CONNEXINS AND CELL SIGNALING IN DEVELOPMENT AND DISEASE 1
|
|
|
- Frederica Webster
- 6 years ago
- Views:
Transcription
1 Annu. Rev. Cell Dev. Biol : doi: /annurev.cellbio First published online as a Review in Advance on July 2, 2004 CONNEXINS AND CELL SIGNALING IN DEVELOPMENT AND DISEASE 1 Chih-Jen Wei, Xin Xu, and Cecilia W. Lo Laboratory of Developmental Biology, National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland 20892; weic@nhlbi.nih.gov; xux@nhlbi.nih.gov; loc@nhlbi.nih.gov Key Words gap junction, knockout mice, motility, mutations, protein interaction Abstract Gap junctions contain hydrophilic membrane channels that allow direct communication between neighboring cells through the diffusion of ions, metabolites, and small cell signaling molecules. They are made up of a hexameric array of polypeptides encoded by the connexin multi-gene family. Cell-cell communication mediated by connexins is crucial to various cellular functions, including the regulation of cell growth, differentiation, and development. Mutations in connexin genes have been linked to a variety of human diseases, including cardiovascular anomalies, peripheral neuropathy, deafness, skin disorders, and cataracts. In addition to their coupling function, recent studies suggest that connexin proteins may also mediate signaling. This could involve interactions with other protein partners that may play a role not only in connexin assembly, trafficking, gating and turnover, but also in the coordinate regulation of cell-cell communication with cell adhesion and cell motility. The integration of these cell functions is likely to be important in the role of gap junctions in development and disease. CONTENTS INTRODUCTION GAP JUNCTIONS Gap Junction Structure and Permeability Properties Connexin Multi-Gene Family and Heteromeric/Heterotypic Gap Junctions Differential Gating of Gap Junction Channels Connexin43 Protein-Protein Interactions Interactions with Adherens Junction Associated Proteins Cytoskeletal Protein Interactions The U.S. Government has the right to retain a nonexclusive, royalty-free license in and to any copyright covering this paper. 811
2 812 WEI XU LO Tight Junction Associated Proteins Caveolin and Membrane Microdomains Protein Kinases and Cx43α1 Phosphorylation Connexins in Development and Disease Connexin Mutations Associated with Peripheral Neuropathy Connexin Mutations Associated with Deafness Connexin Mutations Associated with Skin Disorders Connexin Mutations Associated with Cataracts Connexin Regulation of Germ Cell Development Cx43α1 Mutations and Oculodentodigital Dysplasia CONNEXINS AND CARDIOVASCULAR DEVELOPMENT AND FUNCTION Cx45α7 and Development of the Endocardial Cushions Cx43α1 and Heart Outflow Tract Morphogenesis Cx43α1 and the Modulation of Cell Motility A SPECULATIVE MODEL INTRODUCTION Gap junctions are intercellular membrane channels found in a diverse array of organisms spanning from nematodes, echinoderms, ascidians, mollucs, and arthropods to vertebrates such as frog, chick, rodents, and humans. In vertebrates, gap junctions are made up of a multi-gene family called connexins (Willecke et al. 2002), whereas invertebrate gap junctions are encoded by a separate gene family known as the innexins (Phelan & Starich 2001). Connexins and innexins share an identical membrane topology, but little sequence homology. Given space constraints, this review is focused exclusively on the vertebrate connexins. We begin with a brief overview of the vertebrate gap junction channel structure and physiology, followed by a discussion of connexin protein interactions with other membrane and cytoplasmic proteins. We then provide a brief survey of the role of connexins in development and disease, with emphasis on studies of transgenic and knockout mouse models and the recent discoveries of connexin mutations associated with human diseases. We consider in more detail the specific role of connexins in cardiovascular development, and within this context, propose a role for connexins in mediating cell signaling that may not require the gap junction channel. We hope to stimulate debate and discussion, and no doubt these provocative ideas will require revision and reevaluation as new data emerge. Ultimately, understanding the functional role of connexins in development and disease will likely require paradigm shifts that promise to keep us engaged. We apologize for the inevitable gaps in our review and refer readers to other excellent gap junction reviews (Bruzzone et al. 1996, Evans & Martin 2002, Goodenough &Paul 2003, Harris 2001, Kumar & Gilula 1996, Saez et al. 2003, White & Paul 1999, Willecke et al. 2002).
3 CONNEXINS IN DEVELOPMENT AND DISEASE 813 GAP JUNCTIONS Gap Junction Structure and Permeability Properties Gap junctions are specialized intercellular junctions characterized by the apposition of the plasma membrane of apposing cells such that there is only a narrow 2 3 nm gap. They contain hydrophilic membrane channels that mediate the passage of ions, metabolites, and small cell signaling molecules less than 1 kda in size (Kumar & Gilula 1996). Electron microscopy and X-ray crystallographic studies showed gap junctions are tightly packed in a hexagonal array termed connexons, with each connexon having a hemi-channel made up of six protein subunits known as the connexin (Kumar & Gilula 1996, Yeager 1998) (Figure 1). Thus a gap junction is formed when two connexons are joined end to end in the extracellular space. Hydropathy plots of connexins revealed the presence of two extracellular domains (E1 and E2), four hydrophobic membrane-spanning domains (M1 M4), and three cytoplasmic domains including an intracellular loop, and amino and carboxyl termini (NT and CT, respectively) (Figure 1A). The four transmembrane domains of each connexin subunit exhibit an α-helical structure, whereas the extracellular domains from apposing connexons form a tight seal that prohibits the exchange of substances between the channel lumen and the extracellular milieu (Unger et al. 1999). Gating of the gap junction channel is controlled by multiple factors such as calcium concentration, ph, transjunctional membrane potential and protein phosphorylation (Harris 2001). Connexin Multi-Gene Family and Heteromeric/Heterotypic Gap Junctions To date, 20 connexin genes have been identified in the mouse genome and 21 in the human genome (Sohl & Willecke 2003). These genes show 40% sequence identity. Most of the connexin genes share a common structure: a first exon containing the 5 untranslated region (5 -UTR) followed by an intron of varying length, and a second exon containing the remaining 5 -UTR, the coding sequence, and the 3 -UTR (Willecke et al. 2002). However, several exceptions have been reported, including differential splicing of 5 -UTR and interruption of the coding sequence by introns (Condorelli et al. 1998; Neuhaus et al. 1995; Sohl et al. 1996, 1998, 2001). Connexin proteins have been described using two different nomenclature systems, either by molecular mass or based on sequence homology. Thus a 43 kda connexin protein is refered to as either connexin43 or α 1 connexin. Using the latter nomenclature system, connexin proteins have been subdivided into at least three subgroups referred to as connexin α, β, orγ. Thus Cx43 is referred to as α 1 connexin, Cx32 is β 1 connexin, etc. To minimize confusion, we use a hybrid nomenclature, such that Cx43 or α 1 connexin is referred to as Cx43α1. Most cells and tissues express more than one connexin isotype. Connexons may be composed of six identical connexin subunits (homomeric) or more than one connexin
4 814 WEI XU LO Figure 1 Schematic diagram showing connexin membrane topology and heteromeric/heterotypic arrangements of gap junction contacts. (a) Model showing membrane topology for the connexin polypeptide. M1 M4 represent the four transmembrane domains, E1 and E2 the two intracellular loops; the amino (N) and carboxy (C) termini are intracellular (from Kumar & Gilula 1996). (b) Schematic drawing showing possible arrangements of connexons to form heterotypic and heteromeric gap junction channels. Connexons consisting of six connexin subunits (red and blue) may be homomeric (identical subunits) or heteromeric (more than one connexin isotype), and when associated end to end, form membrane channels that may be homotypic or heterotypic (adapted from Kumar & Gilula 1996). isotype (heteromeric), and two identical connexons can form a homotypic channel, or a heterotypic channel can be generated by two connexons having different connexin isotypes (Figure 1B). The ability to form homomeric/heteromeric and homotypic/heterotypic channels provides greater complexity in the regulation of gap junctional communication. Differential Gating of Gap Junction Channels The gap junction channel can be differentially gated by different mechanisms. Closure of the gap junction channel has been observed in the presence of high
5 CONNEXINS IN DEVELOPMENT AND DISEASE 815 concentration of calcium ion (micromolar range), which suggests that the gap junction channel can be modulated by a variety of calcium-dependent cellular events. It has been proposed that calmodulin serves as a mediator for this calcium effect because it can bind Cx32β1 (Hertzberg & Van Eldik 1987). The depletion of calmodulin has been shown to reduce the sensitivity of gap junctional communication to elevated calcium levels in Xenopus oocytes (Peracchia et al. 1996). Intracellular ph (ph i )isanother factor that can modulate gap junction gating behavior. The regulatory sites for ph gating are believed to be located at the intracellular loop and CT domains of the connexin proteins, a region showing little sequence homology across the connexin multi-gene family. Hence, not surprisingly, different homotypic and heterotypic gap junction channels exhibit a variable degree of sensitivity to intracellular acidification (Ek-Vitorin et al. 1996; Francis et al. 1999; Homma et al. 1998; Morley et al. 1996, 1997; White et al. 1994; Yahuaca et al. 2000). On the basis of a series of studies involving the use of mutagenesis and heterologous expression of connexin CT domains, Delmar and colleagues have proposed a particle-receptor model to describe the mechanism of connexin ph gating. According to this model, intracellular acidification leads to the binding of Cx43α1 CT domain (acting as particle) to a region including histidine 95 (acting as receptor), and this action is proposed to close the channel (Ek-Vitorin et al. 1996, Homma et al. 1998, Morley et al. 1996). Several recent studies suggest that this model may not universally apply to all connexins (Eckert 2002, Stergiopoulos et al. 1999). Gap junction conductance can also be regulated by transjunctional or transmembrane voltage. Most vertebrate connexins are sensitive to transjunctional voltage and form closed channels when large transjunctional voltages are applied (Dahl 1996). Cx26β2, Cx43α1, and Cx45α7 also display transmembrane voltage dependence (Barrio et al. 1991, 1997; White et al. 1994). Different homotypic and heterotypic gap junction channels exhibit voltage gating to different degrees and the voltage-gating properties of a connexin may also be species-specific (Barrio et al. 1991, 1997; White et al. 1995). Mutagenesis studies have identified four separate domains where mutations can affect connexin voltage gating: NT (Verselis et al. 1994), E1 (Rubin et al. 1992), M2 (Suchyna et al. 1993), and CT (Moreno et al. 1992, 2002). Connexin43 Protein-Protein Interactions Connexin proteins have been shown to interact with a diverse array of proteins to form multi-protein complexes (Duffy et al. 2002, Herve et al. 2004). Such interactions are likely to regulate connexin functions at several levels, including connexin assembly, trafficking, turnover, and channel gating. In addition, recent studies suggest connexin protein interactions may modulate connexin function in response to physiological stimulation and pathological conditions (Thomas et al. 2002). Below we summarize the current evidence for interaction between Cx43α1 and other membrane and cytoplasmic proteins and consider whether such interaction may indicate a novel role for connexins in cell signaling. Elucidating connexin
6 816 WEI XU LO protein interactions may also provide mechanistic insights into the role of connexin mutations in human disease. Interactions with Adherens Junction Associated Proteins A number of studies have indicated a close association between gap junctions and cadherin-based adherens junctions. Cadherins are comprised of a major family of transmembrane glycoproteins known to play an important role in the regulation of cell adhesion and cell motility (Juliano 2002; Wheelock & Johnson 2003a,b). Inhibition of cadherin function can disrupt gap junction formation and inhibit cellcell coupling, suggesting that localization of cadherin to cell-cell contact sites may be a prerequiste for gap junction formation (Frenzel & Johnson 1996; Hertig et al. 1996a,b; Kanno et al. 1984; Keane et al. 1988; Kostin et al. 1999; Meyer et al. 1992; Zuppinger et al. 2000). Conversely, inhibition of Cx43α1 can block adherens junction formation (Meyer et al. 1992). Cadherin-mediated cell adhesion can be regulated by signaling through the Rho GTPases, the Wnt pathway, and receptor tyrosine kinases (Wheelock & Johnson 2003a), as well as through a variety of extracellular signals that include signals from gap junctions (Paul et al. 1995). The cytoplasmic domain of cadherins binds α/β-catenins and other F-actin binding proteins including α-actinin, vinculin, and zonula occludens-1 (ZO-1) and thus provide linkage to the actin cytoskeleton (Nagafuchi 2001). In rat cardiac myocytes, β-catenin interacts with Cx43α1 (Ai et al. 2000), and the formation of Cx43α1/ZO-1/β-catenin complex is required for targeting of Cx43α1 to the plasma membrane (Wu et al. 2003). Another recent study also suggests that α-catenin is important for Cx43α1 trafficking and assembly (Govindarajan et al. 2002). Given that N-cadherin and catenins are coassembled in the endoplasmic reticulum/golgi compartments (Wahl et al. 2003), this raises the possibility that Cx43α1 is assembled as part of a multi-protein complex that may coordinately regulate adherens and gap junction assembly. Interestingly, studies in mouse neural crest cells, as well as in NIH3T3 cells, have shown that Cx43α1 is colocalized with N-cadherin and p120ctn at regions of cell-cell contact (Wei et al. 2003, Xu et al. 2001). In Cx43α1 and N-cadherindeficient neural crest cells, the subcellular distribution of p120ctn was altered in conjunction with the perturbation of cell motility, suggesting the possibility that Cx43α1 and N-cadherin may modulate cell motility by engaging in a dynamic cross-talk with the cell s locomotory apparatus through p120ctn signaling (Xu et al. 2001). Cytoskeletal Protein Interactions A number of studies indicate that Cx43α1 gap junctions may be closely associated with cytoskeletal proteins. One study showed that formation of functional Cx43α1 gap junctions required elevated camp and intact microfilaments, which suggests the possibility that clustering of Cx43α1gap junction may involve protein kinase A (PKA) and actin filaments (Wang & Rose 1995). The association of Cx43α1 with actin filaments and perhaps other actin-binding proteins was also implicated
7 CONNEXINS IN DEVELOPMENT AND DISEASE 817 in studies with cultured astrocytes, where microinjection of anti-actin antibody impaired Cx43α1 membrane trafficking and inhibited gap junctional communication (Theiss & Meller 2002). Direct interaction of Cx43α1 with microtubules also has been demonstrated (Giepmans et al. 2001a, Guo et al. 2003). Although the nature of this interaction is still unclear, it is possible that Cx43α1 acts as an anchor to stabilize microtubules, or it may serve to regulate Cx43α1 expression and distribution via the integrin-mediated cell signaling pathway (Giepmans et al. 2001a, Guo et al. 2003). Tight Junction Associated Proteins The CT domain of Cx43α1 has been shown to bind ZO-1 in several cell types, and this interaction is regulated by phosphorylation of Cx43α1 by Src tyrosine kinases (Giepmans et al. 2001a,b; Toyofuku et al. 1998). ZO-1 is a peripheral membrane scaffolding protein that is specifically enriched at the tight junctions of epithelial and endothelial cells (Stevenson et al. 1986). It functions to tether transmembrane proteins to the actin cytoskeleton (Denker & Nigam 1998) and is also part of adherens junctions (Itoh et al. 1993). Although the role of ZO-1 in Cx43α1 function is not clear, it is possible that ZO-1 serves as a scaffold to recruit signaling molecules and/or actin filaments to Cx43α1, which may help in coordinately regulating gap junction formation with modulation of the actin cytoskeleton or with intracellular signaling. Caveolin and Membrane Microdomains Cx43α1 has been shown to interact with caveolin-1, a structural protein that resides in a specialized lipid raft domain known as a caveola (Schubert et al. 2002). Lipid rafts are membrane microdomains enriched in cholesterol and glycosphingolipids. These domains serve as a platform for a number of diverse cellular processes such as signal transduction, endocytosis, and cholesterol trafficking (Pike 2004). The functional significance of Cx43α1 interaction with caveolin-1 is still unclear. Perhaps it recruits Cx43α1 to membrane domains enriched in signaling proteins; for example, a recent study showed that Cx43α1gap junction regulated by PKCγ occurs through caveolin-1-containing lipid rafts (Lin et al. 2003). Protein Kinases and Cx43α1 Phosphorylation All connexins characterized so far are phosphoproteins with the exception of Cx26β2, whose CT domain is relatively short (Herve & Sarrouilhe 2002, Lampe & Lau 2000). Phosphorylation of the connexin CT domains is important in gap junction assembly, trafficking, channel gating, and turnover. Because of the large sequence variation among connexin CT domains, the mechanisms of connexin phosphorylation appear to be complex. For instance, Cx32β1 gap junctional communication is increased by the activated camp-dependent PKA (Saez et al. 1990), whereas intercellular communication is reduced by protein kinase C (PKC) phosphorylation of chick lens Cx56α3 (Berthoud et al. 1997). Connexins can also be differentially phosphorylated in a tissue-specific manner (Kadle et al. 1991), and
8 818 WEI XU LO the communication competence of several cell lines has been correlated with the pattern of protein phosphorylation (Musil et al. 1990). Cx43α1 may be subject to modification by as many as 10 different protein kinases. Among the kinases that phosphorylate Cx43α1, interactions with Src and PKC have been studied most extensively (Herve & Sarrouilhe 2002, Lampe & Lau 2000, Warn-Cramer & Lau 2004). It has been proposed that Src phosphorylation regulates the interaction between Cx43α1 and ZO-1, and disruption of Cx43α1/ZO-1 interaction is suggested to underlie the inhibitory effect of c-src on gap junctional communication (Toyofuku et al. 2001). Cx43α1 phosphorylation by PKC was found to inhibit cell-cell coupling via a reduction in the channel unitary conductance (Lampe et al. 2000) and also altered cell-cycle events (Solan et al. 2003). PKC phosphorylation of Cx43α1 has also been reported with fibroblast growth factor-2 (FGF2) stimulation and was associated with the inhibition of DNA synthesis and cell growth. Interestingly, these changes occurred independently of channel permeability and/or the subcellular distribution of Cx43α1 (Doble et al. 2004). Connexins in Development and Disease Gap junctions have long been speculated as playing a role in development. An attractive hypothesis is that gap junction communication may mediate the formation of morphogen gradients. Consistent with this notion, gap junction communication in the developing embryo shows functional subdivision into communication compartments that coincide with developmentally significant domains (Bagnall et al. 1992; Kalimi & Lo 1988, 1989; Lo & Gilula 1979a,b; Martinez et al. 1992; Warner et al. 1984; Warner & Lawrence 1982; Weir & Lo 1982, 1984, 1985). Such communication compartments may provide the context for generating morphogen gradients that can regulate growth, patterning, and differentiation (Lo & Gilula 1979b, 2000). Although morphogen gradients in development are well described, there is little evidence for gap junctions playing a role in the formation of such gradients. Nevertheless, it is worth noting that recent work by Levin and colleagues indicates that gap junction coupling may modulate left-right patterning (Levin 2002). This was demonstrated using several different methods to alter or inhibit gap junctional communication in Xenopus and chick embryos (Levin & Mercola 1998, 1999). These animal model experiments are in agreement with an earlier report that Cx43α1 mutations can cause visceroatrial heterotaxia (VAH) in humans (Britz-Cunningham et al. 1995), although other groups examining different patient populations did not confirm the latter findings (Casey & Ballabio 1995, Debrus et al. 1997, Gebbia et al. 1996). Recent studies using reverse genetic approaches with the analysis of transgenic and knockout mouse models have provided more conclusive evidence of the requirement for gap junctions in regulating growth and development, and in modulating tissue and organ physiology (Willecke et al. 2002). Thus knockout mouse models have indicated an indispensable role for connexins in the development and function of a variety of tissues and organs (Willecke et al. 2002). Furthermore,
9 CONNEXINS IN DEVELOPMENT AND DISEASE 819 clinical studies have demonstrated a role for connexin mutations in a wide spectra of human diseases, such as in demyelinating neuropathies, various skin disorders, cataracts, sensorineural deafness, and oculodentodigital dysplasia (ODDD) (Willecke et al. 2002). The challenge now is to unravel the mechanism(s) by which connexins contribute to development and disease. Together with transgenic mouse models, human connexin mutations are nature s genetic experiments that can help elucidate connexin protein structure-function relationships. However, it is that the collection of connexin mutations associated with human diseases shows no correlation with protein domains known to be required for the formation or regulation of the gap junction channel itself, the only exception being connexin mutations associated with cataracts. Connexin Mutations Associated with Peripheral Neuropathy Mutations in the connexin protein, Cx32β1, are associated with X-linked form of Charcot-Marie-Tooth (CMTX) disease (Bergoffen et al. 1993a,b). CMTX is a demyelinating syndrome with progressive degeneration of peripheral nerves brought on by a defect in Schwann cells. Over 200 Cx32β1 mutations have been identified in CMTX patients (Nelis et al. 1999). Most of these mutations are situated within the coding region and, surprisingly, span the entire length of the protein. Compared with CMTX patients, Cx32β1 knockout mice develop relatively mild peripheral neuropathy (Nelles et al. 1996, Scherer et al. 1998) that is associated with a distinct pattern of gene dysregulation in Schwann cells (Nicholson et al. 2001). Other defects include reduced nerve stimulation and hormone-induced hepatic glucose release, as well as reduced bile flow following sympathetic stimulation (Nelles et al. 1996, Scherer et al. 1998). In addition, Cx32β1 knockout mice appeared to be more susceptible to chemically induced liver carcinogenesis (Nelles et al. 1996). Connexin Mutations Associated with Deafness At least 5 connexin proteins are reported to be involved in deafness (syndromic and nonsyndromic), including Cx26β2, Cx30β6, Cx31β3, Cx32β1, and Cx43α1 (Kelsell et al. 2001, Richard 2003). Autosomal-recessive (DFNB1) and autosomaldominant (DFNA3) forms of hearing impairment have been associated with more than 50 mutations in the coding region of Cx26β2 (Kelsell et al. 2001, White 2000). The most common mutation is a recessive frame shift mutation (35delG) that causes premature translation termination (Zelante et al. 1997). Recent data indicate that DFNB1 in some patients may be associated with a 342 kb deletion involving Cx30β6 (Del Castillo et al. 2003). Interestingly, as with CMTX, connexin mutations causing deafness are not restricted to any functional domain but are distributed throughout the length of the protein. Knockout mouse studies have provided some insights into the role of Cx26β2 and Cx30β6 in auditory function. Recent work using targeted ablation demonstrated that Cx26β2 is essential for cochlear function and cell survival in the
10 820 WEI XU LO sensory epithelium of the inner ear (Cohen-Salmon et al. 2002), whereas a Cx30β6 knockout mouse model exhibited severe constitutive hearing impairment, suggesting that Cx30β6 plays a role in generating endocochlear potential and mediating survival of the auditory hair cells after the onset of hearing (Teubner et al. 2003). However, it should be noted that connexin mutations associated with hearing loss are not restricted to connexin protein domains required for channel function. Connexin Mutations Associated with Skin Disorders Some of the mutations in Cx26β2, Cx30β6, and Cx31β3 are linked not only with deafness, but also with skin disorders (Richard 2003). In the epidermis, gap junctions appear to play a critical role in regulating keratinocyte growth and differentiation (Choudhry et al. 1997), and studies in mice revealed that at least nine different connexin genes are coexpressed in the epidermis (Richard 2000). Mutations in Cx26β2 have been shown to be linked to Vohwinkel sydrome, an autosomal-dominant condition with mutilating keratoderma accompanied by deafness (Maestrini et al. 1999). Another type of skin disease, erythorokeratoderma variabilis (EKV) was linked to mutations in Cx31β3 (Richard et al. 1998). EKV was recently also reported to be associated with mutations in Cx30.3β4 (Richard et al. 2003). However, Cx31β3-deficient mice exhibit no morphological or functional skin or inner ear defects, which were possibly compensated by other redundantly expressed connexins (Plum et al. 2001). As with mutations associated with CMTX and sensorineural deafness, connexin mutations causing skin disorders are not restricted to any particular connexin protein domain. A recent transgenic mouse study with epidermal expression of a mutant Cx26β2 (D66H) is reported to mimic true Vohwinkel syndrome (Bakirtzis et al. 2003). Such transgenic mice showed keratoderma similar to humans with the same mutation, and they also exhibited premature keratinocyte cell death and marked thickening of the epidermal cornified layers. These findings suggest the possibility that connexin function plays a role in regulating formation of the cornified envelope. It is interesting to note that in this animal model, transgene expression was associated with the cytoplasmic accumulation of both wild-type Cx26β2 and Cx30β6 connexins, indicating a dominant-negative effect of the mutant connexin protein on trafficking of wild-type connexins. Connexin Mutations Associated with Cataracts In the lens, three connexins are expressed: Cx43α1, Cx46α3, and Cx50α8. Fiber cells of the lens are interconnected by an extensive network of gap junctions containing Cx46α3 and Cx50α8, both serving to maintain homeostasis and support cell growth (White 2002). Point mutations in the Cx50α8 and Cx46α3 connexins have been identified in patients with inherited zonular pulverulent cataracts. Perhaps indicative of the unique requirement for gap junction communication in maintaining lens tissue homeostasis, human lens connexin mutations are largely restricted to the extracellular loop or transmembrane domains (Berry et al. 1999,
11 CONNEXINS IN DEVELOPMENT AND DISEASE 821 Berthoud et al. 2003, Graw et al. 2001, Jiang et al. 2003, Mackay et al. 1999, Pal et al. 2000, Rees et al. 2000, Shiels et al. 1998, Steele et al. 1998). Studies using knockout mouse models revealed that loss of Cx46α3 function caused severe cataracts, but otherwise had no effect on lens growth and development (Gong et al. 1997, 1998). In contrast, the deletion of Cx50α8 caused a significant reduction in lens growth together with mild cataracts (Rong et al. 2002, White et al. 1998). Interestingly, knockin replacement of Cx50α8 with Cx46α3 rescued lens clarity but was unable to restore normal growth (Martinez-Wittinghan et al. 2003). In contrast, double Cx43α1/Cx50α8 knockout mice exhibited normal lens development, suggesting that neither Cx43α1 nor Cx50α8 is required for prenatal lens development (White et al. 2001). Overall, these observations indicate that the intrinsic properties of Cx50α8 are likely required for cellular growth, whereas Cx46α3isessential for normal lens differentiation (White 2002). Connexin Regulation of Germ Cell Development Knockout mouse studies have identified an essential role for Cx37α4 and Cx43α1 in germ cell development. Cx37α4 is expressed in oocytes and presumably generates heterotypic gap junctions linking oocytes with granulosa cells (Kidder & Mhawi 2002), whereas Cx43α1 is expressed abundantly in granulosa/granulosa gap junctions (Saez et al. 2003, White & Paul 1999). Cx37α4-deficient mice show defects in oocyte development before competence is reached, together with the perturbation of antral follicle development (Kidder & Mhawi 2002, Simon et al. 1997). In Cx43α1-deficient mice, neonatal mice show germ cell deficiency that arises during fetal life. Grafting experiments show that Cx43α1 deficiency in the testis resulted in a Sertoli cell only phenotype (Roscoe et al. 2001), whereas in the ovary, follicles are arrested early in development with incompetent oocytes (Juneja et al. 1999). Interestingly, the replacement of Cx43α1 with either Cx40α5 or Cx32β1 in Cx40α5 and Cx32β1 knockin mice, respectively, could not rescue the germ cell defect (Plum et al. 2000). Because Cx43α1 is expressed in mouse embryonic ovaries and testes from the earliest stages of development, perhaps it is required for prenatal expansion of germ cells during fetal gonadal development (Perez-Armendariz et al. 2003). Another possibility to consider is whether Cx43α1 may be required for germ cell migration and targeting to the gonads, a possibility worth considering in light of the known role of Cx43α1 modulating the migration of neural crest and proepiardially derived cells (Huang et al. 1998a, Li et al. 2002). Cx43α1 Mutations and Oculodentodigital Dysplasia Another human disease recently shown to be associated with connexin mutations is oculodentodigital dysplasia (ODDD). ODDD is a congenital disorder characterized by developmental abnormalities of the face, eyes, limbs, and dentition and is linked to dominant mutations in Cx43α1(Paznekas et al. 2003). Cx43α1 mutations associated with ODDD are broadly distributed except for the singular absence of
12 822 WEI XU LO any mutation in the CT (Paznekas et al. 2003). In contrast, human patients with VAH have point mutations in the cytoplasmic tail of the Cx43α1 gap junction protein (Britz-Cunningham et al. 1995). CONNEXINS AND CARDIOVASCULAR DEVELOPMENT AND FUNCTION Gap junctions play an essential role in cardiac function, as they mediate the spread of electrical impulses that allows synchronous contraction of the cardiac chambers (Severs 2001). In the adult mammalian working myocardium, Cx43α1 is the major connexin protein expressed and is principally responsible for electrical synchrony. In addition, there are two other connexin isotypes found in the adult heart, Cx45α7 and Cx40α5. Cx45α7 isexpressed in the atrioventricular node and adjoining His bundles, whereas Cx40α5 is expressed in the fast conducting tissues of the His- Purkinje system, nested within the Cx45α7 expression domain (Gourdie et al. 1993). Given that Cx40α5 generates channels with high conductance and Cx45α7 forms voltage-sensitive channels with very low conductance (Veenstra et al. 1992), this compartmentalized connexin expression pattern is expected to provide for the orderly sequential spread of activation from the atrial to ventricular chambers (Gourdie & Lo 1999). Each of these three connexin genes has now been deleted by embryonic stem cell targeting, and analysis of the phenotypes in the respective knockout mouse models shows that all three genes are required for heart conduction. Thus conditional deletion of Cx43α1 restricted to the adult working myocardium showed that Cx43α1 is essential for adult heart conduction, further indicating that Cx43α1 deficiency may provide an arrhythmogenic substrate that could contribute to heart dysfunction (Gutstein et al. 2001). Knockout mice deficient in Cx45α7 died early in gestation, exhibiting conduction block as well as endocardial cushion defects (Kumai et al. 2000), whereas Cx40α5 knockout mice were viable but showed slowed conduction and a partial atrioventricular block (Bevilacqua et al. 2000; Kirchhoff et al. 1998, 2000; Kirchhoff et al. 1998; Simon et al. 1998). Recent knockin gene replacement studies in which Cx40α5 (Cx43KI40 mice) or Cx32β1 (Cx43KI32) were substituted for Cx43α1 showed no conduction abnormalities, indicating that heart conduction is independent of the unitary conductances of the gap junction channel (Plum et al. 2000). In addition to their roles in heart conduction, analysis of the Cx43α1, Cx40α5, and Cx45α7 knockout mouse models showed that these connexin genes also play an essential role in heart morphogenesis. In recent studies reexamining the Cx40α5 knockout mouse phenotype, 16% of newborn homozygous Cx40α5 knockout mice were reported to die at birth with atrioventricular septation defects (Kirchhoff et al. 2000); in a second study, 33% of homozygous Cx40α5 knockout mice were reported to have outflow tract malformations consisting of double outlet right ventricle or Tetralogy of Fallot (Gu et al. 2003). Analysis of the Cx45α7 knockout
13 CONNEXINS IN DEVELOPMENT AND DISEASE 823 mice shows that this connexin gene is required for the development of the endocardial cushions, whereas Cx43α1 plays an essential role in heart outflow tract morphogenesis and development of the coronary arteries (see below). Cx45α7 and Development of the Endocardial Cushions Knockout mice deficient in Cx45α7 die of heart failure at embryonic day 10.5 (E10.5), showing a looping defect, reduced trabeculation, disrupted formation of the endocardial cushions, and a conduction block (Kumai et al. 2000). Significantly, expression of the calcium-dependent transcription factor, NF-ATc1, was inactivated in the endocardium. We note that NF-ATc1-deficient mice have defects associated with formation of endocardially derived valve tissues (de la Pompa et al. 1998, Kruger et al. 2000, Kumai et al. 2000, Ranger et al. 1998), thereby suggesting the possibility that Cx45α7 has a role in modulating calcineurin signaling required for NF-ATc activation. Although another study suggests that Cx45α7 knockout mice die from abnormal vascular development (Kruger et al. 2000), recent experiments using a floxed allele of Cx45α7, together with a myocardial specific Cre, showed that the requirement for Cx45α7 resides in the myocardium (Nishii et al. 2003). It should be noted that these conditional knockout mice did not have endocardial cushion defects, confirming that the cushion defects likely arise from a requirement for Cx45α7inthe endocardium (Nishii et al. 2003). Cx43α1 and Heart Outflow Tract Morphogenesis The gene for Cx43α1 was the first connexin gene to be knocked out (Reaume et al. 1995) and also the first to be linked to a human disease; mutations in the CT of Cx43α1 were reported to be associated with pulmonary atresia and VAH (Britz-Cunningham et al. 1995). Cx43α1 knockout mice survived to term, but expired at birth from pulmonary outflow obstruction. This result was surprising at the time because Cx43α1 was known to be expressed from the 4 8 cell stage and was expected to cause preimplantation lethality (Nishi et al. 1991). However, subsequent studies showed that at least half a dozen connexin genes (and perhaps more) are expressed in the preimplantation mouse embryo (De Sousa et al. 1997). The cardiac phenotype of the Cx43α1 knockout mouse consisted of pulmonary outflow obstruction associated with conotruncal malformations and defects in the patterning of the coronary arteries (Reaume et al. 1995, Ya et al. 1998). The finding of outflow anomalies was unexpected because this particular connexin gene is not expressed in abundance in the outflow tract myocardium (Gourdie et al. 1992, van Kempen et al. 1991). However, as tissue remodeling in this region of the heart is known to require the activities of neural crest cells (Kirby & Waldo 1995), we generated several transgenic mouse models to examine the effects of manipulating Cx43α1 function in the cardiac neural crest cells. Neural crest cells are migratory cells derived via an epithelial-mesenchymal cell transformation from the dorsal neural fold. They migrate in streams and are dispersed throughout the embryo, generating all the cells of the peripheral
14 824 WEI XU LO nervous system, as well as providing melanocytes, cranial mesenchyme, and cells that contribute to the aortic arches and outflow tract septation complex. Cx43α1 is expressed in abundance in migrating neural crest cells and, moreover, migrating neural crest cells are functionally coupled (Lo et al. 1997). Significantly, transgenic mice with gain (CMV43 transgenic mice) or loss (FC transgenic mice) of Cx43α1 function targeted to neural crest cells displayed conotruncal heart defects and outflow obstruction (Ewart et al. 1997, Huang et al. 1998b, Sullivan et al. 1998). These findings suggest that the outflow anomalies in the Cx43α1 knockout mice do arise from the perturbation of cardiac neural crest cells. However, one puzzling fact was that heart defects in the CMV43 or FC transgenic mice were not identical to that of the Cx43α1 knockout mouse. Thus conotruncal pouches typically found at the base of the pulmonary outflow tract in the knockout mice were never observed in either transgenic mouse model (Lo et al. 1999). Additional studies have since shown that the formation of conotruncal pouches likely arises from defects involving not only the cardiac neural crest cells, but also another migratory cell population, the proepicardially derived cells (Li et al. 2002). The proepicardial cells are a mesothelial cell population found near the septum transversum and liver primordia. They delaminate over the surface of the heart to form the epicardium and also invade the heart, providing all of the vascular smooth muscle and endothelial cells of the coronary arteries. Our studies suggest that alterations in the migratory behavior of both cell populations underlie the Figure 2 Altered neural crest cell migration in transgenic mice exhibiting deficiency or overexpression of Cx43α1. (a f ) The distribution of cardiac neural crest cells in E14.5 mouse fetal hearts was examined using a lacz reporter transgene driven by the Cx43α1 promoter (Lo et al. 1997). Shown are the front (a c ) and side views (d f )of X-gal stained hearts from wild-type (a, d), CMV43 (b, e ) and Cx43α1 knockout mice (c, f ). The white asterisk denotes presumptive neural crest cells in the conus in the closing seam of the aorticopulmonary septum. Note the increased abundance of laczlabeled neural crest cells in the CMV43 heart (b, white asterisk); lacz-labeled neural crest cells were reduced in the Cx43α1 knockout heart (c, white asterisk). Black arrows denote bulging of the conotruncus in both CMV43 (e ) and Cx43α1 knockout (c, f ) hearts. Abbreviations: p, pulmonary trunk; a, aorta; rv, right ventricle. (g i ). Altered neural crest cell motility was indicated by time lapse videomicroscopy of neural crest explant cultures derived from E8.5 wild-type (g), CMV43 (h) and Cx43α1 knockout (i ) mouse embryos. A ring of neural crest outgrowth can be observed surrounding the central dense mass of neuroepithelial tissue in the 24-h explant culture (g i ). The color lines represent the migration paths of individual neural crest cells observed over a 20-h interval; the black circles denote the position at time zero. Note that the migratory paths of Cx43α1 knockout neural crest cells were more tortuous than those of the wild-type (g) and CMV43 embryos (h). In addition, the migratory paths of the CMV43 neural crest cells (h) were longer, reflecting an enhanced rate of cell migration compared with that of the wild-type or knockout neural crest cells.
15 CONNEXINS IN DEVELOPMENT AND DISEASE 825 elaboration of the conotruncal heart malformations and coronary artery defects in the Cx43α1 knockout mouse (Huang et al. 1998a, Li et al. 2002). Cx43α1 and the Modulation of Cell Motility Development at the base of the heart, the region most severely affected in the Cx43α1 knockout mouse, requires the precise temporospatial regulation of
16 826 WEI XU LO cardiac neural crest and proepicardial cells deployment to the heart. In the Cx43α1 knockout mice and our transgenic mouse models, the migratory behavior of the cardiac neural crest and proepicardial cells is altered. Neural crest explant experiments showed that CMV43 neural crest cells have an enhanced rate of migration, whereas the FC transgenic and Cx43α1-deficient neural crest cells have a reduced migration rate (Huang et al. 1998a) (Figure 2). These in vitro migration studies were confirmed with in vivo studies using a lacz reporter transgene to track the deployment of neural crest cells (Huang et al. 1998b, Lo et al. 1997). Thus laczlabeled crest cells were increased in abundance in the outflow tract in CMV43 transgenic embryos; in Cx43α1 knockout mouse embryos there was a marked reduction (Huang et al. 1998a) (Figure 2). Overall, these studies indicate that the apparent rate of neural crest cell migration increased or decreased in parallel with the up- or down-regulation of dye coupling in the CMV43 versus Cx43α1-deficient or FC neural crest cells (Huang et al. 1998a). Given this, we previously hypothesized that gap junctions may help coordinate the deployment of neural crest cells to the heart by mediating the cell-to-cell movement of second messengers and other cell signaling molecules involved in regulating cell locomotion (Huang et al. 1998a, Lo & Wessels 1998). However, the tenability of this hypothesis has been called into question given the results of more recent studies using time lapse videomicroscopy, together with motion analysis, to examine more closely the changes in neural crest cell motility in these and other transgenic and knockout mouse models (Xu et al. 2001). These studies showed no consistent correlation between changes in the level of dye coupling and alterations in the directionality versus speed of cell locomotion in neural crest cells derived from the CMV43, FC transgenic, and Cx43α1 knockout mice (Huang et al. 1998a) (Table 1). Such discrepancies were also found in dye coupling levels and cell motile behavior in neural crest cells derived from the N-cadherin and Wnt1 knockout mice (Xu et al. 2001) (Table 1). Particularly striking is the finding that Wnt1-deficient neural crest cells with very low levels of dye coupling nevertheless exhibited cell migration behavior indistinguishable from wild-type neural crest cells (Xu et al. 2001) (Table 1). Significantly, such Wnt1-deficient neural crest cells continued to express Cx43α1 abundantly at the cell surface (Xu et al. 2001). This divergence between changes in neural crest cell motility and alterations in the level of dye coupling suggests that the modulation of cell motility by Cx43α1 may involve a novel function that is independent of gap junction channel activity. We note that several other studies have also shown no consistent correlation between changes in the level of gap junctional communication and alterations in cell proliferation or motile cell behavior (Huang et al. 1998a, Moorby & Patel 2001, Qin et al. 2002). In addition, the ectopic expression of the CT portion of Cx43α1was observed to be as effective as wild-type Cx43α1 in growth suppression (Dang et al. 2003, Moorby & Patel 2001, Zhang et al. 2003). In yet another study, a mutation in the second extracellular loop of Cx43α1 was shown to prevent cell surface trafficking, yet this mutant Cx43α1 protein also retained its ability to suppress growth (Olbina & Eckhart 2003). Below we outline a model in which Cx43α1 may mediate cell signaling independently of its channel
17 CONNEXINS IN DEVELOPMENT AND DISEASE 827 TABLE 1 Alterations in neural crest cell motility not correlated with dye coupling levels Huang et al. 1998a, Xu et al. 2001; C.W. Lo, unpublished data., decrease;, increase;,nochange. function. It should be noted that a dual functional role for a cell junction protein is not unprecedented, as this is well described for a number of cell junction and junction-associated proteins such as E-cadherin and β-catenin (Ben-Ze ev 1999, Chunthapong et al. 2004, Hagen et al. 2004). ASPECULATIVE MODEL Gap junctions, given their hydrophilic membrane channels, are generally assumed to modulate various biological processes through mediating the passive movement of ions and second messengers. Although the gap junction channel undoubtedly is important and essential for some biological processes, a separable signaling function is suggested by the lack of a consistent correlation between changes in the level of gap junctional communication and alterations in cell proliferation or motile cell behavior. We propose that Cx43α1, through interactions with a diverse array of protein binding partners, including signaling proteins (α/β-catenin, p120ctn), structural proteins (ZO-1, caveolin-1), membrane proteins (cadherins), and proteins that interact with or are part of the cell cytoskeleton (α-actinin, microtubule), may cross
18 828 WEI XU LO talk with cell signaling pathways that regulate cell adhesion, cell motility, and the actin cytoskeleton (Figure 3). Several protein kinases, including Src, PKC, and MAPK (PKs) can phosphorylate the CT of Cx43α1, potentially altering not only gating of the channel but also protein interactions that may be important in cell signaling. In addition, transcriptional effects may be elicited via p120ctn/kaiso, or β-catenin-tcf/lef, resulting in additional long-term effects through gene expression changes.
19 CONNEXINS IN DEVELOPMENT AND DISEASE 829 In short, our model places Cx43α1 as an integral member of a large signaling complex that includes β-catenin, p120ctn, N-cadherin, and a host of other proteins, and together, they facilitate cross talk to affect the coordinate regulation of cell-cell communication with cell adhesion, cell motility, and possibly other cell processes such as cell growth and proliferation. The fact that connexin mutations linked with human diseases are generally not restricted to protein domains required for channel function is consistent with a separable nonchannel activity attributable to connexin proteins. One of the important challenges in the future is to examine the functional properties of the various human connexin mutations, and, using tissue culture and animal models, determine how they may contribute to the various disease processes. In the context of this model, it is possible to envision that connexin mutations perturb the assembly or function of this signaling complex, thereby providing a unified mechanism that could account for the wide spectrum of human diseases associated with connexin mutations. ACKNOWLEDGMENTS We thank Dr. Nalin Kumar (University of Illinois at Chicago) for permission to duplicate images used in Figure 1 and Dr. Mark Yeager (Scripps Research Institute) for providing three-dimensional connexin protein structural data used to generate the model shown in Figure 3. We also thank Mr. Donald Bliss for assistance in generating the illustration in Figure 3 and members of the Lo laboratory for Figure 3 Cx43α1 protein interactions and cell-cell signaling. Cx43α1 has a diverse array of potential protein-binding partners, including signaling proteins (α/β-catenin, p120ctn), structural proteins (ZO-1, caveolin-1), membrane proteins (cadherins) and proteins that interact with or are part of the cell cytoskeleton (αactinin, microtubule). Illustrated is the putative arrangement of a Cx43α1/cadherin complex anchored to the actin cytoskeleton through a multi-protein complex containing α-catenin/β-catenin and various other actin-binding proteins such as α-actinin, vinculin, and ZO-1. ZO-1, which can directly bind Cx43α1, may also help anchor Cx43α1 tothe actin cytoskeleton, whereas interactions with caveolin-1 may target Cx43α1 tolipid rafts and recruit Cx43α1 tomembrane domains enriched in other signaling proteins. Rac1/cdc42/IQGAP1, as well as the binding of p120ctn to cadherin s juxtamembrane region may regulate the adhesive strength of adherens junction, and at the same time, these signaling pathways together with p120ctn/rho GTPases may cross talk with Cx43α1toaffect changes in cell motility. Several protein kinases, including Src, PKC, and MAPK (PKs) can phosphorylate the CT of Cx43α1, potentially altering not only gating of the channel but also protein interactions that may be important in cell signaling. It should be noted that p120ctn binding with the transcriptional factor Kaiso or β-catenin interactions with TCF/LEF may elicit downstream transcriptional effects that can result in additional long-term effects through gene expression changes.
20 830 WEI XU LO reviewing the manuscript. This work is supported by National Institutes of Health grant ZO1-HL The Annual Review of Cell and Developmental Biology is online at LITERATURE CITED Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI Wnt-1 regulation of connexin43 in cardiac myocytes. J. Clin. Invest. 105: Bagnall KM, Sanders EJ, Berdan RC Communication compartments in the axial mesoderm of the chick embryo. Anat. Embryol. 186: Bakirtzis G, Choudhry R, Aasen T, Shore L, Brown K, et al Targeted epidermal expression of mutant Connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum. Mol. Genet. 12: Barrio LC, Capel J, Jarillo JA, Castro C, Revilla A Species-specific voltagegating properties of connexin-45 junctions expressed in Xenopus oocytes. Biophys. J. 73: Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, et al Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc. Natl. Acad. Sci. USA 88: Ben-Ze ev A The dual role of cytoskeletal anchor proteins in cell adhesion and signal transduction. Ann. NY Acad. Sci. 886:37 47 Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, et al. 1993a. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262: Bergoffen J, Trofatter J, Pericak-Vance MA, Haines JL, Chance PF, Fischbeck KH. 1993b. Linkage localization of X-linked Charcot- Marie-Tooth disease. Am. J. Hum. Genet. 52: Berry V, Mackay D, Khaliq S, Francis PJ, Hameed A, et al Connexin 50 mutation in a family with congenital zonular nuclear pulverulent cataract of Pakistani origin. Hum. Genet. 105: Berthoud VM, Beyer EC, Kurata WE, Lau AF, Lampe PD The gap-junction protein connexin 56 is phosphorylated in the intracellular loop and the carboxy-terminal region. Eur. J. Biochem. 244:89 97 Berthoud VM, Minogue PJ, Guo J, Williamson EK, Xu X, et al Loss of function and impaired degradation of a cataractassociated mutant connexin50. Eur. J. Cell Biol. 82: Bevilacqua LM, Simon AM, Maguire CT, Gehrmann J, Wakimoto H, et al A targeted disruption in connexin40 leads to distinct atrioventricular conduction defects. J. Interv. Card. Electrophysiol. 4: Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N. Engl. J. Med. 332: Bruzzone R, White TW, Paul DL Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 238:1 27 Casey B, Ballabio A Connexin43 mutations in sporadic and familial defects of laterality. N. Engl. J. Med. 333: Choudhry R, Pitts JD, Hodgins MB Changing patterns of gap junctional intercellular communication and connexin distribution in mouse epidermis and hair follicles during embryonic development. Dev. Dyn. 210: Chunthapong J, Seftor EA, Khalkhali-Ellis Z, Seftor RE, Amir S, et al Dual roles
Cardiac cell-cell Communication Part 1 Alonso P. Moreno D.Sc. CVRTI, Cardiology
 Bioengineering 6003 Cellular Electrophysiology and Biophysics Cardiac cell-cell Communication Part 1 Alonso P. Moreno D.Sc. CVRTI, Cardiology moreno@cvrti.utah.edu November 2010 poster Physiological Relevance
Bioengineering 6003 Cellular Electrophysiology and Biophysics Cardiac cell-cell Communication Part 1 Alonso P. Moreno D.Sc. CVRTI, Cardiology moreno@cvrti.utah.edu November 2010 poster Physiological Relevance
Amneh Auben. Abdulrahman Jabr. Diala Abu-Hassan
 21 Amneh Auben Abdulrahman Jabr Diala Abu-Hassan Matrix polysaccharides Extracellular matrix (ECM): It s a collection of components that fills the spaces outside the cell or between the cells. ---------
21 Amneh Auben Abdulrahman Jabr Diala Abu-Hassan Matrix polysaccharides Extracellular matrix (ECM): It s a collection of components that fills the spaces outside the cell or between the cells. ---------
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Bio 127 Section I Introduction to Developmental Biology. Cell Cell Communication in Development. Developmental Activities Coordinated in this Way
 Bio 127 Section I Introduction to Developmental Biology Cell Cell Communication in Development Gilbert 9e Chapter 3 It has to be EXTREMELY well coordinated for the single celled fertilized ovum to develop
Bio 127 Section I Introduction to Developmental Biology Cell Cell Communication in Development Gilbert 9e Chapter 3 It has to be EXTREMELY well coordinated for the single celled fertilized ovum to develop
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Lecture 3 13/11/2018
 Lecture 3 13/11/2018 1 Plasma membrane ALL cells have a cell membrane made of proteins and lipids. protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump Lipid bilayer allows water, carbon
Lecture 3 13/11/2018 1 Plasma membrane ALL cells have a cell membrane made of proteins and lipids. protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump Lipid bilayer allows water, carbon
Cell Biology Review. The key components of cells that concern us are as follows: 1. Nucleus
 Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Regulation of Connexin40 Gap Junctions
 Georgia State University ScholarWorks @ Georgia State University Biology Dissertations Department of Biology 8-31-2008 Regulation of Connexin40 Gap Junctions Thomas Vinaya Sheela Follow this and additional
Georgia State University ScholarWorks @ Georgia State University Biology Dissertations Department of Biology 8-31-2008 Regulation of Connexin40 Gap Junctions Thomas Vinaya Sheela Follow this and additional
Supplemental table S7.
 Supplemental table S7. GO terms significantly enriched in significantly up-regulated genes of the microarray. K: number of genes from the input cluster in the given category. F: number of total genes in
Supplemental table S7. GO terms significantly enriched in significantly up-regulated genes of the microarray. K: number of genes from the input cluster in the given category. F: number of total genes in
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016
 Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Cell Cell Communication in Development
 Biology 4361 Developmental Biology Cell Cell Communication in Development June 25, 2008 Cell Cell Communication Concepts Cells in developing organisms develop in the context of their environment, including
Biology 4361 Developmental Biology Cell Cell Communication in Development June 25, 2008 Cell Cell Communication Concepts Cells in developing organisms develop in the context of their environment, including
Zool 3200: Cell Biology Exam 5 4/27/15
 Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
CL T123 PKA? Possible Cx26deaf mutation: T123N 2,3 EL2 T177 Intracellular kinases or ecto-kinases 2 EL2/TM4 border
 Name AA MW (Da) Cx26 =GJB2 Cx31 =GJB3 BIP Region P-site Modifying enzyme 226 26,215 9.11 NT D2, T5 or S8 1 # Functional consequences Deafness mutation at S8 1 CL T123 PKA Possible Cx26deaf mutation: T123N
Name AA MW (Da) Cx26 =GJB2 Cx31 =GJB3 BIP Region P-site Modifying enzyme 226 26,215 9.11 NT D2, T5 or S8 1 # Functional consequences Deafness mutation at S8 1 CL T123 PKA Possible Cx26deaf mutation: T123N
7.013 Problem Set
 7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as shown below.
CHAPTER 3. Cell Structure and Genetic Control. Chapter 3 Outline
 CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J INTEGRINS I.V. Yannas, Ph.D. and M. Spector, Ph.D. Regulator
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J INTEGRINS I.V. Yannas, Ph.D. and M. Spector, Ph.D. Regulator
Role of Organizer Chages in Late Frog Embryos
 Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development June 23, 2009 Concepts Cell-Cell Communication Cells develop in the context of their environment, including: - their immediate
Biology 4361 - Developmental Biology Cell-Cell Communication in Development June 23, 2009 Concepts Cell-Cell Communication Cells develop in the context of their environment, including: - their immediate
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Chemical aspects of the cell. Shape and structure of the cell
 Chemical aspects of the cell Shape and structure of the cell Cellular composition https://www.studyblue.com/ 2 Cellular composition Set of videos with basic information: Cell characteristics: https://www.youtube.com/watch?v=urujd5nexc8
Chemical aspects of the cell Shape and structure of the cell Cellular composition https://www.studyblue.com/ 2 Cellular composition Set of videos with basic information: Cell characteristics: https://www.youtube.com/watch?v=urujd5nexc8
Overview of Physiology & Homeostasis. Biological explanations Levels of organization Homeostasis
 Overview of Physiology & Homeostasis 1 Biological explanations Levels of organization Homeostasis 2 Biological Explanations Proximate Proximate causation: an explanation of an animal's behavior based on
Overview of Physiology & Homeostasis 1 Biological explanations Levels of organization Homeostasis 2 Biological Explanations Proximate Proximate causation: an explanation of an animal's behavior based on
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
1. The plasma membrane of eukaryotic cells is supported by a. actin filaments. b. microtubules. c. lamins. d. intermediate filaments.
 ANALYSIS AND MODELING OF CELL MECHANICS Homework #2 (due 1/30/13) This homework involves comprehension of key biomechanical concepts of the cytoskeleton, cell-matrix adhesions, and cellcell adhesions.
ANALYSIS AND MODELING OF CELL MECHANICS Homework #2 (due 1/30/13) This homework involves comprehension of key biomechanical concepts of the cytoskeleton, cell-matrix adhesions, and cellcell adhesions.
What are mitochondria?
 What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Tuesday, December 27, 16
 Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Gap junctional coupling in lenses lacking 3 connexin
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 15303 15308, December 1998 Biophysics Gap junctional coupling in lenses lacking 3 connexin XIAOHUA GONG*, GEORGE J. BALDO, NALIN M. KUMAR*, NORTON B. GILULA*, AND
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 15303 15308, December 1998 Biophysics Gap junctional coupling in lenses lacking 3 connexin XIAOHUA GONG*, GEORGE J. BALDO, NALIN M. KUMAR*, NORTON B. GILULA*, AND
The majority of cells in the nervous system arise during the embryonic and early post
 Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
5- Semaphorin-Plexin-Neuropilin
 5- Semaphorin-Plexin-Neuropilin 1 SEMAPHORINS-PLEXINS-NEUROPILINS ligands receptors co-receptors semaphorins and their receptors are known signals for: -axon guidance -cell migration -morphogenesis -immune
5- Semaphorin-Plexin-Neuropilin 1 SEMAPHORINS-PLEXINS-NEUROPILINS ligands receptors co-receptors semaphorins and their receptors are known signals for: -axon guidance -cell migration -morphogenesis -immune
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
6 Mechanotransduction
 6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Reading. Lecture VI. Making Connections 9/17/12. Bio 3411 Lecture VI. Making Connections. Bio 3411 Monday September 17, 2012
 Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Reception The target cell s detection of a signal coming from outside the cell May Occur by: Direct connect Through signal molecules
 Why Do Cells Communicate? Regulation Cells need to control cellular processes In multicellular organism, cells signaling pathways coordinate the activities within individual cells that support the function
Why Do Cells Communicate? Regulation Cells need to control cellular processes In multicellular organism, cells signaling pathways coordinate the activities within individual cells that support the function
Hole s Human Anatomy and Physiology
 Hole s Human Anatomy and Physiology 1 Chapter 3 Cells vary in size possess distinctive shapes measured in micrometers 2 A Composite Cell hypothetical cell major parts nucleus cytoplasm cell membrane 3
Hole s Human Anatomy and Physiology 1 Chapter 3 Cells vary in size possess distinctive shapes measured in micrometers 2 A Composite Cell hypothetical cell major parts nucleus cytoplasm cell membrane 3
Chapter 16. Cellular Movement: Motility and Contractility. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
ADAM FAMILY. ephrin A INTERAZIONE. Eph ADESIONE? PROTEOLISI ENDOCITOSI B A RISULTATO REPULSIONE. reverse. forward
 ADAM FAMILY - a family of membrane-anchored metalloproteases that are known as A Disintegrin And Metalloprotease proteins and are key components in protein ectodomain shedding Eph A INTERAZIONE B ephrin
ADAM FAMILY - a family of membrane-anchored metalloproteases that are known as A Disintegrin And Metalloprotease proteins and are key components in protein ectodomain shedding Eph A INTERAZIONE B ephrin
7.013 Spring 2005 Problem Set 4
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 4 FRIDAY April 8th,
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 4 FRIDAY April 8th,
Advanced Higher Biology. Unit 1- Cells and Proteins 2c) Membrane Proteins
 Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Domain 6: Communication
 Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Molecular Cell Biology 5068 In Class Exam 1 September 30, Please print your name:
 Molecular Cell Biology 5068 In Class Exam 1 September 30, 2014 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 1 September 30, 2014 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Components of a functional cell. Boundary-membrane Cytoplasm: Cytosol (soluble components) & particulates DNA-information Ribosomes-protein synthesis
 Cell (Outline) - Components of a functional cell - Major Events in the History of Earth: abiotic and biotic phases; anaerobic and aerobic atmosphere - Prokaryotic cells impact on the biosphere - Origin
Cell (Outline) - Components of a functional cell - Major Events in the History of Earth: abiotic and biotic phases; anaerobic and aerobic atmosphere - Prokaryotic cells impact on the biosphere - Origin
MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION
 MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION Drosophila is the best understood of all developmental systems, especially at the genetic level, and although it is an invertebrate it has had an enormous
MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION Drosophila is the best understood of all developmental systems, especially at the genetic level, and although it is an invertebrate it has had an enormous
Intercellular Communication. Department of Physiology School of Medicine University of Sumatera Utara
 Intercellular Communication Department of Physiology School of Medicine University of Sumatera Utara Intercellular Communication and Signal Transduction The ability of cells to communicate with each other
Intercellular Communication Department of Physiology School of Medicine University of Sumatera Utara Intercellular Communication and Signal Transduction The ability of cells to communicate with each other
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
SIGNIFICANCE OF EMBRYOLOGY
 This lecture will discuss the following topics : Definition of Embryology Significance of Embryology Old and New Frontiers Introduction to Molecular Regulation and Signaling Descriptive terms in Embryology
This lecture will discuss the following topics : Definition of Embryology Significance of Embryology Old and New Frontiers Introduction to Molecular Regulation and Signaling Descriptive terms in Embryology
Exam 1 ID#: October 4, 2007
 Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
1- Below is a list of cell cycle phases matched with specific processes. Choose the correct pairing:
 Name: NetID: Exam 4 - Version 2 November 13, 2018 Dr. A. Pimentel Instructions: 1- Select the BEST answer for each question 2- Use pencil to mark your responses in the answer sheet. 3- You can mark your
Name: NetID: Exam 4 - Version 2 November 13, 2018 Dr. A. Pimentel Instructions: 1- Select the BEST answer for each question 2- Use pencil to mark your responses in the answer sheet. 3- You can mark your
Cell (Learning Objectives)
 Cell (Learning Objectives) 1. Understand & describe the basic components necessary for a functional cell. 2. Review the order of appearance of cells on earth and explain the endosymbiotic theory. 3. Compare
Cell (Learning Objectives) 1. Understand & describe the basic components necessary for a functional cell. 2. Review the order of appearance of cells on earth and explain the endosymbiotic theory. 3. Compare
Modulation of intercellular communication by differential regulation and heteromeric mixing of co-expressed connexins
 Brazilian Journal of Medical and Biological Research (2000) 33: 391-397 Co-expression of endothelial connexins ISSN 0100-879X 391 Modulation of intercellular communication by differential regulation and
Brazilian Journal of Medical and Biological Research (2000) 33: 391-397 Co-expression of endothelial connexins ISSN 0100-879X 391 Modulation of intercellular communication by differential regulation and
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
The Cell. C h a p t e r. PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas
 C h a p t e r 2 The Cell PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Introduction
C h a p t e r 2 The Cell PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Introduction
Student Learning Outcomes: Nucleus distinguishes Eukaryotes from Prokaryotes
 9 The Nucleus Student Learning Outcomes: Nucleus distinguishes Eukaryotes from Prokaryotes Explain general structures of Nuclear Envelope, Nuclear Lamina, Nuclear Pore Complex Explain movement of proteins
9 The Nucleus Student Learning Outcomes: Nucleus distinguishes Eukaryotes from Prokaryotes Explain general structures of Nuclear Envelope, Nuclear Lamina, Nuclear Pore Complex Explain movement of proteins
1. What are the three general areas of the developing vertebrate limb? 2. What embryonic regions contribute to the developing limb bud?
 Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Big Idea 1: The process of evolution drives the diversity and unity of life.
 Big Idea 1: The process of evolution drives the diversity and unity of life. understanding 1.A: Change in the genetic makeup of a population over time is evolution. 1.A.1: Natural selection is a major
Big Idea 1: The process of evolution drives the diversity and unity of life. understanding 1.A: Change in the genetic makeup of a population over time is evolution. 1.A.1: Natural selection is a major
AP Curriculum Framework with Learning Objectives
 Big Ideas Big Idea 1: The process of evolution drives the diversity and unity of life. AP Curriculum Framework with Learning Objectives Understanding 1.A: Change in the genetic makeup of a population over
Big Ideas Big Idea 1: The process of evolution drives the diversity and unity of life. AP Curriculum Framework with Learning Objectives Understanding 1.A: Change in the genetic makeup of a population over
Enduring understanding 1.A: Change in the genetic makeup of a population over time is evolution.
 The AP Biology course is designed to enable you to develop advanced inquiry and reasoning skills, such as designing a plan for collecting data, analyzing data, applying mathematical routines, and connecting
The AP Biology course is designed to enable you to develop advanced inquiry and reasoning skills, such as designing a plan for collecting data, analyzing data, applying mathematical routines, and connecting
AP Biology Essential Knowledge Cards BIG IDEA 1
 AP Biology Essential Knowledge Cards BIG IDEA 1 Essential knowledge 1.A.1: Natural selection is a major mechanism of evolution. Essential knowledge 1.A.4: Biological evolution is supported by scientific
AP Biology Essential Knowledge Cards BIG IDEA 1 Essential knowledge 1.A.1: Natural selection is a major mechanism of evolution. Essential knowledge 1.A.4: Biological evolution is supported by scientific
Eukaryotic vs. Prokaryotic genes
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 18: Eukaryotic genes http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Eukaryotic vs. Prokaryotic genes Like in prokaryotes,
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 18: Eukaryotic genes http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Eukaryotic vs. Prokaryotic genes Like in prokaryotes,
BIOH111. o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook
BIOH111 o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook
MEMBRANE STRUCTURE. Lecture 9. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
Host-Pathogen Interaction. PN Sharma Department of Plant Pathology CSK HPKV, Palampur
 Host-Pathogen Interaction PN Sharma Department of Plant Pathology CSK HPKV, Palampur-176062 PATHOGEN DEFENCE IN PLANTS A BIOLOGICAL AND MOLECULAR VIEW Two types of plant resistance response to potential
Host-Pathogen Interaction PN Sharma Department of Plant Pathology CSK HPKV, Palampur-176062 PATHOGEN DEFENCE IN PLANTS A BIOLOGICAL AND MOLECULAR VIEW Two types of plant resistance response to potential
Developmental Biology Lecture Outlines
 Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Advanced Anatomy and Physiology
 Lakeshore Technical College 10806179 Advanced Anatomy and Physiology Course Outcome Summary Course Information Alternate Title Description Total Credits 4 Total Hours 90 Adv Anatomy & Physiology Advanced
Lakeshore Technical College 10806179 Advanced Anatomy and Physiology Course Outcome Summary Course Information Alternate Title Description Total Credits 4 Total Hours 90 Adv Anatomy & Physiology Advanced
Cellular Neuroanatomy I The Prototypical Neuron: Soma. Reading: BCP Chapter 2
 Cellular Neuroanatomy I The Prototypical Neuron: Soma Reading: BCP Chapter 2 Functional Unit of the Nervous System The functional unit of the nervous system is the neuron. Neurons are cells specialized
Cellular Neuroanatomy I The Prototypical Neuron: Soma Reading: BCP Chapter 2 Functional Unit of the Nervous System The functional unit of the nervous system is the neuron. Neurons are cells specialized
Chapter 3: Structure and Function of the Cell
 Chapter 3: Structure and Function of the Cell I. Functions of the Cell A. List and describe the main functions of the cell: 1. 2. 3. 4. 5. II. How We See Cells A. Light microscopes allow us to B. Electron
Chapter 3: Structure and Function of the Cell I. Functions of the Cell A. List and describe the main functions of the cell: 1. 2. 3. 4. 5. II. How We See Cells A. Light microscopes allow us to B. Electron
The Pax and large Maf families of genes in mammalian eye development
 The Pax and large Maf families of genes in mammalian eye development Vertebrate eye development is dependent on the coordinated action of thousands of genes. A specific group of over one hundred of regulatory
The Pax and large Maf families of genes in mammalian eye development Vertebrate eye development is dependent on the coordinated action of thousands of genes. A specific group of over one hundred of regulatory
Drosophila melanogaster- Morphogen Gradient
 NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
RANK. Alternative names. Discovery. Structure. William J. Boyle* SUMMARY BACKGROUND
 RANK William J. Boyle* Department of Cell Biology, Amgen, Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799, USA * corresponding author tel: 805-447-4304, fax: 805-447-1982, e-mail: bboyle@amgen.com
RANK William J. Boyle* Department of Cell Biology, Amgen, Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799, USA * corresponding author tel: 805-447-4304, fax: 805-447-1982, e-mail: bboyle@amgen.com
Biol403 - Receptor Serine/Threonine Kinases
 Biol403 - Receptor Serine/Threonine Kinases The TGFβ (transforming growth factorβ) family of growth factors TGFβ1 was first identified as a transforming factor; however, it is a member of a family of structurally
Biol403 - Receptor Serine/Threonine Kinases The TGFβ (transforming growth factorβ) family of growth factors TGFβ1 was first identified as a transforming factor; however, it is a member of a family of structurally
Intercellular communication
 Intercellular communication Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada Outline General principle of intercellular communicabon Signal molecules
Intercellular communication Dewajani Purnomosari Department of Histology and Cell Biology Faculty of Medicine Universitas Gadjah Mada Outline General principle of intercellular communicabon Signal molecules
Developmental processes Differential gene expression Introduction to determination The model organisms used to study developmental processes
 Date Title Topic(s) Learning Outcomes: Sept 28 Oct 3 1. What is developmental biology and why should we care? 2. What is so special about stem cells and gametes? Developmental processes Differential gene
Date Title Topic(s) Learning Outcomes: Sept 28 Oct 3 1. What is developmental biology and why should we care? 2. What is so special about stem cells and gametes? Developmental processes Differential gene
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
9/4/2015 INDUCTION CHAPTER 1. Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology. Fig 1.
 INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290
 Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290 Question (from Introduction): How does svb control the
Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290 Question (from Introduction): How does svb control the
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-2018 GENETICS BIO-5009A Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-2018 GENETICS BIO-5009A Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Developmental Zoology. Ectodermal derivatives (ZOO ) Developmental Stages. Developmental Stages
 Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Lecture 7. Development of the Fruit Fly Drosophila
 BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Lecture 7 Development of the Fruit Fly Drosophila 1. The fruit fly- a highly successful, specialized organism a. Quick life cycle includes three larval stages
BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Lecture 7 Development of the Fruit Fly Drosophila 1. The fruit fly- a highly successful, specialized organism a. Quick life cycle includes three larval stages
Honors Biology Reading Guide Chapter 11
 Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Organisms are made up of specialized cells.
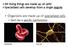 All living things are made up of cells! Specialized cells develop from a single zygote Organisms are made up of specialized cells. Each has a specific job/function red blood cell nerve cell Zygotes (fertilized
All living things are made up of cells! Specialized cells develop from a single zygote Organisms are made up of specialized cells. Each has a specific job/function red blood cell nerve cell Zygotes (fertilized
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Chapter 3: Cells. Lectures by Mark Manteuffel, St. Louis Community College
 Chapter 3: Cells Lectures by Mark Manteuffel, St. Louis Community College Learning Objectives Be able to describe: what a cell is & two main classes of cells. structure & functions of cell membranes. how
Chapter 3: Cells Lectures by Mark Manteuffel, St. Louis Community College Learning Objectives Be able to describe: what a cell is & two main classes of cells. structure & functions of cell membranes. how
1 GO: regulation of cell size E-04 2 GO: negative regulation of cell growth GO:
 Table S2: The biological modulated by mir-5701 Sr. No Term Id 1 Term Name 2 Hit Gene Number 3 P-Value 4 1 GO:0008361 regulation of cell size 9 4.37E-04 2 GO:0030308 negative regulation of cell growth 8
Table S2: The biological modulated by mir-5701 Sr. No Term Id 1 Term Name 2 Hit Gene Number 3 P-Value 4 1 GO:0008361 regulation of cell size 9 4.37E-04 2 GO:0030308 negative regulation of cell growth 8
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
CHAPTER 1 THE STRUCTURAL BIOLOGY OF THE FGF19 SUBFAMILY
 CHAPTER 1 THE STRUCTURAL BIOLOGY OF THE FGF19 SUBFAMILY Andrew Beenken and Moosa Mohammadi* Department of Pharmacology, New York University School of Medicine, New York, New York, USA. *Corresponding Author:
CHAPTER 1 THE STRUCTURAL BIOLOGY OF THE FGF19 SUBFAMILY Andrew Beenken and Moosa Mohammadi* Department of Pharmacology, New York University School of Medicine, New York, New York, USA. *Corresponding Author:
Organization of Genes Differs in Prokaryotic and Eukaryotic DNA Chapter 10 p
 Organization of Genes Differs in Prokaryotic and Eukaryotic DNA Chapter 10 p.110-114 Arrangement of information in DNA----- requirements for RNA Common arrangement of protein-coding genes in prokaryotes=
Organization of Genes Differs in Prokaryotic and Eukaryotic DNA Chapter 10 p.110-114 Arrangement of information in DNA----- requirements for RNA Common arrangement of protein-coding genes in prokaryotes=
From DNA to Diversity
 From DNA to Diversity Molecular Genetics and the Evolution of Animal Design Sean B. Carroll Jennifer K. Grenier Scott D. Weatherbee Howard Hughes Medical Institute and University of Wisconsin Madison,
From DNA to Diversity Molecular Genetics and the Evolution of Animal Design Sean B. Carroll Jennifer K. Grenier Scott D. Weatherbee Howard Hughes Medical Institute and University of Wisconsin Madison,
Roles of Cell Division. Reproduction - Like begets like, more or less. Examples of Cell Numbers. Outline Cell Reproduction
 Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis 4. Sexual Life
Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis 4. Sexual Life
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
A A A A B B1
 LEARNING OBJECTIVES FOR EACH BIG IDEA WITH ASSOCIATED SCIENCE PRACTICES AND ESSENTIAL KNOWLEDGE Learning Objectives will be the target for AP Biology exam questions Learning Objectives Sci Prac Es Knowl
LEARNING OBJECTIVES FOR EACH BIG IDEA WITH ASSOCIATED SCIENCE PRACTICES AND ESSENTIAL KNOWLEDGE Learning Objectives will be the target for AP Biology exam questions Learning Objectives Sci Prac Es Knowl
Initiation of translation in eukaryotic cells:connecting the head and tail
 Initiation of translation in eukaryotic cells:connecting the head and tail GCCRCCAUGG 1: Multiple initiation factors with distinct biochemical roles (linking, tethering, recruiting, and scanning) 2: 5
Initiation of translation in eukaryotic cells:connecting the head and tail GCCRCCAUGG 1: Multiple initiation factors with distinct biochemical roles (linking, tethering, recruiting, and scanning) 2: 5
S1 Gene ontology (GO) analysis of the network alignment results
 1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
1 Supplementary Material for Effective comparative analysis of protein-protein interaction networks by measuring the steady-state network flow using a Markov model Hyundoo Jeong 1, Xiaoning Qian 1 and
