Electron Energy Loss Spectroscopy
|
|
|
- Hilary Payne
- 6 years ago
- Views:
Transcription
1 Electron Energy Loss Spectroscopy EELS: Large signal for Z< 33 90% collection efficiency Spatial resolution is nm composition and bonding information needs very thin samples (< 50 nm) EDX: Low x-ray yield for Z<10, good for detecting heavy elements Poor (1-3%) collection efficiency Spatial resolution is 1-10 nm composition only samples can be up to 100 nm (if you don t mind absorption corrections) Reading and References: Fingerprints of most materials Muller, Singh and Silcox, Phys Rev B57, 8181 (1998) Theory of EELS as a LDOS V. Keast et al, (review of EELS) 1
2 Discrete Energy Spectra for Atoms Unique spectrum for every atom used to identify unknown materials KE=ΔE-E K 3000 M 5 M 4 M 3 M 2 M 1 L 3 L 2 L 1 K 3d 5/2 3d 3/2 3p 3/2 3p 1/2 3s 1/2 2p 3/2 2p 1/2 2s 1/2 1s 1/2 Binding Energy (ev) n=1 n=2 n=3 n=4 E 0 E 0 -ΔE Measured by EELS (Electron Energy Loss Spectroscopy In a TEM) 500 n= Atomic Number Z 2
3 Electron Shells and Transitions 1) Ionization 2a) X-ray Emission 2b) Auger electron KE=ΔE-E K KE = * ( E E ) K L M 2 1 M 5 M 4 M 3 M 2 M 1 3d 5/2 3d 3/2 3p 3/2 3p 1/2 3s 1/2 M 5 M 4 M 3 M 2 M 1 3d 5/2 3d 3/2 3p 3/2 3p 1/2 3s 1/2 hω = E K E L2 M 5 M 4 M 3 M 2 M 1 3d 5/2 3d 3/2 3p 3/2 3p 1/2 3s 1/2 L 3 2p 3/2 L 3 2p 3/2 L 3 2p 3/2 L 2 2p 1/2 L 2 2p 1/2 L 2 2p 1/2 L 1 2s 1/2 L 1 2s 1/2 L 1 2s 1/2 K 1s 1/2 K 1s 1/2 K 1s 1/2 E 0 E 0 -ΔE Measured by EELS (Electron Energy Loss Spectroscopy In a TEM) Measured by EDS/XRF (X-ray fluorescence in an SEM or TEM) Measured by AES (Auger Spectroscopy in a SAM, Leaves atom doubly charged) 3
4 O 6 O 5 O O 4 N M L Electron Shells and Transitions O 8 O 7 5f 7/2 5f 5/2 O 3 O 2 O 1 N 7 4f 7/2 N 6 4f 5/2 N 5 N 4 N 3 N 2 N 1 M M 5 4M M3 2 M 1 L 3 L 2 X-ray lines Lα 1 Lα 2 Lβ1 Lβ 2 Lβ 5 Lγ 3 5d 5/2 5d 3/2 5p 3/2 5p 1/2 5s 1/2 4d 5/2 4d 3/2 4p 3/2 4p 1/2 4s 1/2 3d 5/2 3d 3/2 3p 3/2 3p 1/2 3s 1/2 2p 3/2 2p 1/2 EELS Edges N 6,7 N 4,5 N 2,3 N 1 M 4,5 M 2,3 M 1 L 3 L 2 L 1 K Electron State Notation Principal quantum number Angular momentum state (l) 4 f 5/2 J=L+S quantum number (jj coupling) l=0 s l=1 p l=2 d l=3 f L 1 2s 1/2 Κα 1 Κα 2 Κβ 1 Κβ 3 Κβ 2 K SHELL K EELS transitions are named by the initial state (e.g. 2p 3/2 [3s 1/2 3d 5/2,3d 5/2 ] is just L 3 ) EDX transitions are named by the initial core-hole state, the emission line, and the line strength (1 is the strongest, ) (e.g. 3d 5/2 1s 1/2 is Kα ) 1 AES transitions are named by initial ionization, filling shell, shell of ejected electron (e.g. KL 2 L 3 ) 1s 1/2
5 Electron Energy Loss Spectrum of SiO 2 Each edge sits on the tails of the preceding edges -> Backgrounds are large 10 8 Intensity (arb. units) Incident Beam Valence Excitations Si L edge E 0 O-K edge L 3 L 2 L 1 K 2p 3/2 2p 1/2 2s 1/2 1s 1/2 E 0 -ΔE Energy Loss (ev) 5
6 X-Ray Emission Spectra X-ray Emission Energies for all elements can be found in the X-ray Data Booklet, which is available on line at M 5 M 4 M 3 M 2 M 1 3d 5/2 3d 3/2 3p 3/2 3p 1/2 3s 1/2 L 3 L 2 L 1 2p 3/2 2p 1/2 2s 1/2 K 1s 1/2 X-ray Emission Spectra from Williams and Carter, Electron Microscopy 6
7 Auger Electrons a small signal on the tails Of the secondary electron peak E M 1 L 3 L 2 L 1 2p 3/2 2p 1/2 2s 1/2 K 1s 1/2 Cross section decreases with Energy multiply by E to compensate 2 nd electron background is slowly varying with E, take derivate to remove it -> Easier to see peaks in E. dn(e)/de (price paid: noise is also amplified) From the Auger tutorial on the Charles Evans website 7
8 Auger vs X-ray Yield 1 ~10 kev Fluorescence Yeild ω K ω Auger Z Below ~10 kev, Auger emission dominates and very few x-rays are emitted. X-ray fluorescence is most efficient for detecting high-z elements Eqn 3.13 of Rev. Mod. Phys. 44, (1972) 8
9 X-Ray Absorption e - X-ray detector φ t X t =t/sinφ I esc = I prod exp( -t /λ) (φ should be large to minimize attenuation) X-ray attenuation length λ ~ 10 nm 1 μm Low energy x-rays are strongly attenuated must be corrected for to get quantitative compositions 9
10 10
11 Bremsstrahlung Radiation + X-rays absorbed in sample Cuts off lower energies e - Accelerating charge produces radiation In thicker films and solids, there is a continuum background to the x-ray spectrum 11
12 EDX generated by x-rays (XRF) and electrons (SEM) Xray: No vacuum No charging No Bremsstrahlung 100 μm resolution Electron: 0.5 μm resolution Better low energy excitation efficiency Bremsstrahlung 12
13 Losses in the X-Ray Detector Strong Attenuation for low Z Williams and Carter, Vol 4 13
14 14
15 Comparison EDS Low collection efficiency (small solid angle) Good peak/background Good for high-z elements SEM (~500 nm) or TEM (~10 nm) Stray x-rays in the column can be mistaken for trace elements EELS TEM only & thin samples High collection efficiency (~90%) Poor peak/background (esp in thick samples) Best for low-z elements (large signals) Bonding information for Z<33 High spatial resolution (0.1 1 nm) 15
16 Scanning Transmission Electron Microscopy 200 kv Incident Electron Beam (ΔE= ev) 1 atom wide (0.2 nm) beam is scanned across the sample to form a 2-D image ADF Signal Er M 4 Edge x y 3 Å Elastic Scattering ~ "Z contrast" Distance (nm) Annular Dark Field (ADF) detector Electron Energy Loss Spectrometer Increasing energy loss Single atom Sensitivity: P. Voyles, D. Muller, J. Grazul, P. Citrin, H. Gossmann, Nature (2002) U. Kaiser, D. Muller, J. Grazul, M. Kawasaki, Nature Materials, (2002) 16
17 Energy Filtered Electron Microscopy image 1 energy at a time (as opposed to STEM 1 pixel with all energies at a time) 200 kv Incident Electron Beam ΔE = mev Point Spread Function 10 sec. acquisition time Specimen 150 mv Entrance aperture Energy (ev) Magnetic prism CCD Camera Energy-Selecting Slits 17
18 Energy Filtered TEM: Image at a fixed Energy Loss Ratio maps cancel most of the diffraction contrast (elastic scattering is same for both energy losses) Ti L edge ratio map (post/pre) 18
19 Energy Loss Spectrum of a 100 kev Electron Beam in Si Plasmon mean free path λ~ 120 nm 10 5 Zero Loss Peak 17 nm 210 nm t/λ = 0.14 t/λ = CCD Counts st Plasmon 2 nd Plasmon 3 rd Plasmon Energy Loss (ev) In the thinner film (17 nm thick), only single scattering has occurred, and there is a single peak at the plasma energy (~17 ev) this is also called a plasmon. In the thicker film (210 nm), a significant portion of the electron beam has undergone inelastic scattering many times. In each scattering event it loses ~ 17 ev so those electrons that have scattered twice show up as a peak at 2x17 = 34 ev, those that scattered 3 times at 3x17=51 ev and so on. 19
20 Effect of Thickness on the Si L 23 Edge at 100 kv CCD Counts th Plasmon 5 th Plasmon 6 th Plasmon Si L 2,3 Edge 210 nm 174 nm 148 nm 114 nm 78 nm 38 nm 17 nm Increasing Thickness Plasmon Mean Free Path in Silicon λ p 120 nm. When the thickness t/λ p > 1 multiple plasmon scattering dominates the EELS spectra. At 210 nm, a ratio map at the 100 ev will measure the 6th plasmon, not the Si L edge! Energy Loss (ev) Moral: EELS needs thin samples! 20
21 Effect of Increasing the Illumination Angle ( (α) (by reciprocity: increasing the collector angle in STEM) α<<θ obj α θ obj Phase contrast No Phase contrast Diffraction contrast Diffraction contrast 31 mr 10 2 mr α>>θ obj No Phase contrast No Diffraction contrast 80 3 mr ADF 4 30 nm The incoherent BF image is the complement of the ADF image 21
22 Effect of Increasing the Illumination Angle (by reciprocity: increasing the collector angle in STEM) 1.5 mr 80 mr Si 17 ev (Si Plasmon) Si 23 ev (SiO2 Plasmon) SiO 2 Coherent Illumination: Condenser < Objective Aperture Thickness fringes Fresnel Contrast Incoherent Illumination: Condenser > Objective Aperture Diffraction contrast suppressed 22
23 Core-Level Electron Energy Loss Spectroscopy E Fermi EELS measures a local density of states partitioned by site - as the probe is localized, element - the core level binding energy is unique Dipole transition: s p - probes the conduction band - provides local electronic information O 1s 23
24 EELS Theory Some subtleties as to which density of states is measured see Muller, Singh and Silcox, Phys Rev B57, 8181 (1998) This is very important if you want to measure charge transfers (you don t there is no unique definition). Dipole selection rules: Δl = ± 1, Δj = 0, ± 1 K-edge: 1s p L-edge: L1 : 2s p; L2, 3 : 2 p d, s; 24
25 EELS as a Local Density of States (LDOS) If we project the total density of states on to a local set of states and examine the overlap of each eigenstate with the local state. The probability of finding an electron in the eigenstate at site is, so the local contribution to the density of states from site is The basis set chosen for { i } is not unique, so the amount of charge at site i is also not Unique. (e.g. a sphere of arbitrary size). For EELS, the oscillator strength is proportional to a LDOS with a basis set of Muller, Singh and Silcox, Phys Rev B57, 8181 (1998) 25
26 Don t compare EELS to calculations of charge transfer The charge-transfer problem: Since there is no unique definition of a local density of states, there is also no unique definition for charge transfers between local states To caution against directly comparing EELS whitelines against calculated charges, we show the charge transfer from an atomic sphere surrounding a Ni atom in the B2 NiAl compound, calculated in the LMTO-ASA approximation. The choice of the relative sphere sizes for the Ni and Al sites are a matter of computational convenience, rather than being a physically measurable property of the system. By altering the ratio of the Ni/Al sphere sizes we can change not only the magnitude, but also the sign of the Ni-Al charge transfer. Muller, Singh and Silcox, Phys Rev B57, 8181 (1998)
27 Dipole Approximation is good for Core Level EELS (except when the probe < core orbital size can happen during channeling) K Edge 1s->p dipole 1s->s monopole 1s->d quadrupole L Edge 2p->d dipole 2p->p monopole 2p->s dipole P. Rez, Ultramicroscopy
28 Dipole Approximation is good for Core Level EELS Si L 23 edge for a 100 kev incident electron Collection angles of 12.5 mrad (q=20 nm -1 ), The solid line is the full calculation, the dashed line is the dipole contribution, and the light dotted line is the nondipole 2p-3p term Collection angles of 100 mrad (q= 167 nm -1 ) P. Rez, Ultramicroscopy
29 EELS Fine Structure is Visible in Individual Spectra Ti L 2,3 Edge 0 Intensity (a.u.) La-M 4,5 Distance (nm) Ti-L 2,3 Mn-L 2, Energy Loss (ev) Energy Loss (ev) 29
30 Chemical Imaging in 2D (Integrated counts above background) La Ti EELS map Of LaMnO 3 /SrTiO 3 recorded on the Nion SuperSTEM Mn 28 seconds live time (10 seconds overhead) 30
31 EELS Fine Structure of Transition Metals Ground state interpretation of spectra as a LDOS (ignore core hole) EELS edge L 2 d DOS L 3 L 2 L 3 Pearson, Fultz and Ahn, Phys Rev B47 (1993) 31
32 EELS Fingerprints of Oxidation States Leapman, Grunes, Fejes,Physical Review B (1982) Ti L 2,3 Edge Cu L 2,3 Edge 32
33 Fingerprint EELS from our website : EELS spectra of common semiconductor materials Identify the local environment from the shape of the spectrum (e.g. Cu vs. CuO vs. Cu 2 O) 33
34 Interpreting Experimental Data Intensity (a.u.) Is this real? Intensity (a.u.) Energy (ev) Energy (ev) Always show the pre-edge background. Gives noise level & confidence in background subtraction 34
35 What Happens when Al is added to Ni x Al 1-x? Ni L 2,3 Edge After Deconvolution with Low Loss Ni Ni 3 Al NiAl Area (barns) 514±20 508±20 508± B 10.0 A Energy Loss (ev) The Total Areas under each curve are very similar (no charge-xfer). Ni d is broadened, shifting states from the main band, to the tails increased Ni-Al bonding (Ni p-d hybridization) 35
36 EELS: Final State Effects Initial State Electrons relax to screen core hole metal Excited electron couples to core hole insulator δe ex δe rel δe rel 36
37 Comparison of the Ni L 3 Edge measured by EELS with the calculated, unoccupied d DOS of Ni 0.20 NiAl Measured NiAl Calculated 0.10 G.O.S. (e - /ev/atom) Ni 3 Al Ni Ni 3 Al Ni More Ni-Al bonds Energy (ev) Energy (ev) [D.A. Muller, D.J. Singh, J. Silcox, P.R. B57 (1998) 8181] 37
38 Comparison of the Measured Si-L L Edge with ab-initio Calculations Intensity (arb. units) Experiment Z+1 (core hole) (ground state) Energy (ev) Strong core-hole effects on the silicon-l Edge (it does not reflect the ground state) Neaton et al, Phys. Rev. Lett (2000) 38
39 When are core holes important? When you have good energy resolution (<1 ev) When screening is poor Metals (small), semiconductors(medium), ionic (huge) The effect is larger on anions than cations More noticeable in nanoparticles and clusters than bulk Batson s Rule: core hole effects are more pronounced when the excited electron is confined near the core hole. (It shouldn t work, but it does.) Atoms surrounded by strong scatterers (often nodeless valence wavefunctions 1s, 2p, 3d ) (e.g Si in SiOx, Al in NiAl, TiB 2 out of plane) 39
40 Core Hole Lifetimes Resolution Limits: 0.1 ev Z~14 (Si L 3 Γ i =0.15 ev) 0.2 ev Z~21 (Sc L 3 Γ i =0.19 ev) 0.3 ev Z~25,14 (Na K Γ i =0.30 ev) Better than 0.1 ev is still useful for valence EELS -image electrically active defects, - -Doesn t require sub nm probe Krause and Oliver, J. Phys. Chem. Ref. Dat. 8 (1979)
41 20 ev above the edge, no feature can be sharper than 3 ev 41
42 XAS of the Si L 2,3 Edge at 15 mev Energy Resolution 50 mev Photon Energy Photon Energy Vibrational modes are important at 100 mev resolution Core hole lifetimes are measured at mev (~ % larger than theory) R. Puttner et al, Phys. Rev. A57 297(1998). 42
43 XAS of N-K in N 2 N 2 gas N 2 in Oxidized TiAlN Instrument resolution is 70 mev Vibrational states are resolved, but core-hole lifetime depends on the environment F. Esaka et al, J. Elec. Spectr. and Rel. Phen (1998)
44 Calculation of the Final States Cluster Methods: good for defects & clusters, often easier to run Muffin-Tin Potential (OK for Metals, bad for semiconductors) FEFF7 no self-consistency: must guess charge transfers FEFF8 self consistent: good for metals Full Potential FDMNES no self-consistency, but it can input potentials from WIen2k Bandstructure methods: (3D periodic structures or supercells) Almost all bandstructure codes are self-consistent now Muffin-Tin Potential LMTO good for close-packed structures, esp. metals Full Potential FP-LAPW Wien2k easy to calculate matrix elements & core hole effects Plane-wave codes (faster and less prone to artifacts than APW codes ABINIT (free, open-source and downloadable from abinit.org) VASP (commercial) CASTEP (commercial, fancy user interface) 44
45 The Muffin-Tin Approximation This is a shortcut that makes it easier to solve Schrödinger's equation by approximating the potential From Joly A good approximation for close-packed structures like metals (DOS inside spheres looks like an EELS final states) 45
46 Wien2k vs FEFF8 TiC 0.8 N 0.2 expt FEFF8 Wien2k expt FEFF8 Wien2k 46
47 Many-Body Calculations Strongly-correlated systems, and materials with large core-hole effects cannot be calculated using DFT codes. The options are limited GW : Correct bandgap, but only a few atoms/supercell Configuration-Interaction (CI): very accurate for 1-6 atoms -good for transition metal oxide clusters Multiplet: (de Groot, van der Laan) single-atom in a crystal field good for transition metal oxide crystals. - like CI, except it has adjustable parameters 47
48 Many-Body Corrections for the Ti-L Edge in SrTiO 3 Intensity (arb. units) t 2g Energy (ev) a a L 3 e g t 2g L 3 L 2 L 2 e g Single Particle Theory: (Ogasawara, PRB 2001) includes core-hole self-consistently L 3 : 2p 3/2 Ti 3d (t 2g, e g ) L 2 : 2p 1/2 Ti 3d (t 2g, e g ) Wrong oscillator strengths and positions Configuration-Interaction Theory: (Ogasawara 2001) 4 main peaks analogous to SP theory Peak a is multiplet of the 2p 3/2 t 2g No lifetime broadening (gaussian used) 2p spin-orbit splitting is too small Experiment: XAS: van der Laan 1990 (2 ev too high) EELS: Muller mev resolution 50 mev absolute accuracy 48
49 Spatial Resolution in EELS For E 0 =100 kev electrons, λ=0.037 Å, v=1.64x10 8 m/s 2 d dωde For dipole scattering, the cross section is α f r i ρ ( ΔE) with the characteristic angle at energy loss ΔE of 2 θ 1 + θ σ 2 E 2 E ΔE 2E 0 By analogy with the Raleigh resolution criterion, we might expect a resolution of r inel λ θ (this would assume that all the scattering lies inside θ E, which is not true). E θ f For an energy loss ΔE=20 ev, we get r inel = 6. 5 nm 49
50 How Delocalized is an EELS Signal? Diamond Nanoparticle in ZnS 18 ev 25 ev 34eV An upper limit to the cutoff angle is the maximum momentum transfer in the small-angle approximation. This is also the peak of the Bethe Ridge at Which gives Muller & Silcox, Ultramicroscopy 59 (1995) 195. r min θ B 2θ E λ E ΔE 0 or 0.26 nm for ΔE=20 ev which is a little too small. The real answer seems to lie between r inel and r min (and closer to r min ) 50
51 Dipole Theory Calculations of Inelastic Resolution 4 3 θ θ E R. F. Egerton, Journal of Electron Microscopy 48 (1999)
52 Comparison of Dipole and Full Atomic Calculations For a free atom, agreement is ~10% or better. Crystal channeling could cause problems dipole dipole 52
53 Comparison Nickel Oxide EDS Low collection efficiency (small solid angle) Good peak/background Good for high-z elements SEM (~500 nm) or TEM (~10 nm) EELS TEM only & thin samples High collection efficiency (~90%) Poor peak/background (esp in thick samples) Best for low-z elements (large signals) Bonding information for Z<33 High spatial resolution (0.1 1 nm) 53
Chemical Analysis in TEM: XEDS, EELS and EFTEM. HRTEM PhD course Lecture 5
 Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
IV. Surface analysis for chemical state, chemical composition
 IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
EDS User School. Principles of Electron Beam Microanalysis
 EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
3D and Atomic-resolution Imaging with Coherent Electron Nanobeams - Opportunities and Challenges for X-rays
 3D and Atomic-resolution Imaging with Coherent Electron Nanobeams - Opportunities and Challenges for X-rays David A. Muller Lena Fitting Kourkoutis, Megan Holtz, Robert Hovden, Qingyun Mao, Julia Mundy,
3D and Atomic-resolution Imaging with Coherent Electron Nanobeams - Opportunities and Challenges for X-rays David A. Muller Lena Fitting Kourkoutis, Megan Holtz, Robert Hovden, Qingyun Mao, Julia Mundy,
4. Inelastic Scattering
 1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
Photon Interaction. Spectroscopy
 Photon Interaction Incident photon interacts with electrons Core and Valence Cross Sections Photon is Adsorbed Elastic Scattered Inelastic Scattered Electron is Emitted Excitated Dexcitated Stöhr, NEXAPS
Photon Interaction Incident photon interacts with electrons Core and Valence Cross Sections Photon is Adsorbed Elastic Scattered Inelastic Scattered Electron is Emitted Excitated Dexcitated Stöhr, NEXAPS
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~pchemlab ; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~pchemlab ; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
6. Analytical Electron Microscopy
 Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Supplementary Figure 1: ADF images and profiles for several types of atomic chains encapsulated in DWNTs. (a d) ADF images of NaI, CsF, CsCl, and CsI
 Supplementary Figure 1: ADF images and profiles for several types of atomic chains encapsulated in DWNTs. (a d) ADF images of NaI, CsF, CsCl, and CsI atomic chains encapsulated in DWNTs, respectively.
Supplementary Figure 1: ADF images and profiles for several types of atomic chains encapsulated in DWNTs. (a d) ADF images of NaI, CsF, CsCl, and CsI atomic chains encapsulated in DWNTs, respectively.
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications
 Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications Thomas Neisius Université Paul Cézanne Plan Imaging modes HAADF Example: supported Pt nanoparticles Electron sample interaction
Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications Thomas Neisius Université Paul Cézanne Plan Imaging modes HAADF Example: supported Pt nanoparticles Electron sample interaction
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
An Introduction to XAFS
 An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
Inelastic soft x-ray scattering, fluorescence and elastic radiation
 Inelastic soft x-ray scattering, fluorescence and elastic radiation What happens to the emission (or fluorescence) when the energy of the exciting photons changes? The emission spectra (can) change. One
Inelastic soft x-ray scattering, fluorescence and elastic radiation What happens to the emission (or fluorescence) when the energy of the exciting photons changes? The emission spectra (can) change. One
X-ray Spectroscopy Theory Lectures
 TIMES Lecture Series SIMES-SLAC-Stanford Winter, 2017 X-ray Spectroscopy Theory Lectures J. J. Rehr I. Introduction to the Theory of X-ray spectra II. Real-space Green's function Theory and FEFF III. Inelastic
TIMES Lecture Series SIMES-SLAC-Stanford Winter, 2017 X-ray Spectroscopy Theory Lectures J. J. Rehr I. Introduction to the Theory of X-ray spectra II. Real-space Green's function Theory and FEFF III. Inelastic
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Electron and electromagnetic radiation
 Electron and electromagnetic radiation Generation and interactions with matter Stimuli Interaction with sample Response Stimuli Waves and energy The energy is propotional to 1/λ and 1/λ 2 λ λ 1 Electromagnetic
Electron and electromagnetic radiation Generation and interactions with matter Stimuli Interaction with sample Response Stimuli Waves and energy The energy is propotional to 1/λ and 1/λ 2 λ λ 1 Electromagnetic
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
X-ray Photoelectron Spectroscopy (XPS)
 X-ray Photoelectron Spectroscopy (XPS) As part of the course Characterization of Catalysts and Surfaces Prof. Dr. Markus Ammann Paul Scherrer Institut markus.ammann@psi.ch Resource for further reading:
X-ray Photoelectron Spectroscopy (XPS) As part of the course Characterization of Catalysts and Surfaces Prof. Dr. Markus Ammann Paul Scherrer Institut markus.ammann@psi.ch Resource for further reading:
Generation of X-Rays in the SEM specimen
 Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Name: (a) What core levels are responsible for the three photoelectron peaks in Fig. 1?
 Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
Lecture 20 Auger Electron Spectroscopy
 Lecture 20 Auger Electron Spectroscopy Auger history cloud chamber Although Auger emission is intense, it was not used until 1950 s. Evolution of vacuum technology and the application of Auger Spectroscopy
Lecture 20 Auger Electron Spectroscopy Auger history cloud chamber Although Auger emission is intense, it was not used until 1950 s. Evolution of vacuum technology and the application of Auger Spectroscopy
X-ray Spectroscopy. Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis
 X-ray Spectroscopy Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis Element specific Sensitive to low concentrations (0.01-0.1 %) Why XAS? Applicable under
X-ray Spectroscopy Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis Element specific Sensitive to low concentrations (0.01-0.1 %) Why XAS? Applicable under
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
Electron Spettroscopies
 Electron Spettroscopies Spettroscopy allows to characterize a material from the point of view of: chemical composition, electronic states and magnetism, electronic, roto-vibrational and magnetic excitations.
Electron Spettroscopies Spettroscopy allows to characterize a material from the point of view of: chemical composition, electronic states and magnetism, electronic, roto-vibrational and magnetic excitations.
Lecture 17 Auger Electron Spectroscopy
 Lecture 17 Auger Electron Spectroscopy Auger history cloud chamber Although Auger emission is intense, it was not used until 1950 s. Evolution of vacuum technology and the application of Auger Spectroscopy
Lecture 17 Auger Electron Spectroscopy Auger history cloud chamber Although Auger emission is intense, it was not used until 1950 s. Evolution of vacuum technology and the application of Auger Spectroscopy
Core-Level spectroscopy. Experiments and first-principles calculations. Tomoyuki Yamamoto. Waseda University, Japan
 Core-Level spectroscopy Experiments and first-principles calculations Tomoyuki Yamamoto Waseda University, Japan 22 nd WIEN2k workshop Jun. 26 th, 2015@Singapore Outline What is core-level spectroscopy
Core-Level spectroscopy Experiments and first-principles calculations Tomoyuki Yamamoto Waseda University, Japan 22 nd WIEN2k workshop Jun. 26 th, 2015@Singapore Outline What is core-level spectroscopy
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Auger Electron Spectroscopy
 Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
Introduction to X-ray Photoelectron Spectroscopy (XPS) XPS which makes use of the photoelectric effect, was developed in the mid-1960
 Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Core Level Spectroscopies
 Core Level Spectroscopies Spectroscopies involving core levels are element-sensitive, and that makes them very useful for understanding chemical bonding, as well as for the study of complex materials.
Core Level Spectroscopies Spectroscopies involving core levels are element-sensitive, and that makes them very useful for understanding chemical bonding, as well as for the study of complex materials.
X-ray Energy Spectroscopy (XES).
 X-ray Energy Spectroscopy (XES). X-ray fluorescence as an analytical tool for element analysis is based on 3 fundamental parameters: A. Specificity: In determining an x-ray emission energy E certainty
X-ray Energy Spectroscopy (XES). X-ray fluorescence as an analytical tool for element analysis is based on 3 fundamental parameters: A. Specificity: In determining an x-ray emission energy E certainty
Electron Spectroscopy
 Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Elastic and Inelastic Scattering in Electron Diffraction and Imaging
 Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
SEM. Chemical Analysis in the. Elastic and Inelastic scattering. Chemical analysis in the SEM. Chemical analysis in the SEM
 THE UNIVERSITY Chemical Analysis in the SEM Ian Jones Centre for Electron Microscopy OF BIRMINGHAM Elastic and Inelastic scattering Electron interacts with one of the orbital electrons Secondary electrons,
THE UNIVERSITY Chemical Analysis in the SEM Ian Jones Centre for Electron Microscopy OF BIRMINGHAM Elastic and Inelastic scattering Electron interacts with one of the orbital electrons Secondary electrons,
Spectroscopy of Nanostructures. Angle-resolved Photoemission (ARPES, UPS)
 Spectroscopy of Nanostructures Angle-resolved Photoemission (ARPES, UPS) Measures all quantum numbers of an electron in a solid. E, k x,y, z, point group, spin E kin, ϑ,ϕ, hν, polarization, spin Electron
Spectroscopy of Nanostructures Angle-resolved Photoemission (ARPES, UPS) Measures all quantum numbers of an electron in a solid. E, k x,y, z, point group, spin E kin, ϑ,ϕ, hν, polarization, spin Electron
Energy Spectroscopy. Excitation by means of a probe
 Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
CHEM6416 Theory of Molecular Spectroscopy 2013Jan Spectroscopy frequency dependence of the interaction of light with matter
 CHEM6416 Theory of Molecular Spectroscopy 2013Jan22 1 1. Spectroscopy frequency dependence of the interaction of light with matter 1.1. Absorption (excitation), emission, diffraction, scattering, refraction
CHEM6416 Theory of Molecular Spectroscopy 2013Jan22 1 1. Spectroscopy frequency dependence of the interaction of light with matter 1.1. Absorption (excitation), emission, diffraction, scattering, refraction
MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Interaction X-rays - Matter
 Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Nanoelectronics 09. Atsufumi Hirohata Department of Electronics. Quick Review over the Last Lecture
 Nanoelectronics 09 Atsufumi Hirohata Department of Electronics 13:00 Monday, 12/February/2018 (P/T 006) Quick Review over the Last Lecture ( Field effect transistor (FET) ): ( Drain ) current increases
Nanoelectronics 09 Atsufumi Hirohata Department of Electronics 13:00 Monday, 12/February/2018 (P/T 006) Quick Review over the Last Lecture ( Field effect transistor (FET) ): ( Drain ) current increases
X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES)
 X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES) XPS X-ray photoelectron spectroscopy (XPS) is one of the most used techniques to chemically characterize the surface. Also known
X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES) XPS X-ray photoelectron spectroscopy (XPS) is one of the most used techniques to chemically characterize the surface. Also known
X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu
 X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu X-ray Photoelectron Spectroscopy Introduction Qualitative analysis Quantitative analysis Charging compensation Small area analysis and XPS imaging
X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu X-ray Photoelectron Spectroscopy Introduction Qualitative analysis Quantitative analysis Charging compensation Small area analysis and XPS imaging
Birck Nanotechnology Center XPS: X-ray Photoelectron Spectroscopy ESCA: Electron Spectrometer for Chemical Analysis
 Birck Nanotechnology Center XPS: X-ray Photoelectron Spectroscopy ESCA: Electron Spectrometer for Chemical Analysis Dmitry Zemlyanov Birck Nanotechnology Center, Purdue University Outline Introduction
Birck Nanotechnology Center XPS: X-ray Photoelectron Spectroscopy ESCA: Electron Spectrometer for Chemical Analysis Dmitry Zemlyanov Birck Nanotechnology Center, Purdue University Outline Introduction
Core loss spectra (EELS, XAS)
 Core loss spectra (EELS, XAS) Kevin Jorissen University of Washington (USA) WIENk 013 Penn State 1. Concepts WIENk calculates ELNES / XANES EELS : Electron Energy Loss Spectroscopy XAS: X-ray Absorption
Core loss spectra (EELS, XAS) Kevin Jorissen University of Washington (USA) WIENk 013 Penn State 1. Concepts WIENk calculates ELNES / XANES EELS : Electron Energy Loss Spectroscopy XAS: X-ray Absorption
QUESTIONS AND ANSWERS
 QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
MS482 Materials Characterization ( 재료분석 ) Lecture Note 2: UPS
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 2: UPS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 2: UPS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
Fundamentals of Nanoscale Film Analysis
 Fundamentals of Nanoscale Film Analysis Terry L. Alford Arizona State University Tempe, AZ, USA Leonard C. Feldman Vanderbilt University Nashville, TN, USA James W. Mayer Arizona State University Tempe,
Fundamentals of Nanoscale Film Analysis Terry L. Alford Arizona State University Tempe, AZ, USA Leonard C. Feldman Vanderbilt University Nashville, TN, USA James W. Mayer Arizona State University Tempe,
Electron energy loss spectroscopy (EELS)
 Electron energy loss spectroscopy (EELS) Phil Hasnip Condensed Matter Dynamics Group Department of Physics, University of York, U.K. http://www-users.york.ac.uk/~pjh503 Many slides courtesy of Jonathan
Electron energy loss spectroscopy (EELS) Phil Hasnip Condensed Matter Dynamics Group Department of Physics, University of York, U.K. http://www-users.york.ac.uk/~pjh503 Many slides courtesy of Jonathan
Chapter 4: Bonding in Solids and Electronic Properties. Free electron theory
 Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
X-Ray Emission Spectroscopy
 X-Ray Emission Spectroscopy Axel Knop-Gericke knop@fhi-berlin.mpg.de Core Level Spectroscopy Anders Nilsson. Journal of Electron Spectroscopy and Related Phenomena 126 (2002) 3-42 Creation of core holes
X-Ray Emission Spectroscopy Axel Knop-Gericke knop@fhi-berlin.mpg.de Core Level Spectroscopy Anders Nilsson. Journal of Electron Spectroscopy and Related Phenomena 126 (2002) 3-42 Creation of core holes
Auger Electron Spectroscopy Overview
 Auger Electron Spectroscopy Overview Also known as: AES, Auger, SAM 1 Auger Electron Spectroscopy E KLL = E K - E L - E L AES Spectra of Cu EdN(E)/dE Auger Electron E N(E) x 5 E KLL Cu MNN Cu LMM E f E
Auger Electron Spectroscopy Overview Also known as: AES, Auger, SAM 1 Auger Electron Spectroscopy E KLL = E K - E L - E L AES Spectra of Cu EdN(E)/dE Auger Electron E N(E) x 5 E KLL Cu MNN Cu LMM E f E
Quantum Condensed Matter Physics Lecture 12
 Quantum Condensed Matter Physics Lecture 12 David Ritchie QCMP Lent/Easter 2016 http://www.sp.phy.cam.ac.uk/drp2/home 12.1 QCMP Course Contents 1. Classical models for electrons in solids 2. Sommerfeld
Quantum Condensed Matter Physics Lecture 12 David Ritchie QCMP Lent/Easter 2016 http://www.sp.phy.cam.ac.uk/drp2/home 12.1 QCMP Course Contents 1. Classical models for electrons in solids 2. Sommerfeld
Probing Matter: Diffraction, Spectroscopy and Photoemission
 Probing Matter: Diffraction, Spectroscopy and Photoemission Anders Nilsson Stanford Synchrotron Radiation Laboratory Why X-rays? VUV? What can we hope to learn? 1 Photon Interaction Incident photon interacts
Probing Matter: Diffraction, Spectroscopy and Photoemission Anders Nilsson Stanford Synchrotron Radiation Laboratory Why X-rays? VUV? What can we hope to learn? 1 Photon Interaction Incident photon interacts
5.8 Auger Electron Spectroscopy (AES)
 5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
CHEM-E5225 :Electron Microscopy X-Ray Spectrometry
 CHEM-E5225 :Electron Microscopy X-Ray Spectrometry 2016.11 Yanling Ge Outline X-ray Spectrometry X-ray Spectra and Images Qualitative and Quantitative X-ray Analysis and Imaging Discussion of homework
CHEM-E5225 :Electron Microscopy X-Ray Spectrometry 2016.11 Yanling Ge Outline X-ray Spectrometry X-ray Spectra and Images Qualitative and Quantitative X-ray Analysis and Imaging Discussion of homework
Energy Spectroscopy. Ex.: Fe/MgO
 Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu
 Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Energy-Filtering. Transmission. Electron Microscopy
 Part 3 Energy-Filtering Transmission Electron Microscopy 92 Energy-Filtering TEM Principle of EFTEM expose specimen to mono-energetic electron radiation inelastic scattering in the specimen poly-energetic
Part 3 Energy-Filtering Transmission Electron Microscopy 92 Energy-Filtering TEM Principle of EFTEM expose specimen to mono-energetic electron radiation inelastic scattering in the specimen poly-energetic
Spectroscopies for Unoccupied States = Electrons
 Spectroscopies for Unoccupied States = Electrons Photoemission 1 Hole Inverse Photoemission 1 Electron Tunneling Spectroscopy 1 Electron/Hole Emission 1 Hole Absorption Will be discussed with core levels
Spectroscopies for Unoccupied States = Electrons Photoemission 1 Hole Inverse Photoemission 1 Electron Tunneling Spectroscopy 1 Electron/Hole Emission 1 Hole Absorption Will be discussed with core levels
Soft X-ray Physics DELNOR-WIGGINS PASS STATE PARK
 Soft X-ray Physics Overview of research in Prof. Tonner s group Introduction to synchrotron radiation physics Photoemission spectroscopy: band-mapping and photoelectron diffraction Magnetic spectroscopy
Soft X-ray Physics Overview of research in Prof. Tonner s group Introduction to synchrotron radiation physics Photoemission spectroscopy: band-mapping and photoelectron diffraction Magnetic spectroscopy
IMAGING DIFFRACTION SPECTROSCOPY
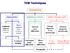 TEM Techniques TEM/STEM IMAGING DIFFRACTION SPECTROSCOPY Amplitude contrast (diffracion contrast) Phase contrast (highresolution imaging) Selected area diffraction Energy dispersive X-ray spectroscopy
TEM Techniques TEM/STEM IMAGING DIFFRACTION SPECTROSCOPY Amplitude contrast (diffracion contrast) Phase contrast (highresolution imaging) Selected area diffraction Energy dispersive X-ray spectroscopy
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN
 KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
Part 1: What is XAFS? What does it tell us? The EXAFS equation. Part 2: Basic steps in the analysis Quick overview of typical analysis
 Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Auger Electron Spectrometry. EMSE-515 F. Ernst
 Auger Electron Spectrometry EMSE-515 F. Ernst 1 Principle of AES electron or photon in, electron out radiation-less transition Auger electron electron energy properties of atom 2 Brief History of Auger
Auger Electron Spectrometry EMSE-515 F. Ernst 1 Principle of AES electron or photon in, electron out radiation-less transition Auger electron electron energy properties of atom 2 Brief History of Auger
X-ray absorption spectroscopy.
 X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ Frank de Groot PhD: solid state chemistry U Nijmegen
X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ Frank de Groot PhD: solid state chemistry U Nijmegen
Ma5: Auger- and Electron Energy Loss Spectroscopy
 Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Spectroscopy at nanometer scale
 Spectroscopy at nanometer scale 1. Physics of the spectroscopies 2. Spectroscopies for the bulk materials 3. Experimental setups for the spectroscopies 4. Physics and Chemistry of nanomaterials Various
Spectroscopy at nanometer scale 1. Physics of the spectroscopies 2. Spectroscopies for the bulk materials 3. Experimental setups for the spectroscopies 4. Physics and Chemistry of nanomaterials Various
EELS Electron Energy Loss Spectroscopy
 EELS Electron Energy Loss Spectroscopy (Thanks to Steve Pennycook, Quan Li, Charlie Lyman, Ondre Krivenak, David Muller, David Bell, Natasha Erdman, Nestor Zaluzec and many others) Nestor Zaluzec,
EELS Electron Energy Loss Spectroscopy (Thanks to Steve Pennycook, Quan Li, Charlie Lyman, Ondre Krivenak, David Muller, David Bell, Natasha Erdman, Nestor Zaluzec and many others) Nestor Zaluzec,
POSITRON AND POSITRONIUM INTERACTIONS WITH CONDENSED MATTER. Paul Coleman University of Bath
 POSITRON AND POSITRONIUM INTERACTIONS WITH CONDENSED MATTER Paul Coleman University of Bath THE FATE OF POSITRONS IN CONDENSED MATTER POSITRON-SURFACE INTERACTIONS positron backscattering BACKSCATTERED
POSITRON AND POSITRONIUM INTERACTIONS WITH CONDENSED MATTER Paul Coleman University of Bath THE FATE OF POSITRONS IN CONDENSED MATTER POSITRON-SURFACE INTERACTIONS positron backscattering BACKSCATTERED
Ultraviolet Photoelectron Spectroscopy (UPS)
 Ultraviolet Photoelectron Spectroscopy (UPS) Louis Scudiero http://www.wsu.edu/~scudiero www.wsu.edu/~scudiero; ; 5-26695 scudiero@wsu.edu Photoemission from Valence Bands Photoelectron spectroscopy is
Ultraviolet Photoelectron Spectroscopy (UPS) Louis Scudiero http://www.wsu.edu/~scudiero www.wsu.edu/~scudiero; ; 5-26695 scudiero@wsu.edu Photoemission from Valence Bands Photoelectron spectroscopy is
Introduction to EDX. Energy Dispersive X-ray Microanalysis (EDS, Energy dispersive Spectroscopy) Basics of EDX
 Introduction to EDX Energy Dispersive X-ray Microanalysis (EDS, Energy dispersive Spectroscopy) EDX Marco Cantoni 1 Basics of EDX a) Generation of X-rays b) Detection Si(Li) Detector, SDD Detector, EDS
Introduction to EDX Energy Dispersive X-ray Microanalysis (EDS, Energy dispersive Spectroscopy) EDX Marco Cantoni 1 Basics of EDX a) Generation of X-rays b) Detection Si(Li) Detector, SDD Detector, EDS
Film Characterization Tutorial G.J. Mankey, 01/23/04. Center for Materials for Information Technology an NSF Materials Science and Engineering Center
 Film Characterization Tutorial G.J. Mankey, 01/23/04 Theory vs. Experiment A theory is something nobody believes, except the person who made it. An experiment is something everybody believes, except the
Film Characterization Tutorial G.J. Mankey, 01/23/04 Theory vs. Experiment A theory is something nobody believes, except the person who made it. An experiment is something everybody believes, except the
X-rays. X-ray Radiography - absorption is a function of Z and density. X-ray crystallography. X-ray spectrometry
 X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
FUNDAMENTAL PARAMETER METHOD FOR THE LOW ENERGY REGION INCLUDING CASCADE EFFECT AND PHOTOELECTRON EXCITATION
 Copyright (c)jcpds-international Centre for Diffraction Data 2002, Advances in X-ray Analysis, Volume 45. 511 FUNDAMENTAL PARAMETER METHOD FOR THE LOW ENERGY REGION INCLUDING CASCADE EFFECT AND PHOTOELECTRON
Copyright (c)jcpds-international Centre for Diffraction Data 2002, Advances in X-ray Analysis, Volume 45. 511 FUNDAMENTAL PARAMETER METHOD FOR THE LOW ENERGY REGION INCLUDING CASCADE EFFECT AND PHOTOELECTRON
MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS. Byungha Shin Dept. of MSE, KAIST
 2015 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2015 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
Transmission Electron Microscopy. Part #2 High Resolution Imaging XEDS EELS spectroscopies Aberration corrected TEM
 Transmission Electron Microscopy Part #2 High Resolution Imaging XEDS EELS spectroscopies Aberration corrected TEM Nicolas Menguy Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie Part
Transmission Electron Microscopy Part #2 High Resolution Imaging XEDS EELS spectroscopies Aberration corrected TEM Nicolas Menguy Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie Part
X-ray Absorption Spectroscopy
 X-ray Absorption Spectroscopy Matthew Newville Center for Advanced Radiation Sources University of Chicago 12-Sept-2014 SES VI SES VI 12-Sept-2014 SES VI What Is XAFS? X-ray Absorption Fine-Structure (XAFS)
X-ray Absorption Spectroscopy Matthew Newville Center for Advanced Radiation Sources University of Chicago 12-Sept-2014 SES VI SES VI 12-Sept-2014 SES VI What Is XAFS? X-ray Absorption Fine-Structure (XAFS)
Lecture 12 Multiplet splitting
 Lecture 12 Multiplet splitting Multiplet splitting Atomic various L and S terms Both valence and core levels Rare earths Transition metals Paramagnetic free molecules Consider 3s level emission from Mn2+
Lecture 12 Multiplet splitting Multiplet splitting Atomic various L and S terms Both valence and core levels Rare earths Transition metals Paramagnetic free molecules Consider 3s level emission from Mn2+
8.6 Relaxation Processes
 CHAPTER 8. INNER SHELLS 175 Figure 8.17: Splitting of the 3s state in Fe which is missing in Zn. Refs. [12,13]. be aligned parallel or antiparallel with the spins of the 3d electrons of iron. 13 Thus we
CHAPTER 8. INNER SHELLS 175 Figure 8.17: Splitting of the 3s state in Fe which is missing in Zn. Refs. [12,13]. be aligned parallel or antiparallel with the spins of the 3d electrons of iron. 13 Thus we
B k k. Fig. 1: Energy-loss spectrum of BN, showing the how K-loss intensities I K (β, ) for boron and nitrogen are defined and measured.
 The accuracy of EELS elemental analysis The procedure of EELS elemental analysis can be divided into three parts, each of which involves some approximation, with associated systematic or statistical errors.
The accuracy of EELS elemental analysis The procedure of EELS elemental analysis can be divided into three parts, each of which involves some approximation, with associated systematic or statistical errors.
ICTP School on Synchrotron Radiation and Applications 2008 Surface Science, Photoemission and Related Techniques Fadley, Goldoni
 ICTP School on Synchrotron Radiation and Applications 2008 Surface Science, Photoemission and Related Techniques Fadley, Goldoni No. 1 Student background questions and study questions from the lectures.
ICTP School on Synchrotron Radiation and Applications 2008 Surface Science, Photoemission and Related Techniques Fadley, Goldoni No. 1 Student background questions and study questions from the lectures.
Photoelectron Peak Intensities in Solids
 Photoelectron Peak Intensities in Solids Electronic structure of solids Photoelectron emission through solid Inelastic scattering Other excitations Intrinsic and extrinsic Shake-up, shake-down and shake-off
Photoelectron Peak Intensities in Solids Electronic structure of solids Photoelectron emission through solid Inelastic scattering Other excitations Intrinsic and extrinsic Shake-up, shake-down and shake-off
Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies.
 PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
Chapter 9. Electron mean free path Microscopy principles of SEM, TEM, LEEM
 Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Lecture 3: Optical Properties of Insulators, Semiconductors, and Metals. 5 nm
 Metals Lecture 3: Optical Properties of Insulators, Semiconductors, and Metals 5 nm Course Info Next Week (Sept. 5 and 7) no classes First H/W is due Sept. 1 The Previous Lecture Origin frequency dependence
Metals Lecture 3: Optical Properties of Insulators, Semiconductors, and Metals 5 nm Course Info Next Week (Sept. 5 and 7) no classes First H/W is due Sept. 1 The Previous Lecture Origin frequency dependence
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
