Testing of Continuous Sampling Air-ICP and Mercury Systems as Continuous Emission Monitors at the Diagnostic Instrumentation and Analysis Laboratory
|
|
|
- Suzanna Quinn
- 5 years ago
- Views:
Transcription
1 Testing of Continuous Sampling Air-ICP and Mercury Systems as Continuous Emission Monitors at the Diagnostic Instrumentation and Analysis Laboratory September 18-26, 2000 D. P. Baldwin, S. J. Bajic, D. E. Eckels, and D. S. Zamzow, Ames Laboratory, and G. P. Miller, S. Tao, and C. A. Waggoner, DIAL Ames Laboratory-USDOE Report IS-5144 February 15, 2001 Revised, March 15, 2001
2 Introduction This report has been prepared to document the performance of the continuous sampling reduced-pressure air-icp-aes (inductively coupled plasma - atomic emission spectroscopy) and mercury-monitor systems developed by Ames Laboratory for use as continuous emission monitors (CEM). This work was funded by the U. S. Department of Energy, Office of Environmental Management, Office of Science and Technology, through the Mixed Waste Focus Area. The purpose of the project is to develop instrumentation and methods for spectroscopic field monitoring applications. During FY00 this included continued work on the development of the continuous sample introduction system and the multi-frequency AOTF-echelle spectrometer, used in conjunction with the reduced-pressure air-icp-aes system as a multi-metal CEM. The assembly, development, and testing of an echelle spectrometer system for the detection of mercury (Hg) by atomic absorption was also completed during FY00. The continuous sampling system and the multi-metal air-icp and mercury-monitor CEM systems were tested at Mississippi State University at the Diagnostic Instrumentation and Analysis Laboratory (DIAL) at the end of FY00. This report describes the characteristics and performance of these systems, and the results of the field tests performed at DIAL. The continuous sampling air-icp and mercury-monitor CEM systems are being developed in response to the need of DOE and other organizations to monitor the heavy-metal or radioactive materials that may be released during the processing or combustion of hazardous or mixed-waste materials. The air-icp system has been designed for the on-line detection and monitoring of heavy metals (beryllium, cadmium, chromium, and lead, in particular) in process or exhaust gas streams by optical emission spectroscopy. Due to the relatively poor limits of detection for Hg by optical emission techniques, the mercury-monitor CEM has been designed specifically for the detection of elemental Hg by optical absorption. A heated pyrolysis tube is used in this system to convert oxidized Hg compounds to elemental Hg prior to analysis, for the determination of total mercury in the gas sample stream. The promulgation of regulations limiting the release of these metals and requiring continuous monitoring of stack gases from combustion and treatment processes would seriously impact the operations of DOE waste incinerators and other facilities. Therefore, it is important to develop and validate techniques that adequately meet proposed sensitivity and accuracy requirements. The most likely form of validation for such a technique will involve comparison of CEM results for a test combustion 2
3 system with EPA Reference Method 29 (RM-29). 1 Therefore, the air-icp and mercury-monitor CEM systems were tested at DIAL by monitoring metal emissions in a fuel oil-air combustion exhaust while simultaneously collecting samples using the RM-29 technique. The CEM results were available continuously during the on-line monitoring that was performed. The results of the RM-29 sampling were received a number of weeks after the testing at DIAL. These results are discussed in this report, with a comparison and evaluation of the RM-29 and air-icp and mercury-monitor CEM data. The continuous sampling reduced-pressure air-icp-aes system was assembled and tested at Ames Laboratory during FY99, and a field test of the system was conducted at the end of FY99 at DIAL. The results for the FY99 test have been reported. 2 For that test, the continuous sampling system and reduced-pressure air-icp were connected to a sampling port on the DIAL test-stand (combustion system), and the system was operated as a CEM for metals analysis while simultaneously collecting samples using RM-29. Reasonable agreement between the continuous sampling air-icp and RM-29 results were obtained for beryllium (Be), chromium (Cr), and lead (Pb) during the FY99 test, using the echelle spectrometer system. Due to a failure of the single-frequency AOTF in the AOTF-echelle spectrometer during the FY99 field test, these results were obtained using a 0.2-m monochromator as a bandpass pre-filter for the echelle, rather than the AOTF. For the FY00 test at DIAL, a multi-frequency AOTF, capable of selecting three wavelength regions simultaneously to allow operation of the AOTF-echelle spectrometer as a simultaneous multi-metals CEM, was used. 3 This was the first field test of the multi-frequency AOTF-echelle spectrometer. This spectrometer was used in conjunction with the reducedpressure air-icp during the test at DIAL for simultaneous detection of the hazardous air pollutant metals Be, cadmium (Cd), Cr, and Pb. After the air-icp CEM tests, the continuous sampling system was used in conjunction with the mercury-monitor system for the detection of Hg. Laboratory testing of the mercury-monitor CEM system was conducted at Ames Laboratory during FY00, and the work performed at DIAL was the first field test of the mercury system. During the test at DIAL, RM-29 sampling was conducted concurrently with the operation of the air-icp and mercury-monitor CEM systems, to allow a comparison of the reference method and CEM results. 3
4 Experimental Continuous Sampling System A description of the continuous sampling system developed for this project has been published, 2,3 and is only summarized here. A schematic diagram of the system is shown in Figure 1. Wall of Exhaust Stack Sampling Probe Differential Pressure Differential Pressure and Thermocouple and Thermocouple Probes Probes Sample Line Sampling Chamber Nebulizer Spike (Air-ICP) Room-Air Valve (Mercury CEM) Ballast to Sampling Pump to Air-ICP or Mercury CEM Figure 1. Schematic diagram of the continuous sampling system, with connection to an exhaust stack. Two heat-traced Teflon sample lines, one 12 and one 24 in length, were used to connect the sampling chamber to the sampling probe, inserted into the stack at DIAL. The continuous sampling system is a dual-stage sampling system. A high-volume primary sample (20-30 standard liters per minute, Lpm) is drawn isokinetically from a process pipe or exhaust stack using a standard commercial EPA isokinetic glass-lined probe with an integral heater (Apex Instruments). The sampling probe is connected to a commercial heat-traced Teflon sample line (Technical Heaters), which is connected to the sampling chamber, a Teflon tube that is 1 -inner diameter and 24 in length. A rotary vane pump (Gast Manufacturing model V4-G180DX) draws gas through the sampling chamber in a laminar-flow arrangement, with over 95% of the sample being removed through an exit port and exhaust line at the end of the chamber. On the inlet side of the sampling chamber, there are two ports (Teflon tees) that are provided for connecting a differential pressure transducer (Validyne model P55D) and a Tefloncoated thermocouple (Omega Engineering) to a Teflon differential pressure flow cell, so that the gas flow rate and temperature of the primary sample can be monitored. For the test at DIAL, a 30-standard-Lpm sample was drawn from the stack, using the sampling pump. Two heat-traced Teflon sample lines (attached in series for a total length of 36 ) were used to connect the sampling chamber (located inside the test facility at DIAL) to the stack sampling port (located outside). The sampling probe, sample lines, and sampling chamber were operated at 4
5 approximately 110 C during the test at DIAL. To address the sample gas flow variations to the axial channel of the air-icp observed during the FY99 test, resulting from pressure fluctuations in the exhaust line at DIAL, 2 a ballast was added to the sampling system in FY00. The ballast is a 6 -inner diameter, 3.5 -long PVC chamber having an internal volume of approximately 19.5 L. The ballast is positioned next to the sampling chamber and connected to the exhaust line of the sampling chamber, which is connected to the sampling pump. The sample gas stream does not enter or flow through the ballast - only the exhaust line of the sampling chamber is connected to the ballast. This arrangement provides for a reduction in pressure variations in the sampling chamber resulting from pressure fluctuations in the stack, since the large-volume (19.5 L) ballast damps the variations in the smaller-volume (approximately 0.3 L) sampling chamber. However, even with the addition of the ballast, apparent air-icp axial channel gas flow fluctuations (and resulting ICP torch flicker) were observed during some of the FY00 air-icp testing at DIAL, as discussed below. However, during testing of the mercury-monitor system, no significant flow fluctuations were observed at the gas flow gauge (oil-filled manometer) on the outlet side of the sampling chamber. A secondary sample is drawn isokinetically from the gas flowing through the sampling chamber using a 1/4 -outer diameter Teflon sampling tube that is inserted approximately 4 into the end of the chamber. The tube currently used has a inner diameter, with a 30 taper at the sampling end. The secondary sample outlet is connected to a 1/4 Teflon Swagelok tee. The in-line port of this tee is followed by another Teflon differential pressure flow cell, to monitor the sample flow rate from the sampling chamber. A Teflon-coated thermocouple (Omega) is inserted into the inlet of this flow cell to measure the gas temperature. The differential pressure is monitored using an oil-filled manometer (Dwyer Instrument model 101) that has a maximum range of 0.5 of water. The primary flow cell, Teflon sampling chamber, and secondary flow cell are heat-traced using electrical heat tape and insulation wrap. For the reduced-pressure air- ICP system, a rotary vane pump (Gast Manufacturing model Q-G608X) is used to draw a slight vacuum on the ICP metal enclosure, drawing a sample flow of approximately 1 standard Lpm out of the sampling chamber, for introduction into the axial channel of the ICP. For the mercury-monitor system, a linear pump (Gast Manufacturing model SPP-6GAS-101) is used to draw a sample flow of approximately 1 standard Lpm from the sampling chamber, for introduction into a 1-m absorption cell. The secondary gas sample drawn from the sampling 5
6 chamber is introduced into either the reduced-pressure air-icp or the mercury-monitor system for the detection of hazardous air pollutant metals. Reduced-Pressure Air-ICP and Multi-Frequency AOTF-Echelle Spectrometer CEM The reduced-pressure air-icp-aes system used for multi-metals emission monitoring has been described in detail previously, 2,3 although some modifications have been made to the system. A photograph of the ICP metal enclosure is shown in Figure 2. Figure 2. Photograph of the reduced-pressure air-icp system, showing the air plasma inside the metal enclosure, part of the connection to the ICP auto-matching network (inside the wire mesh screen on the right), and the front-end of the AOTF-echelle spectrometer (on the left). The system consists of an air-icp that is operated at reduced pressure inside a 6 -diameter metal enclosure, the continuous sampling system, and the AOTF-echelle spectrometer. The bottom flange of the metal enclosure has gas-line connections for the ICP torch plasma and auxiliary gas supplies and for the ICP axial channel (sample) gas, which is connected to the Teflon sampling chamber. A connection point for the Tesla coil discharge required to ignite the ICP is included on the bottom flange of the enclosure. An additional gas-line connection on the bottom flange is provided for the introduction of supplemental air (approximately 50 Lpm), which provides some cooling of the metal enclosure during operation of the 3-kW air plasma. Above the ICP torch, 1/4 -diameter stainless steel tubing is welded onto the 6 -diameter metal enclosure, for watercooling of the upper portion of the enclosure. At the top of the enclosure, 1.5 -diameter stainless steel and copper tubing is connected; water-cooling of this tubing is also provided to reduce the gas temperature of the ICP exhaust to less than 50 C prior to reaching the chamber pump. The chamber pump is connected to this 1.5 -diameter tubing to operate the enclosure at reduced 6
7 pressure, 0 to -5 psig, in order to draw sample continuously into the plasma, from the Teflon sampling chamber, through the axial channel of the ICP torch. This pump has replaced the Roots blower, used in FY99, to decrease the overall size of the reduced-pressure air-icp system. The ICP load coil (a 5-turn coil) is connected to the auto-matching network (Seren Industrial Power Systems solid-state, 27 MHz, 3-kW ICP system) using copper tubing feed-throughs in a Delrin flange on the right side of the metal enclosure. 2,3 The plasma is generated using argon gas at approximately 1-kW RF power and then switched over to air operation, completely replacing the argon with air and increasing the RF power to approximately 3-kW. Optical emission signals from metals introduced into the air-icp are detected using the AOTF-echelle spectrometer. A 2 -diameter, 3 focal-length fused silica lens, mounted in the flange on the left side of the ICP enclosure, forms a 1:1 image of the plasma at the input aperture of the AOTF-echelle spectrometer. A quartz AOTF (MVM Electronics) placed between two crossed polarizers (Casix model PGT8208 α-bbo Glan-Taylor polarizers) acts as an ordersorting pre-filter for the echelle. An RF frequency applied to the AOTF selects a narrow band (approximately 1-nm bandwidth) of optical emission from the ICP, which is introduced into the echelle. The bandpass of the AOTF is smaller than the width (free spectral range) of one order of the echelle grating, so no cross-dispersing optical element such as a prism or grating is required in the echelle spectrometer. This AOTF provides the capability of multi-wavelength simultaneous operation, since a multi-frequency RF driver is incorporated into the system. This allows for monitoring ICP emission signals from multiple elements simultaneously. The collimating and focusing mirrors in the current AOTF-echelle spectrometer are 0.4-m focallength off-axis parabolic mirrors (Atlantis Optical Laboratories, Inc.), not the 0.38-m focallength concave spherical mirrors used in the previous system. 4 The detector is a two-stage thermoelectrically cooled CCD (Hamamatsu model S ). For the test at DIAL, data acquisition for the metals Be, Cd, Cr, and Pb was generally performed in a sequential, multi-element fashion, collecting data for Be and Cr in one data set and Cd and Pb in the following data set (switching the AOTF operating frequencies between data sets), at approximately 10 points per minute. Initial calibration of the air-icp system was performed by introducing aqueous solution standards of metals (Be, Cd, Cr, and Pb at varying concentrations) into a CETAC Technologies U5000-AT ultrasonic nebulizer (USN). The dry aerosol output from the USN was introduced into a tee on the inlet side of the 12 Teflon sample 7
8 line connected to the sampling chamber (Nebulizer Spike in Figure 1), with one port of the tee open to room air. This arrangement was used for introducing metal aerosols into the sampling chamber and the air-icp system for calibration and monitoring experiments with the stack not connected. After calibration, the system was connected to the stack at DIAL, for acquisition of stack-metals monitoring data. Since the USN was used for the introduction of metal aerosols into the stack, on-line metals standard additions, similar to those performed during the FY99 test, were not done. 2 Instead, the response of the air-icp AOTF-echelle system was checked at various intervals by comparing the calibration of the system against the initial non-stackconnected calibration curves. Mercury-Monitor CEM A mercury-monitor CEM system was assembled and tested during FY00 at Ames Laboratory and used for stack-mercury monitoring during the field test at DIAL. Elemental mercury is detected by atomic absorption in a 1-m pathlength absorption cell, using a mercury pen lamp as the source and a 0.38-m focal-length echelle spectrometer with a photodiode array (PDA) as the detector. This echelle spectrometer has no cross-dispersing optical element or order-sorting pre-filter, so all orders of the echelle grating are spatially superimposed at the detector. This spectrometer provides simultaneous detection of all of the strong Hg lines from nm from the mercury pen lamp (diffracted from different orders of the echelle grating), without spectral overlap of these lines at the detector, as shown in Figure 3. Intensity (arb. units) Hg (I) Hg (I) Hg (I) Hg (I) Hg (I) Hg (I) , Hg (I) Hg (I) , Hg (I) Hg (I) Hg (I) Hg (I) Hg (I) Pixel Number Figure 3. Mercury pen lamp spectrum, obtained using the Hg-echelle spectrometer. The Hg (I) nm line is used to measure absorption due to mercury in the 1-m cell. Other Hg lines (non-ground-state electronic transitions not subject to mercury absorption) can be used 8
9 to correct for light source intensity fluctuations, light scattering by particles, and absorption due to species other than Hg. Since all the mercury lines can be monitored simultaneously, this system in effect provides a dual-beam (or multiple-beam ) optical arrangement with a reference channel, using only a single light source, absorption cell, spectrometer, and detector. A schematic diagram and a photograph of the mercury-monitor CEM are shown in Figure 4. Mercury Pen Lamp 1-m Absorption Cell Echelle Spectrometer PDA to Exhaust Sample Pump (1 Lpm) from Stack Room Air Sampling Chamber Tube Furnace with Pyrolysis Tube VICI Dynacalibrator with Mercury Permeation Tube Figure 4. Schematic diagram and photograph of the mercury-monitor CEM system. In the photograph, the 1-m absorption cell is inside the 4 -long insulated tube (2), mounted on the metal frame, along with the mercury pen lamp (1, left side), optics, and 0.38-m echelle spectrometer (3, right side) with PDA detector. The Teflon sampling chamber is inside the 4 -long insulated tube (4), on top of the table. The sample pump draws gas out of the sampling chamber, through the pyrolysis tube in the tube furnace (5), and through the absorption cell. Light from a mercury pen lamp (Oriel model 6035 Hg(Ar) lamp operated at 10-mA AC current, using an Oriel model 6060 power supply) is collected using a 1 -diameter, 6 -focal length fused silica lens, with the lamp emission approximately collimated through the 1-m absorption cell. The lamp is housed in an aluminum block, heated to 35 C using a cartridge heater (Omega Engineering model CIR-1031/120) and temperature controller (Valco Instruments model ITC10399). The absorption cell has quartz windows, and is heated to approximately 125 C using electrical heat tape inside an insulated tube (Accessible Products Company). Light that passes through the cell passes through BG-24A and WG-280 optical filters (Schott Glass Technologies - to attenuate the Hg (I) and nm lines with respect to the other Hg lines) prior to being focused onto the entrance slit of the echelle spectrometer, using a 1 - diameter, 6 -focal length fused silica lens. This spectrometer is a modified version of the 0.38-m echelle spectrometer described previously. 4 The optics in this echelle consist of a 1 -diameter flat turning mirror, two 2 -diameter 0.38-m focal-length concave spherical mirrors (Optics for Research) used as the collimating and focusing mirrors, the grating (Richardson Grating 9
10 Laboratory model echelle, grooves per mm, 69 blaze angle), and a 2 - diameter flat turning mirror. The PDA is an EG&G Princeton Applied Research model 1453 detector. Sample stack gas is introduced into the mercury-monitor CEM using the continuous sampling system described above. A linear pump is used to draw a sample flow of approximately 1 standard Lpm from the Teflon sampling chamber, through a heated pyrolysis tube, and through the 1-m absorption cell. The pyrolysis tube is a 1 -diameter, 22 -long quartz tube, filled with long quartz rings cut from 6-mm OD tubing, that is positioned inside the tube furnace (Lindberg/BlueM model TF55030A, operated at 1000 C). The pyrolysis tube is used for thermal decomposition of oxidized Hg compounds to elemental Hg, prior to introduction into the absorption cell. 5,6 The 1/4 -OD Teflon tubing that connects the sampling chamber to the pyrolysis tube in the tube furnace and to the absorption cell is heat-traced using electrical heating tape and operated at approximately 60 C. A two-way valve is inserted between the sample line and the sampling chamber (see Figure 1) to allow stack gas sampling or zero-checks to be performed. When performing zero-checks, room air (rather than stack gas) is drawn into the sampling chamber and the 1-m absorption cell, by valving off the sample stream from the combustion stack. For the test at DIAL, mercury absorption was measured by ratioing the intensity for the Hg (I) nm line to that for the Hg (I) nm line. Absorption by elemental mercury vapor occurs at the nm line, but not at the nm line. Using both of these lines, an improvement in the accuracy and stability of the system is achieved, since short- and long-term fluctuations in the intensity output from the mercury lamp can be corrected for by using the intensity ratio. This detection scheme also corrects for light scatter from particles in the absorption cell, to the extent that this scattering is comparable at both and nm. Prior to the introduction and analysis of the stack gas sample stream at DIAL, the mercurymonitor CEM was calibrated by measuring the absorption resulting from the introduction of 8.71 µg/m 3 Hg into the 1-m cell, from a permeation tube placed inside the Dynacalibrator (VICI model B-YD). Interference at nm due to absorption by sulfur dioxide (SO 2 ) in the stack gas sample was corrected during mercury-monitor data acquisition. The system was calibrated by introducing a known concentration of SO 2 into the 1-m cell (in the absence of Hg) and measuring the absorption at and nm, using the intensity ratios ( to- 10
11 and to ) for both of these lines. During stack sampling, a correction factor to the measured nm absorption was applied, based on the measured nm SO 2 absorption, to remove the nm SO 2 absorption contribution. During the test at DIAL, Hg was introduced into the stack using the ultrasonic nebulizer (USN) at levels corresponding to approximately 80 and 8 µg/m 3. Hydrogen chloride (HCl) and SO 2 gases were introduced at approximately 100 and 50 ppmv, respectively, during the high-level testing (80 µg/m 3 Hg) and approximately 25 and 500 ppmv during low-level testing (8 µg/m 3 Hg). During the test at DIAL, mercury-monitor CEM data was acquired at a rate of approximately 20 points per minute. Results and Discussion DIAL Stack Operating Conditions During FY00, the combustion test-stand at DIAL was modified by extending the exhaust line approximately ten meters, so that the RM-29 and CEM sampling ports were located outside the facility. This modification allowed the combustion furnace to be operated at its optimum conditions, rather than the fuel-lean conditions used in FY99 to minimize the gas temperature at the sampling ports. During the FY00 test, the furnace was operated with 500 lb/hr of air and 32 lb/hr of fuel oil, which produced a gas flow in DIAL s 6 -diameter, schedule-80 pipe of approximately 4100 standard Lpm. At the sampling point, the gas temperature was 150 C, with a velocity of approximately 6.1 m/s. The CEM and RM-29 sampling probes were installed in a vertical section of the exhaust pipe, several meters downstream from the water-cooled exhaust line. The probes were inserted into the stack through opposing ports with the probe nozzles positioned in equivalent locations, within a few centimeters of each other (see Figure 5). This arrangement minimizes differences in stack metals concentrations introduced into the CEM and RM-29 systems. The equivalent nature of these opposing sampling positions has been demonstrated previously by simultaneous collection of two sets of RM-29 samples using two probes in this configuration. At the sampling point, the exhaust gas was flowing vertically down the pipe. In FY00, the USN was used (rather than the pneumatic air-driven stack nebulizer used in FY99) for introducing appropriate concentrations of Be, Cd, Cr, and Pb (air-icp) and Hg (mercury-monitor CEM) into the exhaust stack gas. For the air-icp testing, the USN output aerosol was introduced into the stack at a port approximately five meters upstream from the 11
12 CEM and RM-29 sampling probes. For the mercury-monitor testing, the USN output aerosol was introduced into the stack afterburner, slightly more than one meter above the sampling port, so that the Hg in the aerosol would be vaporized and potentially oxidized after being introduced into the stack. During RM-29 sampling, the air-icp or mercury-monitor CEM systems were used for on-line measurement of the stack-metals aerosol concentrations. The RM-29 collected samples were sent to an analytical testing laboratory for quantitative analysis. Mercury Introduction CEM and RM-29 Sampling Air-ICP Metals Introduction Primary Burner Gas Flow Wall of Exhaust Stack SO 2 and HCl Introduction CEM Sampling Probe RM-29 Sampling Probe Figure 5. The combustion test-stand at DIAL, showing the port-locations used for the introduction of metal aerosols for the air-icp and mercury-monitor CEM testing and for the introduction of SO 2 and HCl gases during mercury-monitor testing. Sampling from the stack occurred on opposite sides of the same port, using one sampling probe for the air-icp and mercury-monitor CEM systems and a second probe for RM-29 sampling. Reduced-Pressure Air-ICP and Multi-Frequency AOTF-Echelle Spectrometer CEM For the on-line detection of metals (Be, Cd, Cr, and Pb) using the reduced-pressure air- ICP and multi-frequency AOTF-echelle spectrometer during the FY00 test at DIAL, significantly different system performance was observed when the sampling system was connected to the stack compared to the non-stack-connected results. When the sampling system was not connected to the stack (during initial testing and calibration), the four metals Be, Cd, Cr, and Pb were detected using the reduced-pressure air-icp and AOTF-echelle system at levels corresponding to approximately 5, 40, 10, and 50 µg/dscm, respectively. However, when the sampling system was connected to the stack during RM-29 sampling (with metals introduced into the stack at higher concentrations than the initial calibration detection limits), only Be was detected. Some of the difference in system performance is thought to result from the use of compromise ICP plasma conditions during some of the stack-sampling time periods. The 12
13 majority of the difference, however, is believed to result from an increase in the plasma emission background that was not effectively removed from the emission signals for Be, Cd, Cr, and Pb during stack sampling. In particular, the polarizers used with the quartz AOTF were not adequate to eliminate the elevated plasma emission background signal during stack sampling, which resulted in a significant degradation of the detection limits for Be, Cd, Cr, and Pb during the test at DIAL. In the AOTF-echelle spectrometer, the quartz AOTF is placed between crossed polarizers. 3,4 If the polarizers function properly, only light selected by the AOTF (which rotates the polarization of the selected wavelength component in order to pass through the crossed polarizers) reaches the echelle spectrometer and detector. The polarizers that were used during the test at DIAL were ineffective in completely blocking the plasma background emission. The polarizers used are designed to function well in the nm wavelength range (in the ultraviolet, the region of the strongest emission lines for Be, Cd, Cr, and Pb), but not as well in the visible and near-infrared regions. As a result, plasma background optical emission in the visible and near-infrared leaks through these polarizers, increasing the detected background signal. This background is non-specific plasma emission, not selected by the AOTF, and not Be, Cd, Cr, and Pb emission lines. For the air-icp (with the sampling system not connected to the stack), this plasma background is relatively small and manageable. However, with the sampling system connected to the stack, a significant increase in the background signal level occurred, as shown in Figure 6. This increase is thought to arise from the introduction of carbon-containing stack gas into the air-icp, resulting in an increase in CN, CH, and other molecular band plasma emission features. This increase (and the associated noise on this background signal) is believed to have led to the degradation in the AOTF-echelle detection limits for Be, Cd, Cr, and Pb when the reduced-pressure air-icp sampling system was connected to the stack. 13
14 15 Intensity Pixel Number Figure 6. Air-ICP background emission spectra acquired using the multi-frequency AOTF-echelle spectrometer, with the sampling system connected to the stack (upper spectrum) and not connected (lower trace). The difference in AOTF-echelle detection limits for Be, Cd, Cr, and Pb with and without the sampling system connected to the stack is illustrated in Figure 7. These spectra were obtained by nebulizing solution standards containing 0, 10, 50, and 100 ppm Be, Cd, Cr, and Pb using the USN, with the metal aerosol output from the USN introduced into the continuous sampling system. The aerosol introduction point was a tee on the inlet side of the 12 Teflon sample line connected to the sampling chamber (see Figure 1). In Figure 7, the measured signal intensity is plotted as a function of time (point number), as the solution introduced into the USN was switched from water to 10 ppm to 50 ppm to 100 ppm for the four metals, with the stack not connected (A-labeled traces). All four metals are detected at the 10-ppm solution concentration value, corresponding to approximately 140 µg/dscm. With the sampling system connected to the stack, Be and Cr were detected (B-labeled traces) as 0, 100, 50, and 10 ppm solutions were nebulized, but at a much lower signal-to-noise ratio. The elevation in the background signal (and the associated noise) resulted in Cd and Pb being undetectable, even at the 100-ppm solution concentration value, approximately 1400 µg/dscm. It should be noted that a higher ICP RF power and a higher axial channel gas flow rate (approximately 1.7 compared to 1.3 Lpm) were used for the stack-connected data in Figure 7. These modified operating conditions were selected as compromise conditions thought to be necessary due to axial channel gas flow variations and the resulting ICP torch flicker observed during stack sampling on 9/21/00. (On prior days, no significant ICP flicker was observed during stack sampling; however, on these days, ICP torches that had partially-fused axial channels - less than the normal 1-mm diameter aperture - were used.) The plotted concentrations are the values measured using the initial 14
15 calibration curves acquired on 9/19/00, and the offset in concentration values shows differences in the background signal levels measured without and with the stack connected. The effect is most severe for Cd and Pb, in part because the sensitivity for these two metals is lower than that for Be or Cr. For these four metals, Be has the highest sensitivity, with Cr, Cd, and Pb approximately 3, 14, and 50 times less sensitive, based on the slopes of the initial calibration curves obtained during testing at DIAL. However, the relative positions of the plasma background emission peaks (in Figure 6) with respect to the analyte line pixel positions also affects the severity of the effect observed, with the stack connected. 90 Concentration (ppm) Be (B) Concentration (ppm) Cd (B) 10 0 (A) 0 (A) Point Number Point Number Concentration (ppm) (A) Point Number Cr (B) Concentration (ppm) (A) Point Number Figure 7. Air-ICP monitor data for Be (II) nm, Cd (I) nm, Pb (I) nm, and Cr (I) nm lines (clockwise, from upper left) acquired using the AOTF-echelle spectrometer, with metals spiked into the continuous sampling system at the front of the 12 Teflon sample line. The A-labeled traces are signals detected without the stack connected, resulting from the introduction of 0, 10, 50, and 100 ppm solutions using the USN. The B-labeled traces are signals detected with the stack connected, introducing 0, 100, 50, and 10 ppm solutions into the USN. Pb (B) As mentioned above, only beryllium was detected using the reduced-pressure air-icp and multi-frequency AOTF-echelle spectrometer system during RM-29 sampling. An example of the monitor data acquired is shown in Figure 8 for the Be (II) nm line, for the time periods prior to and during RM-29 sampling on 9/21/00. For this particular run, water was initially spiked into the stack (USN output aerosol introduced into the stack at the port indicated in Figure 15
16 5), followed by the multi-metals standard, water, the multi-metals standard (for a period of time slightly longer than the 1-hour RM-29 sampling), and then water (at the end of the monitoring period). For Figure 8, the measured Be concentrations have been converted to µg/dscm values using the USN efficiency measured prior to the test (16%), the solution delivery rate (2.6 ml/min), the stack-gas flow rate (4.1 m 3 /min), and the moisture content (11.7 % for this run). A correction factor for the measured daily response of the air-icp AOTF-echelle system (signal intensity measured compared to that for the initial calibration curves) has also been applied. The initial monitor data in Figure 8 was acquired at a rate of approximately ten points per minute; starting at point number 312, a scan-averaged data acquisition of approximately two points per minute was used, resulting in an improved signal-to-noise ratio. The measured Be aerosol concentration during RM-29 sampling for this run was 145 µg/dscm initially (points ) and 161 µg/dscm at the end of the run (points ). Because of the shift in the baseline over the course of this run, baseline-average values of 49 and 81 µg/dscm, respectively, have been subtracted from the initial and final Be aerosol concentrations. ) m d sc g / (u n a tio n tr e C onc d e u r M eas Point Number Figure 8. Air-ICP monitor data for beryllium using the Be (II) nm line, during RM-29 sampling on 9/21/00; see text for discussion. The measured air-icp CEM results for Be and the determined RM-29 aerosol concentrations are listed in the table below. These air-icp Be concentrations are based on an estimated efficiency for the USN used to calibrate the response of the air-icp system. Prior to the test at DIAL, the nebulizer efficiency was measured under normal operating conditions, and a value of 16% was obtained. However, during the testing at DIAL, modified USN operating conditions were used - higher concentration solutions were nebulized and an extended length of tubing was used as the USN aerosol output line. We have no exact calibration of the USN under these conditions. However, air-icp monitor data acquired during the test indicated that aerosol 16
17 concentrations approximately 2.5 times lower resulted from the use of the extended length of USN output tubing. Based on the solution concentration used to deliver metals to the stack and the RM-29 results for those metals, one calculates an effective nebulizer efficiency of 5.3% when connected to the stack, about three times lower than the 16% value measured prior to the test. Unfortunately, we did not have the opportunity to recalibrate the nebulizer under the conditions that it was used as the calibration source. As a result, the 5.3% effective efficiency was used to calculate the air-icp Be concentrations below, along with the known solution delivery rate, stack-gas flow rate and moisture content, and the measured daily air-icp response factor. The first two runs were conducted on one day (9/20/00), and the reason for the discrepancy in the measured air-icp Be values for run 2 (12 compared to 77 µg/dscm) is unknown. The third run was conducted on the following day, and a value of 51 µg/dscm Be was determined. Since the concentration of the four metals in the multi-metals standard solution nebulized was the same for a given run, one would expect the RM-29 measured values for Be, Cd, and Pb to be the same. (Measured Cr concentrations are routinely elevated due to background Cr detected from the stack, perhaps due to corrosion of some steel components in the stack.) For run 1, the average RM-29 Be-Cd-Pb aerosol concentration is µg/dscm. For run 2, significantly lower values were determined (average Be-Cd-Pb concentration of 80.8 µg/dscm), although this run was nominally a repeat of run 1. Lower aerosol concentrations are expected for run 3 (measured average Be-Cd-Pb concentration of 75.3 µg/dscm), since a slightly lower concentration multimetals standard was nebulized using the USN. The 5.3% nebulizer efficiency used to calculate the tabulated air-icp Be concentrations may be a conservative value; this is an effective efficiency that may include some aerosol losses in the stack in the five meters between the sample introduction and sample extraction ports. The air-icp system was calibrated introducing the USN aerosol at a tee between the 24 and 12 sample lines (not into the stack), so it is conceivable that a slightly higher effective efficiency resulted during calibration of the air-icp. If one assumes that the air-icp and RM-29 values for Be should be the same for run 1, then the effective efficiency for the nebulizer (non-stack-connected) is about 7.4%. Applying this value to run 3 gives a Be value of 71.2 µg/dscm, in reasonably good agreement with the RM-29 value, 67.9 µg/dscm. The air-icp data for Be for run 2 is problematic; the reason for the lower value is unknown. 17
18 Measured Multi-Metals Aerosol Concentrations (µg/dscm) Run 1 Run 2 Run 3 Air-ICP CEM Be RM-29 Be Cd Cr Pb Mercury-Monitor CEM Stability and reproducibility studies for the mercury-monitor CEM were performed at Ames Laboratory prior to the DIAL facility test. The experimental setup was similar to that described for the facility test except that no sampling chamber was used and the mercury/room air sample was pumped through the mercury-monitor CEM using the internal pump of the VICI Dynacalibrator. The sample gas flow rate used in these studies was the same as that for the DIAL test, one standard Lpm. Reproducibility studies were conducted by recording the response of the mercury monitor over a two-week period while introducing mercury vapor into the 1-m absorption cell from a permeation tube having a certified emission rate of 8.71 ng/min Hg. Over the two-week period, the mercury-monitor CEM system yielded a response of 12.1 milliabsorbance units (±3%), after correction for any baseline drift. From this data, the detection limit of the mercury-monitor CEM system was estimated to be 0.51 µg/m 3 Hg. (Throughout this section, the reported mercury concentration is the average plus or minus one standard deviation.) Isokinetic sampling for the mercury-monitor CEM testing at DIAL was performed in the same manner and from the same stack location as that for the reduced-pressure air-icp CEM tests. The response of the mercury monitor was tested at a low level of 8 µg/m 3 and a high level of 80 µg/m 3 in the presence of hydrogen chloride (HCl) and sulfur dioxide (SO 2 ), common interferents present in combustion stacks. The absorption cell was heated to a temperature of 125 C to prevent condensation of moisture. The temperature measured at the cell windows was 69 C, well above the dew-point temperature of the DIAL stack gas. Facility testing of the mercury monitor was conducted over a two-day period. The first day of testing involved introducing mercury at high levels (80 µg/m 3 ) in the presence of approximately 100 ppmv HCl and 50 ppmv SO 2. Figure 9 shows typical data acquired during 18
19 the high-level run during RM-29 sampling on the first day. The SO 2 -corrected average concentration for mercury was 27.3 (±0.93) µg/m 3 for this particular run. For this test, SO 2 was continually introduced at approximately 50 ppmv. The arrows in Figure 9 indicate when HCl gas was introduced into the stack combustion stream. As can be noted from the data in Figure 9, the introduction of HCl did not result in any significant change in the measured mercury concentration. It is presumed that any mercuric chloride that formed in the stack under these conditions was subsequently decomposed in the pyrolyzer into elemental mercury and chlorine gas prior to passing through the absorption cell. Hg Concentration (µg/m 3 ) Figure 9. Mercury-monitor CEM data measured for high Hg concentration (80 µg/m 3 ) in the DIAL stack, when HCl was introduced at approximately 50 and 100 ppmv HCl (indicated by the arrows). The top trace is the SO 2 -uncorrected mercury concentration, and the bottom trace is the SO 2 -corrected value. The SO 2 concentration for this run was approximately 50 ppmv. Although the measured mercury concentration was lower than the expected 80 µg/m 3 value, the determined values were fairly stable. The lower-than-expected measured value is believed to be due to a loose fitting on the sampling chamber that resulted in a dilution of the stack sample gas with room air. This leak was not discovered until the middle of the second day of the mercury-monitor CEM testing. Once the loose fitting was discovered and the leak was sealed, the measured Hg concentrations reached the values expected, as discussed below. As a result, only the mercury-monitor data obtained after the discovery of the leak will be compared to the RM-29 results. The second day of testing involved monitoring mercury at low levels (8 µg/m 3 ) in the presence of approximately 25 ppmv HCl and 500 ppmv SO 2. Figure 10 shows the effect of SO 2 at low mercury levels. This data was acquired prior to the discovery of the sampling chamber 19
20 gas leak, so the measured values are lower than the expected Hg stack concentration. This data was obtained with HCl present at 25 ppmv. Prior to the introduction of SO 2, the measured mercury concentration was 1.51 (±0.21) µg/m 3. It is important to note that the SO 2 -corrected and -uncorrected values are identical at this point. At approximately point 970, SO 2 was starting to be detected by the mercury-monitor CEM, as indicated by the increase in the measured SO 2 and uncorrected mercury concentrations. Sulfur dioxide was initially introduced at a level of approximately 250 ppmv. At this level, the corrected mercury concentration was 0.98 (±0.25) µg/m 3. The SO 2 level was then further increased to 500 ppmv, at about point number 1030 in Figure 10. At this SO 2 level, the corrected mercury concentration was 0.58 (±0.25) µg/m 3. From this data, it would appear that the SO 2 -correction algorithm used was slightly over-correcting for the amount of SO 2 present in the gas sample stream. Hg Concentration (µg/m 3 ) SO2 Concentration (ppmv) Figure 10. Mercury-monitor CEM data at low Hg concentration (8 µg/m 3 ) in the DIAL stack. The dark solid line is the SO 2 -uncorrected mercury concentration, and the lighter solid trace is the value after being corrected for SO 2. The dashed line indicates the measured SO 2 concentration. The measured SO 2 values, approximately 35 and 70 ppmv, are lower than the expected levels, 250 and 500 ppmv, due to dilution in the sampling chamber because of the chamber gas leak. After the problem with the loose fitting on the sampling chamber was discovered and corrected, mercury concentrations closer to the expected values were measured, as shown in Figure 11, a re-run of the high-level stack mercury concentration (80 µg/m 3 ) on the second day of testing. Sealing the leak in the sampling chamber fitting also induced a baseline rise in the absorption measurement, as indicated in Figure 11 by the difference between the room air zerocheck (points 0-15, approximately) and stack gas (points 15-90) baseline values, prior to the introduction of Hg into the stack. It is believed the baseline increase is most likely due to the 20
21 increase of moisture in the absorption cell (i.e., the stack gas sample was no longer being diluted with drier room air), although other interferents potentially present in the stack gas (nitrogen dioxide or organic species) may also contribute. Although the baseline increase was undesirable, once stabilized, it did not present any problems in taking measurements. In order to account for the change in the baseline, an extrapolated baseline was generated and a point-by-point correction was applied to yield the mercury concentration in the combustion stack, for both the SO 2 -corrected and SO 2 -uncorrected data in Figures 11 and 12. When mercury was introduced into the stack (approximately points in Figure 11), the measured SO 2 -corrected mercury concentration was 78.4 (±3.0) µg/m 3. The SO 2 -uncorrected mercury-monitor data yielded a value of 77.8 (±2.9) µg/m 3 mercury for the 10-minute testing period shown in Figure 11. The measured SO 2 concentration was 674 (±4.4) ppmv, higher than the expected 500 ppmv value. While it is possible that there is a low (background) concentration of SO 2 in the combustion stack gas at DIAL, the higher-than-expected measured SO 2 concentration may also be due to absorption at nm by water vapor or other stack gas interferents. Hg Concentration (µg/m 3 ) SO2 Concentration (ppmv) Figure 11. Repeat of a high-level (80 µg/m 3 ) mercury run after sealing the leak in the sampling chamber on the second day of Hg stack monitoring. The increased baseline for the mercury-monitor data is likely due to an increase in the absorption contribution of water at nm measured for the stack gas sample compared to that for room air. Figure 12 shows the response of the mercury monitor during a low-level (8 µg/m 3 ) run on the second day of testing, in the presence of approximately 500 ppmv SO 2 and 25 ppmv HCl. Again, a baseline offset between the room air zero-check and stack gas values (points in Figure 12, prior to the introduction of Hg) is observed. This increase is most likely due to the increase in moisture for the stack gas sample compared to that for room air. For the two-hour 21
22 testing period shown in Figure 12, the measured SO 2 -corrected mercury concentration is 11.8 (±1.2) µg/m 3. The SO 2 -uncorrected data yields a mercury concentration of 11.2 (±1.4) µg/m 3. Some of the variance in these determined values is due to the baseline drift observed over the course of this two-hour run. The source of this drift in the measured values for this mercurymonitor data is unknown. Hg Concentration (µg/m 3 ) SO2 Concentration (ppmv) Figure 12. Low-level (8 µg/m 3 ) mercury test run, in the presence of approximately 25 ppmv HCl and 500 ppmv SO 2. The baseline increase is likely due to the absorption contribution of water at nm measured for the stack gas compared to that for room air. The measured SO 2 concentration is 674 (±4.4) ppmv. The test results for the mercury-monitor CEM and RM-29 sampling are summarized in the table below. Only the mercury CEM concentrations determined during the latter part of the second day are reported (the values measured after correcting the sampling chamber leak). The RM-29 and mercury CEM results are compared as micrograms Hg per dry standard cubic meter, with the percent water in the sample stream determined from the RM-29 sampling procedure. Although only one measurement for the mercury-monitor CEM is reported for each of the high- and low-level mercury tests at DIAL, the values are in very good agreement with the RM-29 results. Day 1 Day 2 RM µg/dscm µg/dscm 9.92 µg/dscm µg/dscm % H 2 O 11.46% 11.21% 10.86% 10.82% Mercury CEM Concentration * 78.4 (±3.0) µg/m (±1.2) µg/m 3 Mercury CEM-H 2 O Corrected * 87.9 (±3.4) µg/dscm (±1.3) µg/dscm * Data taken on day 2 of mercury-monitor testing, using the same operating conditions as those used on day 1. 22
23 Conclusions and Continued Work The work performed during the site-test at DIAL at the end of FY00 was the first fieldtest of both the multi-frequency AOTF-echelle spectrometer and the mercury-monitor CEM systems. A significant amount of information was obtained during the tests, regarding the performance of the reduced-pressure air-icp and mercury-monitor CEM systems in nonlaboratory operating conditions. As with any initial field-test, successful results were obtained for some aspects of the test, some problems were identified, and some changes and modifications to the instrumentation and operating procedures will be made based on the results of the work done at DIAL. For the reduced-pressure air-icp and multi-frequency AOTF-echelle spectrometer, used as a multi-metals CEM system, the most significant problem was the degradation in detection limits for Be, Cd, Cr, and Pb during stack sampling that resulted from the elevation in the detected plasma background emission. Since the test at DIAL, the α-bbo Glan-Taylor polarizers have been replaced with α-bbo Rochon prism polarizers (Casix model PRH8010). The new polarizers function well from nm, significantly reducing the amount of visible and near-infrared plasma background signal that leaks through the polarizers (compared to the Glan-Taylor polarizers). With the incorporation of these new polarizers into the optics of the AOTF-echelle, the performance of the spectrometer is improved considerably. A small amount of non-aotf-selected plasma background is still detected (possibly due to the birefringence of the AOTF crystal itself or scattering from the AOTF), but the situation is significantly improved with the new Rochon polarizers compared to the Glan-Taylor polarizers used during the test at DIAL. Despite the incorporation of the ballast into the continuous sampling system during FY00, apparent air-icp axial channel gas flow fluctuations and resulting ICP torch flicker were observed during testing when an ICP torch having a 1-mm diameter axial channel was used. The inclusion of the ballast has not resulted in a completely stable sample gas flow to the reducedpressure air-icp, at least when sampling from a reduced-pressure exhaust stack such as that at DIAL, using the sampling pump and the ICP chamber pump. However, during times that partially-fused (<1-mm) axial channel ICP torches were used, no obvious torch flicker or significant axial channel gas flow variations were observed. In addition, no significant gas flow fluctuations were observed during testing of the mercury-monitor CEM system using the same 23
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
OES - Optical Emission Spectrometer 2000
 OES - Optical Emission Spectrometer 2000 OES-2000 is used to detect the presence of trace metals in an analyte. The analyte sample is introduced into the OES-2000 as an aerosol that is carried into the
OES - Optical Emission Spectrometer 2000 OES-2000 is used to detect the presence of trace metals in an analyte. The analyte sample is introduced into the OES-2000 as an aerosol that is carried into the
Observation of Self Absorption of Mercury I and Cadmium I Emission in an Atmospheric Microwave Sustained Plasma
 Observation of Self Absorption of Mercury I and Cadmium I Emission in an Atmospheric Microwave Sustained Plasma Kamal Hadidi *, Paul Woskov, Karyn Green, Paul Thomas Plasma Science and Fusion Center Massachusetts
Observation of Self Absorption of Mercury I and Cadmium I Emission in an Atmospheric Microwave Sustained Plasma Kamal Hadidi *, Paul Woskov, Karyn Green, Paul Thomas Plasma Science and Fusion Center Massachusetts
a. An emission line as close as possible to the analyte resonance line
 Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
Lecture 16 Instrumentation for ICP AES-VIII-Instruments
 Inductive Couple Plasma Atomic Emission Spectrometry (ICP-AES) for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture 16 Instrumentation
Inductive Couple Plasma Atomic Emission Spectrometry (ICP-AES) for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture 16 Instrumentation
2101 Atomic Spectroscopy
 2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
Chapter 9. Atomic emission and Atomic Fluorescence Spectrometry Emission spectrophotometric Techniques
 Chapter 9 Atomic emission and Atomic Fluorescence Spectrometry Emission spectrophotometric Techniques Emission Spectroscopy Flame and Plasma Emission Spectroscopy are based upon those particles that are
Chapter 9 Atomic emission and Atomic Fluorescence Spectrometry Emission spectrophotometric Techniques Emission Spectroscopy Flame and Plasma Emission Spectroscopy are based upon those particles that are
10/2/2008. hc λ. νλ =c. proportional to frequency. Energy is inversely proportional to wavelength And is directly proportional to wavenumber
 CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
Hydride Generation for the Determination of As, Sb, Se and Bi Using the Teledyne Leeman Lab s Prodigy 7 ICP-OES
 Application Note - AN1508 Hydride Generation for the Determination of As, Sb, Se and Bi Using the Teledyne Leeman Lab s Prodigy 7 ICP-OES Introduction Page 1 The combination of hydride generation with
Application Note - AN1508 Hydride Generation for the Determination of As, Sb, Se and Bi Using the Teledyne Leeman Lab s Prodigy 7 ICP-OES Introduction Page 1 The combination of hydride generation with
25 Instruments for Optical Spectrometry
 25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
Atomic Emission Spectroscopy
 Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
ICP-3000 Inductively Coupled Plasma Optical Emission Spectrometer
 Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor
 13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
H. R. Hirsch R. J. Hull R. H. Kohler Mrs. Ilana Levitan K. K. Y. Li J. H. Loehlin
 IIIL - -~ V. NUCLEAR MAGNETIC RESONANCE AND HYPERFINE STRUCTURE Prof. F. Bitter Prof. L. C. Bradley III Prof. J. S. Waugh Dr. H. H. Stroke Dr. J. F. Waymouth B. B. Aubrey M. Ciftan H. R. Hirsch R. J. Hull
IIIL - -~ V. NUCLEAR MAGNETIC RESONANCE AND HYPERFINE STRUCTURE Prof. F. Bitter Prof. L. C. Bradley III Prof. J. S. Waugh Dr. H. H. Stroke Dr. J. F. Waymouth B. B. Aubrey M. Ciftan H. R. Hirsch R. J. Hull
PRINCIPLE OF ICP- AES
 INTRODUCTION Non- flame atomic emission techniques, which use electrothermal means to atomize and excite the analyte, include inductively coupled plasma and arc spark. It has been 30 years since Inductively
INTRODUCTION Non- flame atomic emission techniques, which use electrothermal means to atomize and excite the analyte, include inductively coupled plasma and arc spark. It has been 30 years since Inductively
ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW
 ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces
ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces
- A spark is passed through the Argon in the presence of the RF field of the coil to initiate the plasma
 THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
Burner Tubing Specification for the Turbulent Ethylene Non-Premixed Jet Flame
 Burner Tubing Specification for the Turbulent Ethylene Non-Premixed Jet Flame Figure 1 shows a schematic of the burner used to support the turbulent ethylene non-premixed jet flames. The dimensions of
Burner Tubing Specification for the Turbulent Ethylene Non-Premixed Jet Flame Figure 1 shows a schematic of the burner used to support the turbulent ethylene non-premixed jet flames. The dimensions of
Application of Hydroxyl (OH) Radical Ultraviolet Absorption Spectroscopy to Rocket Plumes
 Application of Hydroxyl (OH) Radical Ultraviolet Absorption Spectroscopy to Rocket Plumes M. W. Teague*, Tonya Felix*, M. K. Hudson, and R. Shanks *Department of Chemistry, Hendrix College, Conway, AR
Application of Hydroxyl (OH) Radical Ultraviolet Absorption Spectroscopy to Rocket Plumes M. W. Teague*, Tonya Felix*, M. K. Hudson, and R. Shanks *Department of Chemistry, Hendrix College, Conway, AR
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy
 Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
High Resolution Optical Spectroscopy
 PHYS 3719 High Resolution Optical Spectroscopy Introduction This experiment will allow you to learn a specific optical technique with applications over a wide variety of phenomena. You will use a commercial
PHYS 3719 High Resolution Optical Spectroscopy Introduction This experiment will allow you to learn a specific optical technique with applications over a wide variety of phenomena. You will use a commercial
Spectrometric Methods of Analysis. OCN 633 Fall 2013
 Spectrometric Methods of Analysis OCN 633 Fall 2013 Plasma Emission and Plasma Mass Spectroscopy Two fields of elemental analysis undergoing the most study Myriad analytical applications Three categories
Spectrometric Methods of Analysis OCN 633 Fall 2013 Plasma Emission and Plasma Mass Spectroscopy Two fields of elemental analysis undergoing the most study Myriad analytical applications Three categories
ICP-OES Application Note Number 35
 ICP-OES Application Note Number 35 Rapid measurement of major, minor and trace levels in soils using the Varian 730-ES Vincent Calderon Varian, Inc. Introduction As part of the global strategy for sustainable
ICP-OES Application Note Number 35 Rapid measurement of major, minor and trace levels in soils using the Varian 730-ES Vincent Calderon Varian, Inc. Introduction As part of the global strategy for sustainable
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
Atomic Absorption Spectrophotometry. Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon
 Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Effect of Filter Choice on OH* Chemiluminescence Kinetics at Low and Elevated Pressures
 7 th US National Technical Meeting of the Combustion Institute Hosted by the Georgia Institute of Technology, Atlanta, GA March 20-23, 2011 Effect of Filter Choice on OH* Chemiluminescence Kinetics at
7 th US National Technical Meeting of the Combustion Institute Hosted by the Georgia Institute of Technology, Atlanta, GA March 20-23, 2011 Effect of Filter Choice on OH* Chemiluminescence Kinetics at
Atomization. In Flame Emission
 FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
The Evolution of Inductively Coupled Plasma - Optical Emission Spectroscopy (ICP-OES)
 The Evolution of Inductively Coupled Plasma - Optical Emission Spectroscopy (ICP-OES) Part I: The 1970s Since its commercial inception in 1974, ICP-OES has seen significant technological advancements over
The Evolution of Inductively Coupled Plasma - Optical Emission Spectroscopy (ICP-OES) Part I: The 1970s Since its commercial inception in 1974, ICP-OES has seen significant technological advancements over
Spectroscopy Problem Set February 22, 2018
 Spectroscopy Problem Set February, 018 4 3 5 1 6 7 8 1. In the diagram above which of the following represent vibrational relaxations? 1. Which of the following represent an absorbance? 3. Which of following
Spectroscopy Problem Set February, 018 4 3 5 1 6 7 8 1. In the diagram above which of the following represent vibrational relaxations? 1. Which of the following represent an absorbance? 3. Which of following
Characterisation & Use of Array Spectrometers
 Characterisation & Use of Array Spectrometers Mike Shaw, Optical Technologies & Scientific Computing Team, National Physical Laboratory, Teddington Middlesex, UK 1 Overview Basic design and features of
Characterisation & Use of Array Spectrometers Mike Shaw, Optical Technologies & Scientific Computing Team, National Physical Laboratory, Teddington Middlesex, UK 1 Overview Basic design and features of
Analysis of Cadmium (Cd) in Plastic Using X-ray Fluorescence Spectroscopy
 Analysis of Cadmium (Cd) in Plastic Using X-ray Fluorescence Spectroscopy Hiroshi Onodera Application & Research Center, JEOL Ltd. Introduction um, PBB and PBDE) are subject to usage restrictions in Europe.
Analysis of Cadmium (Cd) in Plastic Using X-ray Fluorescence Spectroscopy Hiroshi Onodera Application & Research Center, JEOL Ltd. Introduction um, PBB and PBDE) are subject to usage restrictions in Europe.
THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION
 JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
First observations of the second solar spectrum with spatial resolution at the Lunette Jean Rösch
 First observations of the second solar spectrum with spatial resolution at the Lunette Jean Rösch Malherbe, J.-M., Moity, J., Arnaud, J., Roudier, Th., July 2006 The experiment setup in spectroscopic mode
First observations of the second solar spectrum with spatial resolution at the Lunette Jean Rösch Malherbe, J.-M., Moity, J., Arnaud, J., Roudier, Th., July 2006 The experiment setup in spectroscopic mode
Atomic emission spectra experiment
 Atomic emission spectra experiment Contents 1 Overview 1 2 Equipment 1 3 Measuring the grating spacing using the sodium D-lines 4 4 Measurement of hydrogen lines and the Rydberg Constant 5 5 Measurement
Atomic emission spectra experiment Contents 1 Overview 1 2 Equipment 1 3 Measuring the grating spacing using the sodium D-lines 4 4 Measurement of hydrogen lines and the Rydberg Constant 5 5 Measurement
INDUCTIVELY COUPLED PLASMA MASS SPECTROMETRY
 INDUCTIVELY COUPLED PLASMA MASS SPECTROMETRY Edited by AKBAR MONTASER George Washington University Washington, D.C. 20052, USA WILEY-VCH New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
INDUCTIVELY COUPLED PLASMA MASS SPECTROMETRY Edited by AKBAR MONTASER George Washington University Washington, D.C. 20052, USA WILEY-VCH New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
Complete the following. Clearly mark your answers. YOU MUST SHOW YOUR WORK TO RECEIVE CREDIT.
 CHEM 322 Name Exam 3 Spring 2013 Complete the following. Clearly mark your answers. YOU MUST SHOW YOUR WORK TO RECEIVE CREDIT. Warm-up (3 points each). 1. In Raman Spectroscopy, molecules are promoted
CHEM 322 Name Exam 3 Spring 2013 Complete the following. Clearly mark your answers. YOU MUST SHOW YOUR WORK TO RECEIVE CREDIT. Warm-up (3 points each). 1. In Raman Spectroscopy, molecules are promoted
Chemistry 524--Final Exam--Keiderling May 4, :30 -?? pm SES
 Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Gaseous CEMS technology
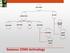 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS)
 Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
CH0302 Process Instrumentation. Lecture 9 Composition Analysis
 CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
ENVG FALL ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analytical Techniques
 ENVG 60500 FALL 2013 ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analytical Techniques HISTORY In the 1940s, arc and high-voltage spark spectrometry became widely utilized for metal analysis
ENVG 60500 FALL 2013 ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analytical Techniques HISTORY In the 1940s, arc and high-voltage spark spectrometry became widely utilized for metal analysis
Measurement Technique and its Application to Trace Components of Atmospheric Gas
 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
Laboratory #29: Spectrometer
 INDIANA UNIVERSITY, DEPARTMENT OF PHYSICS, P309 LABORATORY Laboratory #29: Spectrometer Goal: Learn to adjust an optical spectrometer, use a transmission grating to measure known spectral lines of mercury,
INDIANA UNIVERSITY, DEPARTMENT OF PHYSICS, P309 LABORATORY Laboratory #29: Spectrometer Goal: Learn to adjust an optical spectrometer, use a transmission grating to measure known spectral lines of mercury,
Jumbo Cross Beam Ionizer
 Jumbo Cross Beam Ionizer Version 1.0 September 30, 2008 Page 1 of 19 Table of Contents Cross Beam Ionizer... 1 Table of Contents... 2 1.0 Packing List... 3 1.1 Packing List for Cross Beam Ionizer... 3
Jumbo Cross Beam Ionizer Version 1.0 September 30, 2008 Page 1 of 19 Table of Contents Cross Beam Ionizer... 1 Table of Contents... 2 1.0 Packing List... 3 1.1 Packing List for Cross Beam Ionizer... 3
Astronomy 203 practice final examination
 Astronomy 203 practice final examination Fall 1999 If this were a real, in-class examination, you would be reminded here of the exam rules, which are as follows: You may consult only one page of formulas
Astronomy 203 practice final examination Fall 1999 If this were a real, in-class examination, you would be reminded here of the exam rules, which are as follows: You may consult only one page of formulas
ATOMIC SPECROSCOPY (AS)
 ATOMIC ABSORPTION ANALYTICAL CHEMISTRY ATOMIC SPECROSCOPY (AS) Atomic Absorption Spectroscopy 1- Flame Atomic Absorption Spectreoscopy (FAAS) 2- Electrothermal ( Flame-less ) Atomic Absorption Spectroscopy
ATOMIC ABSORPTION ANALYTICAL CHEMISTRY ATOMIC SPECROSCOPY (AS) Atomic Absorption Spectroscopy 1- Flame Atomic Absorption Spectreoscopy (FAAS) 2- Electrothermal ( Flame-less ) Atomic Absorption Spectroscopy
novaa 800 D Atomic Absorption Spectrometer
 Technical Data Atomic Absorption Spectrometer Cpt : +27 (0) 21 905 0476 Jhb : +27 (0) 11 794 Dbn : +27 (0) 31 266 2454 1/7 General The is a compact atomic absorption spectrometer with deuterium background
Technical Data Atomic Absorption Spectrometer Cpt : +27 (0) 21 905 0476 Jhb : +27 (0) 11 794 Dbn : +27 (0) 31 266 2454 1/7 General The is a compact atomic absorption spectrometer with deuterium background
Fast Analysis of Water Samples Comparing Axially-and Radially- Viewed CCD Simultaneous ICP-OES
 Fast Analysis of Water Samples Comparing Axially-and Radially- Viewed CCD Simultaneous ICP-OES Application Note Inductively Coupled Plasma-Optical Emission Spectrometers Author Tran T. Nham Introduction
Fast Analysis of Water Samples Comparing Axially-and Radially- Viewed CCD Simultaneous ICP-OES Application Note Inductively Coupled Plasma-Optical Emission Spectrometers Author Tran T. Nham Introduction
Agilent 7500a Inductively Coupled Plasma Mass Spectrometer (ICP-MS)
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment Agilent 7500a Inductively Coupled Plasma Mass Spectrometer (ICP-MS) The Agilent
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment Agilent 7500a Inductively Coupled Plasma Mass Spectrometer (ICP-MS) The Agilent
Steven C. DeCaluwe a,b, Paul A. Kienzle b, Pavan Bhargava b,c, Andrew M. Baker b,d, Joseph A. Dura b
 Supplemental Information Neutron Reflectometry Fitting Techniques for Under- determined, Multi- layered Structures: Lamellar Phase Segregation in Ultra- thin Nafion Films Steven C. DeCaluwe a,b, Paul A.
Supplemental Information Neutron Reflectometry Fitting Techniques for Under- determined, Multi- layered Structures: Lamellar Phase Segregation in Ultra- thin Nafion Films Steven C. DeCaluwe a,b, Paul A.
Approval Block. Prepared by: Signature Date Evan Parnell 08 NOV Reviewed by: Signature Date. Approved by: Signature Date
 ATS-SOI-3660 Page: 1 of 6 Approval Block Prepared by: Signature Date Evan Parnell 08 NOV 2013 Reviewed by: Signature Date Brian Flynn 08 NOV 2013 Approved by: Signature Date Kristal Jewell 08 NOV 2013
ATS-SOI-3660 Page: 1 of 6 Approval Block Prepared by: Signature Date Evan Parnell 08 NOV 2013 Reviewed by: Signature Date Brian Flynn 08 NOV 2013 Approved by: Signature Date Kristal Jewell 08 NOV 2013
high temp ( K) Chapter 20: Atomic Spectroscopy
 high temp (2000-6000K) Chapter 20: Atomic Spectroscopy 20-1. An Overview Most compounds Atoms in gas phase high temp (2000-6000K) (AES) (AAS) (AFS) sample Mass-to-charge (ICP-MS) Atomic Absorption experiment
high temp (2000-6000K) Chapter 20: Atomic Spectroscopy 20-1. An Overview Most compounds Atoms in gas phase high temp (2000-6000K) (AES) (AAS) (AFS) sample Mass-to-charge (ICP-MS) Atomic Absorption experiment
EMISSION SPECTROSCOPY
 IFM The Department of Physics, Chemistry and Biology LAB 57 EMISSION SPECTROSCOPY NAME PERSONAL NUMBER DATE APPROVED I. OBJECTIVES - Understand the principle of atomic emission spectra. - Know how to acquire
IFM The Department of Physics, Chemistry and Biology LAB 57 EMISSION SPECTROSCOPY NAME PERSONAL NUMBER DATE APPROVED I. OBJECTIVES - Understand the principle of atomic emission spectra. - Know how to acquire
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Spectroscopic studies of impurities in the LHD plasmas
 Spectroscopic studies of impurities in the LHD plasmas Visitor: Zhenwei Wu (the institute of plasma physics, CAS -ASIPP) Host: Shigeru Morita (the national institute for fusion science -NIFS) Content 1.
Spectroscopic studies of impurities in the LHD plasmas Visitor: Zhenwei Wu (the institute of plasma physics, CAS -ASIPP) Host: Shigeru Morita (the national institute for fusion science -NIFS) Content 1.
CHAPTER III EXPERIMENTAL SYSTEM
 CHAPTER III EXPERIMENTAL SYSTEM 3.1 Introduction The basic design and implementation of laser induced Raman scattering systems is, in general, relatively simple. Laser light from a single laser source
CHAPTER III EXPERIMENTAL SYSTEM 3.1 Introduction The basic design and implementation of laser induced Raman scattering systems is, in general, relatively simple. Laser light from a single laser source
Enhancing the productivity of food sample analysis with the Agilent 7700x ICP-MS
 Enhancing the productivity of food sample analysis with the Agilent 77x ICP-MS Application note Foods testing Authors Sebastien Sannac, Jean Pierre Lener and Jerome Darrouzes Agilent Technologies Paris,
Enhancing the productivity of food sample analysis with the Agilent 77x ICP-MS Application note Foods testing Authors Sebastien Sannac, Jean Pierre Lener and Jerome Darrouzes Agilent Technologies Paris,
Introduction to Blackbody Sources
 Introduction to s This section contains dedicated blackbody sources for low uncertainty calibration of infrared thermometers. A range of portable primary blackbody sources combine high emissivity with
Introduction to s This section contains dedicated blackbody sources for low uncertainty calibration of infrared thermometers. A range of portable primary blackbody sources combine high emissivity with
Atomic Absorption & Atomic Fluorescence Spectrometry
 Atomic Absorption & Atomic Fluorescence Spectrometry Sample Atomization Atomic Absorption (AA) Atomic Fluorescence (AF) - Both AA and AF require a light source - Like Molecular Absorption & Fluorescence,
Atomic Absorption & Atomic Fluorescence Spectrometry Sample Atomization Atomic Absorption (AA) Atomic Fluorescence (AF) - Both AA and AF require a light source - Like Molecular Absorption & Fluorescence,
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Atomic Absorption Spectroscopy
 CH 2252 Instrumental Methods of Analysis Unit IV Atomic Absorption Spectroscopy Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering
CH 2252 Instrumental Methods of Analysis Unit IV Atomic Absorption Spectroscopy Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering
Multiphase Oscillatory Flow Strategy for in Situ Measurement and Screening of Partition Coefficients
 Supporting Information Multiphase Oscillatory Flow Strategy for in Situ Measurement and Screening of Partition Coefficients Milad Abolhasani, Connor W. Coley, and Klavs F. Jensen * Department of Chemical
Supporting Information Multiphase Oscillatory Flow Strategy for in Situ Measurement and Screening of Partition Coefficients Milad Abolhasani, Connor W. Coley, and Klavs F. Jensen * Department of Chemical
The Spectroscopy of Stars
 The Spectroscopy of Stars In this activity you will use a hand held spectroscope to investigate a number of known and unknown light sources. A spectroscope is an instrument that helps to observe the spectrum
The Spectroscopy of Stars In this activity you will use a hand held spectroscope to investigate a number of known and unknown light sources. A spectroscope is an instrument that helps to observe the spectrum
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Optics. Measuring the line spectra of inert gases and metal vapors using a prism spectrometer. LD Physics Leaflets P
 Optics Spectrometer Prism spectrometer LD Physics Leaflets P5.7.1.1 Measuring the line spectra of inert gases and metal vapors using a prism spectrometer Objects of the experiment Adjusting the prism spectrometer.
Optics Spectrometer Prism spectrometer LD Physics Leaflets P5.7.1.1 Measuring the line spectra of inert gases and metal vapors using a prism spectrometer Objects of the experiment Adjusting the prism spectrometer.
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
A simple electric thruster based on ion charge exchange
 A simple electric thruster based on ion charge exchange IEPC-2007-35 Presented at the 30 th International Electric Propulsion Conference, Florence, Italy Joe Khachan and Lachlan Blackhall University of
A simple electric thruster based on ion charge exchange IEPC-2007-35 Presented at the 30 th International Electric Propulsion Conference, Florence, Italy Joe Khachan and Lachlan Blackhall University of
Confirmation of Heat Generation during Hydrogenation of Oil
 Confirmation of Heat Generation during Hydrogenation of Oil T. Mizuno Hydrogen Engineering Application & Development Corporation, 4-3-9-12, Minamimach Makomanai Minamiku, Sapporo 1-16, Japan E-mail: mizuno@qe.eng.hokudai.ac.jp
Confirmation of Heat Generation during Hydrogenation of Oil T. Mizuno Hydrogen Engineering Application & Development Corporation, 4-3-9-12, Minamimach Makomanai Minamiku, Sapporo 1-16, Japan E-mail: mizuno@qe.eng.hokudai.ac.jp
Multi-Element Analysis of Petroleum Crude Oils using an Agilent 7900 ICP-MS
 Multi-Element Analysis of Petroleum Crude Oils using an Agilent 7900 ICP-MS Application note Energy and fuels Authors Jenny Nelson, Agilent Technologies, USA Ed McCurdy, Agilent Technologies, UK Introduction
Multi-Element Analysis of Petroleum Crude Oils using an Agilent 7900 ICP-MS Application note Energy and fuels Authors Jenny Nelson, Agilent Technologies, USA Ed McCurdy, Agilent Technologies, UK Introduction
Atomic Absorption Spectrometer ZEEnit P series
 Atomic Absorption Spectrometer ZEEnit P series Technical Data ZEEnit series Update 07/2014 OBue 1/ 5 ZEEnit P series Variable high-end AA Spectrometer with Deuterium and Zeeman Background Correction with
Atomic Absorption Spectrometer ZEEnit P series Technical Data ZEEnit series Update 07/2014 OBue 1/ 5 ZEEnit P series Variable high-end AA Spectrometer with Deuterium and Zeeman Background Correction with
Defining quality standards for the analysis of solid samples
 Defining quality standards for the analysis of solid samples Thermo Scientific Element GD Plus Glow Discharge Mass Spectrometer Redefine your quality standards for the elemental analysis of solid samples
Defining quality standards for the analysis of solid samples Thermo Scientific Element GD Plus Glow Discharge Mass Spectrometer Redefine your quality standards for the elemental analysis of solid samples
Experiment 24: Spectroscopy
 Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Atomic Spectroscopy AA/ICP/ICPMS:
 Atomic Spectroscopy AA/ICP/ICPMS: A Comparison of Techniques VA AWWA/VWEA Lab Practices Conference July 25, 2016 Dan Davis Shimadzu Scientific Instruments AA/ICP/ICPMS: A Comparison of Techniques Topics
Atomic Spectroscopy AA/ICP/ICPMS: A Comparison of Techniques VA AWWA/VWEA Lab Practices Conference July 25, 2016 Dan Davis Shimadzu Scientific Instruments AA/ICP/ICPMS: A Comparison of Techniques Topics
AE 3051, Lab #16. Investigation of the Ideal Gas State Equation. By: George P. Burdell. Group E3
 AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
The Vacuum Case for KATRIN
 The Vacuum Case for KATRIN Institute of Nuclear Physics, Forschungszentrum Karlsruhe,Germany, for the KATRIN Collaboration Lutz.Bornschein@ik.fzk.de The vacuum requirements of the KATRIN experiment have
The Vacuum Case for KATRIN Institute of Nuclear Physics, Forschungszentrum Karlsruhe,Germany, for the KATRIN Collaboration Lutz.Bornschein@ik.fzk.de The vacuum requirements of the KATRIN experiment have
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
 Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
DAY LABORATORY EXERCISE: SPECTROSCOPY
 AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
Evaluation, Control and Diagnosis of an ICP Through Simple Experiments
 ICP OPTICAL ATOMIC EMISSION SPECTROSCOPY Evaluation, Control and Diagnosis of an ICP Through Simple Experiments Odile Hirsch HORUIBA Scientific, Longjumeau, France Keywords: general 1 Introduction To undertake
ICP OPTICAL ATOMIC EMISSION SPECTROSCOPY Evaluation, Control and Diagnosis of an ICP Through Simple Experiments Odile Hirsch HORUIBA Scientific, Longjumeau, France Keywords: general 1 Introduction To undertake
Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring
 Physical Sciences Inc. VG15-012 Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring January 26, 2015 Krishnan R. Parameswaran, Clinton J. Smith, Kristin L. Galbally-Kinney, William J. Kessler
Physical Sciences Inc. VG15-012 Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring January 26, 2015 Krishnan R. Parameswaran, Clinton J. Smith, Kristin L. Galbally-Kinney, William J. Kessler
9/13/10. Each spectral line is characteristic of an individual energy transition
 Sensitive and selective determination of (primarily) metals at low concentrations Each spectral line is characteristic of an individual energy transition 1 Atomic Line Widths Why do atomic spectra have
Sensitive and selective determination of (primarily) metals at low concentrations Each spectral line is characteristic of an individual energy transition 1 Atomic Line Widths Why do atomic spectra have
Two-Phase Refrigerant Distribution in a Micro- Channel Manifold
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 6 Two-Phase Refrigerant Distribution in a Micro- Channel Manifold Chad D. Bowers
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 6 Two-Phase Refrigerant Distribution in a Micro- Channel Manifold Chad D. Bowers
Chem 310 rd. 3 Homework Set Answers
 -1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
-1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
Chem Homework Set Answers
 Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Sensitive Detection and Identification of Isovanillin Aerosol Particles at the pg/cm 3 Mass Concentration Level Using Raman Spectroscopy*
 Sensitive Detection and Identification of Isovanillin Aerosol Particles at the pg/cm 3 Mass Concentration Level Using Raman Spectroscopy* R. L. Aggarwal 1, S. Di Cecca, L. W. Farrar, Shabshelowitz, A.,
Sensitive Detection and Identification of Isovanillin Aerosol Particles at the pg/cm 3 Mass Concentration Level Using Raman Spectroscopy* R. L. Aggarwal 1, S. Di Cecca, L. W. Farrar, Shabshelowitz, A.,
Overview of X-Ray Fluorescence Analysis
 Overview of X-Ray Fluorescence Analysis AMPTEK, INC., Bedford, MA 01730 Ph: +1 781 275 2242 Fax: +1 781 275 3470 sales@amptek.com 1 What is X-Ray Fluorescence (XRF)? A physical process: Emission of characteristic
Overview of X-Ray Fluorescence Analysis AMPTEK, INC., Bedford, MA 01730 Ph: +1 781 275 2242 Fax: +1 781 275 3470 sales@amptek.com 1 What is X-Ray Fluorescence (XRF)? A physical process: Emission of characteristic
Application Note GA-301E. MBMS for Preformed Ions. Extrel CMS, 575 Epsilon Drive, Pittsburgh, PA I. SAMPLING A CHEMICAL SOUP
 Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Analysis of Trace Metal Impurities in High Purity Hydrochloric Acid Using ICP-QQQ
 Application Note Semiconductor Analysis of Trace Metal Impurities in High Purity Hydrochloric Acid Using ICP-QQQ Authors Kazuo Yamanaka and Kazuhiro Sakai Agilent Technologies, Japan Introduction Hydrochloric
Application Note Semiconductor Analysis of Trace Metal Impurities in High Purity Hydrochloric Acid Using ICP-QQQ Authors Kazuo Yamanaka and Kazuhiro Sakai Agilent Technologies, Japan Introduction Hydrochloric
EXPERIMENTAL DETERMINATION OF SPECTRAL AND ANGULAR DEPENDENT OPTICAL PROPERTIES OF INSULATING GLASSES
 CISBAT 2005, Proceedings, EPFL 2005, p. 441-446 EXPERIMENTAL DETERMINATION OF SPECTRAL AND ANGULAR DEPENDENT OPTICAL PROPERTIES OF INSULATING GLASSES R. Steiner, P. Oelhafen, G. Reber and A. Romanyuk Institute
CISBAT 2005, Proceedings, EPFL 2005, p. 441-446 EXPERIMENTAL DETERMINATION OF SPECTRAL AND ANGULAR DEPENDENT OPTICAL PROPERTIES OF INSULATING GLASSES R. Steiner, P. Oelhafen, G. Reber and A. Romanyuk Institute
Supplemental material for Bound electron nonlinearity beyond the ionization threshold
 Supplemental material for Bound electron nonlinearity beyond the ionization threshold 1. Experimental setup The laser used in the experiments is a λ=800 nm Ti:Sapphire amplifier producing 42 fs, 10 mj
Supplemental material for Bound electron nonlinearity beyond the ionization threshold 1. Experimental setup The laser used in the experiments is a λ=800 nm Ti:Sapphire amplifier producing 42 fs, 10 mj
CHAPTER 4: ANALYTICAL INSTRUMENTATION
 CHAPTER 4: ANALYTICAL INSTRUMENTATION 4.1 INTRODUCTION In this section, a review of the analytical instrumentation used during sample preparation and analysis is presented which includes an overview of
CHAPTER 4: ANALYTICAL INSTRUMENTATION 4.1 INTRODUCTION In this section, a review of the analytical instrumentation used during sample preparation and analysis is presented which includes an overview of
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Problem Solving. radians. 180 radians Stars & Elementary Astrophysics: Introduction Press F1 for Help 41. f s. picture. equation.
 Problem Solving picture θ f = 10 m s =1 cm equation rearrange numbers with units θ factors to change units s θ = = f sinθ fθ = s / cm 10 m f 1 m 100 cm check dimensions 1 3 π 180 radians = 10 60 arcmin
Problem Solving picture θ f = 10 m s =1 cm equation rearrange numbers with units θ factors to change units s θ = = f sinθ fθ = s / cm 10 m f 1 m 100 cm check dimensions 1 3 π 180 radians = 10 60 arcmin
Far UV Performance of the LAMBDA 850/950 UV/Vis and UV/Vis/NIR Research Spectrophotometers
 Far UV Performance of the LAMBDA 850/950 UV/Vis and UV/Vis/NIR Research Spectrophotometers UV/VIS AND UV/VIS/NIR SPECTROSCOPY A P P L I C A T I O N N O T E Summary The far UV region (generally covering
Far UV Performance of the LAMBDA 850/950 UV/Vis and UV/Vis/NIR Research Spectrophotometers UV/VIS AND UV/VIS/NIR SPECTROSCOPY A P P L I C A T I O N N O T E Summary The far UV region (generally covering
