Functional and signaling mechanism analysis of rice CRYPTOCHROME 1
|
|
|
- Stanley Dalton
- 6 years ago
- Views:
Transcription
1 The Plant Journal (2006) 46, doi: /j X x Functional and signaling mechanism analysis of rice CRYPTOCHROME 1 Yan-Chun Zhang, Song-Fu Gong, Qing-Hua Li, Yi Sang and Hong-Quan Yang * National Key Laboratory of Plant Molecular Genetics, Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and Graduate School of the Chinese Academy of Sciences, 300 Fenglin Road, Shanghai , China Received 7 November 2005; revised 7 February 2006; accepted 7 March * For correspondence (fax þ ; hqyang@sibs.ac.cn). Summary Cryptochromes (CRY) are blue-light photoreceptors that mediate various light responses, such as inhibition of hypocotyl elongation, enhancement of cotyledon expansion, anthocyanin accumulation and stomatal opening in Arabidopsis. The signaling mechanism of Arabidopsis CRY is mediated through direct interaction with COP1, a negative regulator of photomorphogenesis. CRY has now been characterized in tomato, pea, moss and fern, but its function in monocots is largely unknown. Here we report the function and basic signaling mechanism of rice cryptochrome 1 (OsCRY1). Overexpresion of OsCRY1b resulted in a blue light-dependent short hypcotyl phenotype in Arabidopsis, and a short coleoptile, leaf sheath and leaf blade phenotype in rice (Oryza sativa). On fusion with b-glucuronidase (GUS), the C-terminal domain of either OsCRY1a (OsCCT1a) or OsCRY1b (OsCCT1b) mediated a constitutive photomorphogenic (COP) phenotype in both Arabidopsis and rice, whereas OsCCT1b mutants corresponding to missense mutations in previously described Arabidopsis cry1 alleles failed to confer a COP phenotype. Yeast two-hybrid and subcellular co-localization studies demonstrated that OsCRY1b interacted physically with rice COP1 (OsCOP1). From these results, we conclude that OsCRY1 is implicated in blue-light inhibition of coleoptile and leaf elongation during early seedling development in rice, and that the signaling mechanism of OsCRY1 involves direct interaction with OsCOP1. Keywords: cryptochrome, constitutive photomorphogenic, light signaling, Arabidopsis, rice. Introduction Light is among the most crucial environmental cues affecting plant growth and development processes from seed germination to flower initiation. Three classes of photoreceptor in higher plants have been identified: phytochromes (Quail, 2002); cryptochromes (CRY) (Cashmore et al., 1999; Lin, 2002); and phototropins (Briggs and Christie, 2002). CRY was first isolated in Arabidopsis (Ahmad and Cashmore, 1993; Lin et al., 1998), and has now been characterized throughout the plant kingdom, Drosophila, and mammals (Cashmore, 2003; Cashmore et al., 1999; Hall, 2000). Arabidopsis CRY (AtCRY1 and AtCRY2) mediates a variety of blue-light responses, including inhibition of hypocotyl/stem elongation, stimulation of leaf expansion, production of anthocyanin, regulation of flowering time, and stomatal opening (Ahmad et al., 1998; Guo et al., 1998; Lin et al., 1996, 1998; Mao et al., 2005; Mockler et al., 1999). It has been demonstrated that Arabidopsis cryptochromes act together with phytochromes to promote photomorphogenesis (Ahmad and Cashmore, 1997; Casal and Mazzella, 1998; Neff and Chory, 1998) and entrain the circadian clock (Somers et al., 1998). The third cryptochrome (CRY3) in Arabidopsis has been found recently, but its exact function remains unknown (Kleine et al., 2003). Cryptochromes show a high degree of similarity to photolyases, flavoproteins that mediate light-dependent DNA repair (Sancar, 1994). However, they have a distinguishing C-terminal domain, which is absent in photolyases, and lack photolyase activity (Cashmore et al., 1999). Transgenic plants expressing the C-terminal domain of AtCRY1 (AtCCT1) and AtCRY2 (AtCCT2) fused to b-glucuronidase (GUS) display a constitutive photomorphogenic (COP) phenotype (Yang et al., 2000), which is similar to that of mutants of both COP1 and COP9 signalosome, the repressor of photomorphogenesis (Deng and Quail, 1992; Deng et al., 971 Journal compilation ª 2006 Blackwell Publishing Ltd
2 972 Yan-Chun Zhang et al. 1991; Misera et al., 1994; Wei et al., 1994a). These findings indicate that the signaling mechanism of AtCRY is mediated through its C-terminal domain. Both AtCCT1 and AtCCT2 are shown to interact physically with COP1 (Wang et al., 2001; Yang et al., 2001). It has been demonstrated recently that the N-terminal domain of AtCRY1 (AtCNT1) mediates homodimerization of AtCRY1, which is required for light activation of CRY1 photoreceptor activity, and that multimerization of GUS is both responsible and required for mediating a COP phenotype on fusion to AtCCT1 (Sang et al., 2005). These studies suggest that activation of AtCCT1 is probably mediated through a blue light-dependent change in the properties of the AtCNT1 dimer. Cryptochromes have recently been characterized in two other dicots: tomato and pea. Four CRY genes have been found in tomato: TCRY1a, TCRY1b, TCRY2 and TCRY3. Both TCRY1a and TCRY2 are shown to promote photomorphogenesis and anthocyanin accumulation in tomato (Ninu et al., 1999; Weller et al., 2001), and TCRY2 was demonstrated to retard flowering under both short- and long-day conditions and to enhance outgrowth of axillary branches (Giliberto et al., 2005). In pea, three cryptochrome genes have been identified: a single CRY1 and two differently regulated CRY2 genes. It has been demonstrated that overexpression of CRY2b confers strong diurnal regulation and rapid blue-light repression of seedling development in pea (Platten et al., 2005). Cryptochromes have also been found in lower plants. Two CRY genes have been isolated in moss: PpCRY1a and PpCRY1b, which are shown to perform an essential role in the mediation of blue light-dependent side-branch formation (Imaizumi et al., 2002); five CRY genes have also been cloned in fern, and it has been demonstrated that fern CRY3 and CRY4 mediate blue lightdependent inhibition of spore germination (Imaizumi et al., 2000). Unlike dicots, monocots do not have hypocotyls and cotyledons. Instead, they have mesocotyls and coleoptiles, elongation of which has been shown to be inhibited by blue light. Rice (Oryza sativa) is the model plant for monocots. In contrast with Arabidopsis, a long-day plant, rice is a shortday plant. Thus it would be interesting to investigate whether CRY is involved in the regulation of photomorphogenic development and photoperiod response in rice. Although rice cryptochrome 1 (OsCRY1) has been isolated, and it has been shown that OsCRY1 fused to green fluorescent protein (GFP) confers short hypocotyl and enhanced anthocyanin phenotype in Arabidopsis (Matsumoto et al., 2003), its function in rice is unknown so far. Here we show, by transgenic, physiological, biochemical, yeast two-hybrid and subcellular co-localization studies, that OsCRY1 performs a role in blue-light regulation of photomorphogenesis in rice, and that the signaling mechanism of OsCRY1 is mediated through direct interaction with rice COP1. Results Cryptochrome gene family in rice By using Arabidopsis CRY1 and CRY2 cdna sequences to conduct NCBI BLAST, we found three full-length rice cryptochrome cdna sequences: OsCRY1a (accession AB073546); OsCRY1b (AB073547); and OsCRY2 (AB103094). Amino acid and nucleotide sequence comparison indicated that OsCRY1a and OsCRY1b were probably the previously described OsCRY1 (AB024337) and OsCRY2 (AB098568) (Lin and Shalitin, 2003; Matsumoto et al., 2003) respectively (data not shown). However, the first exon of OsCRY1b was missing in the previous OsCRY2, and several nucleotide errors occurred throughout the entire sequence of the previously characterized OsCRY1. Specifically, a T was added at nucleotide 1969, resulting in an open reading frame shift and premature termination of the protein. We confirmed the accuracy of OsCRY1a and OsCRY1b sequences by comparing with their genomic sequences (BAC clones OS- JNBb0046L23 and OSJNBa0086B14). Amino acid sequence alignment demonstrated that OsCRY1a and OsCRY1b shared 76.4% and 78.0% similarity, respectively, to Arabidopsis CRY1 (AtCRY1); and 57.6% and 59.9% similarity, respectively, to Arabidopsis CRY2 (AtCRY2). They showed 87.8% overall similarity to each other, and 61.1% and 58.7% similarity, respectively, to the present OsCRY2 (data not shown). Transgenic Arabidopsis seedlings expressing OsCRY1b show hypersensitive response to blue light To investigate the function of OsCRY1, we prepared a construct expressing full-length OsCRY1b (Figure 1b, construct 1), and overexpressed it in the Arabidiopsis cry1 mutant. We obtained 56 independent transgenic lines (OsCRY1b-ovx), 43 of which had dramatically short hypocotyls under blue light (Figure 1c, top, seedling 1). When grown in the dark and in red and far-red light, the OsCRY1b-ovx seedlings displayed a phenotype indistinguishable from that of the wild type (WT) and cry1 mutant (Figure 1c, bottom, seedling 1; and data not shown). Moreover, by visual inspection the OsCRY1b-ovx seedlings were observed to have enhanced levels of anthocyanin under blue light. Measurement of anthocyanin indicated blue light-dependent enhancement of anthocyanin production in OsCRY1-ovx seedlings (data not shown), similar to that observed for AtCRY1-overexpression lines (Lin et al., 1996). By Western blot analysis we examined extracts from two independent OsCRY1b-ovx lines using antibody against the OsCRY1b C-terminal domain (Os- CCT1b), and found that OsCRY1b was expressed at high levels in these lines (Figure 1d, lanes 1 and 2). These results indicated that OsCRY1b was capable of performing a similar
3 Rice CRYPTOCHROME 1 signaling 973 (a) E523 * R585 * (b) (d) (e) (f) (c) (g) Figure 1. Expression of various fusion proteins in Arabidopsis. (a) Comparison of the partial C-terminal amino acid sequences of OsCRY1b and AtCRY1. Asterisks indicate sites that are critical for AtCRY1 photoreceptor activity, also conserved in OsCRY1b. (b) Schematic diagrams showing the various chimeric genes. A number for each construct is shown on the left. OsCRY1b, full-length rice cryptochrome 1b; AtCNT1, Arabidopsis CRY1 N-terminal domain; AtCNT2, Arabidopsis CRY2 N-terminal domain; OsCCT1b, OsCRY1b C-terminal domain; OsCCT1b (R585K) and OsCCT1b (E523K), OsCCT1b mutants containing a R585K and an E523K mutation respectively. (c) Functional analysis of OsCRY1b in Arabidopsis. Six-day-old Arabidopsis seedlings grown under blue light (top) and in darkness (bottom) are shown. Numbers under each seedling represent a transgenic plant expressing the correspondingly numbered construct in (b), cited in the text. All transgenic seedlings were made in the cry1 mutant background. Scale bar, 1 mm. (d g) Western blot analysis of the transgenic Arabidopsis lines expressing OsCRY1b-ovx, AtCNT1-OsCCT1b, AtCNT2-OsCCT1b, GUS-OsCCT1b, GUS-OsCCT1a and GUS-OsCCT1b mutants using antibody against OsCCT1b. Numbers in parentheses represent independent transgenic lines. Transgenic lines OsCRY1b-ovx (11), AtCNT1-OsCCT1b (2), AtCNT2-OsCCT1b (5), GUS-OsCCT1b (8), GUS-OsCCT1a (20), GUS-OsCCT1b(R585K) (2), and GUS-OsCCT1b(E523K) (2) are shown in (c). Degraded protein bands are marked with asterisks. role to AtCRY1 in blue-light inhibition of hypocotyl elongation in Arabidopsis. The N-terminal domain of Arabidopsis CRY can perform the role of that of OsCRY1b in mediation of blue-light response on fusion to the C-terminal domain of OsCRY1b It has been demonstrated that the signaling mechanism of AtCRY is mediated through the C-terminal domain (AtCCT), and that the N-terminal domain (AtCNT1) mediates homodimerization of CRY1, which is required for light activation of CRY1 photoreceptor activity (Sang et al., 2005; Yang et al., 2000). To investigate whether AtCNT is able to perform the OsCNT1 role, we made two constructs expressing OsCCT1b fused to AtCNT1 and AtCNT2 (Figure 1b, constructs 2 and 3), and overexpressed them in Arabidopsis cry1 mutant plants. We obtained more than 10 independent transgenic lines for each of these constructs, which, when grown under blue light, showed short hypocotyl phenotype (Figure 1c, top, seedlings 2 and 3), whereas they showed a WT phenotype when grown in the dark and in red and far-red light (Figure 1c, bottom, seedlings 2 and 3; and results not shown). By Western blot analysis we examined extracts from the transgenic lines using OsCCT1b antibody, and found that all
4 974 Yan-Chun Zhang et al. the lines displaying the short-hypocotyl phenotype expressed high levels of the fusion proteins (Figure 1d, lanes 3, 4, 6 and 7). These data indicate that AtCNT is able to perform the role of OsCNT1b in blue-light activation of OsCRY1b photoreceptor activity. Transgenic Arabidopsis plants expressing GUS, fused to either OsCCT1a or OsCCT1b, show a constitutive photomorphogenic phenotype It has been demonstrated previously that GUS fused to AtCCT mediates a constitutive light response (Yang et al., 2000), and the multimerization of GUS is both responsible and required for constitutive activation of AtCCT1 (Sang et al., 2005). To determine whether OsCCT1 is able to mediate a constitutive light response, we generated two constructs expressing GUS fused to OsCCT1b and OsCCT1a (Figure 1b, constructs 4 and 5), and overexpressed them in the Arabidopsis cry1 mutant. We obtained 32 independent transgenic lines, 22 of which showed a COP phenotype of short hypocotyls, highly accumulated anthocyanin and fully opened and expanded cotyledons in darkness, and displayed a short-hypocotyl phenotype in blue, red and far-red light (Figure 1c, seedlings 4 and 5; and data not shown). Furthermore, GUS-OsCCT1a and GUS-OsCCT1b seedlings accumulated significantly higher levels of anthocyanin than WT in the dark and in blue, red and far-red light (results not shown), similar to that observed for GUS-AtCCT1 seedlings (Yang et al., 2000). Western blot analysis using antibody against OsCCT1b demonstrated that all the lines showing a COP phenotype expressed GUS-OsCCT1a and GUS-OsCCT1b fusion protein (Figure 1d,e). These data suggest that the C-terminal domain of OsCRY1 is involved in OsCRY1 signaling. Point mutations within OsCCT1b failed to confer a COP phenotype in Arabidopsis It has been shown previously that over 30 mutants of hy4/ cry1 show unusually long hypocotyls when grown under blue light (Ahmad et al., 1995; Shalitin et al., 2003). Many of these mutant alleles correspond to mutations within the C-terminal domain, demonstrating the functional importance of this domain. Three of these mutations were conservative arginine-to-lysine substitutions [hy4-10 (R576K); hy4-23 (R581K); hy4-24 (R611K)] and three corresponded to glutamic acid-to-lysine substitutions [hy4-19 (E515K); hy4-20 (E531K); hy4-22 (E559K)] (Ahmad et al., 1995). It has been demonstrated previously that transgenic plants expressing GUS-AtCCT1 mutants corresponding to hy4-10, hy4-19 and hy4-20 alleles failed to show a COP phenotype, indicating that the COP phenotype observed for GUS-CCT1 plants is physiologically meaningful (Yang et al., 2000). Amino acid sequence alignment of OsCRY1b and AtCRY1 revealed that E523 and R585, corresponding to E515 and R576 within At- CCT1, respectively, are conserved within OsCCT1b (Figure 1a). To investigate whether these two sites are important for OsCRY1b functionality, we made two constructs expressing mutant GUS-OsCCT1b (R585K) and GUS-OsCCT1b (E523K) (Figure 1b, constructs 6 and 7), and overexpressed them in the cry1 mutant plants. We examined more than 30 independent transgenic lines for each of these two constructs, and found that none of them showed a COP phenotype in darkness and a shorthypocotyl phenotype in light (Figure 1c, seedlings 6 and 7; and data not shown). Western blot analysis using antibody against OsCCT1b indicated that the mutant GUS-OsCCT1b fusion proteins were expressed normally (Figure 1f,g). These data therefore indicated that the signaling mechanism of OsCRY1 might be analogous to AtCRY1. Transgenic rice plants overexpressing OsCRY1b show short coleoptile and leaf phenotype under blue light It has been shown previously that blue light induces inhibition of coleoptile growth in monocots such as oat (Thornton and Thimann, 1967); maize (Wang and Iino, 1997); and rice (Biswas et al., 2003). To define further the blue-light response of young rice seedlings, we obtained 7-day-old dark- and blue light-grown WT rice seedlings and found that the coleoptile, primary leaf (no leaf blade) and second leaf sheath of the dark-grown seedlings were significantly longer than those of the blue light-grown seedlings (Figure 2a), indicating blue-light inhibition of elongation of the coleoptile and primary leaf. In addition, the second leaf blade of the dark-grown seedlings was folded and unexpanded, and grew vertically, with no angle at the y-axis; whereas that of the blue light-grown seedlings was unfolded and expanded, and grew with an enlarged angle at the y-axis (Figure 2a). To examine whether OsCRY1b is involved in these responses in rice, we generated transgenic rice lines overexpressing OsCRY1b. We obtained nine independent transgenic lines which, when grown under blue light, were significantly shorter than WT; in the dark and in red and far-red light they were indistinguishable from WT (Figure 2b). Western blot analysis using OsCCT1b antibody indicated that all the lines showing a short seedling phenotype accumulated a high level of OsCRY1b (Figure 2c). We measured the length of coleoptile, primary leaf, and second leaf sheath and blade under all light conditions. As shown in Table 1, under blue light these measurements in OsCRY1b-ovx seedlings were significantly shorter than in WT. No difference was observed between OsCRY1b-ovx and WT plants grown in the dark and in red and far-red light. These data indicate that OsCRY1b may function as the blue-light photoreceptor
5 Rice CRYPTOCHROME 1 signaling 975 Figure 2. Expression of OsCRY1b, GUS- OsCRY1b, GUS-OsCCT1b and GUS-OsCCT1a in rice. (a) Seven-day-old blue light-grown (left) and dark-grown (right) wild-type rice seedlings. Arrows indicate leaf structures of young seedlings. a, coleoptile length; b, primary leaf length; c, second leaf sheath length; d, second leaf blade length. a, angle between the second leaf blade and the y-axis. Measurements of coleoptile and leaf length for all the transgenic lines are given in Table 1. Scale bar, 1 cm. (b) OsCRY1b mediates enhanced blue-light response, whereas GUS-OsCRY1b, GUS-OsCCT1b and GUS-OsCCT1a confer a COP phenotype in darkness and hypersensitive light response respectively. Nine-day-old dark-grown seedlings and 7-day-old blue, red and far-red light-grown seedlings are shown. Scale bar, 1 cm. (c f) Western blot analysis of the transgenic rice lines expressing OsCRY1b-ovx, GUS-OsCRY1b, GUS-OsCCT1b and GUS-OsCCT1a using antibody against OsCCT1b. OsCRY1b-ovx (27), GUS- OsCRY1b (2), GUS-OsCCT1b (10) and GUS- OsCCT1a (2) are shown in (c) and used for measurements in Table 1. Degraded protein bands are marked with asterisks. (a) (b) (c) (d) (e) (f) to mediate blue light-dependent inhibition of coleoptile and leaf elongation during early seedling development in rice. GUS-OsCCT1a, GUS-OsCCT1b and GUS-OsCRY1b mediate a COP phenotype in rice With the demonstration that GUS-OsCCT1a and GUS-Os- CCT1b fusion proteins are able to mediate a COP phenotype in Arabidopsis, we asked whether they are able to do so in rice. We overexpressed the constructs expressing GUS- OsCCT1a and GUS-OsCCT1b in WT rice, and obtained 12 and five independent transgenic lines for GUS-OsCCT1b and GUS-OsCCT1a, respectively, which, when grown in darkness, displayed a COP phenotype of fully expanded second leaf blade, shortened sheath and enlarged angle between the second leaf blade and the y-axis in darkness (Figure 2b; Table 1). This morphology is normally observed
6 976 Yan-Chun Zhang et al. Table 1 Measurements of rice coleoptile and leaf length Light quality Genotype of plant Coleoptile (mm) Primary leaf (mm) Second leaf sheath (mm) Second leaf blade (mm) Dark WT OsCRY1b-ovx (27) GUS-OsCRY1b (2) GUS-OsCCT1b (10) Blue WT OsCRY1b-ovx (27) GUS-OsCRY1b (2) GUS-OsCCT1b (10) Red WT OsCRY1b-ovx (27) GUS-OsCRY1b (2) GUS-OsCCT1b (10) Far-red WT OsCRY1b-ovx (27) GUS-OsCRY1b (2) GUS-OsCCT1b (10) Lengths of coleoptile, primary leaf, second leaf sheath and leaf blade were measured for 7-day-old seedlings after growth under different light conditions and for 9-day-old seedlings after growth in the dark. Measurements were performed on 20 seedlings of each genotype. The numbers in brackets signify the transgenic lines analysed. Error values, SD. only for WT in the light (Figure 2a, left). Furthermore, GUS- OsCCT1a and GUS-OsCCT1b seedlings were significantly shorter than WT in blue, red and far-red light (Figure 2b; Table 1). It has been shown that, on fusion with GUS, full-length AtCRY1 is also able to mediate a constitutive light response in Arabidopsis (Sang et al., 2005). To examine whether this is true with OsCRY1, we prepared a construct expressing GUS fused to full-length OsCRY1b, and overexpressed it in WT rice. We obtained 21 independent transgenic lines, which showed a COP phenotype in darkness and a short seedling phenotype in blue, red and far-red light (Figure 2b; Table 1), similar to that observed for GUS-OsCCT1a and GUS-OsCCT1b seedlings. Western blot analysis, using antibody against OsCCT1b, demonstrated that the GUS- OsCRY1b, GUS-OsCCT1b and GUS-OsCCT1a fusion proteins were expressed (Figure 2d f). Mutant GUS-OsCCT1b fusion proteins failed to confer a COP phenotype in rice To explore further the signaling mechanism of OsCRY1 in rice, we overexpressed the mutants of GUS-OsCCT1b in WT rice. We obtained more than 30 independent transgenic lines for each of the two mutant constructs, none of which showed either a COP phenotype in darkness or a short seedling phenotype in blue, red and far-red light (Figure 3a). Western blot assay using OsCCT1b antibody indicated that the mutant GUS-OsCCT1b proteins were expressed normally (Figure 3b,c). These data demonstrated that both E523 and R585 sites within OsCCT1b are also critical for OsCRY1b functionality in rice. GUS-OsCCT1 stimulates rice plastid development in the dark It has been demonstrated previously that GUS-AtCCT1 is capable of initiating chloroplast development in dark-grown Arabidopsis seedlings (Yang et al., 2000). To investigate whether GUS-OsCCT1b affects plastid development in rice in darkness, we examined chloroplast development in darkgrown GUS-OsCCT1b seedlings by electron microscopy. As shown in Figure 4, chloroplast development was indistinguishable for light-grown GUS-OsCCT1b and WT seedlings. However, dark-grown GUS-OsCCT1b seedlings clearly showed signs of chloroplast development, demonstrated by the lack of prolamellar bodies and the presence of parallel thylakoid membranes, many of which were stacked (Figure 4), similarly to those observed for the GUS-AtCCT1, cop1 and det/fus mutant plants (Chory et al., 1989; Deng et al., 1991; Misera et al., 1994; Wei et al., 1994b; Yang et al., 2000). OsCCT1b interacts with OsCOP1 in yeast cells With the demonstration that GUS-OsCCT1a and GUS-Os- CCT1b are able to mediate a COP phenotype in both Arabidopsis and rice, and that GUS-OsCCT1b mutants failed to confer a COP phenotype, we postulated that OsCRY1 and AtCRY1 might have the same downstream signaling partner (COP1). To examine this possibility, we prepared bait constructs expressing the LexA DNA-binding domain fused to OsCCT1b and OsCNT1b (Figure 5a), and prey constructs comprising the B42 transcriptional activation domain (B42 AD) fused to Arabidopsis COP1 (AtCOP1); OsCOP1, lacking
7 Rice CRYPTOCHROME 1 signaling 977 Figure 3. Phenotype of transgenic seedlings expressing GUS-OsCCT1b mutants. (a) Seedlings expressing mutant forms of GUS- OsCCT1b [GUS-OsCCT1b (R585K) and GUS-Os- CCT1b (E523K)] did not display either a COP phenotype in darkness or hypersensitive light response in blue, red and far-red light. Scale bar, 1 cm. (b,c) Western blot analysis of rice plants expressing GUS-OsCCT1b mutants. (a) (b) (c) amino acids (OsCOP1D1 162) but containing the WD40 domain, which is demonstrated to be essential and sufficient to mediate AtCCT1-AtCOP1 interaction (Yang et al., 2001); and OsCRY1b (Figure 5b), and analysed the possible OsCCT1b OsCOP1 interaction in yeast cells. The OsCCT1b bait construct showed strong background, as seen by b-galactosidase activity in yeast cells co-expressing GUS (Figure 5c, sample 1). However, this activity increased dramatically when the OsCCT1b bait was co-expressed with the prey construct containing B42 AD joined to either Os- COP1D1 162 or AtCOP1 (Figure 5c, samples 2 and 3). Os- CNT1b did not interact with OsCOP1D1 162 (Figure 5c, sample 8). However, OsCNT1b interacted with OsCRY1b, indicating the capacity to mediate homodimerization of OsCRY1b. From these results we concluded that OsCCT1b interacts with OsCOP1 in yeast cells. Co-localization of OsCRY1b and OsCOP1 in plant cells With the demonstration that OsCRY1b interacted with OsCOP1 in yeast cells, we asked whether these two proteins are co-localized in plant cells. To examine this possibility, we transiently expressed OsCOP1 tagged with cyan fluorescent protein (CFP) and OsCRY1b tagged with yellow fluorescent protein (YFP), either individually or together, in onion epidermal cells. CFP and YFP served as negative controls, while CFP-AtCOP1 and AtCRY1-YFP fusions served as positive controls. As shown in Figure 6, the nuclear speckles were evident in onion cells co-expressing CFP-AtCOP1 and YFP (row 3) or CFP-OsCOP1 and YFP (row 5), whereas they were absent in those co-expressing CFP and AtCRY1-YFP (row 2) or CFP and OsCRY1b-YFP (row 4). However, as expected, when co-expressed with CFP-AtCOP1, AtCRY1-YFP resulted
8 978 Yan-Chun Zhang et al. Figure 4. GUS-OsCCT1b affects rice plasmid development. Electron micrograph showing a lack of prolamellar bodies and the presence of a parallel thylakoid membrane system in the rice GUS-OsCCT1b dark sample, not observed in the WT, dark control. Scale bar, 1 lm. in clear nuclear speckles (Figure 6, row 6). Consistent with this observation, OsCRY1b-YFP formed nuclear speckles when co-expressed with CFP-OsCOP1 (Figure 6, row 7). These data therefore indicated that OsCRY1b and OsCOP1 co-localized in the same nuclear bodies. Discussion OsCRY1b, GUS-OsCCT1a and GUS-OsCCT1b are functional in Arabidopsis To investigate the function and signaling mechanism of OsCRY1, we first overexpressed OsCRY1b in Arabidopsis cry1 mutant plants. Transgenic seedlings overexpressing OsCRY1b had significantly shortened hypocotyls and accumulated high levels of anthocyanin under blue light, whereas they exhibited a phenotype indistinguishable from WT Arabidopsis seedlings in the dark and in red and far-red light. These results are consistent with previous studies, in which transgenic Arabidopsis overexpressing either Arabidopsis CRY1 or rice CRY1 fused to GFP, and transgenic tobacco seedlings overexpressing Arabidopsis CRY1 were demonstrated to show the same blue light-dependent responses (Lin et al., 1995, 1996; Matsumoto et al., 2003). Therefore OsCRY1b functions like AtCRY1 in Arabidopsis. Our previous studies demonstrated that AtCCT (AtCCT1 and AtCCT2) fused to GUS mediates a COP phenotype in darkness (Yang et al., 2000). In the present study, we obtained transgenic Arabidopsis cry1 plants overexpressing either GUS-OsCCT1a or GUS-OsCCT1b that displayed the same COP phenotype in darkness. However, this phenotype was not observed for transgenic plants overexpressing GUS-OsCCT1b mutants containing either an E523K or a R585K mutation within OsCCT1b, which correspond to missense mutations E515K and R576K within AtCCT1 in hy4-19 and hy4-10 mutant alleles respectively. It was shown previously that transgenic plants overexpressing either AtCNT2- AtCCT1 or AtCNT1-AtCCT2 fusion proteins showed blue light-dependent responses, indicating that the domains of AtCRY1 and AtCRY2 are functionally interchangeable (Ahmad et al., 1998). These earlier results can be explained by previous findings that both CCT1 and CCT2 are able to interact with COP1 (Wang et al., 2001; Yang et al., 2001), and by our recent findings that both CNT1 and CNT2 are capable of mediating self-association (Sang et al., 2005). In the present study, transgenic Arabidopsis seedlings overexpressing OsCCT1b fused to the C-terminus of either AtCNT1 or AtCNT2 showed a blue light-dependent response similar (a) (c) (b) Figure 5. Interaction of OsCCT1b with OsCOP1 in yeast cells. (a) OsCCT1b and OsCNT1b bait constructs. All proteins are fusions with LexA DNA-binding domain (LexA) fused to OsCCT1b and OsCNT1b. OsCNT1b, OsCRY1b N- terminal domain. (b) Prey proteins. All proteins are fusions to the B42 activation domain. AtCOP1, Arabidopsis COP1; OsCOP1D (1 162), rice COP1 lacking amino acids from 1 to 162. (c) OsCCT1b interacts with OsCOP1. The interaction strength was determined by quantitative yeast two-hybrid interaction assay. All vector combinations are given as bait/prey. OsCCT1b/GUS, GUS/OsCRY1b, GUS/AtCOP1 and OsCNT1b/GUS are negative control pairs. OsCCT1b clearly interacted with OsCOP1D (1 162). OsCNT1b interacted with OsCRY1b, but did not interact with OsCOP1D (1 162). Twenty independent original transformants were analysed for each vector combination. Standard deviations indicated by error bars.
9 Rice CRYPTOCHROME 1 signaling 979 rice (Biswas et al., 2003; Thornton and Thimann, 1967; Wang and Iino, 1997), the photoreceptors responsible for this response have not been elucidated. We investigated the role of OsCRY1 in this process as follows. (1) Overexpression of OsCRY1b showed short coleoptile and leaf phenotypes in a blue light-dependent manner. (2) Transgenic rice seedlings expressing either OsCCT1a or OsCCT1b or full-length OsCRY1b fused to GUS, which was demonstrated to be capable of constitutively activating AtCCT in Arabidopsis (Sang et al., 2005; Yang et al., 2000), showed a COP phenotype in darkness, and short coleoptile and leaf phenotype in blue, red and far-red light. (3) The COP phenotype and the light-inhibition effects on coleoptile and leaf elongation were not observed for transgenic rice seedlings expressing GUS fused to mutants of OsCCT1b, indicating the constitutive light response observed for GUS-OsCCT1b was physiologically meaningful. However, to characterize further the OsCRY1 function we need to obtain the loss-of-function mutant of OsCRY1. As the sequence of OsCRY1a and OsCRY1b is highly identical, the function of these two genes is probably largely redundant, and the mutant phenotype might be observed only in the Oscry1a Oscry1b double mutant. Thus it might be very difficult to obtain either the Oscry1a or the Oscry1b mutant from phenotype-based mutant screening. In future studies we will make constructs expressing a variety of doublestranded RNAs in rice, sequences of which are complementary to the same regions of OsCRY1a and OsCRY1b, and overexpress them in WT rice, in the hope of interfering with the expression of both genes and obtaining the loss-of-function phenotype of OsCRY1. Figure 6. Co-localization of OsCRY1b and OsCOP1 in onion cells. Onion peels were co-bombarded with the DNA constructs indicated. Epidermal cells were imaged using CFP and YFP channels of a confocal microscope. Nuclear speckles were not observed for cells co-expressing either CFP and AtCRY1-YFP or CFP and OsCRY1b-YFP, whereas clearly present in cells coexpressing either CFP-AtCOP1 and AtCRY1-YFP; or CFP-OsCOP1 and OsCRY1b-YFP. Dic, differential interference contrast in light microscope mode. Scale bar, 10 lm. to seedlings expressing full-length OsCRY1b, suggesting that the fundamental role of the N-terminal domain of Arabidopsis CRY and rice CRY is probably the same. Taken together, these results suggest that the signaling mechanism of OsCRY1 might be analogous to that of AtCRY1 (see below). OsCRY1b mediates blue-light inhibition of coleoptile and leaf elongation during early seedling development in rice Although it has been demonstrated that blue light mediates inhibition of coleoptile elongation in oat, maize and The basic signaling mechanism of Arabidopsis and rice cryptochromes is conserved Insight into the signaling mechanism of AtCRY was obtained through the demonstration that transgenic plants expressing the C-terminal domain of either AtCRY1 (AtCCT1) or AtCRY2 (AtCCT2), fused to GUS, display a constitutive photomorphogenic phenotype (Yang et al., 2000) similar to that of mutants of both COP1 and COP9 signalosome (Deng and Quail, 1992; Wei et al., 1994a). Both AtCCT1 and AtCCT2 were shown to bind to COP1 (Wang et al., 2001; Yang et al., 2001), indicating that the signaling mechanism of AtCRY1 and AtCRY2 is mediated through negative regulation of COP1 by direct CRY COP1 interaction. Our recent studies demonstrated that the AtCRY1 N-terminal domain mediates homodimerization, which is required for light activation of CCT1, and that multimerization of GUS is both responsible and required for constitutive activation of AtCCT1 (Sang et al., 2005). We have established that the signaling mechanism of AtCRY1 and OsCRY1 is similar, as follows. (1) Transgenic
10 980 Yan-Chun Zhang et al. Arabidopsis seedlings expressing either GUS-OsCCT1a or GUS-OsCCT1b displayed a COP phenotype in darkness and a short-hypocotyl phenotype in blue, red and far-red light, reminiscent of the GUS-AtCCT and cop/det/fus mutant plants. (2) Both AtCNT1 and AtCNT2 fused to OsCCT1b were capable of playing the role of OsCNT1 in blue light-dependent activation of OsCRY1b photoreceptor activity. (3) Transgenic rice seedlings expressing either GUS-OsCCT1a or GUS-OsCCT1b or full-length OsCRY1b displayed a COP phenotype in darkness, and a short-coleoptile and leaf phenotype in blue, red and far-red light. (4) On fusion to GUS, OsCCT1b mutants were not capable of mediating a COP phenotype in either Arabidopsis or rice. (5) The chloroplast in GUS-OsCCT1b seedlings clearly developed under darkness, once again reminiscent of the chloroplastdevelopment phenotype observed for the GUS-AtCCT1 and cop/det/fus mutants. (6) OsCCT1b interacts with OsCOP1 in yeast cells. (7) OsCRY1b co-localized with OsCOP1 in living plant cells. These data suggest that, analogous to AtCRY1, the basic signaling mechanism of OsCRY1b is mediated through direct OsCCT1b OsCOP1 interaction. COP1 has been well characterized to serve as a master negative regulator of photomorphogenesis in Arabidopsis. In the rice genome, OsCOP1 is a single-copy gene (Tsuge et al., 2001). Its encoded protein has the same three signature domains of AtCOP1: the Ring-finger, coiled-coil and WD-40 repeat domains (Ang et al., 1998; Torii et al., 1998; Tsuge et al., 2001). However, to date, the role of OsCOP1 in photomorphogenic development in rice remains unknown. Based on the same COP phenotype observed here for transgenic Arabidopsis GUS-AtCCT (Yang et al., 2000) and GUS-OsCCT1 seedlings, and the similar signaling mechanism of AtCRY1 and OsCRY1, we speculate that the phenotype of the Oscop1 mutant might be analogous to that of the transgenic rice GUS-OsCCT1 plants. Thus OsCOP1 probably acts to constitutively promote coleoptile and leaf elongation, and to inhibit leaf expansion and plastid development during early seedling development in rice. Taken together, we have characterized the in vivo function and signaling mechanism of cryptochrome 1 in rice in the present study. Based on the data presented here, we conclude that OsCRY1 is involved in blue-light inhibition of coleoptile and leaf elongation during early rice seedling development, and that the signaling mechanism of OsCRY1 is mediated through physical interaction with OsCOP1. In future studies, major efforts will be made to obtain either Oscry1 mutant or transgenic rice lines expressing reduced or no endogenous OsCRY1 (OsCRY1a and OsCRY1b) through RNA interference. As soon as these lines are available, it will be possible to characterize further the role of OsCRY1 in photomorphogenesis and other important processes such as photoperiod response and entrainment of the circadian clock in rice. Experimental procedures Cloning of OsCRY1b and construction of expression cassettes Based on the sequence of OsCRY1b (accession AB073547), we obtained an OsCRY1b cdna fragment from nt , containing a native SalI site at the 5 end, by PCR using a rice cdna library DNA as template, and cloned it into SalI and BamHI sites of puc18, resulting in puc18-oscry1bd(1 467). The fragment from nucleotides was synthesized (Shanghai Sangon Co., Shanghai, China) and cloned into HindIII and SalI sites of puc18-oscry1bd(1 467). The full-length OsCRY1b was excised with HindIII and SacI and cloned into pbluescript SK(þ) (pbs). To make AtCNT-OsCCT1b fusions, a PCR-amplified OsCCT1b fragment was cloned into SpeI and SacI sites of pbs and then the PCR-amplified AtCNT1 and AtCNT2 fragments were cloned into SalI and SpeI sites of pbs-oscct1b respectively. To make GUS-OsCCT1a and GUS-CCT1b fusions, based on the sequence of OsCRY1a (accession AB073546), we obtained the OsCCT1a fragment by PCR using rice genomic DNA as template, and the PCR-amplified OsCCT1a and OsCCT1b fragments were cloned into SpeI and SacI sites of pbs-gus (Sang et al., 2005). The resulting construct, pbs-gus-oscct1b, was used as a template to prepare constructs encoding GUS-OsCCT1b (E523K) and GUS-OsCCT1b (R585K) mutants, using the Stratagene In Vitro Mutagenesis Kit. To make GUS-OsCRY1b fusions, the PCR-amplified OsCRY1b fragment was cloned into SpeI and SacI sites of pbs-gus. After confirmation by DNA sequencing, the fragments encoding OsCRY1b, GUS-OsCCT1a, GUS-OsCCT1b, GUS-OsCCT1b (E523K), GUS-OsCCT1b (R585K) and GUS-OsCRY1b were excised with HindIII and SacI and cloned into phb (Mao et al., 2005). The fragments encoding AtCNT1-OsCCT1b and AtCNT2-OsCCT1b were cut with SalI and SacI and cloned into pkyl71. Plant transformation and light source All constructs were transferred into Agrobacterium tumefaciens strain GV3101. The floral dip method was used to conduct transformation of Arabidopsis cry1 (hy4-104) mutant plants (Bruggemann et al., 1996). Homozygous T 4 seeds were used for phenotypic analysis. Arabidopsis seeds were sterilized with 20% bleach and placed in a 4 C refrigerator for 3 5 days, induced for germination in white light for 24 h, and finally transferred to an E-30 LED growth chamber (Percival, Boone, IA, USA) using blue diodes (k max 469 nm); the red diodes (k max 680 nm); or far-red diodes (k max 730 nm) at 22 C in continuous light. The fluence rate of these lights was 30 lmol sec )1 m )2. For rice transformation, all constructs were transferred into A. tumefaciens strain EHA105, and the tissue culture-based transformation method was used to conduct transformation of O. sativa cv. Nipponbare (Hiei et al., 1994). Homozygous T 3 seeds were used for phenotypic analysis. Dehusked caryopses were surface-sterilized for 2 min in 70% (v/v) ethanol and for 40 min in 20% (v/v) bleach. After washing with deionized water, the sterilized caryopses were sown on 0.8% agar Murashige and Skoog (MS) medium supplemented with 2% sucrose and placed in darkness at 30 C for 2 days to induce germination. The germinated seeds were then transferred to MS medium in mm clear acrylic containers (Magenta vessel GA-7 V8505, Sigma-Aldrich, St Louis, MO, USA) and placed in an E-30 LED growth chamber. The blue and red light fluence rate was 35 lmol sec )1 m )2 ; and the far-red light fluence rate was 25 lmol sec )1 m )2. Measurements of light spectra and fluence rates were as described previously (Sang et al., 2005).
11 Rice CRYPTOCHROME 1 signaling 981 OsCCT1b antibody production The PCR-amplified fragment encoding OsCCT1b (from amino acids ) of OsCRY1b was cloned into pet-21a (þ) and the resulting vector was transformed into Escherichia coli BL21 (DE3). Expression, purification and immunization of OsCCT1b were performed according to previously described procedures (Sang et al., 2005). The OsCCT1b antisera were analysed by Western blot using protein extracts prepared from OsCRY1b-ovx and WT plants. Protein-expressing studies Protein gel-blot analysis was performed as described previously, with minor modifications (Lin et al., 1996). Total protein was extracted from 6-day-old blue light-grown Arabidopsis seedlings or 11-day-old blue light-grown rice seedlings. Approximately 50 lg total protein, determined with the DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA), was fractionated in a 10% SDS PAGE minigel and electroblotted to a polyvinylidene difluoride membrane. The primary antibody was OsCCT1b. Detection of proteins was performed using the ECL PLUS kit (Amersham Biosciences, Piscataway, NJ, USA). The exposure time for enhanced chemiluminescence of different immunoblots was not controlled precisely, so the signal intensities from different immunoblots are not directly comparable. Electron microscopy Wild-type and transgenic GUS-OsCCT1b rice seedlings were grown in mm clear acrylic containers for 9 days in either dark or light. Seedlings were collected and then fixed, embedded and stained according to previously described procedures (Deng and Quail, 1992). The samples were examined in a Jeol-1230 electron microscope. Cloning of OsCOP1 cdna Based on the previously published OsCOP1 cdna sequence (Tsuge et al., 2001), we obtained an OsCOP1 cdna fragment encoding amino acids (OsCOP1D1 162) by PCR using a rice cdna library DNA as template. We failed to amplify the fragment encoding amino acids by PCR using either a rice cdna library DNA or the rice genomic DNA as template, possibly due to a high GC content (76%) in this region. We fused the fragment of AtCOP1 encoding amino acids to the fragment encoding OsCOP1D1 162, assuming that this chimeric COP1 functions as OsCOP1. We prepared a construct expressing this chimeric COP1, and expressed it in the Arabidopsis cop1-4 mutant (Deng and Quail, 1992). Phenotype analysis of the transgenic lines indicated a full capacity of this chimeric COP1 to rescue the COP phenotype (data not shown). Thus we referred OsCOP1 to this chimeric COP1 in this study. Construction of vectors for the LexA yeast two-hybrid system Bait constructs. plexa bait vectors expressing AtCCT1 were made previously (Yang et al., 2001). The PCR-amplified OsCCT1b and OsCNT1b fragments were cloned into EcoRI and XhoI sites of plexa. Both constructs used were confirmed by DNA sequencing. Prey constructs. Prey vectors expressing GUS and COP1 were made previously (Ang et al., 1998; Yang et al., 2001). The PCRamplified fragments of OsCRY1b and OsCOP1D1 162 were cloned into EcoRI and XhoI sites of the prey vector pjg4-5. Yeast two-hybrid assay. Yeast transformation and the calculation of relative b-galactosidase activities were as described previously (Yang et al., 2001). Because OsCCT1b had a strong background, we diluted the yeast culture tenfold before using CPRG and b-galactosidase units to quantify the strength of protein protein interaction. Subcellular co-localization study The CFP and YFP coding sequences were fused in-frame to the 5 end of AtCOP1 and OsCOP1, and to the 3 end of AtCRY1 and OsCRY1b, to generate CFP-AtCOP1, CFP-OsCOP1, AtCRY1-YFP and OsCRY1b-YFP fusions. Expression of these fusions was driven by the 35S promoter. Onion epidermal cells were bombarded with different combinations of plasmids using a particle gun-mediated system (Bio-Rad). Bombarded tissues were incubated in darkness for 24 h before visualization of transient expression using a confocal laser-scanning microscope (Carl Zeiss LSM 510) with a standard filter set. Acknowledgements We thank Z.Y. Wang and H.W. Xue for kindly providing us with the rice cdna library DNA template for PCR and the vector for particle bombardment, and S.P. Xu and J. Mao for technical assistance. This work was supported by the 863 Rice Functional Genomics Program from the Ministry of Science and Technology of China and grants from the National Natural Science Foundation of China to H.-Q.Y. ( , , ), the Chinese Academy of Sciences and the Shanghai Government. References Ahmad, M. and Cashmore, A.R. (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature, 366, Ahmad, M. and Cashmore, A.R. (1997) The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 11, Ahmad, M., Lin, C. and Cashmore, A.R. (1995) Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-lightresponsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 8, Ahmad, M., Jarillo, J.A. and Cashmore, A.R. (1998) Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell, 10, Ang, L.H., Chattopadhyay, S., Wei, N., Oyama, T., Okada, K., Batschauer, A. and Deng, X.W. (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell, 1, Biswas, K.K., Neumann, R., Haga, K., Yatoh, O. and Iino, M. (2003) Photomorphogenesis of rice seedlings: a mutant impaired in
12 982 Yan-Chun Zhang et al. phytochrome-mediated inhibition of coleoptile growth. Plant Cell Physiol. 44, Briggs, W.R. and Christie, J.M. (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 7, Bruggemann, E., Handwerger, K., Essex, C. and Storz, G. (1996) Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J. 10, Casal, J.J. and Mazzella, M.A. (1998) Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phya, phyb, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 118, Cashmore, A.R. (2003) Cryptochromes: enabling plants and animals to determine circadian time. Cell, 114, Cashmore, A.R., Jarillo, J.A., Wu, Y.J. and Liu, D. (1999) Cryptochromes: blue light receptors for plants and animals. Science, 284, Chory, J., Peto, C., Feinbaum, R., Pratt, L. and Ausubel, F. (1989) Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell, 58, Deng, X.W. and Quail, P.H. (1992) Genetic and phenotypic characterization of cop1 mutants of Arabidopsis thaliana. Plant J. 2, Deng, X.W., Caspar, T. and Quail, P.H. (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 5, Giliberto, L., Perrotta, G., Pallara, P., Weller, J.L., Fraser, P.D., Bramley, P.M., Fiore, A., Tavazza, M. and Giuliano, G. (2005) Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 137, Guo, H., Yang, H., Mockler, T.C. and Lin, C. (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science, 279, Hall, J.C. (2000) Cryptochromes: sensory reception, transduction, and clock functions subserving circadian systems. Curr. Opin. Neurobiol. 10, Hiei, Y., Ohta, S., Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, Imaizumi, T., Kanegae, T. and Wada, M. (2000) Cryptochrome nucleocytoplasmic distribution and gene expression are regulated by light quality in the fern Adiantum capillus-veneris. Plant Cell, 12, Imaizumi, T., Kadota, A., Hasebe, M. and Wada, M. (2002) Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell, 14, Kleine, T., Lockhart, P. and Batschauer, A. (2003) An Arabidopsis protein closely related to Synechocystis cryptochrome is targeted to organelles. Plant J. 35, Lin, C. (2002) Blue light receptors and signal transduction. Plant Cell, 14, S207 S225. Lin, C.T. and Shalitin, D. (2003) Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 54, Lin, C., Ahmad, M., Gordon, D. and Cashmore, A.R. (1995) Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc. Natl Acad. Sci. USA, 92, Lin, C., Ahmad, M. and Cashmore, A.R. (1996) Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J. 10, Lin, C., Yang, H., Guo, H., Mockler, T., Chen, J. and Cashmore, A.R. (1998) Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl Acad. Sci. USA, 95, Mao, J., Zhang, Y.C., Sang, Y., Li, Q.H. and Yang, H.Q. (2005) A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl Acad. Sci. USA, 102, Matsumoto, N., Hirano, T., Iwasaki, T. and Yamamoto, N. (2003) Functional analysis and intracellular localization of rice cryptochromes. Plant Physiol. 133, Misera, S., Muller, A.J., Weiland-Heidecker, U. and Jurgens, G. (1994) The FUSCA genes of Arabidopsis: negative regulators of light responses. Mol. Gen. Genet. 244, Mockler, T.C., Guo, H., Yang, H., Duong, H. and Lin, C. (1999) Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development, 126, Neff, M.M. and Chory, J. (1998) Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118, Ninu, L., Ahmad, M., Miarelli, C., Cashmore, A.R. and Giuliano, G. (1999) Cryptochrome 1 controls tomato development in response to blue light. Plant J. 18, Platten, J.D., Foo, E., Foucher, F., Hecht, V., Reid, J.B. and Weller, J.L. (2005) The cryptochrome gene family in pea includes two differentially expressed CRY2 genes. Plant Mol. Biol. 59, Quail, P.H. (2002) Photosensory perception and signalling in plant cells: new paradigms? Curr. Opin. Cell Biol. 14, Sancar, A. (1994) Structure and function of DNA photolyase. Biochemistry, 33, 2 9. Sang, Y., Li, Q.H., Rubio, V., Zhang, Y.C., Mao, J., Deng, X.W. and Yang, H.Q. (2005) N-terminal domain-mediated homodimerization is required for photoreceptor activity of Arabidopsis CRYPTOCHROME 1. Plant Cell, 17, Shalitin, D., Yu, X., Maymon, M., Mockler, T. and Lin, C. (2003) Blue light-dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell, 15, Somers, D.E., Devlin, P.F. and Kay, S.A. (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science, 282, Thornton, R.M. and Thimann, K.V. (1967) Transient effects of light on auxin transport in the Avena coleoptile. Plant Physiol. 111, Torii, K.U., McNellis, T.W. and Deng, X.W. (1998) Functional dissection of Arabidopsis COP1 reveals specific roles of its three structural modules in light control of seedling development. EMBO J. 17, Tsuge, T., Inagaki, N., Yoshizumi, T., Shimada, H., Kawamoto, T., Matsuki, R., Yamamoto, N. and Matsui, M. (2001) Phytochromemediated control of COP1 gene expression in rice plants. Mol. Genet. Genomics, 265, Wang, X. and Iino, M. (1997) Blue-light-induced shrinking of protoplasts from maize coleoptiles and its relationship to coleoptile growth. Plant Physiol. 114, Wang, H., Ma, L.G., Li, J.M., Zhao, H.Y. and Deng, X.W. (2001) Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science, 294, Wei, N., Chamovitz, D.A. and Deng, X.W. (1994a) Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell, 78, Wei, N., Kwok, S.F., von Arnim, A.G., Lee, A., McNellis, T.W., Piekos, B. and Deng, X.W. (1994b) Arabidopsis COP8, COP10, and COP11
Figure 18.1 Blue-light stimulated phototropism Blue light Inhibits seedling hypocotyl elongation
 Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Analysis of regulatory function of circadian clock. on photoreceptor gene expression
 Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Life Science Journal 2014;11(9) Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis
 Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis Sung-Il Kim 1, Sang Ik Song 3, Hak Soo Seo 1, 2, 4 * 1 Department of Plant Science and Research Institute of Agriculture
Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis Sung-Il Kim 1, Sang Ik Song 3, Hak Soo Seo 1, 2, 4 * 1 Department of Plant Science and Research Institute of Agriculture
Review. 1 Cryptochrome Signaling in Plants ABSTRACT INTRODUCTION
 P H P 0 0 4 B Dispatch: 14.12.06 Journal: PHP CE: Saranraj Journal Name Manuscript No. Author Received: No. of pages: 8 PE: Saravanan Photochemistry and Photobiology, 2007, 83: 1 8 Review 1 Cryptochrome
P H P 0 0 4 B Dispatch: 14.12.06 Journal: PHP CE: Saranraj Journal Name Manuscript No. Author Received: No. of pages: 8 PE: Saravanan Photochemistry and Photobiology, 2007, 83: 1 8 Review 1 Cryptochrome
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Blue Light Receptors and Signal Transduction
 The Plant Cell, S207 S225, Supplement 2002, www.plantcell.org 2002 American Society of Plant Biologists Blue Light Receptors and Signal Transduction Chentao Lin 1 Department of Molecular, Cell and Developmental
The Plant Cell, S207 S225, Supplement 2002, www.plantcell.org 2002 American Society of Plant Biologists Blue Light Receptors and Signal Transduction Chentao Lin 1 Department of Molecular, Cell and Developmental
Involvement of Rice Cryptochromes in De-etiolation Responses and Flowering
 Plant Cell Physiol. 47(7): 915 925 (2006) doi:10.1093/pcp/pcj064, available online at www.pcp.oxfordjournals.org ß The Author 2006. Published by Oxford University Press on behalf of Japanese Society of
Plant Cell Physiol. 47(7): 915 925 (2006) doi:10.1093/pcp/pcj064, available online at www.pcp.oxfordjournals.org ß The Author 2006. Published by Oxford University Press on behalf of Japanese Society of
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING. AnitaHajdu. Thesis of the Ph.D.
 THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
Light Regulation of Flowering Time in Arabidopsis
 Chapter 38 Light Regulation of Flowering Time in Arabidopsis Xuhong Yu and Chentao Lin Introduction Plant development is dependent on not only endogenous conditions but also environmental factors. One
Chapter 38 Light Regulation of Flowering Time in Arabidopsis Xuhong Yu and Chentao Lin Introduction Plant development is dependent on not only endogenous conditions but also environmental factors. One
Electromagenetic spectrum
 Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in Arabidopsis W
 The Plant Cell, Vol. 21: 2624 2641, September 2009, www.plantcell.org ã 2009 American Society of Plant Biologists Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in
The Plant Cell, Vol. 21: 2624 2641, September 2009, www.plantcell.org ã 2009 American Society of Plant Biologists Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in
Red-Light-Dependent Interaction of phyb with SPA1 Promotes COP1 SPA1 Dissociation and Photomorphogenic Development in Arabidopsis
 Research Article Red-Light-Dependent Interaction of phyb with SPA1 Promotes COP1 SPA1 Dissociation and Photomorphogenic Development in Arabidopsis Xue-Dan Lu 1, Chuan-Miao Zhou 2, Peng-Bo Xu 3, Qian Luo
Research Article Red-Light-Dependent Interaction of phyb with SPA1 Promotes COP1 SPA1 Dissociation and Photomorphogenic Development in Arabidopsis Xue-Dan Lu 1, Chuan-Miao Zhou 2, Peng-Bo Xu 3, Qian Luo
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Blue light affects many aspects of plant growth and development.
 Plant blue-light receptors Chentao Lin Plants have several blue-light receptors, which regulate different aspects of growth and development. Recent studies have identified three such receptors: cryptochrome
Plant blue-light receptors Chentao Lin Plants have several blue-light receptors, which regulate different aspects of growth and development. Recent studies have identified three such receptors: cryptochrome
Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang Chen, Qiang Dong, Jiangqi Wen, Kirankumar S. Mysore and Jian Zhao
 New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
Arabidopsis CONSTANS-LIKE3 Is a Positive Regulator of Red Light Signaling and Root Growth W
 The Plant Cell, Vol. 18, 70 84, January 2006, www.plantcell.org ª 2005 American Society of Plant Biologists Arabidopsis CONSTANS-LIKE3 Is a Positive Regulator of Red Light Signaling and Root Growth W Sourav
The Plant Cell, Vol. 18, 70 84, January 2006, www.plantcell.org ª 2005 American Society of Plant Biologists Arabidopsis CONSTANS-LIKE3 Is a Positive Regulator of Red Light Signaling and Root Growth W Sourav
Report. Blue Light-Dependent Interaction of CRY2 with SPA1 Regulates COP1 activity and Floral Initiation in Arabidopsis
 Current Biology 21, 841 847, May 24, 2011 ª2011 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2011.03.048 Blue Light-Dependent Interaction of CRY2 with SPA1 Regulates COP1 activity and Floral Initiation
Current Biology 21, 841 847, May 24, 2011 ª2011 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2011.03.048 Blue Light-Dependent Interaction of CRY2 with SPA1 Regulates COP1 activity and Floral Initiation
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
Author Manuscript Faculty of Biology and Medicine Publication
 Serveur Académique Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections
Serveur Académique Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections
CBMG688R. ADVANCED PLANT DEVELOPMENT AND PHYSIOLOGY II G. Deitzer Spring 2006 LECTURE
 1 CBMG688R. ADVANCED PLANT DEVELOPMENT AND PHYSIOLOGY II G. Deitzer Spring 2006 LECTURE Photomorphogenesis and Light Signaling Photoregulation 1. Light Quantity 2. Light Quality 3. Light Duration 4. Light
1 CBMG688R. ADVANCED PLANT DEVELOPMENT AND PHYSIOLOGY II G. Deitzer Spring 2006 LECTURE Photomorphogenesis and Light Signaling Photoregulation 1. Light Quantity 2. Light Quality 3. Light Duration 4. Light
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Arabidopsis thaliana. Lucia Strader. Assistant Professor, Biology
 Arabidopsis thaliana Lucia Strader Assistant Professor, Biology Arabidopsis as a genetic model Easy to grow Small genome Short life cycle Self fertile Produces many progeny Easily transformed HIV E. coli
Arabidopsis thaliana Lucia Strader Assistant Professor, Biology Arabidopsis as a genetic model Easy to grow Small genome Short life cycle Self fertile Produces many progeny Easily transformed HIV E. coli
LECTURE 4: PHOTOTROPISM
 http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ LECTURE 4: PHOTOTROPISM LECTURE FLOW 1. 2. 3. 4. 5. INTRODUCTION DEFINITION INITIAL STUDY PHOTROPISM MECHANISM PHOTORECEPTORS
http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ LECTURE 4: PHOTOTROPISM LECTURE FLOW 1. 2. 3. 4. 5. INTRODUCTION DEFINITION INITIAL STUDY PHOTROPISM MECHANISM PHOTORECEPTORS
The role of the N-terminal NTE domain of PHYTOCHROMEs in red and far red light perception
 The role of the N-terminal NTE domain of PHYTOCHROMEs in red and far red light perception Theses of the Ph.D. dissertation János Bindics Supervisor: Dr. Ferenc Nagy Hungarian Academy of Sciences Biological
The role of the N-terminal NTE domain of PHYTOCHROMEs in red and far red light perception Theses of the Ph.D. dissertation János Bindics Supervisor: Dr. Ferenc Nagy Hungarian Academy of Sciences Biological
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
Summary. Introduction
 The Plant Journal (1998) 15(1), 69 77 Combinatorial interaction of light-responsive elements plays a critical role in determining the response characteristics of light-regulated promoters in Arabidopsis
The Plant Journal (1998) 15(1), 69 77 Combinatorial interaction of light-responsive elements plays a critical role in determining the response characteristics of light-regulated promoters in Arabidopsis
Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction
 Development 126, 73-82 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV214 73 Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction
Development 126, 73-82 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV214 73 Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction
BLUE-LIGHT PHOTORECEPTORS
 Annu. Rev. Cell Dev. Biol. 1999.15:33-62. Downloaded from arjournals.annualreviews.org?annu. Rev. Cell Dev. Biol. 1999. 15:33 62 Copyright c 1999 by Annual Reviews. All rights reserved Winslow R. Briggs
Annu. Rev. Cell Dev. Biol. 1999.15:33-62. Downloaded from arjournals.annualreviews.org?annu. Rev. Cell Dev. Biol. 1999. 15:33 62 Copyright c 1999 by Annual Reviews. All rights reserved Winslow R. Briggs
Cryptochromes Are Required for Phytochrome Signaling to the Circadian Clock but Not for Rhythmicity
 The Plant Cell, Vol. 12, 2499 2509, December 2000, www.plantcell.org 2000 American Society of Plant Physiologists Cryptochromes Are Required for Phytochrome Signaling to the Circadian Clock but Not for
The Plant Cell, Vol. 12, 2499 2509, December 2000, www.plantcell.org 2000 American Society of Plant Physiologists Cryptochromes Are Required for Phytochrome Signaling to the Circadian Clock but Not for
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Nature Genetics: doi: /ng Supplementary Figure 1. ssp mutant phenotypes in a functional SP background.
 Supplementary Figure 1 ssp mutant phenotypes in a functional SP background. (a,b) Statistical comparisons of primary and sympodial shoot flowering times as determined by mean values for leaf number on
Supplementary Figure 1 ssp mutant phenotypes in a functional SP background. (a,b) Statistical comparisons of primary and sympodial shoot flowering times as determined by mean values for leaf number on
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
PSEUDO RESPONSE REGULATORs stabilize CONSTANS protein to promote flowering in response to day length
 Published online: March 7, 27 Article PSEUDO RESPONSE REGULATORs stabilize NSTANS protein to promote flowering in response to day length Ryosuke Hayama, Liron Sarid-Krebs, René Richter, Virginia Fernández,
Published online: March 7, 27 Article PSEUDO RESPONSE REGULATORs stabilize NSTANS protein to promote flowering in response to day length Ryosuke Hayama, Liron Sarid-Krebs, René Richter, Virginia Fernández,
Phytochrome A Regulates the Intracellular Distribution of Phototropin 1 Green Fluorescent Protein in Arabidopsis thaliana W
 The Plant Cell, Vol. 20: 2835 2847, October 2008, www.plantcell.org ã 2008 American Society of Plant Biologists Phytochrome A Regulates the Intracellular Distribution of Phototropin 1 Green Fluorescent
The Plant Cell, Vol. 20: 2835 2847, October 2008, www.plantcell.org ã 2008 American Society of Plant Biologists Phytochrome A Regulates the Intracellular Distribution of Phototropin 1 Green Fluorescent
Supplemental material
 Supplemental material Table 1- Segregation analysis of sgt1a sgt1b double mutant plants Parental genotype No. of lines Normal seeds Phenotype Abnormal seeds Ratio of abnormal seeds (%) WT 3 171 2 1.16
Supplemental material Table 1- Segregation analysis of sgt1a sgt1b double mutant plants Parental genotype No. of lines Normal seeds Phenotype Abnormal seeds Ratio of abnormal seeds (%) WT 3 171 2 1.16
Synergistic and Antagonistic Action of Phytochrome (Phy) A and PhyB during Seedling De-Etiolation in Arabidopsis thaliana
 Int. J. Mol. Sci. 2015, 16, 12199-12212; doi:10.3390/ijms160612199 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Synergistic and Antagonistic
Int. J. Mol. Sci. 2015, 16, 12199-12212; doi:10.3390/ijms160612199 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Synergistic and Antagonistic
Positive Regulation of Phytochrome B on Chlorophyll Biosynthesis and Chloroplast Development in Rice
 Rice Science, 2013, 20(4): 243 248 Copyright 2013, China National Rice Research Institute Published by Elsevier BV. All rights reserved DOI: 10.1016/S1672-6308(13)60133-X Positive Regulation of Phytochrome
Rice Science, 2013, 20(4): 243 248 Copyright 2013, China National Rice Research Institute Published by Elsevier BV. All rights reserved DOI: 10.1016/S1672-6308(13)60133-X Positive Regulation of Phytochrome
A Genome-Wide Analysis of Arabidopsis Rop-Interactive CRIB Motif Containing Proteins That Act as Rop GTPase Targets
 The Plant Cell, Vol. 13, 2841 2856, December 2001, www.plantcell.org 2001 American Society of Plant Biologists A Genome-Wide Analysis of Arabidopsis Rop-Interactive CRIB Motif Containing Proteins That
The Plant Cell, Vol. 13, 2841 2856, December 2001, www.plantcell.org 2001 American Society of Plant Biologists A Genome-Wide Analysis of Arabidopsis Rop-Interactive CRIB Motif Containing Proteins That
Leaf Positioning of Arabidopsis in Response to Blue Light
 Molecular Plant Volume 1 Number 1 Pages 15 26 January 2008 Leaf Positioning of Arabidopsis in Response to Blue Light Shin-ichiro Inoue a, Toshinori Kinoshita a,2, Atsushi Takemiya a, Michio Doi b and Ken-ichiro
Molecular Plant Volume 1 Number 1 Pages 15 26 January 2008 Leaf Positioning of Arabidopsis in Response to Blue Light Shin-ichiro Inoue a, Toshinori Kinoshita a,2, Atsushi Takemiya a, Michio Doi b and Ken-ichiro
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Light-Independent Phytochrome Signaling Mediated by Dominant GAF Domain Tyrosine Mutants of Arabidopsis Phytochromes in Transgenic Plants W OA
 The Plant Cell, Vol. 19: 2124 2139, July 2007, www.plantcell.org ª 2007 American Society of Plant Biologists Light-Independent Phytochrome Signaling Mediated by Dominant GAF Domain Tyrosine Mutants of
The Plant Cell, Vol. 19: 2124 2139, July 2007, www.plantcell.org ª 2007 American Society of Plant Biologists Light-Independent Phytochrome Signaling Mediated by Dominant GAF Domain Tyrosine Mutants of
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Supplemental Data. Gao et al. (2012). Plant Cell /tpc
 Supplemental Figure 1. Plant EMP Proteins. (A) The Accession numbers of the 12 EMP members from Arabidopsis. (B) Phylogenetic analysis of EMP proteins from Arabidopsis, human and yeast using the Mac Vector
Supplemental Figure 1. Plant EMP Proteins. (A) The Accession numbers of the 12 EMP members from Arabidopsis. (B) Phylogenetic analysis of EMP proteins from Arabidopsis, human and yeast using the Mac Vector
Supplemental Data. Perea-Resa et al. Plant Cell. (2012) /tpc
 Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Small RNA in rice genome
 Vol. 45 No. 5 SCIENCE IN CHINA (Series C) October 2002 Small RNA in rice genome WANG Kai ( 1, ZHU Xiaopeng ( 2, ZHONG Lan ( 1,3 & CHEN Runsheng ( 1,2 1. Beijing Genomics Institute/Center of Genomics and
Vol. 45 No. 5 SCIENCE IN CHINA (Series C) October 2002 Small RNA in rice genome WANG Kai ( 1, ZHU Xiaopeng ( 2, ZHONG Lan ( 1,3 & CHEN Runsheng ( 1,2 1. Beijing Genomics Institute/Center of Genomics and
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Intracellular trafficking of photoreceptors during lightinduced signal transduction in plants
 COMMENTARY 475 Intracellular trafficking of photoreceptors during lightinduced signal transduction in plants Ferenc Nagy 1,2, Stefan Kircher 3 and Eberhard Schäfer 3, * 1 Plant Biology Institute, Biological
COMMENTARY 475 Intracellular trafficking of photoreceptors during lightinduced signal transduction in plants Ferenc Nagy 1,2, Stefan Kircher 3 and Eberhard Schäfer 3, * 1 Plant Biology Institute, Biological
Arabidopsis SHORT HYPOCOTYL UNDER BLUE1 Contains SPX and EXS Domains and Acts in Cryptochrome Signaling W
 The Plant Cell, Vol. 18, 921 934, April 2006, www.plantcell.org ª 2006 American Society of Plant Biologists Arabidopsis SHORT HYPOCOTYL UNDER BLUE1 Contains SPX and EXS Domains and Acts in Cryptochrome
The Plant Cell, Vol. 18, 921 934, April 2006, www.plantcell.org ª 2006 American Society of Plant Biologists Arabidopsis SHORT HYPOCOTYL UNDER BLUE1 Contains SPX and EXS Domains and Acts in Cryptochrome
Photoperiodic flowering in plants was the first photoperiodism
 Regulation of photoperiodic flowering by Arabidopsis photoreceptors Todd Mockler*, Hongyun Yang, XuHong Yu, Dhavan Parikh, Ying-chia Cheng, Sarah Dolan, and Chentao Lin Department of Molecular, Cell, and
Regulation of photoperiodic flowering by Arabidopsis photoreceptors Todd Mockler*, Hongyun Yang, XuHong Yu, Dhavan Parikh, Ying-chia Cheng, Sarah Dolan, and Chentao Lin Department of Molecular, Cell, and
The photomorphogenic repressors COP1 and DET1: 20 years later
 Review The photomorphogenic repressors and DET1: 20 years later On Sun Lau 1,2 and Xing Wang Deng 1 1 Department of Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06520-8104,
Review The photomorphogenic repressors and DET1: 20 years later On Sun Lau 1,2 and Xing Wang Deng 1 1 Department of Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06520-8104,
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Breker et al., http://www.jcb.org/cgi/content/full/jcb.201301120/dc1 Figure S1. Single-cell proteomics of stress responses. (a) Using
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Breker et al., http://www.jcb.org/cgi/content/full/jcb.201301120/dc1 Figure S1. Single-cell proteomics of stress responses. (a) Using
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Last time: Obtaining information from a cloned gene
 Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
SALT TOLERANCE HOMOLOG2, a B-Box Protein in Arabidopsis That Activates Transcription and Positively Regulates Light-Mediated Development W
 The Plant Cell, Vol. 19: 3242 3255, October 2007, www.plantcell.org ª 2007 American Society of Plant Biologists SALT TOLERANCE HOMOLOG2, a B-Box Protein in Arabidopsis That Activates Transcription and
The Plant Cell, Vol. 19: 3242 3255, October 2007, www.plantcell.org ª 2007 American Society of Plant Biologists SALT TOLERANCE HOMOLOG2, a B-Box Protein in Arabidopsis That Activates Transcription and
Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011) /tpc
 Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011). 10.1105/tpc.110.080788 Supplemental Figure S1. Phylogenetic tree of MYC2-related proteins from Arabidopsis and other plants. Phenogram representation
Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011). 10.1105/tpc.110.080788 Supplemental Figure S1. Phylogenetic tree of MYC2-related proteins from Arabidopsis and other plants. Phenogram representation
Phytochrome E Influences Internode Elongation and Flowering Time in Arabidopsis
 The Plant Cell, Vol. 10, 1479 1487, September 1998, www.plantcell.org 1998 American Society of Plant Physiologists Phytochrome E Influences Internode Elongation and Flowering Time in Arabidopsis Paul F.
The Plant Cell, Vol. 10, 1479 1487, September 1998, www.plantcell.org 1998 American Society of Plant Physiologists Phytochrome E Influences Internode Elongation and Flowering Time in Arabidopsis Paul F.
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development
 A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
Flower Development Pathways
 Developmental Leading to Flowering Flower Development s meristem Inflorescence meristem meristems organ identity genes Flower development s to Flowering Multiple pathways ensures flowering will take place
Developmental Leading to Flowering Flower Development s meristem Inflorescence meristem meristems organ identity genes Flower development s to Flowering Multiple pathways ensures flowering will take place
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
2. Yeast two-hybrid system
 2. Yeast two-hybrid system I. Process workflow a. Mating of haploid two-hybrid strains on YPD plates b. Replica-plating of diploids on selective plates c. Two-hydrid experiment plating on selective plates
2. Yeast two-hybrid system I. Process workflow a. Mating of haploid two-hybrid strains on YPD plates b. Replica-plating of diploids on selective plates c. Two-hydrid experiment plating on selective plates
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Development 143: doi: /dev : Supplementary information
 Supplementary Materials and Methods Plant materials The mutants and transgenic plants used in the present study were as follows: E361 (from Alex Webb s laboratory); tmm-1, ptmm::tmm-gfp and flp-1 (from
Supplementary Materials and Methods Plant materials The mutants and transgenic plants used in the present study were as follows: E361 (from Alex Webb s laboratory); tmm-1, ptmm::tmm-gfp and flp-1 (from
15. PHOTOPERIODISM. 1. Short day plants
 15. PHOTOPERIODISM Photoperiodism is the phenomenon of physiological changes that occur in plants in response to relative length of day and night (i.e. photoperiod). The response of the plants to the photoperiod,
15. PHOTOPERIODISM Photoperiodism is the phenomenon of physiological changes that occur in plants in response to relative length of day and night (i.e. photoperiod). The response of the plants to the photoperiod,
The signal transducing photoreceptors of plants
 Int. J. Dev. Biol. 49: 653-664 (2005) doi: 10.1387/ijdb.051989kf The signal transducing photoreceptors of plants KEARA A. FRANKLIN*, VICTORIA S. LARNER and GARRY C. WHITELAM Department of Biology, University
Int. J. Dev. Biol. 49: 653-664 (2005) doi: 10.1387/ijdb.051989kf The signal transducing photoreceptors of plants KEARA A. FRANKLIN*, VICTORIA S. LARNER and GARRY C. WHITELAM Department of Biology, University
Repression of light signaling by Arabidopsis SPA1 involves post-translational regulation of HFR1 protein accumulation
 The Plant Journal (2005) 43, 131 141 doi: 10.1111/j.1365-313X.2005.02433.x Repression of light signaling by Arabidopsis SPA1 involves post-translational regulation of HFR1 protein accumulation Jianping
The Plant Journal (2005) 43, 131 141 doi: 10.1111/j.1365-313X.2005.02433.x Repression of light signaling by Arabidopsis SPA1 involves post-translational regulation of HFR1 protein accumulation Jianping
EMBO. Phytochrome-mediated photoperception and signal transduction in higher plants. reports. Eberhard Schäfer & Chris Bowler 1,+ Introduction
 EMBO reports Phytochrome-mediated photoperception and signal transduction in higher plants Eberhard Schäfer & Chris Bowler 1,+ Universitat Freiburg, Institut fur Biologie II/Botanik, Schanzlestrasse 1,
EMBO reports Phytochrome-mediated photoperception and signal transduction in higher plants Eberhard Schäfer & Chris Bowler 1,+ Universitat Freiburg, Institut fur Biologie II/Botanik, Schanzlestrasse 1,
The UV-B Photoreceptor UVR8: From Structure to Physiology
 The Plant Cell, Vol. 26: 21 37, January 2014, www.plantcell.org ã 2014 American Society of Plant Biologists. All rights reserved. REVIEW The UV-B Photoreceptor UVR8: From Structure to Physiology Gareth
The Plant Cell, Vol. 26: 21 37, January 2014, www.plantcell.org ã 2014 American Society of Plant Biologists. All rights reserved. REVIEW The UV-B Photoreceptor UVR8: From Structure to Physiology Gareth
Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development
 Development 128, 2291-2299 (21) Printed in Great Britain The Company of Biologists Limited 21 DEV361 2291 Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation
Development 128, 2291-2299 (21) Printed in Great Britain The Company of Biologists Limited 21 DEV361 2291 Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation
Flowering Time Control in Plants -How plants know the time to flower?
 Advanced Molecular and Cell Biology II, 2015/12/04 Flowering Time Control in Plants -How plants know the time to flower? Masaki NIWA Grad. Sch. Biostudies, Kyoto Univ. Why can plants bloom every year in
Advanced Molecular and Cell Biology II, 2015/12/04 Flowering Time Control in Plants -How plants know the time to flower? Masaki NIWA Grad. Sch. Biostudies, Kyoto Univ. Why can plants bloom every year in
Supplemental Data. Yamamoto et al. (2016). Plant Cell /tpc WT + HCO 3. slac1-4/δnc #5 + HCO 3 WT + ABA. slac1-4/δnc #5 + ABA
 I (pa) I (pa) A WT + HCO 3 B 2 V (mv) 15 1 5 5 2 slac14/δnc #5 + HCO 3 4 6 WT (n = 6) 8 WT + HCO 3 (n = 7) slac14/δnc #5 (n = 6) slac14/δnc #5 + HCO 3 (n = 1) C WT + ABA D 2 V (mv) 15 1 5 5 2 slac14/δnc
I (pa) I (pa) A WT + HCO 3 B 2 V (mv) 15 1 5 5 2 slac14/δnc #5 + HCO 3 4 6 WT (n = 6) 8 WT + HCO 3 (n = 7) slac14/δnc #5 (n = 6) slac14/δnc #5 + HCO 3 (n = 1) C WT + ABA D 2 V (mv) 15 1 5 5 2 slac14/δnc
LIGHT SIGNAL TRANSDUCTION IN HIGHER PLANTS
 Annu. Rev. Genet. 2004. 38:87 117 doi: 10.1146/annurev.genet.38.072902.092259 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on June 11, 2004 LIGHT
Annu. Rev. Genet. 2004. 38:87 117 doi: 10.1146/annurev.genet.38.072902.092259 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on June 11, 2004 LIGHT
Model plants and their Role in genetic manipulation. Mitesh Shrestha
 Model plants and their Role in genetic manipulation Mitesh Shrestha Definition of Model Organism Specific species or organism Extensively studied in research laboratories Advance our understanding of Cellular
Model plants and their Role in genetic manipulation Mitesh Shrestha Definition of Model Organism Specific species or organism Extensively studied in research laboratories Advance our understanding of Cellular
Nucleo-cytoplasmic partitioning of the plant photoreceptors phytochromes
 seminars in CELL & DEVELOPMENTAL BIOLOGY, Vol. 11, 2000: pp. 505 510 doi: 10.1006/scdb.2000.0202, available online at http://www.idealibrary.com on Nucleo-cytoplasmic partitioning of the plant photoreceptors
seminars in CELL & DEVELOPMENTAL BIOLOGY, Vol. 11, 2000: pp. 505 510 doi: 10.1006/scdb.2000.0202, available online at http://www.idealibrary.com on Nucleo-cytoplasmic partitioning of the plant photoreceptors
Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells
 Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells By: Patrick Rutledge 1 Dr. Jennifer Lorang 2,3, Dr. Marc Curtis 2,3, Dr. Thomas Wolpert 2,3 BioResource Research 1, Botany and
Looking for LOV: Location of LOV1 function in Nicotiana benthamiana cells By: Patrick Rutledge 1 Dr. Jennifer Lorang 2,3, Dr. Marc Curtis 2,3, Dr. Thomas Wolpert 2,3 BioResource Research 1, Botany and
Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues
 Research Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues Daniel Kirchenbauer 1 *, Andras Viczian 2 *, Eva Adam 2, Zoltan
Research Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues Daniel Kirchenbauer 1 *, Andras Viczian 2 *, Eva Adam 2, Zoltan
Expression Patterns of OsPIL11, a Phytochrome-Interacting Factor in Rice, and Preliminary Analysis of Its Roles in Light Signal Transduction
 Rice Science, 2012, 19(4): 263 268 Copyright 2012, China National Rice Research Institute Published by Elsevier BV. All rights reserved Expression Patterns of OsPIL11, a Phytochrome-Interacting Factor
Rice Science, 2012, 19(4): 263 268 Copyright 2012, China National Rice Research Institute Published by Elsevier BV. All rights reserved Expression Patterns of OsPIL11, a Phytochrome-Interacting Factor
Molecular Genetics of. Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS
 Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Arabidopsis COP8, COPIO, and COPII Genes Are lnvolved in Repression of Photomorphogenic Development in Darkness
 The Plant Cell, Vol. 6, 629-643, May 1994 O 1994 American Society of Plant Physiologists Arabidopsis COP8, COPIO, and COPII Genes Are lnvolved in Repression of Photomorphogenic Development in Darkness
The Plant Cell, Vol. 6, 629-643, May 1994 O 1994 American Society of Plant Physiologists Arabidopsis COP8, COPIO, and COPII Genes Are lnvolved in Repression of Photomorphogenic Development in Darkness
Marcelo J. Yanovsky and Steve A. Kay
 LIVING BY THE CALENDAR: HOW PLANTS KNOW WHEN TO FLOWER Marcelo J. Yanovsky and Steve A. Kay Reproductive processes in plants and animals are usually synchronized with favourable seasons of the year. It
LIVING BY THE CALENDAR: HOW PLANTS KNOW WHEN TO FLOWER Marcelo J. Yanovsky and Steve A. Kay Reproductive processes in plants and animals are usually synchronized with favourable seasons of the year. It
Phytochromes are Involved in Elongation of Seminal Roots and Accumulation of Dry substances in Rice Seedlings
 Rice Science, 2013, 20(1): Copyright 2012, China National Rice Research Institute Published by Elsevier BV. All rights reserved Phytochromes are Involved in Elongation of Seminal Roots and Accumulation
Rice Science, 2013, 20(1): Copyright 2012, China National Rice Research Institute Published by Elsevier BV. All rights reserved Phytochromes are Involved in Elongation of Seminal Roots and Accumulation
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Phytochromes are Involved in Elongation of Seminal Roots and Accumulation of Dry Substances in Rice Seedlings
 Rice Science, 2013, 20(2): 88 94 Copyright 2013, China National Rice Research Institute Published by Elsevier BV. All rights reserved DOI: 10.1016/S1672-6308(13)60115-8 Phytochromes are Involved in Elongation
Rice Science, 2013, 20(2): 88 94 Copyright 2013, China National Rice Research Institute Published by Elsevier BV. All rights reserved DOI: 10.1016/S1672-6308(13)60115-8 Phytochromes are Involved in Elongation
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Photomorphogenesis in Plants and Bacteria 3rd Edition
 Photomorphogenesis in Plants and Bacteria 3rd Edition Function and Signal Transduction Mechanisms Eberhard Schäfer and Ferenc Nagy (Eds.) PHOTOMORPHOGENESIS IN PLANTS AND BACTERIA 3RD EDITION Photomorphogenesis
Photomorphogenesis in Plants and Bacteria 3rd Edition Function and Signal Transduction Mechanisms Eberhard Schäfer and Ferenc Nagy (Eds.) PHOTOMORPHOGENESIS IN PLANTS AND BACTERIA 3RD EDITION Photomorphogenesis
Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport
 Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
GFP WxTP-GFP WxTP-GFP. stromules. DsRed WxTP-DsRed WxTP-DsRed. stromules
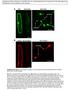 Supplemental Data. Kitajima et al. (2009). The rice -amylase glycoprotein is targeted from the Golgi apparatus through the secretory pathway to the plastids. A GFP WxTP-GFP WxTP-GFP stromules stromules
Supplemental Data. Kitajima et al. (2009). The rice -amylase glycoprotein is targeted from the Golgi apparatus through the secretory pathway to the plastids. A GFP WxTP-GFP WxTP-GFP stromules stromules
The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses
 The Plant Journal (2007) 51, 862 873 doi: 10.1111/j.1365-313X.2007.03187.x The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses Sam-Geun Kong 1,, Toshinori
The Plant Journal (2007) 51, 862 873 doi: 10.1111/j.1365-313X.2007.03187.x The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses Sam-Geun Kong 1,, Toshinori
Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability
 Access the Development most First recent posted version epress online at http://dev.biologists.org/lookup/doi/10.1242/dev.02481 online on 19 July publication 2006 as 10.1242/dev.02481 date 19 July 2006
Access the Development most First recent posted version epress online at http://dev.biologists.org/lookup/doi/10.1242/dev.02481 online on 19 July publication 2006 as 10.1242/dev.02481 date 19 July 2006
** * * * Col-0 cau1 CAU1. Actin2 CAS. Actin2. Supplemental Figure 1. CAU1 affects calcium accumulation.
 Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Functions of Phytochrome in Rice Growth and Development
 Rice Science, 2011, 18(3): 231 237 Copyright 2011, China National Rice Research Institute Published by Elsevier BV. All rights reserved Functions of Phytochrome in Rice Growth and Development GU Jian-wei
Rice Science, 2011, 18(3): 231 237 Copyright 2011, China National Rice Research Institute Published by Elsevier BV. All rights reserved Functions of Phytochrome in Rice Growth and Development GU Jian-wei
