APPENDIX ELEVEN Open Fire Temperature Measurements
|
|
|
- Amelia McKenzie
- 5 years ago
- Views:
Transcription
1 APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers), or with a thermocouple. Alcohol and mercury thermometers are based on the fact that the volume of a liquid changes with its temperature. Such thermometers are suitable for most household and laboratory uses and accurately display temperatures between the freezing and boiling points of methanol, ethanol or mercury (Table 17-1). F C Methanol (CH 3 OH) freezing boiling Ethanol (C 2 H 5 OH) freezing boiling Mercury (Hg) freezing boiling Table 17-1: Freezing and boiling temperatures of liquids commonly used in thermometers. Themistors and resistance temperature detectors (RTDs) are based on the fact that the electrical resistance of metals depend on their temperature. Such thermometers require a small current to operate and an Ohmmeter to measure the electrical resistance in the system. Specific configurations have been developed for different applications and, in the form of digital thermometers, thermistors and RTDs have replaced most other temperature measuring devices. Given the large range and the high maximum reached, the temperatures required to fire pottery, in a kiln or in an open fire, are best measured with a thermocouple (Figure 17-1). A thermocouple is based on the observation by Thomas Johann Seebeck (Tallinn, 9 April 1770-Berlin, 10 December 1831) that a circuit of two different metal wires produces a small, continuous electric current. The voltage of this current, known as the Seebeck voltage, depends on the metals used as well as on the temperature of the joints. If one of the joints is kept at a fixed, known temperature (for instance at 0 C or 32 F in melting ice), or is kept constant by an 'electronic ice point reference', the voltage in the system depends on the temperature of the second joint, which can now be used as a probe (Figure 17-2). It has to be kept in mind that the connection of the leads to the voltmeter, where different metals are connected, results in another thermocouple. If very accurate measurements are needed these connections should be placed in an 'isothermal block'. A large number of different thermocouples, comprising different combinations of metals, are commercially available. A K-type thermocouple, which consists of an alumel (nickel-aluminium alloy) and a chromel (nickelchromium alloy) lead, is a general purpose thermocouple that is suitable to record the temperatures in an open wood fire, as well as in most pottery kilns. The correlation between the temperature of the probe and the current in the system (Figure 17-1) is given in Table 17-3 and Figure Two temperature scales are currently most frequently used (Tables 17-2 and 17-4), one developed by Daniel Gabriel Fahrenheit (Gdansk, 24 May 1686-The Hague, 16 September 1736) and another by Anders Celsius (Uppsala, 27 November April 1744). Fahrenheit identified the temperature of melting ice as 32 F and that of the human body as 96 F. His scale won great popularity because of the quality of the (mercury) thermometers (outfitted with his scale) that he produced. Celsius, on the other hand, was concerned with the universality of the units used in the sciences. He identified the temperature of boiling water as 0 C and that of melting ice as 100 C. How these figures were reversed remains unclear, although it has been suggested that this was done by Carolus Linnaeus (Carl von Linné) in The Celsius scale was chosen as the basis for the Kelvin scale (K = C ), named after William Thomson, Baron Kelvin that places 0 K (without a -sign) at 'absolute zero' ( C or F), which is one of the seven base units of the International System of Units (SI). F C F = ( C x 1.8) + 32 C = ( F 32) x 0.56 Table 17-2: Correlation of F and C (cf. Table 17-4).
2 Appendix XI: Temperature Measurements Figure 17-1: Theoretical lay-out of a thermocouple set to measure large temperature differences (cf. Figures 17-2 and 17-3). Figure 17-2: A K-type thermocouple employed to record the temperature of an open wood fire (cf. Figures 17-1 and 17-4). 213
3 Eastern Desert Ware mv F C mv F C Table 17-3 and Figure 17-3: Correlation between the temperature and the voltage in a K-type thermocouple. 214
4 Appendix XI: Temperature Measurements C F C F C F C F C F C F C F C F C F C F Table 17-4: Conversion table from C into F (cf. Table 17-2). 215
5 Eastern Desert Ware Figure 17-4: Temperature curves in an open wood fire (4 June 2005 and 19 May 2007, cf. Figure 17-2) compared to the temperature of the soil below a continuous wood fire (11 August 2003) and inside an electric pottery kiln, with kiln sitter 012, until just after it shuts off (27 June 2003). 216
4. Thermometry. Temperature and Heat Flow Temperature Scales Thermometers
 4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Thermometry. History. History 1/21/18. The art or science of temperature observation
 Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
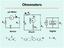 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
Temperature. Sensors. Measuring technique. Eugene V. Colla. 10/25/2017 Physics 403 1
 Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature Measurement
 Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Lecture 2: Zero law of thermodynamics
 Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
I. Introduction and Objectives
 Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Measurements & Instrumentation. Module 3: Temperature Sensors
 Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
MEASURING INSTRUMENTS
 Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Dr.Salwa Alsaleh fac.ksu.edu.sa/salwams
 Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect
 Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
ECNG3032 Control and Instrumentation I
 sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
Exercise 1: Thermocouple Characteristics
 The Thermocouple Transducer Fundamentals Exercise 1: Thermocouple Characteristics EXERCISE OBJECTIVE When you have completed this exercise, you will be able to describe and demonstrate the characteristics
The Thermocouple Transducer Fundamentals Exercise 1: Thermocouple Characteristics EXERCISE OBJECTIVE When you have completed this exercise, you will be able to describe and demonstrate the characteristics
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Thermal energy 7 TH GRADE SCIENCE
 Thermal energy 7 TH GRADE SCIENCE Temperature There s more to temperature than the idea of hot and cold. Remember that all matter is made up of tiny particles that are constantly moving even in solid objects.
Thermal energy 7 TH GRADE SCIENCE Temperature There s more to temperature than the idea of hot and cold. Remember that all matter is made up of tiny particles that are constantly moving even in solid objects.
The Underground Experimental Investigation of Thermocouples
 The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
CHEMICAL ELEMENTS - Aluminum. Bromine. Sodium. pure substances that cannot be decomposed by ordinary means to other substances.
 CHEMICAL ELEMENTS - pure substances that cannot be decomposed by ordinary means to other substances. Aluminum Sodium Bromine The elements, their names, and symbols are given on the PERIODIC TABLE How many
CHEMICAL ELEMENTS - pure substances that cannot be decomposed by ordinary means to other substances. Aluminum Sodium Bromine The elements, their names, and symbols are given on the PERIODIC TABLE How many
Unit 5 Thermodynamics
 Unit 5 Thermodynamics Unit 13: Heat and Temperature Unit 14: Thermal Expansion /Heat Exchange/ Change of Phase Test: Units 13-14 Thermal Energy The total kinetic and potential energy of all the molecules
Unit 5 Thermodynamics Unit 13: Heat and Temperature Unit 14: Thermal Expansion /Heat Exchange/ Change of Phase Test: Units 13-14 Thermal Energy The total kinetic and potential energy of all the molecules
3. EFFECTS OF HEAT. Thus, heat can be defined as a form of energy that gives the sensation of hotness or coldness
 3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
Chapter 6 Temperature Measurement (Revision 2.0, 1/12/2009)
 Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he
Chapter 6 emperature Measurement (Revision 2.0, /2/2009). Introduction his Chapter looks that various methods of temperature measurement. Historically, there are two temperature measurement scales: he
Chapter 14 Heat and Temperature Notes
 Chapter 14 Heat and Temperature Notes Section 1: Temperature The degree of or of an object. Related to the of an object s atoms or molecules What makes something hot? o Particles that make up o They have
Chapter 14 Heat and Temperature Notes Section 1: Temperature The degree of or of an object. Related to the of an object s atoms or molecules What makes something hot? o Particles that make up o They have
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
Heat and temperature are related and often confused, but they are not the same.
 Heat and temperature are related and often confused, but they are not the same. Heat Definition: Heat is energy that is transferred from one body to another as a result of a difference in temperature Symbol:
Heat and temperature are related and often confused, but they are not the same. Heat Definition: Heat is energy that is transferred from one body to another as a result of a difference in temperature Symbol:
ME 105 Mechanical Engineering Laboratory Spring Quarter Experiment #2: Temperature Measurements and Transient Conduction and Convection
 ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2010 Experiment #2: Temperature Measurements and Transient Conduction and Convection Objectives a) To calibrate
ME 105 Mechanical Engineering Lab Page 1 ME 105 Mechanical Engineering Laboratory Spring Quarter 2010 Experiment #2: Temperature Measurements and Transient Conduction and Convection Objectives a) To calibrate
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test
 ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
Physics 207 Lecture 23
 Thermodynamics A practical science initially concerned with economics, industry, real life problems. DYNAMICS -- Concerned with the concepts of energy transfers between a system and its environment and
Thermodynamics A practical science initially concerned with economics, industry, real life problems. DYNAMICS -- Concerned with the concepts of energy transfers between a system and its environment and
Chemistry 1. Worksheet 4. Temperature in Chemistry. 1 MathTutorDVD.com
 Chemistry 1 Worksheet 4 Temperature in Chemistry 1 Conversion factors: Celsius to Kelvin: Add 273.15 Kelvin to Celsius: Subtract 273.15 Celsius to Fahrenheit: T(F) = (T(C) x 1.8) + 32 Fahrenheit to Celsius:
Chemistry 1 Worksheet 4 Temperature in Chemistry 1 Conversion factors: Celsius to Kelvin: Add 273.15 Kelvin to Celsius: Subtract 273.15 Celsius to Fahrenheit: T(F) = (T(C) x 1.8) + 32 Fahrenheit to Celsius:
Mechanical Measurements. Module 2:
 Mechanical Measurements Module : 1. Thermometry Formally we start the study of Mechanical Measurements now! Module will consider the measurement of field quantities like temperature, pressure and fluid
Mechanical Measurements Module : 1. Thermometry Formally we start the study of Mechanical Measurements now! Module will consider the measurement of field quantities like temperature, pressure and fluid
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY
 EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
EM375 MECHANICAL ENGINEERING EXPERIMENTATION THERMOCOUPLE LABORATORY PURPOSE The objective of this laboratory is to introduce the student to the manufacture and use of thermocouples. Thermocouples will
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
I. MEASUREMENT OF TEMPERATURE
 I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
Thermometer Calibration
 Thermometer Calibration Jane Doe: Introduction and references John Smith: Procedure Leo Patel: Results Mike Jones: Discussion/Conclusion Introduction: Thermometers measure the amount of thermal energy
Thermometer Calibration Jane Doe: Introduction and references John Smith: Procedure Leo Patel: Results Mike Jones: Discussion/Conclusion Introduction: Thermometers measure the amount of thermal energy
Tried. Tested. Trusted. A Guide to. Temperature Measurement
 Tried. Tested. Trusted. A Guide to Temperature Measurement Contents 2-3 4 4-5 6 6-7 7-14 15-20 21-23 24-25 26-29 30-33 34-37 38-41 42-45 46-48 49-50 51-52 63-64 Introduction Temperature Temperature Scales
Tried. Tested. Trusted. A Guide to Temperature Measurement Contents 2-3 4 4-5 6 6-7 7-14 15-20 21-23 24-25 26-29 30-33 34-37 38-41 42-45 46-48 49-50 51-52 63-64 Introduction Temperature Temperature Scales
Experiment 5: Thermocouples (tbc 1/14/2007, revised 3/16/2007, 3/22,2007, 2/23/2009, 3/13/2011)
 Experiment 5: Thermocouples (tbc 1/14/2007, revised 3/16/2007, 3/22,2007, 2/23/2009, 3/13/2011) Objective: To implement a thermocouple circuit for temperature sensing. I. Introduction to Thermocouples
Experiment 5: Thermocouples (tbc 1/14/2007, revised 3/16/2007, 3/22,2007, 2/23/2009, 3/13/2011) Objective: To implement a thermocouple circuit for temperature sensing. I. Introduction to Thermocouples
SEN TRONIC AG 1 A 6 6 / "
 1A 66/" 0!"#$%&'() %"*+", - %"*.", - /01234%( 34.+*!54%& 0*%/# "6#,7857.'.0" - 6#)9.:. &%&;! 0 &????'.&% )&" 8" @&& (++ '() %('.('/(#$!!! ' %! %!& ;!;8 ;!;8 0 &&'&&;! C;!C&(D"@@ &;! 0&&+%&;! C&=;!C&(D"@@
1A 66/" 0!"#$%&'() %"*+", - %"*.", - /01234%( 34.+*!54%& 0*%/# "6#,7857.'.0" - 6#)9.:. &%&;! 0 &????'.&% )&" 8" @&& (++ '() %('.('/(#$!!! ' %! %!& ;!;8 ;!;8 0 &&'&&;! C;!C&(D"@@ &;! 0&&+%&;! C&=;!C&(D"@@
PALO VERDE NUCLEAR GENERATING STATION
 PALO VERDE NUCLEAR GENERATING STATION I&C Program Classroom Lesson I&C Program Date: 2/25/2011 1:42:48 PM LP Number: NIA97C000503 Rev Author: DANIEL R. REED Title: Temperature Measurement Technical Review:
PALO VERDE NUCLEAR GENERATING STATION I&C Program Classroom Lesson I&C Program Date: 2/25/2011 1:42:48 PM LP Number: NIA97C000503 Rev Author: DANIEL R. REED Title: Temperature Measurement Technical Review:
Temperature measurement
 emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
13.1 The Nature of Gases (refer to pg )
 13.1 The Nature of Gases (refer to pg. 420-424) Essential Understanding any other state of matter. Temperature and pressure affect gases much more than they affect Lesson Summary Kinetic Theory and a Model
13.1 The Nature of Gases (refer to pg. 420-424) Essential Understanding any other state of matter. Temperature and pressure affect gases much more than they affect Lesson Summary Kinetic Theory and a Model
Veerapong Kanchanawongkul*
 Using LabVIEW to Development of Temperature Measurement System with Thermocouple and Thermistor AIS 08 Veerapong Kanchanawongkul* Department of Mechanical Engineering, Faculty of Engineering, South-East
Using LabVIEW to Development of Temperature Measurement System with Thermocouple and Thermistor AIS 08 Veerapong Kanchanawongkul* Department of Mechanical Engineering, Faculty of Engineering, South-East
4/6/2015. Kelvin. Temperature. Aquatic vs. Air Temperatures. Temperature. 0 K kenetic energy = 0 0 K = -273 C Volume = 0
 Daniel Gabriel Fahrenheit (1686 1736) Fahrenheit scale determined by three reference points 0 F mixture of water, ice, and ammonium chloride 30 F water at the point of surface ice 96 F human body temperature
Daniel Gabriel Fahrenheit (1686 1736) Fahrenheit scale determined by three reference points 0 F mixture of water, ice, and ammonium chloride 30 F water at the point of surface ice 96 F human body temperature
15. Compare the result with the value you have taken above Compare the calculated pressure value with the actual pressure value that you have
 105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
Announcements. Applied Physics. Temperature. Questions
 Announcements Applied Physics 02-14-08 Temperature & Heat Florence Henderson (74) One new quiz up today. Get to work on them NOW! We will be burning food in lab on Tuesday. You may want to wear clothes
Announcements Applied Physics 02-14-08 Temperature & Heat Florence Henderson (74) One new quiz up today. Get to work on them NOW! We will be burning food in lab on Tuesday. You may want to wear clothes
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
 Since ancient times, people have needed thermal energy (heat) to cook their food and to keep them warm. And since the beginning of time, uncontrolled heat has scorched and spoiled the taste of food and
Since ancient times, people have needed thermal energy (heat) to cook their food and to keep them warm. And since the beginning of time, uncontrolled heat has scorched and spoiled the taste of food and
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power).
 Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Measurement in Engineering
 Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Thermodynamics INTRODUCTION AND BASIC CONCEPTS. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics INTRODUCTION AND BASIC CONCEPTS Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. THERMODYNAMICS AND ENERGY Thermodynamics: The science of energy.
Thermodynamics INTRODUCTION AND BASIC CONCEPTS Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. THERMODYNAMICS AND ENERGY Thermodynamics: The science of energy.
LHSE Presents. Temperature
 LHSE Presents Introduction to Chemistry Temperature Conversions Previous lecture Math for Chemistry Temperature Heat Temperature 3 Temperature scales (F o, C o, K) Equations for converting between scale
LHSE Presents Introduction to Chemistry Temperature Conversions Previous lecture Math for Chemistry Temperature Heat Temperature 3 Temperature scales (F o, C o, K) Equations for converting between scale
TEMPERATURE AND THERMAL EXPANSION
 TEMPERATURE AND THERMAL EXPANSION After boiling water, you will feel that the water is hotter than before, or you can say that the water temperature is higher than before. Otherwise, when you pick an ice,
TEMPERATURE AND THERMAL EXPANSION After boiling water, you will feel that the water is hotter than before, or you can say that the water temperature is higher than before. Otherwise, when you pick an ice,
Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund
 Aalborg Universitet Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund Publication date: 2008 Document Version
Aalborg Universitet Temperature Measurements Using Type K Thermocouples and the Fluke Helios Plus 2287A Datalogger Artmann, Nikolai; Vonbank, R.; Jensen, Rasmus Lund Publication date: 2008 Document Version
LYOPHILIZATION. First of Four Lectures. Introduction to Freeze-Drying. Introduction to Freeze-Drying. J. Jeff Schwegman, Ph.D. AB BioTechnologies
 LYOPHILIZATION Introduction to Freeze-Drying First of Four Lectures Introduction to Freeze-Drying J. Jeff Schwegman, Ph.D. AB BioTechnologies P.O. Box 1430 Bloomington, Indiana, 47402 812-327-6898 jjschwegman@ab-biotech.com
LYOPHILIZATION Introduction to Freeze-Drying First of Four Lectures Introduction to Freeze-Drying J. Jeff Schwegman, Ph.D. AB BioTechnologies P.O. Box 1430 Bloomington, Indiana, 47402 812-327-6898 jjschwegman@ab-biotech.com
EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical Simulation and Measurement
 Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Physics 207 Lecture 23. Lecture 23
 Goals: Lecture 3 Chapter 6 Use the ideal-gas law. Use pv diagrams for ideal-gas processes. Chapter 7 Employ energy conservation in terms of st law of TD Understand the concept of heat. Relate heat to temperature
Goals: Lecture 3 Chapter 6 Use the ideal-gas law. Use pv diagrams for ideal-gas processes. Chapter 7 Employ energy conservation in terms of st law of TD Understand the concept of heat. Relate heat to temperature
Dr. Ramy Y. Morjan. Figure 1. PDF created with pdffactory trial version Observations. Quantitative.
 1.1 What is Chemistry? Chemistry can be defined as the science that deals with the materials of the universe and the changes that these materials undergo and the energy associated with those changes. Chemistry
1.1 What is Chemistry? Chemistry can be defined as the science that deals with the materials of the universe and the changes that these materials undergo and the energy associated with those changes. Chemistry
Heat & Temperature. Grade 7 Science - Unit 2 Pgs
 Heat & Temperature Grade 7 Science - Unit 2 Pgs 104-225 4.1 Temperature PTemperature is the measure of how much heat is in a substance. PTemperature is measured in degrees Celcius ( C) PIt is difficult
Heat & Temperature Grade 7 Science - Unit 2 Pgs 104-225 4.1 Temperature PTemperature is the measure of how much heat is in a substance. PTemperature is measured in degrees Celcius ( C) PIt is difficult
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS
 ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
ME 365 EXPERIMENT 5 FIRST ORDER SYSTEM IDENTIFICATION APPLIED TO TEMPERATURE MEASUREMENT SYSTEMS Objectives: In this two week experiment, we will gain familiarity with first order systems by using two
Part Number Range Accuracy Printing WP * Probe Special User LDC with Page Logging included Feature Cal Backlight
 Table of Contents Comparison Chart Introduction Temperature Indicators & Controllers Portable Infrared Printing/Logging Page O2 O3 O10 O14 O30 O33 O1 Part Number Range Accuracy Printing WP * Probe Special
Table of Contents Comparison Chart Introduction Temperature Indicators & Controllers Portable Infrared Printing/Logging Page O2 O3 O10 O14 O30 O33 O1 Part Number Range Accuracy Printing WP * Probe Special
Chapter 17. Temperature. Dr. Armen Kocharian
 Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
Figure 1.1. Relation between Celsius and Fahrenheit scales. From Figure 1.1. (1.1)
 CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
Making Contact with Temperature
 Making Contact with Temperature Here is a look at the phenomenon itself, the basic measurement technologies available, and how industry is presently using them. Jesse Yoder, Flow Research Temperature is
Making Contact with Temperature Here is a look at the phenomenon itself, the basic measurement technologies available, and how industry is presently using them. Jesse Yoder, Flow Research Temperature is
Temperature and Its Measurement
 Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
1. Mark the correct statement(s)
 1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit.
 2 This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit. CONTENTS thermometry what is temperature?, fixed points, Kelvin (Absolute),
2 This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit. CONTENTS thermometry what is temperature?, fixed points, Kelvin (Absolute),
Professional Article. Fire and Ice
 Professional Article Fire and Ice Professional Article Spectrumanalysis Fire and Ice Temperature measurement is important in the industry. In the chemical industry as well as for environmental protection
Professional Article Fire and Ice Professional Article Spectrumanalysis Fire and Ice Temperature measurement is important in the industry. In the chemical industry as well as for environmental protection
Before Statement After
 Thermal Energy Thermal Energy, Temperature, and Heat What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
Thermal Energy Thermal Energy, Temperature, and Heat What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
EXPERIMENT VARIATION OF THERMO-EMF WITH TEMPERATURE. Structure. 7.1 Introduction Objectives
 EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
Solids, Liquids, and Gases. Chapter 14
 Solids, Liquids, and Gases Chapter 14 Matter & Thermal Energy Matter can exist as a solid, a liquid, a gas or a plasma. The Molecular Kinetic Theory of Matter explains their differences and how they can
Solids, Liquids, and Gases Chapter 14 Matter & Thermal Energy Matter can exist as a solid, a liquid, a gas or a plasma. The Molecular Kinetic Theory of Matter explains their differences and how they can
CHE Thermodynamics of Chemical Processes
 CHE 3010 - Thermodynamics of Chemical Processes Venkat Padmanabhan, PhD Department of Chemical Engineering Tennessee Tech University Lecture 2 - Basic Concepts 8/29/2018 CHE 3010 - Thermodynamics Tennessee
CHE 3010 - Thermodynamics of Chemical Processes Venkat Padmanabhan, PhD Department of Chemical Engineering Tennessee Tech University Lecture 2 - Basic Concepts 8/29/2018 CHE 3010 - Thermodynamics Tennessee
General Physics I. Lecture 23: Basic Concepts of Thermodynamics
 General Physics I Lecture 23: Basic Concepts of Thermodynamics Prof. WAN, Xin xinwan@zju.edu.cn http://zimp.zju.edu.cn/~xinwan/ Temperature [Operational definition] Temperature is what you measure with
General Physics I Lecture 23: Basic Concepts of Thermodynamics Prof. WAN, Xin xinwan@zju.edu.cn http://zimp.zju.edu.cn/~xinwan/ Temperature [Operational definition] Temperature is what you measure with
Lecture Notes: Chem 110 Chapter 1
 1 Lecture Notes: Chem 110 Chapter 1 Overview of syllabus: e-mail: vtc101@psu.edu What is chemistry? Some definitions of chemistry (p. 2): Chemistry has been called the central science. This is due to it
1 Lecture Notes: Chem 110 Chapter 1 Overview of syllabus: e-mail: vtc101@psu.edu What is chemistry? Some definitions of chemistry (p. 2): Chemistry has been called the central science. This is due to it
Thermal Process Control Lap 4 Thermal Energy. Notes:
 Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
Thermal Process Control Lap 4 Thermal Energy Notes: 1) Temperature Measurement a) Define temperature i) A measure of the amount of heat contained in a solid, liquid, or gas ii) Result of molecular motion
PROGRAM OF PHYSICS. Lecturer: Dr. DO Xuan Hoi Room A
 PROGRAM OF PHYSICS Lecturer: Dr. DO Xuan Hoi Room A1. 503 E-mail : dxhoi@hcmiu.edu.vn PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) 02 credits (30 periods) Chapter 1 Fluid Mechanics Chapter 2 Heat, Temperature
PROGRAM OF PHYSICS Lecturer: Dr. DO Xuan Hoi Room A1. 503 E-mail : dxhoi@hcmiu.edu.vn PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) 02 credits (30 periods) Chapter 1 Fluid Mechanics Chapter 2 Heat, Temperature
Temperature Sensors & Measurement
 Temperature Sensors & Measurement E80 Spring 2014 Contents Why measure temperature? Characteristics of interest Types of temperature sensors 1. Thermistor 2. RTD Sensor 3. Thermocouple 4. Integrated Silicon
Temperature Sensors & Measurement E80 Spring 2014 Contents Why measure temperature? Characteristics of interest Types of temperature sensors 1. Thermistor 2. RTD Sensor 3. Thermocouple 4. Integrated Silicon
Theory and Design for Mechanical Measurements
 Theory and Design for Mechanical Measurements Third Edition Richard S. Figliola Clemson University Donald E. Beasley Clemson University John Wiley & Sons, Inc. New York / Chichester / Weinheim / Brisbane
Theory and Design for Mechanical Measurements Third Edition Richard S. Figliola Clemson University Donald E. Beasley Clemson University John Wiley & Sons, Inc. New York / Chichester / Weinheim / Brisbane
1 Environment to be measured Gas Mercury. Mercury is added to or removed from flask 1 so that height in flask 2 is constant
 Lecture 2: Thermal Expansion and Ideal Gases Last lecture, we defined temperature and explored the problem of designing and calibrating a thermometer The liquid thermometer was not too bad, but had several
Lecture 2: Thermal Expansion and Ideal Gases Last lecture, we defined temperature and explored the problem of designing and calibrating a thermometer The liquid thermometer was not too bad, but had several
Ch 100: Fundamentals for Chemistry
 Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Physics 121, April 10, Temperature/Heat. Department of Physics and Astronomy, University of Rochester
 Physics 121, April 10, 2008. Temperature/Heat. Physics 121. April 10, 2008. Course Information Topics to be discussed today: Temperature Physics 121. April 10, 2008. Homework set # 8 is due on Saturday
Physics 121, April 10, 2008. Temperature/Heat. Physics 121. April 10, 2008. Course Information Topics to be discussed today: Temperature Physics 121. April 10, 2008. Homework set # 8 is due on Saturday
Chapter 12. Temperature and Heat
 Chapter 12 Temperature and Heat 12.1 Common Temperature Scales Temperatures are reported in degrees Celsius or degrees Fahrenheit. Kelvin Scale 100 o C or 212 o F T K = T + 273.15 Temperature changes,
Chapter 12 Temperature and Heat 12.1 Common Temperature Scales Temperatures are reported in degrees Celsius or degrees Fahrenheit. Kelvin Scale 100 o C or 212 o F T K = T + 273.15 Temperature changes,
1.4 Units of Measurement
 1.4 Units of Measurement Many properties of matter are quantitative; that is, they are associated with numbers. When a number represents a measured quantity, the units of that quantity must always be specified.
1.4 Units of Measurement Many properties of matter are quantitative; that is, they are associated with numbers. When a number represents a measured quantity, the units of that quantity must always be specified.
The Next Step. Mathematics Applications for Adults. Book Measurement
 The Next Step Mathematics Applications for Adults Book 14012 - Measurement OUTLINE Mathematics - Book 14012 Measurement Time demonstrate an understanding of time (minutes, hours, a day, a week, a month).
The Next Step Mathematics Applications for Adults Book 14012 - Measurement OUTLINE Mathematics - Book 14012 Measurement Time demonstrate an understanding of time (minutes, hours, a day, a week, a month).
Using a Mercury itc with thermocouples
 Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
[ ] Sensors for Temperature Measurement, and Their Application 2L R 1 1 T 1 T 2
![[ ] Sensors for Temperature Measurement, and Their Application 2L R 1 1 T 1 T 2 [ ] Sensors for Temperature Measurement, and Their Application 2L R 1 1 T 1 T 2](/thumbs/89/97951344.jpg) Sensors for Temperature Measurement, and Their Application In today s market, it is very rare to see electronic equipment that has not undergone extensive thermal evaluation, either by measurement or simulation.
Sensors for Temperature Measurement, and Their Application In today s market, it is very rare to see electronic equipment that has not undergone extensive thermal evaluation, either by measurement or simulation.
Estimate, for this water, the specific heat capacity, specific heat capacity =... J kg 1 K 1. the specific latent heat of vaporisation.
 1 A kettle is rated as 2.3 kw. A mass of 750 g of water at 20 C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes,
1 A kettle is rated as 2.3 kw. A mass of 750 g of water at 20 C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes,
Very Dynamic! Energy in the Earth s Atmosphere. How Does it Get Here? All Objects Radiate Energy!
 Energy in the Earth s Atmosphere Unit Essential Question: What are the different features of the atmosphere that characterize our weather. How does the atmosphere influence life and how does life influence
Energy in the Earth s Atmosphere Unit Essential Question: What are the different features of the atmosphere that characterize our weather. How does the atmosphere influence life and how does life influence
Test Wednesday, April 12 th 7pm, G20 Ming-Hsieh Bring your calculator and #2 pencil with a good eraser! 20 Multiple choice questions from:
 Test Wednesday, April 12 th 7pm, G20 Ming-Hsieh Bring your calculator and #2 pencil with a good eraser! 20 Multiple choice questions from: Chapter 7 (except 7.6) Rotational motion, Centripetal acceleration,
Test Wednesday, April 12 th 7pm, G20 Ming-Hsieh Bring your calculator and #2 pencil with a good eraser! 20 Multiple choice questions from: Chapter 7 (except 7.6) Rotational motion, Centripetal acceleration,
Period 5: Thermal Energy, the Microscopic Picture
 Name Section Period 5: Thermal Energy, the Microscopic Picture 5.1 How Is Temperature Related to Molecular Motion? 1) Temperature Your instructor will discuss molecular motion and temperature. a) At a
Name Section Period 5: Thermal Energy, the Microscopic Picture 5.1 How Is Temperature Related to Molecular Motion? 1) Temperature Your instructor will discuss molecular motion and temperature. a) At a
Let s Get Digital: A Guide to Unraveling the Tangled World of Digital Thermometers
 Let s Get Digital: A Guide to Unraveling the Tangled World of Digital Thermometers By Maria Knake, Laboratory Assessment Program Manager Posted: November 2011 So, You re Looking to Buy a Digital Thermometer?
Let s Get Digital: A Guide to Unraveling the Tangled World of Digital Thermometers By Maria Knake, Laboratory Assessment Program Manager Posted: November 2011 So, You re Looking to Buy a Digital Thermometer?
What is Temperature?
 What is Temperature? Observation: When objects are placed near each other, they may change, even if no work is done. (Example: when you put water from the hot tap next to water from the cold tap, they
What is Temperature? Observation: When objects are placed near each other, they may change, even if no work is done. (Example: when you put water from the hot tap next to water from the cold tap, they
Community College of Allegheny County Unit 9 Page #1. Thermocouples R1 = 1K
 10K Community College of Allegheny County Unit 9 Page #1 Thermocouples +12V Thermocouple Junction Vin Copper Wire Constantan Wire + - 3 2 741 7 4 1 5-12V 6 V Vout R1 = 1K Rf = 100K Engineers are not expected
10K Community College of Allegheny County Unit 9 Page #1 Thermocouples +12V Thermocouple Junction Vin Copper Wire Constantan Wire + - 3 2 741 7 4 1 5-12V 6 V Vout R1 = 1K Rf = 100K Engineers are not expected
All measurements contain a number and a unit. Every unit is based upon standard.
 All measurements contain a number and a unit. Every unit is based upon standard. Units and Standards A standard is an exact quantity that people agree to use to compare measurements. Measurement Systems
All measurements contain a number and a unit. Every unit is based upon standard. Units and Standards A standard is an exact quantity that people agree to use to compare measurements. Measurement Systems
CHAPTER 9: TEMPERATURE, PRESSURE, STRAIN AND MOTION MEASUREMENTS
 CHAPTER 9: TEMPERATURE, PRESSURE, STRAIN AND MOTION MEASUREMENTS I. MEASUREMENT OF TEMPERATURE 1. Introduction In industrial-process control, temperature is the most frequently controlled and measured
CHAPTER 9: TEMPERATURE, PRESSURE, STRAIN AND MOTION MEASUREMENTS I. MEASUREMENT OF TEMPERATURE 1. Introduction In industrial-process control, temperature is the most frequently controlled and measured
PHYS 352 Assignment #1 Solutions
 PHYS 352 Assignment #1 Solutions 1. Steinhart-Hart Equation for a thermistor In[1]:= a : 0.00128 In[2]:= b : 0.000236 In[3]:= In[4]:= d : 9.27 10^ 8 t r_ : a b Log r d Log r ^3 ^ 1 Note: Mathematica Log
PHYS 352 Assignment #1 Solutions 1. Steinhart-Hart Equation for a thermistor In[1]:= a : 0.00128 In[2]:= b : 0.000236 In[3]:= In[4]:= d : 9.27 10^ 8 t r_ : a b Log r d Log r ^3 ^ 1 Note: Mathematica Log
Measurement of Temperature in the Plastics Industry
 Sawi Mess- und Regeltechnik AG CH 8405 Winterthur-Gotzenwil, Switzerland Telephone +41 52 320 50 50, Fax +41 52 320 50 55 www.sawi.ch Measurement of Temperature in the Plastics Industry Johannes Wild,
Sawi Mess- und Regeltechnik AG CH 8405 Winterthur-Gotzenwil, Switzerland Telephone +41 52 320 50 50, Fax +41 52 320 50 55 www.sawi.ch Measurement of Temperature in the Plastics Industry Johannes Wild,
