Calcium Binding by Synaptotagmin s C 2 A Domain is an Essential Element of the Electrostatic Switch That Triggers Synchronous Synaptic Transmission
|
|
|
- Luke McDowell
- 5 years ago
- Views:
Transcription
1 The Journal of Neuroscience, January 25, (4): Cellular/Molecular Calcium Binding by Synaptotagmin s C 2 A Domain is an Essential Element of the Electrostatic Switch That Triggers Synchronous Synaptic Transmission Amelia R. Striegel, 1 * Laurie M. Biela, 1 * Chantell S. Evans, 2 Zhao Wang, 2 Jillian B. Delehoy, 1 R. Bryan Sutton, 3 Edwin R. Chapman, 2 and Noreen E. Reist 1 1 Department of Biomedical Sciences, Program in Molecular, Cellular, and Integrative Neuroscience, Colorado State University, Fort Collins, Colorado, 8523, 2 Department of Neuroscience, University of Wisconsin, Madison, Wisconsin, 5376, and 3 Department of Cell Physiology and Molecular Biophysics, Texas Tech University Health Sciences Center, Lubbock, Texas, 7949 Synaptotagmin is the major calcium sensor for fast synaptic transmission that requires the synchronous fusion of synaptic vesicles. Synaptotagmin contains two calcium-binding domains: C 2 A and C 2 B. Mutation of a positively charged residue (R233Q in rat) showed that Ca 2 -dependent interactions between the C 2 A domain and membranes play a role in the electrostatic switch that initiates fusion. Surprisingly, aspartate-to-asparagine mutations in C 2 A that inhibit Ca 2 binding support efficient synaptic transmission, suggesting that Ca 2 binding by C 2 A is not required for triggering synchronous fusion. Based on a structural analysis, we generated a novel mutation of a single Ca 2 -binding residue in C 2 A (D229E in Drosophila) that inhibited Ca 2 binding but maintained the negative charge of the pocket. This C 2 A aspartate-to-glutamate mutation resulted in 8% decrease in synchronous transmitter release and a decrease in the apparent Ca 2 affinity of release. Previous aspartate-to-asparagine mutations in C 2 A partially mimicked Ca 2 binding by decreasing the negative charge of the pocket. We now show that the major function of Ca 2 binding to C 2 A is to neutralize the negative charge of the pocket, thereby unleashing the fusion-stimulating activity of synaptotagmin. Our results demonstrate that Ca 2 binding by C 2 Aisa critical component of the electrostatic switch that triggers synchronous fusion. Thus, Ca 2 binding by C 2 B is necessary and sufficient to regulate the precise timing required for coupling vesicle fusion to Ca 2 influx, but Ca 2 binding by both C 2 domains is required to flip the electrostatic switch that triggers efficient synchronous synaptic transmission. Introduction Synaptic transmission occurs when Ca 2 entry into an active nerve terminal triggers the fast, synchronous fusion of synaptic vesicles with the presynaptic membrane. Shortly after the identification of its two C 2 domains, synaptotagmin was postulated to be the Ca 2 sensor that triggers this synchronous fusion of vesicles (Brose et al., 1992). Initial studies suggested that the vesicle proximal C 2 domain, C 2 A, mediated the Ca 2 binding that triggered synchronous vesicle fusion events (Elferink et al., 1993; Fernández-Chacón et al., 21). However, mutations that inhibited Ca 2 binding by C 2 A supported efficient synaptic transmission at excitatory synapses (Fernández-Chacón et al., 22; Received Sept. 11, 211; revised Nov. 13, 211; accepted Nov. 28, 211. Author contributions: E.R.C. and N.E.R. designed research; A.R.S., L.M.B., C.S.E., Z.W., and R.B.S. performed research; A.R.S., L.M.B., C.S.E., Z.W., J.B.D., R.B.S., and N.E.R. analyzed data; A.R.S., C.S.E., and N.E.R. wrote the paper. This work was supported by grants from the National Institutes of Health (NS to N.E.R., MN to E.R.C.), the National Science Foundation (IOS and IOS to N.E.R.), the Microscope Imaging Network, and the College Research Council of Colorado State University. We thank Drs. Gary Pickard and Mike Tamkun for fruitful discussions and Dr. Deborah M. Garrity for comments on this manuscript. E.R.C. is an Investigator of the Howard Hughes Medical Institute. *A.R.S. and L.M.B. contributed equally to this paper. Correspondence should be addressed to Dr. Noreen E. Reist, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO Noreen.Reist@Colostate.edu. DOI:1.1523/JNEUROSCI Copyright 212 the authors /12/ $15./ Robinson et al., 22; Stevens and Sullivan, 23; Yoshihara et al., 21). Mutations in the second C 2 domain, C 2 B, that inhibited Ca 2 binding abolished evoked-transmitter release, demonstrating that Ca 2 binding by C 2 B is essential for synchronous, Ca 2 -triggered transmitter release (Mackler et al., 22). Thus, Ca 2 binding by the C 2 B domain has been thought to be both necessary and sufficient for triggering synchronous transmitter release. Yet mutations that disrupted Ca 2 -dependent interactions by the C 2 A domain decrease synchronous release by 5% and decrease the apparent Ca 2 affinity of release (Fernández-Chacón et al., 21; Wang et al., 23; Paddock et al., 28, 211), suggesting that Ca 2 binding by C 2 A is required for efficient synchronous release. These results raised a now longstanding question: how can Ca 2 -dependent interactions by C 2 A be functionally more significant than C 2 ACa 2 binding itself? When synaptotagmin binds calcium, it alters the electrostatic potential of the calcium-binding pocket (Ubach et al., 1998), enhancing interactions with other presynaptic molecules, such as negatively charged membranes and proteins of the SNARE complex (Brose et al., 1992; Chapman et al., 1995; Schiavo et al., 1997; Chapman and Davis, 1998; Bai et al., 22; Zhang et al., 22). This suggests that both C 2 domains function as an electrostatic switch (Davletov et al., 1998; Ubach et al., 1998; Murray and Honig, 22) such that the bound calcium ions shield, or effectively neutralize, the negative potential of the pocket. Such neu-
2 1254 J. Neurosci., January 25, (4): Striegel et al. C 2 A: Critical Component of Electrostatic Switch tralization permits residues at the tip of both the C 2 A and C 2 B Ca 2 -binding pockets, known to interact with negatively charged phospholipids (Chae et al., 1998; Fernández-Chacón et al., 21; Bai et al., 22; Wang et al., 23), to interact with the presynaptic membrane. Thus, the previously tested aspartate-toasparagine mutations (D3N) in C 2 A, which inhibited Ca 2 binding by removing this negative charge, may result in minimal disruptions of synchronous transmitter release, or even enhance release, because the mutations partially mimic Ca 2 binding (Stevens and Sullivan, 23). Here we directly test the importance of electrostatic repulsion by C 2 A in inhibiting fusion. We designed an aspartate-toglutamate mutation (D3E) to test the function of Ca 2 binding independent of charge neutralization. This mutation inhibited Ca 2 binding but maintained the negative charge (and hence the repulsive force) of the C 2 ACa 2 -binding pocket. Our novel mutation, which cannot mimic the charge-neutralizing function of Ca 2 binding, results in a severe decrease in synchronous synaptic transmission, demonstrating that Ca 2 binding by C 2 Ais required for the electrostatic switch. Materials and Methods Mutagenesis. Drosophila synaptotagmin aspartate residue 229 was mutated to glutamate. Oligonucleotides (cttggtctcgaacttcttcttcttgtcgggcagc aagtacaccttgacatagggctccgaggtac and ctcggagccctatgtcaaggtgtacttgctgccc gacaagaagaagaagttcgagac) were used to create a mutant double-stranded DNA fragment with KpnI and StyI overhangs, which was ligated into a wild-type synaptotagmin cdna construct in pbluescript II KS (Stratagene), sequenced, and subcloned into a puast vector to place the mutant syt gene under the control of the UAS promoter (Brand and Perrimon, 1993); then these were sent to Best Gene to transform Drosophila. Fly lines. Expression of the transgene was localized to the nervous system using elavgal4 to drive pan neuronal expression of the UAS-syt transgenes (Brand and Perrimon, 1993; Yao and White, 1994). The syt null mutation used was syt AD4 (DiAntonio et al., 1993). Standard genetic techniques were used to cross the transgenes into the syt null background to express the transgene in the absence of endogenous synaptotagmin 1 (Loewen et al., 26a). No gender selection was used, thus a mix of male and female larvae were used in all experiments. Experimental flies were yw; syt null elavgal4/syt null ; P[UAS syt A-D229E ]/ (P[syt A-D2E ], transgenic mutant) and yw; syt null elavgal4/syt null ; P[UAS syt WT ]/ (P[syt WT ], transgenic control). Sequence alignments. A ClustalW2 sequence alignment was performed on the C 2 A domain of the following Ca 2 -binding synaptotagmin isoforms: syt 1 from Drosophila melanogaster (NP_ ), Apis mellifera (NP_ ), Manduca sexta (AAK1129.1), Loligo pealei (BAA9866.1), Caenorhabditis elegans (NP_ ), Gallus gallus (NP_9952.1), Mus musculus (NP_ ), and Rattus norvegicus (NP_ ); and Homo sapiens syt 1 (NP_ ), syt 2 (NP_ ), syt 3 (NP_ ), syt 5 (NP_3171.2), syt 6 (NP_ ), syt 7 (NP_ ), syt 9 (NP_ ), and syt 1 (NP_ ). Molecular modeling. The D2E mutation in Figure 1C was modeled using the mutagenesis plugin in PyMOL (The PyMOL Molecular Graphics System, Version 1.3; Schrödinger). Asp-178, from the high-resolution rat synaptotagmin 1 C 2 A x-ray structure (Protein Data Bank, PDB file; 1RSY), was changed to a glutamate. The rotamer with the fewest number of collisions was selected for the figure. Electrophysiology. Excitatory junction potentials (EJPs) and miniature EJPs (mejps) were recorded using standard techniques (Reist et al., 1998; Paddock et al., 211) from L3 muscle fiber 6 of abdominal segments 3 and 4 in HL3 saline containing 7 mm NaCl, 5 mm KCl, 2 mm MgCl 2,1 mm NaHCO 3,5mM Trehalose, 115 mm sucrose, 5 mm HEPES, ph 7.2, and 1.5 mm CaCl 2, unless otherwise indicated. Dissections were performed in Ca 2 -free HL3 saline. Fibers were impaled with a 1 4 M recording electrode containing three parts 2 M potassium citrate to one part 3 M potassium chloride. The resting membrane potential of each fiber was maintained at 55 mv by passing a bias current. To evoke EJPs, segmental nerves were stimulated with a suction electrode filled with 1.5 mm Ca 2 HL3. The Ca 2 dependence curve was generated by averaging 1 EJPs recorded at.5 Hz from fibers bathed in HL3 containing Ca 2 concentrations ranging from.6 to 5. mm. Recordings in multiple Ca 2 concentrations were made from each muscle fiber. With the exception of 1.5 mm Ca 2, 1 13 fibers were recorded at each Ca 2 concentration in each genotype. At 1.5 mm Ca 2, fibers were recorded, since most recording sessions included this concentration. All events were collected using an AxoClamp 2B (Molecular Devices) and digitized using a MacLab4s A/D converter (ADInstruments). The Ca 2 -dependence data were fit with the Hill equation using Kaleidagraph software. The Ca 2 cooperativity coefficient was estimated from the slope of a double-log plot of EJP amplitude versus Ca 2 concentration. EJPs were recorded in Scope software and mejps were recorded in Chart Software (ADInstruments). Immunoblotting and immunohistochemistry. Western analysis was used to determine levels of transgene expression. The CNSs of individual third instar larvae were loaded one CNS per lane and blots were probed with an anti-synaptotagmin antibody [Dsyt-CL1 (Mackler et al., 22)] and an anti-actin antibody (MAB 151; Millipore Bioscience Research Reagents) using standard techniques (Loewen et al., 21). Actin levels were used to normalize for equal protein loading; the synaptotagmin: actin signal ratio was determined for each lane, then normalized to the mean synaptotagmin:actin ratio of the P[syt WT ] lanes on each blot to allow comparison of signal between multiple blots. Transgenic synaptotagmin was localized by immunolabeling third instar larvae with Dsyt-CL1 using standard techniques (Mackler and Reist, 21). Neuromuscular junctions were visualized on a Zeiss Axioplan 2 upright digital imaging microscope. Circular dichroism spectroscopy. Circular dichroism (CD) spectra were measured with an AVIV stop-flow circular dichroism spectropolarimeter at nm using a 1 mmpath-length cell. Samples containing.2 mg/ml of either wild-type or mutant C 2 A domains were assayed at 22 C. For corrections of baseline noise, the signal from a blank run of buffer (5 mm sodium phosphate) was subtracted from all the experimental spectra. Isothermal titration calorimetry. Isothermal titration calorimetry (ITC) data were generated using a GE Healthcare MicroCal itc 2, which, in principle, can detect dissociation constants from 1 mm to 1 nm. Wildtype and mutant C 2 A domains were dialyzed overnight in ITC buffer (5 mm HEPES, ph 7.4, 2 mm NaCl, and 1% glycerol). ITC buffer was further used to make calcium chloride stock and protein dilutions. Before each experiment, samples were degassed and cooled to experimental temperature. Heat of binding was measured from thirty 1.1 l injections of calcium chloride into sample cells containing 1.6 mg/ml of proteins of interest at 15 C. Baseline corrections, for heat of dilution, were made by subtracting the signal of calcium chloride injections into buffer from all experimental traces. Data were analyzed using GE Healthcare MicroCal itc 2 Origin data software package. Statistical analyses. A Student s t test was used to determine whether any statistically significant differences existed between the two independent P[syt A-D2E ] lines (line 3 and line 5). One-way ANOVA and Tukey range tests were used for statistical comparisons of P[syt WT ] and the two P[syt A-D2E ] lines. Results Sequence and structural analyses of C 2 A C 2 domains are a common functional motif found in multiple proteins. Although not all C 2 domains are regulated by Ca 2, many C 2 domains mediate a Ca 2 -dependent translocation of the protein to membranes (Nalefski and Falke, 1996; Cho and Stahelin, 26). In these proteins, Ca 2 is coordinated by five negatively charged residues located in loops 1 and 3 of the C 2 domain -sandwich structure (Fig. 1A,B, D1 D5) (Sutton et al., 1995; Nalefski and Falke, 1996; Ubach et al., 1998). Both aspartate and glutamate residues are used in the Ca 2 -binding motifs of diverse C 2 domains (Nalefski and Falke, 1996). To assess whether Ca 2 binding by C 2 A could be supported by either aspartate or
3 Striegel et al. C 2 A: Critical Component of Electrostatic Switch J. Neurosci., January 25, (4): A Dsyt1 221 Asyt1 184 Ma syt1 182 Lsyt1 216 Csnt1 188 Gsyt1 173 Mu syt1 17 Rsyt1 17 Hsyt1 168 Hsyt2 168 Hsyt3 328 Hsyt5 137 Hsyt6 173 Hsyt7 164 Hsyt9 249 Hsyt1 26 Syt C2A D1M D2 D3 D4 R F D B Loop 1 Loop 3 C C2A WT C2A D2E Figure1. TheC 2 Adomainofsynaptotagmin1hasfivehighlyconservedaspartateresiduesthatcoordinateCa 2.A,AlignmentofC 2 AfromCa 2 -bindingsynaptotagminisoforms:synaptotagmin 1 from Drosophila (Dsyt1), bee (Asyt1), Manduca (Ma syt1), squid (Lsyt1), C. elegans (Csnt1), chicken (Gsyt1), mouse (Mu syt1), rat (Rsyt1), and human synaptotagmins (Hsyt) 1 3, 5 7, 9, and 1. Conserved residues are shown in gray and identical residues are in bold. The five conserved aspartate residues that coordinate the binding of Ca 2 ions are boxed and labeled as D1 D5. The conserved residues that mediate Ca 2 -dependent interactions with negatively charged membranes are also indicated by M, R, and F. B, Schematic representation of loops 1 and 3 that form the Ca 2 -binding pocket of the C 2 A domain. Adapted from Fernandez et al. (21) to highlight the aspartates that coordinate Ca 2 (D1 D5) as well as the residues that interact with membranes (M, R, F). C, Molecular model of the C 2 ACa 2 -binding pocket illustrating the potential effect of the C 2 A D2E mutation. Coloring the oxygen atoms of the aspartate residues that coordinate Ca 2 by elementrevealedthenegativelychargedca 2 -bindingsitesonthesolventaccessiblesurface(red).inratsyt1,asp178(left,d2)participatesinthecoordinationofthefirstca 2 (Ca1siteindicated by dotted line, see also B). Using the mutagenesis function in PyMol, we modeled the consequences of altering asp178 to a glutamate (right, D2E). We predicted that the bulging out of the solvent accessible surface (right, enlarged red bulge above white dotted line) could prevent Ca 2 binding to the Ca1 site. glutamate residues, we compared the sequence of C 2 A domains of synaptotagmin isoforms that bind Ca 2. A sequence alignment revealed that glutamate is excluded from these key positions. In the C 2 ACa 2 -binding motifs of synaptotagmin 1 from many species, as well as of all of the human synaptotagmin isoforms that bind Ca 2, all five aspartate residues are 1% conserved (Fig. 1 A), suggesting that glutamate residues in these positions would not provide full function. Examination of the crystal structure of rat syt 1 C 2 A demonstrates that the deepest parts of this Ca 2 -binding pocket are spatially quite restricted (Sutton et al., 1995). Glutamate, like aspartate, is negatively charged, but glutamate possesses a significantly larger molecular volume (91 Å 3 for Asp vs 19 Å 3 for Glu) (Creighton, 1994). Coupled with the exclusion of glutamate from C 2 ACa 2 -binding motifs, this observation suggests that a D3E mutation may impair Ca 2 binding. In the crystal structure of rat syt1c 2 A, both aspartate residue 178 (Fig. 1A,B, D2) and aspartate residue 23 (Fig. 1A,B, D3) are well ordered and located deep in the Ca 2 -binding pocket (Sutton et al., 1995). To assess whether a D3E mutation in either of these locations may occlude the Ca 2 -binding pocket, we modeled the C 2 A D2E and C 2 A D3E mutations in silico. Results suggest that the C 2 A D2E mutation might occlude Ca 2 -binding site 1 (Fig. 1C, Ca1 site). If true, this novel mutation would directly assess the importance of the electrostatic repulsion provided by C 2 A in inhibiting vesicle fusion since it would inhibit Ca 2 binding without reducing the negative charge of the pocket (Fig. 1C, red). The syt A-D2E mutation inhibits Ca 2 binding to C 2 A To directly measure Ca 2 binding to the C 2 A domain of Drosophila synaptotagmin 1, we used ITC. The titration data indicate three Ca 2 -binding sites in Drosophila C 2 A (Fig. 2A, Table 1). The intrinsic Ca 2 affinities of the three sites measured by ITC (K D values were: M, M, and M, respectively; Table 1) were in the same range, though the K D s were somewhat lower than those in previous reports on murine synaptotagmin using NMR (Shao et al., 1996; Ubach et al., 1998). As predicted, Figure 2A demonstrates that the C 2 A D2E mutation inhibited Ca 2 binding by the C 2 A domain. We assessed protein folding by CD spectroscopy to exclude misfolding of the mutant C 2 A domain. As shown in Figure 2B, the C 2 A D2E mutation does not alter the CD spectrum compared with wild-type C 2 A. Thus,
4 1256 J. Neurosci., January 25, (4): Striegel et al. C 2 A: Critical Component of Electrostatic Switch A.1 mcal/sec A WT ] P[syt P[syt A-D2E] 5mv 2ms C2A WT, Ca 2+ 1mv 2ms B Molecular Ellipticity (deg cm 2 / dmol) Wavelength (nm) C2A D2E, Ca Time (min) C2A WT C2A D2E Figure 2. The C 2 A D2E mutation inhibits Ca 2 binding by C 2 A without disrupting protein folding.a,itcanalysisofca 2 bindingtotheisolatedc 2 AdomainofWTandmutantDrosophila synaptotagmin1;arepresentativeca 2 titrationisshown(n 3).C 2 A WT boundthreecalcium ions (Table 1). The heat of binding of Ca 2 by C 2 A D2E was so small that the data could not be accurately fit. B,C 2 A D2E is correctly folded. The CD spectra of the mutant domain (C 2 A D2E ) was identical to wild-type (C 2 A WT ). Table 1. Thermodynamic properties of calcium binding to C 2 A WT using ITC K D ( M) H (cal/mol) S (cal/mol/k) G (kcal/mol) K D H S G K D H S G K D H S G Data represent mean SD, n 3. K D, Dissociation constant;, change in; H, enthalpy; S, entrophy; G, Gibbs free energy. our novel C 2 A mutation is correctly folded and largely inhibits Ca 2 binding by the C 2 A domain. D2 only directly coordinates the first Ca 2 site (Fig. 1B, D2 Ca1). If the C 2 A D2E mutation does in fact remove all Ca 2 binding, this would support a model in which Ca 2 binding to site 1 is requisite for Ca 2 binding to sites 2 and 3. B EJP Amplitude (mv) mejp Amplitude (mv) C.8 D mejp Frequency (Hz) Line 3 Line WT ] P[syt P[syt A-D2E] WT ] Line 3 Line 2 P[syt P[syt A-D2E ] WT ] * * Line 3 Line 2 P[syt P[syt A-D2E ] E of Maximum Release F% EJP Amplitude (mv) G EJP Amplitude (mv) Extracellular [Ca 2+ ] (mm) Extracellular [Ca 2+ ] (mm) Extracellular [Ca 2+ ] (mm) Figure 3. Synchronous evoked release is severely impaired in C 2 ACa 2 -binding mutants, but spontaneous release remains unchanged. A, Representative traces of EJPs and mejps recorded in saline containing 1.5 mm [Ca 2 ]. B, Mean EJP amplitude was markedly decreased in P[syt A-D2E ] mutants compared with P[syt WT ] controls (mean SEM, *p.1, one-way ANOVA). Neither mejp amplitude (C, mean SEM, p.7, one-way ANOVA) nor frequency (D,mean SEM,p.15,one-wayANOVA)variedsignificantlybetweenP[syt A-D2E ]mutants compared with P[syt WT ] controls. E, EJP amplitude versus [Ca 2 ] fit with the Hill equation. F, Ca 2 dose response data normalized to the maximal response in each line to illustrate the decrease in apparent Ca 2 affinity in the P[syt A-D2E ] mutant. G, EJP amplitudes within the nonsaturating range of Ca 2 on a double log plot demonstrate that the Ca 2 cooperativity of releaseisnotchangedinthep[syt A-D2E ]mutants.alinearregressionlinewasusedtodetermine the slope. Error bars are SEM. Black circles indicate P[syt WT ], white squares indicate P[syt A-D2E ]. C 2 ACa 2 -binding mutant inhibits synchronous transmitter release in vivo To determine the importance of electrostatic repulsion by the C 2 A domain for synchronous synaptic transmission at an intact synapse, we examined evoked transmitter release at Drosophila neuromuscular junctions expressing our mutant transgenic synaptotagmin protein (syt A-D2E ) in the absence of any wild-type synaptotagmin 1. To indicate its transgenic origin, we will refer to the mutant as P[syt A-D2E ] and the transgenic controls as P[syt WT ]. We found that the syt A-D2E Ca 2 -binding motif mutation, which maintains the negative charge of the C 2 A pocket, decreased synchronous evoked release by 8% (Fig. 3 A, B). The amplitude of the EJP in P[syt A-D2E ] was mv (line 3, n 11) or mv (line 2, n 8) compared with (n 8) in P[syt WT ] controls [Fig. 3A (line 3 shown), B, p.1]. There was no significant difference between the independent mutant lines (p.7), demonstrating that the decrease in evoked release is not due to the insertion sites of the transgene, but rather is a direct result of the mutation.
5 Striegel et al. C 2 A: Critical Component of Electrostatic Switch J. Neurosci., January 25, (4): Figure 4. Synaptotagmin expression is similar in P[syt A-D2E ] mutants compared with P[syt WT ] controls. A, Representative Western blots of the CNS of third instar larvae probed with anti-synaptotagmin and anti-actin antibodies. B, Synaptotagmin:actin ratio normalized to the mean ratio of the transgenic control, P[syt WT ]. There was no significant difference between genotypes (mean SEM, p.3, one-way ANOVA; P[syt WT ], n 15; P[syt A-D2E ]: line 3, n 6; line 2, n 7). C, Synaptotagmin is properly localized to the larval neuromuscular junction in both mutant and control transgenic synaptotagmin lines. Scale bar, 2 m. constant mejp amplitude demonstrates that synaptic vesicle filling and the postsynaptic response to neurotransmitter are unimpaired. The frequency of mejps in the C 2 A mutants was also not significantly different at Hz (line 3) or Hz (line 2) in P[syt A-D2E ] compared with Hz in P[syt WT ] controls (Fig. 3D, p.15). Since this C 2 ACa 2 -binding motif mutation results in a large decrease in evoked release (Fig. 3A,B), yet does not affect the rate of spontaneous release (Fig. 3 A, D), the increase in spontaneous release seen in other synaptotagmin point mutants (Mackler and Reist, 21; Mackler et al., 22; Paddock et al., 28) cannot be explained as an indirect developmental artifact resulting from the decrease in evoked release. Rather, our findings support the hypothesis that synaptotagmin plays a direct role in regulating the rate of spontaneous release (Broadie et al., 1994; Morimoto et al., 1995; Mace et al., 29) and that the negative charge of the C 2 A Ca 2 -binding pocket plays a key role in this process. Indeed, when Ca 2 binding is inhibited by D3 N mutations of the C 2 ACa 2 -binding pocket, the rate of spontaneous release is increased sixfold (Yoshihara et al., 21). This dramatic difference in the effect on spontaneous fusion frequency between D3N mutations and our D3E mutation demonstrates that the electrostatic repulsion created by the negative charge in the C 2 ACa 2 -binding pocket must be neutralized to enhance any fusion. Since analogous aspartate-to-asparagine mutations in C 2 A, which also inhibit Ca 2 binding but partially neutralize the negative charge of the pocket, did not significantly impair synchronous evoked release at this same synapse (Robinson et al., 22; Yoshihara et al., 21) or at cultured excitatory synapses (Fernández-Chacón et al., 22; Stevens and Sullivan, 23), our findings demonstrate that the key function of Ca 2 binding to the C 2 A domain is to neutralize the negative charge of the C 2 A Ca 2 -binding pocket. Thus, Ca 2 binding to the C 2 B domain is necessary and sufficient for synchronizing synaptic vesicle fusion to Ca 2 influx (Mackler et al., 22; Robinson et al., 22), as seen by the low level of fast, synchronous, evoked release that remains in our P[syt A-D2E ] mutants (Fig. 3A,B). But it is not sufficient to efficiently trigger the electrostatic switch. Efficient, synchronous release requires Ca 2 binding to the C 2 A and C 2 B domains to neutralize both negatively charged Ca 2 -binding pockets and to flip the electrostatic switch resulting in fast, synchronous vesicle fusion. C 2 A mutant does not impact spontaneous transmitter release The C 2 ACa 2 -binding motif mutation had no significant effect on either the amplitude or frequency of spontaneous transmitter release. The amplitude of mejps in P[syt A-D2E ] was.69.3 mv (line 3, n 12) or.68.3 mv (line 2, n 12) compared with.7.2 mv (n 12) in P[syt WT ] (Fig. 3C, p.7). The C 2 A mutants decrease apparent Ca 2 affinity of release To assess whether the decrease in evoked release resulted from changes in either the apparent Ca 2 affinity or cooperativity of release, we measured EJP amplitudes in extracellular Ca 2 concentrations ranging from.6 to 5 mm. P[syt A-D2E ] had a significantly reduced evoked response compared with the control at every extracellular Ca 2 concentration (Fig. 3E). To facilitate comparison, the data were plotted as a percentage of the maximal response (Fig. 3F). The apparent Ca 2 affinity of release in vivo was decreased in the P[syt A-D2E ] mutants; 45% more Ca 2 was required to trigger a half maximal response (EC mm) compared with P[syt WT ] controls (EC mm). By plotting the mean EJP amplitude against the extracellular Ca 2 concentration at nonsaturating levels on a double log plot (Fig. 3G), we found that the Ca 2 cooperativity of release was not affected by the syt A-D2E mutation [n 2.52 for P[syt A-D2E ] and n 2.69 for P[syt WT ], similar to previously reported values at wild-type neuromuscular junctions in Drosophila (Stewart et al., 2; Okamoto et al., 25)]. The shift in the Ca 2 affinity of release with no effect on the cooperativity of release is consistent with the stochastic model of cooperativity (Dodge and Rahamimoff, 1967; Stewart et al., 2; Fernández-Chacón et al., 21; Mackler et al., 22). However, some synaptotagmin mutations alter the cooperativity of release, which would favor the stoicheiometric model (Dodge and Rahamimoff, 1967; Yoshihara
6 1258 J. Neurosci., January 25, (4): Striegel et al. C 2 A: Critical Component of Electrostatic Switch and Littleton, 22; Tamura et al., 27). Further work will be necessary to discriminate between these models. Regardless, the decrease in the apparent Ca 2 affinity of evoked release is consistent with the finding that the syt A-D2E mutation specifically inhibits Ca 2 binding by the C 2 A domain and demonstrates that Ca 2 binding by C 2 A is essential for fast, synchronous synaptic transmission. Transgene expression and distribution are unaffected Since a decrease in synchronous evoked release could also result from protein misexpression, we assessed transgenic synaptotagmin expression levels in each transgenic line. Western blot analysis of third instar larval CNSs with an anti-synaptotagmin antibody demonstrated that the C 2 ACa 2 -binding motif mutant lines expressed similar levels of transgenic synaptotagmin as the transgenic control (Fig. 4 A, B). In addition, the mutant synaptotagmin was appropriately localized to the neuromuscular junction (Fig. 4C). Therefore, the deficits in evoked release are not due to insufficient protein expression or protein mislocalization. Discussion Our results now clearly demonstrate that Ca 2 binding by the C 2 A domain is a key functional component of the electrostatic switch that triggers synchronous synaptic vesicle fusion. A comparison of multiple C 2 ACa 2 -binding mutants reveals that the key function of Ca 2 binding by C 2 A is to neutralize the negative charge of this pocket. Our D3E mutant inhibits Ca 2 binding but maintains the negative charge of the C 2 A pocket. D3Nmutants also inhibit Ca 2 binding, but they decrease the negative charge of the C 2 A pocket. Neutralization of the C 2 A pocket results in a dramatic increase in the fusion-stimulating activity of synaptotagmin. Our C 2 AD3E mutant inhibits Ca 2 binding but maintains the negative charge of the C 2 A pocket. With the repulsive force of the C 2 A domain intact, both synchronous release and the apparent Ca 2 affinity of release were decreased. In C 2 AD3N mutants, synchronous transmitter release proceeds efficiently with Ca 2 binding to C 2 B, providing the synchronization to Ca 2 influx (Fernández-Chacón et al., 22; Robinson et al., 22; Yoshihara et al., 21). Indeed, C 2 AD3N mutants that include D4N enhanced synchronous fusion as shown by an increase in the apparent Ca 2 affinity of release (Stevens and Sullivan, 23; Pang et al., 26; Yoshihara et al., 21). The major difference between these mutants is the charge of the Ca 2 -binding pocket. Thus, the electrostatic repulsion provided by the C 2 A domain must be neutralized, by either Ca 2 binding or mutation, to activate synaptotagmin s fusion-stimulating function during synchronous transmitter release. Interestingly, one multiple C 2 A mutant (C2A D2,3,4A ) that both inhibits Ca 2 binding and neutralizes the negative charge of the pocket decreased synchronous release by 3% at cultured inhibitory synapses (Shin et al., 29). Yet similar multiple C 2 AD3N mutations (even the C2A D1-5N mutation) do not decrease synchronous release (Stevens and Sullivan, 23). The effect of these mutations on spontaneous release was not reported. This differential effect may indicate that neutral asparagine (vs alanine) residues more closely mimic Ca 2 -bound aspartates. Regardless of the source of these differences, the finding that our C 2 AD3E mutation inhibits synchronous release by 8% demonstrates that the major mechanism used by C 2 A to inhibit synaptotagmin s fusogenic activity is electrostatic repulsion. The effect of mutations on spontaneous vesicle fusion events demonstrates that the negative charge of the C 2 A pocket acts as a clamp to inhibit an inherent fusion-stimulating activity of synaptotagmin. Our C 2 AD3E mutation maintains the ability of synaptotagmin to suppress spontaneous release, while C 2 AD3N mutations result in a massive increase in spontaneous vesicle fusion events (Yoshihara et al., 21). Thus, regardless of the downstream effector interaction(s) that mediate the fusion reaction, the negative charge of the C 2 ACa 2 -binding pocket functionally inhibits synaptic vesicle fusion until neutralized. D3N mutations in both C 2 A and C 2 B increase the rate of spontaneous transmitter release (Mackler et al., 22; Yoshihara et al., 21), in effect removing the need for Ca 2 to unleash the fusion-stimulating activity of synaptotagmin. So why do these mutations impact synchronous release so differently? C 2 BD3N mutants nearly completely block synchronous release (Mackler et al., 22), while C 2 AD3N mutants do not (Fernández- Chacón et al., 22; Robinson et al., 22; Yoshihara et al., 21). Here we propose a possible mechanism (Fig. 5) that may contribute to this differential action, although additional interactions must also be involved, as noted below. C 2 B, by virtue of its Ca 2 -independent priming interaction with the SNARE complex (Rickman et al., 24; Loewen et al., 26b) and being the vesicle distal C 2 domain, would be located immediately adjacent to the presynaptic membrane, while C 2 Ais likely further removed. Before Ca 2 entry, electrostatic repulsion prevents interactions between the C 2 domains and the negatively charged presynaptic membrane (Fig. 5A,B, minus signs). Ca 2 influx initiates the electrostatic switch: an immediate change from electrostatic repulsion (due to the negatively charged residues) to electrostatic attraction of the negatively charged presynaptic membrane (due to the bound Ca 2 and the conserved, positively charged residue; Fig. 5 B, C, blue sticks, plus signs), which pulls the vesicle toward the presynaptic membrane. Hydrophobic residues on the tip of the C 2 domain (Fig. 5B,C, gray sticks) can then penetrate the presynaptic membrane, destabilizing it and promoting the fusion reaction by pulling the presynaptic membrane toward the vesicle in a ring around the site of SNARE-mediated fusion (Fig. 5D). Since C 2 B is located immediately adjacent to the presynaptic membrane, it would attract and penetrate the membrane first (Bai et al., 22; Wang et al., 23; Herrick et al., 26; Fuson et al., 27; Martens et al., 27; Paddock et al., 28, 211; Hui et al., 29). This action may then pivot the vesicle proximal C 2 A domain toward the membrane where it can also then participate in the electrostatic attraction and hydrophobic penetration activities (Fernández-Chacón et al., 21; Bai et al., 22; Wang et al., 23; Herrick et al., 26; Paddock et al., 28, 211). When interactions of the C 2 B domain with the presynaptic membrane are prevented by mutation, the C 2 A domain would not be pivoted into position for interactions with the membrane and synaptic transmission would be blocked (Mackler et al., 22; Paddock et al., 211). When Ca 2 binding by the C 2 A domain is inhibited via D3N mutations, the decreased negative charge of the pocket may partially mimic Ca 2 binding (Stevens and Sullivan, 23), permitting the remaining C 2 A lipid-interacting residues to bind and penetrate the presynaptic membrane when C 2 B pivots the C 2 A domain into position. Thus, these C 2 A mutations would result in little to no disruption in evoked release (Fernández-Chacón et al., 22; Robinson et al., 22; Stevens and Sullivan, 23; Pang et al., 26). On the other hand, removal of the C 2 A positively charged residue (Fig. 5B,C, C 2 A, blue plus sign) or the hydrophobic residues (Fig. 5B,C,C 2 A, gray sticks) by preventing C 2 A from participating in effector interactions with the presynaptic membrane would result in a more
7 Striegel et al. C 2 A: Critical Component of Electrostatic Switch J. Neurosci., January 25, (4): Figure 5. Ca 2 binding by C 2 A is an essential component of the electrostatic switch. The crystal structure of the core complex [PDB file 1SFC, containing syntaxin (red), SNAP-25 (green), and VAMP/synaptobrevin(blue)],theNMRstructuresoftheC 2 A(PDBfile1BYN)andC 2 B(PDBfile1K5W)domainsofsynaptotagmin(yellow),andCa 2 (greencircleswithplussigns)areshowntoscale usingpymol.themembranes,thetransmembranedomains,andthelinkbetweenc 2 AandC 2 BwereaddedinAdobePhotoshop.A,Cross-sectionofadockedvesicleshowingtwoSNAREcomplexes and their associated synaptotagmin molecules (syt). SC, Synaptic vesicle; PM, plasma membrane. B, One synaptotagmin/snare complex viewed from the site of vesicle/presynaptic membrane apposition. A Ca 2 -independent docking/priming interaction between the C 2 B polylysine motif (yellow, space-filled residues) and SNAP-25 (green, space-filled residues) (Rickman et al., 24; Loewen et al., 26b) holds the C 2 BCa 2 -binding site immediately adjacent to the presynaptic membrane with the C 2 ACa 2 -binding site further removed. In the absence of Ca 2, the conserved aspartateresidues(redresidues:syt C2A-D2,space-filled;therestassticks)withinthepocketscreateahighconcentrationofnegativecharge(clusterofminussigns),resultinginelectrostaticrepulsion of the presynaptic membrane that prevents any membrane interactions by the tips of the C 2 domains. C, Upon Ca 2 binding, the electrostatic repulsion of the pockets is neutralized, thereby initiating the electrostatic switch: a strong attraction of the negatively charged membrane by the bound Ca 2 (green circles with plus signs) and the basic residues at the tips of Ca 2 -binding pockets (blue stick residues, blue plus signs). Insertion of the hydrophobic residues (gray stick residues) at the tips of the C 2 domains into the core of the presynaptic membrane then triggers fusion bypromotingalocalca 2 -dependentpositivecurvatureoftheplasmamembrane(martensetal.,27;huietal.,29;paddocketal.,211).thec 2 domaininteractionswiththemembranelikely pull the synaptic vesicle (upper gray membrane) toward the presynaptic membrane (lower gray membrane). The Ca 2 -induced increase in positive charge at the end of the C 2 B domain also likely increases the strength of the electrostatic interaction between the C 2 B polylysine motif and the SNARE complex, resulting in simultaneous binding of the SNARE complex and the presynaptic membrane (Davis et al., 1999; Bhalla et al., 26; Loewen et al., 26a; Dai et al., 27). D, Membrane penetration by multiple synaptotagmins (large gray ovals, arrows) would pull the plasma membrane toward the vesicle in a ring around the SNARE transmembrane domains (small gray circles, arrowheads) facilitating fusion. severe disruption in evoked release (Fernández-Chacón et al., 21; Paddock et al., 28, 211) than the D3N mutations. Studies examining biochemical interactions between C 2 Adomains and negatively charged phospholipids in vitro may not fully reflect interactions in vivo due to the necessarily simplified environment of the in vitro assays. For instance, the positive charge of the Ca 2 bound to C 2 A helps attract and bind negatively charged liposomes in vitro. D3 N mutations in isolated C 2 A domains inhibit this lipid binding despite charge neutralization (Fernández-Chacón et al., 22; Robinson et al., 22). But only one or two of the five negatively charged aspartates are mutated in the D3 N mutations tested. Thus, this partial charge neutralization may be insufficient to actively attract negatively charged liposomes in vitro. Yet in vivo, active attraction by bound Ca 2 may not be necessary to permit near normal membrane interactions mediated by the remaining arginine and hydrophobic residues due to the coordinated action of the SNAREassociated C 2 B domain to pivot the C 2 A pocket onto the presynaptic membrane. Additional interactions not modeled above are undoubtedly also involved. By inhibiting Ca 2 binding yet maintaining the negative charge of the pocket, the syt A-D2E mutation would interrupt all Ca 2 -dependent interactions mediated by C 2 A; the effect would not be limited to the membrane interactions discussed in our model above. Indeed, since the syt A-D2E mutation results in an 8% decrease of synchronous release while the C 2 A positively charged or hydrophobic mutations inhibit only 5% (Fernández- Chacón et al., 21; Paddock et al., 28, 211), the impact of Ca 2 binding to C 2 A clearly influences more than just these interactions with the membrane. Thus, C 2 A likely participates in additional electrostatic interactions upon Ca 2 binding that play a role in triggering synchronous release, perhaps with SNARE complexes or other effector molecules. Regardless of which C 2 A interactions trigger fusion, the finding that C 2 AD3N mutations support efficient synaptic transmission while our C 2 AD3Emutation inhibits transmission by 8% demonstrates the central importance of the change in electrostatic potential of C 2 A for triggering fusion. In summary, our current findings show severe disruption of synchronous synaptic transmission in vivo caused by inhibiting Ca 2 -binding by the C 2 A domain without removal of the negative charge of the pocket. These results demonstrate that this negative charge in C 2 A is a critical component of the electrostatic inhibition that prevents synaptic vesicle fusion. Thus, the essential function of Ca 2 binding to the C 2 A domain of synaptotagmin is to neutralize this charge and, along with C 2 B, to initiate the electrostatic switch mechanism that triggers vesicle fusion. References Bai J, Wang P, Chapman ER (22) C2A activates a cryptic Ca 2 -triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc Natl Acad Sci U S A 99: Bhalla A, Chicka MC, Tucker WC, Chapman ER (26) Ca(2 )-synaptotagmin directly regulates t-snare function during reconstituted membrane fusion. Nat Struct Mol Biol 13: Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: Broadie K, Bellen HJ, DiAntonio A, Littleton JT, Schwarz TL (1994) Absence of synaptotagmin disrupts excitation-secretion coupling during synaptic transmission. Proc Natl Acad Sci U S A 91:
8 126 J. Neurosci., January 25, (4): Striegel et al. C 2 A: Critical Component of Electrostatic Switch Brose N, Petrenko AG, Südhof TC, Jahn R (1992) Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256: Chae YK, Abildgaard F, Chapman ER, Markley JL (1998) Lipid binding ridge on loops 2 and 3 of the C2A domain of synaptotagmin I as revealed by NMR spectroscopy. J Biol Chem 273: Chapman ER, Davis AF (1998) Direct interaction of a Ca2 -binding loop of synaptotagmin with lipid bilayers. J Biol Chem 273: Chapman ER, Hanson PI, An S, Jahn R (1995) Ca2 regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem 27: Cho W, Stahelin RV (26) Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta 1761: Creighton TE (1994) Proteins: structure and molecular properties. New York: W. H. Freeman and Co. Dai H, Shen N, Araç D, Rizo J (27) A quaternary SNARE-synaptotagmin- Ca2 -phospholipid complex in neurotransmitter release. J Mol Biol 367: Davis AF, Bai J, Fasshauer D, Wolowick MJ, Lewis JL, Chapman ER (1999) Kinetics of synaptotagmin responses to Ca2 and assembly with the core SNARE complex onto membranes. Neuron 24: Davletov B, Perisic O, Williams RL (1998) Calcium-dependent membrane penetration is a hallmark of the C2 domain of cytosolic phospholipase A2 whereas the C2A domain of synaptotagmin binds membranes electrostatically. J Biol Chem 273: DiAntonio A, Parfitt KD, Schwarz TL (1993) Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell 73: Dodge FA Jr, Rahamimoff R (1967) Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol 193: Elferink LA, Peterson MR, Scheller RH (1993) A role for synaptotagmin (p65) in regulated exocytosis. Cell 72: Fernandez I, Araç D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Südhof TC, Rizo J (21) Three-dimensional structure of the synaptotagmin 1 C 2 B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32: Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC (21) Synaptotagmin I functions as a calcium regulator of release probability. Nature 41: Fernández-Chacón R, Shin OH, Königstorfer A, Matos MF, Meyer AC, Garcia J, Gerber SH, Rizo J, Südhof TC, Rosenmund C (22) Structure/ function analysis of Ca2 binding to the C2A domain of synaptotagmin I. J Neurosci 22: Fuson KL, Montes M, Robert JJ, Sutton RB (27) Structure of human synaptotagmin 1 C2AB in the absence of Ca2 reveals a novel domain association. Biochemistry 46: Herrick DZ, Sterbling S, Rasch KA, Hinderliter A, Cafiso DS (26) Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry 45: Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER (29) Synaptotagminmediated bending of the target membrane is a critical step in Ca(2 )- regulated fusion. Cell 138: Loewen CA, Mackler JM, Reist NE (21) Drosophila synaptotagmin I null mutants survive to early adulthood. Genesis 31:3 36. Loewen CA, Royer SM, Reist NE (26a) Drosophila synaptotagmin I null mutants show severe alterations in vesicle populations but calciumbinding motif mutants do not. J Comp Neurol 496:1 12. Loewen CA, Lee SM, Shin YK, Reist NE (26b) C2B polylysine motif of synaptotagmin facilitates a Ca2 -independent stage of synaptic vesicle priming in vivo. Molec Biol Cell 17: Mace KE, Biela LM, Sares AG, Reist NE (29) Synaptotagmin I stabilizes synaptic vesicles via its C2A polylysine motif. Genesis 47: Mackler JM, Reist NE (21) Mutations in the second C 2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J Comp Neurol 436:4 16. Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE (22) The C(2)B Ca(2 )-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418: Martens S, Kozlov MM, McMahon HT (27) How synaptotagmin promotes membrane fusion. Science 316: Morimoto T, Popov S, Buckley KM, Poo MM (1995) Calcium-dependent transmitter secretion from fibroblasts: modulation by synaptotagmin I. Neuron 15: Murray D, Honig B (22) Electrostatic control of the membrane targeting of C2 domains. Mol Cell 9: Nalefski EA, Falke JJ (1996) The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci 5: Okamoto T, Tamura T, Suzuki K, Kidokoro Y (25) External Ca2 dependency of synaptic transmission in drosophila synaptotagmin I mutants. J Neurophysiol 94: Paddock BE, Striegel AR, Hui E, Chapman ER, Reist NE (28) Ca2 dependent, phospholipid-binding residues of synaptotagmin are critical for excitation-secretion coupling in vivo. J Neurosci 28: Paddock BE, Wang Z, Biela LM, Chen K, Getzy MD, Striegel A, Richmond JE, Chapman ER, Featherstone DE, Reist NE (211) Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo. J Neurosci 31: Pang ZP, Shin OH, Meyer AC, Rosenmund C, Südhof TC (26) A gain-offunction mutation in synaptotagmin-1 reveals a critical role of Ca2 dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J Neurosci 26: Reist NE, Buchanan J, Li J, DiAntonio A, Buxton EM, Schwarz TL (1998) Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J Neurosci 18: Rickman C, Archer DA, Meunier FA, Craxton M, Fukuda M, Burgoyne RD, Davletov B (24) Synaptotagmin interaction with the syntaxin/ SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J Biol Chem 279: Robinson IM, Ranjan R, Schwarz TL (22) Synaptotagmins I and IV promote transmitter release independently of Ca(2 ) binding in the C(2)A domain. Nature 418: Schiavo G, Stenbeck G, Rothman JE, Söllner TH (1997) Binding of the synaptic vesicle v-snare, synaptotagmin, to the plasma membrane t-snare, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses [see comments]. Proc Natl Acad Sci U S A 94: Shao X, Davletov BA, Sutton RB, Südhof TC, Rizo J (1996) Bipartite Ca 2 - binding motif in C 2 domains of synaptotagmin and protein kinase C. Science 273: Shin OH, Xu J, Rizo J, Südhof TC (29) Differential but convergent functions of Ca2 binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci U S A 16: Stevens CF, Sullivan JM (23) The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron 39: Stewart BA, Mohtashami M, Trimble WS, Boulianne GL (2) SNARE proteins contribute to calcium cooperativity of synaptic transmission. Proc Natl Acad Sci U S A 97: Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR (1995) Structure of the first C2 domain of synaptotagmin I: a novel Ca2 / phospholipid-binding fold. Cell 8: Tamura T, Hou J, Reist NE, Kidokoro Y (27) Nerve-evoked synchronous release and high K -induced quantal events are regulated separately by synaptotagmin I at Drosophila neuromuscular junctions. J Neurophysiol 97: Ubach J, Zhang X, Shao X, Südhof TC, Rizo J (1998) Ca2 binding to synaptotagmin: how many Ca2 ions bind to the tip of a C2-domain? EMBO J 17: Wang P, Wang CT, Bai J, Jackson MB, Chapman ER (23) Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J Biol Chem 278: Yao KM, White K (1994) Neural specificity of elav expression: defining a Drosophila promoter for directing expression to the nervous system. J Neurochem 63: Yoshihara M, Littleton JT (22) Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron 36: Yoshihara M, Guan Z, Littleton JT (21) Differential regulation of synchronous versus asynchronous neurotransmitter release by the C2 domains of synaptotagmin 1. Proc Natl Acad Sci U S A 17: Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF (22) Ca2 -dependent synaptotagmin binding to SNAP-25 is essential for Ca2 -triggered exocytosis. Neuron 34:
Synaptotagmin 2 Mutations Cause Autosomal-Dominant Form of Lambert-Eaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy
 The American Journal of Human Genetics, Volume 95 Supplemental Data Synaptotagmin 2 Mutations Cause AutosomalDominant Form of LambertEaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy David
The American Journal of Human Genetics, Volume 95 Supplemental Data Synaptotagmin 2 Mutations Cause AutosomalDominant Form of LambertEaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy David
Synaptic Vesicle Cycle
 Synaptic Vesicle Cycle proteins SNAREs SNARE complex formation calcium-regulated fusion maintenance lipids (peptidergic vesicles) vesicle budding and fusion specificity? regulation by calcium? --usually
Synaptic Vesicle Cycle proteins SNAREs SNARE complex formation calcium-regulated fusion maintenance lipids (peptidergic vesicles) vesicle budding and fusion specificity? regulation by calcium? --usually
DISCOVERIES OF MACHINERY REGULATING VESICLE TRAFFIC, A MAJOR TRANSPORT SYSTEM IN OUR CELLS. Scientific Background on the Nobel Prize in Medicine 2013
 DISCOVERIES OF MACHINERY REGULATING VESICLE TRAFFIC, A MAJOR TRANSPORT SYSTEM IN OUR CELLS Scientific Background on the Nobel Prize in Medicine 2013 Daniela Scalet 6/12/2013 The Nobel Prize in Medicine
DISCOVERIES OF MACHINERY REGULATING VESICLE TRAFFIC, A MAJOR TRANSPORT SYSTEM IN OUR CELLS Scientific Background on the Nobel Prize in Medicine 2013 Daniela Scalet 6/12/2013 The Nobel Prize in Medicine
Neural Circuits Underlie Brain Function
 Neural Circuits Underlie Brain Function interneuron interneuron pyramidal neurons Neural Circuits Underlie Brain Function interneuron interneuron pyramidal neurons Synapses: the basic computational units
Neural Circuits Underlie Brain Function interneuron interneuron pyramidal neurons Neural Circuits Underlie Brain Function interneuron interneuron pyramidal neurons Synapses: the basic computational units
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Different crystal forms obtained for Sky
 Supplementary Figure 1 Different crystal forms obtained for Sky 1 353. (a) Crystal form 1 obtained in the presence of 20% PEG 3350 and 0.2 M ammonium citrate tribasic ph 7.0. (b) Crystal form 1 of the
Supplementary Figure 1 Different crystal forms obtained for Sky 1 353. (a) Crystal form 1 obtained in the presence of 20% PEG 3350 and 0.2 M ammonium citrate tribasic ph 7.0. (b) Crystal form 1 of the
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
R7.3 Receptor Kinetics
 Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Synaptic vesicle fusion is driven by assembly. Complexin Controls the Force Transfer from SNARE Complexes to Membranes in Fusion REPORTS
 REPORTS roles are also synergistic because the binding of CPX by its central helix strategically positions the accessory helix for clamping. The central helix binds to residues in both the v- and t-snare
REPORTS roles are also synergistic because the binding of CPX by its central helix strategically positions the accessory helix for clamping. The central helix binds to residues in both the v- and t-snare
Overexpression of Cysteine-String Proteins in Drosophila Reveals Interactions with Syntaxin
 The Journal of Neuroscience, December 1, 1999, 19(23):10270 10279 Overexpression of Cysteine-String Proteins in Drosophila Reveals Interactions with Syntaxin Zhiping Nie, Ravi Ranjan, Julia J. Wenniger,
The Journal of Neuroscience, December 1, 1999, 19(23):10270 10279 Overexpression of Cysteine-String Proteins in Drosophila Reveals Interactions with Syntaxin Zhiping Nie, Ravi Ranjan, Julia J. Wenniger,
SNT-1 functions as the Ca 2+ sensor for tonic and evoked neurotransmitter release in C. elegans
 This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. A link to any extended data will be provided when the final version is posted online. Research
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. A link to any extended data will be provided when the final version is posted online. Research
Supplementary Figure 1 Structure of the Orai channel. (a) The hexameric Drosophila Orai channel structure derived from crystallography 1 comprises
 Supplementary Figure 1 Structure of the Orai channel. (a) The hexameric Drosophila Orai channel structure derived from crystallography 1 comprises six Orai subunits, each with identical amino acid sequences
Supplementary Figure 1 Structure of the Orai channel. (a) The hexameric Drosophila Orai channel structure derived from crystallography 1 comprises six Orai subunits, each with identical amino acid sequences
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
!"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%%
 !"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%% !"#$%&'(")*++*%,*'-&'./%/,*#01#%-2)#3&)/% 4'(")*++*% % %5"0)%-2)#3&) %%% %67'2#72'*%%%%%%%%%%%%%%%%%%%%%%%4'(")0/./% % 8$+&'&,+"/7 % %,$&7&/9)7$*/0/%%%%%%%%%%
!"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%% !"#$%&'(")*++*%,*'-&'./%/,*#01#%-2)#3&)/% 4'(")*++*% % %5"0)%-2)#3&) %%% %67'2#72'*%%%%%%%%%%%%%%%%%%%%%%%4'(")0/./% % 8$+&'&,+"/7 % %,$&7&/9)7$*/0/%%%%%%%%%%
Synapses. Electrophysiology and Vesicle release
 Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
How Does Synaptotagmin Trigger Neurotransmitter Release?
 ANNUAL REVIEWS Further Click here for quick links to Annual Reviews content online, including: Other articles in this volume Top cited articles Top downloaded articles rehensive search Annu. Rev. Biochem.
ANNUAL REVIEWS Further Click here for quick links to Annual Reviews content online, including: Other articles in this volume Top cited articles Top downloaded articles rehensive search Annu. Rev. Biochem.
Regulation of Membrane Fusion in Synaptic Excitation-Secretion Coupling: Speed and Accuracy Matter
 Regulation of Membrane Fusion in Synaptic Excitation-Secretion Coupling: Speed and Accuracy Matter Sonja M. Wojcik 1, * and Nils Brose 1 1 Max-Planck-Institut für Experimentelle Medizin, Abteilung Molekulare
Regulation of Membrane Fusion in Synaptic Excitation-Secretion Coupling: Speed and Accuracy Matter Sonja M. Wojcik 1, * and Nils Brose 1 1 Max-Planck-Institut für Experimentelle Medizin, Abteilung Molekulare
Open Syntaxin Docks Synaptic Vesicles
 Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Neuroscience 201A Exam Key, October 7, 2014
 Neuroscience 201A Exam Key, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mv. If the net negativity on the inside of the cell (all
Neuroscience 201A Exam Key, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mv. If the net negativity on the inside of the cell (all
Building a Homology Model of the Transmembrane Domain of the Human Glycine α-1 Receptor
 Building a Homology Model of the Transmembrane Domain of the Human Glycine α-1 Receptor Presented by Stephanie Lee Research Mentor: Dr. Rob Coalson Glycine Alpha 1 Receptor (GlyRa1) Member of the superfamily
Building a Homology Model of the Transmembrane Domain of the Human Glycine α-1 Receptor Presented by Stephanie Lee Research Mentor: Dr. Rob Coalson Glycine Alpha 1 Receptor (GlyRa1) Member of the superfamily
A Genetic Analysis of Complexin Function in Neurotransmitter Release and Synaptic Plasticity
 A Genetic Analysis of Complexin Function in Neurotransmitter Release and Synaptic Plasticity by Sarah Huntwork-Rodriguez B.S. Biological Sciences Stanford University 2003 SUBMITTED TO THE DEPARTMENT OF
A Genetic Analysis of Complexin Function in Neurotransmitter Release and Synaptic Plasticity by Sarah Huntwork-Rodriguez B.S. Biological Sciences Stanford University 2003 SUBMITTED TO THE DEPARTMENT OF
An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding
 NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
by neuronal specific modifications to an evolutionarily conserved vesicle trafficking system. manner (8, 9). In addition, synaptotagmin has been
 Proc. Natd. Acad. Sci. USA Vol. 91, pp. 10888-10892, November 1994 Neurobiology Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin
Proc. Natd. Acad. Sci. USA Vol. 91, pp. 10888-10892, November 1994 Neurobiology Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Table 1. Kinetic data obtained from SPR analysis of domain 11 mutants interacting with IGF-II. Kinetic parameters K D 1.
 Kinetics and Thermodynamics of the Insulin-like Growth Factor II (IGF-II) Interaction with IGF-II/Mannose 6-phosphate Receptor and the function of CD and AB Loop Solvent-exposed Residues. Research Team:
Kinetics and Thermodynamics of the Insulin-like Growth Factor II (IGF-II) Interaction with IGF-II/Mannose 6-phosphate Receptor and the function of CD and AB Loop Solvent-exposed Residues. Research Team:
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
MEMBRANE STRUCTURE. Lecture 9. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
A C1-C2 Module in Munc13 Inhibits Calcium- Dependent Neurotransmitter Release
 Article A C1-C2 Module in Munc13 Inhibits Calcium- Dependent Neurotransmitter Release Highlights d The C1-C2B module of Munc13 inhibits calcium-triggered fusion of primed vesicles Authors Francesco Michelassi,
Article A C1-C2 Module in Munc13 Inhibits Calcium- Dependent Neurotransmitter Release Highlights d The C1-C2B module of Munc13 inhibits calcium-triggered fusion of primed vesicles Authors Francesco Michelassi,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
doi:10.1038/nature17991 Supplementary Discussion Structural comparison with E. coli EmrE The DMT superfamily includes a wide variety of transporters with 4-10 TM segments 1. Since the subfamilies of the
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Nature Methods: doi: /nmeth Supplementary Figure 1. In vitro screening of recombinant R-CaMP2 variants.
 Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
SUPPLEMENTARY FIGURES Supplementary Figure 1 Protein sequence alignment of Vibrionaceae with either a 40-residue insertion or a 44-residue insertion. Identical residues are indicated by red background.
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Structural/functional analysis of synaptotagmin 1 in synaptic transmission using hippocampal autapses
 Structural/functional analysis of synaptotagmin 1 in synaptic transmission using hippocampal autapses PhD Thesis In partial fulfillment of the requirements for the degree Doctor of Philosophy (PhD) in
Structural/functional analysis of synaptotagmin 1 in synaptic transmission using hippocampal autapses PhD Thesis In partial fulfillment of the requirements for the degree Doctor of Philosophy (PhD) in
A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and. Disfavors Mn Binding.
 Supporting information for A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and Disfavors Mn Binding. Anne-Frances Miller* and Ting Wang Department of Chemistry, University
Supporting information for A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and Disfavors Mn Binding. Anne-Frances Miller* and Ting Wang Department of Chemistry, University
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Membrane Potentials, Action Potentials, and Synaptic Transmission. Membrane Potential
 Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Munc13 C 2 B domain is an activity-dependent Ca 2+ regulator of synaptic exocytosis
 Munc C B domain is an activity-dependent Ca regulator of synaptic exocytosis Ok-Ho Shin,,, Jun Lu,,, Jeong-Seop Rhee,4,,, Diana R Tomchick, Zhiping P Pang,5, Sonja M Wojcik 6, Marcial Camacho-Perez 4,7,
Munc C B domain is an activity-dependent Ca regulator of synaptic exocytosis Ok-Ho Shin,,, Jun Lu,,, Jeong-Seop Rhee,4,,, Diana R Tomchick, Zhiping P Pang,5, Sonja M Wojcik 6, Marcial Camacho-Perez 4,7,
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Analysis of nucleotide binding to p97 reveals the properties of a tandem AAA hexameric ATPase
 SUPPLEMENTARY INFORMATION Analysis of nucleotide binding to p97 reveals the properties of a tandem AAA hexameric ATPase Louise C Briggs, Geoff S Baldwin, Non Miyata, Hisao Kondo, Xiaodong Zhang, Paul S
SUPPLEMENTARY INFORMATION Analysis of nucleotide binding to p97 reveals the properties of a tandem AAA hexameric ATPase Louise C Briggs, Geoff S Baldwin, Non Miyata, Hisao Kondo, Xiaodong Zhang, Paul S
Nerve Signal Conduction. Resting Potential Action Potential Conduction of Action Potentials
 Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nobel Lecture. The Principle of Membrane Fusion in the Cell. James E. Rothman Yale University. Karolinska Institutet Stockholm December 7, 2013
 Nobel Lecture James E. Rothman Yale University The Principle of Membrane Fusion in the Cell Karolinska Institutet Stockholm December 7, 2013 Stockholm 10:32 am October 7, 2013 Dr. Göran Hansson Manhattan
Nobel Lecture James E. Rothman Yale University The Principle of Membrane Fusion in the Cell Karolinska Institutet Stockholm December 7, 2013 Stockholm 10:32 am October 7, 2013 Dr. Göran Hansson Manhattan
Membrane Protein Channels
 Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
Presentation Microcalorimetry for Life Science Research
 Presentation Microcalorimetry for Life Science Research MicroCalorimetry The Universal Detector Heat is either generated or absorbed in every chemical process Capable of thermal measurements over a wide
Presentation Microcalorimetry for Life Science Research MicroCalorimetry The Universal Detector Heat is either generated or absorbed in every chemical process Capable of thermal measurements over a wide
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Synaptotagmin-1 Docks Secretory Vesicles to Syntaxin-1/SNAP-25 Acceptor Complexes
 Synaptotagmin-1 Docks Secretory Vesicles to Syntaxin-1/SNAP-25 Acceptor Complexes Heidi de Wit, 1 Alexander M. Walter, 2 Ira Milosevic, 2,5 Attila Gulyás-Kovács, 2,6 Dietmar Riedel, 3 Jakob B. Sørensen,
Synaptotagmin-1 Docks Secretory Vesicles to Syntaxin-1/SNAP-25 Acceptor Complexes Heidi de Wit, 1 Alexander M. Walter, 2 Ira Milosevic, 2,5 Attila Gulyás-Kovács, 2,6 Dietmar Riedel, 3 Jakob B. Sørensen,
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Introduction: actin and myosin
 Introduction: actin and myosin Actin Myosin Myosin V and actin 375 residues Found in all eukaryotes Polymeric Forms track for myosin Many other cellular functions 36 nm pseudo-helical repeat Catalytic
Introduction: actin and myosin Actin Myosin Myosin V and actin 375 residues Found in all eukaryotes Polymeric Forms track for myosin Many other cellular functions 36 nm pseudo-helical repeat Catalytic
7.06 Spring 2004 PS 6 KEY 1 of 14
 7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
b) What is the gradient at room temperature? Du = J/molK * 298 K * ln (1/1000) = kj/mol
 Chem350 Practice Problems Membranes 1. a) What is the chemical potential generated by the movement of glucose by passive diffusion established by a 1000 fold concentration gradient at physiological temperature?
Chem350 Practice Problems Membranes 1. a) What is the chemical potential generated by the movement of glucose by passive diffusion established by a 1000 fold concentration gradient at physiological temperature?
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Insights into the mechanism of botulinum neurotoxin (BoNT) receptor binding and substrate cleavage from a structural perspective
 Insights into the mechanism of botulinum neurotoxin (BoNT) receptor binding and substrate cleavage from a structural perspective Axel Brunger Stanford University Conference on New Frontiers in Microbiology
Insights into the mechanism of botulinum neurotoxin (BoNT) receptor binding and substrate cleavage from a structural perspective Axel Brunger Stanford University Conference on New Frontiers in Microbiology
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
SUPPLEMENTARY INFORMATION doi:10.1038/nature11524 Supplementary discussion Functional analysis of the sugar porter family (SP) signature motifs. As seen in Fig. 5c, single point mutation of the conserved
Protein folding. Today s Outline
 Protein folding Today s Outline Review of previous sessions Thermodynamics of folding and unfolding Determinants of folding Techniques for measuring folding The folding process The folding problem: Prediction
Protein folding Today s Outline Review of previous sessions Thermodynamics of folding and unfolding Determinants of folding Techniques for measuring folding The folding process The folding problem: Prediction
Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization
 Neuron, Vol. 30, 737 749, June, 2001, Copyright 2001 by Cell Press Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization Suzanne Paradis, Sean T. Sweeney, and
Neuron, Vol. 30, 737 749, June, 2001, Copyright 2001 by Cell Press Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization Suzanne Paradis, Sean T. Sweeney, and
Microcalorimetry for the Life Sciences
 Microcalorimetry for the Life Sciences Why Microcalorimetry? Microcalorimetry is universal detector Heat is generated or absorbed in every chemical process In-solution No molecular weight limitations Label-free
Microcalorimetry for the Life Sciences Why Microcalorimetry? Microcalorimetry is universal detector Heat is generated or absorbed in every chemical process In-solution No molecular weight limitations Label-free
Organization of the nervous system. Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2
 Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
Application of density estimation methods to quantal analysis
 Application of density estimation methods to quantal analysis Koichi Yoshioka Tokyo Medical and Dental University Summary There has been controversy for the quantal nature of neurotransmission of mammalian
Application of density estimation methods to quantal analysis Koichi Yoshioka Tokyo Medical and Dental University Summary There has been controversy for the quantal nature of neurotransmission of mammalian
(Received 15 June 1973)
 J. Phyeiol. (1974), 236, pp. 363-371 363 With 5 text-fighurem Printed in Great Britain STOCHASTIC PROPERTIES OF SPONTANEOUS TRANSMITTER RELEASE AT THE CRAYFISH NEUROMUSCULAR JUNCTION BY IRA COHEN, HIROSHI
J. Phyeiol. (1974), 236, pp. 363-371 363 With 5 text-fighurem Printed in Great Britain STOCHASTIC PROPERTIES OF SPONTANEOUS TRANSMITTER RELEASE AT THE CRAYFISH NEUROMUSCULAR JUNCTION BY IRA COHEN, HIROSHI
Supporting information
 Supporting information Fluorescent derivatives of AC-42 to probe bitopic orthosteric/allosteric binding mechanisms on muscarinic M1 receptors Sandrine B. Daval, Céline Valant, Dominique Bonnet, Esther
Supporting information Fluorescent derivatives of AC-42 to probe bitopic orthosteric/allosteric binding mechanisms on muscarinic M1 receptors Sandrine B. Daval, Céline Valant, Dominique Bonnet, Esther
7.06 Cell Biology EXAM #3 KEY
 7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
Biophysical characterization of SMALPs and nanodiscs
 Biophysical characterization of SMALPs and nanodiscs Verna Frasca, Ph.D. Malvern Panalytical Verna.Frasca@Malvern.com Malvern Panalytical 2 SMALP April 11 2018 Malvern Panalytical Biosciences Group Solutions
Biophysical characterization of SMALPs and nanodiscs Verna Frasca, Ph.D. Malvern Panalytical Verna.Frasca@Malvern.com Malvern Panalytical 2 SMALP April 11 2018 Malvern Panalytical Biosciences Group Solutions
TRANSMITTER RELEASE AT THE NEUROMUSCULAR JUNCTION
 TRANSMITTER RELEASE AT THE NEUROMUSCULAR JUNCTION Thomas L. Schwarz Program in Neurobiology, Children s Hospital and Department of Neurobiology Harvard Medical School, Boston, Massachusetts 02115, USA
TRANSMITTER RELEASE AT THE NEUROMUSCULAR JUNCTION Thomas L. Schwarz Program in Neurobiology, Children s Hospital and Department of Neurobiology Harvard Medical School, Boston, Massachusetts 02115, USA
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
A Product of the Drosophila stoned Locus Regulates Neurotransmitter Release
 The Journal of Neuroscience, December 1, 1998, 18(23):9638 9649 A Product of the Drosophila stoned Locus Regulates Neurotransmitter Release Daniel T. Stimson, 1 Patricia S. Estes, 1 Michiko Smith, 2 Leonard
The Journal of Neuroscience, December 1, 1998, 18(23):9638 9649 A Product of the Drosophila stoned Locus Regulates Neurotransmitter Release Daniel T. Stimson, 1 Patricia S. Estes, 1 Michiko Smith, 2 Leonard
Supplementary Information. Overlap between folding and functional energy landscapes for. adenylate kinase conformational change
 Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Dimorphism and Homeostasis of Neuromuscular Synaptic Function
 Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed
 Supplementary Text Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed state It is difficult to examine accessibility of cysteine-substituted mutants in the fully
Supplementary Text Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed state It is difficult to examine accessibility of cysteine-substituted mutants in the fully
Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions:
 Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Interpreting and evaluating biological NMR in the literature. Worksheet 1
 Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
Name: TF: Section Time: LS1a ICE 5. Practice ICE Version B
 Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
BRIEF COMMUNICATION 3,4-DIAMINOPYRIDINE A POTENT NEW POTASSIUM CHANNEL BLOCKER
 BRIEF COMMUNICATION 3,4-DIAMINOPYRIDINE A POTENT NEW POTASSIUM CHANNEL BLOCKER GLENN E. KIRSCH AND ToSHIo NARAHASHI, Department ofpharmacology, Northwestem University Medical School, Chicago, Illinois
BRIEF COMMUNICATION 3,4-DIAMINOPYRIDINE A POTENT NEW POTASSIUM CHANNEL BLOCKER GLENN E. KIRSCH AND ToSHIo NARAHASHI, Department ofpharmacology, Northwestem University Medical School, Chicago, Illinois
Microsystems for Neuroscience and Medicine. Lecture 9
 1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
www.sciencesignaling.org/cgi/content/full/5/243/ra68/dc1 Supplementary Materials for Superbinder SH2 Domains Act as Antagonists of Cell Signaling Tomonori Kaneko, Haiming Huang, Xuan Cao, Xing Li, Chengjun
Potassium channel gating and structure!
 Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
3 Detector vs. Computer
 1 Neurons 1. The detector model. Also keep in mind this material gets elaborated w/the simulations, and the earliest material is often hardest for those w/primarily psych background. 2. Biological properties
1 Neurons 1. The detector model. Also keep in mind this material gets elaborated w/the simulations, and the earliest material is often hardest for those w/primarily psych background. 2. Biological properties
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Molecular Mechanisms of Fast Neurotransmitter Release
 Annu. Rev. Biophys. 2018. 47:469 97 The Annual Review of Biophysics is online at biophys.annualreviews.org https://doi.org/10.1146/annurev-biophys- 070816-034117 Copyright c 2018 by Annual Reviews. All
Annu. Rev. Biophys. 2018. 47:469 97 The Annual Review of Biophysics is online at biophys.annualreviews.org https://doi.org/10.1146/annurev-biophys- 070816-034117 Copyright c 2018 by Annual Reviews. All
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Shangbang Gao, Ph. D
 Shangbang Gao, Ph. D Professor, College of Life Science and Technology, Huazhong University of Science & Technology, 1037 Luoyu Road, Wuhan 430074 China Phone: (027)87792025 E-mail: sgao@hust.edu.cn Lab
Shangbang Gao, Ph. D Professor, College of Life Science and Technology, Huazhong University of Science & Technology, 1037 Luoyu Road, Wuhan 430074 China Phone: (027)87792025 E-mail: sgao@hust.edu.cn Lab
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Wednesday, September 13, 2006 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in
ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Wednesday, September 13, 2006 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in
The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control
 Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
From Fragments to Actin: The Bistramide A Story. March 12 th, 2010
 From Fragments to Actin: The Bistramide A Story March 12 th, 2010 Act I: The Protagonist Synthesis of Bistramide A Gillis, Wang, Davis, Fujii, Bromann Bistramide A Biological Activity Cell Cycle Regulation
From Fragments to Actin: The Bistramide A Story March 12 th, 2010 Act I: The Protagonist Synthesis of Bistramide A Gillis, Wang, Davis, Fujii, Bromann Bistramide A Biological Activity Cell Cycle Regulation
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6
 Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6 Hsuan-Liang Liu* and Chin-Wen Chen Department of Chemical Engineering and Graduate Institute
T H E J O U R N A L O F C E L L B I O L O G Y
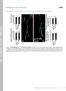 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
REVIEW ARTICLE Role of synaptotagmin in Ca 2 +-triggered exocytosis
 Biochem. J. (2002) 366, 1 13 (Printed in Great Britain) 1 REVIEW ARTICLE Role of synaptotagmin in Ca 2 +-triggered exocytosis Ward C. TUCKER and Edwin R. CHAPMAN 1 Department of Physiology, SMI 129, University
Biochem. J. (2002) 366, 1 13 (Printed in Great Britain) 1 REVIEW ARTICLE Role of synaptotagmin in Ca 2 +-triggered exocytosis Ward C. TUCKER and Edwin R. CHAPMAN 1 Department of Physiology, SMI 129, University
