Bcl-2 Family Proteins Participate in Mitochondrial Quality Control by Regulating Parkin/PINK1-Dependent Mitophagy
|
|
|
- Martina Cummings
- 5 years ago
- Views:
Transcription
1 Article Bcl-2 Family Proteins Participate in Mitochondrial Quality Control by Regulating /-Dependent Mitophagy Emilie Hollville, 1 Richard G. Carroll, 1 Sean P. Cullen, 1 and Seamus J. Martin 1, 1 Molecular Cell Biology Laboratory, Department of Genetics, The Smurfit Institute, Trinity College Dublin, College Green, Dublin 2, Ireland Correspondence: martinsj@tcd.ie SUMMARY Mitophagy facilitates the selective elimination of impaired or depolarized mitochondria through targeting the latter to autophagosomes. becomes localized to depolarized mitochondria in a -dependent manner and polyubiquitinates multiple mitochondrial outer membrane proteins. This permits ubiquitin-binding proteins (e.g., p62 and NBR1) to target impaired mitochondria to autophagosomes via Atg8/LC3II. Bcl-2 family proteins regulate mitochondrial outer membrane permeabilization during apoptosis and can also influence macroautophagy via interactions with Beclin-1. Here, we show that -dependent mitophagy is antagonized by prosurvival members of the Bcl-2 family (e.g., and ) in a Beclin-1-independent manner. Bcl-2 proteins suppressed mitophagy through inhibition of translocation to depolarized mitochondria. Consistent with this, translocation to mitochondria was enhanced by BH3-only proteins or a BH3-only mimetic. Taken together with their role as regulators of apoptosis-associated mitochondrial permeabilization, as well as mitochondrial fission/fusion dynamics, this suggests that Bcl-2 family proteins act as global regulators of mitochondrial homeostasis. INTRODUCTION Macroautophagy promotes recycling of long-lived proteins and organelles and involves entrapment of cytoplasmic material within autophagosomes, followed by fusion of autophagosomes with lysosomes (Mizushima, 7; Chen and Klionsky, 11). Multiple lysosomal proteases promote degradation of the contents of autolysosomes, thus liberating amino acids and other basic building blocks to be reused within the cell. Autophagy can also be invoked to eliminate damaged organelles through selective ubiquitination followed by targeting of the damaged organelle to autophagosomes via ubiquitin (Ub) and LC3-binding adaptor proteins. Mitophagy is an organelle-specific type of autophagy that facilitates the elimination of damaged or depolarized mitochondria through selective targeting of such mitochondria to the autophagic pathway (Youle and Narendra, 11; Ashrafi and Schwarz, 13). However, the molecular events that orchestrate selective recognition and removal of damaged mitochondria are only now emerging. is a Ub E3 ligase that is found in mutant form in recessive familial son s disease, a disorder associated with loss of dopaminergic neurons in the midbrain (Kitada et al., 1998; Shimura et al., ). Due to its role in this neurological condition, the function of has been the subject of intensive study (Kahle and Haass, 4). Phosphatase and tensin homolog deleted on chromosome 1-induced kinase 1 (), which shuttles between the cytosol and mitochondria, has also been linked with son s disease and operates in the same pathway as (Valente et al., 4; Clark et al., 6; Park et al., 6; Weihofen et al., 8). Until recently, the functional role of and remained obscure. However, several studies have shown that, which is normally localized to the cytosol, translocates to depolarized or impaired mitochondria in a -dependent manner (Narendra et al., 8, 1a; Vives-Bauza et al., 1). In healthy cells, is constitutively imported into mitochondria and degraded by mitochondrial proteases but becomes stabilized on the outer membranes of depolarized mitochondria and is required for relocalization to these organelles (Narendra et al., 8). Several laboratories have reported that and directly interact, and loss of either or completely abrogates mitophagy (Shiba et al., 9; Um et al., 9; Narendra et al., 1a; Vives-Bauza et al., 1). is activated upon association with, and recent studies suggest that phosphorylates at Ser65 (Shiba-Fukushima et al., 12; Kondapalli et al., 12), either to trigger its translocation to mitochondria or to activate the Ub ligase activities of the latter. Upon activation of on depolarized mitochondria, numerous proteins within the mitochondrial outer membrane become polyubiquitinated, and this triggers translocation of the Ub- and LC3-binding adaptor protein p62 to these organelles (Lee et al., 1; Narendra et al., 1b; Okatsu et al., 1). Thus, p62 appears to act as a bridge to link damaged mitochondria to autophagosomes. While initial studies suggested that p62 was critical for removal of depolarized mitochondria via mitophagy, more recent studies suggest that p62 may be dispensible for this process (Narendra et al., 1b; Okatsu et al., 1), possibly due to redundancy with the related Ub- and Atg8/ LC3II-binding protein NBR1. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 451
2 A 6 hr B 24 hr C -YFP- TOM TOM (hr): TOM mcherry- mcherry- 7 kda Cyt c COX III HSP6 8 kda 3 kda Actin % of transfected cells D TOM % of transfected cells hr 24 hr % of transfected cells 8 6 HSP6 24 hr E 6 hr F 6 hr G 6 hr Ubiquitin p62 GFP-LC3 TOM TOM TOM mcherry- mcherry- mcherry- % of transfected cells Ubiquitin p % of transfected cells hr 6 hr % of transfected cells 8 6 GFP-LC3 6 hr (legend on next page) 452 Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc.
3 Bcl-2 family proteins regulate entry into apoptosis by influencing the permeability of the mitochondrial outer membrane through controlling the assembly of multimeric BAX/BAK channels (Chipuk et al., 6). BAX/BAK oligomerization permits the efflux of mitochondrial intermembrane space proteins, such as cytochrome c, from mitochondria. The latter event is critical for the formation of the Apaf-1/Caspase-9 apoptosome, which unleashes a cascade of caspase activation events that culminate in apoptosis. Apart from this key role, studies have also shown that prosurvival members of the Bcl-2 family can regulate macroautophagy through binding to the autophagy regulator Beclin-1 and blocking its participation in the triggering of autophagosome formation (Pattingre et al., 5; Erlich et al., 7). However, it is not known whether Beclin-1 is required for mitophagy. Bcl-2 family members have also been implicated in the regulation of mitochondrial fission and fusion dynamics (reviewed in Youle and van der Bliek, 12; Autret and Martin, 9), processes that have been implicated in the quarantining of impaired mitochondria during mitophagy (Twig et al., 8; Gomes et al., 11; Rambold et al., 11). Thus, several members of the Bcl-2 family are localized to mitochondria, affect mitochondrial fission and fusion dynamics, bind to Beclin-1, and regulate mitochondrial permeabilization, an event that leads to mitochondrial depolarization. Because of the multiplicity of events surrounding mitophagy that Bcl-2 family proteins have also been implicated in, we wondered whether members of this family could also regulate /-dependent mitophagy. Here, we show that -dependent elimination of depolarized mitochondria can be inhibited by prosurvival Bcl-2 family members, such as and. However, we found no evidence for the involvement of Beclin-1 in -dependent mitophagy. Instead, Bcl-2 proteins suppressed mitophagy through binding to / complexes and inhibiting translocation to depolarized mitochondria, thereby blocking -dependent ubiquitination of mitochondrial substrates and downstream events. Thus, Bcl-2 family proteins participate in the maintenance of healthy mitochondrial networks through their ability to regulate /-dependent mitophagy. We propose that Bcl-2 proteins act as global regulators of mitochondrial network integrity by regulating mitochondrial permeabilization during apoptosis, steady-state mitochondrial fission/ fusion dynamics, and the removal of impaired mitochondria via mitophagy. RESULTS /PINK-1 Promote Elimination of Depolarized Mitochondria As previously reported by several laboratories, depolarization of mitochondria using the protonophore led to rapid translocation of mcherry- from the cytosol to mitochondria within several hours of treatment (Figure 1A). Coincident with translocation, mitochondrial networks in -treated cells collapsed around the perinuclear region, as revealed by TOM staining (Figure 1A). translocation to mitochondria was followed, within 24 hr, by complete loss of multiple mitochondrial markers, such as TOM, HSP6, COXIII, and cytochrome c (Figures 1B 1D; Figure S1A available online). In -treated cells lacking mitochondrial markers, was again found in the cytosol (Figure 1B). was absolutely required for mitochondrial elimination in response to, as cells transfected with empty vector failed to eliminate these organelles, as did untransfected cells adjoining those transfected with complementary DNA (cdna) (Figure 1B). Consistent with previous results (Narendra et al., 8, 1b; Vives-Bauza et al., 1; Matsuda et al., 1; Geisler et al., 1), depolarized mitochondria in -expressing cells became heavily decorated with Ub (Figure 1E) and were also labeled with the Ub- and LC3-binding adaptor proteins p62 (Figure 1F) and NBR1 (Figure S1B). Furthermore, we also detected relocalization of green fluorescent protein (GFP)-LC3 to depolarized mitochondria within -transfected cells (Figure 1G). Thus, depolarization of mitochondria promoted recruitment of to mitochondria; ubiquitination of mitochondrial substrates; and decoration of depolarized mitochondrial networks with the Ubbinding proteins p62, NBR1, and LC3, followed by elimination of these organelles via mitophagy. Bcl-2 Family Members Inhibit /-Dependent Mitophagy Impaired mitochondria are thought to undergo separation from the remainder of the mitochondrial network during mitophagy through enhanced mitochondrial fission or decreased mitochondrial fusion. Consequently, proteins that regulate mitochondrial fission/fusion dynamics have been implicated as regulators of mitophagy (Twig et al., 8; Gomes et al., 11; Rambold et al., 11). Studies from several laboratories have also implicated members of the Bcl-2 family as regulators of mitochondrial Figure 1. and PINK-1 Promote Elimination of Depolarized Mitochondria (A and B) cells were transfected with mcherry- cdna ( ng) and treated with (1 mm) for 6 hr (A) or 24 hr (B). Mitochondria were immunostained for TOM (green). Colocalization between and mitochondria (A) and the absence of the mitochondrial marker TOM (B) were scored among mcherry--positive cells by confocal microscopy. (C) and cells stably expressing YFP- were treated with (1 mm). Whole-cell lysates were analyzed for TOM, cytochrome c, COX III, HSP6,, and contents by immunoblotting. (D) cells were transfected and treated as in (B). Mitochondria were immunostained for HSP6. MCherry--positive cells were quantified for the absence of the mitochondrial marker HSP6. (E and F) cells were transfected with mcherry- cdna ( ng) and treated with (1 mm) for 6 hr. Cells were coimmunostained for TOM (blue) and Ub (E) or p62 (F) (green). Colocalization between Ub (E) or p62 (F) and mitochondria was scored among mcherry--positive cells. (G) Cells were transfected with plasmids encoding mcherry- ( ng) and GFP-LC3 ( ng) and treated as in (E) and (F). Cells were immunostained for TOM (blue). Colocalization between GFP-LC3 and mitochondria was quantified among mcherry--positive cells. Results shown are representative of at least three independent experiments. Error bars indicate SD of triplicate counts of 1 cells per treatment. See also Figure S1. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 453
4 A TOM 24 hr mcherry- B TOM (-) cells (%) 8 6 TOM 24 hr mcherry- : - (ng) : D - HSP6 24 hr mcherry- C TOM (-) cells (%) mcherry- : - E HSP6 (-) cells (%) 6 6 -Bcl-2 TOM 24 hr - -A1 HSP6 mcherry- : - -Bcl-2 24 hr - -A1 - F (ng) : (ng) : (ng) : hr : - 3 kda 3 kda 3 kda mito-gfp Actin G H TOM (-) cells (%) Valinomycin : - TOM (-) cells (%) TOM TOM Oligomycin Antimycin A : - hr hr (legend on next page) 454 Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc.
5 fission and fusion (Karbowski et al., 6; Delivani et al., 6; Sheridan et al., 8), as well as regulators of macroautophagy (Pattingre et al., 5; Elgendy et al., 11). Thus, we wondered whether members of the Bcl-2 family could also influence clearance of depolarized mitochondria via /-dependent mitophagy. To explore this possibility, we transiently overexpressed Bcl-2 and its close relatives (,,, Bcl-W, and A1), along with followed by treatment, and assessed clearance of depolarized mitochondria in the presence or absence of Bcl-2 family proteins. As Figure 2 illustrates, overexpression of, as well as other Bcl-2 family members, potently inhibited the elimination of mitochondria in expressing cells, as assessed by detection of mitochondrial TOM (Figures 2A 2C), HSP6 (Figures 2D and 2E), or mitochondrially targeted GFP (Figure 2F). Inhibition of -dependent mitophagy by,, and Bcl-W was also observed when mitochondria were depolarized with valinomycin (Figure 2G), as well as due to treatment with a combination of oligomycin and antimycin A (Figure 2H). Similar results were also observed in cells stably expressing yellow fluorescent protein (YFP)- (Figures S2A and S2B), as well as mcherry--transfected SKOV-3 (Figures S2C and S2D) and HaCaT cells (Figure S2E). Thus, / -dependent elimination of depolarized mitochondria can be antagonized by prosurvival members of the Bcl-2 family. It is interesting that Bcl-2 itself did not consistently inhibit mitophagy (Figure 2E); however, we were unable to reliably assess this due to degradation of upon overexpression of Bcl-2 (Figures S2F and S2G). Bcl-2 Proteins Do Not Inhibit Mitochondrial Depolarization or Stabilization One explanation for the ability of prosurvival Bcl-2 family proteins to inhibit mitophagy was the possibility that the latter interfered with depolarization of mitochondria, or with stabilization of on the mitochondrial outer membrane as a consequence of loss of membrane potential. However, staining of mitochondria with the potential-sensitive dye mitotracker revealed that, under conditions of Bcl-2 family protein overexpression, mitochondria were still depolarized in response to treatment (Figure 3A). In agreement with this, depolarization-induced stabilization of endogenous was also unaffected in cells overexpressing Bcl-2 family proteins (Figure 3B). Thus, Bcl-2 proteins do not inhibit mitophagy through interfering with mitochondrial depolarization or stabilization of the receptor,, on depolarized mitochondria. Bcl-2 Family Binding Partners, Beclin-1, BAX, and BAK Are Dispensible for Mitophagy Previous studies have shown that prosurvival Bcl-2 family proteins directly interact with Beclin-1, a protein involved in the initiation of macroautophagy under certain conditions (Liang et al., 1999; Pattingre et al., 5; Funderburk et al., 1; Elgendy et al., 11). Thus, we explored whether Beclin-1 was a possible point of intersection between Bcl-2 proteins and the mitophagy machinery. However, as Figures 3C 3G demonstrate, knockdown of Beclin-1 using two independent small interfering RNAs (sirnas) failed to attenuate /-induced mitophagy. Thus, -dependent clearance of impaired mitochondria appears to be Beclin-1-independent. In contrast, knockdown of robustly inhibited dependent mitophagy under the same conditions (Figures 3C 3G). BAX and BAK are critical binding partners for the prosurvival Bcl-2 proteins in the context of apoptosis (reviewed in Chipuk et al., 1) and have also been implicated in mitochondrial fission and fusion (Karbowski et al., 6; Sheridan et al., 8; Cleland et al., 11; Hoppins et al., 11). Because mitochondrial fission has been implicated in the quarantining of impaired mitochondria prior to their removal via mitophagy (Twig et al., 8; Gomes et al., 11; Rambold et al., 11), we also explored whether BAX or BAK were required for -dependent mitophagy. However, knockdown of BAX or BAK, or both, failed to suppress -dependent mitophagy (Figures 3H and 3I), thereby ruling out a role for these proteins as the targets of Bcl-2 family proteins during mitophagy. In contrast, knockdown of BAX or BAX/BAK robustly suppressed apoptosis induced by daunorubicin (Figure 3J). Thus, while Beclin-1, BAX, and BAK were dispensible for induced mitophagy, was found to be essential for this process. Bcl-2 Family Proteins Antagonize Ubiquitination of Depolarized Mitochondria and Downstream Events To identify the point at which Bcl-2 family proteins intersect with the mitophagy machinery, we explored whether decoration of Figure 2. Bcl-2 Family Proteins Antagonize -Dependent Mitophagy (A) cells, transfected with plasmids encoding mcherry- ( ng) and or ( ng), were treated with (1 mm) for 24 hr. Cells were then immunostained for TOM (green) and (blue). (B) cells were transfected with mcherry- cdna ( ng), along with the indicated amount of plasmid, and treated as in (A). Mitochondria were immunostained for TOM. The percentage of TOM-negative cells among mcherry--positive cells was then scored. (C) cells were transfected with mcherry- cdna ( ng) and the indicated -Bcl-2 construct ( ng). Cells were processed and analyzed as in (B). (D and E) cells were transfected and treated as in (C). Mitochondria were immunostained for HSP6, and mcherry--positive cells were scored for the absence of mitochondria. (F) cells were transfected with plasmids encoding mitochondrially targeted GFP (mito-gfp, ng) and ( ng) along with the indicated amount of -Bcl-2 construct. Cells were treated with (5 mm) for hr and whole-cell lysates were analyzed by immunoblotting. (G and H) cells, transfected with mcherry- cdna ( ng) and the indicated -Bcl-2 construct (8 ng), were treated with valinomycin (1 nm) (G) or oligomycin (25 nm) and antimycin A (25 nm) (H) for hr. Cells were processed and analyzed as in (B). Results shown are representative of at least three independent experiments. Error bars indicate SD of triplicate counts of 1 cells. Statistical significance was assessed by two-tailed paired Student s t test. Asterisks indicate significance: p %.1; p %.1; p %.1. See also Figure S2. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 455
6 A B 4 hr 6 hr 6 hr Actin 6 hr C D E 24 hr 24 hr F 24 hr G 24 hr H I J 24 hr 3 kda 3 kda Figure 3. Bcl-2- Binding Proteins Beclin-1, BAX, and BAK Are Dispensable for -Dependent Mitophagy (A) cells were transfected with plasmids encoding ( ng) and or ( ng) and treated with (1 mm) for 6 hr. Cells were stained with MitoTracker red (5 nm) and immunostained for TOM (green) and or (, blue). MitoTracker-negative cells were scored. (B) SDS-PAGE analysis of endogenous expression. cells were transfected with cdna (1 ng), along with the indicated amount of -Bcl-2 construct. Cells were treated with (5 mm) for 4 hr before analysis by immunoblotting. (legend continued on next page) 456 Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc.
7 depolarized mitochondria with Ub, p62, NBR1, or GFP-LC3 were impaired in -transfected cells expressing or other members of the Bcl-2 family. As Figure 4 shows, decoration of depolarized mitochondria in -expressing cells with Ub (Figure 4A), p62 (Figure 4B), or GFP-LC3 (Figure 4C) was all markedly inhibited upon coexpression of,, and other members of the Bcl-2 family, except Bcl-2 itself. Similarly, translocation of the Ub and LC3II adaptor, NBR1, was also impaired by overexpression of prosurvival Bcl-2 family proteins (Figure S3). This suggested that prosurvival Bcl-2 proteins inhibit /-dependent mitophagy at an early stage in the process, prior to the decoration of depolarized mitochondria with Ub and Ub adaptor proteins involved in targeting mitochondria to autophagosomes. Translocation to Depolarized Mitochondria Is Attenuated by Prosurvival Bcl-2 Family Proteins Because -dependent ubiquitination of mitochondria and its downstream sequelae were blocked by and other Bcl-2 family proteins, we explored whether the -dependent translocation of to mitochondria was affected by Bcl-2 family members. Indeed, during the preceding experiments, we noticed that, in -treated cells, was frequently observed in the cytoplasm, rather than on mitochondria, in cells coexpressing Bcl-2 family proteins (Figures 4A 4C). This was readily appreciated when -treated cells were stained for along with prosurvival Bcl-2 family proteins. As Figure 5A and Figure S4A demonstrate, whereas was clearly cytoplasmic in cells overexpressing or, was sharply localized to mitochondria in neighboring (- or -negative) cells. Thus, we performed time course analyses of translocation after treatment in cells expressing versus empty vector. As Figures 5B and 5C illustrate, rapidly translocated to mitochondria within 1 hr after treatment; however, this was attenuated in the presence of. Similarly, we readily observed impaired translocation of to depolarized mitochondria in cells overexpressing other prosurvival Bcl-2 family proteins (Figures 5D 5E). We also observed delayed translocation in the presence of prosurvival Bcl-2 family proteins upon depolarization of mitochondria with valinomycin (Figure 5F) or the combination of oligomycin/antimycin A (Figure 5G). Furthermore,,, and Bcl-W also inhibited -dependent translocation in response to mitochondrial depolarization in HaCaT (Figure S4B and S4C) and SKOV-3 cells (Figure S4D). It is interesting that we also observed spontaneous translocation, in the absence of mitochondrial depolarization, upon overexpression of in A549,, SKOV-3, and HaCaT cells, and this was also inhibited through coexpression of Bcl-2 family proteins (Figures S4E S4I). Translocation to Mitochondria Is Enhanced by BH3-Only Proteins or a BH3-Mimetic Because endogenous prosurvival Bcl-2 proteins can be neutralized through expression of BH3-only proteins, we also explored whether the latter could have an impact on translocation to depolarized mitochondria. To investigate this, we transiently overexpressed a panel of BH3-only proteins along with, followed by treatment of cells with. These experiments were carried out in the presence of the poly-caspase inhibitor zvad-fmk to block apoptosis mediated by BH3-only proteins. As Figures 5H and 5I show, coexpression of Bad, Bim, Noxa, or Puma all enhanced translocation of to depolarized mitochondria. Furthermore, treatment of cells with the BH3- only mimetic ABT-737, which enhanced apoptosis through neutralization of endogenous prosurvival Bcl-2 family proteins (Figure S4J), also enhanced translocation in response to treatment (Figure 5J). Similar results were also observed in cells stably expressing YFP- (Figures 5K and S4K). In contrast, we failed to observe significant enhancement of mitochondrial translocation upon overexpression of the unconventional BH3-containing proteins, BNIP3, Nix, or Beclin-1 (Figures S4L S4N). Knockdown of Endogenous Prosurvival Bcl-2 Family Proteins Sensitize toward Depolarization-Induced Translocation to Mitochondria Because the preceding experiments utilized overexpression approaches, we also explored whether knockdown of endogenous Bcl-2 family proteins could modulate translocation to mitochondria. As shown in Figures 6A and 6B, whereas knockdown of individual prosurvival Bcl-2 family proteins sensitized toward apoptosis, this had little effect on -induced translocation to mitochondria (Figures 6C and 6D). However, knockdown of multiple prosurvival Bcl-2 proteins enhanced -induced translocation as well as mitophagy (Figures 6C 6G), suggesting a degree of redundancy in this regard. Furthermore, we also found that knockdown of certain Bcl-2 protein pairs (e.g., /Bcl-2; /) was also sufficient to (C) cells were transfected with the indicated sirna for 48 hr before analysis by immunoblotting. (D and E) cells were transfected with the indicated sirna along with mcherry- cdna ( ng). After 24 hr of treatment (1 mm), mitochondria were immunostained for TOM (D) or HSP6 (E). The percentage of TOM-negative (D) or HSP6-negative (E) cells among mcherry--positive cells was scored. (F and G) Confocal images of cells transfected, treated, and immunostained as in (D) and (E). (H) cells were transfected with a plasmid encoding GFP-tagged shrna targeted against control (scrambled) or against BAX or BAK (5 ng) for 48 hr before analysis by immunoblotting. (I) cells were transfected with plasmids encoding the indicated shrna (5 ng) and mcherry- ( ng) for 48 hr. After 24 hr of treatment (1 mm), mitochondria were immunostained for TOM. TOM-negative cells were counted among mcherry--positive cells. (J) cells were transfected with a plasmid encoding the indicated shrna (5 ng) for 48 hr followed by treatment with daunorubicin (2 mm) for 24 hr. Apoptosis was assessed based on standard morphological criteria. Results shown are representative of at least three independent experiments. Error bars indicate sd of triplicate counts of 1 cells. Statistical significance was assessed by two-tailed paired Student s t test. Asterisks indicate significance: p %.1; p %.1; p %.1. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 457
8 A Ubiquitin TOM 6 hr mcherry- 8 Mitochondrial ubiquitin 6 hr mcherry- % of cells 6 mcherry- mcherry- : - -Bcl-2 - -A1 B p62 TOM 6 hr mcherry- 8 Mitochondrial p62 6 hr 6 mcherry- % of cells mcherry- mcherry- : - -Bcl-2 - -A1 C GFP-LC3 TOM 6 hr mcherry- 8 Mitochondrial GFP-LC3 6 hr 6 mcherry- % of cells mcherry- mcherry- : - -Bcl-2 - -A1 Figure 4. Bcl-2 Family Proteins Block -Dependent Decoration of Mitochondria with Ub, p62, and LC3 (A and B) cells were transfected with mcherry- cdna ( ng) and the indicated -Bcl-2 construct ( ng). Cells, treated with (1 mm) for 6 hr, were coimmunostained for mitochondria (TOM, blue) and Ub (green) (A) or p62 (green) (B). Colocalization between Ub (A) or p62 (B) and mitochondria was analyzed by confocal microscopy and scored among mcherry--positive cells. (legend continued on next page) 458 Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc.
9 enhance -induced translocation and mitochondrial ubiquitination (Figures 6H 6J). In contrast, knockdown of endogenous BH3-only proteins, while protecting against apoptosis induced by cisplatin, Fas, or TRAIL (Figures S5A and S5D), failed to influence -induced translocation of to mitochondria in two different cell lines (Figures S5B and S5C). Bcl-2 Family Members Interact with the / Complex The preceding experiments revealed that members of the Bcl- 2 family could influence translocation of to depolarized mitochondria, thereby having an impact on -dependent events such as decoration of mitochondria with Ub, p62, NBR1, and LC3 and their removal by mitophagy. This strongly suggested that members of the Bcl-2 family are involved at an early stage in the sequence that results from depolarization of mitochondria, stabilization of on mitochondrial outer membranes, and formation of the / complex. One possibility was that Bcl-2 family proteins directly antagonized these events through direct interactions with,, or both. Thus, we performed coimmunoprecipitation experiments with,, and Bcl-W to ask whether these could interact with. As Figure 7A shows, we readily detected an interaction between and all three Bcl-2 family proteins, regardless of whether mitochondria were depolarized or not. We carried out similar immunoprecipitation experiments with and also found that we could detect interactions between the latter and overexpressed Bcl-2 family proteins (Figure 7B). Coexpression of,, and Bcl-2 family proteins also coimmunoprecipitated both and with Bcl-2 proteins (Figure 7C, left panel), although capture was diminished upon treatment with, suggesting that might compete with for binding to Bcl-2 family proteins (Figure 7C, right panel). Reciprocal immunoprecipitation experiments with GFP- suggested that the interaction between and was diminished through coexpression with or (Figure 7D). We also performed immunoprecipitation experiments at endogenous levels of Bcl-2 family expression to confirm that the aforementioned results were not due to promiscuous interactions due to overexpression. As Figure 7E shows, immunoprecipitation of endogenous or coimmunoprecipitated endogenous, which was enhanced upon treatment with. However, we failed to detect direct immunoprecipitation of under the same conditions (Figure 7E), suggesting that the interaction seen under overexpression conditions was nonspecific or indirect via endogenous. Thus, prosurvival Bcl-2 family proteins may interfere with the stable recruitment of to mitochondria, through direct binding to, thereby repressing formation of the / complex and setting a threshold for elimination of impaired mitochondria via mitophagy. DISCUSSION Here, we have shown that members of the Bcl-2 family can regulate mitophagy through influencing the recruitment of to depolarized mitochondria.,, and other members of the prosurvival subset of the Bcl-2 family were found to directly interact with and to impede its stable recruitment to depolarized mitochondria, thereby raising the threshold for clearance of the latter via mitophagy. Conversely, BH3-only proteins, such as Bad, Bim, Puma, and Noxa, as well as a BH3- only mimetic, were capable of accelerating recruitment to impaired mitochondria, most likely through neutralization of endogenous Bcl-2 family proteins. BAX and BAK did not appear to be involved in this process, as knockdown of the latter failed to affect translocation or mitophagic clearance of these organelles. Collectively, these data suggest that, in addition to their role as regulators of mitochondrial outer membrane permeabilization during apoptosis and mitochondrial network dynamics, members of the Bcl-2 family can also regulate the clearance of impaired mitochondria via mitophagy. Because is localized to the cytosol and prosurvival Bcl- 2 proteins are localized predominantly to mitochondrial outer membranes, it would seem counterintuitive that the latter can block translocation of to depolarized mitochondria. However, one way in which this may occur is through Bcl-2 proteins interfering with the stable recruitment of to its mitochondrial receptor(s). This may operate in a manner similar to how has been reported to block recruitment of BAX (which is also cytosolic) to mitochondria, with the latter continuously interacting with mitochondrial membranes but undergoing retrotranslocation to the cytosol by (Edlich et al., 11). In a similar vein, may be continuously retrotranslocated to the cytosol via interactions with mitochondria-localized Bcl-2 family proteins (Figure S5E). Alternatively, because there is evidence that the initial pool of that is recruited to depolarized mitochondria may seed a feed-forward amplification loop to promote further recruitment via - interactions (Lazarou et al., 13), Bcl-2 family members may block this feed-forward amplification loop by binding to the initial pool of mitochondria-localized, blocking further recruitment (Figure S5F). The removal of dysfunctional mitochondria is required to maintain a healthy mitochondrial network, and this process must be restricted to impaired mitochondria. Prosurvival Bcl- 2 family proteins may contribute to setting a threshold for ongoing -mediated removal from the mitochondrial network, thereby protecting healthy mitochondria from -mediated ubiquitination and removal. However, the role (C) cells were transfected with plasmids encoding GFP-LC3 ( ng), mcherry- ( ng), and the indicated -Bcl-2 construct ( ng). Cells were treated as in (A) and (B) and immunostained for TOM (blue). Colocalization between GFP-LC3 and mitochondria was scored among mcherry-positive cells. Results shown are representative of at least three independent experiments. Error bars indicate SD of triplicate counts of 1 cells. Statistical significance was assessed by two-tailed paired Student s t test. Asterisks indicate significance: p %.1; p %.1; p %.1. See also Figure S3. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 459
10 A YFP- TOM 2 hr B Mitochondrial 6 % of cells (hr) : C % of cells 8 6 Mitochondrial 2 hr : - (ng) : D H J Mitochondrial % of cells Bad mcherry- : - mcherry- Mitochondrial -Bcl-2 ABT-737 ( M) : hr - -A1 E TOM Mitochondrial Mitochondrial YFP- : - (ng) : (ng) : (ng) : ABT-737 (5 M) : - ABT-737 ( M) : hr F Mitochondrial 6 Valinomycin G Oligomycin/Antimycin A 6 hr 2 hr Valinomycin : - I Mitochondrial : - Bad (ng) : Bim 6 Mitochondrial K 6 Bad : - Bim (ng) : YFP- ABT ( M) : Mitochondrial Mitochondrial Oligomycin Antimycin A : Mitochondrial Mitochondrial 6 - : - Noxa (ng) : Puma 6 Noxa : - Puma (ng) : Mitochondrial -YFP- ABT (5 M) : - ABT-737 ( M) : Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. (legend on next page)
11 of Bcl-2 family members in this process may be especially important under stress conditions where mitochondrial damage is acute. Although we found evidence of redundancy among members of the Bcl-2 family with respect to the regulation of mitophagy, individual Bcl-2 family members may play a greater or lesser role in regulating activation in particular cell types, depending on their relative expression levels in particular tissues. Redundancy of function is also seen within the Bcl-2 family in the context of cell death control, with relative tissue expression levels playing a role in dictating the relative importance of particular Bcl-2 family members in specific tissues. In the context of mitophagy, it is relevant to note that Bcl-W has been implicated to play an important role in the maintenance of mitochondrial network function and integrity in neurons (Liu and Shio, 8; Courchesne et al., 11). Furthermore, has also been implicated in the regulation of mitochondrial biomass in neurons (Berman et al., 9), which may be related to the ability of the latter to regulate mitochondrial turnover via mitophagy. It is also noteworthy that previous studies have suggested that Bcl-2 family members interact with or (Chen et al., 1; Johnson et al., 12; Arena et al., 13). has been shown to monoubiquitinate Bcl-2, enhancing Bcl-2-Beclin-1 interaction (Chen et al., 1). However, given that we could not find any requirement for Beclin-1 in mitophagy, the significance of the latter observation for mitophagy is unclear. has also been reported to phosphorylate in response to mitochondrial depolarization (Arena et al., 13); however, the implications of this phosphorylation event for mitophagy are currently unclear. Accumulating evidence suggests that the role of Bcl-2 family proteins in mitochondrial as well as cellular homeostasis is much broader than previously appreciated (reviewed in Autret and Martin, 9). It is now well established that members of the extended Bcl-2 family play a central role in dictating the onset of apoptosis through regulating the permeability of the mitochondrial outer membrane (Chipuk et al., 1). A network of interactions between BH3-only proteins, BAX and BAK, and the prosurvival members of the Bcl-2 family set a threshold for BAX/BAK oligomerization within the mitochondrial outer membrane, effectively dictating the integrity of the entire mitochondrial network. In addition to this, Bcl-2 family members have also been repeatedly implicated in mitochondrial fission and fusion dynamics (reviewed in Martinou and Youle, 11; Delivani et al., 6; Sheridan et al., 8). In this context, it is relevant to note that the ability to quarantine depolarized mitochondria from the remainder of the mitochondrial network, via increased fission or decreased fusion, may be an important feature of mitophagy (Twig et al., 8; Gomes et al., 11; Rambold et al., 11). has also been implicated as a regulator of mitochondrial respiration (Perciavalle et al., 12). The atypical BH3-only proteins BNIP3 and Nix can regulate autophagy as well as mitophagy in erythrocytes (reviewed in Zhang and Ney, 9). Furthermore, several Bcl-2 family members have also been implicated as regulators of macroautophagy, through their ability to bind to Beclin-1 (Pattingre et al., 5; Elgendy et al., 11). It is striking that many of the processes with which members of the Bcl-2 family have been linked revolve around mitochondrial function and integrity. This suggests that Bcl-2 proteins may function as global regulators of mitochondrial homeostasis in response to cell stress and can regulate several aspects of mitochondrial function, including: mitochondrial permeability (via BAX/BAK pore formation), mitochondrial connectivity (via effects on fission/fusion dynamics), mitochondrial turnover (via mitophagy), and ATP synthesis (via effects on the respiratory chain). It is also highly compelling that perturbation of many of these processes individually can affect the others. For example, inhibition of autophagy is well known to sensitize toward apoptosis; conversely, inhibition of apoptosis increases the likelihood of autophagy (reviewed by Maiuri et al., 7). Similarly, mitochondrial networks fragment during apoptosis, and enforced Figure 5. Translocation of to Depolarized Mitochondria Is Antagonized by Prosurvival Bcl-2 Proteins and Accelerated by BH3-Only Proteins (A) Confocal images of -YFP- cells transfected with or cdna (5 ng). Cells were treated with (5 mm) for 2 hr and coimmunostained for or (, blue) and TOM (red). (B) cells were transfected with plasmids encoding mcherry- ( ng) and ( ng). Cells were treated with (1 mm) and immunostained for mitochondria (TOM). Colocalization between and mitochondria was scored among mcherry--positive cells. (C) cells were transfected with mcherry- cdna ( ng) and the indicated amount of plasmid. Cells were treated, immunostained, and analyzed as in (B). (D) cells were transfected with mcherry- cdna ( ng) along with the indicated -Bcl-2 construct ( ng). Cells were treated, immunostained, and analyzed as in (B). (E) -YFP- cells, transfected with the indicated amount of -Bcl-2 construct, were treated and immunostained as in (A). Cells presenting YFP- colocalized with mitochondria were scored. (F and G) cells were transfected with mcherry- cdna ( ng) and the indicated -Bcl-2 construct (8 ng). Cells, treated with valinomycin (1 nm) for 6 hr (F) or with oligomycin (5 nm) and antimycin A (5 nm) for 2 hr (G), were immunostained for TOM. Colocalization between and mitochondria was scored among mcherry--positive cells. (H) Confocal images of cells transfected with plasmids encoding mcherry- ( ng) and Bad (1 ng) for 8 hr in the presence of zvad-fmk ( mm). Cells were treated with (5 mm) for 1.5 hr and immunostained for mitochondria (TOM, green). (I) cells were transfected with mcherry- cdna ( ng), along with the indicated amount of BH3-only construct for 8 hr in the presence of zvad-fmk ( mm). Cells were treated and immunostained as in (H), and colocalization between and mitochondria was scored among mcherry--positive cells. (J and K) cells transfected with mcherry- cdna ( ng) (J) or -YFP- cells (K) were pretreated with ABT-737 for 1 hr and treated with for 45 min. Mitochondria were immunostained for TOM, and cells presenting associated with mitochondria were scored. Results shown are representative of at least three independent experiments. Error bars indicate SD of triplicate counts of 1 cells. Statistical significance was assessed by two-tailed paired Student s t test. Asterisks indicate significance: p %.1; p %.1; p %.1. See also Figure S4. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 461
12 A E sirna : 3 kda 3 kda 3 kda - Control Bcl-2 Bcl-W Ubiquitin Bcl-2 Bcl-W Bcl-2 Bcl-W Actin B C Cell death (%) Mitochondrial TOM 6 αfas αfas : - sirna : Control Bcl-2 Bcl-W Bcl-2 Bcl-W Mitochondrial : - sirna : - - Control Bcl-2 Bcl-W Bcl-2 Bcl-W Cell death (%) F 8 6 Daunorubicin Daunorubicin : - sirna : - - D Mitochondrial 6 Control Bcl-2 Bcl-W Bcl-2 Bcl-W HEK293T Mitochondrial : - sirna : - - Control 8 Bcl-2 Bcl-W Bcl-2 Bcl-W TOM No sirna Control sirna TOM (-) cells (%) 6 (hr) : Bcl-2 Bcl-W sirna sirna G TOM (-) cells (%) TOM 6 (μm) : H Control sirna : Bcl-2 sirna : sirna : sirna : Bcl-W sirna : kda 3 kda Bcl-2 I % of cells 6 Mitochondrial J % of cells Mitochondrial ubiquitin Bcl-W Actin : - Control sirna : Bcl-2 sirna : sirna : sirna : Bcl-W sirna : : - Control sirna : Bcl-2 sirna : sirna : sirna : Bcl-W sirna : (legend on next page) 462 Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc.
13 mitochondrial fusion has been reported to delay apoptosis (reviewed in Autret and Martin, 9). Furthermore, mitochondrial networks undergo fusion during macroautophagy (Gomes et al., 11) and mitochondrial fission has been shown to facilitate mitophagy (Twig et al., 8; Gomes et al., 11; Rambold et al., 11). It is perhaps highly significant that Bcl-2 family proteins have been implicated as regulators of all of the processes described earlier, suggesting an overarching role for members of this family as regulators of mitochondrial fitness. Thus, Bcl-2 family proteins may police mitochondrial network integrity at a global level, coordinating the isolation (via increased fission or decreased fusion) of impaired mitochondria and their removal (via mitophagy) to minimize the likelihood of complete depolarization of mitochondria and consequent escape of cytochrome c, which would have disastrous consequences for the cell (i.e., apoptosis). On the other hand, in response to severe stress, other members of the Bcl-2 family can directly depolarize mitochondria, via BAX/BAK activation, fragment the mitochondrial network, and permit the escape of mitochondrial intermembrane space proteins to facilitate the elimination of the cell via apoptosis. In conclusion, the ability of Bcl-2 proteins to regulate diverse aspects of mitochondrial function, including the removal of impaired mitochondria via /-dependent mitophagy, as we have shown here, suggests that the Bcl-2 family act as global regulators of mitochondrial network homeostasis, rather than simply as regulators of apoptosis. EXPERIMENTAL PROCEDURES Immunostaining Cells were grown on coverslips before transfection and treatment with, valinomycin, or oligomycin and antimycin A. Cells were washed in PBS, fixed with 3% paraformaldehyde in PBS for 1 min, permeabilized with.15% Triton X-1 in PBS for 15 min, and blocked in 2% BSA in PBS for 3 min. Primary antibodies were used at 1: for 1 hr at room temperature, followed by secondary antibodies at 1:1, for 1 hr at room temperature. Final washing included incubation with 5 nm Hoechst (Sigma-Aldrich) for 1 min. Cells were mounted with Slow Fade (Molecular Probes), and observed on a laser scanning confocal microscope (Olympus FV1) using a 488 nm Argon laser (green fluorescence), a 543 nm HeNe laser (red fluorescence), and a 5 nm LD laser. Confocal images were acquired with a UPlanSApo 63/1.35 NA oil objective lens (zoom, 2.5) using Fluoview 1 V.1 application software. Quantification of mitophagy, translocation, mitochondrial Ub, p62 or GFP- LC3 was estimated by counting a minimum of cells for each treatment. Western Blot Analysis Following treatment or transfection with sirna or small hairpin RNA (shrna) (48 hr), whole-cell lysates were prepared with 1 ml of SDS-PAGE sample buffer. Samples were boiled for 1 min, ran on 1% or 12% SDS- PAGE gels, and transferred on to nitrocellulose membranes. After blocking and incubation with primary and secondary antibodies, immunoreactions were visualized with SuperSignal West Pico (Thermo Scientific) and exposure to autoradiography films. Immunoprecipitation Assays HEK293T cells, transfected for 24 hr, were treated with and lysed at 1 7 cells per milliliter with 1% NP lysis buffer (5 mm Tris-HCl [ph 7.6], 15 mm NaCl, 1% Nonidet P- for immunoprecipitations) or.2% NP lysis buffer ( mm HEPES [ph 7.9], 1 mm dithiothreitol [DTT],.2% Nonidet P- for GFP immunoprecipitations) containing 1 mm phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, 2 mg/ml aprotinin, and 1 mm MG132. Lysates were incubated for 15 min under rotation at 4 C. Following centrifugation at 15, 3 g for 15 min at 4 C, clarified lysates were precleared with ml of agarose-coupled protein A/G (Santa Cruz Biotechnology) and 1 mg of mouse immunoglobulin G (Sigma-Aldrich). Precleared lysates were subjected to immunoprecipitation using 2 mg of the appropriate antibody and 3 ml of agarose-coupled protein A/G at 4 C for 4 hr. Complexes were washed three times in lysis buffer. Immunoprecipitates were then analyzed by immunoblotting. For endogenous immunoprecipitation, HEK293T cells were lysed at cells per milliliter with 1% NP lysis buffer containing 1 mm EDTA, 1 mm DTT, and 1% glycerol. Immunoprecipitation was performed using 4 mg of the appropriate antibody preconjugated to ml of agarose-coupled protein A/G at 4 C for 4 hr. SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at /j.molcel ACKNOWLEDGMENTS We gratefully thank Dr. Richard Youle for provision of mcherry- and YFP- plasmids; Dr. Stephen Tait for provision of cells stably expressing YFP-; Dr. Dennis J. Selkoe for provision of - plasmid; and Dr. Maria Soengas for provision of shrna plasmids targeted against BAX and BAK. The S.J.M. laboratory is supported by Principal Investigator (8/IN.1/B31) and Strategic Research Cluster (7/SRC/B1144) grants from Science Foundation Ireland. S.J.M. is a Science Foundation Ireland Principal Investigator. Figure 6. Knockdown of Bcl-2 Family Proteins Enhances Recruitment to Depolarized Mitochondria and Mitophagy (A) cells were transfected with the indicated sirna for 48 hr before analysis by immunoblotting. (B) cells transfected as in (A) were treated with anti-fas antibody ( ng/ml) for 1 hr or daunorubicin (1 mm) for 12 hr. Apoptosis was assessed based on standard morphological criteria. (C) cells were transfected with the indicated sirna along with cdna (1 ng) for 48 hr. Cells, treated with (4 mm) for 1 hr, were coimmunostained for mitochondria (TOM) and. Colocalization between and mitochondria was scored. (D) HEK293T cells were transfected as in (C) and treated with (1 mm) for 45 min. Cells were processed and analyzed as in (C). (E) Confocal analysis of cells transfected and treated as in (C). Cells were coimmunostained for (green), Ub (red), and TOM (blue). (F and G) cells, transfected with the indicated sirna along with cdna (1 ng) for 48 hr, were treated with (4 mm) for the indicated period of time (F) or with the indicated dose of for 1 hr (G). Following coimmunostaining for mitochondria (TOM) and, TOM-negative cells were scored among -positive cells. (H) cells were transfected with the indicated sirna for 48 hr before analysis by immunoblotting. (I and J) cells were transfected with the indicated sirna along with cdna (1 ng) for 48 hr. Cells were treated with (4 mm) for 45 min before staining for TOM and (I) or for TOM,, and Ub (J). Cells presenting (I) or Ub (J) colocalized with mitochondria were scored. Results shown are representative of at least three independent experiments. Error bars indicate SD of triplicate counts of 1 cells. Statistical significance was assessed by two-tailed paired Student s t test. Asterisks indicate significance: p %.1; p %.1; p %.1. See also Figure S5. Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 463
14 A B C YFP- : 8 kda 3 kda Inputs (2.5%) (Bcl-2) IP Inputs (2.5%) (Bcl-2) IP : 3 kda Inputs (2.5%) (Bcl-2) IP YFP- : : 8 kda YFP- : 8 kda 3 kda Inputs (2.5%) 4 hr (Bcl-2) IP : 3 kda Inputs (2.5%) Inputs (2.5%) 4 hr (Bcl-2) IP 4 hr (Bcl-2) IP YFP- : : 8 kda Figure 7. Bcl-2 Family Proteins Directly Interact with (A) HEK293T cells were transfected with YFP- cdna (1 mg) along with (3 mg), (5 mg), or (5 mg) plasmids. Cells were left untreated (left) or treated with (5 mm) for 4 hr (right).,, and Bcl-W were immunoprecipitated (IP) with an anti- antibody followed by immunoblotting for or,, or Bcl-W (). (B) HEK293T cells were transfected with cdna (1 mg) along with (3 mg), - (5 mg), or (5 mg) plasmids. Immunoprecipitations were performed as in (A). (C) HEK293T cells were transfected with plasmids encoding YFP- (1 mg) and (1 mg) along with (3 mg), (5 mg), or (5 mg) cdna. Immunoprecipitations were performed as in (A). (D) HEK293T cells were transfected with plasmids encoding YFP- (.5 mg) and (.5 mg) along with the indicated amount of or cdna. was immunoprecipitated with an anti-gfp antibody followed by immunoblotting for,, or () and. (E) HEK293T cells were left untreated or treated with (5 mm) for 5 hr. Endogenous (top) and (bottom) were immunoprecipitated. Immunoprecipitates were analyzed by immunoblotting for, and, or. IgG, immunoglobulin G. Results shown are representative of at least three independent experiments. 8 kda 8 kda 3 kda 3 kda D ( g) : ( g) : YFP- : : Inputs (2.5%) E Inputs (4%) Rabbit IgG IP : kda 8 kda 3 kda GFP () IP ( g) : ( g) : YFP- : : - Inputs (4%) Mouse IgG IP : kda 3 kda 8 kda 464 Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc.
15 Received: March 27, 13 Revised: March 24, 14 Accepted: May, 14 Published: July 3, 14 REFERENCES Arena, G., Gelmetti, V., Torosantucci, L., Vignone, D., Lamorte, G., De Rosa, P., Cilia, E., Jonas, E.A., and Valente, E.M. (13). protects against cell death induced by mitochondrial depolarization, by phosphorylating BclxL and impairing its pro-apoptotic cleavage. Cell Death Differ., Ashrafi, G., and Schwarz, T.L. (13). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ., Autret, A., and Martin, S.J. (9). Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol. Cell 36, Berman, S.B., Chen, Y.B., Qi, B., McCaffery, J.M., Rucker, E.B., 3rd, Goebbels, S., Nave, K.A., Arnold, B.A., Jonas, E.A., Pineda, F.J., and Hardwick, J.M. (9). Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J. Cell Biol. 184, Chen, Y., and Klionsky, D.J. (11). The regulation of autophagy unanswered questions. J. Cell Sci. 124, Chen, D., Gao, F., Li, B., Wang, H., Xu, Y., Zhu, C., and Wang, G. (1). mono-ubiquitinates Bcl-2 and regulates autophagy. J. Biol. Chem. 285, Chipuk, J.E., Bouchier-Hayes, L., and Green, D.R. (6). Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 13, Chipuk, J.E., Moldoveanu, T., Llambi, F., Parsons, M.J., and Green, D.R. (1). The BCL-2 family reunion. Mol. Cell 37, Clark, I.E., Dodson, M.W., Jiang, C., Cao, J.H., Huh, J.R., Seol, J.H., Yoo, S.J., Hay, B.A., and Guo, M. (6). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, Cleland, M.M., Norris, K.L., Karbowski, M., Wang, C., Suen, D.F., Jiao, S., George, N.M., Luo, X., Li, Z., and Youle, R.J. (11). Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 18, Courchesne, S.L., Karch, C., Pazyra-Murphy, M.F., and Segal, R.A. (11). Sensory neuropathy attributable to loss of Bcl-w. J. Neurosci. 31, Delivani, P., Adrain, C., Taylor, R.C., Duriez, P.J., and Martin, S.J. (6). Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21, Edlich, F., Banerjee, S., Suzuki, M., Cleland, M.M., Arnoult, D., Wang, C., Neutzner, A., Tjandra, N., and Youle, R.J. (11). Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, Elgendy, M., Sheridan, C., Brumatti, G., and Martin, S.J. (11). Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol. Cell 42, Erlich, S., Mizrachy, L., Segev, O., Lindenboim, L., Zmira, O., Adi-Harel, S., Hirsch, J.A., Stein, R., and Pinkas-Kramarski, R. (7). Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 3, Funderburk, S.F., Wang, Q.J., and Yue, Z. (1). The Beclin 1-VPS34 complex at the crossroads of autophagy and beyond. Trends Cell Biol., Geisler, S., Holmstrom, K.M., Skujat, D., Fiesel, F.C., Rothfuss, O.C., Kahle, P.J., and Springer, W. (1). /-mediated mitophagy is dependent on VDAC1 and p62/sqstm1. Nat. Cell Biol. 12, Gomes, L.C., Di Benedetto, G., and Scorrano, L. (11). During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, Hoppins, S., Edlich, F., Cleland, M.M., Banerjee, S., McCaffery, J.M., Youle, R.J., and Nunnari, J. (11). The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol. Cell 41, Johnson, B.N., Berger, A.K., Cortese, G.P., and Lavoie, M.J. (12). The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc. Natl. Acad. Sci. USA 19, Kahle, P.J., and Haass, C. (4). How does parkin ligate ubiquitin to son s disease? EMBO Rep. 5, Karbowski, M., Norris, K.L., Cleland, M.M., Jeong, S.Y., and Youle, R.J. (6). Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y., and Shimizu, N. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, Kondapalli, C., Kazlauskaite, A., Zhang, N., Woodroof, H.I., Campbell, D.G., Gourlay, R., Burchell, L., Walden, H., Macartney, T.J., Deak, M., et al. (12). is activated by mitochondrial membrane potential depolarization and stimulates E3 ligase activity by phosphorylating Serine 65. Open Biol. 2, 18. Lazarou, M., Narendra, D.P., Jin, S.M., Tekle, E., Banerjee, S., and Youle, R.J. (13). drives self-association and HECT-like E3 activity upstream of mitochondrial binding. J. Cell Biol., Lee, J.Y., Nagano, Y., Taylor, J.P., Lim, K.L., and Yao, T.P. (1). Diseasecausing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell Biol. 189, Liang, X.H., Jackson, S., Seaman, M., Brown, K., Kempkes, B., Hibshoosh, H., and Levine, B. (1999). Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 2, Liu, Q.A., and Shio, H. (8). Mitochondrial morphogenesis, dendrite development, and synapse formation in cerebellum require both Bcl-w and the glutamate receptor delta2. PLoS Genet. 4, e197. Maiuri, M.C., Zalckvar, E., Kimchi, A., and Kroemer, G. (7). Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, Martinou, J.C., and Youle, R.J. (11). Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, Matsuda, N., Sato, S., Shiba, K., Okatsu, K., Saisho, K., Gautier, C.A., Sou, Y.S., Saiki, S., Kawajiri, S., Sato, F., et al. (1). stabilized by mitochondrial depolarization recruits to damaged mitochondria and activates latent for mitophagy. J. Cell Biol. 189, Mizushima, N. (7). Autophagy: process and function. Genes Dev. 21, Narendra, D., Tanaka, A., Suen, D.F., and Youle, R.J. (8). is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, Narendra, D.P., Jin, S.M., Tanaka, A., Suen, D.F., Gautier, C.A., Shen, J., Cookson, M.R., and Youle, R.J. (1a). is selectively stabilized on impaired mitochondria to activate. PLoS Biol. 8, e1298. Narendra, D., Kane, L.A., Hauser, D.N., Fearnley, I.M., and Youle, R.J. (1b). p62/sqstm1 is required for -induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6, Okatsu, K., Saisho, K., Shimanuki, M., Nakada, K., Shitara, H., Sou, Y.S., Kimura, M., Sato, S., Hattori, N., Komatsu, M., et al. (1). p62/sqstm1 cooperates with for perinuclear clustering of depolarized mitochondria. Genes Cells 15, Park, J., Lee, S.B., Lee, S., Kim, Y., Song, S., Kim, S., Bae, E., Kim, J., Shong, M., Kim, J.M., and Chung, J. (6). Mitochondrial dysfunction in Drosophila mutants is complemented by parkin. Nature 441, Pattingre, S., Tassa, A., Qu, X., Garuti, R., Liang, X.H., Mizushima, N., Packer, M., Schneider, M.D., and Levine, B. (5). Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122, Perciavalle, R.M., Stewart, D.P., Koss, B., Lynch, J., Milasta, S., Bathina, M., Temirov, J., Cleland, M.M., Pelletier, S., Schuetz, J.D., et al. (12). Antiapoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat. Cell Biol. 14, Molecular Cell 55, , August 7, 14 ª14 Elsevier Inc. 465
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
TNFα 18hr. Control. CHX 18hr. TNFα+ CHX 18hr. TNFα: 18 18hr (KDa) PARP. Cleaved. Cleaved. Cleaved. Caspase3. Pellino3 shrna. Control shrna.
 Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
Survival ( %) a. TNFα 18hr b. Control sirna Pellino3 sirna TNFα: 18 18hr c. Control shrna Pellino3 shrna Caspase3 Actin Control d. Control shrna Pellino3 shrna *** 100 80 60 CHX 18hr 40 TNFα+ CHX 18hr
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
 http://cwp.embo.org/w09-13/index.html Roads to Ruin is s o t p o ap healthy cell autophagic cell death ne cr os is Thanks to Seamus Martin! Evolution of apoptosis signalling cascades Inhibitor Adopted
http://cwp.embo.org/w09-13/index.html Roads to Ruin is s o t p o ap healthy cell autophagic cell death ne cr os is Thanks to Seamus Martin! Evolution of apoptosis signalling cascades Inhibitor Adopted
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Supplemental Figures S1 S5
 Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells Ines Witte (1), Sebastian Altenhöfer (1), Petra Wilgenbus (1), Julianna Amort (1), Albrecht M. Clement
Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells Ines Witte (1), Sebastian Altenhöfer (1), Petra Wilgenbus (1), Julianna Amort (1), Albrecht M. Clement
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Apoptosis EXTRA REQUIREMETS
 Apoptosis Introduction (SLIDE 1) Organisms develop from a single cell, and in doing so an anatomy has to be created. This process not only involves the creation of new cells, but also the removal of cells
Apoptosis Introduction (SLIDE 1) Organisms develop from a single cell, and in doing so an anatomy has to be created. This process not only involves the creation of new cells, but also the removal of cells
Role of Mitochondrial Remodeling in Programmed Cell Death in
 Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
FSC-W FSC-H CD4 CD62-L
 Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
Supplementary Fig. 1 a SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H CD4 CD62-L b SSC-A FSC-A FSC-W FSC-A FSC-A 7-AAD FSC-A CD4 IL-9 CD4 c SSC-A FSC-A FSC-W FSC-H SSC-W SSC-H 7-AAD KI67 Annexin-V 7-AAD d I L -5
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb97 P ( μm, hours) 1 2 4 P DMSO Figure S1 unningham et al. E 97 65 27 MFN1 GFP-Parkin Opa1 ctin GPDH HEK293 GFP-Parkin 19 115 97 65 27 Mitochondrial Fraction SH-SY5Y GFP-Parkin Mito DMSO Mito
DOI:.38/ncb97 P ( μm, hours) 1 2 4 P DMSO Figure S1 unningham et al. E 97 65 27 MFN1 GFP-Parkin Opa1 ctin GPDH HEK293 GFP-Parkin 19 115 97 65 27 Mitochondrial Fraction SH-SY5Y GFP-Parkin Mito DMSO Mito
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX.
 Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Supplementary Figure 1. AnnexinV FITC and Sytox orange staining in wild type, Nlrp3 /, ASC / and casp1/11 / TEC treated with TNF /CHX. Phase contrast and widefield fluorescence microscopy (20x magnification).
Embedded together: The life and death consequences of interaction of the Bcl-2 family with membranes
 DOI 10.1007/s10495-007-0746-4 Embedded together: The life and death consequences of interaction of the Bcl-2 family with membranes Brian Leber Jialing Lin David W. Andrews Published online: 15 February
DOI 10.1007/s10495-007-0746-4 Embedded together: The life and death consequences of interaction of the Bcl-2 family with membranes Brian Leber Jialing Lin David W. Andrews Published online: 15 February
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
APOPTOSIS REGULATOR BCL-2
 Papers on Anthropology Apoptosis regulator XXII, 2013, BCL-2 pp. 63 67 P. Hussar, M. Žuravskaja, M. Kärner APOPTOSIS REGULATOR BCL-2 Piret Hussar 1, Maria Žuravskaja 2, Martin Kärner 2 1 Institute of Anatomy,
Papers on Anthropology Apoptosis regulator XXII, 2013, BCL-2 pp. 63 67 P. Hussar, M. Žuravskaja, M. Kärner APOPTOSIS REGULATOR BCL-2 Piret Hussar 1, Maria Žuravskaja 2, Martin Kärner 2 1 Institute of Anatomy,
Apoptosis: Death Comes for the Cell
 Apoptosis: Death Comes for the Cell Joe W. Ramos joeramos@hawaii.edu From Ingmar Bergman s The Seventh Seal 1 2 Mutations in proteins that regulate cell proliferation, survival and death can contribute
Apoptosis: Death Comes for the Cell Joe W. Ramos joeramos@hawaii.edu From Ingmar Bergman s The Seventh Seal 1 2 Mutations in proteins that regulate cell proliferation, survival and death can contribute
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Drosophila Apoptosis and the Regulation of the Caspase Cascade
 Drosophila Apoptosis and the Regulation of the Caspase Cascade Kate Stafford March 18, 2005 Abstract The caspase cascade in Drosophila is controlled primarily by DIAP1 (Drosophila inhibitor of apoptosis),
Drosophila Apoptosis and the Regulation of the Caspase Cascade Kate Stafford March 18, 2005 Abstract The caspase cascade in Drosophila is controlled primarily by DIAP1 (Drosophila inhibitor of apoptosis),
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
SUPPLEMENTAL MATERIAL Figure S1. Mitochondrial morphology in Fis1-null, Mff-null and Fis1/Mff-null MEF cells. (A) Western blotting of lysates from Fis1-null, Mff-null and Fis1/Mff-null cells. Lysates were
Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis
 Molecular Biology of the Cell Vol. 15, 5001 5011, November 2004 Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis Yang-ja Lee,* Seon-Yong Jeong,* Mariusz
Molecular Biology of the Cell Vol. 15, 5001 5011, November 2004 Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis Yang-ja Lee,* Seon-Yong Jeong,* Mariusz
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Enterovirus 71 2B induces cell apoptosis by directly inducing the conformational activation of the pro-apoptotic protein Bax
 JVI Accepted Manuscript Posted Online 24 August 2016 J. Virol. doi:10.1128/jvi.01499-16 Copyright 2016, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
JVI Accepted Manuscript Posted Online 24 August 2016 J. Virol. doi:10.1128/jvi.01499-16 Copyright 2016, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
JNK1-Mediated Phosphorylation of Bcl-2 Regulates Starvation-Induced Autophagy
 Article JNK1-Mediated Phosphorylation of Bcl-2 Regulates Starvation-Induced Autophagy Yongjie Wei, 1,2 Sophie Pattingre, 4,5 Sangita Sinha, 2 Michael Bassik, 6,7,8 and Beth Levine 1,2,3, * 1 Howard Hughes
Article JNK1-Mediated Phosphorylation of Bcl-2 Regulates Starvation-Induced Autophagy Yongjie Wei, 1,2 Sophie Pattingre, 4,5 Sangita Sinha, 2 Michael Bassik, 6,7,8 and Beth Levine 1,2,3, * 1 Howard Hughes
Biochimica et Biophysica Acta
 Biochimica et Biophysica Acta 1832 (2013) 453 474 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis PSAP induces a unique
Biochimica et Biophysica Acta 1832 (2013) 453 474 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis PSAP induces a unique
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplementary material
 Supplementary material Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins Vladimir V. Rogov 1,*, Hironori Suzuki 2,3,*, Mija Marinković 4, Verena Lang
Supplementary material Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins Vladimir V. Rogov 1,*, Hironori Suzuki 2,3,*, Mija Marinković 4, Verena Lang
Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain
 SUPPLEMENTAL INFORMATION Supplemental Figure Legends Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain mitochondrial NAD +. A) Extracellular tryptophan, even at 5 µm,
SUPPLEMENTAL INFORMATION Supplemental Figure Legends Figure S1: Extracellular nicotinic acid, but not tryptophan, is sufficient to maintain mitochondrial NAD +. A) Extracellular tryptophan, even at 5 µm,
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1
 Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization
 Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
09/30/2017. Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center
 09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
09/30/2017 Kyu-Sun Lee, Ph.D. Metabolism & Neurophysiology Research Group Hazard Monitoring BNT Res Center Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs
Supporting Information
 Supporting Information Wang et al. 10.1073/pnas.0804871105 SI Materials and Methods Cell Culture and Transfection. Human neuroblastoma M17 cells were grown as described before (1). Transfection was performed
Supporting Information Wang et al. 10.1073/pnas.0804871105 SI Materials and Methods Cell Culture and Transfection. Human neuroblastoma M17 cells were grown as described before (1). Transfection was performed
Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control
 Article Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control Gian-Luca McLelland 1, Vincent Soubannier 2, Carol X Chen 1, Heidi M McBride 2,** & Edward
Article Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control Gian-Luca McLelland 1, Vincent Soubannier 2, Carol X Chen 1, Heidi M McBride 2,** & Edward
Modeling a Snap-Action, Variable-Delay Switch Controlling Extrinsic Cell Death
 Modeling a Snap-Action, Variable-Delay Switch Controlling Extrinsic Cell Death John G. Albeck 1[, John M. Burke 1,2[, Sabrina L. Spencer 1,3, Douglas A. Lauffenburger 2,3, Peter K. Sorger 1,2* PLoS BIOLOGY
Modeling a Snap-Action, Variable-Delay Switch Controlling Extrinsic Cell Death John G. Albeck 1[, John M. Burke 1,2[, Sabrina L. Spencer 1,3, Douglas A. Lauffenburger 2,3, Peter K. Sorger 1,2* PLoS BIOLOGY
Richik N. Ghosh, Linnette Grove, and Oleg Lapets ASSAY and Drug Development Technologies 2004, 2:
 1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
NucView TM 488 Caspase-3 Assay Kit for Live Cells
 NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
BECN1 is involved in the initiation of mitophagy
 Autophagy ISSN: 1554-8627 (Print) 1554-8635 (Online) Journal homepage: https://www.tandfonline.com/loi/kaup20 BECN1 is involved in the initiation of mitophagy Vinay Choubey, Michal Cagalinec, Joanna Liiv,
Autophagy ISSN: 1554-8627 (Print) 1554-8635 (Online) Journal homepage: https://www.tandfonline.com/loi/kaup20 BECN1 is involved in the initiation of mitophagy Vinay Choubey, Michal Cagalinec, Joanna Liiv,
Reconstructing Mitochondrial Evolution?? Morphological Diversity. Mitochondrial Diversity??? What is your definition of a mitochondrion??
 Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial
Bio 3411, Fall 2006, Lecture 19-Cell Death.
 Types of Cell Death Questions : Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can
Types of Cell Death Questions : Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr
 The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
Article. PINK1 Triggers Autocatalytic Activation of Parkin to Specify Cell Fate Decisions
 Current Biology 24, 1854 1865, August 18, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2014.07.014 PINK1 Triggers Autocatalytic Activation of Parkin to Specify Cell Fate
Current Biology 24, 1854 1865, August 18, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2014.07.014 PINK1 Triggers Autocatalytic Activation of Parkin to Specify Cell Fate
MITOCHONDRIAL MARKERS
 1 MITOCHONDRIAL MARKERS www.ptglab.com 2 INTRODUCTION Mitochondria are important cellular organelles that maintain cellular energy balance, contain key regulators of cell death processes, and play a significant
1 MITOCHONDRIAL MARKERS www.ptglab.com 2 INTRODUCTION Mitochondria are important cellular organelles that maintain cellular energy balance, contain key regulators of cell death processes, and play a significant
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
Plant mitochondrial dynamics
 Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Apoptosis in Mammalian Cells
 Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
Apoptosis in Mammalian Cells 7.16 2-10-05 Apoptosis is an important factor in many human diseases Cancer malignant cells evade death by suppressing apoptosis (too little apoptosis) Stroke damaged neurons
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
Yeast Genome-wide Screens to Ascertain the Genetic Landscape for Barth Syndrome. Christopher R. McMaster, PhD Dalhousie University
 Yeast Genome-wide Screens to Ascertain the Genetic Landscape for Barth Syndrome Christopher R. McMaster, PhD Dalhousie University Using Systematic Genetics to Identify Modifies Genes that Affect Fitness
Yeast Genome-wide Screens to Ascertain the Genetic Landscape for Barth Syndrome Christopher R. McMaster, PhD Dalhousie University Using Systematic Genetics to Identify Modifies Genes that Affect Fitness
Mitochondrial dynamics in ischemia and reperfusion
 Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
What are mitochondria?
 What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
What are mitochondria? What are mitochondria? An intracellular organelle. There are 100 to 1000s of mitochondria/cell. Most mitochondria come from the mother. Mitochondria have their own DNA Mitochondria
IAPs. Structure of BIR2 Zn finger domain from XIAP
 IAPs The only known endogenous caspase inhibitors are members of a second family of proteins identified by apoptosis research, the IAP (inhibitor of apoptosis proteins) family. IAPs were originally described
IAPs The only known endogenous caspase inhibitors are members of a second family of proteins identified by apoptosis research, the IAP (inhibitor of apoptosis proteins) family. IAPs were originally described
A Rab5 endosomal pathway mediates Parkin-dependent mitochondrial clearance
 Received 3 Mar 16 Accepted 3 Nov 16 Published 3 Jan 17 DOI: 1.138/ncomms145 A Rab5 endosomal pathway mediates -dependent mitochondrial clearance OPEN Babette C. Hammerling 1,, Rita H. Najor 1,, Melissa
Received 3 Mar 16 Accepted 3 Nov 16 Published 3 Jan 17 DOI: 1.138/ncomms145 A Rab5 endosomal pathway mediates -dependent mitochondrial clearance OPEN Babette C. Hammerling 1,, Rita H. Najor 1,, Melissa
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Zool 3200: Cell Biology Exam 5 4/27/15
 Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Mitochondrial Membrane Potential by Object Spot Counting
 A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility
 PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility Xinnan Wang, 1,3 Dominic Winter, 2 Ghazaleh Ashrafi, 1,4 Julia Schlehe, 5 Yao Liang Wong, 6 Dennis Selkoe,
PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility Xinnan Wang, 1,3 Dominic Winter, 2 Ghazaleh Ashrafi, 1,4 Julia Schlehe, 5 Yao Liang Wong, 6 Dennis Selkoe,
M i t o c h o n d r i a
 M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
USP14 inhibition corrects an in vivo model of impaired mitophagy
 Research Article USP14 inhibition corrects an in vivo model of impaired mitophagy Joy Chakraborty 1, Sophia von Stockum 2, Elena Marchesan 2, Federico Caicci 1, Vanni Ferrari 1, Aleksandar Rakovic 3, Christine
Research Article USP14 inhibition corrects an in vivo model of impaired mitophagy Joy Chakraborty 1, Sophia von Stockum 2, Elena Marchesan 2, Federico Caicci 1, Vanni Ferrari 1, Aleksandar Rakovic 3, Christine
Review Article The Emerging Role of Proteolysis in Mitochondrial Quality Control and the Etiology of Parkinson s Disease
 son s Disease Volume 2012, Article ID 382175, 16 pages doi:10.1155/2012/382175 Review Article The Emerging Role of Proteolysis in Mitochondrial Quality Control and the Etiology of son s Disease Riya Shanbhag,
son s Disease Volume 2012, Article ID 382175, 16 pages doi:10.1155/2012/382175 Review Article The Emerging Role of Proteolysis in Mitochondrial Quality Control and the Etiology of son s Disease Riya Shanbhag,
Bio-Plex Pro RBM Apoptosis Assays
 Acute Phase Apoptosis Cancer Cardiovascular Disease Cytokines Chemokines, Growth Factors Diabetes Gene Expression Genotyping Immunoglobulin Isotyping MicroRNA Expression Bio-Plex Pro RBM Apoptosis Assays
Acute Phase Apoptosis Cancer Cardiovascular Disease Cytokines Chemokines, Growth Factors Diabetes Gene Expression Genotyping Immunoglobulin Isotyping MicroRNA Expression Bio-Plex Pro RBM Apoptosis Assays
Myosin VI-Dependent Actin Cages Encapsulate Parkin-Positive Damaged Mitochondria
 Article Myosin VI-Dependent Actin Cages Encapsulate Parkin-Positive Damaged Mitochondria Graphical Abstract Authors Antonina J. Kruppa, Chieko Kishi-Itakura, Thomas A. Masters,..., James A. Nathan, Michal
Article Myosin VI-Dependent Actin Cages Encapsulate Parkin-Positive Damaged Mitochondria Graphical Abstract Authors Antonina J. Kruppa, Chieko Kishi-Itakura, Thomas A. Masters,..., James A. Nathan, Michal
Journal of Cell Science Accepted manuscript
 2015. Published by The Company of Biologists Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which
2015. Published by The Company of Biologists Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which
Direct Interaction between Survivin and Smac/DIABLO Is Essential for the Anti-apoptotic Activity of Survivin during Taxol-induced Apoptosis*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 278, No. 25, Issue of June 20, pp. 23130 23140, 2003 2003 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Direct Interaction
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 278, No. 25, Issue of June 20, pp. 23130 23140, 2003 2003 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Direct Interaction
Mechanisms. Cell Death. Carol M. Troy, MD PhD August 24, 2009 PHENOMENOLOGY OF CELL DEATH I. DEVELOPMENT 8/20/2009
 Mechanisms of Cell Death Carol M. Troy, MD PhD August 24, 2009 PHENOMENOLOGY OF CELL DEATH I. DEVELOPMENT A. MORPHOGENESIS: SCULPTING/SHAPING STRUCTURES CREATION OF CAVITIES AND TUBES FUSION OF TISSUE
Mechanisms of Cell Death Carol M. Troy, MD PhD August 24, 2009 PHENOMENOLOGY OF CELL DEATH I. DEVELOPMENT A. MORPHOGENESIS: SCULPTING/SHAPING STRUCTURES CREATION OF CAVITIES AND TUBES FUSION OF TISSUE
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with
 Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Parkinson s disease (PD) is the second most common neurodegenerative
 Miro phosphorylation sites regulate Parkin recruitment and mitochondrial motility Evgeny Shlevkov a,b, Tal Kramer a,b, Jason Schapansky c, Matthew J. LaVoie c, and Thomas L. Schwarz a,b,1 a F. M. Kirby
Miro phosphorylation sites regulate Parkin recruitment and mitochondrial motility Evgeny Shlevkov a,b, Tal Kramer a,b, Jason Schapansky c, Matthew J. LaVoie c, and Thomas L. Schwarz a,b,1 a F. M. Kirby
Massive loss of neurons in embryos occurs during normal development (!)
 Types of Cell Death Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can spread) Apoptosis
Types of Cell Death Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can spread) Apoptosis
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity
 Research Article 6535 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity Naotada Ishihara, Yuka Eura and Katsuyoshi Mihara* Department of Molecular Biology, Graduate
Research Article 6535 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity Naotada Ishihara, Yuka Eura and Katsuyoshi Mihara* Department of Molecular Biology, Graduate
Nature Medicine: doi: /nm.3776
 C terminal Hsp90 inhibitors restore glucocorticoid sensitivity and relieve a mouse allograft model of Cushing s disease Mathias Riebold, Christian Kozany, Lee Freiburger, Michael Sattler, Michael Buchfelder,
C terminal Hsp90 inhibitors restore glucocorticoid sensitivity and relieve a mouse allograft model of Cushing s disease Mathias Riebold, Christian Kozany, Lee Freiburger, Michael Sattler, Michael Buchfelder,
PINK1 Stabilized by Mitochondrial Depolarization Recruits Parkin to Damaged Mitochondria and Activates Latent Parkin for Mitophagy
 PINK1 Stabilized by Mitochondrial Depolarization Recruits Parkin to Damaged Mitochondria and Activates Latent Parkin for Mitophagy The Harvard community has made this article openly available. Please share
PINK1 Stabilized by Mitochondrial Depolarization Recruits Parkin to Damaged Mitochondria and Activates Latent Parkin for Mitophagy The Harvard community has made this article openly available. Please share
Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer
 Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer Nexcelom Bioscience LLC. 360 Merrimack Street, Building 9 Lawrence, MA 01843 T: 978.327.5340 F: 978.327.5341
Measuring Mitochondrial Membrane Potential with JC-1 Using the Cellometer Vision Image Cytometer Nexcelom Bioscience LLC. 360 Merrimack Street, Building 9 Lawrence, MA 01843 T: 978.327.5340 F: 978.327.5341
Biology 580 Cellular Physiology Spring 2007 Course Syllabus
 Class # 17416 MW 0800-0850 Biology 580 Cellular Physiology Spring 2007 Course Syllabus Course Text: Course Website: Molecular Biology of the Cell by Alberts et al.; 4th edition, Garland Science, 2002 (ISBN
Class # 17416 MW 0800-0850 Biology 580 Cellular Physiology Spring 2007 Course Syllabus Course Text: Course Website: Molecular Biology of the Cell by Alberts et al.; 4th edition, Garland Science, 2002 (ISBN
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Mitochondrial Quality Control
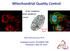 Mitochondrial Quality Control You are here Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 938 Charleston, May 20, 2015 biology4kids.com Cryo-electron microscopy Fluorescent microscopy Yeast Human Semi-autonomous,
Mitochondrial Quality Control You are here Oleh Khalimonchuk, Ph.D. Graduate Course 2214/BIOC 938 Charleston, May 20, 2015 biology4kids.com Cryo-electron microscopy Fluorescent microscopy Yeast Human Semi-autonomous,
Under the Radar Screen: How Bugs Trick Our Immune Defenses
 Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 2: Phagocytosis Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research Salmonella Gram negative bacteria
Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 2: Phagocytosis Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research Salmonella Gram negative bacteria
Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin
 Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin The Harvard community has made this article openly available. Please share how this access benefits you. Your
Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin The Harvard community has made this article openly available. Please share how this access benefits you. Your
Effects of the BH3-only protein human Noxa on mitochondrial dynamics
 FEBS Letters 583 (2009) 2349 2354 journal homepage: www.febsletters.org Effects of the BH3-only protein human Noxa on mitochondrial dynamics Ha-Na Woo a,c,e, Young-Woo Seo b, Ae Ran Moon c, Seon-Yong Jeong
FEBS Letters 583 (2009) 2349 2354 journal homepage: www.febsletters.org Effects of the BH3-only protein human Noxa on mitochondrial dynamics Ha-Na Woo a,c,e, Young-Woo Seo b, Ae Ran Moon c, Seon-Yong Jeong
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Sig2GRN: A Software Tool Linking Signaling Pathway with Gene Regulatory Network for Dynamic Simulation
 Sig2GRN: A Software Tool Linking Signaling Pathway with Gene Regulatory Network for Dynamic Simulation Authors: Fan Zhang, Runsheng Liu and Jie Zheng Presented by: Fan Wu School of Computer Science and
Sig2GRN: A Software Tool Linking Signaling Pathway with Gene Regulatory Network for Dynamic Simulation Authors: Fan Zhang, Runsheng Liu and Jie Zheng Presented by: Fan Wu School of Computer Science and
Hsp90-Cdc37 Chaperone Complex Regulates Ulk1- and Atg13-Mediated Mitophagy
 Article Hsp9- Chaperone Complex Regulates - and -Mediated Mitophagy Joung Hyuck Joo, 1,7 Frank C. Dorsey, 3,7,8 Aashish Joshi, 1,7 Kristin M. Hennessy-Walters, 1,7 Kristie L. Rose, 3 Kelly McCastlain,
Article Hsp9- Chaperone Complex Regulates - and -Mediated Mitophagy Joung Hyuck Joo, 1,7 Frank C. Dorsey, 3,7,8 Aashish Joshi, 1,7 Kristin M. Hennessy-Walters, 1,7 Kristie L. Rose, 3 Kelly McCastlain,
The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression
 Traffic 2010; 11: 827 842 2010 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2010.01055.x The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression Danming Tang, Hebao Yuan and Yanzhuang
Traffic 2010; 11: 827 842 2010 John Wiley & Sons A/S doi:10.1111/j.1600-0854.2010.01055.x The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression Danming Tang, Hebao Yuan and Yanzhuang
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supporting Information
 Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis
 Article Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis Liang Ge 1,,, Min Zhang 1, Samuel J Kenny 2, Dawei Liu 1, Miharu Maeda 3, Kota Saito 3, Anandita Mathur
Article Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis Liang Ge 1,,, Min Zhang 1, Samuel J Kenny 2, Dawei Liu 1, Miharu Maeda 3, Kota Saito 3, Anandita Mathur
Journal of Cell Science Accepted manuscript
 14. Published by The Company of Biologists Ltd. Journal of Cell Science Accepted manuscript 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 21 22 23 24 25 26 27 28 29 30 31 32 33 34, and UBE2D2/3 Ubiquitin-Conjugating
14. Published by The Company of Biologists Ltd. Journal of Cell Science Accepted manuscript 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 21 22 23 24 25 26 27 28 29 30 31 32 33 34, and UBE2D2/3 Ubiquitin-Conjugating
Mechanisms of Cell Death
 Mechanisms of Cell Death CELL DEATH AND FORMATION OF THE SEMICIRCULAR CANALS Carol M. Troy, MD PhD August 24, 2009 FROM: Fekete et al., Development 124: 2451 (1997) PHENOMENOLOGY OF CELL DEATH I. DEVELOPMENT
Mechanisms of Cell Death CELL DEATH AND FORMATION OF THE SEMICIRCULAR CANALS Carol M. Troy, MD PhD August 24, 2009 FROM: Fekete et al., Development 124: 2451 (1997) PHENOMENOLOGY OF CELL DEATH I. DEVELOPMENT
Cell Survival Curves (Chap. 3)
 Cell Survival Curves (Chap. 3) Example of pedigress of EMT6 mouse cells Clone The in vitro cell survival curve Cell Survival Assay Dye exclusion assay: membrane integrity MTT assay: respiratory activity
Cell Survival Curves (Chap. 3) Example of pedigress of EMT6 mouse cells Clone The in vitro cell survival curve Cell Survival Assay Dye exclusion assay: membrane integrity MTT assay: respiratory activity
Opa1-Mediated Cristae Opening Is Bax/Bak and BH3 Dependent, Required for Apoptosis, and Independent of Bak Oligomerization
 Article Opa1-Mediated Cristae Opening Is Bax/Bak and BH3 Dependent, Required for Apoptosis, and Independent of Bak Oligomerization Ryuji Yamaguchi, 1,4 Lydia Lartigue, 1 Guy Perkins, 2 Ray T. Scott, 2
Article Opa1-Mediated Cristae Opening Is Bax/Bak and BH3 Dependent, Required for Apoptosis, and Independent of Bak Oligomerization Ryuji Yamaguchi, 1,4 Lydia Lartigue, 1 Guy Perkins, 2 Ray T. Scott, 2
Biochimica et Biophysica Acta
 Biochimica et Biophysica Acta 1843 (2014) 2100 2113 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbamcr Review Regulating cell death
Biochimica et Biophysica Acta 1843 (2014) 2100 2113 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbamcr Review Regulating cell death
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
Mitochondria are double-membrane
 Mitochondrial Fission, Fusion, and Stress Richard J. Youle 1 * and Alexander M. van der Bliek 2 * Mitochondrial fission and fusion play critical roles in maintaining functional mitochondria when cells
Mitochondrial Fission, Fusion, and Stress Richard J. Youle 1 * and Alexander M. van der Bliek 2 * Mitochondrial fission and fusion play critical roles in maintaining functional mitochondria when cells
Caenorhabditis elegans CED-4 stimulates CED-3 processing and CED-3-induced apoptosis Somasekar Seshagiri* and Lois K. Miller*
 Research Paper 455 Caenorhabditis elegans CED-4 stimulates CED-3 processing and CED-3-induced apoptosis Somasekar Seshagiri* and Lois K. Miller* Background: Programmed cell death or apoptosis is a key
Research Paper 455 Caenorhabditis elegans CED-4 stimulates CED-3 processing and CED-3-induced apoptosis Somasekar Seshagiri* and Lois K. Miller* Background: Programmed cell death or apoptosis is a key
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
12/5/2014. The cell cycle and cell death. The cell cycle: cells duplicate their contents and divide
 The cell cycle and cell death The cell cycle: cells duplicate their contents and divide 1 The cell cycle may be divided into 4 phases Eucaryotic cell division: Mitosis (nuclear division) Cytokinesis (cell
The cell cycle and cell death The cell cycle: cells duplicate their contents and divide 1 The cell cycle may be divided into 4 phases Eucaryotic cell division: Mitosis (nuclear division) Cytokinesis (cell
Importance of Protein sorting. A clue from plastid development
 Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
