Phytochrome A Regulates the Intracellular Distribution of Phototropin 1 Green Fluorescent Protein in Arabidopsis thaliana W
|
|
|
- May Rodgers
- 5 years ago
- Views:
Transcription
1 The Plant Cell, Vol. 20: , October 2008, ã 2008 American Society of Plant Biologists Phytochrome A Regulates the Intracellular Distribution of Phototropin 1 Green Fluorescent Protein in Arabidopsis thaliana W In-Seob Han, a,1 Tong-Seung Tseng, b,1 William Eisinger, c and Winslow R. Briggs b,2 a Department of Biological Sciences, University of Ulsan, Ulsan , Korea b Department of Plant Biology, Carnegie Institution for Science, Stanford, California c Department of Biology, Santa Clara University, Santa Clara, California It has been known for decades that red light pretreatment has complex effects on subsequent phototropic sensitivity of etiolated seedlings. Here, we demonstrate that brief pulses of red light given 2 h prior to phototropic induction by low fluence rates of blue light prevent the blue light induced loss of green fluorescent protein tagged phototropin 1 (PHOT1- GFP) from the plasma membrane of cortical cells of transgenic seedlings of Arabidopsis thaliana expressing PHOT1-GFP in a phot1-5 null mutant background. This red light effect is mediated by phytochrome A and requires ;2 h in the dark at room temperature to go to completion. It is fully far red reversible and shows escape from photoreversibility following 30 min of subsequent darkness. Red light induced inhibition of blue light inducible changes in the subcellular distribution of PHOT1- GFP is only observed in rapidly elongating regions of the hypocotyl. It is absent in hook tissues and in mature cells below the elongation zone. We hypothesize that red light induced retention of the PHOT1-GFP on the plasma membrane may account for the red light induced increase in phototropic sensitivity to low fluence rates of blue light. INTRODUCTION Plants use light as an essential environmental cue. In the course of evolution they have acquired the capacity to respond to changes in light spectral quality, light intensity, light duration, and light direction. These responses regulate developmental and physiological processes in ways that maximize their photosynthetic potential and minimize the possibility of photodamage under unfavorable conditions. To this end, they use a remarkable array of known or putative photoreceptors. In the model plant Arabidopsis thaliana, there are five phytochromes, three cryptochromes, two phototropins, three members of the ZEITLUPE/ ADAGIO family (one of them still a putative photoreceptor), and additional putative photoreceptors for both UV-B and green light. These photoreceptors can act alone, in a cooperative fashion, or in an antagonistic fashion depending on prevailing conditions and developmental stage (see Schäfer and Nagy, 2006). However, we know little about how these interactions might occur and at what level. Phototropism provides us with one of the very first interactions investigated: an interaction between one or more phytochromes and a blue light receptor. It has been know for over half a century that red light treatment of etiolated seedlings can dramatically alter their subsequent 1 These authors contributed equally to this work. 2 Address correspondence to briggs@stanford.edu. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors ( is: Winslow R. Briggs (briggs@stanford.edu). W Online version contains Web-only data. phototropic sensitivity (Briggs, 1963a). Curry (1957) first noted that red light strongly reduced the sensitivity of first positive and first negative curvature of oat (Avena sativa) coleoptiles to unilateral blue light, an effect confirmed by Briggs (1963a) for both maize (Zea mays) and oat coleoptiles. Zimmerman and Briggs (1963) showed that red light treatment decreased phototropic sensitivity for first positive curvature of oat coleoptiles to blue light by a factor of about 10 but simultaneously increased the sensitivity of second positive curvature by a factor of about three. Briggs (1963a) provides a detailed review of these early experiments. Chon and Briggs (1966) subsequently demonstrated the same complex red light effect for maize coleoptiles and showed that it could be reversed by low fluences of far-red light. Higher fluences of far-red light, by contrast, mimicked the effect of red light, a phenomenon likely mediated by a phya-activated highirradiance response (Bae and Choi, 2008). The extreme sensitivity of the system to red light also implicated a phya-mediated response in the very low fluence response range (Mandoli and Briggs, 1981). The response to red light in either maize or oat is not immediate. Although it starts with little lag, it does not reach its maximum effect for between 1 to 2 h either in continuous red light (Briggs, 1963a, maize and oat coleoptiles; Zimmerman and Briggs, 1963, oat coleoptiles) or following a red light pulse (Chon and Briggs, 1966, maize coleoptiles). Briggs (1963b) also noted that continuous red light treatment reduced the total amount of auxin transported from maize coleoptile tips into agar blocks to ;50% of the dark control level. Thus, red light was either reducing auxin synthesis or auxin transport, or increasing auxin destruction, or some combination of the three processes. The decrease followed the same time course as the red
2 2836 The Plant Cell light induced changes in phototropic sensitivity. However, except for the temporal correlation, the physiological relationship between the light-induced changes in auxin levels and phototropic sensitivity was unclear. It was over a quarter of a century later that the Poff laboratory did the first experiments on phototropism with the model plant Arabidopsis, describing mutants that showed impaired phototropic responses (Khurana and Poff, 1989). Two years later, Janoudi and Poff (1991) reported that blue light alone, given either from above or bilaterally, desensitized etiolated hypocotyls of seedlings to unilateral phototropic stimulation with blue light, followed by a refractory period of insensitivity to blue light that lasted between 10 and 20 min. Pretreatment with red light did not cause the desensitization, but either blue or red light pretreatment from above enhanced the subsequent phototropic response to unilateral blue light (an enhancement seen only after the recovery from desensitization if the preirradiation was with blue light). Second positive curvature in dark-grown seedlings is induced only after a certain time threshold of unilateral blue light stimulation in dark-grown seedlings. Janoudi et al. (1992) showed that this time threshold (15 min of blue light stimulation for Arabidopsis and 60 min for tobacco [Nicotiana tabacum]) was greatly reduced if the seedlings were irradiated with red light 2 h prior to the onset of phototropic stimulation (to 4 min of blue light stimulation for Arabidopsis and to 15 min for tobacco). An action spectrum for the enhancement effect over the wavelength range from 350 to 731 nm clearly implicated phytochrome (Janoudi and Poff, 1992). As the appearance of enhancement was gradual (as were the red light induced changes described by the earlier workers), they imposed a 120-min dark period between the preirradiation and the onset of phototropic induction. Janoudi and Poff (1993) then showed that red light not only enhanced the subsequent phototropic sensitivity of Arabidopsis hypocotyls to blue light but also hastened recovery of phototropic sensitivity following blue light induced desensitization. The availability of both phya and phyb mutants of Arabidopsis allowed Parks et al. (1996) to demonstrate that it was phya and not phyb that mediated the red light induced enhancement of phototropic curvature to low fluences of blue light. As with Janoudi and Poff, they used a 2-h dark period between preirradiation and phototropic induction. More detailed studies (Janoudi et al., 1997a) uncovered a more complex picture by investigating the effect of different fluences of red light on enhancement of first positive curvature. They found that the responses to low red light fluences were exclusively attributable to phya, whereas the responses to higher fluences of red light were mediated neither by phya nor by phyb (Janoudi et al., Figure 1. Time Course for Blue Light Induced Relocalization of PHOT1-GFP in Hypocotyl Cortical Cells of 3-d-Old Etiolated Arabidopsis Seedlings without or with Prior Red Light Treatment. Projection images. Blue light (B) source is laser from confocal microscope. Fluence rate ;20 mmol m 2 s 1.Bars=20mm. (A) No red light (R) pretreatment. Note obvious PHOT1-GFP relocalization as early as 10 min after the onset of blue light exposure. (B) Red light pretreatment (5 mmol m 2 s 1 of red light for 10 s followed by 2 h of darkness prior to blue light irradiation). Most of the blue light induced PHOT1-GFP relocalization has been eliminated by the red light treatment.
3 PhyA Modulates Phot1-GFP Distribution 2837 Figure 2. Blue Light Induced Relocalization of PHOT1-GFP in Hypocotyl Cortical Cells from the Plasma Membrane into the Cytoplasm. Seedlings as in Figure 1A, no red light pretreatment. At each time point, 3 z-series single optical sections were taken at three different depths starting just below the surface of the cells illustrated, and at 11-m intervals to demonstrate that blue light induced relocalization is at least partially movement into the cytoplasm and not just redistribution along the plasma membrane. Note obvious relocalization of PHOT1-GFP as early as 12 min after the onset of blue light treatment. Blue light exposure as in Figure 1. Blue light fluence rate: 20 mmol m 2 s 1.Bars=20mm. 1997a). Janoudi et al. (1997b) then studied phototropic responses in Arabidopsis in the absence of preirradiation in the phytochrome mutants. Neither phya nor phyb mutants showed a reduced magnitude for first positive curvature, but first positive curvature was barely detectable in the phya phyb double mutant. Thus, even without red light preirradiation, one or the other of these two phytochromes was required for a normal first positive phototropic response. Neither the phya nor phyb single mutants showed an altered time threshold for second positive curvature. However, the time threshold was increased sixfold in the double mutant, and the magnitude of the response decreased, again indicating a role for the phytochromes in phototropic responses with no preirradiation. Whippo and Hangarter (2004) revisited the role of the various phytochromes in Arabidopsis phototropism. The picture that emerged was even more complex. These workers confirmed that phya is required for normal phototropism in response to very low fluence rates of blue light. At somewhat higher fluences, phyb and phyd function redundantly with phya to promote the phototropic response. At very high blue light fluence rates, phya alone is responsible for attenuating the phototropic response. Until the identification of phototropin 1 (PHOT1) as a photoreceptor for phototropism (Christie et al., 1998), there was no obvious way to investigate the relationship between the phytochromes and the blue light receptor(s) mediating phototropism. The opportunity arose, however, when Sakamoto and Briggs (2002) transformed the null mutant phot1-5 (nph1-5) with a PHOT1 green fluorescent protein (GFP) construct driven by the native PHOT1 promoter. The transgene restored phototropic sensitivity to low fluence rates of blue light and, at the same time, allowed a description of the cellular and subcellular distribution of the PHOT1-GFP protein under different experimental conditions. Sakamoto and Briggs (2002) also demonstrated that blue light treatment of the transformant caused the transformant to lose some of the PHOT1-GFP fluorescence from the plasma membrane, a relocalization phenomenon explored in more detail by Wan et al. (2008). These authors demonstrated the phenomenon in epidermal and mesophyll cells of the etiolated cotyledon, epidermal and cortical cells of the hypocotyl, both elongating and mature, cortical cells of the shoot root transition region, and elongating cortical cells near the root tip. Only developing guard cells failed to show the response. Kong et al. (2006) described a similar phenomenon for PHOT2-GFP. Preliminary experiments indicated that red light pretreatment might interfere with this loss. Thus, this study was designed to characterize this effect of red light and investigate how it might be related to the complex physiological changes that red light pretreatment induces in the subsequent phototropic responses of etiolated Arabidopsis hypocotyls. The working hypothesis was that retention of PHOT1 at the plasma membrane as a consequence of phytochrome photoconversion by red light could cause enhanced phototropic sensitivity by retaining the photoreceptor on the membrane system responsible for auxin transport. RESULTS Effect of Red Light Pretreatment on Blue Light Induced Relocalization of PHOT1-GFP As blue light induces a loss of PHOT1-GFP from the plasma membrane (Sakamoto and Briggs, 2002; Wan et al., 2008), and as red light sensitizes the phototropic response to low fluence rates of blue light, we investigated whether red light treatment would affect the blue light activated phototropin relocalization we previously described (Sakamoto and Briggs, 2002; Wan et al., 2008). We first determined how much blue light is required to saturate the blue light induced PHOT1-GFP relocalization. Using an external light source, we administered blue light fluences ranging from 20 to 2000 mmol m 22 (given at a constant fluence rate of 20 mmol m 22 s 21 with time varied). We then examined the cortical cells of the elongation region of etiolated hypocotyls of Arabidopsis seedlings using the confocal microscope for any changes in PHOT1-GFP distribution after a 1-h dark period. As shown in Supplemental Figure 1 online, the response was not induced by 200 mmol m 22 (10 s 3 20 mmol m 22 s 21 ) but was clearly detectable after exposure to 600 or 2000 mmol m 22,in agreement with our previous results (Wan et al., 2008). Thus,
4 2838 The Plant Cell when we used an external light source in subsequent experiments instead of the microscope laser, we used 2000 mmol m 22 (20 mmol m 22 s 21 for 100 s) of blue light to make certain that the blue light response was saturated. A time course showing the effect of blue light alone is shown by the projection images in Figure 1A. For this experiment, the blue light source was the laser in the confocal microscope (20 mmol m 22 s 21 ). At time zero, PHOT1-GFP is relatively evenly distributed at the plasma membranes of these cortical cells from the elongation region of an etiolated Arabidopsis hypocotyl. Within 10 min of the start of blue light irradiation, some reorganization and mottling of the label is evident, and this mottling increases over the course of an hour in all of the cells shown in the field. By 12 min after the onset of blue light, movement of PHOT1-GFP into the cytoplasm can be observed in single optical sections of the cortical cells (Figure 2). These results confirm our previous results (Wan et al., 2008) and provide a control for experiments investigating possible involvement of phytochrome(s) in the phenomenon (see below). The protein gel blot shown in Supplemental Figure 2 online indicates that a fraction of the PHOT1-GFP released to the cytoplasm on blue light treatment is in the supernatant following centrifugation of seedling extracts for 1 h at 135,000g and is likely soluble. The experiment does not eliminate the possibility that some of the cytoplasmic PHOT1-GFP may still be membrane associated, but the results confirm previous observations by Sakamoto and Briggs (2002) that a fraction of the released PHOT1-GFP cannot be sedimented under the above conditions. The blue light induced movement of PHOT1-GFP into the cytoplasm is not a general property of plasma membrane proteins and is not indicative of a major endocytosis. Figure 3 shows the effect of blue light on three fluorescent-labeled plasma membrane proteins: YFP:PIP2 (yellow fluorescent protein: Plasma Membrane Intrinsic Protein 2), an aquaporin; BRI1-GFP (Brassinosteroid-Insensitive 1:green fluorescent protein), a brassinolide receptor; and GFP:SIMIP (Salt Stress-Induced Major Intrinsic Protein), an aquaporin. None of the three marker proteins shows a change in distribution even after 30 min of blue light treatment (20 mmol m 22 s 21 ) (Figure 3, top and lower left). These results are in sharp contrast with the dramatic effect of the same blue light treatment on PHOT1-GFP (Figure 3, lower right). If a brief pulse of red light (50 mmol m 22 ) is given 2 h prior to the onset of blue light treatment, these dramatic changes in PHOT1- GFP distribution are almost completely eliminated (Figure 1B, projection images). The slight mottling seen in the red light treated cells in Figure 1B after blue light treatment stands in sharp contrast with the extensive relocalization response seen without the prior red light treatment (Figure 1A). Thus, red light Figure 3. Specificity of Blue Light Induced PHOT1-GFP Relocalization. Blue light induced relocalization of PHOT1-GFP is not part of a general blue light induced relocalization of plasma membrane proteins in the cortical cells of etiolated 3-d-old Arabidopsis seedlings. The localization of three fluorescent-labeled intrinsic plasma membrane proteins, YFP:PIP2, GFP: SIMIP, and BRI1:GFP, in cortical cells of Arabidopsis etiolated seedlings is unaffected by blue light treatment sufficient to cause major relocalization of PHOT1-GFP. The blue light source was the laser from a confocal microscope. Blue light fluence rate: 20 mmol m 2 s 1. Bars = 20 mm.
5 PhyA Modulates Phot1-GFP Distribution 2839 level for curvature. When the blue light fluence rate was 20 mmol m 22 s 21, some enhancement occurred, although the enhancement was less than seen in the other lines. The phot1-5 line failed to curve in response to 1 mmol m 22 s 21, as it lacked PHOT1, and the fluence rate was too low to activate PHOT2. We conclude that under the conditions we used to demonstrate a red light effect on blue light induced PHOT1-GFP relocalization, the same red light treatment enhances the phototropic response mediated by PHOT1-GFP (and by PHOT2 alone). Timing and Quantification of the Red Light Effect on PHOT1-GFP Relocalization Figure 4. Effect of Red Light Treatment 2 h before Blue Light Irradiation on Blue Light Induced PHOT1-GFP Relocalization in 3-d-Old Arabidopsis Hypocotyl Cortical Cells. Single z-series images taken from seedlings without (A) or with (B) red light (R) treatment 2 h before the blue light (B) pulse. Red light source as in Figure 1B. The blue light source (fluence rate 20 mmol m 2 s 1, exposure time 100 s) was LEDs. Single z-series optical sections obtained 20 min after the blue light pulse. Note minimal cytoplasmic PHOT1-GFP in red light treated sample. Bar = 20 mm. pretreatment appears to cause retention of PHOT1-GFP at the plasma membrane of these cells. The effect of red light in preventing the movement of PHOT1-GFP into the cytoplasm is illustrated in the single optical sections shown in Figure 4. The 2-h dark period was chosen as it was the time period used in virtually all of the physiological experiments cited in the Introduction investigating the effect of red light preirradiation on subsequent phototropic responses. Effect of Red Light on Phototropism Mediated by PHOT1-GFP To test whether a brief pulse of red light affected phototropism under our growth conditions, we tested the phototropic responses of the PHOT1-GFP, gl1, and phot1-5 lines with or without prior red light treatment (Table 1). A 2-h dark incubation period followed the red treatment. In the PHOT1-GFP line, PHOT1-GFP is the only functional phototropin. In the gl1 line, both PHOT1 and PHOT2 are functional. In the phot1-5 line, only PHOT2 is functional. Furthermore, a blue light fluence rate of 1 mmol m 22 s 21 can only activate PHOT1, whereas 20 mmol m 22 s 21 activates both PHOT1 and PHOT2 (Sakai et al., 2001). At blue light fluence rates of either 1 or 20 mmol m 22 s 21, red light significantly enhanced the response of the PHOT1-GFP line after both 8 and 11 h exposure (Table 1). When PHOT2 alone is functional (phot1-5, blue light = 20 mmol m 22 s 21 ) red light treatment also significantly enhanced the phototropic response. The gl1 line irradiated with 1 mmol m 22 s 21 of blue light (11 h) failed to show strong enhancement, possibly because both red light treated and control seedlings had reached the saturation Since 50 mmol m 22 (5 mmol m 22 s 21 for 10 s) of red light administered 2 h prior to blue light treatment was sufficient to reduce blue light induced relocalization dramatically (Figure 1B), we investigated how much dark time was required between red light pretreatment and a blue light pulse for the red light to be effective. PHOT1-GFP relocalization was then allowed to proceed for 20 min in darkness following the blue light pulse (2000 mmol m 22 ) prior to observation with the confocal microscope. The results are shown in Figure 5. Red light administered 0, 30, or 60 min prior to blue light was essentially ineffective. In all three cases, blue light induced movement of PHOT1-GFP into the cytoplasm. However, by 2 h after red light treatment, the effect of blue light was eliminated. This effect of red light persisted at least for an additional hour. We next asked how much red light was required to prevent the effect of blue light on the subcellular distribution of PHOT1-GFP in the elongating hypocotyl cells. The results are shown in Figure 6 (constant 10-s exposure time for red light with intensity varied) and Figure 7 (constant fluence rate at 10 mmol m 22 s 21 with time varied). The red light effect was saturated between 40 and 100 mmol m 22 in both experiments. This range is well within that to be expected for a classic Table 1. Effect of Red Light (R) on Subsequent Blue-light (B)-Induced Phototropic Curvature Arabidopsis Line Exposure Time (h) R Fluence (mmol m 2 ) B Fluence Rate (mmol m 2 s 1 ) Degrees Curvature 6 SE a PHOT1-GFP ± 1.7 PHOT1-GFP ± 1.8 gl gl phot phot PHOT1-GFP ± 1.7 PHOT1-GFP ± 3.1 gl ± 2.9 gl ± 5.1 phot ± 3.3 phot ± 5.2 PHOT1-GFP ± 2.0 PHOT1-GFP ± 4.7 Dark incubation following R, 2 h. a Values for pairs (6 R) in boldface indicate where R provides significant enhancement by R = 100 mmol m 2 over R = 0 mmol m 2 (t test, P < 0.05). For gl1, 1mmol m 2 s 1 B (P < 0.08).
6 2840 The Plant Cell Figure 5. Duration of Dark Incubation Period after Red Light Treatment Required for Red Light Induced Inhibition of Blue Light Induced PHOT1-GFP Relocalization. Projection images of hypocotyl cortical cells from 3-d-old etiolated Arabidopsis seedlings. Red-light (R) treatment as in Figure 1B. Blue light (B) treatment as in Figure 4. Dark incubation times were 0, 30, 60, 120, and 180 min. A maximum effect of red light in preventing blue light induced PHOT1- GFP relocalization is seen only after 2 or 3 h of dark incubation. Bars = 20 mm. low-fluence phytochrome response (Schäfer and Nagy, 2006). Furthermore, the reciprocity law (Bunsen and Roscoe, 1862) holds under these experimental conditions. Far-Red Reversal of the Red Light Effect If the effect of red light is indeed a low-fluence phytochrome response, one would expect it to be fully far-red reversible. Indeed, 1260 mmol m 22 of far red completely reversed the effect of red light. Figure 8 shows that the effect of blue light (confocal laser) was diminished if 100 mmol m 22 of red light (administered 2 h before blue) was followed immediately by the far-red treatment (Figure 8, bottom right panel). The appearance of the cells 15 min after the blue light pulse is virtually identical to that of blue light without any red pretreatment (Figure 8, bottom left panel) and essentially indistinguishable from that seen in Figure 2A. As Figure 6. Fluence Response Relationship for Red Light Mediated Inhibition of Blue Light Induced PHOT1-GFP Relocalization. Exposure time constant (10 s), fluence rate varied (from 0.4 to 10 mmol m 2 s 1 ). Projection images of PHOT1-GFP in hypocotyl cortical cells from 3-dold etiolated Arabidopsis seedlings. Note that 100 m m 2 (10 s 3 10 mmol m 2 s 1 ) of exposure to red light is sufficient to prevent blue light induced PHOT1-GFP relocalization. The blue light source was the laser from a confocal microscope. Bars = 20 mm.
7 PhyA Modulates Phot1-GFP Distribution 2841 Figure 7. Fluence Response Relationship for Red Light Mediated Inhibition of Blue Light Induced PHOT1-GFP Relocalization. Red light fluence rate constant (10 mmol m 2 s 1 ), exposure time varied (between 0 and 100 s). Projection images of PHOT1-GFP in hypocotyl cortical cells from 3-d-old etiolated Arabidopsis seedlings. Images were obtained 15 min after blue light (B) exposure. Note that 100 mm 2 (10 s 3 10 mmol m 2 s 1 ) red light is sufficient to prevent blue light induced PHOT1-GFP relocalization. Blue light source (fluence rate 20 mmol m 2 s 1, exposure time 100 s), from LEDs. Bars = 20 mm. expected, red light alone almost eliminated the blue light response (Figure 8, middle panels). We next investigated how much far-red light is required to reverse the effect of red light. The results are shown in Figure 9, which presents cells near the bottom of the hypocotyl elongation zone. Far-red light (1260 mmol m 22 ) was sufficient to reverse the effect of red light (Figure 9, center panel). Finally, we investigated the time course for escape from farred reversibility. The results are shown in Figure 10. Regardless of when far-red light was given, the total dark period was always 2 h. With a 15-min dark period between red and far-red treatment, the effect of far-red light in reversing the red-light effect was diminished, and with a 25-min dark period after red light, farred light was completely ineffective. Hence, the consequences of red light treatment become fixed relatively rapidly, although they are not fully expressed until 2 h has passed following red light treatment (Figure 5). Localization of the Red Light Effect to the Elongation Zone We were puzzled that we did not always obtain an effect of red light in preventing subsequent blue light induced PHOT1-GFP relocalization. We identified two reasons for this variability: First, if the hypocotyls are handled a little roughly, some PHOT1-GFP relocalization occurred in the absence of any blue light treatment. We solved this problem by mounting the seedlings carefully on agar (see Methods). However, some variability still persisted. The effect of red light in blocking blue light inducible PHOT1-GFP movement into the cytoplasm had a developmental component. We found that the standard red light fluence had little effect on blue light induced PHOT1-GFP distribution in the hook (Figure 11A) or in the mature cells, below the elongation zone (Figure 11C), but prevented the blue light effect in the elongation zone (Figure 11B). It was logistically possible in these experiments to observe two regions from the same seedlings, but not three. Thus, the images shown in the center and bottom panels of Figure 11 were taken from the same seedlings, but those in the top panel were from a different seedling. Experiments shown in Figure 1A indicate that substantial blue light induced PHOT1- GFP relocalization can be observed well before 30 min from the onset of blue light treatment. However, no effect is detectable after 30 min in the elongation zone cells given prior to red light treatment (Figure 11, center panels). By contrast, by 40 min there is a strong effect of blue light both above and below the elongation zone despite red light pretreatment. Thus, the interaction between phytochrome and PHOT1-GFP develops as cells enter their elongation phase and is lost when they are mature. Since the red light effect is restricted to the hypocotyl elongation zone, we were unable to detect a red light induced decrease in the level of PHOT1-GFP in the soluble fraction. It is likely that a red-induced decrease in the amount of PHOT1-GFP released to the cytoplasm in the elongation zone in response to blue light treatment would be masked by its blue light induced release in the cotyledons, hook, and mature hypocotyl tissues, especially given the large amount of PHOT1-GFP detectable in the cotyledons and hook (Wan et al., 2008). This latter release is unaffected by the red light treatment (see below). Identification of the Phytochrome Mediating the Red Light Effect in Antagonizing Blue Light Induced PHOT1-GFP Relocalization We made crosses for plants carrying the PHOT1-GFP transgene in the phot1-5 null background with strong mutant alleles of phya
8 2842 The Plant Cell as functional phya protein was present (see Supplemental Figure 4 online). DISCUSSION Figure 8. Far-Red Reversal of Red Light Mediated Inhibition of Blue Light Activated PHOT1-GFP Relocalization. Projection images of PHOT1-GFP in hypocotyl cortical cells of 3-d-old etiolated Arabidopsis seedlings without (A) or with (B) red light (R) treatment (100 mmol m 2 ) or red light (100 mmol m 2 ) followed by far-red (FR) treatment (1260 mmol m 2 ) given immediately after red (C). A 2-h dark incubation period followed the red or red/far-red treatment prior to blue light (B) treatment. Note that far-red after red reduces the red light induced inhibition of blue light induced PHOT1-GFP relocalization (cf. [B] and [C]). Blue light source (20 mmol m 2 s 1 ), laser from confocal microscope. Top panels, 2 min of exposure to blue light; bottom panels, 15 min of exposure to blue light. Bars = 20 mm. (phya-211, Reed et al., 1994) or phyb (phyb-9, Reed et al., 1993) and with the phya-211 phyb-9 double mutant to identify the phytochrome(s) that mediated the red light response described above. The seedlings were given 50 mmol m 22 s 21 of red light, 2 h of darkness, a blue light pulse (total fluence 2000 mmol m 22 ), and 30 min of darkness prior to observation with the confocal microscope. In the phyb mutant, red light still blocks blue light induced relocalization of PHOT1-GFP (Figure 12B), whereas in the phya mutant, blue light induces normal relocalization despite the prior red light treatment (Figure 12A). As expected from these results, the response of the phya phyb double mutant is no different from that of the phya mutant: there is no red light effect (see Supplemental Figure 3 online). Thus, the red light induced inhibition of blue light activated PHOT1-GFP relocalization from the plasma membrane is mediated by phya. From following subsequent generations, we identified the three classes of phytochrome mutant lines in which PHOT1-GFP was the only functioning PHOT1. Red light treatment still prevented the blue light induced relocalization of PHOT1-GFP in the absence of wild-type phot1 (the null phot1-5 allele, Huala et al., 1997) as long The action of red light in preventing subsequent blue light induced relocalization of PHOT1-GFP from the plasma membrane in etiolated Arabidopsis hypocotyls has the characteristics of a classic phytochrome effect. The response happens in the low fluence range, is fully far-red reversible, and shows typical escape from far-red photoreversibility during ;30 min of darkness. The above results show that this response is mediated by phya and not phyb (Figure 12; see Supplemental Figure 3 online). Although phya phototransformation must bring about some changes at the plasma membrane to cause the retention of PHOT1-GFP, it is premature to ascribe this effect to a cytoplasmic action of phya. The changes are gradual, requiring 2 h to go to completion, which is more than enough time for the intervention of changes in the transcription of nuclear genes and subsequent proteome changes at the plasma membrane. PHYTOCHROME KINASE SUBSTRATE1 (PKS1) is a cytoplasmic protein that can interact with phytochrome and is phosphorylated by phytochrome (Fankhauser et al., 1999). Lariguet et al. (2006) recently demonstrated that the protein interacts physically with both PHOT1 and plasma membrane associated NONPHO- TOTROPIC HYPOCOTYL3 (NPH3) both in vivo and in vitro. Both NPH3 (Liscum and Briggs, 1995) and PKS1 (Lariguet et al., 2006) are essential for Arabidopsis hypocotyl phototropism. PKS1 is fairly uniformly expressed in the hypocotyls of dark-grown seedlings but becomes concentrated in the root tip and hypocotyl elongation zone after 4 h in the light (Lariguet et al., 2003). Both red and far-red light induce an overall increase in PKS1 protein over a 4-h period, and a few short pulses of far-red light are sufficient to produce a measurable increase. These results suggest that the increase is a very low fluence reaction. It is tempting to postulate that the light-induced increase in PKS1 in the elongation zone is related to the red light induced retention of PHOT1-GFP at the plasma membrane after blue light treatment. Both occur over a few hours, both are mediated exclusively by phya, and both are localized to the elongation zone. The correlation appears to break down, however, in the case of far-red reversibility. The PKS increase is actually induced by far-red light, either continuous or pulsed, whereas the red light effect on PHOT1-GFP localization after blue light is far-red reversible (Figures 8 and 9), and 5 min of far-red light alone 2 h prior to blue light treatment had no effect on PHOT1-GFP relocalization (Figure 9). However, Lariguet et al. (2003) regularly used longer light treatments than those in this study and did not report any results on the effect of single red light pulses or red/ far-red reversibility. Hence, the role of PKS1 in the phenomenon described here remains open. The conditions used here for the experiments in which the red light effect had gone to completion were very similar to those used by Parks et al. (1996), who exposed etiolated Arabidopsis hypocotyls to a brief pulse of red light followed by 2 h of darkness prior to phototropic induction with blue light. A reasonable hypothesis to explain the phototropism enhancement by red
9 PhyA Modulates Phot1-GFP Distribution 2843 Figure 9. Fluence Requirement for Far-Red Light to Reverse the Red Light Induced Inhibition of Blue Light Activated PHOT1-GFP Relocalization. Projection images of PHOT1-GFP in hypocotyl cortical cells of 3-d-old etiolated Arabidopsis seedlings. Irradiation and dark incubation protocols as in Figure 8 but with far-red (FR) exposure time varied. Red light (R) fluence rate 10 mmol m 2 s 1, exposure time 10 s. Far-red (FR) fluence rate 42 mmol m 2 s 1. Blue light (B) source (20 mmol m 2 s 1 ), laser from confocal microscope. Note that 1260 mmol m 2 of far-red light (42 mmol m 2 s s) is sufficient to reverse the red-light effect. Bars = 20 mm. light is that by causing PHOT1-GFP to remain at the plasma membrane where blue light induced lateral auxin transport is presumably taking place, there is more phototropin to impact auxin transport and hence a stronger phototropic response. However, there are other conditions where this hypothesis is not sufficient or where the results actually eliminate it as a possibility. For example, although the changes in phototropic sensitivity of etiolated maize (Chon and Briggs, 1966) or oat (Zimmerman and Briggs, 1963) coleoptiles induced by red light are exquisitely sensitive to red light, it is only the sensitivity of second positive curvature that is increased by red light. That of first positive curvature is actually decreased in both cases by almost an order of magnitude. It is not known at present, however, whether the effect of red light on the subcellular distribution of PHOT1 in Arabidopsis is also seen in maize and oat coleoptiles or indeed whether any PHOT1 moves from the plasma membranes in these organs. The results of Whippo and Hangarter (2004) also suggest a far more complex picture: Even without red light pretreatment, a normal phototropic response to low-fluence-rate blue light requires the presence of either phya or phyb. Presumably the small fraction of the far-red-absorbing form of phytochrome induced by the blue light is needed for a normal phototropic response. While phya is essential for a normal phototropic response when the blue light fluence rate is 0.01 mmol m 22 s 21, for higher blue light fluence rates (above 1.0 mmol m 22 s 21 ), phyb or phyd can serve redundantly. Furthermore, under very high fluence rates of blue light (e.g., 100 mmol m 22 s 21 ), phya actually attenuates the phototropic response. In addition, other work by Whippo and Hangarter (2003) indicates involvement of cryptochromes in modulating phototropic responses as well as phytochromes. Thus, PHOT1 and PHOT2 can both modulate phototropic responses directly and phya, phyb, PhyD, cry1, and cry2 can do so indirectly. No simple model can account for this complexity. A surprising finding is that the regulation of PHOT1-GFP localization and phya, whether direct or indirect, is regulated during development. Red light has little or no effect on the subcellular distribution or redistribution of PHOT1-GFP in the hook tissues or in the mature cells below the elongation zone. The association develops just below the hook and is fully expressed only in the elongation zone. Hence, it occurs only in the phototropically sensitive tissue and not elsewhere in the hypocotyl. Since PHOT1 is well known to mediate a number of other responses (stomatal opening, chloroplast accumulation, chloroplast avoidance, rapid growth inhibition, leaf expansion, and leaf position), it will be interesting to determine whether phya can play a role in modulating one or more of these responses mediated by PHOT1. If so, are these interactions developmentally regulated? The light gradient across an Arabidopsis hypocotyl is steeper in the blue light than in the red region of the spectrum because of both stronger absorption of blue light (e.g., from flavins, carotenoids, and the Soret bands of cytochromes) and greater loss through light scattering. Hence, red light excitation of phya should be more uniform across a hypocotyl than blue light excitation of PHOT1 if both are given unilaterally. Thus, red light induced retention of PHOT1 at the plasma membrane is not likely to show a strong gradient across the tissue regardless of direction of the exciting light. If a small gradient exists for red light, PHOT1 retention at the plasma membrane would be slightly favored on the illuminated side. The response to subsequent blue light might then be enhanced if the blue light came from the same
10 2844 The Plant Cell Figure 10. Escape from Far-Red Reversal of the Red Light Effect on Blue Light Induced PHOT1-GFP Relocalization. Projection images of PHOT1-GFP in hypocotyl cortical cells from etiolated 3-d-old Arabidopsis seedlings. Light sources and irradiation protocols as for Figure 9 except that the time between red light (R) and far-red (FR) pulses was varied from 3 to 60 min. t1, time, min, between red and far-red pulses; t2, time, min, between far-red pulse and beginning of blue light irradiation. t1 +t2 = 120 min. A time course for the B effect is shown for each red/far-red treatment. Note the strong blue light response at 3 and 15 min between red and far red, indicating far-red reversal of the red effect. At 25 and 60 min between red and far-red pulses, the red effect is only weakly reversed. Bars = 20 mm. direction (i.e., more PHOT1 at the plasma membrane to excite and drive lateral auxin transport on the illuminated side than on the shaded side) and slightly inhibited if it came from the opposite direction (i.e., less PHOT1 at the plasma membrane on the illuminated side than the shaded side). Whether this hypothesis is correct would depend on whether the red light effect was cell specific or spread uniformly throughout the hypocotyl tissue. There is physiological evidence for at least a weak gradient of phytochrome photoexcitation across etiolated maize mesocotyls (Iino et al., 1984) and pea (Pisum sativum) epicotyls (Parker et al., 1988). In both cases, phytochrome itself was shown to mediate a weak phototropic response to red light, which suggests some cell specificity. These larger organs would be more suitable for testing the possible role of a gradient in phytochrome photoexcitation on phototropic blue light sensitivity than the Arabidopsis hypocotyl provided that the same relationship between phytochrome and phototropin exists in these species. This work establishes a potential mechanism by which phytochrome might enhance phototropic sensitivity when both red and blue light fluences are low. The mechanism might be advantageous for seedlings exposed to the very first light as they break through the soil, maximizing growth toward the light source. Future study will be required to determine the nature and complexity of the pathways involved in the interaction of the two photoreceptors, PHOT1 and phya. The possible physiological roles of the complicated interactions seen with higher fluence rates both of red and blue light also await further exploration to determine their cellular basis. METHODS Plant Materials and Growth Conditions We used the phot1-5 mutant of Arabidopsis thaliana (Columbia ecotype, gl1 background) transformed with PHOT1-GFP, constructed by Sakamoto and Briggs (2002). Seeds were surface sterilized and plated onto half-strength Murashige and Skoog medium containing 0.8% agar and 1% sucrose. The plates were held in darkness for 3 d at 48C and then exposed to red light (228C, fluence rate of 15 mmol m 22 s 21 )for2hto induce uniform germination. The seedlings were then grown in complete darkness at 228C for;2.5 d. The mutants phya-211, phyb-9, and phya-211 phyb-9, kindly provided by Peter H. Quail, were crossed with PHOT1-GFP plants. F2 seeds were selected under red (8 mmol m 22 s 21 )
11 PhyA Modulates Phot1-GFP Distribution 2845 Figure 11. Restriction of the Red Light Effect on Blue Light Induced PHOT1-GFP Relocalization to the Hypocotyl Elongation Zone. Projection images of cortical cells from the hypocotyl hook (A), the elongation zone (B), and mature hypocotyl zones (C) of etiolated 3-d-old Arabidopsis seedlings. Red light fluence rate 10 mmol m 2 s 1, an exposure time of 10 s followed by a 2-h dark incubation period prior to blue light treatment. The blue light source (20 mmol m 2 s 1 ) is the laser from the confocal microscope. Note that red light treatment eliminates the blue light effect only in the elongation zone. Bars = 20 mm. or far-red (2 mmol m 22 s 21 ) light, and seedlings with long hypocotyls were screened for PHOT1-GFP fluorescence. To confirm the phot1-5 mutant background, we performed PCR genotyping with forward primer 59-GCAGGTACATAGAGCTAGAGC-39 and reverse primer 59-GCTGT- GAGTAATTAGTCCTCC-39. The phot1-5 mutation deleted the primary site for the forward primer and therefore produced no PCR product. The wild-type PHOT1 gene yielded a PCR product of ;1.8 kb, while the PHOT1-GFP fusion produced a band of ;2.4 kb. We selected plants that only produced the 2.4-kb product. Light Sources The red light source to induce germination consisted of two 20-W red fluorescent tubes (Sylvania F20T12/R) filtered through one layer of Rohm and Haas 2444 red Plexiglas. The red light source used to treat the seedlings was a bank of LEDs (630 nm). The source of far-red light was a 12-V, 20-W tungsten halogen lamp filtered through a Rohm and Haas FRF Plexiglas filter. The different fluence rates used in the individual experiments are provided above. For certain experiments, the source of blue light was the laser in the confocal microscope (488 nm). For other experiments, except phototropism, it was a bank of LEDs (470 nm). Unless otherwise indicated, the fluence rate from blue light sources was 20 mmol m 22 s 21. For phototropism experiments, the blue light sources were either two fluorescent lamps (Philips F20T12/CW), with the light passed through a blue Plexiglas filter (Rohm and Haas) (1 mmol m 22 s 21 ), or a Kodak Carousel 4400 slide projector equipped with a Corning blue filter (20 mmol m 22 s 21 ). Sample Preparation for Microscopy As mentioned above, even slight physical damage could cause the loss of some PHOT1-GFP from the plasma membrane to the cytoplasm. To avoid this physical damage, a layer of 1.5% agar dissolved in distilled water was allowed to solidify between two cover slips ( mm). When it had hardened, the upper cover slip was slid off and the seedlings were gently placed on the remaining agar bed on the lower cover slip. A cover slip was then gently placed over the seedling and the assembly was turned over and transferred to the microscope upside down. Uniform plasma membrane distribution of PHOT1-GFP on initial observation with the confocal microscope was taken as evidence that no damage had occurred. Microscopy All images were acquired with a Nikon inverted fluorescence microscope equipped with appropriate Nikon water immersion objective lenses and a Bio-Rad MRC confocal head. All experiments were repeated three or more times with results similar to those presented above. Phototropism Experiments Phototropism experiments were performed as described elsewhere (Cho et al., 2007). Three-day-old seedlings were treated with red light (total fluence 100 mmol m 22 ) and returned to darkness for 2 h prior to blue light treatment. Blue fluence rates were either 1 or 20 mmol m 22 s 21,and
12 2846 The Plant Cell Supplemental Figure 3. In the phya phyb Double Mutant, as in the phya Single Mutant, Red Light Does Not Prevent Blue Light Induced PHOT1-GFP Relocalization. Supplemental Figure 4. In phya but Not phyb Mutants, Red Light Pretreatment Does Not Prevent Blue Light Induced Relocalization of PHOT1-GFP in Lines Lacking Wild-Type PHOT1. ACKNOWLEDGMENTS Figure 12. Red Light Inhibition of Blue Light Induced PHOT1-GFP Is Mediated by phya. Projection images of cortical cells from hypocotyls of 3-d-old etiolated Arabidopsis seedlings. Red light (R) treatment (10 mmol m 2 s 1 for 10 s) was followed by 2 h of incubation in the dark prior to blue light (B) treatment (20 mmol m 2 s 1 for 100 s). Blue light source from LEDs. Bars = 20 mm. (A) In the absence of phya, red light fails to prevent blue light induced PHOT1-GFP relocalization. (B) In the absence of phyb, red light is fully effective in preventing PHOT1-GFP relocalization. exposure times were either 8 or 11 h. The plates of seedlings were scanned and the hypocotyl images were analyzed with NIH ImageJ 1.62 software. Representative data from four different experiments are shown. In all phototropism experiments, the curvatures of 20 or more seedlings were measured for each data point with similar results. Protein Gel Blotting and Gel Electrophoresis Total protein was extracted from control or blue light treated seedlings under dim red light as described elsewhere (Cho et al., 2007). The extract was centrifuged for 10 min at 10,000 rpm in an Eppendorf 5415C tabletop centrifuge to sediment cell walls, larger organelles, and cellular debris not fully broken up. The supernatant was then centrifuged for 1 h at 135,000g.0 to obtain soluble and membrane fractions. Subsequent steps were as described (Cho et al., 2007). Accession Numbers Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: BRI1 (NM ), FKF1 (NM ), LKP2 (NM ), PIP2A (NM ), PHOT1 (NM ), PHOT2 (NM ), PHYA (001,123,784), PHYB (NJM ), and SIMIP (NM ). Supplemental Data The following materials are available in the online version of this article. Supplemental Figure 1. Fluence Requirement for Blue Light Induced Relocalization of PHOT1-GFP from the Plasma Membrane into the Cytoplasm in Cortical Cells of the Hypocotyl of Etiolated Arabidopsis Seedlings. Supplemental Figure 2. Protein Gel Blots Demonstrating Blue Light Induced Release of Both PHOT1 and PHOT1-GFP from the Membrane to the Soluble Fraction of Etiolated Arabidopsis Seedlings. We thank Peter H. Quail for providing the three Arabidopsis phytochrome mutants and David Ehrhardt for providing the YFP:PIP2, GFP: SIMIP, and BRI1:GFP lines and for his help with the confocal microscopy. We also thank Margaret Olney for her careful review of the manuscript. I.-S.H. was supported by the Research Fund of Ulsan University. This work was supported by National Science Foundation Grant to W.R.B. The authors are grateful for this support. Received April 4, 2008; revised September 30, 2008; accepted October 10, 2008; published October 24, REFERENCES Bae, G., and Choi, G. (2008). Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59: Briggs, W.R. (1963a). The phototropic responses of higher plants. Annu. Rev. Plant Physiol. 14: Briggs, W.R. (1963b). Red light, auxin relationships, and the phototropic responses of corn and oat coleoptiles. Am. J. Bot. 50: Bunsen, R., and Roscoe, H. (1862). Photochemische Untersuchungen. Ann. Physiol. Chem. 117: Cho, H.-Y., Tseng, T.-S., Kaiserli, E., Sullivan, S., Christie, J.M., and Briggs, W.R. (2007). Physiological roles of the Light, Oxygen, or Voltage domains of phototropin 1 and phototropin 2 in Arabidopsis. Plant Physiol. 143: Chon, H.P., and Briggs, W.R. (1966). Effect of red light on the phototropic sensitivity of corn coleoptiles. Plant Physiol. 41: Christie, J.M., Raymond, P., Powell, G.K., Bernasconi, P., Raibekas, A.A., Lisum, E., and Briggs, W.R. (1998). Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282: Curry, G.M. (1957). Studies on the Spectral Sensitivity of Phototropism. PhD dissertation (Cambridge, MA: Harvard University). Fankhauser, C., Yeh, K.C., Lagarias, J.C., Zhang, H., Elich, T.D., and Chory, J. (1999). PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284: Huala, H., Oeller, P.W., Liscum, E., Han, I.-S., Larsen, E., and Briggs, W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278: Iino, M., Briggs, W.R., and Schäfer, E. (1984). Phytochrome-mediated phototropism in maize seedling shoots. Planta 160: Janoudi, A.-K., Gordon, W.R., Wagner, D., Quail, P., and Poff, K.L. (1997a). Multiple phytochromes are involved in red-light-induced enhancement of first positive phototropism in Arabidopsis thaliana. Plant Physiol. 113: Janoudi, A.-K., Konjević, R., Apel, P., and Poff, K.L. (1992). Time threshold for second positive phototropism is decreased by a preirradiation with red light. Plant Physiol. 99:
Figure 18.1 Blue-light stimulated phototropism Blue light Inhibits seedling hypocotyl elongation
 Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Analysis of regulatory function of circadian clock. on photoreceptor gene expression
 Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Electromagenetic spectrum
 Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
LECTURE 4: PHOTOTROPISM
 http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ LECTURE 4: PHOTOTROPISM LECTURE FLOW 1. 2. 3. 4. 5. INTRODUCTION DEFINITION INITIAL STUDY PHOTROPISM MECHANISM PHOTORECEPTORS
http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ LECTURE 4: PHOTOTROPISM LECTURE FLOW 1. 2. 3. 4. 5. INTRODUCTION DEFINITION INITIAL STUDY PHOTROPISM MECHANISM PHOTORECEPTORS
CBMG688R. ADVANCED PLANT DEVELOPMENT AND PHYSIOLOGY II G. Deitzer Spring 2006 LECTURE
 1 CBMG688R. ADVANCED PLANT DEVELOPMENT AND PHYSIOLOGY II G. Deitzer Spring 2006 LECTURE Photomorphogenesis and Light Signaling Photoregulation 1. Light Quantity 2. Light Quality 3. Light Duration 4. Light
1 CBMG688R. ADVANCED PLANT DEVELOPMENT AND PHYSIOLOGY II G. Deitzer Spring 2006 LECTURE Photomorphogenesis and Light Signaling Photoregulation 1. Light Quantity 2. Light Quality 3. Light Duration 4. Light
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING. AnitaHajdu. Thesis of the Ph.D.
 THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
Plants are sessile. 10d-17/giraffe-grazing.jpg
 Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Nucleo-cytoplasmic partitioning of the plant photoreceptors phytochromes
 seminars in CELL & DEVELOPMENTAL BIOLOGY, Vol. 11, 2000: pp. 505 510 doi: 10.1006/scdb.2000.0202, available online at http://www.idealibrary.com on Nucleo-cytoplasmic partitioning of the plant photoreceptors
seminars in CELL & DEVELOPMENTAL BIOLOGY, Vol. 11, 2000: pp. 505 510 doi: 10.1006/scdb.2000.0202, available online at http://www.idealibrary.com on Nucleo-cytoplasmic partitioning of the plant photoreceptors
The Role of a Protein in Stomatal Opening Mediated by PHOT2 in Arabidopsis W OA
 The Plant Cell, Vol. 24: 1114 1126, March 2012, www.plantcell.org ã 2012 American Society of Plant Biologists. All rights reserved. The Role of a 14-3-3 Protein in Stomatal Opening Mediated by PHOT2 in
The Plant Cell, Vol. 24: 1114 1126, March 2012, www.plantcell.org ã 2012 American Society of Plant Biologists. All rights reserved. The Role of a 14-3-3 Protein in Stomatal Opening Mediated by PHOT2 in
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
The role of the N-terminal NTE domain of PHYTOCHROMEs in red and far red light perception
 The role of the N-terminal NTE domain of PHYTOCHROMEs in red and far red light perception Theses of the Ph.D. dissertation János Bindics Supervisor: Dr. Ferenc Nagy Hungarian Academy of Sciences Biological
The role of the N-terminal NTE domain of PHYTOCHROMEs in red and far red light perception Theses of the Ph.D. dissertation János Bindics Supervisor: Dr. Ferenc Nagy Hungarian Academy of Sciences Biological
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
15. PHOTOPERIODISM. 1. Short day plants
 15. PHOTOPERIODISM Photoperiodism is the phenomenon of physiological changes that occur in plants in response to relative length of day and night (i.e. photoperiod). The response of the plants to the photoperiod,
15. PHOTOPERIODISM Photoperiodism is the phenomenon of physiological changes that occur in plants in response to relative length of day and night (i.e. photoperiod). The response of the plants to the photoperiod,
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON
 (17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
A copy can be downloaded for personal non-commercial research or study, without prior permission or charge
 Kaiserli, Eirini, Sullivan, Stuart, Jones, Matthew A., Feeney, Kevin A., and Christie, John M. (2009) Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation
Kaiserli, Eirini, Sullivan, Stuart, Jones, Matthew A., Feeney, Kevin A., and Christie, John M. (2009) Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Leaf Positioning of Arabidopsis in Response to Blue Light
 Molecular Plant Volume 1 Number 1 Pages 15 26 January 2008 Leaf Positioning of Arabidopsis in Response to Blue Light Shin-ichiro Inoue a, Toshinori Kinoshita a,2, Atsushi Takemiya a, Michio Doi b and Ken-ichiro
Molecular Plant Volume 1 Number 1 Pages 15 26 January 2008 Leaf Positioning of Arabidopsis in Response to Blue Light Shin-ichiro Inoue a, Toshinori Kinoshita a,2, Atsushi Takemiya a, Michio Doi b and Ken-ichiro
10/4/2017. Chapter 39
 Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
The signal transducing photoreceptors of plants
 Int. J. Dev. Biol. 49: 653-664 (2005) doi: 10.1387/ijdb.051989kf The signal transducing photoreceptors of plants KEARA A. FRANKLIN*, VICTORIA S. LARNER and GARRY C. WHITELAM Department of Biology, University
Int. J. Dev. Biol. 49: 653-664 (2005) doi: 10.1387/ijdb.051989kf The signal transducing photoreceptors of plants KEARA A. FRANKLIN*, VICTORIA S. LARNER and GARRY C. WHITELAM Department of Biology, University
Green Light Stimulates Early Stem Elongation, Antagonizing Light-Mediated Growth Inhibition 1
 Green Light Stimulates Early Stem Elongation, Antagonizing Light-Mediated Growth Inhibition 1 Kevin M. Folta* Plant Molecular and Cellular Biology Program and Horticultural Sciences Department, University
Green Light Stimulates Early Stem Elongation, Antagonizing Light-Mediated Growth Inhibition 1 Kevin M. Folta* Plant Molecular and Cellular Biology Program and Horticultural Sciences Department, University
Low-Fluence Red Light Increases the Transport and Biosynthesis of Auxin 1[C][W][OA]
![Low-Fluence Red Light Increases the Transport and Biosynthesis of Auxin 1[C][W][OA] Low-Fluence Red Light Increases the Transport and Biosynthesis of Auxin 1[C][W][OA]](/thumbs/72/66726940.jpg) Low-Fluence Red Light Increases the Transport and Biosynthesis of Auxin 1[C][W][OA] Xing Liu*, Jerry D. Cohen, and Gary Gardner Plant Biological Sciences Graduate Program, Department of Horticultural Science
Low-Fluence Red Light Increases the Transport and Biosynthesis of Auxin 1[C][W][OA] Xing Liu*, Jerry D. Cohen, and Gary Gardner Plant Biological Sciences Graduate Program, Department of Horticultural Science
LIGHT SIGNAL TRANSDUCTION IN HIGHER PLANTS
 Annu. Rev. Genet. 2004. 38:87 117 doi: 10.1146/annurev.genet.38.072902.092259 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on June 11, 2004 LIGHT
Annu. Rev. Genet. 2004. 38:87 117 doi: 10.1146/annurev.genet.38.072902.092259 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on June 11, 2004 LIGHT
Seeing without eyes-how plants learn from light
 Seeing without eyes-how plants learn from light by STEPHEN DAY 1. INTRODUCTION Plants detect the intensity, direction, colour, and duration of light and use this information to regulate their growth and
Seeing without eyes-how plants learn from light by STEPHEN DAY 1. INTRODUCTION Plants detect the intensity, direction, colour, and duration of light and use this information to regulate their growth and
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Phototropism: Mechanism and Outcomes
 Phototropism: Mechanism and Outcomes Author(s): Ullas V. Pedmale, R. Brandon Celaya, and Emmanuel Liscum Source: The Arabidopsis Book, Published By: The American Society of Plant Biologists https://doi.org/10.1199/tab.0125
Phototropism: Mechanism and Outcomes Author(s): Ullas V. Pedmale, R. Brandon Celaya, and Emmanuel Liscum Source: The Arabidopsis Book, Published By: The American Society of Plant Biologists https://doi.org/10.1199/tab.0125
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses
 The Plant Journal (2007) 51, 862 873 doi: 10.1111/j.1365-313X.2007.03187.x The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses Sam-Geun Kong 1,, Toshinori
The Plant Journal (2007) 51, 862 873 doi: 10.1111/j.1365-313X.2007.03187.x The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses Sam-Geun Kong 1,, Toshinori
expression of AUX1, LAX1, LAX2 and LAX3 in etiolated hypocotyls.
 Table of Contents Supplementary Material and Methods Supplementary Figure S1: Phototropic response of various aux1lax mutants and expression of AUX1, LAX1, LAX2 and LAX3 in etiolated hypocotyls. Supplementary
Table of Contents Supplementary Material and Methods Supplementary Figure S1: Phototropic response of various aux1lax mutants and expression of AUX1, LAX1, LAX2 and LAX3 in etiolated hypocotyls. Supplementary
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I
 BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
Light Regulation of Flowering Time in Arabidopsis
 Chapter 38 Light Regulation of Flowering Time in Arabidopsis Xuhong Yu and Chentao Lin Introduction Plant development is dependent on not only endogenous conditions but also environmental factors. One
Chapter 38 Light Regulation of Flowering Time in Arabidopsis Xuhong Yu and Chentao Lin Introduction Plant development is dependent on not only endogenous conditions but also environmental factors. One
Blue light affects many aspects of plant growth and development.
 Plant blue-light receptors Chentao Lin Plants have several blue-light receptors, which regulate different aspects of growth and development. Recent studies have identified three such receptors: cryptochrome
Plant blue-light receptors Chentao Lin Plants have several blue-light receptors, which regulate different aspects of growth and development. Recent studies have identified three such receptors: cryptochrome
How plants respond to their environment
 Travis Lick Biology How plants respond to their environment Plants, with their roots firmly fixed in the earth, seem immobile and vulnerable compared to animals, but this does not prevent them from reacting
Travis Lick Biology How plants respond to their environment Plants, with their roots firmly fixed in the earth, seem immobile and vulnerable compared to animals, but this does not prevent them from reacting
Plant Development. Chapter 31 Part 1
 Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Growth and development of Arabidopsis thaliana under single-wavelength red
 1 Supplementary Information 2 3 4 Growth and development of Arabidopsis thaliana under single-wavelength red and blue laser light 5 6 7 8 Authors Amanda Ooi 1 *, Aloysius Wong 1 *, Tien Khee Ng 2, Claudius
1 Supplementary Information 2 3 4 Growth and development of Arabidopsis thaliana under single-wavelength red and blue laser light 5 6 7 8 Authors Amanda Ooi 1 *, Aloysius Wong 1 *, Tien Khee Ng 2, Claudius
** * * * Col-0 cau1 CAU1. Actin2 CAS. Actin2. Supplemental Figure 1. CAU1 affects calcium accumulation.
 Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Light-Independent Phytochrome Signaling Mediated by Dominant GAF Domain Tyrosine Mutants of Arabidopsis Phytochromes in Transgenic Plants W OA
 The Plant Cell, Vol. 19: 2124 2139, July 2007, www.plantcell.org ª 2007 American Society of Plant Biologists Light-Independent Phytochrome Signaling Mediated by Dominant GAF Domain Tyrosine Mutants of
The Plant Cell, Vol. 19: 2124 2139, July 2007, www.plantcell.org ª 2007 American Society of Plant Biologists Light-Independent Phytochrome Signaling Mediated by Dominant GAF Domain Tyrosine Mutants of
Flower Development Pathways
 Developmental Leading to Flowering Flower Development s meristem Inflorescence meristem meristems organ identity genes Flower development s to Flowering Multiple pathways ensures flowering will take place
Developmental Leading to Flowering Flower Development s meristem Inflorescence meristem meristems organ identity genes Flower development s to Flowering Multiple pathways ensures flowering will take place
Name: Class: _ Date: ID: A. AP Photosynthesis Test 2012
 Name: Class: _ Date: ID: A AP Photosynthesis Test 2012 Multiple Choice (3 polnts each) _ Directions: Each of the questions or incomplete statements below is followed by four suggested answers or completions.
Name: Class: _ Date: ID: A AP Photosynthesis Test 2012 Multiple Choice (3 polnts each) _ Directions: Each of the questions or incomplete statements below is followed by four suggested answers or completions.
POTASSIUM IN PLANT GROWTH AND YIELD. by Ismail Cakmak Sabanci University Istanbul, Turkey
 POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
Richik N. Ghosh, Linnette Grove, and Oleg Lapets ASSAY and Drug Development Technologies 2004, 2:
 1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
Intracellular trafficking of photoreceptors during lightinduced signal transduction in plants
 COMMENTARY 475 Intracellular trafficking of photoreceptors during lightinduced signal transduction in plants Ferenc Nagy 1,2, Stefan Kircher 3 and Eberhard Schäfer 3, * 1 Plant Biology Institute, Biological
COMMENTARY 475 Intracellular trafficking of photoreceptors during lightinduced signal transduction in plants Ferenc Nagy 1,2, Stefan Kircher 3 and Eberhard Schäfer 3, * 1 Plant Biology Institute, Biological
Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues
 Research Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues Daniel Kirchenbauer 1 *, Andras Viczian 2 *, Eva Adam 2, Zoltan
Research Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues Daniel Kirchenbauer 1 *, Andras Viczian 2 *, Eva Adam 2, Zoltan
Functional characterization of Arabidopsis phototropin 1 in the hypocotyl apex
 The Plant Journal (2016) 88, 907 920 doi: 10.1111/tpj.13313 Functional characterization of Arabidopsis phototropin 1 in the hypocotyl apex Stuart Sullivan 1, Atsushi Takemiya 2,, Eros Kharshiing 1,3, Catherine
The Plant Journal (2016) 88, 907 920 doi: 10.1111/tpj.13313 Functional characterization of Arabidopsis phototropin 1 in the hypocotyl apex Stuart Sullivan 1, Atsushi Takemiya 2,, Eros Kharshiing 1,3, Catherine
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA
 NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
SYLLABUS, BIO 328D: DISCOVERY LABORATORY IN PLANT BIOLOGY
 SYLLABUS, BIO 328D: DISCOVERY LABORATORY IN PLANT BIOLOGY Instructor: Stan Roux; BIO 16; 471-4238; sroux@uts.cc.utexas.edu; Office hrs: TTh, 10-11:30 AM TA: Ashley Cannon; BIO 15; 471-1074; ashleycannon@utexas.edu;
SYLLABUS, BIO 328D: DISCOVERY LABORATORY IN PLANT BIOLOGY Instructor: Stan Roux; BIO 16; 471-4238; sroux@uts.cc.utexas.edu; Office hrs: TTh, 10-11:30 AM TA: Ashley Cannon; BIO 15; 471-1074; ashleycannon@utexas.edu;
GERMINATION OF THE LIGHT-SENSITIVE SEEDS OF OCIMUM AMERICANUM LINN.
 New Phytol. (1968) 67, 125-129. GERMINATION OF THE LIGHT-SENSITIVE SEEDS OF OCIMUM AMERICANUM LINN. BY C. K. VARSHNEY Department of Botany, University of Delhi {Received 30 June 1967) SUMIVT.'\RY A brief
New Phytol. (1968) 67, 125-129. GERMINATION OF THE LIGHT-SENSITIVE SEEDS OF OCIMUM AMERICANUM LINN. BY C. K. VARSHNEY Department of Botany, University of Delhi {Received 30 June 1967) SUMIVT.'\RY A brief
Ch Plant Hormones
 Ch. 39 Plant Hormones I. Plant Hormones Chemical signals that coordinate the parts of an organism. Only minute amounts are needed to get the desired response. Control plant growth and development by affecting
Ch. 39 Plant Hormones I. Plant Hormones Chemical signals that coordinate the parts of an organism. Only minute amounts are needed to get the desired response. Control plant growth and development by affecting
PLANT HORMONES-Introduction
 PLANT HORMONES-Introduction By convention hormone are said to be a substances whose site of synthesis and site of action are different; the two events are separated by space and time. Hormones are known
PLANT HORMONES-Introduction By convention hormone are said to be a substances whose site of synthesis and site of action are different; the two events are separated by space and time. Hormones are known
Chapter 39. Plant Response. AP Biology
 Chapter 39. Plant Response 1 Plant Reactions Stimuli & a Stationary Life u animals respond to stimuli by changing behavior move toward positive stimuli move away from negative stimuli u plants respond
Chapter 39. Plant Response 1 Plant Reactions Stimuli & a Stationary Life u animals respond to stimuli by changing behavior move toward positive stimuli move away from negative stimuli u plants respond
Author Manuscript Faculty of Biology and Medicine Publication
 Serveur Académique Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections
Serveur Académique Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections
Gravity, light and plant form
 Plant, Cell and Environment {^997) 20, 796-800 Gravity, light and plant form R. P. HANGARTER Department of Biology, Indiana University, Bloomington, IN 47405, USA ABSTRACT Plants have evolved highly sensitive
Plant, Cell and Environment {^997) 20, 796-800 Gravity, light and plant form R. P. HANGARTER Department of Biology, Indiana University, Bloomington, IN 47405, USA ABSTRACT Plants have evolved highly sensitive
Chapter 39 Plant Responses to Internal and External Signals
 Chapter 39 Plant Responses to Internal and External Signals Overview: Stimuli and a Stationary Life Plants, being rooted to the ground, must respond to whatever environmental change comes their way For
Chapter 39 Plant Responses to Internal and External Signals Overview: Stimuli and a Stationary Life Plants, being rooted to the ground, must respond to whatever environmental change comes their way For
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormonal and other chemical effects on plant growth and functioning. Bill Davies Lancaster Environment Centre, UK
 Hormonal and other chemical effects on plant growth and functioning Bill Davies Lancaster Environment Centre, UK Integrating the impacts of soil drought and atmospheric stress High radiant load Reduced
Hormonal and other chemical effects on plant growth and functioning Bill Davies Lancaster Environment Centre, UK Integrating the impacts of soil drought and atmospheric stress High radiant load Reduced
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
AP Biology Plant Control and Coordination
 AP Biology Plant Control and Coordination 1. What is the effect of the plant hormone ethylene on fruit ripening? 2. How does fruit change as it ripens? 3. What is the mechanism behind ripening? 4. Why
AP Biology Plant Control and Coordination 1. What is the effect of the plant hormone ethylene on fruit ripening? 2. How does fruit change as it ripens? 3. What is the mechanism behind ripening? 4. Why
Life Science Journal 2014;11(9) Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis
 Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis Sung-Il Kim 1, Sang Ik Song 3, Hak Soo Seo 1, 2, 4 * 1 Department of Plant Science and Research Institute of Agriculture
Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis Sung-Il Kim 1, Sang Ik Song 3, Hak Soo Seo 1, 2, 4 * 1 Department of Plant Science and Research Institute of Agriculture
Plant Stimuli pp Topic 3: Plant Behaviour Ch. 39. Plant Behavioural Responses. Plant Hormones. Plant Hormones pp
 Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Measuring Colocalization within Fluorescence Microscopy Images
 from photonics.com: 03/01/2007 http://www.photonics.com/article.aspx?aid=39341 Measuring Colocalization within Fluorescence Microscopy Images Two-color fluorescence-based methods are uncovering molecular
from photonics.com: 03/01/2007 http://www.photonics.com/article.aspx?aid=39341 Measuring Colocalization within Fluorescence Microscopy Images Two-color fluorescence-based methods are uncovering molecular
Major Plant Hormones 1.Auxins 2.Cytokinins 3.Gibberelins 4.Ethylene 5.Abscisic acid
 Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
PLANT PHYSIOLOGY. a- Photoperiodism c- Vernalization. b- Auxin precursors d- plant development.
 Benha university Faculty of science Botany Department Micro&chem.. 3 th year Exam. 2013 PLANT PHYSIOLOGY Q1: Define the following:- a- Photoperiodism c- Vernalization b- Auxin precursors d- plant development.
Benha university Faculty of science Botany Department Micro&chem.. 3 th year Exam. 2013 PLANT PHYSIOLOGY Q1: Define the following:- a- Photoperiodism c- Vernalization b- Auxin precursors d- plant development.
Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction
 Development 126, 73-82 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV214 73 Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction
Development 126, 73-82 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV214 73 Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction
Phytochromes are Involved in Elongation of Seminal Roots and Accumulation of Dry Substances in Rice Seedlings
 Rice Science, 2013, 20(2): 88 94 Copyright 2013, China National Rice Research Institute Published by Elsevier BV. All rights reserved DOI: 10.1016/S1672-6308(13)60115-8 Phytochromes are Involved in Elongation
Rice Science, 2013, 20(2): 88 94 Copyright 2013, China National Rice Research Institute Published by Elsevier BV. All rights reserved DOI: 10.1016/S1672-6308(13)60115-8 Phytochromes are Involved in Elongation
Chapter 39. Plant Reactions. Plant Hormones 2/25/2013. Plants Response. What mechanisms causes this response? Signal Transduction Pathway model
 Chapter 39 Plants Response Plant Reactions Stimuli & a Stationary life Animals respond to stimuli by changing behavior Move toward positive stimuli Move away from negative stimuli Plants respond to stimuli
Chapter 39 Plants Response Plant Reactions Stimuli & a Stationary life Animals respond to stimuli by changing behavior Move toward positive stimuli Move away from negative stimuli Plants respond to stimuli
Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues
 Edinburgh Research Explorer Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues Citation for published version: Kirchenbauer,
Edinburgh Research Explorer Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-a expressed in different tissues Citation for published version: Kirchenbauer,
Plant Responses to Internal and External Signals
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 39 Plant Responses to Internal
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 39 Plant Responses to Internal
Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in Arabidopsis W
 The Plant Cell, Vol. 21: 2624 2641, September 2009, www.plantcell.org ã 2009 American Society of Plant Biologists Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in
The Plant Cell, Vol. 21: 2624 2641, September 2009, www.plantcell.org ã 2009 American Society of Plant Biologists Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Phytochromes are Involved in Elongation of Seminal Roots and Accumulation of Dry substances in Rice Seedlings
 Rice Science, 2013, 20(1): Copyright 2012, China National Rice Research Institute Published by Elsevier BV. All rights reserved Phytochromes are Involved in Elongation of Seminal Roots and Accumulation
Rice Science, 2013, 20(1): Copyright 2012, China National Rice Research Institute Published by Elsevier BV. All rights reserved Phytochromes are Involved in Elongation of Seminal Roots and Accumulation
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
A. Stimulus Response:
 Plant Hormones A. Stimulus Response: A house plant on a windowsill grows light. If you rotate the plant, it reorients its growth until its leaves face the window again. The growth of a shoot towards light
Plant Hormones A. Stimulus Response: A house plant on a windowsill grows light. If you rotate the plant, it reorients its growth until its leaves face the window again. The growth of a shoot towards light
Molecular Genetic Analysis of Phototropism in Arabidopsis
 Molecular Genetic Analysis of Phototropism in Arabidopsis Tatsuya Sakai* and Ken Haga Graduate School of Science and Technology, Niigata University, Nishi-ku, Niigata, 950-2181 Japan *Corresponding author:
Molecular Genetic Analysis of Phototropism in Arabidopsis Tatsuya Sakai* and Ken Haga Graduate School of Science and Technology, Niigata University, Nishi-ku, Niigata, 950-2181 Japan *Corresponding author:
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Responses to Light. Responses to Light
 Sensory Systems in Plants Chapter 41 Pigments other than those used in photosynthesis can detect light and mediate the plant s response to it Photomorphogenesis refers to nondirectional, light-triggered
Sensory Systems in Plants Chapter 41 Pigments other than those used in photosynthesis can detect light and mediate the plant s response to it Photomorphogenesis refers to nondirectional, light-triggered
What factors, including environmental variables, affect the rate of transpiration in plants?
 Big Idea 4 Interactions investigation 11 TRANSPIRATION* What factors, including environmental variables, affect the rate of transpiration in plants? BACKGROUND Cells and organisms must exchange matter
Big Idea 4 Interactions investigation 11 TRANSPIRATION* What factors, including environmental variables, affect the rate of transpiration in plants? BACKGROUND Cells and organisms must exchange matter
Chapter 35 Regulation and Transport in Plants
 Chapter 35 Regulation and Remember what plants need Photosynthesis light reactions Calvin cycle light sun H 2 O ground CO 2 air What structures have plants evolved to supply these needs? Interdependent
Chapter 35 Regulation and Remember what plants need Photosynthesis light reactions Calvin cycle light sun H 2 O ground CO 2 air What structures have plants evolved to supply these needs? Interdependent
9- #60 5. Photosynthesis. Sixth edition. D. O. Hall. and. K. K. Rao. Published in association with the Institute of Biology CAMBRIDGE UNIVERSITY PRESS
 9- #60 5 Photosynthesis Sixth edition D. O. Hall and K. K. Rao Published in association with the Institute of Biology CAMBRIDGE UNIVERSITY PRESS Contents General preface to the series Preface to the sixth
9- #60 5 Photosynthesis Sixth edition D. O. Hall and K. K. Rao Published in association with the Institute of Biology CAMBRIDGE UNIVERSITY PRESS Contents General preface to the series Preface to the sixth
The involvement of photosynthesis in inducing bud formation on excised leaf segments of Heloniopsis orientalis (Liliaceae)
 Plant & Cell Physiol. 19(5): 791-799 (1978) The involvement of photosynthesis in inducing bud formation on excised leaf of Heloniopsis orientalis (Liliaceae) Yukio Kato Biological Laboratory, Fukui University,
Plant & Cell Physiol. 19(5): 791-799 (1978) The involvement of photosynthesis in inducing bud formation on excised leaf of Heloniopsis orientalis (Liliaceae) Yukio Kato Biological Laboratory, Fukui University,
Embryo Development. Embryo Development. Embryo Development. Embryo Development (Cont.) Vegetative Plant Development
 Vegetative Plant Development Chapter 37 Embryo Development Begins once the egg cell is fertilized -The growing pollen tube enters angiosperm embryo sac and releases two sperm cells -One sperm fertilizes
Vegetative Plant Development Chapter 37 Embryo Development Begins once the egg cell is fertilized -The growing pollen tube enters angiosperm embryo sac and releases two sperm cells -One sperm fertilizes
Amanda Rawson Independent Research Project Report Bio219 Cell Biology. December 3, 2008
 Evidence That Treatment with Cytochalasin B Increases the Average Velocity of Cytoplasmic Streaming in Amoebae Proteus Amanda Rawson Independent Research Project Report Bio219 Cell Biology December 3,
Evidence That Treatment with Cytochalasin B Increases the Average Velocity of Cytoplasmic Streaming in Amoebae Proteus Amanda Rawson Independent Research Project Report Bio219 Cell Biology December 3,
Supplemental material
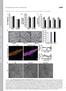 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport
 Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Synergistic and Antagonistic Action of Phytochrome (Phy) A and PhyB during Seedling De-Etiolation in Arabidopsis thaliana
 Int. J. Mol. Sci. 2015, 16, 12199-12212; doi:10.3390/ijms160612199 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Synergistic and Antagonistic
Int. J. Mol. Sci. 2015, 16, 12199-12212; doi:10.3390/ijms160612199 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Synergistic and Antagonistic
Marcelo J. Yanovsky and Steve A. Kay
 LIVING BY THE CALENDAR: HOW PLANTS KNOW WHEN TO FLOWER Marcelo J. Yanovsky and Steve A. Kay Reproductive processes in plants and animals are usually synchronized with favourable seasons of the year. It
LIVING BY THE CALENDAR: HOW PLANTS KNOW WHEN TO FLOWER Marcelo J. Yanovsky and Steve A. Kay Reproductive processes in plants and animals are usually synchronized with favourable seasons of the year. It
Photomorphogenesis in Plants and Bacteria 3rd Edition
 Photomorphogenesis in Plants and Bacteria 3rd Edition Function and Signal Transduction Mechanisms Eberhard Schäfer and Ferenc Nagy (Eds.) PHOTOMORPHOGENESIS IN PLANTS AND BACTERIA 3RD EDITION Photomorphogenesis
Photomorphogenesis in Plants and Bacteria 3rd Edition Function and Signal Transduction Mechanisms Eberhard Schäfer and Ferenc Nagy (Eds.) PHOTOMORPHOGENESIS IN PLANTS AND BACTERIA 3RD EDITION Photomorphogenesis
Antagonistic Regulation of Leaf Flattening by Phytochrome B and Phototropin in Arabidopsis thaliana
 Antagonistic Regulation of Leaf Flattening by Phytochrome B and Phototropin in Arabidopsis thaliana Toshiaki Kozuka 1, Noriyuki Suetsugu 2, Masamitsu Wada 2 and Akira Nagatani 1, * 1 Department of Botany,
Antagonistic Regulation of Leaf Flattening by Phytochrome B and Phototropin in Arabidopsis thaliana Toshiaki Kozuka 1, Noriyuki Suetsugu 2, Masamitsu Wada 2 and Akira Nagatani 1, * 1 Department of Botany,
Phytochrome A is an irradiance-dependent red light sensor
 The Plant Journal (007) 50, 108 117 doi: 10.1111/j.165-1X.007.006.x Phytochrome A is an irradiance-dependent red light sensor Keara A. Franklin *, Trudie Allen and Garry C. Whitelam Department of Biology,
The Plant Journal (007) 50, 108 117 doi: 10.1111/j.165-1X.007.006.x Phytochrome A is an irradiance-dependent red light sensor Keara A. Franklin *, Trudie Allen and Garry C. Whitelam Department of Biology,
LECTURE 04: PHYTOCHROME
 http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ Password: LECTURE 04: PHYTOCHROME Photoreversibility is the most distinctive property of phytochrome 9/19/2017 1 LECTURE OUTCOMES
http://smtom.lecture.ub.ac.id/ Password: https://syukur16tom.wordpress.com/ Password: LECTURE 04: PHYTOCHROME Photoreversibility is the most distinctive property of phytochrome 9/19/2017 1 LECTURE OUTCOMES
Bio 100 Guide 27.
 Bio 100 Guide 27 http://www.offthemarkcartoons.com/cartoons/1994-11-09.gif http://www.cneccc.edu.hk/subjects/bio/album/chapter20/images/plant_growth.jpg http://pgjennielove.files.wordpress.com/2008/06/apical_meristem.png
Bio 100 Guide 27 http://www.offthemarkcartoons.com/cartoons/1994-11-09.gif http://www.cneccc.edu.hk/subjects/bio/album/chapter20/images/plant_growth.jpg http://pgjennielove.files.wordpress.com/2008/06/apical_meristem.png
AP Biology. Transport in plants. Chapter 36. Transport in Plants. Transport in plants. Transport in plants. Transport in plants. Transport in plants
 Chapter 36. Transport in Plants evaporation, adhesion & cohesion negative pressure evaporation, adhesion & cohesion negative pressure transport in phloem bulk flow Calvin cycle in leaves loads sucrose
Chapter 36. Transport in Plants evaporation, adhesion & cohesion negative pressure evaporation, adhesion & cohesion negative pressure transport in phloem bulk flow Calvin cycle in leaves loads sucrose
