Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
|
|
|
- May Nichols
- 5 years ago
- Views:
Transcription
1 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T C C 3F 73.5 Temperature Scale Temperature Concept of temperature A feeling of how hot or cold an object is. Our senses provide us the feeling of temperature. Our senses may be unreliable. Need reliable, qualitative, and reproducible methods for measuring the hotness or coldness of an object. Need a technical definition of temperature. 3 4
2 ( K) (5800 K) Examples of Object Temperatures Thermal Contact Two objects are in thermal contact with each other if energy can be exchanged between the objects. The exchanges is in the form of heat or electromagnetic radiation. The energy is exchanged due to a temperature difference. (77 K) (0 K) (4 K) 5 6 Quick Quiz on Thermal Contact Quick Quiz Two objects, with different sizes, masses, temperatures, and potential energy are placed in thermal contact. In which direction does the energy travel? a) Energy travels from the larger object to the smaller object. b) Energy travels from the object with more mass to the one with less mass. c) Energy travels from the object at higher temperature to the object at lower temperature. d) Energy travels from the object with higher potential energy to the object with lower potential energy. Consider the following pairs of materials. Which pair represents two materials, one of which is twice as hot as the other? (a) boiling water at 00C, a glass of water at 50C; (b) boiling water at 00C, frozen methane at 50C; (c) an ice cube at 0C, flames from a circus fire eater at 33C; (d) none of these pairs. Answer: (c) 7 8
3 Thermal Equilibrium Quick Quiz on Thermal Equilibrium Thermal equilibrium is a situation in which two objects would not exchange energy by heat or electromagnetic radiation if they were placed in thermal contact. The thermal contact does not have to also be physical contact. Is it possible for two objects to be in thermal equilibrium if they are not in contact with each other? Explain. 9 0 Zeroth Law of Thermodynamics Zeroth Law of Thermodynamics If objects A and B are separately in thermal equilibrium with a third object C, then A and B are in thermal equilibrium with each other. Since they are in thermal equilibrium with each other, there is no energy exchanged between them. Object C (thermometer) is placed in contact with A until they achieve thermal equilibrium. The reading on C is recorded. Object C is then placed in contact with object B until they achieve thermal equilibrium. The reading on C is recorded again. If the two readings are the same, A and B are also in thermal equilibrium.
4 Definition of Temperature Demonstration: Temperature Sensors Can you identify the types of temperature sensing? Temperature can be thought of: the property that determines whether an object is in thermal equilibrium with other objects. Two objects in thermal equilibrium with each other are at the same temperature. If two objects have different temperatures, they are not in thermal equilibrium with each other. 3 Temperature Measurements 4 6 Thermal Imager There are many types of sensors used to measure temperature. For different temperature scales, different measurement technique are established as standards. Common types used in physical experiments: Measured Temperature = 34.9 C Background = C Thermometer Resistance temperature detectors RTD (resistive elements) Thermistors (semiconductor resistive elements) Thermocouples (thermoelectric elements) Infrared thermometer (thermal radiation) 5
5 Thermocouple Thermocouples consist of different metals joined together. Seebeck Effect When the junctions of dissimilar metals have different temperatures an EMF is generated in the loop T. Junction Metal A T > T T T EMF Junction Metal B Thermocouple The electromotive force (EMF) is: K A and K B are thermal transportation constants, and is a constant of the metal pair combination (V C ) EMF T EMF EMF e T T T, T T K A K B dt T T 7 8 Standard Thermocouple Standard Thermocouple Curves Type Metal A Metal B Temperature Range J Iron Constantan (Cu 57 Ni 43 ) C 80 E T Copper Constantan (Cu 57 Ni 43 ) C K Chromel (Ni 90 Cr 0 ) Alumel (Ni 94 Mn 3 Al Si ) C Voltage (mv) J K E Chromel (Ni 90 Cr 0 ) Constantan (Cu 57 Ni 43 ) C S Platinum-Rhodium (Pt 90 Rh 0 ) Platinum C 0 R S R Platinum-Rhodium (Pt 87 Rh 3 ) Platinum C Temperature (C) 0
6 Common Configurations of Thermocouple Thermocouple Characteristics In Series: The potential generated is small ~ V C. The potential is only dependent upon the T of the junctions. That is, a junction temperature must be known in order to determine the other junction's temperature. In Parallel: Thermocouple Characteristics Thermocouple Commercial Standards The potential generated by a thermocouple is: e T,T 3 3 T T β T T γ T α T US Standard Connectors For T = 0 (reference temperature at 0C), e T,T e T,T αt βt or αt βt γt γt 3 3 British Standard 3 4
7 Thermocouple Conditioner Resistance Temperature Detectors FEATURES Operates with Type J (AD596) or Type K (AD597) Thermocouples Built In Ice Point Compensation Temperature Proportional Operation: 0 mv/c High Impedance Differential Input Resistance thermometers work on the principle that the electrical resistance of a material varies with temperature. For higher accuracy, second order term can also be used: R T R T R R T T 0C T where and are the first and second order temperature coefficients, respectively. T 0C T 5 6 Resistance Temperature Detectors Resistance Temperature Detectors Resistance elements are commonly calibrated by determining their resistance at 0 C, 00 C, and 78.5 C (sublimation point of CO ). Construction: Most RTD probes consist of a length of coiled wire wrapped around a ceramic or glass core
8 Resistance Temperature Detectors Construction Long thin piece wire coiled and protected by glass or insulated stainless steel tube. Typical resistance R ~ 00 s at room temperature. Platinum is general used even though it is expensive. Platinum is chemically inert and that it produces very repeatable measurements. Resistance Temperature Detectors Typical resistance curves of some common RTD: R( T ) R 0 Copper Nickel 3 Platinum Temperature (C) 9 30 Resistance Temperature Detectors Resistance Temperature Detectors Common metals are platinum, copper and nickel. Metal ( o C - ) Between 0-00 o C Self heating Effect RTD operates by current passing through the sensor. The current will introduce the self heating effect, which causes a rise in temperature T: Platinum Copper Nickel T where P is the Actual Power Dissipated, P D is the Dissipation Constant. Typically, P D ~ 5 mw C. P P D 3 3
9 Resistance Temperature Detectors Self heating Effect For example, current i = 0 ma passing through a 300 RTD, the temperature rise will be: i R T P D o. C 3 Resistance Temperature Detectors Thermal Shunting Altering the measurement temperature by inserting a sensor or transducer. Example: Temperature Measurement of a Drop of Water. If we insert a thermometer into a drop of water and try to measure the temperature, the thermometer will definitely alter the water droplet temperature. T Water Droplet T Thermometer Resistance Temperature Detectors Thermistor Small RTD Fast Response Time Low Thermal Shunting High Self-Heating Effect Large RTD Slow Response Time Poor Thermal Shunting Low Self-Heating Effect Thermistors are resistive semiconductor devices. Thermistors have a very large negative temperature coefficient (NTC). The electrical resistance R (in ) and temperature T (in K) is: R( T ) R o exp T To where R o is resistance at reference temperature T o. is the resistance temperature, typical value ~
10 Thermistor with NTC Thermistor Commercial Thermistors: 00 RT R 98K 5C Thermistors with Negative Temperature Coefficient (NTC) Type: NTC Resistance:.5 k Value: 800 Type: NTC Resistance: 000 k Value: Type: NTC Resistance: 3 k Value: 3988 Type: PTC Resistance: 0 Temperature (C) Thermistor with PTD Positive temperature coefficient (PTC) thermistors are available, but they have short temperature spans. Typically T max ~ T th + 00C (T th is the threshold temperature) Thermistor Signal Conditioning Two common signal conditioning methods for thermistor: Potential Divider Method Positive Temperature Coefficient (PTC ) T r -0 T th T r +0 Temperature ( o C) V ref NTC Thermistor R R + _ R f V out 39 40
11 Thermistor Signal Conditioning Thermistor Practical Application i 0 + Current Source NTC Thermistor Current Source Method R + _ R f V out NTC Thermistor: Temperature measurements. Needs microprocessor to linearize measurements. Self heating effect is a big problem. Self heating reduces the thermistor resistance, results in higher current flowing through the thermistor. R T R 98K 00 5C Thermistors with Negative Temperature Coefficient (NTC) Temperature (C) 4 Thermistor Practical Application Comparison PTC Thermistor: PTC thermistors are used for circuit protection against excessive I R in the event of malfunction or short circuit. I Voltage or Resistance Thermistor (Negative Temperature Coefficient) RTD PTC Thermistor R L Thermocouple Temperature 43 44
12 Thermal Expansion Thermal Expansion Thermal expansion: Increase in the size of an object with an increase in its temperature. Consequence of the change in the average separation between the atoms in an object. If the expansion is small relative to the original dimensions of the object, the change in any dimension is, to a good approximation, proportional to the first power of the change in temperature. Temperature increases, all the dimensions will increase. A cavity in a piece of material expands in the same way as if the cavity were filled with the material Thermal Expansion Linear Expansion Examples to Compensate Solid and Liquid Thermal Expansion: An object with an initial length L. Suppose that the length increases by L in response to a temperature change by T. The coefficient of linear expansion is defined as: L / L T Rearrange: L = L T 47 48
13 Linear Expansion Area Expansion Some materials expand along one dimension, but contract along another as the temperature increases. Example: Calcite (CaCO 3 ), which expands along one dimension (positive ), but contract along another (negative ). Since the linear dimensions change, it follows that the surface area and volume also change with a change in temperature. A cavity in a piece of material expands in the same way as if the cavity were filled with the material. The change in area is proportional to the original area and to the change in temperature: A / A T Volume Expansion On Class Exercise: Thermal Expansion The change in volume is proportional to the original volume and to the change in temperature. V / V T Find the coefficient of area expansion () andthecoefficientof volume expansion (). Express your answers in terms of coefficient of linear expansion (). 5 5
14 Expansion Coefficients Application: Bimetallic Strip Different substances have different characteristic average coefficient of expansion. This physical property can be used to fabricate devices, which are called bimetallic strip Demonstration: Application of Bimetallic Strip Pressure Pressure: Normal force exerted by a fluid per unit area. Unit of Pressure: Force per unit area [ N/m ] [ Pa ] Pa = N/m Other commonly used pressure units: Bar, and Standard Atmosphere 55 56
15 Pressure Pressure Absolute Pressure: Relative to absolute vacuum (absolute zero pressure). Gage Pressure: Relative to atmospheric pressure. Most pressure measuring devices are calibrated to read zero in the atmosphere. Vacuum Pressure: Pressures below atmospheric pressure. Absolute, gage, and vacuum pressures are all positive quantities and are related by: P P gage vac P P abs atm P P atm abs In thermodynamic relations and tables, absolute pressure is almost always used Pressure Variation of Pressure with Depth Illustrations of Absolute, gage, and vacuum pressures. Consider a rectangular fluid element (height z, length x, and unit depth into the page) in equilibrium. Assume =constant, force balance in the z direction: P F z ma z 0 x P x gxz W mg gxz P P P gz
16 Variation of Pressure with Depth Variation of Pressure with Depth For fluids whose density changes significantly with elevation: P P P gage atm or gh gh dp g dz In a room filled with a gas, the variation of pressure with height is negligible. 6 6 Pressure in a fluid at rest is independent of the shape or cross section of the container. Pressure is the same at all points on a horizontal plane in a given fluid. Pressure force exerted by the fluid is always normal to the surface at the specified points. Can you tell at which point the pressure is higher? Exercise: Hydrostatic Pressure on Solar Pond Solar ponds are used to store solar thermal energy. The rise of heated water to the surface is prevented by adding salt at the pond bottom. In a typical salt gradient solar pond, the density of water increases in the gradient zone, and the density () can be expressed as: o tan 4 z H o = density on the water surface z = vertical distance from water surface H = thickness of the gradient zone Suppose H = 4 m, o = 040 kg/m 3, surface zone thickness = 0.8 m, calculate the gage pressure at the bottom of the gradient zone. Hint: tan a axdx sinh tanax C 63 64
17 Exercise: Hydrostatic Pressure on Solar Pond (g/l) Exercise: Hydrostatic Pressure on Solar Pond Hydraulic Lifting P P F F A A Variation of gage pressure with depth in the gradient zone of the solar pond. Dashed line: hydrostatic pressure for the case of constant density at 040 kg/m 3 (for reference). Variation of pressure with depth is not linear when density varies with depth
18 Physical Properties: Water Manometer As temperature increases from 0 o C to 4 o C, water contracts (density increases). Above 4 o C, water expands with increasing temperature (density decreases). The maximum density of water ( g/cm 3 ) occurs at 4 o C. A basic manometer Assume: gravitational effects of gases are negligible. Pressure anywhere in the tank and at position has the same value. Pressure does not vary in the horizontal direction within a fluid, P = P. Pressure is determined directly by P = P atm + gh. the diameter of the tube should be large enough (> a few mm) to ensure that the surface tension effect and thus the capillary rise is negligible Manometer Pascal s Law Some manometers involve multiple immiscible fluids of different densities stacked on top of each other. Such systems can be analyzed by: Pascal's law; pressure change across a fluid column (height h) is P = gh; pressure increases downward in a given fluid and decreases upward (i.e., P bottom > P top ); and two points at the same elevation in a continuous fluid at rest are at the same pressure. Pascal s Law: A change in pressure at any point in an enclosed fluid at rest is transmitted undiminished to all points in the fluid. Pressure in water and air. Pascal's law applies only for fluids. Illustration Source: Wikipedia 7 7
19 Manometer Multiple Immiscible Fluids of Different Densities P atm gh gh gh P 3 3 Other Pressure Measurement Devices Bourdon tube Strain gage pressure transducers Piezoelectric transducers Digital Pressure Meter In stacked up fluid layers, the pressure change across a fluid layer of density and height h is gh Water Phase Diagram Exercise The pendulum of a certain pendulum clock is made of brass. When the temperature increases, does the period of the clock increase, decrease, or remain the same? Give reasons. Solution: 75 76
20 What we will learn: The Balance Equations 77
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
Measurements & Instrumentation. Module 3: Temperature Sensors
 Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Lecture 36: Temperatue Measurements
 Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
1. Mark the correct statement(s)
 1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
4. Thermometry. Temperature and Heat Flow Temperature Scales Thermometers
 4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Lecture 2: Zero law of thermodynamics
 Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
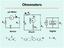 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
Thermodynamics INTRODUCTION AND BASIC CONCEPTS. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics INTRODUCTION AND BASIC CONCEPTS Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. THERMODYNAMICS AND ENERGY Thermodynamics: The science of energy.
Thermodynamics INTRODUCTION AND BASIC CONCEPTS Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. THERMODYNAMICS AND ENERGY Thermodynamics: The science of energy.
MEASURING INSTRUMENTS
 Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Dr.Salwa Alsaleh fac.ksu.edu.sa/salwams
 Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
ECNG3032 Control and Instrumentation I
 sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
VALLIAMMAI ENGINEERING COLLEGE
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6502 -INDUSTRIAL INSTRUMENTATION I Regulation 2013
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6502 -INDUSTRIAL INSTRUMENTATION I Regulation 2013
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect
 Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
15. Compare the result with the value you have taken above Compare the calculated pressure value with the actual pressure value that you have
 105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
Chapter 17. Temperature. Dr. Armen Kocharian
 Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
Control Engineering BDA30703
 Control Engineering BDA30703 Lecture 4: Transducers Prepared by: Ramhuzaini bin Abd. Rahman Expected Outcomes At the end of this lecture, students should be able to; 1) Explain a basic measurement system.
Control Engineering BDA30703 Lecture 4: Transducers Prepared by: Ramhuzaini bin Abd. Rahman Expected Outcomes At the end of this lecture, students should be able to; 1) Explain a basic measurement system.
! =!"#$% exerted by a fluid (liquid or gas) !"#$ =!"# FUNDAMENTAL AND MEASURABLE INTENSIVE PROPERTIES PRESSURE, TEMPERATURE AND SPECIFIC VOLUME
 FUNDAMENTAL AND MEASURABLE INTENSIVE PROPERTIES PRESSURE, TEMPERATURE AND SPECIFIC VOLUME PRESSURE, P! =!"#$%!"#! exerted by a fluid (liquid or gas) Thermodynamic importance of pressure One of two independent
FUNDAMENTAL AND MEASURABLE INTENSIVE PROPERTIES PRESSURE, TEMPERATURE AND SPECIFIC VOLUME PRESSURE, P! =!"#$%!"#! exerted by a fluid (liquid or gas) Thermodynamic importance of pressure One of two independent
I. MEASUREMENT OF TEMPERATURE
 I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
Chapter 6 Temperature Measurement (Revision 2.0, 1/12/2009)
 Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he
Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test
 ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
Part 2. Sensor and Transducer Instrument Selection Criteria (3 Hour)
 Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Temperature measurement
 emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
Thermometry. History. History 1/21/18. The art or science of temperature observation
 Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Using a Mercury itc with thermocouples
 Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
INTRODUCTION AND BASIC CONCEPTS. Chapter 1. Mehmet Kanoglu. Thermodynamics: An Engineering Approach, 6 th Edition. Yunus A. Cengel, Michael A.
 Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
Thermal Process Control Lap 4 Thermal Energy. Notes:
 Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
HEAT AND TEMPERATURE Vikasana-Bridge Course 2012
 HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
Temperature Sensors & Measurement
 Temperature Sensors & Measurement E80 Spring 2014 Contents Why measure temperature? Characteristics of interest Types of temperature sensors 1. Thermistor 2. RTD Sensor 3. Thermocouple 4. Integrated Silicon
Temperature Sensors & Measurement E80 Spring 2014 Contents Why measure temperature? Characteristics of interest Types of temperature sensors 1. Thermistor 2. RTD Sensor 3. Thermocouple 4. Integrated Silicon
Properties of Gases. The perfect gas. States of gases Gas laws Kinetic model of gases (Ch th ed, th ed.) Real gases
 Properties of Gases Chapter 1 of Physical Chemistry - 6th Edition P.W. Atkins. Chapter 1 and a little bit of Chapter 24 of 7th Edition. Chapter 1 and a little bit of Chapter 21 of 8th edition. The perfect
Properties of Gases Chapter 1 of Physical Chemistry - 6th Edition P.W. Atkins. Chapter 1 and a little bit of Chapter 24 of 7th Edition. Chapter 1 and a little bit of Chapter 21 of 8th edition. The perfect
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY
 EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
Engineering Thermodynamics. Chapter 1. Introductory Concepts and Definition
 1.1 Introduction Chapter 1 Introductory Concepts and Definition Thermodynamics may be defined as follows : Thermodynamics is an axiomatic science which deals with the relations among heat, work and properties
1.1 Introduction Chapter 1 Introductory Concepts and Definition Thermodynamics may be defined as follows : Thermodynamics is an axiomatic science which deals with the relations among heat, work and properties
Chapter 16 Temperature and Heat
 Chapter 16 Temperature and Heat 16-1 Temperature and the Zeroth Law of Thermodynamics Definition of heat: Heat is the energy transferred between objects because of a temperature difference. Objects are
Chapter 16 Temperature and Heat 16-1 Temperature and the Zeroth Law of Thermodynamics Definition of heat: Heat is the energy transferred between objects because of a temperature difference. Objects are
Temperature. 3
 Temperature In 1848, Sir William Thomson (Lord Kelvin) stated the zero principle of dynamics. This principle enabled him to define thermodynamic temperature and to establish an objective method of measuring
Temperature In 1848, Sir William Thomson (Lord Kelvin) stated the zero principle of dynamics. This principle enabled him to define thermodynamic temperature and to establish an objective method of measuring
APPENDIX ELEVEN Open Fire Temperature Measurements
 APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers),
APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers),
Theory and Design for Mechanical Measurements
 Theory and Design for Mechanical Measurements Third Edition Richard S. Figliola Clemson University Donald E. Beasley Clemson University John Wiley & Sons, Inc. New York / Chichester / Weinheim / Brisbane
Theory and Design for Mechanical Measurements Third Edition Richard S. Figliola Clemson University Donald E. Beasley Clemson University John Wiley & Sons, Inc. New York / Chichester / Weinheim / Brisbane
Temperature and Heat. Two systems of temperature. Temperature conversions. PHY heat - J. Hedberg
 Temperature and Heat 1. Two systems of temperature 1. Temperature conversions 2. Real science (one scale to rule them all) 3. Temperature scales 2. Effects of temperature on materials 1. Linear Thermal
Temperature and Heat 1. Two systems of temperature 1. Temperature conversions 2. Real science (one scale to rule them all) 3. Temperature scales 2. Effects of temperature on materials 1. Linear Thermal
Heat and temperature are related and often confused, but they are not the same.
 Heat and temperature are related and often confused, but they are not the same. Heat Definition: Heat is energy that is transferred from one body to another as a result of a difference in temperature Symbol:
Heat and temperature are related and often confused, but they are not the same. Heat Definition: Heat is energy that is transferred from one body to another as a result of a difference in temperature Symbol:
1. How much heat was needed to raise the bullet to its final temperature?
 Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Thermodynamics. Atoms are in constant motion, which increases with temperature.
 Thermodynamics SOME DEFINITIONS: THERMO related to heat DYNAMICS the study of motion SYSTEM an object or set of objects ENVIRONMENT the rest of the universe MICROSCOPIC at an atomic or molecular level
Thermodynamics SOME DEFINITIONS: THERMO related to heat DYNAMICS the study of motion SYSTEM an object or set of objects ENVIRONMENT the rest of the universe MICROSCOPIC at an atomic or molecular level
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power).
 Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Chapters 16 Temperature and Heat
 Chapters 16 Temperature and Heat 1 Overview of Chapter 16 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heat Conduction, Convection,
Chapters 16 Temperature and Heat 1 Overview of Chapter 16 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heat Conduction, Convection,
Veerapong Kanchanawongkul*
 Using LabVIEW to Development of Temperature Measurement System with Thermocouple and Thermistor AIS 08 Veerapong Kanchanawongkul* Department of Mechanical Engineering, Faculty of Engineering, South-East
Using LabVIEW to Development of Temperature Measurement System with Thermocouple and Thermistor AIS 08 Veerapong Kanchanawongkul* Department of Mechanical Engineering, Faculty of Engineering, South-East
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
 Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Chapter 1: Basic Concepts of Thermodynamics. Thermodynamics and Energy. Dimensions and Units
 Chapter 1: Basic Concepts of Thermodynamics Every science has its own unique vocabulary associated with it. recise definition of basic concepts forms a sound foundation for development of a science and
Chapter 1: Basic Concepts of Thermodynamics Every science has its own unique vocabulary associated with it. recise definition of basic concepts forms a sound foundation for development of a science and
Chapter 10, Thermal Physics
 CHAPTER 10 1. If it is given that 546 K equals 273 C, then it follows that 400 K equals: a. 127 C b. 150 C c. 473 C d. 1 200 C 2. A steel wire, 150 m long at 10 C, has a coefficient of linear expansion
CHAPTER 10 1. If it is given that 546 K equals 273 C, then it follows that 400 K equals: a. 127 C b. 150 C c. 473 C d. 1 200 C 2. A steel wire, 150 m long at 10 C, has a coefficient of linear expansion
SKMM 2413 Thermodynamics
 SKMM 2413 Thermodynamics Md. Mizanur Rahman, PhD Department of Thermo-Fluids Faculty of Mechanical Engineering Universiti Teknologi Malaysia UTM Office: C23-228 mizanur@fkm.utm.my Semester I, 2016-2017
SKMM 2413 Thermodynamics Md. Mizanur Rahman, PhD Department of Thermo-Fluids Faculty of Mechanical Engineering Universiti Teknologi Malaysia UTM Office: C23-228 mizanur@fkm.utm.my Semester I, 2016-2017
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Heat and Temperature
 Heat and Temperature Temperature What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold? Intro: Discussion A person from Seattle
Heat and Temperature Temperature What does temperature have to do with energy? What three temperature scales are commonly used? What makes things feel hot or cold? Intro: Discussion A person from Seattle
Roll No. :... Invigilator s Signature :.. CS/B.Tech (EIE)/SEM-5/EI-501/ INDUSTRIAL INSTRUMENTATION II
 Name : Roll No. :.... Invigilator s Signature :.. CS/B.Tech (EIE)/SEM-5/EI-501/2011-12 2011 INDUSTRIAL INSTRUMENTATION II Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full
Name : Roll No. :.... Invigilator s Signature :.. CS/B.Tech (EIE)/SEM-5/EI-501/2011-12 2011 INDUSTRIAL INSTRUMENTATION II Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full
Process Control & Design
 458.308 Process Control & Design Lecture 5: Feedback Control System Jong Min Lee Chemical & Biomolecular Engineering Seoul National University 1 / 29 Feedback Control Scheme: The Continuous Blending Process.1
458.308 Process Control & Design Lecture 5: Feedback Control System Jong Min Lee Chemical & Biomolecular Engineering Seoul National University 1 / 29 Feedback Control Scheme: The Continuous Blending Process.1
Heat and Temperature
 Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Transducers. EEE355 Industrial Electronics
 Transducers EEE355 Industrial Electronics 1 Terminology Transducers convert one form of energy into another Sensors/Actuators are input/output transducers Sensors can be passive (e.g. change in resistance)
Transducers EEE355 Industrial Electronics 1 Terminology Transducers convert one form of energy into another Sensors/Actuators are input/output transducers Sensors can be passive (e.g. change in resistance)
PHYS102 Previous Exam Problems. Temperature, Heat & The First Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
Basic Concepts of Thermodynamics
 Basic Concepts of Thermodynamics.. Introduction to kinetic theory of gases... Definition of thermodynamics..3. Thermodynamic systems system, boundary and surroundings closed system open system isolated
Basic Concepts of Thermodynamics.. Introduction to kinetic theory of gases... Definition of thermodynamics..3. Thermodynamic systems system, boundary and surroundings closed system open system isolated
Applied Thermodynamics (Lecture#01)
 Applied Thermodynamics (Lecture#0) Course Outline: Basic Concepts, the system, Open and close system, properties of a system, control volume, working substance, heat and work, state and properties, thermodynamic
Applied Thermodynamics (Lecture#0) Course Outline: Basic Concepts, the system, Open and close system, properties of a system, control volume, working substance, heat and work, state and properties, thermodynamic
Chapter 1 - Temperature and Heat
 Chapter 1 - and Heat and Heat It doesn t make a difference what temperature a room is, it s always room temperature. -Steven Wright David J. Starling Penn State Hazleton Fall 2013 and Heat Thermodynamics
Chapter 1 - and Heat and Heat It doesn t make a difference what temperature a room is, it s always room temperature. -Steven Wright David J. Starling Penn State Hazleton Fall 2013 and Heat Thermodynamics
CHE Thermodynamics of Chemical Processes
 CHE 3010 - Thermodynamics of Chemical Processes Venkat Padmanabhan, PhD Department of Chemical Engineering Tennessee Tech University Lecture 2 - Basic Concepts 8/29/2018 CHE 3010 - Thermodynamics Tennessee
CHE 3010 - Thermodynamics of Chemical Processes Venkat Padmanabhan, PhD Department of Chemical Engineering Tennessee Tech University Lecture 2 - Basic Concepts 8/29/2018 CHE 3010 - Thermodynamics Tennessee
I. Introduction and Objectives
 Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Temperature Measurement
 Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Making Contact with Temperature
 Making Contact with Temperature Here is a look at the phenomenon itself, the basic measurement technologies available, and how industry is presently using them. Jesse Yoder, Flow Research Temperature is
Making Contact with Temperature Here is a look at the phenomenon itself, the basic measurement technologies available, and how industry is presently using them. Jesse Yoder, Flow Research Temperature is
- Copyright Dewesoft d.o.o., all rights reserved. Temperature measurement
 www.dewesoft.com - Copyright 2000-2019 Dewesoft d.o.o., all rights reserved. Temperature measurement Table of Contents Temperature and temperature scales 2 Types of temperature sensors 4 1. Thermocouples
www.dewesoft.com - Copyright 2000-2019 Dewesoft d.o.o., all rights reserved. Temperature measurement Table of Contents Temperature and temperature scales 2 Types of temperature sensors 4 1. Thermocouples
Real-Time & Embedded 1Systems Physical Coupling. Uwe R. Zimmer - The Australian National University
 Real-Time & Embedded 1Systems 2015 Physical Coupling Uwe R. Zimmer - The Australian National University [ Edler2003 ] Edler et al. Noise temperature measurements for the determination of the thermodynamic
Real-Time & Embedded 1Systems 2015 Physical Coupling Uwe R. Zimmer - The Australian National University [ Edler2003 ] Edler et al. Noise temperature measurements for the determination of the thermodynamic
Temperature Measurements
 ME 22.302 Mechanical Lab I Temperature Measurements Dr. Peter Avitabile University of Massachusetts Lowell Temperature - 122601-1 Copyright 2001 A transducer is a device that converts some mechanical quantity
ME 22.302 Mechanical Lab I Temperature Measurements Dr. Peter Avitabile University of Massachusetts Lowell Temperature - 122601-1 Copyright 2001 A transducer is a device that converts some mechanical quantity
Simpo PDF Merge and Split Unregistered Version -
 Simpo PDF Merge and Split Unregistered Version - http://wwwsimpopdfcom 6 If the zeroth law of thermodynamics were not valid, which of the following could not be considered a property of an object? A Pressure
Simpo PDF Merge and Split Unregistered Version - http://wwwsimpopdfcom 6 If the zeroth law of thermodynamics were not valid, which of the following could not be considered a property of an object? A Pressure
HEAT HISTORY. D. Whitehall
 1 HEAT HISTORY 18 th Century In the 18 th century it was assumed that there was an invisible substance called caloric. When objects got it was assumed that they gained caloric, therefore hot objects should
1 HEAT HISTORY 18 th Century In the 18 th century it was assumed that there was an invisible substance called caloric. When objects got it was assumed that they gained caloric, therefore hot objects should
Figure 1.1. Relation between Celsius and Fahrenheit scales. From Figure 1.1. (1.1)
 CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
T h e rm i s t o r s
 Data Pack E Issued March 00 - T h e rm i s t o r s NTC thermistors The R S range of NTC thermistors includes standard tolerance negative temperature coefficient thermistors, a range of small close tolerance
Data Pack E Issued March 00 - T h e rm i s t o r s NTC thermistors The R S range of NTC thermistors includes standard tolerance negative temperature coefficient thermistors, a range of small close tolerance
University of Technology Dr. louay A.Mahdi Department of Machines and Equipments Engineering Branches: General, Refrigeration and Air conditioning,
 1 Introduction: The first recorded temperature measurement was carried out by Galileo at the end of the sixteenth century. His thermometer depended on the expansion of air. Some form of scale was attached
1 Introduction: The first recorded temperature measurement was carried out by Galileo at the end of the sixteenth century. His thermometer depended on the expansion of air. Some form of scale was attached
Energy. E d. Energy Power = time. E t P = E t = P
 Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Slide 1. Temperatures Light (Optoelectronics) Magnetic Fields Strain Pressure Displacement and Rotation Acceleration Electronic Sensors
 Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit.
 2 This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit. CONTENTS thermometry what is temperature?, fixed points, Kelvin (Absolute),
2 This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit. CONTENTS thermometry what is temperature?, fixed points, Kelvin (Absolute),
Siddharth Institute of Engineering & Technology
 SIDDHARTH INSTITUTE OF ENGINEERING & TECHNOLOGY :: PUTTUR (AUTONOMOUS) (Approved by AICTE, New Delhi & Affiliated to JNTUA, Anantapuramu) (Accredited by NBA & Accredited by NAAC with A Grade) (An ISO 9001:2008
SIDDHARTH INSTITUTE OF ENGINEERING & TECHNOLOGY :: PUTTUR (AUTONOMOUS) (Approved by AICTE, New Delhi & Affiliated to JNTUA, Anantapuramu) (Accredited by NBA & Accredited by NAAC with A Grade) (An ISO 9001:2008
Tick the box next to those resources for which the Sun is also the source of energy.
 1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
1 (a) The source of solar energy is the Sun. Tick the box next to those resources for which the Sun is also the source of energy. coal geothermal hydroelectric nuclear wind [2] (b) Fig. 4.1 shows a solar
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Thermodynamics and Statistical Physics
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Week-7 Assignment-1. The due date for submitting this assignment has passed. (1/273.15)th of the normal freezing point of water
 X reviewer2@nptel.iitm.ac.in Courses» Chemical Process Instrumentation Unit 8 - Week 7 Announcements Course Ask a Question Progress Mentor Course outline How to access the portal Week-7 Assignment-1 The
X reviewer2@nptel.iitm.ac.in Courses» Chemical Process Instrumentation Unit 8 - Week 7 Announcements Course Ask a Question Progress Mentor Course outline How to access the portal Week-7 Assignment-1 The
LYOPHILIZATION. First of Four Lectures. Introduction to Freeze-Drying. Introduction to Freeze-Drying. J. Jeff Schwegman, Ph.D. AB BioTechnologies
 LYOPHILIZATION Introduction to Freeze-Drying First of Four Lectures Introduction to Freeze-Drying J. Jeff Schwegman, Ph.D. AB BioTechnologies P.O. Box 1430 Bloomington, Indiana, 47402 812-327-6898 jjschwegman@ab-biotech.com
LYOPHILIZATION Introduction to Freeze-Drying First of Four Lectures Introduction to Freeze-Drying J. Jeff Schwegman, Ph.D. AB BioTechnologies P.O. Box 1430 Bloomington, Indiana, 47402 812-327-6898 jjschwegman@ab-biotech.com
Temperature. Temperature Scales. Temperature (cont d) CHAPTER 14 Heat and Temperature
 Temperature CHAPTER 14 Heat and Temperature The temperature of a substance is proportional to the average kinetic energy of the substance s particles. As the average kinetic energy of the particles in
Temperature CHAPTER 14 Heat and Temperature The temperature of a substance is proportional to the average kinetic energy of the substance s particles. As the average kinetic energy of the particles in
3. EFFECTS OF HEAT. Thus, heat can be defined as a form of energy that gives the sensation of hotness or coldness
 3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
Tried. Tested. Trusted. A Guide to. Temperature Measurement
 Tried. Tested. Trusted. A Guide to Temperature Measurement Contents 2-3 4 4-5 6 6-7 7-14 15-20 21-23 24-25 26-29 30-33 34-37 38-41 42-45 46-48 49-50 51-52 63-64 Introduction Temperature Temperature Scales
Tried. Tested. Trusted. A Guide to Temperature Measurement Contents 2-3 4 4-5 6 6-7 7-14 15-20 21-23 24-25 26-29 30-33 34-37 38-41 42-45 46-48 49-50 51-52 63-64 Introduction Temperature Temperature Scales
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS
 ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
Physical Science Chapter 5 Cont2. Temperature & Heat
 Physical Science Chapter 5 Cont2 Temperature & Heat What are we going to study? Temperature Heat Specific Heat and Latent Heat Heat Transfer Phases of Matter The Kinetic Theory of Gases Thermodynamics
Physical Science Chapter 5 Cont2 Temperature & Heat What are we going to study? Temperature Heat Specific Heat and Latent Heat Heat Transfer Phases of Matter The Kinetic Theory of Gases Thermodynamics
SEN TRONIC AG 1 A 6 6 / "
 1A 66/" 0!"#$%&'() %"*+", - %"*.", - /01234%( 34.+*!54%& 0*%/# "6#,7857.'.0" - 6#)9.:. &%&;! 0 &????'.&% )&" 8" @&& (++ '() %('.('/(#$!!! ' %! %!& ;!;8 ;!;8 0 &&'&&;! C;!C&(D"@@ &;! 0&&+%&;! C&=;!C&(D"@@
1A 66/" 0!"#$%&'() %"*+", - %"*.", - /01234%( 34.+*!54%& 0*%/# "6#,7857.'.0" - 6#)9.:. &%&;! 0 &????'.&% )&" 8" @&& (++ '() %('.('/(#$!!! ' %! %!& ;!;8 ;!;8 0 &&'&&;! C;!C&(D"@@ &;! 0&&+%&;! C&=;!C&(D"@@
Lecture 22. Temperature and Heat
 Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines
 Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
PHYS 352 Assignment #1 Solutions
 PHYS 352 Assignment #1 Solutions 1. Steinhart-Hart Equation for a thermistor In[1]:= a : 0.00128 In[2]:= b : 0.000236 In[3]:= In[4]:= d : 9.27 10^ 8 t r_ : a b Log r d Log r ^3 ^ 1 Note: Mathematica Log
PHYS 352 Assignment #1 Solutions 1. Steinhart-Hart Equation for a thermistor In[1]:= a : 0.00128 In[2]:= b : 0.000236 In[3]:= In[4]:= d : 9.27 10^ 8 t r_ : a b Log r d Log r ^3 ^ 1 Note: Mathematica Log
TOPICS. Density. Pressure. Variation of Pressure with Depth. Pressure Measurements. Buoyant Forces-Archimedes Principle
 Lecture 6 Fluids TOPICS Density Pressure Variation of Pressure with Depth Pressure Measurements Buoyant Forces-Archimedes Principle Surface Tension ( External source ) Viscosity ( External source ) Equation
Lecture 6 Fluids TOPICS Density Pressure Variation of Pressure with Depth Pressure Measurements Buoyant Forces-Archimedes Principle Surface Tension ( External source ) Viscosity ( External source ) Equation
Chapter 10. Thermal Physics
 Chapter 10 Thermal Physics Thermal Physics Thermal physics is the study of Temperature Heat How these affect matter Thermal Physics, cont Descriptions require definitions of temperature, heat and internal
Chapter 10 Thermal Physics Thermal Physics Thermal physics is the study of Temperature Heat How these affect matter Thermal Physics, cont Descriptions require definitions of temperature, heat and internal
Lecture Outlines Chapter 16. Physics, 3 rd Edition James S. Walker
 Lecture Outlines Chapter 16 Physics, 3 rd Edition James S. Walker 2007 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors in
Lecture Outlines Chapter 16 Physics, 3 rd Edition James S. Walker 2007 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors in
Why do we need to study thermodynamics? Examples of practical thermodynamic devices:
 Why do we need to study thermodynamics? Knowledge of thermodynamics is required to design any device involving the interchange between heat and work, or the conversion of material to produce heat (combustion).
Why do we need to study thermodynamics? Knowledge of thermodynamics is required to design any device involving the interchange between heat and work, or the conversion of material to produce heat (combustion).
