Measurement Technique and its Application to Trace Components of Atmospheric Gas
|
|
|
- Hannah Robinson
- 5 years ago
- Views:
Transcription
1 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement techniques that are ideal for measuring trace atmospheric, as in the following examples. To measure carbon monoxide (CO), a non-dispersive infrared absorption method is used. To measure Ozone (O 3 ), an ultraviolet absorption method is used. For nitrogen oxides (NO X ), chemiluminescence. For sulfur dioxide (SO 2 ), ultraviolet fluorescence. For hydrocarbons (HC), a fl ame ionization method is used, and so on. In addition, HORIBA has developed a fl uid modulation method, which is a method to measure concentrations by amplifying the difference of two signals coming from a measurement cell into which the sample and a reference are alternately introduced with a constant time interval, and has achieved absolute zero-point stability with all except SO 2 analyzer. In the case of CO measurement, HORIBA has succeeded in reducing the infl uence of moisture by using an interference compensation detector. In the case of O 3 and SO 2 measurement with a light source, in which light intensity variation over time is not negligible, it compensates for the infl uence by monitoring the light source intensity. Also, a highly productive measurement system has been developed with which it is possible to measure multiple components with a single detector by combining a catalyzer or convertor unit and the three-phase fl uid modulation method (introducing three types of alternately), such as, NO X that can measure NO and NO 2 at the same time and HC that can simultaneously measure methane (CH 4 ) and nonmethane (non-ch 4 ). These various techniques for making comprehensive measurement systems have been adopted also to measure trace quantities of ammonium (NH 3 ) or hydrogen sulfi de (H 2 S) inside the clean room. Introduction Recently, the word "environmental pollution" has not been so frequently used and it seems that concentrations of environmental pollutants such as CO and SO 2 in the atmosphere have been surely reduced. But, the concentration of suspended particle matter (SPM), NO and NO 2 generated by moving source such as automobiles is still staying flat, and in Japan the percentage of emission monitoring stations that have satisfied the national environmental criteria still remains at only 85.7%. As for the ozone (O 3 ) concentration, there is an increasing trend. The environmental problem is one of the important issues to be solved globally not only in Japan but also worldwide while building a recyclingoriented society that can maintain continuous growth. Measurement of atmospheric is different from that emitted from a fixed source such as waste incinerator or power plants. The concentration of target substances to be measured are significantly lower because they have been diffused into the air. Therefore, in order to measure atmospheric components, highly sensitive and stable measurement methods are required. In this paper, we describe the features and measurement principles of the automatic atmospheric measurement equipment developed on the basis of HORIBA's technologies, accumulated through the company's long history. Also we will introduce application examples of measuring NH 3 and H 2 S that are helpful for contamination monitoring in the clean room as an example utilizing such a feature, that is, it is possible to execute stable measurement of low concentration. Air Pollution Monitoring System Environmental pollutants that should be continuously monitored are carbon monoxide (CO), nitrogen oxides (NO X ), sulfur dioxide (SO 2 ), ozone (O 3 ) and suspended particle matter (SPM). HORIBA monitoring system corresponding to each of these pollutants is as follows. 30
2 Technical Reports The APMA-370 uses non-dispersive infrared absorption for measuring carbon monoxide levels. The APNA-370 uses chemiluminescence for measuring levels of nitrogen oxides. The APSA-370 uses ultraviolet fluorescence for measuring sulfur dioxide. The APOA-370 uses ultraviolet absorption for measuring ozone. For measuring hydrocarbon levels, there's the APHA-370 which uses a flame ionization method. There is also SPM analyzer in the form of the APDA-361 which uses a -ray absorption method. Figure 1 shows an external view of APXX-370 analyzer. Figure 1 External View of APXX-370 Analyzer Carbon Monoxide Measurement, Model APMA-370 The APMA-370 measures CO concentration using a nondispersive infrared absorption method that is based on the nature of CO in that it absorbs special infrared light. With this method, stabilizing the output signal is usually achieved by converting the signal from detector into alternative signal by mechanically switching the light on and off. In the APMA-370, stable and highly sensitive measurement has been realized by converting the detector signal into alternative signal using a fluid modulation method that is based on HORIBA's proprietary methodology and by combining this with an interference compensation detector. The fluid modulation method is a technology for measuring the concentration of a target component by amplifying the difference of two signals coming from a measurement cell into which the sample and a reference are alternately introduced with a constant time interval. Zero point is continuously monitored using a reference. Since if the sample does not include CO, no signal is generated, the zero point is always stable. Figure 2 shows the structure of the analyzing unit of the APMA-370 analyzer. The analyzing unit consists of an infrared light source, a measurement cell, an optical filter and a condenser microphone detector with an interference compensation detector. The detector is comprised of two parts, a detector for measuring CO (the target component of the measurement) and an interference compensation section for measuring the interference. When measuring atmospheric, a typical example of interference '' is moisture. and sample are introduced alternately into the measurement cell with an interval of 0.5 seconds through a three-way solenoid valve. Concentration of CO can be obtained by measuring the amplified modulated signal from detector. Conventionally, the sample and the reference have been switched by a rotary valve, but the method was problematic with low sensitivity due to mixing, resulting from the structural issue of using a rotary valve. Further, the lifetime of the motor was short. In recent equipment, since the performance of miniature solenoid valves has been dramatically improved with a high durability of over 100 million switching cycles now possible, a small solenoid valve has been adopted as shown in Figure 2. The reference is created by refining the sample with an oxidation catalyst. By changing CO in the sample to CO 2 using the oxidation catalyst, it becomes possible to obtain an accurate measurement by comparing and canceling out the effects of interference from other es, even if the other es have a large potential of interfering components. However, when the concentration of interference changes, since there is a time gap for the sample and the reference to reach the measurement cell, influence due to interference may occur. In order to address this problem, such as a change in interference concentration, APMA-370 measures the concentration of interference component with an interference compensation detector and corrects its influence on the output signal of the measurement detector, therefore highly accurate measurements are possible. Optical filter Light source Measurement cell Measured signal Interfered signal Figure 2 Structure of Analyzing Unit of APMA-370 Analyzer Compensation Ozone Measurement, Model APOA-370 The APOA-370 measures ozone (O 3 ) concentration using ultraviolet absorption because it is a characteristic of ozone that it absorbs ultraviolet light. This equipment uses a fluid modulation method and, like the APMA-370, it is capable of highly accurate and stable measurement. 31
3 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Figure 3 shows the structure of the analyzing unit of the APOA-370 analyzer. The analyzing unit consists of an ultraviolet light source, a measurement cell, an optical filter and a detector, aligned in a straight line. A pen type low-pressure mercury lamp is used as an ultraviolet light source. Glass is used as the measurement cell material because metal accelerates decomposition of ozone. So as to make use of the reflected light, a special cell which outer surface is coated with Cr by a physical deposition has been used. The optical filter is a bandpass filter that passes only light having wavelength of 254nm, which is in the absorption range of ozone. A silicon photodiode is used as the detector. and sample are introduced alternately into the measurement cell with an interval of 0.5 seconds through a three-way solenoid valve. Ozone concentration can be obtained by measuring the amplified modulated signal from the detector. The is applied after removing the ozone from the sample using an ozone decomposition catalyst. Decay of light intensity is unavoidable if we use an ultraviolet lamp. In general, light intensity decrease to half of the initial intensity in just 6 to 12 months. The detector measures the absolute intensity of light that passes through the measurement cell. Then, the detected signal is divided into an alternating current component and a direct current component. The alternating component is amplified and measured to obtain ozone concentration. The direct current component is measured to obtain the brightness of the ultraviolet lamp. Since the ozone concentration to be measured is very low, a difference of light intensity measured when the reference and the sample are introduced into the measured cell is very small compared to the absolute light intensity. Therefore, the DC component can be assumed to be in proportion with brightness of ultraviolet lamp. Based on this idea, it is possible to suppress an error in measured ozone concentration by compensating for the ozone concentration signal with the DC component, even if the brightness of the ultraviolet light source changes. HORIBA's system is different from conventional methods that measure the light intensity and the ozone concentration signal using independent detectors, since these signals are measured with the same detector. Also the influence of a drift of detector sensitivity can be compensated. Light source Measurement cell Optical filter Amplifier Figure 3 Structure of Analyzing Unit of APOA-370 Analyzer Nitrogen Oxide Measurement, Model APNA-370 APNA-370 measures the concentration of nitrogen oxides by observing the light emitted when excited NO 2 molecules, which come from ozone-oxidized NO, return from the excited state to the ground state. By using a three-phase fluid modulation method, it is possible to measure NO and NO 2 concentrations at the same time with high sensibility and stability. NO + O 3 NO 2 * + O 2 NO 2 * NO 2 + h Here, it should be noted that not all the NO 2 molecules coming from the oxidation are raised up to the excitation state and the ratio of the excited NO 2 to the total number of NO 2 molecules changes with temperature and pressure. Figure 4 shows the structure of the analyzing unit of the APNA-370 analyzer. The analyzing unit consists of a light emission cell that facilitates reaction between the ozone and the sample, a detector and an optical filter for selecting the wavelength to be detected. Light emitted from NO 2 has a broad bandwidth ranging from 600nm to 1000nm. In front of the detector, a bandpass filter covering wavelengths from 600nm to 1400nm is placed to eliminate the influence of light generated by reactions between hydrocarbons and ozone. Since it's impossible to directly measure NO 2 concentrations, the NO 2 has then to be reduced to NO. For reduction of NO 2, a catalyst made of Molybdenum (Mo) supported with activated carbon is used. 32
4 Technical Reports Converter Line A Line B Line C Light emission cell Amplifier Optical filter Figure 4 Structure of Analyzing Unit of APNA-370 Analyzer To path to the light emission cell consists of line-a which includes a converter, line-b through which the sample is introduced, and line-c through which the reference for comparison is introduced, are connected by two-way solenoid valves. From each of lines -A, -B and -C, the sample and the reference are introduced into the light emission cell with a constant time interval. So, the output signal from the detector takes a form arranged as the sequence A, B, C, A, B, C, A, B. C,. By synchronizing the On/Off-timing to switch the solenoid valve with this output signal from the detector, it is possible to divide the output signal into a two-signal processing system as shown below. A, _, C, A, _, C, A, _, C, A, _, C,. Signal processing system (A+C) Figure 5(1) _, B, C, _, B, C, _, B, C, _, B, C,. Signal processing system (B+C) Figure 5(2) Operation of solenoid valve Line-A Line-B Line-C Zero Time Zero Zero difference between line-a's signal and line-c's signal. On the other hand, the signal processing system (B+C) corresponding to Figure 5(2), NO concentration signal can be obtained from the difference between line-b's signal and line-c's signal. Since NO and NO X concentrations are measured using the same detector, it is possible to suppress to a minimum the difference of sensitivity for NO and NO X caused by sensitivity variations of the detector. The sample is utilized as the reference after it has reacted with ozone. Thus, it is not required to prepare special equipment for refining the reference. NO 2 concentration can be calculated by subtracting the NO concentration from the NO X concentration. Sulfur Dioxide Measurement, Model APSA-370 APSA-370 obtains SO 2 concentration with an ultraviolet fluorescence method by measuring the fluorescence intensity generated when SO 2 absorbs ultraviolet light. Since this system is equipped with an excitation light selection system composed of multiple reflection mirrors and a hydrocarbon elimination system to obtain low background noise, stable and highly accurate measurement are now possible even in the comparatively low concentration range. Figure 6 shows a structure of the analyzing unit of APSA-370 analyzer. Light source Reflection type wavelength selection filter unit Light source intensity compensation detector Fluorescence selection filter output signal Calculate NOx concentration (1) Calculate NOx concentration from signal in this period. Calculate NO concentration (2) Calculate NO concentration from signal in this period. Figure 5 Conceptual Diagram of Signal Processing In the signal processing system (A+C) corresponding to Figure 5(1), the NO X concentration signal that is the sum of NO 2 and NO concentrations can be obtained from the Figure 6 Structure of Analyzing Unit of APSA-370 Analyzer The analyzing unit consists of an ultraviolet source for exciting the SO 2, a reflection-type wavelength selection filter unit for selecting excitation light, a fluorescent chamber, a light source intensity compensation detector and a detector. A Xe flash lamp is used as an ultraviolet light source. The Xe lamp has a broad output spectrum including the wavelength range of the necessary bandwidth. For use as an excitation light source, it is required to eliminate unnecessary wavelength components. Otherwise, the background noise level 33
5 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas becomes high and the influences of temperature and noise increase. HORIBA has succeeded in fully suppressing the backlight that causes background noise, without decreasing the excitation light intensity by combining multiple reflection-type wavelength selection filters. Although this equipment does not utilize the fluid modulation method, stable measurement has been realized by using the above-described method. The sample is pre-processed within the pretreatment equipment (hydrocarbon cutter) to eliminate aromatic hydrocarbons that become the interference component in SO 2 measurement. Thereafter the sample is introduced to the fluorescent chamber. In order to minimize a response delay owing to absorption of SO 2, etc, the inner surface of the fluorescent chamber is coated with a fluorocarbon resist. Inside the fluorescent chamber, a detector(photomultiplier) measures fluorescent light generated when part of the SO 2 excited by ultraviolet light returns to the ground state. The intensity of ultraviolet light from the Xe lamp decreases gradually with time. Change of intensity of the light source is measured by a light source intensity compensation detector and the SO 2 concentration data is finally corrected using this result. temperature of CH 4 is higher than that of C 2 H 6 (there is a tendency that the greater the number of carbon atoms in the molecule, the lower the oxidization temperature). By setting the temperature of the catalyst furnace to an appropriate temperature at which CH 4 is not oxidized but C 2 H 6 is oxidized, hydrocarbons other than CH 4 are oxidized and eliminated, generating CO 2 and H 2 O. Gases are introduced into the flame ionization detector via three lines: line-a without the non-methane hydrocarbon cutter, line-b that includes the non-methane hydrocarbon cutter, and line-c that feeds the reference, alternately with a constant time interval. Ion current is measured by the flame ionization detector. The concentration of hydrocarbons ionized by the hydrogen flame can be measured as ion current that is approximately proportional to the number of carbon atoms. THC line CH 4 line Non-methane hydrocarbon cutter Amplifier Hydrocarbon Concentration Measurement, Model APHA-370 APHA-370 measures hydrocarbon concentration using a flame ionization method. This works by measuring the ion current that is generated by ionizing hydrocarbons. By combining this method with the three-phase fluid modulation method and a selective combustion method, it becomes possible to measure both of the methane and non-methane hydrocarbon concentrations with high accuracy in a single unit. Figure 7 shows the structure of analyzing unit of the APHA-370 analyzer. The sample is branched to the CH 4 line including a non-methane hydrocarbon cutter and to the THC-line that connects directly to the valve. Apart from these, reference obtained by refining the atmospheric air which is also connected to the same feeding tube. Each of these es is introduced to the detector with a constant time interval via time- controlled solenoid valves. The non-methane hydrocarbon cutter operates utilizing the characteristic that the oxidization temperatures of hydrocarbons such as CH 4, C 2 H 6, or C 3 H 8 by combustive catalyst are different to each other. The oxidization Figure 7 Structure of Analyzing Unit of APHA-370 Analyzer Features of the APXX-370 Series APXX-370 series is notable not only by its outstanding stability but also by easy-to-use functions due to interactive operation via a touch-panel screen. Users are not required any special knowledge about operation. Since automatic calibration and a self-diagnosis functions (alarm) are installed, anybody can easily execute continuous measurement just by connecting a calibration cylinder and calibration generator (SGGU-610). Measured data can be output as analog and digital signals (optional, RS-232C, TCP-IP). Internal data can be saved to compact flash memory (optional). Through these functions it is possible for users to remotely retrieve data and operate the equipment through a network connection and to download data onto compact flash memory. Applications in Industry General Description Measurement of concentrations within industry is a demanding application not only for measuring the relatively high concentrations of es emitted from fixed sources but also for monitoring clean room contamination, 34
6 Technical Reports or for measuring low concentrations of es in research laboratories, on catalysts, etc. HORIBA is applying and deploying its outstanding measurement technologies acquired through its environmental analysis businesses to industry. Recently, fine patterning and multi-layering have rapidly advanced with the increase of component density in semiconductor integrated circuits. Accompanying this trend, contamination of environmental air in clean rooms has become a major problem. In this section, technology principles for measuring ammonium (NH 3 ) and hydrogen sulfide (H 2 S) concentrations in atmospheric air will be introduced. This is achieved using HORIBA analyzers that are in widespread use for monitoring contamination in clean rooms and so on. Application to Measurement of NH 3 and H 2 S Concentrations It is not easy to measure directly low concentrations of NH 3 and H 2 S in atmospheric air. HORIBA is measuring NH 3 and H 2 S by converting to NO and SO 2, respectively, in a pretreatment stage. Measurement of NH 3 Figure 8 shows a flow diagram for NH 3 pretreatment and nitrogen oxides analyzer. The sample including NO X and NH 3 is divided into two lines. One line's flow is through both the oxidization catalyst and the reduction catalyst, and the other's flow is through only the reduction catalyst. NH 3 introduced into the oxidization catalyst is decomposed to NO 2 and NO and then introduced into the nitrogen oxides analyzer via the NO 2 reduction catalyst. In line-a, with the oxidization catalyst, NH 3 is oxidized to NO according to the following reaction equation. NO X + NH 3 2NO (Line-A) In line-b, that doesn't include the oxidization catalyst, NH 3 is not oxidized. NO X + NH 3 NO + NH 3 (Line-B) In the nitrogen oxides analyzer, is introduced from line-a, line-b and a reference line into the detector alternately with a constant time interval. NH 3 concentration can be obtained by subtracting the signal output when was introduced from line-b from the signal output when was introduced from line-a. Measurement of H 2 S Figure 9 shows the structure of the H 2 S analyzer. Also H 2 S is difficult to measure directly. So, it is oxidized using an oxidization catalyst to produce SO 2, and then the SO 2 is measured by the sulfur dioxide analyzer. sometimes includes SO 2 besides H 2 S. Since SO 2 generates an error in H 2 S measurement, it is removed by a scrubber before it is introduced into the oxidizer. For the SO 2 scrubber, sodium carbonate placed inside a hot container is used. In the oxidizer, hot Vanadium pentoxide is the catalyst. Since it has a small surface area, response time delay caused by SO 2 absorption is short. SO 2 + H 2 S Scrubber H 2 S oxidizer Pretreatment system Sulfur dioxide analyzer Figure 9 Structure of H 2 S Pretreatment Equipment and Hydrogen sulfi de Measurement Equipment Conclusion Today, measurement in the trace range has become an indispensable factor not only in the field of environmental measurement but also in other wide-ranging industrial fields. HORIBA will continue to advance its research and developmental activities as a leading manufacturer of analysis equipment and develop ever more sophisticated, more stable and more accurate measurement systems to contribute to development of industry and also to environmental preservation of the Earth. [1] Norio Kada "Gas analyzer using a dual cross modulation method and its application to atmospheric air monitoring systems", Readout, 1, (1990). Pump NH 3 + NOx NH 3 oxidizer Reduction catalyst Reduction catalyst Pretreatment system Line A Line B Nitrogen oxides analyzer Junji Kato HORIBA, Ltd. Environmental & Process Instruments Systems Division Manager Figure 8 Gas Flow Diagram in NH 3 Pretreatment System and Nitrogen Oxides Analyzer 35
FEATURE ARTICLE. The CS-100 Series High-precision Chemical Solution Monitor in the Semiconductor Cleaning Process. Takaaki Yada.
 FEATURE ARTICLE The CS-100 Series High-precision Chemical Solution Monitor in the Semiconductor Cleaning Process Takaaki Yada Cleaning Cleaning Cleaning Cleaning Wet process Wet Etching process SPM Organic
FEATURE ARTICLE The CS-100 Series High-precision Chemical Solution Monitor in the Semiconductor Cleaning Process Takaaki Yada Cleaning Cleaning Cleaning Cleaning Wet process Wet Etching process SPM Organic
Atomization. In Flame Emission
 FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
MARIYA INTERNATIONAL SCHOOL. Work sheet II. Term II. Level 8 Chemistry [Paper IV] Name: SULFUR AND AIR AND WATER
![MARIYA INTERNATIONAL SCHOOL. Work sheet II. Term II. Level 8 Chemistry [Paper IV] Name: SULFUR AND AIR AND WATER MARIYA INTERNATIONAL SCHOOL. Work sheet II. Term II. Level 8 Chemistry [Paper IV] Name: SULFUR AND AIR AND WATER](/thumbs/87/95831550.jpg) MARIYA INTERNATIONAL SCHOOL Work sheet II Term II Level 8 Chemistry [Paper IV] Name: SULFUR AND AIR AND WATER 1. Nitrogen dioxide and other oxides of nitrogen are formed in car engines. a) Explain how
MARIYA INTERNATIONAL SCHOOL Work sheet II Term II Level 8 Chemistry [Paper IV] Name: SULFUR AND AIR AND WATER 1. Nitrogen dioxide and other oxides of nitrogen are formed in car engines. a) Explain how
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
Catalytic bead sensors are used primarily to detect
 Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
10/2/2008. hc λ. νλ =c. proportional to frequency. Energy is inversely proportional to wavelength And is directly proportional to wavenumber
 CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
MODULE 4.3 Atmospheric analysis of particulates
 MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy
 Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Gaseous CEMS technology
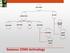 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
THE MEASUREMENT OF SOLAR ULTRAVIOLET SPECTRAL IRRADIANCE PROBLEMS & SOLUTIONS
 THE MEASUREMENT OF SOLAR ULTRAVIOLET SPECTRAL IRRADIANCE PROBLEMS & SOLUTIONS INTRODUCTION In recent years, researchers involved in many unrelated scientific disciplines have acquired an interest in accurately
THE MEASUREMENT OF SOLAR ULTRAVIOLET SPECTRAL IRRADIANCE PROBLEMS & SOLUTIONS INTRODUCTION In recent years, researchers involved in many unrelated scientific disciplines have acquired an interest in accurately
Ch. 9 Atomic Absorption & Atomic Fluorescence Spectrometry
 Ch. 9 Atomic Absorption & Atomic Fluorescence Spectrometry 9.1 9A. Atomization Most fundamental for both techniques. Typical types 1. flame - burner type 2. Electrothermal graphite furnace 3. Specialized
Ch. 9 Atomic Absorption & Atomic Fluorescence Spectrometry 9.1 9A. Atomization Most fundamental for both techniques. Typical types 1. flame - burner type 2. Electrothermal graphite furnace 3. Specialized
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System
 Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Petrochemical. Transformer Oil Gas Analysis - TOGA
 Petrochemical Transformer Oil Gas Analysis - TOGA www.dps-instruments.com The DPS TOGA GC Systems are designed to analyze oil from electrical insulation materials that may have decomposed under thermal,
Petrochemical Transformer Oil Gas Analysis - TOGA www.dps-instruments.com The DPS TOGA GC Systems are designed to analyze oil from electrical insulation materials that may have decomposed under thermal,
Far UV Absorbance Detector
 Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
1 (a) Describe a chemical test which shows the presence of water. Describe how water is treated before it is supplied to homes and industry.
 1 (a) Describe a chemical test which shows the presence of water. test... colour change if water is present...... [3] (b) How could you show that a sample of water is pure?...[1] (c) Describe how water
1 (a) Describe a chemical test which shows the presence of water. test... colour change if water is present...... [3] (b) How could you show that a sample of water is pure?...[1] (c) Describe how water
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
Atomic Absorption Spectrophotometry. Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon
 Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Applications of Laser Spectroscopy to Highly Sensitive Analyses
 G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Applications of Laser Spectroscopy to Highly Sensitive Analyses Cavity Ring-Down Spectroscopy
G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Applications of Laser Spectroscopy to Highly Sensitive Analyses Cavity Ring-Down Spectroscopy
Ch 9 Stoichiometry Practice Test
 Ch 9 Stoichiometry Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A balanced chemical equation allows one to determine the a. mole ratio
Ch 9 Stoichiometry Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A balanced chemical equation allows one to determine the a. mole ratio
Chemistry Instrumental Analysis Lecture 18. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 18 Instrumentation Radiation sources Hollow cathode lamp Most common source Consist of W anode and a cathode sealed in a glass tube filled with Ne or Ar. Hollow
Chemistry 4631 Instrumental Analysis Lecture 18 Instrumentation Radiation sources Hollow cathode lamp Most common source Consist of W anode and a cathode sealed in a glass tube filled with Ne or Ar. Hollow
Industrial In-line and Multi Component Monitor Using Absorption Spectroscopy and Its Application
 FFeature Article Article Industrial In-line and Multi Component Monitor Using Absorption Spectroscopy and Its Application Yoko NAKAI HORIBA s CS-Series chemical concentration monitors that use ultraviolet
FFeature Article Article Industrial In-line and Multi Component Monitor Using Absorption Spectroscopy and Its Application Yoko NAKAI HORIBA s CS-Series chemical concentration monitors that use ultraviolet
OXEA - Online Elemental Analyzer
 02 25 08 OXEA - Online Elemental Analyzer OXEA (Online X-ray Elemental Analyzer) is based on the X-ray fluorescence technology (XRF) which is well known in the laboratory field. With the aid of a patented
02 25 08 OXEA - Online Elemental Analyzer OXEA (Online X-ray Elemental Analyzer) is based on the X-ray fluorescence technology (XRF) which is well known in the laboratory field. With the aid of a patented
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Environmental chemistry
 Page 1 of 5 Environmental chemistry Almost every pollution problem that we face has a chemical basis. Even the qualitative descriptions of such problems as the greenhouse effect, ozone depletion, toxic
Page 1 of 5 Environmental chemistry Almost every pollution problem that we face has a chemical basis. Even the qualitative descriptions of such problems as the greenhouse effect, ozone depletion, toxic
LABORATORY WRITE-UP MICHELSON INTERFEROMETER LAB AUTHOR S NAME GOES HERE STUDENT NUMBER:
 LABORATORY WRITE-UP MICHELSON INTERFEROMETER LAB AUTHOR S NAME GOES HERE STUDENT NUMBER: 111-22-3333 MICHELSON INTERFEROMETER 1. PURPOSE The purpose of this experiment is to give some practice in using
LABORATORY WRITE-UP MICHELSON INTERFEROMETER LAB AUTHOR S NAME GOES HERE STUDENT NUMBER: 111-22-3333 MICHELSON INTERFEROMETER 1. PURPOSE The purpose of this experiment is to give some practice in using
OBSERVATION OF THE HYDROGEN DISPERSION BY USING RAMAN SCATTERING MESURMENT AND INCREASE OF MEASURABLE DISTANCE
 OBSERVATION OF THE HYDROGEN DISPERSION BY USING RAMAN SCATTERING MESURMENT AND INCREASE OF MEASURABLE DISTANCE Yuta Segawa 1, Masahiro Inoue 2, Akihiro Nakamoto 3, Satoshi Umehara 3 1 Natural Resource
OBSERVATION OF THE HYDROGEN DISPERSION BY USING RAMAN SCATTERING MESURMENT AND INCREASE OF MEASURABLE DISTANCE Yuta Segawa 1, Masahiro Inoue 2, Akihiro Nakamoto 3, Satoshi Umehara 3 1 Natural Resource
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
Speed of Light in Air
 Speed of Light in Air Electromagnetic waves represent energy in the form of oscillating electric and magnetic fields which propagate through vacuum with a speed c = 2.9979246x10 8 m/s. Electromagnetic
Speed of Light in Air Electromagnetic waves represent energy in the form of oscillating electric and magnetic fields which propagate through vacuum with a speed c = 2.9979246x10 8 m/s. Electromagnetic
atomic absorption spectroscopy general can be portable and used in-situ preserves sample simpler and less expensive
 Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Reference pg and in Textbook
 Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
(g) 2NH 3. (g) ΔH = 92 kj mol 1
 1 The uses of catalysts have great economic and environmental importance For example, catalysts are used in ammonia production and in catalytic converters (a) Nitrogen and hydrogen react together in the
1 The uses of catalysts have great economic and environmental importance For example, catalysts are used in ammonia production and in catalytic converters (a) Nitrogen and hydrogen react together in the
The Low-Temperature Evaporative Light-Scattering Detector (LT-ELSD)
 The Low-Temperature Evaporative Light-Scattering Detector (LT-ELSD) Basically all compounds which are less volatile than the mobile phase can be detected. Detection is based on a Universal property of
The Low-Temperature Evaporative Light-Scattering Detector (LT-ELSD) Basically all compounds which are less volatile than the mobile phase can be detected. Detection is based on a Universal property of
Cantilever enhanced tunable diode laser photoacoustic spectroscopy in gas purity measurement case study: acetylene in ethylene
 Cantilever enhanced tunable diode laser photoacoustic spectroscopy in gas purity measurement case study: acetylene in ethylene Juho Uotila, Jussi Raittila, Ismo Kauppinen ¹Gasera Ltd., Tykistökatu 4, 20520
Cantilever enhanced tunable diode laser photoacoustic spectroscopy in gas purity measurement case study: acetylene in ethylene Juho Uotila, Jussi Raittila, Ismo Kauppinen ¹Gasera Ltd., Tykistökatu 4, 20520
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
Innovative. Intuitive. Concentration (ppb) R = Versatile. Hydra II C Mercury Analyzer
 Innovative 0.01 0.1 1 10 Intuitive Concentration (ppb) R = 0.99999 Versatile Hydra II C Mercury Analyzer At Teledyne Leeman Labs; atomic spectroscopy is our business our only business. We are industry
Innovative 0.01 0.1 1 10 Intuitive Concentration (ppb) R = 0.99999 Versatile Hydra II C Mercury Analyzer At Teledyne Leeman Labs; atomic spectroscopy is our business our only business. We are industry
Module: 7. Lecture: 36
 Module: 7 Lecture: 36 DIMETHYL FORMAMIDE INTRODUCTION Dimethylformamide is an organic compound and denotes as DMF. The name is derived from the fact that it is a derivative of formamide, the amide of formic
Module: 7 Lecture: 36 DIMETHYL FORMAMIDE INTRODUCTION Dimethylformamide is an organic compound and denotes as DMF. The name is derived from the fact that it is a derivative of formamide, the amide of formic
DAY LABORATORY EXERCISE: SPECTROSCOPY
 AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Determining Chemical Formulas
 Determining Chemical Formulas Scientists estimate that new chemical compounds are being discovered at a rate of two every minute, which is about one million new compounds every year! Many compounds, such
Determining Chemical Formulas Scientists estimate that new chemical compounds are being discovered at a rate of two every minute, which is about one million new compounds every year! Many compounds, such
AT 350 EXAM #1 February 21, 2008
 This exam covers Ahrens Chapters 1 and 2, plus related lecture notes Write the letter of the choice that best completes the statement or answers the question. b_ 1. The Earth s atmosphere is currently
This exam covers Ahrens Chapters 1 and 2, plus related lecture notes Write the letter of the choice that best completes the statement or answers the question. b_ 1. The Earth s atmosphere is currently
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS)
 New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
Module 2. Measurement Systems. Version 2 EE IIT, Kharagpur 1
 Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
 Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Compact Knowledge: Absorbance Spectrophotometry. Flexible. Reliable. Personal.
 L A B O R A T O R Y C O M P E T E N C E Compact Knowledge: Absorbance Spectrophotometry Flexible. Reliable. Personal. The interaction of light with molecules is an essential and well accepted technique
L A B O R A T O R Y C O M P E T E N C E Compact Knowledge: Absorbance Spectrophotometry Flexible. Reliable. Personal. The interaction of light with molecules is an essential and well accepted technique
The RX-6000 Sulfur in Oil Analyzer For the analysis of Sulfur in Oil and other elements from S to U
 Oxford House, 20 Oxford St Newbury, Berkshire. RG14 1JB UK. sales@spectrolab.eu sales@spectrolab.co.uk Offices in UK, EU, Greece, Dubai The RX-6000 Sulfur in Oil Analyzer For the analysis of Sulfur in
Oxford House, 20 Oxford St Newbury, Berkshire. RG14 1JB UK. sales@spectrolab.eu sales@spectrolab.co.uk Offices in UK, EU, Greece, Dubai The RX-6000 Sulfur in Oil Analyzer For the analysis of Sulfur in
Physics of capnography
 Strona 1 z 7 Physics of capnography Bhavani Shankar Kodali MD There are five Physical methods 1. Infra-red Spectrography 2. Molecular Correlation Spectrography 3. Raman Spectrography 4. Mass Spectrography
Strona 1 z 7 Physics of capnography Bhavani Shankar Kodali MD There are five Physical methods 1. Infra-red Spectrography 2. Molecular Correlation Spectrography 3. Raman Spectrography 4. Mass Spectrography
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Questions, Myths and Misconceptions about Using Photoionization Detectors
 Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Importance of in situ Monitoring in MOCVD Process and Future Prospects
 G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Importance of in situ Monitoring in MOCVD Process and Future Prospects Hiroshi Funakubo Tokyo
G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Importance of in situ Monitoring in MOCVD Process and Future Prospects Hiroshi Funakubo Tokyo
Light Emission. Today s Topics. Excitation/De-Excitation 10/26/2008. Excitation Emission Spectra Incandescence
 Light Emission Excitation Emission Spectra Incandescence Absorption Spectra Today s Topics Excitation/De-Excitation Electron raised to higher energy level Electron emits photon when it drops back down
Light Emission Excitation Emission Spectra Incandescence Absorption Spectra Today s Topics Excitation/De-Excitation Electron raised to higher energy level Electron emits photon when it drops back down
Module: 7. Lecture: 36
 Module: 7 Lecture: 36 DIMETHYL FORMAMIDE INTRODUCTION Dimethylformamide is an organic compound and denotes as DMF. The name is derived from the fact that it is a derivative of formamide, the amide of formic
Module: 7 Lecture: 36 DIMETHYL FORMAMIDE INTRODUCTION Dimethylformamide is an organic compound and denotes as DMF. The name is derived from the fact that it is a derivative of formamide, the amide of formic
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications
 Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Matter mass space atoms solid, a liquid, a gas, or plasm elements compounds mixtures atoms Compounds chemically combined Mixtures not chemically
 SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
Experiment #9. Atomic Emission Spectroscopy
 Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1
 ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
Analysis of Cadmium (Cd) in Plastic Using X-ray Fluorescence Spectroscopy
 Analysis of Cadmium (Cd) in Plastic Using X-ray Fluorescence Spectroscopy Hiroshi Onodera Application & Research Center, JEOL Ltd. Introduction um, PBB and PBDE) are subject to usage restrictions in Europe.
Analysis of Cadmium (Cd) in Plastic Using X-ray Fluorescence Spectroscopy Hiroshi Onodera Application & Research Center, JEOL Ltd. Introduction um, PBB and PBDE) are subject to usage restrictions in Europe.
TECHNICAL INFORMATION. Quantum Dot
 Quantum Dot Quantum Dot is the nano meter sized semiconductor crystal with specific optical properties originates from the phenomenon which can be explained by the quantum chemistry and quantum mechanics.
Quantum Dot Quantum Dot is the nano meter sized semiconductor crystal with specific optical properties originates from the phenomenon which can be explained by the quantum chemistry and quantum mechanics.
THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION
 JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
high temp ( K) Chapter 20: Atomic Spectroscopy
 high temp (2000-6000K) Chapter 20: Atomic Spectroscopy 20-1. An Overview Most compounds Atoms in gas phase high temp (2000-6000K) (AES) (AAS) (AFS) sample Mass-to-charge (ICP-MS) Atomic Absorption experiment
high temp (2000-6000K) Chapter 20: Atomic Spectroscopy 20-1. An Overview Most compounds Atoms in gas phase high temp (2000-6000K) (AES) (AAS) (AFS) sample Mass-to-charge (ICP-MS) Atomic Absorption experiment
R O Y G B V. Spin States. Outer Shell Electrons. Molecular Rotations. Inner Shell Electrons. Molecular Vibrations. Nuclear Transitions
 Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Balancing chemical reaction equations (stoichiometry)
 Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
high energy state for the electron in the atom low energy state for the electron in the atom
 Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
CH0302 Process Instrumentation. Lecture 9 Composition Analysis
 CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Beamline practice at BL01B1 (XAFS) In-situ XAFS measurement of catalyst samples
 Beamline practice at BL01B1 (XAFS) In-situ XAFS measurement of catalyst samples ver. 2015/09/18 T. Ina, K. Kato, T. Uruga (JASRI), P. Fons (AIST/JASRI) 1. Introduction The bending magnet beamline, BL01B1,
Beamline practice at BL01B1 (XAFS) In-situ XAFS measurement of catalyst samples ver. 2015/09/18 T. Ina, K. Kato, T. Uruga (JASRI), P. Fons (AIST/JASRI) 1. Introduction The bending magnet beamline, BL01B1,
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine?
 Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
APAS Laboratory { PAGE } Spectroscopy SPECTROSCOPY
 SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
3.2 Alkanes. Refining crude oil. N Goalby chemrevise.org 40 C 110 C 180 C. 250 C fuel oil 300 C 340 C. Fractional Distillation: Industrially
 3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
Cadmium Reduction Method Method to 10.0 mg/l NO 3 N (MR, spectrophotometers) 0.2 to 5.0 mg/l NO 3 N (MR, colorimeters)
 Nitrate, MR DOC316.53.01069 Cadmium Reduction Method Method 8171 0.1 to 10.0 mg/l NO 3 N (MR, spectrophotometers) 0.2 to 5.0 mg/l NO 3 N (MR, colorimeters) Scope and application: For water, wastewater
Nitrate, MR DOC316.53.01069 Cadmium Reduction Method Method 8171 0.1 to 10.0 mg/l NO 3 N (MR, spectrophotometers) 0.2 to 5.0 mg/l NO 3 N (MR, colorimeters) Scope and application: For water, wastewater
a. An emission line as close as possible to the analyte resonance line
 Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
3. Chemical industry. Because of their modular design, the instruments in the TOC-L series can be equipped for any possible measurement
 3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
Experiment Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado
 Experiment 10 1 Introduction Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado Some radioactive isotopes formed billions of years ago have half- lives so long
Experiment 10 1 Introduction Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado Some radioactive isotopes formed billions of years ago have half- lives so long
OH, is an important feedstock for the chemical industry.
 1 Methanol, CH 3 OH, is an important feedstock for the chemical industry. In the manufacture of methanol, carbon dioxide and hydrogen are reacted together in the reversible reaction shown below. CO 2 (g)
1 Methanol, CH 3 OH, is an important feedstock for the chemical industry. In the manufacture of methanol, carbon dioxide and hydrogen are reacted together in the reversible reaction shown below. CO 2 (g)
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Kentaro INOUE. Introduction. Measurement principle (membrane polarographic method)
 FFeature Article Article Performance of the Dissolved Oxygen Monitor Used in the Semiconductor Wet Process; Low Concentration Monitoring, High Temperature, Small Amount of Sampling Volume, Chemical Resistance
FFeature Article Article Performance of the Dissolved Oxygen Monitor Used in the Semiconductor Wet Process; Low Concentration Monitoring, High Temperature, Small Amount of Sampling Volume, Chemical Resistance
Multi-element process analyzer
 Multi-element process analyzer Elemental analysis by X-ray fluorescence Compact multi-element process analyzer for liquid streams or f ixed position web applications Featuring advanced third generation
Multi-element process analyzer Elemental analysis by X-ray fluorescence Compact multi-element process analyzer for liquid streams or f ixed position web applications Featuring advanced third generation
2B Technologies, Inc. An InDevR Company
 2B Technologies, Inc. An InDevR Company Technical Note No. 40 UV-Absorbing Interferences in Ozone Monitors Date: 22 April 2015 Author: John Birks Background Ozone measurements by absorbance of the 253.7-nm
2B Technologies, Inc. An InDevR Company Technical Note No. 40 UV-Absorbing Interferences in Ozone Monitors Date: 22 April 2015 Author: John Birks Background Ozone measurements by absorbance of the 253.7-nm
Application Note S/SL
 Analysis of Ultra low Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatography and Chemiluminescence according to ASTM D Ultra low detection limits Excellent Sensitivity, Repeatability &
Analysis of Ultra low Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatography and Chemiluminescence according to ASTM D Ultra low detection limits Excellent Sensitivity, Repeatability &
EMISSION SPECTROSCOPY
 IFM The Department of Physics, Chemistry and Biology LAB 57 EMISSION SPECTROSCOPY NAME PERSONAL NUMBER DATE APPROVED I. OBJECTIVES - Understand the principle of atomic emission spectra. - Know how to acquire
IFM The Department of Physics, Chemistry and Biology LAB 57 EMISSION SPECTROSCOPY NAME PERSONAL NUMBER DATE APPROVED I. OBJECTIVES - Understand the principle of atomic emission spectra. - Know how to acquire
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
13. CARBON AND CARBONATE ANALYSES, LEG 9
 . CARBON AND CARBONATE ANALYSES, LEG R. E. Boyce and G. W. Bode, Scripps Institute of Oceanography The purpose of these analyses is to determine the amount of acid-soluble and insoluble carbon in the sediment
. CARBON AND CARBONATE ANALYSES, LEG R. E. Boyce and G. W. Bode, Scripps Institute of Oceanography The purpose of these analyses is to determine the amount of acid-soluble and insoluble carbon in the sediment
High Resolution Optical Spectroscopy
 PHYS 3719 High Resolution Optical Spectroscopy Introduction This experiment will allow you to learn a specific optical technique with applications over a wide variety of phenomena. You will use a commercial
PHYS 3719 High Resolution Optical Spectroscopy Introduction This experiment will allow you to learn a specific optical technique with applications over a wide variety of phenomena. You will use a commercial
SC400 Colorimeter. Silica. Photometer-System. Instruction Manual. SiO 2. Bedienungsanleitung Seite Instruction Manual Page 19 33
 Photometer-System SC400 Colorimeter SiO 2 Silica DE GB FR IT ES Bedienungsanleitung Seite 3 17 Instruction Manual Page 19 33 d'emploi Page 35 49 Instruction Manual Istruzioni d'uso Pagina 51 65 Instrucciones
Photometer-System SC400 Colorimeter SiO 2 Silica DE GB FR IT ES Bedienungsanleitung Seite 3 17 Instruction Manual Page 19 33 d'emploi Page 35 49 Instruction Manual Istruzioni d'uso Pagina 51 65 Instrucciones
M06/4/CHEMI/HP3/ENG/TZ0/XX CHEMISTRY HIGHER LEVEL PAPER 3. Candidate session number 0 0. Friday 19 May 2006 (morning) 1 hour 15 minutes
 IB CHEMISTRY HIGHER LEVEL PAPER 3 DIPLOMA PROGRAMME PROGRAMME DU DIPLÔME DU BI PROGRAMA DEL DIPLOMA DEL BI Friday 19 May 2006 (morning) 1 hour 15 minutes M06/4/CHEMI/HP3/ENG/TZ0/XX 22066103 Candidate session
IB CHEMISTRY HIGHER LEVEL PAPER 3 DIPLOMA PROGRAMME PROGRAMME DU DIPLÔME DU BI PROGRAMA DEL DIPLOMA DEL BI Friday 19 May 2006 (morning) 1 hour 15 minutes M06/4/CHEMI/HP3/ENG/TZ0/XX 22066103 Candidate session
Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring
 Physical Sciences Inc. VG15-012 Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring January 26, 2015 Krishnan R. Parameswaran, Clinton J. Smith, Kristin L. Galbally-Kinney, William J. Kessler
Physical Sciences Inc. VG15-012 Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring January 26, 2015 Krishnan R. Parameswaran, Clinton J. Smith, Kristin L. Galbally-Kinney, William J. Kessler
Fast Determination of Impurities in Propane- Propylene Streams Using a Pulsed Flame Photometric Detector (PFPD) and a New Capillary.
 Application Note 36720111 Fast Determination of Impurities in Propane- Propylene Streams Using a Pulsed Flame Photometric Detector (PFPD) and a New Capillary PLOT Column Keywords Pulsed Flame Photometric
Application Note 36720111 Fast Determination of Impurities in Propane- Propylene Streams Using a Pulsed Flame Photometric Detector (PFPD) and a New Capillary PLOT Column Keywords Pulsed Flame Photometric
Practical 1P4 Energy Levels and Band Gaps
 Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
