal siblings at 72 hpf, staining of the fasciculated segments of secondary motor axons with the antibody Zn5 (Fashena and Westerfield, 1999; Trevarrow et al., 1990) shows that secondary motor axons have grown out along the dorsal, ventral, and mediolateral pathways pioneered by primary motor neurons (Fig. 7D; d, v, and ml, respectively). In wdo mutant embryos, secondary motor axons in the dorsal nerve are absent in most myotomal segments (Figs. 7E, F arrows); in some segments, motor axons exit the spinal cord Fig. 8. The number and size of neuromuscular synapses on myotomal muscle fibers are increased in mutant slytherin. (A D) Synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). (BV, DV) Motor axons and synaptic vesicle clusters are labeled with SV2. (BVV, DVV) AChR clusters are labeled with abtx. Scale bar in panel A for panels A and C = 10 Am. Scale bar in panels B BVV and D DVV = 10 Am. (A BVV) At 120 hpf in wild-type embryos, many motor axon branches are present. (B BVV) Higher magnification view of boxed region in panel A; the edges of a typical motor nerve branch indicated by dashed lines. Neuromuscular synapses are small and punctate along motor nerve branches. (C DVV) In srn mutant embryos at 120 hpf, the number and size of neuromuscular synapses are increased. (D, DV) Broad motor axon bundles are present in mutant myotomes (edges indicated by dashed lines). (D DVV) Large synapses are present along motor axon branches (arrows). (E) Quantification of the number of SV2+ and AChR clusters greater than 20 Am 2 in size per myotome. Asterisk indicates significant difference between mutants and normal siblings (Student s t test, P < 0.05; n = 10 mutants, 9 normal siblings examined).
12 J.A. Panzer et al. / Developmental Biology 285 (2005) The number and size of neuromuscular synapses on myotomal muscle fibers are increased in mutant slytherin In srn mutant embryos, initial motor axon branching and neuromuscular synapse formation are increased at 48 hpf, the age at which this mutant was identified. However, the most striking phenotype is apparent by 120 hpf as motor axon branching and the number of presynaptic vesicle and AChR clusters larger than 20 Am 2 in area on myotomal muscle fibers are dramatically increased (Figs. 8C DVV, E) compared to normal siblings (Figs. 8A BVV, E). Interestingly, in srn mutant embryos, an increase in primary motor neuron number was detected by both islet-1 and islet-2 in situ hybridization (Table 2). Two previously identified mutants, mind bomb (Bingham et al., 2003) and deadly seven (Gray et al., 2001), show an increase in primary motor neuron number similar to that observed in srn mutant embryos. Testing for non-complementation with mib b904 (one cross, 0 mutant progeny/100 embryos examined) and des b420 (two crosses, 0 mutant progeny/90 embryos examined) demonstrates that the mutation in srn is not allelic with either of these genes. These observations suggest that srn is a novel mutant that increases primary motor neuron number and the number of neuromuscular synapses. These two phenotypes suggest a lack of competition among primary motor neurons for muscle fiber targets. Dysregulation of neuromuscular synapse location, number, and size in mutant stumpy p37er In sty p37er mutant embryos, motor axons reach but fail to extend beyond the choice point by 28 hpf. This axonal phenotype is similar to that reported for the mutant stumpy (sty; Beattie et al., 1999, 2000), although the sty neuro- Fig. 9. Dysregulation of neuromuscular synapse location, number, and size in mutant stumpy p37er. (A D) Synapses are labeled with antibodies against SV2 (green, presynaptic vesicles) and abtx (red, postsynaptic AChRs). Scale bar in panel A for panels A and C = 10 Am. Scale bar in panel B for panels B and D = 10 Am. (A B) At 120 hpf in wild-type embryos, many motor axon branches are present. (B) Single plane projection perpendicular to the myosepta shown in panel A demonstrates that synapses are present throughout muscle fiber layers within the myotome but that synapses at the myosepta (B, arrows) are present in high density only in the most lateral aspects of the myosepta (bottom of panel). (C D) In sty p37er mutant embryos at 120 hpf, synapses are reduced in the myotome and are increased at the myosepta. (D) Single plane projection perpendicular to the myosepta shown in panel C demonstrating that synapses in mutants located at the myosepta (D, arrows) span the medial (top of panel) to lateral (bottom of panel) extent of the myosepta. (E) Total area of neuromuscular synapses present along the myosepta, presented as synaptic area per micron of myosepta. Asterisks indicate significant difference between mutants and normal siblings (Student s t test, P < 0.05; n = 6 mutants, 6 normal siblings examined). (F) Number of neuromuscular synapses per 100 Am 2 of myotome. Asterisks indicate significant difference between mutants and normal siblings (Student s t test, P < 0.05; n = 6 mutants, 6 normal siblings examined). (G I) Presynaptic vesicles and motor axons are labeled with antibodies against SV2 and neurofilament (green) and abtx (red, postsynaptic AChRs). Scale bar in panel G for panels G and H = 10 Am. (IV) Presynaptic vesicles and motor axons are labeled with antibodies against SV2 and neurofilament. Scale bar in panel I for panels I IVV= 10Am. (IVV) AChR clusters are labeled with abtx. (G IVV) Insty p37er mutant adults, AChR clusters are enlarged. (G GVV) A dramatic increase in postsynaptic AChR cluster area and in the proportion of AChR clusters unoccupied by presynaptic vesicle clusters is seen at many mutant synapses. Compare to wild-type neuromuscular synapses in Fig. 2.
13 352 J.A. Panzer et al. / Developmental Biology 285 (2005) muscular synaptic phenotype has not been previously reported. Testing for non-complementation with sty showed that sty p37er represents a mutation in the sty locus (ca. 25% mutant progeny in two crosses; n = 79 embryos examined). We report here that striking neuromuscular synaptic phenotypes are observed in sty p37er, particularly at 120 hpf and in adults. At 120 hpf, ca. 4-fold fewer neuromuscular synapses are located along myotomal muscle fibers in sty p37er mutants compared to normal siblings (Figs. 9A, C, F), and an increased number of synapses is present at the myosepta (Figs. 9A, C; B, D, arrows). These supernumerary myoseptal synapses are similar in size and morphology to those present in normal siblings, but the number of synapses per micron of myosepta is increased (Fig. 9E). Additionally, these myoseptal synapses cover a larger area of the myosepta and extend further medially into deep muscle fiber layers compared to normal siblings (Figs. 9B, D). Together with the phenotypes observed in xav and lyr, these phenotypes in sty p37er suggest that the mechanisms mediating synaptogenesis at these two target locations are distinct. Sty p37er is the only neuromuscular mutant identified in this screen that is viable as an adult. Mutant adults have grossly normal motility and appear indistinguishable from wild-type fish. Whereas in wild-type adults, the size of presynaptic terminals and postsynaptic AChR clusters are closely matched (Fig. 2), in sty p37er mutant adults, AChR cluster area is often increased and exceeds that of presynaptic terminals (Figs. 9G, H). At some mutant synapses, the mismatch between pre- and postsynaptic specializations is extreme (Fig. 9I IVV). Thus, the neuromuscular synaptic defects seen in sty p37er mutant embryos persist in adult mutant fish. Motility defects in mutant embryos We assessed motor behavior in each mutant at 48 hpf by tapping embryos on the head with a fine probe and then recording the escape response with a high-speed video camera. At 48 hpf, wild-type embryos respond to head tap by performing a large C-bend and a smaller counter bend followed by alternating tail flips (Fig. 10A; Supplementary Fig. 10. Motility in wild-type and mutant zebrafish at 48 hpf. (A) In wild-type larvae at 48 hpf, head tap results in a C-bend (0 25 ms) followed by relaxation ( ms), a counterbend to the opposite side ( ms), and alternating tail flips resulting in a swim trajectory away from the stimulus and out of the field of view ( ms). Panels are shown every 12.5 ms. 0 ms is defined as the time of head tap. Scale bar in panel A for panels A F = 1 mm. (B) In mlh mutant embryos at 48 hpf, head tap results in a single slow sustained bend. Panels are shown every 25 ms. (C) In bos mutant embryos at 48 hpf, head tap results in a C-bend (0 25 ms) followed by multiple smaller bends to the same side ( ms) and uncoordinated swimming ( ms). Panels are shown every 12.5 ms; time gap is indicated by a dashed line. (D) In xav at 50 hpf, mutant embryos are completely paralyzed and fail to respond to head tap. Panels are shown every 25 ms. (E) In lyr mutant embryos at 48 hpf, head tap results in tail flips with a greatly reduced amplitude (arrows). Panels are shown every 25 ms. (F) In srn mutant embryos at 48 hpf, head tap results in a prolonged C-bend (0 60 ms) and multiple sustained muscular contractions ( ms) followed by swimming during which tail bends occur at a normal frequency but are of an abnormally high amplitude. First six panels are shown every 25 ms, last four are shown every 12.5 ms; time gap is indicated by dashed line.
14 J.A. Panzer et al. / Developmental Biology 285 (2005) Data, Movie 1). This results in a rapid turn away from the stimulus followed by normal swimming. These motility behaviors are aberrant in mlh, bos, xav, lyr, and srn mutant embryos at 48 hpf and beyond. In mlh mutant embryos at 48 hpf, mutant embryos often fail to move in response to touch, and the infrequent tail bends that do occur are slow and reduced in amplitude, and embryos become paralyzed by 60 hpf (Fig. 10B; Supplementary Data, Movie 2). In bos mutant embryos, head tap results in a normal C-bend, but this bend is followed by tail bends of abnormally low amplitude (Fig. 10C; Supplementary Data, Movie 3). In xav, while mutant embryos are initially normally motile, by 50 hpf, mutants appear to be paralyzed (Fig. 10D; Supplementary Data, Movie 4). In lyr mutant embryos at 48 hpf, no C-bend response is apparent in the escape response elicited by head or tail tap, and tail bends are greatly decreased in amplitude (Fig. 10E, arrows indicate tail bends; Supplementary Data, Movie 5). In srn mutant embryos, head tap results in a prolonged C-bend, multiple sustained muscular contractions, and larger amplitude tail bends (Fig. 10F; Supplementary Data, Movie 6). Thus, in mlh, bos, xav, and lyr, motility is aberrant and reduced, consistent with the reduction in neuromuscular synaptogenesis observed in these mutants. In srn, mutant embryos are hyperexcitable, consistent with the increased neuromuscular synaptogenesis observed in this mutant. That one-third of the mutants described here, wdo and sty p37er, that exhibit neuromuscular defects do not demonstrate impaired motility suggests that some genes involved in neuromuscular synaptogenesis would be missed in screens based on motility alone. Discussion The results of the phenotypic characterization, mapping, and complementation testing reported here suggest that the mutants we characterize here either have not been previously characterized or reported in zebrafish and/or are distinct from those described in other organisms. To the best of our knowledge, this is the first genetic screen for synaptic phenotypes in vertebrates and the first in any organism to simultaneously assay both pre- and postsynaptic specializations. Many of the mutants have defects in pre- as well as postsynaptic specializations, although surprisingly these defects were not always well correlated. Some mutants with relatively subtle or no presynaptic defects exhibited striking and unexpected postsynaptic defects. For example, in bos, the presynaptic vesicle clusters are normal in number and size, but postsynaptic AChR clustering is impaired. In addition, our identification of a striking postsynaptic phenotype in the previously identified motor axon pathfinding mutant sty clearly demonstrates that axonal defects may be accompanied by unexpected defects in postsynaptic specializations. Characterization of the neuromuscular phenotypes in these mutants, summarized in Fig. 11, has provided several new insights into the cellular events that underlie neuromuscular synaptogenesis during normal development. Prepatterned and non-synaptic AChR clusters in wild-type and mutant embryos Previous studies in mice have shown that AChR clusters are prepatterned along the route of ingrowing motor axons (Morris et al., 1999; Feng et al., 2000; Lin et al., 2001; Yang et al., 2000, 2001). Genetic dissection of prepatterned AChR clusters has demonstrated that these clusters persist in mutant mice lacking agrin or muscle innervation by motor neurons but are absent in mice lacking MuSK, suggesting that clusters are formed via an agrin-independent, MuSK-dependent mechanism (Lin et al., 2001; Yang et al., 2001). While detailed studies of the formation and fate of prepatterned AChR clusters have been hampered by technical limitations imposed by the study of mammalian embryos, the observations we report here in zebrafish myotomal muscle show that prepatterned AChR clusters are present throughout the myotome prior to motor axons exiting the spinal cord. The observation of prepatterned non-synaptic AChRs contrasts with previous work that suggested that AChR clustering required motor neurons and that non-synaptic AChRs are not seen in advance of zebrafish motor axon grow cones (Liu and Westerfield, 1992). This may simply reflect the timing of the observations that were made because, as innervation of the choice point occurs, large prepatterned AChR clusters gradually disappear, well in advance of approaching motor axon growth cones. This suggests that any axon-dependent signal triggering the dispersal of prepatterned AChR clusters can act at a distance of at least several muscle fibers. Such a dispersal signal may be activity-dependent, a likely candidate being acetylcholine release from motor axon growth cones (Misgeld et al., 2002; Brandon et al., 2003). Supporting this hypothesis, in zebrafish twister mutants, which exhibit prolonged AChR open time and excessive postsynaptic activity, there is a failure of synaptic AChR clusters to form during the initial stages of neuromuscular synaptogenesis (Lefebvre et al., 2004). In mlh mutants (Fig. 11, top), although initial synapse formation at the choice point is impaired, the prepattern disperses normally, suggesting that the dispersion of prepatterned AChR clusters is not dependent on the normal formation of these synapses. In vivo imaging suggests that prepatterned AChR clusters rapidly disperse and new clusters rapidly form beneath the tips of outgrowing axons over periods of several minutes (J. Panzer, Y. Song, S. Gibbs and R. Balice-Gordon, unpublished). We are currently studying the formation and fate of prepatterned AChR clusters using this and other approaches.
15 354 J.A. Panzer et al. / Developmental Biology 285 (2005) Fig. 11. Summary of neuromuscular synaptic phenotypes in mutant zebrafish. Shown is a cartoon summary of neuromuscular synaptogenesis in wild-type (wt) zebrafish at 16 20, 20 24, 24 30, 48, and 120 hpf. For each age, two views are shown: a side view of one myotomal segment (top) and a higher magnification view (box, bottom). In each panel, green structures represent motor neuron cell bodies and axons; red structures represent AChR clusters, and yellow represents overlap of pre- and postsynaptic structures and thus a synapse. At the appropriate age, the phenotype of a mutant is shown. Top: at hpf in wild-type zebrafish, when primary motor axons are exiting the spinal cord (green), elongated diffuse AChR clusters are present along medial muscle fibers, well in advance of outgrowing motor axons. The box below illustrates that large diffuse prepatterned clusters coalesce into smaller punctate as yet non-synaptic AChR clusters that are first apparent at this age. At 24 hpf in wild-type zebrafish, motor axons have extended into the myotome and, as shown in the box below, have formed large en passant synapses (yellow) at the choice point on the medial surface of the myotome. Presynaptic vesicle clusters and postsynaptic AChR clusters beyond the choice point are not yet precisely colocalized (note lack of yellow in higher magnification view). In mlh mutants at 24 hpf, AChR clusters on muscle pioneer fibers at the choice point are small, and these are not well colocalized with presynaptic vesicle clusters that are also reduced in size. At hpf in wild-type zebrafish, motor axons have reached the dorsal and ventral edges of the myotome, and AChRs are clustered beneath synaptic vesicle clusters but are less well aligned at newly formed synapses (box, bottom) compared to previously formed synapses (box, top). Non-synaptic AChRs are now present along the myosepta between adjacent myotomes (shown for simplicity only in the rostral myosepta). In bos mutants at hpf, numerous small microclusters of AChRs are present that are poorly aligned with presynaptic vesicle clusters along motor axons. Bottom: at 48 hpf in wild-type zebrafish, motor axons have turned and have grown along the rostral lateral myosepta, and axon branches are present in all muscle fiber layers. Synapses form along all axon branches, and synaptic vesicle clusters and AChR clusters are well colocalized (note yellow in higher magnification view). Between hpf in wild-type embryos, secondary motor axons (thin green axons) have grown out along the pathways pioneered by primary motor neurons (thick green axons). In xav mutant embryos at 48 hpf, motor axon branches are short and synapse number within the myotome is decreased, although the distance that motor axons have grown out along the rostral lateral myosepta is unaffected. In lyr mutant embryos at 48 hpf, AChR clusters and synapse number are reduced along the lateral myosepta. In wdo mutant embryos, secondary motor axons (small green cell bodies) exit at ectopic locations from the ventral spinal cord, and the dorsal secondary motor nerve fails to grow out in most segments. By 120 hpf in wild-type zebrafish, motor axon branching and synapse formation has increased, and the entire length of the rostral lateral myosepta is innervated. In sty p37er mutant embryos at 120 hpf, synapse number is reduced in the myotome but is increased along the myosepta. In srn mutant embryos at 120 hpf, the number and size of neuromuscular synapses are increased, and bundles of motor axon branches are increased in diameter. Neuromuscular synaptogenesis along myotomal muscle fibers and at the myosepta In several of the mutants described here, including sty p37er, xav, and lyr, neuromuscular synaptogenesis along myotomal muscle fibers appears to be different than that at the myoseptal boundaries between segments (Fig. 11). In sty p37er, motor axons fail to form synapses as they extend along the medial surface of the myotome but form an excessive number of synapses at the myosepta. In xav, mutant axons fail to extend branches and form significant numbers of synapses along muscle fibers within the myotome, but synapse formation at the myosepta appears normal. In contrast, in lyr, although motor axon branching and synaptogenesis within muscle fiber layers in unimpaired motor axons do not extend and form synapses along the
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Neuronal Differentiation: Synapse Formation NMJ
 Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons
 Development 114, 825-831 (1992) Printed in Great Britain The Company of Biologists Limited 1992 825 Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons SUSAN H. PIKE, ELOINE F.
Development 114, 825-831 (1992) Printed in Great Britain The Company of Biologists Limited 1992 825 Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons SUSAN H. PIKE, ELOINE F.
SUPPLEMENTARY FIGURES AND TABLES AND THEIR LEGENDS. Transient infection of the zebrafish notochord triggers chronic inflammation
 SUPPLEMENTARY FIGURES AND TABLES AND THEIR LEGENDS Transient infection of the zebrafish notochord triggers chronic inflammation Mai Nguyen-Chi 1,2,5, Quang Tien Phan 1,2,5, Catherine Gonzalez 1,2, Jean-François
SUPPLEMENTARY FIGURES AND TABLES AND THEIR LEGENDS Transient infection of the zebrafish notochord triggers chronic inflammation Mai Nguyen-Chi 1,2,5, Quang Tien Phan 1,2,5, Catherine Gonzalez 1,2, Jean-François
Knockdown of Na v 1.6a Na + channels affects zebrafish motoneuron development
 RESEARCH ARTICLE 3827 Development 133, 3827-3836 (2006) doi:10.1242/dev.02559 Knockdown of Na v 1.6a Na + channels affects zebrafish motoneuron development Ricardo H. Pineda 1, *, Kurt R. Svoboda 1,2,
RESEARCH ARTICLE 3827 Development 133, 3827-3836 (2006) doi:10.1242/dev.02559 Knockdown of Na v 1.6a Na + channels affects zebrafish motoneuron development Ricardo H. Pineda 1, *, Kurt R. Svoboda 1,2,
Plexin A3 and Turnout Regulate Motor Axonal Branch Morphogenesis in Zebrafish
 Plexin A3 and Turnout Regulate Motor Axonal Branch Morphogenesis in Zebrafish Rajiv Sainath, Michael Granato* Department of Cell and Developmental Biology, University of Pennsylvania, Perelman School of
Plexin A3 and Turnout Regulate Motor Axonal Branch Morphogenesis in Zebrafish Rajiv Sainath, Michael Granato* Department of Cell and Developmental Biology, University of Pennsylvania, Perelman School of
Reading: Chapter 5, pp ; Reference chapter D, pp Problem set F
 Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
According to the diagram, which of the following is NOT true?
 Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons
 Development 127, 2653-2662 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV8719 2653 Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons Christine
Development 127, 2653-2662 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV8719 2653 Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons Christine
Zebrafish mutants of the neuromuscular junction: swimming in the gene pool
 J Physiol Sci (2015) 65:217 221 DOI 10.1007/s12576-015-0372-9 MINI-REVIEW Zebrafish mutants of the neuromuscular junction: swimming in the gene pool Eriko Daikoku 1 Masahisa Saito 1 Fumihito Ono 1,2 Received:
J Physiol Sci (2015) 65:217 221 DOI 10.1007/s12576-015-0372-9 MINI-REVIEW Zebrafish mutants of the neuromuscular junction: swimming in the gene pool Eriko Daikoku 1 Masahisa Saito 1 Fumihito Ono 1,2 Received:
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its
 Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
MCN. Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases Regulate Axon Guidance in Drosophila
 MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
Open Syntaxin Docks Synaptic Vesicles
 Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
SUPPLEMENTARY INFORMATION
 Supplementary Discussion Rationale for using maternal ythdf2 -/- mutants as study subject To study the genetic basis of the embryonic developmental delay that we observed, we crossed fish with different
Supplementary Discussion Rationale for using maternal ythdf2 -/- mutants as study subject To study the genetic basis of the embryonic developmental delay that we observed, we crossed fish with different
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity
 Research article 891 Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity Katharine E. Lewis* and Judith S. Eisen Institute of Neuroscience, 1254 University of Oregon, Eugene, OR 97403,
Research article 891 Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity Katharine E. Lewis* and Judith S. Eisen Institute of Neuroscience, 1254 University of Oregon, Eugene, OR 97403,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Supplementary Figure 1. Nature Genetics: doi: /ng.3848
 Supplementary Figure 1 Phenotypes and epigenetic properties of Fab2L flies. A- Phenotypic classification based on eye pigment levels in Fab2L male (orange bars) and female (yellow bars) flies (n>150).
Supplementary Figure 1 Phenotypes and epigenetic properties of Fab2L flies. A- Phenotypic classification based on eye pigment levels in Fab2L male (orange bars) and female (yellow bars) flies (n>150).
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Our patient for the day...
 Muscles Ch.12 Our patient for the day... Name: Eddy Age: Newborn Whole-body muscle contractions No relaxation Severe difficulty breathing due to inadequate relaxation of breathing muscles Diagnosed with
Muscles Ch.12 Our patient for the day... Name: Eddy Age: Newborn Whole-body muscle contractions No relaxation Severe difficulty breathing due to inadequate relaxation of breathing muscles Diagnosed with
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis
 Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
NOTES: CH 48 Neurons, Synapses, and Signaling
 NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
Interactions between muscle fibers and segment boundaries in zebrafish
 Developmental Biology 287 (2005) 346 360 www.elsevier.com/locate/ydbio Interactions between muscle fibers and segment boundaries in zebrafish Clarissa A. Henry a,1, Ian M. McNulty b, Wendy A. Durst a,
Developmental Biology 287 (2005) 346 360 www.elsevier.com/locate/ydbio Interactions between muscle fibers and segment boundaries in zebrafish Clarissa A. Henry a,1, Ian M. McNulty b, Wendy A. Durst a,
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
UNIT 6 THE MUSCULAR SYSTEM
 UNIT 6 THE MUSCULAR SYSTEM I. Functions of Muscular System A. Produces Movement Internal vs. External «locomotion & manipulation «circulate blood & maintain blood pressure «move fluids, food, baby B. Maintaining
UNIT 6 THE MUSCULAR SYSTEM I. Functions of Muscular System A. Produces Movement Internal vs. External «locomotion & manipulation «circulate blood & maintain blood pressure «move fluids, food, baby B. Maintaining
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Zebrafish deadly seven Functions in Neurogenesis
 Developmental Biology 237, 306 323 (2001) doi:10.1006/dbio.2001.0381, available online at http://www.idealibrary.com on Zebrafish deadly seven Functions in Neurogenesis Michelle Gray,*, Cecilia B. Moens,
Developmental Biology 237, 306 323 (2001) doi:10.1006/dbio.2001.0381, available online at http://www.idealibrary.com on Zebrafish deadly seven Functions in Neurogenesis Michelle Gray,*, Cecilia B. Moens,
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
The zebrafish space cadet gene controls axonal pathfinding of neurons that modulate fast turning movements
 Development 128, 2131-2142 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV8791 2131 The zebrafish space cadet gene controls axonal pathfinding of neurons that modulate fast turning
Development 128, 2131-2142 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV8791 2131 The zebrafish space cadet gene controls axonal pathfinding of neurons that modulate fast turning
Unicellular: Cells change function in response to a temporal plan, such as the cell cycle.
 Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Structure of Biological Materials
 ELEC ENG 3BA3: Structure of Biological Materials Notes for Lecture #7 Monday, September 24, 2012 3.2 Muscle biomechanics Organization: skeletal muscle is made up of muscle fibers each fiber is a single
ELEC ENG 3BA3: Structure of Biological Materials Notes for Lecture #7 Monday, September 24, 2012 3.2 Muscle biomechanics Organization: skeletal muscle is made up of muscle fibers each fiber is a single
AP Biology Unit 6 Practice Test 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8
 AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
BIOLOGY 11/10/2016. Neurons, Synapses, and Signaling. Concept 48.1: Neuron organization and structure reflect function in information transfer
 48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
BIOLOGY. 1. Overview of Neurons 11/3/2014. Neurons, Synapses, and Signaling. Communication in Neurons
 CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
Protease Inhibitor Cocktail A (1 tablet / 7 10 ml, Roche Cat# ) Protease inhibitor Cocktail B (0.5ml per 250ml, Calbiochem Cat# )
 Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Supplementary Figures
 Supplementary Figures Supplementary Fig. S1: Normal development and organization of the embryonic ventral nerve cord in Platynereis. (A) Life cycle of Platynereis dumerilii. (B-F) Axonal scaffolds and
Supplementary Figures Supplementary Fig. S1: Normal development and organization of the embryonic ventral nerve cord in Platynereis. (A) Life cycle of Platynereis dumerilii. (B-F) Axonal scaffolds and
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis
 18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
CIE Biology A-level Topic 15: Control and coordination
 CIE Biology A-level Topic 15: Control and coordination Notes Neuron structure The nerve cells called neurones play an important role in coordinating communication within the nervous system. The structure
CIE Biology A-level Topic 15: Control and coordination Notes Neuron structure The nerve cells called neurones play an important role in coordinating communication within the nervous system. The structure
Spinal neurons require Islet1 for subtype-specific differentiation of electrical excitability
 Moreno and Ribera Neural Development 2014, 9:19 RESEARCH ARTICLE Open Access Spinal neurons require Islet1 for subtype-specific differentiation of electrical excitability Rosa L Moreno * and Angeles B
Moreno and Ribera Neural Development 2014, 9:19 RESEARCH ARTICLE Open Access Spinal neurons require Islet1 for subtype-specific differentiation of electrical excitability Rosa L Moreno * and Angeles B
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
GENETIC ANALYSIS OF MOTOR AXON PATHFINDING IN ZEBRAFISH DISSERTATION. Presented in Partial Fulfillment of the Requirements for
 GENETIC ANALYSIS OF MOTOR AXON PATHFINDING IN ZEBRAFISH DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University
GENETIC ANALYSIS OF MOTOR AXON PATHFINDING IN ZEBRAFISH DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University
Solutions to Problem Set 4
 Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
T H E J O U R N A L O F C E L L B I O L O G Y
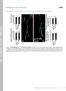 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Neurochemistry 1. Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906
 Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands
 Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands Ashley P. Wright 1, A. Nicole Fox 1, Karl G. Johnson 2, Kai Zinn 1 * 1 Division of Biology,
Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands Ashley P. Wright 1, A. Nicole Fox 1, Karl G. Johnson 2, Kai Zinn 1 * 1 Division of Biology,
AP Biology. Free-Response Questions
 2018 AP Biology Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online home
2018 AP Biology Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online home
RPM-1 is localized to distinct subcellular compartments and regulates axon length in GABAergic motor neurons
 Opperman and Grill Neural Development 14, 9:1 RESERCH RTICLE Open ccess RPM-1 is localized to distinct subcellular compartments and regulates axon length in Gergic motor neurons Karla J Opperman and rock
Opperman and Grill Neural Development 14, 9:1 RESERCH RTICLE Open ccess RPM-1 is localized to distinct subcellular compartments and regulates axon length in Gergic motor neurons Karla J Opperman and rock
Green Fluorescent Protein (GFP) Today s Nobel Prize in Chemistry
 In the news: High-throughput sequencing using Solexa/Illumina technology The copy number of each fetal chromosome can be determined by direct sequencing of DNA in cell-free plasma from pregnant women Confession:
In the news: High-throughput sequencing using Solexa/Illumina technology The copy number of each fetal chromosome can be determined by direct sequencing of DNA in cell-free plasma from pregnant women Confession:
thebiotutor.com A2 Biology Unit 5 Responses, Nervous System & Muscles
 thebiotutor.com A2 Biology Unit 5 Responses, Nervous System & Muscles 1 Response Mechanism tropism Definition A growth movement of part of plant in response to a directional stimulus examples Positive:
thebiotutor.com A2 Biology Unit 5 Responses, Nervous System & Muscles 1 Response Mechanism tropism Definition A growth movement of part of plant in response to a directional stimulus examples Positive:
10/2/2015. Chapter 4. Determination and Differentiation. Neuroanatomical Diversity
 Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
 DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
1. Draw, label and describe the structure of DNA and RNA including bonding mechanisms.
 Practicing Biology BIG IDEA 3.A 1. Draw, label and describe the structure of DNA and RNA including bonding mechanisms. 2. Using at least 2 well-known experiments, describe which features of DNA and RNA
Practicing Biology BIG IDEA 3.A 1. Draw, label and describe the structure of DNA and RNA including bonding mechanisms. 2. Using at least 2 well-known experiments, describe which features of DNA and RNA
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Reading. Lecture VI. Making Connections 9/17/12. Bio 3411 Lecture VI. Making Connections. Bio 3411 Monday September 17, 2012
 Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Information processing. Divisions of nervous system. Neuron structure and function Synapse. Neurons, synapses, and signaling 11/3/2017
 Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Supplementary Information for. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria
 Supplementary Information for Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria Isabella Santi 1 *, Neeraj Dhar 1, Djenet Bousbaine 1, Yuichi Wakamoto, John D.
Supplementary Information for Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria Isabella Santi 1 *, Neeraj Dhar 1, Djenet Bousbaine 1, Yuichi Wakamoto, John D.
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Biology September 2015 Exam One FORM G KEY
 Biology 251 17 September 2015 Exam One FORM G KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology 251 17 September 2015 Exam One FORM G KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology September 2015 Exam One FORM W KEY
 Biology 251 17 September 2015 Exam One FORM W KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology 251 17 September 2015 Exam One FORM W KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Supplementary Information
 Supplementary Information Supplementary figures % Occupancy 90 80 70 60 50 40 30 20 Wt tol-1(nr2033) Figure S1. Avoidance behavior to S. enterica was not observed in wild-type or tol-1(nr2033) mutant nematodes.
Supplementary Information Supplementary figures % Occupancy 90 80 70 60 50 40 30 20 Wt tol-1(nr2033) Figure S1. Avoidance behavior to S. enterica was not observed in wild-type or tol-1(nr2033) mutant nematodes.
Pioneering and pathfinding by an identified neuron in the embryonic leech
 J. Embryol. exp. Morph. 86, 155-167, (1985) 155 Printed in Great Britain The Company of Biologists Limited 1985 Pioneering and pathfinding by an identified neuron in the embryonic leech JOHN Y. KUWADA
J. Embryol. exp. Morph. 86, 155-167, (1985) 155 Printed in Great Britain The Company of Biologists Limited 1985 Pioneering and pathfinding by an identified neuron in the embryonic leech JOHN Y. KUWADA
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning
 MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A)
 Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
TOXICOLOGICAL ENDPOINTS EASILY MEASURED IN DEVELOPING ZEBRAFISH. Robert L. Tanguay Environmental and Molecular Toxicology Oregon State University
 TOXICOLOGICAL ENDPOINTS EASILY MEASURED IN DEVELOPING ZEBRAFISH Robert L. Tanguay Environmental and Molecular Toxicology Oregon State University Why zebrafish for toxicity testing? Vertebrates.. Most importantly:
TOXICOLOGICAL ENDPOINTS EASILY MEASURED IN DEVELOPING ZEBRAFISH Robert L. Tanguay Environmental and Molecular Toxicology Oregon State University Why zebrafish for toxicity testing? Vertebrates.. Most importantly:
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
