Thermocouple Calibrations and Heat Transfer Coefficients
|
|
|
- Arleen Fox
- 6 years ago
- Views:
Transcription
1 Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared by Kimberly Nguyen, A05 Grace Victorine, A05 4/30/15
2 Abstract The objective of this experiment was to calibrate a type K thermocouple to observe the Seebeck Effect and calculate the Biot numbers and heat transfer coefficients of aluminum and copper spheres during both free and forced convection modes of cooling. The offset of the type K thermocouple was determined to be 3.0 ± 0.5. The Biot numbers under free convection were determined to be for aluminum and for copper. Under forced convection, they were determined to be for aluminum and for copper. The heat transfer coefficients for free convection were determined to be 604.0W/(m 2 K) for aluminum and W/(m 2 K) for copper. Finally, the heat transfer coefficients for forced convection were determined to be W/(m 2 K) for aluminum and W/(m 2 K) copper. The rate of heat loss was noticeably faster for forced convection than for free convection. The rate of heat loss was also faster for aluminum than for copper in both convection modes. 1
3 I. Introduction Thermocouples have a copious useful applications, including temperature sensors for control purposes, and the transduction of temperature differences into voltages. This experiment examines a type K thermocouple and the theory behind its operation as well as the rate of heat loss of metal spheres under both free and forced convection. The first portion of this experiment explored the ability of a type K thermocouple to transduce a temperature differential into a potential difference. The cold junction was created by submerging one wire in a temperature bath held at a constant 0. The hot junction was submerged in heated water to produce increasing temperatures so that the difference could be transduced into a measurable voltage. The voltage was displayed digitally while the temperature measurements were calculated via a mercury thermometer. The values produced were then graphed and compared to a given standard to assess both accuracy and precision. The second portion of the experiment was designed to allow determination of the Biot number and heat transfer coefficients of an aluminum and copper sphere under both free and forced convection modes. Each sphere was submerged in hot water until its temperature stabilized at 90. Then it was plunged into the ice bath at 0 so that the rate of cooling could be recorded and graphed. The cooling curves were then examined to determine the Biot numbers and heat transfer coefficients. It was then determined which metal exhibited better heat transfer properties and whether free or forced convection transferred heat more quickly. II. Theory The governing rule behind the operation of a thermocouple was explained to be the Seebeck Effect, according to which, two different metals or semiconductors were necessary to produce a voltage difference in a circuit due to the internal material properties that gave rise to different electron mobilities. In the presence of a temperature gradient, the more mobile electrons were be transferred to the metal with the lower electron mobility. This produced a measurable potential difference. Additionally, Newton s Law of Cooling was examined. It demonstrated that the heat loss rate, Q conv, of a body was directly proportional to the difference in temperature between the system in question and its surroundings. Necessary assumptions made were that temperature gradients inside the sphere were small such that conduction dominated over convection, yielding a Biot number less than 1, and that the heat transfer coefficient was not variable with time. Q conv = ha (T hot T cold ) (1) 2 The Biot number was introduced as a dimensionless number representing the ratio of convection to conduction. When the Biot number was small, conduction was the more prominent means of heat transfer, which was ideal in part II of the experiment as it meant that conductive forces dominated, which met one assumption of Equation (1). R B i = h k (2) 2 Fourier s Law, yielded a relationship from which the heat transfer coefficient was able to be calculated from with knowledge of the temperature difference as well, density, surface area, volume, heat capacity, and time: T s T T o T = e ha [ ]t ρv C (3) 2 2
4 III. Experimental Procedures Part I: Thermocouple Calibration The first part of the experiment sought to calibrate and analyze a type K thermocouple in terms of its voltage produced relative to the change in temperature. One wire was designated the cold junction and submerged in an ice bath in the form of a cooler filled with ice and water to be ideally held at a constant 0. The other wire was made the hot junction and placed in a pan filled to about ⅔ full of room-temperature water. The thermocouple was then connected to the bench multimeter with the BNC adapter, cable, and clips and monitored on the DC setting. The TC junctions were immersed together and stirred to determine the offset. Both junctions were then set in the place so that the multimeter reading increased with in the positive direction with heat added to the hot junction. A thermometer was then suspended in the water pan to record the initial temperature of the hot junction and corresponding voltage difference. The hot plate was then turned to the maximum setting and 12 data points were taken at increasing temperatures stopping at 90. At the last data point, the heat was lowered to avoid overheating the water. The recorded temperatures were then graphed and compared to a standard so that the accuracy and precision as well as the reliability of the type K thermocouple could be assessed. Part II: Determination of Biot number and convective heat transfer coefficients. In the second part of the experiment, after the thermocouples in the metal spheres were determined to be functional, the temperatures of the water in the cooler and the pan were checked for need of adjustment. Then the yellow connector on the sphere was plugged into the thermocouple extension cable, which was in turn connected to the DAS terminal AI0+. The red wire was attached to AI0-, and the ground wire from the stainless steel tube was placed to AI-Gnd. The sphere was fully submerged in the hot water by attachment to a ring clamp so that it did not touch the bottom of the pan as shown in Figure 1. Figure 1: Setup of sphere in hot pan 1 The Thermocouple CJC vi was then used to monitor the temperature change with time. The thermometer was used to read the temperature of the hot water and make note of the offset from the vi s reading. Once a constant hot temperature was observed, the sphere was quickly submerged in the cold water bath and its cooling curve was recorded. The same process was repeated for the second sphere. Then, the process was repeated for both spheres only with the addition of a stirring pump to implement forced convection. 3
5 IV. Data and Results The offset of the thermocouple wires was determined to be 0.00 ± 0.05mV DC. The Seebeck Effect was observed through a voltage produced by the circuit composed of two different metals. The recorded data, and standard data in Figures 2 and 3, respectively show a very close linear relationship. The slope of the best fit line for the recorded data was determined to be /mv while the slope for the standard data was determined to be /mv. Figure 2: Measured Voltage vs Temperature Plot Figure 3: Standard Voltage vs Temperature Plot In part II of the experiment, the Biot numbers and heat transfer coefficients were as displayed in Table 1. Table 1: Heat transfer coefficients and Biot numbers of Al and Cu spheres under free & forced convection Aluminum Free Aluminum Forced Copper Free Copper Forced h (W/(m 2 K)) Biot number Figure 4: Aluminum Free Convection Figure 5: Copper Free Convection Figure 6: Aluminum Forced Convection Figure 6: Copper Forced Convection 4
6 IV. Data and Results (Continued) The Biot numbers and heat transfer coefficients were calculated from their corresponding best fit lines in Figures 4-7. The discrepancy between the thermometer and the LabVIEW vi was found to be 3.0 ± 0.5. The temperature of the ice bath remained at 3.0 ± 0.5 throughout part II of experiment. Figure (7) Aluminum & Copper Free Convection Cooling Figure (8) Aluminum & Copper Forced Convection Cooling 5
7 V. Discussion and Error Analysis In part I, the k thermocouple made of two different metals in a circuit did succeed in producing a voltage in the presence of a temperature differential, thus confirming the Seebeck Effect. Additionally, data recorded for the thermocouple corresponded closely with the standard values. When graphed, the best fit line of the recorded data appeared nearly identical to the graph of the standard data. The slope of the best fit line for the recorded data was determined to be /mv while the slope for the standard data was determined to be /mv. The error between the two was a mere 2.4%, which indicated that the values agreed closely and that the recorded values were therefore most likely reasonably accurate. In part II of the experiment, the Biot number of the spheres in forced convection were higher than of those in free convection, indicating that the increase in convection caused the Biot number to increase. It can be seen in Figures 4-8 how the forced convection curves had steeper curves and higher Biot numbers, indicating higher rates of heat transfer. This substantiates the idea that convection was more efficient at heat transfer than conduction. Additionally, the curve for Copper under free and forced convection is noticeably steeper than that of Aluminum, indicating higher thermal conductivity and heat transfer coefficient. This indicated that the copper sphere more readily transferred heat as compared to the aluminum sphere. Therefore, the copper sphere cooled faster than the aluminum sphere. Copper was therefore at least a slightly better conductor of heat than aluminum. The Biot number remained less than 1, indicating that in all examined cases, conduction forces dominated. It is when the Biot number approached 1 that the convective forces were higher and heat transfer rates were also higher. For Figures 4-7, the values of the best fit lines generally coincided well with the plotted data. It could be observed that the R 2 values were rather close to 1 for each of the graphs, indicating that the best fit line was generally an accurate representation of the trend of the data without large enough error to raise concern. The data collected during the free convection mode of the aluminum sphere had the most poorly fitted line. While it still represented the data well, there was noticeably more deviation in the data. This could have been because it was the first trial conducted. While heating the water, it was noted that there was an odd veneer of film inside the container.this could have presented a source of impurities that may have affected the boiling rate of the water and perhaps the heat transfer rate to a small extent. It was unclear whether or not this was the case, but the existence of subsequent trials with noticeably less error seemed to support that there was more deviation in the first trial than could be attributed to simply random error. That the Biot numbers in the case of forced convection were higher than 0.1 (a 1 to 10 ratio), brought into question their validity. While the best fit lines may have been good fits for the recorded data, whether or not they managed to accurately represent the Biot numbers and heat transfer coefficients of aluminum and copper is questionable. Ideally, it would have been preferable to compare the values with standards or average data of the lab session in an effort to quantify accuracy. Especially since any error in the Biot numbers was propagated to and magnified in the heat transfer coefficients, as they were calculated from the Biot numbers. That there were discernible offsets in parts I and II of the experiment indicated that noise was most likely constantly present, and minute fluctuations in it could have given rise to deviations in recorded and analyzed values. Most likely the trends remain the same, but the precision might have been marred, as random errors such as noise do not affect accuracy, but do impact precision. Concerning the assumptions of Newton s Law of Cooling, it idealized that the temperature gradient inside the sphere was small so that conduction dominated and the heat transfer coefficient did not vary with time. While these were good approximations, they were idealizations and had the heat transfer coefficient varied even slightly with time, then the value obtained would be an average at best. Such assumptions were important to take into consideration as, had they been found not to hold, the laws used would not have yielded accurate calculations. 6
8 VI. Conclusions This experiment successfully demonstrates the validity of the Seebeck Effect in which a two different metals in a circuit subject to a temperature differential will produce a voltage difference. However, the power source and exposed wiring of the thermocouple make it vulnerable to random error such as noise. Additionally, this experiment demonstrates that forced convection significantly increases the rate of heat transfer as opposed to free convection. Finally, it shows that copper is a better conductor than aluminum since copper has a lower Biot number and does not dissipate heat as quickly as aluminum does. Copper s lower heat transfer coefficient, lower Biot number, and ability to retain heat longer than aluminum make it a better conductor. 7
9 VII. References 1. Nicholas Busan, Steve Roberts, and Rahul Kapadia. Experiment 5A: Thermocouple Calibration and Determination of Heat Transfer Coefficients. (2014). UCSD MAE 170. Web. 29 Apr < > 2. Chapter VI. Heat Transfer Coefficients. (2014). UCSD MAE 170. Web. 29 Apr < > VIII. Appendix I: Raw Data Table A2: Temperature and Voltage GV Temperature (C) Voltage (mv) Uncertainty (mv) Table A3: Temperature and Voltage KN Temperature (C) Voltage (mv) Uncertainty (mv)
Calibrating a Pressure Transducer, Accelerometer, and Spring System
 Laboratory Experiment 6: Calibrating a Pressure Transducer, Accelerometer, and Spring System Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE
Laboratory Experiment 6: Calibrating a Pressure Transducer, Accelerometer, and Spring System Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE
Semiconductor thermogenerator
 Semiconductor thermogenerator LEP 4.1.07 Related topics Seebeck effect (thermoelectric effect), thermoelectric e.m.f., efficiency, Peltier coefficient, Thomson coefficient, Seebeck coefficient, direct
Semiconductor thermogenerator LEP 4.1.07 Related topics Seebeck effect (thermoelectric effect), thermoelectric e.m.f., efficiency, Peltier coefficient, Thomson coefficient, Seebeck coefficient, direct
I. Introduction and Objectives
 Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
ME 105 Mechanical Engineering Laboratory Spring Quarter Experiment #2: Temperature Measurements and Transient Conduction and Convection
 ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2010 Experiment #2: Temperature Measurements and Transient Conduction and Convection Objectives a) To calibrate
ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2010 Experiment #2: Temperature Measurements and Transient Conduction and Convection Objectives a) To calibrate
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY
 EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
1) Thermo couple sensor
 1) Thermo couple sensor Fundamental operation. In 1821 Mr. Seebeck found that if you connected 2 wires of different metals, a small Voltage would be generated, when this connection (junction) is heated.
1) Thermo couple sensor Fundamental operation. In 1821 Mr. Seebeck found that if you connected 2 wires of different metals, a small Voltage would be generated, when this connection (junction) is heated.
PURE PHYSICS THERMAL PHYSICS (PART I)
 PURE PHYSICS THERMAL PHYSICS (PART I) 1 The kinetic theory of matter states that all matters are made up of or, which are in and motion. forces hold the atoms or molecules together. The nature of these
PURE PHYSICS THERMAL PHYSICS (PART I) 1 The kinetic theory of matter states that all matters are made up of or, which are in and motion. forces hold the atoms or molecules together. The nature of these
Electricity. Semiconductor thermogenerator Stationary currents. What you need:
 Stationary currents Electricity Semiconductor thermogenerator What you can learn about Seebeck effect (thermoelectric effect) Thermoelectric e.m.f. Efficiency Peltier coefficient Thomson coefficient Seebeck
Stationary currents Electricity Semiconductor thermogenerator What you can learn about Seebeck effect (thermoelectric effect) Thermoelectric e.m.f. Efficiency Peltier coefficient Thomson coefficient Seebeck
Name Group # Date Partners. Specific Heat and Calorimetry
 Specific Heat and Calorimetry Experimental Objective The objective of this experiment is to determine the specific heat of two metals using a calorimetric procedure called the method of mixtures. Theory
Specific Heat and Calorimetry Experimental Objective The objective of this experiment is to determine the specific heat of two metals using a calorimetric procedure called the method of mixtures. Theory
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS
 ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
The Underground Experimental Investigation of Thermocouples
 The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
ME 105 Mechanical Engineering Laboratory Spring Quarter INTRODUCTION TO TRANSIENT CONDUCTION AND CONVECTION
 ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2003 2. INTRODUCTION TO TRANSIENT CONDUCTION AND CONVECTION This set of experiments is designed to provide
ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2003 2. INTRODUCTION TO TRANSIENT CONDUCTION AND CONVECTION This set of experiments is designed to provide
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test
 ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Evaluation copy. The Molar Mass of a Volatile Liquid. computer OBJECTIVES MATERIALS
 The Molar Mass of a Volatile Liquid Computer 3 One of the properties that helps characterize a substance is its molar mass. If the substance in question is a volatile liquid, a common method to determine
The Molar Mass of a Volatile Liquid Computer 3 One of the properties that helps characterize a substance is its molar mass. If the substance in question is a volatile liquid, a common method to determine
Thermal and electrical conductivity of metals
 Thermal and electrical conductivity of metals (Item No.: P2350200) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Thermodynamics Subtopic: Heat, Work, and the First
Thermal and electrical conductivity of metals (Item No.: P2350200) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Thermodynamics Subtopic: Heat, Work, and the First
tp03: The Ideal Gas Law
 tp03: The Ideal Gas Law Jack Lyes 18/01/2014 The main objective of this experiment was to calculate a value for absolute zero, the temperature at which a gas exerts zero pressure. This was achieved by
tp03: The Ideal Gas Law Jack Lyes 18/01/2014 The main objective of this experiment was to calculate a value for absolute zero, the temperature at which a gas exerts zero pressure. This was achieved by
Experiment B6 Thermal Properties of Materials Procedure
 Experiment B6 Thermal Properties of Materials Procedure Deliverables: Checked lab notebook, Brief technical memo Overview In this lab, you will examine the thermal properties of various materials commonly
Experiment B6 Thermal Properties of Materials Procedure Deliverables: Checked lab notebook, Brief technical memo Overview In this lab, you will examine the thermal properties of various materials commonly
The LM741C Integrated Circuit As A Differential Amplifier Building The Electronic Thermometer
 BE 209 Group BEW6 Jocelyn Poruthur, Justin Tannir Alice Wu, & Jeffrey Wu November 19, 1999 The LM741C Integrated Circuit As A Differential Amplifier Building The Electronic Thermometer INTRODUCTION: A
BE 209 Group BEW6 Jocelyn Poruthur, Justin Tannir Alice Wu, & Jeffrey Wu November 19, 1999 The LM741C Integrated Circuit As A Differential Amplifier Building The Electronic Thermometer INTRODUCTION: A
Lab 1f Boiling Heat Transfer Paradox
 Lab 1f Boiling Heat Transfer Paradox OBJECTIVES Warning: though the experiment has educational objectives (to learn about boiling heat transfer, etc.), these should not be included in your report. - Obtain
Lab 1f Boiling Heat Transfer Paradox OBJECTIVES Warning: though the experiment has educational objectives (to learn about boiling heat transfer, etc.), these should not be included in your report. - Obtain
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Harnessing the Power of Arduino for the Advanced Lab
 P P Herbert Jaeger + Harnessing the Power of Arduino for the Advanced Lab (Final Version) ALPhA Immersion Workshop July 27 29, 2017 Department of Physics Indiana University Purdue University Ft. Wayne,
P P Herbert Jaeger + Harnessing the Power of Arduino for the Advanced Lab (Final Version) ALPhA Immersion Workshop July 27 29, 2017 Department of Physics Indiana University Purdue University Ft. Wayne,
Department of Mechanical Engineering ME 96. Free and Forced Convection Experiment. Revised: 25 April Introduction
 CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
4. Heat and Thermal Energy
 4. Heat and Thermal Energy Introduction The purpose of this experiment is to study cooling and heating by conduction, convection, and radiation. Thermal energy is the energy of random molecular motion.
4. Heat and Thermal Energy Introduction The purpose of this experiment is to study cooling and heating by conduction, convection, and radiation. Thermal energy is the energy of random molecular motion.
Using a Mercury itc with thermocouples
 Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation
 IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation Introduction Due to their low cost and ease of use, thermocouples are still a popular means for making temperature measurements up
IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation Introduction Due to their low cost and ease of use, thermocouples are still a popular means for making temperature measurements up
Cool Off, Will Ya! Investigating Effect of Temperature Differences between Water and Environment on Cooling Rate of Water
 Ding 1 Cool Off, Will Ya! Investigating Effect of Temperature Differences between Water and Environment on Cooling Rate of Water Chunyang Ding 000844-0029 Physics HL Ms. Dossett 10 February 2014 Ding 2
Ding 1 Cool Off, Will Ya! Investigating Effect of Temperature Differences between Water and Environment on Cooling Rate of Water Chunyang Ding 000844-0029 Physics HL Ms. Dossett 10 February 2014 Ding 2
Exercise 1: Thermistor Characteristics
 Exercise 1: Thermistor Characteristics EXERCISE OBJECTIVE When you have completed this exercise, you will be able to describe and demonstrate the characteristics of thermistors. DISCUSSION A thermistor
Exercise 1: Thermistor Characteristics EXERCISE OBJECTIVE When you have completed this exercise, you will be able to describe and demonstrate the characteristics of thermistors. DISCUSSION A thermistor
DUBLIN INSTITUTE OF TECHNOLOGY Kevin Street, Dublin 8.
 Question Sheet Page 1 of 5 Instructions for the student: Question 1 is compulsory [40 marks] Attempt any two other questions [30 marks per question] The following must be made available during the examination:
Question Sheet Page 1 of 5 Instructions for the student: Question 1 is compulsory [40 marks] Attempt any two other questions [30 marks per question] The following must be made available during the examination:
Experiment 2: Laboratory Experiments on Ferroelectricity
 Experiment 2: Laboratory Experiments on Ferroelectricity 1. Task: Measure the dielectric constant εr of a TGS crystal as a function of temperature from room temperature up to 70 C at a frequency of about
Experiment 2: Laboratory Experiments on Ferroelectricity 1. Task: Measure the dielectric constant εr of a TGS crystal as a function of temperature from room temperature up to 70 C at a frequency of about
Experiment A4 Sensor Calibration Procedure
 Experiment A4 Sensor Calibration Procedure Deliverables: Checked lab notebook, Brief technical memo Safety Note: Heat gloves and lab coats must be worn when dealing with boiling water. Open-toed shoes
Experiment A4 Sensor Calibration Procedure Deliverables: Checked lab notebook, Brief technical memo Safety Note: Heat gloves and lab coats must be worn when dealing with boiling water. Open-toed shoes
Transient Heat Transfer Experiment. ME 331 Introduction to Heat Transfer. June 1 st, 2017
 Transient Heat Transfer Experiment ME 331 Introduction to Heat Transfer June 1 st, 2017 Abstract The lumped capacitance assumption for transient conduction was tested for three heated spheres; a gold plated
Transient Heat Transfer Experiment ME 331 Introduction to Heat Transfer June 1 st, 2017 Abstract The lumped capacitance assumption for transient conduction was tested for three heated spheres; a gold plated
MYP Year11 Chemistry Electrolysis Lab Annabel Suen 11.5
 MYP Year11 Chemistry Electrolysis Lab Annabel Suen 11.5 Introduction: There are many different factors that can affect the mass of copper deposit on the graphite electrode after electrolysis reaction of
MYP Year11 Chemistry Electrolysis Lab Annabel Suen 11.5 Introduction: There are many different factors that can affect the mass of copper deposit on the graphite electrode after electrolysis reaction of
Measurements & Instrumentation. Module 3: Temperature Sensors
 Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
PAPER 2 THEORY QUESTIONS
 PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
Name Partner. Thermal Physics. Part I: Heat of Vaporization of Nitrogen. Introduction:
 Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Welcome. Functionality and application of RTD Temperature Probes and Thermocouples. Dipl.-Ing. Manfred Schleicher
 Welcome Functionality and application of RTD Temperature Probes and Thermocouples Dipl.-Ing. Manfred Schleicher This presentation informs about the basics of RTD Temperature Probes and thermocouples Functionality
Welcome Functionality and application of RTD Temperature Probes and Thermocouples Dipl.-Ing. Manfred Schleicher This presentation informs about the basics of RTD Temperature Probes and thermocouples Functionality
Current and Resistance
 PHYS102 Previous Exam Problems CHAPTER 26 Current and Resistance Charge, current, and current density Ohm s law Resistance Power Resistance & temperature 1. A current of 0.300 A is passed through a lamp
PHYS102 Previous Exam Problems CHAPTER 26 Current and Resistance Charge, current, and current density Ohm s law Resistance Power Resistance & temperature 1. A current of 0.300 A is passed through a lamp
Thermometry. History. History 1/21/18. The art or science of temperature observation
 Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
EXPERIMENT 14 SPECIFIC HEAT OF WATER. q = m s T
 EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
Temperature Project. Lab: Section K. Masahiro Inano Christopher Casey Adam Tigue
 Temperature Project Lab: Section K Masahiro Inano Christopher Casey Adam Tigue 5/06/2010 Description: For our project a container of instant tomato soup was microwaved according to the given instructions.
Temperature Project Lab: Section K Masahiro Inano Christopher Casey Adam Tigue 5/06/2010 Description: For our project a container of instant tomato soup was microwaved according to the given instructions.
Laboratory 7 Measurement on Strain & Force. Department of Mechanical and Aerospace Engineering University of California, San Diego MAE170
 Laboratory 7 Measurement on Strain & Force Department of Mechanical and Aerospace Engineering University of California, San Diego MAE170 Megan Ong Diana Wu Wong B01 Tuesday 11am May 17 th, 2015 Abstract:
Laboratory 7 Measurement on Strain & Force Department of Mechanical and Aerospace Engineering University of California, San Diego MAE170 Megan Ong Diana Wu Wong B01 Tuesday 11am May 17 th, 2015 Abstract:
The Effect of Temperature and Concentration on Galvanic Cells
 http://www.periodni.com/gallery/galvanic_cell.png Joel Johnson, Yr 12 EEI 3.3, Final, 2/8/16 Page 1 of 12 The Effect of Temperature and Concentration on Galvanic Cells ABSTRACT Standard electrode potentials
http://www.periodni.com/gallery/galvanic_cell.png Joel Johnson, Yr 12 EEI 3.3, Final, 2/8/16 Page 1 of 12 The Effect of Temperature and Concentration on Galvanic Cells ABSTRACT Standard electrode potentials
If there is convective heat transfer from outer surface to fluid maintained at T W.
 Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Electrical and Magnetic Properties of High Temperature Superconductors Using Varying forms of Data Acquisition
 Journal of the Advanced Undergraduate Physics Laboratory Investigation Volume 1 Issue 1 Article 3 2013 Electrical and Magnetic Properties of High Temperature Superconductors Using Varying forms of Data
Journal of the Advanced Undergraduate Physics Laboratory Investigation Volume 1 Issue 1 Article 3 2013 Electrical and Magnetic Properties of High Temperature Superconductors Using Varying forms of Data
Student Experiments HEAT 2. Manual P9160-5C.
 Student Experiments Manual HEAT 2 P9160-5C www.ntl.at INDEX 1. CHANGE OF THE STATE OF AGGREGATION TDS 2.2 Specific heat of water 2. HEAT "QUANTITATIVELY" TDS 3.1 TDS 3.2 TDS 3.3 TDS 3.4 Quantitative heat
Student Experiments Manual HEAT 2 P9160-5C www.ntl.at INDEX 1. CHANGE OF THE STATE OF AGGREGATION TDS 2.2 Specific heat of water 2. HEAT "QUANTITATIVELY" TDS 3.1 TDS 3.2 TDS 3.3 TDS 3.4 Quantitative heat
Ordinary Level Physics Long Questions: TEMPERATURE AND HEAT
 Ordinary Level Physics Long Questions: TEMPERATURE AND HEAT Temperature 2014 Question 7 (a) [Ordinary Level] The temperature of an object can be measured using a thermometer which is based on a suitable
Ordinary Level Physics Long Questions: TEMPERATURE AND HEAT Temperature 2014 Question 7 (a) [Ordinary Level] The temperature of an object can be measured using a thermometer which is based on a suitable
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 3. Electrical Energy. Calculate the electrical power dissipated in a resistor.
 Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
5. TEMPERATURE AND HEAT
 5. TEMPERATURE AND HEAT You will study the concepts of temperature and heat as they apply to a sample of water and you will measure the specific heat capacity of the sample. The measurement of one property
5. TEMPERATURE AND HEAT You will study the concepts of temperature and heat as they apply to a sample of water and you will measure the specific heat capacity of the sample. The measurement of one property
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
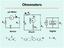 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
LAB. FACTORS INFLUENCING ENZYME ACTIVITY
 AP Biology Date LAB. FACTORS INFLUENCING ENZYME ACTIVITY Background Enzymes are biological catalysts capable of speeding up chemical reactions by lowering activation energy. One benefit of enzyme catalysts
AP Biology Date LAB. FACTORS INFLUENCING ENZYME ACTIVITY Background Enzymes are biological catalysts capable of speeding up chemical reactions by lowering activation energy. One benefit of enzyme catalysts
PHYS102 Previous Exam Problems. Temperature, Heat & The First Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
NEEL Phase Change in Chromium At the Néel Temperature
 University of Toronto ADVANCED PHYSICS LABOATOY NEEL Phase Change in Chromium At the Néel Temperature evisions: January 2018: January/August 2016: October 2005: Original: Young-June Kim David Bailey
University of Toronto ADVANCED PHYSICS LABOATOY NEEL Phase Change in Chromium At the Néel Temperature evisions: January 2018: January/August 2016: October 2005: Original: Young-June Kim David Bailey
Experiment 1. Measurement of Thermal Conductivity of a Metal (Brass) Bar
 Experiment 1 Measurement of Thermal Conductivity of a Metal (Brass) Bar Introduction: Thermal conductivity is a measure of the ability of a substance to conduct heat, determined by the rate of heat flow
Experiment 1 Measurement of Thermal Conductivity of a Metal (Brass) Bar Introduction: Thermal conductivity is a measure of the ability of a substance to conduct heat, determined by the rate of heat flow
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat
 PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
Superconductivity. Never store liquid nitrogen in a container with a tight fitting lid.
 Superconductivity 1 Introduction In this lab we will do some very simple experiments involving superconductors. You will not have to take much data; much of what you do will be qualitative. However, in
Superconductivity 1 Introduction In this lab we will do some very simple experiments involving superconductors. You will not have to take much data; much of what you do will be qualitative. However, in
EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical Simulation and Measurement
 Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Temperature Measurements
 ME 22.302 Mechanical Lab I Temperature Measurements Dr. Peter Avitabile University of Massachusetts Lowell Temperature - 122601-1 Copyright 2001 A transducer is a device that converts some mechanical quantity
ME 22.302 Mechanical Lab I Temperature Measurements Dr. Peter Avitabile University of Massachusetts Lowell Temperature - 122601-1 Copyright 2001 A transducer is a device that converts some mechanical quantity
Modelling the Temperature Changes of a Hot Plate and Water in a Proportional, Integral, Derivative Control System
 Modelling the Temperature Changes of a Hot Plate and Water in a Proportional, Integral, Derivative Control System Grant Hutchins 1. Introduction Temperature changes in a hot plate heating system can be
Modelling the Temperature Changes of a Hot Plate and Water in a Proportional, Integral, Derivative Control System Grant Hutchins 1. Introduction Temperature changes in a hot plate heating system can be
National 5 Physics. Electricity and Energy. Notes
 National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
Comparing 2-D Conduction Experiments with Simulation
 Comparing 2-D Conduction Experiments with Simulation Introduction Simulation techniques are often used in industry as a beneficial part in the development process whether an engineer or scientist is designing
Comparing 2-D Conduction Experiments with Simulation Introduction Simulation techniques are often used in industry as a beneficial part in the development process whether an engineer or scientist is designing
EXPERIMENT VARIATION OF THERMO-EMF WITH TEMPERATURE. Structure. 7.1 Introduction Objectives
 EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
Exercise 1: Thermocouple Characteristics
 The Thermocouple Transducer Fundamentals Exercise 1: Thermocouple Characteristics EXERCISE OBJECTIVE When you have completed this exercise, you will be able to describe and demonstrate the characteristics
The Thermocouple Transducer Fundamentals Exercise 1: Thermocouple Characteristics EXERCISE OBJECTIVE When you have completed this exercise, you will be able to describe and demonstrate the characteristics
Technical Notes. Introduction. PCB (printed circuit board) Design. Issue 1 January 2010
 Technical Notes Introduction Thermal Management for LEDs Poor thermal management can lead to early LED product failure. This Technical Note discusses thermal management techniques and good system design.
Technical Notes Introduction Thermal Management for LEDs Poor thermal management can lead to early LED product failure. This Technical Note discusses thermal management techniques and good system design.
Thermodynamics. We can summarize the four laws of thermodynamics as follows:
 Thermodynamics Objective: To investigate the zeroth and first laws of thermodynamics. To calculate properties such as specific heat. To investigate the ideal gas law. To become familiar with basic P-V
Thermodynamics Objective: To investigate the zeroth and first laws of thermodynamics. To calculate properties such as specific heat. To investigate the ideal gas law. To become familiar with basic P-V
Introduction to Heat and Mass Transfer. Week 1
 Introduction to Heat and Mass Transfer Week 1 This Lecture Course Organization Introduction Basic Modes of Heat Transfer Application Areas Instructor Information Professor Chang-Da Wen 温昌達» Office: 91713
Introduction to Heat and Mass Transfer Week 1 This Lecture Course Organization Introduction Basic Modes of Heat Transfer Application Areas Instructor Information Professor Chang-Da Wen 温昌達» Office: 91713
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Review: Heat, Temperature, Heat Transfer and Specific Heat Capacity
 Name: Block: Date: IP 614 Review: Heat, Temperature, Heat Transfer and Specific Heat Capacity All these questions are real MCAS questions! 1. In a copper wire, a temperature increase is the result of which
Name: Block: Date: IP 614 Review: Heat, Temperature, Heat Transfer and Specific Heat Capacity All these questions are real MCAS questions! 1. In a copper wire, a temperature increase is the result of which
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
5G50.52 Energy Storage with Superconductors
 5G50.52 Energy Storage with Superconductors Abstract Superconductors oppose magnetic fields and are generally considered to have zero resistivity. Theoretically then, a current in a superconducting ring
5G50.52 Energy Storage with Superconductors Abstract Superconductors oppose magnetic fields and are generally considered to have zero resistivity. Theoretically then, a current in a superconducting ring
Comparing the actual value and the experimental value on heat. By conservation of energy
 Topic: Heat 1. Temperature and thermometers a. Temperature: - measure degree of hotness. -measure the average kinetic energy of molecules in random motions. b. Fixed points: -Lower fixed point: temperature
Topic: Heat 1. Temperature and thermometers a. Temperature: - measure degree of hotness. -measure the average kinetic energy of molecules in random motions. b. Fixed points: -Lower fixed point: temperature
Effect of Magnetic and Electric Field Dynamics on Copper-Iron Thermocouple Performance
 Asian Journal of Chemistry Vol. 21, No. 10 (2009), S056-061 Effect of Magnetic and Electric Field Dynamics on Copper-Iron Thermocouple Performance JASPAL SINGH and S.S. VERMA Department of Physics, S.L.I.E.T.,
Asian Journal of Chemistry Vol. 21, No. 10 (2009), S056-061 Effect of Magnetic and Electric Field Dynamics on Copper-Iron Thermocouple Performance JASPAL SINGH and S.S. VERMA Department of Physics, S.L.I.E.T.,
Chapter 1. Blackbody Radiation. Theory
 Chapter 1 Blackbody Radiation Experiment objectives: explore radiation from objects at certain temperatures, commonly known as blackbody radiation ; make measurements testing the Stefan-Boltzmann law in
Chapter 1 Blackbody Radiation Experiment objectives: explore radiation from objects at certain temperatures, commonly known as blackbody radiation ; make measurements testing the Stefan-Boltzmann law in
Part 2. Sensor and Transducer Instrument Selection Criteria (3 Hour)
 Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
EXPERIMENT ET: ENERGY TRANSFORMATION & SPECIFIC HEAT
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01X Fall 2000 EXPERIMENT ET: ENERGY TRANSFORMATION & SPECIFIC HEAT We have introduced different types of energy which help us describe
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01X Fall 2000 EXPERIMENT ET: ENERGY TRANSFORMATION & SPECIFIC HEAT We have introduced different types of energy which help us describe
Chapter 3: Matter and Energy
 Chapter 3: Matter and Energy Convert between Fahrenheit, Celsius, and Kelvin temperature scales. Relate energy, temperature change, and heat capacity. The atoms and molecules that compose matter are in
Chapter 3: Matter and Energy Convert between Fahrenheit, Celsius, and Kelvin temperature scales. Relate energy, temperature change, and heat capacity. The atoms and molecules that compose matter are in
Experiment 5: Thermocouples (tbc 1/14/2007, revised 3/16/2007, 3/22,2007, 2/23/2009, 3/13/2011)
 Experiment 5: Thermocouples (tbc 1/14/2007, revised 3/16/2007, 3/22,2007, 2/23/2009, 3/13/2011) Objective: To implement a thermocouple circuit for temperature sensing. I. Introduction to Thermocouples
Experiment 5: Thermocouples (tbc 1/14/2007, revised 3/16/2007, 3/22,2007, 2/23/2009, 3/13/2011) Objective: To implement a thermocouple circuit for temperature sensing. I. Introduction to Thermocouples
ANALYSIS OF NANOFLUIDS IN LIQUID ELECTRONIC COOLING SYSTEMS
 Proceedings of the ASME 2009 InterPACK Conference IPACK2009 July 19-23, 2009, San Francisco, California, USA Proceedings of InterPACK09 ASME/Pacific Rim Technical Conference and Exhibition on Packaging
Proceedings of the ASME 2009 InterPACK Conference IPACK2009 July 19-23, 2009, San Francisco, California, USA Proceedings of InterPACK09 ASME/Pacific Rim Technical Conference and Exhibition on Packaging
Tick the box next to those resources for which the Sun is also the source of energy.
 1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
Lab E3: The Wheatstone Bridge
 E3.1 Lab E3: The Wheatstone Bridge Introduction The Wheatstone bridge is a circuit used to compare an unknown resistance with a known resistance. The bridge is commonly used in control circuits. For instance,
E3.1 Lab E3: The Wheatstone Bridge Introduction The Wheatstone bridge is a circuit used to compare an unknown resistance with a known resistance. The bridge is commonly used in control circuits. For instance,
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
5. Temperature and Heat
 Leaving Cert Physics Long Questions 2017-2002 5. Temperature and Heat Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Contents Temperature:
Leaving Cert Physics Long Questions 2017-2002 5. Temperature and Heat Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Contents Temperature:
Resistivity vs. Temperature of a Superconductor WARNING
 Resistivity vs. Temperature of a Superconductor 1.0 Expected Learning Outcomes Calibrate a transducer (silicon diode) for low-temperature measurements and measure temperatures down to that of boiling liquid
Resistivity vs. Temperature of a Superconductor 1.0 Expected Learning Outcomes Calibrate a transducer (silicon diode) for low-temperature measurements and measure temperatures down to that of boiling liquid
Per 5 Activity Solutions: Thermal Energy, the Microscopic Picture
 er 5 Activity Solutions: Thermal Energy, the Microscopic icture 5. How Is Temperature Related to Molecular Motion? ) Temperature Your instructor will discuss molecular motion and temperature. a) Watch
er 5 Activity Solutions: Thermal Energy, the Microscopic icture 5. How Is Temperature Related to Molecular Motion? ) Temperature Your instructor will discuss molecular motion and temperature. a) Watch
SPECIFIC HEAT CAPACITY
 SPECIFIC HEAT CAPACITY Apparatus: Thermometer, balance, two large double Styrofoam cups, lid, hooked metal cube, lifting tool, hot plate, boiling pot. Any material is capable of storing some heat or thermal
SPECIFIC HEAT CAPACITY Apparatus: Thermometer, balance, two large double Styrofoam cups, lid, hooked metal cube, lifting tool, hot plate, boiling pot. Any material is capable of storing some heat or thermal
Department of Mechanical and Aerospace Engineering. MAE334 - Introduction to Instrumentation and Computers. Final Examination.
 Name: Number: Department of Mechanical and Aerospace Engineering MAE334 - Introduction to Instrumentation and Computers Final Examination December 17, 2001 Closed Book and Notes 1. Be sure to fill in your
Name: Number: Department of Mechanical and Aerospace Engineering MAE334 - Introduction to Instrumentation and Computers Final Examination December 17, 2001 Closed Book and Notes 1. Be sure to fill in your
Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund
 Aalborg Universitet Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund Publication date: 2008 Document Version
Aalborg Universitet Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund Publication date: 2008 Document Version
Lab 6 Electrostatic Charge and Faraday s Ice Pail
 Lab 6 Electrostatic Charge and Faraday s Ice Pail Learning Goals to investigate the nature of charging an object by contact as compared to charging an object by induction to determine the polarity of two
Lab 6 Electrostatic Charge and Faraday s Ice Pail Learning Goals to investigate the nature of charging an object by contact as compared to charging an object by induction to determine the polarity of two
ECNG3032 Control and Instrumentation I
 sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
Sensors and Actuators Sensors Physics
 Sensors and Actuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems HEMOESISIVE SENSOS (Chapter 16.3) 3 emperature sensors placement excitation
Sensors and Actuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems HEMOESISIVE SENSOS (Chapter 16.3) 3 emperature sensors placement excitation
1. How much heat was needed to raise the bullet to its final temperature?
 Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
