The Xenopus tadpole gut: fate maps and morphogenetic movements
|
|
|
- Rodger Carson
- 5 years ago
- Views:
Transcription
1 Development 127, (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV The Xenopus tadpole gut: fate maps and morphogenetic movements Andrew D. Chalmers and Jonathan M. W. Slack* Developmental Biology Programme, Department of Biology and Biochemistry, University of Bath, Bath BA2 7AY, UK *Author for correspondence ( Accepted 1 November; published on WWW 20 December 1999 SUMMARY We have produced a comprehensive fate map showing where the organs of the gut and respiratory system are derived from in the early Xenopus laevis endoderm. We also show the origin of the associated smooth muscle layer on a separate fate map. Comparison of the two maps shows that for most organs of the gut the prospective epithelium and smooth muscle do not overlie each other in the early embryo but come together at a later stage. These fate maps should be useful for future studies into endoderm specification. It was not previously known how the elongation of the endoderm occurs, how the single-layered dorsal and manylayered ventral endoderm gives rise to the single layered epithelium, and whether or not the archenteron cavity actually gives rise to the gut lumen. Using a variety of labelling procedures we show firstly, that radial intercalation occurs in the gut transforming a short thick tube into a long thin tube; secondly, that the archenteron lining does not become the definitive gut lumen. Instead the archenteron cavity almost closes at tailbud stages before providing a nucleus for the definitive gut cavity, which opens up during elongation. Based on this work we present a model explaining the morphogenesis of the gut. Key words: Xenopus laevis, Endoderm, Mesoderm, Smooth muscle, Fate map, Radial intercalation, Morphogenesis, Gut, Pancreas, Liver, Intestine, Epithelium INTRODUCTION Cells of the endoderm are fated to form the lining of the gut, that is the pharynx, oesophagus, stomach and intestines. The epithelium of the liver, gall bladder, pancreas and the respiratory system also forms from the endoderm. In Xenopus the endoderm originates from the vegetal hemisphere of the embryo (Dale and Slack, 1987), and there has recently been progress showing that both secreted molecules, for example noggin, Vg-1 and cerberus (Sasai et al., 1996; Joseph and Melton, 1998; Bouwmeester et al., 1996) and transcription factors, such as Mix 1, Mixer, Xsox17α and VegT (Lemaire et al., 1998; Henry and Melton, 1998; Hudson et al., 1997; Zhang et al., 1998 and references therein) play a role in endoderm formation. As well as the endoderm-derived epithelium, the organs of the gut also have an outer layer of mesoderm-derived mesenchyme that forms connective tissue and smooth muscle. Work in other vertebrates has shown that signals between the mesenchyme layer and the underlying epithelium are important in the regional specification of the endoderm (reviewed in Haffen et al., 1987; Yasugi and Mizuno, 1990; Rawdon and Andrew, 1993). For example, recombination experiments in the chick have shown that intestinal mesenchyme can cause the respecification of stomach epithelium into intestinal epithelium (Yasugi and Mizuno, 1978; Ishizuya-Oka and Mizuno, 1984; Andrew and Rawdon, 1990). More recent work has started to suggest a molecular basis to these interactions, as the anterior boundaries of Hox gene expression in the intestinal smooth muscle were found to match anatomical boundaries in the intestinal epithelium (Roberts et al., 1995; Yokouchi et al., 1995). This raises the possibility that the Hox genes set up boundaries in the smooth muscle that are somehow transferred onto the underlying epithelium by local inductive signals. Consistent with this, ectopic expression of Hoxd-13 in the anterior intestinal smooth muscle causes a partial transformation of the underlying epithelium into a more posterior fate (Roberts et al., 1998). This corresponds well with the situation in Drosophila where homeotic genes expressed in the visceral mesoderm play a role in patterning the larval midgut (reviewed in Bienz, 1994). Classical recombination experiments in amphibian embryos have also shown that the early mesoderm is able to respecify explanted endoderm to a different regional character (Okada, 1957, 1960). A limitation to these experiments is that they were carried out before the introduction of lineage tracers, making it impossible to eliminate the possibility of contaminating cells in the recombinations. They were also done without an accurate fate map for the endodermal and mesodermal components of the gut. In order to investigate the specification of the endoderm an accurate fate map is required so that the results of any explant or transplantation experiment can be compared with the presumptive fate for that piece of tissue. There are a number of existing amphibian endoderm fate maps (particularly that of Tahara and Nakamura, 1961), but these suffer from the limitations of spreading and fading associated
2 382 A. D. Chalmers and J. M. W. Slack Fig. 1. The stage 46 Xenopus tadpole gut. (A) Schematic diagram (ventral view/anterior at the top). (B) Whole-mount drawing (ventral and lateral views). The gut has been shaded dark grey and the liver and pancreas light grey. oe, oesophagus; lv, liver; gb, gall bladder; pa, pancreas; sia, proximal small intestine; sib, external coil of small intestine; sic, internal coil of small intestine; lic, internal coil of large intestine; lid, distal large intestine; st, stomach; pr, proctodaeum; ht, heart. Figure adapted from Chalmers and Slack (1998), with permission. Bar, 1 mm. with vital dyes and, furthermore, none of these older studies were carried out using Xenopus embryos. In order to understand the development of the endoderm it is important also to understand the origin of the smooth muscle layer and the role it plays in specifying the endoderm. At present it is not known where the smooth muscle layer originates from in the Xenopus mesoderm. As well as regional specification, the development of an organ system also involves morphogenesis: that is, how the shape of the finished organ is formed from the cells of the early embryo. In other systems, cell rearrangements have been shown to be important in morphogenesis (reviewed in Keller, 1987). Little is known about the morphogenesis of the endoderm and there are several important questions that have not been answered. At neurula stages the Xenopus endoderm lines the archenteron cavity. The dorsal endoderm consists of a single cell layer while the ventral endoderm has several layers of large yolky cells. By stage 45 (5-day-old tadpole) the endoderm has undergone enormous elongation and formed the single-cell-layered epithelium of the intestine. It is not known how the elongation of the endoderm is accomplished. It is also not clear how the thin dorsal layer and thick ventral layers of endoderm produce the single layer of cells that forms the gut epithelium. It is sometimes suggested that the large yolky ventral cells disintegrate and are digested during development (Nieuwkoop and Faber, 1967; Mathews and Schoenwolf, 1998), implying that that the outer endodermal cells lying closer to the mesoderm form the gut epithelium, although this has not been proved. Finally, it is not known whether the archenteron cavity of the neurula really gives rise to the gut cavity of the later tadpole. It has been proposed (Goette, 1875), and is generally assumed, that the gut cavity is a continuation of the archenteron. A problem with this model is that the archenteron narrows considerably and appears to close through much of the gut at early tadpole stages. This means there cannot be a simple transition from archenteron to gut cavity. It has also been proposed, based on vital dye experiments, that the gut cavity is not a continuation of the archenteron but opens up de novo during development (Tahara and Nakamura, 1961). So, although it is widely assumed, it is not known whether the archenteron does in fact give rise to the gut cavity. We have used grafts of fluorescent labelled tissue to produce a new and comprehensive fate map showing which parts of the early endoderm give rise to which organs in the gut and respiratory system. This demonstrates that during morphogenesis of the endoderm the axes (anterior/posterior, dorsal/ventral and left/right) of the early embryo are maintained in the later gut. We have also produced a fate map for the smooth muscle layer showing where in the early mesoderm the smooth muscle layer of the gut originates from. Comparison of the two fate maps shows that the origins of the future epithelia and smooth muscle layer are only partially overlapping in the early embryo. Therefore, continuous interactions between the two germ layers could not start until the two layers move into accord later on in development. These two fate maps represent a resource for future explant and transplantation studies into the specification of the endoderm. In order to investigate the morphogenetic movements associated with gut formation we used the lipophilic dye DiI specifically to label the large yolky cells on the floor of the archenteron and the cells in the middle of the ventral endoderm (between the floor of the archenteron and very ventral endoderm that lies next to the mesoderm). Both groups of cells were found to be incorporated into the gut epithelium and so cannot be Fig. 2. The 14 regions used for fate mapping. (A) Left view of a stage 14 whole mount. (B) Dorsal view of a stage 14 whole mount. (C) Parasagittal section of a stage 14 embryo. (D) Transverse section of a stage 14 embryo. The grafting regions are shown on the embryos using the following labels: 1, extreme anterior; 2, anterior dorsal; 3, anterior right; 4, anterior left; 5, anterior ventral; 6, middle dorsal; 7, middle right; 8, middle left; 9, middle ventral; 10, posterior dorsal; 11, posterior right; 12, posterior left, 13; posterior ventral; 14 extreme posterior.
3 Fate maps and morphogenesis in Xenopus gut 383 digested during development. We then used double labelling (DiI + fluorescein dextran amine) to show that these cells are incorporated into the epithelium by a process of radial intercalation. We believe that the occurrence of radial intercalation drives the elongation of the endoderm. Using a second approach, the cells lining the entire surface of the archenteron were labelled with biotin, allowing the embryonic cavity to be followed during development. The archenteron was shown to narrow during development before reopening to split the ventral endoderm and give rise to the definitive gut cavity. Based on this work we present a new model explaining the morphogenesis of the gut. MATERIALS AND METHODS Lineage tracing FDA labelling Xenopus laevis embryos were obtained using standard procedures (Godsave et al., 1988), cultured in normal amphibian medium (NAM) (Beck and Slack, 1999) and staged according to Nieuwkoop and Faber (1967). Fate mapping was carried out using Fluorescein dextran amine (FDA, Molecular Probes) (Gimlich and Braun, 1985). During the first cleavage donor embryos were placed in NAM plus 5% Ficoll and injected once into each of the two blastomeres with 0.25 ng (4.6 nl of 50 mg/ml) FDA. At stage 13/14 an orthotopic graft, a rectangular piece of tissue approximately 400 µm 600 µm containing all three germ layers, was cut from a labelled donor embryo at one of the 14 standard regions (see Results) and used to replace the equivalent region from an unlabelled host embryo. Embryos that developed normally after grafting were fixed at stage 46 in 10% formalin in 70% PBS for 24 or 48 hours at room temperature. They were then embedded, sectioned and mounted in DPX (BDH). The FDA-labelled organs were identified based on our previous work (Chalmers and Slack, 1998). Sections from typical individuals were drawn with the aid of a drawing tube (Leica) and the position of the FDA label was then added to the drawings. A small number of the mesoderm cases were scored in whole-mount guts, as described for the DiI labelling, rather than in sections. DiI labelling To specifically label small populations of endoderm cells a fixable Fig. 3. Examples of FDA labelling. For each example a labelled drawing of a section (A,C,E,G,I) and a photograph of the labelled section (B,D,F,H,J) are shown. A whole-mount drawing is also included at the top of the figure to show the position of the sections in the tadpole. (A,B) Labelled pharynx. (C,D) Labelled pancreas and small intestine. (E,F) Labelled small intestine. (G,H) Labelled proctodaeum. (I,J) Labelled smooth muscle in the large intestine. (K) Smooth muscle layer in the tadpole gut. In B,D,F,H and J, arrowheads highlight labelled epithelia and arrows highlight smooth muscle. ph, pharynx; tn, tongue; sia, proximal small intestine; pa, pancreas; st, stomach; lid, distal large intestine; pr, proctodaeum. Bar, 200 µm (B); 120 µm (F,H); 95 µm (D,J); 30 µm (K).
4 384 A. D. Chalmers and J. M. W. Slack derivative of the lipophilic dye DiI was used (Cell Tracker CM-DiI, Molecular Probes, referred to simply as DiI ). This was dissolved at 3 mg/ml in ethanol mg/ml phosphatidylcholine, heated to 50 C, diluted 1/10 in 0.2 M sucrose at 50 C, centrifuged to remove any precipitate, then a 4.6 nl pulse was fired at the desired position using a Drummond Microinjector. Stage 14 embryos were labelled with DiI at one of four positions (shown in Fig. 6A). (1) Floor of the archenteron: a piece was cut from the middle dorsal position to expose the floor of the archenteron, which was then labelled with DiI before replacing the dorsal piece. (2) The ventral endoderm: a piece of ectoderm and mesoderm was cut from the mid-ventral position exposing the endoderm, which was labelled with DiI and the piece replaced. (3) The mid-ventral endoderm: a thick piece of tissue was cut from the mid-ventral position. This exposed the middle endoderm, which was then labelled with DiI and the piece of tissue replaced. (4) The dorsal endoderm: a piece was cut from the middle dorsal position and turned over to expose the archenteron roof, which was labelled with DiI and then replaced. DiI labelled-embryos at stages 14 and 39/40 were fixed, embedded, sectioned and scored as previously described for the FDA labelling. Stage 45 DiI-labelled embryos were scored as isolated gut preparations. They were dissected as previously described (Chalmers and Slack, 1998) and confocal images were then captured using a Zeiss 510 laser scanning microscope. FDA + DiI labelling To label the floor and dorsal endoderm simultaneously a piece of tissue was cut from the dorsal roof (equivalent to middle and posterior dorsal locations) of a host embryo and the archenteron floor labelled with DiI as above. A graft from an FDA-labelled donor embryo was then used to replace the dorsal roof. To label the floor and ventral endoderm simultaneously a shallow graft (containing ectoderm, mesoderm and approximately 1-2 layers of endoderm cells) was cut from a donor embryo and used to replace the equivalent piece from an unlabelled host. The embryo was left to heal and then the floor was labelled with DiI as described above. To label the middle and ventral positions simultaneously a piece was cut from the mid-ventral position as described before and the middle endoderm labelled with DiI. The ventral piece was then replaced with a graft of FDA-labelled tissue. The three sets of embryos were then cultured, dissected and scored as described for the DiI labelling. Labelling of the archenteron lining The entire external surface of late blastula (stage 9/10) embryos was labelled with biotin using sulfo-nhs-lc-biotin (Pierce) as previously described (Minsuk and Keller, 1997). The only modification was that the sulfo-nhs-lc-biotin was made up in NAM/10 and the labelling time was reduced to 5 minutes. The biotin-labelled embryos were fixed at the required stage and the biotin detected using alkaline phosphatase-conjugated streptavidin (Vector Labs) as previously described (Minsuk and Keller, 1997). RESULTS The Xenopus tadpole gut and respiratory system In our previous work we published a detailed description of the normal development of the Xenopus tadpole gut (Chalmers and Slack, 1998). Similar to the mammalian gut, it consists of the pharynx, oesophagus, stomach, intestines, pancreas and liver (Fig. 1). A striking feature is that the intestine forms a double coiled structure. The double coil is formed by an exterior coil consisting of anticlockwise loops of intestine followed by an interior coil consisting of clockwise loops of intestine. We proposed a nomenclature to describe the different parts of the double coiled intestine, from the proximal to the distal end. sia is the most proximal part of the small intestine that is located before the double coil; sib, is the small intestine that forms the external coil and sic is the small intestine in the internal coil. lic is the proximal part of the large intestine in the internal coil and lid is the distal part of the large intestine that runs to the proctodaeum (pr). In this study we rely on our previous work to identify the organs labelled by the fate mapping and use the nomenclature when describing the coiled intestine. The tadpole respiratory system consists of the gills, which are located in the lateral pharynx, and the trachea, which splits from the posterior pharynx and then bifurcates to give rise to the two lung buds (Nieuwkoop and Faber, 1967; Chalmers and Slack, 1998). The 14 embryonic regions used for the fate mapping The early neurula stage Xenopus embryo was divided up into 14 regions that covered the entire embryo (Fig. 2). Region 1 at the very anterior is called extreme anterior. Posterior to this the embryo was split into 3 anterior/posterior levels, termed anterior, middle and posterior. Each of these levels was split into a dorsal, right, left and ventral region. Finally the most posterior region, the extreme posterior region, number 14, lies opposite the extreme anterior region. Each of these regions was labelled using orthotopic grafts of fluorochrome-labelled tissue and the embryos were left to develop to stage 46. They were then fixed and sectioned and the labelled epithelia scored. Examples of the experiments are shown in Fig. 3, where a drawing of each section is shown along with a photograph of the FDA label (FDA is highlighted by arrowheads). A wholemount drawing shows where the sections lie in the tadpole. The smooth muscle layer in the Xenopus tadpole gut consists of a single very thin layer of cells (Fig. 3K, arrow; Kordylewski, 1983; Chalmers and Slack, 1998). As the grafts also contained the mesoderm layer it was possible to score the label in the smooth muscle layer that surrounds the gut (Fig. 3J, compare with the unlabelled smooth muscle in Fig. 3F). Presenting the endoderm fate map The results for the endoderm fate mapping are presented in three ways. Table 1 shows the organs that were labelled from grafts in each region. If a particular region labelled a particular organ in at least 50% of cases then that organ was considered to arise from that region and was included in the fate map (shaded grey in Table 1). A limitation of this method is that it gives the same score if a graft labels a small or large proportion of cells of an organ. To overcome this we also show the labelling pattern of typical examples for eight of the 14 regions (Fig. 4). The lateral regions label a similar proximal/distal part of the gut to the ventral regions and so were not included here. Drawings of a number of sections from each of the eight typical examples are shown, with the position of the FDA label marked in green. Above the typical examples are a set of standard sections and a drawing of a whole-mount embryo. The organs labelled in each drawing can be identified by reference to the set of standard sections and the position of the section in the tadpole body can then be established by reference to the drawing of the whole mount. These diagrams give an indication of the amount of labelling in each organ. Finally we present the data in two types of summary diagram (Fig. 5A-D). The first shows a drawing of a neurula-stage embryo labelled with the organs that
5 Fate maps and morphogenesis in Xenopus gut 385 Table 1. The endoderm fate map: organs labelled from grafts in each region Position n A ph P ph tn gills tr lungs liver gb pa bd oe st sia sib sic lic lid pr (1) Extreme anterior (2) Anterior dorsal (3) Anterior right (4) Anterior left (5) Anterior ventral (6) Middle dorsal (7) Middle right (8) Middle left (9) Middle ventral (10) Posterior dorsal (11) Posterior right (12) Posterior left (13) Posterior ventral (14) Extreme posterior The percentage of cases where each organ was labelled is shown for each of the 14 regions. Organs that were labelled in at least 50% of the cases were included in the fate map and shaded grey in this table. A ph, anterior pharynx; P ph, posterior pharynx; tn, tongue; tr, trachea; gb, gall bladder; pa, pancreas; bd, bile duct; oe, oesophagus; st, stomach; sia, proximal small intestine; sib, external coil of the small intestine; sic, internal coil of the small intestine; lic, internal coil of the large intestine; lid, distal large intestine; pr, proctodaeum. the extreme anterior, dorsal, ventral and extreme posterior regions are fated to form (Fig. 5A). For the sake of clarity the lateral regions, which gave similar results to the ventral regions, have not been included in the diagram (they are included in Fig. 5D, see later). The second type of diagram shows how the early endoderm projects on to the later gut (Fig. 5B,C). The projection of the ventral endoderm (with extreme anterior and extreme posterior regions) and the dorsal endoderm (with extreme anterior and posterior regions) is shown (Fig. 5B,C). It is important to realise that the shaded regions of the gut are meant to show that labelled cells were found in these regions rather than every cell in these regions was labelled. Overview of the endoderm fate map Each region of the neurula was found to be reproducibly fated to form part of the gut or respiratory system of the tadpole. This shows that all regions of the endoderm do contribute to the tadpole gut or respiratory epithelia. Not surprisingly, the anterior endoderm was found to be fated to form proximal gut epithelia while the posterior endoderm formed distal epithelia. To the resolution of this study there is a smooth projection without discontinuities from early to late stages relative to all anatomical axes: anterior/posterior, dorsal/ventral and left/right. However, there is some quantitative deformation in that the dorsal endoderm was generally found to be fated to form more anterior structures than the ventral endoderm (discussed in more detail below). So while the dorsal endoderm remains opposite the ventral endoderm it ends up opposite ventral cells that originated from a more anterior position in the endoderm. The later gut has a left/right asymmetry and there is currently a lot of interest in how this is established (e.g. Campione et al., 1999; reviewed by Yost, 1998). We were interested in whether there would be any left/right asymmetry in the fate of the endoderm but found no convincing difference between the fates of the left and right grafts. Presumably therefore the asymmetry must arise without large differences in cell fate or migration between the right and left sides of the embryo. Specific regions of the endoderm fate map Anterior regions The extreme anterior region (1) labelled the epithelium of the pharynx and the tongue (orange in Fig. 5). All the anterior grafts (2-5, red in Fig. 5) labelled the epithelium of the pharynx, oesophagus, stomach and proximal small intestine (sia). However, the anterior ventral (5) and lateral grafts (3+4) labelled less pharynx and more distal parts of sia than the anterior dorsal grafts (2) (compare Fig. 5B with C). So, the dorsal endoderm is fated to form more anterior structures than the lateral and ventral endoderm. The anterior ventral (5) grafts also labelled the trachea, lungs, liver, gall bladder, pancreas and bile duct, showing that a large number of organs are fated to form from this small anterior ventral portion of the endoderm. The anterior left and right (3+4) grafts also labelled the pancreas but not the liver. This shows that the ventral pancreatic rudiment but not the liver has a lateral as well as a ventral component. However, finer grain fate mapping studies will be required to establish to what extent the ventral pancreatic rudiment extends laterally and so how different in origin it is from the rudiment of the liver. Middle regions The middle dorsal region (6, blue in Fig. 5) labelled the sia and the pancreas. This means that the dorsal pancreas forms from a more posterior region than the ventral pancreas. The middle right (7), left (8) and ventral (9) regions labelled the distal portion of sia and a large part of sib (blue in Fig. 5). Therefore, like the anterior regions, the middle lateral and ventral grafts labelled more distal parts of the gut than the dorsal grafts. Posterior regions The posterior right (11), left (12) and ventral regions (13)
6 386 A. D. Chalmers and J. M. W. Slack Fig. 4. Representative examples of the fate mapping. Drawings of sections from representative examples of eight of the 14 regions are shown. The position of the FDA label has been added to the drawings (green). A whole-mount drawing and drawings of standard sections are also shown to aid interpretation of the labelled sections (adapted from Chalmers and Slack, 1998). ph, pharynx; tn, tongue; ov, otic vesicle; no, notochord; ht, heart; lv, liver; tr, trachea; sia, proximal small intestine; sib, external coil of small intestine; sic, internal coil of small intestine; lic, internal coil of large intestine; lid, distal large intestine; pa, pancreas; bd, bile duct; lu, lungs; nd, nephritic ducts; pr, proctodaeum. (green in Fig. 5) labelled the distal part of sib, sic and a small part of the large intestine. The posterior dorsal region (13) labelled cells in a large span of the intestine from sia through to sic. Therefore, the fact that the anterior and middle dorsal endoderm is shifted to the anterior compared with the lateral and ventral endoderm is compensated for by the spreading out of the posterior dorsal endoderm over a large part of the intestine (compare Fig. 5B,C). The extreme posterior graft (14, black in Fig. 5) labelled the most distal parts of the gut: the large intestine and the proctodaeum.
7 Fate maps and morphogenesis in Xenopus gut 387 Fig. 5. The endoderm and smooth muscle layer fate maps. (A) The endoderm fate map. The organ rudiments of the extreme anterior (1) (yellow), anterior dorsal (2) and ventral (5) (red), middle dorsal (6) and ventral (9) (blue), posterior dorsal (10) and ventral (13) (green) and extreme posterior (14) (black) endoderm are labelled at the position they form on a drawing of a stage 14 embryo. For the sake of clarity the lateral rudiments have not been included. (B) Projection of the early ventral endoderm onto the tadpole gut. The extreme anterior (1) (yellow), anterior ventral (5) (red), middle ventral (9) (blue), posterior ventral (13) (green) and extreme posterior regions (14) (black) are shaded in the embryo and in the regions they will give rise to in the tadpole gut. The drawings are viewed from the ventral side with anterior at the top. (C) Projection of the early dorsal endoderm. As in B but for the dorsal rather than ventral regions. (D) Location of the dorsal, lateral and ventral digestive tract rudiments for comparison with the smooth muscle layer fate map. The lateral rudiments are shown in the middle of the dorsal/ventral axis to represent their position on the side of the embryo. (E) Smooth muscle layer fate map. The position in the mesoderm of the lateral and ventral rudiments for the smooth muscle layer of the gut is shown on a drawing of a stage 14 embryo. None of the dorsal regions were fated to form smooth muscle. (F) Presumptive gene expression domains in the dorsal and ventral Xenopus endoderm. Regions of the endoderm that will give rise to tissues that express Xlhbox8 or IFABP in normal development are highlighted with coloured diagonal lines. ph, pharynx; tn, tongue; tr, trachea; lu, lungs; lv, liver; gb, gall bladder; pa, pancreas; bd, bile duct; oe, oesophagus; st, stomach; si, small intestine; sia, proximal small intestine; sib, external coil of small intestine; sic, internal coil of small intestine; li, large intestine; lic, internal coil of large intestine; lid, distal large intestine; pr, proctodaeum. The fate map for the smooth muscle layer The fate map for the smooth muscle layer is shown in Table 2 and Fig. 5E. The summary diagram shows which organs of the gut the lateral and ventral mesoderm is fated to cover in smooth muscle (Fig. 5E). The lateral rudiments are shown in the middle of the dorsal/ventral axis to depict their origin from the side of the embryo. The mesoderm fate map can be compared with the endoderm fate map (Fig. 5D). For comparison with the smooth muscle fate map this diagram shows only the organs of the digestive system that are covered in smooth muscle and, unlike Fig. 5A, includes the lateral rudiments, shown in the middle of the embryo. The middle right (7) and left (8) grafts labelled the smooth muscle layer surrounding the oesophagus, stomach and sia. The middle ventral grafts (5) labelled the posterior part of sia and sib. The posterior right (11) and left grafts (12) labelled the distal part of sib, sic and the proximal part of the large intestine. The posterior ventral graft (13) labelled the majority of the large intestine. The other regions were not found reproducibly to contribute to the smooth muscle of the gut but were fated to form other mesodermal tissues such as the notochord (this is consistent with a previous fate map of the early mesoderm; Keller, 1976). These results show that there were several differences between the origins of the epithelia and the smooth muscle. All regions in the endoderm were found to form some part of the gut epithelium while the smooth muscle originated from just a subset of regions in the early mesoderm. Another difference between the mesoderm and endoderm is that the lateral and ventral endoderm labelled the same proximal/distal segment of the gut. In contrast, the lateral mesoderm is fated to form more proximal smooth muscle than the ventral mesoderm. These differences mean that the cells of the future epithelium and smooth muscle layer in the middle of the gut overlay each other in the early embryo. In contrast, the cells of the epithelia and smooth muscle layer at the proximal end, for example oesophagus and stomach, and distal end, for example large intestine, do not.
8 388 A. D. Chalmers and J. M. W. Slack Table 2. Gut smooth muscle fate map: organs labelled from grafts in each region Position n oe st sia sib sic lic lid (1) Extreme anterior 9 (2) Anterior dorsal 8 (3) Anterior right 7(3) (4) Anterior left 7(2) 43 (5) Anterior ventral 6 (6) Middle dorsal (7) Middle right (8) Middle left 5(1) (9) Middle ventral (10) Posterior dorsal 6 (11) Posterior right 6(4) (12) Posterior left 7(3) (13) Posterior ventral (14) Extreme posterior 6 The percentage of cases where the smooth muscle for each organ was labelled is shown for each of the 14 regions. Organs that were labelled in at least 50% of the cases were included in the fate map and shaded grey in this table. The number of cases scored as whole mounts rather than in sections is shown in brackets. oe, oesophagus; st, stomach; sia, proximal small intestine; sib, external coil of the small intestine; sic, internal coil of the small intestine; lic, internal coil of the large intestine; lid, distal large intestine. The yolky cells on the floor of the archenteron are incorporated into the gut epithelium by radial intercalation At neurula stages the Xenopus endoderm consists of a single layer of dorsal cells and many layers of ventral cells (Fig. 6A). At stage 39/40 the archenteron lumen in the trunk region has become a very narrow cavity and often appears completely occluded (Fig. 6B). By stage 45 the endoderm has undergone massive elongation and formed the single layered epithelium that surrounds the gut cavity (Fig. 6C). Measurement of the pharynx and dissected gut from embryos showed that between stage 14 and stage 45 the endoderm increased in length approximately 5 times. From stage 41 to stage 45, when the gut is transformed from a straight tube to a coiled tube, the gut increases in length approximately 3.5 times. It is not clear how this transformation from the short, many layered, embryonic endoderm to the long, single layered, gut epithelia takes place, so we went on to investigate endoderm morphogenesis in more detail. A crucial question of gut formation is whether cells from each of the four positions shown in Fig. 6A are incorporated into the gut epithelium. If the dorsal, floor, middle and ventral cells (Fig. 6A) are all incorporated, then massive cell rearrangement must be occurring. In order to investigate this, a small clump of cells in the middle of the archenteron floor were labelled with DiI (Fig. 6D, arrow; the archenteron is shrunk because of the replacement of the roof). At stage 39/40, when the archenteron has narrowed, the labelled floor cells were still found in a quite dorsal position (Fig. 6E) but had become spread out over a short stretch of the anterior/posterior axis. If a clear archenteron cavity was present the label was normally found abutting the ventral side of the lumen, although occasionally a labelled cell was seen slightly more ventrally, separated from the lumen. At stage 45, once the intestinal epithelium has formed, the labelled cells were found incorporated into the intestinal epithelium (Fig. 6F). The middle endodermal cells that lie between the floor of the archenteron and the very ventral endoderm were then labelled with DiI (Fig. 6G). The middle cells had remained in a middle position by stage 39/40 (Fig. 6H) and were incorporated into the gut at stage 45 (Fig. 6I). This shows that these yolky cells of the archenteron floor and middle endoderm do not disintegrate during development but become an integral part of the gut. The extreme ventral and dorsal endoderm were then labelled to show how these regions move relative to the floor and middle cells. The ventralmost cells of the endoderm that lie next to the mesoderm were labelled (Fig. 6J). At stage 39/40 they were found to have maintained their ventral position next to the mesoderm (Fig. 6K) and at stage 45 were found incorporated into the intestinal epithelium (Fig. 6L). The proximal/distal position of the label in the intestine was consistent with the FDA fate mapping. The dorsal endoderm cells were then labelled (Fig. 6M). These cells were still dorsal at stage 39/40 (Fig. 6N) and were also incorporated into the epithelium at stage 45 (Fig. 6O). As expected from the FDA fate mapping the dorsal endoderm labelled more proximal small intestine than the ventral endoderm, and often labelled the pancreas as well. In each case (dorsal, floor, middle and ventral) a small coherent patch of labelled cells spread out to form a proximal/distal strip of labelled cells interspersed with unlabelled ones. Since cells from each position became incorporated into the single layered epithelium we inferred that radial intercalation of endodermal cells must be occurring. To prove that radial intercalation was occurring and to establish in which directions it was occurring, we carried out double labelling. The floor cells were labelled with DiI and the ventral endoderm labelled with a shallow graft (containing ectoderm, mesoderm and 1-2 layers of endoderm; see Materials and Methods) of FDA-labelled tissue. Surprisingly, this showed that the floor cells and the ventral cells ended up on opposite sides of the gut tube at stage 45 (Fig. 7B). Conversely, we found that the cells of the archenteron floor end up on the same side of the gut tube as those from the dorsal roof. This was shown by labelling the floor cells with DiI and the dorsal cells with a graft of FDA tissue (equivalent to middle and posterior dorsal regions). The dorsal and floor cells were later found intermingled on the same side of the gut tube (Fig. 7C) showing that the floor cells undergo radial intercalation with the dorsal cells as the epithelium is forming. The middle cells were then labelled with DiI and the ventral cells labelled with FDA. At stage 45 these cells were found intermingled on the same side of the gut tube (Fig. 7D). The DiI-labelled cells were also found just lateral to the FDA-labelled cells (Fig. 7D, a couple of DiIlabelled cells can be seen to the left of the gut in a more lateral position). The double labelling showed that radial intercalation was occurring between the floor and dorsal cells and the middle and ventral cells. Radial intercalation increases the surface area of a tissue (Keller, 1980, 1987; Wolpert, 1998) and so could be driving the elongation of the gut tube (see Discussion). The archenteron narrows during development before reopening to split the ventral endoderm and produce the definitive gut cavity The double labelling demonstrates that the cells on the roof and
9 Fate maps and morphogenesis in Xenopus gut 389 Fig. 6. DiI labelling of the Xenopus endoderm. (A) Section of control stage 14 embryo. The four labelling positions are marked with arrows. (B) Section of control stage 40 embryo. (C) Whole-mount stage 45 control gut. (D) Section of floor DiI labelling at stage 14 (the archenteron is shrunk because of the replacement of the roof). Insert shows high magnification view. (E) Section of floor DiI labelling visualised at stage 39. (F) Floor DiI labelling visualised in a stage 45 whole mount. (G) Section of middle labelling at stage 14. (H) Section of middle DiI labelling visualised at stage 39. (I) Middle DiI labelling visualised in a stage 45 whole mount. (J) Section of ventral DiI labelling at stage 14. (K) Section of ventral DiI labelling visualised at stage 39. (L) Ventral DiI labelling visualised in a stage 45 whole mount. (M) Section of dorsal DiI labelling at stage 14. Insert shows high magnification view. (N) Section of dorsal DiI labelling visualised at stage 39. (O) Dorsal DiI labelling visualised in a stage 45 whole mount. Arrows highlight DiI label. no, notochord; ar-r, archenteron roof; ar-f, archenteron floor. floor of the archenteron end up on one side of the gut cavity while the cells of the middle and ventral endoderm end up on the other side. This result is consistent with the archenteron closing and the definitive gut cavity opening up de novo in a position that is ventral and completely separate from the remaining archenteron cavity. However, the results could also be explained by the archenteron narrowing and then widening to split the ventral endoderm and form the definitive gut cavity. To distinguish between these two possibilities it is necessary to label the cells surrounding the archenteron and to follow the progress of the cavity during development. Sulfo-NHS-LC-biotin has previously been used to label the superficial cells of pre-gastrulation Xenopus embryos (Muller and Hausen, 1995; Minsuk and Keller, 1997). After gastrulation these superficial cells will give rise to the cells that line the archenteron cavity (Keller, 1975; Smith and Malacinski, 1983; Minsuk and Keller, 1997) and so this method can be used to label the archenteron cells. The biotin treatment gave good strong labelling of the archenteron cells (Fig. 8B, arrow) with no labelling in the rest of the endoderm or in untreated control embryos (Fig. 8A). At later stages endogenous staining was seen in the epidermis of untreated control embryos but not in the gut (Fig. 8C). By stage 38 the archenteron had closed to a narrow slit through much of the gut (Fig. 8D). At this stage a number of labelled cells can be seen to have separated from the residual archenteron cavity and now lie ventral to it (Fig. 8D,E, arrow). The number of these cells varies slightly between individuals and also along the anterior/posterior axis with fewer isolated cells seen in the posterior gut. This shows that closure of the archenteron occurs by cells becoming separated from the archenteron. At stage 40 the archenteron has narrowed further so that it often appears to have completely closed. Despite this, a small ring of the biotin-labelled cells was always clearly visible (Fig. 8F, arrow). The cells that separated from the closing cavity, clearly visible at stage 38, appear to have lost their label by stage 40. This is probably because as these cells leave the epithelium they lose their polarisation and there is a consequent increase in the turnover of cell surface proteins that would remove the biotin label. In the more posterior regions of the stage 40 intestine the archenteron cavity does not close up so much (Fig. 8G) and it appears that in certain regions it has started to widen in a ventral direction. This gives rise to a cavity lined with a region of labelled cells (arrow) and one of unlabelled cells (arrowhead). At stage 42 the archenteron in the middle of the gut is still very small while in the posterior of the gut the cavity has continued to widen (Fig. 8H). Once the gut cavity has formed the biotin labelling can be seen in one small segment of the circumference (Fig. 8I). This biotin labelling study shows that the archenteron
10 390 A. D. Chalmers and J. M. W. Slack Fig. 7. DiI/FDA double labelling of the Xenopus endoderm. (A) Section of stage 14 control embryo. The four labelling positions are marked with arrows. (B) Floor DiI and ventral FDA double labelling visualised in a stage 45 whole mount. (C) Floor DiI and dorsal FDA double labelling visualised in a stage 45 whole mount. (D) Middle DiI and ventral FDA double labelling visualised in a stage 45 whole mount. narrows to a very small cavity and some of its lining cells become dispersed into the ventral endoderm. Later the definitive gut cavity is formed by enlargement of a split originating from the archenteron remnant. This split divides the endoderm down the middle and the radial intercalation, demonstrated by the DiI labelling, transforms the short thick undifferentiated gut tube into the long thin epithelium. DISCUSSION Origin of the gut and respiratory epithelia The endoderm fate map shows that the axes of the endoderm are maintained in the later gut and that all regions of the endoderm give rise to part of the gut or respiratory epithelium. This means that the mechanisms of gut morphogenesis produce a smooth projection from the neurula to the tadpole stage. Although the axes of the endoderm were maintained, the anterior and middle dorsal endoderm were found to form more proximal gut structures than the ventral endoderm. The anterior shift in the anterior and middle dorsal endoderm was compensated for by the posterior dorsal endoderm, which spread out over a large part of the gut. Convergent extension of the dorsal axial tissues continues to occur during neurula stages (reviewed in Keller, 1987) and could possibly account for this distortion of the dorsal endoderm. A similar anterior shift has also been shown to occur in the Cerberus expressing dorsal leading edge of the gastrulating endomesoderm (Bouwmeester et al., 1996). These cells move anteriorly and then ventrally to end up in the ventral foregut region. In general, the presumptive organ rudiments are found fairly evenly spread across the embryonic endoderm but an exception to this is the anterior ventral region, which gives rise to a large number of organ rudiments. This region is also of interest because it contains the Cerberus expressing cells (see above). It will be important to understand how so many organs are induced to form from the anterior ventral region. For example, it would be interesting to know if this region contains precursor cells with more than one fate or consists of closely spaced small regions with a single fate, two possibilities that this study does not distinguish. The fate map also shows the difference in origin between the rudiments of the dorsal and ventral pancreas. There is currently a lot of progress being made in the study of pancreas development and it will be interesting to see how the different origins of the dorsal and ventral pancreas are reflected in different mechanisms of induction (e.g. Ahlgren et al., 1997; Hebrok et al., 1998; Kim and Melton, 1998; Harrison et al., 1999; Li et al., 1999). The anatomy of the early chick embryo is very different to the anatomy of early Xenopus embryos, but despite these differences there are a number of similarities between the endoderm fate map presented here and the fate map for the chick endoderm (Matsushita, 1996). The anterior/posterior organisation of the Fig. 8. The archenteron cavity during development. The superficial cell layer that gives rise to the cells lining the archenteron was labelled at stage 9/10. The labelled cells lining the archenteron could then be followed during development. (A) Unlabelled stage 14 embryo. (B) Biotin-labelled cells at stage 14 (arrow). (C) Unlabelled stage 38 embryo showing endogenous signal in the epidermis but not the gut. (D,E) Biotin-labelled cells (arrow) at stage 38 showing partial closure of archenteron. (F) Biotinlabelled cells (arrow) at stage 40 showing small persistent archenteron. The label is lost from the internalised cells. (G) Biotin-labelled cells in the posterior gut of a stage 40 embryo showing new cavity opening (arrow, biotin; arrowhead, unlabelled). (H) Biotin-labelled cells in the posterior gut at stage 42 showing cavity is only partially labelled (arrow, biotin; arrowhead, unlabelled). (I) Biotin-labelled cells (arrow) in stage 44 gut showing partial labelling of new cavity. Bar, 100 µm; 50 µm (E,G-I).
11 Fate maps and morphogenesis in Xenopus gut 391 Origin of the smooth muscle layer and signalling between the mesodermal and endodermal tissues The prospective regions for the epithelial tissues are spread throughout the endoderm. In contrast the prospective region for the smooth muscle layer is restricted to only part of the mesoderm, meaning that for most organs of the gut the prospective epithelium and smooth muscle do not overlie each other in the early embryo. The chick fate maps (Matsushita, 1995, 1996) show that the presumptive rudiments of the endoderm are located in a more anterior position than the corresponding ones in the mesoderm. So in both the chick and Xenopus the two sets of rudiments do not overlie each other in the early embryo. This suggests that reciprocal signalling between the two germ layers cannot begin until the two tissue layers come into accord later in development. This does not preclude early signals from the mesoderm to the endoderm, but it does mean that any early signals must be different from the well known late signals responsible for the regional-specific inductions from the mesenchyme (Haffen et al., 1987; Yasugi and Mizuno, 1990; Rawdon and Andrew, 1993). Fig. 9. Morphogenesis of the gut epithelium. From stage 14 to stage 38 the embryonic archenteron narrows to a small cavity. During this time the dorsal (red), floor (blue), middle (black) and ventral (green) cell populations retain their relative positions. After stage 38 the gut cavity starts to open from the remains of the archenteron to split the ventral endoderm. This produces a cavity, part of which originated from the archenteron (solid line) and part is the new cavity (dashed line). By stage 45 the gut cavity is fully open. The splitting of the ventral endoderm places the cells of the archenteron (red + blue) on one side of the cavity and the middle (black) and ventral endoderm cells (green) on the other side. Radial intercalation may drive both the opening of the gut cavity and the elongation of the gut. chick endoderm is maintained in the later gut, and the dorsal endoderm also gives rise to more proximal epithelia than the lateral endoderm. A fate map showing where the endoderm ordinates from in the early Zebrafish embryo has also recently been published (Warga and Nusslein-Volhard, 1999), but it is not possible to make detailed comparisons as it is from a comparatively much earlier stage than our fate map. The endoderm fate map and gene expression domains The intestinal fatty acid binding protein (IFABP) is expressed in the tadpole small intestine and Xlhbox8 is expressed in the tadpole pancreas (Wright et al., 1988; Shi and Hayes, 1994; Chalmers and Slack, 1998). The fate map shows which parts of the endoderm will form these regions of the embryo, making it possible to predict which regions of the endoderm will express these genes in later development (Fig. 5F). As more genes are shown to be expressed in the tadpole gut they can be added to the fate map. A model for morphogenesis of the gut Based on the combined evidence from the endoderm fate map, the DiI labelling and the biotin surface labelling experiments, we propose a model for the morphogenesis of the gut (Fig. 9). From neurula stages to stage 38 the archenteron gradually closes. This is achieved by cells leaving the cavity lining to be incorporated into the deeper endoderm. These movements leave the original dorsal (red), floor (blue), middle (black) and ventral (green) cell groups in similar relative positions. From stage 38 to stage 45 the remains of the archenteron expands to split the ventral endoderm. This gives rise to the definitive gut cavity, which contains the cells of the original archenteron and the more ventral endoderm. Cell rearrangements have been shown to be a powerful force in morphogenesis (reviewed in Keller, 1987; Wolpert, 1998). A good example is gastrulation in Xenopus embryos, where both radial and mediolateral intercalations drive elongation (Keller, 1980, 1984; Keller et al., 1985). The DiI labelling shows that the floor endoderm intercalates with the dorsal endoderm and the middle endoderm intercalates with the ventral/lateral endoderm. This radial intercalation would cause the opening of the gut cavity from the remains of the archenteron and would also increase the surface area of the gut accounting for at least part of the gut elongation. Cell rearrangements can be an active, force-generating process, or a passive process that responds to external forces (Keller, 1987). The DiI labelling shows that the radial intercalation occurs between stage 40 and stage 45, when the gut is transformed from a straight tube to a complex coiled structure and elongates approximately 3.5 times. Once coiling has started the elongation of the endoderm can no longer be linked to the elongation of the body axis and so must occur by a separate active process. We propose that this process is the radial intercalation of the endoderm and that it drives both the elongation of the gut and the opening of the gut cavity. The authors would like to thank the Wellcome Trust for providing a Prize Studentship for A. D. C. and for providing the confocal facility in the department (grant no ). We would also like to thank Caroline Beck, Bea Christen, Marko Horb, Benjamin Rawdon and Andrew Ward for comments on the manuscript.
Endoderm Specification and Differentiation in Xenopus Embryos
 Developmental Biology 236, 330 343 (2001) doi:10.1006/dbio.2001.0347, available online at http://www.idealibrary.com on Endoderm Specification and Differentiation in Xenopus Embryos Marko E. Horb 1 and
Developmental Biology 236, 330 343 (2001) doi:10.1006/dbio.2001.0347, available online at http://www.idealibrary.com on Endoderm Specification and Differentiation in Xenopus Embryos Marko E. Horb 1 and
Mesoderm Induction CBT, 2018 Hand-out CBT March 2018
 Mesoderm Induction CBT, 2018 Hand-out CBT March 2018 Introduction 3. Books This module is based on the following books: - 'Principles of Developement', Lewis Wolpert, et al., fifth edition, 2015 - 'Developmental
Mesoderm Induction CBT, 2018 Hand-out CBT March 2018 Introduction 3. Books This module is based on the following books: - 'Principles of Developement', Lewis Wolpert, et al., fifth edition, 2015 - 'Developmental
Early Development in Invertebrates
 Developmental Biology Biology 4361 Early Development in Invertebrates October 25, 2006 Early Development Overview Cleavage rapid cell divisions divisions of fertilized egg into many cells Gastrulation
Developmental Biology Biology 4361 Early Development in Invertebrates October 25, 2006 Early Development Overview Cleavage rapid cell divisions divisions of fertilized egg into many cells Gastrulation
Gut Tube Development. For it s rare that a man thinks of anything so seriously as his dinner!
 For it s rare that a man thinks of anything so seriously as his dinner! Ben Johnson Gut Tube Development This chapter will follow the development of the embryonic gut caudal to the developing pharynx,
For it s rare that a man thinks of anything so seriously as his dinner! Ben Johnson Gut Tube Development This chapter will follow the development of the embryonic gut caudal to the developing pharynx,
1. What are the three general areas of the developing vertebrate limb? 2. What embryonic regions contribute to the developing limb bud?
 Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Developmental Biology Lecture Outlines
 Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Life Sciences For NET & SLET Exams Of UGC-CSIR. Section B and C. Volume-08. Contents A. BASIC CONCEPT OF DEVELOPMENT 1
 Section B and C Volume-08 Contents 5. DEVELOPMENTAL BIOLOGY A. BASIC CONCEPT OF DEVELOPMENT 1 B. GAMETOGENESIS, FERTILIZATION AND EARLY DEVELOPMENT 23 C. MORPHOGENESIS AND ORGANOGENESIS IN ANIMALS 91 0
Section B and C Volume-08 Contents 5. DEVELOPMENTAL BIOLOGY A. BASIC CONCEPT OF DEVELOPMENT 1 B. GAMETOGENESIS, FERTILIZATION AND EARLY DEVELOPMENT 23 C. MORPHOGENESIS AND ORGANOGENESIS IN ANIMALS 91 0
Introduction to Embryology. He who sees things grow from the beginning will have the finest view of them.
 He who sees things grow from the beginning will have the finest view of them. Aristotle 384 322 B.C. Introduction to Embryology This lecture will introduce you to the science of developmental biology or
He who sees things grow from the beginning will have the finest view of them. Aristotle 384 322 B.C. Introduction to Embryology This lecture will introduce you to the science of developmental biology or
Questions in developmental biology. Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration
 Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
Sonic hedgehog (Shh) signalling in the rabbit embryo
 Sonic hedgehog (Shh) signalling in the rabbit embryo In the first part of this thesis work the physical properties of cilia-driven leftward flow were characterised in the rabbit embryo. Since its discovery
Sonic hedgehog (Shh) signalling in the rabbit embryo In the first part of this thesis work the physical properties of cilia-driven leftward flow were characterised in the rabbit embryo. Since its discovery
10/15/09. Tetrapod Limb Development & Pattern Formation. Developing limb region is an example of a morphogenetic field
 Tetrapod Limb Development & Pattern Formation Figure 16.5(1) Limb Bud Formation derived from lateral plate (somatic) & paraxial (myotome) Fig. 16.2 Prospective Forelimb Field of Salamander Ambystoma maculatum
Tetrapod Limb Development & Pattern Formation Figure 16.5(1) Limb Bud Formation derived from lateral plate (somatic) & paraxial (myotome) Fig. 16.2 Prospective Forelimb Field of Salamander Ambystoma maculatum
BIOLOGY - CLUTCH CH.32 - OVERVIEW OF ANIMALS.
 !! www.clutchprep.com Animals are multicellular, heterotrophic eukaryotes that feed by ingesting their food Most animals are diploid, and produce gametes produced directly by meiosis Animals lack cell
!! www.clutchprep.com Animals are multicellular, heterotrophic eukaryotes that feed by ingesting their food Most animals are diploid, and produce gametes produced directly by meiosis Animals lack cell
Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis
 Development 116, 915-930 (1992) Printed in Great Britain The Company of Biologists Limited 1992 915 Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis JOHN SHIH* and RAY KELLER
Development 116, 915-930 (1992) Printed in Great Britain The Company of Biologists Limited 1992 915 Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis JOHN SHIH* and RAY KELLER
Tail bud determination in the vertebrate embryo
 Tail bud determination in the vertebrate embryo Abigail S. Tucker and Jonathan M.W. Slack ICRF Developmental Biology Unit, Department of Zoology, Oxford University, South Parks Road, Oxford OX1 3PS, UK.
Tail bud determination in the vertebrate embryo Abigail S. Tucker and Jonathan M.W. Slack ICRF Developmental Biology Unit, Department of Zoology, Oxford University, South Parks Road, Oxford OX1 3PS, UK.
Maternal Control of GermLayer Formation in Xenopus
 Maternal Control of GermLayer Formation in Xenopus The zygotic genome is activated at the mid-blastula transition mid-blastula fertilized egg Xenopus gastrulae early-gastrula 7 hrs 10 hrs control not VP
Maternal Control of GermLayer Formation in Xenopus The zygotic genome is activated at the mid-blastula transition mid-blastula fertilized egg Xenopus gastrulae early-gastrula 7 hrs 10 hrs control not VP
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic)
 Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons
Developmental Biology Biology 4361
 Developmental Biology Biology 4361 The Anatomical Tradition 2009 A hen is only an egg s way of making a new egg. Samuel Butler, 1885 The Anatomical Tradition - Overview What is developmental biology? How
Developmental Biology Biology 4361 The Anatomical Tradition 2009 A hen is only an egg s way of making a new egg. Samuel Butler, 1885 The Anatomical Tradition - Overview What is developmental biology? How
Unicellular: Cells change function in response to a temporal plan, such as the cell cycle.
 Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Role of Organizer Chages in Late Frog Embryos
 Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
The Radiata-Bilateria split. Second branching in the evolutionary tree
 The Radiata-Bilateria split Second branching in the evolutionary tree Two very important characteristics are used to distinguish between the second bifurcation of metazoans Body symmetry Germinal layers
The Radiata-Bilateria split Second branching in the evolutionary tree Two very important characteristics are used to distinguish between the second bifurcation of metazoans Body symmetry Germinal layers
Biology 218, practise Exam 2, 2011
 Figure 3 The long-range effect of Sqt does not depend on the induction of the endogenous cyc or sqt genes. a, Design and predictions for the experiments shown in b-e. b-e, Single-cell injection of 4 pg
Figure 3 The long-range effect of Sqt does not depend on the induction of the endogenous cyc or sqt genes. a, Design and predictions for the experiments shown in b-e. b-e, Single-cell injection of 4 pg
Drosophila melanogaster- Morphogen Gradient
 NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
Fish swimbladder: an excellent meso dermal inductor in primary embryonic induction
 /. Embryo/, exp. Morph. Vol 36,, pp. 315-30, 1976 315 Printed in Great Britain Fish swimbladder: an excellent meso dermal inductor in primary embryonic induction IZUMI KAWAKAMI 1 From the Department of
/. Embryo/, exp. Morph. Vol 36,, pp. 315-30, 1976 315 Printed in Great Britain Fish swimbladder: an excellent meso dermal inductor in primary embryonic induction IZUMI KAWAKAMI 1 From the Department of
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning
 MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
Mesoderm Development
 Quiz rules: Spread out across available tables No phones, text books, or (lecture) notes on your desks No consultation with your colleagues No websites open other than the Quiz page No screen snap shots
Quiz rules: Spread out across available tables No phones, text books, or (lecture) notes on your desks No consultation with your colleagues No websites open other than the Quiz page No screen snap shots
!!!!!!!! DB3230 Midterm 2 12/13/2013 Name:
 1. (10 pts) Draw or describe the fate map of a late blastula stage sea urchin embryo. Draw or describe the corresponding fate map of the pluteus stage larva. Describe the sequence of gastrulation events
1. (10 pts) Draw or describe the fate map of a late blastula stage sea urchin embryo. Draw or describe the corresponding fate map of the pluteus stage larva. Describe the sequence of gastrulation events
Paraxial and Intermediate Mesoderm
 Biology 4361 Paraxial and Intermediate Mesoderm December 6, 2007 Mesoderm Formation Chick Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of
Biology 4361 Paraxial and Intermediate Mesoderm December 6, 2007 Mesoderm Formation Chick Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of
Introduction to Animals
 Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
Designation of the Anterior/Posterior Axis in Pregastrula Xenopus laevis
 Developmental Biology 225, 37 58 (2000) doi:10.1006/dbio.2000.9803, available online at http://www.idealibrary.com on Designation of the Anterior/Posterior Axis in Pregastrula Xenopus laevis Mary Constance
Developmental Biology 225, 37 58 (2000) doi:10.1006/dbio.2000.9803, available online at http://www.idealibrary.com on Designation of the Anterior/Posterior Axis in Pregastrula Xenopus laevis Mary Constance
SIGNIFICANCE OF EMBRYOLOGY
 This lecture will discuss the following topics : Definition of Embryology Significance of Embryology Old and New Frontiers Introduction to Molecular Regulation and Signaling Descriptive terms in Embryology
This lecture will discuss the following topics : Definition of Embryology Significance of Embryology Old and New Frontiers Introduction to Molecular Regulation and Signaling Descriptive terms in Embryology
Paraxial and Intermediate Mesoderm
 Biology 4361 Paraxial and Intermediate Mesoderm December 7, 2006 Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of BMPs more lateral mesoderm
Biology 4361 Paraxial and Intermediate Mesoderm December 7, 2006 Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of BMPs more lateral mesoderm
Chapter 10 Development and Differentiation
 Part III Organization of Cell Populations Chapter Since ancient times, people have wondered how organisms are formed during the developmental process, and many researchers have worked tirelessly in search
Part III Organization of Cell Populations Chapter Since ancient times, people have wondered how organisms are formed during the developmental process, and many researchers have worked tirelessly in search
The epithelium of the dorsal marginal zone of Xenopus has organizer properties
 Development 116, 887-899 (1992) Printed in Great Britain The Company of Biologists Limited 1992 887 The epithelium of the dorsal marginal zone of Xenopus has organizer properties JOHN SHIH* and RAY KELLER
Development 116, 887-899 (1992) Printed in Great Britain The Company of Biologists Limited 1992 887 The epithelium of the dorsal marginal zone of Xenopus has organizer properties JOHN SHIH* and RAY KELLER
Outline. v Definition and major characteristics of animals v Dividing animals into groups based on: v Animal Phylogeny
 BIOSC 041 Overview of Animal Diversity: Animal Body Plans Reference: Chapter 32 Outline v Definition and major characteristics of animals v Dividing animals into groups based on: Body symmetry Tissues
BIOSC 041 Overview of Animal Diversity: Animal Body Plans Reference: Chapter 32 Outline v Definition and major characteristics of animals v Dividing animals into groups based on: Body symmetry Tissues
Chapter 32. Objectives. Table of Contents. Characteristics. Characteristics, continued. Section 1 The Nature of Animals
 Introduction to Animals Table of Contents Objectives Identify four important characteristics of animals. List two kinds of tissues found only in animals. Explain how the first animals may have evolved
Introduction to Animals Table of Contents Objectives Identify four important characteristics of animals. List two kinds of tissues found only in animals. Explain how the first animals may have evolved
BIOLOGY 340 Exam Study Guide All Exams Comparative Embryology Dr. Stuart S. Sumida California State University San Bernardino; Department of Biology
 BIOLOGY 340 Exam Study Guide All Exams Comparative Embryology Dr. Stuart S. Sumida California State University San Bernardino; Department of Biology Midterm and final exams may include materials studied
BIOLOGY 340 Exam Study Guide All Exams Comparative Embryology Dr. Stuart S. Sumida California State University San Bernardino; Department of Biology Midterm and final exams may include materials studied
Biosc 41 9/10 Announcements
 Biosc 41 9/10 Announcements v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal Body Plans
Biosc 41 9/10 Announcements v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal Body Plans
The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organiser
 Development 1992 Supplement, 81-91 (1992) Printed in Great Britain C The Company of Biologist! Limited 1992 81 The patterning and functioning of protrusive activity during convergence and extension of
Development 1992 Supplement, 81-91 (1992) Printed in Great Britain C The Company of Biologist! Limited 1992 81 The patterning and functioning of protrusive activity during convergence and extension of
ANIMAL DIVERSITY AND THE EVOLUTION OF BODY PLANS
 ANIMAL DIVERSITY AND THE EVOLUTION OF BODY PLANS GENERAL FEATURES OF ANIMALS Heterotrophy - obtain energy and organic molecules by ingesting other organisms Multicellularity - Many have complex bodies
ANIMAL DIVERSITY AND THE EVOLUTION OF BODY PLANS GENERAL FEATURES OF ANIMALS Heterotrophy - obtain energy and organic molecules by ingesting other organisms Multicellularity - Many have complex bodies
1. Why Dissect. Why are frogs a good model to use when studying the digestive system (as well as other systems)?
 Name: Date: Period: Frog Dissection Virtual Lab Use the frog Dissection link that follows to answer the questions. http://www.mhhe.com/biosci/genbio/virtual_labs/bl_16/bl_16.html Introduction 1. Why Dissect.
Name: Date: Period: Frog Dissection Virtual Lab Use the frog Dissection link that follows to answer the questions. http://www.mhhe.com/biosci/genbio/virtual_labs/bl_16/bl_16.html Introduction 1. Why Dissect.
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Question Set # 4 Answer Key 7.22 Nov. 2002
 Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
v Scientists have identified 1.3 million living species of animals v The definition of an animal
 Biosc 41 9/10 Announcements BIOSC 041 v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal
Biosc 41 9/10 Announcements BIOSC 041 v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Name KEY. Biology Developmental Biology Winter Quarter Midterm 3 KEY
 Name KEY 100 Total Points Open Book Biology 411 - Developmental Biology Winter Quarter 2009 Midterm 3 KEY All of the 25 multi-choice questions are single-answer. Choose the best answer. (4 pts each) Place
Name KEY 100 Total Points Open Book Biology 411 - Developmental Biology Winter Quarter 2009 Midterm 3 KEY All of the 25 multi-choice questions are single-answer. Choose the best answer. (4 pts each) Place
Developmental Biology 3230 Midterm Exam 1 March 2006
 Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Section 4 Professor Donald McFarlane
 Characteristics Section 4 Professor Donald McFarlane Lecture 11 Animals: Origins and Bauplans Multicellular heterotroph Cells lack cell walls Most have nerves, muscles, capacity to move at some point in
Characteristics Section 4 Professor Donald McFarlane Lecture 11 Animals: Origins and Bauplans Multicellular heterotroph Cells lack cell walls Most have nerves, muscles, capacity to move at some point in
What Is an Animal? Section 25.1 Typical Animal Characteristics. I. Characteristics of Animals. Biology II Mrs. Michaelsen
 What Is an Animal? Section 25.1 Typical Animal Characteristics Biology II Mrs. Michaelsen I. Characteristics of Animals A. All animals are eukaryotic, multicellular, have ways of moving to reproduce, obtain
What Is an Animal? Section 25.1 Typical Animal Characteristics Biology II Mrs. Michaelsen I. Characteristics of Animals A. All animals are eukaryotic, multicellular, have ways of moving to reproduce, obtain
Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues
 Developmental Biology 280 (2005) 87 99 www.elsevier.com/locate/ydbio Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues Kimberly D. Tremblay
Developmental Biology 280 (2005) 87 99 www.elsevier.com/locate/ydbio Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues Kimberly D. Tremblay
Physiology. Organization of the Body. Assumptions in Physiology. Chapter 1. Physiology is the study of how living organisms function
 Introduction to Physiology and Homeostasis Chapter 1 Physiology Physiology is the study of how living organisms function On the street explanations are in terms of meeting a bodily need Physiologic explanations
Introduction to Physiology and Homeostasis Chapter 1 Physiology Physiology is the study of how living organisms function On the street explanations are in terms of meeting a bodily need Physiologic explanations
Drosophila Life Cycle
 Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
Developmental processes Differential gene expression Introduction to determination The model organisms used to study developmental processes
 Date Title Topic(s) Learning Outcomes: Sept 28 Oct 3 1. What is developmental biology and why should we care? 2. What is so special about stem cells and gametes? Developmental processes Differential gene
Date Title Topic(s) Learning Outcomes: Sept 28 Oct 3 1. What is developmental biology and why should we care? 2. What is so special about stem cells and gametes? Developmental processes Differential gene
2. Fertilization activates the egg and bring together the nuclei of sperm and egg
 2. Fertilization activates the egg and bring together the nuclei of sperm and egg Sea urchins (what phylum?) are models for the study of the early development of deuterostomes (like us, right?). Sea urchin
2. Fertilization activates the egg and bring together the nuclei of sperm and egg Sea urchins (what phylum?) are models for the study of the early development of deuterostomes (like us, right?). Sea urchin
Name. Biology Developmental Biology Winter Quarter 2013 KEY. Midterm 3
 Name 100 Total Points Open Book Biology 411 - Developmental Biology Winter Quarter 2013 KEY Midterm 3 Read the Following Instructions: * Answer 20 questions (5 points each) out of the available 25 questions
Name 100 Total Points Open Book Biology 411 - Developmental Biology Winter Quarter 2013 KEY Midterm 3 Read the Following Instructions: * Answer 20 questions (5 points each) out of the available 25 questions
7.013 Problem Set
 7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
Maternal VegT and b-catenin: Patterning the Xenopus Blastula
 CHAPTER 1 Maternal VegT and b-catenin: Patterning the Xenopus Blastula Matthew Kofron, Jennifer Xanthos, and Janet Heasman 1 1.1 Introduction Loss of the maternal T-box transcription factor VegT has a
CHAPTER 1 Maternal VegT and b-catenin: Patterning the Xenopus Blastula Matthew Kofron, Jennifer Xanthos, and Janet Heasman 1 1.1 Introduction Loss of the maternal T-box transcription factor VegT has a
Kingdom Animalia. Zoology the study of animals
 Kingdom Animalia Zoology the study of animals Summary Animals are multicellular and eukaryotic. consume and digest organic materials thereby being heterotrophs. Most are motile at some time in their lives.
Kingdom Animalia Zoology the study of animals Summary Animals are multicellular and eukaryotic. consume and digest organic materials thereby being heterotrophs. Most are motile at some time in their lives.
Paraxial and Intermediate Mesoderm
 Biology 4361 Paraxial and Intermediate Mesoderm December 6, 2007 Mesoderm Formation Chick Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of
Biology 4361 Paraxial and Intermediate Mesoderm December 6, 2007 Mesoderm Formation Chick Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of
Are these organisms. animals or not?
 1 2 3 4 5 Are these organisms 6 7 8 animals or not? 9 10 11 12 13 14 15 16 1 2 3 4 5 6 7 8 9 10 11 12 Typical Animal Characteristics Eukaryotic Multicellular Ability to move Reproduce Obtain food (heterotrophic)
1 2 3 4 5 Are these organisms 6 7 8 animals or not? 9 10 11 12 13 14 15 16 1 2 3 4 5 6 7 8 9 10 11 12 Typical Animal Characteristics Eukaryotic Multicellular Ability to move Reproduce Obtain food (heterotrophic)
PRACTICE EXAM. 20 pts: 1. With the aid of a diagram, indicate how initial dorsal-ventral polarity is created in fruit fly and frog embryos.
 PRACTICE EXAM 20 pts: 1. With the aid of a diagram, indicate how initial dorsal-ventral polarity is created in fruit fly and frog embryos. No Low [] Fly Embryo Embryo Non-neural Genes Neuroectoderm Genes
PRACTICE EXAM 20 pts: 1. With the aid of a diagram, indicate how initial dorsal-ventral polarity is created in fruit fly and frog embryos. No Low [] Fly Embryo Embryo Non-neural Genes Neuroectoderm Genes
Urodeles Remove Mesoderm from the Superficial Layer by Subduction through a Bilateral Primitive Streak
 Developmental Biology 248, 220 239 (2002) doi:10.1006/dbio.2002.0718 Urodeles Remove Mesoderm from the Superficial Layer by Subduction through a Bilateral Primitive Streak David R. Shook, 1 Christina Majer,
Developmental Biology 248, 220 239 (2002) doi:10.1006/dbio.2002.0718 Urodeles Remove Mesoderm from the Superficial Layer by Subduction through a Bilateral Primitive Streak David R. Shook, 1 Christina Majer,
Revision Based on Chapter 25 Grade 11
 Revision Based on Chapter 25 Grade 11 Biology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A cell that contains a nucleus and membrane-bound organelles
Revision Based on Chapter 25 Grade 11 Biology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A cell that contains a nucleus and membrane-bound organelles
Axis determination in flies. Sem 9.3.B.5 Animal Science
 Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Developmental Zoology. Ectodermal derivatives (ZOO ) Developmental Stages. Developmental Stages
 Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
9/4/2015 INDUCTION CHAPTER 1. Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology. Fig 1.
 INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
Unit 1: Body Plan & Organization Test Review 1. Define anatomy and contrast it with physiology.
 Name: Period: Unit 1: Body Plan & Organization Test Review 1. Define anatomy and contrast it with physiology. 2. Arrange and identify, in order, the six levels of structural organization of the human body.
Name: Period: Unit 1: Body Plan & Organization Test Review 1. Define anatomy and contrast it with physiology. 2. Arrange and identify, in order, the six levels of structural organization of the human body.
Mesoderm induction in Xenopus laevis \ a quantitative study using a cell lineage label and tissue-specific antibodies
 /. Embryol. exp. Morph. 89, 289-312 (1985) 289 Printed in Great Britain The Company of Biologists Limited 1985 Mesoderm induction in Xenopus laevis \ a quantitative study using a cell lineage label and
/. Embryol. exp. Morph. 89, 289-312 (1985) 289 Printed in Great Britain The Company of Biologists Limited 1985 Mesoderm induction in Xenopus laevis \ a quantitative study using a cell lineage label and
 DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Caudalization of neural fate by tissue recombination and bfgf
 Development 121, 4349-4358 (1995) Printed in Great Britain The Company of Biologists Limited 1995 DEV9434 4349 Caudalization of neural fate by tissue recombination and bfgf Wm. Gregory Cox and Ali Hemmati-Brivanlou
Development 121, 4349-4358 (1995) Printed in Great Britain The Company of Biologists Limited 1995 DEV9434 4349 Caudalization of neural fate by tissue recombination and bfgf Wm. Gregory Cox and Ali Hemmati-Brivanlou
Chapter 32, 10 th edition Q1.Which characteristic below is shared by plants, fungi, and animals? ( Concept 32.1)
 Chapter 32, 10 th edition Q1.Which characteristic below is shared by plants, fungi, and animals? ( Concept 32.1) A) They are multicellular eukaryotes. B) They are heterotrophs. C) Their cells are supported
Chapter 32, 10 th edition Q1.Which characteristic below is shared by plants, fungi, and animals? ( Concept 32.1) A) They are multicellular eukaryotes. B) They are heterotrophs. C) Their cells are supported
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its
 Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Independent Induction and Formation of the Dorsal and Ventral Fins in Xenopus laevis
 DEVELOPMENTAL DYNAMICS 230:461 467, 2004 RESEARCH ARTICLE Independent Induction and Formation of the Dorsal and Ventral Fins in Xenopus laevis A.S. Tucker 1 * and J.M.W. Slack 2 It has been known since
DEVELOPMENTAL DYNAMICS 230:461 467, 2004 RESEARCH ARTICLE Independent Induction and Formation of the Dorsal and Ventral Fins in Xenopus laevis A.S. Tucker 1 * and J.M.W. Slack 2 It has been known since
Biology 11. The Kingdom Animalia
 Biology 11 The Kingdom Animalia Objectives By the end of the lesson you should be able to: Describe the 5 ways we classify animals Symmetry Germ layers Body plan Segmentation Animal Evolution Hank Video
Biology 11 The Kingdom Animalia Objectives By the end of the lesson you should be able to: Describe the 5 ways we classify animals Symmetry Germ layers Body plan Segmentation Animal Evolution Hank Video
Faculty of Science Course Syllabus Department of Biology BIOL 3050: Developmental Biology Fall 2018
 Faculty of Science Course Syllabus Department of Biology BIOL 3050: Developmental Biology Fall 2018 Instructor(s): Margaret Cooper Margaret.Cooper@dal.ca LSC 4014 Lectures: 1:35 2:25 MWF LSC Common Area
Faculty of Science Course Syllabus Department of Biology BIOL 3050: Developmental Biology Fall 2018 Instructor(s): Margaret Cooper Margaret.Cooper@dal.ca LSC 4014 Lectures: 1:35 2:25 MWF LSC Common Area
Unit 4 Evaluation Question 1:
 Name: Unit 4 Evaluation Question 1: /7 points A naturally occurring dominant mutant in mice is the Doublefoot (Dbf) mutant. Below is an image of the bones from a wildtype (wt) and Doublefoot mutant mouse.
Name: Unit 4 Evaluation Question 1: /7 points A naturally occurring dominant mutant in mice is the Doublefoot (Dbf) mutant. Below is an image of the bones from a wildtype (wt) and Doublefoot mutant mouse.
Animal Origins and Evolution
 Animal Origins and Evolution Common Features of Animals multicellular heterotrophic motile Sexual reproduction, embryo Evolution of Animals All animals are multicellular and heterotrophic, which means
Animal Origins and Evolution Common Features of Animals multicellular heterotrophic motile Sexual reproduction, embryo Evolution of Animals All animals are multicellular and heterotrophic, which means
Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida. Overview of Pre-Metazoan. and Protostome Development (Insects)
 Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida Overview of Pre-Metazoan and Protostome Development (Insects) Plants Fungi Animals In1998 fossilized animal embryos were reported from the
Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida Overview of Pre-Metazoan and Protostome Development (Insects) Plants Fungi Animals In1998 fossilized animal embryos were reported from the
Exam 2 ID#: November 9, 2006
 Biology 4361 Name: KEY Exam 2 ID#: November 9, 2006 Multiple choice (one point each) Circle the best answer. 1. Inducers of Xenopus lens and optic vesicle include a. pharyngeal endoderm and anterior neural
Biology 4361 Name: KEY Exam 2 ID#: November 9, 2006 Multiple choice (one point each) Circle the best answer. 1. Inducers of Xenopus lens and optic vesicle include a. pharyngeal endoderm and anterior neural
Paraxial and Intermediate Mesoderm
 Biology 4361 Paraxial and Intermediate Mesoderm July 28, 2008 Paraxial and Intermediate Mesoderm Overview Development of major mesodermal lineages Somites: formation specification and differentiation Mesodermal
Biology 4361 Paraxial and Intermediate Mesoderm July 28, 2008 Paraxial and Intermediate Mesoderm Overview Development of major mesodermal lineages Somites: formation specification and differentiation Mesodermal
Why Flies? stages of embryogenesis. The Fly in History
 The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
1. General Features of Animals
 Chapter 32: An Overview of Animal Diversity 1. General Features of Animals 2. The History of Animals 1. General Features of Animals General Characteristics of Animals animals are multicellular eukaryotic
Chapter 32: An Overview of Animal Diversity 1. General Features of Animals 2. The History of Animals 1. General Features of Animals General Characteristics of Animals animals are multicellular eukaryotic
1/30/2009. Copyright The McGraw Hill Companies, Inc. Permission required for reproduction or display.
 CHAPTER 9 Architectural Pattern of an Animal New Designs for Living Zoologists recognize 34 major phyla of living multicellular animals Survivors of around 100 phyla that appeared 600 million years ago
CHAPTER 9 Architectural Pattern of an Animal New Designs for Living Zoologists recognize 34 major phyla of living multicellular animals Survivors of around 100 phyla that appeared 600 million years ago
Exam 3 (Final Exam) December 20, 2007
 Biology 4361 Exam 3 (Final Exam) December 20, 2007 Name: ID: Multiple choice (1 point each. Indicate the best answer.) 1. During Drosophila gastrulation, mesoderm moves in through the a. primitives streak.
Biology 4361 Exam 3 (Final Exam) December 20, 2007 Name: ID: Multiple choice (1 point each. Indicate the best answer.) 1. During Drosophila gastrulation, mesoderm moves in through the a. primitives streak.
Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants
 Development 105, 155-166 (1989) Printed in Great Britain The Company of Biologists Limited 1989 155 Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants PAUL A. WILSON
Development 105, 155-166 (1989) Printed in Great Britain The Company of Biologists Limited 1989 155 Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants PAUL A. WILSON
18. Which body system is needed for the exchange of oxygen and carbon dioxide? A. Respiratory B. Integumentary C. Digestive D. Urinary 19.
 1 Student: 1. Which of the following is NOT a part of the study of anatomy? A. The structure of body parts B. Predicting the body's responses to stimuli C. Microscopic organization D. The relationship
1 Student: 1. Which of the following is NOT a part of the study of anatomy? A. The structure of body parts B. Predicting the body's responses to stimuli C. Microscopic organization D. The relationship
Introduction to Animal Diversity Lecture 7 Winter 2014
 Introduction to Animal Diversity Lecture 7 Winter 2014 Evolution of Animals 1 Prokaryotes Eukaryotes Prokaryotes No nucleus Nucleoid region Simple No membrane bound organelles Smaller (1-5 nm) Evolutionarily
Introduction to Animal Diversity Lecture 7 Winter 2014 Evolution of Animals 1 Prokaryotes Eukaryotes Prokaryotes No nucleus Nucleoid region Simple No membrane bound organelles Smaller (1-5 nm) Evolutionarily
Chapter 1: Introduction to Anatomy and Physiology
 Chapter 1: Introduction to Anatomy and Physiology MULTIPLE CHOICE 1. The anatomic term means toward the midline. a. anterior b. posterior c. medial d. cranial The term medial indicates an anatomic direction
Chapter 1: Introduction to Anatomy and Physiology MULTIPLE CHOICE 1. The anatomic term means toward the midline. a. anterior b. posterior c. medial d. cranial The term medial indicates an anatomic direction
Later embryogenesis: regulatory circuitry in morphogenetic fields
 Development 118, 665-690 (1993) Printed in Great Britain The Company of Biologists Limited 1993 Review Article 665 Later embryogenesis: regulatory circuitry in morphogenetic fields Eric H. Davidson Division
Development 118, 665-690 (1993) Printed in Great Britain The Company of Biologists Limited 1993 Review Article 665 Later embryogenesis: regulatory circuitry in morphogenetic fields Eric H. Davidson Division
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Applegate: The Anatomy and Physiology Learning System, 3 rd Edition
 Applegate: The Anatomy and Physiology Learning System, 3 rd Edition Chapter 1: Introduction to the Human Body TRUE/FALSE 1. The cell is the simplest living unit of organization within the human body. T
Applegate: The Anatomy and Physiology Learning System, 3 rd Edition Chapter 1: Introduction to the Human Body TRUE/FALSE 1. The cell is the simplest living unit of organization within the human body. T
Chapter 1- An Orientation to the Human Body NOTES
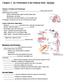 Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
Workshop: The Evolution of Animalia body symmetry embryonic germ layers ontogenetic origins I. What is an Animal? II. Germ Layers
 Workshop: The Evolution of Animalia by Dana Krempels Perhaps even more than the other Eukarya, Animalia is characterized by a distinct progression of complexity in form and function as one moves from the
Workshop: The Evolution of Animalia by Dana Krempels Perhaps even more than the other Eukarya, Animalia is characterized by a distinct progression of complexity in form and function as one moves from the
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
MBios 401/501: Lecture 14.2 Cell Differentiation I. Slide #1. Cell Differentiation
 MBios 401/501: Lecture 14.2 Cell Differentiation I Slide #1 Cell Differentiation Cell Differentiation I -Basic principles of differentiation (p1305-1320) -C-elegans (p1321-1327) Cell Differentiation II
MBios 401/501: Lecture 14.2 Cell Differentiation I Slide #1 Cell Differentiation Cell Differentiation I -Basic principles of differentiation (p1305-1320) -C-elegans (p1321-1327) Cell Differentiation II
Animal Diversity. Features shared by all animals. Animals are multicellular, heterotrophic eukaryotes with tissues that develop from embryonic layers
 Animal Diversity Animals are multicellular, heterotrophic eukaryotes with tissues that develop from embryonic layers Nutritional mode Ingest food and use enzymes in the body to digest Cell structure and
Animal Diversity Animals are multicellular, heterotrophic eukaryotes with tissues that develop from embryonic layers Nutritional mode Ingest food and use enzymes in the body to digest Cell structure and
