Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development
|
|
|
- Spencer Franklin
- 5 years ago
- Views:
Transcription
1 Development 129, (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development Kelly Beumer, Heinrich J. G. Matthies, Amber Bradshaw and Kendal Broadie* Department of Biology, University of Utah, Salt Lake City, UT , USA *Author for correspondence ( broadie@biology.utah.edu) Accepted 11 April 2002 SUMMARY Calcium/calmodulin dependent kinase II (CaMKII), PDZ-domain scaffolding protein Discs-large (DLG), immunoglobin superfamily cell adhesion molecule Fasciclin 2 (FAS2) and the position specific (PS) integrin receptors, including βps and its alpha partners (αps1, αps2, αps3/αvolado), are all known to regulate the postembryonic development of synaptic terminal arborization at the Drosophila neuromuscular junction (NMJ). Recent work has shown that DLG and FAS2 function together to modulate activity-dependent synaptic development and that this role is regulated by activation of CaMKII. We show that PS integrins function upstream of CaMKII in the development of synaptic architecture at the NMJ. βps integrin physically associates with the synaptic complex anchored by the DLG scaffolding protein, which contains CaMKII and FAS2. We demonstrate an alteration of the FAS2 molecular cascade in integrin regulatory mutants, as a result of CaMKII/integrin interactions. Regulatory βps integrin mutations increase the expression and synaptic localization of FAS2. Synaptic structural defects in βps integrin mutants are rescued by transgenic overexpression of CaMKII (proximal in pathway) or genetic reduction of FAS2 (distal in pathway). These studies demonstrate that βps integrins act through CaMKII activation to control the localization of synaptic proteins involved in the development of NMJ synaptic morphology. Key words: Drosophila, CaMKII, FAS2, Neuromuscular junction, Synaptic proteins INTRODUCTION Molecular mechanisms mediating the initial establishment and elaboration of neuronal synapses during embryogenesis are likely to be retained for postembryonic, experience-dependent development of synaptic architecture. Indeed, several molecules that mediate embryonic pathfinding, target recognition and synaptogenesis are known to play roles in later synaptic modulation events (reviewed by Zhang et al., 2001). One class of cell adhesion molecules (CAM) important in both synaptic development and later modulation is the immunoglobin (Ig) superfamily, including NCAM and L1 in vertebrates (Dahme et al., 1997; Luthl et al., 1994), apcam in Aplysia (Bailey and Chen, 1989; Mayford et al., 1992) and Fasciclin 2 (FAS2) in Drosophila/grasshopper. FAS2 is involved in axon sorting, fasciculation/ defasciculation and target selection in the embryo (Bastiani et al., 1987; Davis et al., 1997; Grenningloh et al., 1991; Lin et al., 1994), and is later necessary for synaptic stabilization and growth modulation in the postembryonic neuromuscular junction (NMJ) (Schuster et al., 1996a; Schuster et al., 1996b; Stewart et al., 1996; Thomas et al., 1997). Synaptic localization of FAS2 is regulated by Discs Large (DLG) through a C-terminal PDZ consensus binding site (Thomas et al., 1997; Zito et al., 1997). Drosophila DLG, like its mammalian homologue PSD95, is a PDZ-domain scaffolding protein with multiple binding sites that are capable of mediating the formation of large molecular complexes. PSD95 binds NMDA receptors, K + channels and semaphorins at central glutamatergic synapses (Chung et al., 2000; Inagaki et al., 2001; Kim et al., 1996; Kim et al., 1995; Kornau et al., 1995; Niethammer et al., 1996; Xia et al., 2000). Similarly, DLG binds both FAS2 and Shaker K + channels, and is responsible for their synaptic localization at the Drosophila glutamatergic NMJ (Tejedor et al., 1997; Thomas et al., 1997; Zito et al., 1997). DLG-dependent localization of FAS2 to the Drosophila NMJ is negatively regulated by Ca 2+ /calmodulin dependent kinase II (CaMKII). Phosphorylated DLG disassociates from the synaptic protein complex, leading to the synaptic loss of FAS2 (Koh et al., 1999b). NMJs in animals expressing a CaMKII inhibiting peptide (ala) display impaired synaptic modulation and altered synaptic morphology (Griffith et al., 1994; Wang et al., 1994). Thus, the current model suggests that synaptic activity leads to CaMKII activation via Ca 2+ -dependent auto-phosphorylation. CaMKII, in turn,
2 3382 K. Beumer and others phosphorylates DLG to release FAS2 from the synaptic complex, allowing developmental growth in response to increased synaptic activity (Koh et al., 1999b). An independent line of investigation has shown that integrins also regulate activity-dependent development of Drosophila NMJ architecture (Beumer et al., 1999; Rohrbough et al., 2000). Integrins are heterodimeric transmembrane receptors for the extracellular matrix with both adhesion and bi-directional transmembrane signaling functions. During embryogenesis, integrins play roles in neuronal pathfinding in vertebrates, C. elegans and Drosophila (Baum and Garriga, 1997; Hoang and Chiba, 1998; Ivins et al., 2000). Moreover, at least ten different integrin receptors are found in postembryonic vertebrate synapses, and at least three in larval Drosophila NMJs (Beumer et al., 1999; Burkin et al., 1998; Cohen et al., 2000; Fernandes et al., 1996; Martin et al., 1996; Pinkstaff et al., 1999; Rodriguez et al., 2000; Rohrbough et al., 2000), suggesting a persistent synaptic function. Drosophila integrin mutants display disrupted short-term memory and a loss of functional synaptic plasticity (Grotewiel et al., 1998; Rohrbough et al., 2000). Mutations of the Drosophila β integrin (βps) subunit, or one α integrin partner (αvolado), cause multiple alterations in synaptic architecture, indicating a role for integrins in synaptic morphological development (Beumer et al., 1999; Rohrbough et al., 2000). Do integrins affect synaptic morphological development via CaMKII or via an independent, parallel pathway? We propose the hypothesis that integrins may act through CaMKII at the Drosophila NMJ to modulate synaptic structure. Integrins regulate CaMKII activation in cultured cells (Bilato et al., 1997; Blystone et al., 1999), and this regulation has been proposed to mediate communication between heterologous integrin receptors expressed together in single tissues, a condition seen at the Drosophila NMJ (Beumer et al., 1999; Rohrbough et al., 2000). We show here that transgenic overexpression of CaMKII completely rescues synaptic structural defects in integrin mutants, indicating that integrins act through alteration of CaMKII expression or activity. We also show that synaptic FAS2, whose regulation is downstream of CaMKII activity, is misregulated in integrin mutants and that genetic compensation for this misregulation rescues synaptic structural defects in integrin mutants. We therefore propose that synaptic integrin receptors act upstream of CaMKII to regulate NMJ morphological development, largely via regulation of FAS2 expression, and that this integrin function is required for synaptic structural alterations. MATERIALS AND METHODS Fly stocks and genetic manipulations Drosophila melanogaster stocks were reared in well-yeasted, uncrowded vials on standard medium at 25 C (Ashburner, 1989) unless otherwise noted. Oregon-R or Canton S flies were used as wildtype control animals. An extensive allelic series of myospheroid (mys; encodes βps integrin) mutant stocks were used: mys xg43 (Bunch et al., 1992), an embryonic lethal genetic null mutant; cm ct 6 mys ts1 (Bunch et al., 1992), a regulatory mutant with greatly reduced pre- and postsynaptic βps expression at the NMJ (Beumer et al., 1999); sn mys b9 v (D. Brower) (FlyBase, 1998), a hypomorphic mutant with moderately reduced βps expression at the NMJ due primarily to postsynaptic mislocalization (Beumer et al., 1999); and a series of mys olfc alleles, olfc X5, olfc X10, olfc X14 and olfc X17 (C. Ayyub) (Ayyub et al., 2000), which have been less thoroughly characterized. For some experiments, sn mys b9 v and cm ct 6 mys ts1 were backcrossed a minimum of eight times to a Canton S (CS) stock, removing all marker mutations and producing a uniform genetic background that is conservatively 96% CS. To generate double mutants, yw was crossed onto cm ct 6 mys ts1,and sn mys b9 v. CaMKII protein levels were manipulated by crossing P[w +, UASCAMKII + ] R3, located on chromosome 3 (V. Budnik) (Koh et al., 1999b) into mys mutant backgrounds. To drive the inducible UAS constructs in muscle and nerve, we used previously described GAL4 constructs (FlyBase, 1998). One GAL4, elavgal4 (Lin and Goodman, 1994) is located on the tip of the X chromosome, and was crossed onto each of the mys mutant chromosomes and the stock tested for proper genotype by testing for viability and wing blister phenotypes over mys xg43. To inhibit CaMKII, we used the heat-shock inducible construct P[hsp70-ala]2, which expresses the CaMKII inhibitor peptide (ala) at a low level without heat shock induction (Griffith et al., 1993; Wang et al., 1994). For descriptions of the marker mutations used in this study see Lindsley and Zimm (Lindsley and Zimm, 1992). To genetically reduce FAS2 levels in integrin mutant backgrounds, we recombined mys chromosomes with the P-element alleles fas2 e76, which reduces wild-type FAS2 to ~10%, and fas2 e86, which reduces FAS2 protein ~50% (C. Goodman) (Grenningloh et al., 1991). Recombinants were tested for proper expression of the FAS2 protein by western blot analysis of flies putatively homozygous for the appropriate fas2 mutant allele but heterozygous for the integrin allele (i.e., fas2 e76 sn mys b9 v / fas2 e76 ). The continued presence of the mys allele was reconfirmed in each new stock by testing for viability and wing blister phenotypes over mys xg43. Immunocytochemistry and imaging Wandering third instar larvae were dissected and immunologically stained as previously reported (Beumer et al., 1999; Broadie and Bate, 1993). To examine NMJ morphology, preparations were probed with a mouse monoclonal anti-cysteine string protein (CSP) antibody (1:200) (Zinsmaier et al., 1990). Staining was visualized using a Vectastain ABC Elite kit with NiCl 2 and CoCl 2 enhancement (Broadie and Bate, 1993). Images were captured digitally. For clarity, different focal planes were occasionally combined in one picture using Adobe PhotoShop. In confocal preparations, either rabbit anti-dlg (1:1000) (Woods and Bryant, 1991), rabbit anti-synaptotagmin (1:500) (Littleton et al., 1993), mouse anti-csp (1:500) (Zinsmaier et al., 1990), rabbit anti-hrp (1:500; Cappel/Oreon Teknica Corp.) or Texas-Red-conjugated goat anti-hrp (1:500; Jackson Laboratories) was used to mark synaptic arbors. Integrin expression was examined with the following antibodies: mouse monoclonal anti-βps integrin antibody CF6G11 (1:500) or CF6G11 ascites (1:300) (Brower et al., 1984); rabbit polyclonal 185E (1:1000; R. Hynes) (Zusman et al., 1993), rat polyclonal anti-αps2, PS2hc2 (1:5) (Bogaert et al., 1987) and rabbit rabps21 (1:1000) (Bloor and Brown, 1998). All anti-integrin antibodies were independently tested for their well-characterized embryonic staining patterns prior to probing the larval preparations (Bloor and Brown, 1998; Brower et al., 1984). FAS2 was visualized using mouse monoclonal anti-fas2 1D4G9 (1:10; C. Goodman) (Zito et al., 1999). CaMKII was visualized with rabbit anti-camkii (1:4,000; V. Budnik) (Koh et al., 1999b). Fluorescent secondaries used were Alexa-red 594- and Alexa-green 488-conjugated goat anti-mouse and goat anti-rabbit (1:500; Molecular Probes). Confocal microscopy was performed on either a BioRad MRC-600 or a Zeiss LSM 510. ImageQuant software from Molecular Dynamics was used to quantify staining intensity. Statistical significance of all measurements was determined with the ANOVA test using InStat software.
3 Integrin-mediated synaptic structural development 3383 Western blot analysis To prepare proteins for western blot quantification, 10 third instar larvae were homogenized in 100 µl NP40 buffer (150 mm NaCl, 1% IGEPAL CA630, 50 mm Tris (ph 8), 1 Complete Protease inhibitor (Boehringer Mannheim), incubated on ice for 45 minutes and centrifuged at high speed for 20 minutes at 4 C. The supernatant was removed to a fresh tube, and the pellet was resuspended in an additional 100 µl NP40 and spun again for 20 minutes. The supernatants were combined and 25 µl of non-reducing sample buffer was added to 100 µl of the supernatant. The sample was incubated at 85 C for 10 minutes and separated on a 10% SDS-PAGE gel, then transferred onto PVDF membrane. Immunoblotting was performed with anti-fas2 monoclonal 34B3C2 (1:100; C. Goodman) (G. Helt and C. Goodman, unpublished) and visualized with chemiluminescence reagents (Amersham). Anti-actin was used as a loading control (developed by J. J.-C. Lin and obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Department of Biological Sciences, Iowa City, IA under contract NO1-HD from the NICHD). Bands were quantified using NIH Image. Immunoprecipitation Immunoprecipitation assays were performed essentially as described by Thomas et al. (Thomas et al., 1997); however, antibodies were coupled as described by Zhang et al. (Zhang et al., 2001) to recombinant Protein A sepharose beads. Briefly, beads were washed in phosphate-buffered saline (PBS) 0.2% NP40, 5% bovine serum albumin (BSA) and incubated for 2 hours with rabbit anti-dlg (Woods and Bryant, 1991). Beads were washed, coupled with disuccinimidyl suberate, washed again and finally blocked with PBS 0.2% NP40 and 5% BSA. These beads were gently rocked with Drosophila head extract prepared in the following manner. OR flies were frozen in liquid N 2, agitated and heads isolated by sieving. Heads were homogenized in 50 mm Tris (ph 7.2) 150 mm NaCl 2 mm EGTA 0.5% Triton X-100 and 2 complete protease inhibitors (Roche). The supernatants were pre-cleared for 45 minutes with protein A Sepharose at 4 C. The cleared supernatant was incubated with Protein A beads coupled to either anti-kinesin (SUK4), anti-dlg (Woods and Bryant, 1991) or naïve beads for 1 hour at 4 C. The beads were washed six times in 800 µl PBS-NP40 buffer once with 5 ml PBS and resuspended in 2 SDS-sample buffer. The proteins were separated on a 4-20% SDS-PAGE gel and transferred to PVDF membranes. Membranes were blocked (5% powdered milk), and probed with either rabbit anti-dlg (1:1000; D. Woods) (Woods and Bryant, 1991) or rabbit anti-βps (1:1500; R. Hynes) (Zusman et al., 1993). Bands were visualized with alkaline phosphate using NBT/BCIP as a substrate. RESULTS Differential NMJ structural growth defects caused by postsynaptic versus pre/postsynaptic βps misregulation A single β integrin receptor family is present on both sides of the Drosophila NMJ, comprising the βps subunit encoded by myospheroid (mys) and its α-integrin partners αps1 (multiple edematous wings; mew), αps2 (inflated; if) and αps3 (Volado; Vol) (Beumer et al., 1999; Rohrbough et al., 2000). Mutations in mys alter the synaptic level of the heterodimeric integrin receptors in parallel (Beumer et al., 1999), allowing the assay of total PS integrin synaptic function. Null mys alleles are embryonic lethal because of catastrophic failure of development (i.e. dorsal closure failure, gut defects and muscle detachment), precluding analysis of mutant phenotypes at the postembryonic NMJ. Therefore, we used two wellcharacterized regulatory hypomorphic mys mutant alleles in these analyses; mys b9, which causes loss of ~50% of synaptic integrin, and mys ts1, which causes a more severe ~80% integrin loss (Beumer et al., 1999). Phenotypically, mys b9 NMJ synapses display profound undergrowth with a reduced number of small, tightly spaced type I boutons, and an increased number of satellite boutons (Fig. 1A, Fig. 3A), which probably represent developmentally arrested type I boutons (Beumer et al., 1999; Zito et al., 1999). The mys ts1 synapses display the opposite phenotype of structural overgrowth, with an increased number of boutons in a highly branched, over-elaborated Fig. 1. Synaptic bouton quantification in mys olfc alleles. (A) Representative muscle 12 NMJ morphology in mature third instars of wild-type, mys b9, mys ts1 and mys olfcx17 alleles. Black arrowheads indicate type I boutons. White arrowheads indicate type II boutons. Scale bar indicates 20 µm. (B) Quantification of boutons for each allele and for mys olfcx17 /mys XG43, a null allele. Note that mys ts1 is among the least severe of the mys overgrowth alleles, whereas the mys b9 undergrowth allele is clearly very different. Error bars indicate s.e.m. (*P<0.05; **, P<0.01; ***, P<0.001).
4 3384 K. Beumer and others synaptic arbor (Fig. 1A, Fig. 3A) (Beumer et al., 1999). Thus, two integrin mutant alleles, which differentially alter integrin expression at the NMJ, cause opposite morphological growth phenotypes. Our results suggest that the mys b9 mutation causes specific mislocalization of postsynaptic (muscle) integrin, whereas the mys ts1 mutation results in globally decreased synaptic expression in both the pre- and postsynaptic membranes (Beumer et al., 1999). In support of this conclusion, many mys b9 phenotypes, including NMJ undergrowth, reduced bouton number and reduced physiological function, improve over a mys deficiency, while mys ts1 morphological and functional phenotypes are either unchanged or more severe over the same deficiency (Beumer et al., 1999). For further support, we analyzed the olfc locus, which has recently been shown to be allelic to myospheroid (Rodrigues and Siddiqi, 1978; Ayyub et al., 2000). Mutants in olfc are recessive, with nervous system deficiencies in responding to a variety of odorants (Ayyub et al., 2000). Two alleles, olfc x3 and olfc x14, have been assayed for βps protein expression and, like mys ts1 and mys b9, express normal protein when assayed by western blot, and are therefore proposed to be regulatory mutants. We assayed olfc mutants for changes in NMJ morphology. Of five mutant combinations assayed, four (olfc x17, olfc x17xg43, olfc x10 and olfc x5 ) showed NMJ overgrowth phenotypes that were more severe than mys ts1 (Fig. 1), and the fifth (olfc x14 ) showed less overgrowth than mys ts1. No allelic combinations showed undergrowth. These results suggest that synaptic overgrowth represents a simpler genetic lesion that represents loss of function. Thus, the mys b9 phenotype is probably attributable to a more complex gain-of-function alteration of the normal βps integrin expression pattern, which reduces postsynaptic integrin while increasing extrasynaptic integrin (Beumer et al., 1999). To test this idea further, we used tissue-specific GAL4 drivers (Brand and Dormand, 1995) to overexpress βps integrin specifically either in muscle [myosin heavy chain (MHC)-GAL4] or neurons [embryonic lethal, abnormal vision (elav)-gal4], and assayed NMJ structure in mys mutants. The undergrowth phenotype of the mys b9 NMJ can be completely rescued by postsynaptic muscle expression, which increases the amount of integrin localized to the synapse, of either βps alone or with its alpha partners (Table 1). This observation provides good evidence that the mys b9 synaptic undergrowth is Genotype Table 1. Rescue by Gal4 expression Wild type 57.9 mys b *** mys b9 ;; UASβPS UASPS *** mys b9 ; mhcgal4; UASβPS; UASPS mys ts * mys ts1 ; UASβPS; UASPS mys ts1 ; mhcgal4; UASβPS; UASPS n, average bouton number, muscle 12. A minimum of 20 A2 NMJ synapses per genotype were assayed. Data are for only one set of rescue combinations. Data for rescue with mhcgal4; UASβPS; UASPS1 or mhcgal4; UASβPS are essentially identical. Asterisks indicate significance relative to wild type. Circles indicate significance relative to each mys mutant without manipulated integrin levels. Asterisks indicate significance (*P<0.05; *** or, P<0.0001). n due to postsynaptic misregulation of βps. By contrast, expression of βps integrin in muscle alone provided no rescue of the mys ts1 phenotype (Table 1). We also attempted to rescue the mys ts1 phenotype by overexpressing βps integrin in the presynaptic neuron, but were unable to do so because overexpression of either βps alone or with its α partners in the nervous system caused extensive NMJ overgrowth, even in wild-type animals (data not shown). The observation that both presynaptic overexpression (elavgal4; UAS-βPS) and underexpression (mys ts1 ) causes structural overgrowth suggests that βps integrins are involved in a regulatory mechanism sensitive to both over- and underactivation and pre- and postsynaptic balance. Thus, we suggest that mys ts1 most probably represents the integrin loss-of-function phenotype, whereas the mys b9 synaptic undergrowth defect is due to alteration in the βps integrin expression pattern in postsynaptic muscle. Integrins and DLG are associated in a neuromuscular junction complex βps integrin is a transmembrane receptor present in both preand postsynaptic membranes at the larval NMJ (Beumer et al., 1999). DLG is a synaptic scaffolding protein associated with both pre-and postsynaptic membranes and is also involved in the regulation of synaptic morphology, through the localization of diverse transmembrane proteins (including FAS2) (Guan et al., 1996; Lahey et al., 1994). We wished to determine whether βps integrin associates with the DLG complex to tie together these disparate receptor components in a common molecular machine. In confocal analyses, βps integrin and DLG co-localize at the larval NMJ (Fig. 2A). DLG clearly has less extensive expression, more tightly localized at NMJ boutons, whereas βps is more extensive through the subsynaptic reticulum (SSR) and also localized at extrasynaptic sites in the muscle, including the muscle sarcomere (Volk et al., 1990) and attachment sites (Leptin et al., 1989). However, both proteins are most intensively expressed at the NMJ pre-/postsynaptic interface, where they co-localize (Fig. 2A). Therefore, we tested to determine if βps integrins form part of the synaptic complex linked by DLG. We performed protein immunoprecipitation assays using rabbit anti-dlg to probe Oregon R head extracts. DLG antibodies clearly coimmunoprecipitate DLG and βps integrin protein (Fig. 2C), consistent with co-localization observed in confocal analysis. Inspection of the ratio of both bound proteins and proteins not bound to beads (immunoprecipitation versus flow through lanes, Fig. 2C) indicates that a large portion of the βps integrin protein associates with a complex containing DLG. Control experiments with either naïve protein beads or beads coupled to anti-kinesin antibodies failed to immunoprecipitate sufficient levels of βps integrin protein to be detected (Fig. 2B), demonstrating the specificity of the coimmunoprecipitation. The fact that βps was found to coimmunoprecipitate with the complex mediated by DLG provides support for integrins existing in a synaptic complex with FAS2 and CaMKII at the synapse. Genetically increasing CaMKII levels rescues mys phenotypes at the NMJ Animals with directly reduced CaMKII activity show altered
5 Integrin-mediated synaptic structural development 3385 synaptic structure at the larval NMJ (Griffith et al., 1993; Wang et al., 1994), comparable with mys ts1 mutant phenotypes. Our hypothesis is that integrins may mediate their regulation of NMJ architecture through the modulation of CaMKII, both pre- and postsynaptically. If integrins modulate CaMKII in regulating synaptic structure, then transgenically manipulating CaMKII levels in integrin mutants should ameliorate mys synaptic structural defects. We first inhibited synaptic CaMKII activity in mys mutants with constructs that constitutively expressed the inhibitory peptide ala (Griffith et al., 1993; Wang et al., 1994). In wild-type animals, expression of the ala peptide results in a ~40% increase in NMJ bouton number (Wang et al., 1994) (data not shown). Expression of ala in integrin mutants resulted in similar, though smaller, increases in bouton number, with 20% and 16% increases in mys b9 and mys ts1 mutants animals, respectively (data not shown). Thus, integrin mutant phenotypes cannot be rescued by inhibition of CaMKII activity. Indeed, inhibition of CaMKII has less effect in altering NMJ structure in mys mutants than in controls. We next overexpressed CaMKII + through introduction of an inducible UAS-CaMKII + construct (Koh et al., 1999b). We again used a muscle driver (MHC-GAL4) and neuronal driver (elav-gal4), and also expressed CaMKII ubiquitously (GAL4-e22c) (Koh et al., 1999a). Overexpression of CaMKII ubiquitously, or either pre- or postsynaptically, in wild-type animals produced no significant alteration in synaptic architecture (Fig. 3). Similarly, presynaptic CaMKII over-expression in mys b9 mutants did not detectably alter the NMJ undergrowth phenotype. By contrast, transgenic expression of CaMKII in postsynaptic muscle completely rescued the mys b9 undergrowth phenotype (Fig. 3A). The mys b9 NMJ is spatially contracted and contains 35% fewer type 1 boutons than normal, but CaMKII expression in muscle rescued both the abnormal growth and the reduced number of type I boutons to normal levels (Fig. 3B). This finding reinforces our conclusion that the mys b9 defect of NMJ undergrowth is due entirely to a postsynaptic impairment. In the mys ts1 mutant, expressing CaMKII in the presynaptic terminal caused no detectable alteration in the overgrowth phenotype (Fig. 3A). Expressing CaMKII in postsynaptic muscle caused a slight, but insignificant, decrease in the overgrowth phenotype, to partially rescue the mys ts1 mutant overproduction of type I boutons (Fig. 3A,B). By contrast, transgenically expressing CaMKII ubiquitously completely rescued the mys ts1 overgrowth phenotype (Fig. 3A). The mys ts1 NMJ is overly expansive and contains 25% more type 1 boutons than normal, but combined pre/postsynaptic CaMKII expression rescued the abnormal growth and reduced the number of type I boutons to normal (Fig. 3B). Ubiquitous CaMKII overexpression in wild-type animals had no effect on Fig. 2. βps integrin interacts with DLG. (A) Co-localization of DLG (red) and βps integrin (green) in the third instar NMJ. βps staining overlaps, but is not restricted to, the domain of DLG staining. (B) Immunoprecipitations of fly head extracts using protein A beads or beads coupled to either anti-kinesin or DLG were examined by western analysis. Note detectable βps is found only in the IP with DLG beads validating the DLG IP assay. (C) Anti-DLG immunoprecipitation (IP) experiments from adult heads were performed and western blots of total lysate (LYS), material on beads (IP-resuspended to same volume as lysate) and unbound proteins or flow through (FT) were probed with either anti-dlg or anti-βps antibodies. These results indicate that quantitative immunodepletion of DLG leads to a substantial reduction of βps from the lysate. synapse morphology, but also rescued mys b9, as expected (Koh et al., 1999a) (Fig. 3B). We conclude that mys b9 synaptic undergrowth can be rescued by genetically increasing CaMKII only postsynaptically, whereas CaMKII must be increased on both sides of the synapse to completely rescue mys ts1 synaptic overgrowth. These results are consistent with integrins regulating synaptic growth through a CaMKII-dependent pathway, and with mys ts1 having defects in both pre- and postsynaptic CaMKII regulation, whereas mys b9 is specifically defective in postsynaptic CaMKII regulation. Fasciclin 2 expression is increased in myospheroid integrin mutants Although CaMKII is known to have multiple synaptic targets, one of the most clearly understood is DLG. The ultimate result of CaMKII phosphorylation of DLG is to limit the localization of the homophilic adhesion receptor FAS2, which dictates the directionality and degree of synaptic elaboration. As we have demonstrated that βps integrin lies upstream of CaMKII, we
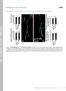
6 3386 K. Beumer and others Fig. 3. Rescue of mys mutant phenotypes by genetic manipulation of CaMKII expression. (A) Phenotypic rescue of structural defects at the third instar NMJ on muscle 4. Black arrowheads indicate type Ib boutons; open arrowheads indicate type 1s boutons. Scale bar: 20 µm. (B) Quantification of synaptic bouton number in mys and CaMKII overexpression mutant animals. In all cases, type I boutons on a minimum of 20 muscle 12 NMJs in hemisegment A2 were quantified. Error bars indicate s.e.m. Asterisks indicate significance relative to wild type. Circles indicate significance relative to each mys mutant without manipulated CaMKII levels. (* or P<0.05; *** or, P<0.001). hypothesized an alteration in FAS2 localization. We therefore assayed FAS2 expression in mys b9 and mys ts1 alleles in two ways: (1) quantitative confocal immunocytochemistry to assay FAS2 localization at the NMJ (Fig. 4A,B), and (2) western blot analysis to assay FAS2 levels in whole animal protein extracts (Fig. 4C,D). FAS2 levels at the larval NMJ were clearly elevated in both mys b9 and mys ts1 mutants (Fig. 4A). To quantify this elevated expression, we measured the average anti-fas2 fluorescent intensity (green) relative to the internal control of anti-hrp (red), a neuronal membrane marker. In both mys alleles, FAS2 synaptic localization was very significantly increased relative to the internal control (Fig. 4B). The increase was most marked for mys ts1 mutants, with a 64% (P<0.0001) increase in FAS2 synaptic expression. In mys b9 mutants, FAS2 expression was also greatly increased (48%; P<0.0001). The two mys alleles were also significantly different from each other (P<0.002). In the reverse experiment, βps integrin expression and localization levels were unaltered in fas2 mutant animals (data not shown). When we measured FAS2 levels in the ubiquitous CaMKII transgenic line, which rescues both mys b9 and mys ts1 structural defects (Fig. 3), we found that FAS2 expression was reduced in both mys b9 and mys ts1 to levels comparable with controls (Fig. 4C). Indeed, overexpression of CaMKII in wild-type, mys b9 and mys ts1 reduced FAS2 levels in all three genotypes, to a uniform level that represented ~80% of wild-type levels (Fig. 4C) (Koh et al., 1999b). We next assayed total FAS2 protein levels using quantified western blot analysis of staged third instar larva (Fig. 4D,E). As a control and verification test for subsequent experiments (see below), we quantified FAS2 levels in two fas2 mutants, fas2 e76 and fas2 e86. We observed a ~90% and ~50% reduction of FAS2, respectively, in these alleles which is consistent with published levels of expression (Grenningloh et al., 1991). In both mys b9 and mys ts1, quantification of the FAS2 band relative to an actin internal control band supported the increased FAS2 expression observed at the synapse in confocal immunocytochemistry experiments (Fig. 4E). We measured a 35% increase in FAS2 in mys ts1 animals and a 17% increase in mys b9 animals. Note that the relative change of FAS2 levels in mys b9 and mys ts1 mutants is the same using both techniques. Thus, at least part of the increased synaptic FAS2 levels could be explained by more abundant FAS2 protein in the mys mutants. Taken together, these data are consistent with the hypothesis that the βps integrin receptor, together with its α partners, functions via CaMKII to regulate both FAS2 expression and its localization at the NMJ synapse. By contrast, FAS2 has no detectable effect on the expression or localization of βps integrin. Genetically reducing FAS2 expression in mys mutants rescues synaptic structural defects As the synaptic structural defects of mys b9 and mys ts1 are accompanied by a striking increase in FAS2 expression at the synapse, we next determined if genetically reducing FAS2 expression would rescue these developmental phenotypes. We recombined two fas2 regulatory alleles, fas2 e76 (~90% loss of FAS2) and fas2 e86 (~50% loss of FAS2) (Fig. 4E) (Grenningloh et al., 1991) onto chromosomes carrying the βps integrin mutations, generating four stocks: fas2 e76 mys b9, fas2 e76 mys ts1,
7 Integrin-mediated synaptic structural development 3387 Fig. 4. FAS2 is increased in mys mutant NMJ synapses. (A) Anti-FAS2 staining (green) and anti-dlg staining (red) at the third instar NMJ. FAS2 staining is significantly increased in both mys alleles. Arrowhead marks a type Ib bouton; arrow indicates a satellite bouton. Scale bar: 12 µm. High magnification views of FAS2 in NMJ boutons. Scale bar: 3 µm. (B) Quantification of the average intensity of FAS2 staining normalized to HRP (neuronal marker). Each bar represents the average of boutons for each genotype. Error bars indicate s.e.m. Asterisks indicate significance. (C) Quantification of FAS2 in animals overexpressing wild type CaMKII. (***, P<0.001) (D) Sample western blots of FAS2 in the same mys mutants and the FAS2 mutants, fas e76 and fas e86. (E) Graph quantifying band intensities for western blot experiments. Staining intensities are normalized to wild type. fas2 e86 mys b9 and fas2 e86 mys ts1. Each genotype was then tested for alterations in the synaptic architecture phenotype at the third instar NMJ. Animals doubly mutant for fas2 e76 and either mys allele reduced FAS2 expression to very low levels, below 20% of wild type (Fig. 4E), and showed reduced NMJ growth phenotypes, consistent with those published for fas2 e76 mutants alone (data not shown) (Schuster et al., 1996a; Schuster et al., 1996b; Stewart et al., 1996). By contrast, fas2 e86 in conjunction with either mys b9 or mys ts1, produced FAS2 protein at near normal levels, as measured by western blot analysis (Fig. 4E). Most strikingly, NMJ synaptic architecture, including bouton size, neurite branch length and number, and synaptic area, is altered towards normal by genetically reducing FAS2 levels in both mys b9 and mys ts1 animals (Fig. 5A). In mys ts1 mutants, the reduced FAS2 level resulted in a highly significant (P=0.0002) decrease in bouton number to near normal numbers (Fig. 5B), resulting in no significant difference between wild-type and mys ts1 fas2 e86 bouton numbers. This result is particularly striking because either mys ts1 or fas2 e86 (Stewart et al., 1996) alone causes excessive NMJ overgrowth. By contrast, in mys b9 mutants, the reduced FAS2 level resulted in an increase in bouton number towards normal, although the rescue was not complete (Fig. 5B). Taken together, these data are consistent with regulation of FAS2 being a primary mechanism by which postsynaptic integrins regulate synaptic growth at the NMJ. However, the overgrowth of mys ts1 in the presence of high expression of FAS2, and the incomplete rescue of mys b9 by restoring FAS2 levels, indicates that other mechanisms must be involved. DISCUSSION Integrins regulate CaMKII activity and FAS2 expression during postembryonic synaptic development of the NMJ Several studies in the laboratories of Budnik, Goodman, Griffith and Wu have shown that activity-dependent development at the Drosophila NMJ is mediated by the Ca 2+ - dependent kinase CaMKII phosphorylating the scaffolding
8 3388 K. Beumer and others Fig. 5. Rescue of mys synaptic structure defects by genetic manipulation of FAS2 expression. (A) Phenotypic rescue at the third instar muscle 4 NMJ. mys b9 causes undergrowth, whereas mys ts1 causes overgrowth. Other phenotypic characteristics include increased satellite boutons and irregular bouton size. Black arrowheads indicate type Ib boutons. Open arrowheads indicate type 1s boutons. Scale bar: 20µm. (B) Quantification of NMJ synaptic growth in mys and fas mutant animals. In all genotypes, type I boutons on a minimum of 20 muscle- 12 NMJs in hemisegment A2 were quantified. Error bars indicate s.e.m. Asterisks indicate significance relative to wild type. Circles indicate significance relative to each genotype without manipulated FAS2 levels. (* or, P<0.05; ** or, P<0.01; *** or, P<0.001). protein DLG, which, in turn, tethers the homophilic adhesion molecule FAS2 to control its synaptic localization (Budnik et al., 1996; Koh et al., 1999b; Tejedor et al., 1997; Thomas et al., 1997; Zito et al., 1997). Independently, integrins have been implicated in activity-dependent synaptic development following the isolation of integrin mutants that affect shortterm memory in Drosophila and in our subsequent demonstration that synaptic integrins modulate NMJ architecture in a manner reminiscent of alterations in the CaMKII-DLG-FAS2 pathway (Beumer et al., 1999; Grotewiel et al., 1998; Rohrbough et al., 2000). The purpose of this study was to determine whether integrins function to regulate FAS2 synaptic expression by the CaMKII pathway, or rather affect morphology via an independent, parallel mechanism. If the former were true, our aims were to determine where in the CaMKII-DLG-FAS2 pathway the integrins function and to assess whether disruption of CaMKII-mediated regulation of FAS2 is sufficient to account for the structural defects observed in integrin mutant synapses. To assay the mys requirement in synaptic development comprehensively, we compared and contrasted two regulatory alleles with opposing structural phenotypes: mys b9, which causes NMJ undergrowth, and mys ts1, which causes NMJ overgrowth (Beumer et al., 1999). In both mutants, we have observed a correlation between the morphological alterations and synaptic function (Beumer et al., 1999), in contrast to the homeostasis seen in fas2 mutant synapses (Davis et al., 1997; Stewart et al., 1996) but consistent with the parallel alterations seen in CaMKII-inhibited synapses (Wang et al., 1994). However, the correlation between synaptic structural and functional alterations in mys mutants is not striking, and it is clear that integrins primarily mediate architectural regulation. Therefore, our focus in this study was exclusively on the mechanism(s) by which integrins modulate NMJ structural development. This article presents molecular and genetic evidence that strongly support the hypothesis that synaptic integrin receptors containing βps modulate CaMKII activation on both sides of the synaptic cleft and, through CaMKII, control both the expression and the synaptic localization of FAS2 at the synapse. The primary experimental results supporting these conclusions are detailed below. First, transgenic expression of CaMKII is sufficient to completely rescue all synaptic structure defects in mys mutants. Genetically increasing CaMKII in postsynaptic muscle, but not presynaptic neuron, completely rescues the structural undergrowth of the mys b9 integrin mutant, whereas it is necessary to elevate CaMKII both pre- and postsynaptically to rescue structural overgrowth in the mys ts1 mutant. These results are consistent with the postsynaptic mislocalization of βps integrin in the mys b9 mutant, as opposed to the global loss of synaptic integrin in the mys ts1 mutant (Beumer et al., 1999). These data indicate that coordinate regulation of CaMKII in the muscle and motoneuron is necessary for proper development of synaptic architecture. Second, at the distal end of the cascade, both the expression and synaptic localization of FAS2 are increased in mys mutants, although the extent of FAS2 misregulation was significantly different between the two regulatory mutants. Importantly, both the NMJ overgrowth (mys ts1 ) and undergrowth (mys b9 ) phenotypes are rescued towards wild-type structure by genetically reducing the amount of FAS2 available at the synapse to near normal levels. In mys ts1 mutants, correcting for FAS2 level suppresses the synaptic overgrowth, while in mys b9 mutants, correcting the FAS2 level suppresses the synaptic undergrowth. These results support the conclusion that FAS2 is centrally involved in mediating synaptic growth,
9 Integrin-mediated synaptic structural development 3389 but suggest that the FAS2-mediated mechanism is more complex than previously thought. Do integrins do more than regulate FAS2 at the synapse? Our results demonstrate that integrins regulate morphological growth at the postembryonic NMJ through activation of CaMKII in both pre- and postsynaptic compartments. One important target of CaMKII is FAS2 and it is clear that regulation of FAS2 expression and localization is an important component of integrin regulation. However, the modulation of FAS2 levels alone is unlikely to account fully for the alterations in synaptic architecture in integrin mutants. In particular, both mys ts1 and mys b9 upregulate synaptic FAS2 levels, albeit to different degrees, yet show opposite alteration in synaptic growth. Moreover, reducing FAS2 levels rescues both underand overgrowth defects, but the rescue is not perfect. Precise control of FAS2 levels finely tunes morphological development at the NMJ. Different degrees of reduced FAS2 expression can either facilitate or inhibit the growth/ maintenance of the NMJ, and reduced FAS2 expression has been demonstrated in other overgrowth mutants (Schuster et al., 1996b). However, overexpression of FAS2 in specific muscles drives increased NMJ elaboration/bouton differentiation and selective preference for a high-expressing muscle over a low-expressing muscle (Davis and Goodman, 1998). How can this complexity be interpreted? One likely explanation is interaction between FAS2 and other developmental pathways regulated by CaMKII. To date, the only known FAS2-independent regulation of synaptic morphology involves a deubiquitinating protease encoded by fat facets (faf), and a putative ubiquitin ligase encoded by highwire (hiw), which have been shown to work together to modulate the degree of NMJ growth (DiAntonio et al., 2001; Wan et al., 2000). Loss-of-function hiw mutants display NMJ structural overgrowth, importantly without a concomitant decrease in FAS2. Indeed, overexpression of FAS2 cannot suppress the overgrowth seen in hiw mutants (Wan et al., 2000). These observations are consistent with the overgrowth combined with overexpression of FAS2 observed in mys ts1 mutants in this study. However, any putative interaction between FAS2-dependent and -independent mechanisms of morphological growth regulation at the Drosophila NMJ synapse remain to be elucidated. We expect that further study will reveal an additional structural control mechanism regulated by CaMKII, acting in parallel to and interacting with FAS2. The ubiquination mechanism discussed here is one candidate mechanism, but as CaMKII is known to have many synaptic targets, it is clearly not the only candidate. Presynaptic CaMKII has previously been shown to be important for behavioral change, including learning in mice, and habituation in Drosophila (Griffith et al., 1993; Soderling, 2000). In mice, animals null for αcamkii are unable to learn, and do not manifest experience-dependent plasticity (Frankland et al., 2001). Animals missing just one copy of αcamkii learn normally, but are unable to form long-term memories, which is accompanied by an inability to maintain LTP in the cortex. In Drosophila, presynaptic CaMKII has been shown to bidirectionally regulate habituation of a simple motor response (Jin et al., 1998). Inhibition of CaMKII activation by expression of the ala peptide reduces the initial response, which is then followed by facilitation instead of habituation. Moreover, presynaptically targeted expression of a constitutively active form of CaMKII eliminated habituation. Thus, appropriate regulation of CaMKII appears necessary for many aspects of developmental plasticity. In summary, we conclude that architectural developmental defects observed in NMJ synapses mutant for βps integrin are due to the loss of the ability to regulate synaptic CaMKII properly. One function of CaMKII is to phosphorylate the scaffolding protein DLG, and thus regulate the proteins synaptically localized by this scaffold. FAS2 is the central known output of this regulatory cascade. Loss of this regulation is central to the mys mutant defects in the postembryonic elaboration of NMJ structure. It is clear from this study, however, that regulation of FAS2 localization via CaMKII is only one facet of how integrins function at the synapse. We are particularly grateful to Drs Danny Brower, Nick Brown, Vivian Budnik and Corey Goodman for providing stocks and antibodies that made this study possible. We also most gratefully acknowledge the extensive advice freely given by Dr Christoph Schuster and by members of the Budnik laboratory. We thank Sean Speese, Keri Swearingen, Jay Newby and Gini Walters in the Broadie Lab for extensive technical assistance. This work was supported by an NIH postdoctoral fellowship to K. Beumer, and an NSF CAREER grant, an EJLB Fellowship and NIH grant GM54544 to K. Broadie. REFERENCES Ashburner, M. (1989). Drosophila. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. Ayyub, C., Rodrigues, V., Hasan, G. and Siddiqi, O. (2000). Genetic analysis of olfc demonstrates a role for the position-specific integrins in the olfactory system of Drosophila melanogaster. Mol. Gen. Genet. 263, Bailey, C. H. and Chen, M. (1989). Structural plasticity at identified synapses during long-term memory in Aplysia. J. Neurobiol. 20, Bastiani, M. J., Harrelson, A. L., Snow, P. M. and Goodman, C. S. (1987). Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell 48, Baum, P. D. and Garriga, G. (1997). Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19, Beumer, K. J., Rohrbough, J., Prokop, A. and Broadie, K. (1999). A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development 126, Bilato, C., Curto, K. A., Monticone, R. E., Pauly, R. R., White, A. J. and Crow, M. T. (1997). The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the alphavbeta3 integrin complex is reversed by activated calcium/calmodulin-dependent protein kinase II. J. Clin. Invest. 100, Bloor, J. W. and Brown, N. H. (1998). Genetic analysis of the Drosophila alphaps2-integrin subunit reveals discrete adhesive, morphogenetic and sarcomeric functions. Genetics 148, Blystone, S. D., Slater, S. E., Williams, M. P., Crow, M. T. and Brown, E. J. (1999). A molecular mechanism of integrin crosstalk: alphavbeta3 suppression of calcium/calmodulin-dependent protein kinase II regulates alpha5beta1 function. J. Cell Biol. 145, Bogaert, T., Brown, N. and Wilcox, M. (1987). The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell 51, Brand, A. H. and Dormand, E. L. (1995). The GAL4 system as a tool for unravelling the mysteries of the Drosophila nervous system. Curr. Opin. Neurobiol. 5, Broadie, K. S. and Bate, M. (1993). Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J. Neurosci. 13, Brower, D. L., Wilcox, M., Piovant, M., Smith, R. J. and Reger, L. A.
10 3390 K. Beumer and others (1984). Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. USA 81, Budnik, V., Koh, Y. H., Guan, B., Hartmann, B., Hough, C., Woods, D. and Gorczyca, M. (1996). Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17, Bunch, T. A., Salatino, R., Engelsgjerd, M. C., Mukai, L., West, R. F. and Brower, D. L. (1992). Characterization of mutant alleles of myospheroid, the gene encoding the β-subunit of the Drosophila PS integrins. Genetics 132, Burkin, D. J., Gu, M., Hodges, B. L., Campanelli, J. T. and Kaufman, S. J. (1998). A functional role for specific spliced variants of the alpha7beta1 integrin in acetylcholine receptor clustering. J. Cell Biol. 143, Chung, H. J., Xia, J., Scannevin, R. H., Zhang, X. and Huganir, R. L. (2000). Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci. 20, Cohen, M. W., Hoffstrom, B. G. and DeSimone, D. W. (2000). Active zones on motor nerve terminals contain alpha 3beta 1 integrin. J. Neurosci. 20, Dahme, M., Bartsch, U., Martini, R., Anliker, B., Schachner, M. and Mantei, N. (1997). Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 17, Davis, G. W. and Goodman, C. S. (1998). Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr. Opin. Neurobiol. 8, Davis, G. W., Schuster, C. M. and Goodman, C. S. (1997). Genetic analysis of the mechanisms controlling target selection: target- derived Fasciclin II regulates the pattern of synapse formation. Neuron 19, DiAntonio, A., Haghighi, A. P., Portman, S. L., Lee, J. D., Amaranto, A. M. and Goodman, C. S. (2001). Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, Fernandes, J. J., Celniker, S. E. and VijayRaghavan, K. (1996). Development of the indirect flight muscle attachment sites in Drosophila: Role of the PS-integrins and the stripe gene. Dev. Biol. 176, FlyBase (1998). Flybase: A Drosophila database. Nucleic Acids Res. 26, Frankland, P. W., O Brien, C., Ohno, M., Kirkwood, A. and Silva, A. J. (2001). Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature 411, Grenningloh, G., Rehm, E. J. and Goodman, C. S. (1991). Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67, Griffith, L. C., Verselis, L. M., Aitken, K. M., Kyriacou, C. P., Danho, W. and Greenspan, R. J. (1993). Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron 10, Griffith, L. C., Wang, J., Zhong, Y., Wu, C. F. and Greenspan, R. J. (1994). Calcium/calmodulin-dependent protein kinase II and potassium channel subunit Eag similarly affect plasticity in Drosophila. Proc. Natl. Acad. Sci. USA 91, Grotewiel, M. S., Beck, C. D., Wu, K. H., Zhu, X. R. and Davis, R. L. (1998). Integrin-mediated short-term memory in Drosophila. Nature 391, Guan, B., Hartmann, B., Kho, Y. H., Gorczyca, M. and Budnik, V. (1996). The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr. Biol. 6, Hoang, B. and Chiba, A. (1998). Genetic analysis of the role of integrin during axon guidance in Drosophila. J. Neurosci. 18, Inagaki, S., Ohoka, Y., Sugimoto, H., Fujioka, S., Amazaki, M., Kurinami, H., Miyazaki, N., Tohyama, M. and Furuyama, T. (2001). Sema4c, a transmembrane semaphorin, interacts with a post-synaptic density protein, psd-95. J. Biol. Chem. 276, Ivins, J. K., Yurchenco, P. D. and Lander, A. D. (2000). Regulation of neurite outgrowth by integrin activation. J. Neurosci. 20, Jin, P., Griffith, L. C. and Murphey, R. K. (1998). Presynaptic calcium/ calmodulin-dependent protein kinase II regulates habituation of a simple reflex in adult Drosophila. J. Neurosci. 18, Kim, E., Niethammer, M., Rothschild, A., Jan, Y. N. and Sheng, M. (1995). Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 378, Kim, E., Cho, K. O., Rothschild, A. and Sheng, M. (1996). Heteromultimerization and NMDA receptor-clustering activity of Chapsyn- 110, a member of the PSD-95 family of proteins. Neuron 17, Koh, Y., Popova, E., Thomas, U., Griffith, U. and Budnick, V. (1999a). Role of CaMKII-dependent phosphorylation on the selective localization of DLG to synaptic junctions (abstract). 40th Annual Drosophila Research Conference Programs and Abstracts 1, 204. Koh, Y. H., Popova, E., Thomas, U., Griffith, L. C. and Budnik, V. (1999b). Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell 98, Kornau, H. C., Schenker, L. T., Kennedy, M. B. and Seeburg, P. H. (1995). Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269, Lahey, T., Gorczyca, M., Jia, X. X. and Budnik, V. (1994). The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron 13, Leptin, M., Bogaert, T., Lehmann, R. and Wilcox, M. (1989). The function of PS-integrins during Drosophila embryogenesis. Cell 56, Lin, D. M. and Goodman, C. S. (1994). Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13, Lin, D. M., Fetter, R. D., Kopczynski, C., Grenningloh, G. and Goodman, C. S. (1994). Genetic analysis of Fasciclin II in Drosophila: defasciculation, refasciculation, and altered fasciculation. Neuron 13, Lindsley, D. L. and Zimm, G. (1992). The Genome of Drosophila melanogaster. San Diego: Academic Press. Littleton, J. T., Bellen, H. J. and Perin, M. S. (1993). Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development 118, Luthl, A., Laurent, J. P., Figurov, A., Muller, D. and Schachner, M. (1994). Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature 372, Martin, P. T., Kaufman, S. J., Kramer, R. H. and Sanes, J. R. (1996). Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7a, and alpha7b integrins with the neuromuscular junction. Dev. Biol. 174, Mayford, M., Barzilai, A., Keller, F., Schacher, S. and Kandel, E. R. (1992). Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science 256, Niethammer, M., Kim, E. and Sheng, M. (1996). Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J. Neurosci. 16, Pinkstaff, J. K., Detterich, J., Lynch, G. and Gall, C. (1999). Integrinsubunit gene expression is regionally differentiated in adult brain. J. Neurosci. 19, Rodrigues, V. and Siddiqi, O. (1978). Genetic analysis of chemosensory pathway. Proc. Indian Acad. Sci. USA(B) 87B, Rodriguez, M. A., Pesold, C., Liu, W. S., Kriho, V., Guidotti, A., Pappas, G. D. and Costa, E. (2000). Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc. Natl. Acad. Sci. USA 97, Rohrbough, J., Grotewiel, M. S., Davis, R. L. and Broadie, K. (2000). Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J. Neurosci. 20, Schuster, C. M., Davis, G. W., Fetter, R. D. and Goodman, C. S. (1996a). Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, Schuster, C. M., Davis, G. W., Fetter, R. D. and Goodman, C. S. (1996b). Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17, Soderling, T. R. (2000). CaM-kinases: modulators of synaptic plasticity. Curr. Opin. Neurobiol. 10, Stewart, B. A., Schuster, C. M., Goodman, C. S. and Atwood, H. L. (1996). Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J. Neurosci. 16, Tejedor, F. J., Bokhari, A., Rogero, O., Gorczyca, M., Zhang, J., Kim, E., Sheng, M. and Budnik, V. (1997). Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J. Neurosci. 17, Thomas, U., Kim, E., Kuhlendahl, S., Koh, Y. H., Gundelfinger, E. D., Sheng, M., Garner, C. C. and Budnik, V. (1997). Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron 19, Volk, T., Fessler, L. I. and Fessler, J. H. (1990). A role for integrin in the formation of sarcomeric cytoarchitecture. Cell 63,
A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila
 Development 126, 5833-5846 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8655 5833 A role for PS integrins in morphological growth and synaptic function at the postembryonic
Development 126, 5833-5846 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8655 5833 A role for PS integrins in morphological growth and synaptic function at the postembryonic
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Shaggy, the Homolog of Glycogen Synthase Kinase 3, Controls Neuromuscular Junction Growth in Drosophila
 The Journal of Neuroscience, July 21, 2004 24(29):6573 6577 6573 Brief Communication Shaggy, the Homolog of Glycogen Synthase Kinase 3, Controls Neuromuscular Junction Growth in Drosophila Bénédicte Franco,
The Journal of Neuroscience, July 21, 2004 24(29):6573 6577 6573 Brief Communication Shaggy, the Homolog of Glycogen Synthase Kinase 3, Controls Neuromuscular Junction Growth in Drosophila Bénédicte Franco,
Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction
 Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction Myung-Jun Kim, Michael B. O Connor* Department of Genetics, Cell
Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction Myung-Jun Kim, Michael B. O Connor* Department of Genetics, Cell
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control
 Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Highwire Regulates Synaptic Growth in Drosophila
 Neuron, Vol. 26, 313 329, May, 2000, Copyright 2000 by Cell Press Highwire Regulates Synaptic Growth in Drosophila Hong I. Wan,* Aaron DiAntonio,* Richard D. Fetter,* Kendra Bergstrom,* Roland Strauss,
Neuron, Vol. 26, 313 329, May, 2000, Copyright 2000 by Cell Press Highwire Regulates Synaptic Growth in Drosophila Hong I. Wan,* Aaron DiAntonio,* Richard D. Fetter,* Kendra Bergstrom,* Roland Strauss,
Bypass and interaction suppressors; pathway analysis
 Bypass and interaction suppressors; pathway analysis The isolation of extragenic suppressors is a powerful tool for identifying genes that encode proteins that function in the same process as a gene of
Bypass and interaction suppressors; pathway analysis The isolation of extragenic suppressors is a powerful tool for identifying genes that encode proteins that function in the same process as a gene of
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Neuronal Differentiation: Synapse Formation NMJ
 Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Overexpression of Cysteine-String Proteins in Drosophila Reveals Interactions with Syntaxin
 The Journal of Neuroscience, December 1, 1999, 19(23):10270 10279 Overexpression of Cysteine-String Proteins in Drosophila Reveals Interactions with Syntaxin Zhiping Nie, Ravi Ranjan, Julia J. Wenniger,
The Journal of Neuroscience, December 1, 1999, 19(23):10270 10279 Overexpression of Cysteine-String Proteins in Drosophila Reveals Interactions with Syntaxin Zhiping Nie, Ravi Ranjan, Julia J. Wenniger,
Neurite initiation. Neurite formation begins with a bud that sprouts from the cell body. One or several neurites can sprout at a time.
 Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Open Syntaxin Docks Synaptic Vesicles
 Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development
 RESEARCH ARTICLE 2337 Development 134, 2337-2347 (2007) doi:10.1242/dev.004242 Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development Zhiyu
RESEARCH ARTICLE 2337 Development 134, 2337-2347 (2007) doi:10.1242/dev.004242 Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development Zhiyu
Supplementary Figures Supplementary Tables 1 and 2. Supplementary References
 The Nuclear Import of a Non-Canonical Frizzled2 Signal by Importins-β11 and α2 Promotes Postsynaptic Development Timothy J. Mosca and Thomas L. Schwarz * The F.M. Kirby Neurobiology Center, Children s
The Nuclear Import of a Non-Canonical Frizzled2 Signal by Importins-β11 and α2 Promotes Postsynaptic Development Timothy J. Mosca and Thomas L. Schwarz * The F.M. Kirby Neurobiology Center, Children s
A complementation test would be done by crossing the haploid strains and scoring the phenotype in the diploids.
 Problem set H answers 1. To study DNA repair mechanisms, geneticists isolated yeast mutants that were sensitive to various types of radiation; for example, mutants that were more sensitive to UV light.
Problem set H answers 1. To study DNA repair mechanisms, geneticists isolated yeast mutants that were sensitive to various types of radiation; for example, mutants that were more sensitive to UV light.
The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development. Kyle Pemberton
 The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development Kyle Pemberton Acknowledgement Dr. Xu Lab Members Brittany Mersman Nicki Patel Pallavi Mhaskar Jason Cocjin Committee
The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development Kyle Pemberton Acknowledgement Dr. Xu Lab Members Brittany Mersman Nicki Patel Pallavi Mhaskar Jason Cocjin Committee
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
7.06 Spring 2004 PS 6 KEY 1 of 14
 7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
Varicose and cheerio collaborate with pebble to mediate semaphorin-1a reverse signaling in Drosophila
 Varicose and cheerio collaborate with pebble to mediate semaphorin-1a reverse signaling in Drosophila Sangyun Jeong a,1, Da-som Yang a, Young Gi Hong a, Sarah P. Mitchell b,c, Matthew P. rown b,c, and
Varicose and cheerio collaborate with pebble to mediate semaphorin-1a reverse signaling in Drosophila Sangyun Jeong a,1, Da-som Yang a, Young Gi Hong a, Sarah P. Mitchell b,c, Matthew P. rown b,c, and
Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry,adrosophila pax3/7 Homolog
 The Journal of Neuroscience, June 16, 2010 30(24):8071 8082 8071 Development/Plasticity/Repair Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry,adrosophila pax3/7 Homolog Bruno Marie,
The Journal of Neuroscience, June 16, 2010 30(24):8071 8082 8071 Development/Plasticity/Repair Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry,adrosophila pax3/7 Homolog Bruno Marie,
Human Coagulation Factor X Total Antigen ELISA Kit
 Human Coagulation Factor X Total Antigen ELISA Kit Catalog No: IHFXKT-TOT Lot No: SAMPLE INTENDED USE This human coagulation Factor X antigen assay is intended for the quantitative determination of total
Human Coagulation Factor X Total Antigen ELISA Kit Catalog No: IHFXKT-TOT Lot No: SAMPLE INTENDED USE This human coagulation Factor X antigen assay is intended for the quantitative determination of total
Reading: Chapter 5, pp ; Reference chapter D, pp Problem set F
 Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
5- Semaphorin-Plexin-Neuropilin
 5- Semaphorin-Plexin-Neuropilin 1 SEMAPHORINS-PLEXINS-NEUROPILINS ligands receptors co-receptors semaphorins and their receptors are known signals for: -axon guidance -cell migration -morphogenesis -immune
5- Semaphorin-Plexin-Neuropilin 1 SEMAPHORINS-PLEXINS-NEUROPILINS ligands receptors co-receptors semaphorins and their receptors are known signals for: -axon guidance -cell migration -morphogenesis -immune
Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization
 Neuron, Vol. 30, 737 749, June, 2001, Copyright 2001 by Cell Press Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization Suzanne Paradis, Sean T. Sweeney, and
Neuron, Vol. 30, 737 749, June, 2001, Copyright 2001 by Cell Press Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization Suzanne Paradis, Sean T. Sweeney, and
Drosophila Plexin B is a Sema-2a receptor required for axon guidance
 RESEARCH ARTICLE 2125 Development 133, 2125-2135 (2006) doi:10.1242/dev.02380 Drosophila Plexin B is a Sema-2a receptor required for axon guidance Joseph C. Ayoob 1, Jonathan R. Terman 1,2 and Alex L.
RESEARCH ARTICLE 2125 Development 133, 2125-2135 (2006) doi:10.1242/dev.02380 Drosophila Plexin B is a Sema-2a receptor required for axon guidance Joseph C. Ayoob 1, Jonathan R. Terman 1,2 and Alex L.
Axon guidance I. Paul Garrity March 15, /9.013
 Axon guidance I Paul Garrity March 15, 2004 7.68/9.013 Neuronal Wiring: Functional Framework of the Nervous System Stretch reflex circuit Early theories of axonogenesis Schwann: many neurons link to form
Axon guidance I Paul Garrity March 15, 2004 7.68/9.013 Neuronal Wiring: Functional Framework of the Nervous System Stretch reflex circuit Early theories of axonogenesis Schwann: many neurons link to form
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
The Transmembrane Semaphorin Sema I Is Required in Drosophila for Embryonic Motor and CNS Axon Guidance
 Neuron, Vol. 20, 207 220, February, 1998, Copyright 1998 by Cell Press The Transmembrane Semaphorin Sema I Is Required in Drosophila for Embryonic Motor and CNS Axon Guidance Hung-Hsiang Yu, Houmam H.
Neuron, Vol. 20, 207 220, February, 1998, Copyright 1998 by Cell Press The Transmembrane Semaphorin Sema I Is Required in Drosophila for Embryonic Motor and CNS Axon Guidance Hung-Hsiang Yu, Houmam H.
Human Coagulation Factor XII Total Antigen ELISA Kit
 Human Coagulation Factor XII Total Antigen ELISA Kit Catalog No: IHFXIIKT-TOT Lot No: SAMPLE INTENDED USE This human coagulation Factor XII antigen assay is intended for the quantitative determination
Human Coagulation Factor XII Total Antigen ELISA Kit Catalog No: IHFXIIKT-TOT Lot No: SAMPLE INTENDED USE This human coagulation Factor XII antigen assay is intended for the quantitative determination
Supporting Information
 Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Protease Inhibitor Cocktail A (1 tablet / 7 10 ml, Roche Cat# ) Protease inhibitor Cocktail B (0.5ml per 250ml, Calbiochem Cat# )
 Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
Report. Functional Diversity of Robo Receptor Immunoglobulin Domains Promotes Distinct Axon Guidance Decisions
 Current Biology 20, 567 572, March 23, 2010 ª2010 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2010.02.021 Functional Diversity of Robo Receptor Immunoglobulin Domains Promotes Distinct Axon Guidance
Current Biology 20, 567 572, March 23, 2010 ª2010 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2010.02.021 Functional Diversity of Robo Receptor Immunoglobulin Domains Promotes Distinct Axon Guidance
Developmental Biology
 Developmental Biology 366 (2012) 204 217 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Developmental changes in
Developmental Biology 366 (2012) 204 217 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Developmental changes in
Axon Guidance. Multiple decision points along a growing axon s trajectory Different types of axon guidance cues:
 Axon Guidance Multiple decision points along a growing axon s trajectory Different types of axon guidance cues: Contact mediated - requires direct contact by growth cone Long range - growth cone responds
Axon Guidance Multiple decision points along a growing axon s trajectory Different types of axon guidance cues: Contact mediated - requires direct contact by growth cone Long range - growth cone responds
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis
 Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
According to the diagram, which of the following is NOT true?
 Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
The majority of cells in the nervous system arise during the embryonic and early post
 Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
with%dr.%van%buskirk%%%
 with%dr.%van%buskirk%%% How$to$do$well?$ Before$class:$read$the$corresponding$chapter$ Come$to$class$ready$to$par9cipate$in$Top$Hat$ Don t$miss$an$exam!!!!!!!!!!!!!!!!!!!!!!!!!!$ But$I m$not$good$with$science
with%dr.%van%buskirk%%% How$to$do$well?$ Before$class:$read$the$corresponding$chapter$ Come$to$class$ready$to$par9cipate$in$Top$Hat$ Don t$miss$an$exam!!!!!!!!!!!!!!!!!!!!!!!!!!$ But$I m$not$good$with$science
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses
 Developmental Biology 229, 55 70 (2001) doi:10.1006/dbio.2000.9983, available online at http://www.idealibrary.com on Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses Bao Hoang 1 and Akira
Developmental Biology 229, 55 70 (2001) doi:10.1006/dbio.2000.9983, available online at http://www.idealibrary.com on Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses Bao Hoang 1 and Akira
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
MCN. Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases Regulate Axon Guidance in Drosophila
 MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
RPM-1 is localized to distinct subcellular compartments and regulates axon length in GABAergic motor neurons
 Opperman and Grill Neural Development 14, 9:1 RESERCH RTICLE Open ccess RPM-1 is localized to distinct subcellular compartments and regulates axon length in Gergic motor neurons Karla J Opperman and rock
Opperman and Grill Neural Development 14, 9:1 RESERCH RTICLE Open ccess RPM-1 is localized to distinct subcellular compartments and regulates axon length in Gergic motor neurons Karla J Opperman and rock
The Ras1 Mitogen-Activated Protein Kinase Signal Transduction Pathway Regulates Synaptic Plasticity through Fasciclin II-Mediated Cell Adhesion
 The Journal of Neuroscience, April 1, 2002, 22(7):2496 2504 The Ras1 Mitogen-Activated Protein Kinase Signal Transduction Pathway Regulates Synaptic Plasticity through Fasciclin II-Mediated Cell Adhesion
The Journal of Neuroscience, April 1, 2002, 22(7):2496 2504 The Ras1 Mitogen-Activated Protein Kinase Signal Transduction Pathway Regulates Synaptic Plasticity through Fasciclin II-Mediated Cell Adhesion
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
Reading. Lecture VI. Making Connections 9/17/12. Bio 3411 Lecture VI. Making Connections. Bio 3411 Monday September 17, 2012
 Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Different crystal forms obtained for Sky
 Supplementary Figure 1 Different crystal forms obtained for Sky 1 353. (a) Crystal form 1 obtained in the presence of 20% PEG 3350 and 0.2 M ammonium citrate tribasic ph 7.0. (b) Crystal form 1 of the
Supplementary Figure 1 Different crystal forms obtained for Sky 1 353. (a) Crystal form 1 obtained in the presence of 20% PEG 3350 and 0.2 M ammonium citrate tribasic ph 7.0. (b) Crystal form 1 of the
Review. Wiring by Fly: The Neuromuscular System of the Drosophila Embryo. Neuron, Vol. 15, , September, 1995, Copyright 1995 by Ceil Press
 Neuron, Vol. 15, 513-525, September, 1995, Copyright 1995 by Ceil Press Wiring by Fly: The Neuromuscular System of the Drosophila Embryo Michael Bate and Kendal Broadie Department of Zoology University
Neuron, Vol. 15, 513-525, September, 1995, Copyright 1995 by Ceil Press Wiring by Fly: The Neuromuscular System of the Drosophila Embryo Michael Bate and Kendal Broadie Department of Zoology University
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Types of biological networks. I. Intra-cellurar networks
 Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Types of biological networks I. Intra-cellurar networks 1 Some intra-cellular networks: 1. Metabolic networks 2. Transcriptional regulation networks 3. Cell signalling networks 4. Protein-protein interaction
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Lecture 2: mrna localisation and decay in cells and in tissues
 Lecture 2: mrna localisation and decay in cells and in tissues Prof. Ilan Davis, Department of Biochemistry. Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com References
Lecture 2: mrna localisation and decay in cells and in tissues Prof. Ilan Davis, Department of Biochemistry. Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com References
The Control of Semaphorin-1a-Mediated Reverse Signaling by Opposing Pebble and RhoGAPp190 Functions in Drosophila
 Article The Control of Semaphorin-1a-Mediated Reverse Signaling by Opposing Pebble and RhoGAPp190 Functions in Drosophila Sangyun Jeong, 1 Katarina Juhaszova, 1 and Alex L. Kolodkin 1, 1 Solomon H. Snyder
Article The Control of Semaphorin-1a-Mediated Reverse Signaling by Opposing Pebble and RhoGAPp190 Functions in Drosophila Sangyun Jeong, 1 Katarina Juhaszova, 1 and Alex L. Kolodkin 1, 1 Solomon H. Snyder
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
Zool 3200: Cell Biology Exam 5 4/27/15
 Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
7.06 Cell Biology EXAM #3 KEY
 7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Neuron. Detector Model. Understanding Neural Components in Detector Model. Detector vs. Computer. Detector. Neuron. output. axon
 Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
What I do. (and what I want to do) Berton Earnshaw. March 1, Department of Mathematics, University of Utah Salt Lake City, Utah 84112
 What I do (and what I want to do) Berton Earnshaw Department of Mathematics, University of Utah Salt Lake City, Utah 84112 March 1, 2008 Current projects What I do AMPA receptor trafficking Synaptic plasticity
What I do (and what I want to do) Berton Earnshaw Department of Mathematics, University of Utah Salt Lake City, Utah 84112 March 1, 2008 Current projects What I do AMPA receptor trafficking Synaptic plasticity
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J INTEGRINS I.V. Yannas, Ph.D. and M. Spector, Ph.D. Regulator
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J INTEGRINS I.V. Yannas, Ph.D. and M. Spector, Ph.D. Regulator
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Original Article Overexpression of ter94, Drosophila VCP, improves motor neuron degeneration induced by knockdown of TBPH, Drosophila TDP-43
 Am J Neurodegener Dis 2018;7(1):11-31 www.ajnd.us /ISSN:2165-591X/AJND0069303 Original Article Overexpression of ter94, Drosophila VCP, improves motor neuron degeneration induced by knockdown of TBPH,
Am J Neurodegener Dis 2018;7(1):11-31 www.ajnd.us /ISSN:2165-591X/AJND0069303 Original Article Overexpression of ter94, Drosophila VCP, improves motor neuron degeneration induced by knockdown of TBPH,
A Hierarchy of Cell Intrinsic and Target-Derived Homeostatic Signaling
 Article A Hierarchy of Cell Intrinsic and Target-Derived Homeostatic Signaling Sharon Bergquist, 1 Dion K. Dickman, 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics, Program in Neuroscience,
Article A Hierarchy of Cell Intrinsic and Target-Derived Homeostatic Signaling Sharon Bergquist, 1 Dion K. Dickman, 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics, Program in Neuroscience,
Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function
 Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function Nina Tang Sherwood 1, Qi Sun 1 1, Mingshan Xue 2 2, Bing Zhang 2, Kai Zinn 1 * Open access, freely available
Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function Nina Tang Sherwood 1, Qi Sun 1 1, Mingshan Xue 2 2, Bing Zhang 2, Kai Zinn 1 * Open access, freely available
Role of Neural Activity during Synaptogenesis in Drosophila
 The Journal of Neuroscience, December 1995, 15( 12): 8177-8190 Role of Neural Activity during Synaptogenesis in Drosophila Jill Jareckil and Haig Keshishian* Departments of Genetics and 2Biology, Yale
The Journal of Neuroscience, December 1995, 15( 12): 8177-8190 Role of Neural Activity during Synaptogenesis in Drosophila Jill Jareckil and Haig Keshishian* Departments of Genetics and 2Biology, Yale
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition
 Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses is regulated by Mind the gap
 RESEARCH ARTICLE 3523 Development 137, 3523-3533 (2010) doi:10.1242/dev.047878 2010. Published by The Company of Biologists Ltd Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses
RESEARCH ARTICLE 3523 Development 137, 3523-3533 (2010) doi:10.1242/dev.047878 2010. Published by The Company of Biologists Ltd Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses
RACK-1 Acts with Rac GTPase Signaling and UNC-115/ ablim in Caenorhabditis elegans Axon Pathfinding and Cell Migration
 RACK-1 Acts with Rac GTPase Signaling and UNC-115/ ablim in Caenorhabditis elegans Axon Pathfinding and Cell Migration Rafael S. Demarco, Erik A. Lundquist* Programs in Genetics and Molecular, Cellular,
RACK-1 Acts with Rac GTPase Signaling and UNC-115/ ablim in Caenorhabditis elegans Axon Pathfinding and Cell Migration Rafael S. Demarco, Erik A. Lundquist* Programs in Genetics and Molecular, Cellular,
Dimorphism and Homeostasis of Neuromuscular Synaptic Function
 Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Synapses. Electrophysiology and Vesicle release
 Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Connectin Mediates Adhesion in Drosophila
 Neuron, Vol. 18, 873 880, June, 1997, Copyright 1997 by Cell Press Connectin Mediates Adhesion in Drosophila Srikala Raghavan and Robert A. H. White in little observable perturbation of the normal axonal
Neuron, Vol. 18, 873 880, June, 1997, Copyright 1997 by Cell Press Connectin Mediates Adhesion in Drosophila Srikala Raghavan and Robert A. H. White in little observable perturbation of the normal axonal
