Chapter 6 Temperature Measurement (Revision 2.0, 1/12/2009)
|
|
|
- Dorcas Tyler
- 5 years ago
- Views:
Transcription
1 Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he Celsius (of which the absolute range is the Kelvin scale) and the Fahrenheit (of which the absolute range is the Rankine scale). here are three principal methods of measuring temperature: temperature measurement by mechanical effects, temperature measurement by electrical effects; and temperature measurement by radiation effects. 2. Mechanical Effects Measuring temperature by mechanical effects is based on the fact that materials expand as their temperature increases. here are number of applications of this method. 2. Liquid-in-glass thermometer he liquid-in-glass thermometer is one of the most widely used devices in measuring temperature used in every-day use. It is based on the principle of the expansion of a liquid (such as mercury or alcohol) in a capillary glass tube. he rise of the fluid in the glass tube can be read off against a scale that indicated the temperature. An example of such a device is shown in Figure. he liquid is stored in a reservoir to provide enough fluid to fill the capillary tube. A safety bulb is provided at the top of the capillary tube to protect against the breakage of the glass in case of excessive expansion of the fluid. Safety bulb Proper depth of immersion Capillary tube emperature sensing bulb Fluid to be measured Figure : Diagram showing an example of a liquid-in-glass thermometer. It is worth mentioning the temperature indication on such a device is affected not only by the expansion of the fluid but also by the expansion of the glass Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page of 22
2 (in effect the rise in the fluid level depends on the difference between the expansion of the fluid and the expansion of the glass). In order to overcome this problem, manufacturers will alter the scale in order to take this effect into consideration based on a certain immersion level of the device. A mark will then be shown on the capillary tube showing the correct point of immersion that makes the scale correct (as shown in Figure ). he most widely used fluids are mercury and alcohol. Mercury has the disadvantage that is freezes at C so it cannot measure temperature below this value. Alcohol has a high coefficient of expansion (which is desirable to increase the sensitivity of the device) but is limited to measuring lower temperatures. he Mercury-in-glass device can used to measure temperature up to 35 C. If the space is filled with Nitrogen, then this can be increased to 538 C. 2.2 Bimetallic Strip Another mechanically based method exploits the fact that different metals have different thermal coefficients of expansion. If two strips of different metals are bonded together, then as the temperature rises from that at which they were bonded, one strip will elongate more than the other strip and the whole unit will bend with a certain radius of curvature. If one end of the device is fixed then the other end will move as the temperature changes. he following formula can be used to calculate the radius of curvature of the bonded strip as follows: Where: t 3 r = 6 2 ( + m) + ( + m n) ( α α ) ( ) ( + m) m 2 + m n t is the combined thickness (in m) m is the ratio of thickness of the two strips (dimensionless) n ratio of the moduli of elasticity of the two strips (dimensionless) α is the lower coefficient of expansion (in units of K - ) α 2 is the higher coefficient of expansion (in units of K - ) is the temperature at which the radius of curvature is required ( C) 0 is the initial bonding temperature ( C) Some examples of materials used in bimetallic strips are shown below: hermal coefficient of Modulus of elasticity Material expansion (K - ) (GPa) Invar.7x Yellow Brass 2.02x Stainless steel.6x he bimetallic strip is used widely in room thermostat devices and in electric water heaters (for controlling the temperature). Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 2 of 22
3 2.3 Fluid expansion hermometers hese devices work on the principle that a fluid will expand when heated. But if the volume on the container is fixed this will lead to an increase in pressure. If this pressure is measured, it could be used as an indication of the increase in temperature. A block diagram of such a system is shown in Figure 2 below. It shows a container with a fluid in it connected to a Bourdon pressure gauge using a capillary tube. he tube can be up to 60 m long. tube emperature Vapour Bourdon Pressure Gauge Liquid Figure 2: Diagram showing a fluid expansion thermometer. his device in effect converts the temperature into an expansion, the expansion into pressure and the pressure into a displacement (in the pressure gauge). he device has a low cost, is stable in operation and is widely used in industrial applications. It can provide an accuracy of around ± C. he dynamic response of the device depends on the volume of the bulb and the characteristics of the tube connecting it to the pressure gauge. 3. emperature Measurement by Electrical Effects here are six methods that can be used to measure temperature by electrical effects. hese are discussed in more detail in this section. 3. Resistance emperature Detector (RD) Whenever a metallic material is heated its resistivity will change. Most metals have a positive temperature coefficient (PC). his is due to the fact that metals in general have sufficient electrical carriers. So an increase in temperature will lead to an increase in the collisions between the carriers, thus increasing the resistivity of the material. A resistance temperature detector (RD) is a device that is used to detect the temperature change as a change in its resistance. he coefficient of temperature changes is calculated as follows: α = R R 2 R ( ) 2 Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 3 of 22
4 he unit of are K - (or more clearly can be thought of as Ω Ω - K - ). In effect it represents a percentage change in resistance (or resistivity) for every one degree change in temperature. For example Nickel has a value of equal to K - which means that its resistivity (or resistance) will increase by 0.67% for every one degree kelvin increase in temperature. A linear relationship is assumed between resistance and temperature and this is shown graphically in Figure 3. his linearity is only valid provided that it is applied over a narrow temperature range. R 2 Resistance R 2 emperature Figure 3: Approximate relationship between resistance and temperature for the RD. he coefficient is shown below for various materials. able : hermal coefficient of resistance for various materials. Material α (Ω Ω - K - ) Nickel ungsten Platinum Manganin ± Carbon Semiconductors to +0.4 For wider temperature variations the linearity is no longer valid, and a nonlinear model has to be used. 2 ( + a + b ) R = R0 Where a and b are constants that depend on the material. It is important to protect the wire element from moisture coming into contact with it and from mechanical stresses, as both of these disturbances would change the value of the resistance. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 4 of 22
5 In practice an RD is built by winding a wire around a glass or ceramic bobbin (former) and then sealing it with molten glass. he molten glass protects the wire element from moisture, but it introduces stresses into the material caused by temperature variations (i.e., strain gauge effect). A RD is usually installed within a dc bridge. As opposed to the use of four strain gauges in the case of force or strain measurements, only one element is used within a bridge for temperature measurements. As this element might be installed far away from the bridge, an error develops caused by the lead wires connecting the temperature sensor to the bridge. his requires socalled lead-wire compensation. A number of methods are available including the three wire Siemens method, the Callender 4 wire method, and the floating wire method. Practical problems in the use of RD s are:. he error caused by the leads. 2. Its bulky size that slows down its speed of response and thus increases its time constant. 3. Its fragile construction (as it contains glass). 4. he risk of self heating (I 2 R effects) when placed in the bridge. 3.2 hermistor hermistors, as opposed to RDs, have a negative temperature coefficient of resistance, but are much more sensitive that RD s. heir resistance can be modelled in accordance with the following exponential equation: R = R 0 e β 0 he value of β ranges from 3500 to 4600 K (note that its unit must be K in order to balance the equation above dimensionally). It is worth noting that the equation above can also be expressed in terms of resistivity (rather than resistance) as follows: ρ l R = = R A ρ = ρ e 0 0 e β 0 β 0 ρ0 l = e A β 0 It is worth noting that the slope of this curve is negative (i.e., it has a negative temperature coefficient, NC). he sensitivity at a certain point can be found by differentiating the function above at that point. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 5 of 22
6 Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 6 of 22 = = e dt d e β ρ ρ ρ ρ β β Example Calculate the sensitivity of a thermistor at =00 C=373 K, if 00 ρ =0 Ω cm=. Ω m and, β=420 K at =00 C Solution Substituting the values in the equation above, gives: K m e. e S C 00 0 Ω = = β = ρ β = he sign of the answer is obviously negative confirming the negative slope of the function and the NC property. A thermistor is usually used as one arm of a dc bridge to detect temperature. he two outputs of the bridge are connected to the inputs of an instrumentation amplifier. A thermistor usually consists of a semiconductor material, and hence cannot withstand high temperatures. It cannot be used for measuring temperatures above 300 C. hermistors for example are used for measuring the temperature inside motor windings, for temperatures up to around 80 C (F class insulation). 3.3 hermoelectric Effects (thermocouple) he third electric method of temperature measurement makes use of the socalled temperature effect. When two different metals are placed against each other, an emf is produced that is a function of the temperature of the junction (Figure 4). he junction is called a thermocouple.
7 θ Material Material 2 + emf - Figure 4: A thermocouple is a junction of two different materials. here are three effects that interact within the thermocouple []:. Seebeck effect: his is the most important effect. It is the fact that a voltage (emf) is produced as a function of the junction temperature. 2. Peltier effect: If a current is drawn from the junction, the original voltage (emf) is altered. 3. homson effect: If a temperature gradient exists along either or both materials, then an addition alteration of the emf takes place. Various types of thermocouples are shown below showing the letter designation and the materials there are made of (starting with the positive material) [2]: able 2: Various thermocouples with their materials. ype E J K N S Material (positive listed first) Chromel-Constantan Iron-Constantan Chromel-Alumel Nicrosil-Nisil Platinum/0% Rhodium-Platinum Copper-Constantan When attempting to measure the voltage from a thermocouple, a practical problem is encountered. When the thermocouple terminals are connected to the voltmeter, for example, they have to be connected via a wire that is a different material that the materials making the thermocouple (most likely the connecting wire is copper). But this in effect creates two new junctions: One between the first material of the thermocouple and the first copper wire; the second between the second material and the other copper wire. his will create an error in the reading of the voltmeter due to the two new thermocouples introduced into the circuit. his can be further complicated by the other two new junctions formed from the copper wires connecting to the voltmeter itself which might have different materials internally. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 7 of 22
8 θ J Material J2 J3 Material 2 Copper Copper J4 J5 Material 3 Material V/M Figure 5: he five junctions that form when trying to measure the voltage output of a thermocouple by the use of a voltmeter. he full setup is shown in Figure 5. Five junctions are shown. J is intentional, but junctions 2 to 5 are unintentional. Provided J4 and J5 are at the same temperature, their effect will cancel out as they have opposing polarities. his leave J2 and J3 to deal with, as their effect will be to alter the voltage ready by the voltmeter. So the circuit will simplify to that shown in Figure 6 below. θ J Material J2 J3 Material 2 Copper Copper + - V/M Figure 6: the two junctions J4 and J5 can now be removed as they cancel out. In order to further simplify the model above we can use the following two thermocouple laws:. he law of intermediate metals: If a metal is placed between the two materials of the thermocouples, the net voltage is the same if the intermediate metal is removed. his is shown graphically in below. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 8 of 22
9 θ Material θ Material θ J Material 3 = J3 J2 Material 2 Material 2 Figure 7: the two junctions J4 and J5 can now be removed as they cancel out. By using this law, we can further simplify the circuit as shown below: θ J Material Material 2 J2 Material V/M Figure 8: Using the law of intermediate metals, we can remove the copper from the circuit model. In this setup we call J the hot junction and J2 the reference junction. Obviously the temperature of this junction will affect the final reading of the voltmeter. We next use the law of intermediate temperatures. 2. he law of intermediate temperatures: he voltage produced by the hot junction is equal to the sum of the voltage shown by the voltmeter and the voltage produced by the reference junction. his consequence of this law is that if we can measure the voltage at the voltmeter, add to it the voltage produced by the reference junction (knowing the temperature of the reference junction), then we can use the resultant voltage to infer the temperature of the hot junction. his can be done by the use of tables. For each thermocouple the relationship between the junction temperature and the voltage from it are tabulated, with reference to a Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 9 of 22
10 reference junction of 0 C. he table, by definition shows 0 V for a junction temperature junction of 0 C. Example 2 Assume you are using a K-type thermocouple. he voltmeter is measuring a voltage of 2.5 mv. If you know that the reference junction (i.e., the two connections between the two thermocouple wires and the two Copper terminals) are at room temperature, which is known to be 25 C. What is the temperature of the hot junction, if:. You ignored the error caused by the reference junction? 2. You took the reference junction into consideration? Use the attached K type thermocouple table that provides the value of emf for each temperature in Celsius. Solution If we ignore the reference junction, then we can use the table to find the temperature for a K-type thermocouple when it produces a voltage of 2.5 mv. his gives a temperature of 62 C. However, if we take the reference junction into consideration, then we must first add to the voltage measured the corresponding voltage for the 25 C temperature to the reference junction, which from the table is (using interpolation).000 mv. hus the total voltage for the hot junction is = mv. Using this value in the table again (and also using interpolation) gives a hot junction temperature of 86 C. Note that the answer is no 87 C as would be incorrectly inferred by adding the two temperatures corresponding to the reference junction and the temperature found from the table for the read voltage. his is due the non-linearity in the relationship. Also note that the error from ignoring the reference junction is very large (around 24 C!). One way to overcome the problem of having to know the temperature f the reference junction is use a reference junction that is kept at 0 C. One way of doing this is to immerse the reference junction (in practice two junctions, one for each of the thermocouple terminals) in an ice-water mixture. Such a mixture will remain at 0 C provided:. he ice has not all melted. 2. he pressure is exactly one atmospheric pressure. 3. he water is distilled and saturated with air. Appendix B shows a simple experiment conducted to show how the water/ice mixture stays at a constant temperature until all the ice has melted. In this case the temperature at which it stays constant is not 0 C but 4 C due the imperfections in the conditions required above (e.g., water not distilled, pressure not at atmospheric pressure) in addition to errors in the measuring device (liquid-in-glass thermometer). Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 0 of 22
11 If such an accurate setup is available to keep the reference junction at 0 C, then the temperature that is read on the voltmeter can be directly use to infer the hot junction temperature, without the need for any correction. he following are the problems usually encountered with the use of thermocouples:. Junctions could be formed using high temperatures or faulty soldering. 2. hermocouples could be used outside their applicable temperature range. 3. Faulty reference junction may be employed. 4. Faults in the installation. 5. Wrong type of the thermocouple for the readout instrument (i.e., each readout instrument must be matched to the thermocouple type). hermocouples could also be connected in series to increase the output sensitivity or be used to detect the temperature difference between two objects. hey are then called thermopiles. If an even number of junctions is used, this removes the need for a reference junction. But care must be taken to ensure they are all kept insulated from each other. Parallel connection is also possible; although this presents the problem that they may not equally share the current. 3.4 emperature sensitive semiconductor devices Semiconductor devices can also be used for accurate temperature measurement. hey offer the advantage of a linear output that is already signal conditioned. A widely used example device is the LM35 that is shown in Appendix C. 3.5 Quartz Crystal hermometers he resonant frequency of a quartz crystal is sensitive to its temperature. Provided the correct angle is cut, a very linear relationship is obtained between the frequency and the temperature. his can be used to accurately measure the temperature. Since it relies on frequency measurement, the device is less sensitive to noise pickup in the connecting cables. he device has a sensitivity of around 00 Hz K -, and can measure temperature in the range of -40 to 230 C. 3.6 Liquid Crystal hermography Certain materials exhibit different colours depending on their temperature. hese can be used to indicate temperature changes. Certain esters of cholesterol exhibit this phenomenon. he advantage is that this change is reversible and repeatable, and it can be made to work from 0 C up to several hundred C. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page of 22
12 4. hermal time constant he thermal time constant of a thermocouple will be derived in this section. It is based on the assumption that heat will only be transferred by convection and that none is transferred by radiation. he rate of flow of heat into the thermocouple will lead to a change in its temperature. his can be expressed as follows (note that the left hand side represents the flow of heat into the thermocouple, where it depends on the area, the heat transfer coefficient and the difference in temperature the right hand side is the rise in the temperature of the thermocouple due to the received heat): h A ( ) = m c Where: h is the heat transfer coefficient (W m -2 K - ) A is the surface area of the thermocouple exposed to the surrounding fluid (m 2 ) is the temperature of the surrounding fluid (K) is the temperature of the thermocouple (K) m is the mass of the thermocouple (kg). c is the specific heat capacity of the thermocouple material (J kg - K - ) t is time (s) Rearranging: d dt m c d dt + h A = h A aking Laplace transforms, gives the transfer function: ( s ) Where the time constant is equal to m c s + h A = m c h A and the steady state gain is equal to. his shows that the time constant does not only depend on the characteristics of the thermocouple (h, A and c), but also on the medium (h: the heat transfer coefficient that depends on the fluid surrounding the thermocouple). Compensation can be applied to improve the response of the thermocouple by the use of the RC network shown below. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 2 of 22
13 R C V i C R V o Figure 9: Dynamic Compensation Network. It can be shown that its response is: F( s ) + τ s = α + α τ s where : τ = R C C R α = R + R C If τ is made to be equal to the time constant of the thermocouple, then the effect of this dynamic compensator is to reduce the time constant by multiplying it by a number that is less than (i.e., α) and reducing the steady state gain by multiplying by α as well. So the dynamic response is speeded up at the expense of steady state gain. 5. Radiation Effects emperature can also be measured by the use of radiation effects. he main advantage of this method is that no contact is required between the measurement device and the object. his is very useful in hazardous environments. hermal radiation is electromagnetic radiation emitted by a body by virtue of its temperature. his should be distinguished from other forms of electromagnetic radiation such as radio waves and X-rays that are not propagated as a result of temperature. It is worth noting that electromagnetic radiation in general does not need a medium for transport. 5. Radiation Pyrometry (Emittance Determination) Any object when subjected to thermal radiation will either absorb some of the energy, reflect some of the energy and transmit some of the energy (transmission is only applicable to transparent objects that will allow the energy to continue to the other side). his can be summarised in the following equation: = α + ρ + τ Where these three parameters are all dimensionless α is the absorptivity ρ is the reflectivity Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 3 of 22
14 τ is the transmissivity (only applicable to transparent objects) For the special case of opaque objects, this reduces to: = α + ρ In other words, the received thermal energy must be either reflected or absorbed. A further special case of this is the case of a perfect black body, that does not reflect any energy (the perfect black body must have a black colour and a rough surface). In this case, the equation further reduces to: = α We shall define the emissive power (E) as the amount of thermal radiation energy emitted by a body per unit area at a certain temperature. he units of E are W m -2. As the black body has the highest emissivity (as it has zero reflectivity) we can compare the emissive power of any body by dividing it by the emissive power of a black body at the same temperature. his parameter is called the emissivity (denoted by ε) and is very important in temperature measurement by radiation effects. ε = It is worth noting that the emissive power of a black body varies with wavelength. Under thermal equilibrium, any thermal energy received by the body will raise its temperature. hus, under thermal equilibrium conditions for an opaque body: ε = E = E E b E E b = α = ρ ( ρ) Eb = ε Eb But the emissive power of a black body at a certain temperature is related to the absolute temperature of the body as follows: E b = σ 4 Where σ is the Stefan Boltzmann constant (5.669x0-8 W m -2 K -4 ) [his should be distinguished from the Boltzmann constant used in calculating the thermal noise from a resistor.37x0-23 J K - ] Combining the last two equations gives us the basis for measuring the temperature of any body by thermal radiation: Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 4 of 22
15 E = ε σ E = ε σ 4 4 So the temperature of a body can be measured by radiation by measuring the emissive power from it and knowing the emissivity. he emissivity is the item that need determining and could lead to an error. he emissivity of a body depends on the following:. Surface finish. 2. Colour. 3. Oxidisation. 4. Aging. 5. Other factors. his method is thus not suitable for measuring the temperature of very shiny and smooth objects (e.g., polished stainless steel). Most of the radiation meters will allow the user to enter the value of emissivity for the object he/she is measuring. he meter usually has a default value of 0.95 for emissivity. Adjustment for material with different emissivities Most radiation thermometers will have an assumed emissivity that is used within the device, usually If the emissivity of the body the temperature of which is being measured has a different emissivity the user can make an adjustment in the reading to account for such a different. he method of adjustment is discussed below. Let us assume the following: E is the emissive power per unit area of the body being measured in units of W m -2 ε def is the emissivity that is assumed within the device (default emissivity) ε act is the emissivity of the object being measured (actual emissivity) σ is the Stefan Boltzmann constant (5.669x0-8 W m -2 K -4 ) ind is the indicated temperature by the device in K act is the actual temperature of the body in K 4 ind E ind = ε def σ 4 = E ind ε def σ E E ε def = ε = ε def σ ε def σ ε act ε 4 act def E ε = ε act σ ε act def But: Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 5 of 22
16 E = ε act σ Substituting in the previous equation gives: 4 act 4 ind = 4 act ε ε act def Or ind act ε = ε act def 4 Rearranging gives the important result: = act ind ε ε def act 4 So by knowing the indicated temperature by the thermometer, the actual emissivity of the body and the default emissivity assumed in the device (usually given in the manual) the actual correct temperature of the body can be found. A list of the emissivities of some common materials can be used for the correction. 5.2 Optical Pyrometry his method is based on the principle that material when heated up to high temperatures will change colours in accordance with the temperature (staring with dark red, then orange and then white). By using a special optical device, a user can adjust the device until a reading can be taken of the colour emitted. his method is only suitable for high temperatures. 6. Comparison of methods Appendix A contains a table from [] that shows a comparison of the various methods with their typical parameters. It is worth noting the following:. he S-type thermocouple is the most resistant to oxidising environments, but has a high cost. 2. he RD is the most accurate device. 3. he thermistor has a good dynamic response. 4. he radiation methods are the only methods that are contact-less. References Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 6 of 22
17 [] Experimental Methods for Engineers, J.P. Holman, 7 th Edition, McGraw Hill International Edition. [2] Measurement & Instrumentation Principles, Alan S. Morris, Elsevier, 200. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 7 of 22
18 Appendix A: Comparison of different types of temperature measurement devices (adapted from able 8.7 of J.P.Holman, Experimental Methods for Engineers []) Effect Principle Mechanical Device Electrical Applicable emperature Range ( C) Approximate accuracy ( C) ransient Response Cost Comments Liquid in glass Fluid expansion thermometer Bimetallic Strip RD (electrical resistance) Alcohol Mercury Glass filled Mercury Liquid or gas Vapourpressure Bimetallic Strip RD (electrical resistance) -70 to to to to to to to 000 ±0.5 Poor Low ±0.25 Poor Variable ±0.25 Poor Variable ± Poor Low ± Poor Low ±0.25 Poor Low ± Fair to good depending on size of element Readout equipment can be rather expensive for highprecision work Used as inexpensive low cost thermometers. Accuracy of ±0.05 C may be obtained with special calibrated thermometers Widely used for industrial temperature measurements Widely used in simple temperature-control devices Most accurate of all methods Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 8 of 22
19 Effect Principle Device Applicable emperature Range ( C) Approximate accuracy ( C) ransient Response Cost Comments hermistor hermoelectric hermistor -type J-ype K-ype S-ype -70 to to to to to 650 ±0.0 Very good ±0.25 Good, depends on wire size Low, but readout equipment may be rather expensive for highprecision work Low Low Low High Useful for temperature compensation circuits; thermistor beads may be obtained in very small sizes. Superior in reducing atmospheres Resistant to oxidation at high temperatures Low output; most resistant to oxidation at high temperatures; accuracy of ±0.5 may be obtained in carefully controlled conditions Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 9 of 22
20 Effect Principle Device Applicable emperature Range ( C) Approximate accuracy ( C) ransient Response Radiation Cost Comments Optical Pyrometry Radiation Pyrometry Optical Pyrometer Radiation Pyrometer 650 and above -5 and above ±0 Poor Medium ±0.5 at low ranges* Good, depending on type Medium to high Widely used for measurement of industrial furnace temperatures Increased applications resulting from new high precision devices being developed * ±2.5 to 0 at high temperatures; depends on blackbody conditions and type of pyrometer. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 20 of 22
21 Appendix B: Experimental investigation into the temperature of an ice-water mixture against time compared to water. Figure 0: ime plot of temperature of ice-water mixture. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 2 of 22
22 Appendix C: Part of a datasheet for a semiconductor type of temperature sensor, LM35. Copyright held by the author 2009: Dr. Lutfi R. Al-Sharif Page 22 of 22
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
MEASURING INSTRUMENTS
 Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Lecture 36: Temperatue Measurements
 Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
Temperature measurement
 emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
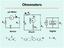 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
Introduction. Measurement of temperature is generally considered to be one of the simplest and most accurate measurements performed in engineering.
 Slide Nr. 0 of 15 Slides Introduction Measurement of temperature is generally considered to be one of the simplest and most accurate measurements performed in engineering. Some important considerations
Slide Nr. 0 of 15 Slides Introduction Measurement of temperature is generally considered to be one of the simplest and most accurate measurements performed in engineering. Some important considerations
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
 Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Physics 17 Spring 2003 The Stefan-Boltzmann Law
 Physics 17 Spring 2003 The Stefan-Boltzmann Law Theory The spectrum of radiation emitted by a solid material is a continuous spectrum, unlike the line spectrum emitted by the same material in gaseous form.
Physics 17 Spring 2003 The Stefan-Boltzmann Law Theory The spectrum of radiation emitted by a solid material is a continuous spectrum, unlike the line spectrum emitted by the same material in gaseous form.
Control Engineering BDA30703
 Control Engineering BDA30703 Lecture 4: Transducers Prepared by: Ramhuzaini bin Abd. Rahman Expected Outcomes At the end of this lecture, students should be able to; 1) Explain a basic measurement system.
Control Engineering BDA30703 Lecture 4: Transducers Prepared by: Ramhuzaini bin Abd. Rahman Expected Outcomes At the end of this lecture, students should be able to; 1) Explain a basic measurement system.
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test
 ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
1. How much heat was needed to raise the bullet to its final temperature?
 Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Part 2. Sensor and Transducer Instrument Selection Criteria (3 Hour)
 Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
VALLIAMMAI ENGINEERING COLLEGE
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6502 -INDUSTRIAL INSTRUMENTATION I Regulation 2013
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6502 -INDUSTRIAL INSTRUMENTATION I Regulation 2013
1 THE CONCEPT OF TEMPERATURE
 1 THE CONCEPT OF TEMPERATURE 1 1.1 Historical Perspective, 2 1.2 Early Definitions of Temperature, 9 1.3 A Simple Qualitative Definition of Temperature, 10 1.4 Units of Temperature for Various Temperature
1 THE CONCEPT OF TEMPERATURE 1 1.1 Historical Perspective, 2 1.2 Early Definitions of Temperature, 9 1.3 A Simple Qualitative Definition of Temperature, 10 1.4 Units of Temperature for Various Temperature
Handout 10: Heat and heat transfer. Heat capacity
 1 Handout 10: Heat and heat transfer Heat capacity Consider an experiment in Figure 1. Heater is inserted into a solid substance of mass m and the temperature rise T degrees Celsius is measured by a thermometer.
1 Handout 10: Heat and heat transfer Heat capacity Consider an experiment in Figure 1. Heater is inserted into a solid substance of mass m and the temperature rise T degrees Celsius is measured by a thermometer.
15. Compare the result with the value you have taken above Compare the calculated pressure value with the actual pressure value that you have
 105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Lecture 22. Temperature and Heat
 Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
4. Thermometry. Temperature and Heat Flow Temperature Scales Thermometers
 4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
Temperature Measurement
 Temperature Measurement Dr. Clemens Suter, Prof. Sophia Haussener Laboratory of Renewable Energy Sciences and Engineering Suter Temperature Measurement Mar, 2017 1/58 Motivation Suter Temperature Measurement
Temperature Measurement Dr. Clemens Suter, Prof. Sophia Haussener Laboratory of Renewable Energy Sciences and Engineering Suter Temperature Measurement Mar, 2017 1/58 Motivation Suter Temperature Measurement
Sensors and Actuators Sensors Physics
 Sensors and Actuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems HEMOESISIVE SENSOS (Chapter 16.3) 3 emperature sensors placement excitation
Sensors and Actuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems HEMOESISIVE SENSOS (Chapter 16.3) 3 emperature sensors placement excitation
I. MEASUREMENT OF TEMPERATURE
 I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
Temperature. 3
 Temperature In 1848, Sir William Thomson (Lord Kelvin) stated the zero principle of dynamics. This principle enabled him to define thermodynamic temperature and to establish an objective method of measuring
Temperature In 1848, Sir William Thomson (Lord Kelvin) stated the zero principle of dynamics. This principle enabled him to define thermodynamic temperature and to establish an objective method of measuring
Thermometry. History. History 1/21/18. The art or science of temperature observation
 Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Process Control & Design
 458.308 Process Control & Design Lecture 5: Feedback Control System Jong Min Lee Chemical & Biomolecular Engineering Seoul National University 1 / 29 Feedback Control Scheme: The Continuous Blending Process.1
458.308 Process Control & Design Lecture 5: Feedback Control System Jong Min Lee Chemical & Biomolecular Engineering Seoul National University 1 / 29 Feedback Control Scheme: The Continuous Blending Process.1
National 5 Physics. Electricity and Energy. Notes
 National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
INSTRUMENTATION ECE Fourth Semester. Presented By:- Sumit Grover Lect., Deptt. of ECE
 INSTRUMENTATION ECE Fourth Semester Presented By:- Sumit Grover Lect., Deptt. of ECE Detailed Contents Objectives Sensors and transducer Classification of transducers Temperature transducers Resistance
INSTRUMENTATION ECE Fourth Semester Presented By:- Sumit Grover Lect., Deptt. of ECE Detailed Contents Objectives Sensors and transducer Classification of transducers Temperature transducers Resistance
Lecture 2: Zero law of thermodynamics
 Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Sensing, Computing, Actuating
 Sensing, Computing, Actuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems HEMOESISIVE SENSOS AND LINEAIZAION (Chapter.9, 5.11) 3 Applications discharge air temperature
Sensing, Computing, Actuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems HEMOESISIVE SENSOS AND LINEAIZAION (Chapter.9, 5.11) 3 Applications discharge air temperature
Week-7 Assignment-1. The due date for submitting this assignment has passed. (1/273.15)th of the normal freezing point of water
 X reviewer2@nptel.iitm.ac.in Courses» Chemical Process Instrumentation Unit 8 - Week 7 Announcements Course Ask a Question Progress Mentor Course outline How to access the portal Week-7 Assignment-1 The
X reviewer2@nptel.iitm.ac.in Courses» Chemical Process Instrumentation Unit 8 - Week 7 Announcements Course Ask a Question Progress Mentor Course outline How to access the portal Week-7 Assignment-1 The
Chapter 24 Photonics Question 1 Question 2 Question 3 Question 4 Question 5
 Chapter 24 Photonics Data throughout this chapter: e = 1.6 10 19 C; h = 6.63 10 34 Js (or 4.14 10 15 ev s); m e = 9.1 10 31 kg; c = 3.0 10 8 m s 1 Question 1 Visible light has a range of photons with wavelengths
Chapter 24 Photonics Data throughout this chapter: e = 1.6 10 19 C; h = 6.63 10 34 Js (or 4.14 10 15 ev s); m e = 9.1 10 31 kg; c = 3.0 10 8 m s 1 Question 1 Visible light has a range of photons with wavelengths
Chapter 18. Temperature, Heat, and the First Law of Thermodynamics Temperature
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
Measurements & Instrumentation. Module 3: Temperature Sensors
 Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
1 Written and composed by: Prof. Muhammad Ali Malik (M. Phil. Physics), Govt. Degree College, Naushera
 CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
Temperature. Sensors. Measuring technique. Eugene V. Colla. 10/25/2017 Physics 403 1
 Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
TRANSMISSION OF HEAT
 TRANSMISSION OF HEAT Synopsis :. In general heat travels from one point to another whenever there is a difference of temperatures.. Heat flows from a body at higher temperature to a lower temperature..
TRANSMISSION OF HEAT Synopsis :. In general heat travels from one point to another whenever there is a difference of temperatures.. Heat flows from a body at higher temperature to a lower temperature..
14CH406. Chemical Engineering Scheme of Valuation Process Instrumentation Maximum : 60 Marks (1X12 = 12 Marks) Answer ONE question from each unit.
 April, 2018 Fourth Semester Time: Three Hours Answer Question No.1 compulsorily. II/IV B.Tech. (Regular/Supplementary) DEGREE EXAMINATION 14CH406 Chemical Engineering Scheme of Valuation Process Instrumentation
April, 2018 Fourth Semester Time: Three Hours Answer Question No.1 compulsorily. II/IV B.Tech. (Regular/Supplementary) DEGREE EXAMINATION 14CH406 Chemical Engineering Scheme of Valuation Process Instrumentation
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Tick the box next to those resources for which the Sun is also the source of energy.
 1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
Chapter 16 Temperature and Heat
 Chapter 16 Temperature and Heat 16-1 Temperature and the Zeroth Law of Thermodynamics Definition of heat: Heat is the energy transferred between objects because of a temperature difference. Objects are
Chapter 16 Temperature and Heat 16-1 Temperature and the Zeroth Law of Thermodynamics Definition of heat: Heat is the energy transferred between objects because of a temperature difference. Objects are
Making Contact with Temperature
 Making Contact with Temperature Here is a look at the phenomenon itself, the basic measurement technologies available, and how industry is presently using them. Jesse Yoder, Flow Research Temperature is
Making Contact with Temperature Here is a look at the phenomenon itself, the basic measurement technologies available, and how industry is presently using them. Jesse Yoder, Flow Research Temperature is
Energy. E d. Energy Power = time. E t P = E t = P
 Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Chapter 6. Fiber Optic Thermometer. Ho Suk Ryou
 Chapter 6. Fiber Optic Thermometer Ho Suk Ryou Properties of Optical Fiber Optical Fiber Composed of rod core surrounded by sheath Core: conducts electromagnetic wave Sheath: contains wave within the core
Chapter 6. Fiber Optic Thermometer Ho Suk Ryou Properties of Optical Fiber Optical Fiber Composed of rod core surrounded by sheath Core: conducts electromagnetic wave Sheath: contains wave within the core
Real-Time & Embedded 1Systems Physical Coupling. Uwe R. Zimmer - The Australian National University
 Real-Time & Embedded 1Systems 2015 Physical Coupling Uwe R. Zimmer - The Australian National University [ Edler2003 ] Edler et al. Noise temperature measurements for the determination of the thermodynamic
Real-Time & Embedded 1Systems 2015 Physical Coupling Uwe R. Zimmer - The Australian National University [ Edler2003 ] Edler et al. Noise temperature measurements for the determination of the thermodynamic
Theory and Design for Mechanical Measurements
 Theory and Design for Mechanical Measurements Third Edition Richard S. Figliola Clemson University Donald E. Beasley Clemson University John Wiley & Sons, Inc. New York / Chichester / Weinheim / Brisbane
Theory and Design for Mechanical Measurements Third Edition Richard S. Figliola Clemson University Donald E. Beasley Clemson University John Wiley & Sons, Inc. New York / Chichester / Weinheim / Brisbane
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Thermodynamics and Statistical Physics
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Latest Heat Transfer
 Latest Heat Transfer 1. Unit of thermal conductivity in M.K.S. units is (a) kcal/kg m2 C (b) kcal-m/hr m2 C (c) kcal/hr m2 C (d) kcal-m/hr C (e) kcal-m/m2 C. 2. Unit of thermal conductivity in S.I. units
Latest Heat Transfer 1. Unit of thermal conductivity in M.K.S. units is (a) kcal/kg m2 C (b) kcal-m/hr m2 C (c) kcal/hr m2 C (d) kcal-m/hr C (e) kcal-m/m2 C. 2. Unit of thermal conductivity in S.I. units
= (fundamental constants c 0, h, k ). (1) k
 Introductory Physics Laboratory, Faculty of Physics and Geosciences, University of Leipzig W 12e Radiation Thermometers Tasks 1 Measure the black temperature T s of a glowing resistance wire at eight different
Introductory Physics Laboratory, Faculty of Physics and Geosciences, University of Leipzig W 12e Radiation Thermometers Tasks 1 Measure the black temperature T s of a glowing resistance wire at eight different
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
PALO VERDE NUCLEAR GENERATING STATION
 PALO VERDE NUCLEAR GENERATING STATION I&C Program Classroom Lesson I&C Program Date: 2/25/2011 1:42:48 PM LP Number: NIA97C000503 Rev Author: DANIEL R. REED Title: Temperature Measurement Technical Review:
PALO VERDE NUCLEAR GENERATING STATION I&C Program Classroom Lesson I&C Program Date: 2/25/2011 1:42:48 PM LP Number: NIA97C000503 Rev Author: DANIEL R. REED Title: Temperature Measurement Technical Review:
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Measurement in Engineering
 Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Temperature Measurement
 Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Thermal Process Control Lap 4 Thermal Energy. Notes:
 Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation
 IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation Introduction Due to their low cost and ease of use, thermocouples are still a popular means for making temperature measurements up
IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation Introduction Due to their low cost and ease of use, thermocouples are still a popular means for making temperature measurements up
Solar Flat Plate Thermal Collector
 Solar Flat Plate Thermal Collector INTRODUCTION: Solar heater is one of the simplest and basic technologies in the solar energy field. Collector is the heart of any solar heating system. It absorbs and
Solar Flat Plate Thermal Collector INTRODUCTION: Solar heater is one of the simplest and basic technologies in the solar energy field. Collector is the heart of any solar heating system. It absorbs and
High temperature He is hot
 Lecture 9 What is Temperature and Heat? High temperature He is hot Some important definitions * Two objects are in Thermal contact with each other if energy can be exchanged between them. Thermal equilibrium
Lecture 9 What is Temperature and Heat? High temperature He is hot Some important definitions * Two objects are in Thermal contact with each other if energy can be exchanged between them. Thermal equilibrium
SENSORS AND TRANSDUCERS
 Electrical Measurements International Program Department of Electrical Engineering UNIVERSITAS INDONESIA ANDRITTO ABDUL GHAFFAR ANDHIKA ADIEL INSANI Lecturer : Ir. Chairul Hudaya, ST, M.Eng., Ph.D., IPM
Electrical Measurements International Program Department of Electrical Engineering UNIVERSITAS INDONESIA ANDRITTO ABDUL GHAFFAR ANDHIKA ADIEL INSANI Lecturer : Ir. Chairul Hudaya, ST, M.Eng., Ph.D., IPM
ME 105 Mechanical Engineering Laboratory Spring Quarter Experiment #2: Temperature Measurements and Transient Conduction and Convection
 ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2010 Experiment #2: Temperature Measurements and Transient Conduction and Convection Objectives a) To calibrate
ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2010 Experiment #2: Temperature Measurements and Transient Conduction and Convection Objectives a) To calibrate
Transducers. EEE355 Industrial Electronics
 Transducers EEE355 Industrial Electronics 1 Terminology Transducers convert one form of energy into another Sensors/Actuators are input/output transducers Sensors can be passive (e.g. change in resistance)
Transducers EEE355 Industrial Electronics 1 Terminology Transducers convert one form of energy into another Sensors/Actuators are input/output transducers Sensors can be passive (e.g. change in resistance)
Dr.Salwa Alsaleh fac.ksu.edu.sa/salwams
 Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Department of Mechanical Engineering ME 96. Free and Forced Convection Experiment. Revised: 25 April Introduction
 CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
University of Technology Dr. louay A.Mahdi Department of Machines and Equipments Engineering Branches: General, Refrigeration and Air conditioning,
 1 Introduction: The first recorded temperature measurement was carried out by Galileo at the end of the sixteenth century. His thermometer depended on the expansion of air. Some form of scale was attached
1 Introduction: The first recorded temperature measurement was carried out by Galileo at the end of the sixteenth century. His thermometer depended on the expansion of air. Some form of scale was attached
Chapter 1. Blackbody Radiation. Theory
 Chapter 1 Blackbody Radiation Experiment objectives: explore radiation from objects at certain temperatures, commonly known as blackbody radiation ; make measurements testing the Stefan-Boltzmann law in
Chapter 1 Blackbody Radiation Experiment objectives: explore radiation from objects at certain temperatures, commonly known as blackbody radiation ; make measurements testing the Stefan-Boltzmann law in
1. Mark the correct statement(s)
 1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
Part Number Range Accuracy Printing WP * Probe Special User LDC with Page Logging included Feature Cal Backlight
 Table of Contents Comparison Chart Introduction Temperature Indicators & Controllers Portable Infrared Printing/Logging Page O2 O3 O10 O14 O30 O33 O1 Part Number Range Accuracy Printing WP * Probe Special
Table of Contents Comparison Chart Introduction Temperature Indicators & Controllers Portable Infrared Printing/Logging Page O2 O3 O10 O14 O30 O33 O1 Part Number Range Accuracy Printing WP * Probe Special
Force and Displacement Measurement
 Force and Displacement Measurement Prof. R.G. Longoria Updated Fall 20 Simple ways to measure a force http://scienceblogs.com/dotphysics/200/02/diy_force_probe.php Example: Key Force/Deflection measure
Force and Displacement Measurement Prof. R.G. Longoria Updated Fall 20 Simple ways to measure a force http://scienceblogs.com/dotphysics/200/02/diy_force_probe.php Example: Key Force/Deflection measure
PHYS 352 Assignment #1 Solutions
 PHYS 352 Assignment #1 Solutions 1. Steinhart-Hart Equation for a thermistor In[1]:= a : 0.00128 In[2]:= b : 0.000236 In[3]:= In[4]:= d : 9.27 10^ 8 t r_ : a b Log r d Log r ^3 ^ 1 Note: Mathematica Log
PHYS 352 Assignment #1 Solutions 1. Steinhart-Hart Equation for a thermistor In[1]:= a : 0.00128 In[2]:= b : 0.000236 In[3]:= In[4]:= d : 9.27 10^ 8 t r_ : a b Log r d Log r ^3 ^ 1 Note: Mathematica Log
TEST METHOD FOR STILL- AND FORCED-AIR JUNCTION-TO- AMBIENT THERMAL RESISTANCE MEASUREMENTS OF INTEGRATED CIRCUIT PACKAGES
 SEMI G38-0996 N/A SEMI 1987, 1996 TEST METHOD FOR STILL- AND FORCED-AIR JUNCTION-TO- AMBIENT THERMAL RESISTANCE MEASUREMENTS OF INTEGRATED CIRCUIT PACKAGES 1 Purpose The purpose of this test is to determine
SEMI G38-0996 N/A SEMI 1987, 1996 TEST METHOD FOR STILL- AND FORCED-AIR JUNCTION-TO- AMBIENT THERMAL RESISTANCE MEASUREMENTS OF INTEGRATED CIRCUIT PACKAGES 1 Purpose The purpose of this test is to determine
Thermal Sensors and Actuators
 Thermal Sensors and Actuators Part I Fundamentals of heat transfer Heat transfer occurs where there is a temperature gradient until an equilibrium is reached. Four major mechanism Thermal conduction Natural
Thermal Sensors and Actuators Part I Fundamentals of heat transfer Heat transfer occurs where there is a temperature gradient until an equilibrium is reached. Four major mechanism Thermal conduction Natural
Temperature Measurements
 ME 22.302 Mechanical Lab I Temperature Measurements Dr. Peter Avitabile University of Massachusetts Lowell Temperature - 122601-1 Copyright 2001 A transducer is a device that converts some mechanical quantity
ME 22.302 Mechanical Lab I Temperature Measurements Dr. Peter Avitabile University of Massachusetts Lowell Temperature - 122601-1 Copyright 2001 A transducer is a device that converts some mechanical quantity
INSTRUMENTATION AND CONTROL
 INSTRUMENTATION AND CONTROL This tutorial provides minimal engineering science necessary to complete the rest of the tutorials. Greater depth of the individual topics can be found on the web site. It is
INSTRUMENTATION AND CONTROL This tutorial provides minimal engineering science necessary to complete the rest of the tutorials. Greater depth of the individual topics can be found on the web site. It is
Science In Action 7 Heat and Temperature Section Quiz
 Section 2 Heat affects Matter in different ways 2.1 States of Matter and The Particle Model 1. Water has a distinct characteristic that sets it apart from other liquids on Earth. Water expands when it
Section 2 Heat affects Matter in different ways 2.1 States of Matter and The Particle Model 1. Water has a distinct characteristic that sets it apart from other liquids on Earth. Water expands when it
Thermal Radiation: The Stefan-Boltzmann Law
 Thermal Radiation: The Stefan-Boltzmann Law Andy Chmilenko, 20310799 Instructor: Tan Dinh Section 1 (Dated: 2:30 pm Wednesday June 26, 2013) I. PURPOSE The purpose of this experiment is to verify the Stefan-
Thermal Radiation: The Stefan-Boltzmann Law Andy Chmilenko, 20310799 Instructor: Tan Dinh Section 1 (Dated: 2:30 pm Wednesday June 26, 2013) I. PURPOSE The purpose of this experiment is to verify the Stefan-
Resistivity and Temperature Coefficients (at 20 C)
 Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
AN INTRODUCTION TO INFRARED TEMPERATURE MEASUREMENT LEVEL 1 TRAINING
 AN INTRODUCTION TO INFRARED TEMPERATURE MEASUREMENT LEVEL 1 TRAINING WWW.LANDINST.COM WWW.AMETEK-LAND.COM AMETEK Land Instruments Infrared Training Notes Level 1 Introduction This document is intended
AN INTRODUCTION TO INFRARED TEMPERATURE MEASUREMENT LEVEL 1 TRAINING WWW.LANDINST.COM WWW.AMETEK-LAND.COM AMETEK Land Instruments Infrared Training Notes Level 1 Introduction This document is intended
Mechanical Measurements. Module 2:
 Mechanical Measurements Module : 1. Thermometry Formally we start the study of Mechanical Measurements now! Module will consider the measurement of field quantities like temperature, pressure and fluid
Mechanical Measurements Module : 1. Thermometry Formally we start the study of Mechanical Measurements now! Module will consider the measurement of field quantities like temperature, pressure and fluid
TEMPERATURE. 8. Temperature and Heat 1
 TEMPERATURE Heat is the energy that is transferred between objects because of a temperature difference Terms such as transfer of heat or heat flow from object A to object B simply means that the total
TEMPERATURE Heat is the energy that is transferred between objects because of a temperature difference Terms such as transfer of heat or heat flow from object A to object B simply means that the total
Answer: The relation between kelvin scale and Celsius scale is TK =TC => TC=TK
 Question The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Answer: The relation between kelvin scale and
Question The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Answer: The relation between kelvin scale and
Heat and Temperature
 Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *7904310746* PHYSICS 5054/21 Paper 2 Theory October/November 2012 1 hour 45 minutes Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *7904310746* PHYSICS 5054/21 Paper 2 Theory October/November 2012 1 hour 45 minutes Candidates answer
10 Measurement of Acceleration, Vibration and Shock Transducers
 Chapter 10: Acceleration, Vibration and Shock Measurement Dr. Lufti Al-Sharif (Revision 1.0, 25/5/2008) 1. Introduction This chapter examines the measurement of acceleration, vibration and shock. It starts
Chapter 10: Acceleration, Vibration and Shock Measurement Dr. Lufti Al-Sharif (Revision 1.0, 25/5/2008) 1. Introduction This chapter examines the measurement of acceleration, vibration and shock. It starts
Thermocouple Calibrations and Heat Transfer Coefficients
 Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared
Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared
CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS
 50 CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS 4.1 INTRODUCTION In the development of any energy-efficient heat transfer fluids for enhanced heat transfer performance, in practical applications,
50 CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS 4.1 INTRODUCTION In the development of any energy-efficient heat transfer fluids for enhanced heat transfer performance, in practical applications,
Thermal Radiation Heat Transfer Mechanisms
 18-6 Heat Transfer Mechanisms Thermal Radiation Radiation is an energy transfer via the emission of electromagnetic energy. The rate P rad at which an object emits energy via thermal radiation is Here
18-6 Heat Transfer Mechanisms Thermal Radiation Radiation is an energy transfer via the emission of electromagnetic energy. The rate P rad at which an object emits energy via thermal radiation is Here
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Practical 1P4 Energy Levels and Band Gaps
 Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
THERMAL PROPERTIES OF MATTER
 CHP # 8 HERMA PROPERIES OF MAER Q.1 Differentiate between heat and temperature? (Ans) Heat It can be defined as "the sum of kinetic energy of the molecules present in a substance is called heat". Heat
CHP # 8 HERMA PROPERIES OF MAER Q.1 Differentiate between heat and temperature? (Ans) Heat It can be defined as "the sum of kinetic energy of the molecules present in a substance is called heat". Heat
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS
 ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
Energy and Radiation. GEOG/ENST 2331 Lecture 3 Ahrens: Chapter 2
 Energy and Radiation GEOG/ENST 2331 Lecture 3 Ahrens: Chapter 2 Last lecture: the Atmosphere! Mainly nitrogen (78%) and oxygen (21%)! T, P and ρ! The Ideal Gas Law! Temperature profiles Lecture outline!
Energy and Radiation GEOG/ENST 2331 Lecture 3 Ahrens: Chapter 2 Last lecture: the Atmosphere! Mainly nitrogen (78%) and oxygen (21%)! T, P and ρ! The Ideal Gas Law! Temperature profiles Lecture outline!
The LM741C Integrated Circuit As A Differential Amplifier Building The Electronic Thermometer
 BE 209 Group BEW6 Jocelyn Poruthur, Justin Tannir Alice Wu, & Jeffrey Wu November 19, 1999 The LM741C Integrated Circuit As A Differential Amplifier Building The Electronic Thermometer INTRODUCTION: A
BE 209 Group BEW6 Jocelyn Poruthur, Justin Tannir Alice Wu, & Jeffrey Wu November 19, 1999 The LM741C Integrated Circuit As A Differential Amplifier Building The Electronic Thermometer INTRODUCTION: A
PROGRAM OF PHYSICS. Lecturer: Dr. DO Xuan Hoi Room A
 PROGRAM OF PHYSICS Lecturer: Dr. DO Xuan Hoi Room A1. 503 E-mail : dxhoi@hcmiu.edu.vn PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) 02 credits (30 periods) Chapter 1 Fluid Mechanics Chapter 2 Heat, Temperature
PROGRAM OF PHYSICS Lecturer: Dr. DO Xuan Hoi Room A1. 503 E-mail : dxhoi@hcmiu.edu.vn PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) 02 credits (30 periods) Chapter 1 Fluid Mechanics Chapter 2 Heat, Temperature
