Unit 2 - The Lithosphere and the Atmosphere
|
|
|
- Stuart Wright
- 6 years ago
- Views:
Transcription
1 Unit 2 - The Lithosphere and the Atmosphere Key concept - Systems - How do different environmental systems interact with each other on Earth? Related concepts - Models and environment - How can we use scientific models to explain changes in our environment? Global concept - Orientations in time and space - Why is the position of Earth in the solar system essential for our survival? Unit 2 KEYWORDS: Lithosphere Inner core Outer core Mantle Crust Periodic table Elements Minerals Gems Mining Diamond Global warming Carbon dioxide Fossil fuels Energy Rising sea levels Climate change Nitrogen Atmosphere Greenhouse effect Climate Our planet The photo to the right is called "The Pale Blue Dot". It is a photo taken on the 14th February, 1990 by the Voyager 1 Space probe from 6 billion km away. It shows the Earth in a scattered ray of light from the Sun as the space probe was leaving our solar system. Click on the photo to hear a famous quote by Carl Sagan. What is the Earth made of? To fully understand how our Earth works, it is important to know the chemicals that make up the different systems. In science, we organise all of the chemicals into the periodic table (below). Each type of chemical has a name and a symbol. This table contains all of the chemical elements that we know. You will need to learn some of these names and symbols. The documents below contain the names and symbol that you need to learn.
2 Task 2a: You will notice that some of the symbols are very different to the names of the elements. This is because the symbols are derived from the LATIN names not the ENGLISH ones. 1. Use the documents to write the names or symbols of these elements: F, Br, Na, Fe, H, chlorine, neon, potassium, oxygen and silver. Fluorine, Bromine, Sodium, Iron, Hydrogen. Cl, Ne, K, O, Ag 2. Work out the English names from these Latin names and your knowledge of the symbols: cuprum,natrium, ferrum, stannum, kalium, argentum, aurum. Copper, Sodium, Iron, Tim, Potassium, Silver, Gold 3. Chemical compounds are made from different types of element. Which elements do you think are in these chemical compounds: carbon dioxide, magnesium chloride, beryllium sulfide, water. Carbon + Oxygen, Magnesium + Chlorine, Beryllium + Sulfur, Hydrogen + Oxygen
3 The lithosphere Definition: The lithosphere is the rocky part of the Earth The hard, rocky part of the Earth consists of 4 layers as shown in the diagram. The inner and outer core are formed mainly from the chemical elements iron (Fe) and nickel (Ni) and can have a temperature of up to 5,500 ºC. The mantle and the crust are solid and contain a large mixture of elements, including: Si, Al, Ca, Na, Kand Mg. Task 2b: (Bbc.co.uk, 2015) 1. State which chemical elements have the symbols above? Silicon, Aluminium, Calcium, Sodium, Potassium, Magnesium 2. Find out which are the 2 most abundant (common) elements in the Earth s crust? Oxygen and Silicon 3. Although the mantle and crust are both solid, the mantle has a property that is more like a liquid. Which property is it? The rocks in the mantle are under high pressure and temperatures and so can flow like a liquid. Extension: This property is responsible for earthquakes. Explain how? The solid crust is divided into different plates that move on the liquid-like mantle. When they rub together an earthquake can happen. What are minerals? Definition: Minerals are naturally occurring substances with a definite chemical composition and a regular internal structure. Note: Most minerals are crystals, like salt and diamonds. Many minerals are very hard so have been used
4 throughout human history for things such as building. Rare and beautiful minerals such as emeralds and diamonds are called gems. The piece of art to the right is a skull made with over 8,000 gems and was sold in 2007 for 75,000,000! You will need to be able to describe certain properties of minerals: (Fivestarstoneinc.com, 2014) Colour Hardness - This is measured on Mohs scale from Diamond is the hardest mineral on the scale --> Transparency - We can describe a mineral as: Transparent - Light can pass through it and you can see through it. Translucent - Light can pass through it but you cannot see objects through it. Opaque - No light can pass through it. Task 2c: Image - (Thehappyscientist.com, 2015) 1. Make a table in your NSD with 8 rows and 3 columns. Label each column - Mineral letter, Colour and Transparency. Complete the table using the photo to the left. 2. How do we compare the hardness of different minerals? Minerals are ranked by their hardness between 1 and 10 on the Mohs Hardness scale. 1 is soft like talc and 10 is hard like diamond. 3. Can you identify any of the minerals shown? How did you work it out?
5 (Stuffpoint.com, 2015) Rocks Definition: A rock is a naturally occurring solid material containing 1 or more minerals. There is a huge range of uses for rock that depend on their properties. You should be able to suggest uses according to the properties of a rock. You will see the rock cycle in Unit 8. Task 2d: 1. Use the following interactive link to suggest which rock would be the best to make a: kitchen surface; make tiles for a roof; and use to mark distances on a road. kitchen surface: marble make tiles for a roof: slate use to mark distances on a road: chalk 2. A new type of rock has the following properties: is multi-coloured, shiny, easy to shape, resistant to rain. Suggest a use for this rock and explain your choice. 3. The photo below shows the Bingham mine in America. It is the largest open mine in the world and is used to extract rocks containing copper (Cu). Evaluate the advantages and disadvantages of mining for rocks and minerals? Hints: Think about environmental, economic and human issues.
6 (Wired UK, 2015) The atmosphere Definition: The atmosphere is the gaseous part of the Earth The Earth s atmosphere is essential in providing the conditions for life on our planet. Without it, the average surface temperature would be -20 ºC and the amount of dangerous radiation from the Sun would cause large amounts of damage to living things. We would also have no oxygen to breathe! Task 2e: Read the text below and make a bar graph using the information in it: It is clear from the pie chart that the main gas is nitrogen at 78 percent. Oxygen is the next most abundant gas at 21 percent. This is the gas that allows animals and plants to respire, and fuels to burn. All the other gases make up only 1 percent. These include carbon dioxide, water vapour and argon. (Bbc.co.uk, 2015)
7 All the gases in the atmosphere are made from chemical elements. Oxygen for example, has the chemical symbol O and nitrogen has the symbol N. Carbon dioxide actually contains one carbon (C) and two oxygens. As the composition of the atmosphere is different in different places, scientists have named different layers. There are many layers that have been named, but the most common are the ones in this diagram. You might also hear of other layers such as the ionosphere and the exosphere. Task 2b: 1. Find a different diagram of these 4 layers and copy it to your NSD. 2. In which layer do we find most meteorological phenomena (weather)? Most of our weather happens in the troposphere. 3. In which layer can we find the ozone layer? The ozone layer is found in the stratosphere. 4. What important role does the ozone layer have in our safety? The ozone layer protects us from harmful radiation from the sun. It absorbs the UV radiation which can damage our DNA. (Lamb, 2015) Extension: Copy this map to your NSD andexplain where you think the ozone layer has been destroyed the most in the world. The most damage is along the equator, that is why Australia is red on the map.
8 (HubPages, 2015) The Greenhouse Effect The most important role of the atmosphere is something called thegreenhouse effect. A greenhouse works by letting in the heat and light energy from the Sun but then preventing it from leaving again so that the temperature remains warm. The Earth s atmosphere works in a similar way. It allows the Sun s energy to pass through but then prevents most of it from escaping. Without the atmosphere, the energy would be lost back into space immediately. (Climatekids.nasa.gov, 2015) Certain gases are very good at absorbing the heat energy from the Sun so we call them greenhouse gases. The most important greenhouse gases are: Water (H2O) Carbon dioxide (CO2) Methane (CH4)
9 Image - (Qyuhouse.website, 2015) Click the link to see the video. Try the simulation to the right. Investigate the effect on temperature of: The amount of greenhouse gases The number of clouds The number of glass slides (on the second tab) Global warming Over the million years, the average temperature of the Earth s atmosphere has fluctuated (moved up and down) between cold periods and warm periods (right). Recently, however, human activities have started causing a particularly fast increase in the temperature of the Earth s atmosphere (below). This increase in temperature is called. global warming.
10 (Geocraft.com, 2015) Which human actvities do you know that have been causing these changes? Three of the most important human activities that are causing global warming are: Burning fossil fuels Deforestation Livestock (breeding animals) We will focus on the burning of fossil fuels to provide energy. This releases large amounts ofgreenhouse gases, especially carbon dioxide, into the atmosphere. (Savage, 2009)
11 The more greenhouse gases that we release into the atmosphere, the more heat energy is trapped and this causes global warming. The problems with global warming The increasing temperature of the Earth s atmosphere is already having a range of negative effects on the environment and systems that function in it. The video below explains global warming and the problems that it is causing. (Qyuhouse.website, 2015) Video and activities, see web page to finish and complete the notes. References: ReferencesClimatekids.nasa.gov,. (2015). NASA's Climate Kids :: What is the greenhouse effect?. Retrieved 2 July 2015, from Fivestarstoneinc.com,. (2014). 3 Criteria To Consider When You Go For A Granite Countertop Five Star Stone Inc Countertops. Retrieved 2 July 2015, from Geocraft.com,. (2015). Global Warming:A Chilling Perspective. Retrieved 2 July 2015, from Jones, J. (2011). Damien Hirst's skull tasteless? That's the point Jonathan Jones. the Guardian. Retrieved 1 July 2015, from n-hirst-diamond-skull
12 News.bbc.co.uk,. (2015). BBC NEWS Entertainment Hirst's diamond skull raises 50m. Retrieved 2 July 2015, from Qyuhouse.website,. (2015). Greenhouse Effect Simple - Qyu House. Retrieved 2 July 2015, from Savage, M. (2009). Life Sustaining Organizations: June Artofthefutureofwork.blogspot.com.es. Retrieved 2 July 2015, from Skeptical Science,. (2015). IPCC Draft Report Leaked, Shows Global Warming is NOT Due to the Sun. Retrieved 3 July 2015, from Thehappyscientist.com,. (2015). Study Unit : Minerals Around You The Happy Scientist. Retrieved 2 July 2015, from Wired UK,. (2015). Bingham copper mine. Retrieved 2 July 2015, from
Elements and the Periodic Table
 Chapter 7 Elements and the Periodic Table What are metals like? Think of things that are made with metals like aluminum, copper, iron, and gold. What do they have in common? They are usually shiny, and
Chapter 7 Elements and the Periodic Table What are metals like? Think of things that are made with metals like aluminum, copper, iron, and gold. What do they have in common? They are usually shiny, and
2 Complete the following sentences to describe the properties of the different layers of the Earth s structure. Use the words given below.
 The Earth Task 1: Structure of the Earth 1 Label the diagram below, which shows the structure of the Earth. 2 Complete the following sentences to describe the properties of the different layers of the
The Earth Task 1: Structure of the Earth 1 Label the diagram below, which shows the structure of the Earth. 2 Complete the following sentences to describe the properties of the different layers of the
Chemistry Summer Holiday Homework Year Y9 & 10
 Chemistry Summer Holiday Homework Year Y9 & 10 1. An atom of aluminium has the symbol (a) Give the number of protons, neutrons and electrons in this atom of aluminium. Number of protons... Number of neutrons...
Chemistry Summer Holiday Homework Year Y9 & 10 1. An atom of aluminium has the symbol (a) Give the number of protons, neutrons and electrons in this atom of aluminium. Number of protons... Number of neutrons...
Fundamentals of General, Organic & Biological Chemistry 4 th Edition. Matter and Life
 Fundamentals of General, Organic & Biological Chemistry 4 th Edition Chapter One Matter and Life Mohammed Hashmat Ali Southeast Missouri State University 2003 Prentice Hall, Inc. 1.1 Chemistry: The Central
Fundamentals of General, Organic & Biological Chemistry 4 th Edition Chapter One Matter and Life Mohammed Hashmat Ali Southeast Missouri State University 2003 Prentice Hall, Inc. 1.1 Chemistry: The Central
-discovered set of patterns that applied to all elements published 1st periodic table. -wrote properties of each on note cards (density, color)
 Dmitri Mendeleev -discovered set of patterns that applied to all elements -1869 published 1st periodic table -total of 63 elements discovered -wrote properties of each on note cards (density, color) -noticed
Dmitri Mendeleev -discovered set of patterns that applied to all elements -1869 published 1st periodic table -total of 63 elements discovered -wrote properties of each on note cards (density, color) -noticed
3.1 Classification of Matter. Copyright 2009 by Pearson Education, Inc.
 Chapter 3 Atoms and Elements 3.1 Classification of Matter Copyright 2009 by Pearson Education, Inc. 1 Matter Matter is the stuff that makes up all things. Copyright 2009 by Pearson Education, Inc. 2 Pure
Chapter 3 Atoms and Elements 3.1 Classification of Matter Copyright 2009 by Pearson Education, Inc. 1 Matter Matter is the stuff that makes up all things. Copyright 2009 by Pearson Education, Inc. 2 Pure
The bar chart shows the composition of a sample of dry air from the Earth s atmosphere. Name the three gases shown in the bar chart.
 Q1. (a) The diagram shows the Earth s layered structure. Name parts (i) and (ii). (b) The bar chart shows the composition of a sample of dry air from the Earth s atmosphere. Name the three gases shown
Q1. (a) The diagram shows the Earth s layered structure. Name parts (i) and (ii). (b) The bar chart shows the composition of a sample of dry air from the Earth s atmosphere. Name the three gases shown
The Earth. Overall Structure of Earth
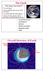 The Earth Why Study The Earth??? It s our home! Where did life come from, where is it going. To understand the other planets. Study of other planets will, in turn, help us understand the Earth. Overall
The Earth Why Study The Earth??? It s our home! Where did life come from, where is it going. To understand the other planets. Study of other planets will, in turn, help us understand the Earth. Overall
Common Elements: Nitrogen, 78%
 Chapter 23 Notes Name: Period: 23.1 CHARACTERISTICS OF THE ATMOSPHERE The atmosphere is a layer of that surrounds the earth and influences all living things. Meteorology is the study of the. WHAT S IN
Chapter 23 Notes Name: Period: 23.1 CHARACTERISTICS OF THE ATMOSPHERE The atmosphere is a layer of that surrounds the earth and influences all living things. Meteorology is the study of the. WHAT S IN
Earth s Atmosphere. Composition
 Earth s Atmosphere Earth s atmosphere is a layer of gases surrounding the planet that is held in place by gravity. The atmosphere protects life on Earth by absorbing ultraviolet radiation, warming the
Earth s Atmosphere Earth s atmosphere is a layer of gases surrounding the planet that is held in place by gravity. The atmosphere protects life on Earth by absorbing ultraviolet radiation, warming the
Section 2: The Atmosphere
 Section 2: The Atmosphere Preview Classroom Catalyst Objectives The Atmosphere Composition of the Atmosphere Air Pressure Layers of the Atmosphere The Troposphere Section 2: The Atmosphere Preview, continued
Section 2: The Atmosphere Preview Classroom Catalyst Objectives The Atmosphere Composition of the Atmosphere Air Pressure Layers of the Atmosphere The Troposphere Section 2: The Atmosphere Preview, continued
INSIDE OUR EARTH. The Earth is primarily composed of rocks. They can be in solid, semiplastic GEOGRAPHY. Chapter
 Chapter 2 INSIDE OUR EARTH Unit-1 : OUR ENVIRONMENT GEOGRAPHY 12 Continental Crust and Oceanic Crust The Earth is primarily composed of rocks. They can be in solid, semiplastic (semi molten) or liquid
Chapter 2 INSIDE OUR EARTH Unit-1 : OUR ENVIRONMENT GEOGRAPHY 12 Continental Crust and Oceanic Crust The Earth is primarily composed of rocks. They can be in solid, semiplastic (semi molten) or liquid
Chapter 1. Matter. 1.1 What is Chemistry. 1.2 The Scientific Method:
 Chapter 1. Matter 1.1 What is Chemistry CHEMISTRY The study of the structure, composition, properties and reactions of matter and the energy changes associated with matter. In other words the study of
Chapter 1. Matter 1.1 What is Chemistry CHEMISTRY The study of the structure, composition, properties and reactions of matter and the energy changes associated with matter. In other words the study of
Atmospheric Layers. Ionosphere. Exosphere. Thermosphere. Mesosphere. Stratosphere. Troposphere. mi (km) above sea level 250 (400) 50 (80) 30 (50)
 mi (km) above sea level Atmospheric Layers Exosphere 250 (400) Thermosphere Ionosphere 50 (80) Mesosphere Ozone Layer 30 (50) 7 (12) Stratosphere Troposphere Atmospheric Layers Earth s atmosphere is held
mi (km) above sea level Atmospheric Layers Exosphere 250 (400) Thermosphere Ionosphere 50 (80) Mesosphere Ozone Layer 30 (50) 7 (12) Stratosphere Troposphere Atmospheric Layers Earth s atmosphere is held
The Fundamental Ideas in Chemistry
 The Fundamental Ideas in Chemistry Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C1 The Fundamental Ideas in Chemistry Silver Level Question Paper Time
The Fundamental Ideas in Chemistry Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C1 The Fundamental Ideas in Chemistry Silver Level Question Paper Time
Chapter 3-1. proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small
 Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Q1. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed.
 Q. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed. (a) Millions of years ago the Earth s atmosphere was probably just like
Q. Scientists study the atmosphere on planets and moons in the Solar System to understand how the Earth s atmosphere has changed. (a) Millions of years ago the Earth s atmosphere was probably just like
Introduction to Atoms
 Introduction to Atoms Understanding Main Ideas Answer the following questions on a separate sheet of paper. 1. What three particles are found in an atom? 2. Which two particles are found in an atom s nucleus?
Introduction to Atoms Understanding Main Ideas Answer the following questions on a separate sheet of paper. 1. What three particles are found in an atom? 2. Which two particles are found in an atom s nucleus?
National 4 Unit Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes. Homework
 National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1]
![...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1] ...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1]](/thumbs/76/74051985.jpg) High Demand Questions QUESTIONSHEET 1 The boxes represent particles of different gases. One box shows the particles of elements in group 0 (group 8). A B C D What name is given to group 0 (8) elements?
High Demand Questions QUESTIONSHEET 1 The boxes represent particles of different gases. One box shows the particles of elements in group 0 (group 8). A B C D What name is given to group 0 (8) elements?
Earth as Planet. Earth s s Magnetic Field. The Earth s s Crust. Earth s s Interior
 Earth as Planet Earth s s Interior The Earth is a medium size planet with a diameter of 12,756 kilometers (7926 miles) Composed primarily of iron, silicon, and oxygen Nearly circular orbit and just the
Earth as Planet Earth s s Interior The Earth is a medium size planet with a diameter of 12,756 kilometers (7926 miles) Composed primarily of iron, silicon, and oxygen Nearly circular orbit and just the
1 Characteristics of the Atmosphere
 CHAPTER 1 1 Characteristics of the Atmosphere SECTION The Atmosphere BEFORE YOU READ After you read this section, you should be able to answer these questions: What is Earth s atmosphere made of? How do
CHAPTER 1 1 Characteristics of the Atmosphere SECTION The Atmosphere BEFORE YOU READ After you read this section, you should be able to answer these questions: What is Earth s atmosphere made of? How do
CLASS COPY Structure and Properties of Matter Parts of the atom
 CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
Atomic Structure Chapter 4 Mr. Hines
 Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
Final Examination ( ) Date: 19/ 06/ 2014
 Class: F.3 ( ) Baptist Lui Ming Choi Secondary School Final Examination (2013-2014) Date: 19/ 06/ 2014 ame: Form 3 Chemistry Time: 8:40-9:50a.m.(70min) Total number of pages: 10 Answer ALL the questions.
Class: F.3 ( ) Baptist Lui Ming Choi Secondary School Final Examination (2013-2014) Date: 19/ 06/ 2014 ame: Form 3 Chemistry Time: 8:40-9:50a.m.(70min) Total number of pages: 10 Answer ALL the questions.
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:
 ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
Table of Contents. Chapter: Atmosphere. Section 1: Earth's Atmosphere. Section 2: Energy Transfer in the Atmosphere. Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter 4: Atmosphere Section 1: Earth's Atmosphere
Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter 4: Atmosphere Section 1: Earth's Atmosphere
Elements. Chem101 - Lecture 2. Elements. Elements. Elements. Elements. Atoms and Molecules
 Chem101 - Lecture 2 Atoms and Molecules Elements Elements are pure substances containing only one kind of atom (homoatomic). There are at last count 114 elements. (Your book indicates 112.). - 88 of these
Chem101 - Lecture 2 Atoms and Molecules Elements Elements are pure substances containing only one kind of atom (homoatomic). There are at last count 114 elements. (Your book indicates 112.). - 88 of these
2.3 Elements and Compounds > Chapter 2 Matter and Change. 2.3 Elements and Compounds. 2.1 Properties of Matter 2.2 Mixtures. 2.4 Chemical Reactions
 Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
3/1/2010. created by Ms Janelle Tay\2010. Learning Objectives
 1 Learning Objectives Define what elements, compounds and mixtures are. Give the names and symbols of common elements. State how elements are classified. State what the building block of an element is.
1 Learning Objectives Define what elements, compounds and mixtures are. Give the names and symbols of common elements. State how elements are classified. State what the building block of an element is.
Year 8 Chemistry Knowledge Organiser Topic 1: Periodic Table
 KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20]
![The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20] The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20]](/thumbs/89/99385122.jpg) The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20] TOPICS: DICHOTOMOUS KEY, ELEMENTS AND COMPOUNDS, MIXTURES Q-1:
The City School PAF Chapter First Term 2 nd Comprehensive Worksheet October 2015 Subject: Science Class 7 Time: 40 minutes Total Marks [20] TOPICS: DICHOTOMOUS KEY, ELEMENTS AND COMPOUNDS, MIXTURES Q-1:
Geosphere Classwork. 5 th Grade PSI. 1. Define geosphere. 2. Where is the oldest part of the Earth located?
 Geosphere Classwork 1. Define geosphere. 2. Where is the oldest part of the Earth located? 3. What are the four layers of the Earth? List them in order from the outermost to the innermost. a. b. c. d.
Geosphere Classwork 1. Define geosphere. 2. Where is the oldest part of the Earth located? 3. What are the four layers of the Earth? List them in order from the outermost to the innermost. a. b. c. d.
A pure substance that cannot be broken down into simpler substances Elements are made up of identical atoms
 Atoms and Elements Objective: S1-2-03 Define element and identify symbols of some common elements & S1-2-04 Explain the atomic structure of an element in terms of the number of protons, electrons, and
Atoms and Elements Objective: S1-2-03 Define element and identify symbols of some common elements & S1-2-04 Explain the atomic structure of an element in terms of the number of protons, electrons, and
Carbon Cycling Internal
 Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Earth s Atmosphere. Describing Earth s Atmosphere
 CHAPTER 4 Earth s Atmosphere LESSON 1 Describing Earth s Atmosphere What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column
CHAPTER 4 Earth s Atmosphere LESSON 1 Describing Earth s Atmosphere What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column
Year 10 Revision. Atomic Structure C minutes. 75 marks. Page 1 of 28
 Year 0 Revision Atomic Structure C.-5 75 minutes 75 marks Page of 28 Q. A substance made of only one type of atom is called an element. The chemical symbols and positions of six elements in the periodic
Year 0 Revision Atomic Structure C.-5 75 minutes 75 marks Page of 28 Q. A substance made of only one type of atom is called an element. The chemical symbols and positions of six elements in the periodic
The Earth System Connections among the great spheres
 Why should we discuss the Earth System? The Earth System Connections among the great spheres Before we delve into the connection between geology, health, and forensics, we must gain an appreciation of
Why should we discuss the Earth System? The Earth System Connections among the great spheres Before we delve into the connection between geology, health, and forensics, we must gain an appreciation of
UNIT 2: Matter and its changes. Mrs. Turner
 UNIT 2: Matter and its changes Mrs. Turner Preassessment Take out a sheet of paper and number it from 1-25. Write down your answers to plug them into your clickers. Don t worry about not knowing an answer
UNIT 2: Matter and its changes Mrs. Turner Preassessment Take out a sheet of paper and number it from 1-25. Write down your answers to plug them into your clickers. Don t worry about not knowing an answer
Layers of the Earth, Ozone Layer and Spheres of the Earth
 Layers of the Earth, Ozone Layer and Spheres of the Earth Importance of the Atmosphere: Earth s atmosphere is a thin layer of air that forms a protective covering around the planet, without which days
Layers of the Earth, Ozone Layer and Spheres of the Earth Importance of the Atmosphere: Earth s atmosphere is a thin layer of air that forms a protective covering around the planet, without which days
Chapter 2 Energy, Force, and Motion Lesson 6 Describing Motion C, D; 8.2C, D; 8.4A; 8.6B
 Table of Contents Texas Essential Knowledge and Skills Correlation Chart....... 7 Chapter 1 Matter..................................... 11 Lesson 1 Atoms and Elements.......................... 12 6.5A*,
Table of Contents Texas Essential Knowledge and Skills Correlation Chart....... 7 Chapter 1 Matter..................................... 11 Lesson 1 Atoms and Elements.......................... 12 6.5A*,
Unit 3 Review Guide: Atmosphere
 Unit 3 Review Guide: Atmosphere Atmosphere: A thin layer of gases that forms a protective covering around the Earth. Photosynthesis: Process where plants take in carbon dioxide and release oxygen. Trace
Unit 3 Review Guide: Atmosphere Atmosphere: A thin layer of gases that forms a protective covering around the Earth. Photosynthesis: Process where plants take in carbon dioxide and release oxygen. Trace
The Earth Fast Facts. Outline. The Solar System is Ours! Astronomy 210. Section 1 MWF Astronomy Building
 Astronomy 210 Section 1 MWF 1500-1550 134 Astronomy Building This Class (Lecture 19): The Earth Night Observations! Next Class: The Earth-Moon System Music: Amy Hit the Atmosphere Counting Crows The Solar
Astronomy 210 Section 1 MWF 1500-1550 134 Astronomy Building This Class (Lecture 19): The Earth Night Observations! Next Class: The Earth-Moon System Music: Amy Hit the Atmosphere Counting Crows The Solar
Unit 7: Being a Chemist Homework
 Unit 7: Being a Chemist Homework Homework Date due Parent/Guardian Signature Mark 1 - Space /10 2 - Life on other planets /10 3 Rock Formation /15 4 - The Earth /10 5 - Metals from the Earth /10 6 Reactivity
Unit 7: Being a Chemist Homework Homework Date due Parent/Guardian Signature Mark 1 - Space /10 2 - Life on other planets /10 3 Rock Formation /15 4 - The Earth /10 5 - Metals from the Earth /10 6 Reactivity
Atomic Structure Chapter 4 Mr. Hines
 Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
CHEMISTRY ELEMENTS, COMPOUNDS & MIXTURES
 CHEMISTRY ELEMENTS, COMPOUNDS & MIXTURES Lesson Intentions In this lesson we will classify substances as Elements, Compounds, Mixtures Key Words 1. Compounds 2. Mixtures 3. Elementary 4. Symbols 5. Reaction
CHEMISTRY ELEMENTS, COMPOUNDS & MIXTURES Lesson Intentions In this lesson we will classify substances as Elements, Compounds, Mixtures Key Words 1. Compounds 2. Mixtures 3. Elementary 4. Symbols 5. Reaction
Chem101 - Lecture 2. Atoms and Molecules
 Chem101 - Lecture 2 Atoms and Molecules Elements Elements are pure substances containing only one kind of atom (homoatomic). There are at last count 114 elements. - 88 of them are naturally occurring.
Chem101 - Lecture 2 Atoms and Molecules Elements Elements are pure substances containing only one kind of atom (homoatomic). There are at last count 114 elements. - 88 of them are naturally occurring.
All atoms of an element must have the same number of protons but not neutrons.
 Counting Atoms Key Terms atomic number nuclide mole isotope unified atomic mass unit Avogadro s number mass number average atomic mass molar mass Consider neon, Ne, the gas used in many illuminated signs.
Counting Atoms Key Terms atomic number nuclide mole isotope unified atomic mass unit Avogadro s number mass number average atomic mass molar mass Consider neon, Ne, the gas used in many illuminated signs.
H Li. Mass Number. Number of Electrons Hydrogen He Draw diagrams to show the electronic structure of the elements above.
 AQA Knowledge test Unit 1 Chemistry C1 C1.1 The fundamental Ideas in Chemistry C1.1.1 Atoms 1. What is an atom? 2. What is an element? 3. Match the name of the element with the symbol Element Oxygen Sodium
AQA Knowledge test Unit 1 Chemistry C1 C1.1 The fundamental Ideas in Chemistry C1.1.1 Atoms 1. What is an atom? 2. What is an element? 3. Match the name of the element with the symbol Element Oxygen Sodium
Surface Processes and the Hydrosphere Unit Heating the Earth s Atmosphere Chapter 11 (pg )
 Name: Block: Surface Processes and the Hydrosphere Unit Heating the Earth s Atmosphere Chapter 11 (pg. 352 385) 11.1: Focus on the Atmosphere: Weather and Climate What is the difference between the weather
Name: Block: Surface Processes and the Hydrosphere Unit Heating the Earth s Atmosphere Chapter 11 (pg. 352 385) 11.1: Focus on the Atmosphere: Weather and Climate What is the difference between the weather
Atmosphere. Earth's atmosphere is a mixture of gases, solids, and liquids that surround the planet.
 Atmosphere Atmosphere- a thin layer of air that forms a protective covering around the planet. If Earth had no atmosphere, days would be extremely hot and nights would be extremely cold. Earth's atmosphere
Atmosphere Atmosphere- a thin layer of air that forms a protective covering around the planet. If Earth had no atmosphere, days would be extremely hot and nights would be extremely cold. Earth's atmosphere
The Atmosphere. Composition of the Atmosphere. Section 2
 The Atmosphere Earth is surrounded by a mixture of gases known as the Nitrogen, oxygen, carbon dioxide, and other gases are all parts of this mixture. Earth s atmosphere changes constantly as these gases
The Atmosphere Earth is surrounded by a mixture of gases known as the Nitrogen, oxygen, carbon dioxide, and other gases are all parts of this mixture. Earth s atmosphere changes constantly as these gases
Atoms and Elements Class Notes and Class Work
 Atoms and Elements Class Notes and Class Work Introduction to Matter Property: Characteristics matter has. Law: A rule nature seems to follow. It s been observed regularly. Theory: Tries to explain the
Atoms and Elements Class Notes and Class Work Introduction to Matter Property: Characteristics matter has. Law: A rule nature seems to follow. It s been observed regularly. Theory: Tries to explain the
Chapter 4 Atoms Practice Problems
 Chapter 4 Atoms Practice Problems 1) The primary substances of which all other things are composed are A) molecules. B) compounds. C) elements. D) electrons. E) protons. 2) Which of the following is a
Chapter 4 Atoms Practice Problems 1) The primary substances of which all other things are composed are A) molecules. B) compounds. C) elements. D) electrons. E) protons. 2) Which of the following is a
Lesson 9 The Physical Earth
 Lesson 9 The Physical Earth I. Form questions using the phrases below and then discuss with a partner. 1. the most beautiful place on the Earth 2. seeing the Earth from space 3. the application Google
Lesson 9 The Physical Earth I. Form questions using the phrases below and then discuss with a partner. 1. the most beautiful place on the Earth 2. seeing the Earth from space 3. the application Google
(i) an element which is gaseous at room temperature and pressure ... [1] (ii) an element which forms an oxide that is a reactant in photosynthesis
![(i) an element which is gaseous at room temperature and pressure ... [1] (ii) an element which forms an oxide that is a reactant in photosynthesis (i) an element which is gaseous at room temperature and pressure ... [1] (ii) an element which forms an oxide that is a reactant in photosynthesis](/thumbs/87/96894203.jpg) 1 (a) For each of the following, give the name of an element from Period 2 (lithium to neon), which matches the description. Elements may be used once, more than once or not at all. (i) an element which
1 (a) For each of the following, give the name of an element from Period 2 (lithium to neon), which matches the description. Elements may be used once, more than once or not at all. (i) an element which
Classification of Matter
 Classification of Matter All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that
Classification of Matter All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that
Matter and Energy. Section 2.1 Chapter 2. Representations of Matter: Models and Symbols. Goal 1. Goal 2
 Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
Classification of Matter. Elements, Compounds, Mixtures
 Classification of Matter Elements, Compounds, Mixtures Introducing Little Miss Element Hi! I am Little Miss Element I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a
Classification of Matter Elements, Compounds, Mixtures Introducing Little Miss Element Hi! I am Little Miss Element I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a
Our Planet Earth. Earth Systems
 Our Planet Earth Earth Systems What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement or
Our Planet Earth Earth Systems What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement or
Unit 3. Atoms and molecules
 Unit 3. Atoms and molecules Index. s and compounds...2.. Dalton's Atomic theory...2 2.-The atom...2 3.-Atomic number and mass number...2 4.-Isotopes, atomic mass unit and atomic mass...3 5.- configuration...3
Unit 3. Atoms and molecules Index. s and compounds...2.. Dalton's Atomic theory...2 2.-The atom...2 3.-Atomic number and mass number...2 4.-Isotopes, atomic mass unit and atomic mass...3 5.- configuration...3
Cool Video Clip Image Source: Google
 Cool Video Clip 1. 2. 1 2 The theme for Seventh Grade Science is structure. Structure is how things are built. It often refers to the physical characteristics of something. 3 All substances are made of
Cool Video Clip 1. 2. 1 2 The theme for Seventh Grade Science is structure. Structure is how things are built. It often refers to the physical characteristics of something. 3 All substances are made of
ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are oxygen and...
 Chemistry C1 Foundation Questions Q1. Choose words from this list to complete the sentences, ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are
Chemistry C1 Foundation Questions Q1. Choose words from this list to complete the sentences, ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are
Acids and Bases 2 Science Notes JC-Learn. JC-Learn. Science Notes Acids and Bases 2. 1 P a g e
 JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
EARTH TAKES SHAPE 1. Define all vocabulary words. Crust: The thin and solid outermost layer of the Earth above the mantle. Mantle: The layer of rock
 EARTH TAKES SHAPE 1. Define all vocabulary words. Crust: The thin and solid outermost layer of the Earth above the mantle. Mantle: The layer of rock between the Earth s crust and core Core: The central
EARTH TAKES SHAPE 1. Define all vocabulary words. Crust: The thin and solid outermost layer of the Earth above the mantle. Mantle: The layer of rock between the Earth s crust and core Core: The central
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
 THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) Time: 3 Hours Thursday, 06 th November
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) Time: 3 Hours Thursday, 06 th November
How Elements Bond. 578 CHAPTER 19 Chemical Bonds. Figure 11 Sodium and chlorine react forming white crystalline sodium chloride. Vocabulary SECTION
 SECTION ow Elements Bond Compare and contrast ionic and covalent bonds. Identify the difference between polar and nonpolar covalent bonds. Interpret chemical shorthand. Vocabulary ion ionic bond compound
SECTION ow Elements Bond Compare and contrast ionic and covalent bonds. Identify the difference between polar and nonpolar covalent bonds. Interpret chemical shorthand. Vocabulary ion ionic bond compound
10/11/2010. Acceleration due to gravity, a. Bulk Properties Mass = 6 x kg Diameter = 12,756 km Density = 5515 kg/m 3 (mix of rock and iron)
 Acceleration due to gravity, a Bulk Properties Mass = 6 x 10 24 kg Diameter = 12,756 km Density = 5515 kg/m 3 (mix of rock and iron) Escape Velocity, v e Albedo Amount of sunlight reflected back into space
Acceleration due to gravity, a Bulk Properties Mass = 6 x 10 24 kg Diameter = 12,756 km Density = 5515 kg/m 3 (mix of rock and iron) Escape Velocity, v e Albedo Amount of sunlight reflected back into space
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds. What is an atmosphere? About 10 km thick
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds What is an atmosphere? Sources of Gas Losses of Gas Thermal Escape Earth s Atmosphere About 10 km thick Consists mostly of molecular
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds What is an atmosphere? Sources of Gas Losses of Gas Thermal Escape Earth s Atmosphere About 10 km thick Consists mostly of molecular
Clever Catch Weather Ball Question and Answer Sheets
 Clever Catch Weather Ball Question and Answer Sheets 1. Too much exposure to can cause skin cancer. B. Ultraviolet radiation 2. The layer of the atmosphere closest to the Earth s surface is the 3. Some
Clever Catch Weather Ball Question and Answer Sheets 1. Too much exposure to can cause skin cancer. B. Ultraviolet radiation 2. The layer of the atmosphere closest to the Earth s surface is the 3. Some
I T A T I O N H B I T B T V A O C J K M R S A T M O S P H E R E
 Word Search Directions: Below are definitions of vocabulary terms. Figure out each term and then find and circle it in the puzzle. Words may appear horizontally, vertically, or diagonally. K E M I S S
Word Search Directions: Below are definitions of vocabulary terms. Figure out each term and then find and circle it in the puzzle. Words may appear horizontally, vertically, or diagonally. K E M I S S
Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry
 c Grade 9 Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry Duration: 01:30 Hours Index No:- PART I Underline the correct answers 1) Identify the correct statement about
c Grade 9 Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry Duration: 01:30 Hours Index No:- PART I Underline the correct answers 1) Identify the correct statement about
Element Names Chem Worksheet 1-1
 Element Names Chem Worksheet 1-1 Use a textbook or the website http://www.webelements.com to write the name of the element described below. phosphorus calcium iron nitrogen chlorine helium oxygen neon
Element Names Chem Worksheet 1-1 Use a textbook or the website http://www.webelements.com to write the name of the element described below. phosphorus calcium iron nitrogen chlorine helium oxygen neon
Lithosphere: (Rocky Sphere) Solid, rocky, outer layer of the Earth. Includes the crust and part of the upper mantle. Lithosphere
 Lithosphere: (Rocky Sphere) Solid, rocky, outer layer of the Earth. Includes the crust and part of the upper mantle. Lithosphere Minerals: Solid, inorganic substances that have a clearly defined composition
Lithosphere: (Rocky Sphere) Solid, rocky, outer layer of the Earth. Includes the crust and part of the upper mantle. Lithosphere Minerals: Solid, inorganic substances that have a clearly defined composition
An atom is the smallest particle of an element which still retains the properties of that element
 Chemistry: 4.The Atom (and introduction to the periodic table) Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC39 Describe the structure
Chemistry: 4.The Atom (and introduction to the periodic table) Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC39 Describe the structure
SCH 3UI Unit 1 Outline Introduction to Chemistry: Review and Labs
 SCH 3UI Unit 1 Outline Introduction to Chemistry: Review and Labs Lesson Topics Covered Homework Questions and Assignments 1 Introduction to SCH 3UI Course Outline, expectations SCH 3UI website: pattersonscience@weebly.com
SCH 3UI Unit 1 Outline Introduction to Chemistry: Review and Labs Lesson Topics Covered Homework Questions and Assignments 1 Introduction to SCH 3UI Course Outline, expectations SCH 3UI website: pattersonscience@weebly.com
Today. Events. Terrestrial Planet Geology - Earth. Terrestrial Planet Atmospheres. Homework DUE next time
 Today Terrestrial Planet Geology - Earth Terrestrial Planet Atmospheres Events Homework DUE next time Ring of Fire Boundaries of plates traced by Earthquakes and Volcanos Plate Motions Measurements of
Today Terrestrial Planet Geology - Earth Terrestrial Planet Atmospheres Events Homework DUE next time Ring of Fire Boundaries of plates traced by Earthquakes and Volcanos Plate Motions Measurements of
3 Families of Elements
 CHAPTER 5 3 Families of Elements SECTION The Periodic Table KEY IDEAS As you read this section, keep these questions in mind: What makes up a family of elements? What properties do the elements in a group
CHAPTER 5 3 Families of Elements SECTION The Periodic Table KEY IDEAS As you read this section, keep these questions in mind: What makes up a family of elements? What properties do the elements in a group
Chapter 2 Atoms and the Periodic Table
 Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
2/15/2013. Chapter 6 6.1
 Chapter 6 In a self-service store, the products are grouped according to similar characteristics. With a logical classification system, finding and comparing products is easy. You will learn how elements
Chapter 6 In a self-service store, the products are grouped according to similar characteristics. With a logical classification system, finding and comparing products is easy. You will learn how elements
Q1. Methane and oxygen react together to produce carbon dioxide and water.
 Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
What are plants used for?
 1 of 48 Boardworks Ltd 2007 2 of 48 Boardworks Ltd 2007 What are plants used for? 3 of 48 Boardworks Ltd 2007 How many different uses of plants can you spot? Using plants 4 of 48 Boardworks Ltd 2007 How
1 of 48 Boardworks Ltd 2007 2 of 48 Boardworks Ltd 2007 What are plants used for? 3 of 48 Boardworks Ltd 2007 How many different uses of plants can you spot? Using plants 4 of 48 Boardworks Ltd 2007 How
8/24/2018. Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry. Chapter 2: Essential Chemistry for Biology
 1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
Grade 9 End semester exam Revision sheet Answer key. Kingdom of Bahrain Ministry of Education Ahlia School -ABCD
 Grade 9 End semester exam Revision sheet Answer key Question 1: Directions: Put a check mark in the column that each type of matter describes. 1. Oxygen Substances Element Compound Mixtures 2. Granite
Grade 9 End semester exam Revision sheet Answer key Question 1: Directions: Put a check mark in the column that each type of matter describes. 1. Oxygen Substances Element Compound Mixtures 2. Granite
Name: Group: Date: d) lead turning into gold A nuclear transformation, because atomic nuclei are rearranged.
 ST Questions 1 7, 17, 18, 20 23, C and E Checkup 1 WHAT ARE CHANGES IN MATTER? (p. 108) 1. Does each of the following phenomena describe a physical change, a chemical change or a nuclear transformation?
ST Questions 1 7, 17, 18, 20 23, C and E Checkup 1 WHAT ARE CHANGES IN MATTER? (p. 108) 1. Does each of the following phenomena describe a physical change, a chemical change or a nuclear transformation?
Determine Chemical Behavior
 Fun with the Periodic Table Activity 7 CHEM POETRY A sodium atom walks onto the scene, His valence electron s feeling keen, Positive that he will ionically bond With a halogen of whom he is fond. How Electrons
Fun with the Periodic Table Activity 7 CHEM POETRY A sodium atom walks onto the scene, His valence electron s feeling keen, Positive that he will ionically bond With a halogen of whom he is fond. How Electrons
5E Essential Lesson-SC.8.P.8.6. Element Name: Hydrogen (H) Element Name: Helium (He) Number of orbitals: 1. Number of valence electrons: 2
 Element Name: Hydrogen (H) Number of orbitals: 1 Number of protons: 1 Atomic Mass: 1.01 AMU Properties: gas, bonds with other elements, flammable Element Name: Helium (He) Number of orbitals: 1 Number
Element Name: Hydrogen (H) Number of orbitals: 1 Number of protons: 1 Atomic Mass: 1.01 AMU Properties: gas, bonds with other elements, flammable Element Name: Helium (He) Number of orbitals: 1 Number
Periodic Table of Elements
 Periodic Table of Elements chlorine nitrogen helium gold oxygen silver mercury hydrogen neodymium sodium niobium carbon Elements Science has come along way since Aristotle s theory of Air, Water, Fire,
Periodic Table of Elements chlorine nitrogen helium gold oxygen silver mercury hydrogen neodymium sodium niobium carbon Elements Science has come along way since Aristotle s theory of Air, Water, Fire,
1. In the diagram below, letters A and B represent locations near the edge of a continent.
 1. In the diagram below, letters A and B represent locations near the edge of a continent. A geologist who compares nonsedimentary rock samples from locations A and B would probably find that the samples
1. In the diagram below, letters A and B represent locations near the edge of a continent. A geologist who compares nonsedimentary rock samples from locations A and B would probably find that the samples
SIXTH FORM AES CHEMISTRY TRANSITION UNIT. Name: Secondary School
 SIXTH FORM AES CHEMISTRY TRANSITION UNIT Name: Secondary School 0 1 Contents Introduction 2 Task 1: The structure of atoms 3 Task 2: Atoms and ions 4 Task 3: Writing formulas 5 Task 4: Relative masses
SIXTH FORM AES CHEMISTRY TRANSITION UNIT Name: Secondary School 0 1 Contents Introduction 2 Task 1: The structure of atoms 3 Task 2: Atoms and ions 4 Task 3: Writing formulas 5 Task 4: Relative masses
~ C\J Co Co :J :J (9 (9
 I Under normal conditions, hydrogen and oxygen are colourless, odourless gases. If you ignite a mixture of Period 1 hydrogen and oxygen, it burns Period 2 explosively, forming water. P. d 3 Water's physical
I Under normal conditions, hydrogen and oxygen are colourless, odourless gases. If you ignite a mixture of Period 1 hydrogen and oxygen, it burns Period 2 explosively, forming water. P. d 3 Water's physical
Chapter 7 Earth and the Terrestrial Worlds
 Chapter 7 Earth and the Terrestrial Worlds Guest Lecture by Chris Kelso Please pick up one notecard of each color (5 total) Outline The Earth s Interior The Earth s Surface The Earth s Atmosphere Concept
Chapter 7 Earth and the Terrestrial Worlds Guest Lecture by Chris Kelso Please pick up one notecard of each color (5 total) Outline The Earth s Interior The Earth s Surface The Earth s Atmosphere Concept
Welcome to Honors Chemistry!
 Welcome to Honors Chemistry! Congratulations on taking Honors Chemistry next year! This summer assignment is designed to assess your reading comprehension and interpreting graph skills; review how to make
Welcome to Honors Chemistry! Congratulations on taking Honors Chemistry next year! This summer assignment is designed to assess your reading comprehension and interpreting graph skills; review how to make
SNC1P - Chemistry Test Review
 SNC1P - Chemistry Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is an example of a physical property? a. solubility
SNC1P - Chemistry Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is an example of a physical property? a. solubility
Knox Academy Science Department. S1 Science
 Knox Academy Science Department S1 Science Our Material World Part 2 Write on Booklet 1 1. Chemical Elements the Builders How many materials are there? There are millions upon millions of different materials,
Knox Academy Science Department S1 Science Our Material World Part 2 Write on Booklet 1 1. Chemical Elements the Builders How many materials are there? There are millions upon millions of different materials,
Key Concept Heat in Earth s atmosphere is transferred by radiation, conduction, and convection.
 Section 2 Atmospheric Heating Key Concept Heat in Earth s atmosphere is transferred by radiation, conduction, and convection. What You Will Learn Solar energy travels through space as radiation and passes
Section 2 Atmospheric Heating Key Concept Heat in Earth s atmosphere is transferred by radiation, conduction, and convection. What You Will Learn Solar energy travels through space as radiation and passes
Ch. 3 Answer Key. O can be broken down to form two atoms of H and 1 atom of O. Hydrogen and oxygen are elements.
 Ch. 3 Answer Key 1. The Greeks believed that all matter is made of elements. We currently believe the same thing. However, the Greeks believed that there were 4 elements: earth, water, air and fire. Instead,
Ch. 3 Answer Key 1. The Greeks believed that all matter is made of elements. We currently believe the same thing. However, the Greeks believed that there were 4 elements: earth, water, air and fire. Instead,
ASTR-101 Section 004 Lecture 9 Rare Earth? John T. McGraw, Professor
 ASTR-101 Section 004 Lecture 9 Rare Earth? John T. McGraw, Professor Rare Earth Long-lived sun Rocky world C, O, Si, materials for soil, tools and subsistence Near circular orbit Not too warm not too cold
ASTR-101 Section 004 Lecture 9 Rare Earth? John T. McGraw, Professor Rare Earth Long-lived sun Rocky world C, O, Si, materials for soil, tools and subsistence Near circular orbit Not too warm not too cold
GCSE Additional Science
 GCSE Additional Science Module C5 Chemicals of the Natural Environment: What you should know Name: Science Group: Teacher: each of the statements to help focus your revision: R = Red: I don t know this
GCSE Additional Science Module C5 Chemicals of the Natural Environment: What you should know Name: Science Group: Teacher: each of the statements to help focus your revision: R = Red: I don t know this
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
