Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor
|
|
|
- Samson Oliver
- 5 years ago
- Views:
Transcription
1 13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey L. Hahn Covanta Energy 40 Lane Road Fairfield, New Jersey, USA KEYWORDS: FTIR Spectroscopy, Emissions Measurements, Method Validation, HCl, Relative Accuracy, RATA, CO, S02, NO ABSTRACT EPA Test Methods 301 and 320 include statistical techniques for validating sampling methods in specific gas matrices. Several of these techniques were used to analyze extractive FTIR data collected at a municipal solid waste incinerator and to validate the method for hydrogen chloride (HCl) measurements. FTIR results for carbon monoxide (CO), sulfur dioxide (S02), and nitric oxide (NO) were also compared to measurements recorded by the facility's continuous emissions monitors (CEMs). Presented are discussions of the equipment, spectral analyses, and statistical comparisons of the various test methods. INTRODUCTION The work described here was performed in October, 2004, at two sampling locations of a municipal waste combustor. The major goal of the study was to validate extractive Fourier transform infrared (FTIR) spectrometry for measurements of gaseous hydrogen chloride (HCl) as described in Environmental Protection Agency (EPA) Test Methods and Three different validation procedures were successfully performed; they were direct comparisons with EPA's "manual" Test Method 26 3, "single instrument" dynamic spiking, and "dual instrument" dynamic spiking. A secondary goal of the study was the comparison of CO, NO, and S02 results obtained using the standard CEMs installed at the facility to those obtained using FTIR techniques. Four of the six Relative Accuracy Test Audits (RATAs) performed for these criteria pollutants were successful according to the statistical criteria required by EPA 4; the average measured analyte concentrations for those (two) audits which failed were at the extremes of the CEM and FTIR instrument calibration ranges. A complete description of the method requirements and calculations is given in References 1 through 4; for brevity, only summaries are provided in this work. The referenced methods are available at the website
2 TEST LOCATIONS AND EQUIPMENT TEST FACILITY The test facility is a typical municipal waste combustor/generating station utilizing refuse-derived fuel (RDF). The plant consists of several independent "units," two of which (referred to as "Unit 1" and "Unit 2" below) were sampled during this work. Each unit includes a boiler and a set of emissions control devices, notably a lime-slurry injection system for reducing the HCl emissions. All validation testing was performed at one of the two controldevice outlet locations; the sampling ports were located in rectangular crosssection ducts connecting the outlets of the induced-draft fans to the base of the stack. SAMPLING SYSTEMS The sample streams passed through heated probe assemblies, heat-traced (-350 F) Teflon. samples line, and the absorption cells of each of two FTIR spectrometers; a pump connected to the cell's outlet ports continuously drew sample gases through the cells at approximately 10 liters per minute (Lpm). The only materials used in the sampling systems were Pyrex, Type 316 stainless steel, and Teflon. All Method 26 sampling was performed using equipment and techniques described in Reference 3; the resulting samples were submitted to Testar, Inc. (Raleigh, NC) for HCl analysis. FTIR ANALYTICAL SYSTEMS The two FTIR spectrometers used in this work are MIDAC Corporation Model instruments (see Figure 1) with nominal one-half wavenumber (0.5 cm-l) spectral resolution; they are designated below as Instruments "A" and "B." Both systems employ Michelson interferometers, beam splitters and cell windows of zinc selenide (ZnSe), hotwire infrared radiation sources, frontsurface optical transfer mirrors, and multi-pass absorption cells. MCT detectors were used for the testing described here. In all cases, the detectors were cooled with liquid nitrogen and their temperatures maintained at 77 K. The -la-meter path length "White" absorption cells use goldsurface internal mirrors, and their interior cell walls are made of polished nickel in order to minimize chemical interactions with the sample gas. Transducers and thermocouples connected directly to the insulated sample cells provide the pressures and temperatures of the sample streams. During testing, the temperature of the absorption cells was 1810 C; the elevated temperature prevents gas condensation within the cell and minimizes analyte adhesion to the cell walls and mirrors. The volumes of the absorption cells are 2.0 liters, so at a sample gas flow rate of 10 Lpm the sample gas in each cell is refreshed approximately five times per minute. Interferograms consisting of 50 co-added scans were recorded nearly continuously during the test periods, and provided oneminute average concentrations. 106
3 FIGURE 1. TYPICAL FTIR SPECTROMETER SYSTEM DURING TESTING FTIR SPECTRAL ANALYSES The program AutoquantPro (hereafter "AQ," version ), was used to collect and analyze all the infrared field data. The program allows the development and storage of analytical "methods" for analysis of spectral data (absorbance) files; various "methods" were generated for the instrumentdetector combinations described above and used to for determine the absorption path lengths and the concentrations of the gaseous analytes. On every test day, at least one 128-scan background spectrum was recorded using nitrogen gas. Before and after each test run, spectra of a calibration transfer standard (CTS) gas were obtained and used to determine the effective absorption path lengths of the FTIR systems. A cylinder of ethylene in nitrogen (103 ppm) served as the CTS, and the absorption path lengths were determined by comparing the field CTS spectra to a wellcharacterized laboratory ethylene spectrum. All the absorption path lengths (for each instrument detector combination) varied by less than 5% over the entire test period; the analytical results presented in this work are based on the average path length determined for each instrument-detector combination over the course of the field tests. 107
4 The HCI concentrations in the extracted FTIR samples were determined by mathematically comparing the samples' absorbance spectra to a number of HCI reference spectra; these reference spectra were recorded during the test period using the two field instruments and a cylinder standard of 86.2 ppm HCI and 5.03 ppm SF6 (balance nitrogen). Details of the "classical least squares" mathematical comparison employed are described in Reference 5. Using both a NIST -calibrated mass flow controller and standard barometric techniques, the reference samples were made by diluting the HCl/SF6 standard gas to four lower volumetric concentrations. The absolute HCI concentration of the cylinder standard was determined in Enthalpy's laboratory according to the sampling and analytical procedures of EPA Method 26; only the relative SF6 concentrations are required for the validation procedures described below, so the SF6 cylinder concentration was not independently confirmed. Concentrations of three other analytes (CO, S02, and NO) were also determined using quantitative FTIR reference libraries prepared in Enthalpy's laboratory, on Instrument B, from barometric dilutions of certified calibration gas standards. Table 1 presents the infrared wavenumber (cm- I ) analytical ranges and concentration-path length (ppmmeter) limits of the reference libraries employed to date. Spectra representing the absorbance interferences from water and carbon dioxide were also employed as required over these same regions. TABLE 1. FTIR REFERENCE SPECTRA AND ANALYTICAL REGIONS ANALYTE WA VENUMBER RANGE NUMBER OF SPECTRA PPM-MRANGE HCL CM PPMM SF CM PPM CO CM PPM-M NO CM PPM-M S CM PPM-M TABLE 2. METHOD 301 VALIDATION RESULTS(PPM) SAMPLE PAIR M 26 RESULTS SR FTIR RESULTS Sp ABS (SR - Sp) I MEANS
5 HCL METHODV ALIDATIONS EPA's Test Methods 301 (Reference 1) and 320 (Reference 2) both provide statistical techniques for the validation of emissions test procedures. Three validation procedures for FTIR measurements of HCI were performed and are described below. METHOD 301: REFERENCE METHOD COMPARISON EPA Method 301 (Sections 5.2 and 6.2 ) allows the validation of a proposed test method (here, FTIR measurements of HCI) by a pair-wise comparison between the results of a simultaneously performed EPA Reference Method 26. Briefly, the results SR of nine one-hour test runs using the reference method are compared to the corresponding results SP of the proposed method. Next, a bias B and a correction factor CF for the proposed method are calculated. A t-test is applied to determine whether the bias of proposed method is statistically significant, and the relative standard deviation SOP of the set of nine corrected differences (SR - SP) is compared via an F-test to the published standard deviation of the reference method3 (here, SOR = 3.2% at 15.3 ppm and SOR = 6.2% at 3.9 ppm). The criteria required for validation of the proposed method are a) SOP < SOR and b) 0.9 < CF < 1.1. Table 2 presents the nine pairs of concentration results and the (uncorrected) absolute differences used in these calculations. All these results presented in this section were calculated on a "dry, 7% 02" basis using Method 26 results for H20 and O2. Although the t-test shows that the proposed method bias (B = 2.0 ppm) is significant (t = 2.148), the F-test (F = 0.108) indicates good precision, and the correction factor (CF = 1.031) falls in the acceptable range 0.9 < CF < The corrected differences between the paired results of Table 2 yield a relative standard deviation SOP = 4.3%, which lies between the two published values (3.9% and 6.2%) of the reference method. METHOD 320: DUAL INSTRUMENT SPIKING VALIDATION EPA Method 320 (Reference 2, Section 13) allows validation of FTIR-based measurements by a pairwise comparison between the results of two independent FTIR systems. One of these systems must provide the "native" (or "unspiked") concentrations in 12 independent samples, and the other must provide simultaneous measurements of samples which have been dynamically "spiked" to provide a calculable change in the analyte concentrations. Briefly, the means of the spiked results and calculated spiked levels provide a bias estimate for the FTIR measurements, and a t-test is applied to the (12) differences in these values measurements to determine whether the bias is significant. If the bias is found significant, a correction factor is calculated and must lie in the range 0.7 < CF < 1.3. In this work, one of the two FTIR spectrometers (Instrument A) was used to collect and analyze 12 unspiked gas samples. Simultaneously, Instrument B analyzed spiked samples collected from the same outlet sample port. Spike gas was introduced into the Instrument B sampling system by injecting gas from a cylinder standard of 86.2 ppm HCI and 5.03 ppm SF6 (balance nitrogen) directly into the sample probe; the spike gas flow rate was adjusted (by monitoring the observed SF6 concentration) so that the spike gas represented 10% to 20% of the total Instrument B sample flow rate. Table 3 presents the HCI and SF6 results
6 of these measurements for the spiked and un-spiked samples. The results presented in this section are "wet-basis" concentrations - that is, they have not been adjusted for the presence of water vapor nor corrected to an oxygen standard. From the mean of the Ss (SF6) values and the cylinder SF 6 value concentration, the expected mean spiked concentration is CS = 16.5 ppm; the actual mean spiked concentration is, so the bias in the proposed method is B = 3.1 ppm. The t-test using the standard deviations of the values Ss - Su yields t = 5.76, indicating that a correction CF = factor is required; this value falls within the allowed range (0.7 < CF < 1.3). TABLE 3. METHOD 320 DUAL-INSTRUMENT SPIKING RESULTS (PPM) SAMPLE PAIR) Ss (HCL) Su(HCL Ss (SF6) MEANS METHOD 320: SINGLE INSTRUMENT SPIKING VALIDATION EPA Method 320 (Reference 2, Section 13) also allows validation of FTIR-based measurements by a pair-wise comparison between the results of a single FTIR system. The procedures and analyses are identical to those described above for the dual-instrument validation, except that the spikedlunspiked sample pairs are the results of sequential measurements on a single system (rather than simultaneous measurements on a pair of system). Table 4 presents the HCI and SF6 results of these ("wet-basis") measurements for the spiked and un-spiked samples. Briefly, from the mean of the Ss (SF6) values and the cylinder SF6 value concentration, the expected mean spiked concentration is CS = 17.6 ppm; the actual mean spiked concentration is 14.0, so the bias in the proposed method is B = 3.6 ppm. The t-test using the standard deviations of the values Ss - Su yields t = 4.19, indicating that a correction factor CF = is required; this correction factor falls within the allowed range (0.7 < CF < 1.3). 110
7 TABLE 4. METHOD 320 SINGLE-INSTRUMENT SPIKING RESULTS (PPM) SAMPLE PAIR Ss (HCL) Su (HCL) Ss (SF6) MEANS RELATIVE ACCURACY TEST AUDITS The FTIR spectra recorded during the Method 301 validation procedures described above were also analyzed for the presence of CO, NO, and S02. The FTIR - based concentrations of these compounds were corrected for the water vapor and adjusted to an oxygen standard (7%) of the sample stream (as determined by the concurrent EPA Method 26 tests) and compared to the corresponding results of the 8 two CEM systems operated by the facility. As required during the RAT As performed periodically on each unit, the one-minute concentration averages of the two independent systems (a single FTIR spectrometer, and one set of three singlecomponent gas analyzers) were obtained over nine 21-minute test periods, for each unit and for each analyte. Table 5 (Unit 1) and Table 6 (Unit 2) present the FTIR-based and CEM-based average concentration values over selected 21- minute subsets of the available data. The nine pairs of run-average concentrations, for each analyte and each unit, were then mathematically compared as prescribed by Reference 4. Briefly, the mean absolute difference between the FTIR based results and those of the facility CEM systems was calculated, as well as both the standard deviations in those absolute differences and a related confidence coefficient. Both an "Absolute Relative Accuracy" (ARA, based on the run-averaged results of the reference method) and a "Standard-Based Relative Accuracy" (SRA, based on the assumed emissions standards, taken as 100 ppm, 180 ppm, and 30 ppm for the compounds CO, NO, and S02, respectively) were calculated for each analyte. Each compound passes the audit, for a specific unit, if either ARA :5 20% or SRA :5 10%. In the current case, Reference 4 provides no clear guidance as to which system (CEM or FTIR) is to be regarded as the "reference" and which as the "facility" method. The results quoted below are based on the assumptions that the CEM- 111
8 based results comprise the "reference" system and that the FTIR-based results are those of the "facility" system. For the data collected during this work, this conservative assumption leads to the largest possible ARA and SRA values, but, as it turns out, affects none of the "pass/fail" audit results cited below. Table 7 presents the RA T A results obtained for the two units, averaged over all nine runs. Each set of results was obtained using a separate pair of FTIR CEM units; gas samples for each of the four analytical systems were supplied by four separate sampling systems. As mentioned above, the audit is passed if either ARA ::; 20% or SRA ::; 10%. Of the six sets of results presented in Table 7, four meet this criterion. Though is not possible to draw any definitive conclusions based on the available data, the following points are of possible interest: All three compounds passed the audit for Unit 1, and on Unit 2 only one (NO) passed. Of the four passing results, only one, that for CO on Unit 1, passed the audit on the basis of the "Standard Based Relative Accuracy" (SRA). Three passing results (NO on both Units and S02 on Unit 1 only) are based on only the "Absolute Relative Accuracy" (ARA) value. For three of the four passing results (NO on both Units and S02 on Unit 1), the average FTIR concentrations fall within the FTIR calibration range. For both failing results (CO and S02 on Unit 2), the mean FTIR results fall outside the ppm calibration range. CONCLUSIONS Three validation studies of FTIR spectrometry based on EPA reference methods indicate the suitability of the technique for the measurement of gaseous HCI in the emissions from municipal waste combustors using refuse-derived fuel. The three validation techniques were a) direct comparisons with EPA Method 26 according to EPA Method 301, b) dual-ftir spiking procedures according to EPA Method 320, and c) single-ftir spiking procedures according to EPA Method 320. Significant method bias was indicated in every case, but in all three cases the associated correction factors lie between and 1.257, and all three fall within the limits allowed by the EPA validation procedures. The results of six relative accuracy test audits performed using the same FTIR spectra and data from two of the facility's CEM systems were good; the CO, NO, and S02 channels from one system (Unit 1) passed the audit requirements. While for Unit 2 only the NO results met the audit criteria, a planned recalibration of the FTIR instruments over wider ranges of CO and S02 concentration channels is expected to improve these results. ACKNOWLEDGEMENTS I want to thank Grant Plummer, Todd Grosshandler and Peter Zewek for their support. 112
9 TAB LE 5. CEM AND FTIR RUN-A VERAGE RESULTS FOR UNIT 1 (PP M) CO NO S02 RUN CEM FTIR CEM FTIR CEM FTIR TAB LE 6. CEM AND FTIR RUN-A VERAGE RESULTS FOR UNIT 2 (P PM) CO NO S02 RUN CEM FTIR CEM FTIR CEM FTIR TABLE 7. RATA RESULTS AVERAGED OVER ALL RUNS UNIT 1 UNIT 2 PARAMETER CO NO S02 CO NO S02 EMISSION STANDARD (PPM) O2 (%) H2O%) FTIR RESULT (PPM) CEM RESULT (PPM) ABS. VALUE [CEM-FTIR] (PPM) SRA 7.7% 10.8% 15.2% 24.4% 16.2% 33.6% AUDIT STATUS - SRA PASS FAIL FAIL FAIL FAIL FAIL ARA 14.6% 10.8% 14.4% 29.8% 1401% 58.2% AUDIT STATUS - ARA PASS PASS PASS FAIL PASS FAIL OVERALL AUDIT STATUS PASS PASS PASS FAIL PASS FAIL UPPER FTIR CAL. (PPM) LOWER FTIR CAL. (PPM)
10 REFERENCES 1. EPA Test Method 301, "Validation of Pollutant Measurement Methods from Various Waste Media", u.s. Code of Federal Regulations (40 CFR 61, Appendix B). 2. EPA Test Method 320, "Measurement of Vapor Phase Organic and Inorganic Emissions By Extractive FTIR Spectroscopy", U.S. Code of Federal Regulations (40 CFR 61, Appendix B). 3. EPA Test Method 26, "Determination of Hydrogen Chloride Emissions from Stationary Sources", U.S. Code of Federal Regulations (40 CFR 61, Appendix B) EPA, "CEMS Performance Specification 2 for S02 and NOx" and "CEMS Performance Specification 4 for CO", U.S. Code of Federal Regulations (40 CFR 61, Appendix B). 5. D.M. Haaland, R.G. Easterling and D.A. Vopicka, "Multivariate Least Squares Methods Applied to the Quantitative Spectral Analysis of Multicomponent Samples," Alml. Spectrosc. 39(1), 1985, pp
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
Draft PS 18 APPENDIX A Dynamic Spiking Procedure ( ) 1.1 This appendix to Performance Specification 18
 Draft PS 18 APPENDIX A Dynamic Spiking Procedure (4-4-2013) A1. Scope and Application 1.1 This appendix to Performance Specification 18 describes the procedure and performance requirements for dynamic
Draft PS 18 APPENDIX A Dynamic Spiking Procedure (4-4-2013) A1. Scope and Application 1.1 This appendix to Performance Specification 18 describes the procedure and performance requirements for dynamic
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System
 Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
FTIR CEM Performance Specification Modification Considerations
 FTIR CEM Performance Specification Modification Considerations EPA/ICAC Emissions Measurements Roundtable Meeting September 17, 2013 Peter G. Zemek MKS Instruments Marty Spartz Prism Analytical MultiGas
FTIR CEM Performance Specification Modification Considerations EPA/ICAC Emissions Measurements Roundtable Meeting September 17, 2013 Peter G. Zemek MKS Instruments Marty Spartz Prism Analytical MultiGas
Gaseous CEMS technology
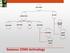 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Pilot-Plant Performance Testing to Support Development of a Dilution Extractive HCl Monitoring System
 Pilot-Plant Performance Testing to Support Development of a Dilution Extractive HCl Monitoring System Ty Smith Cemtek Group Inc. and Ken Greaves Cemtek Instruments Inc. Background Cemtek is researching
Pilot-Plant Performance Testing to Support Development of a Dilution Extractive HCl Monitoring System Ty Smith Cemtek Group Inc. and Ken Greaves Cemtek Instruments Inc. Background Cemtek is researching
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
Appendix A - Test Methods Method 301--Field Validation of Pollutant Measurement Methods from Various Waste Media
 Appendix A - Test Methods Method 301--Field Validation of Pollutant Measurement Methods from Various Waste Media 1. APPLICABILITY AND PRINCIPLE 1.1 Applicability. This method, as specified in the applicable
Appendix A - Test Methods Method 301--Field Validation of Pollutant Measurement Methods from Various Waste Media 1. APPLICABILITY AND PRINCIPLE 1.1 Applicability. This method, as specified in the applicable
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions
 Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions Laura L. Kinner and James W. Peeler Emission Monitoring Inc. Raleigh, NC 27612 Daniel
Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions Laura L. Kinner and James W. Peeler Emission Monitoring Inc. Raleigh, NC 27612 Daniel
TO-15 Checklist Determination of VOCs in Air by GC-MS
 LAB ID: DATE: LAB NAME: ASSESSOR NAME: Method Number: TO-15 Checklist Determination of VOCs in Air by GC-MS SOP Number: Revision Number: SOP Date: Personnel records observed: Data records observed: Revision
LAB ID: DATE: LAB NAME: ASSESSOR NAME: Method Number: TO-15 Checklist Determination of VOCs in Air by GC-MS SOP Number: Revision Number: SOP Date: Personnel records observed: Data records observed: Revision
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION
 JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure
 ANLECR&D project 6 0215 0234 Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure Sub contract to Macquarie University Report of ANLECR&D Project 6 0215 0243
ANLECR&D project 6 0215 0234 Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure Sub contract to Macquarie University Report of ANLECR&D Project 6 0215 0243
Robustness of the QAL2 calibration (EN 14181) Uncertainty on the results given by a calibrated AMS
 Robustness of the 2 calibration (EN 14181) Uncertainty on the results given by a calibrated Jean.Poulleau*¹, Cécile.Raventos 1, Philippe.Fayolle², Emmanuel.Fiani 3 ¹ INERIS, Parc technologique ALATA BP
Robustness of the 2 calibration (EN 14181) Uncertainty on the results given by a calibrated Jean.Poulleau*¹, Cécile.Raventos 1, Philippe.Fayolle², Emmanuel.Fiani 3 ¹ INERIS, Parc technologique ALATA BP
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
METHOD 7060A ARSENIC (ATOMIC ABSORPTION, FURNACE TECHNIQUE)
 METHOD 7060A ARSENIC (ATOMIC ABSORPTION, FURNACE TECHNIQUE) 1.0 SCOPE AND APPLICATION 1.1 Method 7060 is an atomic absorption procedure approved for determining the concentration of arsenic in wastes,
METHOD 7060A ARSENIC (ATOMIC ABSORPTION, FURNACE TECHNIQUE) 1.0 SCOPE AND APPLICATION 1.1 Method 7060 is an atomic absorption procedure approved for determining the concentration of arsenic in wastes,
FTIR GAS ANALYSIS WHITE PAPER INTRODUCTION TYPICAL APPLICATIONS
 WHITE PAPER W FTIR GAS ANALYSIS INTRODUCTION FTIR (Fourier Transform InfraRed) spectroscopy is the most popular analytical technology for industrial applications requiring the continuous measurement of
WHITE PAPER W FTIR GAS ANALYSIS INTRODUCTION FTIR (Fourier Transform InfraRed) spectroscopy is the most popular analytical technology for industrial applications requiring the continuous measurement of
In-Situ FTIR Spectroscopy and Metrology of a Tungsten CVD Process
 In-Situ FTIR Spectroscopy and Metrology of a Tungsten CVD Process A. Singhal, L. Henn-Lecordier and J. N. Kidder Jr. University of Maryland, College Park, MD C.A. Gogol, J.F. Kushneir Inficon, Inc. East
In-Situ FTIR Spectroscopy and Metrology of a Tungsten CVD Process A. Singhal, L. Henn-Lecordier and J. N. Kidder Jr. University of Maryland, College Park, MD C.A. Gogol, J.F. Kushneir Inficon, Inc. East
Fourier Transform Infrared. Spectrometry
 Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
Testing of Continuous Sampling Air-ICP and Mercury Systems as Continuous Emission Monitors at the Diagnostic Instrumentation and Analysis Laboratory
 Testing of Continuous Sampling Air-ICP and Mercury Systems as Continuous Emission Monitors at the Diagnostic Instrumentation and Analysis Laboratory September 18-26, 2000 D. P. Baldwin, S. J. Bajic, D.
Testing of Continuous Sampling Air-ICP and Mercury Systems as Continuous Emission Monitors at the Diagnostic Instrumentation and Analysis Laboratory September 18-26, 2000 D. P. Baldwin, S. J. Bajic, D.
Assessing Unknown Odors and Chemicals
 Treat yourself to better rental service. Thank You for Attending Today s Webinar: Assessing Unknown Odors and Chemicals Your Host Matt DeLacluyse Operations Mgr RAECO Rents mattd@raecorents.com Featured
Treat yourself to better rental service. Thank You for Attending Today s Webinar: Assessing Unknown Odors and Chemicals Your Host Matt DeLacluyse Operations Mgr RAECO Rents mattd@raecorents.com Featured
Chemistry Instrumental Analysis Lecture 15. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature
 CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature Charles A. Lockert, Breen Energy Solution, 104 Broadway Street, Carnegie, PA 15106 Peter C. Hoeflich and Landis S. Smith, Progress Energy
CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature Charles A. Lockert, Breen Energy Solution, 104 Broadway Street, Carnegie, PA 15106 Peter C. Hoeflich and Landis S. Smith, Progress Energy
Zinc Metal Determination Perkin Elmer Atomic Absorption Spectrometer AAnalyst Procedures
 Villanova University Date: Oct 2011 Page 1 of 9 Villanova University Villanova Urban Stormwater Partnership Watersheds Laboratory Standard Operating Procedure VUSP F Zinc Metal Determination Perkin Elmer
Villanova University Date: Oct 2011 Page 1 of 9 Villanova University Villanova Urban Stormwater Partnership Watersheds Laboratory Standard Operating Procedure VUSP F Zinc Metal Determination Perkin Elmer
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS)
 Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
PAPER No. 12: ORGANIC SPECTROSCOPY MODULE No. 4: Basic principles and Instrumentation for IR spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Module 4: Basic principles and Instrumentation for IR spectroscopy CHE_P12_M4_e-Text TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Module 4: Basic principles and Instrumentation for IR spectroscopy CHE_P12_M4_e-Text TABLE OF CONTENTS
Hach Method Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis
 Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
Chem Homework Set Answers
 Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
UV Quality Assurance. Dark Current Correction
 UV Quality Assurance This document presents the Quality Assurance protocol used for the measurement of broad spectrum UV spectra according to the guidelines given by the United States Environmental Protection
UV Quality Assurance This document presents the Quality Assurance protocol used for the measurement of broad spectrum UV spectra according to the guidelines given by the United States Environmental Protection
Industrial In-line and Multi Component Monitor Using Absorption Spectroscopy and Its Application
 FFeature Article Article Industrial In-line and Multi Component Monitor Using Absorption Spectroscopy and Its Application Yoko NAKAI HORIBA s CS-Series chemical concentration monitors that use ultraviolet
FFeature Article Article Industrial In-line and Multi Component Monitor Using Absorption Spectroscopy and Its Application Yoko NAKAI HORIBA s CS-Series chemical concentration monitors that use ultraviolet
Supporting Information
 1 Supporting Information 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Materials and Methods Experiment In this study, an alloy IR probe which allowed us to get access to spectral
1 Supporting Information 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Materials and Methods Experiment In this study, an alloy IR probe which allowed us to get access to spectral
Fourier Transform Infrared Spectrometry Prelab Last modified: June 17, 2014
 Fourier Transform Infrared Spectrometry Prelab Recommended reading: AirUCI Lab Manual: Environmental Chemistry Text: FTIR Lab Pages: 6, 7, 13 16 on Light Absorption Pages: 175 177, 184, 185 on Molecular
Fourier Transform Infrared Spectrometry Prelab Recommended reading: AirUCI Lab Manual: Environmental Chemistry Text: FTIR Lab Pages: 6, 7, 13 16 on Light Absorption Pages: 175 177, 184, 185 on Molecular
SULFUR DIOXIDE REMOVAL
 Report No. 63 A SULFUR DIOXIDE REMOVAL FROM STACK GASES Supplement A by EARL D. OLIVER August 1973 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE MENLO PARK, CALIFORNIA CONTENTS
Report No. 63 A SULFUR DIOXIDE REMOVAL FROM STACK GASES Supplement A by EARL D. OLIVER August 1973 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE MENLO PARK, CALIFORNIA CONTENTS
Fourier transform infrared spectroscopy (FTIR) is a method used to obtain an infrared
 Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
Prima PRO Process Mass Spectrometer
 APPLICATION NOTE Prima PRO Process Mass Spectrometer No. xxxx Controlling emissions by multi-component analysis of Volatile Organic Compounds (VOC) Authors: Peter Traynor, Thermo Fisher Scientific, Franklin,
APPLICATION NOTE Prima PRO Process Mass Spectrometer No. xxxx Controlling emissions by multi-component analysis of Volatile Organic Compounds (VOC) Authors: Peter Traynor, Thermo Fisher Scientific, Franklin,
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Bombardier SMP 800-C Toxic Gas Generation of "Moniflex as manufactured by Isoflex AB"
 UNCONTROLLED ELECTRONIC COPY Bombardier SMP 800-C of "Moniflex as manufactured by Isoflex AB" A Report To: Ciucevich 7200 Vermilion Ct. Wake Forest, NC 27587 USA Phone: (919) 570-291 Email: Robert.Ciucevich@celanese.com
UNCONTROLLED ELECTRONIC COPY Bombardier SMP 800-C of "Moniflex as manufactured by Isoflex AB" A Report To: Ciucevich 7200 Vermilion Ct. Wake Forest, NC 27587 USA Phone: (919) 570-291 Email: Robert.Ciucevich@celanese.com
Project S66 Benchscale Testing of Gas Absorption in Wet Scrubbers. SEMATECH Technology Transfer A-ENG
 Project S66 Benchscale Testing of Gas Absorption in Wet Scrubbers Technology Transfer 95052814A-ENG and the logo are registered service marks of, Inc. Apple and Macintosh are registered trademarks of Apple
Project S66 Benchscale Testing of Gas Absorption in Wet Scrubbers Technology Transfer 95052814A-ENG and the logo are registered service marks of, Inc. Apple and Macintosh are registered trademarks of Apple
METHOD 8033 ACETONITRILE BY GAS CHROMATOGRAPHY WITH NITROGEN-PHOSPHORUS DETECTION
 METHOD 80 ACETONITRILE BY GAS CHROMATOGRAPHY WITH NITROGEN-PHOSPHORUS DETECTION 1.0 SCOPE AND APPLICATION 1.1 Method 80 may be used to determine the concentration of acetonitrile (CAS No. 75-05-8) in aqueous
METHOD 80 ACETONITRILE BY GAS CHROMATOGRAPHY WITH NITROGEN-PHOSPHORUS DETECTION 1.0 SCOPE AND APPLICATION 1.1 Method 80 may be used to determine the concentration of acetonitrile (CAS No. 75-05-8) in aqueous
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
3. Chemical industry. Because of their modular design, the instruments in the TOC-L series can be equipped for any possible measurement
 3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
United States Patent (19)
 United States Patent (19) Rajic et al. 54 FTIR SPECTROMETER WITH SOLID-STATE DRIVE SYSTEM 75 Inventors: Slobodan Rajic, Knoxville; Roland D. Seals; Charles M. Egert, both of Oak Ridge, all of Tenn. 73
United States Patent (19) Rajic et al. 54 FTIR SPECTROMETER WITH SOLID-STATE DRIVE SYSTEM 75 Inventors: Slobodan Rajic, Knoxville; Roland D. Seals; Charles M. Egert, both of Oak Ridge, all of Tenn. 73
Boeing BSS 7239 Toxic Gas Generation of "PH-63 ( ) Item Number "
 UNCONTROLLED ELECTRONIC COPY Boeing BSS 7239 of "PH-63 (6477-54) Item Number 1009-08151" A Report To: Uniroyal Engineered Products LLC 501 South Water Street Stoughton, WI 53589 USA Phone: (608) 873-6631
UNCONTROLLED ELECTRONIC COPY Boeing BSS 7239 of "PH-63 (6477-54) Item Number 1009-08151" A Report To: Uniroyal Engineered Products LLC 501 South Water Street Stoughton, WI 53589 USA Phone: (608) 873-6631
Determination the elemental composition of soil samples
 4. Experiment Determination the elemental composition of soil samples Objectives On this practice you will determine the elemental composition of soil samples by Inductively Coupled Plasma Optical Emission
4. Experiment Determination the elemental composition of soil samples Objectives On this practice you will determine the elemental composition of soil samples by Inductively Coupled Plasma Optical Emission
Hydride Generation for the Determination of As, Sb, Se and Bi Using the Teledyne Leeman Lab s Prodigy 7 ICP-OES
 Application Note - AN1508 Hydride Generation for the Determination of As, Sb, Se and Bi Using the Teledyne Leeman Lab s Prodigy 7 ICP-OES Introduction Page 1 The combination of hydride generation with
Application Note - AN1508 Hydride Generation for the Determination of As, Sb, Se and Bi Using the Teledyne Leeman Lab s Prodigy 7 ICP-OES Introduction Page 1 The combination of hydride generation with
Method 323 Measurement of Formaldehyde Emissions From Natural Gas-Fired Stationary Sources Acetyl Acetone Derivitization Method
 While we have taken steps to ensure the accuracy of this Internet version of the document, it is not the official version. Please refer to the official version in the FR publication, which appears on the
While we have taken steps to ensure the accuracy of this Internet version of the document, it is not the official version. Please refer to the official version in the FR publication, which appears on the
FTIR Spectrometer. Basic Theory of Infrared Spectrometer. FTIR Spectrometer. FTIR Accessories
 FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
STANDARD OPERATING PROCEDURES SOP: 1828 PAGE: 1 of 14 REV: 0.0 DATE: 05/12/95 ANALYSIS OF METHYL PARATHION IN CARPET SAMPLES BY GC/MS
 PAGE: 1 of 14 1.0 SCOPE AND APPLICATION 2.0 METHOD SUMMARY CONTENTS 3.0 SAMPLE PRESERVATION, CONTAINERS, HANDLING AND STORAGE 4.0 INTERFERENCES AND POTENTIAL PROBLEMS 5.0 EQUIPMENT/APPARATUS 6.0 REAGENTS
PAGE: 1 of 14 1.0 SCOPE AND APPLICATION 2.0 METHOD SUMMARY CONTENTS 3.0 SAMPLE PRESERVATION, CONTAINERS, HANDLING AND STORAGE 4.0 INTERFERENCES AND POTENTIAL PROBLEMS 5.0 EQUIPMENT/APPARATUS 6.0 REAGENTS
(918) or (888)
 Corporate Headquarters 5634 S. 122nd E. Ave. Ste. F Tulsa, Oklahoma 74146 Las Vegas Office 5925 E. Lake Mead Blvd. Las Vegas, Nevada 89156 Philadelphia Office 8900 State Road Philadelphia, Pennsylvania
Corporate Headquarters 5634 S. 122nd E. Ave. Ste. F Tulsa, Oklahoma 74146 Las Vegas Office 5925 E. Lake Mead Blvd. Las Vegas, Nevada 89156 Philadelphia Office 8900 State Road Philadelphia, Pennsylvania
CHEMISTRY DEPARTMENT
 POINT LEPREAU GENERATING STATION GADOLINIUM ARSENAZO III Issued By: Date: APPROVED BY: of 6 REVISION RECORD AUTHOR REV DESCRIPTION DATE 0 Initial Issue W. Mawhinney Not Tracked Oct./9 W. T. Underhill 2
POINT LEPREAU GENERATING STATION GADOLINIUM ARSENAZO III Issued By: Date: APPROVED BY: of 6 REVISION RECORD AUTHOR REV DESCRIPTION DATE 0 Initial Issue W. Mawhinney Not Tracked Oct./9 W. T. Underhill 2
Alternative Monitoring Technologies: A Brief Overview
 Alternative Monitoring Technologies: A Brief Overview a technical solution to meet every need Cemtek Environmental Inc. 3041 S. Orange Ave. Santa Ana, CA 92707 800-400-0200 www.cemteks.com 1 What s New?
Alternative Monitoring Technologies: A Brief Overview a technical solution to meet every need Cemtek Environmental Inc. 3041 S. Orange Ave. Santa Ana, CA 92707 800-400-0200 www.cemteks.com 1 What s New?
Laboratory ID. Laboratory Name. Analyst(s) Auditor. Date(s) of Audit. Type of Audit Initial Biennial Special ELCP TNI/NELAP.
 NEW JERSEY DEPARTMENT OF ENVIRONMENTAL PROTECTION OFFICE OF QUALITY ASSURANCE ENVIRONMENTAL LABORATORY CERTIFICATION PROGRAM ON-SITE LABORATORY EVALUATION RADIOCHEMISTRY PROCEDURES Gross Alpha-Gross Beta
NEW JERSEY DEPARTMENT OF ENVIRONMENTAL PROTECTION OFFICE OF QUALITY ASSURANCE ENVIRONMENTAL LABORATORY CERTIFICATION PROGRAM ON-SITE LABORATORY EVALUATION RADIOCHEMISTRY PROCEDURES Gross Alpha-Gross Beta
Fourier Transform IR Spectroscopy
 Fourier Transform IR Spectroscopy Absorption peaks in an infrared absorption spectrum arise from molecular vibrations Absorbed energy causes molecular motions which create a net change in the dipole moment.
Fourier Transform IR Spectroscopy Absorption peaks in an infrared absorption spectrum arise from molecular vibrations Absorbed energy causes molecular motions which create a net change in the dipole moment.
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j
 UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
ICP-OES Application Note Number 35
 ICP-OES Application Note Number 35 Rapid measurement of major, minor and trace levels in soils using the Varian 730-ES Vincent Calderon Varian, Inc. Introduction As part of the global strategy for sustainable
ICP-OES Application Note Number 35 Rapid measurement of major, minor and trace levels in soils using the Varian 730-ES Vincent Calderon Varian, Inc. Introduction As part of the global strategy for sustainable
Near-Infrared Spectroscopy of Nitride Heterostructures EMILY FINAN ADVISOR: DR. OANA MALIS PURDUE UNIVERSITY REU PROGRAM AUGUST 2, 2012
 Near-Infrared Spectroscopy of Nitride Heterostructures EMILY FINAN ADVISOR: DR. OANA MALIS PURDUE UNIVERSITY REU PROGRAM AUGUST 2, 2012 Introduction Experimental Condensed Matter Research Study of large
Near-Infrared Spectroscopy of Nitride Heterostructures EMILY FINAN ADVISOR: DR. OANA MALIS PURDUE UNIVERSITY REU PROGRAM AUGUST 2, 2012 Introduction Experimental Condensed Matter Research Study of large
INDUCTIVELY COUPLED PLASMA MASS SPECTROMETRY
 INDUCTIVELY COUPLED PLASMA MASS SPECTROMETRY Edited by AKBAR MONTASER George Washington University Washington, D.C. 20052, USA WILEY-VCH New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
INDUCTIVELY COUPLED PLASMA MASS SPECTROMETRY Edited by AKBAR MONTASER George Washington University Washington, D.C. 20052, USA WILEY-VCH New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
METHOD 8030A ACROLEIN AND ACRYLONITRILE BY GAS CHROMATOGRAPHY
 METHOD 8030A ACROLEIN AND ACRYLONITRILE BY GAS CHROMATOGRAPHY 1.0 SCOPE AND APPLICATION 1.1 Method 8030 is used to determine the concentration of the following volatile organic compounds: Compound Name
METHOD 8030A ACROLEIN AND ACRYLONITRILE BY GAS CHROMATOGRAPHY 1.0 SCOPE AND APPLICATION 1.1 Method 8030 is used to determine the concentration of the following volatile organic compounds: Compound Name
A New On-line Cyanide Analyzer for Measurement of Cyanide in Hydrometallurgical Processing of Precious Metal Ores
 Application Note 37890312 A New On-line Cyanide Analyzer for Measurement of Cyanide in Hydrometallurgical Processing of Precious Metal Ores Keywords CNSolution 9310 On-line Cyanide Analyzer Cyanidation
Application Note 37890312 A New On-line Cyanide Analyzer for Measurement of Cyanide in Hydrometallurgical Processing of Precious Metal Ores Keywords CNSolution 9310 On-line Cyanide Analyzer Cyanidation
New Test Results For Physical Separation Of Tritium From Noble Gases And It s Implications For Sensitivity And Accuracy In Air And Stack Monitoring
 New Test Results For Physical Separation Of Tritium From Noble Gases And It s Implications For Sensitivity And Accuracy In Air And Stack Monitoring -Robert Goldstein, Ivan Mitev, Dell Williamson, Overhoff
New Test Results For Physical Separation Of Tritium From Noble Gases And It s Implications For Sensitivity And Accuracy In Air And Stack Monitoring -Robert Goldstein, Ivan Mitev, Dell Williamson, Overhoff
Fourier Transform Infrared Spectroscopy (Perkin Elmer - Spectrum One)
 Fourier Transform Infrared Spectroscopy (Perkin Elmer - Spectrum One) This operating procedure intends to provide guidance for transmission/absorbance measurements with the FTIR. For additional modes of
Fourier Transform Infrared Spectroscopy (Perkin Elmer - Spectrum One) This operating procedure intends to provide guidance for transmission/absorbance measurements with the FTIR. For additional modes of
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Using FIMS to Determine Mercury Content in Sewage Sludge, Sediment and Soil Samples
 A P P L I C A T I O N N ot e Atomic Absorption Using FIMS to Determine Mercury Content in Sewage Sludge, Sediment and Soil Samples Introduction The Flow Injection Mercury System (FIMS) is a dedicated system
A P P L I C A T I O N N ot e Atomic Absorption Using FIMS to Determine Mercury Content in Sewage Sludge, Sediment and Soil Samples Introduction The Flow Injection Mercury System (FIMS) is a dedicated system
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy
 Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
TECHNETIUM-99 IN WATER
 Analytical Procedure TECHNETIUM-99 IN WATER (TEVA DISC METHOD) 1. SCOPE 1.1. This procedure describes a method to separate and measure technetium-99 in water. 1.2. This method does not address all aspects
Analytical Procedure TECHNETIUM-99 IN WATER (TEVA DISC METHOD) 1. SCOPE 1.1. This procedure describes a method to separate and measure technetium-99 in water. 1.2. This method does not address all aspects
METHOD MIDGET IMPINGER HCl/Cl EMISSION SAMPLING TRAIN
 METHOD 0051 MIDGET IMPINGER HCl/Cl EMISSION SAMPLING TRAIN 1.0 SCOPE AND APPLICATION This method describes the collection of hydrogen chloride (HCl, CAS Registry Number 7647010) and chlorine (Cl, CAS Registry
METHOD 0051 MIDGET IMPINGER HCl/Cl EMISSION SAMPLING TRAIN 1.0 SCOPE AND APPLICATION This method describes the collection of hydrogen chloride (HCl, CAS Registry Number 7647010) and chlorine (Cl, CAS Registry
It is the purpose of this paper to demonstrate that the detection limit is directly related to the sample size, for both TG and TG/FTIR.
 BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Spectroscopy Problem Set February 22, 2018
 Spectroscopy Problem Set February, 018 4 3 5 1 6 7 8 1. In the diagram above which of the following represent vibrational relaxations? 1. Which of the following represent an absorbance? 3. Which of following
Spectroscopy Problem Set February, 018 4 3 5 1 6 7 8 1. In the diagram above which of the following represent vibrational relaxations? 1. Which of the following represent an absorbance? 3. Which of following
Atomic Absorption Spectrophotometry. Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon
 Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
The Application of Method TO-15 to Naphthalene Measurements in Indoor Air
 The Application of Method TO-15 to Naphthalene Measurements in Indoor Air Extended Abstract #13 Heidi C. Hayes and Diane J. Benton Air Toxics Ltd. 180 Blue Ravine Rd. Ste. B Folsom, CA 95630 INTRODUCTION
The Application of Method TO-15 to Naphthalene Measurements in Indoor Air Extended Abstract #13 Heidi C. Hayes and Diane J. Benton Air Toxics Ltd. 180 Blue Ravine Rd. Ste. B Folsom, CA 95630 INTRODUCTION
Interaction of Hydrogen on a Lanthanum hexaboride (111) Surface Jenna Cameli, Aashani Tillekaratne, Michael Trenary Department of Chemistry,
 Interaction of Hydrogen on a Lanthanum hexaboride (111) Surface Jenna Cameli, Aashani Tillekaratne, Michael Trenary Department of Chemistry, University of Illinois at Chicago, Chicago, IL 60607 1 Abstract
Interaction of Hydrogen on a Lanthanum hexaboride (111) Surface Jenna Cameli, Aashani Tillekaratne, Michael Trenary Department of Chemistry, University of Illinois at Chicago, Chicago, IL 60607 1 Abstract
QUALITY CONTROL CRITERIA FOR CHEMISTRY EXCEPT RADIOCHEMISTRY.
 1 REVISOR 4740.2100 4740.2100 QUALITY CONTROL CRITERIA FOR CHEMISTRY EXCEPT RADIOCHEMISTRY. Subpart 1. Scope. This part applies to laboratories performing testing under the inorganic chemistry, metals,
1 REVISOR 4740.2100 4740.2100 QUALITY CONTROL CRITERIA FOR CHEMISTRY EXCEPT RADIOCHEMISTRY. Subpart 1. Scope. This part applies to laboratories performing testing under the inorganic chemistry, metals,
PRINCIPLE OF ICP- AES
 INTRODUCTION Non- flame atomic emission techniques, which use electrothermal means to atomize and excite the analyte, include inductively coupled plasma and arc spark. It has been 30 years since Inductively
INTRODUCTION Non- flame atomic emission techniques, which use electrothermal means to atomize and excite the analyte, include inductively coupled plasma and arc spark. It has been 30 years since Inductively
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
New good practices for emission measurements and relevance of the SRM with respect to these requirements/practice
 New good practices for emission measurements and relevance of the SRM with respect to these requirements/practice INERIS Parc Technologique Alata, BP 2, 60550 Verneuil-en-Halatte France, Jean Poulleau,
New good practices for emission measurements and relevance of the SRM with respect to these requirements/practice INERIS Parc Technologique Alata, BP 2, 60550 Verneuil-en-Halatte France, Jean Poulleau,
Vibrations of Carbon Dioxide and Carbon Disulfide
 Vibrations of Carbon Dioxide and Carbon Disulfide Purpose Vibration frequencies of CO 2 and CS 2 will be measured by Raman and Infrared spectroscopy. The spectra show effects of normal mode symmetries
Vibrations of Carbon Dioxide and Carbon Disulfide Purpose Vibration frequencies of CO 2 and CS 2 will be measured by Raman and Infrared spectroscopy. The spectra show effects of normal mode symmetries
METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD)
 683 METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and
683 METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and
Supplementary Material for. Reactive Uptake of Ammonia by Secondary Organic Aerosols: Implications for Air Quality
 Supplementary Material for Reactive Uptake of Ammonia by Secondary Organic Aerosols: Implications for Air Quality Jeremy R. Horne, 1 Shupeng Zhu, 1 Julia Montoya-Aguilera, 2 Mallory L. Hinks, 2 Lisa M.
Supplementary Material for Reactive Uptake of Ammonia by Secondary Organic Aerosols: Implications for Air Quality Jeremy R. Horne, 1 Shupeng Zhu, 1 Julia Montoya-Aguilera, 2 Mallory L. Hinks, 2 Lisa M.
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
New good practices for emission measurements. and relevance of the SRM with respect to these requirements/practices CEM 2014
 New good practices for emission measurements and relevance of the SRM with respect to these requirements/practices CEM 2014 Jean Poulleau Cécile Raventos Evolution of French rules for emission measurements
New good practices for emission measurements and relevance of the SRM with respect to these requirements/practices CEM 2014 Jean Poulleau Cécile Raventos Evolution of French rules for emission measurements
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Warning!! Chapter 5 Gases. Chapter Objectives. Chapter Objectives. Chapter Objectives. Air Pollution
 Warning!! Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 5 Gases These slides contains visual aids for learning BUT they are NOT the actual lecture notes! Failure to attend to lectures most
Warning!! Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 5 Gases These slides contains visual aids for learning BUT they are NOT the actual lecture notes! Failure to attend to lectures most
Monitoring Emulsion Polymerization by Raman Spectroscopy
 An Executive Summary Monitoring Emulsion Polymerization by Raman Spectroscopy Why process analytical matters to process development R&D. Serena Stephenson, PhD Senior R&D Analytical Manager Kishori Deshpande,
An Executive Summary Monitoring Emulsion Polymerization by Raman Spectroscopy Why process analytical matters to process development R&D. Serena Stephenson, PhD Senior R&D Analytical Manager Kishori Deshpande,
Thermo Scientific Prima PRO Process Mass Spectrometer
 APPLICATION NOTE Thermo Scientific Prima PRO Process Mass Spectrometer No. xxxx Fast On-line monitoring of flare gases Author: Graham Lewis, Thermo Fisher Scientific, Winsford, Cheshire, United Kingdom
APPLICATION NOTE Thermo Scientific Prima PRO Process Mass Spectrometer No. xxxx Fast On-line monitoring of flare gases Author: Graham Lewis, Thermo Fisher Scientific, Winsford, Cheshire, United Kingdom
Module: 7. Lecture: 36
 Module: 7 Lecture: 36 DIMETHYL FORMAMIDE INTRODUCTION Dimethylformamide is an organic compound and denotes as DMF. The name is derived from the fact that it is a derivative of formamide, the amide of formic
Module: 7 Lecture: 36 DIMETHYL FORMAMIDE INTRODUCTION Dimethylformamide is an organic compound and denotes as DMF. The name is derived from the fact that it is a derivative of formamide, the amide of formic
ABB Remote Sensing Atmospheric Emitted Radiance Interferometer AERI system overview. Applications
 The ABB Atmospheric Emitted Radiance Interferometer AERI provides thermodynamic profiling, trace gas detection, atmospheric cloud aerosol study, air quality monitoring, and more. AERI high level overview
The ABB Atmospheric Emitted Radiance Interferometer AERI provides thermodynamic profiling, trace gas detection, atmospheric cloud aerosol study, air quality monitoring, and more. AERI high level overview
DEVELOPMENT OF MEASUREMENT AND
 DEVELOPMENT OF MEASUREMENT AND CHEMOMETRIC METHODS IN THE FOURIER TRANSFORM INFRARED AND RAMAN SPECTROSCOPY PhD Theses Written by: Brunó Hren Chemical engineer Supervisor: Prof. Dr. János Mink Made in
DEVELOPMENT OF MEASUREMENT AND CHEMOMETRIC METHODS IN THE FOURIER TRANSFORM INFRARED AND RAMAN SPECTROSCOPY PhD Theses Written by: Brunó Hren Chemical engineer Supervisor: Prof. Dr. János Mink Made in
ORGANIC AND INORGANIC GASES BY 3800 EXTRACTIVE FTIR SPECTROMETRY
 ORGANIC AND INORGANIC GASES BY 3800 EXTRACTIVE FTIR SPECTROMETRY FORMULA: Table 1 MW: Table 1 CAS: Table 1 RTECS: Table 1 METHOD: 3800, Issue 1 EVALUATION: FULL Issue 1: 15 March 2003 OSHA : Table 1 NIOSH:
ORGANIC AND INORGANIC GASES BY 3800 EXTRACTIVE FTIR SPECTROMETRY FORMULA: Table 1 MW: Table 1 CAS: Table 1 RTECS: Table 1 METHOD: 3800, Issue 1 EVALUATION: FULL Issue 1: 15 March 2003 OSHA : Table 1 NIOSH:
Fundamentals of Combustion
 Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Introduction to Gas Phase FTIR Spectroscopy
 Introduction to Gas Phase FTIR Spectroscopy Introduction to FTIR spectroscopy FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared (IR) spectroscopy, radiation
Introduction to Gas Phase FTIR Spectroscopy Introduction to FTIR spectroscopy FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared (IR) spectroscopy, radiation
2012 Parts Catalog Phone (919) URG
 2012 Parts Catalog Phone (919)942-2753 www.urgcorp.com 5 URG Air Samplers Ambient Ion Monitor Time Resolved Direct Measurement of Nitrate, Sulfate and Ammonium State-of-the-Art Sampler with the Ability
2012 Parts Catalog Phone (919)942-2753 www.urgcorp.com 5 URG Air Samplers Ambient Ion Monitor Time Resolved Direct Measurement of Nitrate, Sulfate and Ammonium State-of-the-Art Sampler with the Ability
