Research. M. Aguila Ruiz Sola, Mario Coiro, Simona Crivelli, Samuel C. Zeeman, Signe Schmidt Kjølner Hansen and Elisabeth Truernit.
|
|
|
- Kory Montgomery
- 5 years ago
- Views:
Transcription
1 Research OCTOPUS-LIKE 2, a novel player in Arabidopsis root and vascular development, reveals a key role for OCTOPUS family genes in root metaphloem sieve tube differentiation M. Aguila Ruiz Sola, Mario Coiro, Simona Crivelli, Samuel C. Zeeman, Signe Schmidt Kjølner Hansen and Elisabeth Truernit Department of Biology, Institute of Molecular Plant Biology, ETH Zurich, Universit atsstrasse 2, 8092 Zurich, Switzerland Author for correspondence: Elisabeth Truernit Tel: etruerni@ethz.ch Received: 4 April 2017 Accepted: 16 July 2017 doi: /nph Key words: Arabidopsis, cell differentiation, development, metaphloem, OCTOPUS-LIKE 2 (OPL2), phloem, root, vasculature. Summary Protophloem and metaphloem sieve tubes are essential for transporting carbohydrates and signalling molecules towards sink tissues. OCTOPUS (OPS) was previously identified as an important regulator of protophloem differentiation in Arabidopsis roots. Here, we investigated the role of OCTOPUS-LIKE 2 (OPL2), a gene homologous to OPS. OPL2 expression patterns were analysed, and functional equivalence of OPS and OPL2 was tested. Mutant and double mutant phenotypes were investigated. OPS and OPL2 displayed overlapping expression patterns and a high degree of functional overlap. A mutation in OPL2 revealed redundant functions of OPS and OPL2 in developmental processes in which OPS was known to play a role, notably cotyledon vascular patterning and protophloem development. Moreover, we also uncovered redundant roles for OPS and OPL2 in leaf vascular patterning and, most interestingly, metaphloem sieve tube differentiation. Our results reveal a novel OPS-like protein that, together with OPS, is an important regulator of vascular patterning, root growth and phloem development. OPS and OPL2 are the first genes identified that play a role in metaphloem sieve tube differentiation. Introduction The evolution of a vascular system was a major innovation in the history of land plants. Vascular bundles interconnect all plant organs and consist of two specialized tissue types for longdistance transport phloem and xylem. While xylem transports mainly water and minerals, the phloem is essential for the transport of sugars and signalling molecules. In Arabidopsis thaliana, like in other angiosperms, the vasculature of cotyledons and leaves forms a reticulate network with a complex and highly regulated developmental origin (Scarpella et al., 2006). Arabidopsis roots, in contrast, have one central vascular bundle that branches out into lateral roots. At the root tip, the root meristem cells maintain indeterminate growth by dividing to produce new cells. In Arabidopsis root meristems, vascular stem cells add new cells to a bilaterally symmetric vascular bundle with a central xylem axis flanked by two phloem poles (Bonke et al., 2003) (Fig. 1). Before secondary growth, each phloem pole consists of two sieve elements (protophloem and metaphloem sieve elements), the actual conducting units of the phloem. These are flanked by two companion cells which are responsible for sucrose loading into the sieve element companion cell complex (Stadler & Sauer, 1996; Gottwald et al., 2000; Gould et al., 2012) (Fig. 1). Protophloem and metaphloem sieve tube cells within one phloem pole are ultimately derived from the same stem cell in the root meristem (Rodriguez- Villalon, 2016) (Fig. 1). Companion cells develop from a different cell lineage adjacent to the sieve tube cells (M ah onen et al., 2000). In older parts of the root, in a process called secondary growth, procambial cells resume meristematic activity and add new phloem and xylem cells to the existing vascular bundles (Nieminen et al., 2015) (Fig. 1). Both protophloem and metaphloem sieve tubes are indispensable for the transport of sugars and other solutes towards the root meristem. Solutes are transported in the metaphloem sieve tubes towards the root tip, where they are transferred into the protophloem sieve tubes (Ross-Elliott et al., 2017). Unloading of solutes then occurs through the protophloem sieve tubes (Oparka et al., 1994; Stadler et al., 2005). Therefore, to meet the energy demand of the growing root meristem, protophloem sieve elements differentiate closer to the root tip than all other root cell types. While protophloem sieve tubes lose their transport capacity in the more differentiated parts of the root, the metaphloem sieve tube cells differentiate further away from the root tip and stay functional for long periods (Verbelen et al., 2006;Lucas et al., 2013; Furuta et al., 2014; Rodriguez-Villalon et al., 2014) (Fig. 1). The maturation of protophloem and metaphloem sieve tubes may occur in similar ways (Esau, 1969). To date, however, we 1191
2 1192 Research New Phytologist Fig. 1 A model of Arabidopsis root phloem development. Top, longitudinal section through an Arabidopsis root depicting the phloem sieve tube cell lineage. Starting from the phloem initial, two consecutive divisions lead to a protophloem sieve tube cell file (PP), a metaphloem sieve tube cell file (MP), and a procambium cell file (PC), which eventually starts dividing and is then called cambium (C). Bottom, cross-sections through the root stele at the respective positions in the longitudinal section. Blue, protophloem cell lineage; red, metaphloem cell lineage; green, cambium; yellow, companion cells; dark grey, differentiated xylem cells. The grade of differentiation of protophloem sieve tube members and metaphloem sieve tube members is indicated by colour intensity. have considerably more information about Arabidopsis protophloem cell differentiation. When protophloem cells start to differentiate, their cell walls stain more intensely with the flourescent dye propidium iodide (Truernit et al., 2008, 2012). This easily detectable feature was previously interpreted as an indication of sieve tube cell wall thickening (Truernit et al., 2008). However, recent findings suggest that propidium iodide binds to demethoxylated pectins in cell walls, the presence of which is an indication of imminent cell growth (Rounds et al., 2011). This represents a very early event during the protophloem cell differentiation process (Furuta et al., 2014). In later steps, the nucleus and other organelles are degraded, and cytoplasmic density is reduced, eventually leading to the formation of the highly specialized sieve tube cells (Esau, 1969; Furuta et al., 2014). Recent studies have identified a small number of genetic determinants of protophloem differentiation. OCTOPUS (OPS) has been established as a key regulator of protophloem differentiation (Truernit et al., 2012). In ops, root protophloem cells show two features of irregular differentiation: (1) in the root meristem transition zone, some cells within the differentiating protophloem cell files do not display more intense propidium iodide staining and also retain other features of nondifferentiated protophloem cells, such as an intact nucleus (Truernit et al., 2012) (these cells are hereafter called gap cells ); (2) in sections through the differentiated part of ops roots, some protophloem cells appear undifferentiated, as judged by their positive staining with toluidine blue, which does not stain the lumen of mature sieve elements because of their relatively low cytoplasmic density (Rodriguez- Villalon et al., 2014). As it is technically difficult to follow the development of individual gap cells during root growth, it is unknown whether gap cells are identical to the undifferentiated protophloem sieve tube cells seen in the mature root. Consistent with protophloem defects, ops mutants display long-distance transport problems (Truernit et al., 2012). Moreover, the impaired protophloem sieve tube cell differentiation in ops seems to affect companion cell function. Although ops companion cells appear to have normal morphology, some do not express the gene encoding the sucrose transporter AtSUC2, which is responsible for active sucrose loading into the phloem (Truernit & Sauer, 1995; Truernit et al., 2012; Rodriguez- Villalon et al., 2014). Decreased transport of sugars and signalling molecules, notably auxin, towards the root meristem probably leads to the reduced root growth and increased lateral root density observed in the mutant (Truernit et al., 2012; Rodriguez-Villalon et al., 2015). In addition to the phloemspecific role of OPS in roots, OPS also seems to be involved in the specification of provascular cells in cotyledons, as ops cotyledons have a simpler vascular pattern (Truernit et al., 2012). OPS associates with the plasma membrane, showing polar shootward localization in the developing protophloem and metaphloem cell files of the root (Truernit et al., 2012). While its subcellular localization suggests a role for OPS in polar signalling processes, the actual molecular function of OPS remains largely elusive. It was recently shown (Anne et al., 2015) that OPS can interact with BRASSINOSTEROID INSENSITIVE 2 (BIN2), a key component of the brassinosteroid signalling pathway (Li & Nam, 2002). However, as brassinosteroids apparently have a limited role in protophloem development, it seems unlikely that this interaction is crucial for protophloem sieve tube differentiation (Kang et al., 2017). In recent years, some other mutants with phloem defects similar to ops have been identified. Loss of BREVIX RADIX (BRX), a putative transcription factor, or of COTYLEDON VASCULAR PATTERN 2 (CVP2) together with its homologue CVP2-LIKE 1 (CVL1), two phosphoinositide 5-phosphatases, also results in the appearance of gap cells and nondifferentiated protophloem sieve tube cells in the mature root (Rodriguez-Villalon et al., 2014, 2015). Although loss-of-function phenotypes are similar, current evidence suggests that OPS acts in parallel to these genes (Rodriguez-Villalon et al., 2014, 2015). Moreover, among these genes, OPS seems to be a master regulator of the commitment to protophloem cell fate. First, of all known genes regulating phloem development, OPS is expressed earliest in the phloem cell lineage of roots, that is, already in the phloem lineage stem cell (Rodriguez-Villalon et al., 2014; Kondo et al., 2016). Second, a higher dosage of OPS-GFP expressed in the phloem cell lineage can rescue the gap cell phenotype of brx and cvp2 cvl1, but not vice versa (Truernit et al., 2012; Rodriguez-Villalon et al., 2014, 2015). The fact that sieve elements still develop in ops may point towards genetic redundancy. OPS is part of a small gene family (Nagawa et al., 2006) and closely related to At2g38070, whose function has not been described so far. In this work, we investigate
3 New Phytologist Research 1193 the role of At2g38070, which we call OCTOPUS-LIKE 2 (OPL2). OPS and OPL2 have partially overlapping expression patterns, and the phenotype of ops opl2 double mutants suggests a high degree of functional redundancy between the two genes. Most interestingly, metaphloem sieve tube differentiation is also impaired in the double mutants, pointing towards a hitherto undiscovered role of the two homologous proteins in metaphloem development. Materials and Methods Plant material and growth conditions Arabidopsis thaliana (L.) Heynh (ecotype Columbia) was used as the wild type (WT) in all experiments. ops-2 (SALK_139316) and opl2-1 (SALK_004773C) mutants were from the Salk collection (Salk Institute, San Diego, CA, USA) (Alonso et al., 2003). Plants were grown in growth chambers (16 h : 8 h, light : dark) on soil, or on Murashige and Skoog (MS) medium (half-strength MS salt mixture with vitamins and 0.5 g l 1 2-(Nmorpholino)ethanesulfonic acid, ph 5.7) with 0.6% plant agar for horizontally orientated plates or 1.2% for vertically orientated plates in continuous light. Mutant characterization The information on T-DNA insertions was obtained from the SIGnAL website at Primers for PCR-based genotyping of the mutants are indicated in Supporting Information Table S1. Gene expression levels in the mutants were evaluated by RT-PCR. Seedling RNA was isolated using the RNeasy Mini Kit (Qiagen). A RevertAid First Strand cdna Synthesis Kit (ThermoFisher Scientific, Waltham, MA, USA) was used to generate cdna. RT-PCR primers used are listed in Table S1. Root length, meristem length, and vascular complexity measurements were performed using the public domain NIH IMAGE program (developed at the US National Institutes of Health and available at Constructs for plant transformation Cloning strategies are summarized in Table S2. The Gateway Technology (Invitrogen) was used for all cloning. Destination vectors pb7m24gw.3 and pb7m34gw.0 were used (Karimi et al. 2005; Final vectors were used for stable Agrobacterium tumefaciens-mediated Arabidopsis transformation (Bechtold et al., 1993). Transgenic plants were screened for single insertion lines (by segregation analysis), and three representative lines out of 10 or more were chosen for all further analyses. Tissue preparation and microscopy Floral stem sections were obtained for confocal microscopy as follows: pieces of stem were embedded in 2% agarose, and 60-lm sections were cut with a vibratome (Leica VT1200S) and stained using the modified Pseudo-Schiff-Propidium Iodide technique (Truernit et al., 2008). For staining of roots for confocal microscopy, roots were incubated for 5 min in a 10 lg ml 1 aqueous solution of propidium iodide (Molecular Probes, Eugene, OR, USA) and imaged within 30 min. Roots expressing trans-membrane GFP under control of the AtSUC2 promoter (proatsuc2-tmgfp) (Stadler et al., 2005) were cleared according to Kurihara et al. (2015). To study venation patterns, cotyledons or leaves were cleared in a saturated chloral hydrate/glycerol solution and imaged using dark field microscopy. For GUS staining, tissue was fixed for 20 min in 90% ice-cold acetone and washed once with staining solution. Tissue was then immersed in a 5-bromo-4-chloro-3-indolyl-D-glucuronic acid (X-Gluc)-containing staining solution (50 mm sodium phosphate buffer, ph 7.2, 0.2% Triton X-100, 2 mm potassium ferrocyanide and ferricyanide, and 2 mm X-Gluc), vacuum treated for min and incubated at 37 C until blue staining became visible. For root cross-sections, seedlings were fixed (in 25% glutaraldehyde and 37% formaldehyde in 50 mm sodium phosphate buffer, ph 7.2), embedded in Historesin (Technovit 71100; Heraeus Kulzer, Hanau, Germany), sectioned (8 lm) on a rotary microtome (Leica RM 2155) and visualized by light microscopy. Single and double mutant root cross-sections were stained with toluidine blue (0.25% w/v) for 1 min on the slides before visualization. For iodine staining, rosettes of plants grown in 12 h : 12 h, light : dark for 30 d were kept in 80% ethanol until chlorophyll had been completely removed, and were subsequently stained with Lugol s iodine solution (Sigma) for 90 min and de-stained in water. 5(6)Carboxyfluorescein diacetate (CFDA) experiments were performed according to Oparka et al. (1994). For confocal microscopy, an LSM 780 (Carl Zeiss) was used. Light microscopy was performed with an Imager Z2 (Carl Zeiss). Dark field images were taken with an AxioZoomV16 (Carl Zeiss). Statistical analysis For all box-plots, significant differences were determined using one-way analysis of variance (ANOVA) followed by a Tukey s multiple comparison post hoc test. Significance values for column graphs were calculated using a Student s two-tailed t-test. The significance of differences in venation complexity between lines was determined by categorizing venation complexity into five levels and using Fisher s exact test as implemented in R v.3.3.0, with post hoc testing using Bonferroni correction as implemented in the function pairwisenominalindependence in the package RCOM- PANION ( html). Results OPS and OPL2 have partially overlapping expression patterns The Arabidopsis OPS gene family consists of five members, which can be divided into two classes (Fig. S1) (Nagawa et al.,
4 1194 Research New Phytologist 2006). The three class I genes (OPS, OPL1 and OPL2) were described as being expressed in vascular tissue (Nagawa et al., 2006). However, in an initial analysis, we only found OPL2 to be expressed in developing phloem cells, while OPL1 seemed to be expressed in the xylem in mature plant tissue. Thus, to further understand the role of OPS and its homologues in phloem development, we concentrated our investigations on OPL2. To compare directly OPS and OPL2 expression, we cloned the promoter regions of the two genes, and fused each promoter upstream of the coding sequence of two different reporter genes, one encoding an endoplasmic reticulum-targeted YELLOW FLUORESCENT PROTEIN (YFPER), and the other b-glucuronidase (GUS). In addition, we generated constructs in which the respective promoters were used to drive expression of translational fusions of OPS and OPL2 with GREEN FLUORESCENT PROTEIN (GFP). For OPS, we used the same promoter region as described in Truernit et al. (2012). For OPL2 we isolated a 2704-bp fragment directly upstream of the start codon of the gene. We generated transgenic Arabidopsis lines harbouring the reporter constructs, and investigated the expression patterns of the reporter genes at different developmental stages, mainly focussing on those tissues where OPS was known to play a role (Truernit et al., 2012). Expression patterns in all reporter lines (n 10) were similar, and three lines each were chosen for more careful analyses. As described previously (Truernit et al., 2012), in developing embryos OPS expression was restricted to provascular cells in cotyledons and hypocotyls from the early torpedo stage onwards (Fig. 2a,b). By contrast, OPL2 promoter activity was seen in all subprotodermal layers and only in the cotyledons during the torpedo stage (Fig. 2g). From the walking stick stage onwards, OPL2 expression was stronger in cotyledon provascular cells than in surrounding tissues (Fig. 2h). A similar expression trend was observed in young leaf primordia, where OPS expression was confined to provascular tissue (Fig. 2c) while OPL2 was expressed strongly in all subepidermal layers (Fig. 2i). In older leaf primordia, OPS was expressed specifically in vascular bundles, while OPL2 was still expressed in the whole central part of the leaf (Fig. 2d,j). In more mature leaves, however, expression of both genes was confined to the vasculature, and expression of OPL2 seemed to be weaker than that of OPS (Fig. 2e,k). To corroborate this, we looked at stem sections, where vascular bundles can be analysed more easily. Here, both genes were expressed specifically in the phloem, and OPS was also expressed at a higher level than OPL2 (Fig. 2f,l). In root tips, we investigated expression from 2 to 5 d after germination using the translational fusion reporter constructs. As expected, OPS was strongly expressed at all developmental stages in the protophloem cell files, starting from the phloem initial, and more weakly in the incipient metaphloem cell file (Fig. 2m, n). In proopl2:opl2-gfp lines we saw weak but clear expression of the fusion protein throughout the whole root meristem, including the developing protophloem and metaphloem cell files (Fig. 2q,r). In the mature part of the root, both genes were strongly expressed in two vascular files (Fig. 2o,s). Sections through the mature root revealed OPS and OPL2 expression in cells directly adjacent to the differentiated primary phloem cells. These cells probably give rise to secondary phloem cells (Fig. 2p,t). To summarize, in all growing organs investigated, expression of the two genes overlapped in the OPS expression domain. In developing cotyledons and leaves, OPL2 was initially expressed in a broader area than OPS, which was already confined to the vascular tissue. At more mature stages, both genes were expressed specifically in the phloem. In roots, both genes were expressed in developing secondary phloem cells in the differentiated part of the root, but in the root meristem only OPS was distinctly expressed in primary phloem cells, while OPL2 showed weak expression throughout the meristem, including the developing phloem cell files. Functional overlap of OPS and OPL2 OPS and OPL2 amino acid sequences share 58% identity. Many parts of the two proteins are similar, suggesting that they possibly have a similar biochemical role (Fig. 3a). To test this hypothesis, we first studied the OPL2 subcellular localization in more detail. Interestingly, just as was shown for OPS (Truernit et al., 2012), the OPL2 promoter-driven OPL2-GFP fusion appeared to be predominantly associated with the plasma membrane (Figs 2q,r, 3b d). Like OPS, it was localized to the shoot-ward side of the developing phloem cells and in secondary phloem files (Fig. 3b d). Also, in the other cell types of the root meristem OPL2-GFP fluorescence appeared to be stronger on the shoot-ward side of the cells, but lateral localization was also observed in some cells (Figs 2q,r, S2). The strongest evidence for functional overlap of OPL2 and OPS, however, came from a complementation experiment, in which we expressed OPL2-GFP under control of the OPS promoter in ops plants. The roots of these plants were longer than in ops, and almost as long as in WT, demonstrating that OPL2 can at least partially take over the function of OPS in root growth when expressed in the OPS expression domain (Fig. 3e,f). In addition, cotyledon vascular complexity was also partially recovered (Fig. 3g). Taken together, these findings demonstrate functional overlap between OPL2 and OPS, suggesting that the most distinguishing feature between the two proteins is their expression pattern. Leaf venation patterning and root growth are more severely affected in the ops opl2 double mutant Functional overlap and partially overlapping expression patterns between OPS and OPL2 prompted us to investigate the mutant phenotype of OPL2, as well as that of double mutants lacking both proteins. We obtained a T-DNA knockout line for OPL2, which we named opl2-1 (called opl2 hereafter), predicted to contain an insertion at 299 base pairs after the translational start codon of the gene and thus very unlikely to produce a functional OPL2 protein. Indeed, no full-length OPL2 transcript was found in this line (Fig. S3). Next, we generated ops opl2 double mutants using the well-characterized OPS mutant allele ops-2 (called ops hereafter) (Truernit et al., 2012; Rodriguez-Villalon et al., 2014).
5 New Phytologist Research 1195 (a) (c) (d) (f) (b) (e) Fig. 2 Expression patterns of OCTOPUS (OPS) and OCTOPUS-LIKE 2 (OPL2). (a c, g i) Arabidopsis lines expressing the gene encoding for endoplasmic reticulum targeted YELLOW FLUORESCENT PROTEIN (YFPER) under control of the OPS promoter (a c, o) or the OPL2 promoter (g i, s). (d f, j l) Lines expressing b-glucuronidase (GUS) under control of the OPS promoter (d f, p) or the OPL2 promoter (j l, t). (m, n, q, r) Lines expressing OPS-Green Fluorescent Protein (GFP) (m, n) or OPL2-GFP (q, r) under control of the respective promoter. (a, g) Late torpedo stage embryos showing OPL2 expression in a broader domain within the cotyledon than OPS expression. (b, h) Cotyledons of late walking stick stage embryos showing OPS expression in the vasculature and OPL2 expression predominantly in the vasculature. (c, i) Leaf primordia imaged 3 d after germination (dag) with OPS expression being restricted to the provascular cells, while OPL2 expression is broader. (d, j) Expression in sections of leaf primordia at 5 dag. (e, k) Expression in young leaves at 10 dag. (f, l) Expression in the phloem of vascular bundles of floral stems. (m, n) Five-day-old root tips show protophloem expression of OPS-GFP, while OPL2 is weakly expressed throughout the meristem (q, r). *, protophloem cell file; +, metaphloem cell file. (m, q) Overlay images of GFP expression (green) and red staining of cell walls with propidium iodide. (n, r) GFP channel images only. (o, s) Mature part of a 5-d-old root shows expression of both genes in the phloem files. (p, t) Expression in sections through the mature part of 5-d-old roots. Blue triangles, phloem poles; red triangles, xylem poles. Protoxylem and first two metaxylem cells are already differentiated at this stage. Bars, 50 lm. (g) (i) (j) (h) (k) (m) (n) (o) * * (p) (q) (r) (s) * * (t) (l) Root growth in opl2 was not affected, while ops roots were shorter than WT roots, as previously reported (Truernit et al., 2012). However, the ops opl2 double mutant roots were significantly shorter than ops roots (Fig. 4a,b). This was consistent with a more severe reduction in root meristem size in the double mutant (Fig. 4c). Also, lateral root density was significantly higher in the double mutant than in ops, which showed the expected small increase in lateral root density (Rodriguez-Villalon et al., 2015) (Fig. 4d). Complementation analysis demonstrated that the mutation in OPL2 was the underlying cause of the observed phenotypes (Fig. S4). Cotyledon vascular complexity was also more heavily affected in the double mutant than either single mutant. In ops plants, the majority (57%) of cotyledons showed two closed vascular loops, while WT cotyledons had most frequently four closed vascular loops (47%). The majority (52%) of ops opl2 mutants had only two open loops, and only two of 79 examined plants had two completed vascular loops which was the most complex
6 1196 Research New Phytologist (a) (b) (e) (c) (d) (g) (f) Fig. 3 Functional overlap of OCTOPUS (OPS) and OCTOPUS-LIKE 2 (OPL2). (a) Alignment of the amino acid sequences of OPS and OPL2. The top row shows all amino acids that are identical between OPL2 and OPS. (b d) Polar membrane-associated localization of OPL2-GFP in proopl2:opl2-gfp lines in (b) a secondary phloem file in the mature root part of a 6-d-old plant (arrows indicate OPL2-GFP localization), and (c, d) a developing protophloem file (asterisk) in the root meristem of a 5-d-old plant. (d) The same image as (c) but here the rainbow lookup table was used, which colour-codes fluorescence intensity levels (dark blue, low; red, high; see bar on the right hand side of (d)). (e) Example of seedlings used for root length measurements shown in (f). (f) Root length of 8-d-old wild-type (WT) and ops roots, as well as two ops lines expressing proops:opl2-gfp ( OPL2 complemented lines numbers 2.4 and 6.3). The horizontal lines show the median of the data sets. The whiskers represent interquartile range. Statistically significant (P < 0.05) differences are indicated by different lowercase letters. Number of roots measured: WT, 52; ops, 53; 2.4, 49; line 6.3, 51. OPL2 complemented lines have significantly longer roots than ops (P = for 2.4 and for 6.3). (g) Per cent occurrence of cotyledon vascular phenotypes depicted on the x-axis (8-d-old plants; same lines as in e). Number of cotyledons analysed: WT, 97; ops, 104; line 2.4, 93; line 6.3, 95. Cotyledon venation of OPL2 complemented lines is significantly more complex when compared to ops (P = for 2.4 and for 6.3). The right panels show typical examples of cotyledon venation in the different genotypes. Bars: (b d) 50 lm; (e) 500 lm. cotyledon vascular phenotype seen in the double mutant. opl2 cotyledons showed a phenotype between those of WT and ops, with 7% of cotyledons having two completed loops, 14% four completed loops, and the rest showing intermediate phenotypes (Fig. 5a). Cotyledon size in the different genotypes was similar, thus excluding the possibility that the reduced vascular complexity was simply attributable to a reduced cotyledon size (Fig. S5). Prompted by our findings in cotyledons, we also looked at the vascular complexity (branch points per mm 2 ) of true leaves numbers 2 and 3, which were fully expanded at the time of analysis (Fig. S5). Indeed, the leaf vasculature of the double mutant was notably less complex than in WT or the single mutants (Fig. 5b, c). We also found a small but significant decrease in vascular complexity in ops (Fig. 5b,c). In general, ops opl2 plants grew more slowly and never reached the bolt height of WT plants, but they were viable and set seed (Fig. 5d). Leaves in the double mutant were shorter and also grew more slowly (Fig. S5). We also investigated vascular bundles in floral stem sections. Like in ops-1 (Truernit et al., 2012), the phloem : xylem ratio was unaffected in any of our single and double mutants (Fig. S6).
7 New Phytologist Research 1197 Fig. 4 Root phenotypes of wild type (WT), octopus (ops), octopus-like2 (opl2) and ops opl2. (a) Phenotypes of 8-d-old Arabidopsis seedlings. (b) Root length of 8-d-old roots. Number of roots measured: WT, 61; ops, 62; opl2, 62; ops opl2, 67. Double mutant roots are significantly (P = ) shorter than ops roots. (c) Meristem size of 6-d-old roots measured from the quiescent centre to first elongated cortex cell (2 times as long as the cell before). Number of roots measured: WT, 17; ops, 20; opl2, 32; ops opl2, 28. Double mutant meristems are significantly (P = ) shorter than ops meristems. (d) Number of lateral (lat.) roots per cm of lateral root zone in 12-d-old plants. Number of roots quantified: WT, 22; ops, 12; opl2, 22; ops opl2, 12. Lateral root density is significantly (P = ) increased in double mutants when compared with ops. The horizontal lines in (b d) show the median of the data sets. The whiskers represent interquartile range. Statistically significant (P < 0.05) differences are indicated by different lowercase letters. Bar, 500 lm. In summary, the root and shoot phenotypes of ops opl2 mutants were more severe than ops phenotypes, thus revealing a high degree of redundancy between OPS and OPL2 in root growth and cotyledon and leaf vascular complexity. Nevertheless, our results indicate a greater role for OPS in these processes, as the opl2 mutant was like the WT in all aspects examined, except for the minor alterations in cotyledon vascular patterning. At the same time, we can rule out the possibility that potentially altered OPS expression could compensate for loss of OPL2 and vice versa. Expression pattern and localization of OPS were not changed in opl2, and OPL2 expression was also normal in ops (Fig. S7). Protophloem differentiation in ops opl2 is delayed relative to other root cell types The most conspicious phenotype of ops is its failure to produce continuously differentiated root protophloem sieve tube cell files (Truernit et al., 2012). The failure of some cells (gap cells) in ops protophloem files in the root transition zone to stain intensely with propidium iodide is usually regarded as a first indication of defects in protophloem differentiation (Truernit et al., 2012; Rodriguez-Villalon et al., 2014). Considering the observed redundancy of OPS and OPL2 in regulating root growth, we expected to find a higher frequency of gap cells in the double mutant. However, this was not the case. While 50% of ops roots in our experiment displayed gap cells, only 6% of the double mutants had this defect (Fig. 6a d,i). The same observation was made when we introduced the Phloem Development 1 (PD1) marker line (Bauby et al., 2007) into the mutant backgrounds. In root tips, PD1 marker expression is specific to the developing protophloem cell file and starts shortly before protophloem cells commence elongation (Fig. 6e). Protophloem files in ops frequently displayed cells which did not express the PD1 marker (32/68), while this feature was only rarely seen in ops opl2 protophloem files (4/34), and never in WT (0/41) or opl2 (0/45) (Fig. 6e h). Yet, in WT and opl2 root meristems, stronger protophloem propidium iodide cell wall staining was observed at about half the distance between the quiescent centre (QC) and the start of rapid cortex cell elongation (Fig. 6a,c,j). By contrast, intense propidium iodide staining of protophloem cell walls and cortex cell elongation occurred roughly at the same distance from the QC in ops opl2 roots, while ops roots showed an intermediary phenotype (Fig. 6b,d,j). Thus, while protophloem cells in WT and the mutant lines displayed features of differentiation (marker expression, stronger cell wall propidium iodide staining, and elongation) at roughly the same distance from the QC (Fig. 6a h), other root cell types in ops and the double mutant were elongating considerably closer to the QC than in WT and opl2 (Fig. 6a h,j). This can be interpreted as a relative delay in protophloem differentiation in ops and even more in the double mutant. A novel role for OPS and OPL2 in metaphloem development As a consequence of their reduced cytoplasmic density, fully differentiated sieve tube cells appear white in toluidine blue-stained root sections (Rodriguez-Villalon et al., 2014). To investigate if phloem sieve tube cells appeared normally differentiated in the mature root of the mutants, we generated root sections at a
8 1198 Research New Phytologist (a) (c) (d) (b) Fig. 5 Shoot phenotypes of Arabidopsis wild-type (WT), octopus (ops), octopus-like2 (opl2) and ops opl2. (a) Frequency of cotyledon vascular phenotypes depicted on the x-axis (8-d-old plants). Number of cotyledons analysed: WT, 95; ops, 105; opl2, 74; ops opl2, 79. The cotyledon vascular pattern is significantly less complex in the double mutant when compared with ops (P = ). The right panels show typical examples of cotyledon venation in the genotypes indicated. (b) Typical examples of leaf no. 2 venation phenotypes in the genotypes indicated. In the right half of the leaf, branch points are labelled with light orange dots. (c) Number of branch points per mm 2 in leaf no. 2 and leaf no. 3. The horizontal lines show the median of the data sets. The whiskers represent interquartile range. Statistically significant (P < 0.05) differences are indicated by different lowercase letters. ops leaves have significantly fewer branch points than WT (P = 0.03 (leaf 2), 0.01 (leaf 3)), and double mutant leaves have significantly fewer branch points than ops leaves (P = 0.17 (leaf 2), (leaf 3)). Number of leaves analysed (leaf 2/leaf 3): WT, 8/6; ops, 5/5; opl2, 9/9; ops opl2, 5/6. (d) Phenotypes of 50-d-old plants grown on soil. Bar, 5 cm. position where proto- and metaphloem sieve tubes are usually mature (where protoxylem and the first two metaxylem cells are differentiated) and analysed an average of 70 sections in two experiments (Fig. 7a d). Although there was a small but significant reduction in the number of cells in the double mutant stele (Fig. 7f), the organization of the phloem poles in the mutants looked like that of WT. To our surprise, we not only found an increase in failed protophloem differentiation in the ops opl2 double mutant (28% in ops opl2, 12%inops, and 0% in opl2 and WT), but also a considerably higher percentage of failed metaphloem sieve tube cell differentiation: cells that had the shape and position of metaphloem sieve tube cells were not differentiated properly, as judged from their positive staining with toluidine blue (31% in ops opl2 compared with between 6% and 8% in ops, opl2, and WT) (Fig. 7g). This phenotype was not only seen at this position, but also in even more mature parts of the root, excluding the possibility that metaphloem sieve tube differentiation was just delayed (Fig. 7e). We also observed an increase in the relative length of AtSUC2 expression gaps in roots from c. 7%inops to c. 14% in the double mutant (Fig. 7h). Using plants expressing membrane-targeted GFP under control of the companion cell-specific AtSUC2 promoter (Stadler et al., 2005), we monitored all four companion cell files in mature root parts. Expression in WT and opl2 was always seen in all four files, while expression in the mutants was frequently interrupted in one or more of the files, with ops opl2 showing the most severe interruptions (Fig. 7i l). Taken together, the results demonstrate that ops opl2 mutants showed an increase in developmental defects of all phloem cell types. Protophloem and companion cell differentiation defects in the mature root were more severe than in ops. Moreover, our results show that OPS and OPL2 also play an important role in metaphloem sieve tube differentiation. Physiological consequences of phloem defects We reasoned that the phloem defects in the double mutant would also lead to severe phloem transport problems (Truernit et al., 2012). To monitor this, we crossed lines expressing soluble GFP under control of the AtSUC2 promoter (Imlau et al., 1999) into our mutant lines. Here, considerably less GFP was arriving in the root tips of ops opl2 when compared with the other genotypes and WT (20 plants each analysed) (Fig. 8a). To obtain direct evidence of phloem transport defects, we used the fluorescent phloem-mobile dye CFDA (Grignon et al., 1989), which has been widely used to monitor phloem transport (Oparka et al., 1994; Knoblauch & van Bel, 1998). When applied to cotyledons, CFDA clearly labelled the two phloem strands in WT (10 of 10), opl2 (9 of 10), and most ops roots (8 of 10) within 1 h after application (Fig. 8b). In the double mutant,
9 New Phytologist Research 1199 (a) (b) (c) (d) (e) (f) (g) (h) Fig. 6 Protophloem defects in root tips of Arabidopsis wild-type (WT), octopus (ops), octopus-like2 (opl2) and ops opl2. (a d) Examples of 6-d-old propidium iodidestained roots used for quantifications shown in (i, j). Genotypes are indicated in the pictures. (e h) Roots expressing the protophloem-specific Phloem Development 1 (PD1) green fluorescent protein (GFP) marker. Genotypes are indicated in the pictures. Asterisks mark the protophloem cell file, and the bracket shows a gap. Yellow arrowheads mark the first protophloem cell with more intense propidium iodide staining in (a d). White arrowheads mark the first protophloem cell expressing GFP in (e h). Green arrowheads mark the first rapidly elongating cortex cell in (a h). (i) Number of gaps in protophloem cell files in the different genotypes (gaps/number of samples analysed). (j) Ratio calculated using the length from the quiescent centre to the first elongated cortex cell, divided by the length from the quiescent centre to the first elongated protophloem (PP) cell. The horizontal lines show the median of the data sets. The whiskers represent interquartile range. Statistically significant (P < 0.05) differences are indicated by different lowercase letters. Number of samples analysed: WT, 17; ops, 20; opl2, 32; ops opl2, 28(P = (WT ops) and (ops ops opl2)). Bars, 20 lm. (i) (j) we frequently (5 of 10) found that only one phloem strand was transporting the dye. Moreover, there seemed to be less dye moving in the phloem strands (judged by fluorescence intensity), and the labelled area was more diffuse than in WT, indicating a potential leaking of solutes out of the phloem files. As phloem transport problems lead to accumulation of carbohydrates in leaves (Sonnewald et al., 1991; Krapp & Stitt, 1995), we visualized leaf starch content at the end of a 12-h night by iodine staining. In Arabidopsis leaves, starch accumulates during the day and serves as a carbohydrate source during the night, thus
10 1200 Research New Phytologist being fully degraded by the end of the night (Stitt & Zeeman, 2012). As expected, we did not detect any starch in WT or opl2 leaves at the end of the night, but we found slightly more starch in ops leaves and a considerable amount of starch in older double mutant leaves (five plants per genotype) (Fig. 8c). These results indicate that, in the double mutant, starch was not fully consumed by the end of the night, which also points to defects in carbohydrate distribution. Altogether, these observations are consistent with severe phloem transport problems in the double mutant. Discussion In this work, we examined the role of OPL2, a homologue of OPS, whose function was unknown. In growing organs, the expression of the two genes overlapped in the OPS expression domain, and OPL2, like OPS, was found to be a predominantly membrane-associated polar-localized protein. Moreover, OPL2 could substitute for OPS when expressed under the control of the OPS promoter. A high degree of functional redundancy of the two genes was also revealed in our analysis of the ops opl2 mutant. Altogether, these results suggest that the molecular functions of the two proteins are very similar. Consistent with a role in vascular patterning, OPS and OPL2 were expressed in developing cotyledons and leaves before the visible appearance of procambium cells (West & Harada, 1993; Scarpella, 2004). It was previously shown that OPS is important for proper cotyledon vascular patterning (Truernit et al., 2012; Roschzttardtz et al., 2014). Here, we found that opl2 plants also had a mild cotyledon vein patterning phenotype, and ops opl2 displayed a drastic reduction in cotyledon vascular complexity when compared with the single mutants. We also discovered a role for OPS and OPL2 in determining the vascular complexity of true leaves. ops opl2 leaf vasculature had fewer branch points than the WT or either single mutant. In this developmental process, OPS plays the major role, as the ops mutant already shows a mild mutant vascular phenotype, while opl2 plants look like WT. In root meristems, OPS and OPL2 expression overlapped in the developing proto- and metaphloem sieve tube cell files. Root growth of opl2 was normal. However, the loss of OPL2 exacerbated the root growth defects in the ops mutant. Thus, although OPL2 is expressed more broadly in the root meristem, it seems that expression of both genes solely in the phloem cell lineage is important for proper root growth. Moreover, ops root growth was restored by expressing OPL2 in the OPS expression domain. Therefore, a higher dose of an OPS-like protein in the developing phloem cell files compared with the surrounding tissues appears to be sufficient for proper root development to occur. These observations are in line with the finding that a higher dose of OPS in the phloem cell files can also rescue root growth defects in other phloem mutants (Rodriguez-Villalon et al., 2014, 2015). Taken together, our results are further evidence for a key role of OPS(-like) genes in root growth through promoting vascular development. Considering the overlapping expression patterns in the root phloem files, and the high degree of redundancy between OPS and OPL2, it was not too surprising that the number of undifferentiated protophloem sieve tube cells in ops opl2 root sections was increased relative to ops. In addition, however, the double mutant also revealed a previously unknown function for OPS and OPL2 in root metaphloem sieve tube cell differentiation: at a longitudinal position where metaphloem sieve tube cells are usually differentiated, they appeared to be immature in c. 30% of double mutant individuals analysed. Thus, OPS and OPL2 regulate differentiation entry of both proto- and metaphloem sieve tube cells. To our knowledge, no molecular determinants of metaphloem sieve tube differentiation have been pinpointed and no mutants defective in metaphloem sieve tube development have been identified to date. Thus, OPS and OPL2 may represent a first entry point towards identifying the factors that control metaphloem sieve tube development. Our lack of information about metaphloem development may be a consequence of the essential nature of this tissue, which does not allow for the identification of strong phloem development mutants. Moreover, metaphloem cells are difficult to visualize, and, to our knowledge, no marker gene specific for the metaphloem sieve tube cell lineage has been identified. For these reasons, metaphloem development is largely unstudied. Although proto- and metaphloem sieve tube cells in roots originate from the same initial, there is evidence that differentiation of these two cell types is driven by independent positional cues. The most obvious argument for this is that metaphloem sieve tube cells differentiate later than protophloem sieve tube cells (Esau, 1969). Moreover, in ops and brx, the second division in the phloem cell lineage, which leads to the generation of proto- and metaphloem cell files, is frequently missing. Nonetheless, cells with the characteristics of metaphloem sieve tube cells appear in the correct location in these mutants, suggesting that other cells, probably procambial cells, can adopt metaphloem sieve tube cell function when located in the right position (Rodriguez-Villalon et al., 2014). Although differentiation signals for the two cell types may be distinct, the fact that OPS together with OPL2 can influence both proto- and metaphloem sieve tube differentiation suggests that there may be similar mechanisms acting downstream of these signals, which drive the differentiation of both cell types. Considering all this, it may well be that more factors affecting protophloem development also play a yet undetected role in metaphloem development. Our results also further support the idea that companion cell differentiation is dependent on proper sieve tube development (Rodriguez-Villalon et al., 2014), as the decreased AtSUC2 marker gene expression in companion cells correlated with the increase in sieve tube defects in ops opl2 compared with ops. For ops it was shown that lack of AtSUC2 expression and/or the occurrence of undifferentiated protophloem cells have an impact on phloem transport (Truernit et al., 2012; Rodriguez-Villalon et al., 2015). The increased lateral root density, the higher amount of starch in leaves, and the weaker accumulation of a phloem mobile dye in the phloem strands in the double mutant relative to ops are all indications for even more severe phloem transport problems in the double mutant. It was proposed that functional protophloem at the start of the transition zone drives
11 New Phytologist Research 1201 (a) (f) (g) (b) (i) (j) (c) (d) (k) (l) (e) (h) Fig. 7 Metaphloem defects in mature root parts of wild type (WT), octopus (ops), octopus-like2 (opl2) and ops opl2. (a e) Toluidine blue-stained root sections of 5-d-old roots at a position where protoxylem and the first two metaxylem cells are already differentiated (a d) and at a position where secondary growth has started (e). Black arrowheads, protophloem sieve tube cell positions; white arrowheads, metaphloem sieve tube cell positions. Genotypes are indicated in the pictures. (f) Number of cells in the pericycle and stele in sections from the same stage as those shown in (a d). Error bars show 95% confidence intervals. Number of sections analysed: WT, 10; ops, 20; opl2, 20; ops opl2, 20. The double mutant has a significantly (P = 0.003) lower stele cell number than ops. (g) Quantification of nondifferentiated proto- and metaphloem sieve tube cells observed in sections. Error bars, SD. Number of sections analysed (total of two experiments): WT, 82; ops, 70; opl2, 73; ops opl2, 69. (h) Missing Arabidopsis thaliana SUCROSE TRANSPORTER 2 (AtSUC2) marker gene expression relative to total root length. AtSUC2 marker expression in the double mutant is more affected than in ops (P = 0.03). Error bars show 95% confidence intervals. Number of roots analysed: WT, 9; ops, 14; opl2, 10; ops opl2, 17. (i l) Maximum intensity projections of confocal laser-scanning images taken at different z-positions parallel to the longitudinal axis of roots expressing membrane-localized green fluorescent protein (GFP) under control of the AtSUC2 promoter. The inserts on the right show optical cross-sections through the stele of these roots at different positions along their axis (indicated with arrows). All four companion cells are in the picture. Orange arrows, positions where AtSUC2 expression is missing. Bars, 20 lm.
12 1202 Research New Phytologist (a) (b) (c) Fig. 8 Physiological consequences of phloem defects in octopus octopus-like2 (ops opl2). (a) Seven-day-old Arabidopsis root tips of plants expressing soluble green fluorescent protein (GFP) under control of the Arabidopsis thaliana SUCROSE TRANSPORTER 2 (AtSUC2) promoter. Shown are a transmitted light image in the top row and the corresponding GFP image in the bottom row. More GFP arrives in wildtype (WT) and opl2 root tips. (b) Mature root parts of 19-d-old plants displaying the distribution of 5(6)carboxyfluorescein diacetate (CFDA) applied to individual leaves. (c) Thirty-day-old plants of indicated genotypes stained for the presence of starch with iodine (dark blue colour) at the end of an 8-h night period. Genotypes are indicated in the figure. Exposure time and microscope settings for all images of the same experiment were the same. Bars: (a, b) 100 lm; (c) 1 cm. elongation of other root cell types by delivering sugars and signalling molecules to the root tip (Zhu et al., 1998; Stadler et al., 2005; Verbelen et al., 2006). Lower efficiency in phloem transport is thus also likely to result in slower root meristem growth, which is one of the phenotypes observed in ops, and even more strongly in ops opl2. Considering the higher number of nondifferentiated protophloem cells in the mature part of ops opl2 roots, the rarer occurrence of gap cells in the root tip of these mutants seems to be counterintuitive. However, while it is a reasonable assumption that the gap cells in the ops protophloem transition zone correlate with the protophloem defects seen in the mature part of the root, because of technical limitations this link has never been demonstrated and may not exist (Truernit et al., 2012; Rodriguez- Villalon et al., 2014). Gap cells were defined as cells within the developing protophloem cell file whose cell walls do not stain intensely with propidium iodide. The differentiation state of phloem cells in the mature root, in contrast, was scored by toluidine blue staining. This stain is used to monitor cytoplasmic density, the reduction of which represents a later step of phloem sieve tube differentiation. Therefore, the absence of gap cells in the root transition zone may not necessarily mean that protophloem development proceeds normally. Given the interdependance between phloem transport and root growth, it is difficult to discriminate between causes and consequences of the phloem defects in the root tips of our mutants. As discussed, problems in phloem transport will probably lead to reductions in root growth, and this is what can be seen in ops opl2. In particular, the roots grow more slowly, root meristem size is reduced, and thus the cells surrounding the protophloem cell file stop dividing closer to the root tip than in WT. In this scenario, even if protophloem differentiation is impaired, slower root growth may allow more time for developing protophloem cells to complete the first steps of differentiation before they are displaced out of the root tip. This theory is consistent with the observed absence of gap cells close to the meristem in spite of clearly visible phloem defects in sections through the differentiated part of ops opl2 roots. We speculate that the slower root meristem growth in ops opl2 may compensate for the delayed protophloem differentiation in the transition zone. ops mutants, in contrast, may represent an intermediate state, in which phloem transport is efficient enough to drive root meristem activity sufficiently fast, so that a delay in protophloem differentiation results in the occurrence of gap cells, whereby some cells in the developing protophloem cell file are displaced from the protophloem transition zone prematurely, and thus cannot complete the first steps of differentiation (i.e. cell wall loosening) in time. Taken together, our results suggest that, while the appearance of gap cells in the root tip may be a general indication for defective or delayed phloem differentiation, it may be the later stages of protophloem and metaphloem differentiation that are actually impaired in ops and ops opl2. Acknowledgements We acknowledge ScopeM (ETH Zurich) for providing microscopy facilities. N. Kornet kindly provided us with the NK102 vector. We appreciate the help of Susanne Meile with the opl2 complementation experiments. We are very grateful to Antia Rodriguez-Villalon and Elizabeth Kastanaki for help with producing root sections, and to Michael Knoblauch for advice on CFDA experiments. We also wish to thank Antia Rodriguez- Villalon and Bojan Gujas for scientific discussions, Leo B urgy for help with statistics, and David Seung for critical reading of the manuscript. This work was supported by ETH Zurich Research Grant ETH
The mode of development in animals and plants is different
 The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
Lecture 4: Radial Patterning and Intercellular Communication.
 Lecture 4: Radial Patterning and Intercellular Communication. Summary: Description of the structure of plasmodesmata, and the demonstration of selective movement of solutes and large molecules between
Lecture 4: Radial Patterning and Intercellular Communication. Summary: Description of the structure of plasmodesmata, and the demonstration of selective movement of solutes and large molecules between
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
C MPETENC EN I C ES LECT EC UR U E R
 LECTURE 7: SUGAR TRANSPORT COMPETENCIES Students, after mastering the materials of Plant Physiology course, should be able to: 1. To explain the pathway of sugar transport in plants 2. To explain the mechanism
LECTURE 7: SUGAR TRANSPORT COMPETENCIES Students, after mastering the materials of Plant Physiology course, should be able to: 1. To explain the pathway of sugar transport in plants 2. To explain the mechanism
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Arabidopsis NPCC6/NaKR1 Is a Phloem Mobile Metal Binding Protein Necessary for Phloem Function and Root Meristem Maintenance C W
 This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Introduction to Plant Transport
 Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
** * * * Col-0 cau1 CAU1. Actin2 CAS. Actin2. Supplemental Figure 1. CAU1 affects calcium accumulation.
 Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Topic 14. The Root System. II. Anatomy of an Actively Growing Root Tip
 Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Primary Plant Body: Embryogenesis and the Seedling
 BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Translocation 11/30/2010. Translocation is the transport of products of photosynthesis, mainly sugars, from mature leaves to areas of growth and
 Translocation Translocation is the transport of products of photosynthesis, mainly sugars, from mature leaves to areas of growth and storage. Phloem is the tissue through which translocation occurs. Sieve
Translocation Translocation is the transport of products of photosynthesis, mainly sugars, from mature leaves to areas of growth and storage. Phloem is the tissue through which translocation occurs. Sieve
Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems.
 Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems. Fig. 35.8 Plant Cells pp.798-802 Types of plant cells Include:
Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems. Fig. 35.8 Plant Cells pp.798-802 Types of plant cells Include:
Introduction to Plant Transport
 Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
Introduction to Plant Transport The algal ancestors of plants were completely immersed in water and dissolved minerals. The adaptation to land involved the differentiation of the plant body into roots,
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Bio Factsheet. Transport in Plants. Number 342
 Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
UNIT 6 - STRUCTURES OF FLOWERING PLANTS & THEIR FUNCTIONS
 6.1 Plant Tissues A tissue is a group of cells with common function, structures or both. In plants we can find 2 types of tissues: Meristem Permanent tissues Meristem is found in regions with continuous
6.1 Plant Tissues A tissue is a group of cells with common function, structures or both. In plants we can find 2 types of tissues: Meristem Permanent tissues Meristem is found in regions with continuous
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
POTASSIUM IN PLANT GROWTH AND YIELD. by Ismail Cakmak Sabanci University Istanbul, Turkey
 POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
POTASSIUM IN PLANT GROWTH AND YIELD by Ismail Cakmak Sabanci University Istanbul, Turkey Low K High K High K Low K Low K High K Low K High K Control K Deficiency Cakmak et al., 1994, J. Experimental Bot.
The Science of Plants in Agriculture Pl.Sci 102. Getting to Know Plants
 The Science of Plants in Agriculture Pl.Sci 102 Getting to Know Plants Growth and Development of Plants Growth and Development of Plants Why it s important to have knowledge about plant development. What
The Science of Plants in Agriculture Pl.Sci 102 Getting to Know Plants Growth and Development of Plants Growth and Development of Plants Why it s important to have knowledge about plant development. What
Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA
 Himber et al. Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA silencing SUPPLEMENTAL MATERIAL Supplemental material 1. Short-range movement of GFP silencing affects a nearly
Himber et al. Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA silencing SUPPLEMENTAL MATERIAL Supplemental material 1. Short-range movement of GFP silencing affects a nearly
Plant Tissues and Organs. Topic 13 Plant Science Subtopics , ,
 Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
BOTANY LAB #1 MITOSIS AND PLANT TISSUES
 Mitosis and cytokinesis in plants BOTANY LAB #1 MITOSIS AND PLANT TISSUES In plants the formation of new cells takes place in specialized regions of meristematic tissue. Meristematic tissues contain immature,
Mitosis and cytokinesis in plants BOTANY LAB #1 MITOSIS AND PLANT TISSUES In plants the formation of new cells takes place in specialized regions of meristematic tissue. Meristematic tissues contain immature,
Chapter 29: Plant Tissues
 Chapter 29: Plant Tissues Shoots and Roots Shoots (Leaves and Stem) Produce food by photosynthesis Carry out reproductive functions Roots Anchor the plant Penetrate the soil and absorb water and dissolved
Chapter 29: Plant Tissues Shoots and Roots Shoots (Leaves and Stem) Produce food by photosynthesis Carry out reproductive functions Roots Anchor the plant Penetrate the soil and absorb water and dissolved
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Lab Exercise 4: Primary Growth and Tissues in Stems
 Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Plants. Tissues, Organs, and Systems
 Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 m
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
IX. PRIMARY STEM STRUCTURE AND DEVELOPMENT Bot 404 Fall 2004
 IX. PRIMARY STEM STRUCTURE AND DEVELOPMENT Bot 404 Fall 2004 A. Shoot apex -plants have an open system of growth, therefore the ability (at least potentially) to continue growth because there is a meristem
IX. PRIMARY STEM STRUCTURE AND DEVELOPMENT Bot 404 Fall 2004 A. Shoot apex -plants have an open system of growth, therefore the ability (at least potentially) to continue growth because there is a meristem
Chapter 28 Active Reading Guide Plant Structure and Growth
 Name: AP Biology Mr. Croft Chapter 28 Active Reading Guide Plant Structure and Growth In this unit on plants, the challenge for students will be to learn the new vocabulary. As we work through this unit,
Name: AP Biology Mr. Croft Chapter 28 Active Reading Guide Plant Structure and Growth In this unit on plants, the challenge for students will be to learn the new vocabulary. As we work through this unit,
2.5 : Cells are grouped into tissue
 2.5 : Cells are grouped into tissue 1 CELL STRUCTURE AND FUNCTIONS Prokaryotic and eukaryotic cells Structures & functions: Cell membrane and organelles Animal Cells are grouped into tissue Plant Cell
2.5 : Cells are grouped into tissue 1 CELL STRUCTURE AND FUNCTIONS Prokaryotic and eukaryotic cells Structures & functions: Cell membrane and organelles Animal Cells are grouped into tissue Plant Cell
Major Plant Hormones 1.Auxins 2.Cytokinins 3.Gibberelins 4.Ethylene 5.Abscisic acid
 Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter
 Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter 9/10/2008 1 Learning Objectives Explain similarities and differences between fungal, mammalian and plant cell cycles Explain
Plant Molecular and Cellular Biology Lecture 10: Plant Cell Cycle Gary Peter 9/10/2008 1 Learning Objectives Explain similarities and differences between fungal, mammalian and plant cell cycles Explain
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
Ginkgo leaf. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts.
 Ginkgo leaf Figure 22-30 Ginkgo tree. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts. The vein pattern is dichotomous: Divided
Ginkgo leaf Figure 22-30 Ginkgo tree. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts. The vein pattern is dichotomous: Divided
Ethylene is critical to the maintenance of primary root growth and Fe. homeostasis under Fe stress in Arabidopsis
 Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis Guangjie Li, Weifeng Xu, Herbert J. Kronzucker, Weiming Shi * Supplementary Data Supplementary
Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis Guangjie Li, Weifeng Xu, Herbert J. Kronzucker, Weiming Shi * Supplementary Data Supplementary
Forms strands that conduct water, minerals, and organic compounds. Much of the inside of nonwoody parts of plants. Includes roots, stems, and leaves
 Biology II Vascular plants have 3 tissue systems: Dermal Protective outer layer of plant Vascular Forms strands that conduct water, minerals, and organic compounds Ground Much of the inside of nonwoody
Biology II Vascular plants have 3 tissue systems: Dermal Protective outer layer of plant Vascular Forms strands that conduct water, minerals, and organic compounds Ground Much of the inside of nonwoody
Identification of factors involved in Xylem Cell Differentiation Aarush Mohit Mittal 1, 2
 Identification of factors involved in Xylem Cell Differentiation Aarush Mohit Mittal 1, 2 1 Department of Biological Sciences and Bio-Engineering, Indian Institute of Technology, Kanpur, India 2 Department
Identification of factors involved in Xylem Cell Differentiation Aarush Mohit Mittal 1, 2 1 Department of Biological Sciences and Bio-Engineering, Indian Institute of Technology, Kanpur, India 2 Department
Anatomy of Plants Student Notes
 Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
Ron et al SUPPLEMENTAL DATA
 Ron et al SUPPLEMENTAL DATA Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model Mily Ron, Kaisa Kajala,
Ron et al SUPPLEMENTAL DATA Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model Mily Ron, Kaisa Kajala,
Name: TF: Section Time: LS1a ICE 5. Practice ICE Version B
 Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Transport of substances in plants
 Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
The Plant body has a hierarch of organs, tissues, and cells. [2]
![The Plant body has a hierarch of organs, tissues, and cells. [2] The Plant body has a hierarch of organs, tissues, and cells. [2]](/thumbs/77/75254697.jpg) GUIDED READING - Ch. 35 PLANT STRUCTURE NAME: Please print out these pages and HANDWRITE the answers directly on the printouts. Typed work or answers on separate sheets of paper will not be accepted. Importantly,
GUIDED READING - Ch. 35 PLANT STRUCTURE NAME: Please print out these pages and HANDWRITE the answers directly on the printouts. Typed work or answers on separate sheets of paper will not be accepted. Importantly,
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
(Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, )
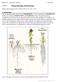 BIOL 221 Concepts of Botany Fall 2007 Topic 07: Primary Plant Body: The Root System (Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, 9.5 9.23) A. Introduction The root has the primary functions of
BIOL 221 Concepts of Botany Fall 2007 Topic 07: Primary Plant Body: The Root System (Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, 9.5 9.23) A. Introduction The root has the primary functions of
PLANT TISSUES 12 MARCH 2014
 PLANT TISSUES 12 MARCH 2014 Lesson Description In this lesson we: Identify the different types of plant tissue Be able to relate the different structures with the different functions Plant Tissue Summary
PLANT TISSUES 12 MARCH 2014 Lesson Description In this lesson we: Identify the different types of plant tissue Be able to relate the different structures with the different functions Plant Tissue Summary
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds
 HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
PHARMACOBOTANY LECTURE 5. PLANT TISSUES III.
 PHARMACOBOTANY LECTURE 5. PLANT TISSUES III. VASCULAR TISSUES VASCULAR TISSUES Xylem transporting water and mineral substances from the root upwards to other plant organs Phloem carries photosynthetic
PHARMACOBOTANY LECTURE 5. PLANT TISSUES III. VASCULAR TISSUES VASCULAR TISSUES Xylem transporting water and mineral substances from the root upwards to other plant organs Phloem carries photosynthetic
CONTROL OF GROWTH BY HORMONES
 CONTROL OF GROWTH BY HORMONES Growth and organogenesis are controlled......by genes (independent of environment): e.g., number of primary vascular bundles, general shape of a leaf or flower...by genes
CONTROL OF GROWTH BY HORMONES Growth and organogenesis are controlled......by genes (independent of environment): e.g., number of primary vascular bundles, general shape of a leaf or flower...by genes
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I
 BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
BIO1PS 2012 Plant Science Lecture 4 Hormones Pt. I Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Hormones and Ghost gum Eucalyptus papuana Coordination ~3 Lectures Leaves
Figure 18.1 Blue-light stimulated phototropism Blue light Inhibits seedling hypocotyl elongation
 Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Chapter 6. Biology of Flowering Plants. Anatomy Seedlings, Meristems, Stems, and Roots
 BOT 3015L (Outlaw/Sherdan/Aghoram); Page 1 of 6 Chapter 6 Biology of Flowering Plants Anatomy Seedlings, Meristems, Stems, and Roots Objectives Seedling germination and anatomy. Understand meristem structure
BOT 3015L (Outlaw/Sherdan/Aghoram); Page 1 of 6 Chapter 6 Biology of Flowering Plants Anatomy Seedlings, Meristems, Stems, and Roots Objectives Seedling germination and anatomy. Understand meristem structure
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
ARE YOU familiar with the sayings Get to
 Root Anatomy ARE YOU familiar with the sayings Get to the root of the problem or the root of all evil? Both these sayings suggest that the root is an essential part of something. With plants, the essential
Root Anatomy ARE YOU familiar with the sayings Get to the root of the problem or the root of all evil? Both these sayings suggest that the root is an essential part of something. With plants, the essential
Useful Propagation Terms. Propagation The application of specific biological principles and concepts in the multiplication of plants.
 Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Plant Structure and Organization - 1
 Plant Structure and Organization - 1 In our first unit of Biology 203 we will focus on the structure and function of the higher plants, in particular the angiosperms, or flowering plants. We will look
Plant Structure and Organization - 1 In our first unit of Biology 203 we will focus on the structure and function of the higher plants, in particular the angiosperms, or flowering plants. We will look
A developmental geneticist s guide to roots Find out about the hidden half of plants
 the Centre for Plant Integrative Biology A developmental geneticist s guide to roots Find out about the hidden half of plants What do roots look like from the inside? How do roots form? Can we improve
the Centre for Plant Integrative Biology A developmental geneticist s guide to roots Find out about the hidden half of plants What do roots look like from the inside? How do roots form? Can we improve
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Plant Structure. Lab Exercise 24. Objectives. Introduction
 Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Supplemental Data. Perea-Resa et al. Plant Cell. (2012) /tpc
 Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Plants. Plant Form and Function. Tissue Systems 6/4/2012. Chapter 17. Herbaceous (nonwoody) Woody. Flowering plants can be divided into two groups:
 Monocots Dicots 6/4/2012 Plants Plant Form and Function Chapter 17 Herbaceous (nonwoody) In temperate climates, aerial parts die back Woody In temperate climates, aerial parts persist The Plant Body Functions
Monocots Dicots 6/4/2012 Plants Plant Form and Function Chapter 17 Herbaceous (nonwoody) In temperate climates, aerial parts die back Woody In temperate climates, aerial parts persist The Plant Body Functions
13.2 The Vascular Plant Body (textbook p )
 13.2 The Vascular Plant Body (textbook p544 550) Learning Goal: Label and explain the anatomy of the Vascular Plant and it's Tissue Types Plants are classified into two main groups: and. Vascular plants
13.2 The Vascular Plant Body (textbook p544 550) Learning Goal: Label and explain the anatomy of the Vascular Plant and it's Tissue Types Plants are classified into two main groups: and. Vascular plants
Anatomy of Flowering Plants. K C Meena PGT Biology
 Anatomy of Flowering Plants K C Meena PGT Biology Tissues A group of similar cells performing same function. Types of plant tissues - Meristematic tissues and permanent tissues. Meristematic tissues Have
Anatomy of Flowering Plants K C Meena PGT Biology Tissues A group of similar cells performing same function. Types of plant tissues - Meristematic tissues and permanent tissues. Meristematic tissues Have
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
Name the tube-like tissue found in part C in which water moves. Name the cells which are responsible for controlling the size of the opening at Z
 Past Questions on Plant Structure Note: You need to be able to draw and label all the diagrams in this worksheet for your exam. Name the parts labelled B, C and E. C E Give one main function each for the
Past Questions on Plant Structure Note: You need to be able to draw and label all the diagrams in this worksheet for your exam. Name the parts labelled B, C and E. C E Give one main function each for the
Plant Anatomy and Tissue Structures
 Plant Anatomy and Tissue Structures The Two Major Plant Systems Reproductive shoot (flower) Terminal bud Node Internode Angiosperm plants have threse major organs: Roots Stems Leaves & Flowers Terminal
Plant Anatomy and Tissue Structures The Two Major Plant Systems Reproductive shoot (flower) Terminal bud Node Internode Angiosperm plants have threse major organs: Roots Stems Leaves & Flowers Terminal
DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Other cellular processes (17%)
 Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
SMALL AUXIN UP RNA (SAUR) genes are the largest class of primary auxin (growth
 Abstract SMALL AUXIN UP RNA (SAUR) genes are the largest class of primary auxin (growth hormone) responsive genes in all land plants, many of which are expressed in actively growing plant tissues. This
Abstract SMALL AUXIN UP RNA (SAUR) genes are the largest class of primary auxin (growth hormone) responsive genes in all land plants, many of which are expressed in actively growing plant tissues. This
Plant Structure and Growth
 Plant Structure and Growth A. Flowering Plant Parts: The flowering plants or are the most diverse group of plants. They are divided into 2 classes and. Examples of monocots: Examples of dicots: The morphology
Plant Structure and Growth A. Flowering Plant Parts: The flowering plants or are the most diverse group of plants. They are divided into 2 classes and. Examples of monocots: Examples of dicots: The morphology
Bring Your Text to Lab!!!
 Bring Your Text to Lab!!! Vascular Plant Anatomy: Flowering Plants Objectives: 1. To observe what the basic structure of vascular plants is, and how and where this form originates. 2. To begin to understand
Bring Your Text to Lab!!! Vascular Plant Anatomy: Flowering Plants Objectives: 1. To observe what the basic structure of vascular plants is, and how and where this form originates. 2. To begin to understand
Topic 15. The Shoot System
 Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Plant Anatomy Lab 7 - Stems II
 Plant Anatomy Lab 7 - Stems II This exercise continues the previous lab in studying primary growth in the stem. We will be looking at stems from a number of different plant species, and emphasize (1) the
Plant Anatomy Lab 7 - Stems II This exercise continues the previous lab in studying primary growth in the stem. We will be looking at stems from a number of different plant species, and emphasize (1) the
Non Permanent Tissues - Meristematic Tissue
 PLANT TISSUES Non Permanent Tissues - Meristematic Tissue Undifferentiated plant cells that are continually dividing by mitosis Large thin walled cells No vacuole Dense cytoplasm Large nucleus Found at
PLANT TISSUES Non Permanent Tissues - Meristematic Tissue Undifferentiated plant cells that are continually dividing by mitosis Large thin walled cells No vacuole Dense cytoplasm Large nucleus Found at
IB Bio: Plant Biology. Topic 9
 IB Bio: Plant Biology Topic 9 9.1: Transport in xylem How and why does water move up a plant? How do plants conserve water? 9.2: Transport in phloem How and why and where does food move in a plant? 9.3:
IB Bio: Plant Biology Topic 9 9.1: Transport in xylem How and why does water move up a plant? How do plants conserve water? 9.2: Transport in phloem How and why and where does food move in a plant? 9.3:
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Molecular Genetics of. Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS
 Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
THREE MITOSIS AND MEIOSIS OVERVIEW OBJECTIVES INTRODUCTION
 THREE MITOSIS AND MEIOSIS OVERVIEW In this lab you will investigate the processes of mitosis and rneiosis: 1. You will use prepared slides of onion root tips to study plant mitosis and to calculate the
THREE MITOSIS AND MEIOSIS OVERVIEW In this lab you will investigate the processes of mitosis and rneiosis: 1. You will use prepared slides of onion root tips to study plant mitosis and to calculate the
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Plant Stimuli pp Topic 3: Plant Behaviour Ch. 39. Plant Behavioural Responses. Plant Hormones. Plant Hormones pp
 Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Reading Assignments. A. Genes and the Synthesis of Polypeptides. Lecture Series 7 From DNA to Protein: Genotype to Phenotype
 Lecture Series 7 From DNA to Protein: Genotype to Phenotype Reading Assignments Read Chapter 7 From DNA to Protein A. Genes and the Synthesis of Polypeptides Genes are made up of DNA and are expressed
Lecture Series 7 From DNA to Protein: Genotype to Phenotype Reading Assignments Read Chapter 7 From DNA to Protein A. Genes and the Synthesis of Polypeptides Genes are made up of DNA and are expressed
Plant Structure and Function
 Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Plant Development. Chapter 31 Part 1
 Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Types of Plants. Unit 6 Review 5/2/2011. Plants. A. pine B. moss C. corn plant D. bean plant E. liverwort
 Unit 6 Review Plants Initial questions are worth 1 point each. Each question will be followed by an explanation All questions will be asked a second time at the very end, each of those questions will be
Unit 6 Review Plants Initial questions are worth 1 point each. Each question will be followed by an explanation All questions will be asked a second time at the very end, each of those questions will be
The plant body has a hierarchy of organs, tissues, and cells. Plants, like multicellular animals:
 Chapter 28 The plant body has a hierarchy of organs, tissues, and cells Plants, like multicellular animals: o Have organs composed of different tissues, which are in turn composed of cells 3 basic organs:
Chapter 28 The plant body has a hierarchy of organs, tissues, and cells Plants, like multicellular animals: o Have organs composed of different tissues, which are in turn composed of cells 3 basic organs:
Plant and animal cells (eukaryotic cells) have a cell membrane, cytoplasm and genetic material enclosed in a nucleus.
 4.1 Cell biology Cells are the basic unit of all forms of life. In this section we explore how structural differences between types of cells enables them to perform specific functions within the organism.
4.1 Cell biology Cells are the basic unit of all forms of life. In this section we explore how structural differences between types of cells enables them to perform specific functions within the organism.
10/4/2017. Chapter 39
 Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
Chapter 39 1 Reception 1 Reception 2 Transduction CYTOPLASM CYTOPLASM Cell wall Plasma membrane Phytochrome activated by light Cell wall Plasma membrane Phytochrome activated by light cgmp Second messenger
Chapter C3: Multicellular Organisms Plants
 Chapter C3: Multicellular Organisms Plants Multicellular Organisms Multicellular organisms have specialized cells of many different types that allow them to grow to a larger size than single-celled organisms.
Chapter C3: Multicellular Organisms Plants Multicellular Organisms Multicellular organisms have specialized cells of many different types that allow them to grow to a larger size than single-celled organisms.
AP Biology Chapter 36
 Chapter 36 Chapter 36 Transport in Plants 2006-2007 Transport in plants - Overview H2O & minerals transport in xylem transpiration evaporation, adhesion & cohesion negative pressure Sugars transport in
Chapter 36 Chapter 36 Transport in Plants 2006-2007 Transport in plants - Overview H2O & minerals transport in xylem transpiration evaporation, adhesion & cohesion negative pressure Sugars transport in
