Thermometry. History. History 1/21/18. The art or science of temperature observation
|
|
|
- Alyson Robinson
- 5 years ago
- Views:
Transcription
1 Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and coldness of air caused water in a closed tube partially filled with air to expand and contract; 11 th century Likely conducted the first air temperature measurement History Galileo invented the thermoscope (galileo thermometer); reflected changes in sensible heat Galileo s thermoscope is comprised of glass sphere s filled with aqueous alcohol of slightly different densities Guiseppe Biancani Developed first clear diagram of a thermoscope
2 History Robert Fludd (1638) First thermoscope containing a scale (thermometer) vertical tube, with a bulb at the top and the end immersed in water water level in the tube is controlled by the expansion and contraction of the air One major drawback History Ferdinando II de' Medici (Grand Duke of Tuscany) Invented first modern-style thermometer with a sealed tube partly filled with alcohol, with a bulb and stem Each inventor and each thermometer was unique there was no standard scale History Christiaan Huygens (1665) - suggested using the melting and boiling points of water as standards Carlo Renaldini (1694) - proposed using them as fixed points on a universal scale Isaac Newton (1701) - proposed a scale of 12 degrees between the melting point of ice and body temperature 2
3 History Daniel Gabriel Fahrenheit (1724) Invented Fahrenheit scale based on three fixed points of temperature. Used Hg for first time. First: frigorific mixture of ice, water, and ammonium chloride, a salt, and waiting for it to reach equilibrium. Reading was 0F Second: reading taken in still water as ice is just forming on the surface. 32F Third: reading when placed under the arm or in the mouth. 96F History Anders Celsius (1742) - proposed a scale with zero at the boiling point and 100 degrees at the melting point of water Jean Pierre Cristin (1743) proposed to flip the Celsius scale and named it the Centigrade scale Thermometry Air temperature is one of the most fundamental of all meteorological measurements, also most influenced by exposure Accurate measurements can be made, but can be hard due to cost, reliability and power requirements Errors in measurement are very common across networks 3
4 Temperature What is meant by the term temperature? What are you actually measuring? Common Misnomer Temperature is NOT hot or cold, it is high or low Hot and cold applicable to measurements against some set-point Effects of Errors Errors in excess of 2 to 3 degrees Celsius not uncommon Numerical models greatly impacted by a difference of 1 degrees Celsius Climate models greatly impacted by a difference of 0.2 degrees Celsius 4
5 Measurement Quality Accuracy not limited by technology Limited by ability to use it while avoiding exposure error Temperature Scales Preferred scales are Kelvin and Celsius Can be used almost interchangeably Fahrenheit mainly used just in the U.S. What are the 4 th and 5 th scales? Defunct Scale Joseph-Nicolas Delisle (1732) used Mercury and developed the Delisle scale (Degrees De or D) Boiling point was 0, freezing point was 150 Ranged up to 2400 degrees Popular in Russia in the 19 th century 5
6 Triple point Temperature and pressure where all three phases can coexist Water (6.106 hpa) Kelvin 0.01 Celsius Fahrenheit Why is this important in temperature measurement? Requirements for Proper Measurements What precautions must be taken when measuring temperature during the day? What precautions must be taken when measuring temperature during the night? We know air is a very effective insulator. What does this mean for measuring temperature? Requirements for Proper Measurements What effect can precipitation have on temperature measurement? What type of shielding is ideal for temperature measurement? What level of sensitivity should be expected from a thermometer used to measure air temperature? 6
7 Requirements for Proper Measurements Sensor should be robust, stable in calibration, easy to use. How long should it s operational life be (on average?) What effects can air pollution have on the sensor? What types of error(s) can this lead to? What is the standard height above ground for temperature measurements? Minimizing Exposure Error Sensor needs to be completely shielded by shield Radiation errors can reach extremes when there is maximum solar radiation, light winds and a highly reflective ground surface (ie snow) Site and Exposure Requirements WMO Guidance: The best site for [temperature] measurements is over level ground, freely exposed to sunshine and wind and not shielded by, or close to, trees buildings and other obstructions. Why avoid trees? How far away from obstructions should the thermometer be? 7
8 Bad Siting Areas Very sheltered positions. Why? Locations which may result in significant additional reflected radiation. Rooftop, chimneys, eaves. Why? Sites with significant topographic shelter. Why? Bad Siting Areas Masts or towers where the screen is significantly sheltered by the mast structure or where height is above 2 meters Areas of tarmac or concrete Minimizing Exposure Error, Thermometer Shielding Aspirated Radiation Shield Protects sensor from the elements Fan forces air over the sensor Passive shield No fan Impedes natural airflow as little as possible Only blocks 80% of incoming solar radiation 8
9 Thermometer Shielding Stevenson Screen First described by Thomas Stevenson in 1866 Louvered Screen, passive Still widely used today Typically house a max/min Thermometer What is the typical cost? AWS Screen What does AWS stand for? Come in various sizes and shapes Does size/shape of this type of screen matter? Why or why not? How do you pick the best screen? 9
10 Aspirated Shield Provide constant flow of air across the sensor Why is this advantageous to nonaspirated shields? Why not switch all observations over to aspirated shields? What are the disadvantages to using this shield? Temperature Sensors Categorized according to the physical principal they use Thermal expansion* Thermoelectric* Electrical resistance* Electrical capacitance Thermal Expansion Temperature is determined through the use of bimetallic strips and liquid-in-glass thermometers 10
11 Linear Expansion One-dimensional length change with temperature ΔL = Volume Expansion Change in volume with temperature ΔV = Bimetallic Strips Pair of metals with different thermal expansion coefficients Strip maintains it s shape at temperature bonding took place Temperature change causes bending of the metal Typically found in thermostats 11
12 Bimetallic Strip Deflection y = Liquid-in-Glass Thermometer Glass tube with a bulb at one end filled with liquid and attached to, or etched with, a scale Liquid is typically either mercury or alcohol Sheathed vs unsheathed Sheathed Encased in outer glass sheath, with scale engraved Unsheathed Graduations marked on thermometer stem or attached scale Advantages/disadvantages to these? Liquid-in-Glass Thermometer Three classifications for these thermometers determined by immersion requirements Partial Total Complete 12
13 Partial Immersion Should be placed in a bath liquid (water or oil) until the bulb and a small portion of stem (typically indicated by an immersion line) are immersed in the liquid to be measured Can be used for calibrating other sensors Total Immersion Bulb and portion of the stem containing the thermometric fluid are immersed Can also be used for calibrating other sensors Complete Immersion Bulb and stem are completely immersed in the liquid Used for air measurement 13
14 Special Types of Liquid-in-Glass Thermometers Minimum thermometer Uses alcohol with a dumbbell in the stem Thermometer is mounted horizontally Alcohol flows around the dumbbell as temperature increases As temperature decreases, the alcohol miniscus drags the dumbbell down to the minimum measurement Special Types of Liquid-in-Glass Thermometers Maximum thermometer Uses mercury and has a constriction in the stem Bulb is mounted slightly higher than the rest of the column Temperature increase causes volume increase and mercury is forced through constriction Temperature decrease causes break at constriction point Expansion As temperature increases, the glass and thermometric fluid expand according to: ΔV d = 14
15 Static Sensitivity of Liquid-in-Glass Thermometer S = assuming r is constant What are the units of static sensitivity? What does it mean? Thermocouples Thermocouple is an electrical apparatus that can generate a current proportional to the amount of heat it is exposed to Thomas Johann Seebeck ( 1821) - discovered that when any conductor (such as a metal) is subjected to a thermal gradient, it will generate a voltage (Seebeck effect) Thermocouples Two dissimilar metals are joined at each end (at points known as junctions) If each end experiences a different temperature (i.e. temperature gradient), a voltage will develop 15
16 Thermocouples the magnitude of the effect depends on the metal in use Using a dissimilar metal to complete the circuit creates a circuit in which the two legs generate different voltages, leaving a small difference in voltage available for measurement Thermocouples Thermocouples measure the temperature difference between two points, not absolute temperature In traditional applications, one of the junctions (cold junction) is maintained at a known (reference) temperature Thermocouples Having available known cold junctions is impractical outside laboratory environments Artificial cold junctions (thermistor or diode) have been have been implemented as an artificial cold junction Leads must be composed of same wires How can you increase their sensitivity? 16
17 Common Thermocouple Metals Type T Copper Constantan Type J Iron and Constantan Type E Nickel (10% Chromium) and Constantan Type K Nickel (10% Chromium) and Nickel (5% aluminum and silicon) Transfer Equation Mathematical equation in terms of spatial or temporal frequency, of the relation between the input and output of a (linear timeinvariant) system Thermocouple Transfer Equation ΔV = 17
18 Electrical Resistance Sensors Two types Resistance Temperature Detectors (RTDs) Thermistors RTDs Many materials exhibit variations in their properties with changing temperature Metals or semiconductors that show variation in electrical resistance can be used as temperature sensors RTDs Platinum is the most commonly used metal Stable Resists Corrosion Easily Workable High Melting Point Can be Highly Purified Simple and Stable Resistance-Temperature Relationship Drawback Sensitive to strain; bending can change resistance 18
19 R T = Platinum Sensor Resistance Thermistors An electrical resistor whose resistance is greatly reduced by heating Typically composed of metallic oxides Popular because their high resistance makes them less sensitive to lead wire resistance and are available in many configurations Temperature Sensor Exposure If the temperature sensor is exposed to the air, the instrument will indicate a temperature Temperature sensor may differ from the actual air temperature Heat flows toward or away from a temperature sensor by conduction, convection, and radiation 19
20 Radiative properties Sensor exists in radiative environment Sensor radiates heat to cooler surfaces or has heat radiated to the sensor from hotter surfaces Accurate Temperature Measurement Sensor must be in good thermal contact with the air (air is a poor conductor) Usually requires air circulation to promote heat transfer by convection Sensor must be protected from heat flow along the sensor support and radiative heat transfer Central problem for immersion sensing instruments in meteorology is coupling Heat Transfer Equations Conduction (H C ) = Radiation (H R ) = Convection (H V ) = What are the units of all three? 20
21 k M = A M = T S, T M, T A = ΔX = D, L = Heat Transfer Variables α S = R = c = V A = Heat Transfer Variables 21
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
4. Thermometry. Temperature and Heat Flow Temperature Scales Thermometers
 4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
Temperature Measurement
 Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Lecture 2: Zero law of thermodynamics
 Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
APPENDIX ELEVEN Open Fire Temperature Measurements
 APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers),
APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers),
Dr.Salwa Alsaleh fac.ksu.edu.sa/salwams
 Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Temperature measurement
 emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Control Engineering BDA30703
 Control Engineering BDA30703 Lecture 4: Transducers Prepared by: Ramhuzaini bin Abd. Rahman Expected Outcomes At the end of this lecture, students should be able to; 1) Explain a basic measurement system.
Control Engineering BDA30703 Lecture 4: Transducers Prepared by: Ramhuzaini bin Abd. Rahman Expected Outcomes At the end of this lecture, students should be able to; 1) Explain a basic measurement system.
MEASURING INSTRUMENTS
 Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Heat and temperature are related and often confused, but they are not the same.
 Heat and temperature are related and often confused, but they are not the same. Heat Definition: Heat is energy that is transferred from one body to another as a result of a difference in temperature Symbol:
Heat and temperature are related and often confused, but they are not the same. Heat Definition: Heat is energy that is transferred from one body to another as a result of a difference in temperature Symbol:
Introduction. Measurement of temperature is generally considered to be one of the simplest and most accurate measurements performed in engineering.
 Slide Nr. 0 of 15 Slides Introduction Measurement of temperature is generally considered to be one of the simplest and most accurate measurements performed in engineering. Some important considerations
Slide Nr. 0 of 15 Slides Introduction Measurement of temperature is generally considered to be one of the simplest and most accurate measurements performed in engineering. Some important considerations
Heat & Temperature. Grade 7 Science - Unit 2 Pgs
 Heat & Temperature Grade 7 Science - Unit 2 Pgs 104-225 4.1 Temperature PTemperature is the measure of how much heat is in a substance. PTemperature is measured in degrees Celcius ( C) PIt is difficult
Heat & Temperature Grade 7 Science - Unit 2 Pgs 104-225 4.1 Temperature PTemperature is the measure of how much heat is in a substance. PTemperature is measured in degrees Celcius ( C) PIt is difficult
JSUNIL TUTORIAL,SAMASTIPUR PH: CBSE Class-7 Science Heat and temperature solve questions and Notes
 CBSE Class-7 Science Heat and temperature solve questions and Notes Fill in the blanks : (a) The hotness of an object is determined by its temperature. (b) Temperature of boiling water cannot be measured
CBSE Class-7 Science Heat and temperature solve questions and Notes Fill in the blanks : (a) The hotness of an object is determined by its temperature. (b) Temperature of boiling water cannot be measured
Part 2. Sensor and Transducer Instrument Selection Criteria (3 Hour)
 Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Science In Action 7 Heat and Temperature Section Quiz
 Section 2 Heat affects Matter in different ways 2.1 States of Matter and The Particle Model 1. Water has a distinct characteristic that sets it apart from other liquids on Earth. Water expands when it
Section 2 Heat affects Matter in different ways 2.1 States of Matter and The Particle Model 1. Water has a distinct characteristic that sets it apart from other liquids on Earth. Water expands when it
1. How much heat was needed to raise the bullet to its final temperature?
 Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
University of Technology Dr. louay A.Mahdi Department of Machines and Equipments Engineering Branches: General, Refrigeration and Air conditioning,
 1 Introduction: The first recorded temperature measurement was carried out by Galileo at the end of the sixteenth century. His thermometer depended on the expansion of air. Some form of scale was attached
1 Introduction: The first recorded temperature measurement was carried out by Galileo at the end of the sixteenth century. His thermometer depended on the expansion of air. Some form of scale was attached
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test
 ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
Heat and Temperature
 Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
VALLIAMMAI ENGINEERING COLLEGE
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6502 -INDUSTRIAL INSTRUMENTATION I Regulation 2013
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6502 -INDUSTRIAL INSTRUMENTATION I Regulation 2013
Lecture 6. Temperature and Heat 27 September 2018
 Lecture 6. Temperature and Heat 27 September 2018 Wannapong Triampo, Ph.D. Korey Stringer 7-31-01 27 Yrs Old 6 3 335 lbs Eraste Autin 7-25-01 18 Yrs Old 6 2 250 lbs Preston Birdsong 8-13-00 18 Yrs Old
Lecture 6. Temperature and Heat 27 September 2018 Wannapong Triampo, Ph.D. Korey Stringer 7-31-01 27 Yrs Old 6 3 335 lbs Eraste Autin 7-25-01 18 Yrs Old 6 2 250 lbs Preston Birdsong 8-13-00 18 Yrs Old
3. EFFECTS OF HEAT. Thus, heat can be defined as a form of energy that gives the sensation of hotness or coldness
 3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
Measurements & Instrumentation. Module 3: Temperature Sensors
 Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurement in Engineering
 Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Al-Saudia Virtual Academy Online tuiton Pakistan Online Tutor Pakistan. Heat
 Al-Saudia Virtual Academy Online tuiton Pakistan Online Tutor Pakistan Heat Nature of Heat: Heat is the transfer of energy (every in transit) from one body to another due to the temperature difference
Al-Saudia Virtual Academy Online tuiton Pakistan Online Tutor Pakistan Heat Nature of Heat: Heat is the transfer of energy (every in transit) from one body to another due to the temperature difference
Lecture 36: Temperatue Measurements
 Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Recap: Bernoulli s Principle
 Recap: Bernoulli s Principle The sum of pressure plus kinetic energy per unit volume of a flowing fluid is constant. P + ½ρv 2 = constant pressure K.E. per unit volume (ρ = mass vol ) Result: Relates pressure
Recap: Bernoulli s Principle The sum of pressure plus kinetic energy per unit volume of a flowing fluid is constant. P + ½ρv 2 = constant pressure K.E. per unit volume (ρ = mass vol ) Result: Relates pressure
Thermal Process Control Lap 4 Thermal Energy. Notes:
 Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
ECNG3032 Control and Instrumentation I
 sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
* Defining Temperature * Temperature is proportional to the kinetic energy of atoms and molecules. * Temperature * Internal energy
 * Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
* Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY
 EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
8th Grade. Thermal Energy Study Guide.
 1 8th Grade Thermal Energy Study Guide 2015 10 09 www.njctl.org 2 Thermal Energy Study Guide www.njctl.org 3 Part 1 Define the following terms and/or concepts 4 1 Temperature 5 2 Kinetic Energy 6 3 Thermal
1 8th Grade Thermal Energy Study Guide 2015 10 09 www.njctl.org 2 Thermal Energy Study Guide www.njctl.org 3 Part 1 Define the following terms and/or concepts 4 1 Temperature 5 2 Kinetic Energy 6 3 Thermal
Slide 1 / 67. Slide 2 / 67. 8th Grade. Thermal Energy Study Guide Slide 3 / 67. Thermal Energy. Study Guide.
 Slide 1 / 67 Slide 2 / 67 8th Grade Thermal Energy Study Guide 2015-10-09 www.njctl.org Slide 3 / 67 Thermal Energy Study Guide www.njctl.org Slide 4 / 67 Part 1 Define the following terms and/or concepts
Slide 1 / 67 Slide 2 / 67 8th Grade Thermal Energy Study Guide 2015-10-09 www.njctl.org Slide 3 / 67 Thermal Energy Study Guide www.njctl.org Slide 4 / 67 Part 1 Define the following terms and/or concepts
Temperature. Temperature Scales. Temperature (cont d) CHAPTER 14 Heat and Temperature
 Temperature CHAPTER 14 Heat and Temperature The temperature of a substance is proportional to the average kinetic energy of the substance s particles. As the average kinetic energy of the particles in
Temperature CHAPTER 14 Heat and Temperature The temperature of a substance is proportional to the average kinetic energy of the substance s particles. As the average kinetic energy of the particles in
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
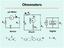 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 6: Thermal Physics
 Unit 6: Thermal Physics 6.1 Thermal Expansion Objectives Describe qualitatively the thermal expansion of solids, liquids, and gases at constant pressure Identify and explain some of the everyday applications
Unit 6: Thermal Physics 6.1 Thermal Expansion Objectives Describe qualitatively the thermal expansion of solids, liquids, and gases at constant pressure Identify and explain some of the everyday applications
INSTRUMENTATION ECE Fourth Semester. Presented By:- Sumit Grover Lect., Deptt. of ECE
 INSTRUMENTATION ECE Fourth Semester Presented By:- Sumit Grover Lect., Deptt. of ECE Detailed Contents Objectives Sensors and transducer Classification of transducers Temperature transducers Resistance
INSTRUMENTATION ECE Fourth Semester Presented By:- Sumit Grover Lect., Deptt. of ECE Detailed Contents Objectives Sensors and transducer Classification of transducers Temperature transducers Resistance
Unit 5 Thermodynamics
 Unit 5 Thermodynamics Unit 13: Heat and Temperature Unit 14: Thermal Expansion /Heat Exchange/ Change of Phase Test: Units 13-14 Thermal Energy The total kinetic and potential energy of all the molecules
Unit 5 Thermodynamics Unit 13: Heat and Temperature Unit 14: Thermal Expansion /Heat Exchange/ Change of Phase Test: Units 13-14 Thermal Energy The total kinetic and potential energy of all the molecules
Peltier Application Note
 Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Observing Climate. How do we measure climate parameters? Surface Measurements. - Temperature > Wind Chill Temperature Index > Heat Index.
 Observing Climate 3-1 How do we measure climate parameters? Surface Measurements - Temperature > Wind Chill Temperature Index > Heat Index - Pressure Observing - To see a world in a grain of sand, And
Observing Climate 3-1 How do we measure climate parameters? Surface Measurements - Temperature > Wind Chill Temperature Index > Heat Index - Pressure Observing - To see a world in a grain of sand, And
I. Introduction and Objectives
 Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
PALO VERDE NUCLEAR GENERATING STATION
 PALO VERDE NUCLEAR GENERATING STATION I&C Program Classroom Lesson I&C Program Date: 2/25/2011 1:42:48 PM LP Number: NIA97C000503 Rev Author: DANIEL R. REED Title: Temperature Measurement Technical Review:
PALO VERDE NUCLEAR GENERATING STATION I&C Program Classroom Lesson I&C Program Date: 2/25/2011 1:42:48 PM LP Number: NIA97C000503 Rev Author: DANIEL R. REED Title: Temperature Measurement Technical Review:
Part Number Range Accuracy Printing WP * Probe Special User LDC with Page Logging included Feature Cal Backlight
 Table of Contents Comparison Chart Introduction Temperature Indicators & Controllers Portable Infrared Printing/Logging Page O2 O3 O10 O14 O30 O33 O1 Part Number Range Accuracy Printing WP * Probe Special
Table of Contents Comparison Chart Introduction Temperature Indicators & Controllers Portable Infrared Printing/Logging Page O2 O3 O10 O14 O30 O33 O1 Part Number Range Accuracy Printing WP * Probe Special
Chapter 6 Temperature Measurement (Revision 2.0, 1/12/2009)
 Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he
Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he
Chapter 14: Temperature and Heat
 Chapter 14 Lecture Chapter 14: Temperature and Heat Goals for Chapter 14 To study temperature and temperature scales. To describe thermal expansion and its applications. To explore and solve problems involving
Chapter 14 Lecture Chapter 14: Temperature and Heat Goals for Chapter 14 To study temperature and temperature scales. To describe thermal expansion and its applications. To explore and solve problems involving
Temperature. Sensors. Measuring technique. Eugene V. Colla. 10/25/2017 Physics 403 1
 Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
SMS-204: Integrative marine sciences.
 SMS-204: Integrative marine sciences. Lecture 4: Heat and temperature The study of heat transfer is called thermodynamics. In thermodynamics, we talk about systems, a collection of molecule in a container,
SMS-204: Integrative marine sciences. Lecture 4: Heat and temperature The study of heat transfer is called thermodynamics. In thermodynamics, we talk about systems, a collection of molecule in a container,
Thermometry And Thermal Expansion
 Thermometry And Thermal Expansion 1. Define heat (a) on conventional basis (b) on the basis of kinetic model. Ans. (a) On the conventional basis heat is the from of energy which causes in us sensation
Thermometry And Thermal Expansion 1. Define heat (a) on conventional basis (b) on the basis of kinetic model. Ans. (a) On the conventional basis heat is the from of energy which causes in us sensation
Chapter 18. Temperature, Heat, and the First Law of Thermodynamics Temperature
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
15. Compare the result with the value you have taken above Compare the calculated pressure value with the actual pressure value that you have
 105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
Heat and Temperature
 Heat and Temperature Temperature What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold? Intro: Discussion A person from Seattle
Heat and Temperature Temperature What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold? Intro: Discussion A person from Seattle
HEAT AND TEMPERATURE Vikasana-Bridge Course 2012
 HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
TEMPERATURE AND THERMAL EXPANSION
 TEMPERATURE AND THERMAL EXPANSION After boiling water, you will feel that the water is hotter than before, or you can say that the water temperature is higher than before. Otherwise, when you pick an ice,
TEMPERATURE AND THERMAL EXPANSION After boiling water, you will feel that the water is hotter than before, or you can say that the water temperature is higher than before. Otherwise, when you pick an ice,
Thermocouple Calibrations and Heat Transfer Coefficients
 Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared
Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared
Lecture 22. Temperature and Heat
 Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
WELCOME TO PERIOD 5: THERMAL ENERGY, THE MICROSCOPIC PICTURE. Homework #4 is due today at the beginning of class.
 WELCOME TO PERIOD 5: THERMAL ENERGY, THE MICROSCOPIC PICTURE Homework #4 is due today at the beginning of class. PHYSICS 1104 PERIOD 5 How are temperatures measured? How do atoms and molecules act at different
WELCOME TO PERIOD 5: THERMAL ENERGY, THE MICROSCOPIC PICTURE Homework #4 is due today at the beginning of class. PHYSICS 1104 PERIOD 5 How are temperatures measured? How do atoms and molecules act at different
Period 5: Thermal Energy, the Microscopic Picture
 Name Section Period 5: Thermal Energy, the Microscopic Picture 5.1 How Is Temperature Related to Molecular Motion? 1) Temperature Your instructor will discuss molecular motion and temperature. a) At a
Name Section Period 5: Thermal Energy, the Microscopic Picture 5.1 How Is Temperature Related to Molecular Motion? 1) Temperature Your instructor will discuss molecular motion and temperature. a) At a
Introduction to Heat and Mass Transfer. Week 1
 Introduction to Heat and Mass Transfer Week 1 This Lecture Course Organization Introduction Basic Modes of Heat Transfer Application Areas Instructor Information Professor Chang-Da Wen 温昌達» Office: 91713
Introduction to Heat and Mass Transfer Week 1 This Lecture Course Organization Introduction Basic Modes of Heat Transfer Application Areas Instructor Information Professor Chang-Da Wen 温昌達» Office: 91713
Q11: WHAT IS A MEANT GOOD ELECTRICAL CONNECTION?
 Q1. How to check for zero error in a: (i) Vernier caliper (ii) Micrometer screw gauge (iii) Meter rule (iv) Stopwatch Ans: (i) Close the jaws of the vernier caliper fully. When the zeros of both MAIN SCALE
Q1. How to check for zero error in a: (i) Vernier caliper (ii) Micrometer screw gauge (iii) Meter rule (iv) Stopwatch Ans: (i) Close the jaws of the vernier caliper fully. When the zeros of both MAIN SCALE
[ ] Sensors for Temperature Measurement, and Their Application 2L R 1 1 T 1 T 2
![[ ] Sensors for Temperature Measurement, and Their Application 2L R 1 1 T 1 T 2 [ ] Sensors for Temperature Measurement, and Their Application 2L R 1 1 T 1 T 2](/thumbs/89/97951344.jpg) Sensors for Temperature Measurement, and Their Application In today s market, it is very rare to see electronic equipment that has not undergone extensive thermal evaluation, either by measurement or simulation.
Sensors for Temperature Measurement, and Their Application In today s market, it is very rare to see electronic equipment that has not undergone extensive thermal evaluation, either by measurement or simulation.
Chapter 17. Temperature. Dr. Armen Kocharian
 Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold?
 Heat and Temperature Section 1: Temperature What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold? 1 Intro: Discussion A person
Heat and Temperature Section 1: Temperature What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold? 1 Intro: Discussion A person
Tick the box next to those resources for which the Sun is also the source of energy.
 1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
Chapter 14 Heat and Temperature Notes
 Chapter 14 Heat and Temperature Notes Section 1: Temperature The degree of or of an object. Related to the of an object s atoms or molecules What makes something hot? o Particles that make up o They have
Chapter 14 Heat and Temperature Notes Section 1: Temperature The degree of or of an object. Related to the of an object s atoms or molecules What makes something hot? o Particles that make up o They have
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
SENSORS AND TRANSDUCERS
 Electrical Measurements International Program Department of Electrical Engineering UNIVERSITAS INDONESIA ANDRITTO ABDUL GHAFFAR ANDHIKA ADIEL INSANI Lecturer : Ir. Chairul Hudaya, ST, M.Eng., Ph.D., IPM
Electrical Measurements International Program Department of Electrical Engineering UNIVERSITAS INDONESIA ANDRITTO ABDUL GHAFFAR ANDHIKA ADIEL INSANI Lecturer : Ir. Chairul Hudaya, ST, M.Eng., Ph.D., IPM
Thermal Energy. Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures.
 Thermal Energy Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures. And remember: heat will always transfer from a warm object to a cold object. HEAT
Thermal Energy Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures. And remember: heat will always transfer from a warm object to a cold object. HEAT
Temperature and Heat. Two systems of temperature. Temperature conversions. PHY heat - J. Hedberg
 Temperature and Heat 1. Two systems of temperature 1. Temperature conversions 2. Real science (one scale to rule them all) 3. Temperature scales 2. Effects of temperature on materials 1. Linear Thermal
Temperature and Heat 1. Two systems of temperature 1. Temperature conversions 2. Real science (one scale to rule them all) 3. Temperature scales 2. Effects of temperature on materials 1. Linear Thermal
Simpo PDF Merge and Split Unregistered Version -
 Simpo PDF Merge and Split Unregistered Version - http://wwwsimpopdfcom 6 If the zeroth law of thermodynamics were not valid, which of the following could not be considered a property of an object? A Pressure
Simpo PDF Merge and Split Unregistered Version - http://wwwsimpopdfcom 6 If the zeroth law of thermodynamics were not valid, which of the following could not be considered a property of an object? A Pressure
Slide 1. Temperatures Light (Optoelectronics) Magnetic Fields Strain Pressure Displacement and Rotation Acceleration Electronic Sensors
 Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
Solar Flat Plate Thermal Collector
 Solar Flat Plate Thermal Collector INTRODUCTION: Solar heater is one of the simplest and basic technologies in the solar energy field. Collector is the heart of any solar heating system. It absorbs and
Solar Flat Plate Thermal Collector INTRODUCTION: Solar heater is one of the simplest and basic technologies in the solar energy field. Collector is the heart of any solar heating system. It absorbs and
Physics 207 Lecture 23
 Thermodynamics A practical science initially concerned with economics, industry, real life problems. DYNAMICS -- Concerned with the concepts of energy transfers between a system and its environment and
Thermodynamics A practical science initially concerned with economics, industry, real life problems. DYNAMICS -- Concerned with the concepts of energy transfers between a system and its environment and
INSTRUMENTATION AND CONTROL SYSTEMS LAB
 INSTRUMENTATION AND CONTROL SYSTEMS LAB INDEX S.No. Name of the Experiment Page No. 1 Linear Variable Differential Transformer (L.V.D.T) 2 Speed Measurement Module 3 Capacitive Pickup 4 Thermister Module
INSTRUMENTATION AND CONTROL SYSTEMS LAB INDEX S.No. Name of the Experiment Page No. 1 Linear Variable Differential Transformer (L.V.D.T) 2 Speed Measurement Module 3 Capacitive Pickup 4 Thermister Module
Unit 57: Mechatronic System
 Unit 57: Mechatronic System Unit code: F/60/46 QCF level: 4 Credit value: 5 OUTCOME 2 TUTORIAL 2 - SENSOR TECHNOLOGIES 2 Understand electro-mechanical models and components in mechatronic systems and products
Unit 57: Mechatronic System Unit code: F/60/46 QCF level: 4 Credit value: 5 OUTCOME 2 TUTORIAL 2 - SENSOR TECHNOLOGIES 2 Understand electro-mechanical models and components in mechatronic systems and products
the energy of motion!
 What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
Chapter 10. Thermal Physics
 Chapter 10 Thermal Physics Thermal Physics Thermal physics is the study of Temperature Heat How these affect matter Thermal Physics, cont Descriptions require definitions of temperature, heat and internal
Chapter 10 Thermal Physics Thermal Physics Thermal physics is the study of Temperature Heat How these affect matter Thermal Physics, cont Descriptions require definitions of temperature, heat and internal
I. MEASUREMENT OF TEMPERATURE
 I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Cryogenic Instrumentation I Thermometry OUTLINE Thermometry Pt (pure metal) Temperature Ranges of Thermometer Application Typical Resistive Thermal
 Cryogenic Instrumentation I 1. Thermometry 2. anges of Application 3. Constant Volume 4. Thermocouples 5. Time esponse Data 6. 4 Terminal esistance Measurement OUTLINE 8. Pt (pure metal) 9. Typical esistive
Cryogenic Instrumentation I 1. Thermometry 2. anges of Application 3. Constant Volume 4. Thermocouples 5. Time esponse Data 6. 4 Terminal esistance Measurement OUTLINE 8. Pt (pure metal) 9. Typical esistive
Week-7 Assignment-1. The due date for submitting this assignment has passed. (1/273.15)th of the normal freezing point of water
 X reviewer2@nptel.iitm.ac.in Courses» Chemical Process Instrumentation Unit 8 - Week 7 Announcements Course Ask a Question Progress Mentor Course outline How to access the portal Week-7 Assignment-1 The
X reviewer2@nptel.iitm.ac.in Courses» Chemical Process Instrumentation Unit 8 - Week 7 Announcements Course Ask a Question Progress Mentor Course outline How to access the portal Week-7 Assignment-1 The
Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund
 Aalborg Universitet Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund Publication date: 2008 Document Version
Aalborg Universitet Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund Publication date: 2008 Document Version
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS
 ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors
 Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors 1. Scope... 2 2. Introduction... 2 3. Selection of thermocouples
Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors 1. Scope... 2 2. Introduction... 2 3. Selection of thermocouples
Applications. Remote Weather Station with Telephone Communications. Tripod Tower Weather Station with 4-20 ma Outputs
 Tripod Tower Weather Station with 4-20 ma Outputs Remote Weather Station with Telephone Communications NEMA-4X Enclosure with Two Translator Boards and Analog Barometer Typical Analog Output Evaporation
Tripod Tower Weather Station with 4-20 ma Outputs Remote Weather Station with Telephone Communications NEMA-4X Enclosure with Two Translator Boards and Analog Barometer Typical Analog Output Evaporation
Technical Notes. Introduction. PCB (printed circuit board) Design. Issue 1 January 2010
 Technical Notes Introduction Thermal Management for LEDs Poor thermal management can lead to early LED product failure. This Technical Note discusses thermal management techniques and good system design.
Technical Notes Introduction Thermal Management for LEDs Poor thermal management can lead to early LED product failure. This Technical Note discusses thermal management techniques and good system design.
We call the characteristic of a system that determines how much its temperature will change heat capacity.
 3/3 Measuring Heat If all we do is add heat to a system its temperature will rise. How much the temperature rises depends on the system. We call the characteristic of a system that determines how much
3/3 Measuring Heat If all we do is add heat to a system its temperature will rise. How much the temperature rises depends on the system. We call the characteristic of a system that determines how much
Thermal energy. Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules.
 Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
