Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells
|
|
|
- Dayna Lang
- 5 years ago
- Views:
Transcription
1 RESEARCH ARTICLE 493 Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells Hiroki Oda 1, * and Shoichiro Tsukita 1,2 1 Tsukita Cell Axis Project, Exploratory Research for Advanced Technology, Japan Science and Technology Corporation, Kyoto Research Park, Chudoji Minami-machi, Shimogyo-ku, Kyoto , Japan 2 Department of Cell Biology, Faculty of Medicine, Kyoto University, Yoshidakonoe-cho, Sakyo-ku, Kyoto , Japan *Author for correspondence ( hoda@cell.tsukita.jst.go.jp) Accepted 15 November 2000 Journal of Cell Science 114, The Company of Biologists Ltd SUMMARY Invagination of the epithelial cell sheet of the prospective mesoderm in Drosophila gastrulation is a well-studied, relatively simple morphogenetic event that results from dynamic cell shape changes and cell movements. However, these cell behaviors have not been followed at a sufficiently short time resolution. We examined mesoderm invagination in living wild-type embryos by real-time imaging of fluorescently labeled cell-cell adherens junctions, which are located at the apical zones of cell-cell contact. Low-light fluorescence video microscopy directly visualized the onset and progression of invagination. In an initial period of approximately 2 minutes, cells around the ventral midline reduced their apical surface areas slowly in a rather synchronous manner. Next, the central and more lateral cells stochastically accelerated or initiated their apical constriction, giving rise to random arrangements of cells with small and relatively large apices. Thus, we found that mesoderm invagination began with slow synchronous and subsequent fast stochastic phases of cell apex constriction. Furthermore, we showed that the mesoderm invagination of folded gastrulation mutant embryos lacked the normal two constriction phases, and instead began with asynchronous, feeble cell shape changes. Our observations suggested that Folded gastrulation-mediated signaling enabled synchronous activation of the contractile cortex, causing competition among the individual mesodermal cells for apical constriction. Movies available on-line: Key words: Mesoderm, Invagination, Gastrulation, Apical constriction, Adherens junction, Cadherin, Folded gastrulation, GFP, Drosophila melanogaster INTRODUCTION Invagination or folding of an epithelial cell sheet is seen in a variety of morphogenetic events in metazoan development. Such epithelial morphogenesis often begins with constriction of the apices of specific cell populations. For example, avian neural tube formation and amphibian bottle cell formation, which have been analyzed extensively, initially exhibit cells with small apices in defined regions of the epithelia (Schoenwolf and Franks, 1984; Hardin and Keller, 1988). Constriction of cell apices appears to play an important role in the initiation of epithelial invagination or folding. Invagination of the prospective mesoderm in Drosophila gastrulation is a well-studied morphogenetic event that begins with constriction of cell apices. At about 3 hours of development, a Drosophila embryo has about 6000 cells, all of which, with the exception of the germ cells, are essentially uniform in size and shape, and are organized in an epithelial layer. In the cellular blastoderm, a band of cells corresponding to the ventral one-quarter of the embryo (approximately 18 cells in width) is destined to be the mesoderm. The prospective mesoderm begins morphogenetic movement earlier than all other regions of the embryo, and is brought into the interior of the embryo within 20 minutes at 25 C. Although there have been many detailed studies of the morphology of the mesodermal cells during the invagination (Turner and Mahowald, 1977; Fullilove et al., 1978; Leptin and Grunewald, 1990; Kam et al., 1991; Parks and Wieschaus, 1991; Sweeton et al., 1991; Costa et al., 1993; Oda et al., 1998), most were performed with fixed embryos. Detailed observations using scanning electron microscopy and epi-illumination video microscopy (Sweeton et al., 1991) suggested that the process of mesoderm invagination is divided into four phases: (1) flattening of the cell apices and initial random apical constrictions; (2) a shallow groove stage associated with increased constriction and cell elongation; (3) cell shortening and the subsequent inward movement; (4) closing over of the invagination and germband extension. In addition, examination of living embryos using a combination of injection of fluorescent reagents that visualize cell outlines and nuclei, and time-lapse three-dimensional fluorescence microscopy, supported the idea of initial random initiation of cell shape changes and the subsequent groove formation stage, although the data sets were only acquired at 1-minute intervals (Kam et al., 1991). It was also suggested that cell-cell communication mediated by Folded gastrulation may involve a rapid, orderly
2 494 JOURNAL OF CELL SCIENCE 114 (3) progression of constriction initiations from the center to the periphery of the mesoderm (Costa et al., 1994). These earlier observations implied that the mesodermal cell behavior during gastrulation is too dynamic to be thoroughly described without examining living embryos at much higher time resolutions. The classic cadherin-based cell-cell adhesion system has been shown to play a fundamental role in controlling cell behavior during morphogenesis of both vertebrates and invertebrates (Tepass, 1999; Steinberg and McNutt, 1999). In epithelial cells of embryos or tissues, E-type classic cadherin, a membrane-spanning protein, constitutes cell-cell adherens junctions together with many other components including β- and α-catenin (Yap et al., 1997; Steinberg and McNutt, 1999; Provost and Rimm, 1999). The adherens junctions, linking apical edges of epithelial cells, interact with cortical actin bundles (Provost and Rimm, 1999), and are thought to contribute mechanically to the constriction of cell apices in epithelial invagination or folding. In the Drosophila cellular blastoderm, adherens junctions are formed in the apical areas of the lateral surfaces of the cells (Tepass and Hartenstein, 1994). Drosophila E-cadherin (DE-cadherin) is present in the adherens junctions together with Armadillo and Dα-catenin (Oda et al., 1993; Oda et al., 1994; Müller and Wieschaus, 1996). In the ventralmost prospective mesodermal cells that have just begun to constrict their apices, adherens junctions are observed at the apical poles of the lateral cell surfaces (Oda et al., 1998). As the invagination proceeds, the adherens junctions appear to be disrupted in parallel with transition of the mesodermal cells from the epithelial to the mesenchymal state. Contraction of a cortical actin-myosin network may mediate apical constriction at the start of mesoderm invagination (Young et al., 1991; Leptin et al., 1992). Concertina (G protein α-subunit), DRhoGEF2 (guanine nucleotide exchange factor) and Rho1 (GTPase) may be involved in the Folded gastrulation (Fog) signaling pathway, which coordinates cell shape changes (Parks and Wieschaus, 1991; Costa et al., 1994; Barrett et al., 1997; Häcker and Perrimon, 1998). In this study, we expressed DE-cadherin tagged with green fluorescent protein in early Drosophila embryos to enable realtime imaging of cell-cell adherens junctions before and during gastrulation. Low-light fluorescence video microscopy, with which we recorded images every second, directly visualized the onset of mesoderm invagination in addition to the rest of this event in wild-type embryos. Our observations revealed dynamic aspects of cell behavior, some of which might be difficult to recognize clearly in low-time resolution analyses. We found that Drosophila mesoderm invagination began with slow synchronous and subsequent fast stochastic phases of cell apex constriction. Furthermore, we observed defective cell behavior of the prospective mesoderm in living fog mutant embryos, directly demonstrating the importance of Fogmediated signaling in the two phases of cell apex constriction. MATERIALS AND METHODS DNA constructs and transgenic flies cdna containing the open reading frame and the 3 untranslated region for DE-cadherin was modified by addition of a BspHI site at the translational start site and of a NcoI site in front of the stop codon, and cloned into the NcoI/XbaI site of pup2m, which contains the promoter region of the ubiquitin gene (Lee et al., 1988). The resulting plasmid was named pup-dech-nc. A red-shifted mutant GFP cdna (pqbi25, excitation peak 473 nm; emission peak 509 nm, Quantum Biotechnologies) was inserted into the NcoI site of pup-dech-nc, and then the KpnI-XbaI fragment was transferred to pcasper4-sv40 (Oda and Tsukita, 1999) to produce pcasper-ubi-de-cad-gfp. Transgenic flies were produced as described previously (Robertson et al., 1988). Several independent homozygous lines were established, and examined with a Zeiss Axiophot II microscope or an Edge directview 3-D microscope. In all the lines, cell-cell adherens junctions were clearly visible. Although the selected line of ubi-de-cad-gfp laid many unfertilized eggs (about 50%) for as yet unknown reasons, most fertilized eggs from the flies developed normally. Western blotting and antibodies For western blotting, anti-de-cadherin (Oda et al., 1994), anti-αspectrin (Pesacreta et al., 1989) and anti-gfp (Clontech) antibodies were used at dilutions of 1:50, 1:1000 and 1:1000, respectively. Flies Canton-S was used as the wild-type Drosophila melanogaster strain. Df(2R)E2, shg R69 and fog 4a6 were described previously (Uemura et al., 1996; Godt and Tepass, 1998; Costa et al., 1994). The recombinant, Df(2R)E2, ubi-de-cad-gfp, did not complement Df(2R)E2, Df(2R)D17, insc P49 or insc P72 (Uemura et al., 1996; Kraut and Campos-Ortega, 1996), but did complement shg 2, shg g317 and shg E17B in addition to shg R69 (Uemura et al., 1996; Tepass et al., 1996). Southern blotting Genomic DNA was isolated from adult flies with the indicated genotypes, and digested with HindIII. Digoxigenin (DIG)-labeled nucleotide probes were generated using a PCR DIG Probe Synthesis Kit (Boehringer Mannheim), and used for hybridization. Signals were detected using a combination of peroxidase-conjugated anti-dig antibody (Boehringer Mannheim) and the ECL detection system (Amersham Pharmacia Biotech). Video microscopy ubi-de-cad-gfp embryos at 2.5 hours of development (25 C) were dechorionated, placed on coverslips in the appropriate orientation, set in place with vacuum silicone grease (Beckman) and mounted in halocarbon oil 700 (Sigma) on special slides with an oxygenpermeable membrane (Yellow Spring Instrument) as described (Girdham and O Farrell, 1994). The embryos were then viewed under an inverted fluorescence microscope (Carl Zeiss, Axiovert 135) equipped with a highly sensitive SIT video camera (C , Hamamatsu Photonics) using a 100 NA1.4 Plan-Apochromat oil immersion objective (Carl Zeiss) and a filter set for GFP (Chroma, 41017). Excitation light generated from a 100 W mercury arc fluorescent-light source was attenuated to 1.5-6% using 50, 25 and 12% ND filters. Images were subjected to real-time averaging by Argus 20 (FRM=32, Hamamatsu Photonics), and recorded every second on a laser disc using a laser videodisc recorder (LVR-3000AN, Sony). During the minute period of observation of embryos at 23±1 C, the focus was manually adjusted to the level of the embryo surface. The observed embryos were further incubated to check that they developed normally to hatching. Some images were transferred to a Power Macintosh, and processed using Adobe Potoshop 4.0J, NIH image 1.61 and Adobe Premiere 4.2.1J to produce the figures and the QuickTime movies. Measurement of area was performed using Adobe Photoshop 4.0J. To observe fog mutant embryos, a confocal microscope (Bio-rad, MRC1024) was used. Images were taken at 10 second intervals with 3% of the maximum laser power. This condition did not affect gastrulation and germband extension in wild-type embryos. Embryos from fog 4a6 /FM7 and Y/FM7; ubi-de-cad-gfp were randomly taken and observed. During and after the observations, we ascertained
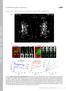
3 Drosophila mesoderm invagination 495 whether the individual embryos exhibited characteristic fog phenotypes as described previously (Costa et al., 1994). RESULTS AND DISCUSSION Visualization of adherens junctions in living early embryos To visualize the adherens junctions in living Drosophila embryos, we constructed a chimeric gene encoding DEcadherin tagged at its C terminus with green fluorescent protein (DE-cadherin-GFP), and used it to transform flies. The constructed gene, designated as ubi-de-cad-gfp, contained the ubiquitin promoter (Lee et al., 1988) (Fig. 1A), which allowed ubiquitous expression of the gene product throughout development. We established several independent homozygous lines, each of which had two copies of ubi-de-cad-gfp in addition to two copies of the endogenous DE-cadherin gene, encoded in the shotgun (shg) locus (Uemura et al., 1996; Tepass et al., 1996), and designated these flies as ubi-de-cad- GFP. Of these, we selected one line not only bearing the transgene on the second chromosome, where shg is located, but also expressing DE-cadherin-GFP at a level comparable to that of endogenous DE-cadherin (Fig. 1B), and used it throughout this study. When living ubi-de-cad-gfp embryos were examined by fluorescence microscopy, cell-cell adherens junctions, which form three-dimensional networks linking apical edges of epithelial cells, were clearly visible in blastoderm cells and later epithelial cells (Fig. 2). ubi-de-cad-gfp complements the shg null mutant We next examined whether ubi-de-cad-gfp can function in development. Zygotic mutations in shg lead to severe defects in morphogenetically active epithelia of the embryo (Uemura et al., 1996; Tepass et al., 1996). To perform rescue assays of shg mutants with ubi-de-cad-gfp, we generated a chromosome containing ubi-de-cad-gfp and a deletion, Df(2R)E2, which removes at least shg and a nearby essential gene, inscuteable (insc) (Uemura et al., 1996; Kraut and Campos-Ortega, 1996). Flies with this chromosome were crossed with those carrying a shg null allele, shg R69 (Godt and Tepass, 1998), to produce embryos with the zygotic genotype of Df(2R)E2, ubi-de-cad-gfp/shg R69. These embryos developed into morphologically normal adult flies, the genotype of which was confirmed by Southern blotting (Fig. 1C). In addition, the progeny of Df(2R)E2, ubi-de-cad- GFP/shg R69 flies were maintained for more than 5 generations, and homozygotes for shg R69, ubi-de-cad-gfp developed normally. These results indicated that ubi-de-cad-gfp complemented the shg null allele, and that DE-cadherin-GFP Fig. 1. Construction and characterization of ubi- DE-cad-GFP. (A) Schematic representation of the structures of the shg and ubi-de-cad-gfp genes and the DE-cadherin-GFP protein. Arrowheads indicate cleavage sites for maturation of DE-cadherin (Oda and Tsukita, 1999). Matured DE-cadherin-GFP consists of a 150 kda polypeptide containing the CRs and a 110 kda polypeptide containing the GFP, noncovalently bound to each other. CRs, cadherin repeats; NC, nonchordate motif; CE, cysteine-rich EGF-like motif; LAG, laminin A globular domain-like motif; PCCD, primitive classic cadherin domain; CP, cytoplasmic domain. (B) Western blot analysis of DEcadherin-GFP. Lysates of 4-6 hour embryos of the wild-type (Wt) and ubi-de-cad-gfp were resolved on 7.5% SDS gels. The indicated number of embryos was loaded in each lane. The antibodies used are indicated to the right of each blot. Anti-α-spectrin antibody (Anti-αSp) was used as a control to check that the expected amounts of protein were loaded in each lane. The squares indicate the precursor form of endogenous DE-cadherin, whereas the asterisks indicate the precursor form of DE-cadherin- GFP. As judged by the intensities of the detected bands, the quantity of the product from the ubi-de-cad-gfp gene was comparable to that from the shg gene. (C) Southern blotting analysis of HindIII-digested genomic DNA derived from wild-type (lane 1), Df(2R)E2/CyO (lane 2), Df(2R)E2, ubi-de-cad-gfp/cyo (lane 3) and Df(2R)E2, ubi-de-cad-gfp/shg R69 (lane 4) adult flies. The probe and HindIII sites are shown in A. The lower bands (2.5 kb) correspond to shg, and the upper bands (4.5kb) to ubi-de-cad-gfp.
4 496 JOURNAL OF CELL SCIENCE 114 (3) Fig. 2. Visualization of cell-cell adherens junctions in a living early embryo. A stereo micrograph of a lateral view of a stage 8 ubi-de-cad-gfp embryo revealing a three-dimensional network of cell-cell adherens junctions linking apical edges of surface epithelial cells. As, apical surface; gb, germband. Bars, 20 µm. can function normally even in the complete absence of intact DE-cadherin. Also, it was suggested that the behavior of DEcadherin-GFP, visible as fluorescence in the living flies, is the same as that of intact DE-cadherin. ubi-de-cad-gfp, therefore, could be an ideal tool not only for visualizing the adherens junctions but also for analyzing the dynamics of the functional cadherin molecule. Video microscopy revealed the time course of changes in cell shape and cell movements during mesoderm invagination To follow the changes in shape and position of the prospective mesodermal cells before and during gastrulation, we examined living ubi-de-cad-gfp embryos. A highly sensitive video camera enabled us to detect sufficient levels of GFP signals continuously with excitation light largely attenuated by ND filters. Exposure of early embryos to the excitation light for minutes did not affect gastrulation or later development. Blastoderm embryos were mounted on slides so that their ventral side, corresponding to the prospective mesoderm region, faced the objective lens (Fig. 3A). Since the mounted embryos were slightly flattened by compression, a larger part of the embryo surface was set in focus than in intact embryos. This deformation did not essentially affect cell behaviors in the developing embryos. The observed embryos on the slides developed normally to hatching, suggesting that they had undergone no serious damage while being observed. Images were recorded every second from before the onset of gastrulation to the start of germband extension, at 23±1 C. We obtained four sets of sequential images from different embryos, and found that they showed consistent results with only small variations. Of the four, a representative set of images is shown in Fig. 3B. We traced back the cells positioned at the site of ventral closure, corresponding to the mesectodermal cells, and determined which cells were destined to form the mesoderm. The prospective mesodermal cells, positioned more ventrally than the mesectodermal cells, showed dynamic changes in shape and position. Onset of movement in the prospective mesoderm was detected around a time point corresponding to the third panel of Fig. 3B in a quantitative analysis described below (Fig. 4), and this time point was therefore designated time 0. The process of mesoderm invagination was roughly divided into the following four stages. First, the majority of cells in the centralmost (or midventral) region, corresponding to about two thirds of the total mesoderm, constricted their apices, while the apices of more lateral mesodermal cells, designated as the lateral mesodermal cells, were elongated along the mediolateral axis (Fig. 3B, 0-7 minutes). DE-cadherin-GFP was strongly concentrated at the reduced apical zones of cellcell contact. This constriction stage was subdivided into slow and fast phases on the basis of the analysis described below. Second, the midventral cells gradually moved apart from the tangential plane to form a shallow furrow, called the ventral furrow, along the anterior-posterior axis (Fig. 3B, 7-14 minutes). Many of the lateral mesodermal cells were still in the exterior, while their apices were further elongated. Third, the lateral mesodermal cells rapidly migrated toward the ventral midline, and then entered the interior of the embryo, presumably pushing the midventral cells inward (Fig. 3B, minutes). Finally, the prospective mesectodermal cells coming from both sides met at the ventral midline, and maximized their contacts, where DE-cadherin-GFP was accumulated (Fig. 3B, minutes). This sealing of the ventral midline by the mesectodermal cells was accompanied by the start of germband extension. Most published images of fixed embryos at various gastrulation stages could be correlated with the images obtained here. Our observations, therefore, provide an example of time scales for cell shape changes and cell movements during mesoderm invagination, although the timing of the cellular behaviors varied slightly between embryos. The stages defined above may be comparable to those defined previously using a combination of epi-illumination video microscopy and scanning electron microscopy (Sweeton et al., 1991). Quantitative analysis revealed slow and fast phases of cell apex constriction following the onset of mesoderm invagination The prospective mesodermal cells in the cellular blastoderm were essentially uniform in shape and area, with only small variations. We found no cells that increased or decreased their apical surface areas at a significant rate in an irreversible manner before the onset of mesoderm invagination. After only 5 minutes, however, the apical surfaces of the mesodermal cells exhibited large differences in shape and area, as they began to change their shapes (Fig. 3B). To examine the relationship between the position of the mesodermal cells relative to the medio-lateral axis and the timing of their shape changes, we identified four adjoining groups each containing ten cells in mediolateral order as shown in Fig. 4A, and followed the temporal changes in the apical surface area of each group (Fig. 4B). The most ventral group, Group 1, began to show a reduction of the apical area earlier than all the other groups. The adjacent, more lateral group, Group 2, showed a rapid reduction in apical area with a delay of about 2.5 minutes. In contrast to Groups 1 and 2, Group 3 showed a significant increase in apical area followed by a rapid reduction in the area.
5 Drosophila mesoderm invagination 497 Fig. 3. Video microscopic observation of mesoderm invagination. The surface of a living ubi-de-cad-gfp embryo was observed by low-light video microscopy. Images were recorded at 1 second intervals. (A) Schematic illustration showing the prospective mesodermal region of the embryo. Red dots indicate mesectodermal cells and green lines the ventral midline. (B) Images were selected at 1-minute intervals. Time (minutes) is indicated at the bottom of each panel. Time 0 corresponds to the onset of mesoderm invagination detected quantitatively (see Fig. 4B). The ventral midline is marked by green bars. 15 mesectodermal cells were traced back, and are marked by red dots. The anterior is to the left. See also Movie 1. (C) Schematic illustration of cross-sections of gastrulating embryos corresponding to the images shown in B. The most lateral group, Group 4, did not decrease but increased its apical area from the onset of apical constriction, eventually reaching a plateau. These observations indicated that apical constrictions start first in the center of the mesoderm followed by lateral propagation of apical constrictions, while the most lateral cells (about 3 cells in width) are forced to enlarge their apical surfaces. The behavior of the cells around the ventral midline, including those in Group 1, involved two phases of apical constriction. During the first phase, lasting for about 2 minutes, the apical area of Group 1 decreased slowly with only slight differences between the individual cells (Fig. 4A,B). Most cells in the central region reduced their apical areas. No cells were found that showed rapid constriction of their apices compared to their surrounding cells. During the second phase, the apical area of Group 1 decreased about 2.5-fold faster than in the preceding phase, and the differences between the cells became progressively apparent (Fig. 4B,C). Many, but not all, cells around the ventral midline showed accelerated constriction of their apices. Many rapidly constricting cells were also found in Group 2 and in similar positions, with fewer in positions corresponding to Group 3 during the second phase (Fig. 4A). In contrast to the rapidly constricting cells, the remaining cells failed to accelerate their constriction, and some were slightly
6 498 JOURNAL OF CELL SCIENCE 114 (3) Fig. 4. Cell behavior at the start of mesoderm invagination. (A) High magnification images from the same set of data as used for Fig. 3. Four adjoining groups each consisting of 10 cells were set in the prospective mesodermal region, and numbered 1 to 4 in order of mediolateral position, as shown in the first panel. The outline of each group was drawn on the images selected at 5-second intervals, and some of the drawings are shown here. Time (seconds) is indicated at the bottom of each panel. Color code is the same as in Fig. 3B. Arrows indicate two examples of cells constricting their apices rapidly in Group 3. (B) Temporal changes in the apical surface area of each group. (C) Random arrangement of cells with small (arrows) and relatively large (asterisks) apices became progressively apparent during the fast phase. The images shown correspond to the boxed region in A, panel 0. See also Movie 2. (D) Temporal changes in the apical surface area of each group in a fog mutant embryo shown in Fig. 5A. Bars, 10 µm. widened, coinciding with constriction of nearby cells (Fig. 4C), although they soon began again to reduce their apical areas. In the middle of the second phase, cells with small and others with relatively large apices were randomly arranged, and the individual embryos showed different patterns. In all the embryos observed, cell behaviors characteristic of both the slow and fast phases were observed. These observations suggested that the onset of mesoderm invagination was followed by slow synchronous and subsequent fast stochastic phases of cell apex constriction. Fig. 5. Defective cell behavior in a fog mutant embryo at the start of mesoderm invagination. (A) The surface of an embryo with the genotype of fog/y; ubi-de-cad-gfp was observed by time-lapse confocal microscopy. Images were recorded at 10-second intervals, and are shown here at 1-minute intervals. Time (minutes) is indicated at the bottom of each panel. Color code is the same as in Fig. 3B. Anterior is to the left. The cell groups (the apical surface areas of which were measured for Fig. 4D) are indicated in the first panel. The brackets indicate a central 8 cellwide region corresponding to Groups 1 and 2. Five cells are arbitrarily marked by + to make it easier to follow changes in cell arrangement. From around time 0, reductions in the surface areas of the central cell groups were quantitatively detected (Fig. 4D). In the central 8-cell-wide region, some cells in random positions began to constrict their apices very slowly (arrows), and other cells remained large with no initial constriction (asterisks). Some of the constricted cells enlarged their apices again later (arrows). In this example, onset of folding took place from an anterior region of the embryo (large arrow in the panel 12), while many of the central cells still had large apices. As a result, in panel 13, the apices of the cells marked by the asterisks were forced to become enlarged or distorted. Note that the shapes of lateral mesodermal cells were less affected by the constriction of central cells at earlier time points compared to normal embryos. (B) Normal embryo with the genotype of either fog/fm7 or FM7/Y; ubi-de-cad-gfp. Anterior is to the right. In this embryo, all the central mesodermal cells reduced their apices to a sufficiently small size before the cell sheet folded. See also Movies 3 and 4. Bars, 20 µm.
7 Drosophila mesoderm invagination 499
8 500 JOURNAL OF CELL SCIENCE 114 (3) Some previous observations of living and fixed embryos suggested that mesoderm invagination begins with random initiation of apical constriction of individual cells (Sweeton et al., 1991; Kam et al., 1991). However, we found no cells showing prominently distinct behaviors from those of surrounding cells just after the onset of mesoderm invagination. In the initial 2 minutes, the cell shape changes were slight, and might be difficult to recognize in fixed embryo preparations or in low-time resolution analyses. Our observations suggested that the random apical constrictions observed previously (Sweeton et al., 1991) corresponded to the second phase of apical constriction defined in this study. It was previously shown that basal migration of the nuclei of the mesodermal cells also occurs in a random manner at the beginning of mesoderm invagination (Kam et al., 1991), but the relationship between apical constriction and nuclear migration was not analyzed in this study. Defects of fog mutant embryos in the apical constriction We introduced ubi-de-cad-gfp into fog mutant embryos, in which ventral furrow formation was previously shown to be disordered (Costa et al., 1994), and observed the behavior of the prospective mesodermal cells in the living mutant embryos. We took images at 10 second intervals using time-lapse confocal microscopy, and present some of the images obtained from a fog mutant embryo together with those from a normal embryo in Fig. 5. In fog mutant embryos, the onset of mesoderm invagination was recognized when some cells randomly positioned in a central 8-cell-wide region corresponding to Groups 1 and 2 began to constrict their apices very slowly (Fig. 5A, arrows). The apices of many other cells in the central region remained large with no initial constriction (Fig. 5A, asterisks). The temporal changes in the apical areas of the cell groups were measured as was done for the normal embryo (Figs 4D, 5A). Even when the apical areas of the central mesodermal cells became varied, only slight decreases in the apical areas of Groups 1 and 2 were detected. Several minutes after the onset of mesoderm invagination, a random arrangement of cells with apices of various sizes became more apparent (Fig. 5A, 4-7 minutes). This mode of cell shape change was in contrast to that seen in wild-type embryos, in which stochastic acceleration of constriction of cell apices took place. Although the numbers of cells with relatively small apices slowly increased, the cell shape changes were reversible in fog mutant embryos. Some of the constricted cells enlarged their apices again later (Fig. 5, arrows). In addition, the apices of the lateral mesodermal cells were little enlarged in at least the first 6 minutes (Figs 4D, 5A), indicating that the total contraction force generated in the central mesodermal cells may be markedly weak. Although the initiation of folding of the mesodermal cell sheet was delayed in fog mutant embryos, when it did suddenly take place the apices of most central mesodermal cells were not fully constricted. During the folding, some cells around the ventral midline were forced to expand their apices (Fig. 5, asterisks). Thus, the prospective mesoderm of fog mutant embryos lacked the normal two constriction phases seen in wild-type embryos, and instead exhibited weak, asynchronous cell shape changes. Fog is a secreted protein that may function as a signal to induce apical constriction of neighboring cells (Costa et al., 1994; Morize et al., 1998). Our high-temporal resolution observations of wild-type and fog mutant embryos support the idea that Fog-mediated signaling enables synchronous initiation of the contraction forces exerted in central populations of the prospective mesodermal cells and rapid propagation of this initiation of contraction to more lateral cells. Without Fog activity, some prospective mesodermal cells did initiate apical constrictions, although their cell shape changes were feeble. The constrictions in fog mutants were likely to be caused by relatively weak contraction forces continuously or discontinuously generated in the cells in a rather independent manner. In fog mutant embryos, failure to minimize the apical areas of the central mesodermal cells resulted in an abnormal mode of folding of the mesodermal sheet. Some previous studies stressed independent initiation of apical constriction in the individual cells (Sweeton et al., 1991; Kam et al., 1991). However, our observations suggest the importance of a Fog-mediated mechanism by which the central mesodermal cells synchronously initiate contraction forces to drive apical constriction at the onset of invagination. These contraction forces may give rise to high levels of tension among the central mesodermal cells. The apical area of each cell may depend on the balance between the force generated in the cell and that pulled from the surrounding cells. The irregular changes in cell shape observed in the second phase of apical constriction may result from imbalance of the elevated contraction forces among the individual cells rather than from random initiations of apical constriction. In other words, the synchronous activation of the contractile cortex by Fogmediated signaling may cause competition among the individual mesodermal cells for apical constriction. During the second phase, lateral progression of initiation of apical constriction was detected by quantitative analysis (Fig. 4B). The changes in the apical areas of the grouped cells partially mimicked the situation proposed in the purse-string model, in which a wave of contractions propagating from cell to cell is postulated (Odell et al., 1981). This study enabled real-time imaging of cell-cell adherens junctions by making flies with ubiquitous expression of GFPtagged DE-cadherin. We used these flies to examine dynamic cell behavior in Drosophila mesoderm invagination in wildtype and mutant embryos. The new tools are easily applicable to phenotypic analysis of other Drosophila mutants that have defects in epithelial cell polarity and morphogenesis. This kind of analysis may allow one to detect the earliest defects in such mutants, contributing to a better understanding of the molecular and cellular mechanisms controlled by the genes. We thank D. Branton, J. Lee, U. Tepass and E. Wieschaus for providing the antibody, plasmids and flies, V. Verkhusha for discussion, and Y. Akiyama-Oda for critical reading of this manuscript. We are also grateful to S. Okajima and M. Okubo for technical assistance. REFERENCES Barrett, K., Leptin, M. and Settleman, J. (1997). The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, Costa, M., Sweeton, D. and Wieschaus, E. (1993). Gastrulation in
9 Drosophila mesoderm invagination 501 Drosophila: Cellular mechanisms of morphogenetic movements. The Development of Drosophila melanogaster (ed. M. Bate and A. Martinez- Arias), pp Cold Spring Harbor: Cold Spring Harbor Laboratory Press. Costa, M., Wilson, E. T. and Wieschaus, E. (1994). A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76, Fullilove, S. L., Jacobson, A. G. and Turner, F. R. (1978). Embryonic development: descriptive. In Genetics and Biology of Drosophila, vol. 2c (ed. M. Ashburner and T. R. F. Wright), pp New York: Academic Press. Girdham, C. H. and O Farrell, P. H. (1994). The use of photoactivatable reagents for the study of cell lineage in Drosophila embryogenesis. In Methods in Cell Biology (ed. L. S. B. Goldstein and E. A. Fyrberg), pp San Diego: Academic Press. Godt, D. and Tepass, U. (1998). Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature 395, Häcker, U. and Perrimon, N. (1998). DRhoGEF2 encodes a member of the dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12, Hardin, J. and Keller, R. (1988). The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development 103, Kam, Z., Minden, J. S., Agard, D. A., Sedat, J. W. and Leptin, M. (1991). Drosophila gastrulation: analysis of cell shape changes in living embryos by three-dimensional fluorescence microscopy. Development 112, Kraut, R. and Campos-Ortega, J. A. (1996). inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol. 174, Lee, H. S., Simon, J. A. and Lis, J. T. (1988). Structure and expression of ubiquitin genes of Drosophila melanogaster. Mol. Cell Biol. 8, Leptin, M., Casal, J., Grunewald, B. and Reuter, R. (1992). Mechanisms of early Drosophila mesoderm formation. Development Supplement, Leptin, M. and Grunewald, B. (1990). Cell shape changes during gastrulation in Drosophila. Development 110, Morize, P., Christiansen, A. E., Costa, M., Parks, S. and Wieschaus, E. (1998). Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development 125, Müller, H. A. J. and Wieschaus, E. (1996). armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134, Oda, H. and Tsukita, S. (1999). Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev. Biol. 216, Oda, H., Tsukita, S. and Takeichi, M. (1998). Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev. Biol. 203, Oda, H., Uemura, T., Harada, Y., Iwai, Y. and Takeichi, M. (1994). A Drosophila homolog of cadherin associated with Armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 165, Oda, H., Uemura, T., Shiomi, K., Nagafuchi, A., Tsukita, S. and Takeichi, M. (1993). Identification of a Drosophila homologue of α-catenin and its association with the armadillo protein. J. Cell Biol. 121, Odell, G. M., Oster, G., Alberch, P. and Burnside, B. (1981). The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev. Biol. 85, Parks, S. and Wieschaus, E. (1991). The Drosophila gastrulation gene concertina encodes a Gα-like protein. Cell 64, Pesacreta, T. C., Byers, T. J., Dubreuil, R., Kiehart, D. P. and Branton, D. (1989). Drosophila spectrin: the membrane skeleton during embryogenesis. J. Cell Biol. 108, Provost, E. and Rimm, D. L. (1999). Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr. Opin. Cell Biol. 11, Robertson, H. M., Preston, C. R., Phillis, R. W., Johnson-Schlitz, D. M., Benz, W. K. and Engels, W. R. (1988). A stable genomic source of P- element transposase in Drosophila melanogaster. Genetics 118, Schoenwolf, G. C. and Franks, M. V. (1984). Quantitative analysis of changes in cell shapes during bending of the avian neural plate. Dev. Biol. 105, Steinberg, M. S. and McNutt, P. M. (1999). Cadherins and their connections: adhesion junctions have broader functions. Curr. Opin. Cell Biol. 11, Sweeton, D., Parks, S., Costa, M. and Wieschaus, E. (1991). Gastrulation in Drosophila: The formation of the ventral furrow and posterior midgut invaginations. Development 112, Tepass, U. (1999). Genetic analysis of cadherin function in animal morphogenesis. Curr. Opin. Cell Biol. 11, Tepass, U., Gruszynski-DeFeo, E., Haag, T. A., Omatyar, L., Torok, T. and Hartenstein, V. (1996). shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neuroectoderm and other morphogenetically active epithelia. Genes Dev. 10, Tepass, U. and Hartenstein, V. (1994). The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161, Turner, F. R. and Mahowald, A. P. (1977). Scanning electron microscopy of Drosophila melanogaster embryogenesis. II. Gastrulation and segmentation. Dev. Biol. 57, Uemura, T., Oda, H., Kraut, R., Hayashi, S., Kataoka, Y. and Takeichi, M. (1996). Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, Yap, A. S., Brieher, W. M. and Gumbiner, B. M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, Young, P. E., Pesacreta, T. C. and Kiehart, D. P. (1991). Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development 111, 1-14.
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Cell biology: Death drags down the neighbourhood
 Cell biology: Death drags down the neighbourhood The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Vasquez,
Cell biology: Death drags down the neighbourhood The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Vasquez,
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
998 Biophysical Journal Volume 107 August
 998 Biophysical Journal Volume 107 August 2014 998 1010 Article Passive Mechanical Forces Control Cell-Shape Change during Drosophila Ventral Furrow Formation Oleg Polyakov, 1, * Bing He, 2 Michael Swan,
998 Biophysical Journal Volume 107 August 2014 998 1010 Article Passive Mechanical Forces Control Cell-Shape Change during Drosophila Ventral Furrow Formation Oleg Polyakov, 1, * Bing He, 2 Michael Swan,
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
 DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
Unicellular: Cells change function in response to a temporal plan, such as the cell cycle.
 Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Mechanical Signals Trigger Myosin II Redistribution and Mesoderm Invagination in. Drosophila Embryos
 Mechanical Signals Trigger Myosin II Redistribution and Mesoderm Invagination in Drosophila Embryos Philippe-Alexandre Pouille, Padra Ahmadi, Anne-Christine Brunet and Emmanuel Farge* UMR 168, CNRS, Institut
Mechanical Signals Trigger Myosin II Redistribution and Mesoderm Invagination in Drosophila Embryos Philippe-Alexandre Pouille, Padra Ahmadi, Anne-Christine Brunet and Emmanuel Farge* UMR 168, CNRS, Institut
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo.
 DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. Padash, Mojgan; Rogers, Stephen; Häcker, Udo Published in: Journal of Cell Biology DOI: 10.1083/jcb.200407124
DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. Padash, Mojgan; Rogers, Stephen; Häcker, Udo Published in: Journal of Cell Biology DOI: 10.1083/jcb.200407124
An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1
 2594 Research Article An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1 Dhianjali Chandraratna 1, Nicola Lawrence 1, *, David P. Welchman 2 and Bénédicte
2594 Research Article An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1 Dhianjali Chandraratna 1, Nicola Lawrence 1, *, David P. Welchman 2 and Bénédicte
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Mesoderm Induction CBT, 2018 Hand-out CBT March 2018
 Mesoderm Induction CBT, 2018 Hand-out CBT March 2018 Introduction 3. Books This module is based on the following books: - 'Principles of Developement', Lewis Wolpert, et al., fifth edition, 2015 - 'Developmental
Mesoderm Induction CBT, 2018 Hand-out CBT March 2018 Introduction 3. Books This module is based on the following books: - 'Principles of Developement', Lewis Wolpert, et al., fifth edition, 2015 - 'Developmental
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Early Development in Invertebrates
 Developmental Biology Biology 4361 Early Development in Invertebrates October 25, 2006 Early Development Overview Cleavage rapid cell divisions divisions of fertilized egg into many cells Gastrulation
Developmental Biology Biology 4361 Early Development in Invertebrates October 25, 2006 Early Development Overview Cleavage rapid cell divisions divisions of fertilized egg into many cells Gastrulation
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Actin dynamics in lamellipodia of migrating border cells in the Drosophila ovary revealed by a GFP-actin fusion protein
 FEBS 21595 FEBS Letters 445 (1999) 395^401 Actin dynamics in lamellipodia of migrating border cells in the Drosophila ovary revealed by a GFP-actin fusion protein Vladislav V. Verkhusha a; *, Shoichiro
FEBS 21595 FEBS Letters 445 (1999) 395^401 Actin dynamics in lamellipodia of migrating border cells in the Drosophila ovary revealed by a GFP-actin fusion protein Vladislav V. Verkhusha a; *, Shoichiro
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis
 18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular iology of the Cell Figure S1 Krüger et al. Arabidopsis Plasmodium H. sapiens* 1) Xenopus* 1) Drosophila C.elegans S.cerevisae S.pombe L.major T.cruzi T.brucei DCP5 CITH
Supplemental Materials Molecular iology of the Cell Figure S1 Krüger et al. Arabidopsis Plasmodium H. sapiens* 1) Xenopus* 1) Drosophila C.elegans S.cerevisae S.pombe L.major T.cruzi T.brucei DCP5 CITH
178 Part 3.2 SUMMARY INTRODUCTION
 178 Part 3.2 Chapter # DYNAMIC FILTRATION OF VARIABILITY WITHIN EXPRESSION PATTERNS OF ZYGOTIC SEGMENTATION GENES IN DROSOPHILA Surkova S.Yu. *, Samsonova M.G. St. Petersburg State Polytechnical University,
178 Part 3.2 Chapter # DYNAMIC FILTRATION OF VARIABILITY WITHIN EXPRESSION PATTERNS OF ZYGOTIC SEGMENTATION GENES IN DROSOPHILA Surkova S.Yu. *, Samsonova M.G. St. Petersburg State Polytechnical University,
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Questions in developmental biology. Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration
 Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Chapter 10 Development and Differentiation
 Part III Organization of Cell Populations Chapter Since ancient times, people have wondered how organisms are formed during the developmental process, and many researchers have worked tirelessly in search
Part III Organization of Cell Populations Chapter Since ancient times, people have wondered how organisms are formed during the developmental process, and many researchers have worked tirelessly in search
7.013 Spring 2005 Problem Set 4
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 4 FRIDAY April 8th,
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 4 FRIDAY April 8th,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Rho1 regulates Drosophila adherens junctions independently of p120ctn
 First posted online on 5 October 2005 as 10.1242/dev.02056 Access the most recent version epress at online http://dev.biologists.org/lookup/doi/10.1242/dev.02056 publication date 5 October 2005 4819 Rho1
First posted online on 5 October 2005 as 10.1242/dev.02056 Access the most recent version epress at online http://dev.biologists.org/lookup/doi/10.1242/dev.02056 publication date 5 October 2005 4819 Rho1
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Development of Drosophila
 Development of Drosophila Hand-out CBT Chapter 2 Wolpert, 5 th edition March 2018 Introduction 6. Introduction Drosophila melanogaster, the fruit fly, is found in all warm countries. In cooler regions,
Development of Drosophila Hand-out CBT Chapter 2 Wolpert, 5 th edition March 2018 Introduction 6. Introduction Drosophila melanogaster, the fruit fly, is found in all warm countries. In cooler regions,
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A)
 Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Describe the process of cell division in prokaryotic cells. The Cell Cycle
 The Cell Cycle Objective # 1 In this topic we will examine the cell cycle, the series of changes that a cell goes through from one division to the next. We will pay particular attention to how the genetic
The Cell Cycle Objective # 1 In this topic we will examine the cell cycle, the series of changes that a cell goes through from one division to the next. We will pay particular attention to how the genetic
Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida. Overview of Pre-Metazoan. and Protostome Development (Insects)
 Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida Overview of Pre-Metazoan and Protostome Development (Insects) Plants Fungi Animals In1998 fossilized animal embryos were reported from the
Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida Overview of Pre-Metazoan and Protostome Development (Insects) Plants Fungi Animals In1998 fossilized animal embryos were reported from the
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Chemical aspects of the cell. Shape and structure of the cell
 Chemical aspects of the cell Shape and structure of the cell Cellular composition https://www.studyblue.com/ 2 Cellular composition Set of videos with basic information: Cell characteristics: https://www.youtube.com/watch?v=urujd5nexc8
Chemical aspects of the cell Shape and structure of the cell Cellular composition https://www.studyblue.com/ 2 Cellular composition Set of videos with basic information: Cell characteristics: https://www.youtube.com/watch?v=urujd5nexc8
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
downstream (0.8 kb) homologous sequences to the genomic locus of DIC. A DIC mutant strain (ro- 6
 A B C D ts Figure S1 Generation of DIC- mcherry expressing N.crassa strain. A. N. crassa colony morphology. When a cot1 (top, left panel) strain is grown at permissive temperature (25 C), it exhibits straight
A B C D ts Figure S1 Generation of DIC- mcherry expressing N.crassa strain. A. N. crassa colony morphology. When a cot1 (top, left panel) strain is grown at permissive temperature (25 C), it exhibits straight
Reading. Lecture VI. Making Connections 9/17/12. Bio 3411 Lecture VI. Making Connections. Bio 3411 Monday September 17, 2012
 Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Control of cell flattening and junctional remodeling during squamous epithelial morphogenesis in Drosophila
 Access the Development most First recent posted version epress online at http://dev.biologists.org/lookup/doi/10.1242/dev.019802 on online 28 May publication 2008 as 10.1242/dev.019802 date 28 May 2008
Access the Development most First recent posted version epress online at http://dev.biologists.org/lookup/doi/10.1242/dev.019802 on online 28 May publication 2008 as 10.1242/dev.019802 date 28 May 2008
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
Drosophila Life Cycle
 Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Supplementary Figure 1. Nature Genetics: doi: /ng.3848
 Supplementary Figure 1 Phenotypes and epigenetic properties of Fab2L flies. A- Phenotypic classification based on eye pigment levels in Fab2L male (orange bars) and female (yellow bars) flies (n>150).
Supplementary Figure 1 Phenotypes and epigenetic properties of Fab2L flies. A- Phenotypic classification based on eye pigment levels in Fab2L male (orange bars) and female (yellow bars) flies (n>150).
Supplementary Information for. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria
 Supplementary Information for Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria Isabella Santi 1 *, Neeraj Dhar 1, Djenet Bousbaine 1, Yuichi Wakamoto, John D.
Supplementary Information for Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria Isabella Santi 1 *, Neeraj Dhar 1, Djenet Bousbaine 1, Yuichi Wakamoto, John D.
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Question Set # 4 Answer Key 7.22 Nov. 2002
 Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
Fibroblast growth factor signalling controls successive cell behaviours during mesoderm layer formation in Drosophila
 RESEARCH ARTICLE 2705 Development 138, 2705-2715 (2011) doi:10.1242/dev.060277 2011. Published by The Company of Biologists Ltd Fibroblast growth factor signalling controls successive cell behaviours during
RESEARCH ARTICLE 2705 Development 138, 2705-2715 (2011) doi:10.1242/dev.060277 2011. Published by The Company of Biologists Ltd Fibroblast growth factor signalling controls successive cell behaviours during
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA
 Himber et al. Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA silencing SUPPLEMENTAL MATERIAL Supplemental material 1. Short-range movement of GFP silencing affects a nearly
Himber et al. Transitivity-dependent and transitivity-independent cell-to-cell movement of RNA silencing SUPPLEMENTAL MATERIAL Supplemental material 1. Short-range movement of GFP silencing affects a nearly
MCB 141 Midterm I Feb. 14, 2012
 Write your name and student ID# on EVERY PAGE of your exam MCB 141 Midterm I Feb. 14, 2012 Question #1 Question #2 Question #3 Question #4 BONUS / 28 pts / 27 pts / 25 pts / 20 pts / 1 pt TOTAL / 100 pts
Write your name and student ID# on EVERY PAGE of your exam MCB 141 Midterm I Feb. 14, 2012 Question #1 Question #2 Question #3 Question #4 BONUS / 28 pts / 27 pts / 25 pts / 20 pts / 1 pt TOTAL / 100 pts
Reading Assignments. A. Systems of Cell Division. Lecture Series 5 Cell Cycle & Cell Division
 Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division
 Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Death Read Chapter 19 Cell Division Read Chapter 20 pages 659-672 672 only (Benefits of Sex & Meiosis sections)
Lecture Series 5 Cell Cycle & Cell Division
 Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Division Read Chapter 19 pages 651-663 663 only (Benefits of Sex & Meiosis sections these are in Chapter
Lecture Series 5 Cell Cycle & Cell Division Reading Assignments Read Chapter 18 Cell Cycle & Cell Division Read Chapter 19 pages 651-663 663 only (Benefits of Sex & Meiosis sections these are in Chapter
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Civelekoglu-Scholey et al., http://www.jcb.org/cgi/content/full/jcb.200908150/dc1 Figure S1. Spindle dynamics in Drosophila embryos and in vitro gliding
Supplemental material THE JOURNAL OF CELL BIOLOGY Civelekoglu-Scholey et al., http://www.jcb.org/cgi/content/full/jcb.200908150/dc1 Figure S1. Spindle dynamics in Drosophila embryos and in vitro gliding
Why Flies? stages of embryogenesis. The Fly in History
 The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
sma-1 encodes a β H -spectrin homolog required for Caenorhabditis elegans
 Development 125, 2087-2098 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV6324 2087 sma-1 encodes a β H -spectrin homolog required for Caenorhabditis elegans morphogenesis Caroline
Development 125, 2087-2098 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV6324 2087 sma-1 encodes a β H -spectrin homolog required for Caenorhabditis elegans morphogenesis Caroline
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
Chapter 3. Results rho-1 regulates epidermal elongation in C. elegans embryogenesis
 Chapter 3. Results 3.1. rho-1 regulates epidermal elongation in C. elegans embryogenesis 3.1.1 Zygotic rho-1 is required for elongation Elongation is driven by actin-myosin contraction in the seam cells,
Chapter 3. Results 3.1. rho-1 regulates epidermal elongation in C. elegans embryogenesis 3.1.1 Zygotic rho-1 is required for elongation Elongation is driven by actin-myosin contraction in the seam cells,
Three Myosins Contribute Uniquely to the Assembly and Constriction of the Fission Yeast Cytokinetic Contractile Ring
 Current Biology Supplemental Information Three Myosins Contribute Uniquely to the Assembly and Constriction of the Fission Yeast Cytokinetic Contractile Ring Caroline Laplante, Julien Berro, Erdem Karatekin,
Current Biology Supplemental Information Three Myosins Contribute Uniquely to the Assembly and Constriction of the Fission Yeast Cytokinetic Contractile Ring Caroline Laplante, Julien Berro, Erdem Karatekin,
Exam 1 ID#: October 4, 2007
 Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
AP3162D: Lecture 4 - Basic modelling frameworks for developmental biology and cell-fate decisions
 AP162D: Lecture 4 - Basic modelling frameworks for developmental biology and cell-fate decisions Hyun Youk Delft University of Technology (Dated: March 15, 2018) In this lecture, we will derive the Berg-Purcell
AP162D: Lecture 4 - Basic modelling frameworks for developmental biology and cell-fate decisions Hyun Youk Delft University of Technology (Dated: March 15, 2018) In this lecture, we will derive the Berg-Purcell
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus:
 m Eukaryotic mrna processing Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus: Cap structure a modified guanine base is added to the 5 end. Poly-A tail
m Eukaryotic mrna processing Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus: Cap structure a modified guanine base is added to the 5 end. Poly-A tail
Solutions to Problem Set 4
 Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
The Cell Cycle. Chapter 12
 The Cell Cycle Chapter 12 Why are cells small? As cells get bigger they don t work as well WHY? Difficulties Larger Cells Have: More demands on its DNA Less efficient in moving nutrients/waste across its
The Cell Cycle Chapter 12 Why are cells small? As cells get bigger they don t work as well WHY? Difficulties Larger Cells Have: More demands on its DNA Less efficient in moving nutrients/waste across its
Anisotropy of Crumbs and apkc Drives Myosin Cable Assembly during Tube Formation
 Article Anisotropy of Crumbs and apkc Drives Myosin Cable Assembly during Tube Formation Katja Röper 1, * 1 MRC-Laboratory of Molecular Biology, Cambridge CB2 0QH, UK *Correspondence: kroeper@mrc-lmb.cam.ac.uk
Article Anisotropy of Crumbs and apkc Drives Myosin Cable Assembly during Tube Formation Katja Röper 1, * 1 MRC-Laboratory of Molecular Biology, Cambridge CB2 0QH, UK *Correspondence: kroeper@mrc-lmb.cam.ac.uk
Fertilization of sperm and egg produces offspring
 In sexual reproduction Fertilization of sperm and egg produces offspring In asexual reproduction Offspring are produced by a single parent, without the participation of sperm and egg CONNECTIONS BETWEEN
In sexual reproduction Fertilization of sperm and egg produces offspring In asexual reproduction Offspring are produced by a single parent, without the participation of sperm and egg CONNECTIONS BETWEEN
Drosophila melanogaster- Morphogen Gradient
 NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
Chapter 18 Regulation of Gene Expression
 Chapter 18 Regulation of Gene Expression Differential gene expression Every somatic cell in an individual organism contains the same genetic information and replicated from the same original fertilized
Chapter 18 Regulation of Gene Expression Differential gene expression Every somatic cell in an individual organism contains the same genetic information and replicated from the same original fertilized
Cell Division. Mitosis 11/8/2016
 Cell division consists of two phases, nuclear division followed by cytokinesis. Nuclear division divides the genetic material in the nucleus, while cytokinesis divides the cytoplasm. There are two kinds
Cell division consists of two phases, nuclear division followed by cytokinesis. Nuclear division divides the genetic material in the nucleus, while cytokinesis divides the cytoplasm. There are two kinds
Rap1 and Canoe/afadin are essential for establishment of apical basal polarity in the Drosophila embryo
 MBoC ARTICLE Rap1 and Canoe/afadin are essential for establishment of apical basal polarity in the Drosophila embryo Wangsun Choi a, *, Nathan J. Harris a, *, Kaelyn D. Sumigray b, and Mark Peifer a,b
MBoC ARTICLE Rap1 and Canoe/afadin are essential for establishment of apical basal polarity in the Drosophila embryo Wangsun Choi a, *, Nathan J. Harris a, *, Kaelyn D. Sumigray b, and Mark Peifer a,b
Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant
 9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
Chapter 8. The Continuity of Life: How Cells Reproduce. Gregory Ahearn. Lectures by. Ammended by John Crocker. University of North Florida
 Chapter 8 The Continuity of Life: How Cells Reproduce Lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc. Review Questions for Chapters
Chapter 8 The Continuity of Life: How Cells Reproduce Lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc. Review Questions for Chapters
MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION
 MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION Drosophila is the best understood of all developmental systems, especially at the genetic level, and although it is an invertebrate it has had an enormous
MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION Drosophila is the best understood of all developmental systems, especially at the genetic level, and although it is an invertebrate it has had an enormous
Chapter 8 Lectures by Gregory Ahearn University of North Florida
 Chapter 8 The Continuity of Life: How Cells Reproduce Lectures by Gregory Ahearn University of North Florida Copyright 2009 Pearson Education, Inc. 8.1 Why Do Cells Divide? Cells reproduce by cell division.
Chapter 8 The Continuity of Life: How Cells Reproduce Lectures by Gregory Ahearn University of North Florida Copyright 2009 Pearson Education, Inc. 8.1 Why Do Cells Divide? Cells reproduce by cell division.
SOALAN ULANGKAJI BAB 5 BIOLOGI TINGKATAN 4
 SOALAN ULANGKAJI BAB 5 BIOLOGI TINGKATAN 4 SECTION A: OBJECTIVES QUESTIONS. Diagram shows the phases in a cell cycle. Diagram 3 Diagram What is V? A Mitosis B Cytokinesis C Stage S D Stage G What is the
SOALAN ULANGKAJI BAB 5 BIOLOGI TINGKATAN 4 SECTION A: OBJECTIVES QUESTIONS. Diagram shows the phases in a cell cycle. Diagram 3 Diagram What is V? A Mitosis B Cytokinesis C Stage S D Stage G What is the
Three different fusions led to three basic ideas: 1) If one fuses a cell in mitosis with a cell in any other stage of the cell cycle, the chromosomes
 Section Notes The cell division cycle presents an interesting system to study because growth and division must be carefully coordinated. For many cells it is important that it reaches the correct size
Section Notes The cell division cycle presents an interesting system to study because growth and division must be carefully coordinated. For many cells it is important that it reaches the correct size
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Developmental Biology Lecture Outlines
 Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
Green Fluorescent Protein (GFP) Today s Nobel Prize in Chemistry
 In the news: High-throughput sequencing using Solexa/Illumina technology The copy number of each fetal chromosome can be determined by direct sequencing of DNA in cell-free plasma from pregnant women Confession:
In the news: High-throughput sequencing using Solexa/Illumina technology The copy number of each fetal chromosome can be determined by direct sequencing of DNA in cell-free plasma from pregnant women Confession:
The Microscopic Observation of Mitosis in Plant and Animal Cells
 The Microscopic Observation of Mitosis in Plant and Animal Cells Prelab Assignment Before coming to lab, read carefully the introduction and the procedures for each part of the experiment, and then answer
The Microscopic Observation of Mitosis in Plant and Animal Cells Prelab Assignment Before coming to lab, read carefully the introduction and the procedures for each part of the experiment, and then answer
Developmental Biology 3230 Midterm Exam 1 March 2006
 Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Maternal Control of GermLayer Formation in Xenopus
 Maternal Control of GermLayer Formation in Xenopus The zygotic genome is activated at the mid-blastula transition mid-blastula fertilized egg Xenopus gastrulae early-gastrula 7 hrs 10 hrs control not VP
Maternal Control of GermLayer Formation in Xenopus The zygotic genome is activated at the mid-blastula transition mid-blastula fertilized egg Xenopus gastrulae early-gastrula 7 hrs 10 hrs control not VP
Supporting Information
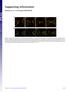 Supporting Information Fleissner et al. 10.1073/pnas.0907039106 Fig. S1. (A) MAK-2-GFP localized to CATs tips is not bound by membrane. his-3::pccg1 mak-2-gfp; mak-2 strain labeled with membrane dye FM4
Supporting Information Fleissner et al. 10.1073/pnas.0907039106 Fig. S1. (A) MAK-2-GFP localized to CATs tips is not bound by membrane. his-3::pccg1 mak-2-gfp; mak-2 strain labeled with membrane dye FM4
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology September 19, 2006 Major Research Questions How do forces outside the embryo affect its development? (Environmental Developmental
Biology 4361 Developmental Biology Principles of Experimental Embryology September 19, 2006 Major Research Questions How do forces outside the embryo affect its development? (Environmental Developmental
Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity
 Research article 2849 Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity Christine M. Field 1,, Margaret Coughlin 1, Steve Doberstein 2, Thomas
Research article 2849 Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity Christine M. Field 1,, Margaret Coughlin 1, Steve Doberstein 2, Thomas
Genetics 275 Notes Week 7
 Cytoplasmic Inheritance Genetics 275 Notes Week 7 Criteriafor recognition of cytoplasmic inheritance: 1. Reciprocal crosses give different results -mainly due to the fact that the female parent contributes
Cytoplasmic Inheritance Genetics 275 Notes Week 7 Criteriafor recognition of cytoplasmic inheritance: 1. Reciprocal crosses give different results -mainly due to the fact that the female parent contributes
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
THREE MITOSIS AND MEIOSIS OVERVIEW OBJECTIVES INTRODUCTION
 THREE MITOSIS AND MEIOSIS OVERVIEW In this lab you will investigate the processes of mitosis and rneiosis: 1. You will use prepared slides of onion root tips to study plant mitosis and to calculate the
THREE MITOSIS AND MEIOSIS OVERVIEW In this lab you will investigate the processes of mitosis and rneiosis: 1. You will use prepared slides of onion root tips to study plant mitosis and to calculate the
Adventures in Multicellularity
 Adventures in Multicellularity The social amoeba (a.k.a. slime molds) Dictyostelium discoideum Dictyostelium discoideum the most studied of the social amoebae / cellular slime molds predatory soil amoeba
Adventures in Multicellularity The social amoeba (a.k.a. slime molds) Dictyostelium discoideum Dictyostelium discoideum the most studied of the social amoebae / cellular slime molds predatory soil amoeba
